Highly Efficient Production of Diacylglycerols via Enzymatic Glycerolysis Catalyzed by Immobilized MAS1-H108W Lipase
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of MAS1-H108W Lipase
2.3. Immobilization of MAS1-H108W Lipase
2.4. Characterizations of Free and Immobilized MAS1-H108W Lipase
2.5. Production of DAG by Enzymatic Glycerolysis
2.6. Reusability and Applicability of MAS1-H108W @XAD1180
2.7. Analysis of the Composition of the Reaction Mixtures
2.8. Statistical Analysis
3. Results and Discussions
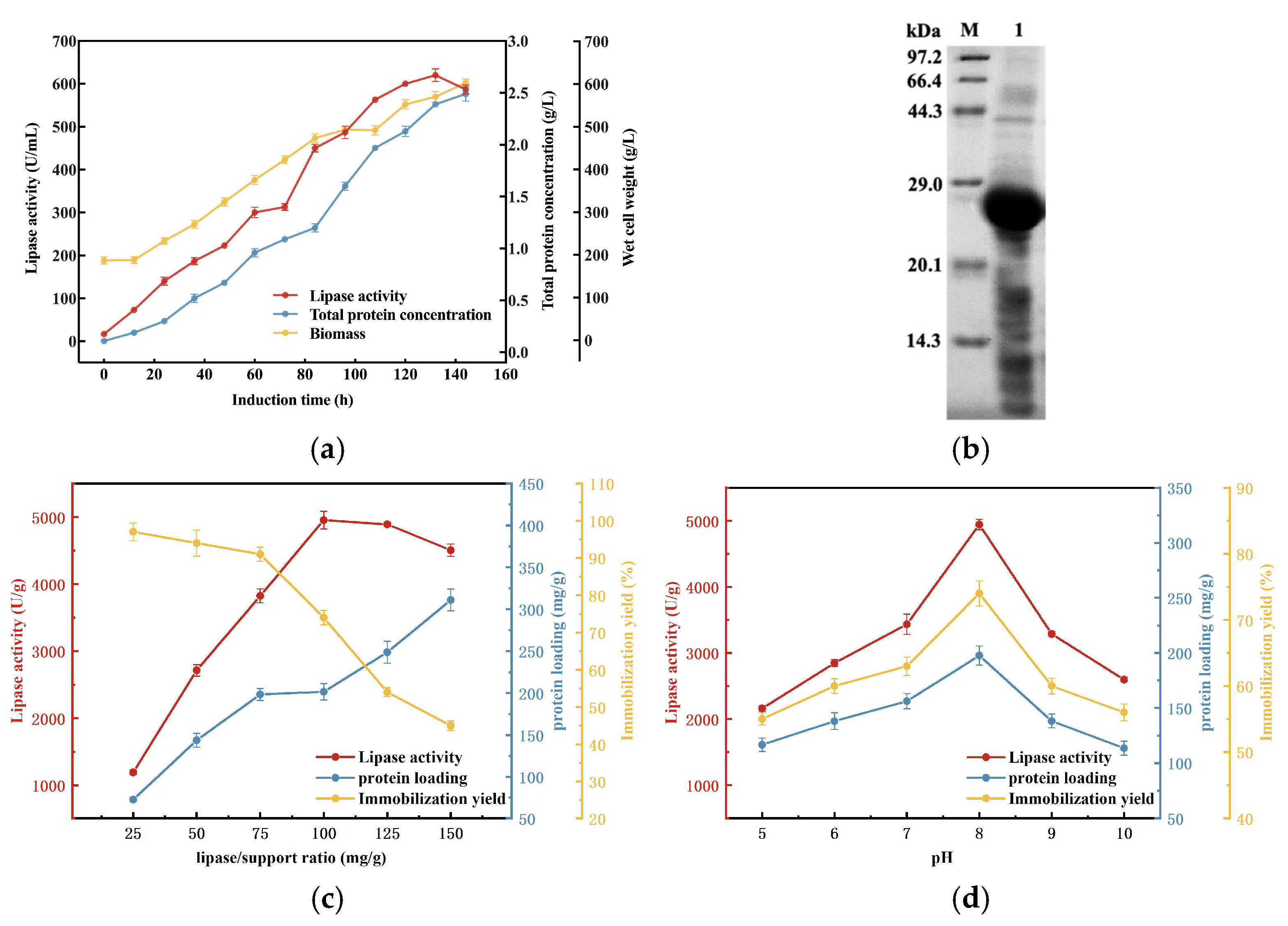
3.1. Expression and Immobilization of MAS1-H108W Lipase
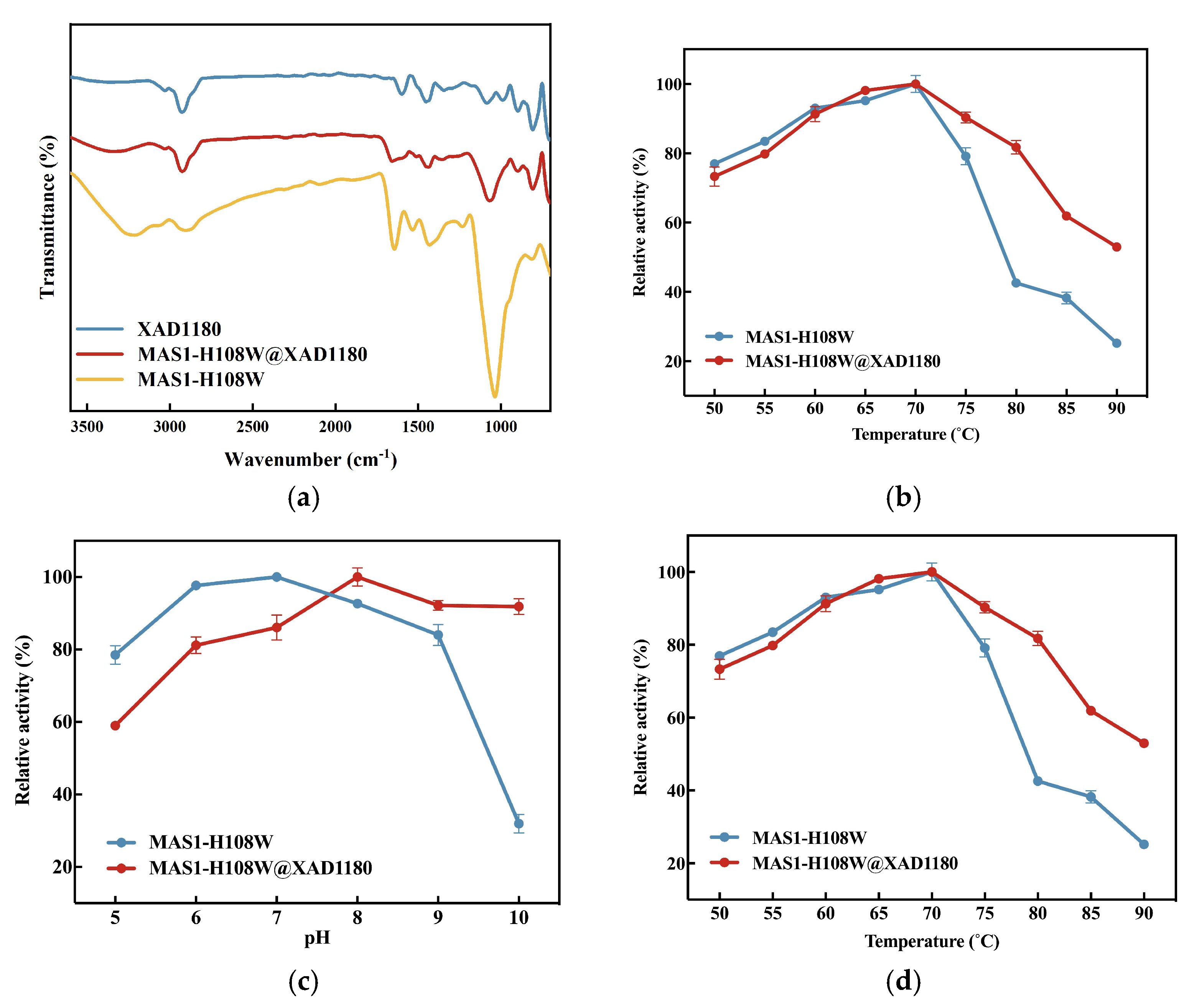
3.2. Characterizations of Free and Immobilized MAS1-H108W Lipase
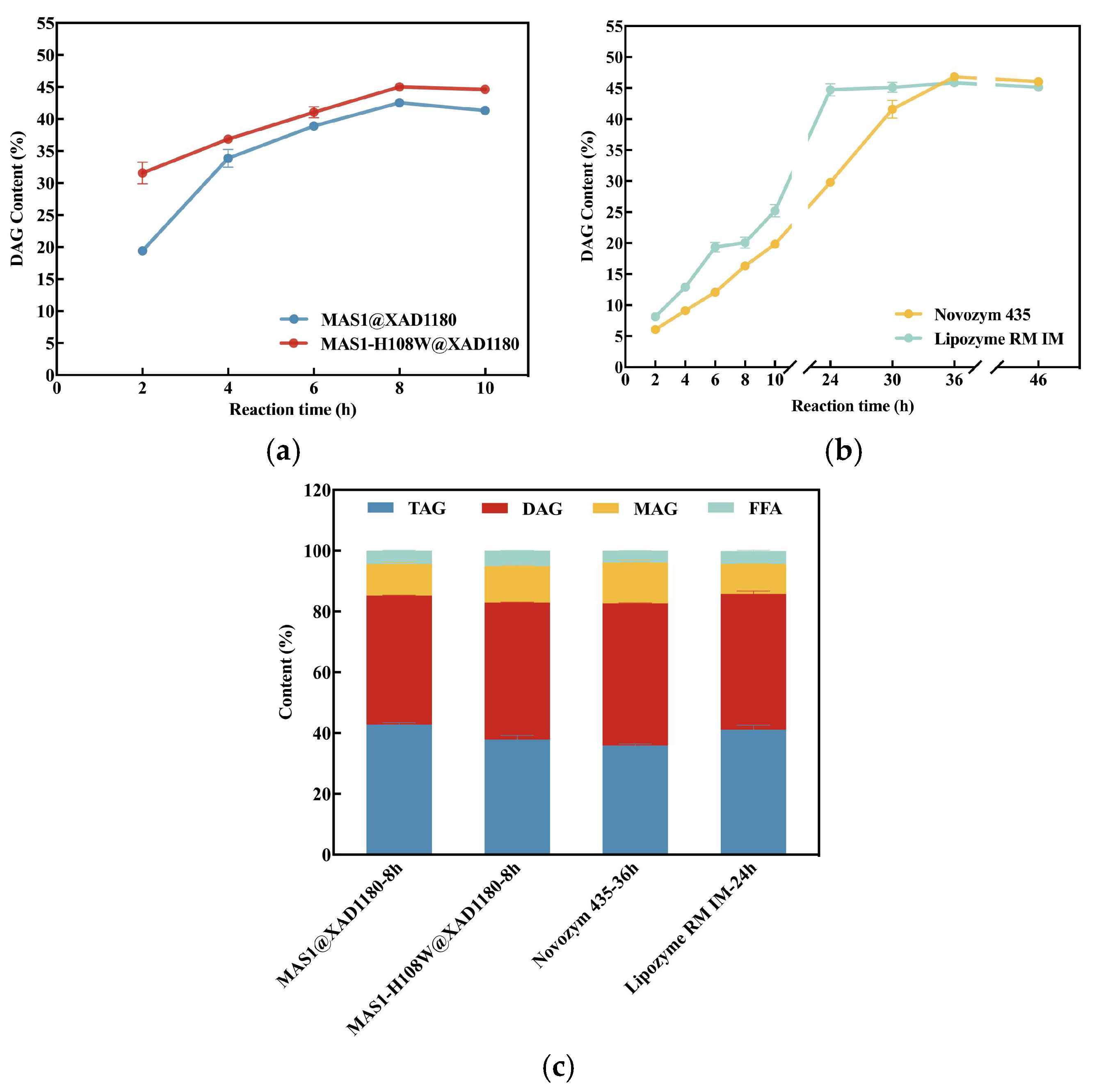
3.3. Comparison of DAG Production by Different Immobilized Lipases-Catalyzed Glycerolysis Reaction
3.4. Optimization of DAG Production by MAS1-H108W@XAD1180-Catalyzed Glycerolysis Reaction
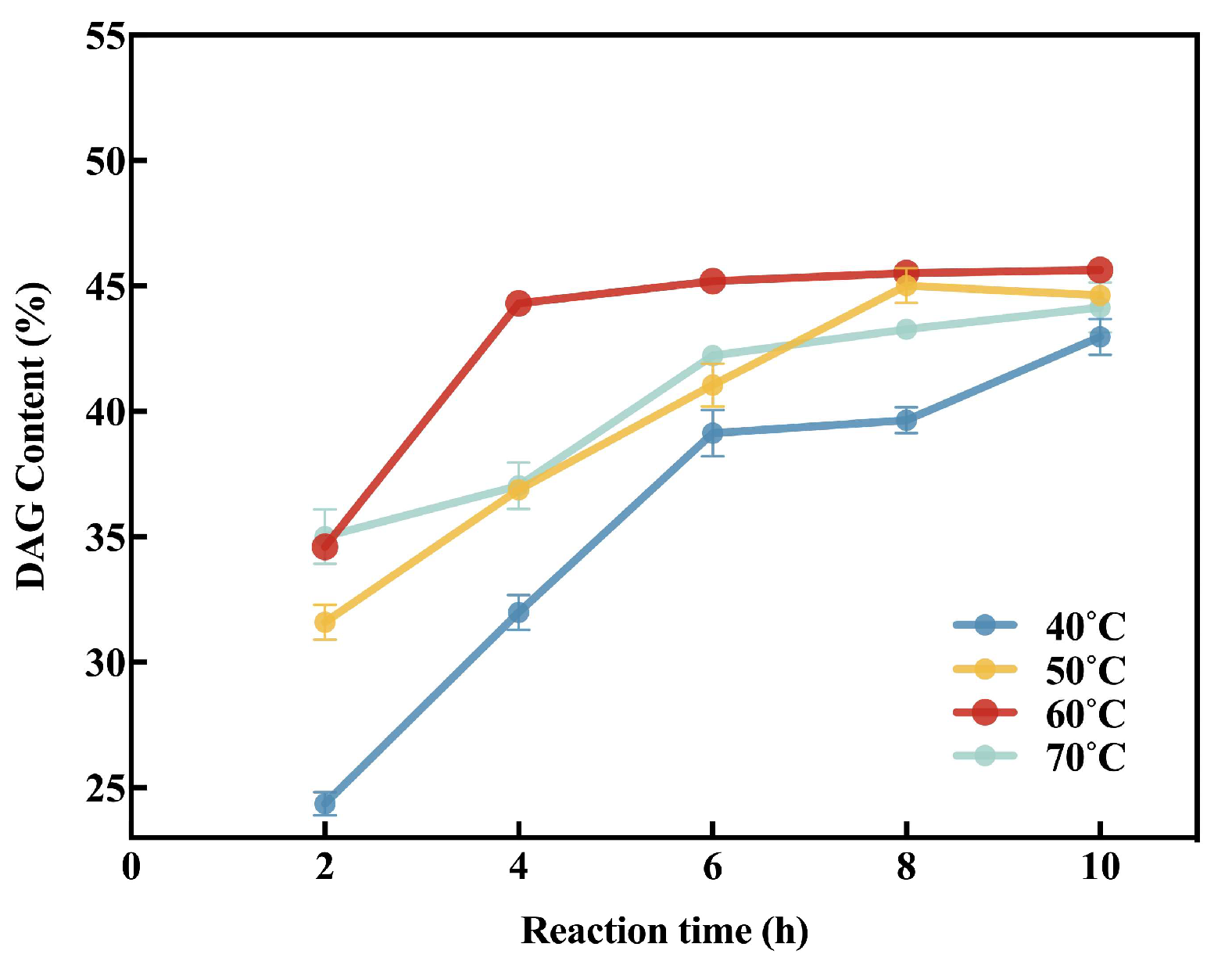
3.4.1. Reaction Temperature
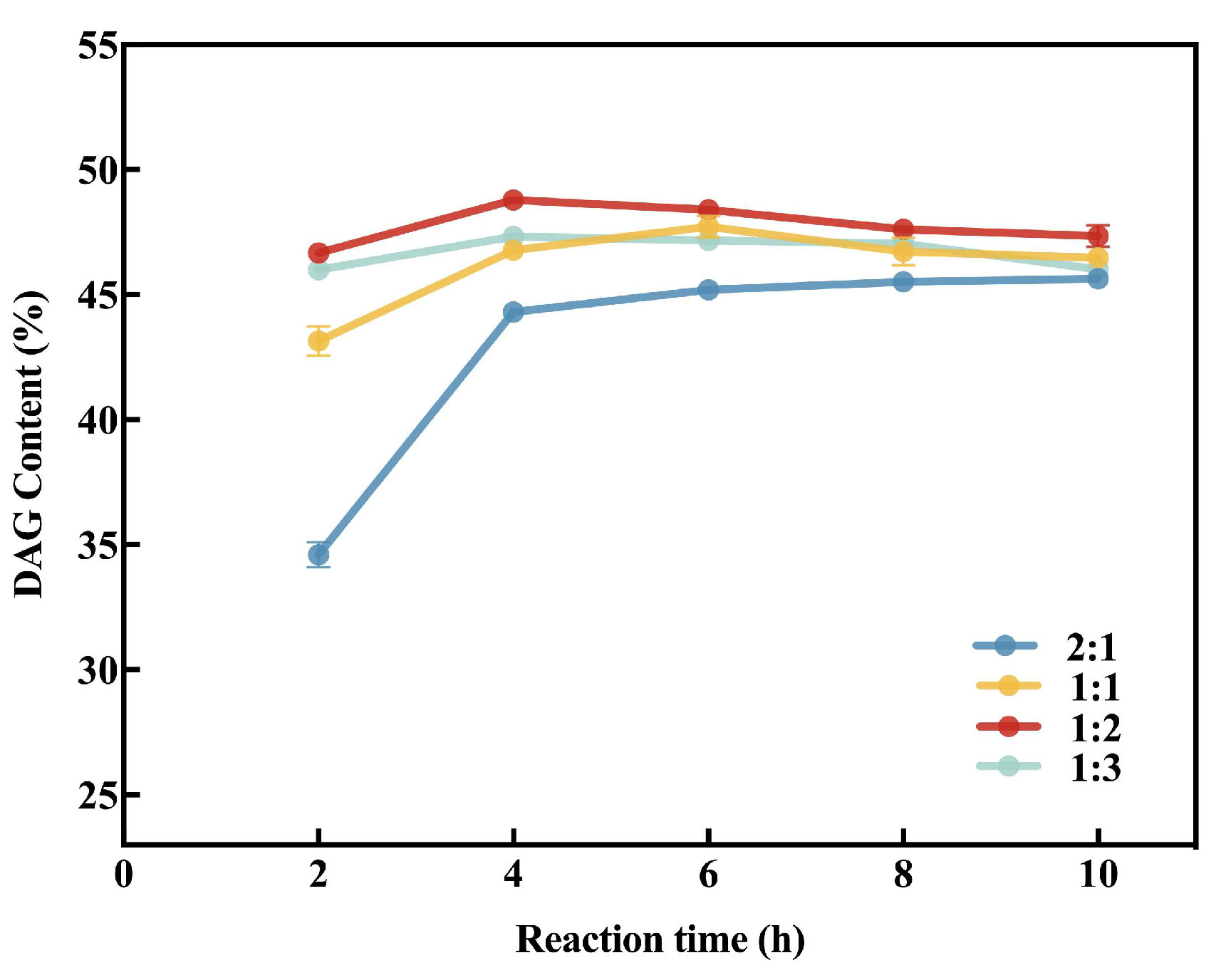
3.4.2. Molar Ratio of Substrate
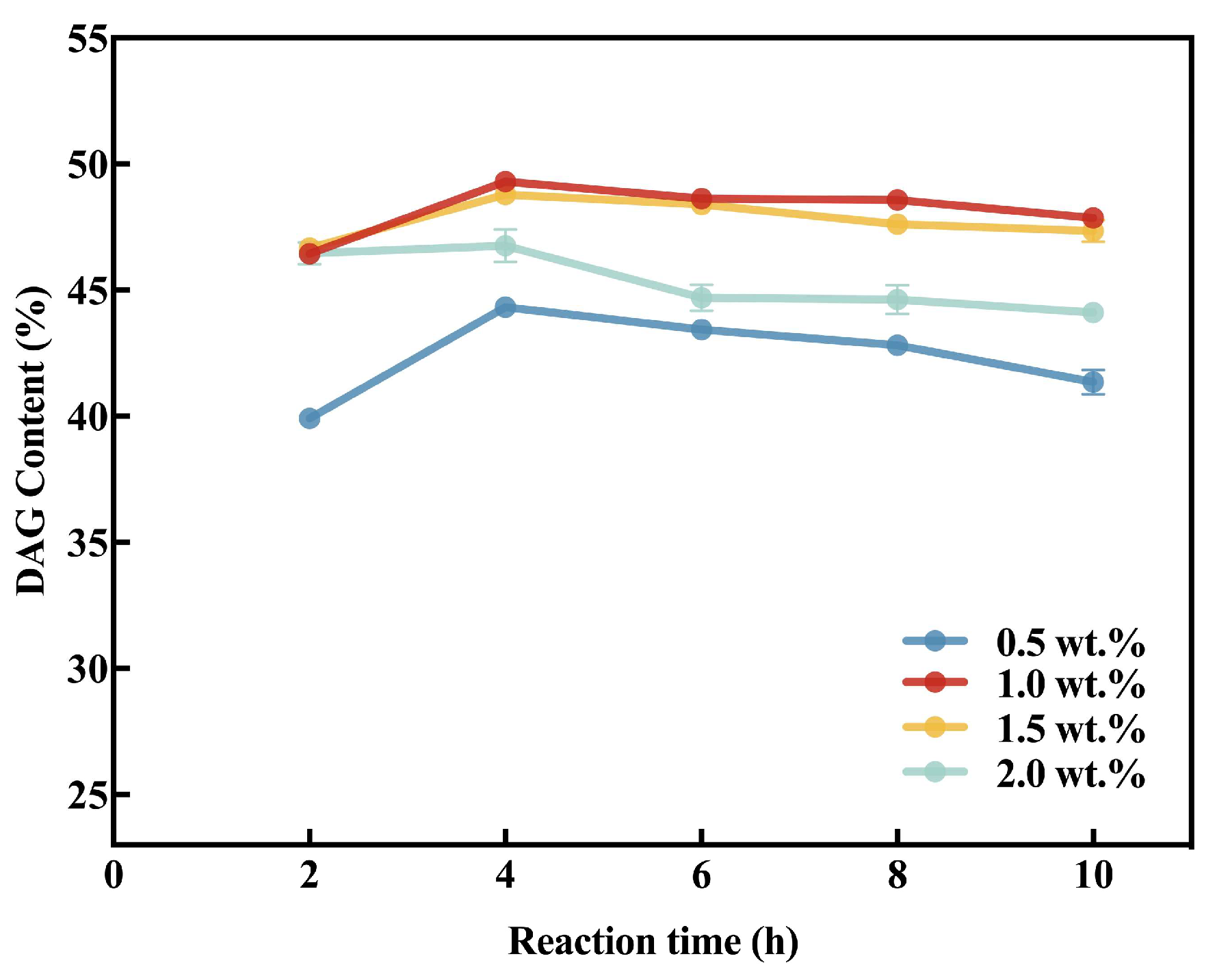
3.4.3. Adding Amount of MAS1-H108W@XAD1180
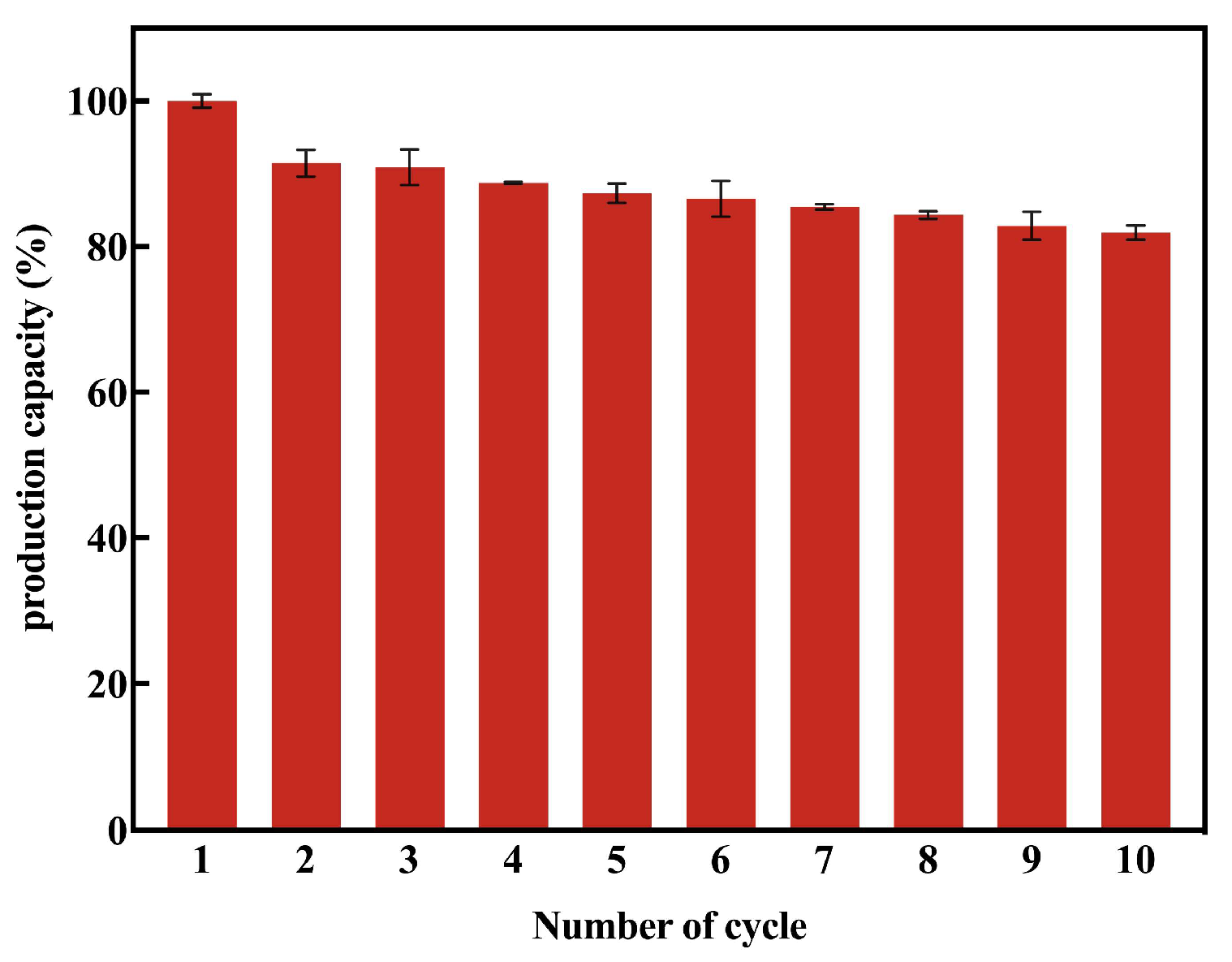
3.5. Reusability and Applicability of MAS1-H108W@XAD1180
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DAG | diacylglycerol |
| FFA | free fatty acids |
| TAG | triacylglycerols |
| FT-IR | Fourier transform infrared |
| HPLC | High Performance Liquid Chromatography |
References
- Lee, W.J.; Zhang, Z.; Lai, O.M.; Tan, C.P.; Wang, Y. Diacylglycerol in food industry: Synthesis methods, functionalities, health benefits, potential risks and drawbacks. Trends Food Sci. Technol. 2020, 97, 114–125. [Google Scholar] [CrossRef]
