Abstract
The tension in the relationship between water and energy seriously restricts the harmonious coexistence between man and the ecological environment. The solar-powered interface evaporation technology emerging in recent years has shown good application prospects in high-salt wastewater treatment for achieving the zero-discharge treatment of wastewater. In this review, advanced solar-driven interfacial evaporation is primarily focused on its mechanisms, photothermal materials optimization, and the structure of solar evaporators for salt removal. The high wide-spectrum solar absorption rate of photothermal materials determines the total energy that can be utilized in the evaporation system. The light-to-heat conversion capacity of photothermal materials directly affects the efficiency and performance of solar interface evaporators. We highlight the microstructures enabled by the nanophotonic designs of photothermal material-based solar absorbers, which can achieve highly efficient light harvesting across the entire solar irradiance spectral range with weighted solar absorptivity. Finally, based on current research, existing problems, and future development directions for high-salt wastewater evaporation research are proposed. The review provides insights into the effective treatment of high-salt wastewater.
1. Introduction
High-salt wastewater generally refers to sewage with a salt-containing mass fraction greater than or equal to 1% originating from engineering, pharmacology, and the papermaking industry. Salt-rich wastewater, if discharged directly, will lead to adverse effects on the ecological and environmental problems. On the one hand, the inorganic salt will cause freshwater mineralization, soil salinization, and water eutrophication. Moreover, the accumulation of heavy metal elements in the food chain will largely affect the drinking water safety [1]. On the other hand, salt infiltration into the soil will change the soil structure and permeability, and reduce the soil moisture permeability, increasing the possibility of being flooded [2]. In addition, irrigation with brine can lead to irreversible soil salinization. It hinders the crops’ growth, and the agricultural productivity is significantly reduced [3]. The treatment of high-salinity wastewater is more problematic than that of other industrial wastewaters. Due to the complex and diverse composition of high-salinity wastewater, it is often necessary to select appropriate pretreatment schemes based on actual situations. The excessive inorganic salts in high-salinity wastewater inhibit the growth and reproduction of microorganisms, greatly reducing the treatment efficiency, which makes it difficult for traditional biological technologies to treat high-salinity wastewater [4]. Traditional physical and chemical treatment methods also have certain limitations, such as excessive use of chemicals, equipment corrosion, and high operating costs [5,6,7,8,9]. Furthermore, the purification of waste salt after desalination remains very challenging [10]. If this high-salinity wastewater is discharged directly without treatment, it will further burden municipal sewage treatment plants [11]. Accelerating the promotion of the recycling of industrial high-salt wastewater, turning it from waste into a resource, has become the key to promoting green industrial development and alleviating the contradiction between water supply and demand [12]. Research on high-salt wastewater treatment technologies is urgent, and exploring efficient and economical high-salt wastewater treatment technologies has become one of the hotspots in current high-salt wastewater treatment [13,14,15,16,17,18,19]. At the same time, it is necessary to vigorously develop key core technologies such as the zero-discharge and resource-oriented treatment of saline wastewater, to achieve a new pattern of recycling and resource recovery utilization [20].
The treatment process for high-salinity wastewater typically employs thermal desalination technology and membrane separation technology. Thermal desalination technology separates distilled water from saline water through heating. The most widely used methods are multi-stage flash evaporation and multi-effect distillation. Thermal desalination technology consumes a significant amount of fossil fuels, which may lead to increased air pollution and greenhouse gas emissions [21]. Membrane separation technology includes methods of reverse/forward osmosis, electrodialysis, and membrane distillation. Among these, the most commonly used reverse osmosis devices consume approximately 100 TWh of energy annually, and if this energy is provided by coal combustion, it would result in 60 to 100 tons of carbon dioxide emissions [22]. Therefore, the research and development of novel water purification materials and technologies, along with the utilization of renewable energy to drive seawater desalination in order to reduce energy consumption, have attracted significant attention in the scientific community. Among them, solar-driven interfacial water evaporation technology, as a green, clean, simple, and efficient water treatment method, is one of the frontier hotspots in the field of materials and environmental research.
Solar energy, as a clean, environmentally friendly, and renewable energy source, is considered an effective alternative to traditional fossil fuels. It has been extensively researched [23] and plays a significant role in energy transition. Through solar energy technology, we can convert sunlight into electrical or thermal energy to meet the needs of human life and industrial production. The conversion and utilization of solar energy is one of the important strategies proposed to alleviate the current global energy crisis and environmental issues [24]. Solar photothermal interfacial evaporation technology is a sustainable water treatment technology, exploiting solar energy to realize water evaporation and purification. It heats the photothermal layer using the heat provided by solar energy. After absorbing sunlight, the photothermal layer converts it into thermal energy on the water-contacting surface. Once the surface water temperature reaches a certain level, water molecules begin to evaporate into water vapor, and pure water is obtained by collecting and condensing the water vapor. In brief, heat can be sealed on the surface of seawater, and the diffusion of a large amount of heat in other directions can be inhibited [25]. This is conducive to maximizing the “boiling” of seawater and high-salinity wastewater, improving the utilization efficiency of solar energy. This technology has attracted widespread attention once reported. As shown in Figure 1 [26,27], solar photothermal interfacial evaporation technology has the advantages of being environmentally friendly, low-cost, and easy to maintain, and is therefore widely used in seawater desalination, wastewater treatment, drinking water purification, agricultural irrigation, and other fields [28]. Solar-powered water evaporation technologies are highly dependent on significant advancements in the design and manufacture of multifunctional photothermal materials. In terms of photothermal materials selection, researchers focus on developing photothermal materials with high efficiency and broad absorption spectra in solar-driven seawater desalination technology, such as metal nanomaterials, semiconductor materials, carbon-based substrates, and conductive polymers [29], which promote the light-to-heat conversion of desalinated seawater.
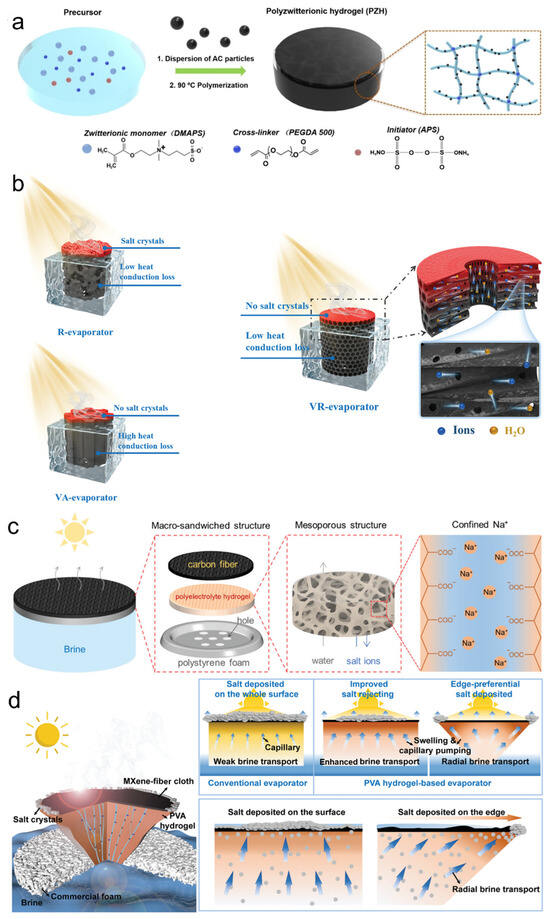

Figure 1.
Different types of solar photothermal evaporators: (a) Salt-responsive PZH evaporators. Reproduced with permission [30]. Copyright 2022, Wiley-VCH. (b) Three-dimensional hydrogel evaporators. Reproduced with permission [30]. Copyright 2022, Wiley-VCH. (c) Macro-sandwiched structure of salt-tolerant solar evaporators. Reproduced with permission [27]. Copyright 2021, Wiley-VCH. (d) High-salt-tolerant 3D hydrogel evaporators. Reproduced with permission [31]. Copyright 2021, Wiley-VCH.
Traditional solar water evaporation involves directly exposing the entire water body to sunlight to obtain water vapor and complete water purification. However, since water molecules can only absorb infrared solar light and the water surface scatters light, this method is time-consuming and inefficient (30–45%), failing to achieve the desired results [32]. Therefore, improving the evaporation device to enhance evaporation efficiency has become a key issue in solar water evaporation technology. In recent years, researchers have leveraged the advantage of photothermal materials, which can efficiently convert solar energy into thermal energy, and applied them to the field of solar water evaporation. By additionally heating the water through photothermal materials, a higher water evaporation efficiency is achieved [33]. Meanwhile, the structural design of solar water evaporation devices has also undergone further development, with the working area of photothermal materials being moved from the entire water body to the air–water interface. This interfacial evaporation method increases the light absorption area of the system, avoids the majority of heat loss, and facilitates the diffusion of water vapor, thereby effectively enhancing the photothermal conversion efficiency of the system (reaching over 97%).
In this review, a comprehensive understanding of advanced solar-driven interfacial evaporation is primarily focused on in terms of its mechanisms, photothermal materials optimization, and the structure of solar evaporators for salt removal. The high wide-spectrum solar absorption rate of photothermal materials determines the total energy that can be utilized in the evaporation system. The light-to-heat conversion capacity of photothermal materials directly affects the efficiency and performance of interfacial solar evaporators. Therefore, the delicate design of nano-/microstructured photothermal materials is of great importance for attaining highly efficient solar absorptivity across the entire solar irradiance spectral range. Finally, the present problems and future prospective directions for high-salt wastewater evaporation are anticipated. The review will pave the way for designing effective solar-driven interfacial evaporation systems in wastewater treatment and provide insights for future advancement.
