Photoelectrochemical CO2 Reduction Measurements of a BiOI Coating Deposited onto a Non-Conductive Glass Support as a Platform for Environmental Remediation
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Bismuth Oxyiodide
2.2. Preparation of the Electrodes with a Conductive Support
2.3. Preparation of the Electrodes with a Non-Conductive Support
2.4. Instrumentation
3. Results and Discussion
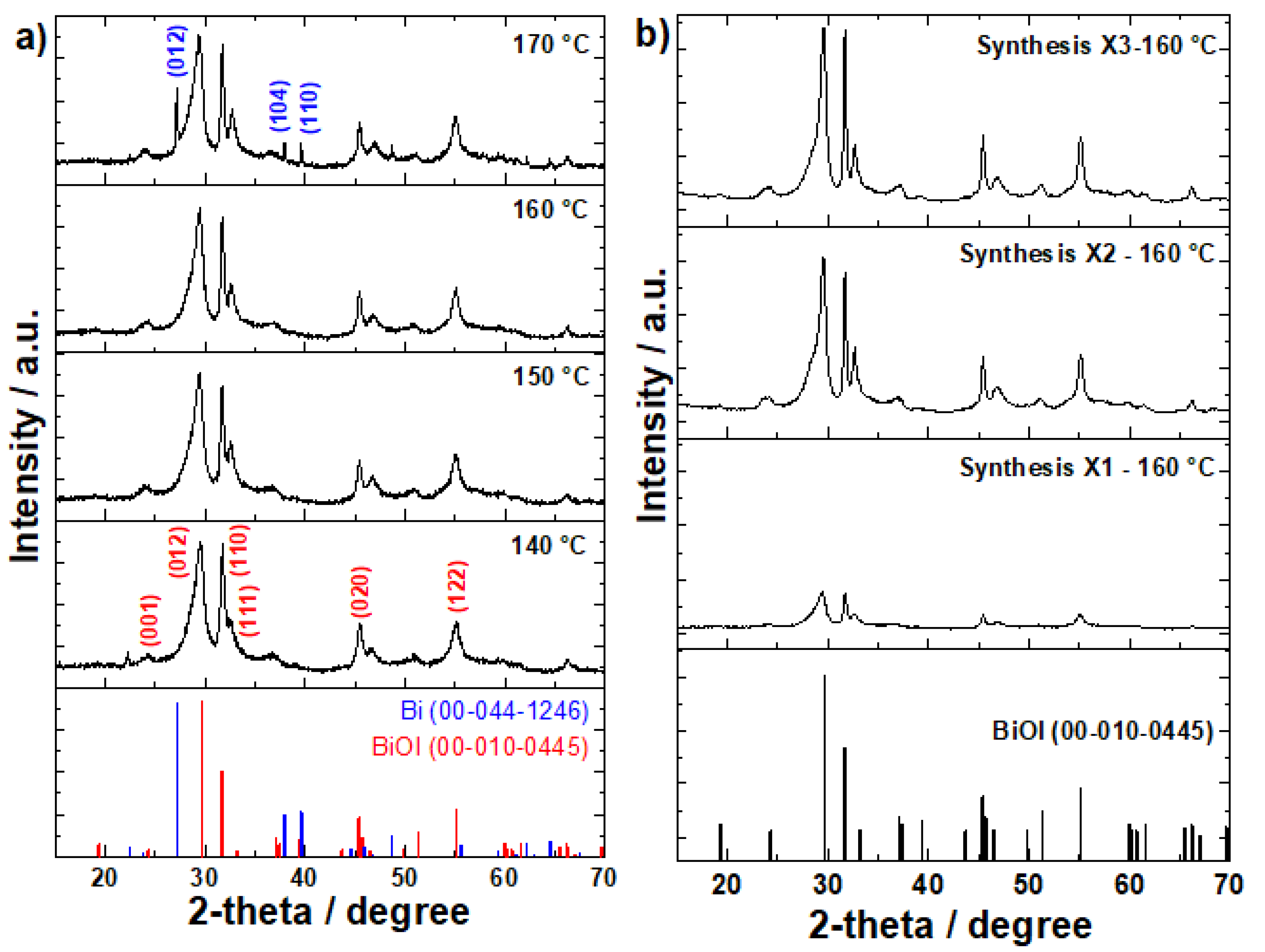
3.1. X-Ray Diffraction Analysis
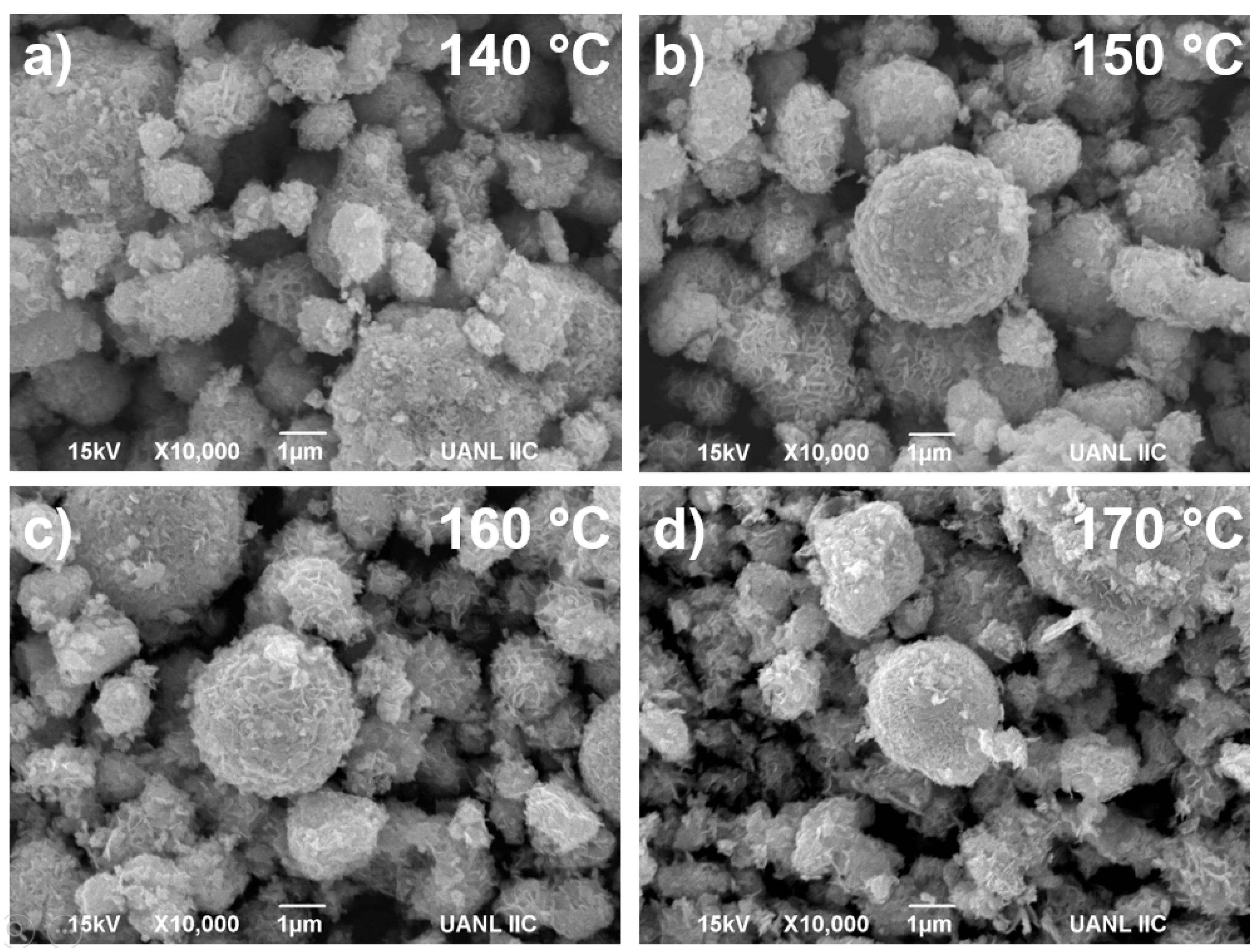
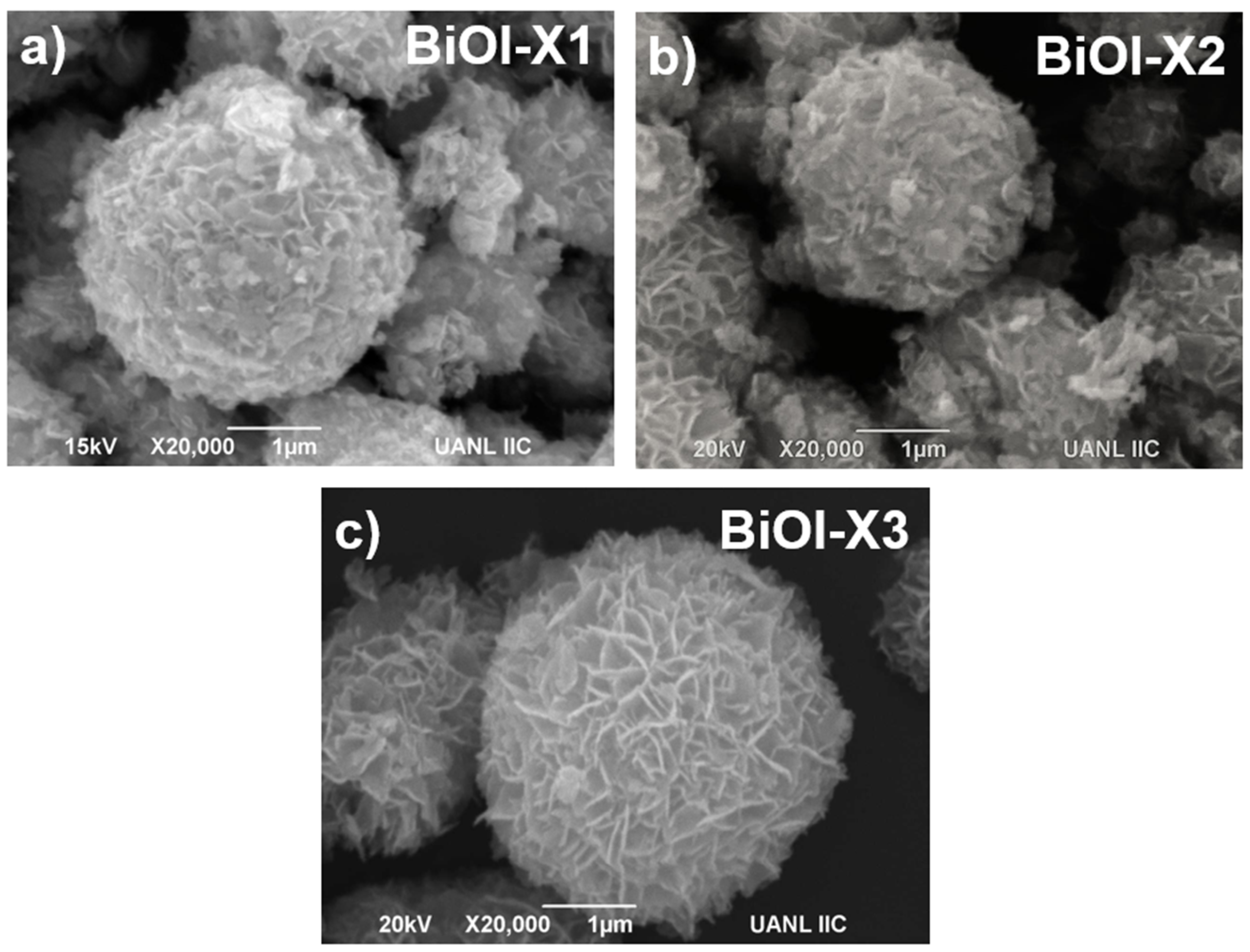
3.2. Microscopy Characterization
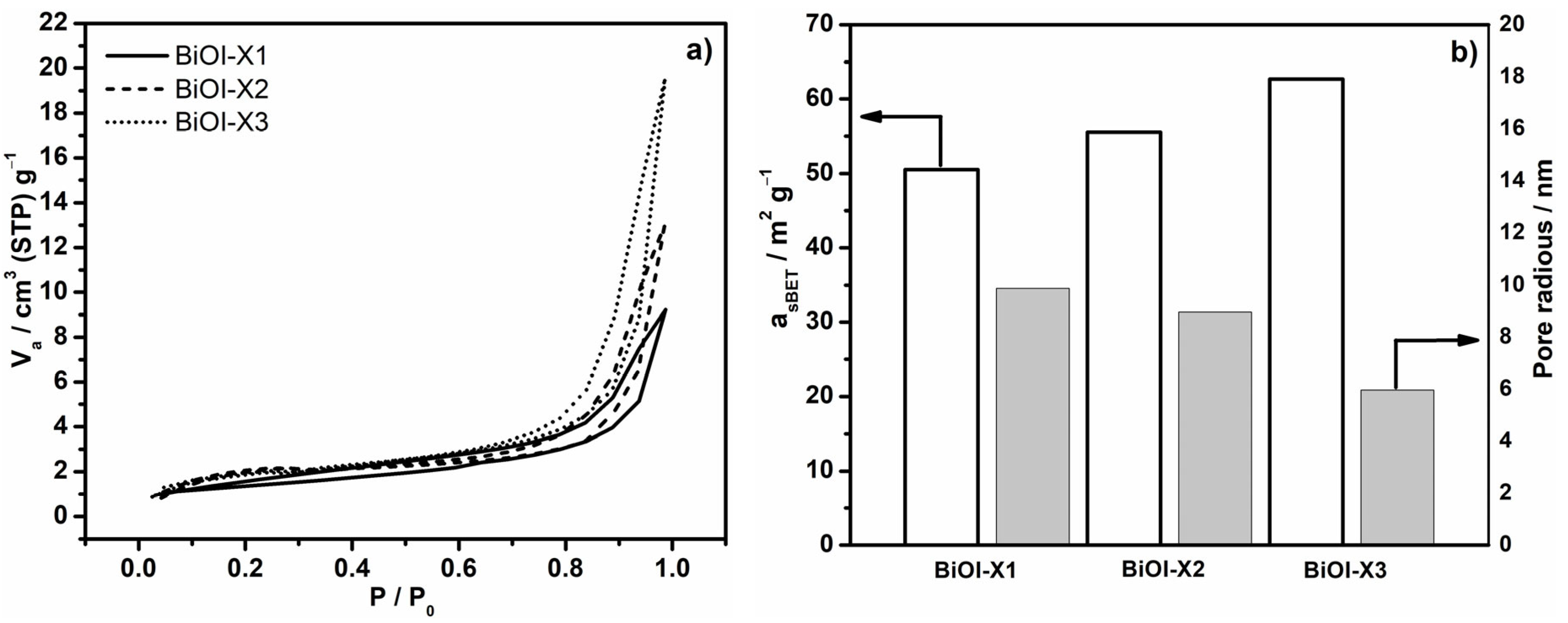
3.3. BET Area (asBET) and Pore Radius Estimations
3.4. Electrochemical Characterization
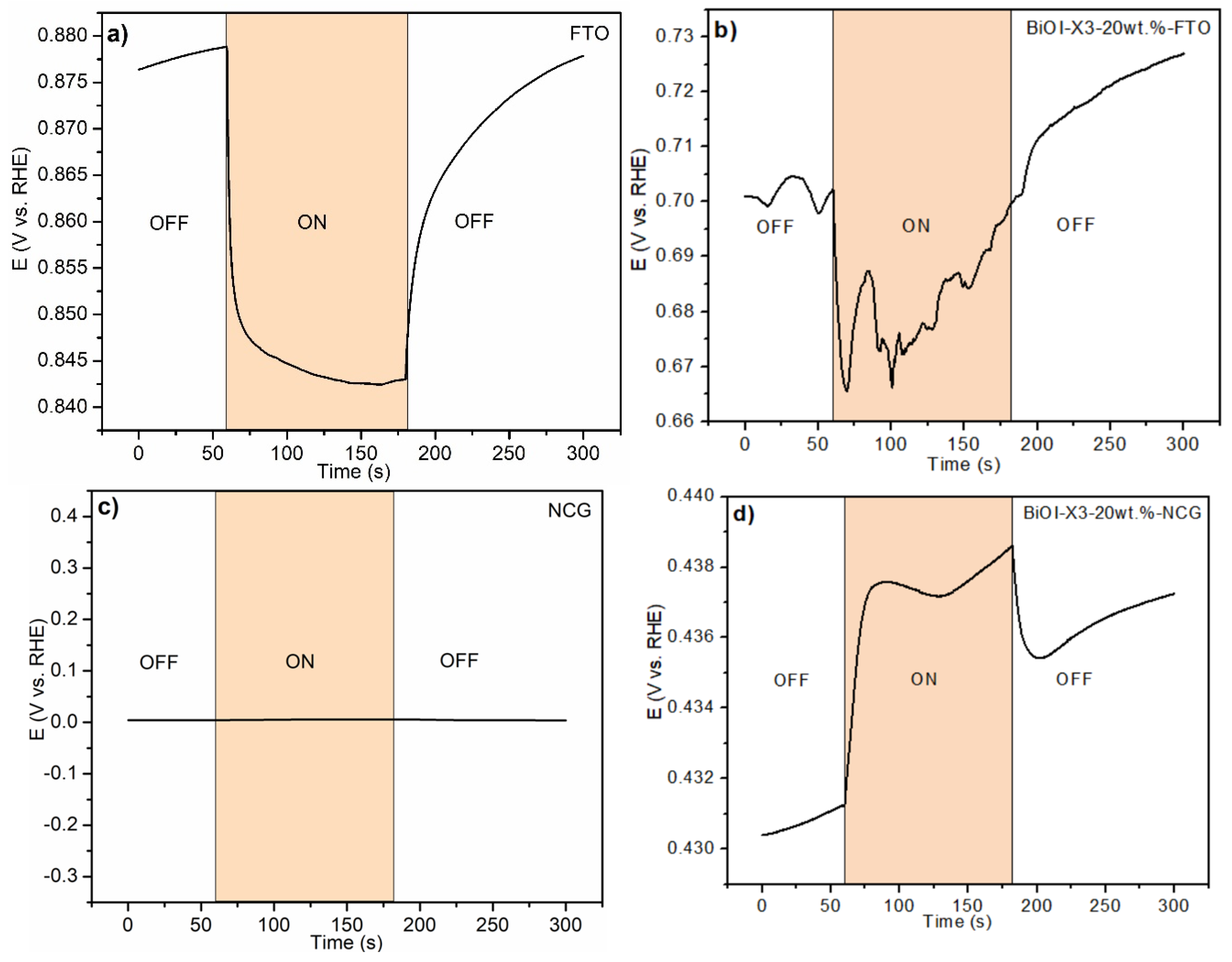
3.4.1. Open-Circuit Potential Transients
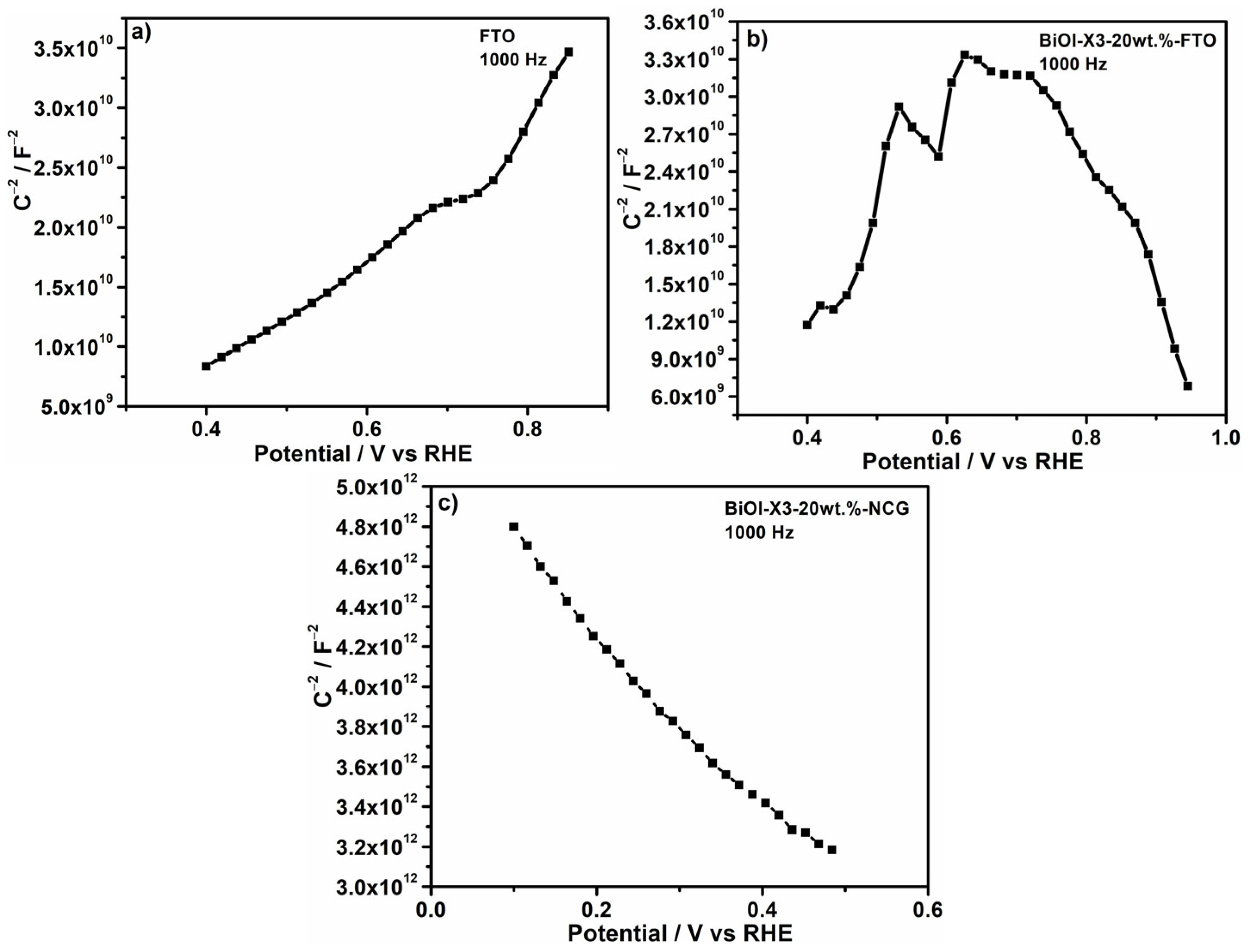
3.4.2. Mott–Schottky Measurements
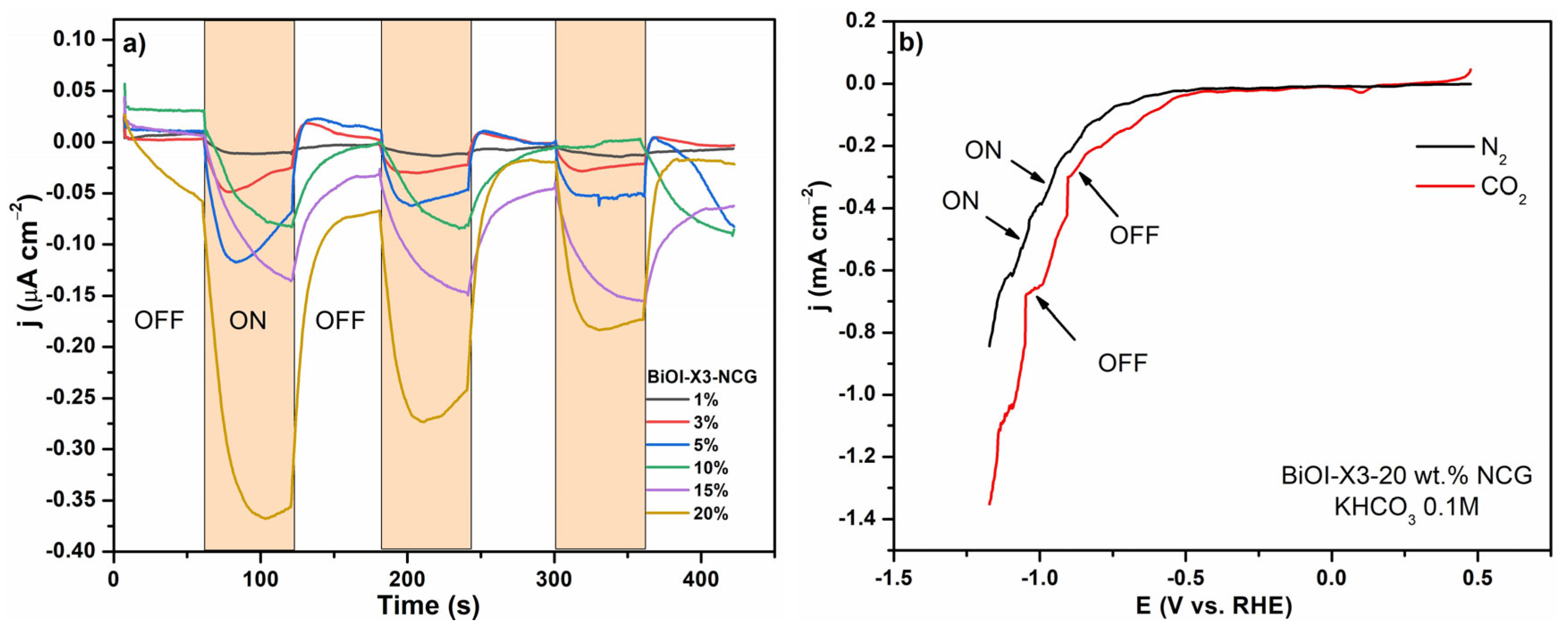
3.4.3. Photo(electro)chemical Response
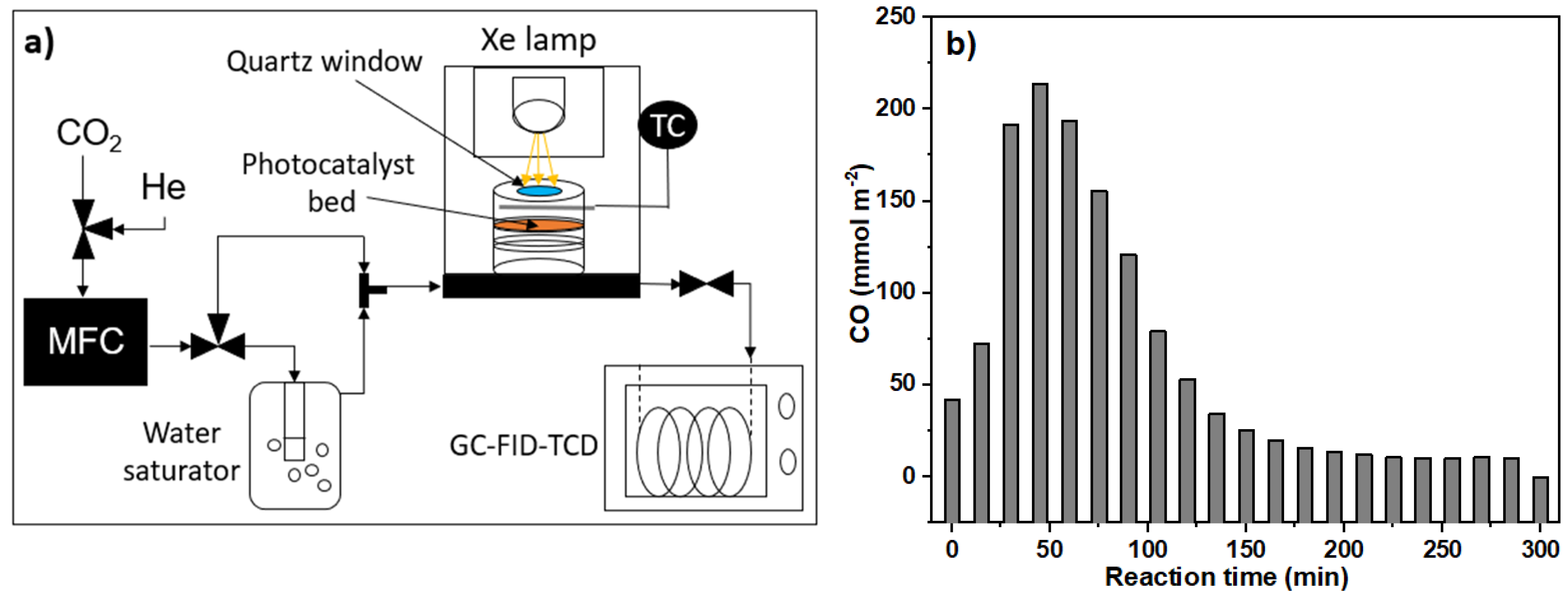
3.4.4. Photocatalytic Activity in Gaseous Media
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernandez-Guzman, D.; Lavarello, R.; Yglesias-González, M.; Hartinger, S.M.; Rojas-Rueda, D. A scoping review of the health co-benefits of climate mitigation strategies in South America. Lancet Reg. Health Am. 2023, 26, 100602. [Google Scholar] [CrossRef]
- Filonchyk, M.; Peterson, M.P.; Zhang, L.; Hurynovich, V.; He, Y. Greenhouse gases emissions and global climate change: Examining the influence of CO2, CH4, and N2O. Sci. Total. Environ. 2024, 935, 173359. [Google Scholar] [CrossRef]
