Systematic Design and Evaluation of an Extraction Process for Traditionally Used Herbal Medicine on the Example of Hawthorn (Crataegus monogyna JACQ.)
Abstract
1. Introduction
2. Material and Methods
2.1. Raw Material
2.2. Solvent Screening
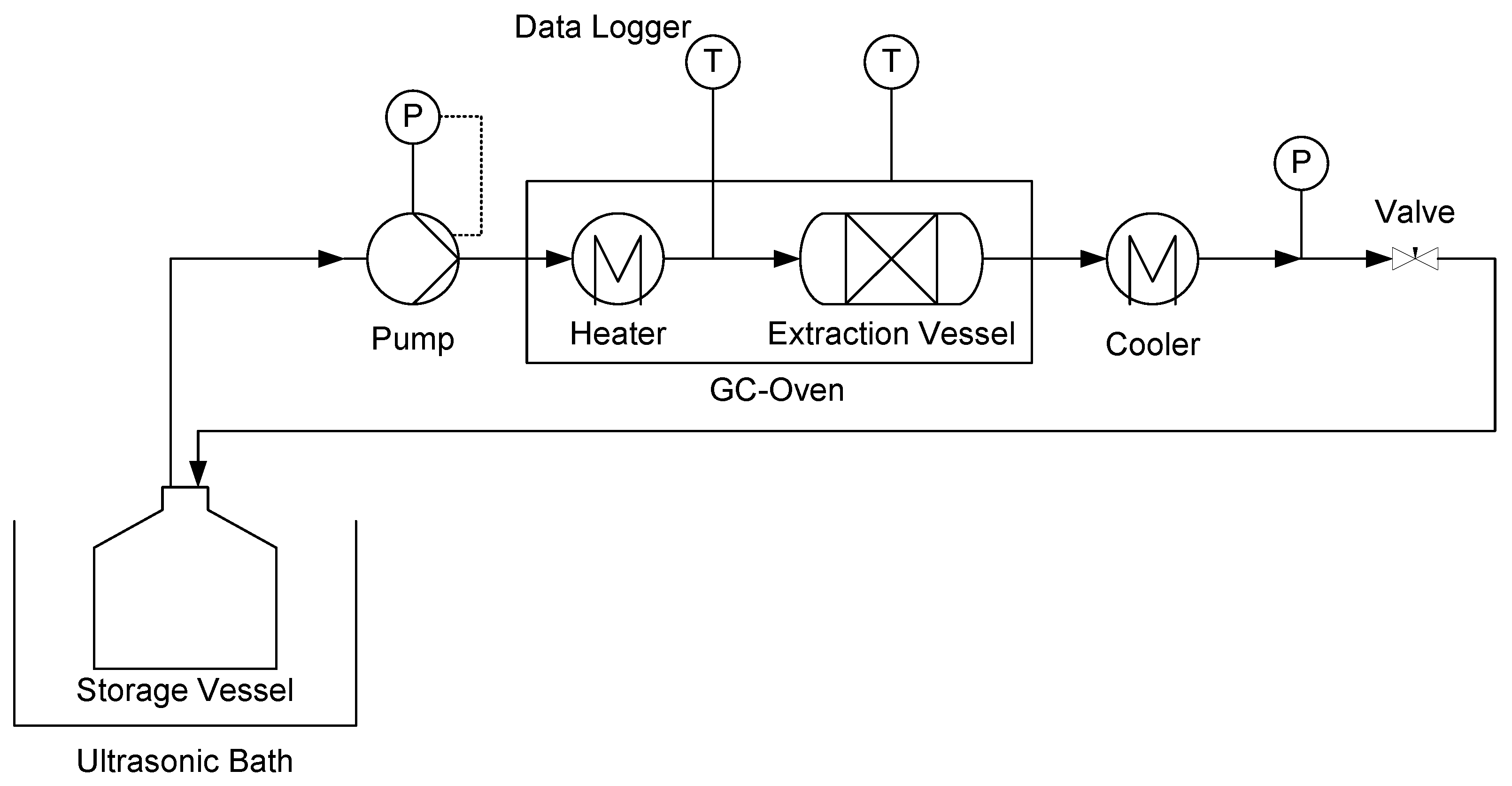
2.3. Solid-Liquid Extraction
2.4. Pressurized Hot Water Extraction (PHWE)
2.5. Analytics
3. Results and Discussion
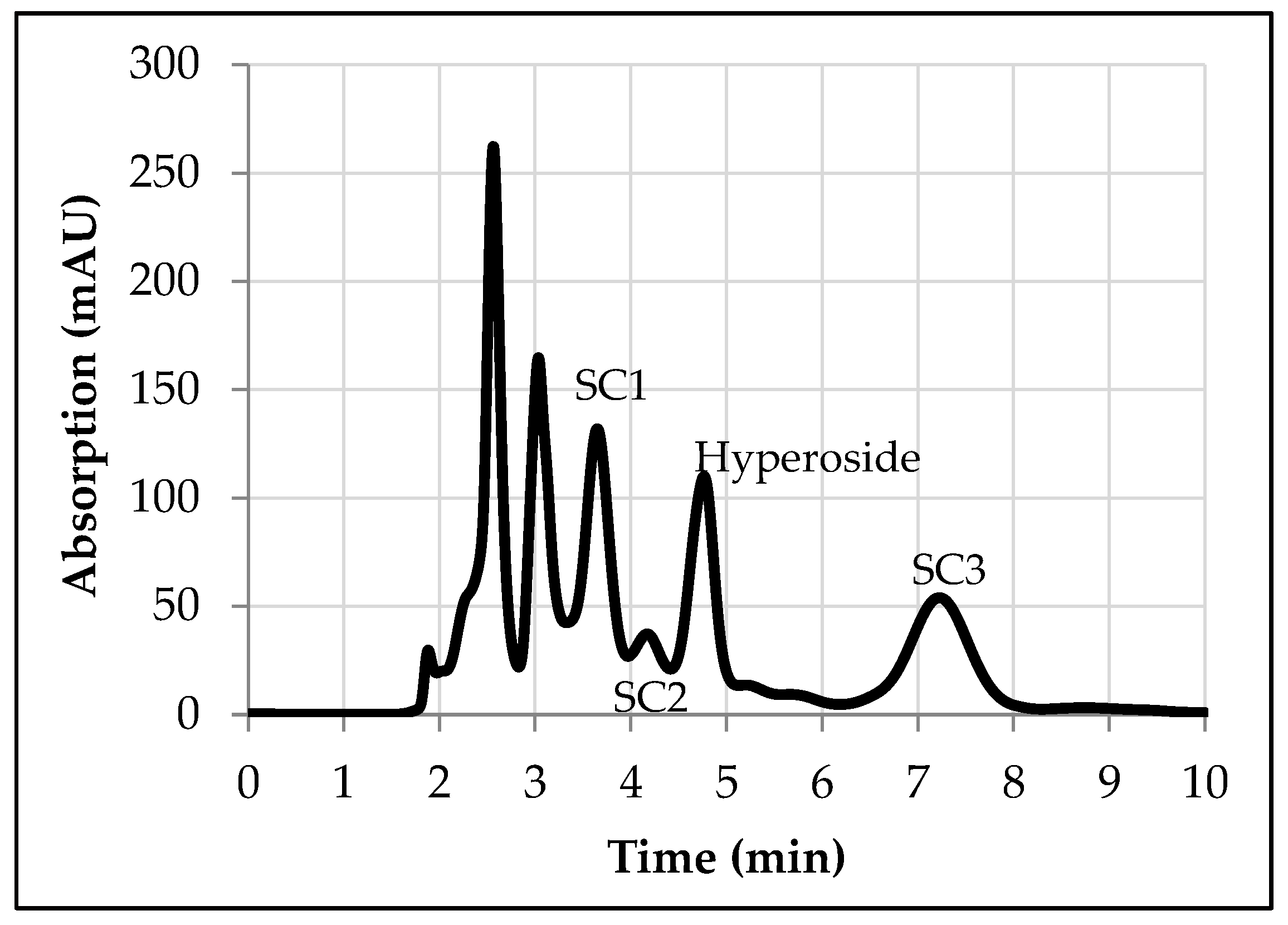