- Tada, N. Physiological actions of diacylglycerol outcome. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 145–149. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Tang, T.K.; Phuah, E.T.; Tan, C.P.; Wang, Y.; Li, Y.; Cheong, L.Z.; Lai, O.M. Production, safety, health effects and applications of diacylglycerol functional oil in food systems: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 2509–2525. [Google Scholar] [CrossRef]
- Matsuo, N. Nutritional characteristics and health benefits of diacylglycerol in foods. Food Sci. Technol. Res. 2007, 10, 103–110. [Google Scholar] [CrossRef]
- Sikorski, D. Application of Diacylglycerol Oil in Baked Goods, Nutritional Beverages/Bars, Sauces, and Gravies. In Diacylglycerol Oil, 1st ed.; Katsuragi, Y., Yasukawa, T., Matsuo, N., Flickinger, B.D., Tokimitsu, I., Matlock, M.G., Eds.; AOCS Publishing: New York, NY, USA, 2019; pp. 223–252. [Google Scholar]
- Anikisetty, M.; Krishna, A.G.; Panneerselvam, V.; Kamatham, A. Diacylglycerol (DAG) rich rice bran and sunflower oils modulate lipid profile and cardiovascular risk factors in Wistar rats. J. Funct. Foods 2018, 40, 117–127. [Google Scholar] [CrossRef]
- Flickinger, B.D.; Matsuo, N. Nutritional characteristics of DAG oil. Lipids 2003, 38, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Feltes, M.M.C.; de Oliveira, D.; Block, J.M.; Ninow, J.L. The production, benefits, and applications of monoacylglycerols and diacylglycerols of nutritional interest. Food Bioprocess Technol. 2013, 6, 17–35. [Google Scholar] [CrossRef]
- Das, K.; Ghosh, M. Comparative qualitative assessment of DAG production from medium chain fatty acids mediated by enzymatic and chemical catalysts under individually optimized conditions. Biocatal. Agric. Biotechnol. 2019, 22, 101422. [Google Scholar] [CrossRef]
