Ultrasound-Assisted Extraction Processes of Benzopyrans from Hypericum polyanthemum: COSMO-RS Prediction and Mass Transfer Modeling
Abstract
1. Introduction
2. Materials and Methods
2.1. Solubility Screening of Target Compounds in DES
Geometry Optimization of Molecules
2.2. Experimental Extraction
2.2.1. DES Preparation
2.2.2. Plant Material
2.3. Ultrasound-Assisted Extraction from H. polyanthenum
2.4. Analysis of the Extracts
2.4.1. Total Phenolic Content
2.4.2. Quantitative Analysis of Benzopyrans
2.5. Mathematical Modeling
2.6. Statistical Analysis
3. Results and Discussion
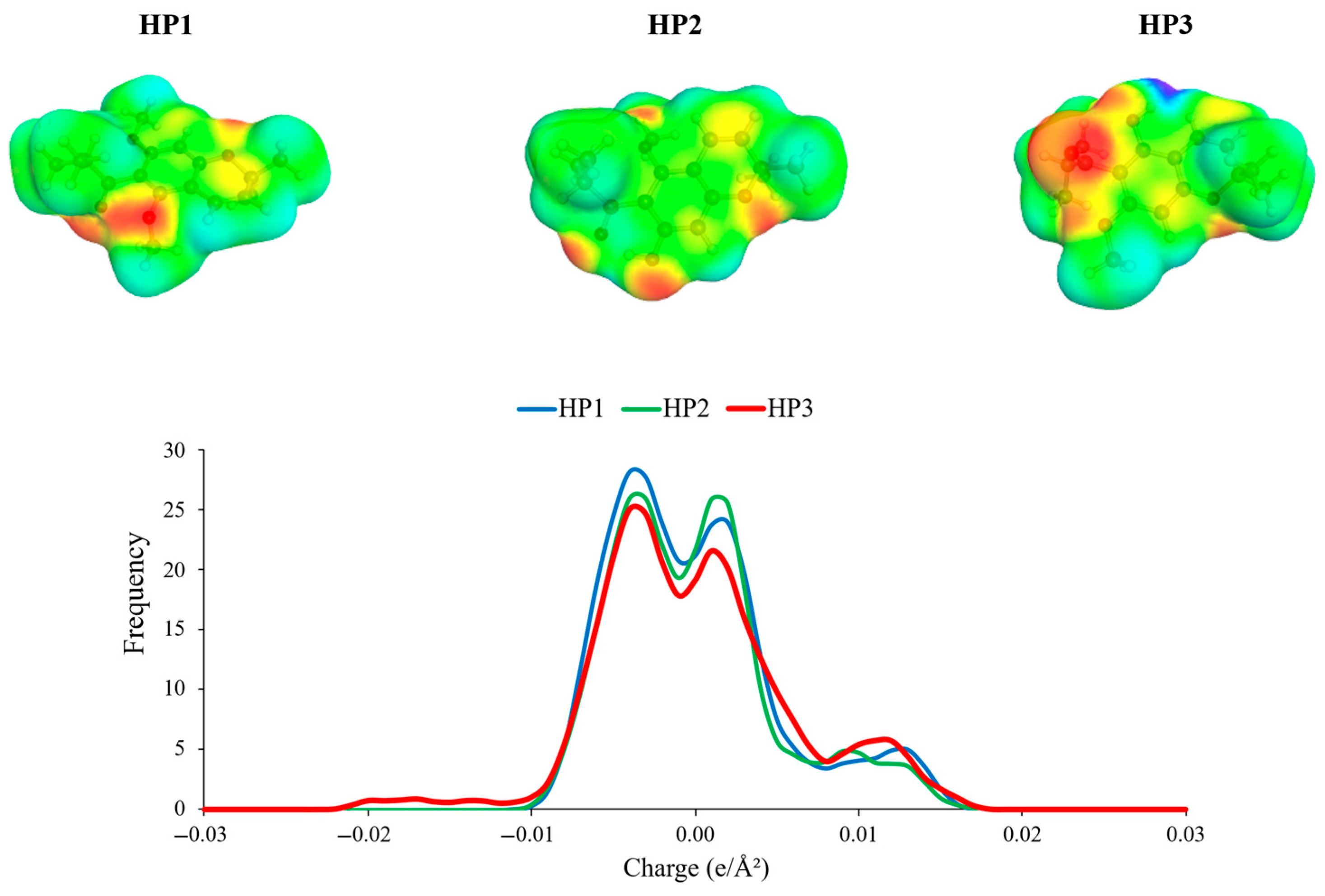
3.1. COSMO-RS Solubility Screening
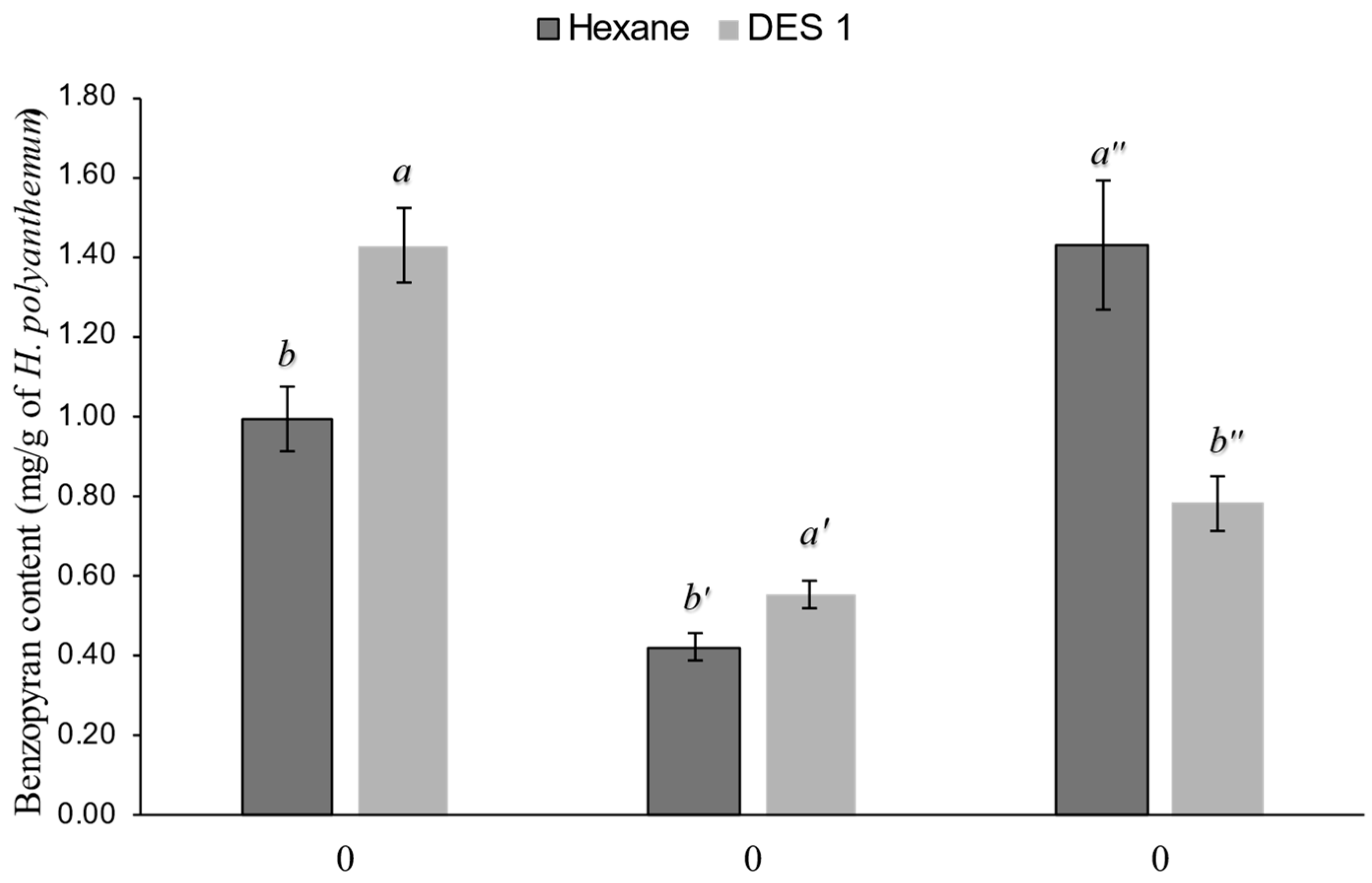
3.2. Extractions with Deep Eutectic Solvents
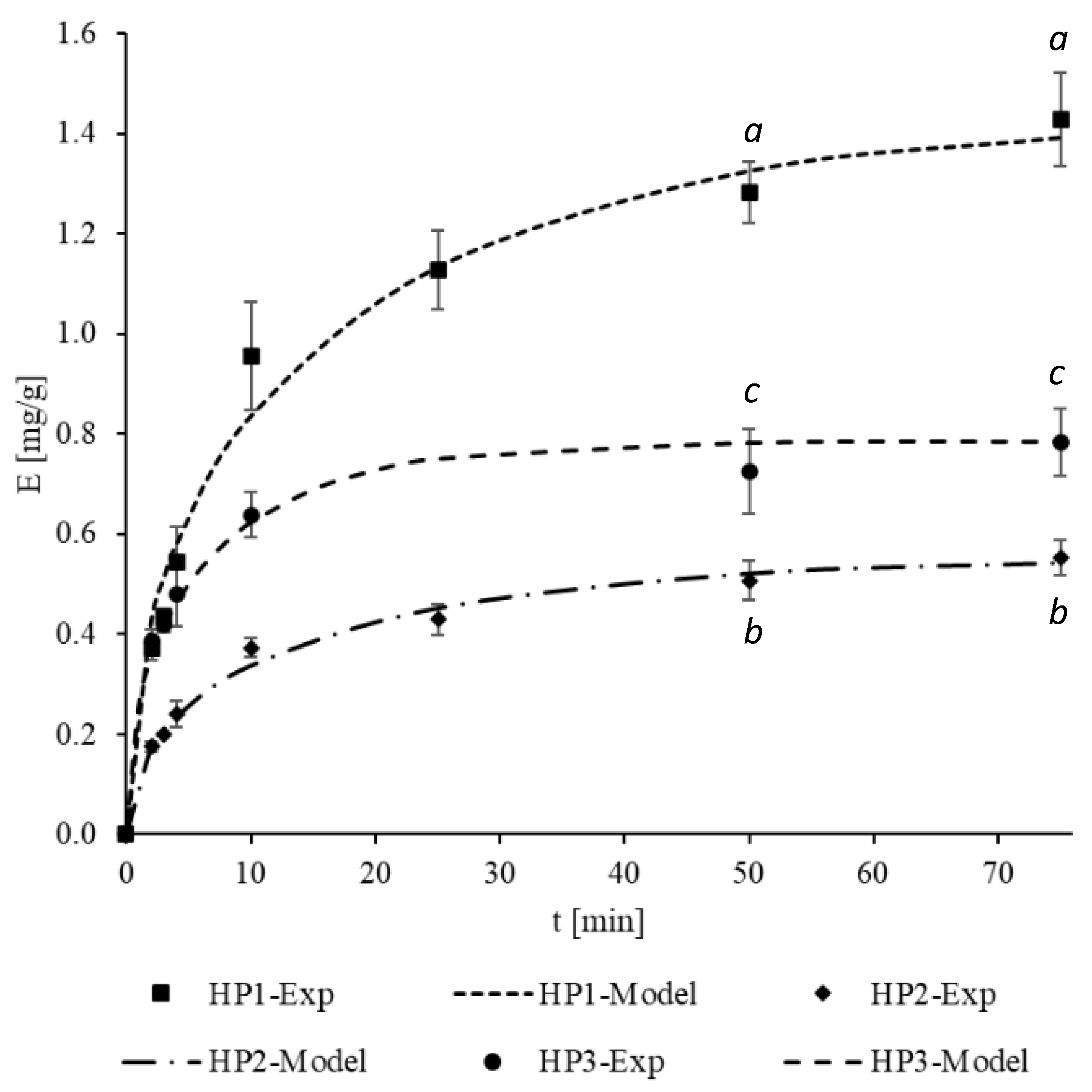
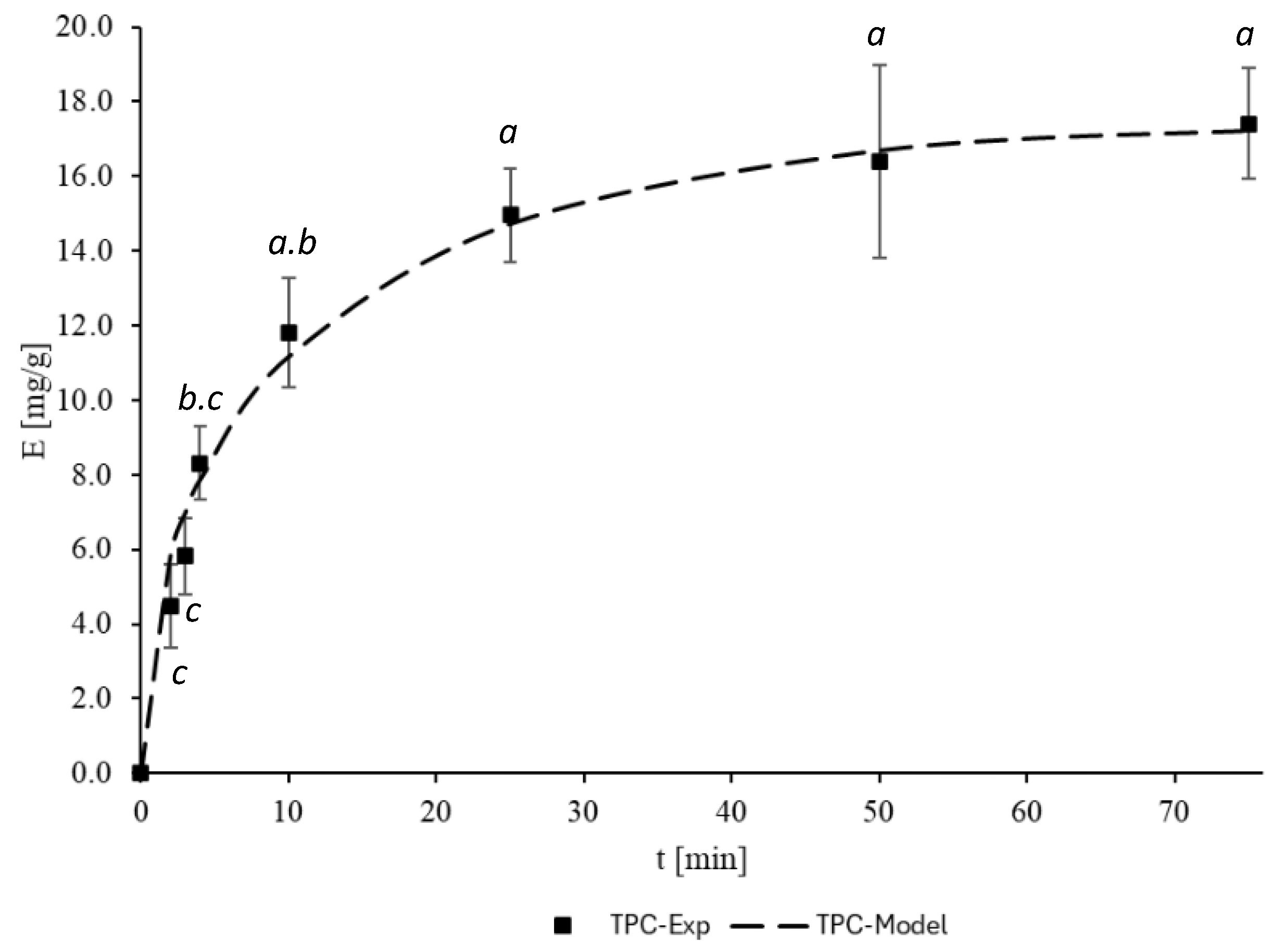
3.3. Mass Transfer Modeling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kartnig, T.; Göbel, I.; Heydel, B. Production of Hypericin, Pseudohypericin and Flavonoids in Cell Cultures of Various Hypericum Species and Their Chemotypes. Planta Med. 1996, 62, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Bennett, G.J.; Lee, H.H. Xanthones from Guttiferae. Phytochemistry 1989, 28, 967–998. [Google Scholar] [CrossRef]
- Rocha, L.; Marston, A.; Potterat, O.; Kaplan, M.A.C.; Stoeckli-Evans, H.; Hostettmann, K. Antibacterial Phloroglucinols and Flavonoids from Hypericum brasiliense. Phytochemistry 1995, 40, 1447–1452. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, A.P.M.; López-Alarcón, C.; Aspee, A.; Rech, S.; von Poser, G.L.; Bride, R.; Lissp, E. Antioxidant activity of flavonoids isolated from Hypericum ternum. J. Chil. Chem. Soc. 2007, 52, 1326–1329. [Google Scholar] [CrossRef]
- Ccana-Ccapatinta, G.V.; von Poser, G.L. Acylphloroglucinol Derivatives from Hypericum laricifolium Juss. Phytochem. Lett. 2015, 12, 63–68. [Google Scholar] [CrossRef]
- Bhattacharya, S.K. Activity Profiles of Two Hyperforin-Containing Hypericum Extracts in Behavioral Models. Pharmacopsychiatry 1998, 31, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Verotta, L.; Appendino, G.; Belloro, E.; Jakupovic, J.; Bombardelli, E. Furohyperforin, a Prenylated Phloroglucinol from St. John’s Wort (Hypericum perforatum). J. Nat. Prod. 1999, 62, 770–772. [Google Scholar] [CrossRef] [PubMed]
