Agent-Based Modeling of Immune Response to Study the Effects of Regulatory T Cells in Type 1 Diabetes
Abstract
1. Introduction
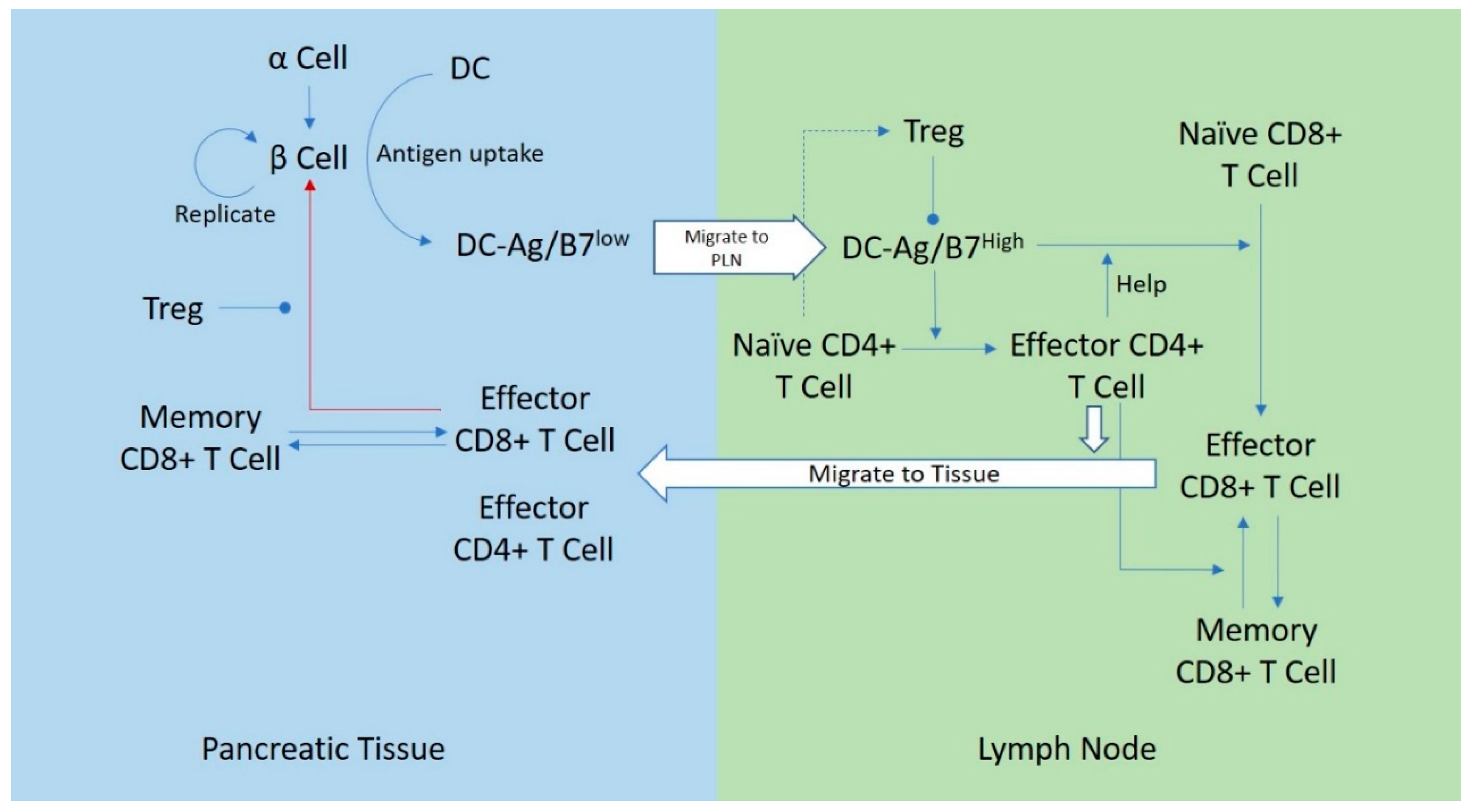
2. The Model
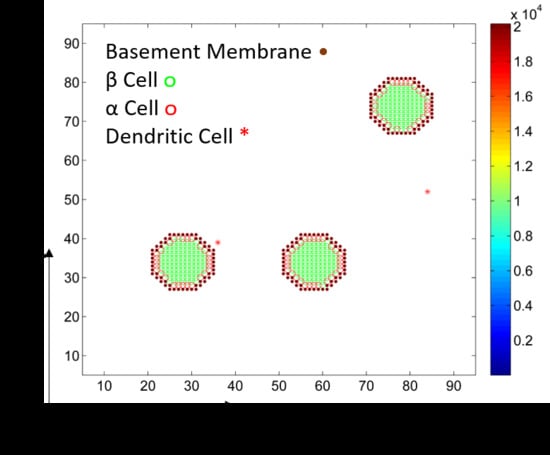
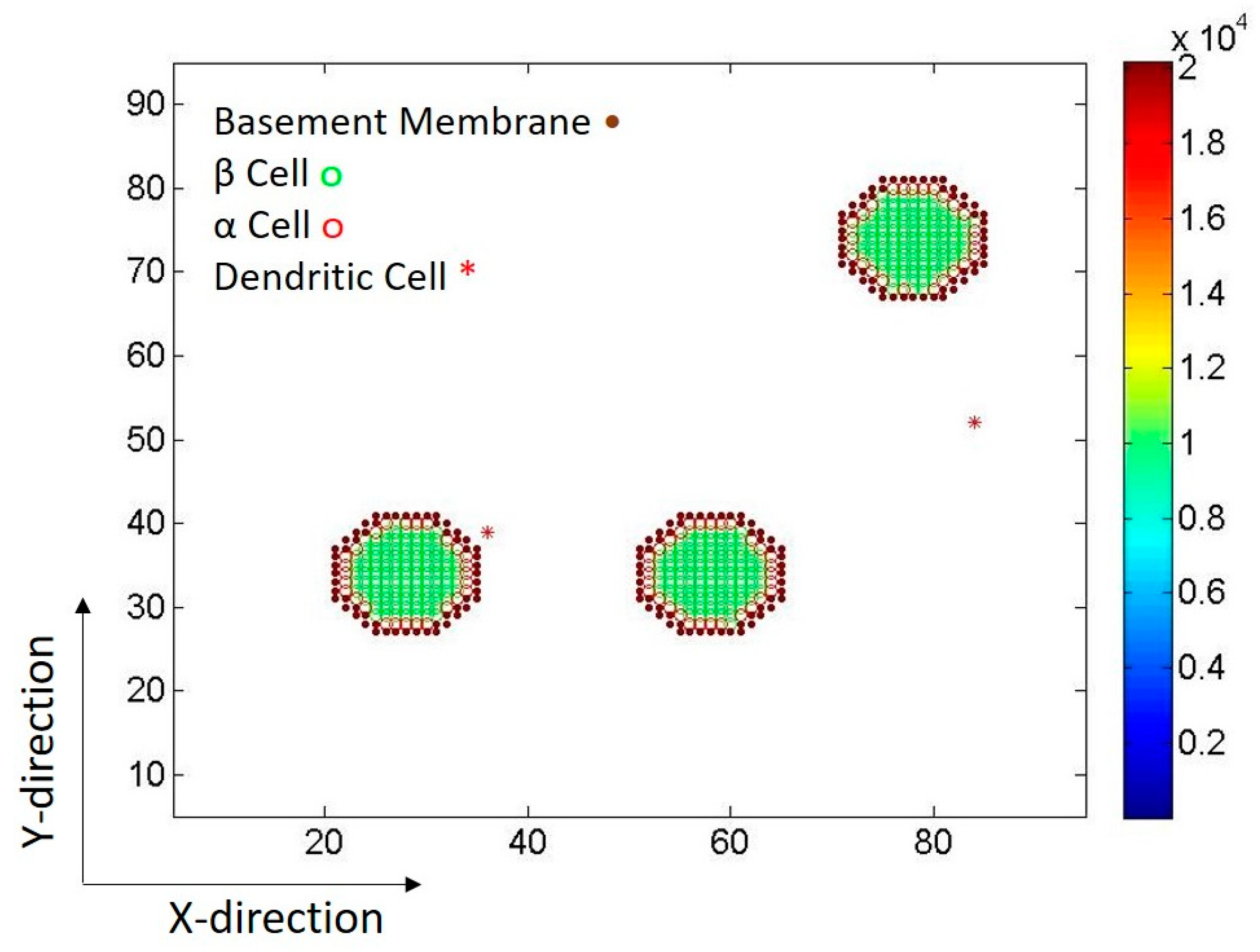
2.1. Islets of Langerhans
2.2. Dendritic Cells
2.3. CD8+ T Cells
2.4. CD4+ T Cells
2.5. Compartments
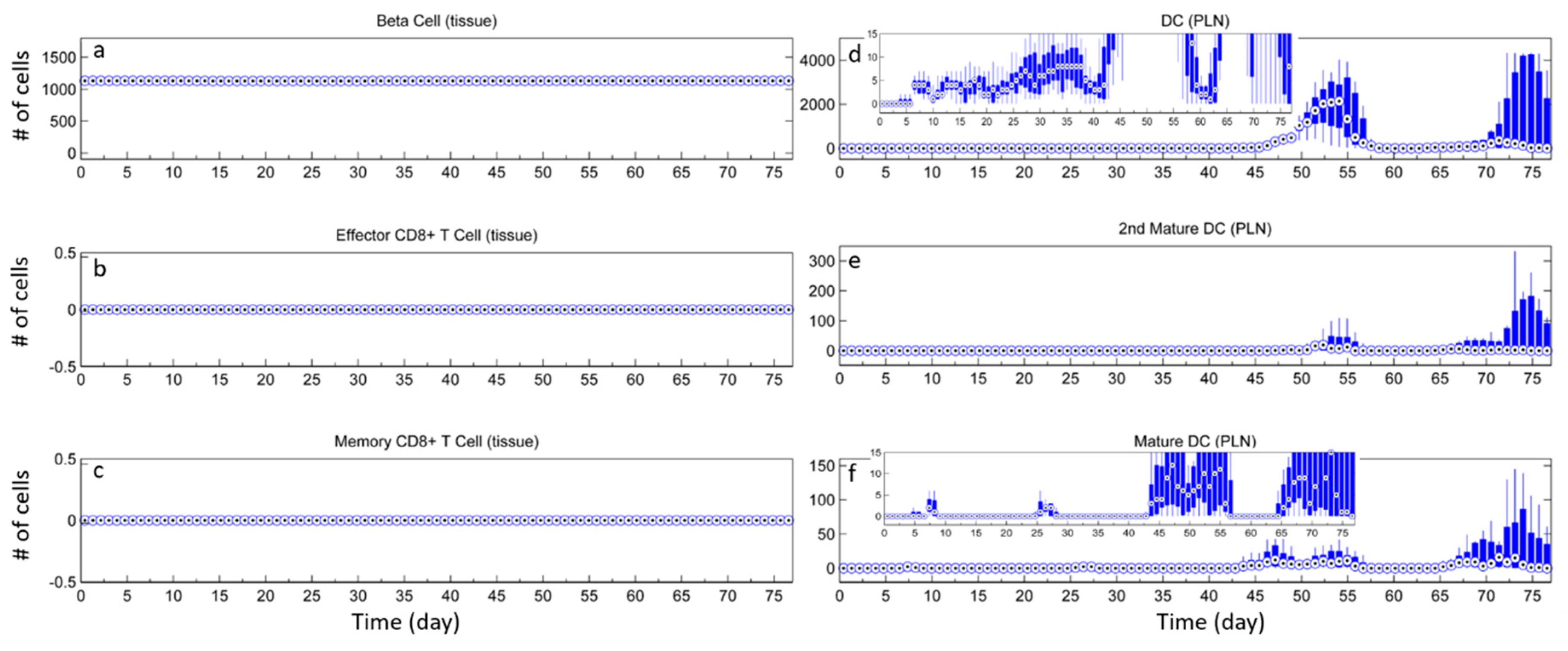
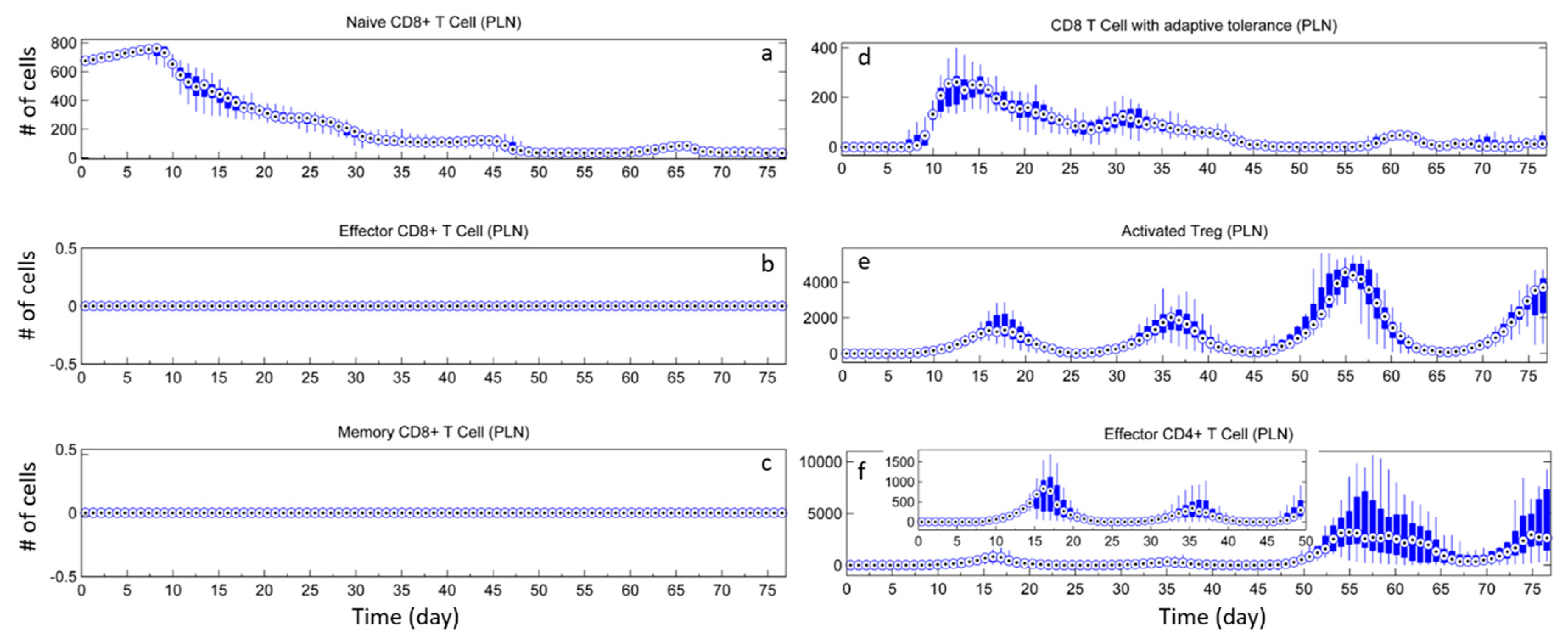
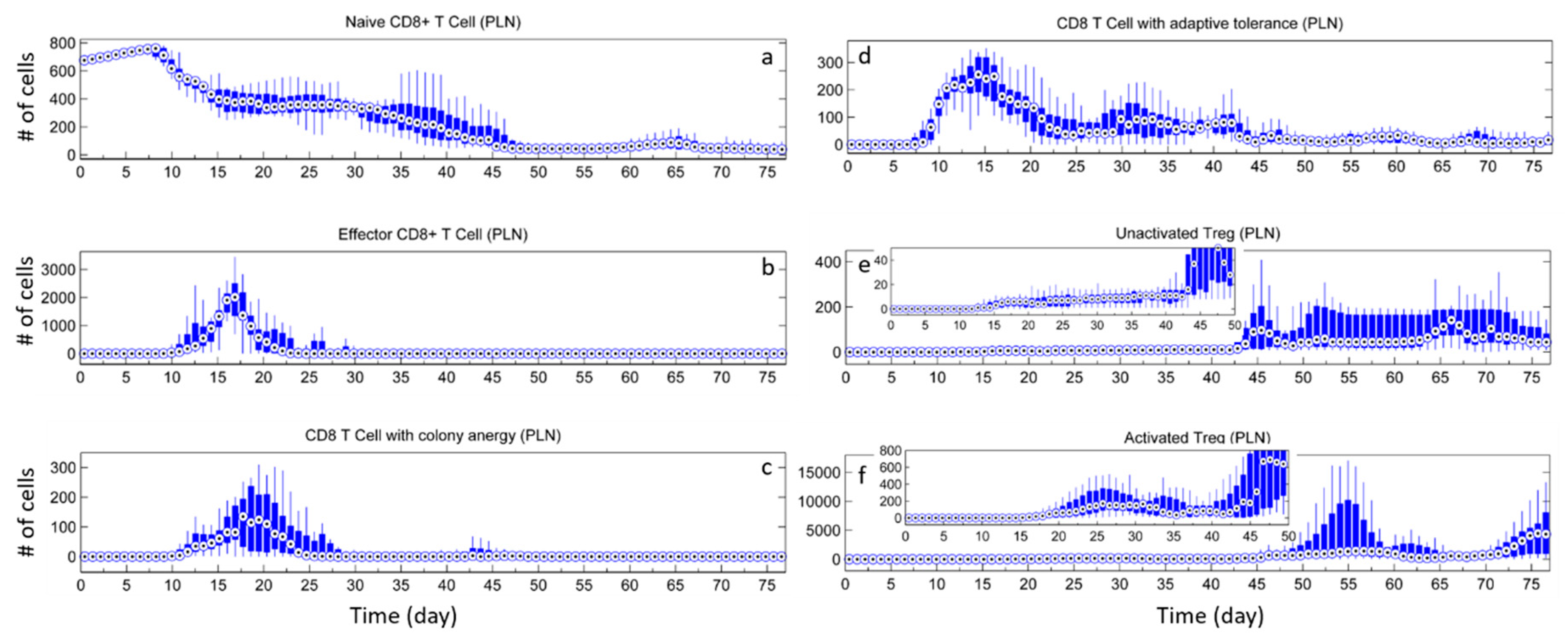
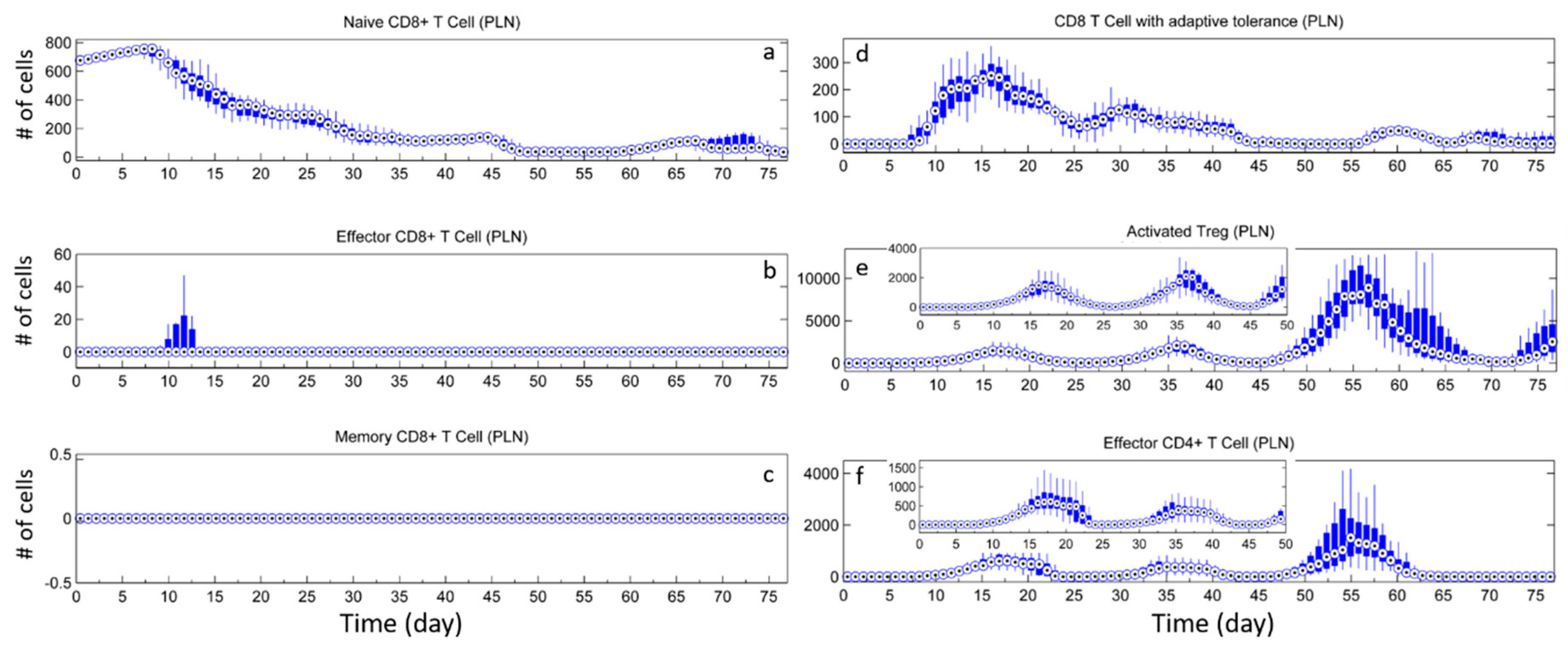
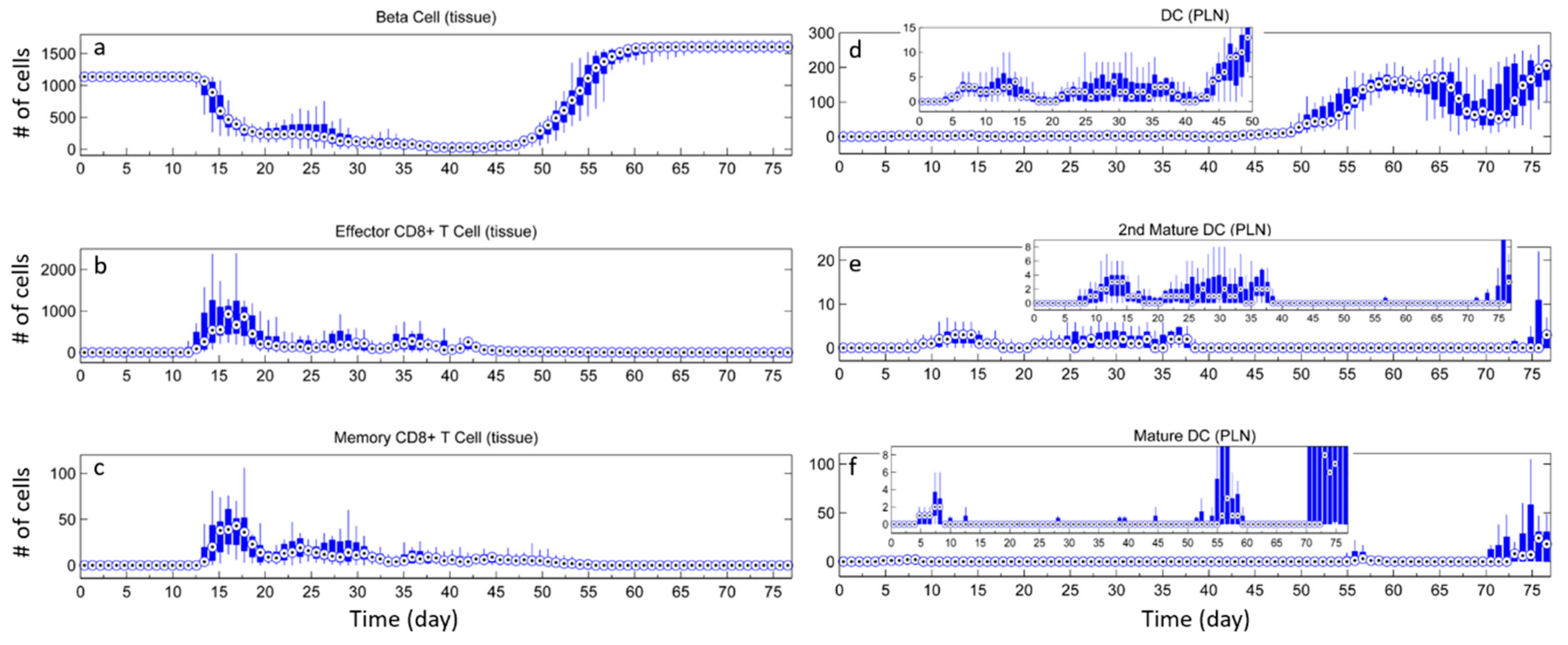
3. Results
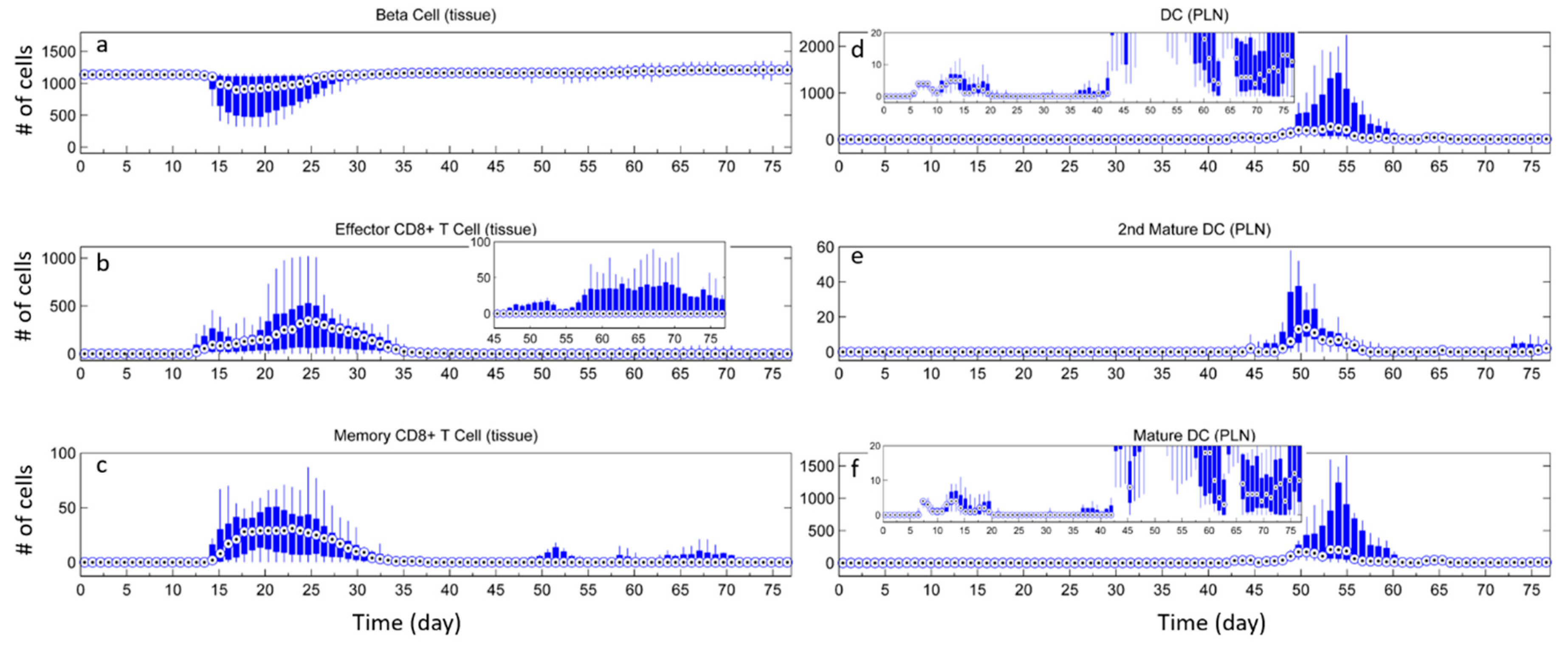
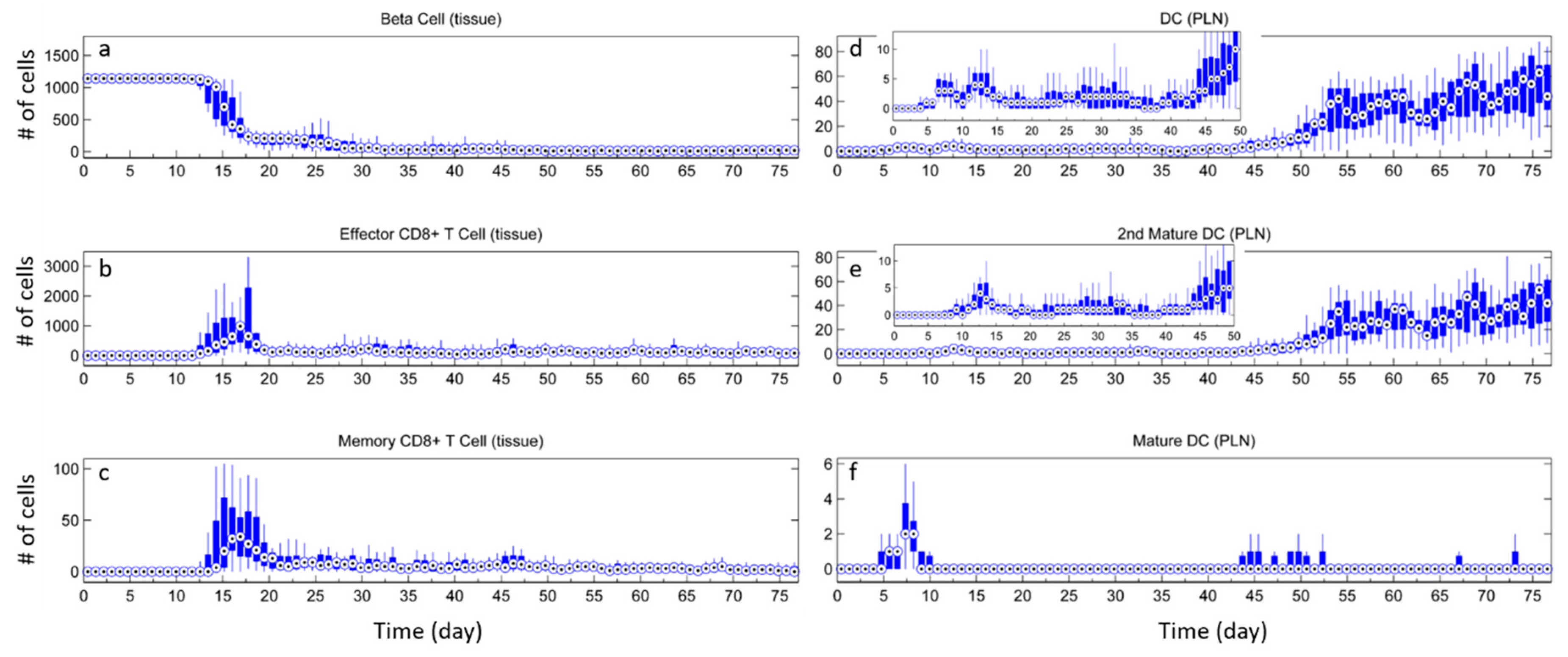
3.1. Without Tregs
3.2. Effect of Tregs
3.3. Effects of Tregs from Different Sources
3.4. External Addition of Tregs
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A


References
- ElEssawy, B.; Li, X.C. Type 1 diabetes and T regulatory cells. Pharmacol. Res. 2015, 98, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Morel, P.A. Dendritic cell subsets in type 1 diabetes: Friend or foe? Front. Immunol. 2013, 4, 415. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, C.A.; Shevach, E.M. Cutting edge: Control of CD8+ T cell activation by CD4+ CD25+ immunoregulatory cells. J. Immunol. 2001, 167, 1137–1140. [Google Scholar] [CrossRef] [PubMed]
- Waid, D.M.; Vaitaitis, G.M.; Pennock, N.D.; Wagner, D.H., Jr. Disruption of the homeostatic balance between autoaggressive (CD4+ CD40+) and regulatory (CD4+ CD25+ FoxP3+) T cells promotes diabetes. J. Leukoc. Biol. 2008, 84, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Bluestone, J.A.; Buckner, J.H.; Fitch, M.; Gitelman, S.E.; Gupta, S.; Hellerstein, M.K.; Herold, K.C.; Lares, A.; Lee, M.R.; Li, K.; et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci. Transl. Med. 2015, 7, 315ra189. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lin, B.; Darflinger, R.; Zhang, Y.; Holterman, M.J.; Skidgel, R.A. Human cord blood stem cell-modulated regulatory T lymphocytes reverse the autoimmune-caused type 1 diabetes in nonobese diabetic (NOD) mice. PLoS ONE 2009, 4, e4226. [Google Scholar] [CrossRef] [PubMed]
