Coupled Evolution of Clay Minerals and Organic Matter During Diagenesis: Mechanisms of Smectite Illitization in Organic-Rich Shale
Abstract
1. Introduction
2. Samples and Methods
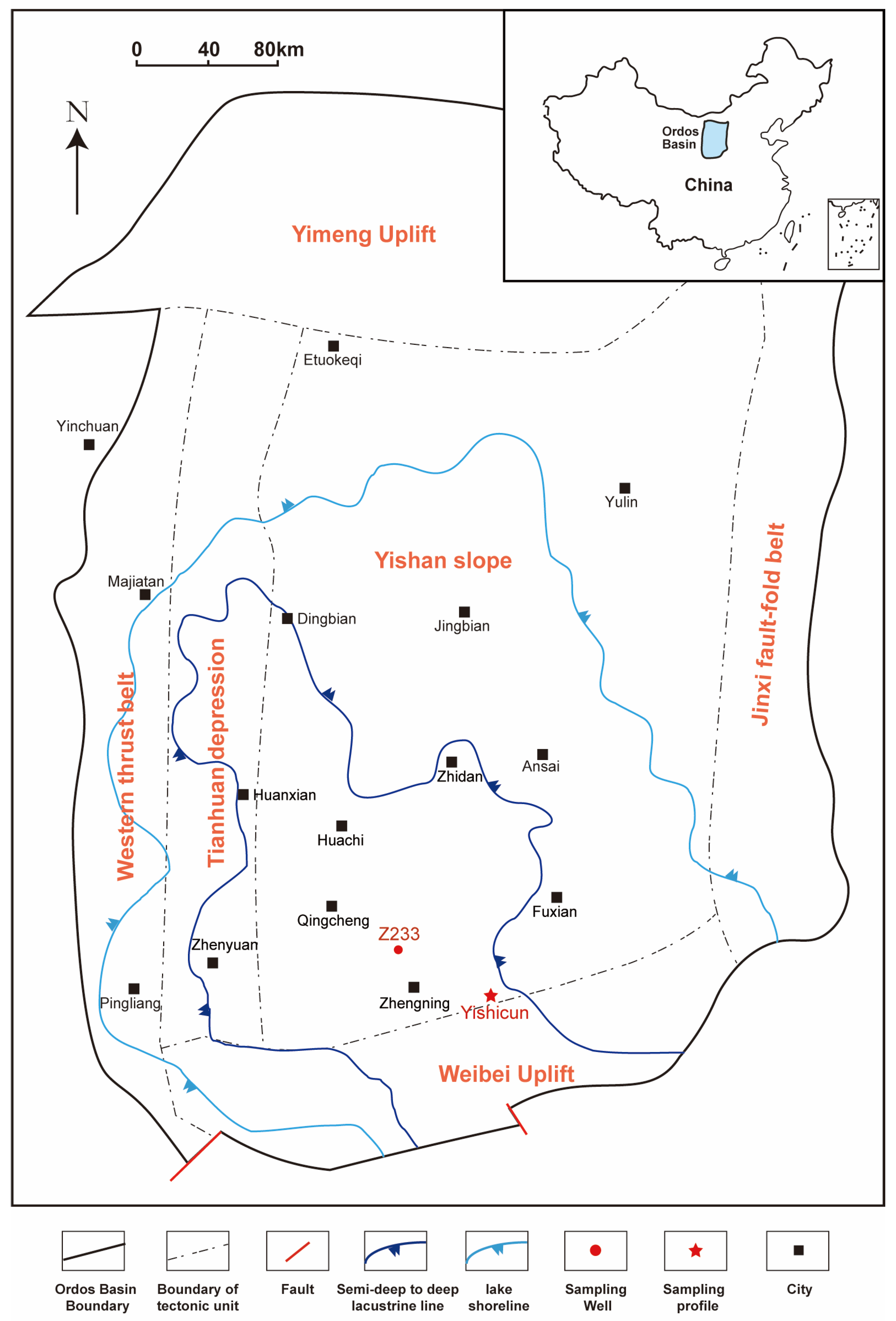
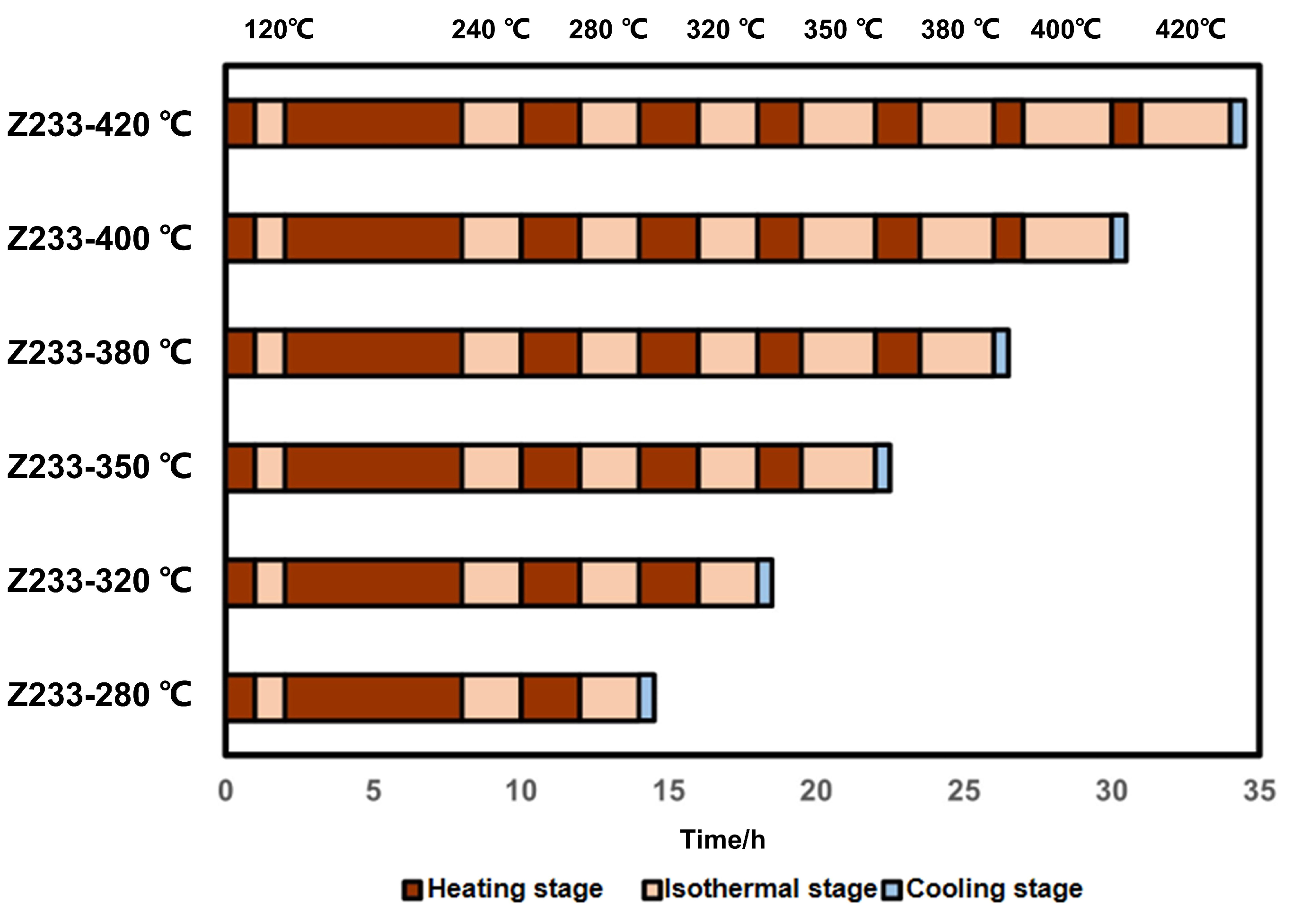
2.1. Natural Sample: Semi-Open Pyrolysis Experiments
2.2. Synthetic Samples: Hydrothermal Experiments
2.3. Organic-Inorganic Composition Analyses