- Bornscheuer, U.T. Enzymes in lipid modification. Annu. Rev. Food Sci. Technol. 2018, 9, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Peng, X.; Lee, Y.Y.; Xie, P.; Tan, C.P.; Wang, Y.; Zhang, Z. Enzymatic preparation of diacylglycerols: Lipase screening, immobilization, characterization and glycerolysis performance. J. Sci. Food Agric. 2025, 105, 816–828. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.L.; Fang, H.Y.; Liu, G.; Dai, W.J.; Song, M.Y.; Fu, J.Y.; Wen, L.F.; Kan, Q.X.; Chen, Y.J.; Li, Y.Y.; et al. Enzymatic synthesis of diacylglycerol-enriched oil by two-step vacuum-mediated conversion of fatty acid ethyl ester and fatty acid from soy sauce by-product oil as lipid-lowering functional oil. Front. Nutr. 2022, 9, 884829. [Google Scholar] [CrossRef]
- Lai, O.M.; Lee, Y.Y.; Phuah, E.T.; Tang, T.K.; Wang, Y.; Cheong, L.Z.; Tan, C.P. Diacylglycerol Oil: Health Benefits, Synthesis and Applications. In Recent Advances in Edible Fats and Oils Technology; Springer: Singapore, 2022; pp. 249–264. [Google Scholar]
- Wang, W.F.; Li, T.; Ning, Z.X.; Wang, Y.H.; Yang, B.; Yang, X.Q. Production of extremely pure diacylglycerol from soybean oil by lipase-catalyzed glycerolysis. Enzym. Microb. Technol. 2011, 49, 192–196. [Google Scholar] [CrossRef]
- Plou, F.J.; Barandiarán, M.; Calvo, M.V.; Ballesteros, A.; Pastor, E. High-yield production of mono-and di-oleylglycerol by lipase-catalyzed hydrolysis of triolein. Enzym. Microb. Technol. 1996, 18, 66–71. [Google Scholar] [CrossRef]
- Arcos, J.A.; Otero, C. Enzyme, medium, and reaction engineering to design a low-cost, selective production method for mono- and dioleoylglycerols. J. Am. Oil Chem. Soc. 1996, 73, 673–682. [Google Scholar] [CrossRef]
