Physicochemical Characteristics of Copper Smelting Slags from Kazakhstan and Their Potential for Secondary Resource Recovery
Abstract
1. Introduction
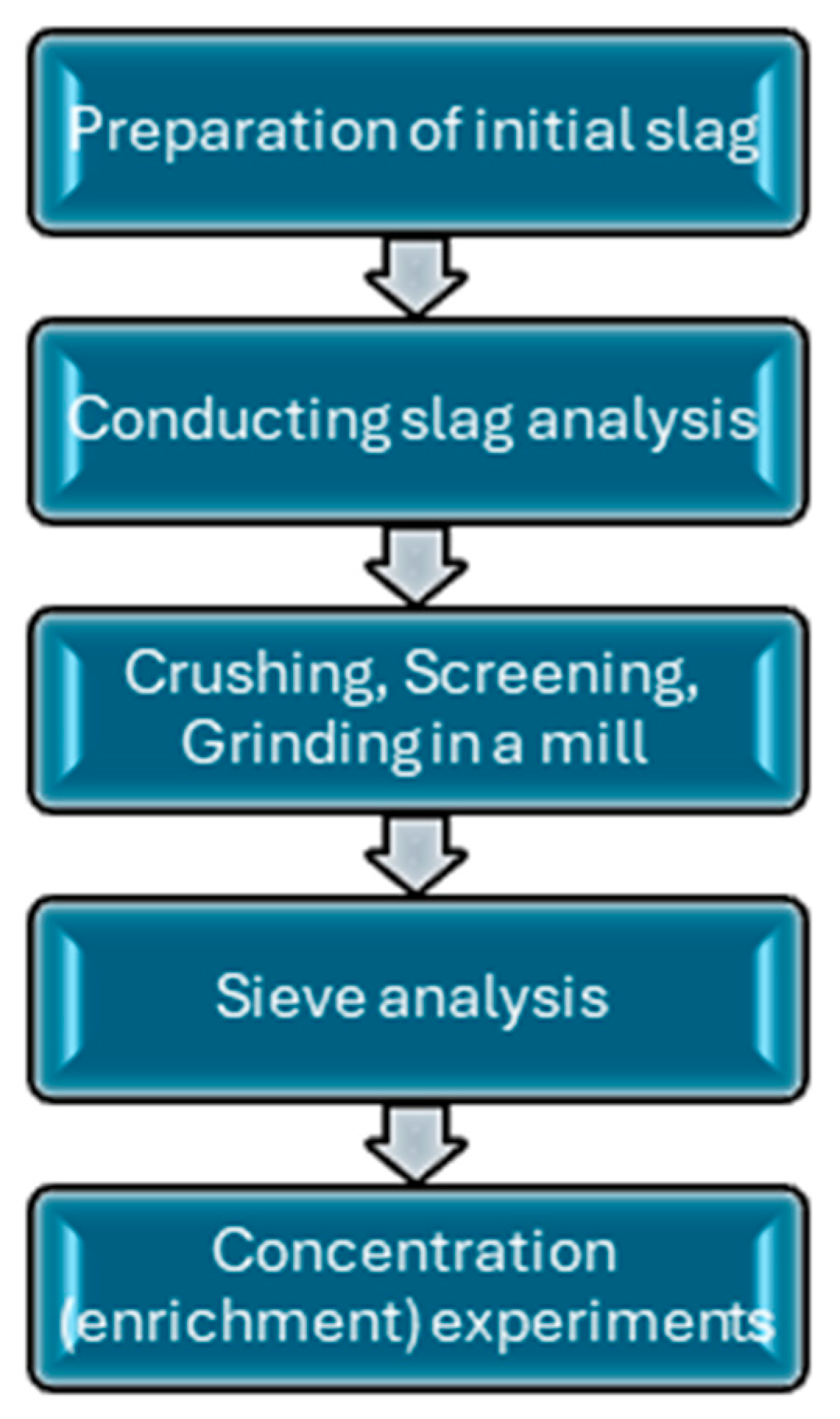
2. Materials and Methods
2.1. Mineralogical Analysis
2.2. Chemical and Granulometric Analysis
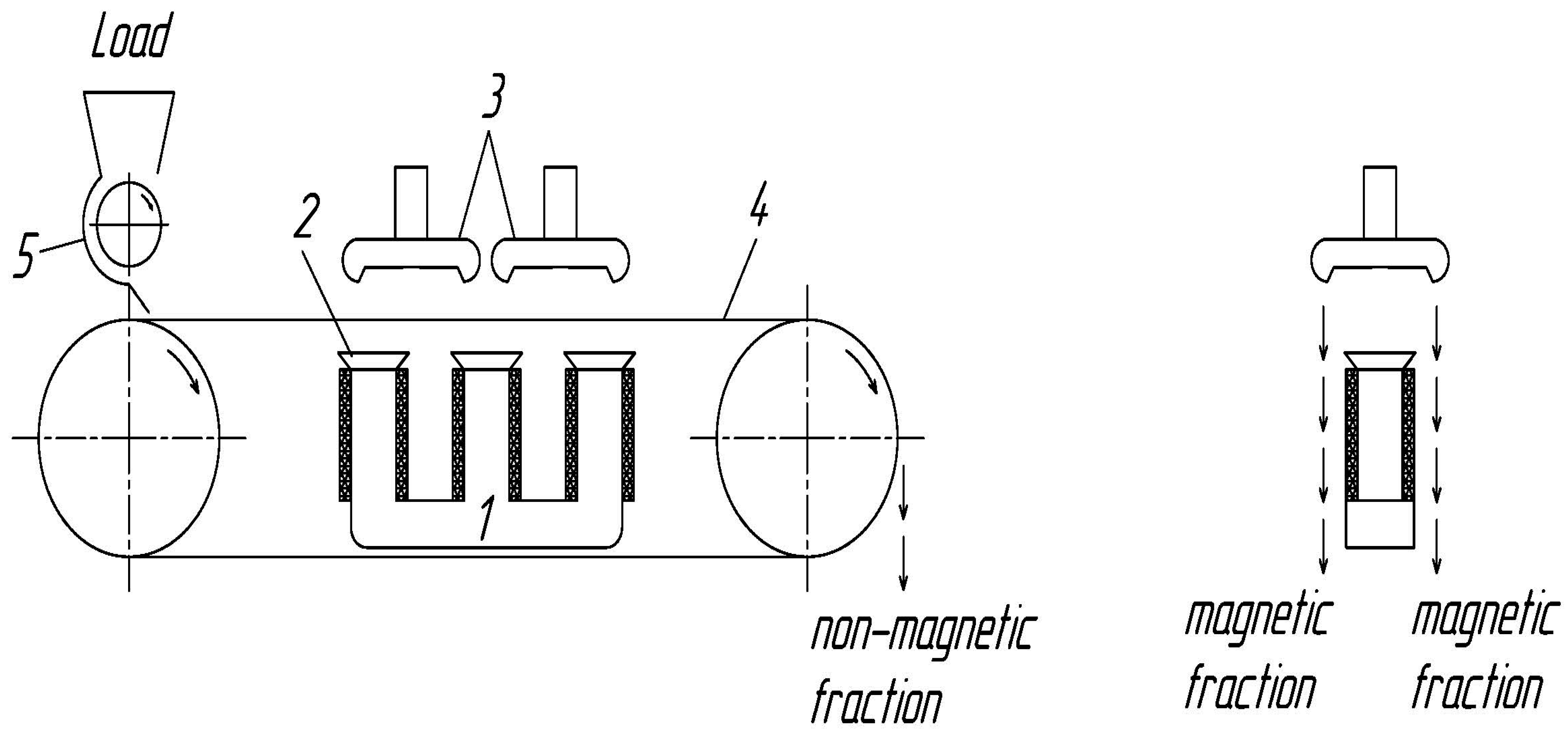
