Mass Transfer in Multiphasic Gas/Liquid/Liquid Systems. KLa Determination Using the Effectiveness-Number of Transfer Unit Method
Abstract
:1. Introduction
2. ε-NTU Method for KLa Determination
2.1. Single Gas-Liquid Countercurrent Absorber
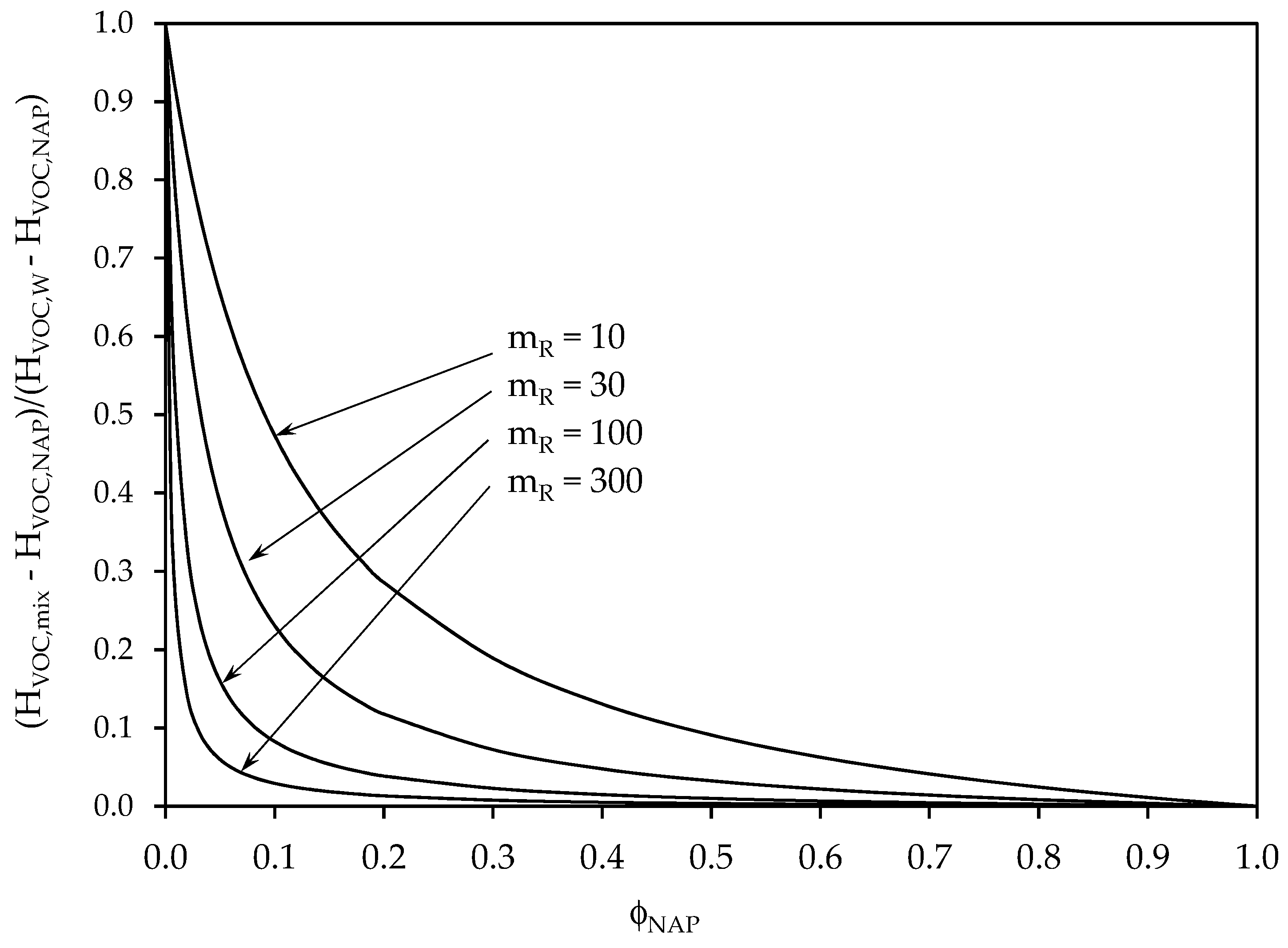
2.2. Gas/Liquid/Liquid Systems. Use of the “Equivalent Absorption Capacity” Concept
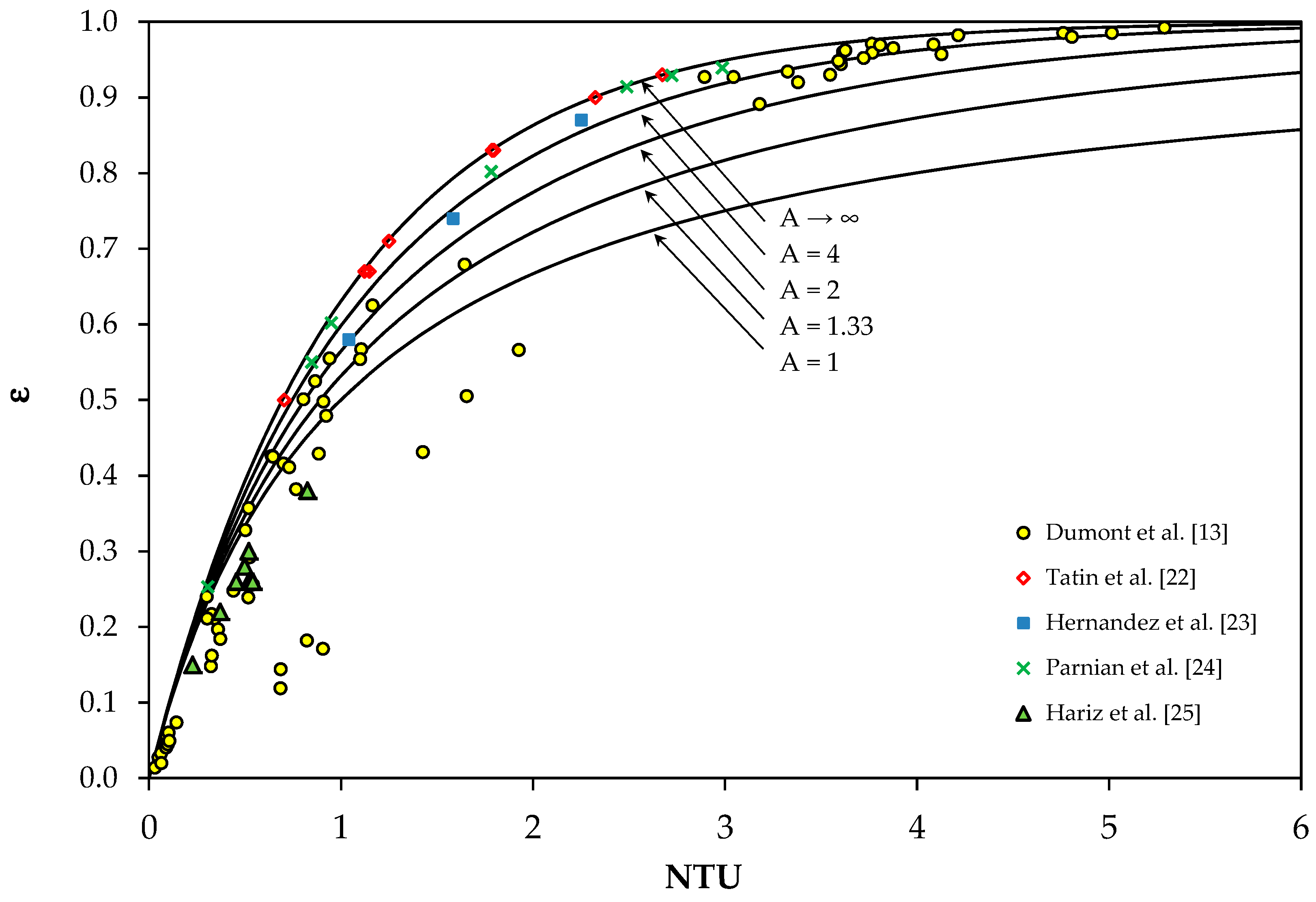
3. Validation of the ε-NTU Method for Countercurrent Scrubbers (CS)
3.1. Data from Tatin et al.
3.2. Data from Hariz et al.
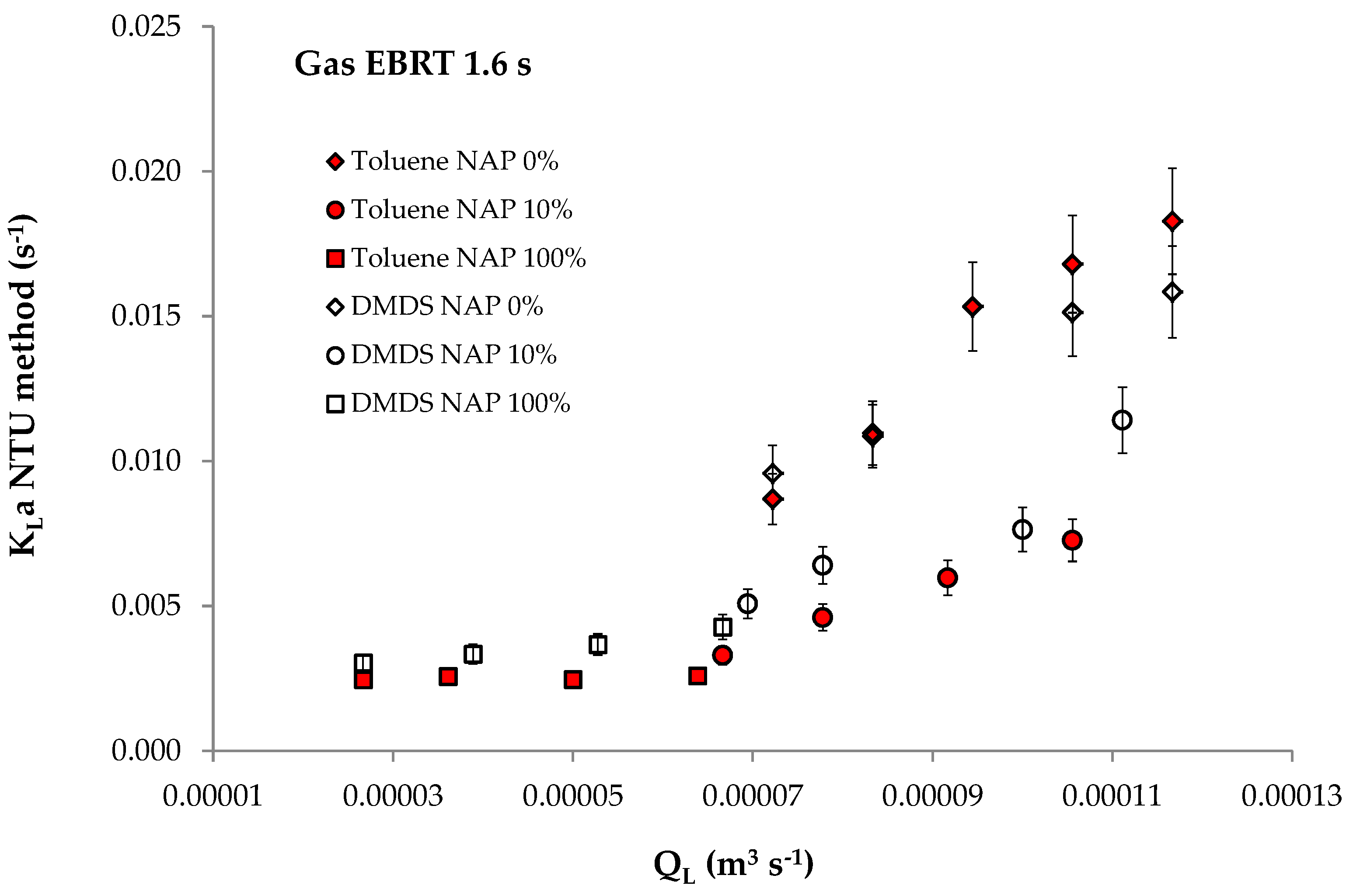
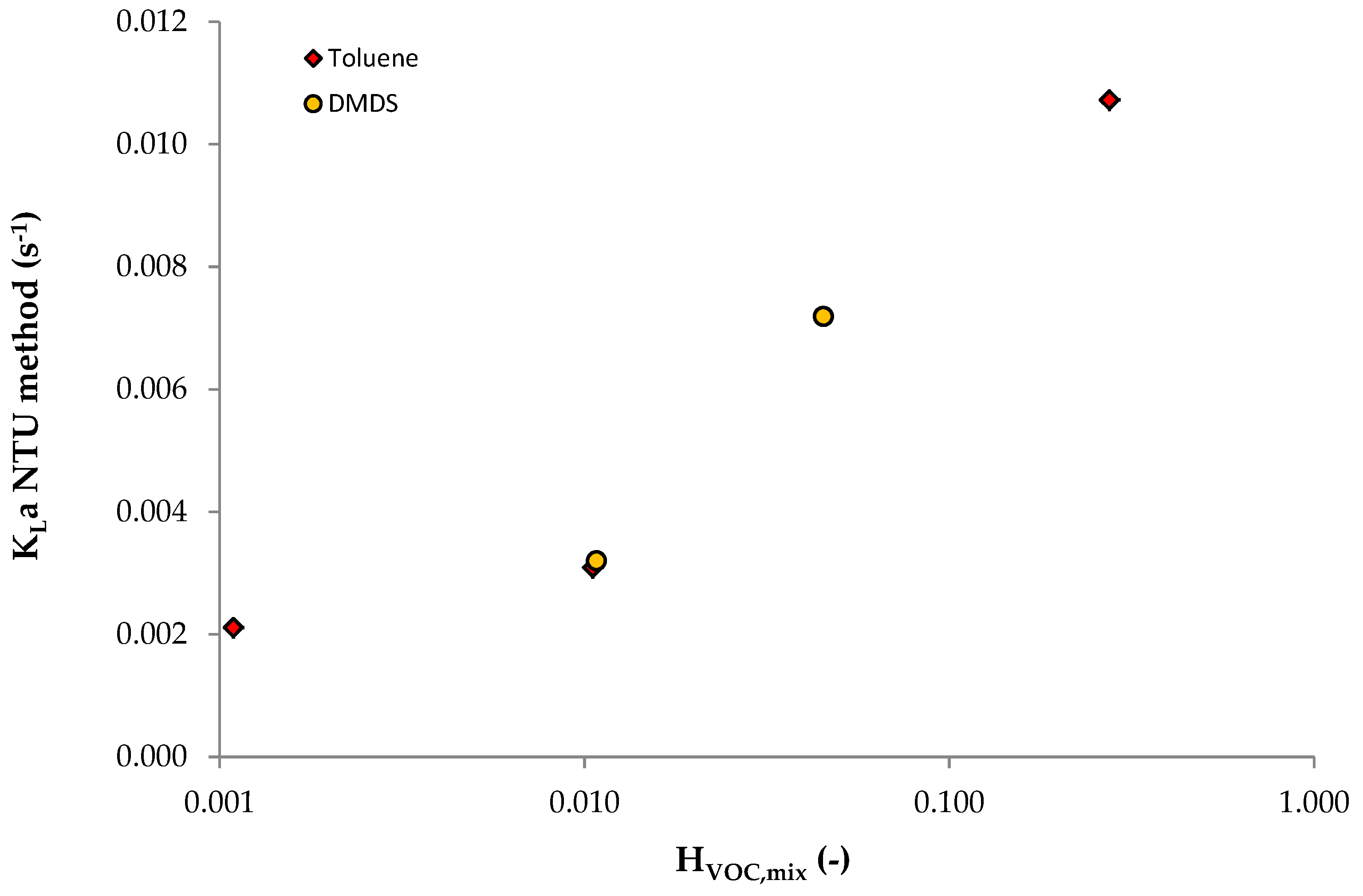
3.3. Data from Dumont et al.
3.4. Data from Hernandez et al. and from Parnian et al.
3.5. Conclusions
4. Validation of the ε-NTU Method for Stirred Tank Reactor (STR)
5. Conclusions
Funding
Conflicts of Interest
Nomenclature
| A | absorption factor |
| CG | gaseous concentration (mol/m−3) |
| H | Henry coefficient |
| KLa | overall mass transfer coefficient (s−1) |
| mR | solubility ratio |
| NTU | Number of Transfer Unit |
| QG | gas flow rate (m3/s) |
| QL | liquid flow rate (m3/s) |
| V | absorber volume (m3) |
| Greek Letters | |
| ε | absorber effectiveness |
| φ | NAP volume fraction |
| Subscripts | |
| in | inlet |
| L | liquid |
| mix | water/NAP mixture |
| NAP | Non Aqueous Phase |
| out | outlet |
| VOC | Volatile Organic Compound |
| W | water |
References
- Dumont, E.; Delmas, H. Mass transfer enhancement of gas absorption in oil-in-water systems: A review. Chem. Eng. Process. Process Intensif. 2003, 42, 419–438. [Google Scholar] [CrossRef]
- Dumont, E.; Darracq, G.; Couvert, A.; Couriol, C.; Amrane, A.; Thomas, D.; Andrès, Y.; Le Cloirec, P. VOC absorption in a countercurrent packed-bed column using water/silicone oil mixtures: Influence of silicone oil volume fraction. Chem. Eng. J. 2011, 168, 241–248. [Google Scholar] [CrossRef]
