Abstract
Non-catalytic partial oxidation (POX) of natural gas is gaining importance in low-carbon energy systems for methane conversion to acetylene, syngas, and olefins. However, uncontrolled polycyclic aromatic hydrocarbons (PAHs) and soot formation remain challenges. This work developed a Hierarchical-Integrated Mechanism (HI-Mechanism) by constructing detailed C0-C6, C5-C15 and C16 mechanisms, and then hierarchically simplifying C5-C15 subsystems, ultimately integrating them into a final mechanism with 397 species and 5135 reactions. The HI-Mechanism accurately predicted shock tube ignition delays and major species concentrations. Microkinetic analyses, including production rates and reaction sensitivity, revealed key pathways and enabled reliable product distribution prediction. The HI-Mechanism provides theoretical guidance for optimizing POX of natural gas processes and can be extended to complex systems like heavy oil cracking, supporting clean energy technology development.
1. Introduction
The global energy transition, driven by climate change mitigation and carbon neutrality objectives, is fundamentally reshaping energy systems. As low-carbon development gains international consensus [1], natural gas emerges as a critical transitional energy source, progressively displacing coal in industrial applications with projected consumption growth over the next two decades [2,3]. This trend has accelerated advancements in natural gas conversion technologies, among which Non-catalytic Partial Oxidation (POX) stands out due to its distinctive advantages. The POX process efficiently produces acetylene while co-generating syngas with tunable H2/CO ratios [4] and other valuable products like methanol [5]. Its remarkable feedstock flexibility enables processing of me-thane-rich gases including natural gas, coke oven gas [6], shale gas [7], and biogas [8], conferring both technical sophistication and economic viability for chemical feedstock production.
The POX process of natural gas involves complex reactions, including oxidation, cracking, water–gas shift, and polymerization, characterized by high temperatures, turbulent flows, and millisecond-scale reactions. However, while producing target products like acetylene, this process simultaneously generates undesirable byproducts such as soot and coke. Researchers have investigated the POX process in various experimental systems including flow reactors [9], shock tubes [10], premixed flames [11], and counterflow diffusion flames [12] to better understand and optimize the technology. Notably, Kohler et al. [13] conducted experiments in a high-temperature flow reactor in 2016, employing mass spectrometry for quantitative product analysis, which provided reliable data for chemical kinetic model validation. Furthermore, studies confirm that polycyclic aromatic hydrocarbons (PAHs) serve as key precursors for soot formation. Their generation not only reduces process efficiency but also contributes to air pollution [14] and global warming, necessitating suppression during POX operations [15].
While experimental data can validate kinetic models, significant limitations remain in elucidating complex reaction networks. Advances in computational and analytical techniques [4] now enable the development of more detailed kinetic models. Recent years have witnessed remarkable progress in POX kinetic modeling, yielding several representative model frameworks. GRI-Mech 3.0 [16] demonstrates excellent performance for C1-C3 species simulations, with validated reliability across multiple experimental datasets including ignition delay times, shock tube species profiles, and laminar flame speeds. However, it shows limited accuracy in predicting aromatic species formation under fuel-rich conditions. In comparison, AramcoMech2.0 [17,18,19], FFCM-1 [20] and USC-II [21] exhibit superior performance under specific operating conditions. Significant advances have been made in kinetic mechanisms for first-ring aromatics (e.g., benzene) formation [22]. Skjøth-Rasmussen et al. [23] developed a PAH formation mechanism up to pyrene through GC-MS quantification in laminar reactors, though their model underpredicted soot volume fractions by two to three orders of magnitude. The Blanquart mechanism [22] and its improved Narayanaswamy version [24] extended fuel applicability from methane to isooctane through validation with aromatic compounds like toluene and ethylbenzene. Chernov et al. [22] enhanced the Slavinskaya mechanism [25] by incorporating PAH growth pathways, substantially improving soot volume fraction predictions in methane/ethylene-rich flames. Current models still face limitations including narrow temperature applicability and inadequate pressure dependence considerations. Nevertheless, researchers continue refining existing mechanisms within their applicable ranges as fundamental research tools. Savchenko et al. [26] employed light hydrocarbon oxidation mechanisms [18] with thermodynamic equilibrium principles to predict maximum product yields and evaluate coking risks. Voloshchuk et al. [27] developed reduced-order models based on GRI-Mech 3.0 for POX reactor simulations. Zhang et al. [28] conducted CFD simulations of POX reactors using GRI-Mech 3.0 to examine how reaction conditions affect acetylene yields. Given the critical temperature-pressure coupling effects, future work must develop comprehensive PAH growth models to provide theoretical foundations for process optimization and reactor design.
To more accurately elucidate the formation mechanisms of both target products and PAHs in POX of natural gas, this study proposes an innovative hierarchical composite mechanism construction methodology. The detailed mechanisms for hydrocarbons and hydrogen with carbon numbers ranging from 1 to 6 (C0-C6), hydrocarbons with carbon numbers ranging from 5 to 15 (C5-C15), and hydrocarbons with a carbon number of 16 (C16) were constructed using Reaction Mechanism Generator (RMG) [29], followed by product-directed simplification for C5-C15 through Ansys Workbench. These subsystems were integrated into a final HI-Mechanism containing 397 species and 5135 reactions. Experimental validation confirmed the HI-Mechanism’s accuracy. Chemkin-Pro analyses including production rates, sensitivity, and pathway elucidated key reactions governing product selectivity and PAHs formation. The HI-Mechanism provides reactor optimization guidance while its hierarchical approach reduces computational cost without sacrificing C1-C6 accuracy. This methodology enables new research avenues for complex systems and promotes cleaner natural gas conversion.
2. Computational Details
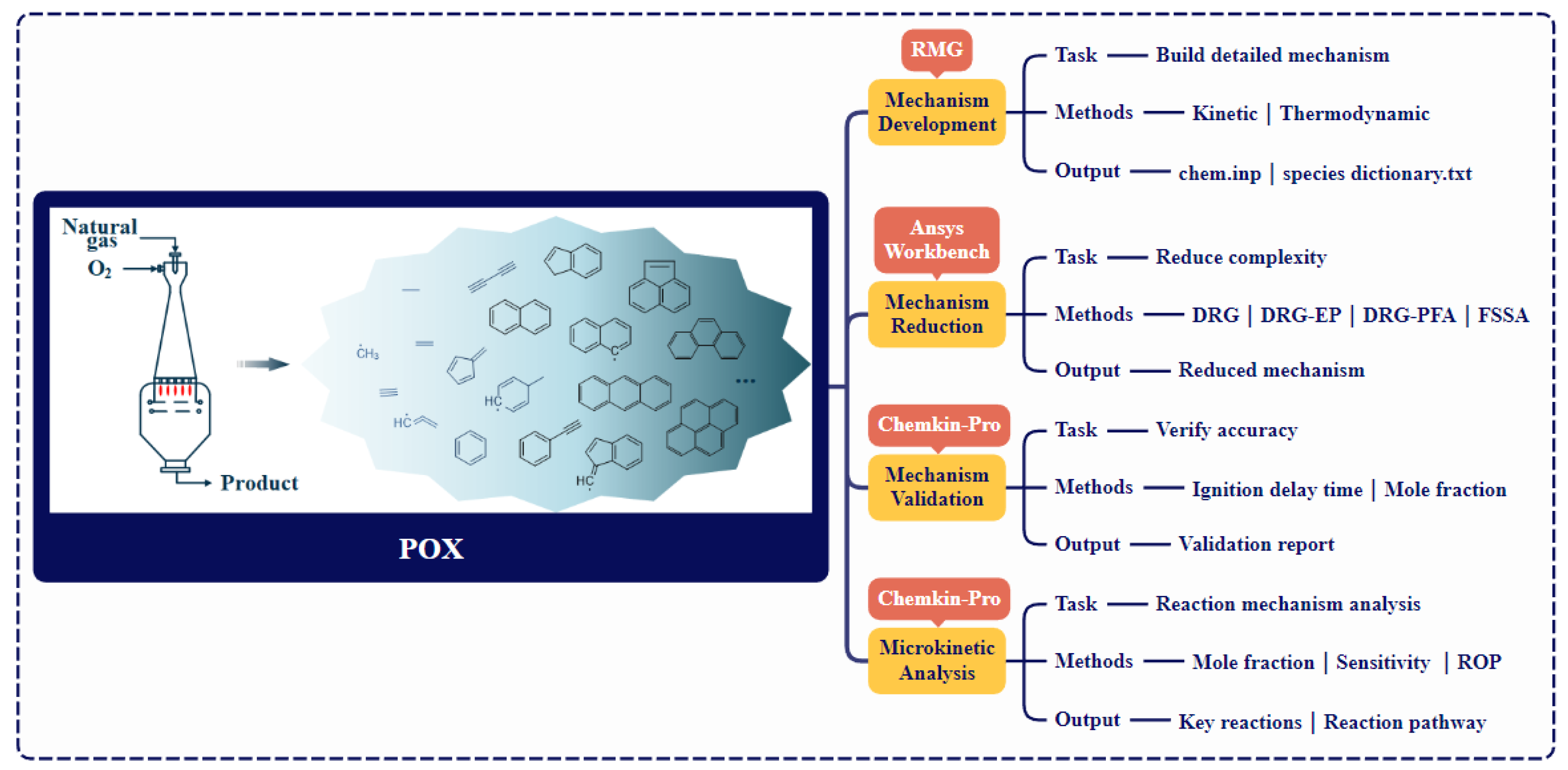
A systematic four-step methodology was implemented (Figure 1). A detailed gas-phase mechanism was developed using RMG [29]. For input file format and detailed specifications, please visit https://rmg.mit.edu/. Product-directed simplification was then performed using Ansys Workbench, yielding the final Hierarchical-Integrated Mechanism. Subsequently, the HI-Mechanism was validated against experimental data using Chemkin-Pro 2021. Finally, microkinetic analyses identified dominant pathways for target products and PAHs formation.
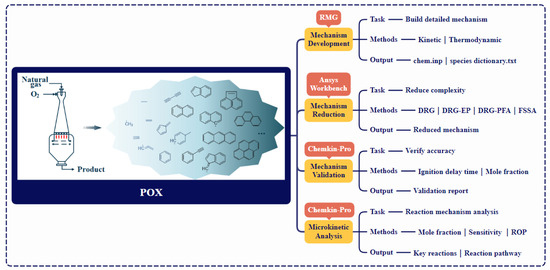
Figure 1.
Computational framework for modeling POX of natural gas.
2.1. Reactor System and Process Parameters
The adiabatic isobaric batch reactor model in Chemkin-Pro was employed to simulate POX of natural gas processes. Two key assumptions were adopted: (i) steady-state adiabatic operation (no heat exchange); (ii) negligible coke-induced fouling effects. These simplifications allowed isolation of intrinsic POX kinetics by excluding transient heat transfer and multiphase coke dynamics. It should be noted that while theoretically justified, industrial applications must account for coke fouling effects on reactor performance.
The HI-Mechanism was designed to simulate POX of natural gas with emphasis on condition-dependent product/soot precursor yields. The parametric study examined: temperatures (1000–2000 K, Δ200 K); 1 bar pressure; O2/CH4 molar ratio (0.4–0.8, Δ0.05).