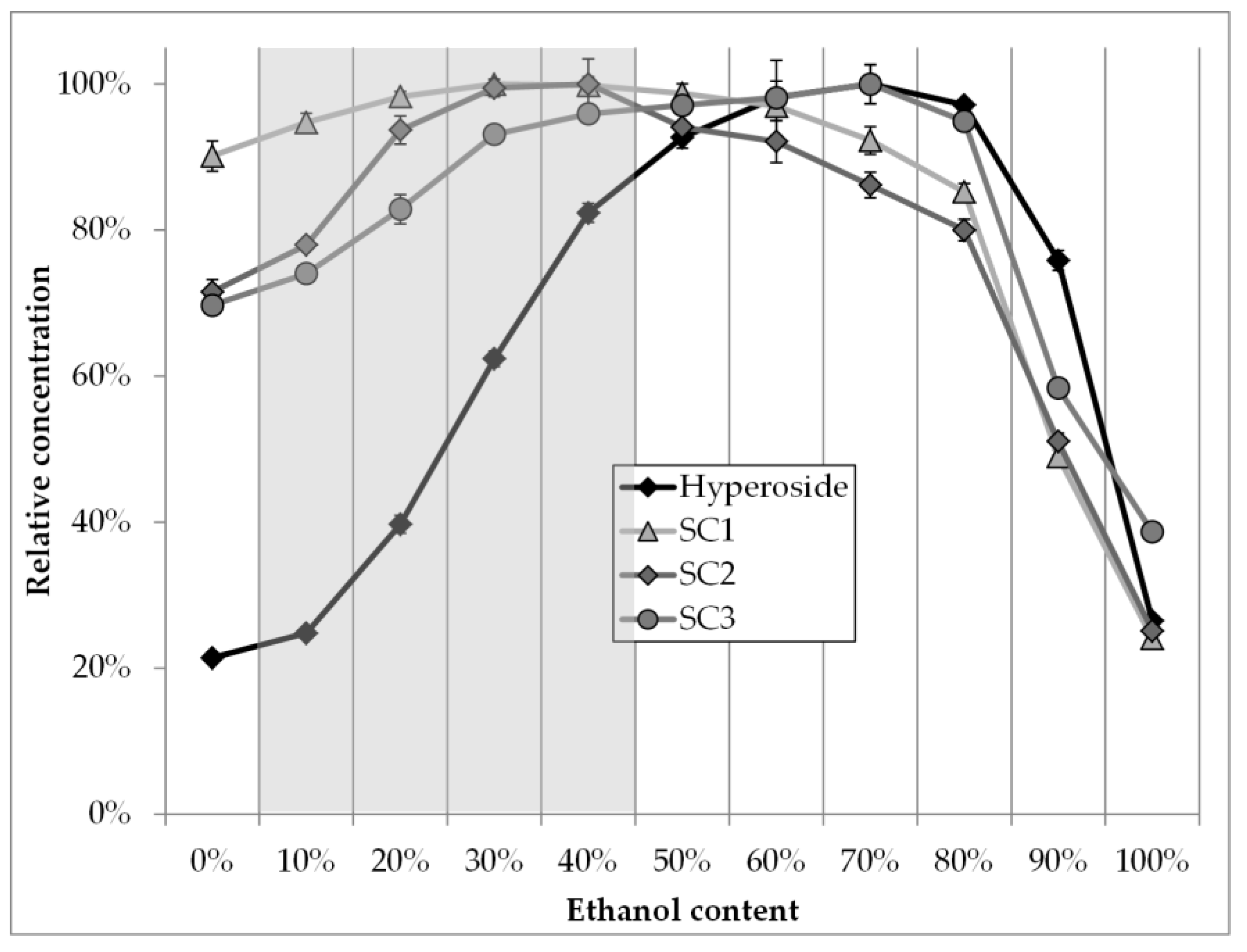
3.1. Solvent Screening
- Hyperoside, 70 vol. % ethanol, rest water
- SC1, 30 vol. % ethanol, rest water
- SC2, 30–40 vol. % ethanol, rest water
- SC3, 70 vol. % ethanol, rest water
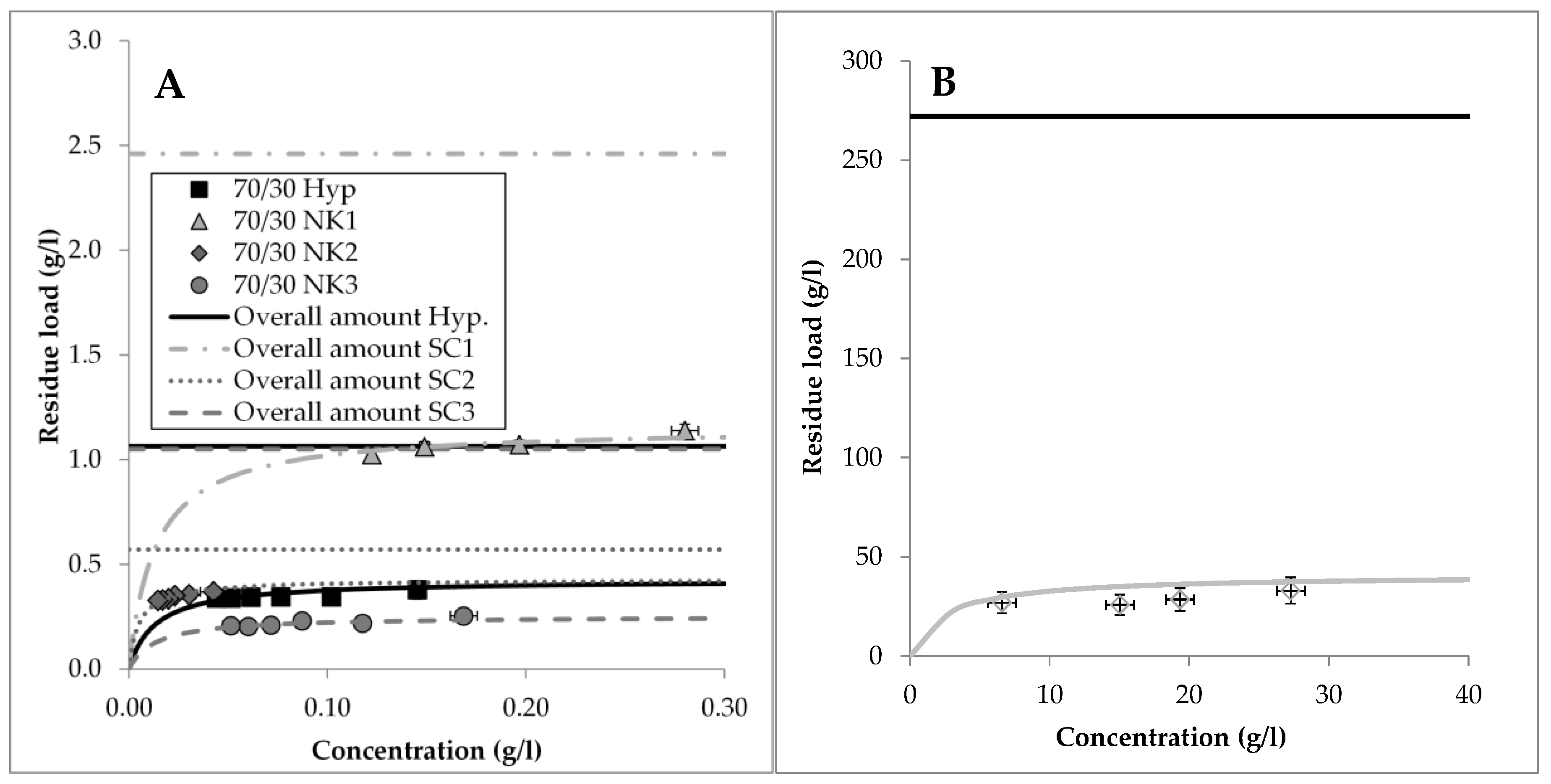
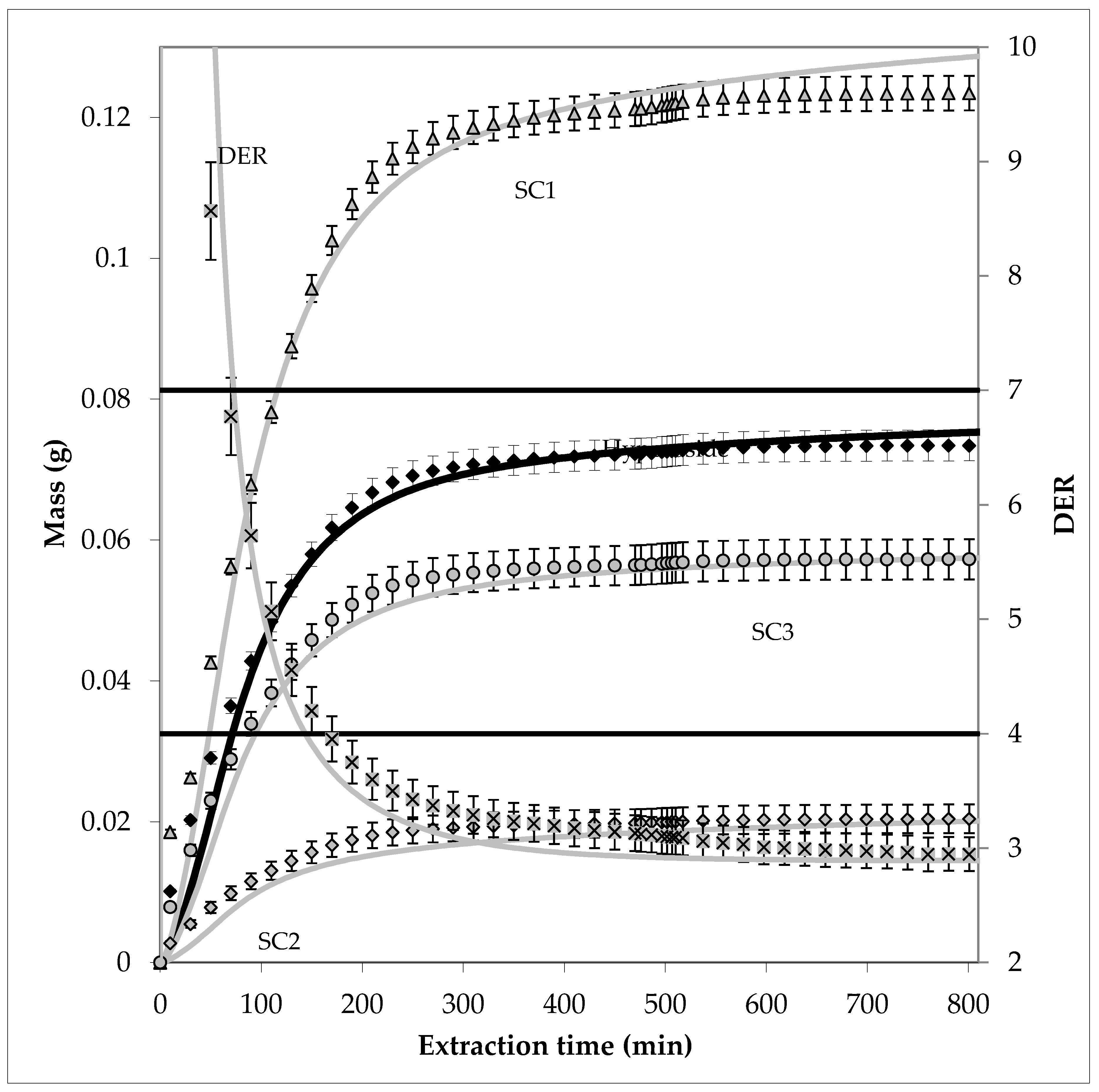
3.2. Model Parameter Determination for Conventional Extraction
3.3. Model Parameter Determination for PHWE
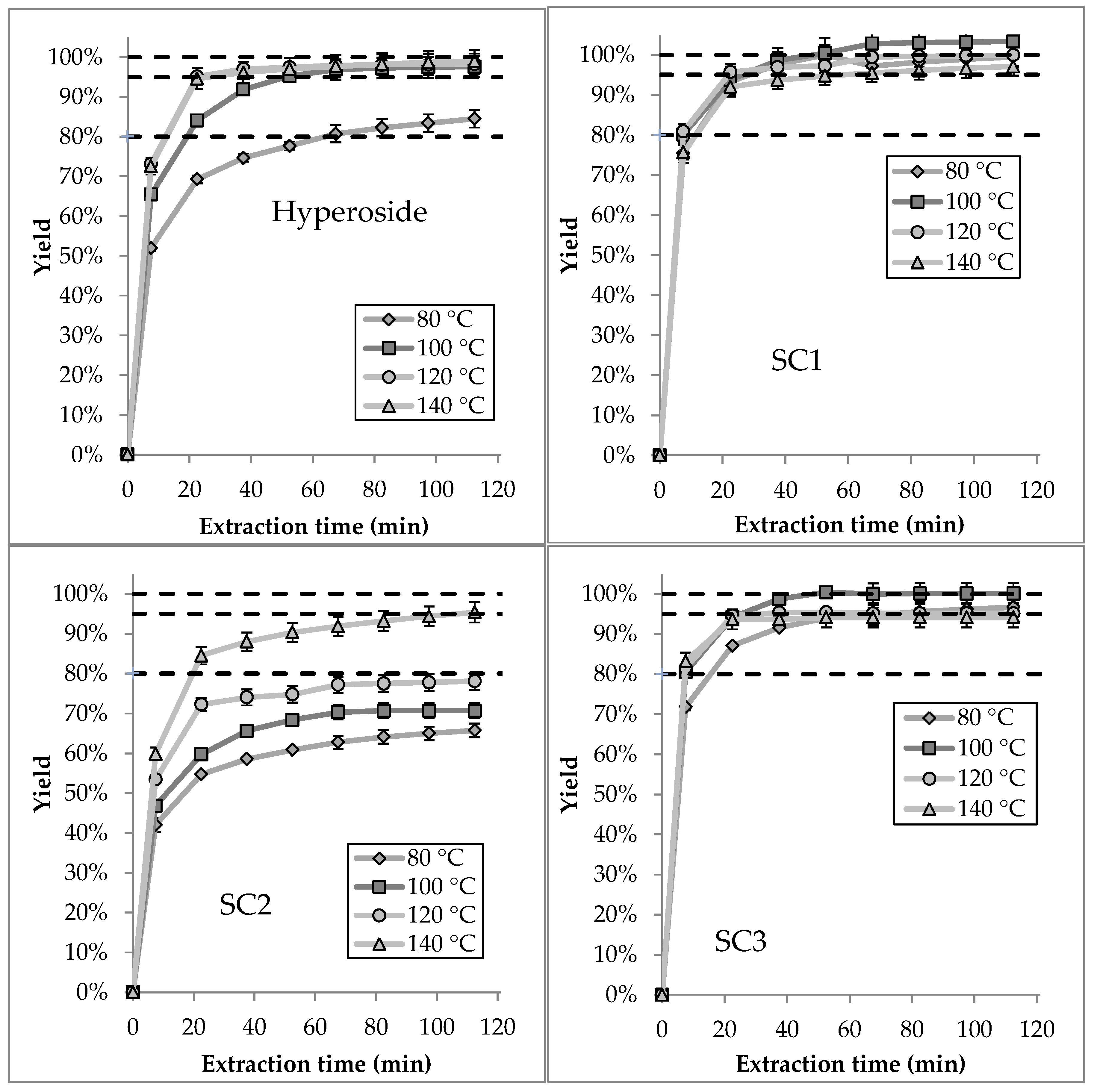
3.3.1. Extraction Temperature
- Hyperoside: The screening shows that hyperoside is almost entirely extracted after 60 min at 100 °C. A Yield of 95% is reached after about 20 min at 120 °C and 140 °C. At that temperature, the productivity is 250% higher compared to 100 °C and 700% higher compared to 80 °C. A decline in yield with rising temperature cannot be observed, indicating a high thermal stability of hyperoside.