- Dumont, E.; Darracq, G.; Couvert, A.; Couriol, C.; Amrane, A.; Thomas, D.; Andrès, Y.; Le Cloirec, P. Determination of partition coefficients of three volatile organic compounds (dimethylsulphide, dimethyldisulphide and toluene) in water/silicone oil mixtures. Chem. Eng. J. 2010, 162, 927–934. [Google Scholar] [CrossRef]
- Fair, J.; Steinmeyer, D.; Penney, W.; Corcker, B. Gas absorption and gas-liquid system design. In Perry’s Chemical Engineers’ Handbook, 8th ed.; Perry, R.H., Green, D.W., Eds.; McGraw-Hill: New York, NY, USA, 2008; pp. 14-1–14-98. [Google Scholar]
- Mackowiak, J. Fluid Dynamics of Packed Columns: Principles of the Fluid Dynamic Design of Columns for Gas/Liquid and Liquid/Liquid Systems; Chemische Technik/Verfahrenstechnik; Springer-Verlag: Berlin/Heidelberg, Germany, 2010; ISBN 978-3-540-88780-5. [Google Scholar]
- Sinnott, R. Coulson and Richardson’s Chemical Engineering Volume 6 (Design); Pergamon Press: Oxford, UK, 1994; Volume 6, ISBN 0-08-041865-1. [Google Scholar]
- Dumont, E. VOCs mass transfer in countercurrent absorbers using viscous solvents. KLa determination using the Effectiveness-NTU Method. J. Ind. Eng. Chem. 2018. submitted. [Google Scholar]
- Garcia-Ochoa, F.; Gomez, E. Bioreactor scale-up and oxygen transfer rate in microbial processes: An overview. Biotechnol. Adv. 2009, 27, 153–176. [Google Scholar] [CrossRef] [PubMed]
- Linek, V.; Vacek, V. Chemical engineering use of catalyzed sulfite oxidation kinetics for the determination of mass transfer characteristics of gas—Liquid contactors. Chem. Eng. Sci. 1981, 36, 1747–1768. [Google Scholar] [CrossRef]
