Ionic Liquid (1-Butyl-3-Metylimidazolium Methane Sulphonate) Corrosion and Energy Analysis for High Pressure CO2 Absorption Process
Abstract
1. Introduction
2. Materials and Methods
3. Results & Discussion
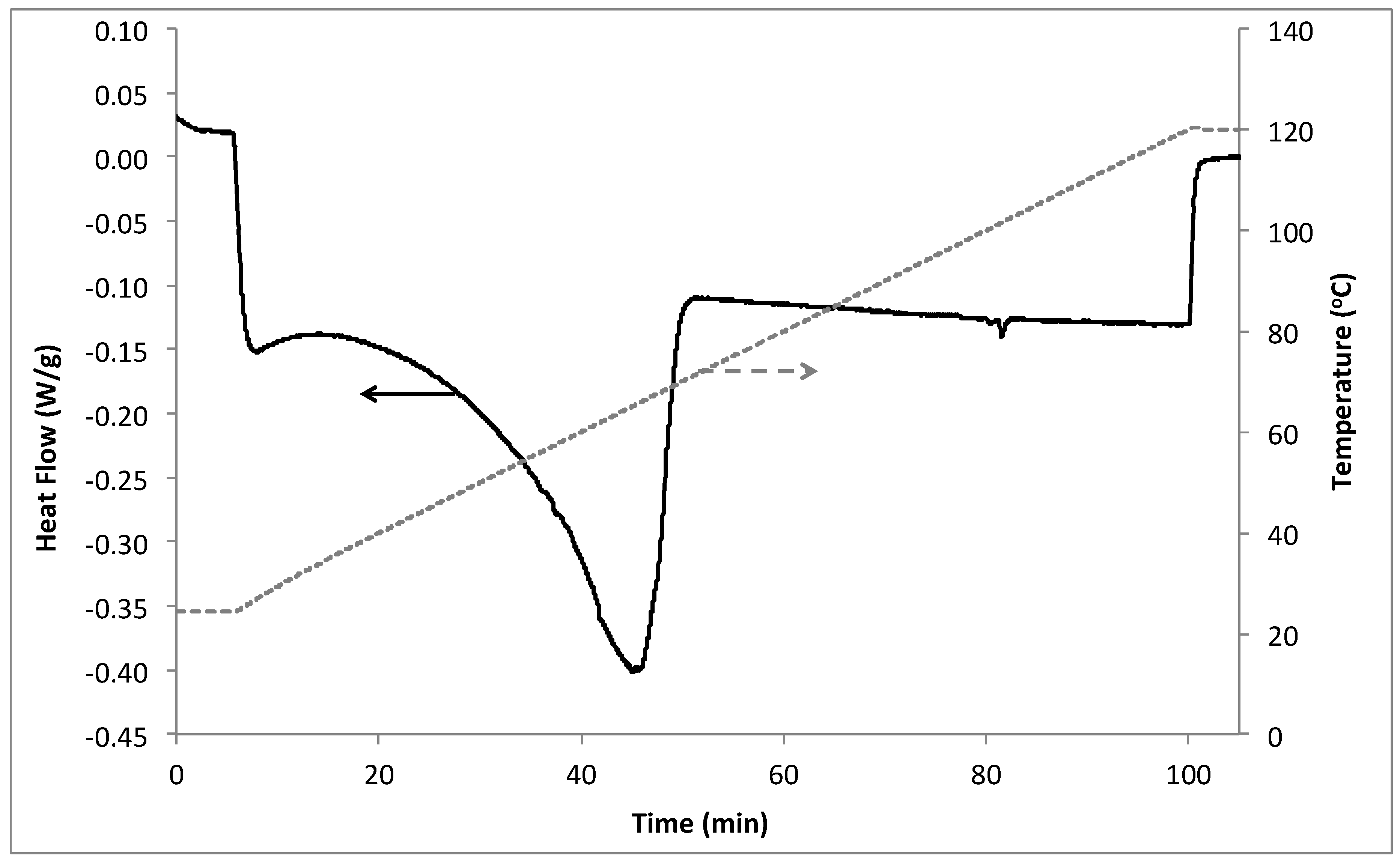
3.1. Differential Scanning Calorimetry
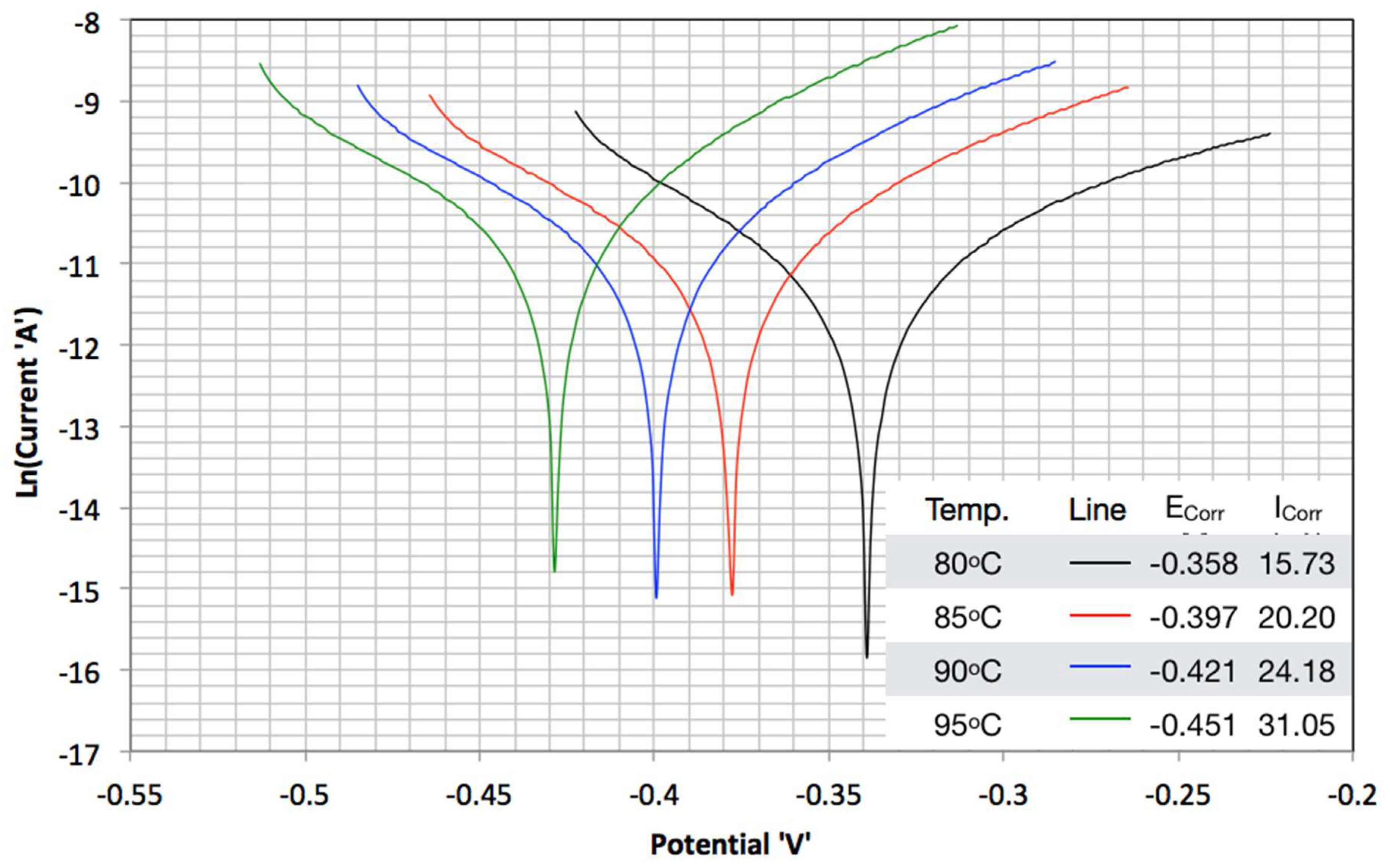
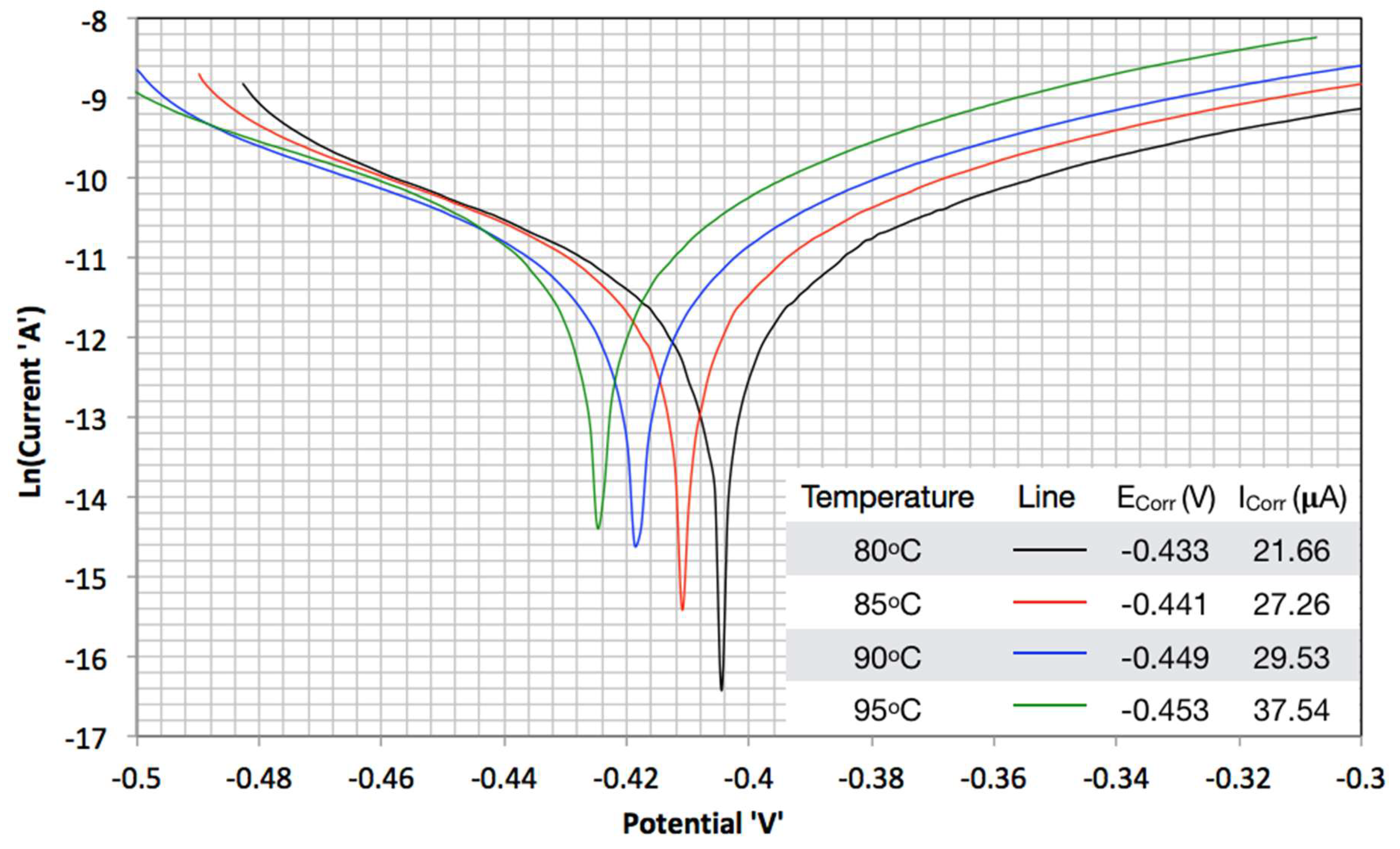
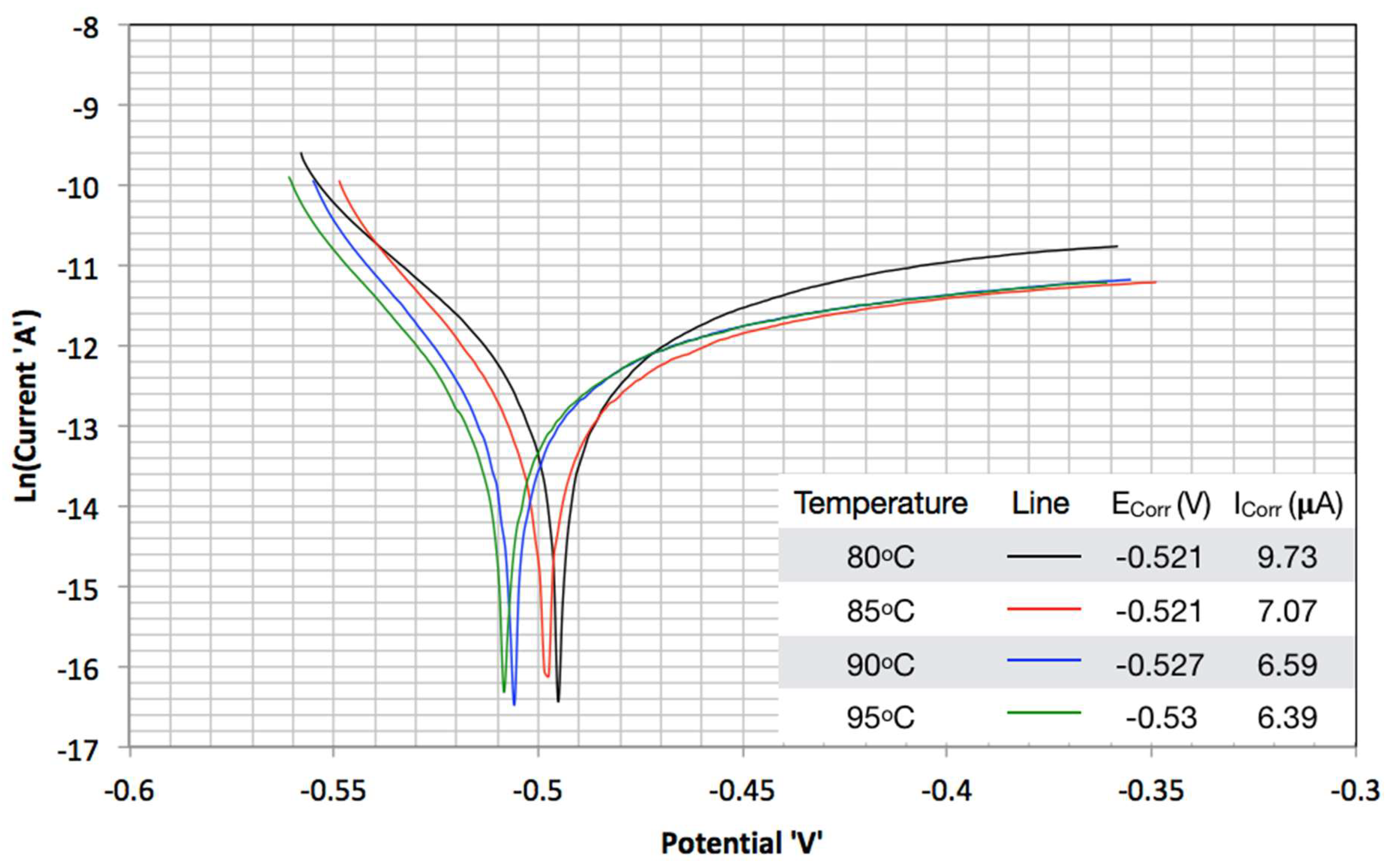