- Décosterd, L.; Stoeckli-Evans, H.; Msonthi, J.D.; Hostettmann, K. A New Antifungal Chromene and a Related Di-Chromene from Hypericum revolutum. Planta Med. 1986, 52, 429. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, K.; Yamaki, M.; Kashihara, M.; Takagi, S.; Isoi, K. A Chromene from Hypericum japonicum. Phytochemistry 1990, 29, 1010–1011. [Google Scholar] [CrossRef]
- Bridi, H.; Meirelles, G.D.C.; Bordignon, S.A.D.L.; Rates, S.M.K.; von Poser, G.L. Denudatin A, a Dimeric Acylphloroglucinol from Hypericum denudatum Presents an Antinociceptive Effect in Mice. Planta Med. 2017, 83, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Kitanov, G.M.; Nedialkov, P.T. Benzophenone O-Glucoside, a Biogenic Precursor of 1,3,7-Trioxygenated Xanthones in Hypericum annulatum. Phytochemistry 2001, 57, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, A.P.M.; Ferraz, A.B.F.; Albring, D.V.; Bordignon, S.A.L.; Schripsema, J.; Bridi, R.; Dutra-Filho, C.S.; Henriques, A.T.; von Poser, G.L. Benzophenones from Hypericum carinatum. J. Nat. Prod. 2005, 68, 784–786. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, A.P.M.; López-Alarcón, C.; Aspée, A.; Rech, S.B.; von Poser, G.L.; Bridi, R.; Dutrafilho, C.S.; Lissi, E. Antioxidant activityin southern brazil Hypericum species. J. Chil. Chem. Soc. 2008, 53, 1658–1662. [Google Scholar] [CrossRef]
- Proksch, P.; Proksch, M.; Towers, G.H.N.; Rodriguez, E. Phototoxic and Insecticidal Activities of Chromenes and Benzofurans from Encelia. J. Nat. Prod. 1983, 46, 331–334. [Google Scholar] [CrossRef]
- Uth, J.F.; Börgel, F.; Lehmkuhl, K.; Schepmann, D.; Kaiser, M.; Jabor, V.A.P.; Nonato, M.C.; Krauth-Siegel, R.L.; Schmidt, T.J.; Wünsch, B. Synthesis and Biological Evaluation of Natural-Product-Inspired, Aminoalkyl-Substituted 1-Benzopyrans as Novel Antiplasmodial Agents. J. Med. Chem. 2021, 64, 6397–6409. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, A.B.F.; Bordignon, S.A.L.; Staats, C.; Schripsema, J.; von Poser, G.L. Benzopyrans from Hypericum polyanthemum. Phytochemistry 2001, 57, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Haas, J.S.; Viana, A.F.; MacHado Heckler, A.P.; von Poser, G.L.; Kuze Rates, S.M. The Antinociceptive Effect of a Benzopyran (HP1) Isolated from Hypericum polyanthemum in Mice Hot-Plate Test Is Blocked by Naloxone. Planta Med. 2010, 76, 1419–1423. [Google Scholar] [CrossRef] [PubMed]
- Cargnin, S.T.; de Matos Nunes, J.; Haas, J.S.; Baladão, L.F.; Cassel, E.; Vargas, R.F.; Rech, S.B.; von Poser, G.L. Supercritical Fluid Extraction and High Performance Liquid Chromatographic Determination of Benzopyrans and Phloroglucinol Derivative in Hypericum polyanthemum. J. Chromatogr. B 2010, 878, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Meirelles, G.C.; Mendes, C.; Caon, T.; Teixeira, H.F.; von Poser, G.; Ponchel, G. Intestinal Permeability Enhancement of Benzopyran HP1-Loaded Nanoemulsions. Eur. J. Pharm. Sci. 2019, 127, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Gnerre, C.; von Poser, G.L.; Ferraz, A.; Viana, A.; Testa, B.; Rates, S.M.K. Monoamine Oxidase Inhibitory Activity of Some Hypericum Species Native to South Brazil. J. Pharm. Pharmacol. 2001, 53, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Ivanković, A.; Dronjić, A.; Bevanda, A.M.; Talić, S. Review of 12 Principles of Green Chemistry in Practice. Int. J. Sustain. Green Energy 2017, 6, 39–48. [Google Scholar] [CrossRef]
- Shah, P.A.; Chavda, V.; Hirpara, D.; Sharma, V.S.; Shrivastav, P.S.; Kumar, S. Exploring the Potential of Deep Eutectic Solvents in Pharmaceuticals: Challenges and Opportunities. J. Mol. Liq. 2023, 390, 123171. [Google Scholar] [CrossRef]
- Lu, W.; Liu, S. Choline Chloride–Based Deep Eutectic Solvents (Ch-DESs) as Promising Green Solvents for Phenolic Compounds Extraction from Bioresources: State-of-the-Art, Prospects, and Challenges. Biomass Convers. Biorefin. 2020, 12, 2949–2962. [Google Scholar] [CrossRef]
- Al-Maari, M.A.; Hizaddin, H.F.; Hayyan, A. COSMO-RS-Based Screening of Organic Solvents for Efficient Extraction of Rubber Seed Oil: Computational Prediction and Experimental Verification. Chem. Eng. Trans. 2023, 106, 1147–1152. [Google Scholar] [CrossRef]