- Roncarolo, M.-G.; Battaglia, M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat. Rev. Immunol. 2007, 7, 585–598. [Google Scholar] [CrossRef] [PubMed]
- The Mount Hood 4 Group. Computer modeling of diabetes and its complications. Diabetes Care 2007, 30, 1638–1646. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, N.P.; Rangaiah, G.P.; Samavedham, L. Review and analysis of blood glucose (BG) models for type 1 diabetic patients. Ind. Eng. Chem. Res. 2011, 50, 12041–12066. [Google Scholar] [CrossRef]
- Henriksson, M.; Jindal, R.; Sternhufvud, C.; Bergenheim, K.; Sorstadius, E.; Willis, M. A systematic review of cost-effectiveness models in type 1 diabetes mellitus. Pharmacoeconomics 2016, 34, 569–585. [Google Scholar] [CrossRef] [PubMed]
- Makroglou, A.; Li, J.; Kuang, Y. Mathematical models and software tools for the glucose-insulin regulatory system and diabetes: An overview. Appl. Numer. Math. 2006, 56, 559–573. [Google Scholar] [CrossRef]
- Shoda, L.; Kreuwel, H.; Gadkar, K.; Zheng, Y.; Whiting, C.; Atkinson, M.; Bluestone, J.; Mathis, D.; Young, D.; Ramanujan, S. The type 1 diabetes physiolab® platform: A validated physiologically based mathematical model of pathogenesis in the non-obese diabetic mouse. Clin. Exp. Immunol. 2010, 161, 250–267. [Google Scholar] [CrossRef] [PubMed]
- Martínez, I.V.; Hernando, M.E.; Gómez, E.J.; Villares, R.; Mellado, M. Definition of an agent-based model of the autoimmune response in type 1 diabetes. In Proceedings of the 7th Iberian Conference on Information Systems and Technologies (CISTI 2012), Madrid, Spain, 20–23 June 2012; pp. 1–4. [Google Scholar]
- Wedgwood, K.C.; Richardson, S.J.; Morgan, N.G.; Tsaneva-Atanasova, K. Spatiotemporal dynamics of insulitis in human type 1 diabetes. Front. Physiol. 2016, 7, 633. [Google Scholar] [CrossRef] [PubMed]
- Bonabeau, E. Agent-based modeling: Methods and techniques for simulating human systems. Proc. Natl. Acad. Sci. USA 2002, 99, 7280–7287. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, M.C.; Xu, Q.; Cinar, A. Agent-based modeling of the interaction between CD8+ T cells and beta cells in type 1 diabetes. PLoS ONE 2018, 13, e0190349. [Google Scholar] [CrossRef] [PubMed]