- Dumont, E.; Darracq, G.; Couvert, A.; Couriol, C.; Amrane, A.; Thomas, D.; Andrès, Y.; Le Cloirec, P. Hydrophobic VOC absorption in two-phase partitioning bioreactors; influence of silicone oil volume fraction on absorber diameter. Chem. Eng. Sci. 2012, 71, 146–152. [Google Scholar] [CrossRef] [Green Version]
- Dumont, E.; Couvert, A.; Amrane, A.; Couriol, C.; Darracq, G.; Le Cloirec, P. Equivalent Absorption Capacity (EAC) concept applied to the absorption of hydrophobic VOCs in a water/PDMS mixture. Chem. Eng. J. 2016, 287, 205–216. [Google Scholar] [CrossRef] [Green Version]
- Dumont, E.; Darracq, G.; Couvert, A.; Couriol, C.; Amrane, A.; Thomas, D.; Andrès, Y.; Le Cloirec, P. Volumetric mass transfer coefficients characterising VOC absorption in water/silicone oil mixtures. Chem. Eng. J. 2013, 221, 308–314. [Google Scholar] [CrossRef] [Green Version]
- Clarke, K.G.; Correia, L.D.C. Oxygen transfer in hydrocarbon–aqueous dispersions and its applicability to alkane bioprocesses: A review. Biochem. Eng. J. 2008, 39, 405–429. [Google Scholar] [CrossRef]
- Cesário, M.T.; Beverloo, W.A.; Tramper, J.; Beeftink, H.H. Enhancement of gas-liquid mass transfer rate of apolar pollutants in the biological waste gas treatment by a dispersed organic solvent. Enzyme Microb. Technol. 1997, 21, 578–588. [Google Scholar] [CrossRef]
- Sander, R. Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmos. Chem. Phys. 2015, 15, 4399–4981. [Google Scholar] [CrossRef]
- Staudinger, J.; Roberts, P.V. A critical compilation of Henry’s law constant temperature dependence relations for organic compounds in dilute aqueous solutions. Chemosphere 2001, 44, 561–576. [Google Scholar] [CrossRef]