- Panić, M.; Gunjević, V.; Radošević, K.; Bubalo, M.C.; Ganić, K.K.; Redovniković, I.R. COSMOtherm as an Effective Tool for Selection of Deep Eutectic Solvents Based Ready-To-Use Extracts from Graševina Grape Pomace. Molecules 2021, 26, 4722. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, V.H.S.; Vladic, J.; Pereira, J.; Monteiro, H.; Paiva, A.; Vargas, R.M.F.; Cassel, E.; Duarte, A.R.C. Green Approach for Obtaining Bioactive Compounds from Pterocaulon Polystachyum through Ultrasound-Assisted Deep Eutectic Solvent Extraction. Clean. Chem. Eng. 2025, 11, 100166. [Google Scholar] [CrossRef]
- Al-Maari, M.A.; Hizaddin, H.F.; Hayyan, A.; Hadj-Kali, M.K. Screening Deep Eutectic Solvents as Green Extractants for Oil from Plant Seeds Based on COSMO-RS Model. J. Mol. Liq. 2024, 393, 123520. [Google Scholar] [CrossRef]
- Wojeicchowski, J.P.; Ferreira, A.M.; Abranches, D.O.; Mafra, M.R.; Coutinho, J.A.P. Using COSMO-RS in the Design of Deep Eutectic Solvents for the Extraction of Antioxidants from Rosemary. ACS Sustain. Chem. Eng. 2020, 8, 12132–12141. [Google Scholar] [CrossRef]
- Zurob, E.; Cabezas, R.; Villarroel, E.; Rosas, N.; Merlet, G.; Quijada-Maldonado, E.; Romero, J.; Plaza, A. Design of Natural Deep Eutectic Solvents for the Ultrasound-Assisted Extraction of Hydroxytyrosol from Olive Leaves Supported by COSMO-RS. Sep. Purif. Technol. 2020, 248, 117054. [Google Scholar] [CrossRef]
- Xu, D.; Chow, J.; Weber, C.; Packer, M.; Baroutian, A.; Shahbaz, K. Evaluation of Deep Eutectic Solvents for the Extraction of Fucoxanthin from the Alga Tisochrysis lutea—COSMO-RS Screening and Experimental Validation. J. Environ. Chem. Eng. 2022, 10, 108370. [Google Scholar] [CrossRef]
- Fan, C.; Shan, Y.; Wen, L.; Cao, X. Extraction of Artemisinin Using Natural Deep Eutectic Solvent Selected by COSMO-RS. Sustain. Chem. Pharm. 2023, 33, 101096. [Google Scholar] [CrossRef]
- Melreles, M.A.A.; Zahedi, G.; Hatami, T. Mathematical Modeling of Supercritical Fluid Extraction for Obtaining Extracts from Vetiver Root. J. Supercrit. Fluids 2009, 49, 23–31. [Google Scholar] [CrossRef]