- Ghaffari-Moghaddam, M.; Eslahi, H.; Aydin, Y.A.; Saloglu, D. Enzymatic processes in alternative reaction media: A mini review. J. Biol. Methods 2015, 2, e25. [Google Scholar] [CrossRef]
- Zheng, J.W.; Liang, Y.D.; Li, J.X.; Lin, S.P.; Zhang, Q.Y.; Zuo, K.H.; Zhong, N.J.; Xu, X.B. Enzymatic preparation of mono-and diacylglycerols: A review. Grain Oil Sci. Technol. 2023, 6, 185–205. [Google Scholar]
- Yuan, D.J.; Lan, D.M.; Xin, R.P.; Yang, B.; Wang, Y.H. Screening and characterization of a thermostable lipase from marine Streptomyces sp. strain W007. Biotechnol. Appl. Biochem. 2016, 63, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, D.; Wang, W.; Yang, B.; Wang, Y. A highly efficient immobilized MAS1 lipase for the glycerolysis reaction of n-3 PUFA-rich ethyl esters. J. Mol. Catal. B Enzym. 2016, 134, 25–31. [Google Scholar]
- Wang, X.; Li, D.; Qu, M.; Durrani, R.; Yang, B.; Wang, Y. Immobilized MAS1 lipase showed high esterification activity in the production of triacylglycerols with n-3 polyunsaturated fatty acids. Food Chem. 2017, 216, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, J.B.; Xu, X.; Mu, H. Diacylglycerol synthesis by enzymatic glycerolysis: Screening of commercially available lipases. J. Am. Oil Chem. Soc. 2005, 82, 329–334. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, S.; Xu, L.; Cai, J.; Wang, J.; Wang, Y. Structural basis for the regiospecificity of a lipase from Streptomyces sp. W007. Int. J. Mol. Sci. 2022, 23, 5822. [Google Scholar] [CrossRef]