- Prăvălie, R. Major perturbations in the Earth’s forest ecosystems. Possible implications for global warming. Earth-Sci. Rev. 2018, 185, 544–571. [Google Scholar] [CrossRef]
- Deng, H.-M.; Liang, Q.-M.; Liu, L.-J.; Anadon, L.D. Co-benefits of greenhouse gas mitigation: A review and classification by type, mitigation sector, and geography. Environ. Res. Lett. 2017, 12, 123001. [Google Scholar] [CrossRef]
- Chakravorty, A.; Roy, S. A review of photocatalysis, basic principles, processes, and materials. Sustain. Chem. Environ. 2024, 8, 100155. [Google Scholar] [CrossRef]
- Atacan, K.; Güy, N.; Özacar, M. Recent advances in photocatalytic coatings for antimicrobial surfaces. Curr. Opin. Chem. Eng. 2022, 36, 100777. [Google Scholar] [CrossRef]
- Pichat, P. 4.16—Photocatalytic Coatings. In Comprehensive Materials Processing; Hashmi, S., Ed.; Elsevier: Oxford, UK, 2014; pp. 413–423. [Google Scholar]
- Brattich, E.; Barbano, F.; Pulvirenti, B.; Pilla, F.; Bacchetti, M.; Di Sabatino, S. The effect of photocatalytic coatings on NOx concentrations in real-world street canyons. Build. Environ. 2021, 205, 108312. [Google Scholar] [CrossRef]
- Gandolfo, A.; Bartolomei, V.; Truffier-Boutry, D.; Temime-Roussel, B.; Brochard, G.; Bergé, V.; Wortham, H.; Gligorovski, S. The impact of photocatalytic paint porosity on indoor NOx and HONO levels. Phys. Chem. Chem. Phys. 2020, 22, 589–598. [Google Scholar] [CrossRef]