2.2. Development of HI-Mechanism
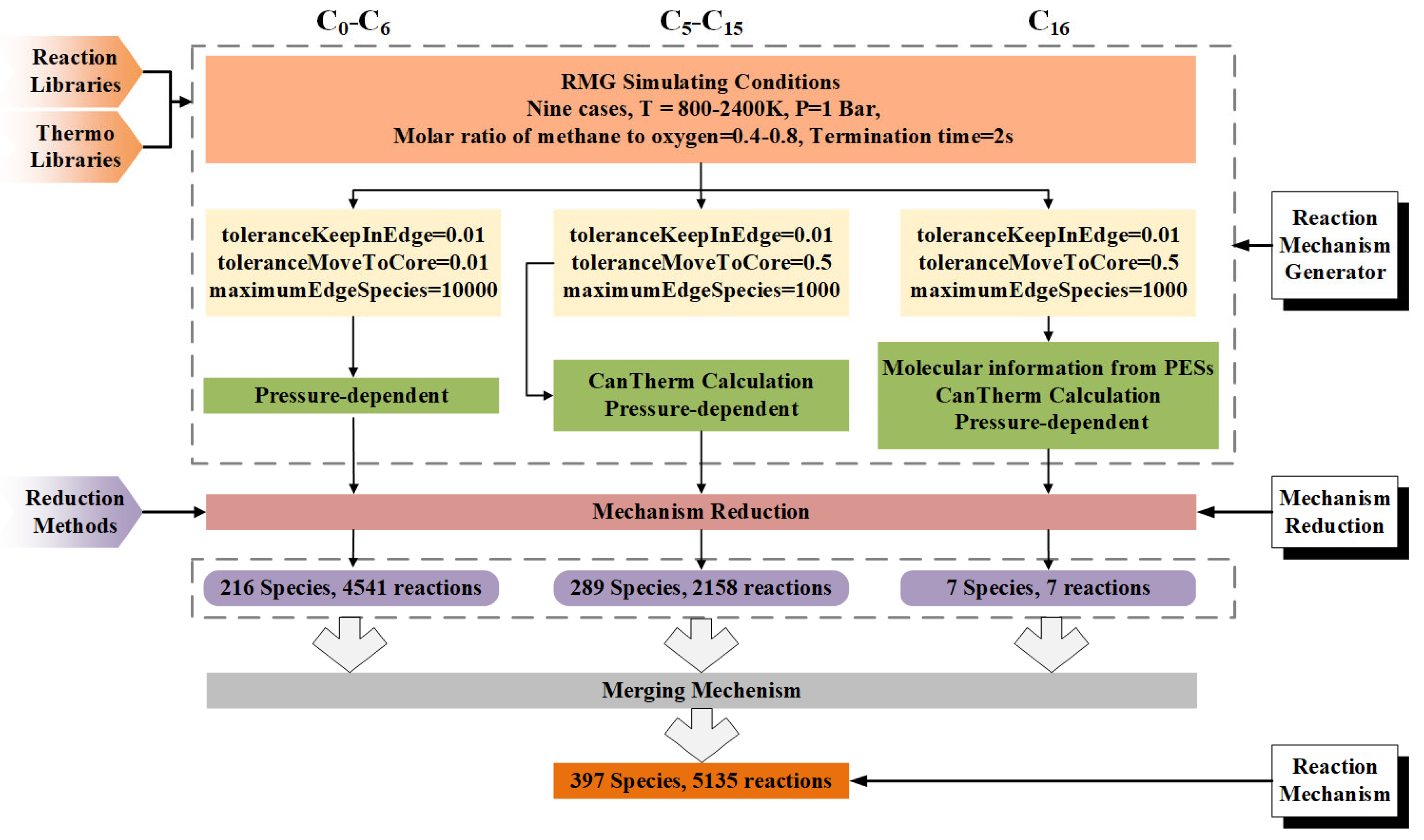
The HI-Mechanism was structured with three reaction stages: (i) C0-C6 to mono-aromatics, (ii) mono- to di-aromatics, and (iii) di-aromatics to PAHs. As illustrated in Figure 2, detailed mechanisms for C0-C6, C5-C15 and C16 hydrocarbons were first developed using RMG. The procedure included: (a) complete pathway identification, (b) rate constant calculation, and (c) thermodynamic parameter determination to ensure mechanistic completeness. The C5-C15 mechanism underwent product-oriented simplification via Ansys Workbench. The integration of these sub-mechanisms produced the final HI mechanism, consisting of 397 species and 5135 reactions. All mechanistic data, including thermodynamic and kinetic parameters, are provided in Supporting Information S1, while molecular structures (adjacency lists) are detailed in Supporting Information S2. The complete parameter sets (kinetic/thermodynamic) are presented in the following section.
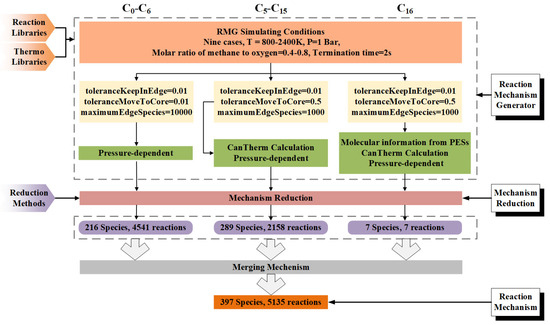
Figure 2.
Workflow of the Hierarchical-Integrated Mechanism (HI-Mechanism) development.
2.2.1. Kinetic Parameter Database Development
The Reaction Mechanism Generator (RMG), while typically estimating kinetic parameters using template-based rate rules, may yield substantial errors for sensitive reactions. Key reaction parameters were refined through high-level quantum chemistry calculations and experimental validation to enhance RMG’s predictive accuracy. Importantly, C0-C2 reaction kinetics were identified as critical for POX of natural gas mechanism fidelity. The FFCM-1 mechanism [20] was adopted as the C0-C2 reference due to its established reliability. The mechanism was augmented with Burke’s H2/O2 kinetics [30] and Senosiain’s C2/H2 + OH network [31] for comprehensive small-molecule coverage.
Propargyl (C3H3) radical recombination was identified as the critical initiation step for aromatic growth in combustion systems. Miller’s propargyl recombination mechanism [32] was implemented for smono-aromatic formation pathways. Aromatic growth pathways were constructed based on Kislov’s G3(MP2,CC)//B3LYP toluene/indene reactions [33] and Mebel’s TST calculations [34,35] for indene → naphthalene conversion. Naphthalene formation pathways were reinforced with Vervust’s and Long’s CBS-QB3 calculations [36,37].
Higher-ring PAHs formation incorporated Kislov’s pressure-dependent rates for acenaphthylene/anthracene [38]. The complete network included Aguilera’s phenanthrene pathways [39]. The main pathways for pyrene synthesis under POX conditions were two: (a) Pyrene radical + C2H2 electrophilic addition [25], (b) Ring expansion reaction based on HACA [40,41]. Therefore, the reaction kinetics of pyrene was added by Zhao [42] based on G3(MP2,CC)//B3LYP calculations, providing comprehensive soot precursor characterization.
2.2.2. Thermodynamic Parameter Database Development
The base reaction network was established using FFCM-1 and Burke’s H2/O2 mecha-nisms [20,30] for small hydrocarbon conversion to monoaromatics. These foundational mechanisms provided the initial thermodynamic data for the core species and reactions. Thermodynamic consistency was achieved through primaryThermoLibrary integration, which leverages group additivity methods to ensure consistent thermochemical properties across the network and corrects any inconsistencies inherent in the base mechanisms. For methane-rich systems, the DFT-QCI library [41] was added specifically to address missing propargyl recombination pathways and cyclic singlet carbene intermediate reactions, providing higher fidelity thermochemistry for these critical low temperature intermediates and pathways. High pressure-limit benzene formation via propargyl recombination was incorporated using CBS-QB3 calculations [41,43,44,45,46,47,48,49], a high-level quantum chemistry method chosen for its accuracy in predicting thermochemistry and barrier heights for this key initiation step.
Di-aromatic formation was modeled via three steps [35]: (i) mono-aromatic-unsaturated hydrocarbon coupling, (ii) adduct cyclization, and (iii) β-scission stabilization. Accurate thermochemistry for the complex intermediates involved in these ring-growth steps was essential. The thermodynamic library was augmented with Vervust’s critical species [36] for ring-growth accuracy, providing benchmark-quality thermochemical data (e.g., enthalpies of formation) derived from high-level theoretical calculations for key polycyclic aromatic hydrocarbon (PAH) radicals and intermediates. PAH growth via HACA (Hydrogen Abstraction Acetylene Addition) mechanisms was characterized using Cantherm-level quantum methods [40], which computed thermodynamic properties (e.g., ΔHf°, S°, Cp(T)) for the relevant radical and stable PAH species involved in these sequential growth steps based on optimized geometries and vibrational frequencies. Thermodynamic consistency across the entire mechanism, from small core species to large PAHs, was achieved through integration of SABIC-aromatics [50] and heavy-oil libraries [1,36,51,52,53], which provided validated thermochemical data for a wide range of aromatic and polyaromatic species, ensuring the reliability of the thermodynamic driving forces governing PAH growth. The systematic parameter optimization strategy significantly improved model predictive capability for aromatic growth. The current model focuses on predicting gaseous products and aromatic precursors (up to 4 rings), without explicitly including soot formation pathways.
2.3. Target-Oriented Mechanism Reduction
The implementation of detailed reaction mechanisms in complex systems presents three fundamental challenges: prohibitive computational cost, excessive memory demands, and convergence difficulties. These challenges necessitate advanced mechanism reduction techniques that preserve essential chemistry while improving computational tractability—a key research priority in modern reaction kinetics.
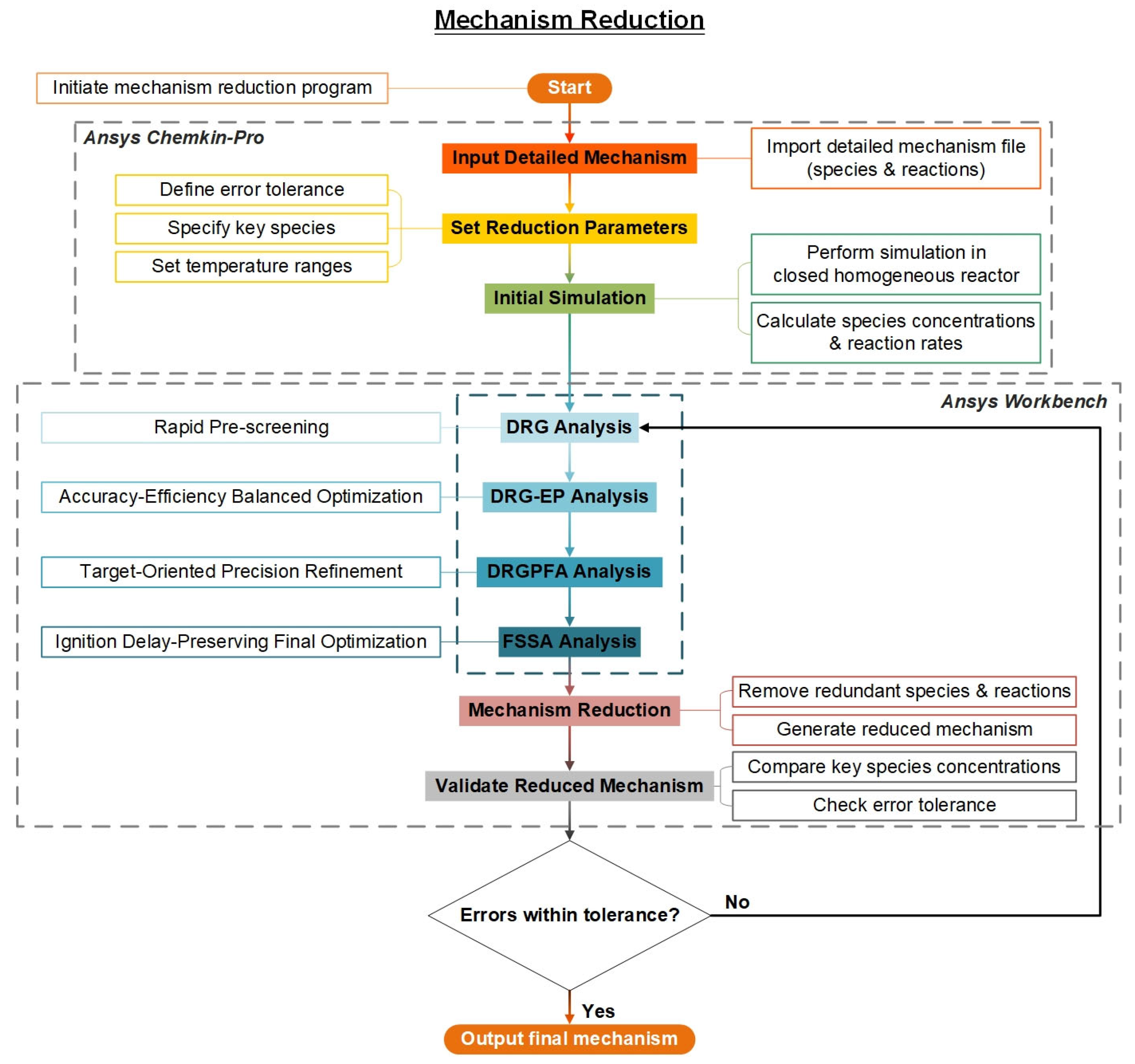
The primary objective of mechanism reduction is to preserve prediction accuracy of key parameters (e.g., species concentrations, reaction rates) while substantially reducing computational complexity. Species were classified into three categories: critical (e.g., C2H6, C2H4, C2H2, C6H6 in POX), condition-dependent, and negligible based on production rates and pathway connectivity. Effective reduction requires three key steps: retaining all critical species/pathways, selectively removing minor/negligible species, and quantifying error impacts on global sensitive parameters against preset thresholds. A tiered reduction framework was implemented in Ansys Workbench combining four methods: directed relation graph (DRG) [54], DRG with error propagation (DRG-EP) [55], path flux analysis DRG (DRG-PFA) [56], and full species sensitivity analysis (FSSA) [56].