2. Solar-Driven Interfacial Evaporation
2.1. Photothermal Conversion
The light absorption capacity of photothermal materials refers to the extent to which the material absorbs sunlight or other light sources. Two critical factors are involved as follows: the absorption range over the full solar spectrum and the corresponding intensity per wavelength. A higher light absorption capacity translates to more efficient absorption of sunlight by the material. Photothermal materials selected for interfacial heating should exhibit high absorptance, minimal transmittance, and low reflectance in the whole solar irradiance spectral range from 300 to 2500 nm [34]. Therefore, the high broad-spectrum solar absorptance of photothermal materials determines the total energy that can be utilized in the evaporation system.
Secondly, the excellent light-to-heat conversion capability of photothermal materials directly affects the efficiency and performance of interfacial solar evaporators. Below is the formula for calculating the photothermal conversion efficiency [35]:
where m is the mass flux of evaporated water; h is the latent heat of water evaporation at the specific temperature; C is the specific heat capacity of water, with a constant value of 4.2 J·g−1·K−1; T is the evaporation temperature; T0 is the initial temperature of the water; and Ps is the incident solar radiation power.
2.2. Interfacial Evaporation
Solar-driven interfacial evaporation technology utilizes sunlight as the energy source. Through photothermal conversion materials (solar absorbers), light energy is converted into heat energy. Water molecules at the interface between the photothermal conversion material and the water body absorb sufficient heat energy and escape in gaseous form through internal channels of the photothermal conversion material, while impurities and solutes in the water remain in the water body, thereby achieving water purification [36], as shown in Figure 2. This technology leverages green and sustainable solar energy to drive the acquisition of clean water, addressing both water scarcity issues and alleviating the energy conflicts arising from the water purification process.
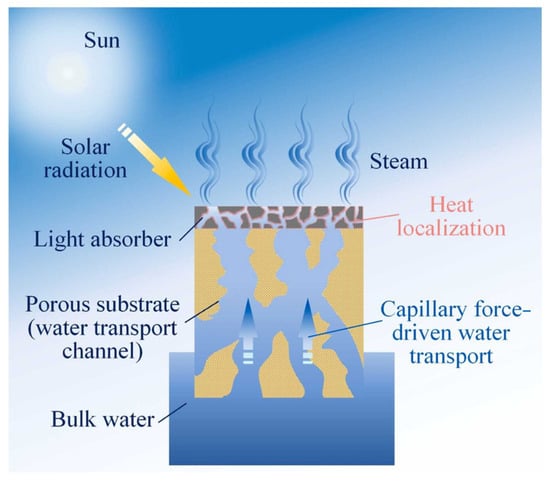

Figure 2.
Schematic of the operating principle in a solar-driven interfacial evaporation (SDIE) unit. Reproduced with permission [36]. Copyright 2024, Elsevier.
In the solar-driven water evaporation system, the solar absorber is its main component. The first stage of the development of solar–thermal water evaporation technology is bottom heating [37]. As shown in Figure 3a, the heating device is placed at the bottom of the water body. Due to the scattering of water, a significant amount of light energy is lost, and heat cannot be concentrated on the surface of the solar absorber, resulting in slow evaporation. The second stage is volumetric heating, as shown in Figure 3b. The photothermal conversion particles are uniformly dispersed in the water body. The heat generated during the contact between the photothermal conversion particles and the water is conducted to the non-evaporating water body, leading to substantial heat loss. The third stage is interfacial heating. As shown in Figure 3c, the solar absorber is located at the water–air interface, concentrating the heat at the interface and largely avoiding heat loss. Interfacial evaporation technology does not require high-temperature or high-pressure conditions and can achieve water desalination or liquid concentration with low energy consumption [38]. The operation is relatively simple, eliminating the need for complex equipment and control systems, and is easy to maintain and manage. It can also be combined with renewable energy sources, such as solar thermal energy or waste heat utilization, contributing to reducing dependence on traditional energy sources and demonstrating good sustainability. Therefore, the emerging SDIE technology significantly improves evaporation efficiency and drives the rapid development of solar-driven interfacial evaporation technology [39].
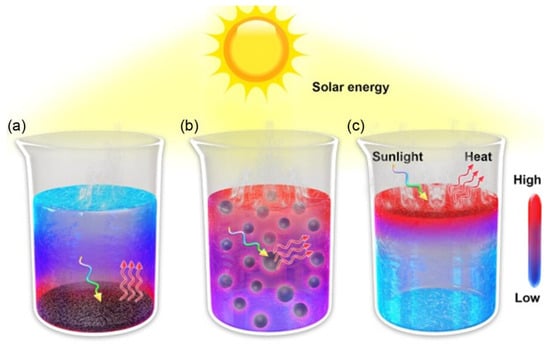

Figure 3.
Different solar absorbers in the light–heat water evaporation field. (a) Bottom heating. (b) Volumetric heating. (c) Interfacial heating. Reproduced with permission [37]. Copyright 2023, Wiley-VCH.
3. Micro-Nano Photothermal Materials
High-performance photothermal materials are key to achieving efficient photothermal evaporation. Photothermal materials can generally be classified into four categories: metal nanomaterials, inorganic semiconductor materials, carbon-based light-absorbing materials, and polymer materials [24,29], as shown in Figure 4.
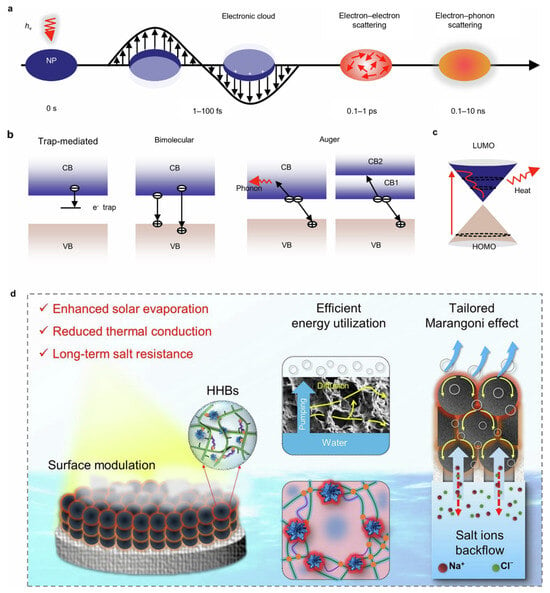

Figure 4.
Photothermal conversion mechanism of diverse photothermal materials. (a) Thermal energy generation through plasmonic resonance in metallic light-to-thermal conversion materials (LTCMs). (b) Schematic illustration of photogenerated exciton recombination in semiconductors. (c) Depiction of carbon-based LTCMs generating heat through molecular thermal vibrations. (d) Operational mechanism of a hydrogel evaporator. Reproduced with permission [40]. Copyright 2025, Nature Publishing Group.
3.1. Metal-Based Photothermal Materials
For metal-based plasmonic materials, efforts have been made to carefully regulate their size, shape, orientation, and structure at the nanoscale, thereby improving their absorption rate of sunlight and the wavelength range covered [41,42]. Precious metals such as gold and silver are the two typical metallic materials in hot plasmas, exhibiting significant surface plasmon effects in the visible and near-infrared regions [43]. Taking gold nanostructures as an example, various structural gold nanostructures have been obtained through different synthesis methods. Bae et al. [44] achieved solar steam generation by heating surface plasmons using metal nanoshells or nanoparticles with narrow absorption bandwidths. Thermal nanofocusing in self-assembled metal nanowire bundles achieved 91% average absorption across 400–2500 nm, whereas microscale funnel structures maintained 7% average reflection over 2.5–17 μm. However, Ag is prone to oxidation or sulfurization in atmospheric environments, which greatly reduces its plasma characteristics and ultimately affects its photothermal conversion capability. Additionally, as a rare metal, the high cost of Ag hinders its large-scale industrial application. Copper (Cu) and aluminum (Al), due to their abundant reserves and excellent cost-effectiveness, are expected to become the best substitutes for Au and Ag [45]. Al exhibits good light absorption across the entire UV–Vis–NIR region. Even though a 3 nm thick Al2O3 passivation layer can form on its surface after oxidation, this does not affect Al’s photothermal effect but rather prevents further oxidation of Al [46,47]. Considering cost, stability, and practicality, the further application of precious metals remains challenging. Recently, research has focused on combining plasmonic materials with substrate materials of complex structures to create evaporators [37,44], aiming to further enhance light absorption and reduce material costs.
3.2. Inorganic Semiconductor-Based Photothermal Materials
Photothermal conversion in inorganic semiconductors relies on two mechanisms: photogenerated carrier dynamics and thermal conduction [48]. When inorganic semiconductor materials are illuminated, photon energy is absorbed, exciting valence band electrons to the conduction band, creating electron–hole pairs [49]. These carriers diffuse within the material, with some being trapped and undergoing recombination processes, while others are transported to the material’s surface. At the surface, these photogenerated carriers can participate in surface reactions or subsequently undergo thermalization and non-radiative recombination, releasing energy as heat for local heating [50]. This photothermal effect can be utilized in fields such as solar photovoltaics, photothermal conversion, and photocatalysis. Metal chalcogenide compounds of nanoscale size are commonly used as photon absorbers in optoelectronic devices, yet research on these semiconductor materials in photothermal conversion is still lacking [51]. Yang et al. [52] synthesized anti-fouling copper zinc tin selenide (CZTSe) nanospheres and deposited them onto a membrane, achieving efficient and stable photothermal evaporation. In practical applications, this device exhibits excellent photothermal conversion performance, abundant nanochannels, anti-fouling properties, timely water supply, and low heat loss. The photothermal evaporation rate of this evaporator can reach 1.528 kg·m−2·h−1, and it can maintain stable and continuous operation for 30 days under saline water conditions.
