1. Introduction
Polyphenols are organic compounds produced by plants to provide them protection to different kinds of attacks such as UV radiation, parasites, insect pests and many other environmental threats. Close to 10,000 different polyphenols have already been identified. Their incidence changes among the different plant species, as well as the kind of vegetable parts (roots, stalks, barks, leaves, fruits, skin-fruits, shells, seeds, stones, etc.) inside the same species [
1].
It is well known that polyphenols present important antioxidant and anti-inflammatory activities, thus providing beneficial effects on human health, such as the protection of the cardiovascular system, anti-cancer effects or anti-aging actions. Besides the preventive effects on human health, polyphenols are also being considered in the treatment of several diseases [
1]. Owing to these useful properties, a growing interest on these natural compounds is being observed namely by the pharmaceutical, biomedical, biotechnological and cosmetics industries [
1]. Hence, the development of techniques and processes to perform the efficient extraction, purification, fractionation, isolation and concentration of polyphenols is also a subject attracting wide interest from the scientific community and the above-mentioned industries. Extraction methods for the polyphenols present in the vegetable matrices can range from the use of conventional solvents (e.g., hydro-alcoholic solutions) at different operation temperatures (e.g., up to 100 °C) in refluxing extraction [
2], microwave assisted and sonication extractions [
3], Soxhlet processes [
4] to the application of supercritical fluids [
5] (usually allowing the efficient operation at lower temperatures, such as 40 °C, making it a “green” process). Due to their particular chemical structures, polyphenols tend to undergo sorption in different kinds of materials, namely activated carbons (by adsorption) and synthetic resins (mainly by dissolution), which are therefore often chosen for the purification, fractionation and isolation of such compounds (see e.g., chapter 16 in [
1] and references therein and review works [
6] and [
7,
8,
9,
10] for examples of application systems using different combinations of resins and extracts).
Indeed, a sorption process is a relatively simple option, namely concerning the operation requirements and scale-up, which can be considered for separating compounds present in dilute solutions. Moreover, this is a low-cost technology, allowing, in principle, the facile regeneration of active sorbents. It usually avoids the use of toxic solvents and prevents the degradation of the target compounds. Owing to these benefits, many important processes used in practice to recover and concentrate phenolic compounds are based upon sorption technology [
6]. Recent studies also explore the coupled use of ultrafiltration/diafiltration and sorption or membrane technology to recover polyphenols from vegetable extracts [
4,
10].
Activated carbons, minerals (e.g., siliceous materials or clay), synthetic resins (e.g., styrene/divinylbenzene or acrylic polymer networks) and materials originated from industrial or agricultural wastes (e.g., fly ash, lignocellulosic materials or polysaccharides) are examples of different of adsorbents used to recover and concentrate/purify phenolic compounds [
6]. Among these classes, synthetic polymeric adsorbents are inert and durable materials, presenting high stability, selectivity and sorption capability. Ease of regeneration and limited toxicity are also generally associated to adsorbents based on synthetic resins [
6]. Furthermore, tailored adsorbents can in principle be designed when synthetic polymers are considered, namely through the control of the hydrophilic and hydrophobic moieties or the inclusion of specific functional groups to improve the selectivity and efficiency (e.g., anionic or cationic groups as in the ion-exchange resins, hydrogen bonding functionalities, etc.).
Molecularly imprinted polymers (MIPs) are polymer networks with tailor-made cavities, having high specificity and affinity with respect to a specific target molecule, thus playing the role of artificial antibodies [
11,
12,
13]. Sorption and separation, among other important applications (e.g., biological and biomedical processes, development of sensors, catalysis, etc. [
14]), are potential areas where MIPs features can yield important gains comparatively to alternative materials. Indeed, after network formation (often through a free radical polymerization using a high crosslinker content), the generated binding sites should preserve geometrical stability, allowing the uptake and release of target molecules in several cycles. Note, however, that performance of MIPs is affected by several factors, namely their preparation process (e.g., chemical composition, target/functional monomer interactions, final morphology, etc.), as discussed in the reference works [
11,
12,
13,
14] and evidenced with imprinted hydrogels [
15] or precipitation/suspension products [
16]. Furthermore, specific molecular recognition capabilities of MIPs depend also on their application conditions, particularly the extent of hydrophilic and hydrophobic interactions between the MIPs, the solvents considered and the different molecules present in the solutions. Currently, new strategies are being implemented by the scientific community to address some shortcomings of MIPs, namely through the design of materials combining molecular recognition capabilities and stimulation or by modification of their surface properties (e.g., tuning of the hydrophobic/hydrophilic balance). The grafting of functional polymer brushes on the surface of molecularly imprinted particles (e.g., using RAFT polymerization) is an example of such approaches [
17,
18,
19,
20,
21,
22,
23,
24]. Indeed, the improvement of water compatibility of MIPs for biological applications [
17,
18,
19,
21], the design of multifunctional materials allowing stimulation [
20,
22] and the development of drug delivery carriers [
23,
24] are some new possibilities being explored in this context.
Considering the aforementioned potential affinity of MIPs towards the selected template molecules, adsorbents in this class have been chosen for the selective uptake of phenolic compounds in past research works aiming at different application fields, as shown in works [
25,
26,
27,
28,
29,
30,
31,
32,
33,
34,
35,
36,
37,
38,
39,
40,
41,
42,
43] and references therein, considering diverse retention materials and plant extracts. Indeed, the isolation and purification of polyphenols present in different vegetable sources [
25,
26,
27,
28,
29,
30,
31,
32,
33,
34,
35] (e.g., resveratrol in
Polygonum Cuspidatum, giant knotweed or grape skin) or the detection and quantification of these compounds in beverages [
36,
37,
38,
39] (e.g., in wine or fruit juices) are examples of such approaches. Resveratrol—due to its popular benefits for human heath—was targeted in many of these studies but the same principles can be applied to other kinds of polyphenols present in vegetable extracts, such as emodin [
29], catechin, piceid or A-type procyanidins [
31], quercetin and other flavonoids [
34,
35,
37,
38], etc. Furthermore, the synthesis of tailored adsorbents based on the molecular imprinting technique can be used for the retention of other kinds of phenolic compounds, namely bisphenol A [
40,
41,
42,
43], whose presence in beverages, water and food constitutes an important health damaging issue. It should also be emphasized that MIPs show promising capabilities for application in continuous processes, as reported in recent research involving ions separation [
44], the extraction of melamine from milk [
45], the treatment of wastewater membranes [
46] and the valorization of agricultural wastes through the isolation of the biophenol oleuropein from olive leaves [
47].
Here, we present results of an experimental research concerning the effect of the molecular imprinting synthesis conditions on the performance of the resulting materials for the retention of polyphenols, in particular when making use of continuous processes. Polydatin was selected as a template molecule and different kinds of MIPs were produced and characterized, namely concerning the targeting of polyphenols present in plant extracts, as described in the next sections.
2. Materials and Methods
2.1. Reagents
Acrylic acid (AA), methacrylic acid (MAA), acrylamide (AAm), 2-(dimethylamino)ethyl methacrylate (DMAEMA), N-vinylpyrrolidone (NVP), ethylene glycol dimethacrylate (EGDMA), trimethylolpropane triacrylate (TMPTA) were purchased from Sigma Aldrich and 4-vinylpyridine (4VP) from Alfa Aesar. Analytical reagent grade acetonitrile (ACN), dimethylformamide (DMF) and methanol (MeOH) were bought from Fisher Chemical (Loughborough, Leicestershire, UK). Azobisisobutyronitrile (AIBN), acetic acid and n-heptane were purchased from Sigma-Aldrich (Steinheim, Germany). The surfactant sorbitan mono-oleate (Span 80) was purchased from Panreac (Barcelona, Spain). Millipore water (Milli-Q quality) was used in all the experiments unless otherwise mentioned. Polydatin (purity ≥ 95%), trans-resveratrol (purity ≥ 99%), gallic acid (assay 97.5–102.5%, titration), tannic acid (Chinese natural gall nuts) and bisphenol A (purity ≥ 99%) were also purchased from Sigma Aldrich. Caffeine (98.5% purity) and quercetin (hydrate, 95% purity) were bought from Acros Organics (Geel, Belgium). Catechin (hydrate, purity ≥ 98%) was purchased from Cayman Chemical Company (Ann Arbor, MI, USA).
2.2. Synthesis of Molecularly Imprinted and Non-Imprinted Polymer Particles
Molecularly imprinted (MIP), as well as non-imprinted (NIP) polymer particles, have been synthesized following procedures similar to previous works [
16]. Briefly, for MIP synthesis with precipitation polymerization, the template polyphenol (polydatin) was mixed with the selected functional monomer and the solvent (a binary mixture was used) up to the formation of a clear solution. Note that, owing to the limited solubility of polydatin (and of many polyphenols in general) in common pure solvents, an appropriate amount of MeOH was generally used to compose the final solvent, as will be discussed in the next section. Then, the template–functional monomer (T-FM) interaction was promoted in an ultrasounds bath during 30 min. A solution containing the required crosslinker and initiator was then added and the final homogeneous mixture was transferred to a glass vessel and purged with a flow of dry argon for 30 min. The vessel was then sealed and polymerization was started, using magnetic stirring at low speed (c.a. 100 rpm), in a thermostatic oil bath pre-set at 60 °C. The reaction was allowed to run for 24 h.
For MIP synthesis with suspension polymerization, Span 80 was first dissolved in n-heptane under vigorous stirring using a thermostatic oil bath pre-set at 70 °C. In parallel, a solution containing the polydatin, selected functional monomer, chosen crosslinker, AIBN and the selected solvent was prepared, following a procedure similar to that above described for precipitation polymerization, but at higher monomer concentrations, as will be discussed below. Then, this solution was drop wise fed to the reaction vessel containing the oil phase (n-heptane/Span 80), under magnetic vigorous stirring (c.a. 1000 rpm) and polymerization was also carried out during 24 h.
NIP particles were also synthesized by precipitation and suspension polymerization, using procedures similar to those above described, but in the absence of the template molecule (polydatin).
The yield of the preparation was estimated using gravimetric analysis and values in the range 90% to 100% were generally observed. Note, however, that some dispersion in these data occurs due to the small particle sizes of certain products and the number of cleaning steps performed.
2.3. Isolation and Purification of the Polymer Particles
The synthesized polymer particles were submitted to several cleaning steps in order to perform their isolation and purification. Centrifugation in a large excess of MeOH (c.a. 1/10 v/v) was used in the first stages. The extraction and the cleaning with a methanol/acetic acid mixture (90:10) were afterwards carried out in order to remove impurities and the bonded polyphenol template. The washing processes were monitored through UV–vis spectroscopy measurements up to the time at which the level of the template molecule became lower than the detection limit. Finally, the purified MIP and NIP materials were dried in a vacuum oven at 40 °C.
2.4. SEM Analysis
The morphology of the different kinds of synthesized MIP and NIP particles was analyzed by SEM of the dried products, at the Materials Center of the University of Porto (CEMUP). A SEM FEI Quanta 400FEG instrument with the EDS (Energy Dispersive Spectroscopy) system Edax Genesis X4M was used in these analyses.
2.5. FTIR Analysis
Characterization of the dried MIP and NIP particles was also performed using IR spectroscopy with an ABB Bomem Fourier Transform Infra-Red instrument, model FTLA2000-104. Particles were mixed with KBr and pressed into pellets in order to collect the correspondent IR spectra.
2.6. Solid Phase Extraction Measurements
Solid phase extraction (SPE) measurements were carried out using packing extraction cartridges containing the MIP or NIP particles (e.g., 150 mg of dried products). The cartridges were first eluted with a methanol/acetic acid mixture (90:10), then with methanol and finally conditioned with the solvent selected for the polyphenols uptake assessment during 24 h. A final elution step with this same solvent was performed before testing. Afterwards, cartridges were loaded with the solution containing a specific phenolic compound at the desired concentration (or a mixture phenolic compounds in the competitive testing), using a constant percolation flow rate [
16]. After loading, the washing step was performed by percolating the same solvent through the particles. At the end, the elution step was carried out using pure MeOH. The collected fractions correspondent to loading, washing and elution steps were monitored using batch UV–vis spectrometry or HPLC analysis also with UV detection (this analysis was mainly used with SPE competitive polyphenols sorption). From these data, the retention capabilities (
) of the different materials were calculated. These SPE measurements were performed at least in duplicate.