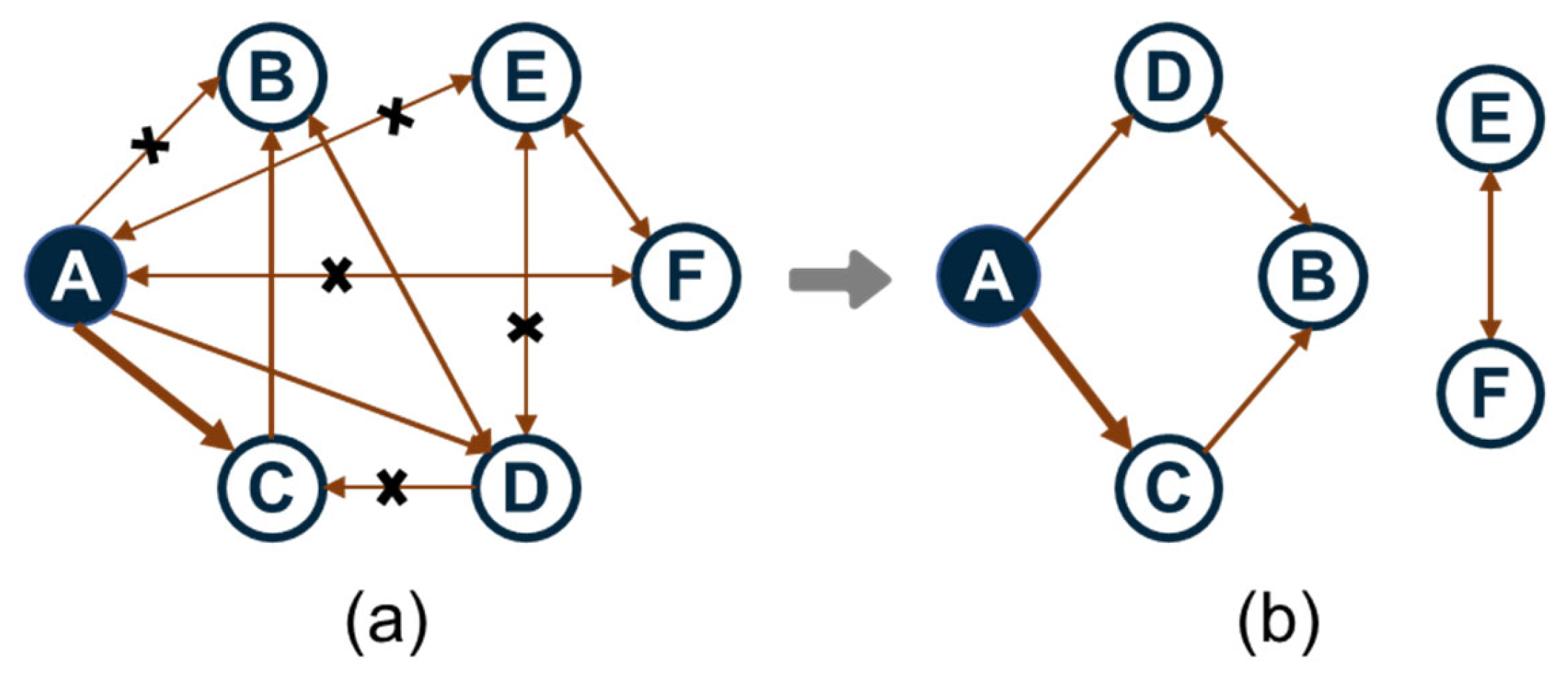
Among all these methods, the DRG method is the backbone which efficiently identifies redundant species by quantifying direct coupling strength between species through reaction rates and stoichiometric coefficients. As schematized in Figure 3, each vertex represents a species in the full mechanism and each directed edge denotes the immediate dependence of one species to another. The interaction coefficient εAB quantities the contribution of species B to the production rate of species A:
where ξA,i is the stoichiometric coefficient, γi is the reaction rate, and δB,i is the participation flag. Species with εAB below threshold are removable.
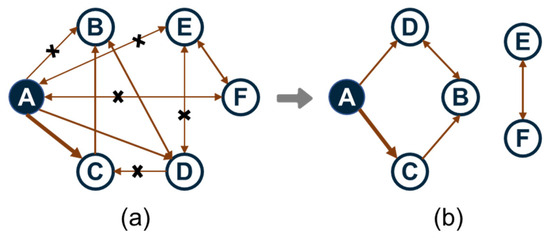
Figure 3.
Schematic diagram of a directed relation graph: (a) before dimensional reduction; (b) after dimensional reduction.
Obviously, εAB measures the normalized error of the production rate of species A incurred by the elimination of all the reactions that involve species B. Then, a directed relation graph is constructed by a search procedure in which there is a directed edge from A to B if and only if εAB exceeds or equal to a certain threshold value as displayed in Figure 3b.
Regarding the definition of dependencies between species, Pepiot-Desjardins and Pitsch [57] argued that a more accurate way is to evaluate the production and consumption separately instead of the net contribution, Therefore, DRG-EP extends DRG by considering multi-step path effects through error propagation factors:
where PA and CA represent the production and consumption rates of species A, respectively, which are expressed with:
In the DRG-EP method, the effect of removing species group is also included, since the previously removed species are considered recursively. In contrast to the DRG method, the DRG-EP incorporates the notion of error propagation in evaluating the error RAB which takes the length of the path the error has to propagate into account:
where rij represents direct error between adjacent species. This prevents oversimplification of long-range dependencies.
By definition, the DRG and DRG-EP methods build upon the absolute and net reaction rates for active species selection. However, the use absolute reaction rates in DRG makes the relation index not conservative, while DRG-EP only picks up the strongest reaction path which cannot identify the species flux physically. DRG-PFA evaluates pathway contributions to target species, eliminating paths below flux thresholds. Particularly effective for unsteady POX simulations with varying T/P conditions.
FSSA employs Monte Carlo sampling or adjoint methods to compute global sensitivity indices. Ensures mechanism robustness across operating conditions.
The simplification procedure is designed based on two fundamental principles: (1) a hierarchical error control strategy progressing from local to global scales (DRG→DRG-EP→FSSA), and (2) pathway optimization following structural simplification (DRG-PFA). The implementation involves four key steps: First, direct weak interactions are eliminated using DRG. Second, multi-step error propagation is addressed through DRG-EP. Subsequently, pathway fluxes are optimized via DRG-PFA after completing the structural simplification. Finally, global sensitivity validation is performed using FSSA.
As shown in Figure 4, the simplified process was divided into four stages. (i) Reference simulation: The detailed mechanism was executed in a closed homogeneous batch reactor to establish baseline profiles of temperature, pressure and species concentrations (0D simulation with 500+ output time points). (ii) Hierarchical reduction: Sequential application of DRG (mechanism scale reduction), DRG-EP (depth reduction), DRG-PFA (superior critical path optimization) and FSSA (global sensitivity validation). (iii) Error control: Key combustion parameters (ignition delay, peak T, major species) were compared between detailed and reduced mechanisms, enforcing <5% deviation (normalized RMS error). (iv) Verification of the robustness of the simplified mechanism over a wide range of operating conditions.
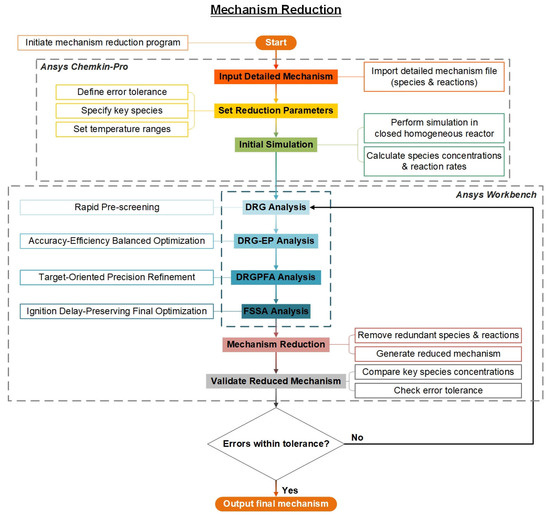
Figure 4.
Workflow of the HI-Mechanism reduction.
3. Mechanism Validation and Microkinetic Analysis Methods
Accurate prediction of mixture ignition characteristics and key species concentration profiles is essential for developing reliable chemical kinetic mechanisms in POX of natural gas simulations. This study established a multi-dimensional validation framework incorporating three critical aspects: ignition delay times, key species concentration profiles, and industrial operating conditions. Furthermore, the predictive reliability of the HI-Mechanism for POX of natural gas was systematically evaluated through comprehensive validation.
Sensitivity analysis numerically computes normalized influence coefficients of kinetic parameters (pre-exponential factors, activation energies) on target species concentrations [58], identifying the top 5% most sensitive elementary reactions through Morris screening with 10% parameter perturbation. Production rate analysis quantifies pathway significance by evaluating each reaction’s contribution to species formation or consumption. Time-integrated analysis preserves cumulative contribution while applying a threshold for individual pathways. Reaction path analysis reconstructs complete networks from light hydrocarbons to PAHs by tracking key intermediates, visualized through multi-level pathway maps. The framework establishes rate-determining steps, pathway dominance, and network connectivity. Cross-validated results demonstrate robust mechanism coverage with minimal discrepancy.
3.1. Ignition Delay Time
Ignition is defined as the transition of fuel-oxidizer mixtures from chemically inert state → slow oxidation → stable exothermic state, culminating in flame formation at specific spatiotemporal coordinates. This critical transition in reaction kinetics manifests as an abrupt shift from quasi-steady state to rapid chain-branching reactions within microseconds [59]. Ignition delay time quantifies the interval between initial state and significant heat release under specified T/P/Φ conditions, involving three key processes: accelerated chain-branching, exponential radical growth, and sustained heat release. Measured via shock tubes (5–20 μs resolution) or rapid compression machines (RCMs) [60,61]. In acetylene production reactors, mixture residence time in the premixing zone must be maintained below the autoignition delay (typically <50 ms at 800–1000 K) to prevent premature ignition hazards. This requires three critical measures: strict temperature/pressure monitoring, turbulent mixing enhancement, and radical scavenger injection.
3.2. Key Component Concentrations
The HI-Mechanism was employed in 0D simulations of CH4/O2/N2 premixed flames to examine two key aspects: radical chain reaction dynamics and product formation pathways through concentration profile validation. In addition, the variations in selectivity and molar yield of key products with temperature were investigated. Given the large number of species present in the reaction system, the methane selectivity (Si) for key product i was calculated based on molar carbon basis, as follows:
To objectively evaluate the predictive capability of the reaction mechanism, this study employs the following statistical metrics to quantify deviations between simulated predictions and experimental observations:
Mean Absolute Error (MAE) measures the average absolute deviation between predicted and observed values (units: mol/mol). As a robust metric insensitive to outliers, it effectively characterizes typical prediction errors:
where and represent the ith predicted and experimental values, respectively, and n denotes the total number of samples.
Root Mean Squared Error (RMSE) amplifies the contribution of larger errors through squaring while maintaining dimensional consistency (units: mol/mol). This metric demonstrates higher sensitivity to extreme deviations:
A value approaching zero indicates superior prediction accuracy.
Root Mean Squared Logarithmic Error (RMSLE) provides stabilized error measurement through logarithmic transformation, particularly suitable for datasets with wide-ranging or skewed distributions:
The Coefficient of Determination (R2) quantifies the model’s explanatory power for data variability (dimensionless, range: [0, 1]):
where denotes the mean of experimental observations. R2 = 1 indicates perfect fitting, while R2 = 0 suggests the model performs no better than mean-value prediction.
3.3. Sensitivity Analysis
Recent computational advances have established sensitivity analysis as the primary tool for investigating multiparameter reaction systems. Sensitivity coefficients were obtained by systematically perturbing three fundamental kinetic parameters: pre-exponential factors, activation energies, and rate constants, while maintaining minimal variation in other parameters [58]. The ODE system for ideal reactors yields sensitivity coefficients through:
where y is the vector of variables (e.g., mole fractions), x the independent variable, and α the parameter vector. For unsteady systems, the sensitivity coefficient of the ith variable to the jth parameter is defined as:
with normalized form:
where S and nS denote sensitivity and normalized sensitivity coefficients.