- Yu, X.; Yang, J.; Ye, K.; Fu, X.; Zhu, Y.; Zhang, Y. Facile one-step synthesis of BiOCl/BiOI heterojunctions with exposed {001} facet for highly enhanced visible light photocatalytic performances. Inorg. Chem. Commun. 2016, 71, 45–49. [Google Scholar] [CrossRef]
- Jagt, R.A.; Huq, T.N.; Börsig, K.M.; Sauven, D.; Lee, L.C.; MacManus-Driscolla, J.L.; Hoye, R.L.Z. Controlling the preferred orientation of layered BiOI solar absorbers. J. Mater. Chem. C 2020, 8, 10791–10797. [Google Scholar] [CrossRef]
- Liu, J.; Shi, J.; Deng, H. Current status of research on BiOX-based heterojunction photocatalytic systems: Synthesis methods, photocatalytic applications and prospects. J. Environ. Chem. Eng. 2023, 11, 110311. [Google Scholar] [CrossRef]
- Zhang, X.; Lou, B.; Li, D.; Hong, W.; Yu, Y.; Li, J.; Wang, E. A universal method for the preparation of functional ITO electrodes with ultrahigh stability. Chem. Commun. 2015, 51, 6788–6791. [Google Scholar] [CrossRef] [PubMed]
- de la Cruz, P.T.S.; Irikura, K.; Lachgar, A.; Cardoso, J.C.; Cavero, H.A.; Zanoni, M.V.B. Preparation of FTO/CU2O Electrode Protected by PEDOT:PSS and Its Better Performance in the Photoelectrocatalytic Reduction of CO2 to Methanol. Electrocatalysis 2020, 11, 546–554. [Google Scholar] [CrossRef]
- Chang, C.; Zhu, L.; Fu, Y.; Chu, X. Highly active Bi/BiOI composite synthesized by one-step reaction and its capacity to degrade bisphenol A under simulated solar light irradiation. Chem. Eng. J. 2013, 233, 305–314. [Google Scholar] [CrossRef]
