Dual Population Balance Monte Carlo Simulation of Particle Synthesis by Flame Spray Pyrolysis
Abstract
:1. Introduction
2. Population Balance Modeling
3. Droplet Combustion
3.1. Heat and Mass Transport
3.2. Average Liquid and Gas-Phase Properties
3.3. Droplet Events
4. Results and Discussion
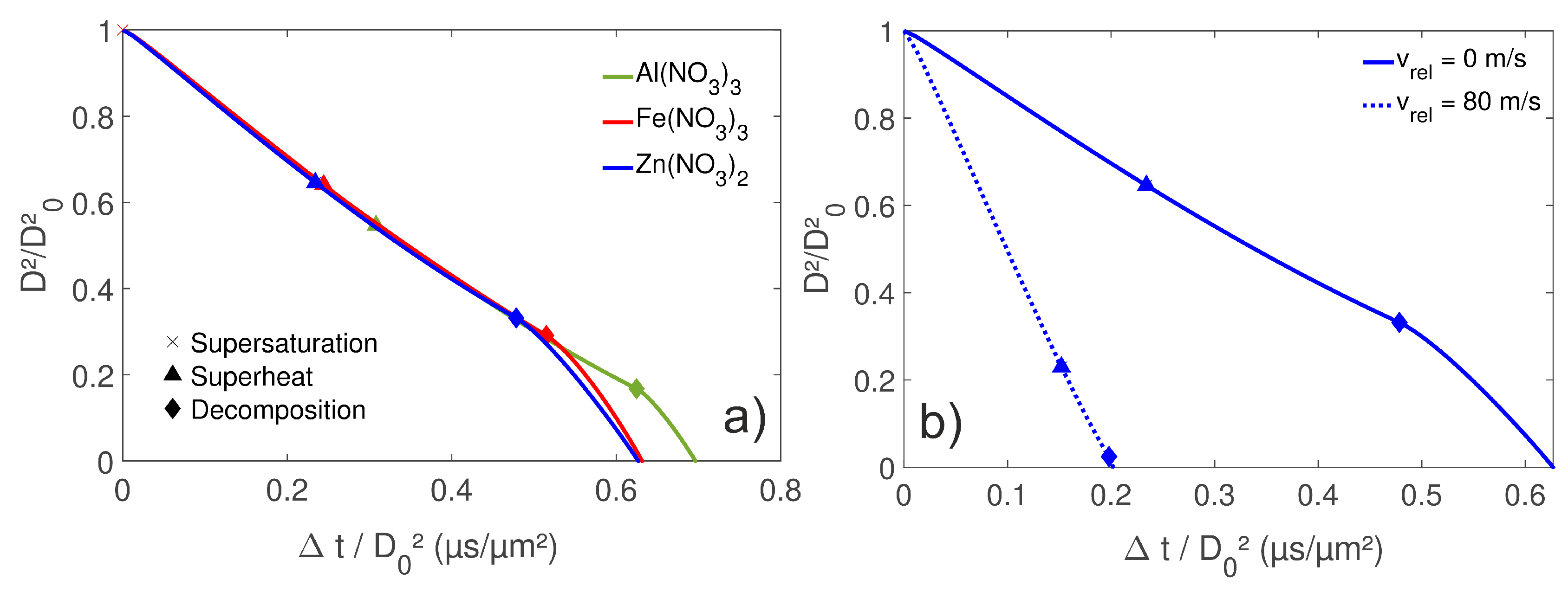
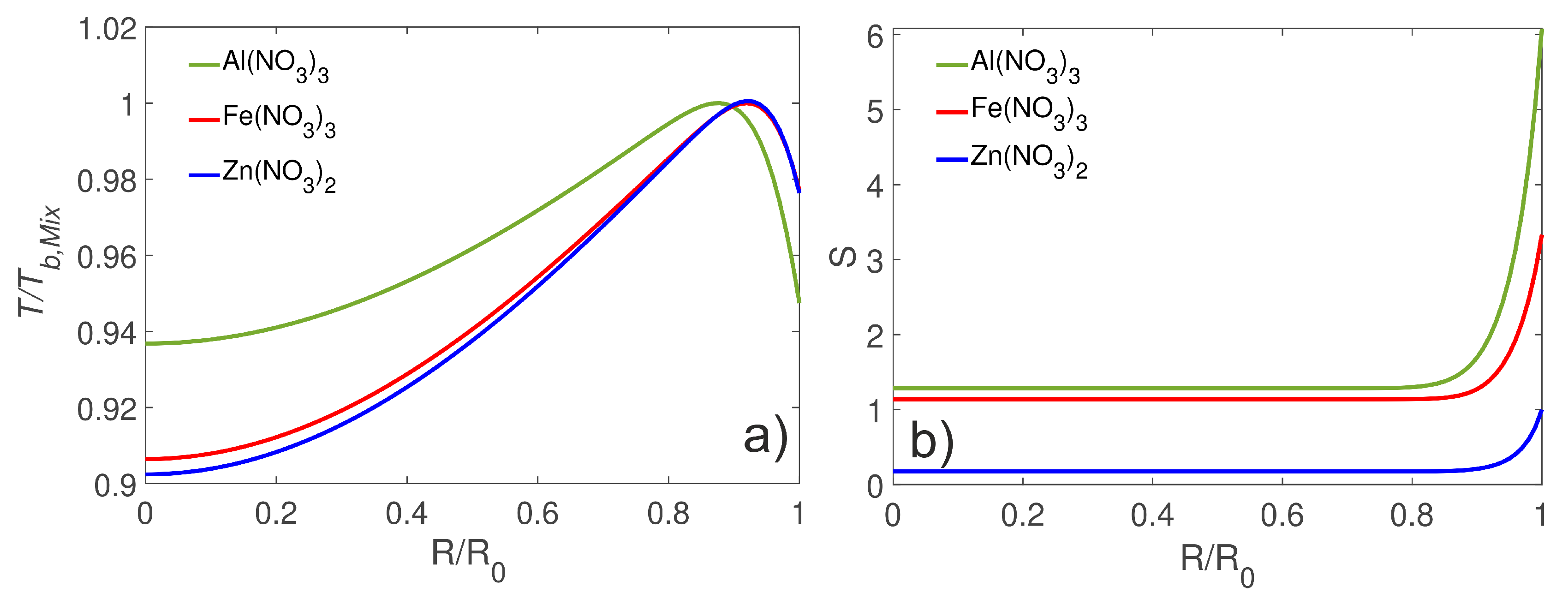
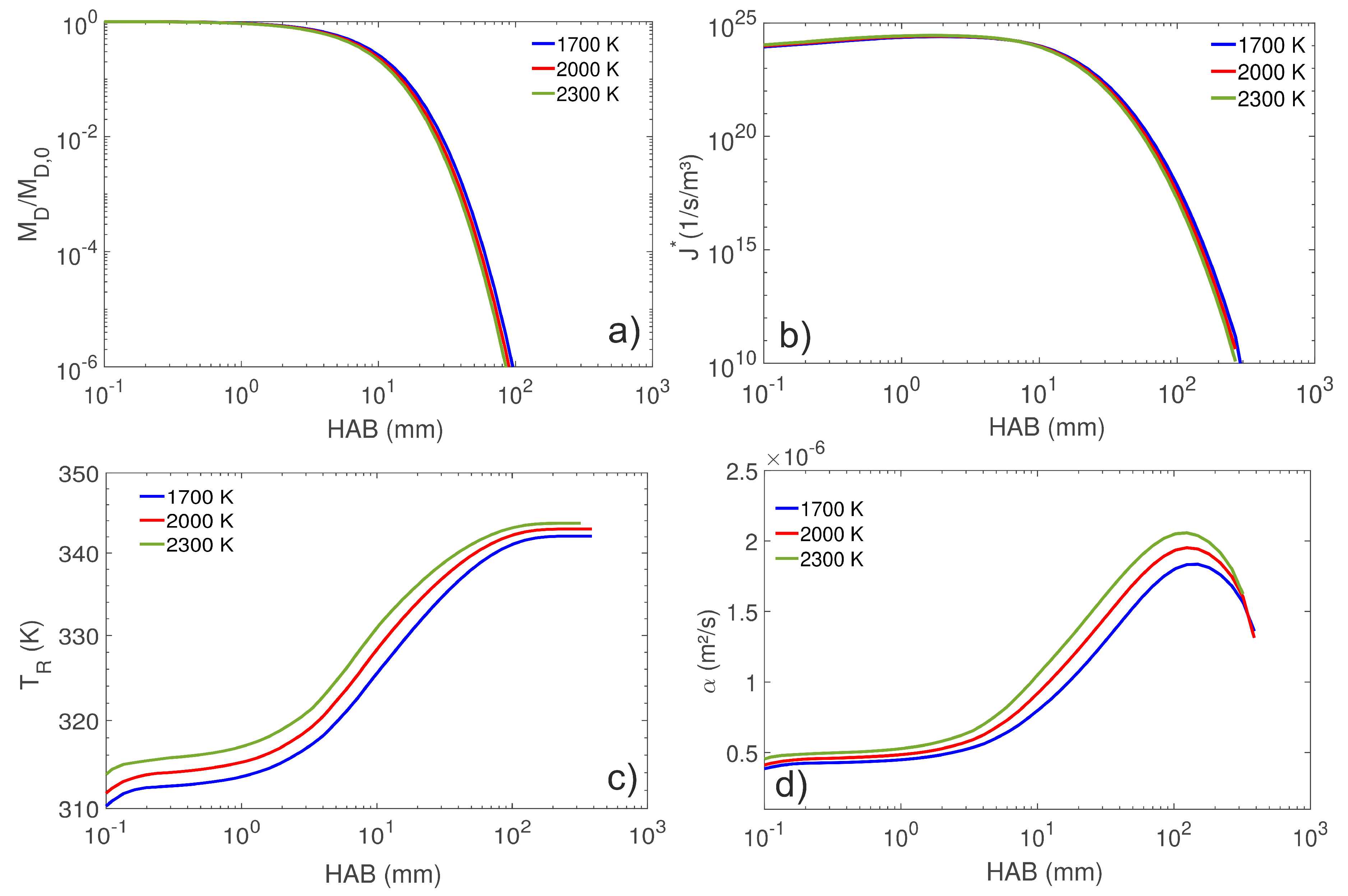
4.1. Droplet Temperature and Concentration Fields
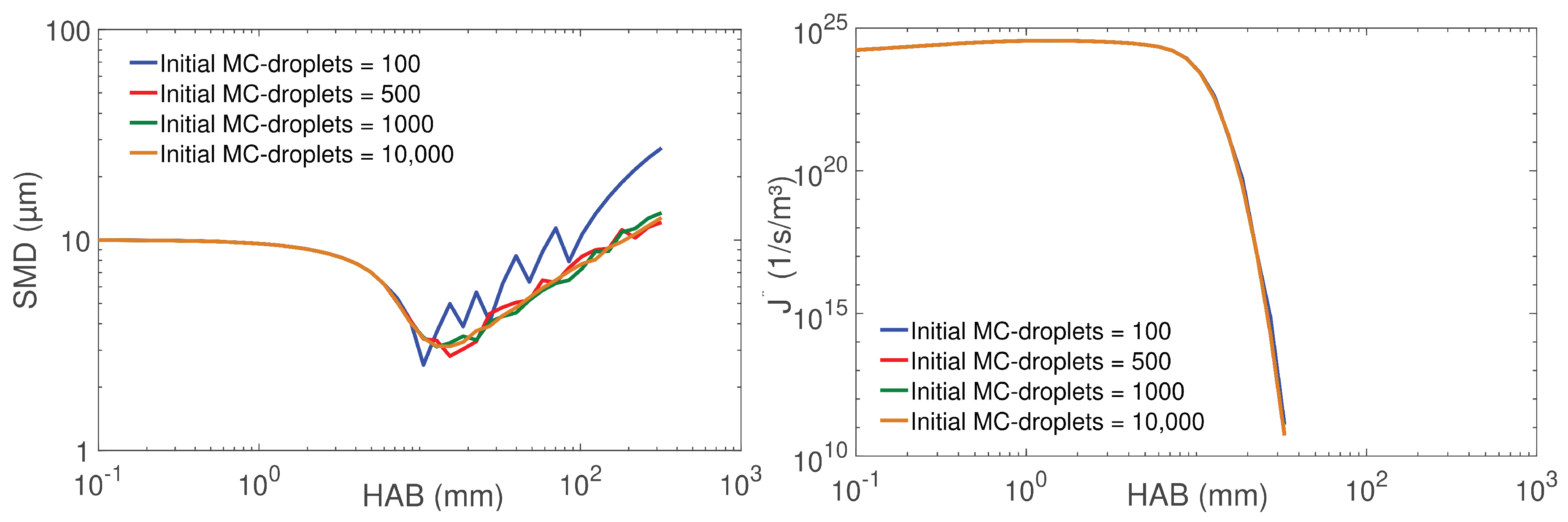
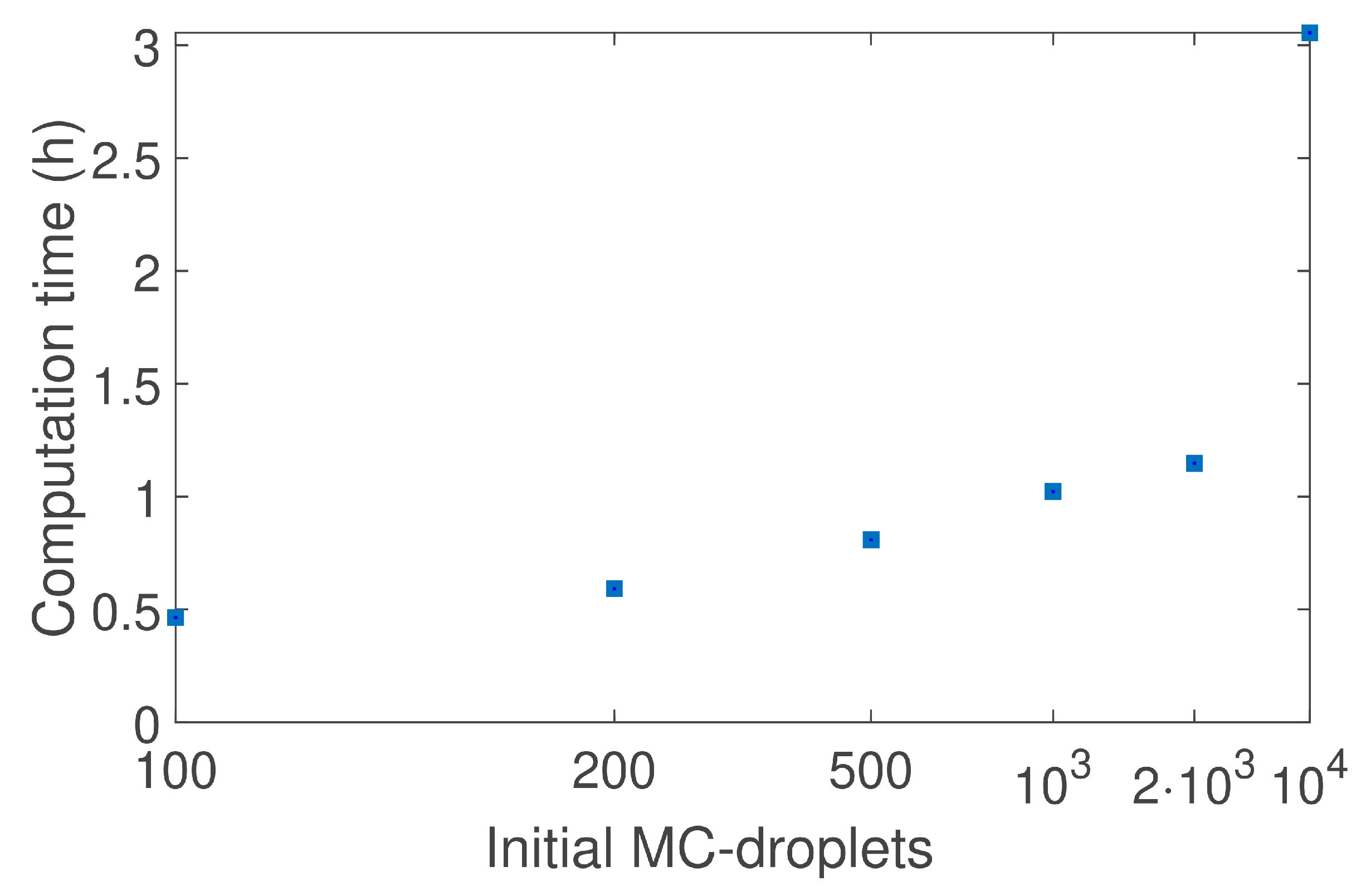
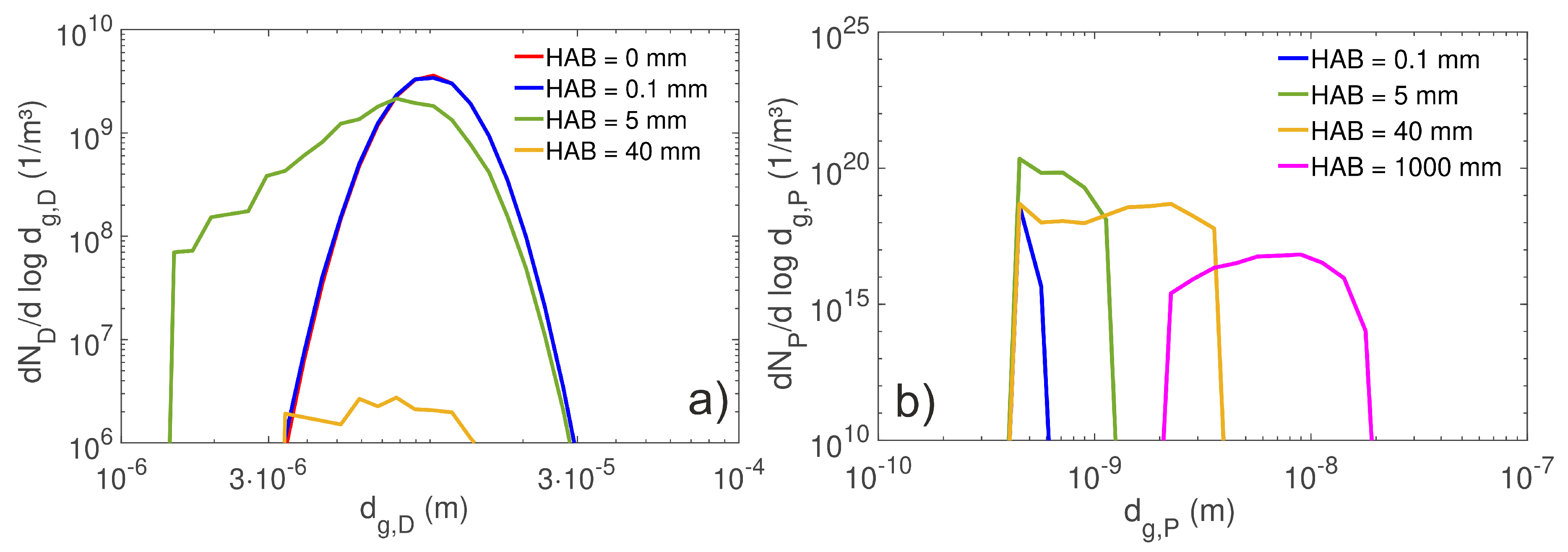
4.2. Droplet Population Evolution
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DPBMC | Dual population balance Monte Carlo |
| DSD | Droplet size distribution |
| FD | Finite Difference |
| FTCS-FD | Forward time centered space finite difference |
| FSP | Flame spray pyrolysis |
| GPU | Graphics processing unit |
| HAB | Height above burner |
| MC | Monte Carlo |
| PB | Population balance |
| PDA | Phase Doppler Anemometry |
| PSD | Particle size distribution |
List of Symbols
| B | Spalding transfer number |
| Specific heat | |
| Geometric mean diameter | |
| D | Droplet Diameter |
| Thermal diffusivity | |
| Mass diffusivity | |