- Collier, N.; North, M. Repast HPC: A platform for large-scale agent-based modeling. In Large-Scale Computing; Dubitzky, W., Kurowski, K., Schott, B., Eds.; John Wiley & Sons, Inc.: New York, NY, USA, 2012. [Google Scholar]
- Zhang, X.; Giangreco, L.; Broome, H.E.; Dargan, C.M.; Swain, S.L. Control of CD4 effector fate: Transforming growth factor beta 1 and interleukin 2 synergize to prevent apoptosis and promote effector expansion. J. Exp. Med. 1995, 182, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Den Braber, I.; Mugwagwa, T.; Vrisekoop, N.; Westera, L.; Mogling, R.; de Boer, A.B.; Willems, N.; Schrijver, E.H.; Spierenburg, G.; Gaiser, K.; et al. Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity 2012, 36, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Matheu, M.P.; Othy, S.; Greenberg, M.L.; Dong, T.X.; Schuijs, M.; Deswarte, K.; Hammad, H.; Lambrecht, B.N.; Parker, I.; Cahalan, M.D. Imaging regulatory T cell dynamics and CTLA4-mediated suppression of T cell priming. Nat. Commun. 2015, 6, 6219. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, I.; Krueger, A.; Baumann, S.; Schulze-Bergkamen, H.; Krammer, P.H.; Kirchhoff, S. An IL-2-dependent switch between CD95 signaling pathways sensitizes primary human T cells toward CD95-mediated activation-induced cell death. J. Immunol. 2003, 171, 2930–2936. [Google Scholar] [CrossRef] [PubMed]
- Coppieters, K.; Amirian, N.; von Herrath, M. Intravital imaging of CTLs killing islet cells in diabetic mice. J. Clin. Investig. 2012, 122, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Kinjyo, I.; Qin, J.; Tan, S.Y.; Wellard, C.J.; Mrass, P.; Ritchie, W.; Doi, A.; Cavanagh, L.L.; Tomura, M.; Sakaue-Sawano, A.; et al. Real-time tracking of cell cycle progression during CD8+ effector and memory T-cell differentiation. Nat. Commun. 2015, 6, 6301. [Google Scholar] [CrossRef] [PubMed]
- Bousso, P. T-cell activation by dendritic cells in the lymph node: Lessons from the movies. Nat. Rev. Immunol. 2008, 8, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Kamath, A.T.; Henri, S.; Battye, F.; Tough, D.F.; Shortman, K. Developmental kinetics and lifespan of dendritic cells in mouse lymphoid organs. Blood 2002, 100, 1734–1741. [Google Scholar] [PubMed]