- Garcez, J.J.; Barros, F.; Lucas, A.M.; Xavier, V.B.; Fianco, A.L.; Cassel, E.; Vargas, R.M.F. Evaluation and Mathematical Modeling of Processing Variables for a Supercritical Fluid Extraction of Aromatic Compounds from Anethum graveolens. Ind. Crops Prod. 2017, 95, 733–741. [Google Scholar] [CrossRef]
- Bonfigli, M.; Godoy, E.; Reinheimer, M.A.; Scenna, N.J. Comparison between Conventional and Ultrasound-Assisted Techniques for Extraction of Anthocyanins from Grape Pomace. Experimental Results and Mathematical Modeling. J. Food Eng. 2017, 207, 56–72. [Google Scholar] [CrossRef]
- Lanjekar, K.J.; Rathod, V.K. Application of Ultrasound and Natural Deep Eutectic Solvent for the Extraction of Glycyrrhizic Acid from Glycyrrhiza glabra: Optimization and Kinetic Evaluation. Ind. Eng. Chem. Res. 2021, 60, 9532–9538. [Google Scholar] [CrossRef]
- Bassani, A.; García-Roldán, A.; Spigno, G.; Jauregi, P. Extraction of Phenolic Compounds from Spent Coffee Ground Using Natural Deep Eutectic Solvents: New Modeling Approach. J. Food Process Eng. 2024, 47, e14584. [Google Scholar] [CrossRef]
- Benítez-Correa, E.; Bastías-Montes, J.M.; Acuña-Nelson, S.; Quevedo-León, R. Deep Eutectic Solvent-Based Cocoa (Theobroma cacao L.) Bean Shell Flavonoid Release: A Comparative Study of Extraction Kinetic Models. J. Food Process Eng. 2025, 48, e70054. [Google Scholar] [CrossRef]
- Faisal, T.F.A.; Tun, H.; Ismail, F.; Amil, M.; Benjamin, Z.; Awang, M.A. Morinda citrifolia L. (Noni) Fruits: Optimising Extraction Kinetics of Yield and Flavonoids for Enhanced Antioxidant Activity at Different Temperatures. Eng. Appl. Sci. Res. 2024, 51, 626–638. [Google Scholar] [CrossRef]
- Han, C.; Yang, Z.; Chen, X.; Yan, L.; Zhang, Y. Ultrasound-Assisted Natural Deep Eutectic Solvents Extraction of Flavonoids from Hippophae rhamnoides: Optimization and Kinetics Studies. Chem. Eng. Process. Process Intensif. 2025, 211, 110244. [Google Scholar] [CrossRef]
- Wang, J.; Guo, Y.; Liu, F.; Zhang, X.; Wang, W.; Peng, Q. COSMO-RS Prediction and Experimental Verification of Deep Eutectic Solvents for Water Insoluble Pesticides with High Solubility. J. Mol. Liq. 2022, 349, 118139. [Google Scholar] [CrossRef]
- Klamt, A.; Eckert, F. COSMO-RS: A Novel and Efficient Method for the a Priori Prediction of Thermophysical Data of Liquids. Fluid Phase Equilib. 2000, 172, 43–72. [Google Scholar] [CrossRef]