3.4. Rate-of-Production and Reaction Pathway Analysis
ROP analysis quantifies elementary reaction contributions through net production rates and consumption pathway weights. 0D systems enable faster computation compared to spatial models, handling of numerous elementary reactions, and high dominant pathway identification accuracy. In 0D homogeneous systems, the molar production rate Pk of species k is defined as:
where vk,i is the stoichiometric coefficient and ωi the reaction rate. Normalized contributions satisfy:
Normalized contributions are defined for cross-comparison:
Transient simulations employ time-discretized ROP calculations, where iterative methods ensure accurate real-time dynamics tracking.
4. Results and Discussion
4.1. Mechanism Validation
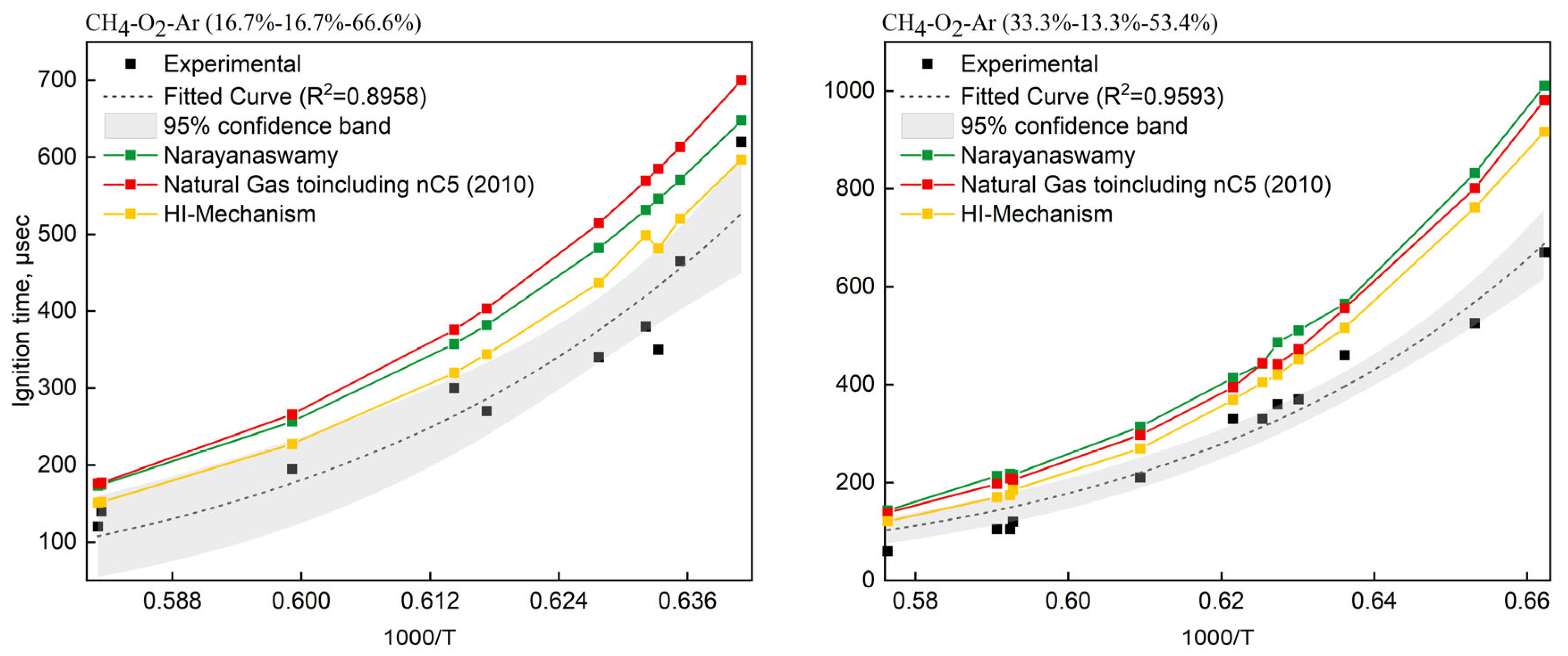
Two CH4/O2/Ar mixtures from Seery et al. [62] were selected: (i) 16.7% CH4—16.7% O2—66.6% Ar (O2/CH4 molar ratio = 1.0, 13.4 mol/m3) and (ii) 33% CH4—13% O2—54% Ar (O2/CH4 molar ratio = 0.4, 29.1 mol/m3). The HI-Mechanism was validated against Narayanaswamy [24] and NG-nC5(2010) [17,19,63,64] as reference mechanisms. Figure 5 presents the temperature-dependent ignition delays, comparing experimental data (scatter points ±95% CI) with simulated results from three mechanisms. Comparisons revealed systematic overprediction of ignition delays by all three mechanisms (HI-Mechanism, Narayanaswamy [24], NG-nC5(2010) [17,19,63,64]) versus experimental data. Through systematic comparative studies and literature validation, we attribute the overestimation of ignition delay time by the model to the following key factors: First, the reaction between methyl radicals and molecular oxygen is one of the critical steps controlling ignition. Research indicates that this reaction may proceed through two competing pathways: one directly forming CH3O2 (methylperoxy), and the other generating CH2O (formaldehyde) and OH radicals via hydrogen abstraction [65]. The relative importance of these pathways varies with temperature and pressure, but the aforementioned mechanism fails to accurately describe this dynamic variation, leading to deviations in simulation results under specific conditions. Second, in actual combustion systems, nitrogen not only acts as a diluent but may also participate in reactions, thereby affecting radical concentrations. Lastly, real experimental setups involve wall heat losses, whereas the simulation assumes an ideal reactor, which is insufficient to capture ignition characteristics. Zhang et al. [66] also noted that the assumption of an ideal constant-volume reactor can lead to overestimation of ignition delay times in low-temperature regions.
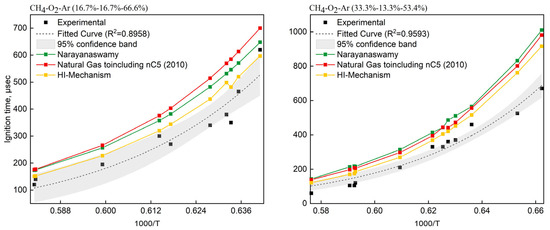
Figure 5.
Comparison of experimental [62] and simulated ignition delay times for CH4/O2 mixtures in shock tube.
Moreover, we observed a distinct phenomenon where the methane ignition delay time exhibited a significant decrease at T = 1579 K (1000/T = 0.6333), representing a characteristic kinetic feature. This phenomenon was attributed to the synergistic effects of two key mechanisms: (1) The accelerated decomposition of HO2 radicals (HO2→OH+O) substantially increased the concentration of OH radicals in the system; (2) The CH3 oxidation pathway transitioned from the CH3O2-dominated mode at low temperatures to the fast CH2O/CO route at high temperatures. The dramatic increase in OH radical concentration significantly enhanced chain-branching reactions, thereby markedly accelerating the methane oxidation process. These observations were fully consistent with experimental findings. Compared with conventional mechanisms, the HI-Mechanism successfully predicted this critical kinetic transition, demonstrating its superior mechanistic performance.
To address these challenges, the HI-Mechanism significantly improves prediction accuracy by introducing quantum chemistry corrections. We employed the DFT_QCI and CBS_QB3 methods to correct the thermodynamic parameters of key reactions, including the methyl radical and molecular oxygen reaction (see Section 2.2.2 for details). Additionally, we incorporated reactions involving nitrogen to accurately capture the regulatory effect of NO formation on the radical pool under lean combustion conditions. These enhancements allow the model to maintain computational efficiency while significantly improving predictive accuracy.
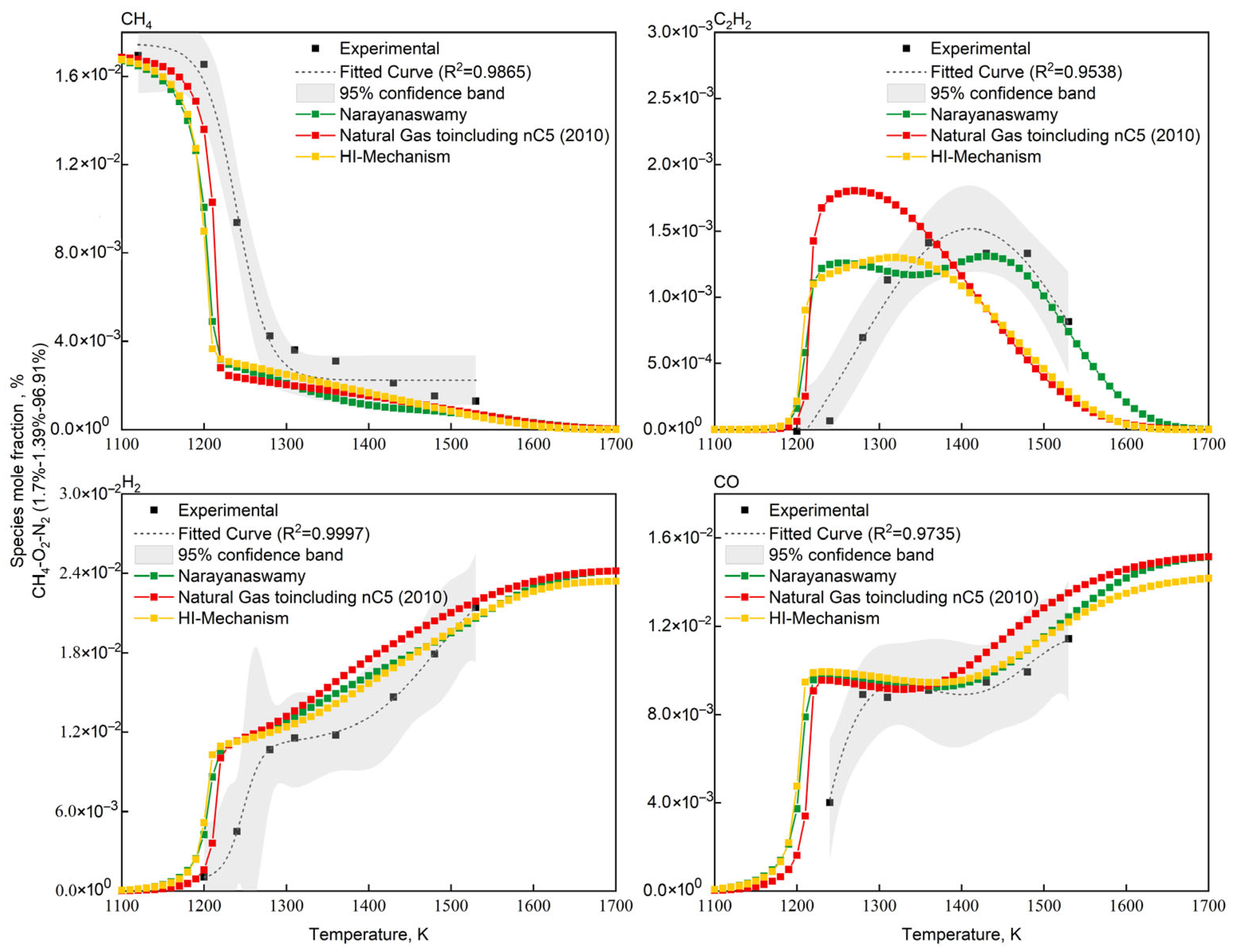
Validation of the HI-Mechanism was performed using Konnov’s lean CH4/O2/N2 systems [67] with mixtures: 1.7% CH4—1.39% O2—96.91% N2 (O2/CH4 molar ratio = 0.82) in adiabatic batch reactors. The validation data were selected based on the characteristic conditions of non-catalytic partial oxidation of natural gas, where methane is in excess with an O2/CH4 molar ratio below 1, to better represent actual industrial operating conditions. Consistent with Figure 5 methodology, Narayanaswamy and NG-nC5(2010) served as benchmarks. Figure 6 demonstrates the CH4/C2H2/H2/CO concentration evolution, showing both experimental measurements and three mechanism predictions. Figure 7 showed the quantitative assessment of predicted key species concentrations in the methane/oxygen mixture.
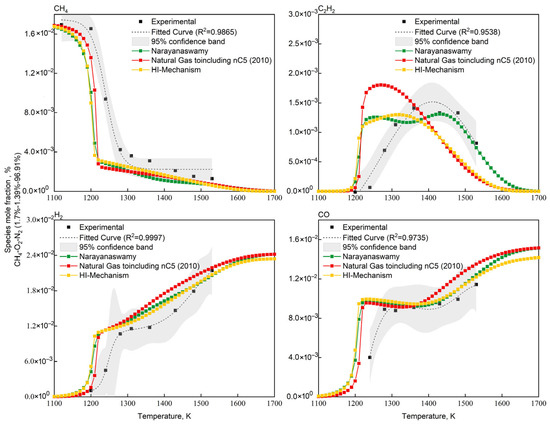
Figure 6.