| H | Energy per kg of fuel used for droplet heating |
| Nucleation rate | |
| L | Heat of vaporization |
| m | Evaporation rate |
| Monomer release rate | |
| Total droplet mass | |
| droplet number density function | |
| particle number density function | |
| N | Number of species or MC-droplets/particles |
| Q | Heat of combustion |
| R | Droplet radius |
| Specific gas constant | |
| t | Time |
| T | Temperature |
| v | Volume |
| X | Mole fraction |
| Y | Mass fraction |
Greek letters
| Droplet surface regression rate | |
| Coagulation rate | |
| Dirac-Delta function | |
| Fractional mass evaporation rate | |
| Thermal conductivity | |
| Stoichiometric oxygen to fuel mass ratio | |
| Density | |
| Geometric standard deviation | |
| Weight |
Subscripts
| b | Boiling state |
| D | Droplet |
| Decomposition state | |
| f | Flame |
| F | Fuel |
| g | Gas phase |
| l | Liquid phase |
| Mixture | |
| O | Oxidizer |
| P | Particle |
| Reference state | |
| 0 | Initial state |
| 1 | Monomer |
| ∞ | Ambience |
| + | Gas side of droplet surface |
| − | Liquid side of droplet surface |
Superscripts
| Dimensionless | |
| Species weighted |
Appendix A
| Parameter | Value |
|---|---|
| Material System | in ethanol |
| Temperature | 2300 K |
| Pressure | 1 bar |
| Spray load | /3 |