- Sriwong, C.; Choojun, K.; Sriwong, S. High photocatalytic performance of 3D porous-structured TiO2@natural rubber hybrid sheet on the removal of indigo carmine dye in water. SN Appl. Sci. 2019, 1, 864. [Google Scholar] [CrossRef]
- Burton, A.W.; Ong, K.; Rea, T.; Chan, I.Y. On the estimation of average crystallite size of zeolites from the Scherrer equation: A critical evaluation of its application to zeolites with one-dimensional pore systems. Microporous Mesoporous Mater. 2009, 117, 75–90. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Zaen, R.; Oktiani, R. Correlation between crystallite size and photocatalytic performance of micrometer-sized monoclinic WO3 particles. Arab. J. Chem. 2020, 13, 1283–1296. [Google Scholar] [CrossRef]
- Devi, L.G.; Kottam, N.; Kumar, S.G. Preparation and Characterization of Mn-Doped Titanates with a Bicrystalline Framework: Correlation of the Crystallite Size with the Synergistic Effect on the Photocatalytic Activity. J. Phys. Chem. C 2009, 113, 15593–15601. [Google Scholar] [CrossRef]
- Devi, L.G.; Murthy, B.N.; Kumar, S.G. Photocatalytic activity of TiO2 doped with Zn2+ and V5+ transition metal ions: Influence of crystallite size and dopant electronic configuration on photocatalytic activity. Mater. Sci. Eng. B 2010, 166, 1–6. [Google Scholar] [CrossRef]