- Biard, P.-F.; Coudon, A.; Couvert, A.; Giraudet, S. A simple and timesaving method for the mass-transfer assessment of solvents used in physical absorption. Chem. Eng. J. 2016, 290, 302–311. [Google Scholar] [CrossRef] [Green Version]
- Bruneel, J.; Walgraeve, C.; Van Huffel, K.; Van Langenhove, H. Determination of the gas-to-liquid partitioning coefficients using a new dynamic absorption method (DynAb method). Chem. Eng. J. 2016, 283, 544–552. [Google Scholar] [CrossRef]
- Patel, M.J.; Popat, S.C.; Deshusses, M.A. Determination and correlation of the partition coefficients of 48 volatile organic and environmentally relevant compounds between air and silicone oil. Chem. Eng. J. 2017, 310, 72–78. [Google Scholar] [CrossRef]
- Tatin, R.; Moura, L.; Dietrich, N.; Baig, S.; Hébrard, G. Physical absorption of volatile organic compounds by spraying emulsion in a spray tower: Experiments and modelling. Chem. Eng. Res. Des. 2015, 104, 409–415. [Google Scholar] [CrossRef]
- Biard, P.-F.; Couvert, A.; Giraudet, S. Volatile organic compounds absorption in packed column: Theoretical assessment of water, DEHA and PDMS 50 as absorbents. J. Ind. Eng. Chem. 2018, 59, 70–78. [Google Scholar] [CrossRef]
- Hernández, M.; Quijano, G.; Muñoz, R.; Bordel, S. Modeling of VOC mass transfer in two-liquid phase stirred tank, biotrickling filter and airlift reactors. Chem. Eng. J. 2011, 172, 961–969. [Google Scholar] [CrossRef]
- Parnian, P.; Zamir, S.M.; Shojaosadati, S.A. Styrene vapor mass transfer in a biotrickling filter: Effects of silicone oil volume fraction, gas-to-liquid flow ratio, and operating temperature. Chem. Eng. J. 2016, 284, 926–933. [Google Scholar] [CrossRef]
- Hariz, R.; del Rio Sanz, J.I.; Mercier, C.; Valentin, R.; Dietrich, N.; Mouloungui, Z.; Hébrard, G. Absorption of toluene by vegetable oil–water emulsion in scrubbing tower: Experiments and modeling. Chem. Eng. Sci. 2017, 157, 264–271. [Google Scholar] [CrossRef]