- Zhao, Z.; Hou, S.; Lan, D.; Wang, X.; Liu, J.; Khan, F.I.; Wang, Y. Crystal structure of a lipase from Streptomyces sp. strain W007–implications for thermostability and regiospecificity. FEBS J. 2017, 284, 3506–3519. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Y.; Qin, X.; Lan, D.; Yang, B.; Wang, Y. Immobilization of lipase SMG1 and its application in synthesis of partial glycerides. Eur. J. Lipid Sci. Technol. 2014, 116, 1063–1069. [Google Scholar] [CrossRef]
- Yang, B.; Wang, Y.H.; Yang, J.G. Optimization of enzy matic degumming process for rapeseed oil. J. Am. Oil Chem. Soc. 2006, 83, 653–658. [Google Scholar] [CrossRef]
- Kielkopf, C.L.; Bauer, W.; Urbatsch, I.L. Bradford assay for determining protein concentration. Cold Spring Harb. Protoc. 2020, 2020, pdb-prot102269. [Google Scholar] [CrossRef]
- Liu, N.; Fu, M.; Wang, Y.; Zhao, Q.; Sun, W.; Zhao, M. Immobilization of Lecitase® Ultra onto a novel polystyrene DA-201 resin: Characterization and biochemical properties. Appl. Biochem. Biotechnol. 2012, 168, 1108–1120. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, X.; Qin, X.; Zhao, Z.; Yang, B.; Wang, Y. Properties of immobilized MAS1-H108A lipase and its application in the efficient synthesis of n-3 PUFA-rich triacylglycerols. Bioprocess Biosyst. Eng. 2021, 44, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Asemani, M.; Rabbani, A.R. Detailed FTIR spectroscopy characterization of crude oil extracted asphaltenes: Curve resolve of overlapping bands. J. Pet. Sci. Eng. 2020, 185, 106618. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of infrared spectra, a practical approach. Encycl. Anal. Chem. 2000, 12, 10815–10837. [Google Scholar]
- Liu, N.; Li, D.M.; Wang, W.F.; Hollmann, F.; Xu, L.; Ma, Y.J.; Yang, B.; Bai, W.D.; Sun, X.T.; Wang, Y.H. Production and immobilization of lipase PCL and its application in synthesis of α-Linolenic acid-Rich diacylglycerol. J. Food Biochem. 2018, 42, e12574. [Google Scholar]