3.3. Carbon-Based Photothermal Materials
Owing to their optical transitions in the π-band, carbon-based materials can significantly reduce Fresnel reflection [53]. To further increase light absorption, nanostructures of carbon-based materials with low refractive indices have been developed [54]. Fu et al. [55] developed a method to prepare graphene aerogel (GA) solely through photoreduction from graphene oxide. The material demonstrated intensity-dependent solar–steam efficiencies at 1 kW·m−2 (53.6 ± 2.5%) and 10 kW·m−2 (82.7 ± 2.5%). Moreover, after 10 cycles of testing, the efficiency remained high, and the morphology of GA was almost undamaged. Carbon nanotubes, for example, are widely used in the field of photothermal evaporation due to their high specific surface area, high light absorption, and high photothermal conversion efficiency [56]. However, the preparation of carbon nanotubes through methods such as arc discharge [57], chemical vapor deposition [58], and laser ablation [59] often requires high temperatures, high energy consumption, and inert gas protection, making the preparation conditions stringent and costly. Additionally, carbonization—the high-temperature pyrolysis synthesis of carbon materials—commonly converts low-cost, accessible organic precursors (e.g., biomass, biochar, polymer foams, sugarcane, lotus seedpods, bamboo mushroom, and wood) into different photothermal converters [60,61,62,63,64]. These materials’ solar energy absorption efficiency is significantly improved after carbonization treatment. Nevertheless, such synthesis processes also have the disadvantages of high energy consumption and high costs [65].
3.4. Photothermal Polymers
Conjugated organic polymers are long-established photothermal evaporation materials due to their exceptional light absorption, low cost, lightweight nature, and tunable chemistry [48]. Polyaniline’s (PANI’s) strong broadband absorption further minimizes energy dissipation during solar-to-thermal conversion, owing to its abundant π-electron delocalized structure. Polypyrrole (PPy) is also a commonly used polymer material, particularly notable in a wide wavelength region (200–2500 nm), and it exhibits better photostability in comparison [34]. Zhang et al. [66] developed a PPy-based solar evaporator, which was modified with fluoroalkylsilane and coated on a stainless-steel mesh to impart ideal hydrophobicity to the evaporator. This evaporator floats on the water surface for interfacial heating. The pure water photothermal evaporation rate is 0.92 kg·m−2·h−1, and the energy conversion efficiency is 58%. In addition, polydopamine (PDA) materials, which possess excellent light absorption properties and photothermal conversion efficiency, also make certain contributions to water evaporation applications. Xu et al. [67], inspired by the self-expansion and contraction cycle of river dolphins when facing danger, developed a highly elastic and light-responsive solar absorption gel (SAG). The introduction of PDA into SAG can modify N-isopropylacrylamide (PNIPAm) hydrogels to achieve solar light enrichment. Researchers conducted multiple tests on the water purification capability of SAG and found that it has great potential in treating various types of wastewater, holding promise for sustainable water resource recycling. Yang et al. [68] prepared a fiber membrane coated with tobramycin-doped PDA nanoparticles (PDA/TOB@CA). Under 1 kW·m−2 of illumination, this membrane achieved a high water evaporation rate of 1.61 kg·m−2·h−1 and an evaporation efficiency of greater than 90%. Furthermore, this composite membrane also exhibited excellent antibacterial activity, enabling efficient and sustained solar water evaporation performance even in bacteria-rich environments. Zhang et al. [69] prepared a bio-inspired aerogel (PAS) using PDA and attapulgite (ATP) as carriers. Under 1 kW·m−2 of illumination, the evaporation rate of this PAS was 1.71 kg·m−2·h−1, with an evaporation efficiency of 98.7%.
Polymer photothermal materials, due to their unique light-absorbing properties, ease of molding and processing, stable performance during use, and ultimate degradability under natural conditions after disposal, have emerged as a novel material in the field of solar water evaporation and are widely studied. However, polymer photothermal materials still have several drawbacks, such as the high structural stability of the polymers themselves, which makes direct modification and structural design challenging, as well as limitations like complex synthesis processes and high costs. Consequently, polymer photothermal materials have been facing issues of intrinsic low photothermal conversion efficiency and long-term cyclic stability in interfacial evaporation applications. Therefore, improving the photothermal conversion performance and long-term stability of these materials is the primary task for the future development of polymer photothermal materials.
4. Design Principles of High-Efficiency Interfacial Evaporators
Solar-driven interfacial evaporation features three energy flows: incident solar radiation, vapor enthalpy output, and environmental heat exchange [70]. Consequently, efficient systems require the following: (1) high-efficiency solar harvesting/conversion, (2) optimized water transport from bulk reservoirs to active evaporation fronts, and (3) suppressed parasitic heat losses at the vaporization interface. Currently, broadband solar absorption and high-efficiency photothermal conversion are largely obstructed by the accumulation of salt on the surface of photothermal materials [71]. To further boost evaporation rates in solar-driven systems, research focus is shifting from material-centric development toward integrated system design with multiscale energy management [72,73,74]. Advanced photothermal architectures and optimized system configurations are emerging to boost energy capture and enable precision thermal management [75,76]. Assuming good photothermal conversion efficiency, researchers have proposed the following strategies to design high-efficiency solar evaporation systems [54,70,77,78,79,80,81,82,83,84], including (1) enhancing light capture through surface morphology; (2) restricting heat conduction; (3) increasing the specific evaporation area; (4) creating hydrophilic surfaces; and (5) reducing the enthalpy change in evaporation.
4.1. Optical Enhancement
As the main component of the interfacial structured evaporator, the light absorber, with its surface-modified photothermal material, determines the absorption and conversion of light, thereby influencing the system’s steam generation efficiency. Although photothermal materials inherently possess high photothermal conversion efficiency, diffuse reflection and thermal radiation inevitably occur during practical operation, potentially affecting the system’s ability to capture sunlight. Additionally, under actual conditions, variations in the angle of the sun may pose another challenge to solar light absorption and steam generation. To maximize light collection, it is particularly important to design and regulate surface structures at both macro and nanoscales.
(1) Enhancing light absorption through (i) multiple reflections or (ii) matrix formation: (i) Macroscopically, a three-dimensional microstructure can be formed on the surface of traditional two-dimensional evaporators, allowing multiple interactions between sunlight and the material to enhance light collection. Compared to two-dimensional planes, the absorbance of three-dimensional evaporators increases from ~93% to 99%, as exemplified by origami and cup-like structures [85]. Angular tuning of PPy/PVDF cones [86] revealed inverse apex angle–temperature dependence: sharper geometries concentrated heat, achieving 1.7× higher evaporation than planar interfaces. This demonstrated the effectiveness of achieving the multiple utilization of light by regulating the surface structure of solar absorbers. Li et al. [87] designed a foldable evaporator with a flower-like surface morphology and found that the origami rose with maximized petal count and steepest folds achieved record internal light reflection, yielding 99% solar absorption and 2.12 kg·m−2·h−1 evaporation—the highest reported for biomimetic evaporators (Figure 5a–c). However, despite achieving peak energy utilization efficiency, the origami rose evaporator exhibited significantly lower surface temperatures than its flat counterpart. This finding challenges the conventional view correlating elevated surface temperatures with enhanced evaporation performance. The researchers speculated that the origami rose with an enlarged surface area could achieve evaporation over a larger surface, thus resulting in lower heat accumulation than in the flat device. In addition, the lower surface temperature also helps reduce radiative energy losses and improve energy utilization efficiency.
Currently, while surface architecture now dominates photothermal performance optimization, significant challenges persist in achieving scalable and stable evaporation systems. For instance, although increasing the height of the sidewalls can reabsorb more dissipated energy and thus generate higher temperatures, the resulting high internal humidity within the solar absorber can inhibit steam generation [88]. Consequently, unresolved structural challenges threaten the operational sustainability of solar evaporation systems. (ii) In practical applications, large-scale desalination and freshwater production often require the implementation of evaporation unit matrices. Hong et al. [89] engineered a paper-based solar evaporator featuring periodic surface topography with alternating peaks and valleys (Figure 5d). They found that light capture performance and the energy recovered from heat loss increased with the increasing surface area density, leading to an increase in steam production, surpassing planar counterparts by 50%. Additionally, under both sunny and shady conditions, it was observed that the paper with a periodic surface structure exhibited an uneven spatial temperature distribution in a wet state, with the temperatures of valley folds always being higher than those of peak folds. Therefore, such concave structures are conducive to reducing light reflection, and the resulting reverse heat flux facilitates further reuse of thermal energy. Nature employs periodic surface architectures for photothermal efficiency, exemplified by Sun et al. [90] who leveraged carbonized sunflower heads—biomimetic radial structures achieving efficient solar vaporization. Due to the large number of three-dimensional cavities on its top surface, the evaporation efficiency reached up to ~100% by minimizing diffuse reflection and thermal radiation. Additionally, effective light capture can be achieved by hollowing out the initially compacted material to form periodic cavities. Therefore, Mizuno et al. [91] demonstrated through research that the light absorptance of the surface of a vertically aligned single-walled carbon nanotube (SWCNT) array (0.98) exhibits 29% greater absorptance than planar laminates (0.76). Thus, the photothermal efficiency in real-world evaporation systems depends critically on matrix topography optimization. Further exploration is needed into the density–shape relationship of solar absorber units and the system’s light absorptance and steam generation.