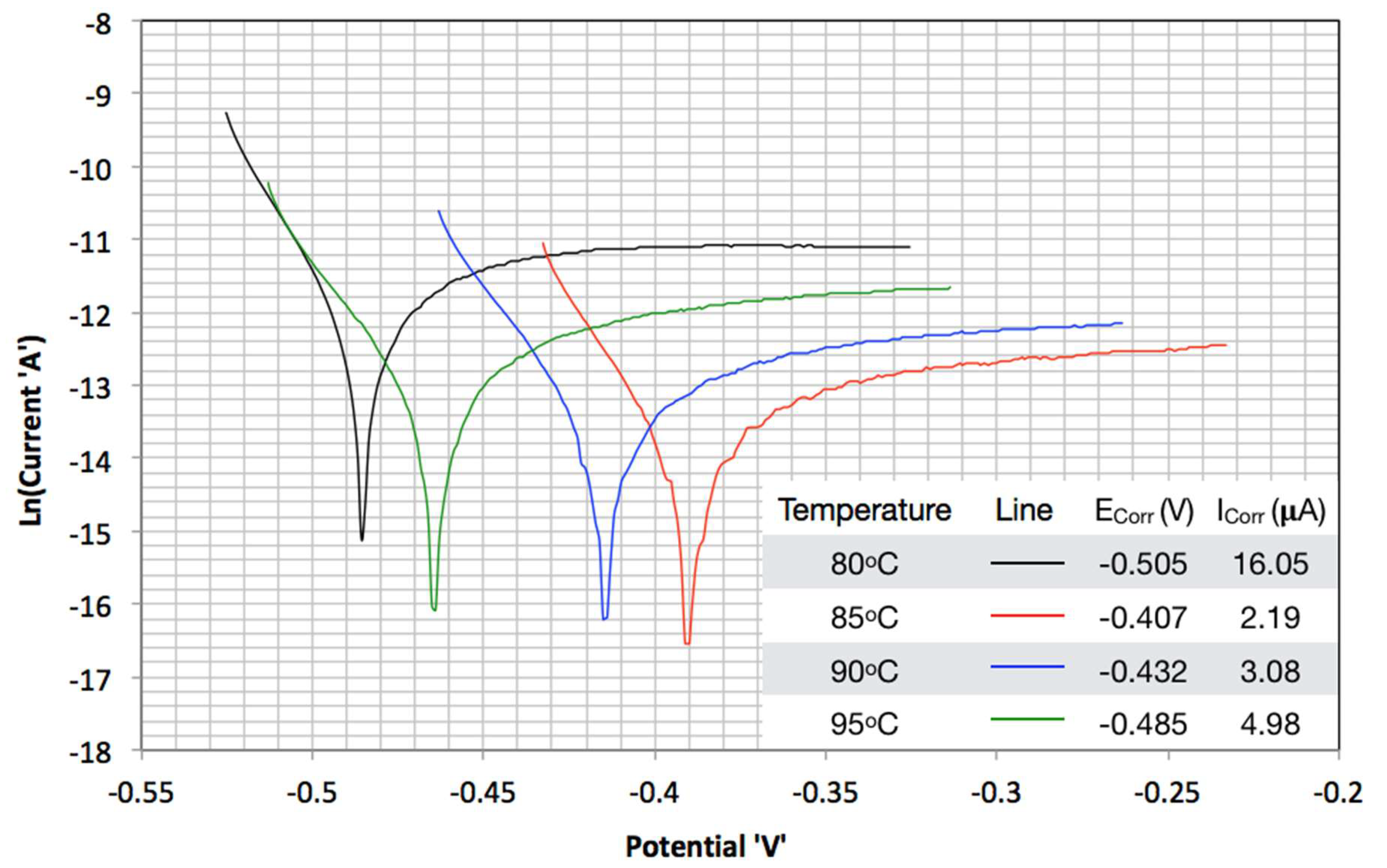
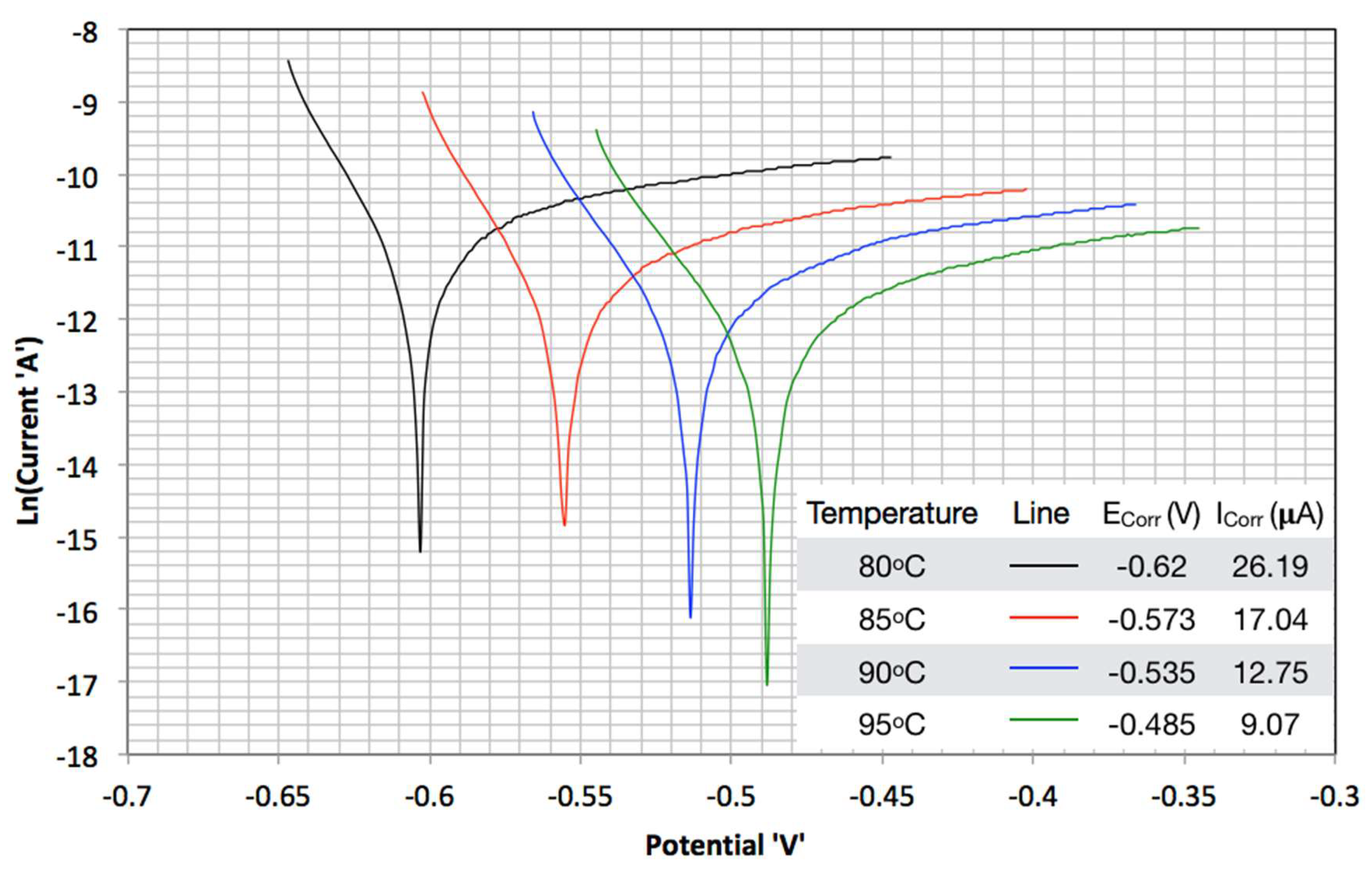
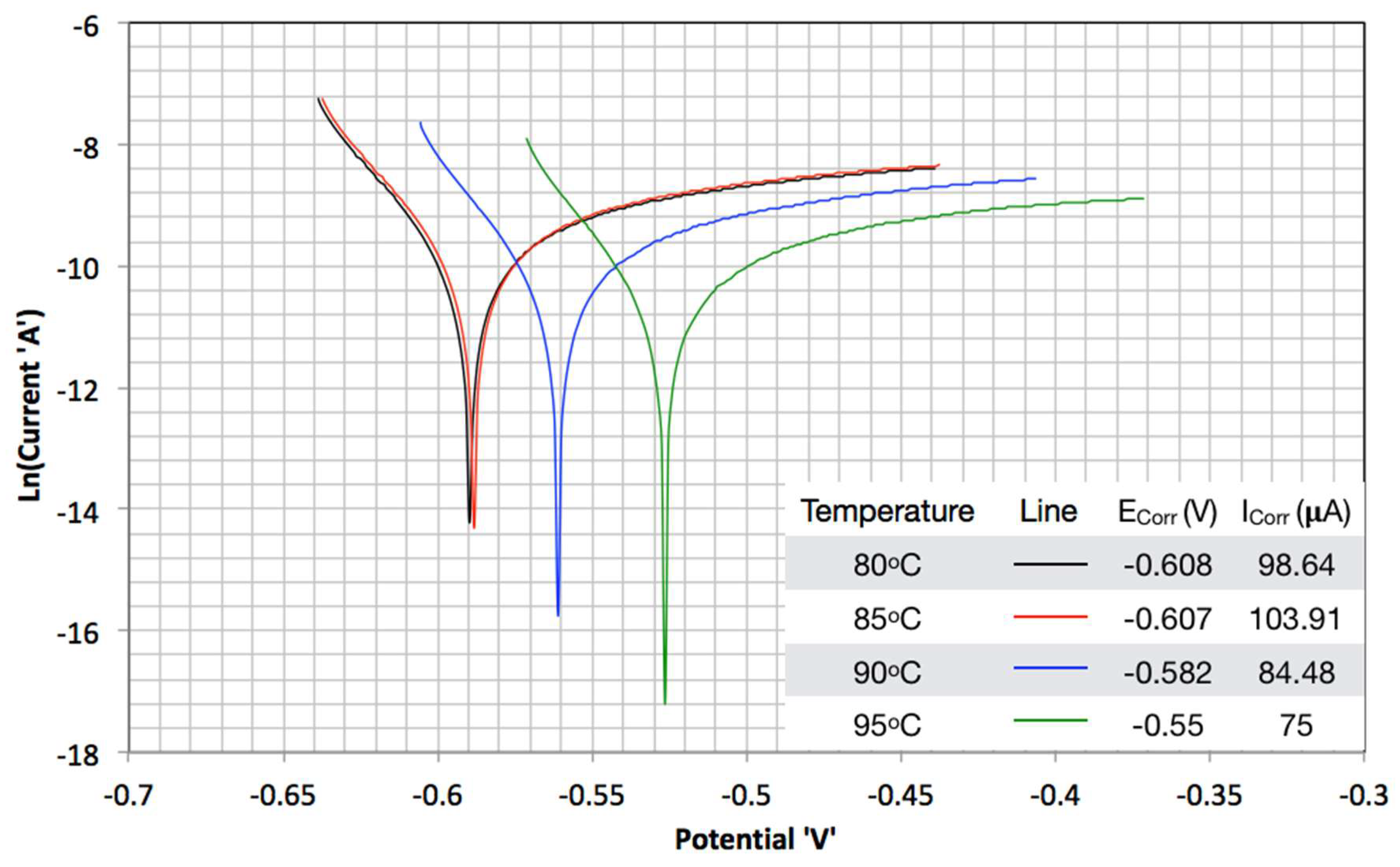
3.2. TAFEL Curves
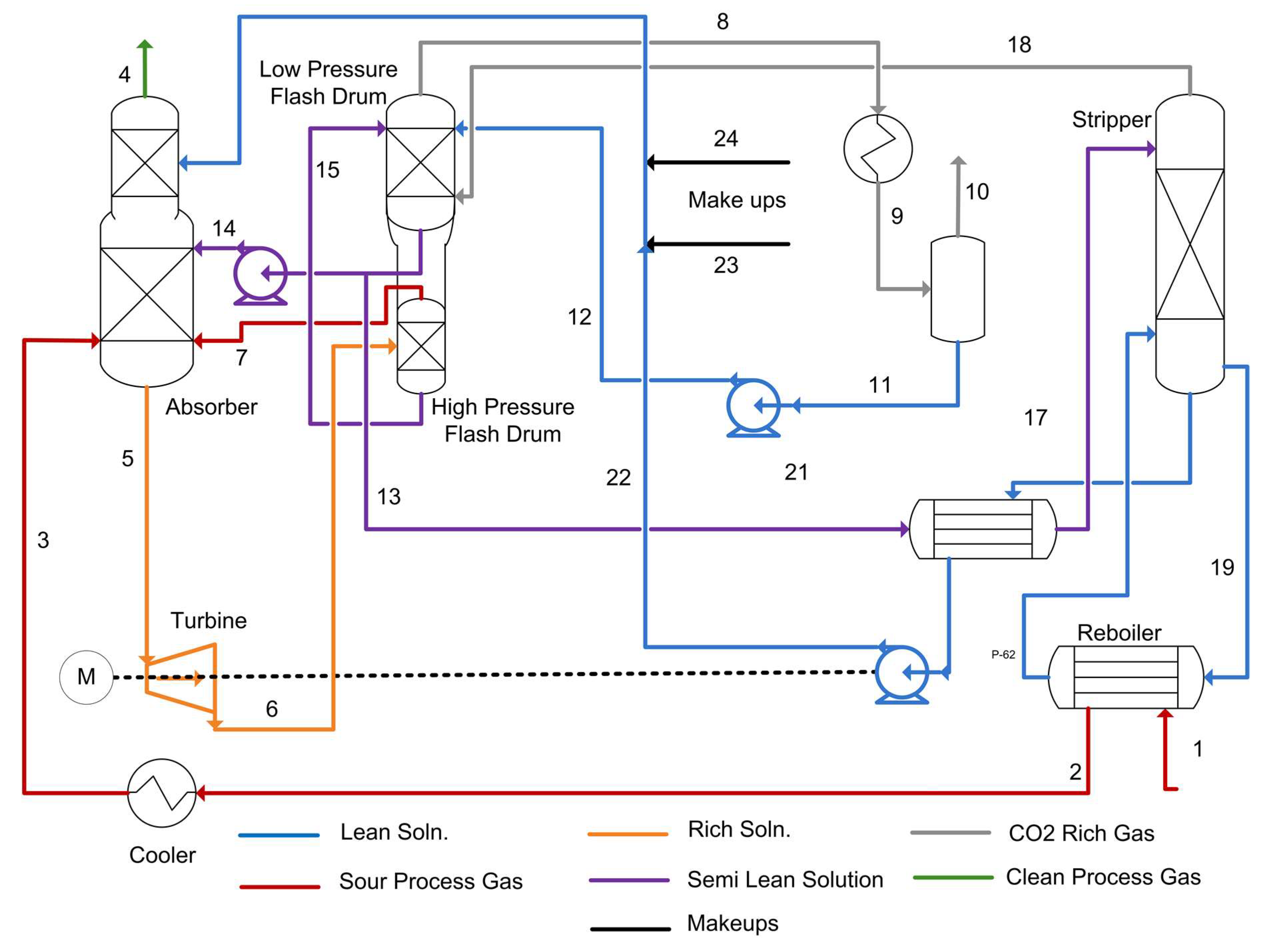
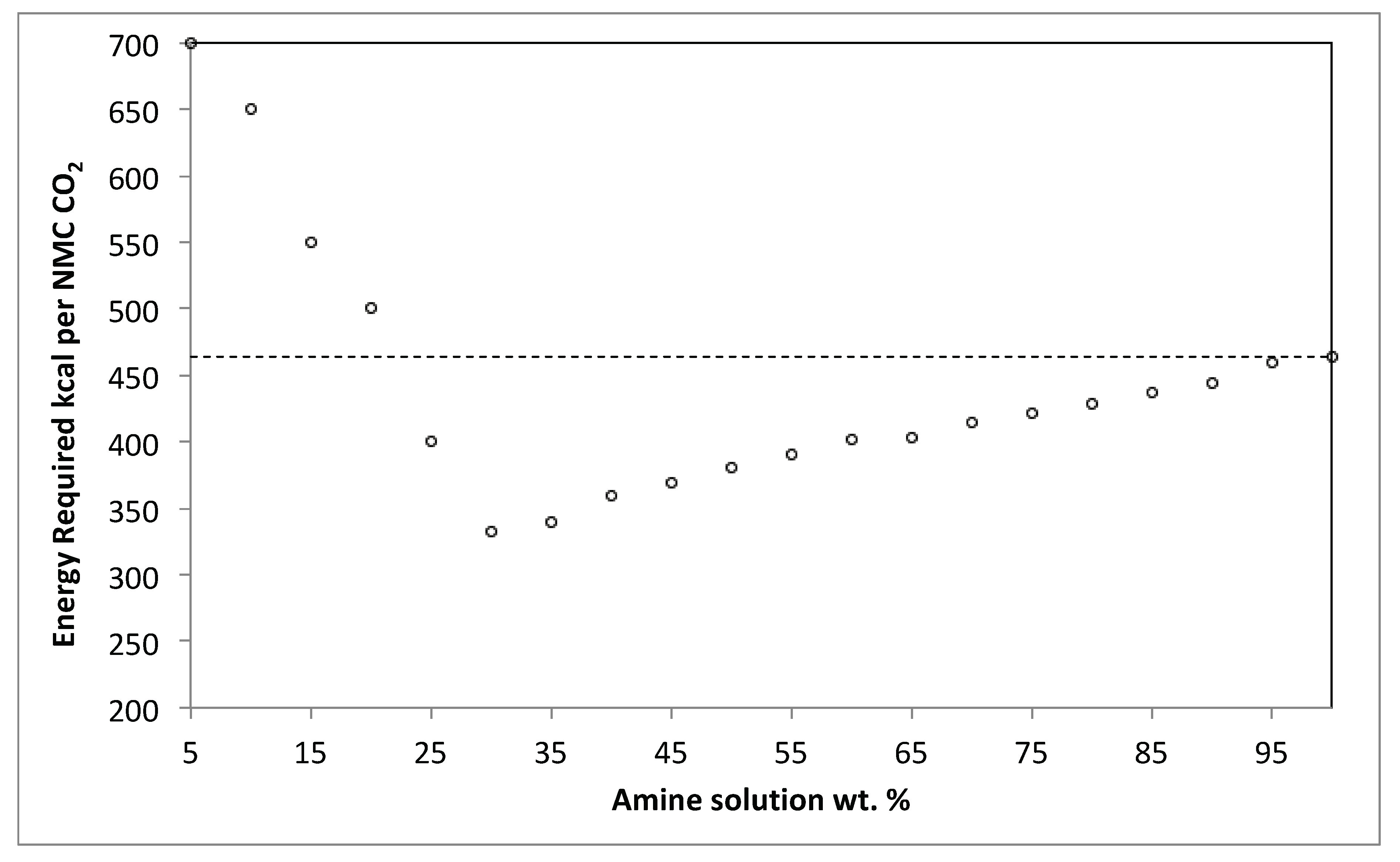
3.3. Process Simulation and Validation
3.3.1. Existing Facility Model
3.3.2. Absorption Process with Ionic Liquid
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yang, H.; Xu, Z.; Fan, M.; Gupta, R.; Slimane, R.B.; Bland, A.; Wright, I. Progress