- Bourgois, D.; Vanderschuren, J.; Thomas, D. Study of mass transfer of VOCs into viscous solvents in a pilot-scale cables-bundle scrubber. Chem. Eng. J. 2009, 145, 446–452. [Google Scholar] [CrossRef]
- Dumont, E.; Andrès, Y.; Le Cloirec, P. Mass transfer coefficients of styrene into water/silicone oil mixtures: New interpretation using the “equivalent absorption capacity” concept. Chem. Eng. J. 2014, 237, 236–241. [Google Scholar] [CrossRef] [Green Version]
- Hollis, P.G.; Clarke, K.G. A systematic quantification and correlation of oxygen transfer coefficients and interfacial area in simulated model hydrocarbon-based bioprocesses in stirred tank reactors: Oxygen transfer coefficients and area in bioprocesses. J. Chem. Technol. Biotechnol. 2016, 91, 2720–2728. [Google Scholar] [CrossRef]
- Bordel, S.; Hernandez, M.; Villaverde, S.; Muñoz, R. Modelling gas–liquid VOCs transport in two-liquid phase partitioning bioreactors. Int. J. Heat Mass Transf. 2010, 53, 1139–1145. [Google Scholar] [CrossRef]
- Dumont, E.; Andrès, Y. Styrene absorption in water/silicone oil mixtures. Chem. Eng. J. 2012, 200–202, 81–90. [Google Scholar] [CrossRef]
| COVs | NAP | HVOC,W | HVOC,NAP | mR | Operating Conditions | Ref |
|---|---|---|---|---|---|---|
| Hexane | Silicone oil 190 mPa s | 61 | 5.8 × 10−3 | 10,500(30 °C) | V = 2 × 10−3 m3 QL = 3.01 × 10−6 m3/s QG: 1.67 × 10−5; 3.33 × 10−5; 5.00 × 10−5 m3/s (gas EBRT: 120; 60; 40 s) φNAP: 0; 20% | [22] |
| Toluene | Silicone oil 340 mPa s | 2.69 × 10−4 T2 + 3.83 × 10−4 T + 9.1 × 10−2 (5–50 °C) | 6.35 × 10−7 T2 + 6.63 × 10−6 T + 3.75 × 10−4 (5–60 °C) | 235(5 °C) 261(17 °C) 341(50 °C) | V = 5.52 × 10−1 m3 QL = 5.0 × 10−3; 7.22 × 10−3 m3/s QG: 2.78 × 10−2; 5.56 × 10−2; 9.72 × 10−2 m3/s (gas EBRT: 20; 10; 6 s) φNAP: 10; 20% | [20] |