- Wang, X.; Sø, L.; Su, R.; Wendt, S.; Hald, P.; Mamakhel, A.; Yang, C.; Huang, Y.; Iversen, B.B.; Besenbacher, F. The influence of crystallite size and crystallinity of anatase nanoparticles on the photo-degradation of phenol. J. Catal. 2014, 310, 100–108. [Google Scholar] [CrossRef]
- Murillo-Sierra, J.; Hernández-Ramírez, A.; Pino-Sandoval, D.; Ruiz-Ruiz, E.; Martínez-Hernández, A. Promoting multielectron CO2 reduction using a direct Z-scheme WO3/ZnS photocatalyst. J. CO2 Util. 2022, 63, 102122. [Google Scholar] [CrossRef]
- Chico-Vecino, M.; Murillo-Sierra, J.C.; Pino-Sandoval, D.A.; Hinojosa-Reyes, L.; Maya-Treviño, M.L.; Contreras, D.; Hernández-Ramírez, A. Preparation of WO3/In2O3 heterojunctions and their performance on the CO2 photocatalytic conversion in a continuous flow reactor. J. Environ. Chem. Eng. 2023, 11, 110372. [Google Scholar] [CrossRef]
- Rahman, M.; Shafiullah, A.Z.; Pal, A.; Islam, A.; Jahan, I.; Saha, B.B. Study on Optimum IUPAC Adsorption Isotherm Models Employing Sensitivity of Parameters for Rigorous Adsorption System Performance Evaluation. Energies 2021, 14, 7478. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Rosa, D.; Cimini, G.; Bracciale, M.P.; Felici, A.C.; Di Palma, L. Iron-doped titania nanoparticles supported on polystyrene for photocatalytic treatment of contaminated water in a continuous system. J. Photochem. Photobiol. A Chem. 2024, 447, 115241. [Google Scholar] [CrossRef]