in carbon dioxide separation and capture: A review. J. Environ. Sci. 2008, 20, 14–27. [Google Scholar] [CrossRef]
- Taimoor, A.A.; Pitault, I.; Meunier, F.C. Correlation between deactivation and Pt-carbonyl formation during toluene hydrogenation using a H2/CO2 mixture. J. Catal. 2011, 278, 153–161. [Google Scholar] [CrossRef]
- Aaron, D.; Tsouris, C. Separation of CO2 from Flue Gas: A. Review. Sep. Sci. Technol. 2005, 40, 321–348. [Google Scholar] [CrossRef]
- Shi, X.; Xiao, H.; Lackner, K.S.; Chen, X. Capture CO2 from ambient air using nanoconfined ion hydration. Angew. Chem. Int. Ed. Eng. 2016, 55, 4026–4029. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Li, Q.; Wang, T.; Lackner, K.S. Kinetic analysis of an anion exchange absorbent for CO2 capture from ambient air. PLoS ONE 2017, 12, e0179828. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-H. A Review of CO2 Capture by Absorption and Adsorption. Aerosol Air Qual. Res. 2012, 12, 745–769. [Google Scholar] [CrossRef]
- Gomes, J.; Santos, S.; Bordado, J. Choosing amine-based absorbents for CO2 capture. Environ. Technol. 2015, 36, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.; Johnson, D.; Gilmartin, J. Fluor’s Econamine FG Plus Technology for CO2 Capture at Coal-fired Power Plants. In Proceedings of the Power Plant Air Pollutant Control “Mega” Symposium, Baltimore, MD, USA, 25–28 August 2008; pp. 1–17. [Google Scholar]
- Johnson, D.W.; Reddy, S.; Brown, J.H. Commercially Available CO2 Capture Technology. Available online: http://www.powermag.com/commercially-available-co2-capture-technology/?printmode=1 (accessed on 9 February 2016).