- Coppieters, K.; Martinic, M.M.; Kiosses, W.B.; Amirian, N.; Herrath, M.V. A novel technique for the in vivo imaging of autoimmune diabetes development in the pancreas by two-photon microscopy. PLoS ONE 2010, 5, e15732. [Google Scholar] [CrossRef] [PubMed]
- Fisson, S.; Darrasse-Jeze, G.; Litvinova, E.; Septier, F.; Klatzmann, D.; Liblau, R.; Salomon, B.L. Continuous activation of autoreactive CD4+ CD25+ regulatory T cells in the steady state. J. Exp. Med. 2003, 198, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, S.; Iyoda, T.; Tarbell, K.; Olson, K.; Velinzon, K.; Inaba, K.; Steinman, R.M. Direct expansion of functional CD25+ CD4+ regulatory T cells by antigen-processing dendritic cells. J. Exp. Med. 2003, 198, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.H.; Hao, E.; Piran, R.; Keinan, E.; Levine, F. Pancreatic β-cell neogenesis by direct conversion from mature alpha-cells. Stem Cells 2010, 28, 1630–1638. [Google Scholar] [CrossRef] [PubMed]
- Magnuson, A.M.; Thurber, G.M.; Kohler, R.H.; Weissleder, R.; Mathis, D.; Benoist, C. Population dynamics of islet-infiltrating cells in autoimmune diabetes. Proc. Natl. Acad. Sci. USA 2015, 112, 1511–1516. [Google Scholar] [CrossRef] [PubMed]
- Chintinne, M.; Stange, G.; Denys, B.; Ling, Z.; Veld, P.I.; Pipeleers, D. Beta cell count instead of beta cell mass to assess and localize growth in beta cell population following pancreatic duct ligation in mice. PLoS ONE 2012, 7, e43959. [Google Scholar] [CrossRef] [PubMed]
- Wing, K.; Sakaguchi, S. Regulatory t cells exert checks and balances on self tolerance and autoimmunity. Nat. Immunol. 2010, 11, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yang, X.; Chen, B.; Xu, X. Pancreas β cell regeneration and type 1 diabetes (review). Exp. Ther. Med. 2015, 9, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Collombat, P.; Xu, X.; Ravassard, P.; Sosa-Pineda, B.; Dussaud, S.; Billestrup, N.; Madsen, O.D.; Serup, P.; Heimberg, H.; Mansouri, A. The ectopic expression of pax4 in the mouse pancreas converts progenitor cells into α and subsequently β cells. Cell 2009, 138, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Marcela, B.; Michael, J.F.; Wendell, E.N.; Anita, C.; Boaz, H.; David, M.H.; Alvin, C.P. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J. Histochem. Cytochem. 2005, 53, 1087–1097. [Google Scholar] [CrossRef]