2.7. Batch Sorption Measurements
Fixed amounts of the purified MIPs and NIPs in the dried state (e.g., 20 mg) were replicated and first subjected to a conditioning process in SPE tubes (similar to above described the processes with SPE measurements). Tubing ends were after sealed and the particles were mixed with the required amount of the solution (e.g., 2 mL) containing the polyphenol at different concentrations. A blank solution was also included in this procedure. The sorption systems were then equilibrated at room temperature for 24 h under agitation using an orbital shaker. At the end, the supernatant was collected by centrifugation and the concentration measured by UV–vis monitoring. From these absorbance data, the adsorbed amount of polyphenol was calculated using the previously reported calculation method [
16,
23].
2.8. Experiments with Continuous Sorption Processes
Different kinds of continuous sorption processes have also been considered in this research in order to assess the performance of the synthesized materials for the retention and release of different kinds of polyphenols. Generically, the polymer particles were packed in HPLC columns (different column sizes were considered, ranging from 10 to 300 mm bed lengths and 4.6 to 8 mm of internal diameter) and then submitted to saturation and cleaning steps with selected polyphenols, mixtures of these molecules or natural extracts (red wine was used). A frontal analysis procedure was used in these studies, considering an approach similar to that before described [
16], but continuous sorption with recycling was also here tried, as discussed below. Continuous sorption with the particles placed in transparent syringe tubes was also carried out in order to visually observe the possible retention of anthocyanins in the adsorbents. In-line and off-line UV measurements were both used for putting into evidence the dynamics of retention and release of the polyphenols during these continuous processes.
2.9. HPLC Analysis for Polyphenols Identification and Quantification
HPLC analysis needed for polyphenols identification and quantification (e.g., with the competitive polyphenols sorption/desorption runs) were performed using an Ascentis
® C18 column, 5 µ particle size and with dimensions L × ID = 25 cm × 4.6 mm. This column was assembled in a Viscotek GPC pumping system—the module Viscotek TDA 305 and UV detection was used for these purposes. HPLC analysis were performed in isocratic conditions, with H
2O/ACN 70/30 (
v/v) at pH = 3 (adjusted with acetic acid), T = 45 °C and at a constant flow rate Q = 1 mL/min. Note that a straightforward HPLC analysis of the solutions containing polyphenols was here considered. Improved separation and identification of similar polyphenol molecules demand much more complex analysis conditions, as reported in the literature; see e.g., [
48,
49] and references therein for the discussion of the complexities involved in such kinds of protocols.
3. Results and Discussion
In this research, the polydatin molecule (also known as piceid) was selected as a template polyphenol for the molecular imprinting studies. This choice was driven by the hybrid hydrophilic/hydrophobic character of this molecule, namely in comparison with resveratrol, owing to the presence of a glucoside group. Additionally, polydatin is a molecule with a relatively high size. Besides the hydrophilic and hydrophobic interactions, molecular size is a parameter with an important effect on molecular imprinting efficiency through the formation of specific cavities inside the polymer network. Moreover, polydatin is a natural precursor of resveratrol, which is present in many vegetable extracts (e.g., vineyard products,
Polygonum cuspidatum extracts, etc.), with possible important beneficial effects on human health, namely the protection against hemorrhagic shock, ischemia/reperfusion, heart failure, endometriosis and cancer (e.g., see [
50] and references therein).
Different kinds of MIPs were synthesized by changing the functional monomers (acrylic acid, methacrylic acid, 4-vinylpyridine, acrylamide, 2-(dimethylamino)ethyl methacrylate and N-vinylpyrrolidone were alternatively used), the crosslinkers considered for the polymer network generation (ethylene glycol dimethacrylate and trimethylolpropane triacrylate were chosen for that purpose) and also the solvents (toluene, acetonitrile, methanol, dimethylformamide and water, or their mixtures, were used). Diverse types of interactions between the template molecule and the functional monomers were thence tried. The effect of hydrophilic/hydrophobic interactions on molecular imprinting efficiency was also assessed. Precipitation and suspension polymerization were both considered for MIP particles production using AIBN as free radical initiator.
The morphology of the products was studied by SEM and the chemical composition was determined by FTIR. The assessment of the synthesized MIPs with respect to polyphenols uptake was carried out using different techniques, namely batch sorption, individual/competitive solid phase extraction and individual/competitive sorption/desorption in continuous processes (e.g., by packing the MIP particles in HPLC columns). In these assays, solvents with different polarities were considered in order to highlight the effect of the hydrophilic and hydrophobic interactions on the MIPs capability to perform the selective uptake and release of different kinds of polyphenols. Finally, the usefulness of the MIPs for the recovering, isolation and concentration of polyphenols present in natural extracts was assessed through the analysis of sorption and desorption processes with red wine.
The above described strategy was aimed at elucidating possible links between the molecular imprinting conditions (template, functional monomers, solvent, polymerization mechanisms), the structure of the products (morphology, chemical composition) and their performance on polyphenols retention, as thoroughly discussed in the next sections.
3.1. Rationale for MIP Synthesis
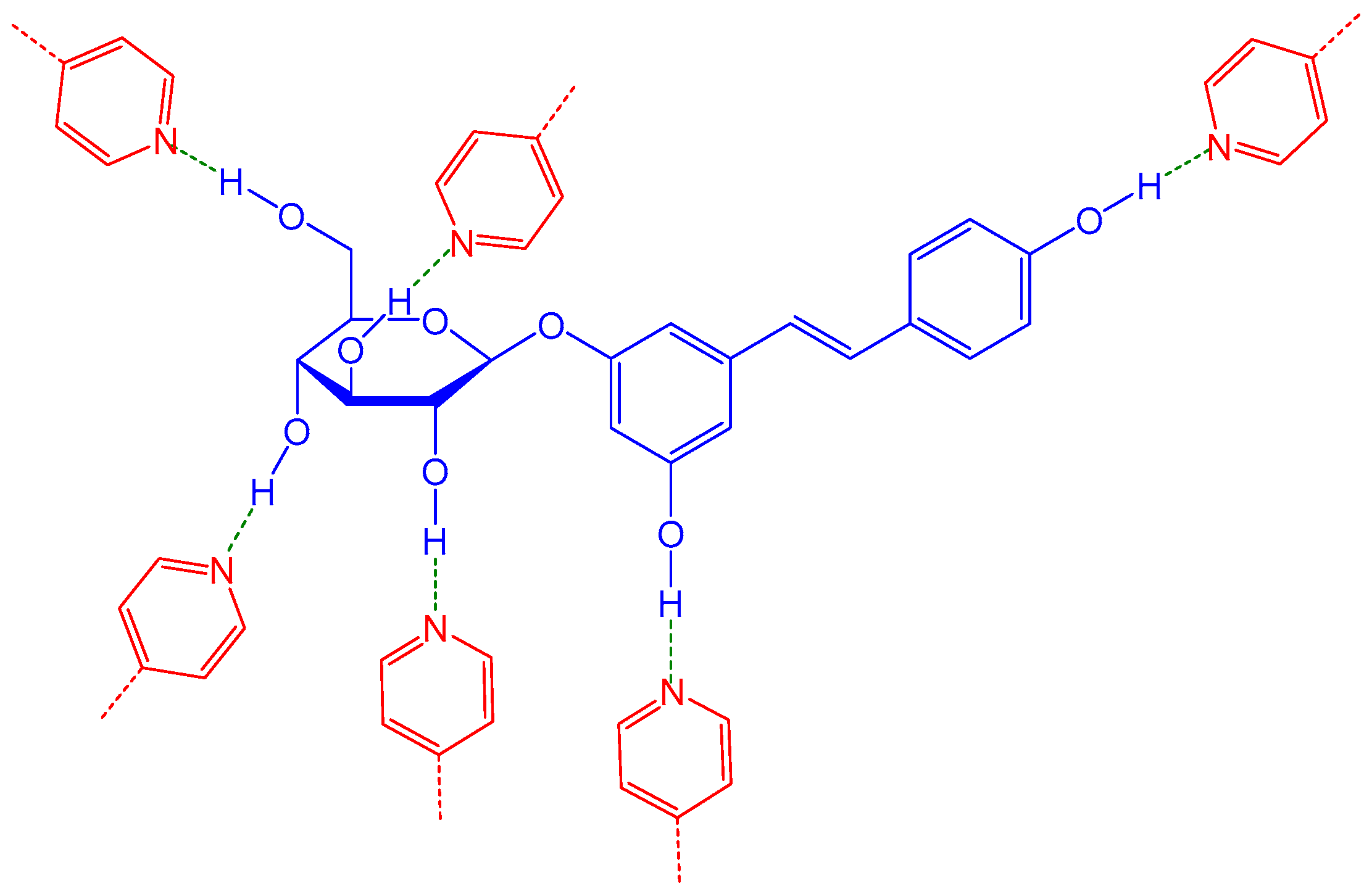
The molecular imprinting process is based on the promotion of specific interactions (e.g., hydrogen bonding) between the template molecule and a functional monomer. Through the polymerization with a crosslinker, stereo-specific three-dimensional cavities could form in the polymer network. An example of such kinds of interactions in the framework of the present MIP research is presented in
Figure 1, with polydatin as the template (T) molecule and 4VP as a possible functional monomer (FM), highlighting the plausible role of hydrogen bonding in this imprinting system.
However, the imprinting efficiency (effective formation of selective binding sites towards the target molecule) strongly depends on many factors such as the strength of the interactions between the template and the functional monomer (different functional monomers lead to MIPs with dissimilar performance) or the solvent being used in the synthesis process [
11,
12,
13,
16]. For instance, many solvents (e.g., water) can break the preferential assembly T/FM by hydrogen bonding, which justifies the use solvents with poor H-bond capacity (e.g., ACN) in many imprinting systems [
11,
12,
13,
16]. Additional kinds of intermolecular forces such as hydrophobic interactions, π-π stacking and others, can also enhance or damage the imprinting process, depending on the particular chemical system addressed [
11,
12,
13]. Moreover, the common solubility of all the components within the reaction locus for molecular imprinting is another issue with important impact on the performance of the produced MIPs.
The behavior of MIPs in molecular recognition is also affected by the morphology of the obtained materials due to possible limitation of the involved mass transfer mechanisms (the size of the template molecule plays here an important role) and the available surface areas, such as in other kinds of adsorbents (e.g., activated carbons, etc.). The morphology of the MIPs can also be tailored (in principle) by the choice of the polymerization conditions: nanoparticles, microparticles or macroscopic monoliths can alternatively be synthesized. Finally, it should also be stressed that the performance of the MIPs in selective uptake and release process is also strongly dependent on the working conditions and the same material can show different capabilities at different environments. One of the most important parameters in this context is the polarity of the solvent considered in the sorption/desorption processes. Non-specific retention mechanisms are often inevitable due to hydrophilic/hydrophobic effects. Thus, the design of amphiphilic MIPs and the change of their surface properties (e.g., inclusion of hydrophilic grafted chains) are also important issues to tailor these materials.
Considering the above discussed effects of the synthesis conditions on MIPs performance, different kinds of materials were produced in our research with polydatin as template, as described in
Table 1. MAA, 4VP, AAm, AA, DMAEMA and NVP were alternatively used as functional monomers in order to explore possible differences in T/FM assembly and a concomitant effect on MIPs performance. EGDMA and TMPTA were tried as crosslinkers leading to the formation of networks with dissimilar crosslinking topology (bi and tri-functional, respectively). Assessment of the change of solvent polarity on molecular imprinting was studied considering different mixtures, namely ACN/MeOH, TOL/MeOH, MeOH/H
2O and DMF. Note that a small amount of MeOH was generally needed in order to obtain total solubility of polydatin in the reaction medium. Modification in the morphology of the MIPs was tried considering precipitation (usually at a very low monomer concentration) and suspension polymerization (higher concentrations can therefore be specified).
Definitions for the composition parameters described in
Table 1 are the following:
—mass fraction of FM + crosslinker in the solution (%).
—mole fraction of initiator comparatively to FM + crosslinker (%).
—mole fraction of crosslinker in the mixture FM + crosslinker (%).