Comparison of experimental [67] and simulated concentration profiles of key species for CH4/O2 mixtures in adiabatic batch reactor.
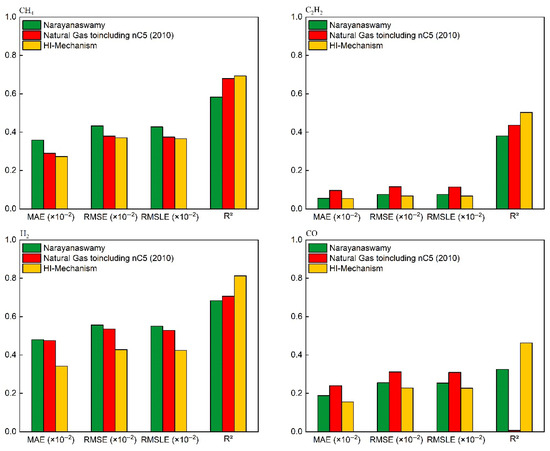
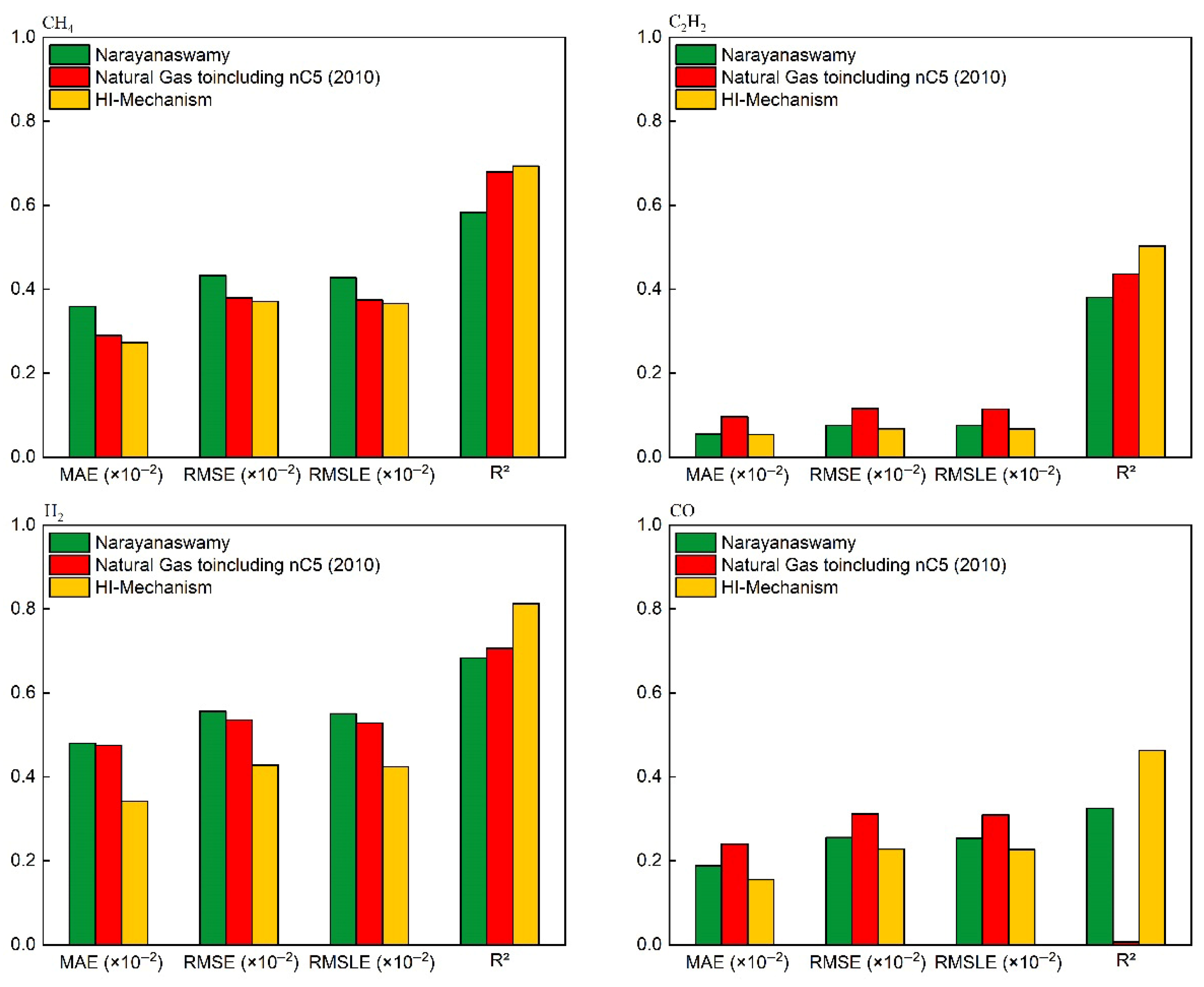
Figure 7.
Quantitative performance evaluation of key species concentration predictions for CH4/O2 mixtures (comparison with experimental–simulation results in Figure 6).
As shown in Figure 6 and Figure 7, all three mechanisms exhibit consistent deviations: underprediction of CH4 concentration and overprediction of CO/H2 concentrations. This systematic discrepancy fundamentally arises from structural differences between the idealized conditions in numerical simulations (adiabatic boundaries and homogeneous mixing) and the non-ideal experimental conditions (wall heat loss, mixing delays, and kinetic delays during quenching). The simulations assume that the homogeneous mixing of methane and oxygen remains unaffected by ongoing reactions, whereas actual reaction processes involve local concentration gradients. The additional time required for physical mixing leads to higher residual CH4 concentrations in experiments. During experimental quenching, the water-gas shift reaction continues to occur, resulting in lower measured concentrations of CO and H2, as well as a delayed peak concentration of C2H2. To address these issues, future studies could incorporate heat loss models and quenching models to correct the simulation process, while avoiding sampling delays in experiments. Nevertheless, the HI mechanism demonstrates superior accuracy in predicting acetylene concentrations compared to other mechanisms, primarily due to corrections made to the thermodynamic parameters of acetylene-related reactions through quantum chemical calculations (see Section 2.2). These results indicate that the HI model offers distinct advantages in simulating the chemical processes of methane oxidation reactions.
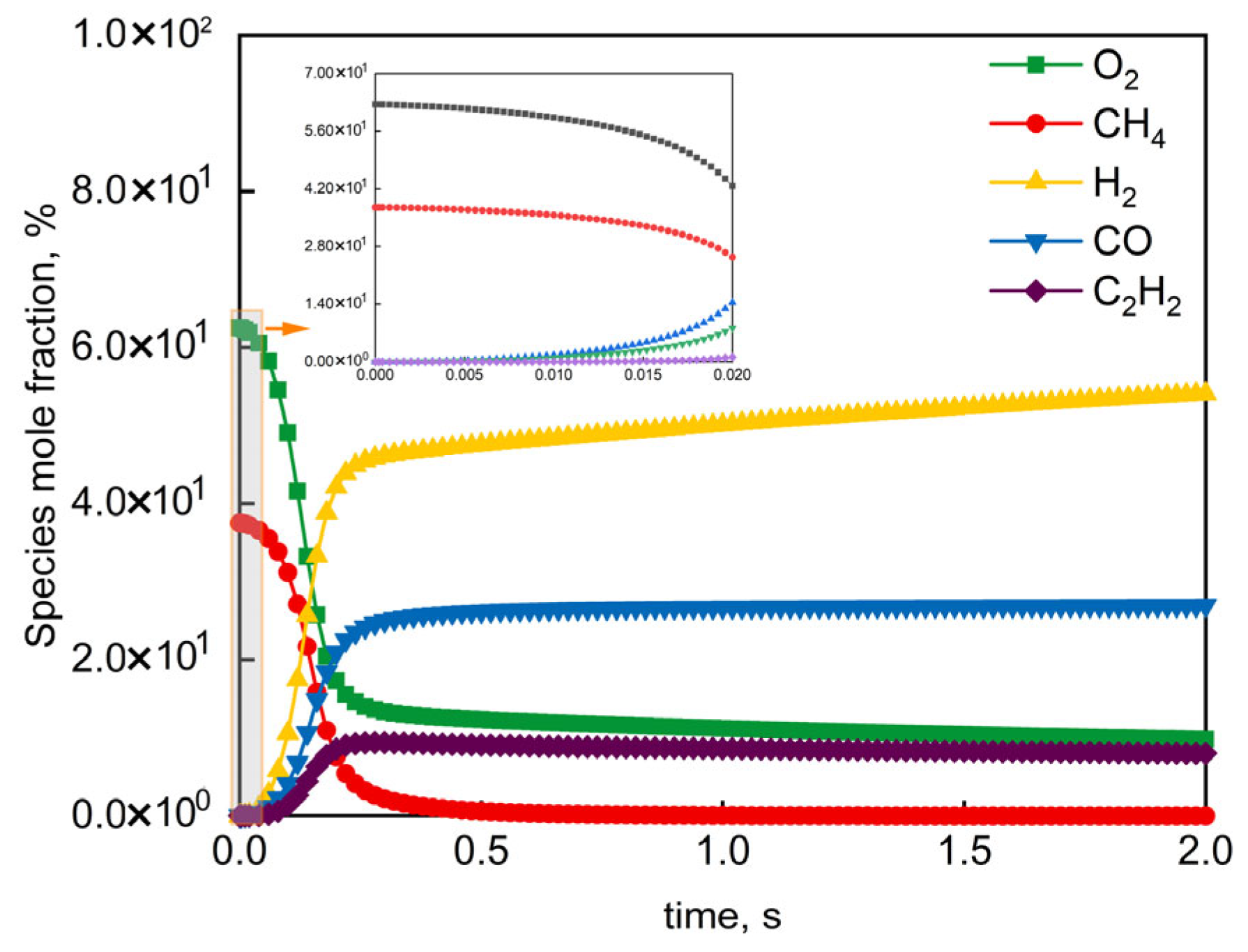
The HI-Mechanism was evaluated at representative industrial reactor conditions [68] (O2/CH4 molar ratio = 0.60, 1 atm). Figure 8 shows the HI-Mechanism accurately captured two-stage autoignition dynamics: (i) slow reactant (CH4/O2) consumption during induction (0–5 ms), and (ii) rapid product (H2/CO) formation in flame development (5–15 ms). The model replicated the characteristic “rapid-rise/slow-growth” trends of H2/CO during flame development, matching industrial observations with <5% maximum deviation. These results validate both the HI-Mechanism’s accuracy in complex network description and its reliability for industrial process prediction.
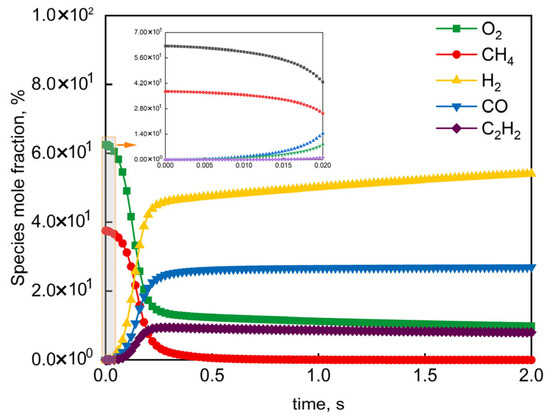
Figure 8.
Simulated mole fraction profiles of reactants and products in CH4/O2 mixture under industrial conditions (O2/CH4 molar ratio = 0.60, atmospheric pressure).