| Carrier gas velocity | 70 m/s |
| 10 | |
| 1.1; 1.3; 1.7 | |
| MC-droplets/particles | 1000/1000 |
| MC-simulations | 100 |
References
- Teoh, W.Y.; Amal, R.; Mädler, L. Flame spray pyrolysis: An enabling technology for nanoparticles design and fabrication. Nanoscale 2010, 2, 1324–1347. [Google Scholar] [CrossRef] [PubMed]
- Gröhn, A.J.; Pratsinis, S.E.; Sánchez-Ferrer, A.; Mezzenga, R.; Wegner, K.S.D. Scale-up of nanoparticle synthesis by flame spray pyrolysis. Ind. Eng. Chem. Res. 2014, 26, 10734–10742. [Google Scholar] [CrossRef]
- Heine, M.C.; Pratsinis, S.E. Droplet and particle dynamics during flame spray synthesis of nanoparticles. Ind. Eng. Chem. Res. 2005, 16, 6222–6232. [Google Scholar] [CrossRef]
- Widiyastuti, W.; Wang, W.N.; Lenggoro, I.W.; Iskandar, F.; Okuyama, K. Simulation and experimental study of spray pyrolysis of polydispersed droplets. J. Mater. Res. 2007, 7, 1888–1898. [Google Scholar] [CrossRef]
- Jossen, R.; Pratsinis, S.E.; Stark, W.J.; Mädler, L. Criteria for flame-spray synthesis of hollow, shell-like, or inhomogeneous oxides. J. Am. Ceram. Soc. 2005, 6, 1388–1393. [Google Scholar] [CrossRef]
- Mueller, R.; Jossen, R.; Kammler, H.K.; Pratsinis, S.E.; Akhtar, M.K. Growth of zirconia particles made by flame spray pyrolysis. AIChE J. 2004, 12, 3085–3094. [Google Scholar] [CrossRef]