—mole ratio between the FM and the template molecule.
—mass ratio between the oil phase and the monomer phase in suspension polymerization.
Differences in the specified monomer concentrations for precipitation and suspension polymerization become evident when the values for
are compared (around 5% with precipitation polymerization and up to c.a. 60% with suspension polymerization). Besides their possible impact on the imprinting process (e.g., due to the effect on products morphology), these differences are also important when the large-scale production of MIPs is intended [
16]. Note, however, that thermodynamic solubility effects involving polymers, monomers and solvents present a critical impact on the precipitation and suspension reaction mechanisms, as also below discussed in the framework of the entropic and enthalpic phase segregation.
3.2. SEM Analysis
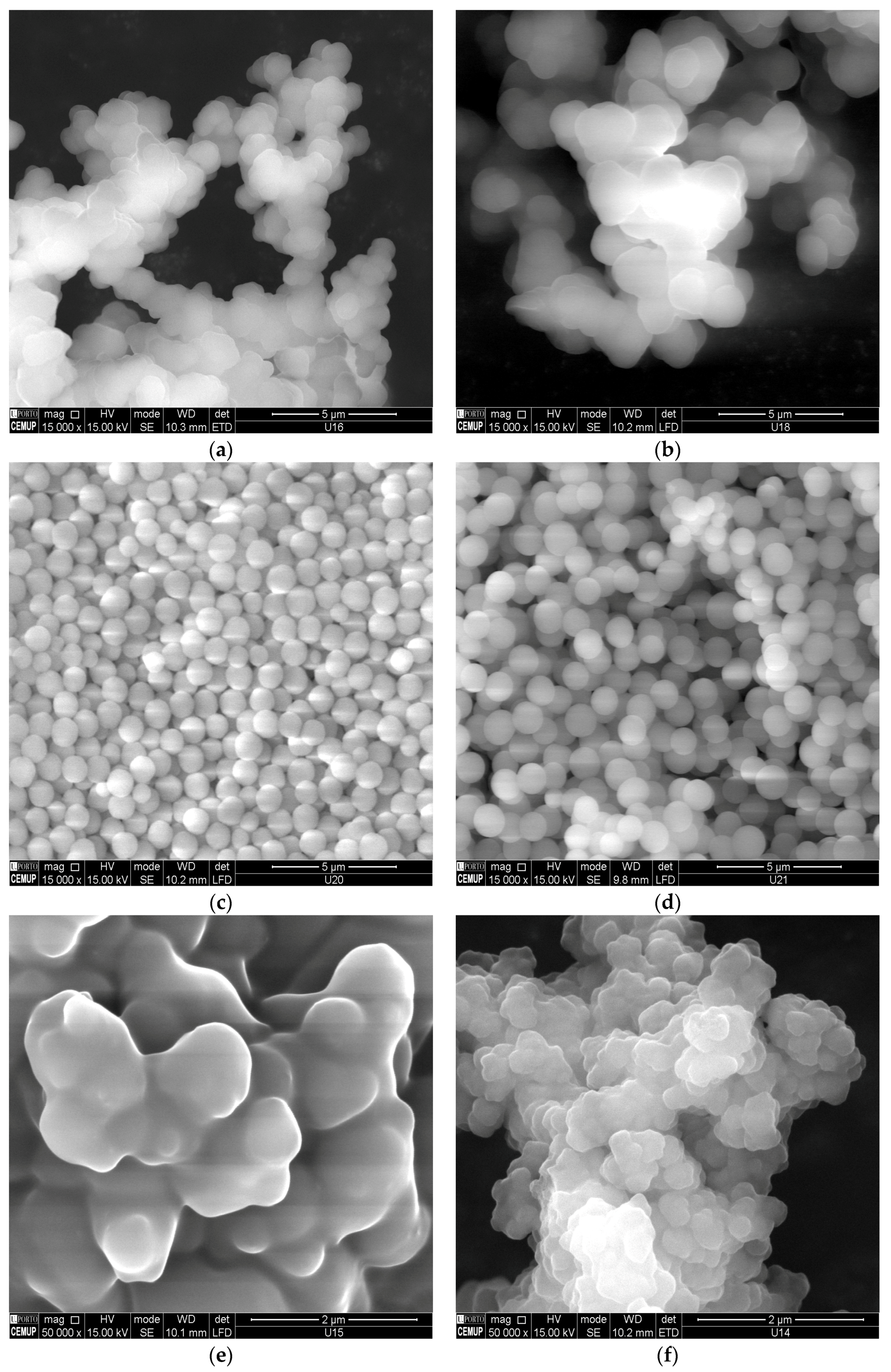
In
Figure 2 are presented the SEM micrographs for some products obtained in this research, highlighting the effect of the synthesis conditions on MIPs morphology (see also the
Supplementary Materials for additional SEM images). Particles with diameter smaller than 1 µm were formed with MIP1 and MIP2 (MAA and 4VP as FM, respectively, both with ACN/MeOH as solvent) but a higher agglomeration seems to have occurred in the latter case. Aggregates consisting of particles with particle diameter ~2 µm were formed with MIP3 (AAm as FM in ACN/MeOH) and a moderate agglomeration phenomena was observed. MIP4 shows a very different morphology (almost plain surface at the µm scale) due to the effect of the solvent used (TOL/MeOH) with 4VP as FM. Conversely, individual particles with particle diameter ~1 µm, without agglomeration effects, were formed with 4VP as FM and MeOH/H
2O as solvent (MIP5). Formation of similar particles was also possible with suspension polymerization with 4VP as FM and DMF as solvent in the monomer phase (MIP6). Stronger agglomeration effects should have occurred for MIP7, resulting also from suspension polymerization with 4VP as FM but MeOH/H
2O as solvent in the monomer phase. Aggregates consisting of particles with diameter <1 µm, thus also exhibiting some degree of product agglomeration, have also been observed with other imprinting systems, such as MIP8 (AA as FM in ACN/MeOH and TMPTA as crosslinker). Note that, besides the concentration effect on particles agglomeration, other issues such as the thermodynamics of phase segregation or even the stirring conditions can regulate the formation of such aggregates.
Interesting features concerning the impact of polymerization conditions on products morphologies were also observed with other reaction systems (see also the
Supplementary Materials). A much higher agglomeration extent is observed for MIP9 (DMAEMA as FM), namely comparatively to MIP8, highlighting the important effect of the FM on products morphology, even at a low content (
). The SEM micrograph for NIP1, obtained with conditions similar to MIP1, but in the absence of polydatin, shows the formation of individual particles with lower diameter and without agglomeration effects. A possible impact of the template molecule on particles formation is thus evidenced. The morphologies correspondent to EGDMA particles and MAA particles, respectively, obtained with conditions similar to MIP1/NIP1, highlight the entropic and enthalpic precipitation processes involved in the formation of such kind of polymer structures [
51,
52]. Indeed, in these kinds of polymerization processes, the growing polymer chains can phase-separate from the continuous medium by precipitation due to adverse enthalpic polymer-solvent interactions, such as in the case of MAA polymerization in ACN/MeOH. Additionally, precipitation can also be a consequence of an entropic effect due to the crosslinking action that prevents the mixing of solvent and polymer, such in the case of EGDMA polymerization. Note, however, that in many cases, such as MIPs/NIPs formation, both entropic and enthalpic precipitation mechanisms could be involved with concomitant effects on products morphology. Important insights on these precipitation/dispersion processes can also be obtained through polymer reaction engineering modeling studies (see e.g., [
53,
54,
55] and references therein) but these issues are beyond the scope of the present paper.
3.3. FTIR Analysis
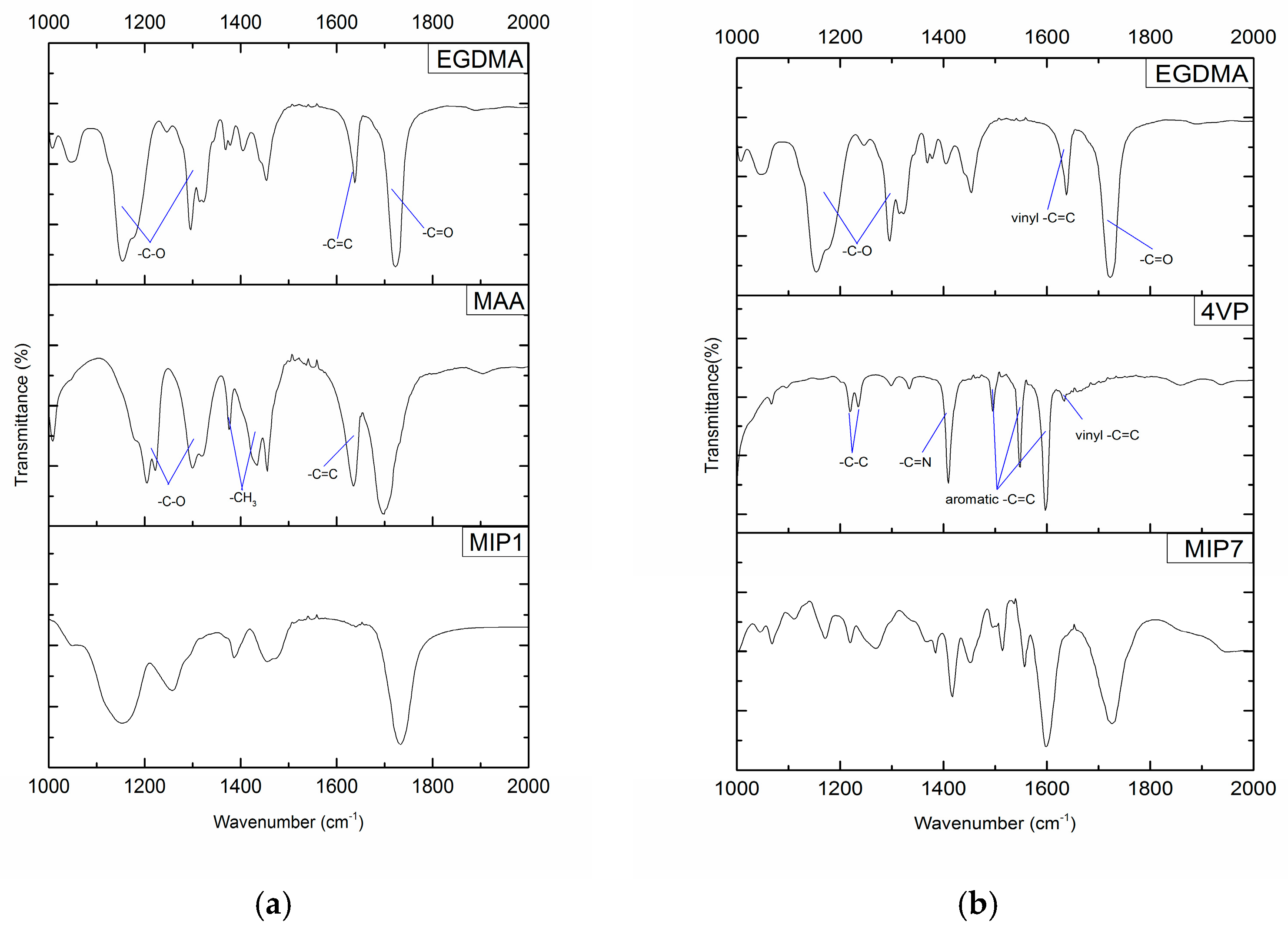
Important information concerning MIPs formation can be obtained through FTIR analysis, namely the extent of carbon-carbon double bonds depletion, functionalization and the incorporation of both the functional monomer and crosslinker in the final polymer network. Examples of such kinds of analysis are presented in
Figure 3 for two imprinting systems explored in this research (MIP1 and MIP7 were selected for illustration purposes). Besides the FTIR spectra for the isolated and purified MIPs, the spectra for the constitutive functional monomers (MAA or 4VP) and crosslinker (EGDMA) are also included in the plots.
Observation of
Figure 3a,b allows to conclude that a very high conversion of carbon-carbon double bonds must have occurred since only a very small peak is observed for the final materials at around 1630 cm
−1 (a well-known C=C assignment). Moreover, the incorporation of the functional monomer in the final MIP (an important issue in view of the molecular imprinting efficiency) is also clearly put into evidence in these analyses. Indeed, peak assignments correspondent to the FM (e.g., aromatic C=C in 4VP at ~1550 cm
−1 or ~1600 cm
−1), which are not observed in the crosslinker, can be indubitably identified in the synthesized MIPs.