4.2. Microkinetic Analysis
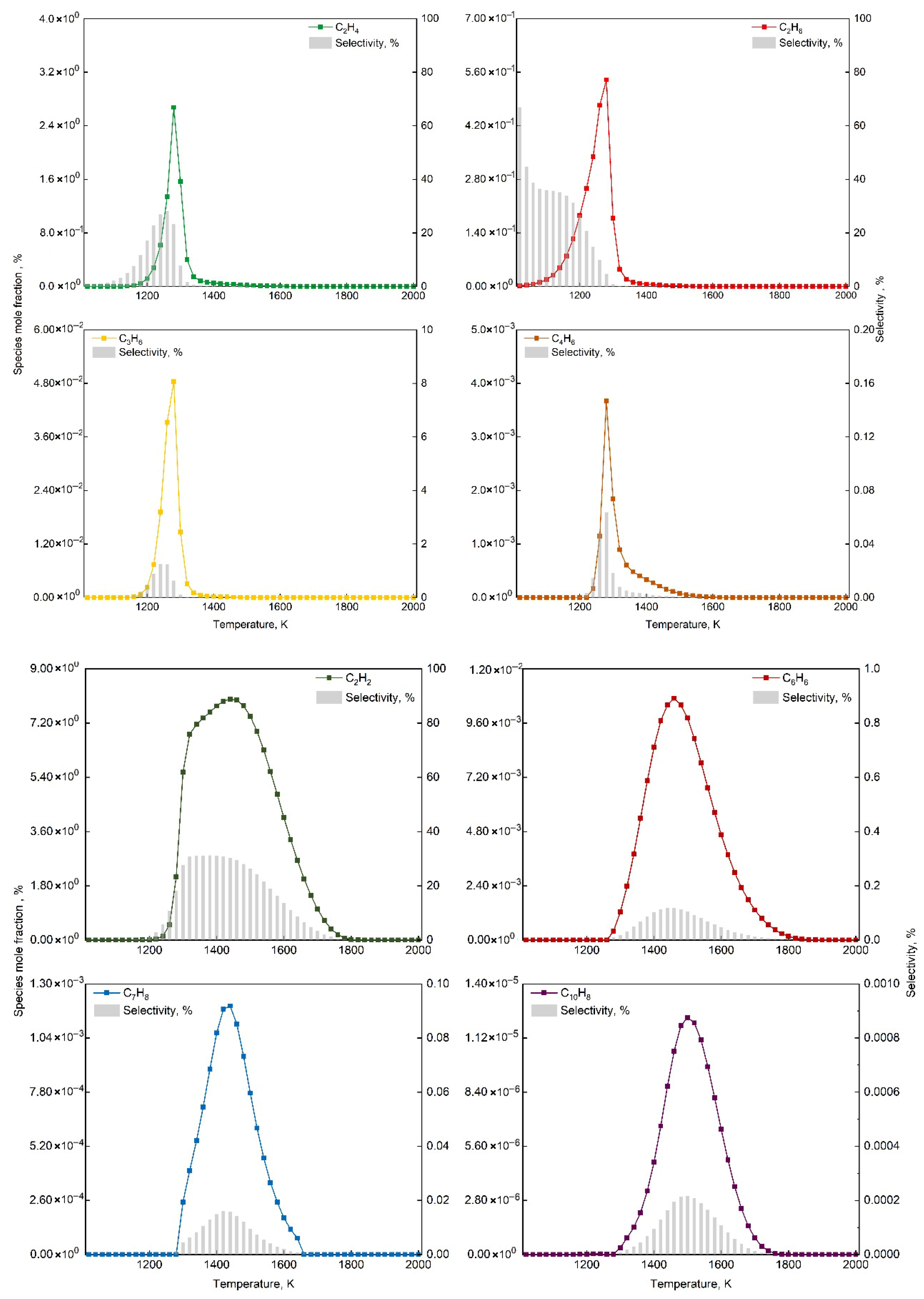
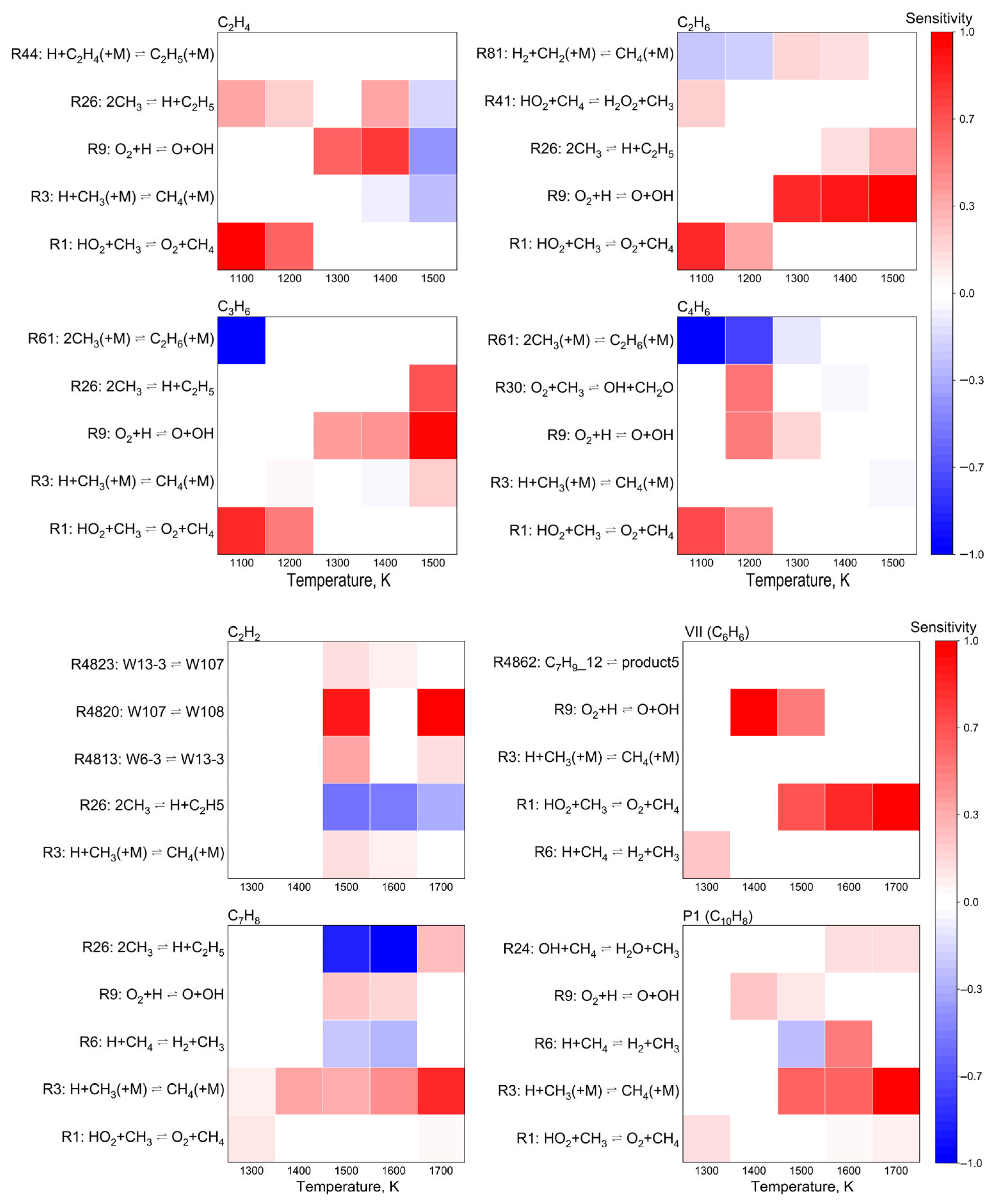
This study employed Chemkin-Pro to analyze the HI-Mechanism, focusing on product formation characteristics at 1 atm with O2/CH4 molar ratio 0.6. Figure 9 identifies optimal temperature ranges for C2H6, C2H4, C3H6, C4H6, C2H2, C6H6, C7H8 and C10H8 formation, showing distinct temperature dependencies between light hydrocarbons and aromatics. Figure 10 analyzes five key elementary reactions per product via normalized sensitivity coefficients (positive: enhancing, negative: inhibiting). Unimolecular reactions (dissociation/coupling) are governed by molecular collisions and energy transfer, while third-body (+M) reactions strongly depend on system pressure and local conditions [69,70].
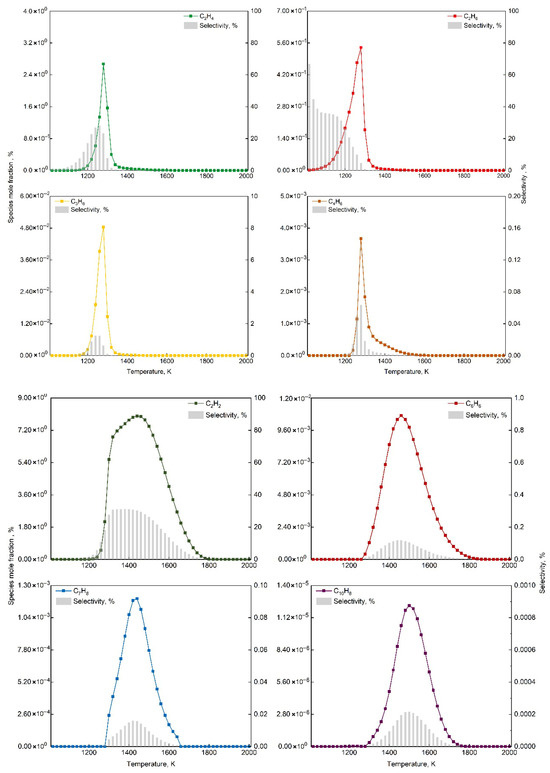
Figure 9.
Temperature-dependent mole fractions of target products and methane selectivity at O2/CH4 molar ratio = 0.6 and 1 bar.
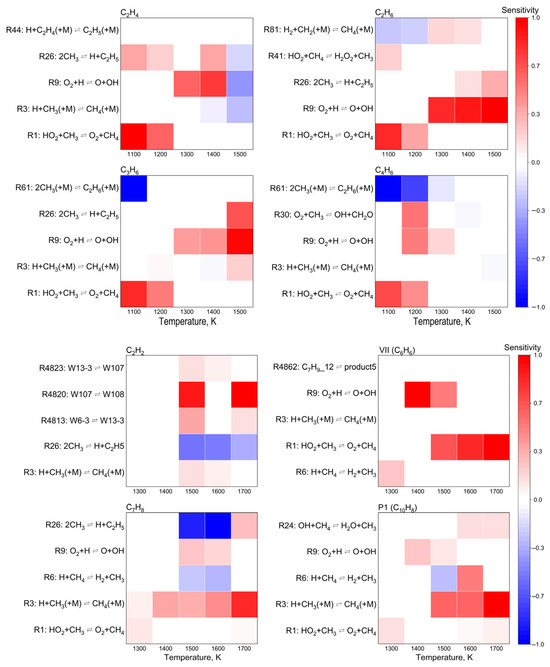
Figure 10.
Normalized sensitivity coefficients of target products at 1.0 atm and O2/CH4 molar ratio = 0.6.
Figure 9 illustrates the temperature-dependent variations in mole fractions of key products and methane selectivity, revealing distinct formation temperature distributions among different hydrocarbon species. The plot clearly demonstrates the rapid formation of ethane and its subsequent dehydrogenation characteristics, along with the temperature distributions for various products. As shown, ethane selectivity remains relatively high during the initial reaction stage but exhibits a significant decreasing trend with increasing temperature. This phenomenon originates from ethane’s generation through the bimolecular recombination pathway of methyl radicals (CH3), followed by sequential dehydrogenation to form other products. This reaction mechanism leads to the characteristic initial increase followed by decrease in methane selectivity of other products with rising temperature.
Figure 9 also shows distinct temperature distributions: light hydrocarbons (C2H4, C2H6, C3H6, C4H6) form at 1100–1500 K (peak 1280 K), while aromatics (C6H6, C7H8, C10H8) require 1300–1700 K (peaks at 1460 K, 1440 K, 1500 K, respectively). This temperature difference reflects activation energy variations: light hydrocarbons form via chain growth (lower Ea), while aromatics require cyclization/condensation. Although classified as light hydrocarbon, C2H2 shows similar Ea to aromatics (peak at 1440 K), due to unique reaction mechanisms and molecular properties. C2H2 formation requires C2H4 deep dehydrogenation, with C-H bond breaking Ea comparable to benzene cyclization initiation. As aromatic precursor, C2H2 formation requires sustained high-energy radical participation, linking its pathways to aromatic production. The narrow 20 K peak gap (C2H2:1440 K vs. C6H6:1460 K) versus 160 K (C2H4:1280 K) reflects <5 kJ/mol Ea difference. The stable C≡C triple bond demands higher energy input, making C2H2 thermodynamically favored at high temperatures like aromatics. Detailed Ea data were available in Supplementary File.