- Law, C.K. Recent advances in droplet vaporization and combustion. Prog. Energy Combust. Sci. 1982, 12, 171–201. [Google Scholar] [CrossRef]
- Rosebrock, C.D.; Wriedt, T.; Mädler, L.; Wegner, K. The role of microexplosions in flame spray synthesis for homogeneous nanopowders from low-cost metal precursors. AIChE J. 2016, 2, 381–398. [Google Scholar] [CrossRef]
- Li, H.; Rosebrock, C.D.; Wu, Y.; Wriedt, T. Single droplet combustion of precursor/solvent solutions for nanoparticle production. Proc. Combust. Inst. 2018. [Google Scholar] [CrossRef]
- Lee, K.F.; Patterson, R.; Wagner, W.; Kraft, M. Stochastic weighted particle methods for population balance equations with coagulation, fragmentation and spatial inhomogeneity. J. Comput. Phys. 2015, 303, 1–18. [Google Scholar] [CrossRef]
- Kotalczyk, G.; Devi, J.; Kruis, F.E. A time-driven constant-number Monte Carlo method for the GPU-simulation of particle breakage based on weighted simulation particles. Powder Technol. 2017, 317, 417–429. [Google Scholar] [CrossRef]
- Kotalczyk, G.; Skenderovic, I.; Kruis, F.E. A GPU-based Monte Carlo Technique for the simulation of simultaneous nucleation, coagulation and growth based on weighted simulation particles. In Proceedings of the AIChE Annual Meeting, San Francisco, CA, USA, 13–18 November 2016; pp. 490–497. [Google Scholar]