3.4. SPE Testing
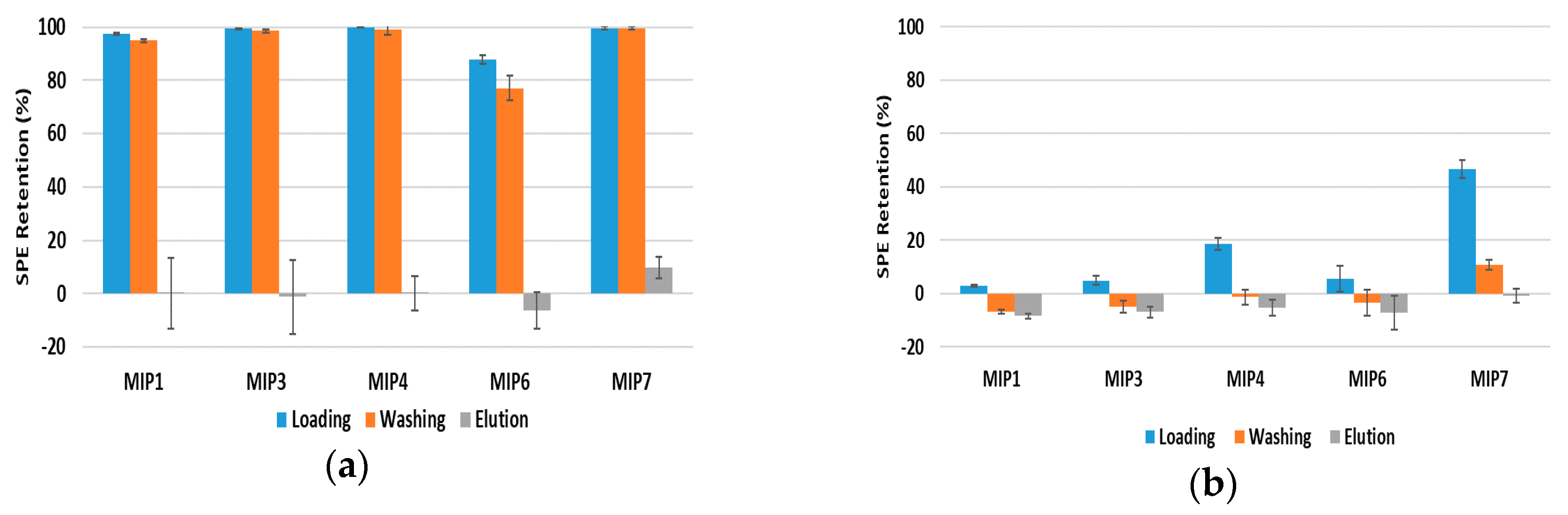
Due to its simplicity and the fast analysis procedure involved, solid phase extraction (SPE) was considered as a starting point in screening studies regarding the performance of the different materials synthesized (MIPs and NIPs) for different polyphenols uptake and release. In view of the expectable influence of the work conditions in the retention and liberation of phenolic compounds, assays with change on solvent polarity were also performed. Below are presented the most relevant results obtained in the framework of these SPE assays.
3.4.1. Study of the MIPs Performance in Low Polarity Solvent
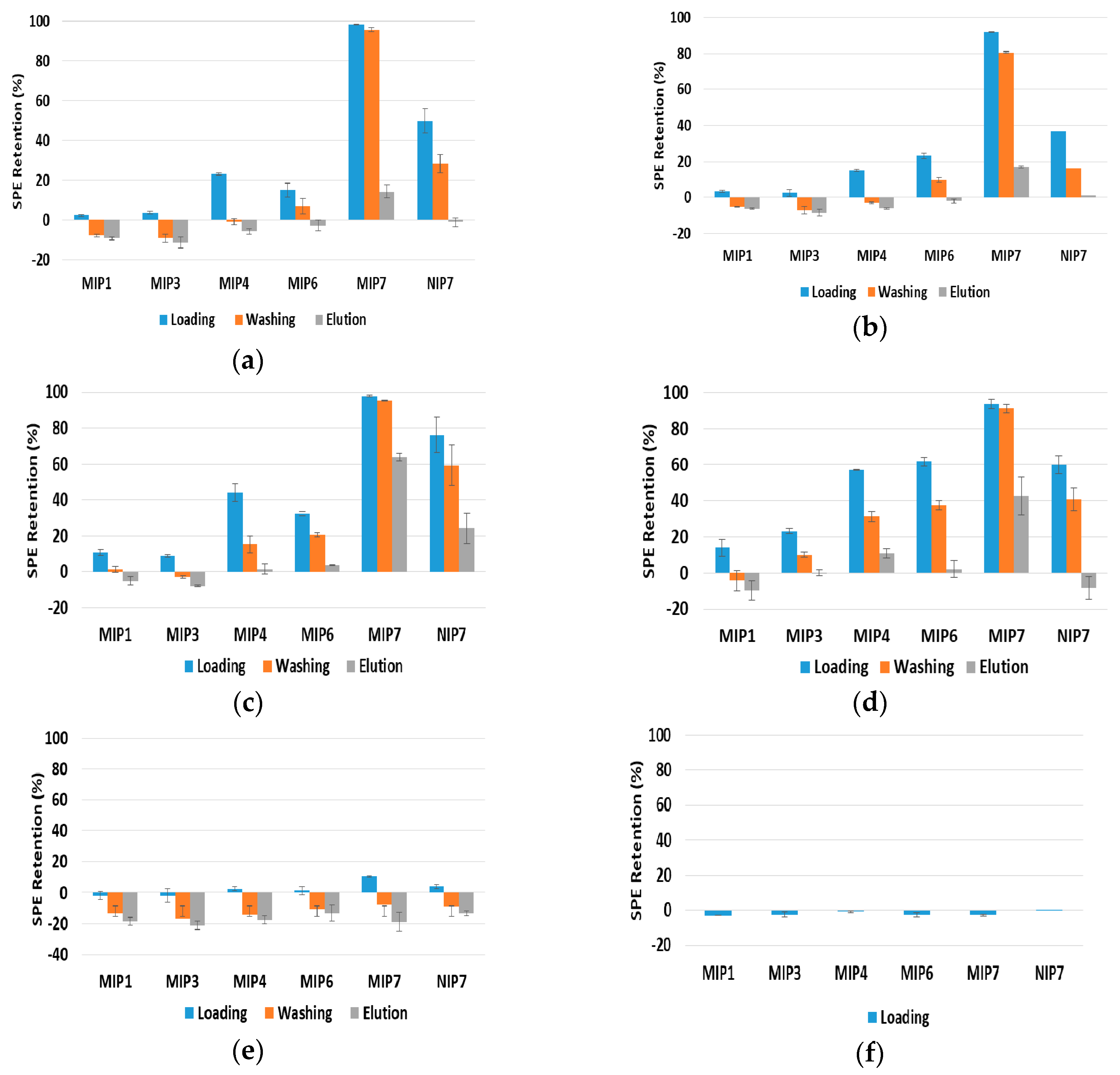
A common strategy for MIPs evaluation consists in using the same solvent of the molecular imprinting step to measure their retention capability. Additionally, in order to avoid breaking possible specific hydrogen bonding effects between the MIP and the target molecules, solvents lacking H-bond capacity, such as ACN, are often used. Thus, in
Figure 4 are presented results for the SPE analysis of different materials with ACN/MeOH as solvent together with different polyphenols (note that, as above stated, the use of a small fraction of MeOH (e.g.,) is necessary due to solubility limitations of many compounds). The obtained results show a clearly higher capacity of MIP4, MIP6 and MIP7 to retain polyphenols such as polydatin, resveratrol, quercetin or gallic acid (see
Figure 4a–d). However, MIP7 show a superior performance for the uptake of these polyphenols. Also, the relatively high retained amount of these molecules also after the washing process puts into evidence the plausible benefits of the molecular imprinting mechanism. Nonetheless, these MIPs were produced with polydatin as the template and these results show that non-specific retention of other polyphenols in the solid sorbent is unavoidable, even with a favorable solvent. The fitting of small molecules in cavities generated by molecular imprinting of polydatin is a possible explanation for such observations. Additional molecular-imprinting experiments with other template molecules (e.g., gallic acid) and considering the same polymerization conditions used in this work are needed to clarify these issues. Moreover, NIP7 also presents a very high retention capability for these polyphenols, which seems to indicate an enhancement of uptake properties when the polymerization process considered with MIP7/NIP7 is used (suspension polymerization with 4VP as FM and MeOH/H
2O as solvent), as will be further discussed below.
On the other hand, results presented in
Figure 4e,f, concerning the retention of bisphenol A and caffeine in these same materials, show that almost no affinity with these molecules is observed when ACN/MeOH is used as solvent ; only a very small holding capacity is observed in these conditions for these species. Therefore, some of the synthesized materials, especially MIP4, MIP6 and MIP7, present a good selective ability for polyphenols uptake with working conditions leading to the total elution of other kind of species (e.g., bisphenol A or caffeine).
3.4.2. Effect of the Solvent Polarity on MIPs Sorption Capabilities
As observed with many other sorbents, the retention capability of MIPs and their selectivity are strongly dependent on the chosen operating conditions, namely the polarity of the solvent; the temperature is also often used to tune the sorption capabilities. Indeed, due to hydrophilic and/or hydrophobic interactions critical changes on the uptake of a target molecule by a MIP can be observed, leading to possible impairing of the designed selectivity of the material (see e.g., the high retention of caffeine in a 5-fluouracil MIP reported in [
16] when water was used as solvent).
These issues are highlighted in
Figure 5, where the SPE performance of different MIPs for polydatin retention is compared considering two solvents with different polarities, namely H
2O/MeOH (9/1) and MeOH/H
2O (4/1). A very high retention capability of polydatin in all materials is observed with H
2O/MeOH (9/1), namely when measurements presented in
Figure 5a are compared with the results shown in
Figure 4a. This outcome is a consequence of the strong hydrophobic interactions between the MIPs and the polydatin molecule arising when H
2O/MeOH (9/1) is used as solvent. Note that very high retention is observed in conditions similar to other molecules (e.g., bisphenol A, namely in comparison with
Figure 4e), due to hydrophobic interactions, thus breaking a possible selectivity of the adsorbent.
Still, results presented in
Figure 5b show that a strong elution effect is observed when MeOH/H
2O (4/1) is used as solvent (see also
Figure 4a). However, even with these unfavorable conditions for sorption, a significant uptake capability is observed with MIP7 (c.a. 50% retention of polydatin), indicating an indisputable ability of this material for polyphenols retention.