2.3. Magnetic Concentration
2.4. Gravity Concentration
2.5. Jigging
2.6. Concentration on a Concentrating Table
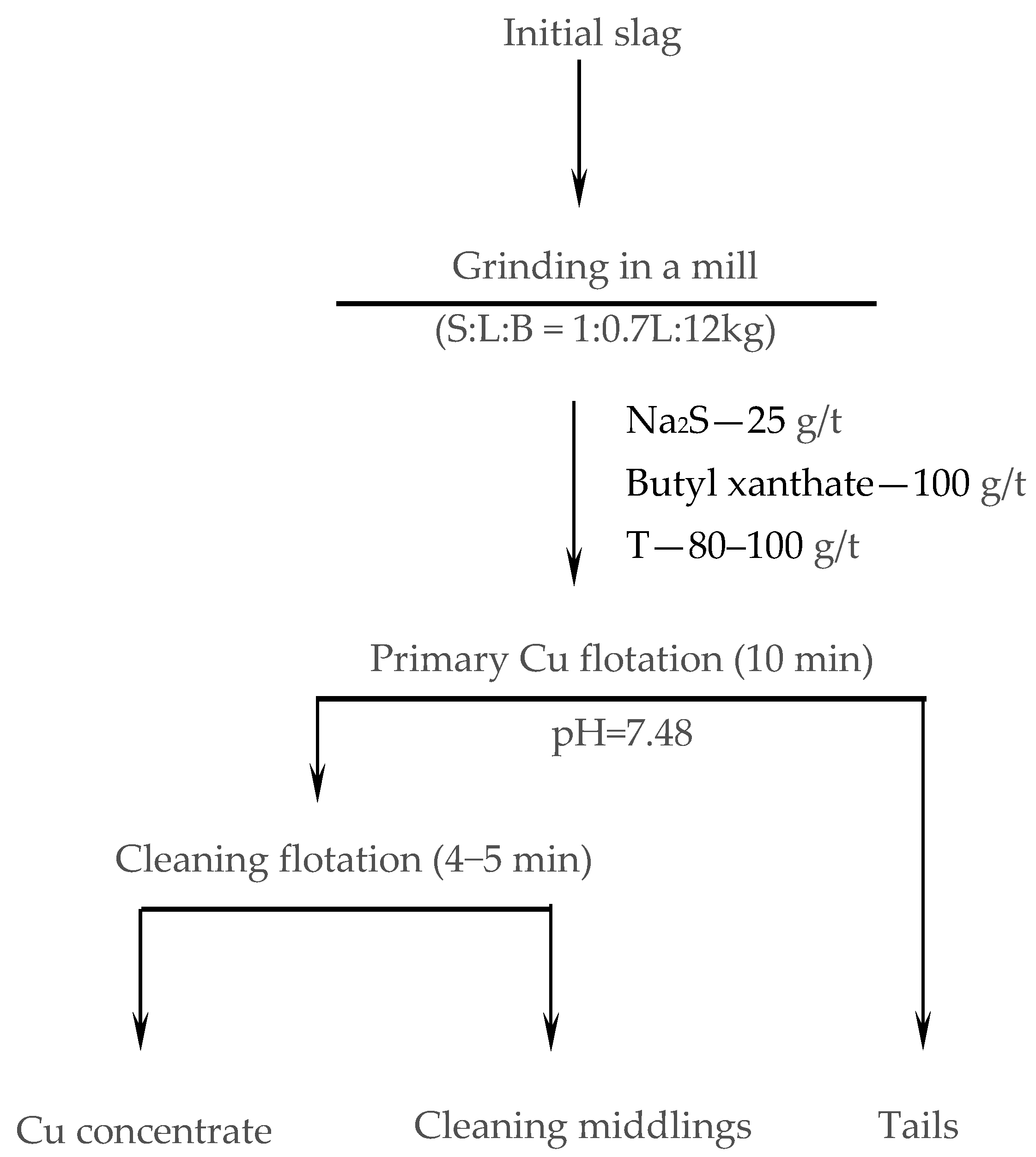
2.7. Flotation
3. Results
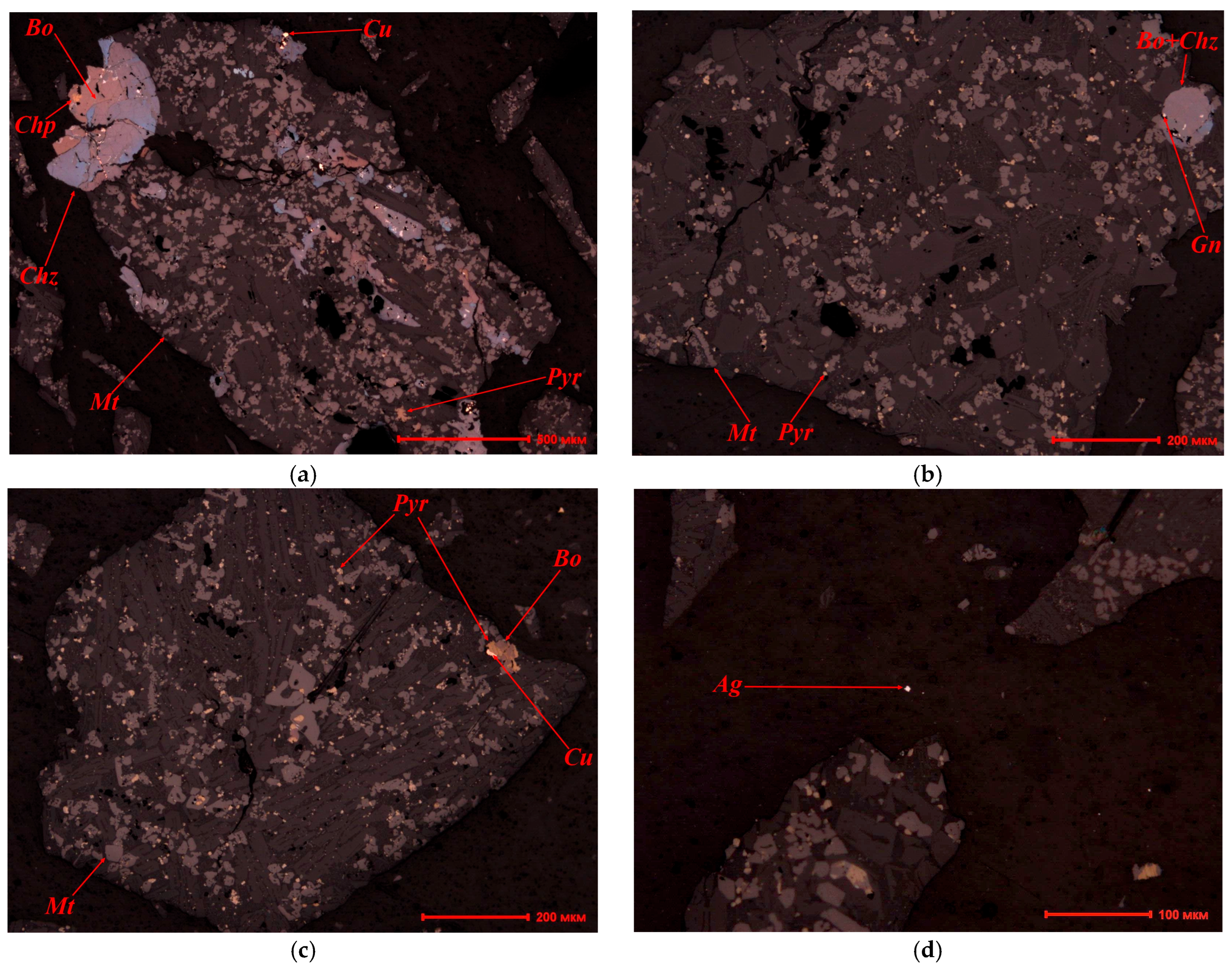
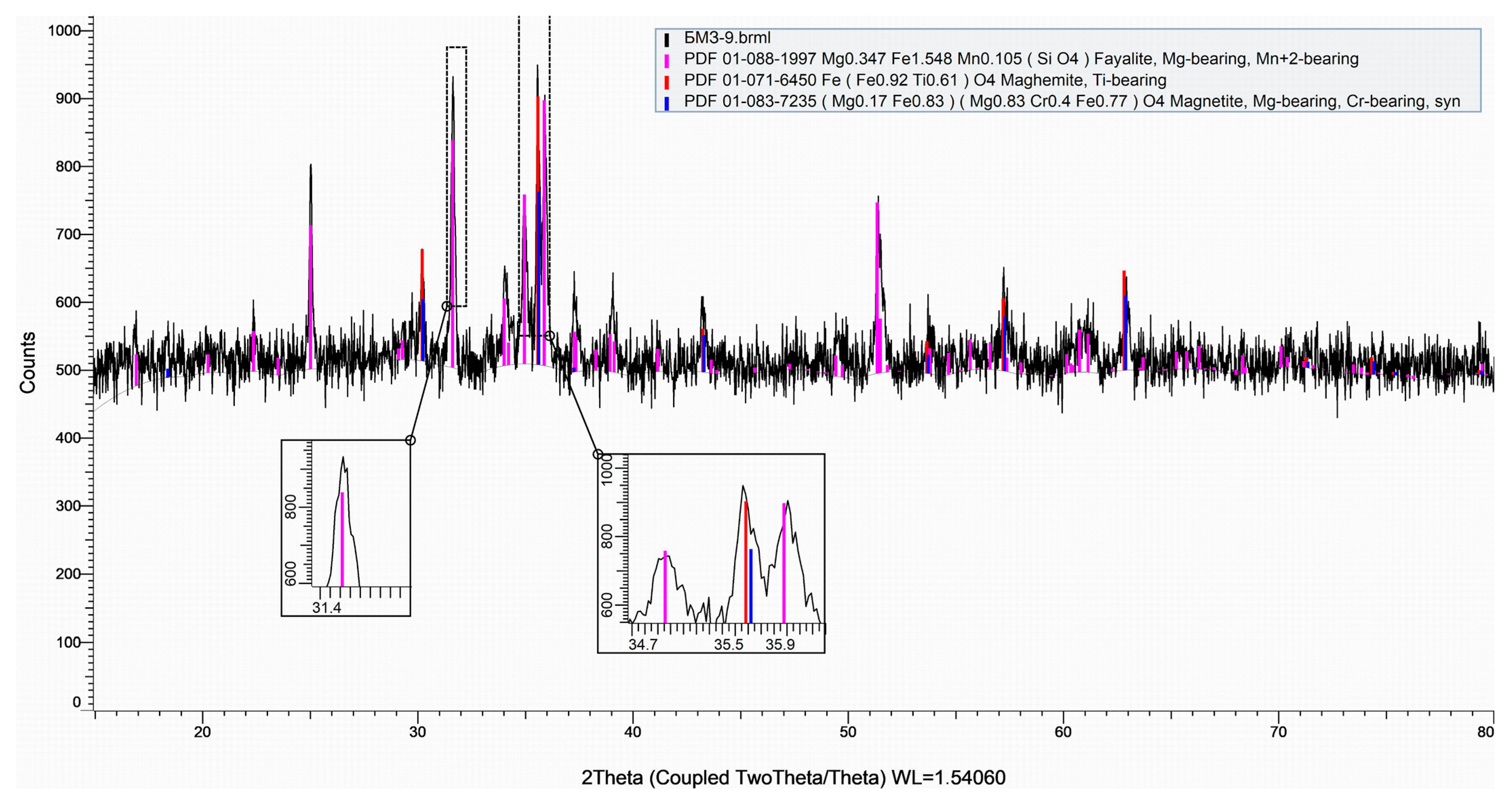
3.1. Mineralogical Analysis
3.2. Chemical and Granulometric Analysis
3.3. Magnetic Concentration