- Milidovich, S.; Zbacnik, E. Increasing Efficiency of Hot Potassium Carbonate CO2 Removal Systems; UOP LLC: Des Plaines, IL, USA, 2013; pp. 11–14. [Google Scholar]
- Eickmeyer, A.G. The Catalysis of Potassium Carbonate Solutions in Carbon Dioxide Absorption. In Proceedings of the Fall Meeting of the Society of Petroleum Engineers of AIME, Dallas, TX, USA, 8–11 October 1961. [Google Scholar]
- DOW oil and Gas Solutions. Available online: http://www.dow.com/en-us/oil-gas-mining/oil-and-gas-solutions/gas-processing (accessed on 9 February 2016).
- BASF. OASE Gas Treating Excellence. Available online: http://www.intermediates.basf.com/chemicals/web/en/function/conversions:/publish/content/news-and-publications/brochures/download/BASF_Oase_Gas-Treatment.pdf (accessed on 26 April 2018).
- Mumford, K.A.; Wu, Y.; Smith, K.H.; Stevens, G.W. Review of solvent based carbon-dioxide capture technologies. Front. Chem. Sci. Eng. 2015, 9, 125–141. [Google Scholar] [CrossRef]
- Karande, R.; Halan, B.; Schmid, A.; Buehler, K. Segmented flow is controlling growth of catalytic biofilms in continuous multiphase microreactors. Biotechnol. Bioeng. 2014, 111, 1831–1840. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Fang, M.; Lv, Z.; Wang, Z.; Luo, Z. Selection of Blended Solvents for CO2 Absorption from Coal-Fired Flue Gas. Part 1: Monoethanolamine (MEA)-Based Solvents. Energy Fuels 2012, 26, 147–153. [Google Scholar] [CrossRef]
- Cousins, A.; Wardhaugh, L.T.; Feron, P.H.M. A survey of process flow sheet modifications for energy efficient CO2 capture from flue gases using chemical absorption. Int. J. Greenh. Gas Control 2011, 5, 605–619. [Google Scholar] [CrossRef]
- Reddick, C.; Li, C.; Sorin, M.; Sapoundjiev, H. Lowering the energy cost of carbon dioxide capture using ejectors for waste heat upgrading. Energy Procedia 2014, 63, 715–726. [Google Scholar] [CrossRef]
- Ding, J.; Lin, Y.-J.; Rochelle, G.T. Optimization of stripping piperazine with variable rich loading. Energy Procedia 2014, 63, 1842–1853. [Google Scholar] [CrossRef][Green Version]
- Sachde, D.; Rochelle, G.T. Absorber Intercooling Configurations using Aqueous Piperazine for Capture from Sources with 4 to 27% CO2. Energy Procedia 2014, 63, 1637–1656. [Google Scholar] [CrossRef]
- Kothandaraman, A.; Nord, L.; Bolland, O.; Herzog, H.J.; McRae, G.J. Comparison of solvents for post-combustion capture of CO2 by chemical absorption. Energy Procedia 2009, 1, 1373–1380. [Google Scholar] [CrossRef]
- Desideri, U.; Paolucci, A. Performance modelling of a carbon dioxide removal system for power plants. Energy Convers. Manag. 1999, 40, 1899–1915. [Google Scholar] [CrossRef]
- Kumar, P.S.; Hogendoorn, J.A.; Feron, P.H.M.; Versteeg, G.F. New absorption liquids for the removal of CO2 from dilute gas streams using membrane contactors. Chem. Eng. Sci. 2002, 57, 1639–1651. [Google Scholar] [CrossRef]
- Scovazzo, P. Regular Solution Theory and CO2 Gas Solubility in Room-Temperature Ionic Liquids. Ind. Eng. Chem. Res. 2004, 43, 6855–6860. [Google Scholar] [CrossRef]
- Karadas, F.; Atilhan, M.; Aparicio, S. Review on the use of ionic liquids (ILs) as alternative fluids for CO2 capture and natural gas sweetening. Energy Fuels 2010, 24, 5817–5828. [Google Scholar] [CrossRef]
- Camper, D.; Bara, J.E.; Gin, D.L.; Noble, R.D. Room-temperature ionic liquid-amine solutions: Tunable solvents for efficient and reversible capture of CO2. Ind. Eng. Chem. Res. 2008, 47, 8496–8498. [Google Scholar] [CrossRef]
- Budzianowski, W.M. Energy Efficient Solvents for CO2 Capture by Gas-Liquid Absorption: Compounds, Blends and Advanced Solvent Systems; Springer International Publishing: New York, NY, USA, 2016. [Google Scholar]
- Brennecke, J.F.; Gurkan, B.E. Ionic Liquids for CO2 Capture and Emission Reduction. J. Phys. Chem. Lett. 2010, 1, 3459–3464. [Google Scholar] [CrossRef]
- Veawab, A.; Tontiwachwuthikul, P.; Amit, C. Corrosion Behavior of Carbon Steel in the CO2 Absorption Process Using Aqueous Amine Solutions. Ind. Eng. Chem. Res. 1999, 38, 3917–3924. [Google Scholar] [CrossRef]
- DuPart, M.; Bacon, T.; Edwards, D. Understanding corrosion in alkanolamine gas treating plants: Part 1. Hydrocarb. Process. 1993, 72, 75–80. [Google Scholar]
- Hasib-ur-rahman, M.; Bouteldja, H.; Fongarland, P.; Siaj, M. Corrosion Behavior of Carbon Steel in Alkanolamine/Room-Temperature Ionic Liquid Based CO2 Capture Systems. Ind. Eng. Chem. Res. 2012, 51, 8711–8718. [Google Scholar] [CrossRef]