(2) Compensation for the effect of the incident angle: Despite laboratory success in thermal management and evaporation efficiency, additional parameters must be considered for water evaporation in outdoor environments, such as unstable solar intensity. Real-world solar absorption is limited to 50–90% due to atmospheric conditions: approximately 10–20% irradiance loss occurs under clear skies, escalating to 50% during cloud cover. Stochastic cloud movement further disrupts consistent energy flux [92]. In such situations, solar evaporation systems rarely have the opportunity to fully realize their potential. Li et al. [1] designed an umbrella-shaped evaporation device synthesized from a GO membrane and proposed that the three-dimensional device can capture sunlight from different incident angles. They found that the steam generation of the three-dimensional device was 1.43 times higher than that of the planar device. Chen et al. [92] designed a three-dimensional hemispherical solar-driven evaporation device to compensate for changes in the solar altitude angle. Under medium intensity (0.75 kW·m−2), when irradiating the three-dimensional hemispherical device at wide incident angles of 90, 80, and 65°, they found that its water evaporation rate and energy efficiency remained constant, while the performance of two-dimensional planar and cylindrical devices decreased sharply as the incident angle decreased. Bian et al. [93] prepared an evaporation system by carbonizing the tree-like hairy magnolia fruits. The study found that this evaporation system, through its large surface area and additional available space (interwoven pod-like peels), provides omnidirectional solar absorption and enables rapid steam escape, thereby increasing daily steam production of 3.15 kg·m−2·h−1 (Figure 5e). Overcoming changes in the solar angle is of great significance in practical applications. Early research primarily focused on the photothermal characteristics of the solar absorber layer, but some surfaces of the absorber inevitably fall into shadow, preventing them from fully harnessing their light absorption potential. Dynamic solar-tracking systems [94] theoretically achieve peak absorption but face structural fragility barriers in real-world environments.
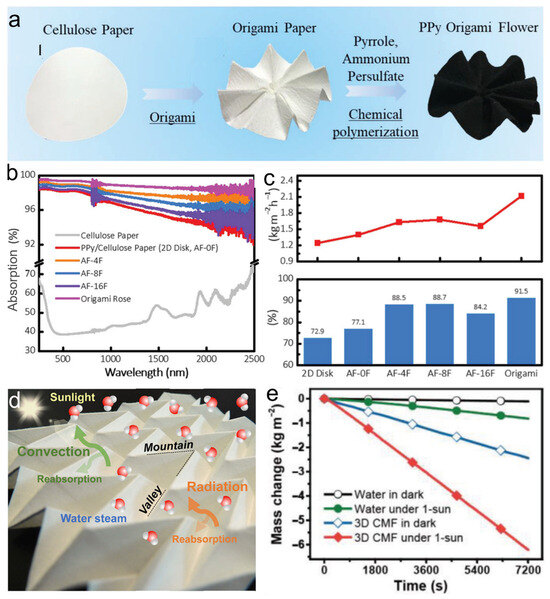

Figure 5.
(a) Fabrication process of PPy origami. (b) Optical absorption of cellulose paper and different PPy origami structures. (c) Solar steam water evaporation rate and solar–thermal energy conversion efficiency under 1 sun. Reproduced with permission [87]. Copyright 2019, Wiley-VCH. (d) Miura-ori tessellation for enhanced photothermal vaporization. Reproduced with permission [89]. Copyright 2018, American Chemical Society. (e) Mass change in water generation rate of 3D CMF in dark and under 1 sun. Reproduced with permission [93]. Copyright 2019, Wiley-VCH.
(3) Reducing or utilizing thermal radiation: Generally, an excellent solar absorber often needs to have a high solar absorptance and a low thermal emissivity, while a blackbody absorber has both high solar absorptance and high thermal emissivity. Therefore, when dealing with high-temperature steam, a spectrally selective absorbing surface can be used to replace the blackbody absorber to reduce thermal radiation losses while maintaining a high solar absorptance [95]. Studies have found that, assuming the absorber surface temperature is 100 °C and the ambient temperature is 20 °C, by replacing the blackbody absorber with a spectrally selective absorbing surface, radiant heat loss can be reduced from 680 to 50 W m−2 [19]. Thus, spectrally selective absorbing surfaces that reduce system energy losses by inhibiting thermal radiation have great potential [19]. According to Stefan–Boltzmann’s law, radiative heat loss scales as T4, dominating thermal dissipation in high-temperature systems. It is feasible to recover energy generated by long-wavelength thermal radiation from high-temperature absorbers. To achieve this goal, Wang et al. [96] developed a solar collector based on a reasonably thick, low-scattering silica aerogel layer integrated with a non-vacuum flat plate (Figure 6a). This device can recover thermal radiation by capturing long-wavelength radiation while maintaining high solar absorptance. However, the additional coating inevitably reduces transmittance and leads to a decrease in efficiency, thus prioritizing this strategy for systems dominated by radiative heat loss. Further research is needed for its application in evaporation systems.
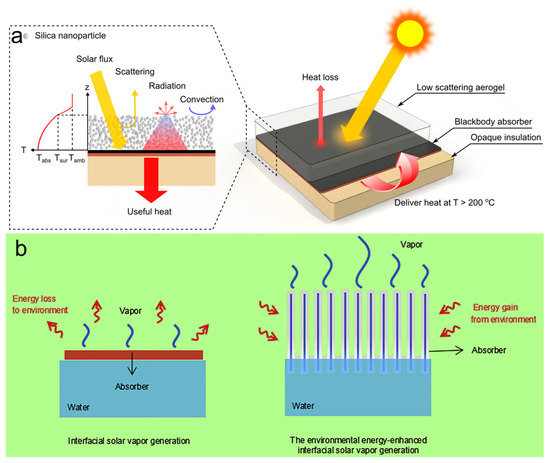

Figure 6.
(a). The aerogel allows sunlight to transmit through but blocks thermal radiation from the blackbody absorber by strong IR absorption. Reproduced with permission [96]. Copyright 2019, American Chemical Society. (b) Schematics of (left) the traditional interfacial solar vapor generator and (right) the environmental energy-enhanced interfacial solar vapor generator. Reproduced with permission [70]. Copyright 2018, Elsevier.
4.2. Limiting Heat Conduction
Reducing conductive heat loss—critical for evaporation enhancement—requires minimizing thermal conductivity in inherently conductive solar absorbers. The high thermal conductivity of solar absorbers makes a significant portion of the heat dissipate into the bulk water through heat conduction. To address this, reasonably coupling the solar absorber with a thermal insulation structure becomes a viable solution. Two primary strategies mitigate conductive heat losses: inserting a low-thermal-conductivity hydrophilic insulation layer beneath the absorber to decouple absorption from heat dissipation. These substrates, owing to their hydrophilic capillaries, can provide a sufficient water supply for evaporation while reducing downward heat conduction [97]. However, water-saturated hydrophilic substrates form 3D hydration networks but compromise insulation: water’s thermal conductivity (0.6 W·m−1·K−1) exceeds air’s (0.023 W·m−1·K−1) by 26×, enabling conductive losses through the wetted insulation layer. Another method is to adopt structures with limited water transport paths (two-dimensional and one-dimensional water paths) and thermal insulation layers. While this approach enables air-trapping insulation and controlled hydration to suppress conductive losses [98], it also sacrifices the salt rejection path, and limiting the water path can lead to salt accumulation issues. Therefore, when using limited water paths for seawater desalination applications, the continuing operational system stability must also be considered.
4.3. Increasing the Evaporation Rate
Water evaporation dissipates substantial thermal energy via the latent heat of vaporization, resulting in a phenomenon where the surface temperature is lower than the ambient temperature. For three-dimensional evaporators, the evaporation interface irradiated by sunlight typically has a higher temperature than the environment due to the photothermal conversion effect, while the dark surface is the opposite. Therefore, expanding the dark surface area enables radiative thermal regulation, potentially inverting the evaporator–environment temperature gradient and reversing net heat flux direction. Given this, researchers have initially explored the following three strategies: (1) increasing the side area of photothermal materials that are not exposed to sunlight; (2) introducing macroscopic structures on the evaporation surface; and (3) utilizing the inner surface for evaporation [99,100,101,102]. Zhu et al. [70] proposed the use of the lateral surface of an array of cylindrical evaporators to collect energy from the environment, thereby enhancing the evaporation rate (Figure 6b). For specific macrostructures, due to beam divergence, the surfaces at different heights receive varying intensities of sunlight, leading to the formation of distinct temperature zones. To address this, the evaporation rate can be accelerated by enhancing convective contributions from the surrounding environment. Typically, the evaporation process is determined by two consecutive steps: phase change and diffusion. Although conventional evaporators have a large specific surface area for the phase change process, a small internal surface area may hinder vapor diffusion. Therefore, activating the pore structure of the evaporator’s internal surface is crucial for optimizing vapor diffusion. Interconnected porous architectures simultaneously optimize the phase change interface and facilitate rapid vapor transport, achieving evaporation rates higher than the theoretical values. Although increasing the specific evaporation area to enhance the evaporation rates has been preliminarily validated as effective, most of the water vapor has a temperature lower than the surrounding environment and cannot condense into droplets [70]. Therefore, a key challenge in hypothermal evaporation systems is the disparity between highly efficient vapor generation and inefficient condensation. Optimizing system design for effective low-temperature vapor condensation is crucial to achieving productive desalination and water harvesting.
4.4. Creating a Hydrophilic Surface
Local thermal positioning of the photothermal interface is of great significance for gas–liquid interface heating, and the wettability of the photothermal interface is equally important for obtaining high-efficiency steam. Hydrophilic surfaces promote water spreading into thin films, whereas hydrophobic surfaces induce droplet beading [103]. Therefore, from the perspective of achieving high-efficiency water evaporation, hydrophilic surfaces are often the preferred design for photothermal interfaces, ensuring a higher evaporation area and adequate water supply. Studies have shown that, under identical roughness conditions, hydrophilic surfaces exhibit a significantly larger effective evaporation area than hydrophobic surfaces, resulting in an increased evaporation rate of up to 10% [104]. Notably, the thin hydrophilic film enhances thermal conduction, elevating the three-phase contact temperature above the surrounding regions. This surface property also sustains a continuous water supply from the bulk reservoir to the evaporation front, preventing interfacial dry-out and associated performance losses [74]. Research confirms hydrophobic evaporator interfaces repel underlying water, whereas hydrophilic materials drive spontaneous upward wicking via capillarity. Hydrophobic surfaces also promote vapor cavity formation beneath the evaporator, further restricting vertical liquid conveyance [105]. From the perspective of salt crystallization, hydrophobic evaporators can reduce salt adhesion, serving as an effective method to address salt deposition. Consequently, evaporator wettability must be application-tailored.