- Kotalczyk, G.; Skenderovic, I.; Kruis, F.E. Modeling of particle formation in arc discharges by Monte Carlo based population balance modeling. MRS Adv. 2017, 1511–1518. [Google Scholar] [CrossRef]
- Wei, J.; Kruis, F.E. A GPU-based parallelized Monte Carlo method for particle coagulation using an acceptance-rejection strategy. Chem. Eng. Sci. 2013, 104, 451–459. [Google Scholar] [CrossRef]
- Girshick, S.L.; Chiu, C.P. Modelling particle formation and growth in a plasma synthesis reactor. Plasma Chem. Plasma Proc. 1988, 28, 145–157. [Google Scholar] [CrossRef]
- Kotalczyk, G.; Kruis, F.E. A Monte Carlo method for the simulation of coagulation and nucleation based on weighted particles and the concepts of stochastic resolution and merging. J. Comput. Phys. 2017, 340, 276–296. [Google Scholar] [CrossRef]
- Ulrich, G.D. Theory of particle formation and growth in oxide synthesis flames. Combust. Sci. Technol. 1971, 4, 47–57. [Google Scholar] [CrossRef]
- Rosebrock, C.D.; Shirinzadeh, S.; Soeken, M.; Riefler, N.; Wriedt, T.; Drechsler, R.; Mädler, L. Time-resolved detection of diffusion limited temperature gradients inside single isolated burning droplets using Rainbow Refractometry. Combust. Flame 2016, 168, 255–269. [Google Scholar] [CrossRef]
- Law, C.K. Unsteady Droplet Combustion with Droplet Heating—II: Conduction limit. Combust. Flame 1977, 28, 175–186. [Google Scholar] [CrossRef]
- Law, C.K. Internal boiling and superheating in vaporizing multicomponent droplets. AIChE J. 1978, 4, 626–632. [Google Scholar] [CrossRef]
- Sirignano, W.A. Fluid Dynamics and Transport of Droplets and Sprays; Cambridge University Press: New York, NY, USA, 2010; pp. 19–133. ISBN 978-0-521-88489-1. [Google Scholar]
- Ranz, W.E.; Marshall, W.R. Evaporation from Drops. Chem. Eng. Prog. 1952, 48, 173–180. [Google Scholar]
- Carslaw, H.S.; Jaeger, J.C. Conduction of Heat in Solids; Oxford University Press: New York, NY, USA, 1986; pp. 466–468. ISBN 9780198533689. [Google Scholar]
- Law, C.K.; Williams, F.A. Kinetics and convection in the combustion of alkane droplets. Combust. Flame 1972, 3, 393–405. [Google Scholar] [CrossRef]
- Abramzon, B.; Sirignano, W.A. Droplet vaporization model for spray combustion calculations. Int. J. Heat Mass Transf. 1989, 9, 393–405. [Google Scholar] [CrossRef]