- Korsgren, E.; Korsgren, O. An apparent deficiency of lymphatic capillaries in the islets of langerhans in the human pancreas. Diabetes 2016, 65, 1004–1008. [Google Scholar] [CrossRef] [PubMed]
- Dieu, M.-C.; Vanvervliet, B.; Vicari, A.; Bridon, J.-M.; Oldham, E.; Ait-Yahia, S.; Briere, F.; Zlotnik, A.; Lebecque, S.; Caux, C. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J. Exp. Med. 1998, 188, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Caux, C.; Vanbervliet, B.; Massacrier, C.; Ait-Yahia, S.; Vaure, C.; Chemin, K.; Dieu-Nosjean, M.-C.; Vicari, A. Regulation of dendritic cell recruitment by chemokines. Transplantation 2002, 73, S7–S11. [Google Scholar] [CrossRef] [PubMed]
- Caux, C.; Dubois, B. Antigen uptake by dendritic cells. Methods Mol. Med. 2001, 64, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Pletinckx, K.; Dohler, A.; Pavlovic, V.; Lutz, M.B. Role of dendritic cell maturity/costimulation for generation, homeostasis, and suppressive activity of regulatory T cells. Front. Immunol. 2011, 2, 39. [Google Scholar] [CrossRef] [PubMed]
- Price, J.D.; Tarbell, K.V. The role of dendritic cell subsets and innate immunity in the pathogenesis of type 1 diabetes and other autoimmune diseases. Front. Immunol. 2015, 6, 288. [Google Scholar] [CrossRef] [PubMed]
- Regoli, M.; Bertelli, E.; Orazioli, D.; Fonzi, L.; Bastianini, A. Pancreatic lymphatic system in rodents. Anat. Rec. 2011, 263, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Randolph, G.J.; Angeli, V.; Swartz, M.A. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat. Rev. Immunol. 2005, 5, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Vasilevko, V.; Ghochikyan, A.; Holterman, M.J.; Agadjanyan, M.G. CD80 (B7-1) and CD86 (B7-2) are functionally equivalent in the initiation and maintenance of CD4+ T-cell proliferation after activation with suboptimal doses of PHA. DNA Cell Biol. 2002, 21, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Schildknecht, A.; Miescher, I.; Yagita, H.; van den Broek, M. Priming of CD8+ T cell responses by pathogens typically depends on CD70-mediated interactions with dendritic cells. Eur. J. Immunol. 2007, 37, 716–728. [Google Scholar] [CrossRef] [PubMed]