- Side component 1: The productivity of extraction is nearly constant in the regarded temperature range from 80 °C to 140 °C. Thermal decay does not seem to appear, as well.
- Side component 2: SC2 shows the greatest dependency on temperature among the regarded substances. At 80 °C a yield of only about 70% is reached after 120 min. This yield is reached at 140 °C after just 15 min. The increase in productivity is approximately 800%. Thermal degradation cannot be traced based on this data.
- Side component 3: SC3 shows high productivity among the whole temperature field. Besides that, the yields at 80 °C and 140 °C are under the level of 100 °C (90% yield after 120 min). At a temperature of 140 °C this can indicate thermal decomposition, which is investigated in the following section. At 80 °C the productivity is limited because the temperature is not well-chosen for this substance.
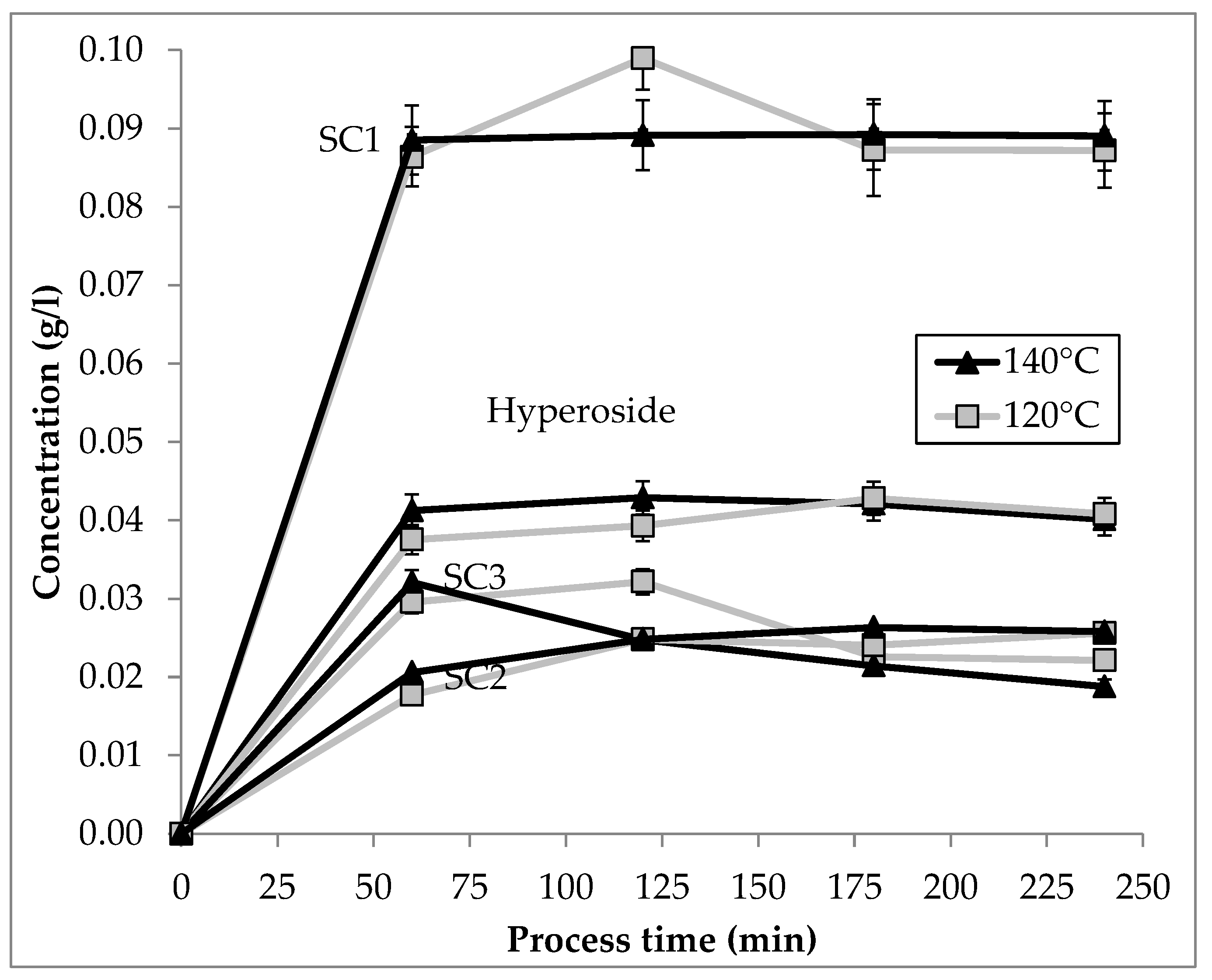
3.3.2. Thermal Degradation
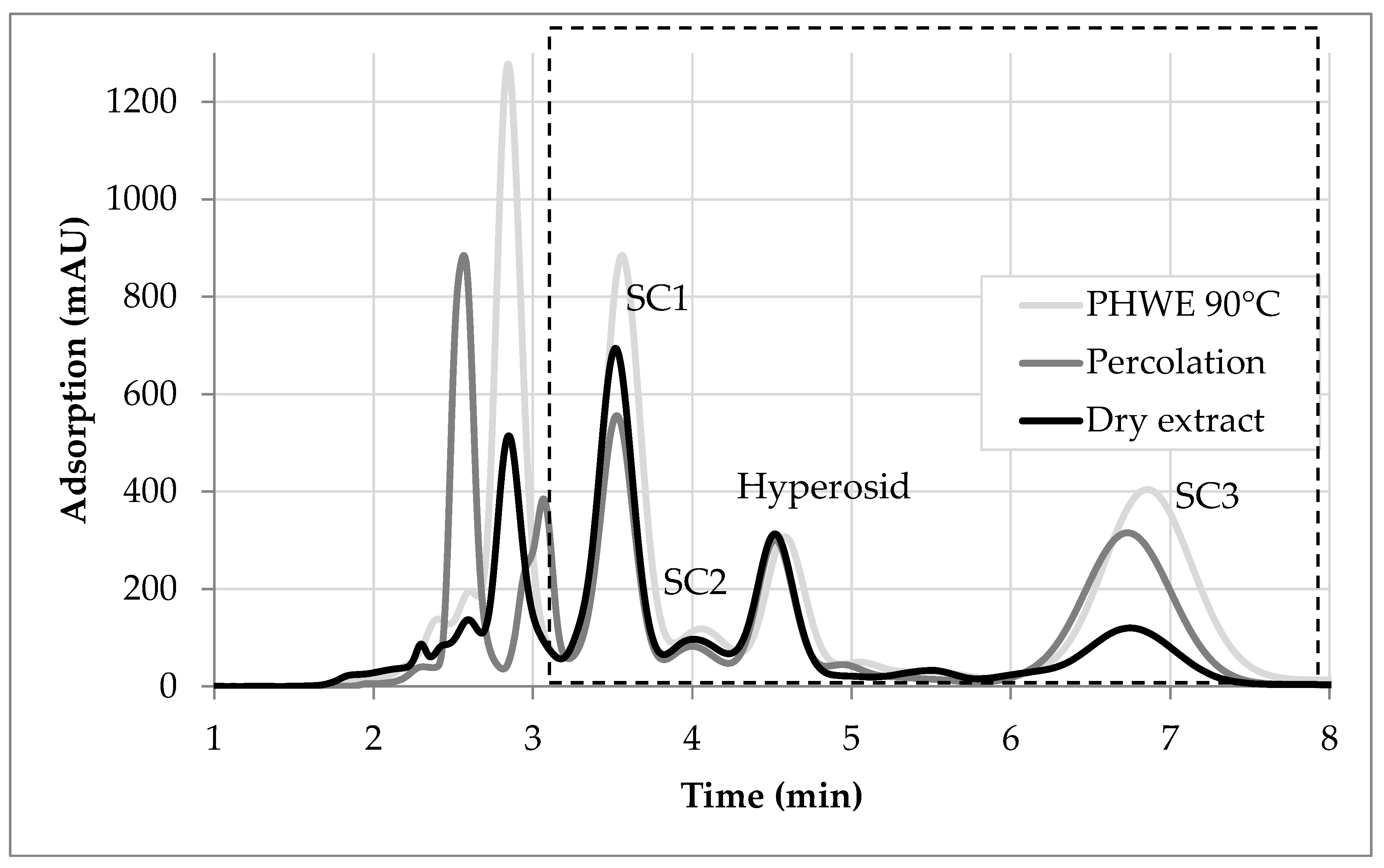
3.4. Evaluation of Hot Water Extraction and Solvent-Based Percolation
- Side component 1: SC1 shows a minor content in percolation compared to the commercially available product. According to the solvent screening, SC1 dissolves better in the solvent of the reference product. A significantly higher amount of SC1 is extracted during hot water extraction.