3.4. Gravity Concentration
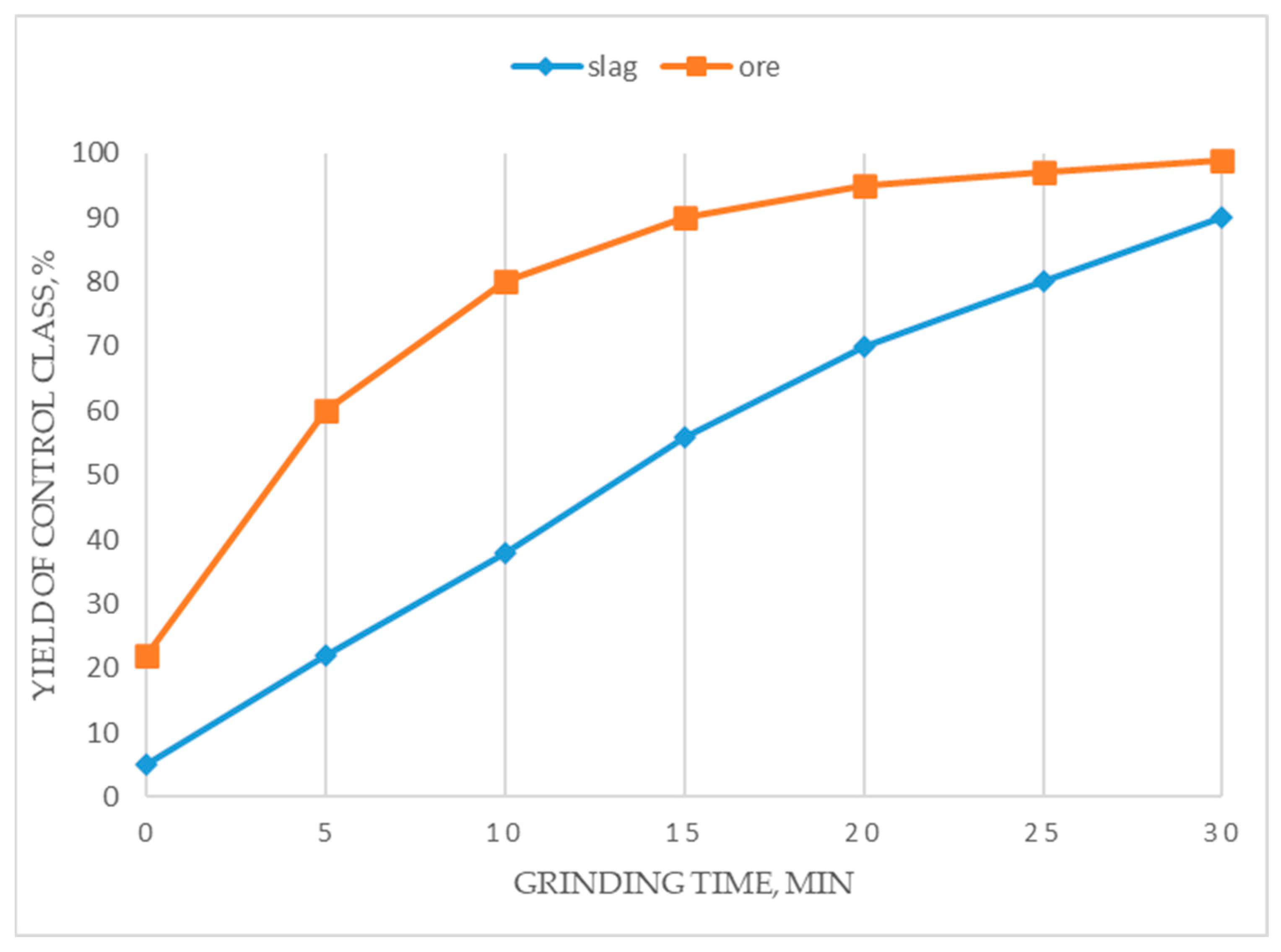
3.5. Jigging and Concentration Table Enrichment
3.6. Flotation
4. Discussion
4.1. Factors Limiting the Efficiency of Physical Beneficiation of Copper Smelting Slags
4.2. Recommended Processing Routes for the Recovery of Valuable Metals in the Slag
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Icsg Copper Bulletin. Available online: https://icsg.org/publications-list/ (accessed on 12 December 2025).
- IEA. Global Critical Minerals Outlook 2025. IEA, Paris. 2025. Available online: https://www.iea.org/reports/global-critical-minerals-outlook-2025 (accessed on 12 December 2025).