- Shi, X.; Xiao, H.; Chen, X.; Lackner, K.S. The Effect of Moisture on the Hydrolysis of Basic Salts. Chem. Eur. J. 2016, 22, 18326–18330. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.-H.; Jung, J.-Y.; Jin, Y.-R.; Lee, B.-C.; Baek, I.-H.; Kim, S.-H. Solubility of Carbon Dioxide in Imidazolium-Based Ionic Liquids with a Methanesulfonate Anion. J. Chem. Eng. Data 2012, 57, 3321–3329. [Google Scholar] [CrossRef]
- Paul, S.; Panda, A.K. Physicochemical investigations on the aqueous solution of an ionic liquid, 1-butyl-3-methylimidazolium methanesulfonate, [bmim][MS], in a concentrated and dilute regime. Colloids Surf. A Physicochem. Eng. Asp. 2012, 404, 1–11. [Google Scholar] [CrossRef]
- Kärkkäinen, J. Preperation and Characterization of Some Ionic Liquids and Their Use in the Dimerization Recation of 2-Methylpropene. Ph.D. Thesis, University of Oulu, Oulu, Finland, 9 March 2007. [Google Scholar]
- Caplow, M. Kinetics of carbamate formation and breakdown. J. Am. Chem. Soc. 1968, 90, 6795–6803. [Google Scholar] [CrossRef]
- Danckwerts, P.V. The reaction of CO2 with ethanolamines. Chem. Eng. Sci. 1979, 34, 443–446. [Google Scholar] [CrossRef]
- Greer, T.; Bedelbayev, A.; Igreja, J.M.; Gomes, J.F.; Lie, B. A simulation study on the abatement of CO2 emissions by de-absorption with monoethanolamine. Environ. Technol. 2010, 31, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Pereiro, A.B.; Verdía, P.; Tojo, E.; Rodríguez, A. Physical properties of 1-butyl-3-methylimidazolium methyl sulfate as a function of temperature. J. Chem. Eng. Data 2007, 52, 377–380. [Google Scholar] [CrossRef]
- Bates, E.D.; Mayton, R.D.; Ntai, I.; Davis, J.H., Jr. CO2 Capture by a Task-Specific Ionic Liquid. J. Am. Chem. Soc. 2002, 9, 926–927. [Google Scholar] [CrossRef]
- Galán Sánchez, L.M.G.; Meindersma, G.W.; de Haan, A.B. Solvent properties of Functionalized Ionic Liquids for CO2 Absorption. Chem. Eng. Res. Des. 2007, 85, 31–39. [Google Scholar] [CrossRef]
- Gutowski, K.E.; Maginn, E.J. Amine-Functionalized Task-Specific Ionic Liquids: A Mechanistic Explanation for the Dramatic Increase in Viscosity upon Complexation with CO2 from Molecular Simulation. J. Am. Chem. Soc. 2008, 130, 14690–14704. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, X.; Dong, H.; Zhao, Z.; Zhang, S.; Huang, Y. Carbon capture with ionic liquids: Overview and progress. Energy Environ. Sci. 2012, 5, 6668–6681. [Google Scholar] [CrossRef]
- Sobrino, M.; Concepción, E.I.; Gómez-Hernández, Á.; Martín, C.M.; Segovia, J.J. Viscosity and density measurements of aqueous amines at high pressures: MDEA-water and MEA-water mixtures for CO2 capture. J. Chem. Thermodyn. 2016, 98, 231–241. [Google Scholar] [CrossRef]
- Seddon, K.R.; Stark, A.; Torres, M.-J. Influence of chloride, water, and organic solvents on the physical properties of ionic liquids. Pure Appl. Chem. 2000, 72, 2275–2287. [Google Scholar] [CrossRef]
| No | Reactions | Forward Reaction | Backward Reaction |
|---|---|---|---|
| 1 | Quasi-state equilibrium | ||
| 2 | Quasi-state equilibrium | ||
| 3 | Quasi-state equilibrium | ||
| 4 | Quasi-state equilibrium | ||
| 5 | Quasi-state equilibrium | ||
| 4 | |||
| 5 | |||
| 6 | |||
| 7 | |||
| Description | Model | Industrial Data | |
|---|---|---|---|
| Min | Max | ||
| CO2 slippage (ppm) | 500 | 200 | 500 |
| Specific Energy Consumption kcal/NMC of CO2 removed | 464 | 452 | 486 |
| Lean solution loading | 4.2 | 3.4 | 3.47 |
| * Process gas temperature | 78 | 70 | 78 |
| High pressure flash drum temperature | 72 | 70 | 74 |
| Semilean solution to stripper temperature | 87 | 87 | 95 |
| Absorber top temperature | 57 | 51 | 58 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taimoor, A.A.; Al-Shahrani, S.; Muhammad, A. Ionic Liquid (1-Butyl-3-Metylimidazolium Methane Sulphonate) Corrosion and Energy Analysis for High Pressure CO2 Absorption Process. Processes 2018, 6, 45. https://doi.org/10.3390/pr6050045
Taimoor AA, Al-Shahrani S, Muhammad A. Ionic Liquid (1-Butyl-3-Metylimidazolium Methane Sulphonate) Corrosion and Energy Analysis for High Pressure CO2 Absorption Process. Processes. 2018; 6(5):45. https://doi.org/10.3390/pr6050045
Chicago/Turabian StyleTaimoor, Aqeel Ahmad, Saad Al-Shahrani, and Ayyaz Muhammad. 2018. "Ionic Liquid (1-Butyl-3-Metylimidazolium Methane Sulphonate) Corrosion and Energy Analysis for High Pressure CO2 Absorption Process" Processes 6, no. 5: 45. https://doi.org/10.3390/pr6050045
APA StyleTaimoor, A. A., Al-Shahrani, S., & Muhammad, A. (2018). Ionic Liquid (1-Butyl-3-Metylimidazolium Methane Sulphonate) Corrosion and Energy Analysis for High Pressure CO2 Absorption Process. Processes, 6(5), 45. https://doi.org/10.3390/pr6050045