2.3.1. Mineralogical Characterization
2.3.2. Organic Geochemical Analyses
3. Results
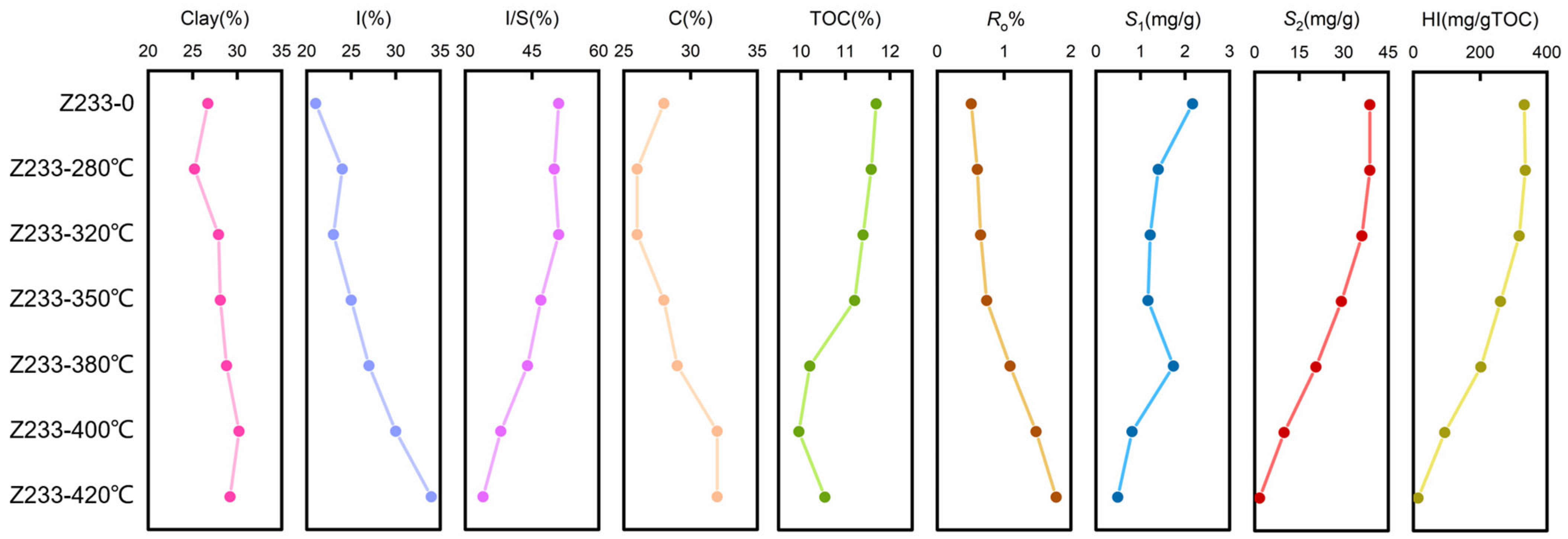
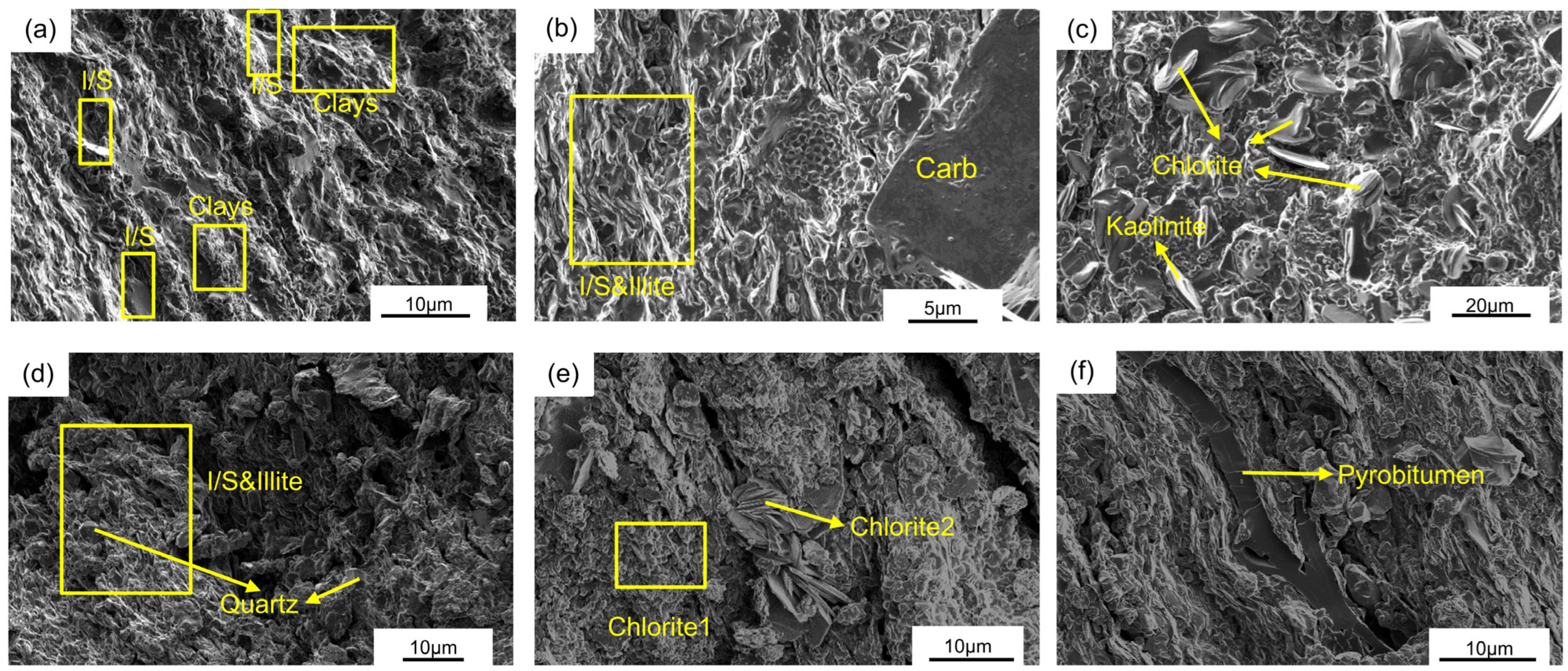
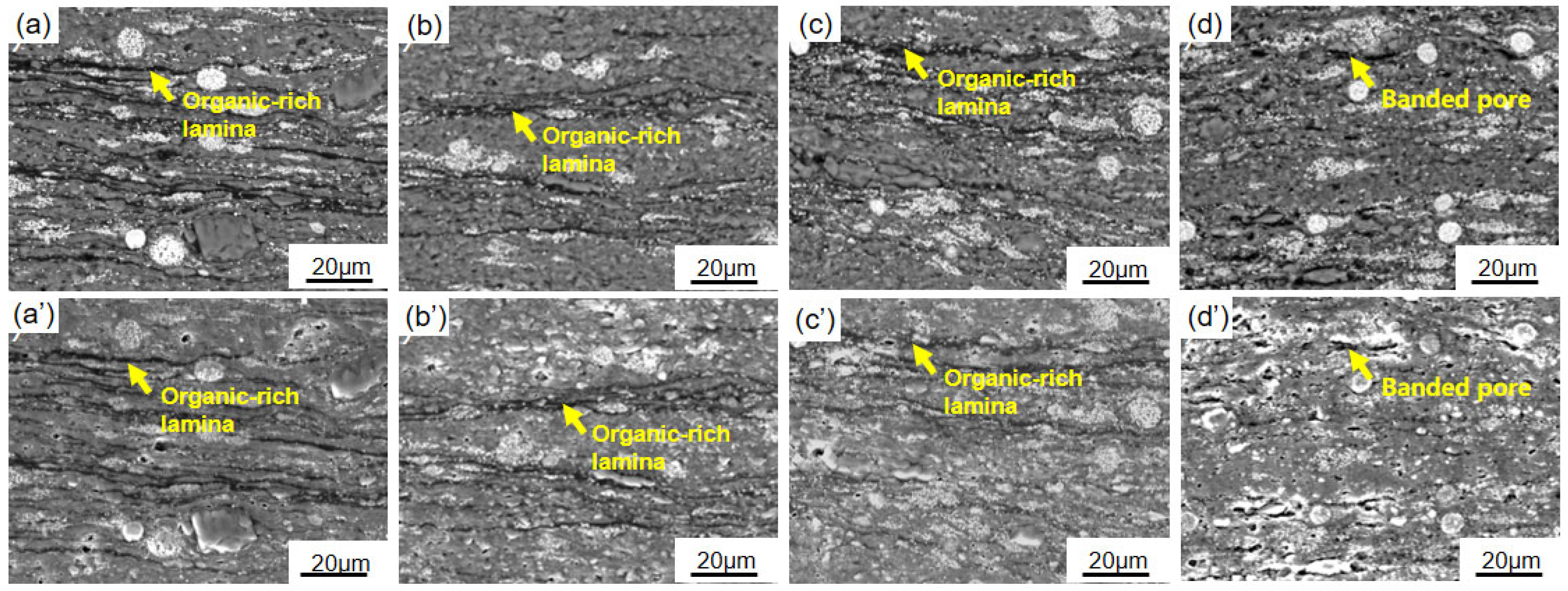
3.1. Mineralogical Characteristics of Semi-Open Pyrolysis Products
3.2. Organic Geochemical Analyses of Semi-Open Pyrolysis Products