- Non-Ferrous Metals in Kazakhstan: Current State and Prospects. Available online: https://geoportal-kz.org/non-ferrous-metals/ (accessed on 12 December 2025).
- Kenzhaliyev, B.; Kvyatkovskiy, S.; Kozhakhmetov, S.; Sokolovskaya, L.V.; Semenova, A.S. Deparation of dump slags at the Balkhash copper smelting plant. Kompleks. Ispolz. Miner. Syra Complex Use Miner. Resour. 2018, 306, 45–53. [Google Scholar] [CrossRef]
- Kurmangaliyev, D.; Abdulina, S.; Mamyachenkov, S. Promising methods for hydrometallurgical processing of copper slag. Kompleks. Ispolz. Miner. Syra Complex Use Miner. Resour. 2022, 323, 46–50. [Google Scholar] [CrossRef]
- Qu, Y.; Tan, K.; Zhao, B.; Xie, S. Recovery of Cu-Fe Alloy from Copper Smelting Slag. Metals 2023, 13, 271. [Google Scholar] [CrossRef]
- Valderrama, L.; Tapia, J.; Pavez, O.; Santander, M.; Rivera, V.; Gonzalez, M. Recovery of Copper from Slags Through Flotation at the Hernán Videla Lira Smelter. Minerals 2024, 14, 1228. [Google Scholar] [CrossRef]
- Dzinomwa, G.; Mapani, B.; Nghipulile, T.; Maweja, K.; Kurasha, J.T.; Amwaama, M.; Chigayo, K. Mineralogical Characterization of Historic Copper Slag to Guide the Recovery of Valuable Metals: A Namibian Case Study. Materials 2023, 16, 6126. [Google Scholar] [CrossRef]
- Phiri, T.C.; Singh, P.; Nikoloski, A.N. Mineralogical Characterisation of Copper Slag and Phase Transformation after Carbocatalytic Reduction for Hydrometallurgical Extraction of Copper and Cobalt. Metals 2024, 14, 1119. [Google Scholar] [CrossRef]
- Nasab, S.M.; Bafti, B.S.; Yarahmadi, M.R.; Maymand, M.M.; Khorasani, J.K. Mineralogical Properties of the Copper Slags from the SarCheshmeh Smelter Plant, Iran, in View of Value Recovery. Minerals 2022, 12, 1153. [Google Scholar] [CrossRef]
- Sayitov, S.S.; Tsoi, V.D.; Rasulov, S.H.M.; Pechersky, R.D.; Rasulova, A.V.; Abduvaitov, A.K.; Asrorov, A.A. Material composition of copper slag of the Almalyk copper-smelting plant (Uzbekistan). Bull. Tomsk. Polytech. Univ. Geo Assets Eng. 2024, 335, 148–158. [Google Scholar] [CrossRef]
- Nadirov, R.K.; Turan, M.D.; Karamyrzayev, G.A. Copper Ammonia Leaching from Smelter Slag. Int. J. Biol. Chem. 2020, 12, 135–140. [Google Scholar] [CrossRef]
- Turan, M.D.; Karamyrzayev, G.; Nadirov, R. Recovery of zinc from copper smelter slag by sulfuric acid leaching in an aqueous and alcoholic environment. Chem. Bull. Kazakh Natl. Univ. 2021, 103, 4–11. [Google Scholar] [CrossRef]
- Baimbetov, B.S.; Moldabayeva, G.Z.; Taimassova, A.N.; Kunilova, I.V. Processing of copper smelting slag using sulfuric acid leaching: Technological aspects and solutions. Rudomet J. 2025, 1, 83–85. [Google Scholar] [CrossRef]
- Kurmangaliyev, D.B.; Abdulina, S.A.; Krykpaeva, A.A.; Mamyachenkov, S.V. A Method for Extracting Valuable Components from Copper Smelter Slag. Bull. D. Serikbayev EKTU 2023, 3, 80–87. [Google Scholar] [CrossRef]
- Zheng, J.; Xiao, K. The research on using dispersant agent to lron recovery in copper slag. Nonferr. Met. Sci. Eng. 2011, 6, 71–73. (In Chinese) [Google Scholar]
- Zhou, X.L.; Zhu, D.Q.; Pan, J.; Wu, T.J. Utilization of waste copper slag to produce directly reduced iron for weathering resistant steel. ISIJ Int. 2015, 55, 1347–1352. [Google Scholar] [CrossRef]
- Tong, X.; Han, B.; Ren, S.P.; Yang, B. Recovery of Copper from Copper Smelter Slag by Flotation. Appl. Mech. Mater. 2014, 496–500, 406–409. [Google Scholar] [CrossRef]
- Mu, X.; Kang, D.; Chen, J.H. Flotation Behavior of Smelting Abandoned Copper Slags. Adv. Mater. Res. 2013, 734–737, 1045–1048. [Google Scholar] [CrossRef]
- Štirbanović, Z.; Urošević, D.; Đorđević, M.; Sokolović, J.; Aksić, N.; Živadinović, N.; Milutinović, S. Application of Thionocarbamates in Copper Slag Flotation. Metals 2022, 12, 832. [Google Scholar] [CrossRef]