| Styrene | Silicone oil 10 mPa s | 0.153(NaCl solution) | 6.0 × 10−4 | 255(30 °C) | V = 2 × 10−3 m3 QG: 1.67 × 10−5 m3/s (gas EBRT: 120 s; QG/QL = 7.5 and 16) QG: 3.33 × 10−5 m3/s (gas EBRT: 60 s; QG/QL = 32) φNAP: 0; 5; 10; 20% | [23] |
| Toluene DMDS | Silicone oil 5 mPa s | 0.274 0.045 | 1.09 × 10−3 1.37 × 10−3 | 252(25 °C) 33(25 °C) | V = 1.13 × 10−2 m3 QL = [2.67 × 10−5–1.39 × 10−4] m3/s QG: 5.25 × 10−3; 7.30 × 10−3; 9.25 × 10−3 m3/s (gas EBRT: 2.2; 1.6; 1.3 s) φNAP: 0; 10; 100% | [11] |
| Toluene | HOSO (sunflower oil) 77 mPa s Commercial sunflower oil 57 mPa s | 0.274 | 0.0147 0.017 | 19(24 °C) 16(24 °C) | V = 2.2 × 10−2 m3 QL = [1.33 × 10−5–2.07 × 10−5] m3/s QG: 2.33 × 10−4; 3.00 × 10−4; 3.83 × 10−4 m3/s (gas EBRT: 94; 73; 57 s) φNAP: 5% | [24] |
| T (°C) | φNAP | HVOC,mix | QG (m3/s) | A | ε | NTU | KLa (s−1) | EBRT (s) |
|---|---|---|---|---|---|---|---|---|
| 5 | 0.1 | 0.0041 | 2.78 × 10−2 | 63.7 | 0.90 | 2.325 | 4.78 × 10−4 | 20 |
| 5.56 × 10−2 | 31.8 | 0.67 | 1.123 | 4.61 × 10−4 | 10 | |||
| 9.72 × 10−2 | 18.2 | 0.50 | 0.704 | 5.06 × 10−4 | 6 | |||
| 5 | 0.1 | 0.0041 | 2.78 × 10−2 | 63.7 | 0.90 | 2.325 | 4.78 × 10−4 | 20 |
| 17 | 0.0065 | 40.1 | 0.83 | 1.796 | 5.86 × 10−4 | |||
| 50 | 0.0224 | 11.6 | 0.67 | 1.148 | 1.29 × 10−3 | |||
| 5 | 0.1 | 0.0041 | 2.78 × 10−2 | 63.7 | 0.90 | 2.325 | 4.78 × 10−4 | 20 |
| 0.2 | 0.0021 | 124.7 | 0.93 | 2.673 | 2.80 × 10−4 |
| NAP | φNAP | HVOC,mix | QL (m3/s) | QG (m3/s) | A | ε | NTU | KLa (s−1) | EBRT (s) |
|---|---|---|---|---|---|---|---|---|---|
| HOSO | 0.05 | 0.146 | 1.33 × 10−5 | 2.33 × 10−4 | 0.392 | 0.22 | 0.371 | 5.72 × 10−4 | 94 |
| 1.92 × 10−5 | 0.564 | 0.30 | 0.520 | 8.04 × 10−4 | |||||