- Karthik, P.; Vinoth, R.; Babu, S.G.; Wen, M.; Kamegawa, T.; Yamashita, H.; Neppolian, B. Synthesis of highly visible light active TiO2-2-naphthol surface complex and its application in photocatalytic chromium(vi) reduction. RSC Adv. 2015, 5, 39752–39759. [Google Scholar] [CrossRef]
- Berglund, S.P.; Abdi, F.F.; Bogdanoff, P.; Chemseddine, A.; Friedrich, D.; van de Krol, R. Comprehensive Evaluation of CuBi2O4 as a Photocathode Material for Photoelectrochemical Water Splitting. Chem. Mater. 2016, 28, 4231–4242. [Google Scholar] [CrossRef]
- Muhibbullah, M.; Haleem, A.M.A. Estimation of the Open Circuit Voltage of a pn Junction Based on Photoelectrochemical Measurements. Trans. Mater. Res. Soc. Jpn. 2015, 40, 247–252. [Google Scholar] [CrossRef]
- Juodkazis, K.; Juodkazytė, J.; Šebeka, B.; Savickaja, I.; Juodkazis, S. Photoelectrochemistry of silicon in HF solution. J. Solid State Electrochem. 2013, 17, 2269–2276. [Google Scholar] [CrossRef]
- Hu, E.; Chen, Q.; Gao, Q.; Fan, X.; Luo, X.; Wei, Y.; Wu, G.; Deng, H.; Xu, S.; Wang, P.; et al. Cyano-Functionalized Graphitic Carbon Nitride with Adsorption and Photoreduction Isosite Achieving Efficient Uranium Extraction from Seawater. Adv. Funct. Mater. 2024, 34, 2312215. [Google Scholar] [CrossRef]
- Zare, M.; Solaymani, S.; Shafiekhani, A.; Kulesza, S.; Ţălu, Ş.; Bramowicz, M. Evolution of rough-surface geometry and crystalline structures of aligned TiO2 nanotubes for photoelectrochemical water splitting. Sci. Rep. 2018, 8, 10870. [Google Scholar] [CrossRef] [PubMed]