- Li, S.; Guo, Z.; Pan, J.; Zhu, D.; Dong, T.; Lu, S. Stepwise Utilization Process to Recover Valuable Components from Copper Slag. Minerals 2021, 11, 211. [Google Scholar] [CrossRef]
- Reddy, R.G.; Prabhu, V.L.; Mantha, D. Zinc Fuming from Lead Blast Furnace Slag. High Temp. Mater. Process. 2002, 21, 377–386. [Google Scholar] [CrossRef]
- Altundoǧan, H.S.; Tümen, F. Metal recovery from copper converter slag by roasting with ferric sulphate. Hydrometallurgy 1997, 44, 261–267. [Google Scholar] [CrossRef]
- Tümen, F.; Bailey, N.T. Recovery of metal values from copper smelter slags by roasting with pyrite. Hydrometallurgy 1990, 25, 317–328. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, B.; Wang, Q.; Li, Z.; Tian, Q. Recovery of Zinc and Lead from Copper Smelting Slags by Chlorination Roasting. JOM 2021, 73, 1861–1870. [Google Scholar] [CrossRef]
- Nadirov, R.K.; Syzdykova, L.I.; Zhussupova, A.K.; Usserbaev, M.T. Recovery of value metals from copper smelter slag by ammonium chloride treatment. Int. J. Miner. Process. 2013, 124, 145–149. [Google Scholar] [CrossRef]
- Silwamba, M.; Ito, M.; Hiroyoshi, N.; Tabelin, C.B.; Hashizume, R.; Fukushima, T.; Park, I.; Jeon, S.; Igarashi, T.; Sato, T.; et al. Alkaline Leaching and Concurrent Cementation of Dissolved Pb and Zn from Zinc Plant Leach Residues. Minerals 2022, 12, 393. [Google Scholar] [CrossRef]
| Suspension Density, g/cm3 | Viscosity, cP |
|---|---|
| 3.2 | 6–7 |
| 3.0 | 4–5 |
| 2.8 | 3.4–4.5 |
| 2.6 | 3–3.5 |
| Parameter | Value |
|---|---|
| Impeller speed, rpm | 1800 |
| Aeration rate, l/min | 3 |
| Pulp temperature, °C | 25 |
| Estimated number of revolutions of the foam extractor, rpm | 14 |
| Component Name | Absolute, % | Relative, % |
|---|---|---|
| Forms of Copper Occurrence | ||
| Water-soluble | 0.004 | 0.5 |
| Oxides | 0.039 | 4.8 |
| Sulfides | 0.660 | 82.1 |
| Ferrites | 0.101 | 12.6 |
| Total | 0.804 | 100.0 |
| Forms of Iron Occurrence | ||
| Oxidized divalent | 32.84 | 82.6 |
| Oxidized trivalent | 6.86 | 17.3 |
| Sulfide | 0.04 | 0.1 |
| Total | 39.74 | 100.0 |
| Forms of Zinc Occurrence | ||
| Oxidized | 1.14 | 46.15 |
| Sulfide | 0.26 | 10.53 |
| Sparingly soluble | 1.07 | 43.32 |
| Total | 2.47 | 100.0 |
| Classes, mm | Yield | Element | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Grams | % | Cu | Pb | Zn | As | SiO2 | Fe | Stotal | Au | Ag | |
| −2 + 1 | 5940.0 | 57.9 | 0.72 | 0.43 | 2.48 | 0.36 | 26.1 | 40.39 | 1.27 | 0.05 | 2.5 |
| −1 + 0.5 | 2755.0 | 26.8 | 0.74 | 0.43 | 2.50 | 0.36 | 25.62 | 40.39 | 1.24 | 0.12 | 2.6 |
| −0.5 + 0.315 | 111.5 | 1.1 | 0.73 | 0.42 | 2.53 | 0.38 | 26.32 | 40.63 | 1.24 | 0.075 | 2.5 |
| −0.315 + 0.2 | 536.0 | 5.2 | 0.74 | 0.41 | 2.46 | 0.38 | 25.88 | 40.31 | 1.23 | 0.066 | 2.6 |
| −0.2 + 0.1 | 513.0 | 5 | 0.76 | 0.41 | 2.47 | 0.39 | 26.40 | 40.47 | 1.23 | 0.062 | 3.0 |
| −0.1 + 0.074 | 113.5 | 1.1 | 0.78 | 0.39 | 2.39 | 0.35 | 25.72 | 40.63 | 1.25 | 0.079 | 3.2 |
| −0.074 + 0.044 | 77.7 | 0.8 | 0.82 | 0.39 | 2.38 | 0.32 | 25.70 | 40.23 | 1.21 | 0.13 | 3.4 |
| −0.044 | 217.2 | 2.1 | 0.89 | 0.42 | 2.30 | 0.35 | 27.46 | 39.83 | 1.24 | 0.22 | 4.2 |
| Initial slag | 10264.4 | 100.0 | 0.73 | 0.43 | 2.48 | 0.362 | 26 | 40.39 | 1.26 | 0.075 | 2.6 |
| Name | Yield | Element | ||||
|---|---|---|---|---|---|---|
| Grams | % | Cu (wt.%) | Fe (wt.%) | Au (g/t) | Ag (g/t) | |
| Experiment 1 | ||||||
| I m.f. H = 500 Oe | 11.8 | 1.19 | 0.97 | 39.84 | 0.123 | 3.08 |
| II m.f. H = 800 Oe | 968.4 | 98.02 | 0.78 | 39.45 | 0.106 | 2.23 |
| III m.f. H = 2500 Oe | 4.8 | 0.49 | 1.19 | 38.70 | 0.399 | 9.90 |
| IV m.f. H = 2900 Oe | 1.8 | 0.18 | 1.33 | 37.26 | 0.481 | 2.35 |
| Non-magnetic fraction | 1.2 | 0.12 | 0.76 | 26.13 | 0.290 | 7.39 |
| Total | 988 | 100.0 | 0.79 | 39.43 | 0.109 | 2.37 |
| Experiment 2 | ||||||
| I m.f. H = 500 Oe | 20.1 | 2.05 | 1.01 | 40.21 | 0.120 | 3.04 |