- Onishi, Y.; Fehervari, Z.; Yamaguchi, T.; Sakaguchi, S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc. Natl. Acad. Sci. USA 2008, 105, 10113–10118. [Google Scholar] [CrossRef] [PubMed]
- Taraban, V.Y.; Rowley, T.F.; Al-Shamkhani, A. Cutting edge: A critical role for CD70 in CD8 T cell priming by CD40-licensed APCs. J. Immunol. 2004, 173, 6542–6546. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, P.J.; McWilliams, J.A.; Haluszczak, C.; Yagita, H.; Kedl, R.M. Combined TLR/CD40 stimulation mediates potent cellular immunity by regulating dendritic cell expression of CD70 in vivo. J. Immunol. 2007, 178, 1564–1572. [Google Scholar] [CrossRef] [PubMed]
- Eizirik, D.L.; Mandrup-Poulsen, T. A choice of death-the signal-transduction of immune-mediated beta-cell apoptosis. Diabetologia 2001, 44, 2115–2133. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.H. T cell anergy. Annu. Rev. Immunol. 2003, 21, 305–334. [Google Scholar] [CrossRef] [PubMed]
- Feau, S.; Garcia, Z.; Arens, R.; Yagita, H.; Borst, J.; Schoenberger, S.P. The CD4+ T-cell help signal is transmitted from APC to CD8+ T-cells via CD27-CD70 interactions. Nat. Commun. 2012, 3, 948. [Google Scholar] [CrossRef] [PubMed]
- Bevan, M.J. Helping the CD8+ T-cell response. Nat. Rev. Immunol. 2004, 4, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Van Kooten, C.; Banchereau, J. CD40-CD40 ligand. J. Leukoc. Biol. 2000, 67, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Davidson, T.S.; DiPaolo, R.J.; Andersson, J.; Shevach, E.M. Cutting edge: IL-2 is essential for TGF-β-mediated induction of Foxp3+ T regulatory cells. J. Immunol. 2007, 178, 4022–4026. [Google Scholar] [CrossRef] [PubMed]