3.3. XRD Spectral Characteristics of Hydrothermal Experiment Products
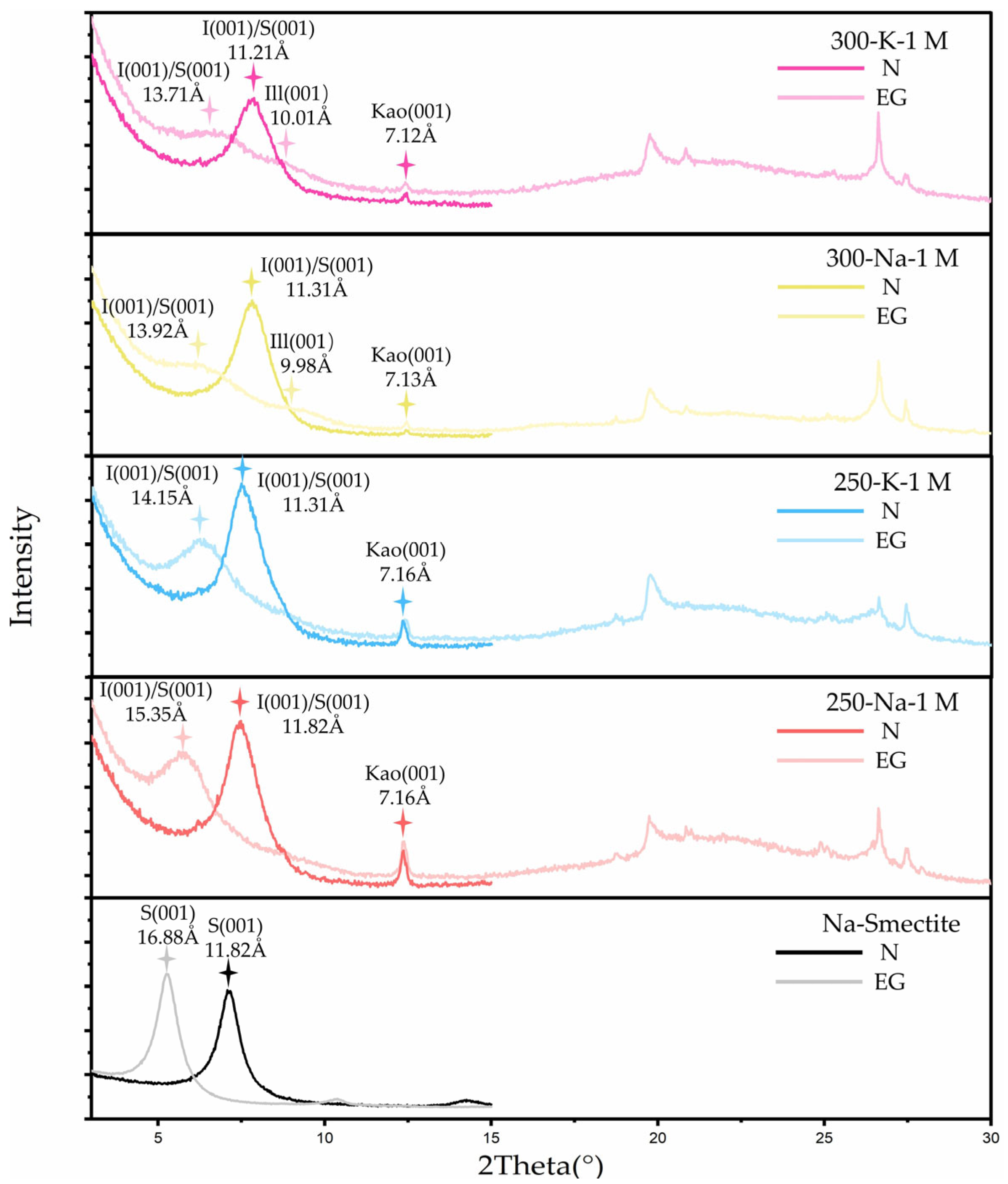
3.3.1. Reference Samples
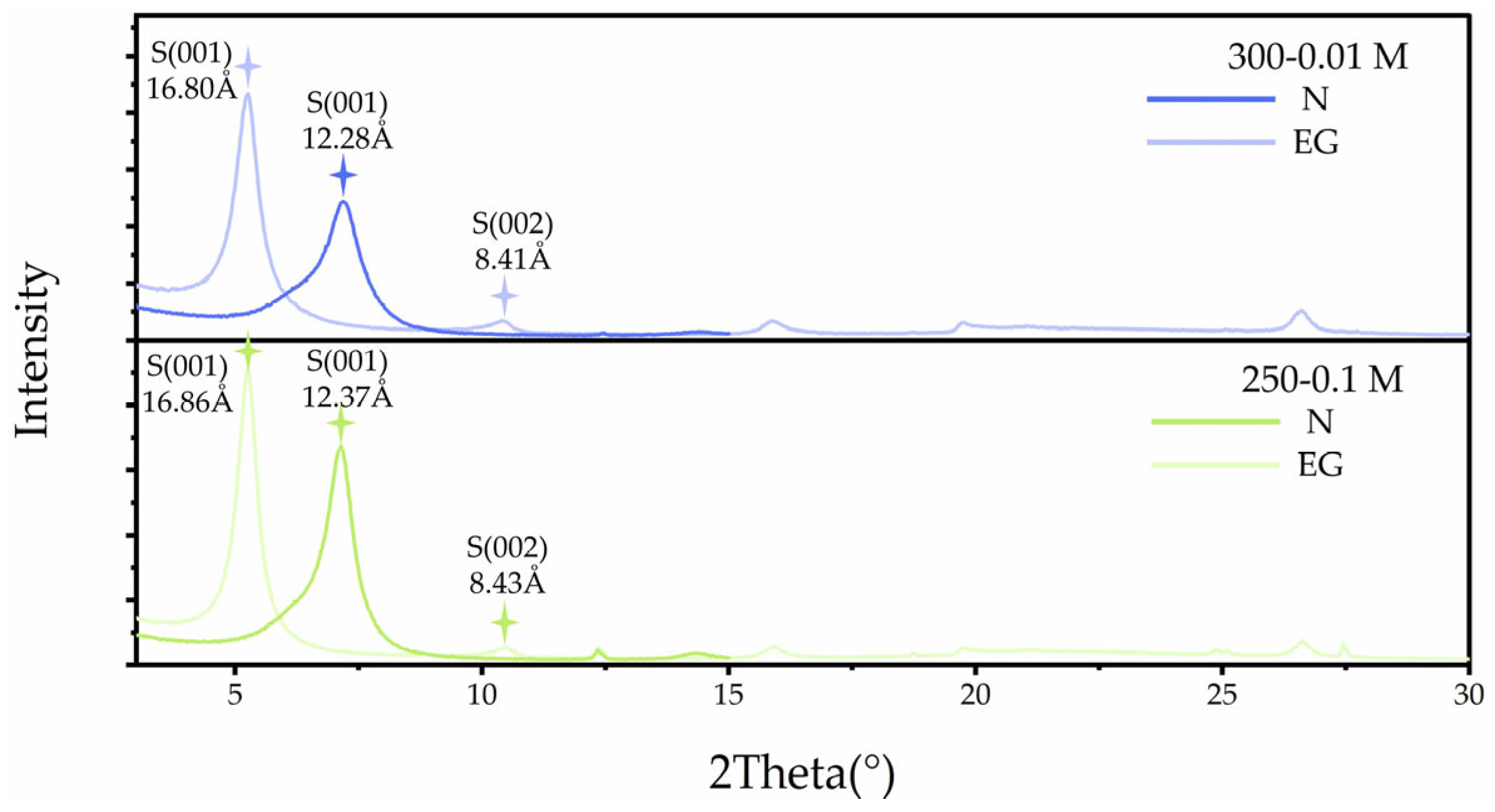
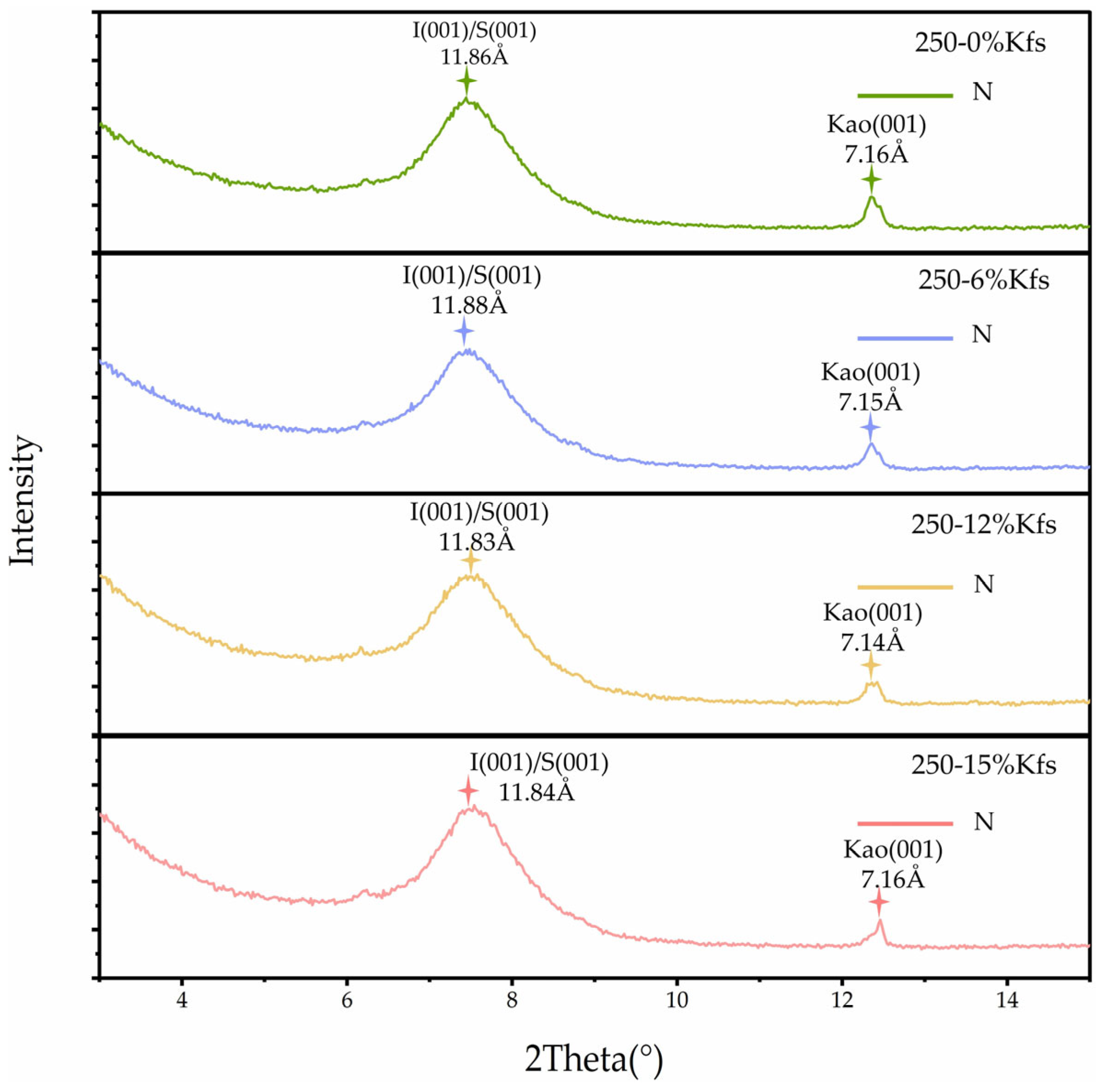
3.3.2. Effects of KCl Solution and K-Feldspar
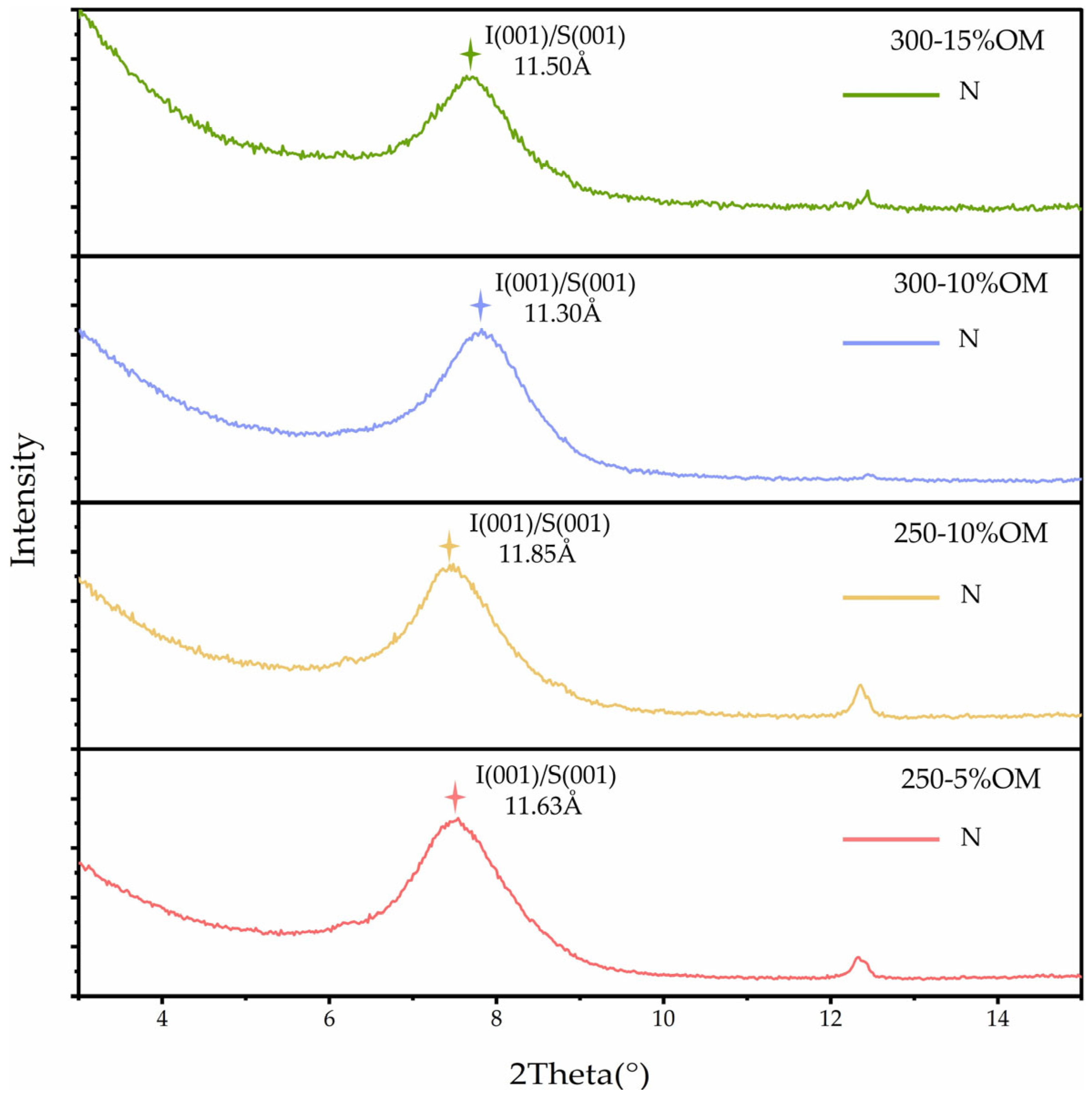
3.3.3. Effects of Organic Matter
4. Discussion
4.1. Thermally Driven but Organically Mediated Smectite Illitization
4.2. Organic Regulation of K+ Availability and Cation Exchange Dynamics
5. Conclusions
- When aqueous media are deficient in potassium ions, the smectite-to-illite transformation is nearly stagnant. Given that potassium ion (K+) supply is essential for this process, the extensive illitization observed in low-salinity depositional environments (e.g., the Chang-7 shale) strongly suggests that K-feldspar dissolution plays a critical role in the transformation.
- Illitization progresses with increasing temperature and is accompanied by the thermal evolution of organic matter. As the primary kinetic regulator, temperature also exerts indirect influences on critical diagenetic processes—including organic matter thermal evolution and K-feldspar dissolution.