- Li, X.; Li, D.; Wang, W.; Durrani, R.; Yang, B.; Wang, Y. Immobilization of SMG1-F278N lipase onto a novel epoxy resin: Characterization and its application in synthesis of partial glycerides. J. Mol. Catal. B Enzym. 2016, 133, 154–160. [Google Scholar] [CrossRef]
- Zeng, H.Y.; Liao, K.B.; Deng, X.; Jiang, H.; Zhang, F. Characterization of the lipase immobilized on Mg–Al hydrotalcite for biodiesel. Process Biochem. 2009, 44, 791–798. [Google Scholar] [CrossRef]
- Ranjbakhsh, E.; Bordbar, A.K.; Abbasi, M.; Khosropour, A.R.; Shams, E. Enhancement of stability and catalytic activity of immobilized lipase on silica-coated modified magnetite nanoparticles. Chem. Eng. J. 2012, 179, 272–276. [Google Scholar] [CrossRef]
- Monte Blanco, S.F.M.; Santos, J.S.; Feltes, M.M.C.; Dors, G.; Licodiedoff, S.; Lerin, L.A.; de Oliveira, D.; Ninow, L.; Furigo, A. Optimization of diacylglycerol production by glycerolysis of fish oil catalyzed by Lipozyme TL IM with Tween 65. Bioprocess Biosyst. Eng. 2015, 38, 2379–2388. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Sun, Q.; Qin, Z.; Liu, Q.; Kong, B. Ultrasonic pretreatment promotes diacylglycerol production from lard by lipase-catalysed glycerolysis and its physicochemical properties. Ultrason. Sonochem. 2018, 48, 11–18. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Liu, R.; Liu, Z.; Goh, K.L.; Zivkovic, V. Solvent-free synthesis of diacylglycerols via enzymatic glycerolysis between edible oils and glycerol catalyzed by self-made immobilized lipase PS@ LXTE-1000. Oil Crop Sci. 2024, 9, 225–233. [Google Scholar] [CrossRef]