4.5. Reducing the Enthalpy of Evaporation
The energy absorbed per unit mass of liquid water during evaporation (latent heat of vaporization) is as high as 2455.6 kJ kg−1 (at ambient temperature) [106]. The high enthalpy of vaporization is typically caused by the strong interactions between water molecules, and, therefore, the evaporation rate of the system can be increased by reducing the enthalpy of water. One feasible strategy is to regulate the enthalpy of liquid water by controlling the state of water in the solar absorber [80]. Depending on the interaction strength between the solar absorber and surrounding molecules, water can exist in the states of free water (weak), intermediate water (moderate), or bound water (strong). Compared to bulk water in the bound state, intermediately bound water molecules have fewer intermolecular contacts and a weaker hydrogen bond network, resulting in a lower enthalpy of vaporization [104]. An alternative strategy involves evaporating water as molecular clusters rather than single molecules. Evidence suggests water within hydrogen-bonding networks preferentially evaporates as small clusters [80], requiring less energy to dissociate bonded units. This collective evaporation mechanism substantially lowers the enthalpy of vaporization. As a result, materials including hydrogels, aerogels, inorganic nanosheets, wood, and metals can evaporate in the form of water clusters, leading to enhanced water evaporation performance. Although it has been demonstrated that exceptional performance can be achieved by reducing the evaporation enthalpy, researchers still need to further consider and unify relevant experiments and calculations.
5. Conclusions and Prospective
In summary, the photothermal conversion mechanism of solar-driven interfacial water evaporation devices is summarized in detail. Meanwhile, we comprehensively report the recent design principles for photothermal materials and evaporators. It provides a detailed introduction to the design methods for photothermal materials and the structural design of solar evaporation devices, and analyzes and compares various strategies for improving the evaporation performance of the devices.
Despite significant achievements in the design of photothermal materials and device structures, the development and application of most evaporators are still at the laboratory stage, and outdoor practical applications are only at the prototype testing stage, with insufficient preparation for large-scale outdoor use. Several factors restrict their widespread use as follows: for instance, obtaining low-cost, high-efficiency photothermal materials remains challenging; the design of stable and salt-resistant evaporation devices with good anti-fouling properties is still relatively complex; furthermore, the collection efficiency of condensed water in practical applications is relatively low. Therefore, further in-depth research is needed to address the issues faced by solar water evaporation devices in practical applications. Specifically, considerations can be made from the following aspects.
- (1)
- Further development of photothermal materials. Semiconductor materials have high photothermal conversion efficiency but weak light absorption capacity, while carbon-based materials are the opposite, and metal nanoparticles and polymer materials have relatively high costs. Therefore, considering factors such as cost, light absorption rate, photothermal conversion efficiency, and the instability of light intensity during outdoor practical use, further research and development of photothermal materials are needed to enable them to have good light absorption capacity, excellent photothermal conversion ability under any light intensity (including low light intensity), and be obtainable through simple preparation processes.
- (2)
- Optimization and integrated design of evaporation devices. To enhance energy utilization efficiency, the interplay of various factors such as light absorption, water transport, thermal management, and salt tolerance and rejection capabilities should be considered when designing the structure of evaporators. Sometimes, there may be conflicting relationships among these factors. For instance, an increased water transport rate inevitably leads to higher energy losses, resulting in poorer thermal management capabilities of the evaporation device. Therefore, it is necessary to comprehensively consider all factors in the design of evaporation devices to maintain a balance in device performance. This requires researchers to have a thorough understanding of the theoretical mechanisms underlying interfacial evaporation, which is crucial for optimizing and integrating interfacial solar evaporators. Additionally, it is essential to comprehensively consider potential issues that may arise when constructing and deploying evaporation devices outdoors on a large scale.
- (3)
- Stability enhancement of evaporation devices. When solar evaporators are applied to desalination, the stability and service life of the evaporators need to be considered. In current research reports, the investigation of the device’s salt resistance/anti-scaling performance is not sufficient, and the salt resistance of some devices still needs further improvement. To ensure that the photothermal materials do not detach and release into the environment during long-term use, it is necessary to develop solar evaporators with biocompatibility, good corrosion resistance, and structural stability, and ensure their long-term usability.
- (4)
- Techno-economic analysis and practical application challenges. Research topics on interfacial solar evaporation typically focus on experimental results, and challenges still exist in terms of scaling up and practical applications [18]. As a result, economic analysis is not always conducted. Consequently, the cost-effectiveness of evaporators is not regularly obvious. Economic analysis is fundamental when expanding interfacial solar evaporation systems, encompassing materials, manufacturing, and operational costs [107]. Considering factors such as the structure, shape, substrate type, and salt resistance of interfacial solar evaporation systems, biomass-based evaporators, compared to other materials [108], have the lowest cost due to their relatively lower prices.
In general, for this field, both fundamental research and practical exploration are indispensable. We look forward to the eventual commercialization of this green, clean, and sustainable solar evaporation device to serve human life.
Author Contributions
Writing—review and editing, Y.P.; supervision, Y.P.; conceptualization, investigation, S.J., Z.Z., M.Z., Z.Y., Y.F. and L.C.; supervision, J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Shandong Provincial Natural Science Foundation, grant number ZR2022QB079.
Data Availability Statement
The original contributions presented in this study are included in the article.
Conflicts of Interest
Authors Shunjian Ji, Zhihong Zhang, and Juan Zhang were employed by Shanghai Power Equipment Research Institute Co., Ltd.; authors Meijie Zhang, Zexin Yang, and Yaguang Fan were employed by Wucaiwan Power Generation Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Li, X.; Lin, R.; Ni, G.; Xu, N.; Hu, X.; Zhu, B.; Lv, G.; Li, J.; Zhu, S.; Zhu, J. Three-Dimensional Artificial Transpiration for Efficient Solar Waste-Water Treatment. Natl. Sci. Rev. 2018, 5, 70–77. [Google Scholar] [CrossRef]
- Jesus, J.M.; Cassoni, A.C.; Danko, A.S.; Fiúza, A.; Borges, M.-T. Role of Three Different Plants on Simultaneous Salt and Nutrient Reduction from Saline Synthetic Wastewater in Lab-Scale Constructed Wetlands. Sci. Total Environ. 2017, 579, 447–455. [Google Scholar] [CrossRef]
- Foglia, A.; Akyol, Ç.; Frison, N.; Katsou, E.; Eusebi, A.L.; Fatone, F. Long-Term Operation of a Pilot-Scale Anaerobic Membrane Bioreactor (AnMBR) Treating High Salinity Low Loaded Municipal Wastewater in Real Environment. Sep. Purif. Technol. 2020, 236, 116279. [Google Scholar] [CrossRef]