- Organic matter exerts a temporally evolving influence on smectite-to-illite transformation. During early diagenesis, enrichment in organic matter correlates with delayed illitization, likely due to reduced K+ accessibility. At higher thermal maturity stages, however, organic matter degradation coincides with significantly enhanced transformation rates, facilitated by secondary porosity generation and increased mineral surface exposure. This transition demonstrates a feedback mechanism whereby organic matter functions as both a mediator and a modulator within the diagenetic system.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cai, L.; Yang, T.; Tian, J.; Yi, J.; Ren, Q. Advances in Studies of Development and Growth Mechanisms of Clay Minerals in Tight Sandstone Reservoirs. Acta Sedimentol. Sin. 2023, 41, 1859–1889. [Google Scholar] [CrossRef]
- Tannenbaum, E.; Huizinga, B.J.; Kaplan, I.R. Role of Minerals in Thermal Alteration of Organic Matter—II: A Material Balance. AAPG Bull. 1986, 70, 1156–1165. [Google Scholar] [CrossRef]
- Pollastro, R.M. Considerations and Applications of the Illite/Smectite Geothermometer in Hydrocarbon-Bearing Rocks of Miocene to Mississippian Age. Clays Clay Miner. 1993, 41, 119–133. [Google Scholar] [CrossRef]
- Ola, P.S.; Aidi, A.K.; Bankole, O.M. Clay Mineral Diagenesis and Source Rock Assessment in the Bornu Basin, Nigeria: Implications for Thermal Maturity and Source Rock Potential. Mar. Pet. Geol. 2018, 89, 653–664. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, C.; Keeling, J.; Tong, D.; Yu, W. Towards an Understanding of the Role of Clay Minerals in Crude Oil Formation, Migration and Accumulation. Earth-Sci. Rev. 2012, 115, 373–386. [Google Scholar] [CrossRef]
- Rahman, H.M.; Kennedy, M.; Löhr, S.; Dewhurst, D.N. Clay-Organic Association as a Control on Hydrocarbon Generation in Shale. Org. Geochem. 2017, 105, 42–55. [Google Scholar] [CrossRef]
- Bjørlykke, K. Clay Mineral Diagenesis in Sedimentary Basins—A Key to the Prediction of Rock Properties. Examples from the North Sea Basin. Clay Miner. 1998, 33, 15–34. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, S.; Yu, Z.; Liu, X.; Wang, S.; Miao, H.; Liu, D.; Tian, J.; Wang, H. Controls of Clay Mineral Transformation and Organic Matter on Pore Networks of the Paleogene Lacustrine Shale Oil System in the Yitong Basin, NE China. J. Asian Earth Sci. 2025, 280, 106469. [Google Scholar] [CrossRef]
- Mills, M.M.; Sanchez, A.C.; Boisvert, L.; Payne, C.B.; Ho, T.A.; Wang, Y. Understanding Smectite to Illite Transformation at Elevated (>100 °C) Temperature: Effects of Liquid/Solid Ratio, Interlayer Cation, Solution Chemistry and Reaction Time. Chem. Geol. 2023, 615, 121214. [Google Scholar] [CrossRef]
- Zanoni, G.; Egvi, B.; Sweet, D.E. Clay Mineral Diagenesis in Alternating Mudstone-sandstone Beds from Late Palaeozoic Strata of the Anadarko Basin, United States. Geol. J. 2023, 58, 108–130. [Google Scholar] [CrossRef]
- Eberl, D.; Hower, J. Kinetics of Illite Formation. Geol. Soc. Am. Bull. 1976, 87, 1326–1330. [Google Scholar] [CrossRef]
- Roaldset, E.; Wei, H.; Grimstad, S. Smectite to Illite Conversion by Hydrous Pyrolysis. Clay Miner. 1998, 33, 147–158. [Google Scholar] [CrossRef]
- Huang, W.-L.; Longo, J.M.; Pevear, D.R. An Experimentally Derived Kinetic Model for Smectite-to-Illite Conversion and Its Use as a Geothermometer. Clays Clay Miner. 1993, 41, 162–177. [Google Scholar] [CrossRef]
- Elliott, W.C.; Edenfield, A.M.; Wampler, J.M.; Matisoff, G.; Long, P.E. The Kinetics of the Smectite to Illite Transformation in Cretaceous Bentonites, Cerro Negro, New Mexico. Clays Clay Miner. 1999, 47, 286–296. [Google Scholar] [CrossRef]
- Ramseyer, K.; Boles, J.R. Mixed-Layer Illite/Smectite Minerals in Tertiary Sandstones and Shales, San Joaquin Basin, California. Clays Clay Miner. 1986, 34, 115–124. [Google Scholar] [CrossRef]
- Whitney, G. Role of Water in the Smectite-to-Illite Reaction. Clays Clay Miner. 1990, 38, 343–350. [Google Scholar] [CrossRef]
- Bettison-Varga, L.; Mackinnon, I.D. The Role of Randomly Mixed-Layered Chlorite/Smectite in the Transformation of Smectite to Chlorite. Clays Clay Miner. 1997, 45, 506–516. [Google Scholar] [CrossRef]
- Ge, C. Compression Behavior of Smectitic vs. Illitic Mudrocks. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2019. [Google Scholar]
- Kennedy, M.J.; Löhr, S.C.; Fraser, S.A.; Baruch, E.T. Direct Evidence for Organic Carbon Preservation as Clay-Organic Nanocomposites in a Devonian Black Shale; from Deposition to Diagenesis. Earth Planet. Sci. Lett. 2014, 388, 59–70. [Google Scholar] [CrossRef]
- Gu, Y.; Wan, Q.; Li, X.; Han, T.; Yang, S.; Hu, Q. Structure and Evolution of Clay-Organic Nanocomposites in Three Leading Shales in China. J. Earth Sci. 2023, 34, 824–837. [Google Scholar] [CrossRef]
- Wilson, M.J.; Wilson, L.; Shaldybin, M.V. Clay Mineralogy and Unconventional Hydrocarbon Shale Reservoirs in the USA. II. Implications of Predominantly Illitic Clays on the Physico-Chemical Properties of Shales. Earth-Sci. Rev. 2016, 158, 1–8. [Google Scholar] [CrossRef]
- Du, J.; Cai, J.; Lei, T.; Li, Y. Diversified Roles of Mineral Transformation in Controlling Hydrocarbon Generation Process, Mechanism, and Pattern. Geosci. Front. 2021, 12, 725–736. [Google Scholar] [CrossRef]
- Corma, A. Inorganic Solid Acids and Their Use in Acid-Catalyzed Hydrocarbon Reactions. Chem. Rev. 1995, 95, 559–614. [Google Scholar] [CrossRef]
- Gooßen, L.; Döhring, A. Lewis Acids as Highly Efficient Catalysts for the Decarboxylative Esterification of Carboxylic Acids with Dialkyl Dicarbonates. Adv. Synth. Catal. 2003, 345, 943–947. [Google Scholar] [CrossRef]