- Paduszyn’ski, K.; Paduszyn’ski, P. An Overview of the Performance of the COSMO-RS Approach in Predicting the Activity Coefficients of Molecular Solutes in Ionic Liquids and Derived Properties at Infinite Dilution. Phys. Chem. Chem. Phys. 2017, 19, 11835–11850. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvents as New Potential Media for Green Technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, L.; Gea, T.; Artola, A.; Sánchez, A. Air Filled Porosity Measurements by Air Pycnometry in the Composting Process: A Review and a Correlation Analysis. Bioresour. Technol. 2009, 100, 2655–2666. [Google Scholar] [CrossRef] [PubMed]
- Rente, D.; Cvjetko Bubalo, M.; Panić, M.; Paiva, A.; Caprin, B.; Radojčić Redovniković, I.; Duarte, A.R.C. Review of Deep Eutectic Systems from Laboratory to Industry, Taking the Application in the Cosmetics Industry as an Example. J. Clean Prod. 2022, 380, 135147. [Google Scholar] [CrossRef]
- Mohammadpour, H.; Sadrameli, S.M.; Eslami, F.; Asoodeh, A. Optimization of Ultrasound-Assisted Extraction of Moringa Peregrina Oil with Response Surface Methodology and Comparison with Soxhlet Method. Ind. Crops Prod. 2019, 131, 106–116. [Google Scholar] [CrossRef]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H.; et al. A Comprehensive Review of Ultrasonic Assisted Extraction (UAE) for Bioactive Components: Principles, Advantages, Equipment, and Combined Technologies. Ultrason. Sonochem. 2023, 101, 106646. [Google Scholar] [CrossRef] [PubMed]
- Rebocho, S.; Mano, F.; Cassel, E.; Anacleto, B.; Bronze, M.d.R.; Paiva, A.; Duarte, A.R.C. Fractionated Extraction of Polyphenols from Mate Tea Leaves Using a Combination of Hydrophobic/ Hydrophilic NADES. Curr. Res. Food Sci. 2022, 5, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough Study of Reactivity of Various Compound Classes toward the Folin-Ciocalteu Reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef] [PubMed]
- Pedras, B.; Salema-Oom, M.; Sá-Nogueira, I.; Simões, P.; Paiva, A.; Barreiros, S. Valorization of White Wine Grape Pomace through Application of Subcritical Water: Analysis of Extraction, Hydrolysis, and Biological Activity of the Extracts Obtained. J. Supercrit. Fluids 2017, 128, 138–144. [Google Scholar] [CrossRef]