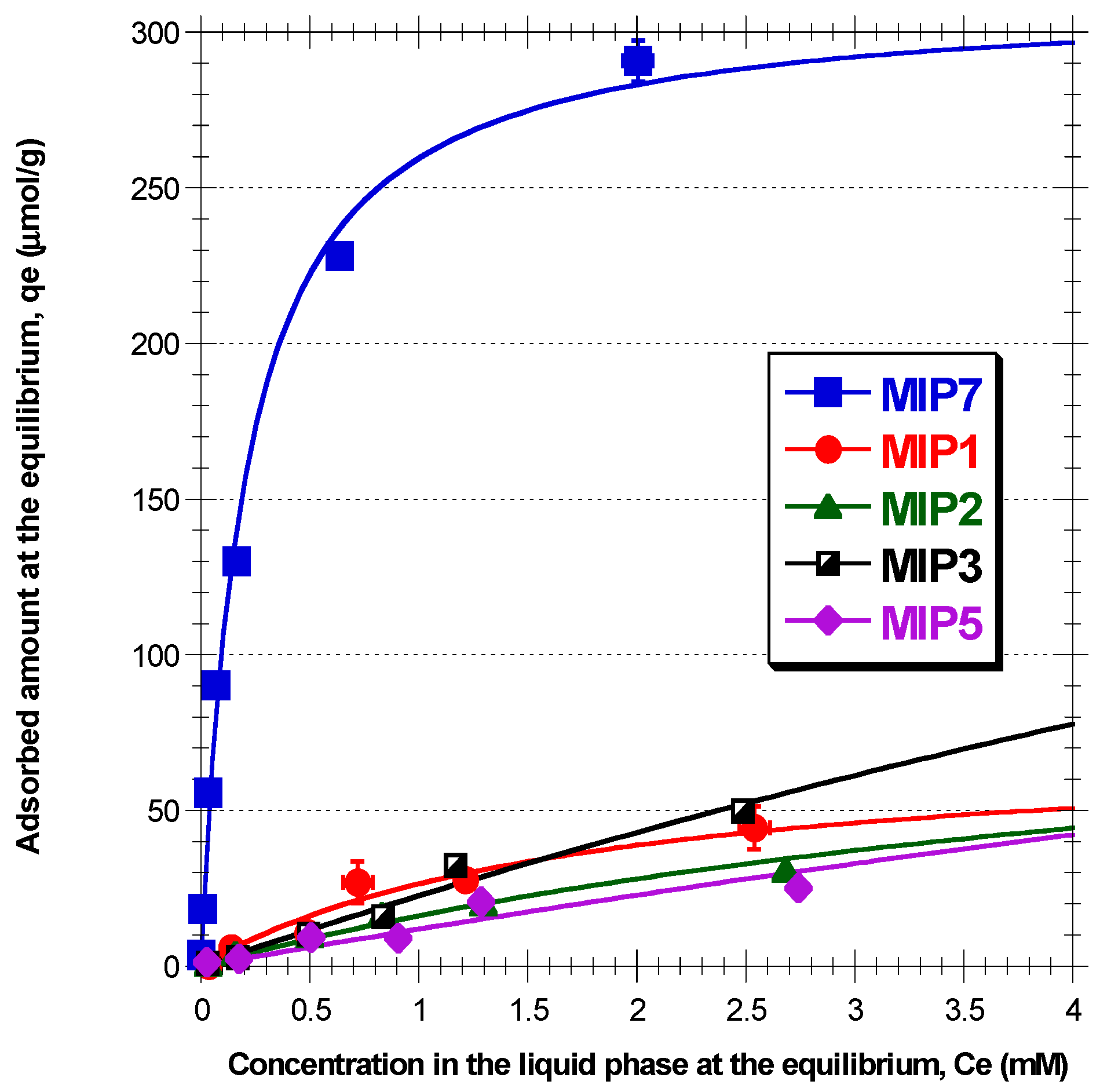
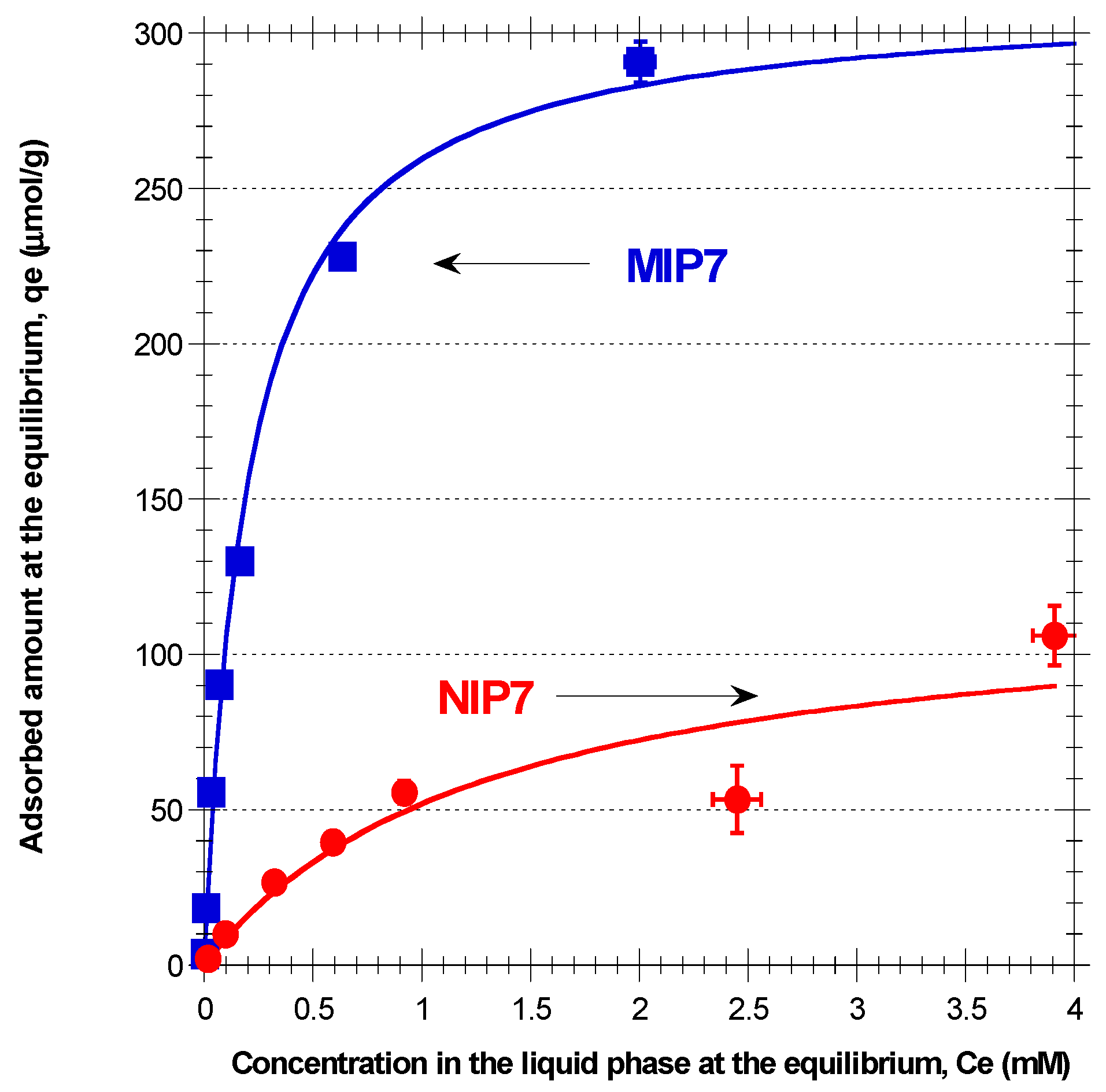
3.5. Batch Sorption
Batch sorption is a technique regularly considered for MIPs evaluation and some correspondent results for polydatin retention in different materials with ACN/MeOH 10/1 as solvent are presented in
Figure 6 and
Figure 7. Globally, these results confirm the superior ability of MIP7 for the retention of polydatin (and other polyphenols, as presented in
Figure 4), namely in comparison with remaining prepared materials. With the working conditions used, a maximum retention capability
300 µmol/g is estimated for MIP7, which is much higher than the sorption capabilities reported in the bibliography for related systems. Indeed,
116 µmol/g [
26],
20 µmol/g [
27],
83 µmol/g [
33] and
30 µmol/g [
39] are examples of reported values for the maximum binding capacity of resveratrol in different MIPs, considering ACN as solvent (contribution of non-specific sites is included in these values). As expected, a smaller value
3 µmol/g was measured for the retention of resveratrol in a MIP choosing ethanol as solvent [
32].
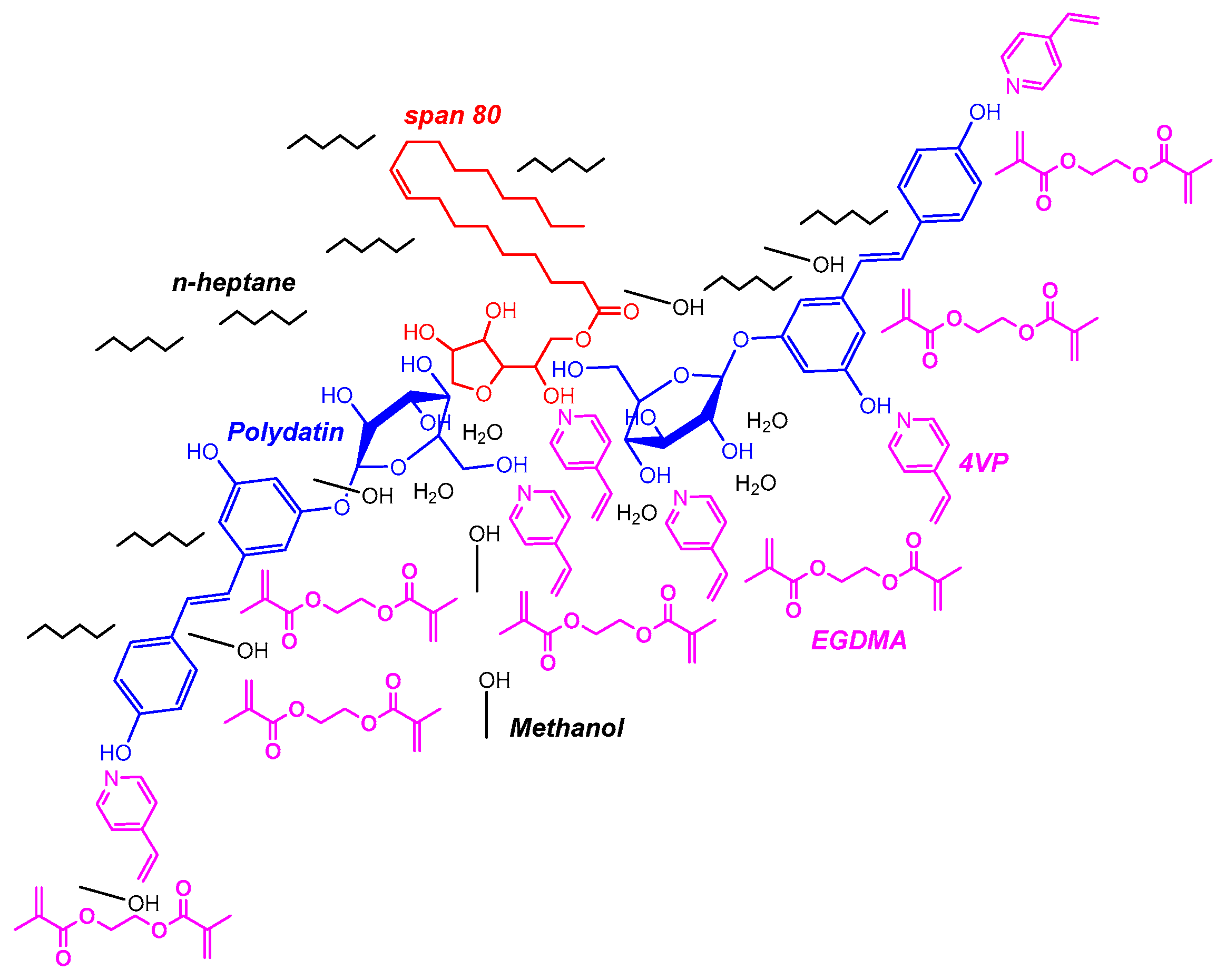
In
Figure 8 it is presented a possible explanation for the very high sorption capability for polyphenols measured with MIP7. Indeed, the hybrid hydrophilic/hydrophobic (amphiphilic) character of polydatin (a glucoside moiety is present in this molecule in contrast with resveratrol), can lead to its simultaneous interaction with the hydrophobic and the hydrophilic phases in a suspension polymerization process, as depicted in
Figure 8. The hydrophilic part of polydatin should interact preferentially with the aqueous phase containing water, MeOH, monomer 4VP (the FM) and EGDMA. Note that these three later species have affinity with the hydrophobic phase and should also be distributed along the interface layer. Conversely, the hydrophobic moieties of polydatin tend to shun water, thus migrating to the hydrophobic phase containing n-heptane. Moreover, Span 80 should also be located at the interface layer between the two phases (see
Figure 8), with the correspondent water-liking group interacting with hydrophilic moieties (e.g., water, glucoside group of polydatin, MeOH). After polymerization of this assembly and removal of the template (and also Span 80), the resulting polymer network particles should bear surface cavities with high affinity for polydatin and related polyphenols. Mass transfer mechanisms should also be enhanced owing to these surface binding sites, contributing to the higher performance observed with MIP7.