- Rosebrock, C.D.; Riefler, N.; Wriedt, T.; Mädler, L.; Tse, S.D. Disruptive burning of precursor/solvent droplets in flame-spray synthesis of nanoparticles. AIChE J. 2013, 12, 4553–4566. [Google Scholar] [CrossRef]
- Widiyastuti, W.; Balgis, R.; Iskandar, F.; Okuyama, K. Nanoparticle formation in spray pyrolysis under low-pressure conditions. Chem. Eng. Sci. 2008, 5, 1846–1854. [Google Scholar] [CrossRef]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skenderović, I.; Kotalczyk, G.; Kruis, F.E. Dual Population Balance Monte Carlo Simulation of Particle Synthesis by Flame Spray Pyrolysis. Processes 2018, 6, 253. https://doi.org/10.3390/pr6120253
Skenderović I, Kotalczyk G, Kruis FE. Dual Population Balance Monte Carlo Simulation of Particle Synthesis by Flame Spray Pyrolysis. Processes. 2018; 6(12):253. https://doi.org/10.3390/pr6120253
Chicago/Turabian StyleSkenderović, Ivan, Gregor Kotalczyk, and Frank Einar Kruis. 2018. "Dual Population Balance Monte Carlo Simulation of Particle Synthesis by Flame Spray Pyrolysis" Processes 6, no. 12: 253. https://doi.org/10.3390/pr6120253
APA StyleSkenderović, I., Kotalczyk, G., & Kruis, F. E. (2018). Dual Population Balance Monte Carlo Simulation of Particle Synthesis by Flame Spray Pyrolysis. Processes, 6(12), 253. https://doi.org/10.3390/pr6120253