- Kafle, B.P. Effect of Precursor Fluorine Concentration Optical and Electrical Properties of Fluorine Doped Tin Oxide thin Films. Mater. Today Proc. 2021, 47, 6389–6397. [Google Scholar] [CrossRef]
- Kumar, S.; Ahirwar, S.; Satpati, A.K. Insight into the PEC and interfacial charge transfer kinetics at the Mo doped BiVO4photoanodes. RSC Adv. 2019, 9, 41368–41382. [Google Scholar] [CrossRef] [PubMed]
- Karazehir, T.; Ates, M.; Sarac, A.S. Mott–Schottky and Morphologic Analysis of Poly(Pyrrole-N-Propionic Acid) in various electrolyte systems. Int. J. Electrochem. Sci. 2015, 10, 6146–6163. [Google Scholar] [CrossRef]
- Xin, C.; Wang, Y.; Zhang, S.; Xu, L.; Yu, Y.; Xiang, H.; Wu, W.; Hua, J. Energy Band Transition and Voltage Compensation via Surface Stoichiometry Alteration in p-Type Dye-Sensitized Solar Cells. Phys. Status Solidi (RRL)—Rapid Res. Lett. 2017, 11, 1700258. [Google Scholar] [CrossRef]
- Saber, S.; Mollar, M.; El Nahrawy, A.; Khattab, N.; Eid, A.; Abo-Aly, M.; Marí, B. Annealing study of electrodeposited CuInSe2 and CuInS2 thin films. Opt. Quantum Electron. 2018, 50, 248. [Google Scholar] [CrossRef]
- Zhong, H.; Fujii, K.; Nakano, Y. Electroactive species study in the electrochemical reduction of CO2 in KHCO3 solution at elevated temperature. J. Energy Chem. 2016, 25, 517–522. [Google Scholar] [CrossRef]
- Zhong, H.; Fujii, K.; Nakano, Y. Effect of KHCO3 Concentration on Electrochemical Reduction of CO2 on Copper Electrode. J. Electrochem. Soc. 2017, 164, F923. [Google Scholar] [CrossRef]