- Bu, H.; Yuan, P.; Liu, H.; Liu, D.; Liu, J.; He, H.; Zhou, J.; Song, H.; Li, Z. Effects of Complexation between Organic Matter (OM) and Clay Mineral on OM Pyrolysis. Geochim. Cosmochim. Acta 2017, 212, 1–15. [Google Scholar] [CrossRef]
- Cai, J.; Du, J.; Chen, Z.; Lei, T.; Zhu, X. Hydrothermal Experiments Reveal the Influence of Organic Matter on Smectite Illitization. Clays Clay Miner. 2018, 66, 28–42. [Google Scholar] [CrossRef]
- Connan, J. Time-Temperature Relation in Oil Genesis: Geologic Notes. AAPG Bull. 1974, 58, 2516–2521. [Google Scholar] [CrossRef]
- Tissot, B.P.; Pelet, R.; Ungerer, P.H. Thermal History of Sedimentary Basins, Maturation Indices, and Kinetics of Oil and Gas Generation. AAPG Bull. 1987, 71, 1445–1466. [Google Scholar] [CrossRef]
- Waples, D.W. Time and Temperature in Petroleum Formation: Application of Lopatin’s Method to Petroleum Exploration. AAPG Bull. 1980, 64, 916–926. [Google Scholar] [CrossRef]
- Ungerer, P. State of the Art of Research in Kinetic Modelling of Oil Formation and Expulsion. Org. Geochem. 1990, 16, 1–25. [Google Scholar] [CrossRef]
- Behar, F.; Kressmann, S.; Rudkiewicz, J.L.; Vandenbroucke, M. Experimental Simulation in a Confined System and Kinetic Modelling of Kerogen and Oil Cracking. Org. Geochem. 1992, 19, 173–189. [Google Scholar] [CrossRef]
- Ma, W.; Hou, L.; Luo, X.; Liu, J.; Tao, S.; Guan, P.; Cai, Y. Generation and Expulsion Process of the Chang 7 Oil Shale in the Ordos Basin Based on Temperature-Based Semi-Open Pyrolysis: Implications for in-Situ Conversion Process. J. Pet. Sci. Eng. 2020, 190, 107035. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.; Liu, Z.; Dang, H.; Guo, J.; Zhang, C. Semi-Open System Simulation of Organic-Rich Shale to Produce Organic Acids. ACS Earth Space Chem. 2024, 8, 2420–2427. [Google Scholar] [CrossRef]
- Lewan, M.D.; Roy, S. Role of Water in Hydrocarbon Generation from Type-I Kerogen in Mahogany Oil Shale of the Green River Formation. Org. Geochem. 2011, 42, 31–41. [Google Scholar] [CrossRef]
- Huang, Y.; Liao, Y.; Pan, Y.; Hsu, C.S.; Wang, Y.; Peng, P. Improved Light Hydrocarbons Collection Method for the Pyrolysis of Crude Oil in Gold Tube Closed System Experiments. Org. Geochem. 2022, 168, 104432. [Google Scholar] [CrossRef]
- Salisu, A.M.; Bello, A.M.; Amao, A.O.; Lander, R.H.; Al-Ramadan, K. Experimental Study of Smectite Authigenesis and Its Subsequent Illitization: Insights from Modern Estuary Sediments. J. Sediment. Res. 2025, 95, 562–588. [Google Scholar] [CrossRef]
- Bentabol, M.; Lamarca-Irisarri, D.; Van Driessche, A.E.S.; Ryan, P.C.; Huertas, F.J. Illitization of Montmorillonite in Ammonium Solutions under Hydrothermal Conditions. Appl. Clay Sci. 2024, 258, 107478. [Google Scholar] [CrossRef]
- Wu, S.; Zhu, R.; Cui, J.; Cui, J.; Bai, B.; Zhang, X.; Xu, J.; Zhu, D.; You, J.; Li, X. Characteristics of Lacustrine Shale Porosity Evolution, Triassic Chang 7 Member, Ordos Basin, NW China. Pet. Explor. Dev. 2015, 42, 185–195. [Google Scholar] [CrossRef]
- Wang, F.; Guo, S. Influential Factors and Model of Shale Pore Evolution: A Case Study of a Continental Shale from the Ordos Basin. Mar. Pet. Geol. 2019, 102, 271–282. [Google Scholar] [CrossRef]
- Yuan, G.; Cao, Y.; Zan, N.; Schulz, H.-M.; Gluyas, J.; Hao, F.; Jin, Q.; Liu, K.; Wang, Y.; Chen, Z.; et al. Coupled Mineral Alteration and Oil Degradation in Thermal Oil-Water-Feldspar Systems and Implications for Organic-Inorganic Interactions in Hydrocarbon Reservoirs. Geochim. Cosmochim. Acta 2019, 248, 61–87. [Google Scholar] [CrossRef]
- Ma, J.; Ma, Z.; Miao, J.; Zheng, L.; Wang, Q.; He, C. Co-Evolution Simulation Experiment of Source Rock Fluid and Reservoir Rock and Its Geological Implications: A Case Study of Upper Triassic Xujiahe Formation, Western Sichuan Basin. Pet. Geol. Exp. 2022, 44, 698–704. [Google Scholar] [CrossRef]
- Shan, C.; Shi, Y.; Liang, X.; Zhang, L.; Wang, G.; Jiang, L.; Zou, C.; He, F.; Mei, J. Diagenetic Characteristics and Microscopic Pore Evolution of Deep Shale Gas Reservoirs in Longmaxi Formation, Southeastern Sichuan Basin, China. Unconv. Resour. 2024, 4, 100090. [Google Scholar] [CrossRef]
- Wang, G.; Jin, Z.; Zhang, Q.; Zhu, R.; Tang, X.; Liu, K.; Dong, L. Effects of Clay Minerals and Organic Matter on Pore Evolution of the Early Mature Lacustrine Shale in the Ordos Basin, China. J. Asian Earth Sci. 2023, 246, 105516. [Google Scholar] [CrossRef]
- Zhang, D.; Cao, H.; Lei, Y.; Wu, H.; Wang, X.; Guo, X.; Yan, J.; Wang, S.; Shi, T.; Li, H. A Study on Molecular Structural Evolution of Type II Kerogen in a Gold Tube Thermal System: Insights from Solid-State 13C NMR. Fuel 2023, 331, 125898. [Google Scholar] [CrossRef]
- Pan, C.; Jiang, L.; Liu, J.; Zhang, S.; Zhu, G. The Effects of Pyrobitumen on Oil Cracking in Confined Pyrolysis Experiments. Org. Geochem. 2012, 45, 29–47. [Google Scholar] [CrossRef]
- National Energy Administration. Analysis Method for Clay Minerals and Ordinary Non-Clay Minerals in Sedimentary Rocks by the X-Ray Diffraction: SY/T 5163-2018; Petroleum Industry Press: Beijing, China, 2018. [Google Scholar]
- Wang, Z.; Dong, L.; Jin, Z.; Zou, S.; Fu, J.; Zhu, R. Efforts to Untie the Multicollinearity Knot and Identify Factors Controlling Macropore Structures in Shale Oil Reservoirs. Adv. Geo-Energy Res. 2024, 11, 194–207. [Google Scholar] [CrossRef]
- Wang, R.; Wang, G.; Zhao, G.; Qian, M.; Liu, Y.; He, W.; Li, Z. Geological Characteristics and Resources Potential of Shale Oil in Chang 7 Member of Upper Triassic Yanchang Formation in Fuxian Area, Southern Ordos Basin, Western China. Unconv. Resour. 2023, 3, 237–247. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, M.; Misch, D.; Sachsenhofer, R.F.; Wu, Y.; Li, J. Mineral Diagenesis in Lacustrine Organic-Rich Shales: Evolution Pathways and Implications for Reservoir Characteristics. J. Asian Earth Sci. 2024, 263, 106026. [Google Scholar] [CrossRef]