- Xie, R.; Lee, Y.Y.; Xie, P.; Tan, C.P.; Wang, Y.; Zhang, Z. Immobilization of Lipase from Thermomyces Lanuginosus and Its Glycerolysis Ability in Diacylglycerol Preparation. Molecules 2024, 29, 4141. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, F.; Zhong, N. Production of diacylglycerols through glycerolysis with sba-15 supported thermomyces lanuginosus lipase as catalyst. J. Sci. Food Agric. 2020, 100, 1426–1435. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, N.J.; Cheong, L.Z.; Huang, J.R.; Chen, H.X.; Lin, S.Y. Immobilization of candida antarctica lipase b onto organically-modified sba-15 for efficient production of soybean-based mono and diacylglycerols. Int. J. Biol. Macromol. 2018, 120, 886–895. [Google Scholar] [CrossRef]
- Zhong, N.; Chen, W.; Liu, L.; Chen, H. Immobilization of rhizomucor miehei lipase onto the organic functionalized sba-15: Their enzymatic properties and glycerolysis efficiencies for diacylglycerols production. Food Chem. 2019, 271, 739–746. [Google Scholar] [CrossRef]
- Liu, N.; Wang, Y.; Zhao, Q.; Cui, C.; Fu, M.; Zhao, M. Immobilisation of lecitase® ultra for production of diacylglycerols by glycerolysis of soybean oil. Food Chem. 2012, 134, 301–307. [Google Scholar] [CrossRef]
- Duan, Z.Q.; Du, W.; Liu, D.H. Novozym 435-catalyzed 1, 3-diacylglycerol preparation via esterification in t-butanol system. Process Biochem. 2010, 45, 1923–1927. [Google Scholar] [CrossRef]
- Kuo, C.H.; Liu, Y.C.; Chang, C.M.J.; Chen, J.H.; Chang, C.; Shieh, C.J. Optimum conditions for lipase immobilization on chitosan-coated Fe3O4 nanoparticles. Carbohydr. Polym. 2012, 87, 2538–2545. [Google Scholar] [CrossRef]