- Mora-Hernandez, J.M.; Herrera, L.A.A.; Garay-Rodriguez, L.F.; Torres-Martínez, L.M.; Hernandez-Perez, I. An enhanced photo(electro)catalytic CO2 reduction onto advanced BiOX (X = Cl, Br, I) semiconductors and the BiOI–PdCu composite. Heliyon 2023, 9, e20605. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mora-Hernandez, J.M.; Hernández-Ramírez, A. Photoelectrochemical CO2 Reduction Measurements of a BiOI Coating Deposited onto a Non-Conductive Glass Support as a Platform for Environmental Remediation. Processes 2025, 13, 2292. https://doi.org/10.3390/pr13072292
Mora-Hernandez JM, Hernández-Ramírez A. Photoelectrochemical CO2 Reduction Measurements of a BiOI Coating Deposited onto a Non-Conductive Glass Support as a Platform for Environmental Remediation. Processes. 2025; 13(7):2292. https://doi.org/10.3390/pr13072292
Chicago/Turabian StyleMora-Hernandez, J. Manuel, and A. Hernández-Ramírez. 2025. "Photoelectrochemical CO2 Reduction Measurements of a BiOI Coating Deposited onto a Non-Conductive Glass Support as a Platform for Environmental Remediation" Processes 13, no. 7: 2292. https://doi.org/10.3390/pr13072292
APA StyleMora-Hernandez, J. M., & Hernández-Ramírez, A. (2025). Photoelectrochemical CO2 Reduction Measurements of a BiOI Coating Deposited onto a Non-Conductive Glass Support as a Platform for Environmental Remediation. Processes, 13(7), 2292. https://doi.org/10.3390/pr13072292