- Voutchkov, N. Energy Use for Membrane Seawater Desalination—Current Status and Trends. Desalination 2018, 431, 2–14. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, H.; Kang, Z.; Fan, J. 3D Cellular Solar Crystallizer for Stable and Ultra-efficient High-salinity Wastewater Treatment. Adv. Sci. 2024, 11, 2305313. [Google Scholar] [CrossRef]
- Gu, R.; Yu, Z.; Su, Y.; Li, Y.; Cheng, S. High-Salinity Brine Desalination and Wastewater Treatment Strategies Based on Solar-Driven Interfacial Evaporators. Sep. Purif. Technol. 2023, 322, 124322. [Google Scholar] [CrossRef]
- Lu, Y.; Fan, D.; Shen, Z.; Zhang, H.; Xu, H.; Yang, X. Design and Performance Boost of a MOF-Functionalized-Wood Solar Evaporator through Tuning the Hydrogen-Bonding Interactions. Nano Energy 2022, 95, 107016. [Google Scholar] [CrossRef]
- Sheng, M.; Yang, Y.; Bin, X.; Zhao, S.; Pan, C.; Nawaz, F.; Que, W. Recent Advanced Self-Propelling Salt-Blocking Technologies for Passive Solar-Driven Interfacial Evaporation Desalination Systems. Nano Energy 2021, 89, 106468. [Google Scholar] [CrossRef]
- Li, H.; Yan, Z.; Li, Y.; Hong, W. Latest Development in Salt Removal from Solar-Driven Interfacial Saline Water Evaporators: Advanced Strategies and Challenges. Water Res. 2020, 177, 115770. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Gao, Y.; Zhao, H.; Zhou, W. Seawater Toilet Flushing Sewage Treatment and Nutrients Recovery by Marine Bacterial-Algal Mutualistic System. Chemosphere 2018, 195, 70–79. [Google Scholar] [CrossRef]
- Liu, X.; Dai, J.; Wu, D.; Jiang, F.; Chen, G.; Chui, H.-K.; van Loosdrecht, M.C.M. Sustainable Application of a Novel Water Cycle Using Seawater for Toilet Flushing. Engineering 2016, 2, 460–469. [Google Scholar] [CrossRef]
- Zhang, C.; Liang, H.; Xu, Z.; Wang, Z. Harnessing Solar-driven Photothermal Effect toward the Water–Energy Nexus. Adv. Sci. 2019, 6, 1900883. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, S.; Jin, B.; Yu, Z.; Gu, R. Extendable Solar Interfacial Evaporator with High Salt Resistance Achieved by Managing the Evaporation Region. Sol. RRL 2023, 7, 2300242. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, S.; Yu, Z.; Gu, R.; Li, Y.; Chen, H. Enhanced Evaporation Performance of Solar Interface Evaporator by Modifying the Evaporation Layer Surface with Hydrophobic Fumed Silica. J. Clean. Prod. 2023, 392, 136302. [Google Scholar] [CrossRef]
- Genduso, G.; Missinne, A.; Ali, Z.; Ogieglo, W.; Van der Bruggen, B.; Pinnau, I. Hydrophobic Polydimethylsiloxane Thin-Film Composite Membranes for the Efficient Pervaporative Desalination of Seawater and Brines. Sep. Purif. Technol. 2022, 280, 119819. [Google Scholar] [CrossRef]
- Wang, W.; Aleid, S.; Shi, Y.; Zhang, C.; Li, R.; Wu, M.; Zhuo, S.; Wang, P. Integrated Solar-Driven PV Cooling and Seawater Desalination with Zero Liquid Discharge. Joule 2021, 5, 1873–1887. [Google Scholar] [CrossRef]
- Ghenai, C.; Kabakebji, D.; Douba, I.; Yassin, A. Performance Analysis and Optimization of Hybrid Multi-Effect Distillation Adsorption Desalination System Powered with Solar Thermal Energy for High Salinity Sea Water. Energy 2021, 215, 119212. [Google Scholar] [CrossRef]
- Tao, P.; Ni, G.; Song, C.; Shang, W.; Wu, J.; Zhu, J.; Chen, G.; Deng, T. Solar-Driven Interfacial Evaporation. Nat. Energy 2018, 3, 1031–1041. [Google Scholar] [CrossRef]
- Ni, G.; Li, G.; Boriskina, S.V.; Li, H.; Yang, W.; Zhang, T.; Chen, G. Steam Generation under One Sun Enabled by a Floating Structure with Thermal Concentration. Nat. Energy 2016, 1, 16126. [Google Scholar] [CrossRef]
- Gu, R.; Yu, Z.; Sun, Y.; Xie, P.; Li, Y.; Cheng, S. Enhancing Stability of Interfacial Solar Evaporator in High-Salinity Solutions by Managing Salt Precipitation with Janus-Based Directional Salt Transfer Structure. Desalination 2022, 524, 115470. [Google Scholar] [CrossRef]
- Sharon, H.; Reddy, K.S. A Review of Solar Energy Driven Desalination Technologies. Renew. Sustain. Energy Rev. 2015, 41, 1080–1118. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Hashaikeh, R.; Hilal, N. Solar Powered Desalination—Technology, Energy and Future Outlook. Desalination 2019, 453, 54–76. [Google Scholar] [CrossRef]
- Aytaç, E.; Ahmed, F.E.; Aziz, F.; Khayet, M.; Hilal, N. A Metadata Survey of Photothermal Membranes for Solar-Driven Membrane Distillation. Sep. Purif. Technol. 2025, 364, 132565. [Google Scholar] [CrossRef]
- Lv, F.; Miao, J.; Hu, J.; Mantecon, D.O. 3D Solar Evaporation Enhancement by Superhydrophilic Copper Foam Inverted Cone and Graphene Oxide Functionalization Synergistic Cooperation. Small 2023, 19, 2208137. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.W.; Park, S.H.; Lee, S.J. 3D Thermoresponsive Hydrogel with Enhanced Water Uptake and Active Evaporation for Effective Interfacial Solar Steam Generation. Desalination 2023, 550, 116368. [Google Scholar] [CrossRef]
- Liu, X.; Chen, F.; Li, Y.; Jiang, H.; Mishra, D.D.; Yu, F.; Chen, Z.; Hu, C.; Chen, Y.; Qu, L.; et al. 3D Hydrogel Evaporator with Vertical Radiant Vessels Breaking the Trade-off between Thermal Localization and Salt Resistance for Solar Desalination of High-salinity. Adv. Mater. 2022, 34, 2203137. [Google Scholar] [CrossRef]
- Zhao, W.; Gong, H.; Song, Y.; Li, B.; Xu, N.; Min, X.; Liu, G.; Zhu, B.; Zhou, L.; Zhang, X.; et al. Hierarchically Designed Salt-resistant Solar Evaporator Based on Donnan Effect for Stable and High-performance Brine Treatment. Adv. Funct. Mater. 2021, 31, 2100025. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, H.; Yin, Z.; Zhao, J.; Yin, X.; Li, N.; Yin, D.; Li, Y.; Lei, B.; Du, Y.; et al. A General Salt-Resistant Hydrophilic/Hydrophobic Nanoporous Double Layer Design for Efficient and Stable Solar Water Evaporation Distillation. Mater. Horiz. 2018, 5, 1143–1150. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, X.; Guo, P.; Yao, X.; Cong, H.; Xu, B. Solar Interfacial Evaporation at the Water–Energy Nexus: Bottlenecks, Approaches, and Opportunities. Sol. RRL 2023, 7, 2201098. [Google Scholar] [CrossRef]
- Lei, C.; Guan, W.; Guo, Y.; Shi, W.; Wang, Y.; Johnston, K.P.; Yu, G. Polyzwitterionic Hydrogels for Highly Efficient High Salinity Solar Desalination. Angew. Chem. Int. Ed. 2022, 61, e202208487. [Google Scholar] [CrossRef]
- Li, L.; He, N.; Jiang, B.; Yu, K.; Zhang, Q.; Zhang, H.; Tang, D.; Song, Y. Highly Salt-resistant 3D Hydrogel Evaporator for Continuous Solar Desalination via Localized Crystallization. Adv. Funct. Mater. 2021, 31, 2104380. [Google Scholar] [CrossRef]
- Chen, C.; Kuang, Y.; Hu, L. Challenges and Opportunities for Solar Evaporation. Joule 2019, 3, 683–718. [Google Scholar] [CrossRef]
- Neumann, O.; Urban, A.S.; Day, J.; Lal, S.; Nordlander, P.; Halas, N.J. Solar Vapor Generation Enabled by Nanoparticles. ACS Nano 2013, 7, 42–49. [Google Scholar] [CrossRef]
- Wu, X.; Chen, G.Y.; Owens, G.; Chu, D.; Xu, H. Photothermal Materials: A Key Platform Enabling Highly Efficient Water Evaporation Driven by Solar Energy. Mater. Today Energy 2019, 12, 277–296. [Google Scholar] [CrossRef]
- Wang, W.; Cai, Y.; Du, M.; Hou, X.; Liu, J.; Ke, H.; Wei, Q. Ultralight and Flexible Carbon Foam-Based Phase Change Composites with High Latent-Heat Capacity and Photothermal Conversion Capability. ACS Appl. Mater. Interfaces 2019, 11, 31997–32007. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-F.; Li, Y.-Z.; Xi, Y.-A.-M.; Xu, J.-L.; Zhang, Y. Key Technology Developments for Solar-Driven Interface Evaporation on Structural Innovation and Thermal Design. Nano Energy 2024, 132, 110369. [Google Scholar] [CrossRef]
- Asghar, M.S.; Arshad, N.; Tao, J.; Irshad, M.S.; Li, J.; Wang, X. Recent Advances in Multifunctional Photothermal Materials for Solar-driven Steam and Energy Generation. Energy Technol. 2023, 11, 2300500. [Google Scholar] [CrossRef]
- Xia, Y.; Li, Y.; Yuan, S.; Kang, Y.; Jian, M.; Hou, Q.; Gao, L.; Wang, H.; Zhang, X. A Self-Rotating Solar Evaporator for Continuous and Efficient Desalination of Hypersaline Brine. J. Mater. Chem. A 2020, 8, 16212–16217. [Google Scholar] [CrossRef]
- Li, J.; Jing, Y.; Xing, G.; Liu, M.; Cui, Y.; Sun, H.; Zhu, Z.; Liang, W.; Li, A. Solar-Driven Interfacial Evaporation for Water Treatment: Advanced Research Progress and Challenges. J. Mater. Chem. A 2022, 10, 18470–18489. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Z.; Chen, X.; Ji, J.; Zhang, L.; Meng, C. Advancing Robust All-Weather Desalination: A Critical Review of Emerging Photothermal Evaporators and Hybrid Systems. Commun. Mater. 2025, 6, 29. [Google Scholar] [CrossRef]