- Meirelles, G.; Bridi, H.; Stolz, E.D.; Teixeira, H.F.; von Poser, G.L.; Rates, S.M.K. Nanoemulsion Improves Antinociceptive Activity of HP1, a Benzopyran from Hypericum polyanthemum. Planta Med. Int. Open 2017, 4, e82–e88. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion; Oxford University Press: New York, NY, USA, 1975. [Google Scholar]
- Wang, Y.; Hu, Y.; Wang, H.; Tong, M.; Gong, Y. Green and Enhanced Extraction of Coumarins from Cortex Fraxini by Ultrasound-Assisted Deep Eutectic Solvent Extraction. J. Sep. Sci. 2020, 43, 3441–3448. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Tan, J.; Li, Q.; Tang, J.; Cai, X. Optimization Ultrasound-Assisted Deep Eutectic Solvent Extraction of Anthocyanins from Raspberry Using Response Surface Methodology Coupled with Genetic Algorithm. Foods 2020, 9, 1409. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.H.; Yusoff, R.; Ngoh, G.C. Modeling and Kinetics Study of Conventional and Assisted Batch Solvent Extraction. Chem. Eng. Res. Des. 2014, 92, 1169–1186. [Google Scholar] [CrossRef]
- So, G.C.; Macdonald, D.G. Kinetics of Oil Extraction from Canola (Rapeseed). Can. J. Chem. Eng. 1986, 64, 80–86. [Google Scholar] [CrossRef]
- Xavier, L.; Freire, M.S.; González-Álvarez, J. Modeling and Optimizing the Solid–Liquid Extraction of Phenolic Compounds from Lignocellulosic Subproducts. Biomass Convers. Biorefin. 2019, 9, 737–747. [Google Scholar] [CrossRef]
- Reche, C.; Rosselló, C.; Umaña, M.M.; Eim, V.; Simal, S. Mathematical Modelling of Ultrasound-Assisted Extraction Kinetics of Bioactive Compounds from Artichoke by-Products. Foods 2021, 10, 931. [Google Scholar] [CrossRef] [PubMed]
- da Silva, C.G.F.; Cassel, E.; Vargas, R.M.F.; Marczak, L.D.F. Selective Extraction of Saponins from Ilex paraguariensis St.-Hil. Unripe Fruits. Food Bioprod. Process. 2018, 111, 72–82. [Google Scholar] [CrossRef]


| DES | No. | ||
|---|---|---|---|
| HBA | HBD | Molar Ratio | |
| Lactic Acid | Glycine/Water | 3:1:3 | 1 |
| Choline Chloride | Glycerol | 1:2 | 2 |