- Side component 2: SC2 shows the same behavior as SC1. According to the solvent screening, it dissolves better in the solvent used to produce the reference extract.
- Side component 3: The relative amount of SC3 in the reference extract is smaller than in the percolation performed in this study. According to the solvent screening the used mixture of 70 vol. % ethanol and 30 vol. % water has a much higher capacity towards this substance compared to the solution of the reference product. Again, the PHWE delivers an even higher amount.
3.5. Batch Variablity
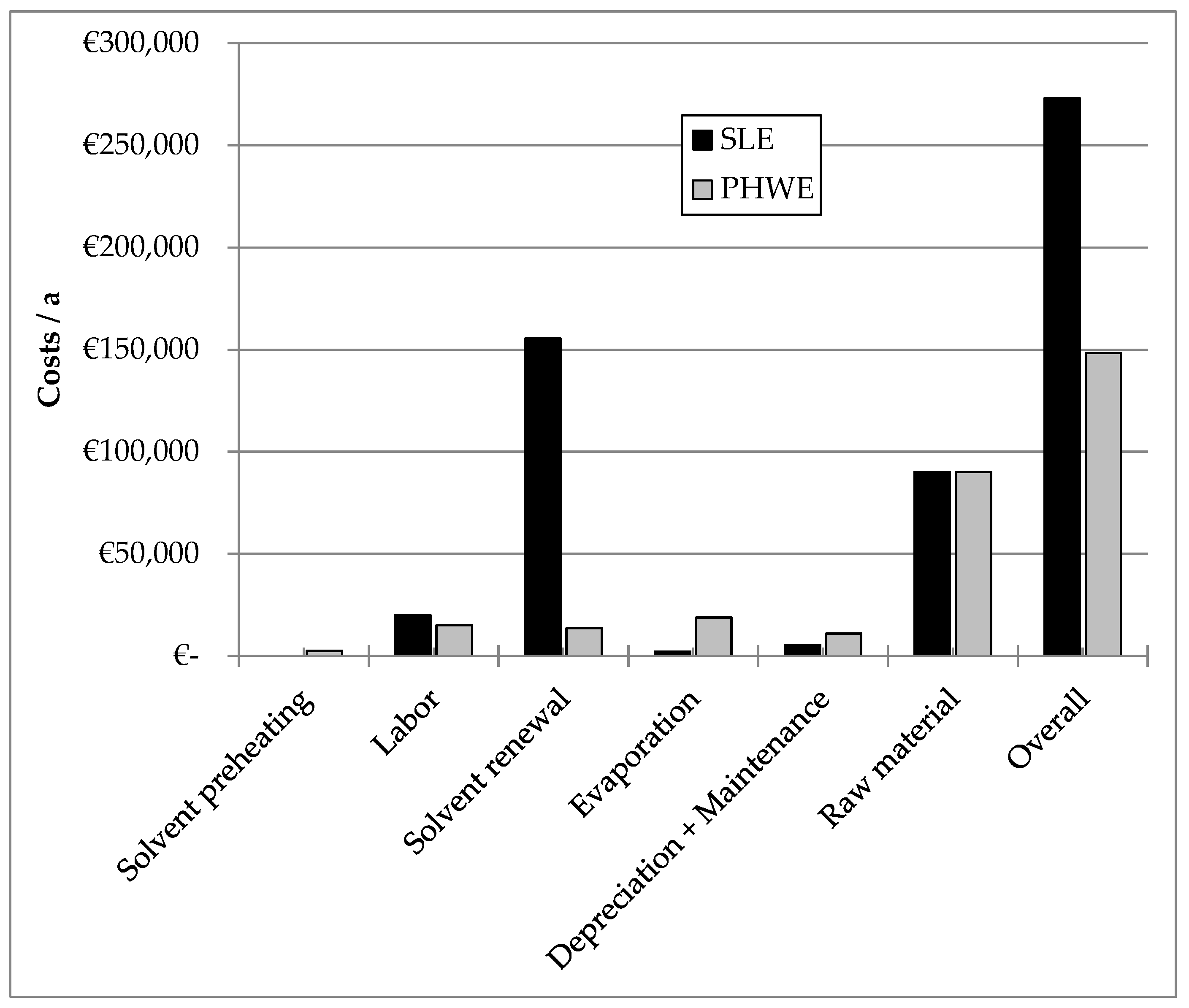
4. Economic Feasibility Study
- 30 tons of hawthorn leaves are to be extracted annually in 60 batches on a multi-product device. The purchase price is 3 €/kg. The annual occupancy of the system with this product should be 25%.
- For the SLE as well as for the PHWE, an extractor of 2 m3 empty volume is available. The investment costs for the SLE amount to 200,000 €. The high-pressure equipment is 100% more expensive than the conventional technology and amounts to 400,000 €.
- Operating parameters and process times up to 95% yield are directly taken from the laboratory studies.
- The energy costs are split into solvent preheating (here PHWE only) and evaporation. To operate the evaporators and the preheating steam (120 °C, 5 bar, 2.7 MJ/kg) is used at a price of 13 €/t.
- 10% of the solvent quantity per extraction is lost due to losses and must be renewed continuously. In addition, once a year, the entire pre-existing solvent, which is assumed to be 20 m3, is replaced. For the ethanol, a price of 3 €/L and for water 0.08 €/L is assumed.
- A worker is scheduled for the operation of the plant. The labor costs amount to 100,000 €/a and are weighted according to the total process time of the extraction of hawthorn added (multi-product plant!).
- The costs for depreciation and maintenance for this product amount to 2.5% of the total system costs (multi-product investment!).
5. Effort Analysis
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| aP | Specific surface area, 1/m |
| cL | Concentration in the liquid phase, kg/m3 |
| cP | Concentration in the porous particle, kg/m3 |
| Dax | Axial dispersion coefficient, m/s2 |
| Deff | Effective diffusion coefficient, m2/s |
| KL | Equilibrium constant, m3/kg |
| Pe | Péclet number |
| q | Loading, kg/m3 |
| qmax | Maximum Loading, kg/m3 |
| Re | Reynolds number |
| R | Radius, m |
| Sc | Schmidt number |
| SC | Side Component |
| Sh | Sherwood number |
| t | Time, s |
| uz | Superficial velocity, m/s |
| z | Coordinate in axial direction, m |
| ε | Dielectric constant, - |
| ε | Voids fraction, - |
| DPF | Distributed plug flow |
| HPLC | High performance liquid chromatography |
| i.d. | Inner diameter |
| PHWE | Pressurized hot water extraction |
| PTFE | Polytetrafluoroethylene |
| SLE | Solid-liquid extraction |
References
- Vuorela, P.; Leinonen, M.; Saikku, P.; Tammela, P.; Rauha, J.; Wennberg, T.; Vuorela, H. Natural Products in the process of finding new drug candidates. Curr. Med. Chem. 2004, 11, 1375–1389. [Google Scholar] [CrossRef]
- Koehn, F.E.; Carter, G.T. The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 2005, 4, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Teo, C.C.; Tan, S.N.; Yong, J.W.H.; Hew, C.S.; Ong, E.S. Pressurized hot water extraction (PHWE). J. Chromatogr. A 2010, 1217, 2484–2494. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.; Turner, C. Pressurized hot water extraction of bioactives. TrAC Trends Anal. Chem. 2015, 71, 39–54. [Google Scholar] [CrossRef]
- Sixt, M.; Strube, J. Systematic and Model-Assisted Evaluation of Solvent Based- or Pressurized Hot Water Extraction for the Extraction of Artemisinin from Artemisia annua L. Processes 2017, 5, 86. [Google Scholar] [CrossRef]
- Sixt, M.; Strube, J. Pressurized hot water extraction of 10-deacetylbaccatin III from yew for industrial application. Resour. Effic. Technol. 2017, 3, 177–186. [Google Scholar] [CrossRef]
- Hiller, K.; Melzig, M.F. Lexikon der Arzneipflanzen und Drogen, 2. Auflage; Spektrum Akademischer: Heidelberg, Germany, 2010. [Google Scholar]
- Europäisches Arzneibuch. Europäisches Arzneibuch DVD-ROM 8. Ausgabe, Grundwerk 2014 (Ph. Eur. 8.0) inkl. 1. bis 8. Nachtrag (Ph.Eur. 8.1 bis 8.8). Amtliche deutsche Ausgabe, 1. Auflage; Deutscher Apotheker Verlag: Stuttgart, Germany, 2016. [Google Scholar]
- Hevert. Bomacorin 450 mg Weißdorntabletten N: Nussbaum. 2004. Available online: https://www.docmorris.de/medias/sys_master/8452192362128000/3137679.pdf (accessed on 7 January 2018).