- Baffou, G.; Quidant, R.; Girard, C. Heat Generation in Plasmonic Nanostructures: Influence of Morphology. Appl. Phys. Lett. 2009, 94, 153109. [Google Scholar] [CrossRef]
- Huang, P.; Lin, J.; Li, W.; Rong, P.; Wang, Z.; Wang, S.; Wang, X.; Sun, X.; Aronova, M.; Niu, G.; et al. Biodegradable Gold Nanovesicles with an Ultrastrong Plasmonic Coupling Effect for Photoacoustic Imaging and Photothermal Therapy. Angew. Chem. Int. Ed. 2013, 52, 13958–13964. [Google Scholar] [CrossRef]
- Zhu, L.; Gao, M.; Peh, C.K.N.; Ho, G.W. Solar-Driven Photothermal Nanostructured Materials Designs and Prerequisites for Evaporation and Catalysis Applications. Mater. Horiz. 2018, 5, 323–343. [Google Scholar] [CrossRef]
- Bae, K.; Kang, G.; Cho, S.K.; Park, W.; Kim, K.; Padilla, W.J. Flexible Thin-Film Black Gold Membranes with Ultrabroadband Plasmonic Nanofocusing for Efficient Solar Vapour Generation. Nat. Commun. 2015, 6, 10103. [Google Scholar] [CrossRef]
- Marimuthu, A.; Zhang, J.; Linic, S. Tuning Selectivity in Propylene Epoxidation by Plasmon Mediated Photo-Switching of Cu Oxidation State. Science 2013, 339, 1590–1593. [Google Scholar] [CrossRef]
- Lalisse, A.; Tessier, G.; Plain, J.; Baffou, G. Quantifying the Efficiency of Plasmonic Materials for Near-Field Enhancement and Photothermal Conversion. J. Phys. Chem. C 2015, 119, 25518–25528. [Google Scholar] [CrossRef]
- Clark, B.D.; Jacobson, C.R.; Lou, M.; Yang, J.; Zhou, L.; Gottheim, S.; DeSantis, C.J.; Nordlander, P.; Halas, N.J. Aluminum Nanorods. Nano Lett. 2018, 18, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Tao, F.; Green, M.; Garcia, A.V.; Xiao, T.; Van Tran, A.T.; Zhang, Y.; Yin, Y.; Chen, X. Recent Progress of Nanostructured Interfacial Solar Vapor Generators. Appl. Mater. Today 2019, 17, 45–84. [Google Scholar] [CrossRef]
- Li, J.; Zhou, X.; Chen, G.; Wang, F.; Mao, J.; Long, Y.; Sun, H.; Zhu, Z.; Liang, W.; Li, A. Evaporation Efficiency Monitoring Device Based on Biomass Photothermal Material for Salt-Resistant Solar-Driven Interfacial Evaporation. Sol. Energy Mater. Sol. Cells 2021, 222, 110941. [Google Scholar] [CrossRef]
- Su, L.; Hu, Y.; Ma, Z.; Miao, L.; Zhou, J.; Ning, Y.; Chang, Z.; Wu, B.; Cao, M.; Xia, R.; et al. Synthesis of Hollow Copper Sulfide Nanocubes with Low Emissivity for Highly Efficient Solar Steam Generation. Sol. Energy Mater. Sol. Cells 2020, 210, 110484. [Google Scholar] [CrossRef]
- Sheikh, M.; Pazirofteh, M.; Dehghani, M.; Asghari, M.; Rezakazemi, M.; Valderrama, C.; Cortina, J.-L. Application of ZnO Nanostructures in Ceramic and Polymeric Membranes for Water and Wastewater Technologies: A Review. Chem. Eng. J. 2020, 391, 123475. [Google Scholar] [CrossRef]
- Yang, Y.; Que, W.; Zhao, J.; Han, Y.; Ju, M.; Yin, X. Membrane Assembled from Anti-Fouling Copper-Zinc-Tin-Selenide Nanocarambolas for Solar-Driven Interfacial Water Evaporation. Chem. Eng. J. 2019, 373, 955–962. [Google Scholar] [CrossRef]
- Dao, V.; Choi, H. Carbon-based Sunlight Absorbers in Solar-driven Steam Generation Devices. Glob. Chall. 2018, 2, 1700094. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhao, F.; Guo, Y.; Zhang, Y.; Yu, G. A Hydrogel-Based Antifouling Solar Evaporator for Highly Efficient Water Desalination. Energy Environ. Sci. 2018, 11, 1985–1992. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, G.; Mei, T.; Li, J.; Wang, J.; Wang, X. Accessible Graphene Aerogel for Efficiently Harvesting Solar Energy. ACS Sustain. Chem. Eng. 2017, 5, 4665–4671. [Google Scholar] [CrossRef]
- Han, S.; Yang, J.; Li, X.; Li, W.; Zhang, X.; Koratkar, N.; Yu, Z.-Z. Flame Synthesis of Superhydrophilic Carbon Nanotubes/Ni Foam Decorated with Fe2O3 Nanoparticles for Water Purification via Solar Steam Generation. ACS Appl. Mater. Interfaces 2020, 12, 13229–13238. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, B.; Ma, Y.; Huang, Y.; Li, N.; Zhang, F.; Chen, Y. Efficient and Large-Scale Synthesis of Few-Layered Graphene Using an Arc-Discharge Method and Conductivity Studies of the Resulting Films. Nano Res. 2010, 3, 661–669. [Google Scholar] [CrossRef]
- Li, C.; Li, Z.; Zhu, H.; Wang, K.; Wei, J.; Li, X.; Sun, P.; Zhang, H.; Wu, D. Graphene nano-“patches” on a carbon nanotube network for highly transparent/conductive thin film applications. J. Phys. Chem. C 2010, 114, 14008–14012. [Google Scholar] [CrossRef]
- Yudasaka, M.; Komatsu, T.; Ichihashi, T.; Iijima, S. Single-Wall Carbon Nanotube Formation by Laser Ablation Using Double-Targets of Carbon and Metal. Chem. Phys. Lett. 1997, 278, 102–106. [Google Scholar] [CrossRef]
- Xue, G.; Liu, K.; Chen, Q.; Yang, P.; Li, J.; Ding, T.; Duan, J.; Qi, B.; Zhou, J. Robust and Low-Cost Flame-Treated Wood for High-Performance Solar Steam Generation. ACS Appl. Mater. Interfaces 2017, 9, 15052–15057. [Google Scholar] [CrossRef]
- Xu, N.; Hu, X.; Xu, W.; Li, X.; Zhou, L.; Zhu, S.; Zhu, J. Mushrooms as Efficient Solar Steam-generation Devices. Adv. Mater. 2017, 29, 1606762. [Google Scholar] [CrossRef]
- Bian, Y.; Du, Q.; Tang, K.; Shen, Y.; Hao, L.; Zhou, D.; Wang, X.; Xu, Z.; Zhang, H.; Zhao, L.; et al. Carbonized Bamboos as Excellent 3D Solar Vapor-generation Devices. Adv. Mater. Technol. 2019, 4, 1800593. [Google Scholar] [CrossRef]
- Ibrahim, I.; Bhoopal, V.; Seo, D.H.; Afsari, M.; Shon, H.K.; Tijing, L.D. Biomass-Based Photothermal Materials for Interfacial Solar Steam Generation: A Review. Mater. Today Energy 2021, 21, 100716. [Google Scholar] [CrossRef]
- Fillet, R.; Nicolas, V.; Fierro, V.; Celzard, A. A Review of Natural Materials for Solar Evaporation. Sol. Energy Mater. Sol. Cells 2021, 219, 110814. [Google Scholar] [CrossRef]
- Li, Y.; Shi, Y.; Wang, H.; Liu, T.; Zheng, X.; Gao, S.; Lu, J. Recent Advances in Carbon-based Materials for Solar-driven Interfacial Photothermal Conversion Water Evaporation: Assemblies, Structures, Applications, and Prospective. Carbon Energy 2023, 5, e331. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, B.; Wu, J.; Li, R.; Wang, P. Hydrophobic Light-to-Heat Conversion Membranes with Self-Healing Ability for Interfacial Solar Heating. Adv. Mater. Deerfield 2015, 27, 4889–4894. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ozden, S.; Bizmark, N.; Arnold, C.B.; Datta, S.S.; Priestley, R.D. A Bioinspired Elastic Hydrogel for Solar-driven Water Purification. Adv. Mater. 2021, 33, 2007833. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, L.; Yang, F.; Bai, W.; Zhang, X.; Li, H.; Duan, G.; Xu, Y.; Li, Y. A Bioinspired Antibacterial and Photothermal Membrane for Stable and Durable Clean Water Remediation. Mater. Horiz. 2023, 10, 268–276. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, S.; Wen, B.; Su, Z. Sugarcane Inspired Polydopamine @ Attapulgite-Based Aerogels with Vertical Pore Structure for Solar-Driven Steam Generation. Sep. Purif. Technol. 2023, 321, 124188. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Lu, J.; Xu, N.; Chen, C.; Min, X.; Zhu, B.; Li, H.; Zhou, L.; Zhu, S.; et al. Enhancement of Interfacial Solar Vapor Generation by Environmental Energy. Joule 2018, 2, 1331–1338. [Google Scholar] [CrossRef]
- Menon, A.K.; Haechler, I.; Kaur, S.; Lubner, S.; Prasher, R.S. Enhanced Solar Evaporation Using a Photo-Thermal Umbrella for Wastewater Management. Nat. Sustain. 2020, 3, 144–151. [Google Scholar] [CrossRef]
- Luo, Y.; Yao, X.; Zhao, L.; Liu, L.; Zhou, Y.; Jiang, L.; Ju, J. Lubricant-interface Induced Mobile Crystallization for Hypersaline Wastewater Management. Adv. Funct. Mater. 2023, 33, 2301086. [Google Scholar] [CrossRef]
- Sun, S.; Shi, C.; Kuang, Y.; Li, M.; Li, S.; Chan, H.; Zhang, S.; Chen, G.; Nilghaz, A.; Cao, R.; et al. 3D-Printed Solar Evaporator with Seashell Ornamentation-Inspired Structure for Zero Liquid Discharge Desalination. Water Res. 2022, 226, 119279. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, Y.; Wu, P.; Zhao, J.; Lu, Y.; Yang, X.; Xu, H. Dual-zone Photothermal Evaporator for Antisalt Accumulation and Highly Efficient Solar Steam Generation. Adv. Funct. Mater. 2021, 31, 2102618. [Google Scholar] [CrossRef]