| Choline Chloride | Ethylene glycol | 1:2 | 3 |
| Betaine | Glycerol | 1:2 | 4 |
| Betaine | Glycerol | 1:3 | 5 |
| Betaine | Lactic Acid | 1:2 | 6 |
| Proline | Glycerol | 1:2 | 7 |
| Proline | Ethylene glycol | 1:2 | 8 |
| Proline | Lactic Acid | 1:2 | 9 |
| Leucine | Glycerol | 1:2 | 10 |
| Compound | CAS Number | Source | Supplier Purity a | Molar Mass (g/mol) |
|---|---|---|---|---|
| Lactic acid | 50-21-5 | Sigma-Aldrich (St. Louis, MO, USA) | ≥85.0% | 90.1 |
| Glycine | 56-40-6 | Sigma-Aldrich (St. Louis, MO, USA) | ≥98.5% | 75.1 |
| HP1 | HP2 | HP3 | TPC | |
|---|---|---|---|---|
| D [m2/s] | 6.09 × 10−12 | 6.67 × 10−12 | 1.46 × 10−11 | 7.40 × 10−12 |
| k | 0.61 | 0.72 | 0.88 | 0.88 |
| R2 | 0.98 | 0.99 | 0.99 | 0.98 |
| MAPE [%] | 7.41% | 3.09% | 2.96% | 8.37% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salerno, V.M.J.; Meirelles, G.d.C.; Tavares, H.M.; Rodrigues, V.H.S.; Cassel, E.; von Poser, G.L.; Vargas, R.M.F. Ultrasound-Assisted Extraction Processes of Benzopyrans from Hypericum polyanthemum: COSMO-RS Prediction and Mass Transfer Modeling. Processes 2025, 13, 2351. https://doi.org/10.3390/pr13082351
Salerno VMJ, Meirelles GdC, Tavares HM, Rodrigues VHS, Cassel E, von Poser GL, Vargas RMF. Ultrasound-Assisted Extraction Processes of Benzopyrans from Hypericum polyanthemum: COSMO-RS Prediction and Mass Transfer Modeling. Processes. 2025; 13(8):2351. https://doi.org/10.3390/pr13082351
Chicago/Turabian StyleSalerno, Victor Mateus Juchem, Gabriela de Carvalho Meirelles, Henrique Martins Tavares, Victor Hugo Silva Rodrigues, Eduardo Cassel, Gilsane Lino von Poser, and Rubem Mário Figueiró Vargas. 2025. "Ultrasound-Assisted Extraction Processes of Benzopyrans from Hypericum polyanthemum: COSMO-RS Prediction and Mass Transfer Modeling" Processes 13, no. 8: 2351. https://doi.org/10.3390/pr13082351
APA StyleSalerno, V. M. J., Meirelles, G. d. C., Tavares, H. M., Rodrigues, V. H. S., Cassel, E., von Poser, G. L., & Vargas, R. M. F. (2025). Ultrasound-Assisted Extraction Processes of Benzopyrans from Hypericum polyanthemum: COSMO-RS Prediction and Mass Transfer Modeling. Processes, 13(8), 2351. https://doi.org/10.3390/pr13082351