A systematic analysis of temperature-dependent sensitivity coefficients revealed that temperature exerted a profound influence on reaction pathway selectivity. Within the lower temperature regime (1100–1300 K), the formation of light hydrocarbons (C2H4, C2H6, C3H6, and C4H6) was predominantly governed by chain initiation and propagation reactions, with the elementary reaction O2 + ∙H ⇌ ∙O + ∙OH exhibiting the highest sensitivity coefficient, thereby serving as the rate-determining step. Upon increasing the temperature beyond 1300 K, a marked elevation in radical concentrations was observed, which subsequently enhanced the production of acetylene (C2H2) and aromatic compounds. Notably, acetylene demonstrated dual functionality in the reaction network: (i) acting as a crucial precursor for aromatization reactions, while (ii) undergoing competitive inhibition via methyl radical addition. This intricate kinetic interplay accounted for the characteristic maximum in acetylene concentration observed within the intermediate temperature range (1300–1500 K), followed by a subsequent decline at elevated temperatures due to enhanced secondary reactions. Furthermore, the hydrogen abstraction reaction H + CH3(+M) ⇌ CH4(+M) was identified as a pivotal “regulatory switch” in the reaction network. This elementary step not only directly influenced methane formation kinetics but also indirectly modulated aromatic production pathways through its control of methyl radical concentrations. These mechanistic insights provided a fundamental basis for process optimization, suggesting that precise regulation of both temperature and pressure could effectively manipulate the equilibrium position of this key reaction, thereby enabling selective control over product distributions.
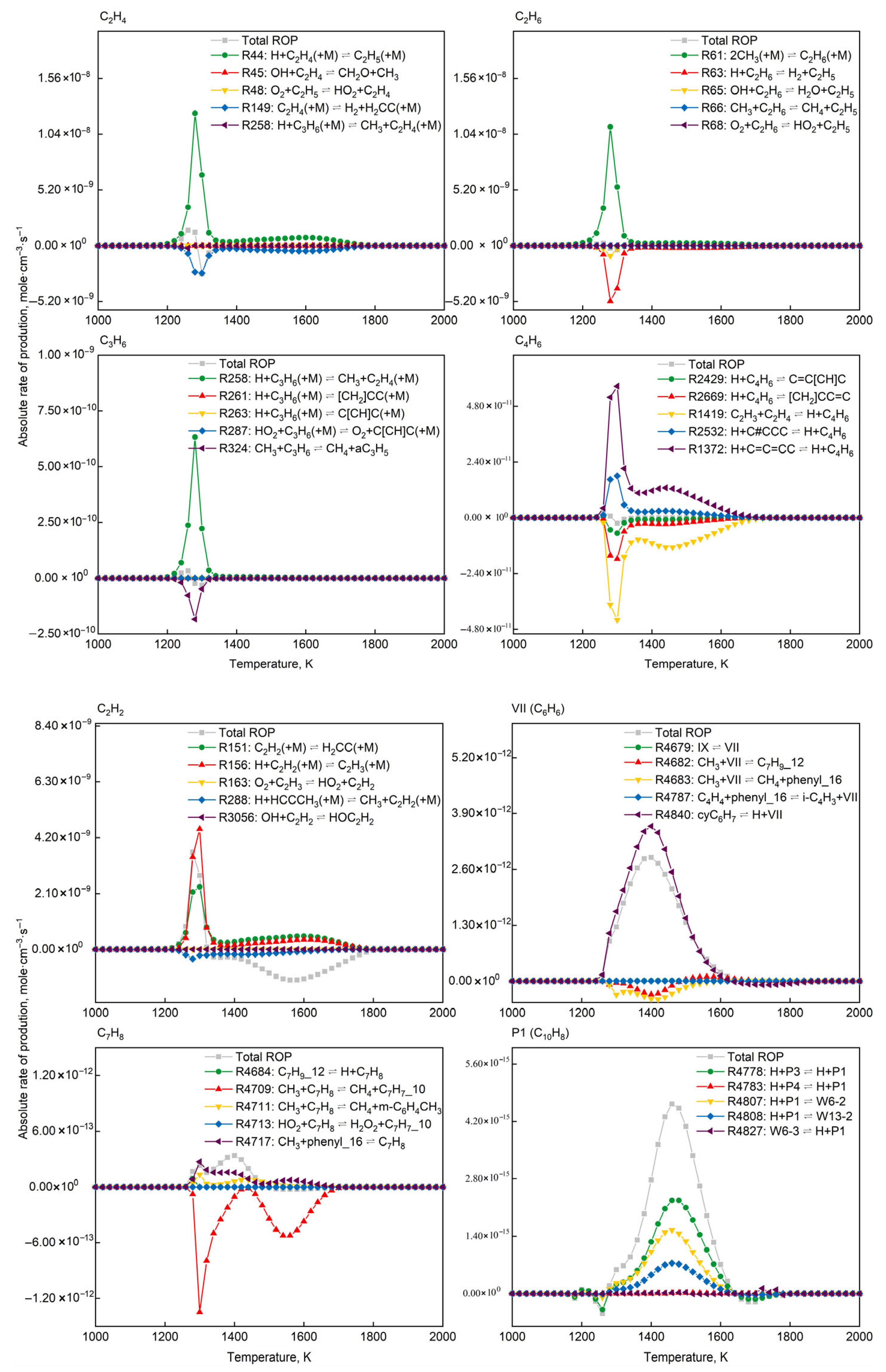
Accurate prediction of target product yields and formation kinetics under high-temperature conditions is crucial for reactor optimization and process control in natural gas non-catalytic partial oxidation. This study employed absolute reaction rate-based quantitative analysis to systematically investigate target product formation pathways.