- Vignali, D.A.; Collison, L.W.; Workman, C.J. How regulatory T cells work. Nat. Rev. Immunol. 2008, 8, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Mempel, T.R.; Pittet, M.J.; Khazaie, K.; Weninger, W.; Weissleder, R.; von Boehmer, H.; von Andrian, U.H. Regulatory T cells reversibly suppress cytotoxic T cell function independent of effector differentiation. Immunity 2006, 25, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Par, S.; Amrani, A.; Yamanouchi, J.; Han, B.; Thiessen, S.; Utsugi, T.; Verdaguer, J.; Santamaria, P. CD40 ligation releases immature dendritic cells from the control of regulatory CD4+ CD25+ T cells. Immunity 2003, 19, 877–889. [Google Scholar] [CrossRef]
- Belkaid, Y. Regulatory T cells and infection: A dangerous necessity. Nat. Rev. Immunol. 2007, 7, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory t cells and immune tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Roncarolo, M.G.; Gregori, S.; Battaglia, M.; Bacchetta, R.; Fleischhauer, K.; Levings, M.K. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol. Rev. 2006, 212, 28–50. [Google Scholar] [CrossRef] [PubMed]
- Burzyn, D.; Benoist, C.; Mathis, D. Regulatory T cells in nonlymphoid tissues. Nat. Immunol. 2013, 14, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Bluestone, J.A.; Herold, K.; Eisenbarth, G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature 2010, 464, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Adams, J.Y.; Tooley, A.J.; Bi, M.; Fife, B.T.; Serra, P.; Santamaria, P.; Locksley, R.M.; Krummel, M.F.; Bluestone, J.A. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat. Immunol. 2006, 7, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Chin, R.K.; Christiansen, P.; Sun, Y.; Tumanov, A.V.; Wang, J.; Chervonsky, A.V.; Fu, Y.X. Recruitment and activation of naive T cells in the islets by lymphotoxin β receptor-dependent tertiary lymphoid structure. Immunity 2006, 25, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Thorel, F.; Népote, V.; Avril, I.; Kohno, K.; Desgraz, R.; Chera, S.; Herrera, P.L. Conversion of adult pancreatic α-cells to β-cells after extreme β-cell loss. Nature 2010, 464, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fava, G.E.; Wang, H.; Mauvais-Jarvis, F.; Fonseca, V.A.; Wu, H. PAX4 gene transfer induces α-to-β cell phenotypic conversion and confers therapeutic benefits for diabetes treatment. Mol. Ther. 2016, 24, 251–260. [Google Scholar] [CrossRef] [PubMed]