| 1.67 × 10−5 | 0.491 | 0.28 | 0.498 | 7.69 × 10−4 | |||||
| 1.67 × 10−5 | 2.33 × 10−4 | 0.491 | 0.28 | 0.498 | 7.69 × 10−4 | 94 | |||
| 3.00 × 10−4 | 0.382 | 0.26 | 0.520 | 1.03 × 10−3 | 73 | ||||
| 3.83 × 10−4 | 0.299 | 0.15 | 0.228 | 5.78 × 10−4 | 57 | ||||
| Commercial oil | 0.05 | 0.156 | 1.33 × 10−5 | 2.33 × 10−4 | 0.366 | 0.26 | 0.541 | 8.96 × 10−4 | 94 |
| 1.67 × 10−5 | 0.458 | 0.26 | 0.454 | 7.52 × 10−4 | |||||
| 2.07 × 10−5 | 0.568 | 0.38 | 0.826 | 1.37 × 10−3 |
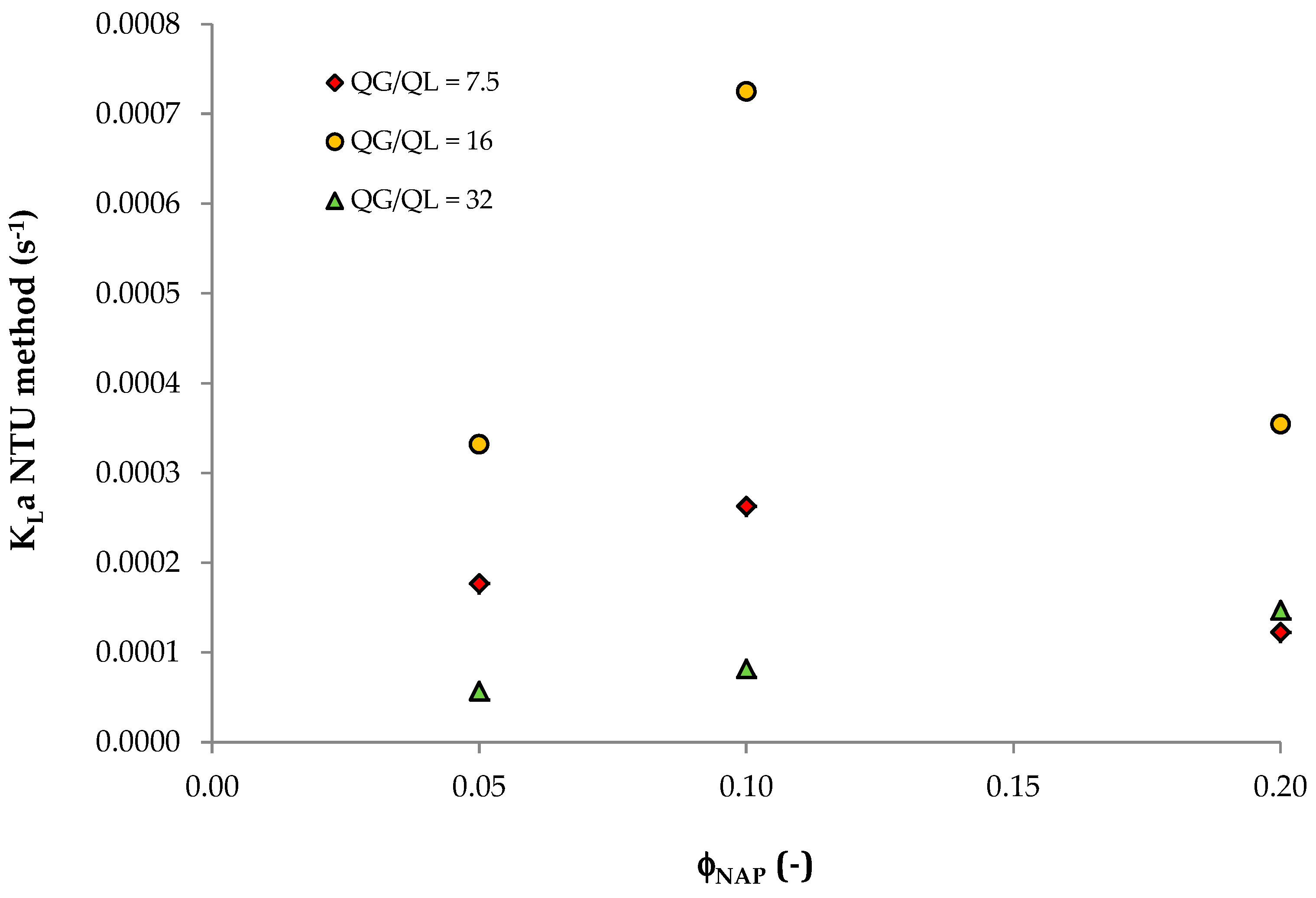
| φNAP | HVOC,mix | QL (m3/s) | QG (m3/s) | A | ε | NTU | KLa (s−1) |
|---|---|---|---|---|---|---|---|
| 0 | 0.1530 | 4.44 × 10−6 | 3.33 × 10−5 | 0.87 | 0.999 | no calculable | |
| 0.05 | 0.0112 | 11.94 | 0.602 | 0.95 | 1.77 × 10−4 | ||
| 0.1 | 0.0058 | 23.01 | 0.929 | 2.72 | 2.63 × 10−4 | ||
| 0.2 | 0.0030 | 45.14 | 0.914 | 2.49 | 1.22 × 10−4 | ||
| 0 | 0.1530 | 2.08 × 10−6 | 3.33 × 10−5 | 0.41 | 0.972 | no calculable | |
| 0.05 | 0.0112 | 5.60 | 0.802 | 1.78 | 3.32 × 10−4 | ||
| 0.1 | 0.0058 | 10.78 | 0.999 | 7.51 | 7.25 × 10−4 | ||
| 0.2 | 0.0030 | 21.16 | 0.999 | 7.20 | 3.54 × 10−4 | ||
| 0 | 0.1530 | 1.04 × 10−6 | 3.33 × 10−5 | 0.20 | 0.815 | no calculable | |
| 0.05 | 0.0112 | 2.80 | 0.253 | 0.31 | 5.70 × 10−5 | ||
| 0.1 | 0.0058 | 5.39 | 0.55 | 0.85 | 8.19 × 10−5 | ||
| 0.2 | 0.0030 | 10.58 | 0.939 | 2.99 | 1.47 × 10−4 | ||
| VOCs | NAP | HVOC,W | HVOC,NAP | mR | Operating Conditions | Ref |
|---|---|---|---|---|---|---|
| Hexane | Silicone oil 190 mPa s | 61 | 5.8 × 10−3 | 10,500 (30 °C) | V = 2 × 10−3 m3 QG = 1.67 × 10−5 m3/s Stirring rates: 100; 200; 300 rpm φ: 5; 7.5; 10; 20% | [28] |
| Hexane | Silicone oil 190 mPa s | 61 | 5.8 × 10−3 | 10,500 (30 °C) | V = 2 × 10−3 m3 QG: 1.67 × 10−5; 3.33 × 10−5; 5.00 × 10−5 m3/s (EBRT: 120; 60; 40 s) Stirring rates: 200 rpm φ: 0; 20% | [22] |