- Schwabe. Gebrauchsinformation. Crataegutt® novo 450 mg: Karlsruhe. 2006. Available online: https://www.docmorris.de/medias/sys_master/h91/hdf/8845213073438/GI-Crataegutt-novo-450-mg.pdf (accessed on 7 January 2018).
- Hexal. Gebrauchsinformation: Information für den Anwender. Craegium novo 450 mg, Holzkirchen. 2007. Available online: https://m.apotal.de/images/ecommerce/01/35/01359849_2007-07_de_o.pdf (accessed on 7 January 2018).
- Aliud Pharma. Gebrauchsinformation: Information für Anwender. Crataegus® AL 450 mg Filmtabletten, Laichingen. 2012. Available online: https://www.aliva.de/images/ecommerce/00/01/00013178_2012-05_de_o.pdf (accessed on 7 January 2018).
- Schaper und Brümmer. Gebrauchsinformation: Information für den Anwender. Esbericard® novo Dragees: Salzgitter. 2013. Available online: http://www.schaper-bruemmer.de/fileadmin/docs/beipackzettel/Gebrauchsinformationen_Esbericard_novo_Dragees.pdf (accessed on 7 January 2018).
- Kaßing, M. Process Development for Plant-Based Extract Production; Shaker: Aachen, Germany, 2012. [Google Scholar]
- Kaßing, M.; Jenelten, U.; Schenk, J.; Hänsch, R.; Strube, J. Combination of Rigorous and Statistical Modeling for Process Development of Plant-Based Extractions Based on Mass Balances and Biological Aspects. Chem. Eng. Technol. 2012, 35, 109–132. [Google Scholar] [CrossRef]
- Both, S.; Koudous, I.; Jenelten, U.; Strube, J. Model-based equipment-design for plant-based extraction processes—Considering botanic and thermodynamic aspects. C. R. Chim. 2014, 17, 187–196. [Google Scholar] [CrossRef]
- Both, S. Systematische Verfahrensentwicklung für Pflanzlich Basierte Produkte im Regulatorischen Umfeld; Shaker: Aachen, Germany, 2015. [Google Scholar]
- Sixt, M.; Koudous, I.; Strube, J. Process design for integration of extraction, purification and formulation with alternative solvent concepts. C. R. Chim. 2016, 19, 733–748. [Google Scholar] [CrossRef]
- Uhlenbrock, L.; Sixt, M.; Strube, J. Quality-by-Design (QbD) process evaluation for phytopharmaceuticals on the example of 10-deacetylbaccatin III from yew. Resour. Effic. Technol. 2017, 3, 137–143. [Google Scholar] [CrossRef]