Note that a close association between polydatin and functional monomer should occur with chosen MIP7 synthesis conditions, due to the higher mass fraction used in the suspended phase, leading to an expected increase of molecular imprinting efficiency. Moreover, polydatin is c.a. 50 times more soluble in DMF than in water/methanol, which is likely to avoid the above depicted surface molecular imprinting process in MIP6.
The relatively high capability of NIP7 (non-imprinted material) for retention of polyphenols (see
Figure 4 and
Figure 7), should also be a consequence of an interfacial phenomena similar to the above depicted surface-molecular imprinting process. Actually, NIP7 was produced in the absence of polydatin but in the presence of Span 80. Therefore, it is also likely the formation of surface cavities owing to the hydrophilic moiety of Span 80 (containing OH groups) that should be effective (at a lower extent comparatively to MIP7) for polyphenols retention.
3.6. Competitive Sorption with SPE
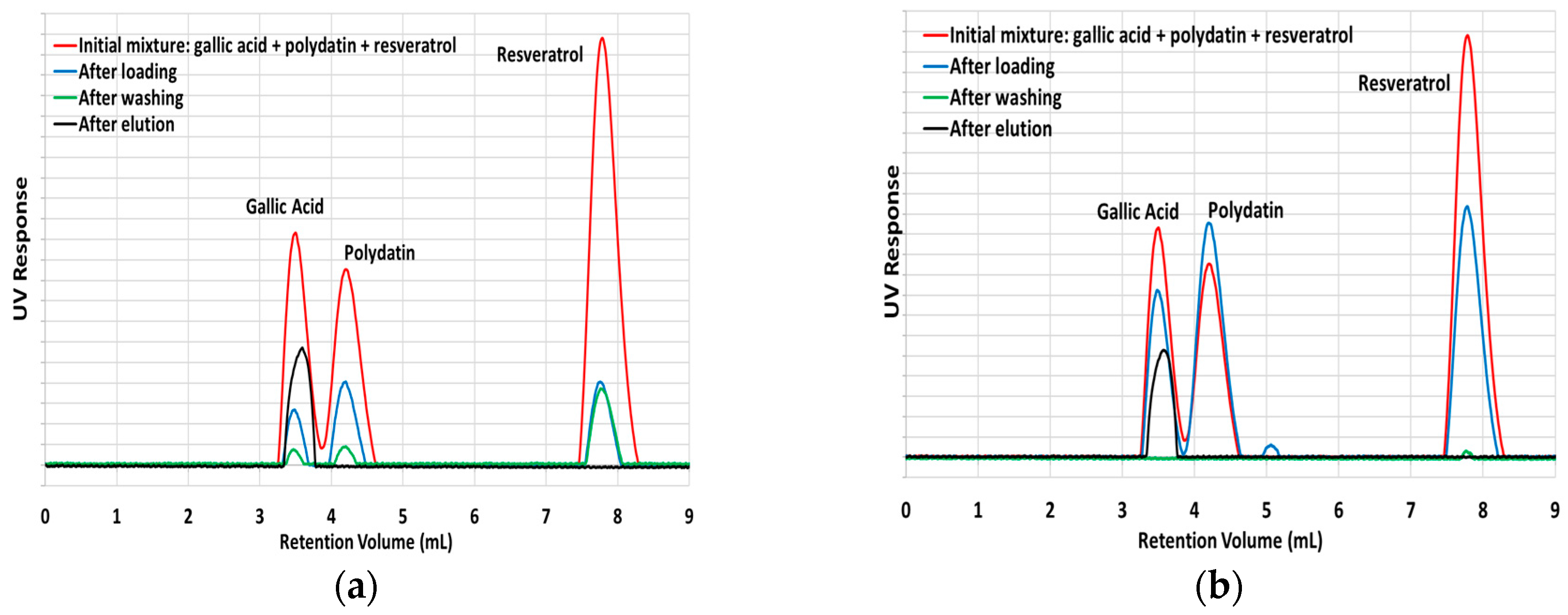
Considering the superior performance of MIP7 concerning polyphenols retention, this material was selected for the main further characterization studies, having in view final applications with natural extracts. At a first stage, the competitive sorption in SPE of a mixture containing gallic acid + polydatin + resveratrol was chosen to provide information concerning the selectivity of this material and correspondent non-imprinted analogue, with different polyphenols. Results presented in
Figure 9 confirm the previous findings with individual sorption assays, namely the high ability of MIP7 and NIP7 to uptake different kinds of polyphenols (eventually present in natural extracts). However, these results also highlight the benefits of molecular imprinting for polyphenols retention because a much higher and stronger uptake ability is shown for MIP7 comparatively to NIP7 (see the results for the washing step).
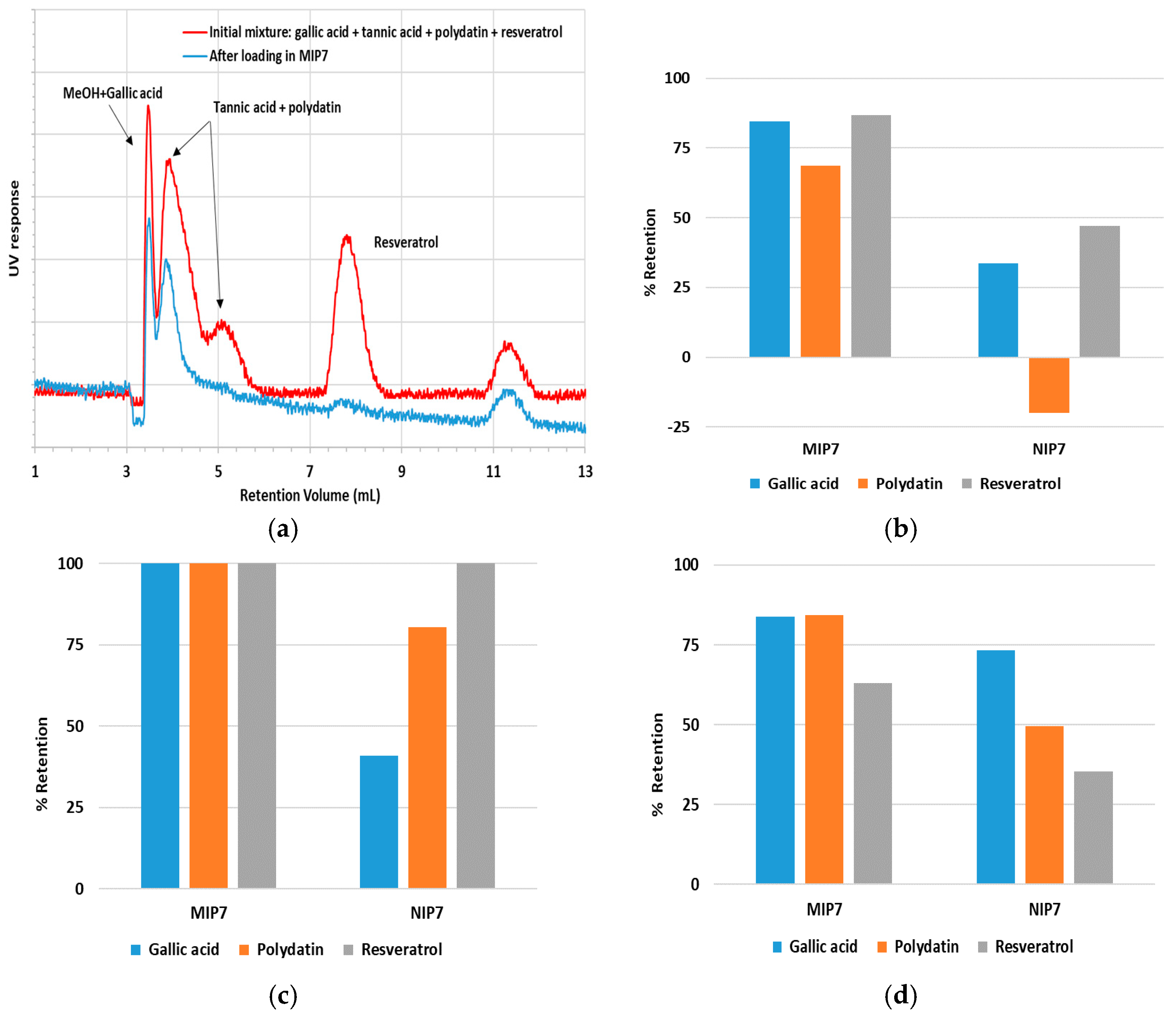
In
Figure 10a are presented additional results concerning the competitive sorption of different polyphenols in MIP7 and tannic acid, a plausible compound in many natural extracts, which was now included in the mixture. These results show again the likely ability of this material to uptake polyphenols eventually present in more complex natural matrices. Note that the peak appearing at about 11 mL should be due to some component of tannic acid, which is not a well-defined compound. Elution of other impurities present in the used polyphenols (e.g., in gallic acid, polydatin, resveratrol) is another possible cause for those unexpected peaks.
Previously (see
Figure 4a and
Figure 5), it was put into evidence the influence of solvent polarity on MIPs performance, namely owing to the interference of hydrophilic and hydrophobic effects. Results presented in
Figure 10b–d show similar outcomes with competitive sorption in three solvents with different polarities (H
2O/ACN 50/50, H
2O/ACN 90/10 and ACN/MeOH 10/1). Note, again, the superior retention in MIP7 comparatively to NIP7 in all the solvents and the noticeable influence of the hydrophobic effects when a polar solvent in used (total retention for the three polyphenols in MIP7 when H
2O/ACN 90/10 is considered). These results also show that the tuning of the polarity of the solvent is a critical issue if these materials are intended to separate different kinds of polyphenols because the non-specific retention is inevitable owing to the hydrophobic interactions. The use of some gradient of solvent composition is a possible way to address this issue, as discussed below.
3.7. Continuous Sorption Processes
Continuous sorption processes are important if industrial applications are sought. They also make easier the study of many fundamental mechanisms, such as mass transfer phenomena and equilibrium conditions. This approach was also considered in the present research and two different kinds of operating conditions we used (with/without recycling) are depicted in the
Supplementary Materials file. In these experimental systems, the dynamics of polyphenols retention/release was measured using in-line, off-line UV detection or HPLC analysis of collected samples. Note that much more elaborated continuous processes for the isolation of polyphenols from plant extracts were recently reported in the literature, namely concerning the recovery of oleuropein from olive leaf extracts with MIPs [
47]. In that work, besides a sophisticated experimental set-up, contour plots were used for the selection of the solvent and adsorbent (ethyl acetate and a MIP based on 1-(4-vinylphenyl)-3-(3,5-bis(trifluoromethyl)phenyl)urea/styrene with q ~ 228 µmol/g and IF ~ 12.2 were chosen) [
47]. Here, we only present first assessment studies concerning the performance of MIP7 in continuous processes.