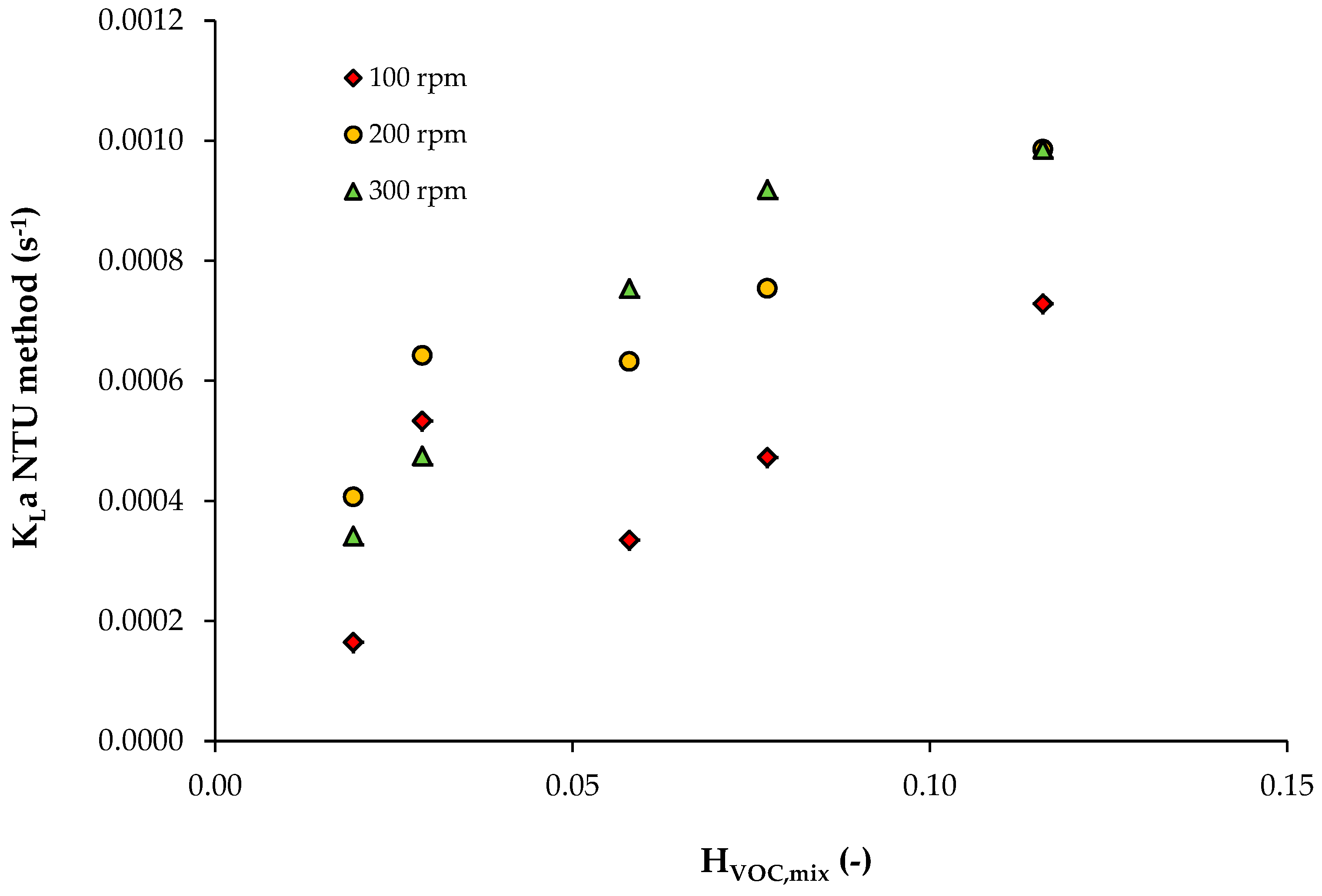
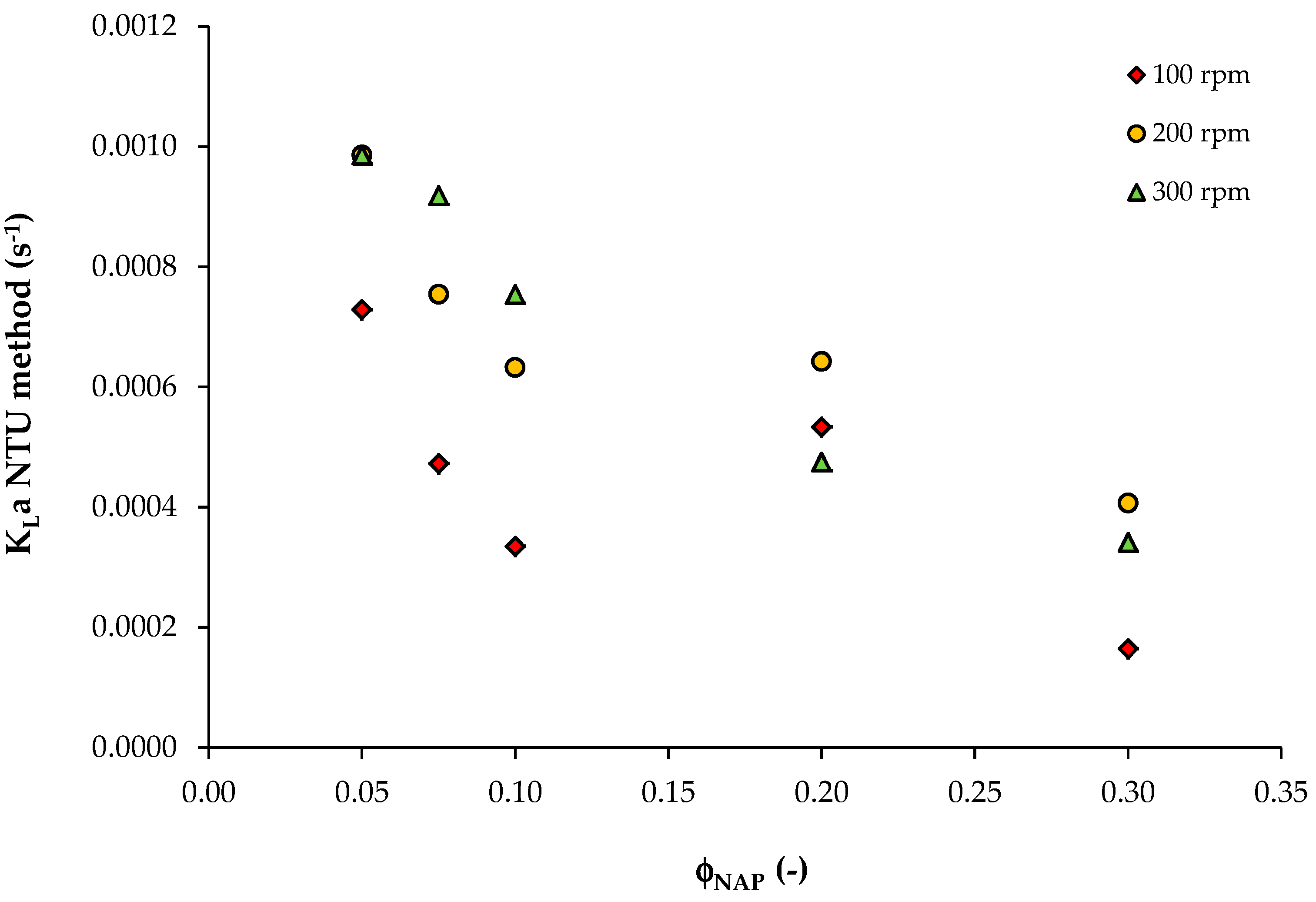
| φNAP | HVOC,mix | 100 rpm | 200 rpm | 300 rpm | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ε | NTU | KLa (s−1) | ε | NTU | KLa (s−1) | ε | NTU | KLa (s−1) | ||
| 0.05 | 0.116 | 0.53 | 0.755 | 7.29 × 10−4 | 0.64 | 1.022 | 9.86 × 10−4 | 0.64 | 1.022 | 9.86 × 10−4 |
| 0.075 | 0.077 | 0.52 | 0.734 | 4.72 × 10−4 | 0.69 | 1.171 | 7.54 × 10−4 | 0.76 | 1.427 | 9.19 × 10−4 |
| 0.1 | 0.058 | 0.50 | 0.693 | 3.35 × 10−4 | 0.73 | 1.309 | 6.32 × 10−4 | 0.79 | 1.561 | 7.54 × 10−4 |
| 0.2 | 0.029 | 0.89 | 2.207 | 5.33 × 10−4 | 0.93 | 2.659 | 6.42 × 10−4 | 0.86 | 1.966 | 4.75 × 10−4 |
| 0.3 | 0.019 | 0.64 | 1.022 | 1.65 × 10−4 | 0.92 | 2.526 | 4.07 × 10−4 | 0.88 | 2.120 | 3.42 × 10−4 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumont, É. Mass Transfer in Multiphasic Gas/Liquid/Liquid Systems. KLa Determination Using the Effectiveness-Number of Transfer Unit Method. Processes 2018, 6, 156. https://doi.org/10.3390/pr6090156
Dumont É. Mass Transfer in Multiphasic Gas/Liquid/Liquid Systems. KLa Determination Using the Effectiveness-Number of Transfer Unit Method. Processes. 2018; 6(9):156. https://doi.org/10.3390/pr6090156
Chicago/Turabian StyleDumont, Éric. 2018. "Mass Transfer in Multiphasic Gas/Liquid/Liquid Systems. KLa Determination Using the Effectiveness-Number of Transfer Unit Method" Processes 6, no. 9: 156. https://doi.org/10.3390/pr6090156
APA StyleDumont, É. (2018). Mass Transfer in Multiphasic Gas/Liquid/Liquid Systems. KLa Determination Using the Effectiveness-Number of Transfer Unit Method. Processes, 6(9), 156. https://doi.org/10.3390/pr6090156