- Sixt, M.; Strube, J. In-Line Raman Spectroscopy and Advanced Process Control for the Extraction of Anethole and Fenchone from Fennel Fruits (Foeniculum vulgare L. MILL.). C. R. Chim. 2018, 21, 97–103. [Google Scholar] [CrossRef]
- Kaßing, M.; Jenelten, U.; Schenk, J.; Strube, J. A New Approach for Process Development of Plant-Based Extraction Processes. Chem. Eng. Technol. 2010, 33, 377–387. [Google Scholar] [CrossRef]
- Koudous, I.; Both, S.; Gudi, G.; Schulz, H.; Strube, J. Process design based on physicochemical properties for the example of obtaining valuable products from plant-based extracts. C. R. Chim. 2014, 17, 218–231. [Google Scholar] [CrossRef]
- Both, S.; Eggersgluess, J.K.; Lehnberger, A.; Schulz, T.; Strube, J. Optimizing Established Processes like Sugar Extraction from Sugar Beets—Design of Experiments versus Physicochemical Modeling. Chem. Eng. Technol. 2013, 36, 2125–2136. [Google Scholar] [CrossRef]
- Wang, C.-H.; Wang, Y.-X.; Liu, H.-J. Validation and application by HPLC for simultaneous determination of vitexin-2″-O-glucoside, vitexin-2″-O-rhamnoside, rutin, vitexin, and hyperoside. J. Pharm. Anal. 2011, 1, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Gudi, G.; Krähmer, A.; Koudous, I.; Strube, J.; Schulz, H. Infrared and Raman spectroscopic methods for characterization of Taxus baccata L.—Improved taxane isolation by accelerated quality control and process surveillance. Talanta 2015, 143, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Fintelmann, V.; Weiß, R.F. Lehrbuch der Phytotherapie; Hippokrates-Verlag: Stuttgart, Germany, 2002. [Google Scholar]
- Sovová, H. Rate of the vegetable oil extraction with supercritical CO2-I. Modelling of extraction curves. Chem. Eng. Sci. 1994, 49, 409–414. [Google Scholar] [CrossRef]
- Perrut, M.; Clavier, J.Y.; Poletto, M.; Reverchon, E. Mathematical Modeling of Sunflower Seed Extraction by Supercritical CO2. Ind. Eng. Chem. Res. 1997, 36, 430–435. [Google Scholar] [CrossRef]
- Sovová, H. Mathematical model for supercritical fluid extraction of natural products and extraction curve evaluation. J. Supercrit. Fluids 2005, 33, 35–52. [Google Scholar] [CrossRef]
- Koudous, I. Stoffdatenbasierte Verfahrensentwicklung zur Isolierung von Wertstoffen aus Pflanzenextrakten, 1. Auflage; Shaker: Herzogenrath, Germany, 2017. [Google Scholar]