- Huang, S.; Huang, K.; Feng, W.; Tong, H.; Liu, L.; Zhang, X. Mass Exchanges among Feldspar, Kaolinite and Illite and Their Influences on Secondary Porosity Formation in Clastic Diagenesis—A Case Study on the Upper Paleozoic, Ordos Basin and Xujiahe Formation, Western Sichuan Depression. Geochimica 2009, 38, 498–506. [Google Scholar] [CrossRef]
- Zhao, X.; He, D. Clay Mineral and Application in Oil and Gas Exploration and Development; Petroleum Industry Press: Beijing, China, 2016; ISBN 978-7-5183-0360-1. [Google Scholar]
- Green, H.; Šegvić, B.; Zanoni, G.; Omodeo-Salé, S.; Adatte, T. Evaluation of Shale Source Rocks and Clay Mineral Diagenesis in the Permian Basin, USA: Inferences on Basin Thermal Maturity and Source Rock Potential. Geosciences 2020, 10, 381. [Google Scholar] [CrossRef]
- Yang, Y.; Adhikari, S.; Xu, G. Molecular Dynamics Simulation in the Interlayer of Mixed-Layer Clays Due to Hydration and Swelling Mechanism. Crystals 2021, 11, 586. [Google Scholar] [CrossRef]
- Ling, K.; Wang, Z.; Cao, Y.; Liu, Y.; Dong, L. Clay Mineral Characteristics and Smectite-to-Illite Transformation in the Chang-7 Shale, Ordos Basin: Processes and Controlling Factors. Minerals 2025, 15, 951. [Google Scholar] [CrossRef]
- Ding, F.; Cai, J.; Song, M.; Yuan, P. The Relationship between Organic Matter and Specific Surface Area in <2 μm Clay Size Fraction of Muddy Source Rock. Sci. China Earth Sci. 2013, 56, 1343–1349. [Google Scholar] [CrossRef]
- Kaiser, K.; Guggenberger, G. Mineral Surfaces and Soil Organic Matter. Eur. J. Soil Sci. 2010, 54, 219–236. [Google Scholar] [CrossRef]
- Löhr, S.C.; Kennedy, M.J. Organomineral Nanocomposite Carbon Burial during Oceanic Anoxic Event 2. Biogeosciences 2014, 11, 4971–4983. [Google Scholar] [CrossRef]
- Chang, J.; Fan, X.; Jiang, Z.; Wang, X.; Chen, L.; Li, J.; Zhu, L.; Wan, C.; Chen, Z. Differential Impact of Clay Minerals and Organic Matter on Pore Structure and Its Fractal Characteristics of Marine and Continental Shales in China. Appl. Clay Sci. 2022, 216, 106334. [Google Scholar] [CrossRef]
- Kleber, M.; Eusterhues, K.; Keiluweit, M.; Mikutta, C.; Mikutta, R.; Nico, P.S. Mineral–Organic Associations: Formation, Properties, and Relevance in Soil Environments. Adv. Agron. 2015, 130, 1–140. [Google Scholar] [CrossRef]
- Yu, W.H.; Li, N.; Tong, D.S.; Zhou, C.H.; Lin, C.X. (Cynthia); Xu, C.Y. Adsorption of Proteins and Nucleic Acids on Clay Minerals and Their Interactions: A Review. Appl. Clay Sci. 2013, 80–81, 443–452. [Google Scholar] [CrossRef]
- Ganor, J.; Reznik, I.; Rosenberg, Y. Organics in Water-Rock Interactions. Rev. Mineral. Geochem. 2009, 70, 259–369. [Google Scholar] [CrossRef]
- Harpreet, K. Forms of Potassium in Soil and Their Relationship with Soil Properties—A Review. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1580–1586. [Google Scholar] [CrossRef]
- Wang, F.L.; Huang, P.M. Effects of Organic Matter on the Rate of Potassium Adsorption by Soils. Can. J. Soil Sci. 2001, 81, 325–330. [Google Scholar] [CrossRef]
| Original Rock | |
|---|---|
| TOC (%) | 11.69 |
| Tmax (°C) | 435 |
| S1 (mg/g) | 2.17 |
| S2 (mg/g) | 38.81 |
| S3 (mg/g) | 0.25 |
| RO (%) | 0.51 |
| Kerogen type | Type-II |
| Naming Convention | Simulated Temperature (°C) | Solution | Powder Sample Composition Content (wt%) | |||||
|---|---|---|---|---|---|---|---|---|
| Kerogen | Smectite * | Kaolinite | Chlorite | K-Feldspar | Quartz | |||
| 250-Na-1 M | 250 | 1 M KCl | 10 | 36 (Na) | 3 | 3 | 12 | 36 |
| 250-K-1 M | 1 M KCl | 10 | 36 (K) | 3 | 3 | 12 | 36 | |
| 250-0.1 M | 0.1 M KCl | 10 | 36 (Na) | 3 | 3 | 12 | 36 | |
| 250-0.01 M | 0.01 M KCl | 10 | 36 (Na) | 3 | 3 | 12 | 36 | |
| 250-0Kfs | 1 M KCl | 10 | 36 (Na) | 3 | 3 | 0 | 48 | |
| 250-6%Kfs | 1 M KCl | 10 | 36 (Na) | 3 | 3 | 6 | 42 | |
| 250-15%Kfs | 1 M KCl | 10 | 36 (Na) | 3 | 3 | 15 | 33 | |
| 250-5%OM | 1 M KCl | 5 | 36 (Na) | 3 | 3 | 12 | 41 | |
| 250-15%OM | 1 M KCl | 15 | 36 (Na) | 3 | 3 | 12 | 31 | |
| 300-Na-1 M | 300 | 1 M KCl | 10 | 36 (Na) | 3 | 3 | 12 | 36 |
| 300-K-1 M | 1 M KCl | 10 | 36 (K) | 3 | 3 | 12 | 36 | |
| 300-0.1 M | 0.1 M KCl | 10 | 36 (Na) | 3 | 3 | 12 | 36 | |
| 300-0.01 M | 0.01 M KCl | 10 | 36 (Na) | 3 | 3 | 12 | 36 | |
| 300-0Kfs | 1 M KCl | 10 | 36 (Na) | 3 | 3 | 0 | 48 | |
| 300-6%Kfs | 1 M KCl | 10 | 36 (Na) | 3 | 3 | 6 | 42 | |
| 300-15%Kfs | 1 M KCl | 10 | 36 (Na) | 3 | 3 | 15 | 33 | |
| 300-5%OM | 1 M KCl | 5 | 36 (Na) | 3 | 3 | 12 | 41 | |
| 300-15%OM | 1 M KCl | 15 | 36 (Na) | 3 | 3 | 12 | 31 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ling, K.; Wang, Z.; Zhang, C.; Dong, L. Coupled Evolution of Clay Minerals and Organic Matter During Diagenesis: Mechanisms of Smectite Illitization in Organic-Rich Shale. Processes 2025, 13, 2966. https://doi.org/10.3390/pr13092966
Ling K, Wang Z, Zhang C, Dong L. Coupled Evolution of Clay Minerals and Organic Matter During Diagenesis: Mechanisms of Smectite Illitization in Organic-Rich Shale. Processes. 2025; 13(9):2966. https://doi.org/10.3390/pr13092966
Chicago/Turabian StyleLing, Kun, Ziyi Wang, Changhu Zhang, and Lin Dong. 2025. "Coupled Evolution of Clay Minerals and Organic Matter During Diagenesis: Mechanisms of Smectite Illitization in Organic-Rich Shale" Processes 13, no. 9: 2966. https://doi.org/10.3390/pr13092966
APA StyleLing, K., Wang, Z., Zhang, C., & Dong, L. (2025). Coupled Evolution of Clay Minerals and Organic Matter During Diagenesis: Mechanisms of Smectite Illitization in Organic-Rich Shale. Processes, 13(9), 2966. https://doi.org/10.3390/pr13092966