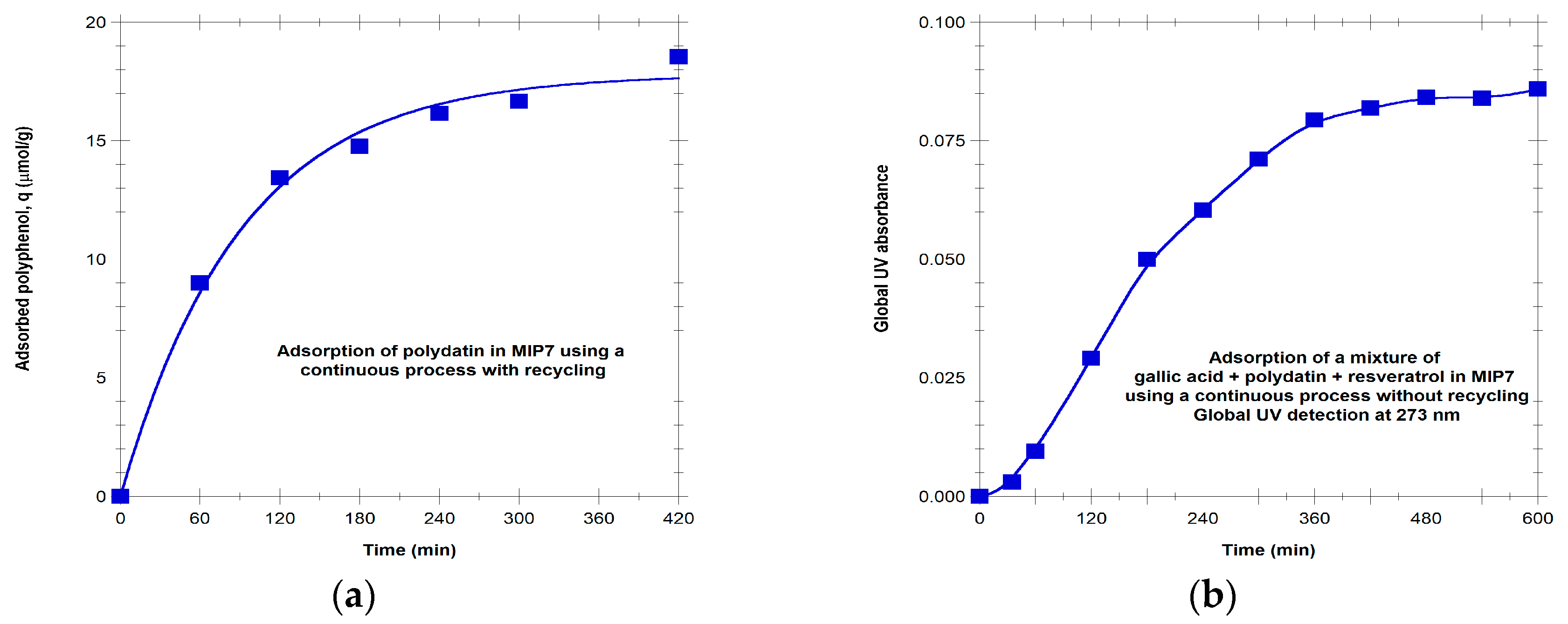
Typical profiles measured for the dynamics of sorption in MIP7 of individual polydatin or a mixture of gallic acid + polydatin + resveratrol are presented in
Figure 11a,b, respectively. Note the saturation of the MIP adsorbent that it is attained in both situations. For the individual polydatin sorption, equilibrium is reached with q ~ 19 µmol/g (with H
2O/MeOH 50/50 as solvent), while the global UV absorbance is used to show the column saturation with the polyphenols mixture.
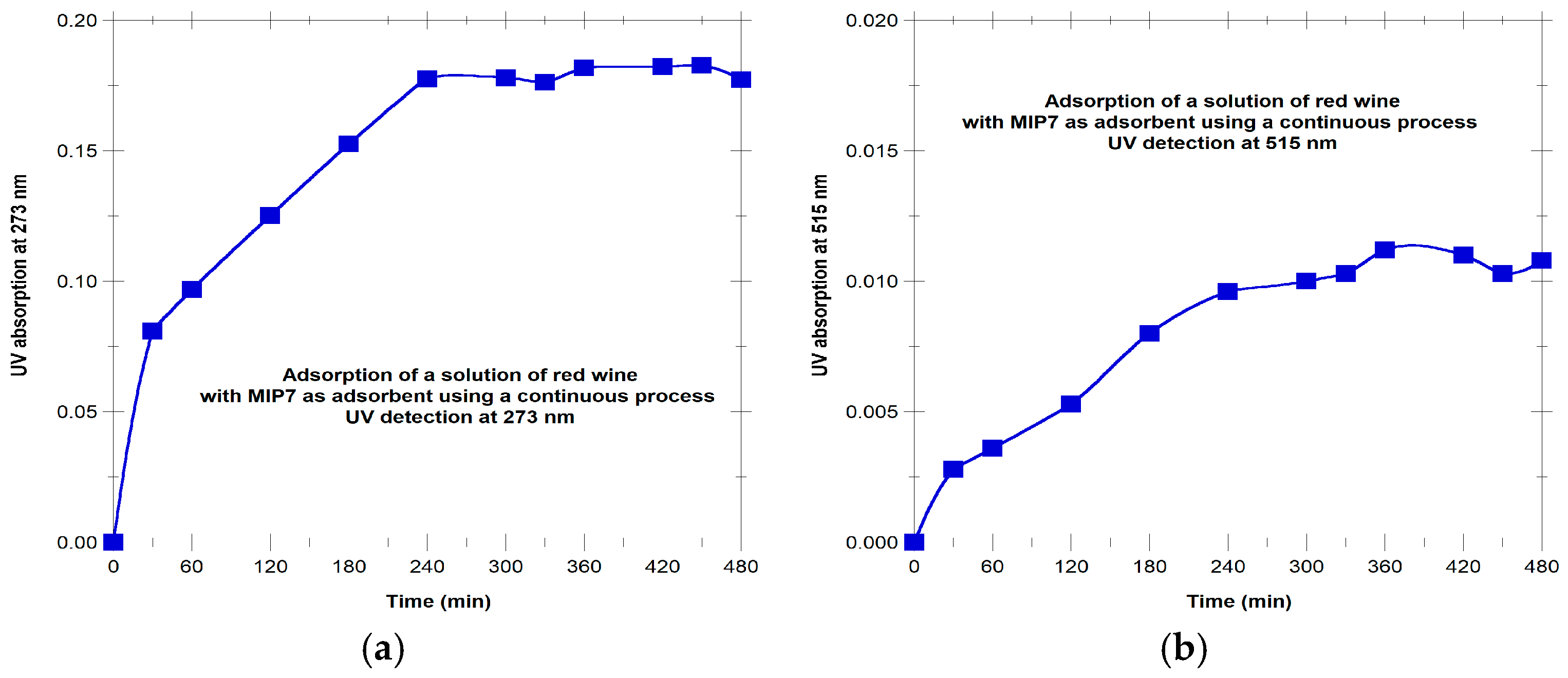
The potential use of MIP7 as adsorbent in a continuous process for a natural extract containing polyphenols is showed in
Figure 12. Red wine (Portuguese red wine from the Douro region) was directly diluted in H
2O/ACN 70/30 at 6% (
v/v) and the solution was percolated through MIP7. Results for the dynamics of sorption are presented in
Figure 12 with UV detection at 273 nm and also at 515 nm. The ability of the material to retain compounds with response at both wavelengths is shown.
Gallic acid, tannic acid, polydatin or resveratrol are examples of compounds probably present in the red wine with response at 273 nm, while anthocyanins are the likely molecules in red wine with absorbance at 515 nm. Indeed, the high ability of MIP7 to retain anthocyanins (compounds with important applications in bioprocesses) was visually confirmed through the elution of the red wine solution with the adsorbent placed in a transparent syringe tube. An obvious coloration of the polymer particles and discoloration of the solution was thus observed.
Having demonstrated the good performance of MIP7 on the global retention of polyphenols present in composed mixtures, or eventually in natural extracts, it is important to get insights on the selectivity of the uptake process and the subsequent release from the adsorbent. Actually, these issues are critical if the application of the materials is also aimed at the identification, separation and purification of the individual molecules.
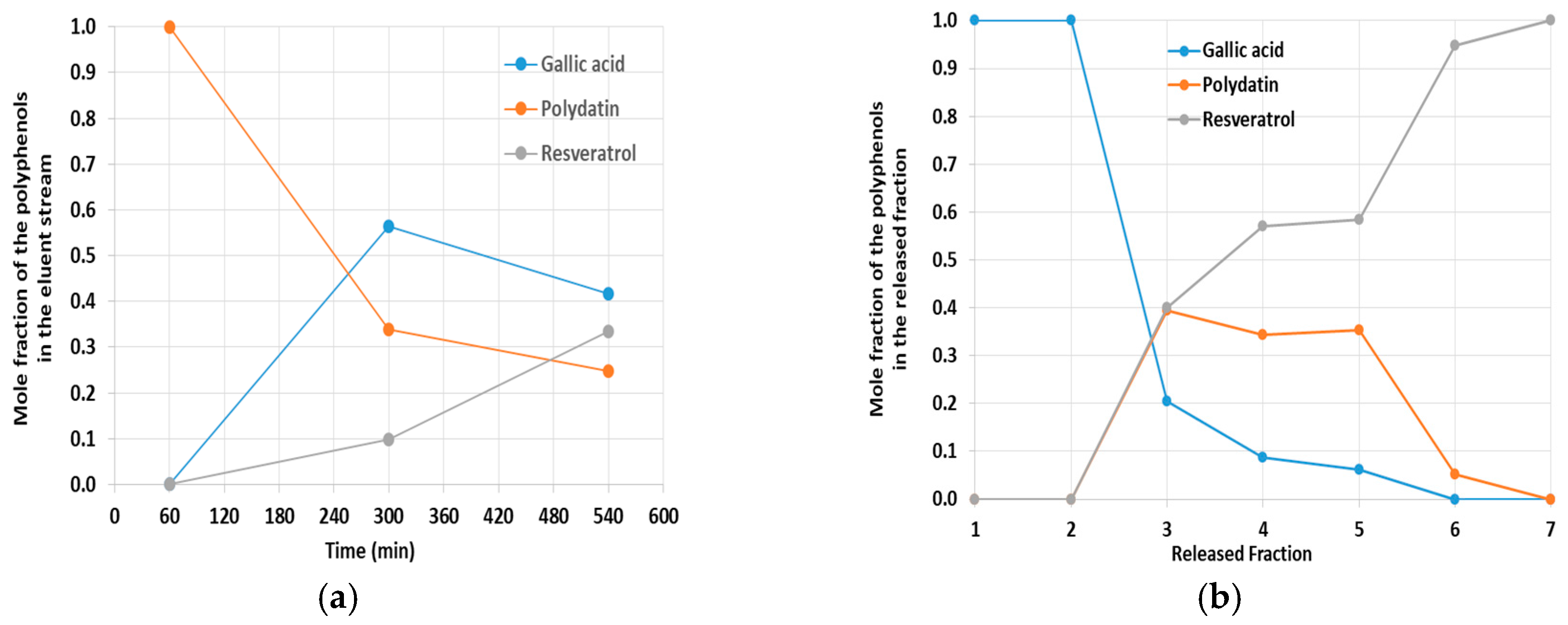
Some results obtained in this context for the composed mixture gallic acid + polydatin + resveratrol are presented in
Figure 13. Information concerning the individual amounts of each molecule in the collected samples was obtained by HPLC analysis. The dynamics for the composition of the eluent stream in a continuous process, with H
2O/ACN 70/30 as solvent (see also
Figure 11b), is shown in
Figure 13a. A preferential initial retention of resveratrol (probably due to hydrophobic interactions) and gallic acid seems to occur but close to saturation the retention order appears to be polydatin > resveratrol > gallic acid and some selectivity of the material through the three components is observed.
After saturation, the selectivity on the release of the three components was assessed and a kind of gradient of solvents with different polarities was considered, owing to the expected effect of the hydrophobic interactions on the release of the individual species. Measurements for the composition of the successive fractions obtained are presented in
Figure 13b. Fractions 1/2 were obtained with water as eluent and only gallic acid was recovered. With fractions 3/4/5, H
2O/ACN 50/50 was used as eluent and products with mixed compositions were recovered, but with prevalence to resveratrol + polydatin. Finally, with fractions 6/7, ACN/MeOH 10/1 was considered and almost only resveratrol was recovered.
The results above presented confirm the usefulness of the MIP7 adsorbent in the uptake and subsequent release of polyphenols, having in mind the concentration, separation and purification of such kinds of compounds. However, the effect of concomitant hydrophilic and hydrophobic interactions is inevitable in these processes and the tuning of the solvents polarity is a critical issue to achieve good separation of the individual species when complex mixtures are targeted.