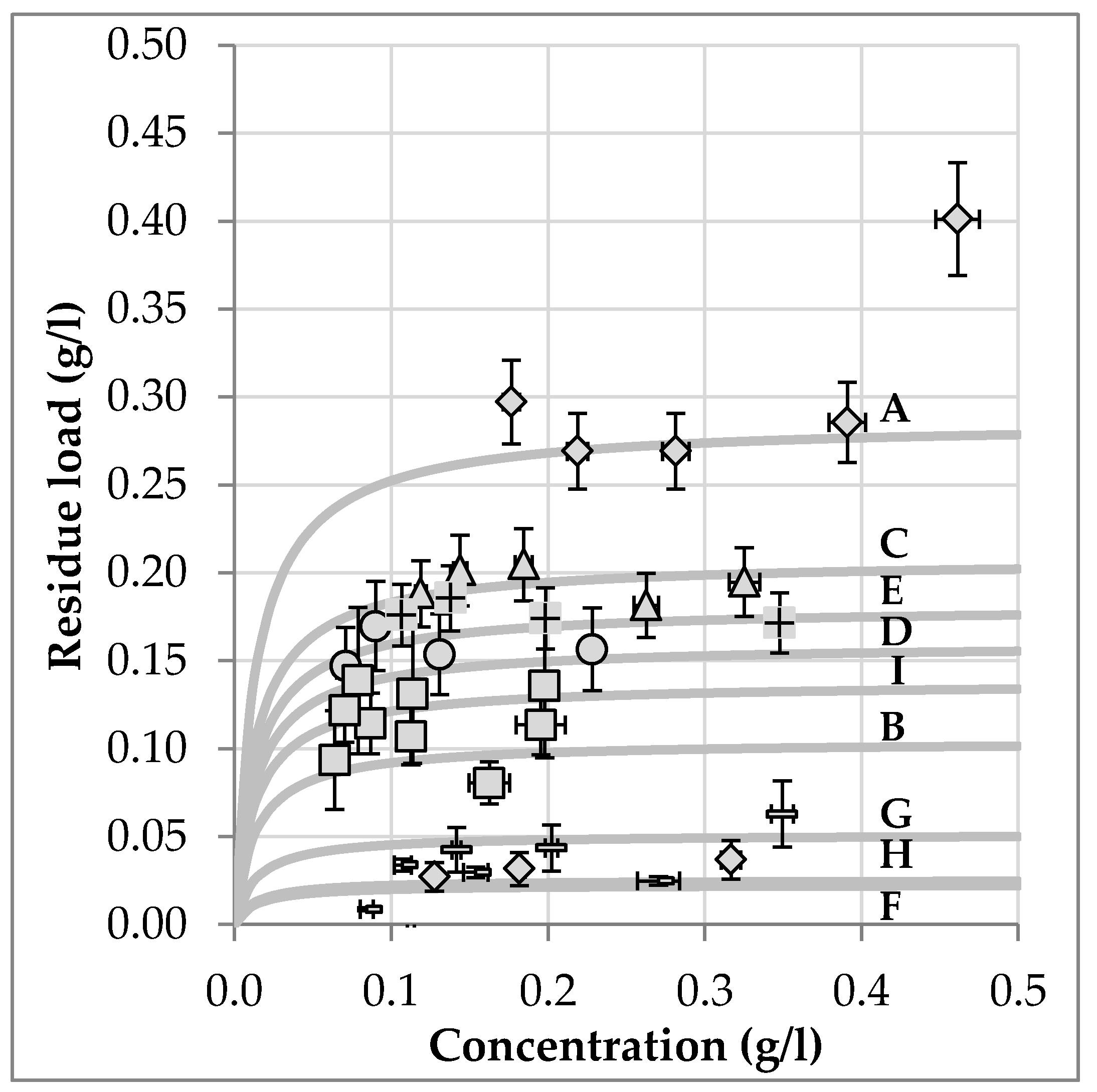
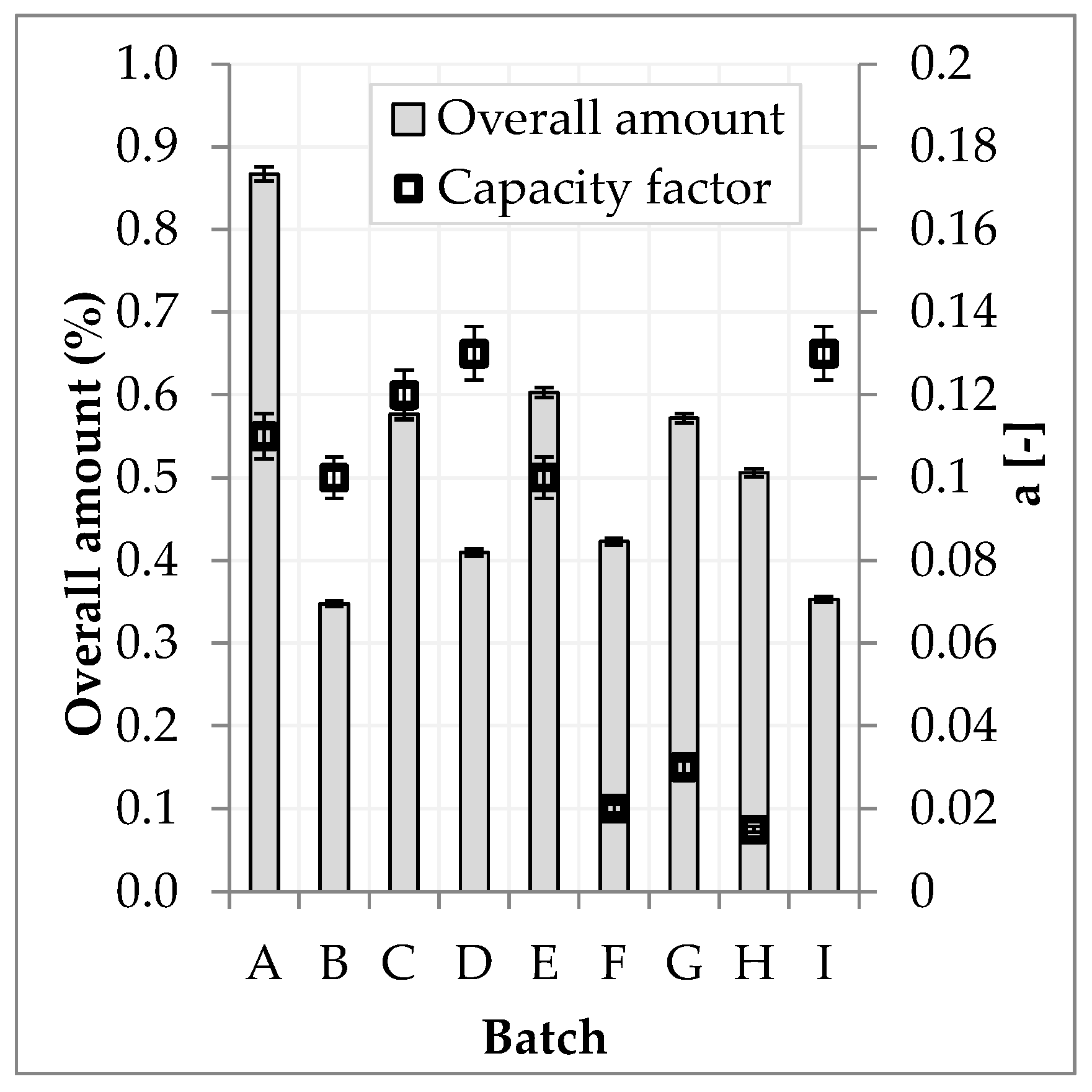
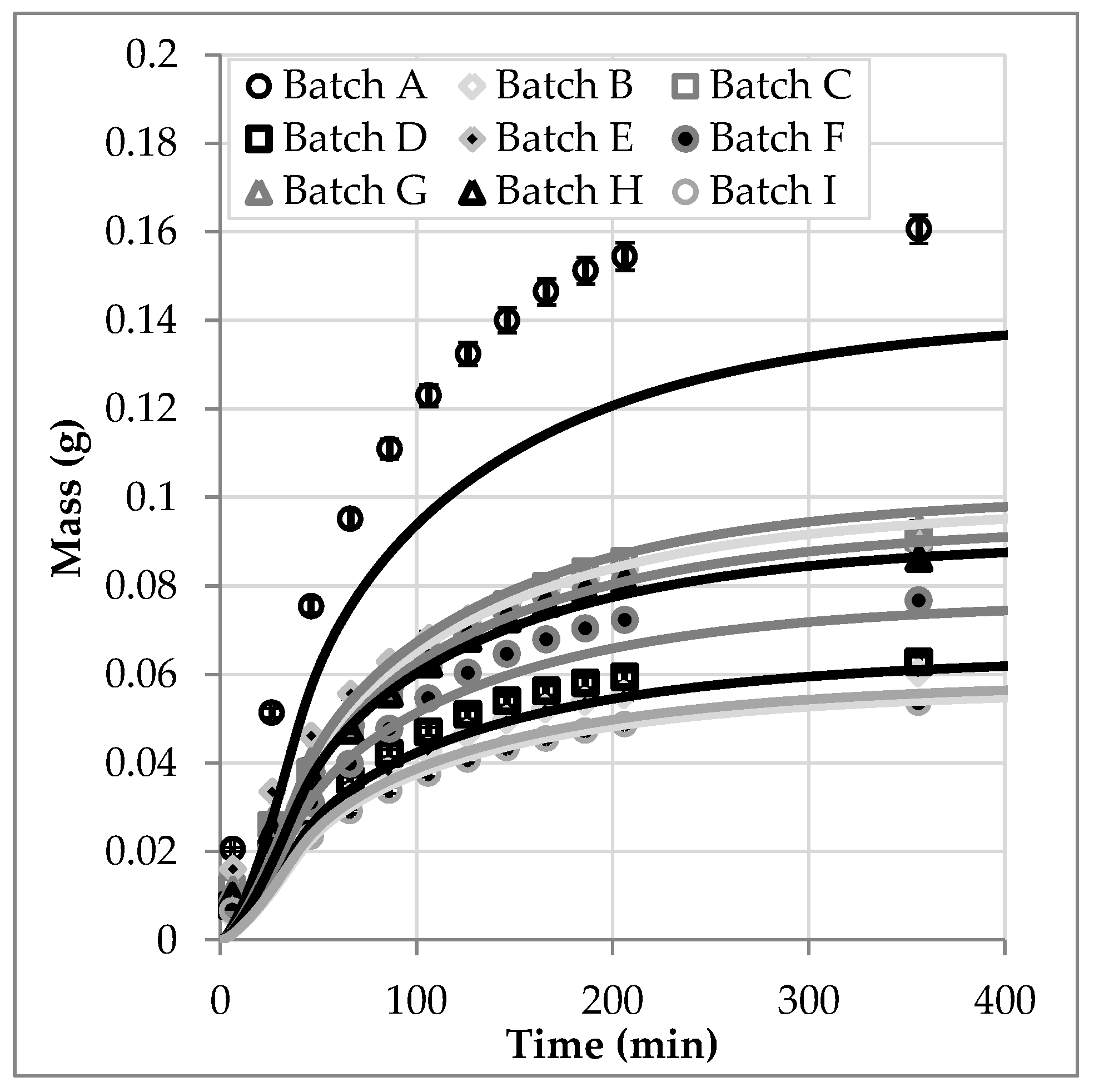
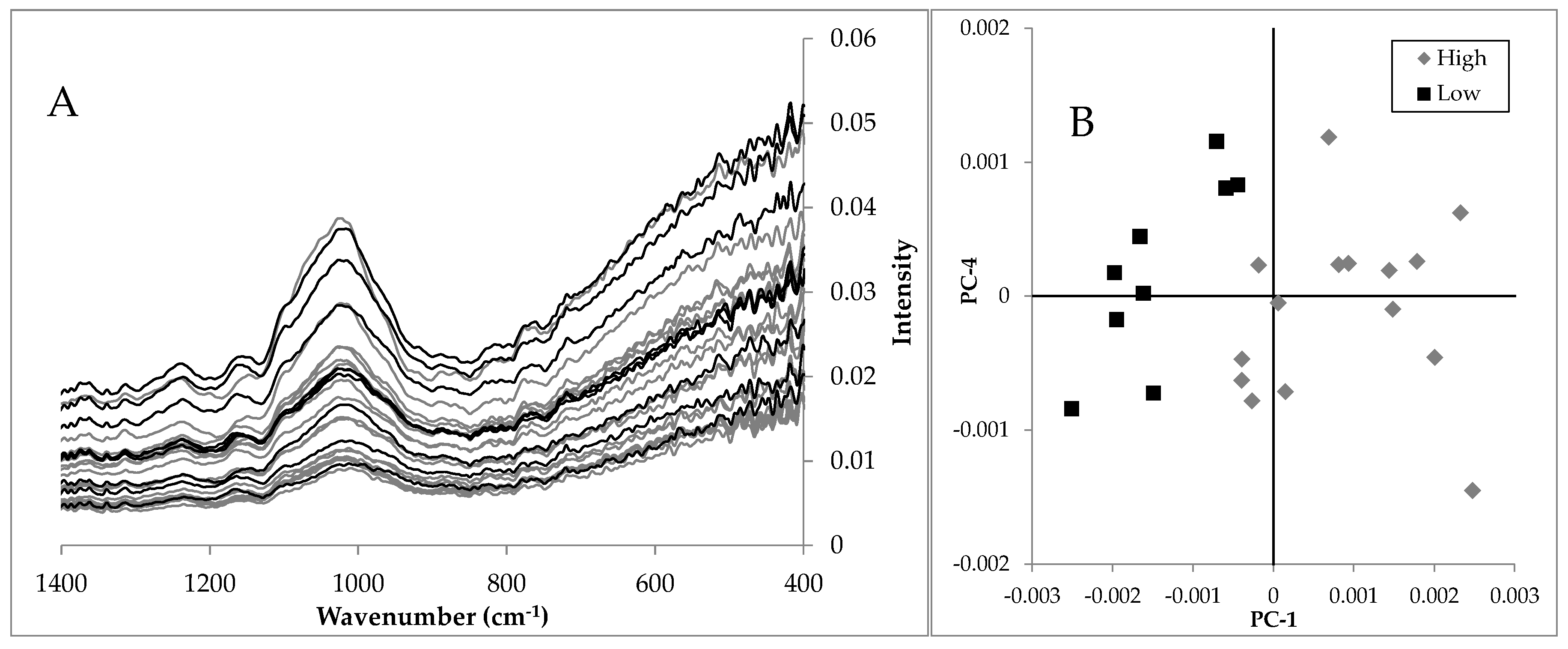

| Batch | Harvest Year | Origin | Overall Amount | Deviation to Reference | a | Deviation to Reference |
|---|---|---|---|---|---|---|
| A | 2017 | Southeast Europe | 0.87% | 146% | 0.11 | −15% |
| B | 2016 | Macedonia | 0.35% | −1% | 0.10 | −23% |
| C | 2017 | Bulgaria | 0.58% | 64% | 0.12 | −8% |
| D | 2017 | Romania | 0.41% | 16% | 0.13 | 0% |
| E | 2014 | Bulgaria | 0.60% | 71% | 0.10 | −23% |
| F | 2017 | Albania | 0.42% | 20% | 0.02 | −84% |
| G | 2017 | Southeast Europe | 0.57% | 62% | 0.03 | −77% |
| H | 2017 | Serbia | 0.51% | 43% | 0.015 | −88% |
| I (Reference) | 2017 | Germany | 0.35% | - | 0.13 | - |
| Batch | Mass Experiment (g) | Mass Simulation (g) | Difference (g) | Deviation |
|---|---|---|---|---|
| A | 0.135 | 0.161 | −0.026 | −16% |
| B | 0.054 | 0.060 | −0.006 | −10% |
| C | 0.090 | 0.091 | −0.001 | −1% |
| D | 0.061 | 0.063 | −0.002 | −3% |
| E | 0.094 | 0.090 | 0.004 | 5% |
| F | 0.074 | 0.077 | −0.003 | −4% |
| G | 0.097 | 0.093 | 0.004 | 4% |
| H | 0.087 | 0.086 | 0.000 | 0% |
| I (Reference) | 0.056 | 0.054 | 0.002 | 3% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sixt, M.; Strube, J. Systematic Design and Evaluation of an Extraction Process for Traditionally Used Herbal Medicine on the Example of Hawthorn (Crataegus monogyna JACQ.). Processes 2018, 6, 73. https://doi.org/10.3390/pr6070073
Sixt M, Strube J. Systematic Design and Evaluation of an Extraction Process for Traditionally Used Herbal Medicine on the Example of Hawthorn (Crataegus monogyna JACQ.). Processes. 2018; 6(7):73. https://doi.org/10.3390/pr6070073
Chicago/Turabian StyleSixt, Maximilian, and Jochen Strube. 2018. "Systematic Design and Evaluation of an Extraction Process for Traditionally Used Herbal Medicine on the Example of Hawthorn (Crataegus monogyna JACQ.)" Processes 6, no. 7: 73. https://doi.org/10.3390/pr6070073
APA StyleSixt, M., & Strube, J. (2018). Systematic Design and Evaluation of an Extraction Process for Traditionally Used Herbal Medicine on the Example of Hawthorn (Crataegus monogyna JACQ.). Processes, 6(7), 73. https://doi.org/10.3390/pr6070073