- Dong, X.; Li, H.; Gao, L.; Chen, C.; Shi, X.; Du, Y.; Deng, H. Janus Fibrous Mats Based Suspended Type Evaporator for Salt Resistant Solar Desalination and Salt Recovery. Small 2022, 18, 2107156. [Google Scholar] [CrossRef]
- Xu, W.; Hu, X.; Zhuang, S.; Wang, Y.; Li, X.; Zhou, L.; Zhu, S.; Zhu, J. Flexible and Salt Resistant Janus Absorbers by Electrospinning for Stable and Efficient Solar Desalination. Adv. Energy Mater. 2018, 8, 1702884. [Google Scholar] [CrossRef]
- Huang, Z.; Wei, J.; Wan, Y.; Li, P.; Yu, J.; Dong, J.; Wang, S.; Li, S.; Lee, C. Aligned Millineedle Arrays for Solar Power Seawater Desalination with Site-specific Salt Formation. Small 2021, 17, 2101487. [Google Scholar] [CrossRef]
- Gu, Y.; Mu, X.; Wang, P.; Wang, X.; Liu, J.; Shi, J.; Wei, A.; Tian, Y.; Zhu, G.; Xu, H.; et al. Integrated Photothermal Aerogels with Ultrahigh-Performance Solar Steam Generation. Nano Energy 2020, 74, 104857. [Google Scholar] [CrossRef]
- Zhao, F.; Guo, Y.; Zhou, X.; Shi, W.; Yu, G. Materials for Solar-Powered Water Evaporation. Nat. Rev. Mater. 2020, 5, 388–401. [Google Scholar] [CrossRef]
- Zhao, F.; Zhou, X.; Shi, Y.; Qian, X.; Alexander, M.; Zhao, X.; Mendez, S.; Yang, R.; Qu, L.; Yu, G. Highly Efficient Solar Vapour Generation via Hierarchically Nanostructured Gels. Nat. Nanotechnol. 2018, 13, 489–495. [Google Scholar] [CrossRef]
- Guo, Y.; Lu, H.; Zhao, F.; Zhou, X.; Shi, W.; Yu, G. Biomass-derived Hybrid Hydrogel Evaporators for Cost-effective Solar Water Purification. Adv. Mater. 2020, 32, 1907061. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, F.; Guo, Y.; Rosenberger, B.; Yu, G. Architecting Highly Hydratable Polymer Networks to Tune the Water State for Solar Water Purification. Sci. Adv. 2019, 5, eaaw5484. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Q.; Wu, S.; Xu, B.; Xu, H. Multilayer Polypyrrole Nanosheets with Self-organized Surface Structures for Flexible and Efficient Solar–Thermal Energy Conversion. Adv. Mater. 2019, 31, 1807716. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, X.; Yang, X.; Owens, G.; Xu, H. Reversing Heat Conduction Loss: Extracting Energy from Bulk Water to Enhance Solar Steam Generation. Nano Energy 2020, 78, 105269. [Google Scholar] [CrossRef]
- Xia, Y.; Kang, Y.; Wang, Z.; Yuan, S.; Li, Y.; Gao, L.; Wang, H.; Zhang, X. Rational Designs of Interfacial-Heating Solar-Thermal Desalination Devices: Recent Progress and Remaining Challenges. J. Mater. Chem. A 2021, 9, 6612–6633. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Song, X.; Huang, M.; Megarajan, S.K.; Shaukat, S.F.; Jiang, H. Improved Light-Harvesting and Thermal Management for Efficient Solar-Driven Water Evaporation Using 3D Photothermal Cones. J. Mater. Chem. A 2018, 6, 9874–9881. [Google Scholar] [CrossRef]
- Li, W.; Li, Z.; Bertelsmann, K.; Fan, D.E. Portable Low-pressure Solar Steaming-collection Unisystem with Polypyrrole Origamis. Adv. Mater. 2019, 31, 1900720. [Google Scholar] [CrossRef] [PubMed]
- Cooper, T.A.; Zandavi, S.H.; Ni, G.W.; Tsurimaki, Y.; Huang, Y.; Boriskina, S.V.; Chen, G. Contactless Steam Generation and Superheating under One Sun Illumination. Nat. Commun. 2018, 9, 5086. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Shi, Y.; Li, R.; Zhang, C.; Jin, Y.; Wang, P. Nature-Inspired, 3D Origami Solar Steam Generator toward near Full Utilization of Solar Energy. ACS Appl. Mater. Interfaces 2018, 10, 28517–28524. [Google Scholar] [CrossRef]
- Sun, P.; Zhang, W.; Zada, I.; Zhang, Y.; Gu, J.; Liu, Q.; Su, H.; Pantelić, D.; Jelenković, B.; Zhang, D. 3D-Structured Carbonized Sunflower Heads for Improved Energy Efficiency in Solar Steam Generation. ACS Appl. Mater. Interfaces 2020, 12, 2171–2179. [Google Scholar] [CrossRef]
- Mizuno, K.; Ishii, J.; Kishida, H.; Hayamizu, Y.; Yasuda, S.; Futaba, D.N.; Yumura, M.; Hata, K. A Black Body Absorber from Vertically Aligned Single-Walled Carbon Nanotubes. Proc. Natl. Acad. Sci. USA 2009, 106, 6044–6047. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, N.; Li, N.; Yu, L.; Xu, X. A 3D Hemispheric Steam Generator Based on an Organic–Inorganic Composite Light Absorber for Efficient Solar Evaporation and Desalination. Adv. Mater. Interfaces 2020, 7, 1901715. [Google Scholar] [CrossRef]
- Bian, Y.; Shen, Y.; Tang, K.; Du, Q.; Hao, L.; Liu, D.; Hao, J.; Zhou, D.; Wang, X.; Zhang, H.; et al. Carbonized Tree-like Furry Magnolia Fruit-based Evaporator Replicating the Feat of Plant Transpiration. Glob. Chall. 2019, 3, 1900040. [Google Scholar] [CrossRef]
- He, C.; Li, Z.; Zhao, P.; Jiang, H.; Zhou, Z.; Gao, R.; La, P.; Wang, L.; Gao, X. High-efficiency Solar Selective Absorber: Using the High-entropy Nitride MoTaTiCrN Nanoceramics as a Perspective Strategy. EcoEnergy 2025, 3, 156–169. [Google Scholar] [CrossRef]
- Cao, F.; McEnaney, K.; Chen, G.; Ren, Z. A Review of Cermet-Based Spectrally Selective Solar Absorbers. Energy Environ. Sci. 2014, 7, 1615–1627. [Google Scholar] [CrossRef]
- Zhao, L.; Bhatia, B.; Yang, S.; Strobach, E.; Weinstein, L.A.; Cooper, T.A.; Chen, G.; Wang, E.N. Harnessing Heat beyond 200 °C from Unconcentrated Sunlight with Nonevacuated Transparent Aerogels. ACS Nano 2019, 13, 7508–7516. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tian, Y.; Caratenuto, A.; Chen, F.; Zheng, Y. Biomass-based Materials for Sustainably Sourced Solar-driven Interfacial Steam Generation. Adv. Eng. Mater. 2023, 25, 2300778. [Google Scholar] [CrossRef]
- Meng, S.; Zhao, X.; Tang, C.-Y.; Yu, P.; Bao, R.-Y.; Liu, Z.-Y.; Yang, M.-B.; Yang, W. A Bridge-Arched and Layer-Structured Hollow Melamine Foam/Reduced Graphene Oxide Composite with an Enlarged Evaporation Area and Superior Thermal Insulation for High-Performance Solar Steam Generation. J. Mater. Chem. A 2020, 8, 2701–2711. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Lin, Z.; Xu, N.; Li, X.; Liang, J.; Zhao, W.; Lin, R.; Zhu, B.; Liu, G.; et al. Over 10 kg m−2 h−1 Evaporation Rate Enabled by a 3D Interconnected Porous Carbon Foam. Joule 2020, 4, 928–937. [Google Scholar] [CrossRef]
- Xu, Y.; Tang, C.; Ma, J.; Liu, D.; Qi, D.; You, S.; Cui, F.; Wei, Y.; Wang, W. Low-Tortuosity Water Microchannels Boosting Energy Utilization for High Water Flux Solar Distillation. Environ. Sci. Technol. 2020, 54, 5150–5158. [Google Scholar] [CrossRef]
- Xu, W.; Xing, Y.; Liu, J.; Wu, H.; Cui, Y.; Li, D.; Guo, D.; Li, C.; Liu, A.; Bai, H. Efficient Water Transport and Solar Steam Generation via Radially, Hierarchically Structured Aerogels. ACS Nano 2019, 13, 7930–7938. [Google Scholar] [CrossRef]
- Song, H.; Liu, Y.; Liu, Z.; Singer, M.H.; Li, C.; Cheney, A.R.; Ji, D.; Zhou, L.; Zhang, N.; Zeng, X.; et al. Cold Vapor Generation beyond the Input Solar Energy Limit. Adv. Sci. 2018, 5, 1800222. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, F.; Zhou, X.; Chen, Z.; Yu, G. Tailoring Nanoscale Surface Topography of Hydrogel for Efficient Solar Vapor Generation. Nano Lett. 2019, 19, 2530–2536. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, Y.; Yang, C.; Tian, Y.; Han, Y.; Liu, J.; Yin, X.; Que, W. A Hydrophobic Surface Enabled Salt-Blocking 2D Ti3C2 MXene Membrane for Efficient and Stable Solar Desalination. J. Mater. Chem. A 2018, 6, 16196–16204. [Google Scholar] [CrossRef]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel Slopes from a Microkinetic Analysis of Aqueous Electrocatalysis for Energy Conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Song, H.; Ji, D.; Li, C.; Cheney, A.; Liu, Y.; Zhang, N.; Zeng, X.; Chen, B.; Gao, J.; et al. Extremely Cost-effective and Efficient Solar Vapor Generation under Nonconcentrated Illumination Using Thermally Isolated Black Paper. Glob. Chall. 2017, 1, 1600003. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Wang, P.; Che, H.; Chen, J.; Liu, B.; Ao, Y. Solar-Driven Interfacial Water Evaporation for Wastewater Purification: Recent Advances and Challenges. Chem. Eng. J. 2023, 477, 147158. [Google Scholar] [CrossRef]
- AlMallahi, M.N.; Selim, M.Y.E.; Elgendi, M. Interfacial Solar Evaporation Using Biomass: Environmental Impact, Financial Feasibility, and a Bibliometric Perspective. Desalination 2025, 614, 119160. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).