3.8. Application to Polyphenols Identification and Separation in Natural Extracts
The above described strategy for the synthetic mixtures of polyphenols was also tried with natural extracts, namely a diluted solution of red wine (6%
v/v) in H
2O/ACN 70/30 (see also
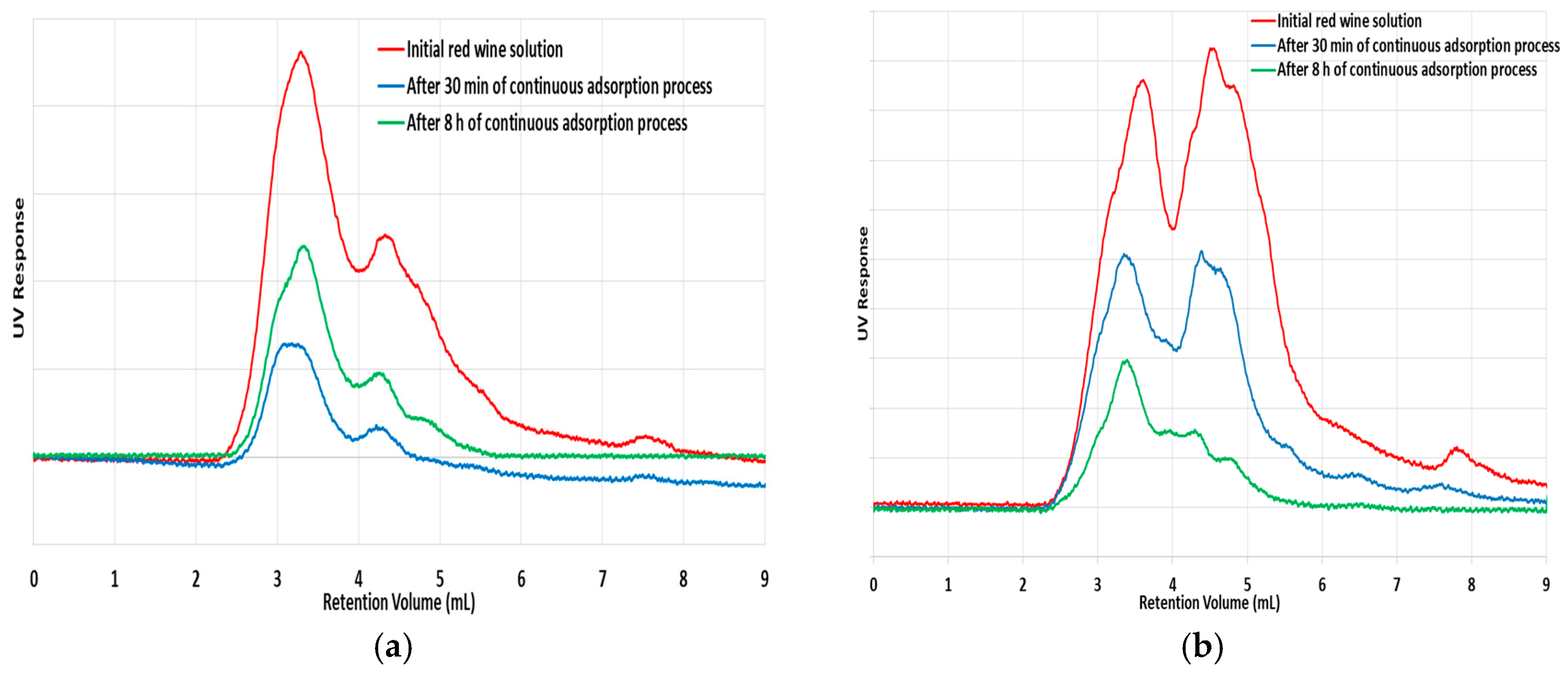
Figure 12). The dynamics of sorption of such solution of red wine in MIP7 is further put into evidence in
Figure 14, through HPLC analysis of samples collected at the column outlet at different operation times. Note the clear change of the UV absorbance for the samples collected at the column end, namely when compared with the feeding solution, showing the sorption of species with UV response at 273 nm (e.g., gallic acid, tannic acid, polydatin, resveratrol, etc.) and also at 306 nm (resveratrol and polydatin have stronger absorbance at this wavelength).
After column saturation, the release of the adsorbed species was studied by collecting fractions with solvents of different polarities, as above described for the mixture gallic acid + polydatin + resveratrol. Some of the results thus obtained are presented in
Figure 15, considering again the HPLC analysis of the fractions collected. In
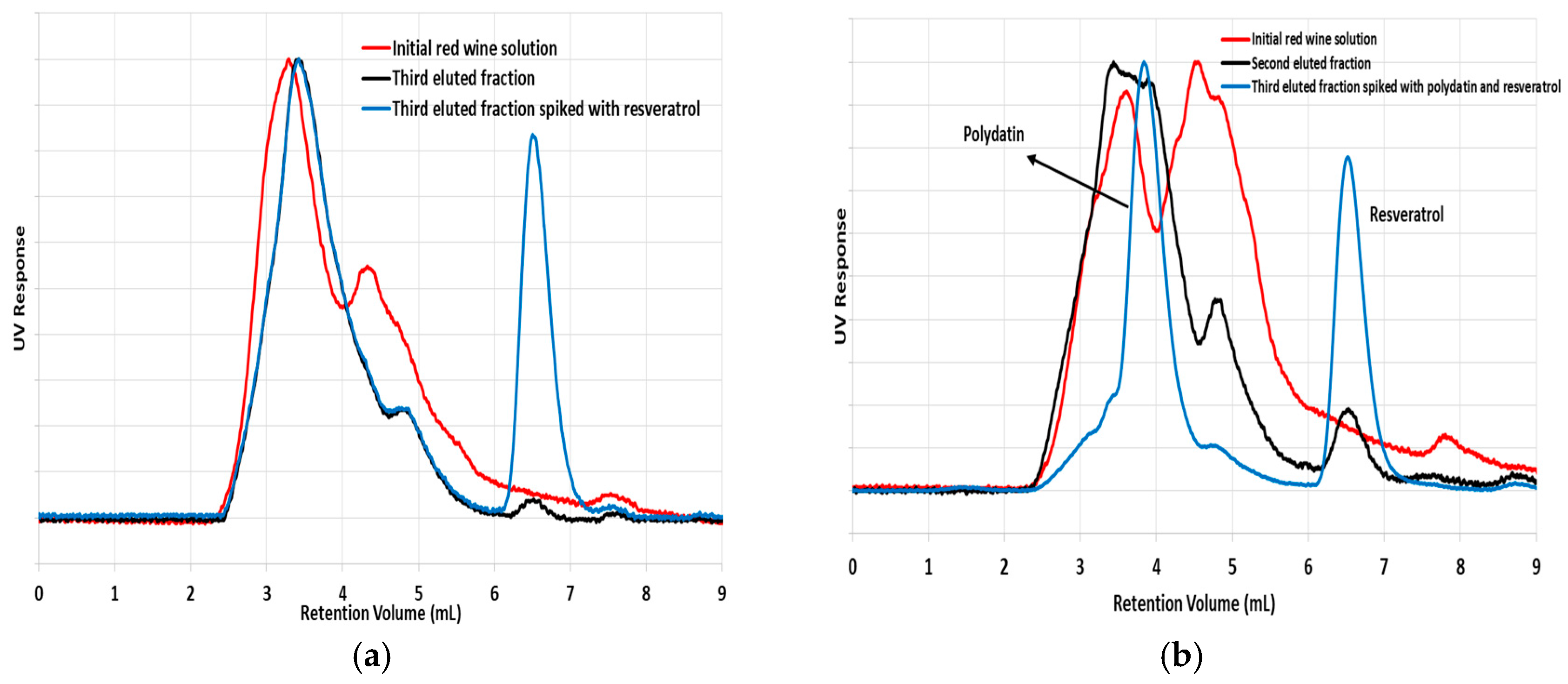
Figure 15a are compared the chromatograms correspondent to the initial red wine solution and to a fraction collected with H
2O/ACN 50/50. Differences observed for the two chromatograms are indicative of composition changes achieved with the sorption process. Among other differences, a new peak at around 6.5 mL retention volume is clearly identified in the chromatogram of the eluted fraction but not in the original extract. This outcome is a plausible consequence of the concentration process achieved with the MIP for some specie present in the original red wine. Indeed, a third chromatogram presented in
Figure 15a shows that the concentrated species should be resveratrol. This third chromatogram concerns the same eluted fraction spiked with resveratrol. The matching of the new peak identified in the collected fraction with the one correspondent to the spike of resveratrol is indicative of the likely concentration process achieved for this polyphenol. Similar results are presented in
Figure 15b, considering detection at 306 nm and also a sample spiked with polydatin + resveratrol. Besides the clear peak arising at 6.5 mL retention volume, consistent with resveratrol concentration, comparison of the three chromatograms is also indicative of polydatin (or similar species) concentration in consequence of the sorption process performed in the MIP (see change in the chromatogram of the original red wine extract at around 4 mL, that is the region correspondent to polydatin).
As described in
Section 2.9, a simple isocratic HPLC analysis was here considered and some changes in peak positions are possible due to eluent composition variations (see e.g., resveratrol in
Figure 10a and
Figure 15). Injection of the pure components and spiking studies were used to identify individual molecules in more complex mixtures. However, improved results for the identification and quantification of such compounds can be achieved using more elaborated HPLC gradient strategies, as reported in the literature (see e.g., [
48,
49] and references therein).
Results here presented are indisputable evidence for the usefulness of the tested MIP for the uptake, identification and separation of polyphenols present in natural extracts. However, many process upgrades are possible in order to improve the results obtained with complex natural extracts where a huge number of different species can be present. A possible strategy is the designing of different MIPs to target diverse polyphenols and consider a train of columns in a continuous sorption process (see also [
31]). Indeed, a MIP such as MIP9 can undoubtedly be used to selectively retain acidic species present in natural extracts, such as gallic acid and tannic acid, due to ionic interactions with the functional monomer DMAEMA (see also [
56], where additional characterization data for some materials here addressed are also available). Other tailored materials can in principle be synthesized with expected outcomes in the simplification of these complex mixtures. The long-term stability and reusability of these MIPs is also an important issue to assure the reliability and economic viability of the continuous sorption processes [
57].
4. Conclusions
This work was devoted to the development of molecularly imprinted polymers to target polyphenols present in vegetable extracts. Polydatin was selected as a template polyphenol due to the relatively high size of the molecule and its hybrid hydrophilic/hydrophobic character. Different kinds of MIPs were synthesized in order to explore preferential interactions between the functional monomers (methacrylic acid, 4-vinylpyridine or 2-(dimethylamino)ethyl methacrylate, among others, were alternatively used) and the template molecule. The role of the polarity of the selected solvent on the molecular imprinting efficiency, namely owing to hydrophobic interactions, was also assessed. Precipitation and suspension polymerization were also considered in order to change the products morphology with potential effects on MIPs performance. Different techniques (SPE, batch sorption, continuous sorption processes) were used to measure the ability of the prepared MIPs to uptake and release polyphenols. Individual and competitive assays (e.g., with composed mixtures of gallic acid + tannic acid + polydatin + resveratrol) were both considered.
Among all prepared MIPs, a material resulting from a suspension polymerization process, with 4VP as functional monomer, EGDMA as crosslinker, water/methanol as solvent, n-heptane as oil and Span 80 as surfactant showed a superior performance on polyphenols uptake. A maximum retention capability 300 µmol/g is estimated for this MIP, which is much higher than sorption capabilities reported in the literature for related systems. The origin of such significant outcome is the likely surface imprinting process caused by the amphiphilic properties of polydatin. The better performances observed with this suspension product comparatively to the precipitation particles should also rely on these interfacial phenomena. The uptake and release of polyphenols present in natural extracts, considering a red wine solution as a case study, was also assessed using this material as sorbent. The ability of the MIP to uptake polyphenols present in the red wine was put into evidence and the subsequent selective release of the adsorbed species was also demonstrated. However, hydrophilic/hydrophobic interactions are inevitable in such processes, especially with complex natural extracts and the tuning of the polarity of the solvents used (eventually in a gradient process) is an important issue to achieve the improved isolation of the different polyphenols.
Despite the promising results obtained with the above-described MIP, further studies are needed to confirm the likely surface imprinting process. New synthesis tasks with similar suspension processes, but involving other template molecules (changing the amphiphilic interactions) and the measurement of the surface area and pore size distribution (e.g., using BET) for such products and analogues (e.g., correspondent NIPs) are some issues to be addressed in the future. Additionally, many other developments are possible to tailor materials and processes devoted to polyphenols targeting. Tuning of the hydrophilic/hydrophobic balance of the MIPs by changing the polymerization conditions (e.g., composition) or designing more complex architectures (e.g., MIP particles with RAFT surface—grafted hydrophilic chains) are strategies being explored. Use of a train of different MIPs in continuous sorption processes in order to address complex natural extracts or the designing of solvent gradient formulations are other ongoing studies in this context. Valorization of different kinds of agriculture and forest residues is the final goal of this research line.