- Yücel, Y.; Demir, C.; Dizge, N.; Keskinler, B. Lipase immobilization and production of fatty acid methyl esters from canola oil using immobilized lipase. Biomass Bioenergy 2011, 35, 1496–1501. [Google Scholar] [CrossRef]
- He, L.; Zheng, J.; Feng, S.; Xu, L.; Zhong, N. Immobilization of Candida Antarctica lipase A onto macroporous resin NKA-9: Esterification and glycerolysis performance study. J. Oleo Sci. 2022, 71, 1337–1348. [Google Scholar] [CrossRef]
| Vegetable Oils | Olive Oil | Corn Oil | Rapeseed Oil | Peanut Oil | Sunflower Oil | Soybean Oil |
|---|---|---|---|---|---|---|
| Initial DAG | 1.752 ± 0.034 | 3.465 ± 0.06 | 1.527 ± 0.087 | 2.670 ± 0.091 | 1.692 ± 0.052 | 0.820 ± 0.011 |
| DAG in theglycerolysis reactions | 49.041 ± 0.144 | 48.446 ± 1.01 | 48.749 ± 0.663 | 49.455 ± 0.886 | 49.753 ± 0.446 | 50.021 ± 0.115 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Yu, S.; Xiao, Q.; Cai, J.; Zhao, Z. Highly Efficient Production of Diacylglycerols via Enzymatic Glycerolysis Catalyzed by Immobilized MAS1-H108W Lipase. Processes 2025, 13, 2937. https://doi.org/10.3390/pr13092937
Zhou L, Yu S, Xiao Q, Cai J, Zhao Z. Highly Efficient Production of Diacylglycerols via Enzymatic Glycerolysis Catalyzed by Immobilized MAS1-H108W Lipase. Processes. 2025; 13(9):2937. https://doi.org/10.3390/pr13092937
Chicago/Turabian StyleZhou, Ling, Siqin Yu, Qingqing Xiao, Jun Cai, and Zexin Zhao. 2025. "Highly Efficient Production of Diacylglycerols via Enzymatic Glycerolysis Catalyzed by Immobilized MAS1-H108W Lipase" Processes 13, no. 9: 2937. https://doi.org/10.3390/pr13092937
APA StyleZhou, L., Yu, S., Xiao, Q., Cai, J., & Zhao, Z. (2025). Highly Efficient Production of Diacylglycerols via Enzymatic Glycerolysis Catalyzed by Immobilized MAS1-H108W Lipase. Processes, 13(9), 2937. https://doi.org/10.3390/pr13092937