| II m.f. H = 800 Oe | 939.7 | 95.99 | 0.77 | 39.67 | 0.102 | 2.20 |
| III m.f. H = 2500 Oe | 6.8 | 0.69 | 1.54 | 37.48 | 0.393 | 9.96 |
| IV m.f. H = 2900 Oe | 4.9 | 0.50 | 1.92 | 36.96 | 0.491 | 2.45 |
| Non-magnetic fraction | 7.5 | 0.77 | 1.17 | 19.52 | 0.295 | 7.48 |
| Total | 979 | 100.0 | 0.79 | 39.50 | 0.108 | 2.36 |
| Suspension Density, g/cm3 | Yield | Element | Concentration Efficiency, % | ||||
|---|---|---|---|---|---|---|---|
| Grams | % | Cu (wt.%) | Fe (wt.%) | Au (g/t) | Ag (g/t) | ||
| Experiment 1 | |||||||
| +3.0 | 868.1 | 87.1 | 0.82 | 39.67 | 0.109 | 2.40 | Cu = 0.8 Fe = −0.1 |
| −3.0 + 2.9 | 114.6 | 11.5 | 0.76 | 39.30 | 0.103 | 2.10 | |
| Σ | 982.7 | 98.6 | 0.81 | 39.63 | 0.108 | 2.37 | |
| −2.9 | 13.9 | 1.4 | 0.72 | 36.78 | 0.101 | 2.10 | |
| Total | 996.6 | 100.0 | 0.81 | 39.59 | 0.108 | 2.36 | |
| Experiment 2 | |||||||
| +3.0 | 835.1 | 84.0 | 0.83 | 4.05 | 0.109 | 2.40 | Cu = 1.3 Fe = 0.2 |
| −3.0 + 2.9 | 145.2 | 14.6 | 0.72 | 39.22 | 0.103 | 2.10 | |
| Σ | 980.3 | 98.6 | 0.81 | 39.93 | 0.108 | 2.37 | |
| −2.9 | 13.9 | 1.4 | 0.70 | 36.64 | 0.096 | 2.10 | |
| Total | 994.2 | 100.0 | 0.81 | 39.88 | 0.107 | 2.39 | |
| Experiment 3 | |||||||
| +3.0 | 763.3 | 76.6 | 0.83 | 39.99 | 0.107 | 2.45 | Cu = 0.8 Fe = 0.5 |
| −3.0 + 2.9 | 202.3 | 20.3 | 0.72 | 39.15 | 0.105 | 2.10 | |
| Σ | 965.6 | 96.9 | 0.81 | 39.81 | 0.107 | 2.38 | |
| −2.9 | 30.9 | 3.1 | 0.70 | 37.85 | 0.104 | 2.10 | |
| Total | 996.5 | 100.0 | 0.81 | 39.75 | 0.107 | 2.37 | |
| Suspension Density, g/cm3 | Yield | Element | Concentration Efficiency, % | ||||
|---|---|---|---|---|---|---|---|
| Grams | % | Cu (wt.%) | Fe (wt.%) | Au (g/t) | Ag (g/t) | ||
| Experiment 1 | |||||||
| +3.0 | 986 | 98.3 | 0.81 | 39.60 | 0.108 | 2.36 | Cu = 0.8 Fe = −0.2 |
| −3.0 | 17 | 1.7 | 0.72 | 39.28 | 0.101 | 2.09 | |
| Total | 1003 | 100.0 | 0.81 | 39.59 | 0.108 | 2.36 | |
| Experiment 2 | |||||||
| +3.0 | 920 | 91.6 | 0.82 | 39.94 | 0.108 | 2.42 | Cu = 1.8 Fe = 0.3 |
| −3.0 | 84 | 8.4 | 0.72 | 39.19 | 0.100 | 2.10 | |
| Total | 1004 | 100.0 | 0.71 | 39.88 | 0.107 | 2.39 | |
| Experiment 3 | |||||||
| +3.0 | 876.7 | 87.6 | 0.82 | 39.86 | 0.107 | 2.41 | Cu = 1.1 Fe = 0.5 |
| −3.0 | 124.1 | 12.4 | 0.72 | 39.00 | 0.105 | 2.10 | |
| Total | 1000.8 | 100.0 | 0.81 | 39.75 | 0.107 | 2.37 | |
| Name | Yield | Element | ||||
|---|---|---|---|---|---|---|
| Grams | % | Cu (wt.%) | Fe (wt.%) | Au (g/t) | Ag (g/t) | |
| Experiment 1: Grinding time—11 min; 40% class −0.074 mm | ||||||
| Class + 1.0 | 72.7 | 7.5 | 0.75 | 38.56 | 0.059 | 2.20 |
| Table concentrate | 58.1 | 6.0 | 1.11 | 40.60 | 0.360 | 5.00 |
| Table tails | 837.9 | 86.5 | 0.77 | 39.74 | 0.092 | 2.20 |
| Total | 968.7 | 100.0 | 0.79 | 39.70 | 0.106 | 2.37 |
| Experiment 2: Grinding time—16 min; 60% class −0.074 mm | ||||||
| Class + 1.0 | 36.1 | 3.6 | 0.78 | 38.35 | 0.062 | 2.20 |
| Table concentrate | 56.1 | 5.6 | 1.12 | 40.28 | 0.380 | 5.11 |
| Table tails | 910.5 | 90.8 | 0.77 | 39.74 | 0.094 | 2.23 |
| Total | 1002.7 | 100.0 | 0.79 | 39.70 | 0.106 | 2.39 |
| Experiment 3: Grinding time—23 min; 80% class −0.074 mm | ||||||
| Class + 1.0 | 16 | 1.6 | 0.78 | 38.30 | 0.067 | 2.25 |
| Table concentrate | 25 | 2.5 | 1.24 | 40.18 | 0.570 | 5.50 |
| Table tails | 959.8 | 95.9 | 0.78 | 39.72 | 0.096 | 2.30 |
| Total | 1000.8 | 100.0 | 0.79 | 39.71 | 0.107 | 2.38 |
| Name | Yield | Element | ||||
|---|---|---|---|---|---|---|
| Grams | % | Cu (wt.%) | Fe (wt.%) | Au (g/t) | Ag (g/t) | |
| Heavy fraction 1st chamber | 1766.8 | 88.4 | 0.79 | 39.73 | 0.102 | 2.35 |
| Heavy fraction 2nd chamber | 45.9 | 2.3 | 0.78 | 39.84 | 0.110 | 2.60 |
| Σ | 1812.7 | 90.7 | 0.79 | 39.73 | 0.102 | 2.36 |
| Tails of jigging | 185.9 | 9.3 | 0.91 | 39.54 | 0.115 | 2.80 |
| Total | 1998.6 | 100.0 | 0.81 | 39.71 | 0.103 | 2.40 |