| Cells/Micro-Organ | Initial Amount 1 | Lifespan (Days) | Move Speed (µm/min) | Proliferation Rate (hours/cell) | ||
|---|---|---|---|---|---|---|
| Effector | Naive | PLN 2 | Tissue | |||
| CD4+ T cells | 1576 | 6 [18] | 27 [19] | 15 3 [20] | 10 | 36 |
| CD8+ T cells | 676 | 6 [21] | 70 [19] | 15 [22] | 10 [22] | ~14.3 [23] |
| DCs | 6 | 3 d in PLN [24,25] | 7.5 | 5 [26] | / | |
| Tregs | 176 | 5 d | 61 [27] | 15 [20] | 10 | 36 [28] |
| β cells/islets | ~1100/11 | / | / | 42 4 [29] | ||
| Week | 3~9 | 9~10 | 10~12 | 12~14 |
|---|---|---|---|---|
| DC number (cell) 1 | 6 | 68 | 136 | 170 |
| Type | Affected Cell | Effector Function | Proliferation | Reactivation |
|---|---|---|---|---|
| Clonal anergy | Activated T cell | Normal | Inhibited | / |
| Adaptive tolerance | Naïve T cell | Reduce killing probability to 10% | 3 times longer cell cycle | Persist under strong stimulation |
| Death under weak stimulation |
| Sources | Condition |
|---|---|
| nTregs | Dendritic cell (DC) with strong B7 |
| Naive CD4+ T cells | Step 1 DC with weak B7 and IL-2 |
| Step 2 DC with strong B7 | |
| Naive CD4+ T cells | DC with strong B7 and IL-10 |
| Effector CD4+ T cells | DC with strong B7 and IL-10 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Q.; Ozturk, M.C.; Cinar, A. Agent-Based Modeling of Immune Response to Study the Effects of Regulatory T Cells in Type 1 Diabetes. Processes 2018, 6, 141. https://doi.org/10.3390/pr6090141
Xu Q, Ozturk MC, Cinar A. Agent-Based Modeling of Immune Response to Study the Effects of Regulatory T Cells in Type 1 Diabetes. Processes. 2018; 6(9):141. https://doi.org/10.3390/pr6090141
Chicago/Turabian StyleXu, Qian, Mustafa Cagdas Ozturk, and Ali Cinar. 2018. "Agent-Based Modeling of Immune Response to Study the Effects of Regulatory T Cells in Type 1 Diabetes" Processes 6, no. 9: 141. https://doi.org/10.3390/pr6090141
APA StyleXu, Q., Ozturk, M. C., & Cinar, A. (2018). Agent-Based Modeling of Immune Response to Study the Effects of Regulatory T Cells in Type 1 Diabetes. Processes, 6(9), 141. https://doi.org/10.3390/pr6090141