| Name | Yield, % | Element | |||
|---|---|---|---|---|---|
| Cu (wt.%) | Fe (wt.%) | Au (g/t) | Ag (g/t) | ||
| Experiment 1: Grinding Time—15 min; 56% class −0.074 | |||||
| −0.315 + 0.2 | 8.0 | 0.79 | 39.84 | 0.070 | 2.00 |
| −0.2 + 0.1 | 19.1 | 0.70 | 39.89 | 0.081 | 2.00 |
| −0.1 + 0.074 | 16.9 | 0.73 | 39.90 | 0.092 | 2.12 |
| −0.074 + 0.044 | 13.0 | 0.73 | 39.40 | 0.195 | 2.00 |
| −0.044 | 43.0 | 0.89 | 39.86 | 0.109 | 2.11 |
| Σ | 100.0 | 0.79 | 39.81 | 0.109 | 2.72 |
| Experiment 2: Grinding Time—20 min; 71.7% class −0.074 | |||||
| −0.315 + 0.2 | 4.2 | 0.72 | 39.41 | 0.066 | 2.10 |
| −0.2 + 0.1 | 10.4 | 0.67 | 39.28 | 0.083 | 1.90 |
| −0.1 + 0.074 | 13.7 | 0.70 | 39.54 | 0.094 | 1.90 |
| −0.074 + 0.044 | 15.8 | 0.73 | 39.58 | 0.124 | 2.20 |
| −0.044 | 55.9 | 0.85 | 40.04 | 0.111 | 2.60 |
| Σ | 100.0 | 0.79 | 39.79 | 0.106 | 2.35 |
| Experiment 3: Grinding Time—30 min; 90.2% class −0.074 | |||||
| −0.315 + 0.2 | - | - | - | - | - |
| −0.2 + 0.1 | 4.2 | 0.70 | 38.37 | 0.082 | 2.00 |
| −0.1 + 0.074 | 5.6 | 0.65 | 38.37 | 0.100 | 1.85 |
| −0.074 + 0.044 | 13.3 | 0.70 | 39.51 | 0.110 | 2.33 |
| −0.044 | 76.9 | 0.82 | 39.77 | 0.100 | 2.42 |
| Σ | 100.0 | 0.79 | 39.60 | 0.101 | 2.36 |
| Name | Yield | Element | ||||
|---|---|---|---|---|---|---|
| Grams | % | Cu (wt.%) | Fe (wt.%) | Au (g/t) | Ag (g/t) | |
| Experiment 1: Grinding time—15 min; 62.0% class −0.074 mm | ||||||
| Cu concentrate | 80.3 | 8.2 | 4.02 | 38.08 | 0.620 | 14.90 |
| Intermediate product | 31.3 | 3.2 | 0.56 | 39.58 | 0.095 | 2.05 |
| Σ | 111.6 | 11.4 | 3.05 | 38.50 | 0.473 | 11.30 |
| Tails | 867.6 | 88.6 | 0.50 | 39.82 | 0.059 | 1.21 |
| Initial slag | 979.2 | 100.0 | 0.79 | 39.67 | 0.106 | 2.37 |
| Experiment 2: Grinding time—20 min; 71.0% class −0.074 mm | ||||||
| Cu concentrate | 75.3 | 8.2 | 4.23 | 38.36 | 0.640 | 15.06 |
| Intermediate product | 35.8 | 3.9 | 0.57 | 39.60 | 0.095 | 1.95 |
| Σ | 111.1 | 12.1 | 3.05 | 38.76 | 0.464 | 10.83 |
| Tails | 806.9 | 87.9 | 0.48 | 39.83 | 0.058 | 1.21 |
| Initial slag | 918 | 100.0 | 0.79 | 39.70 | 0.107 | 2.37 |
| Experiment 3: Grinding time—24 min; 80.0% class −0.074 mm | ||||||
| Cu concentrate | 82.3 | 8.3 | 4.57 | 38.43 | 0.670 | 15.30 |
| Intermediate product | 45.6 | 4.6 | 0.60 | 39.60 | 0.094 | 1.90 |
| Σ | 127.9 | 12.9 | 3.15 | 38.85 | 0.464 | 10.51 |
| Tails | 863.9 | 87.1 | 0.45 | 39.85 | 0.053 | 1.18 |
| Initial slag | 991.8 | 100.0 | 0.80 | 39.72 | 0.106 | 2.39 |
| Experiment 4: Grinding time—30 min; 90.0% class −0.074 mm | ||||||
| Cu concentrate | 110.9 | 12.0 | 3.5 | 37.21 | 0.540 | 10.87 |
| Intermediate product | 69.3 | 7.5 | 0.49 | 39.65 | 0.090 | 1.88 |
| Σ | 180.2 | 19.5 | 2.39 | 38.15 | 0.367 | 7.41 |
| Tails | 744.1 | 80.5 | 0.40 | 40.10 | 0.043 | 1.13 |
| Initial slag | 924.3 | 100.0 | 0.79 | 39.72 | 0.106 | 2.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Kurmangaliyev, D.; Abdulina, S. Physicochemical Characteristics of Copper Smelting Slags from Kazakhstan and Their Potential for Secondary Resource Recovery. Processes 2026, 14, 113. https://doi.org/10.3390/pr14010113
Kurmangaliyev D, Abdulina S. Physicochemical Characteristics of Copper Smelting Slags from Kazakhstan and Their Potential for Secondary Resource Recovery. Processes. 2026; 14(1):113. https://doi.org/10.3390/pr14010113
Chicago/Turabian StyleKurmangaliyev, Damir, and Saule Abdulina. 2026. "Physicochemical Characteristics of Copper Smelting Slags from Kazakhstan and Their Potential for Secondary Resource Recovery" Processes 14, no. 1: 113. https://doi.org/10.3390/pr14010113
APA StyleKurmangaliyev, D., & Abdulina, S. (2026). Physicochemical Characteristics of Copper Smelting Slags from Kazakhstan and Their Potential for Secondary Resource Recovery. Processes, 14(1), 113. https://doi.org/10.3390/pr14010113