Figure 11 presents the absolute rate distributions of elementary reactions for target products (C2H4, C2H6, C3H6, C4H6, C2H2, C6H6, C7H8, C10H8) at different temperatures. Forward rates quantify formation kinetics, while negative rates reflect consumption pathways. Comparative analysis of rate distributions across temperatures identified dominant formation pathways and their temperature dependencies.
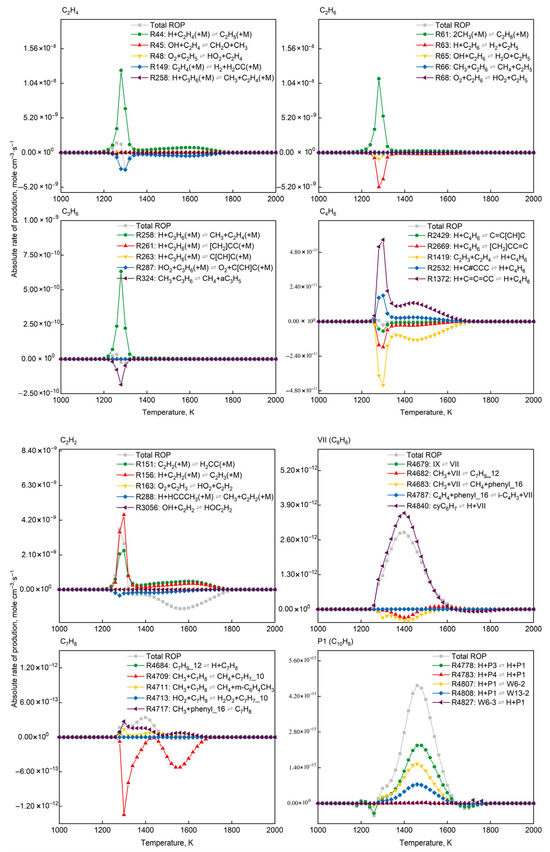
Figure 11.
Absolute rate-of-production distribution of top 5 dominant elementary reactions for target product formation (P = 1.0 atm, O2/CH4 molar ratio = 0.6).
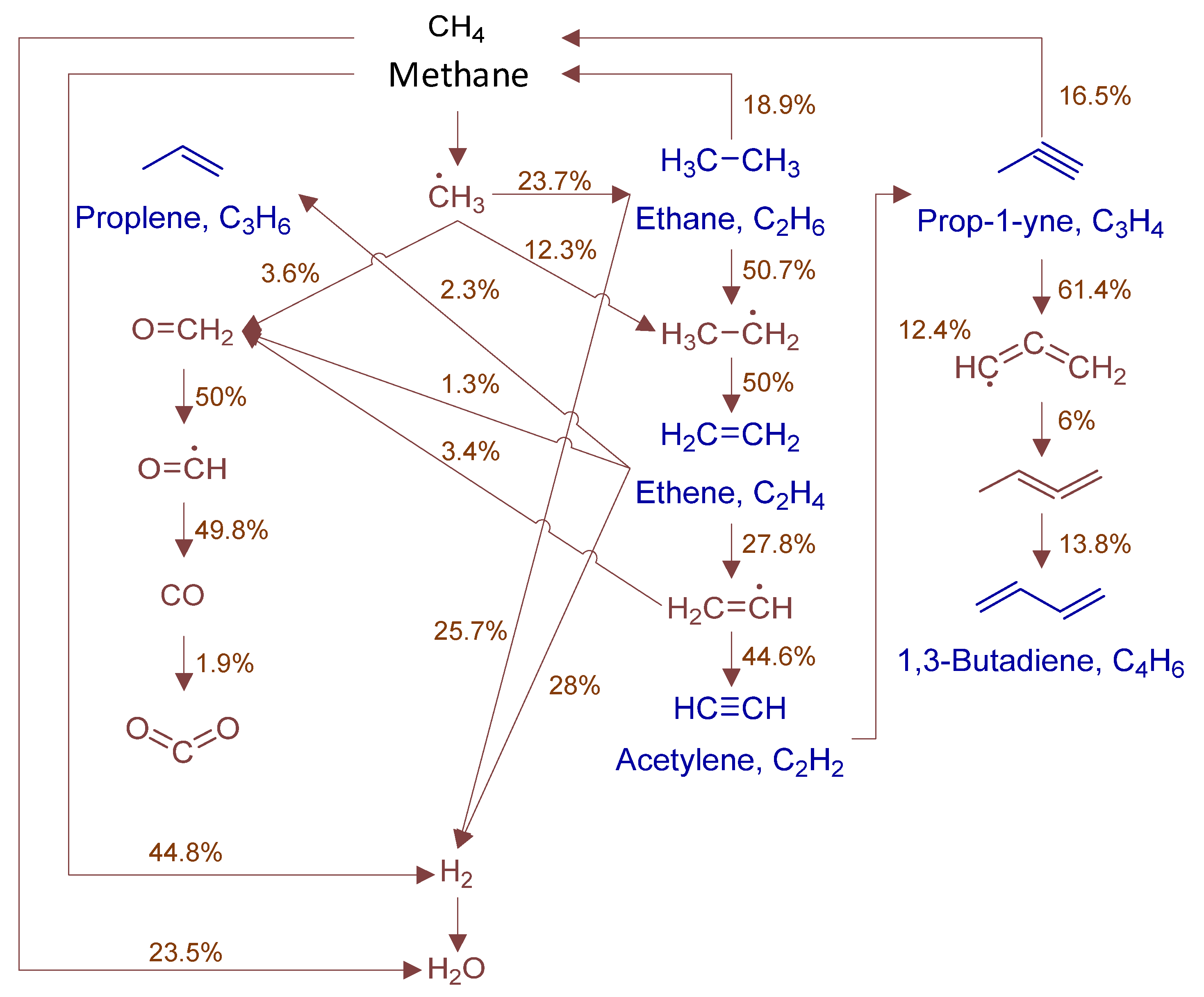
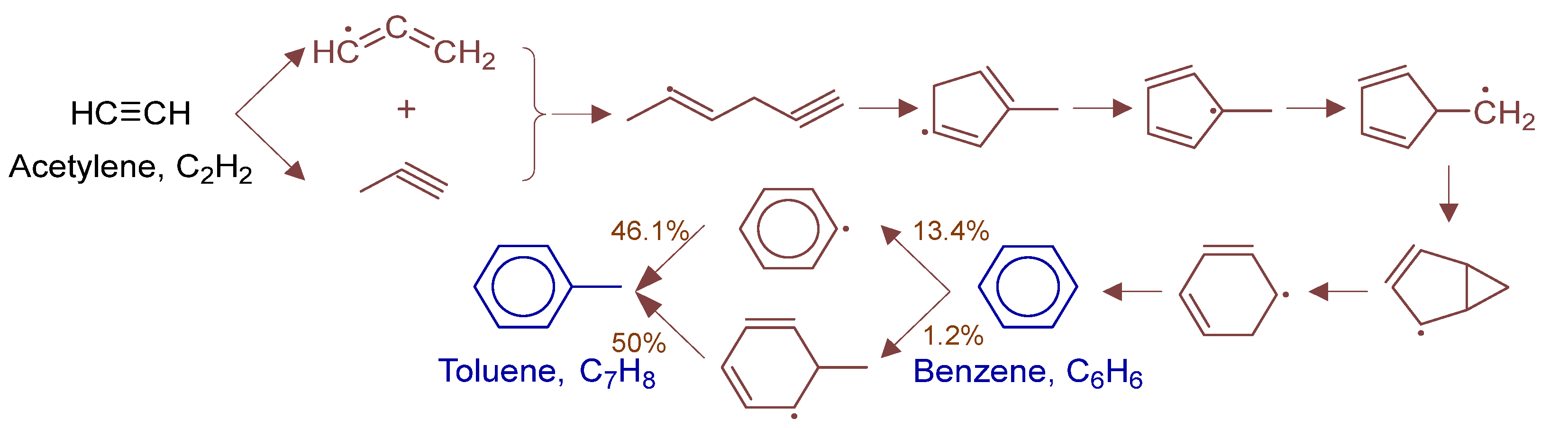
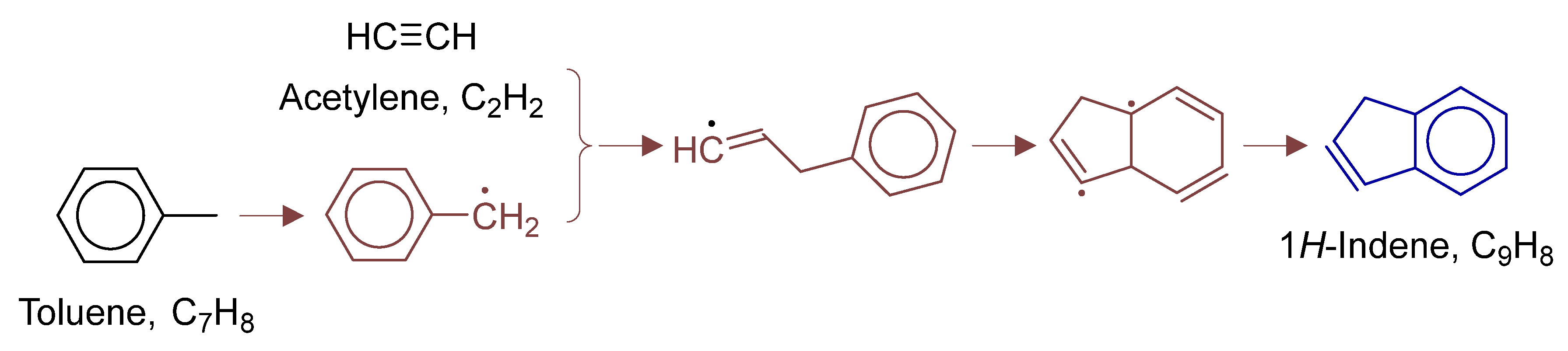
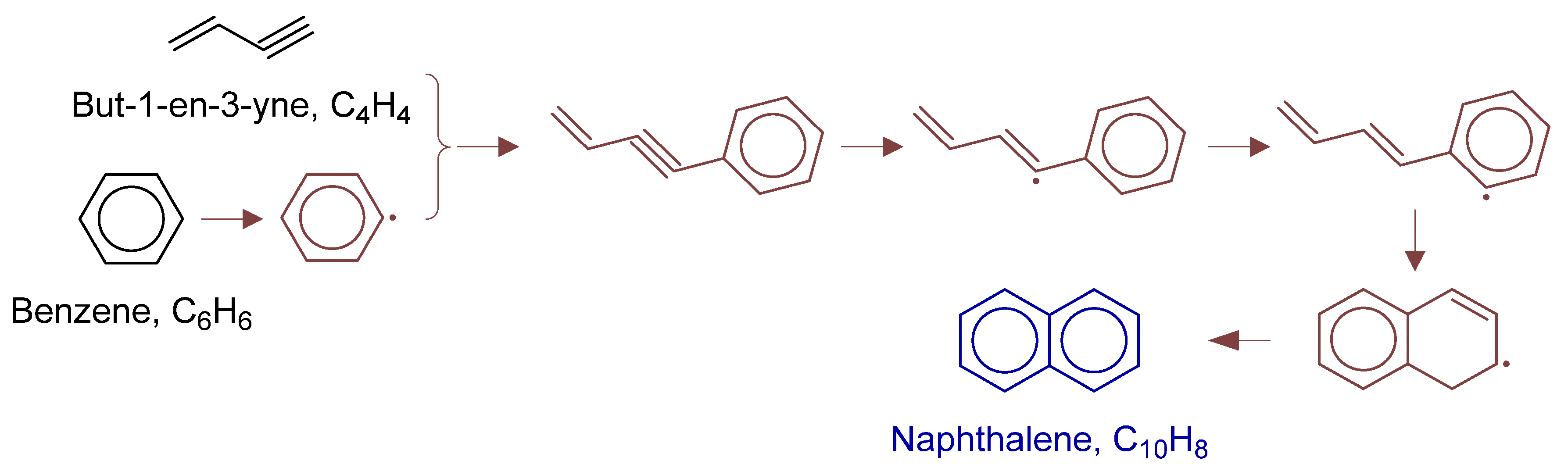
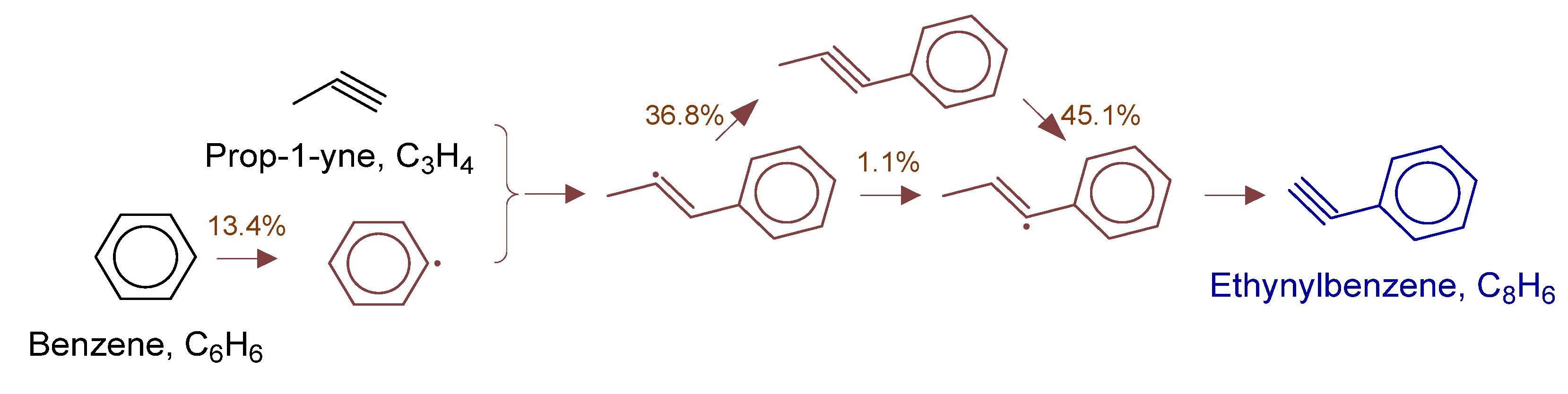
To fully elucidate formation mechanisms of target products and soot precursors, reaction pathway diagrams (Figure 12, Figure 13, Figure 14, Figure 15, Figure 16, Figure 17, Figure 18 and Figure 19) were constructed for relevant species (styrylcyclohexane, indene, phenanthrene, anthracene, acenaphthylene, pyrene) based on absolute rate distribution calculations of key elementary reactions from Figure 11. All pathways were constructed at peak concentration conditions, retaining only major pathways (>1% flux contribution) with reactants (black), intermediates (brown), and target products (blue) color-coded.
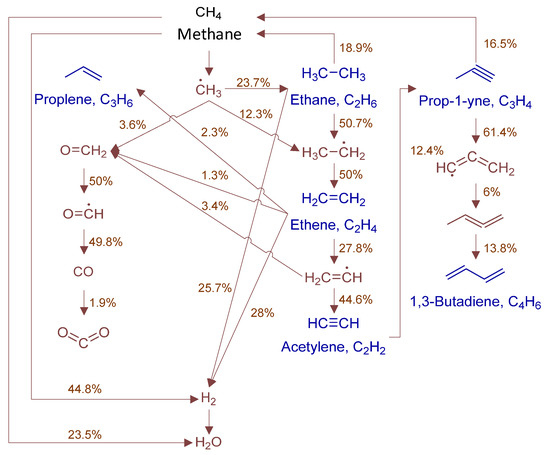
Figure 12.
Primary formation pathways of C0-C4 light hydrocarbons.
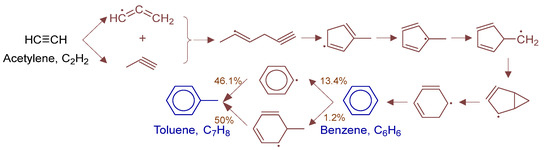
Figure 13.
Primary formation pathways of Benzene and Toluene.
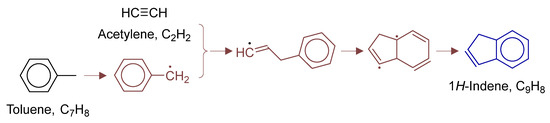
Figure 14.
Primary formation pathways of indene formation.
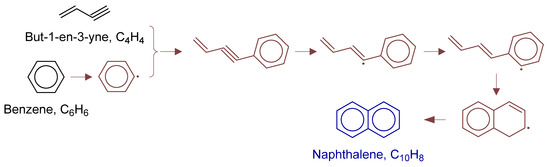
Figure 15.
Primary formation pathways of naphthalene formation.
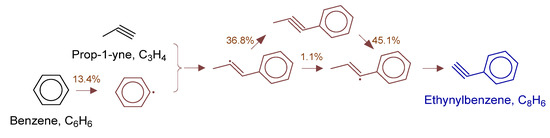
Figure 16.
Primary formation pathways of ethynylbenzene formation.
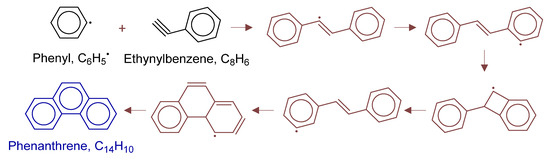
Figure 17.
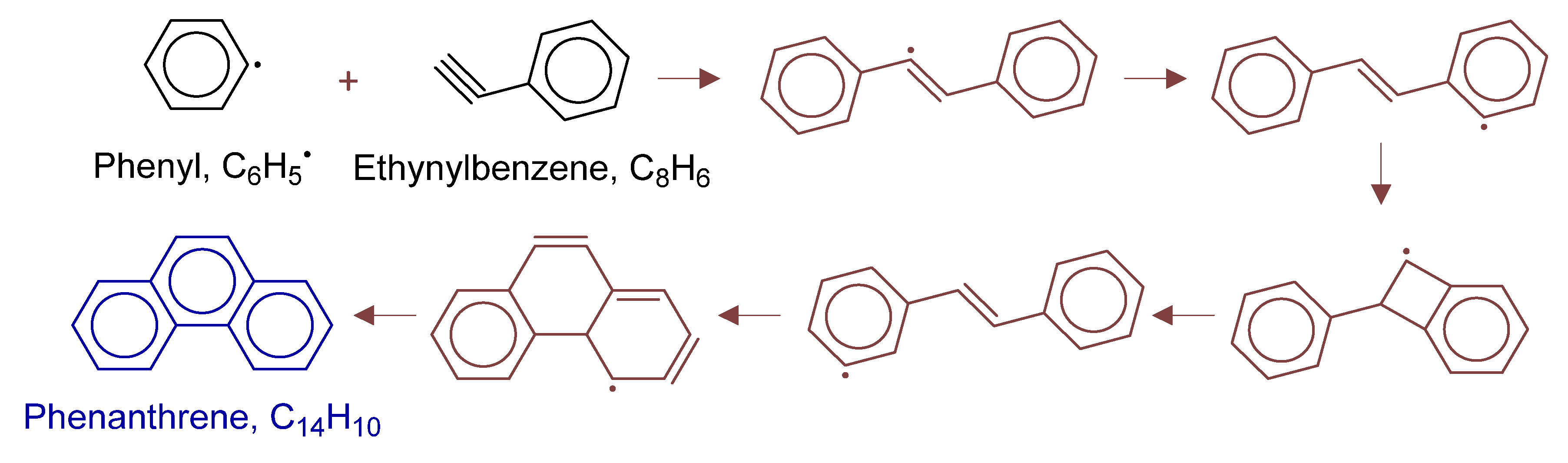
Primary formation pathways of phenanthrene formation.
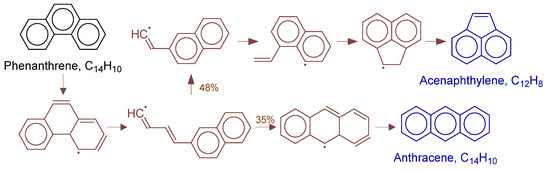
Figure 18.
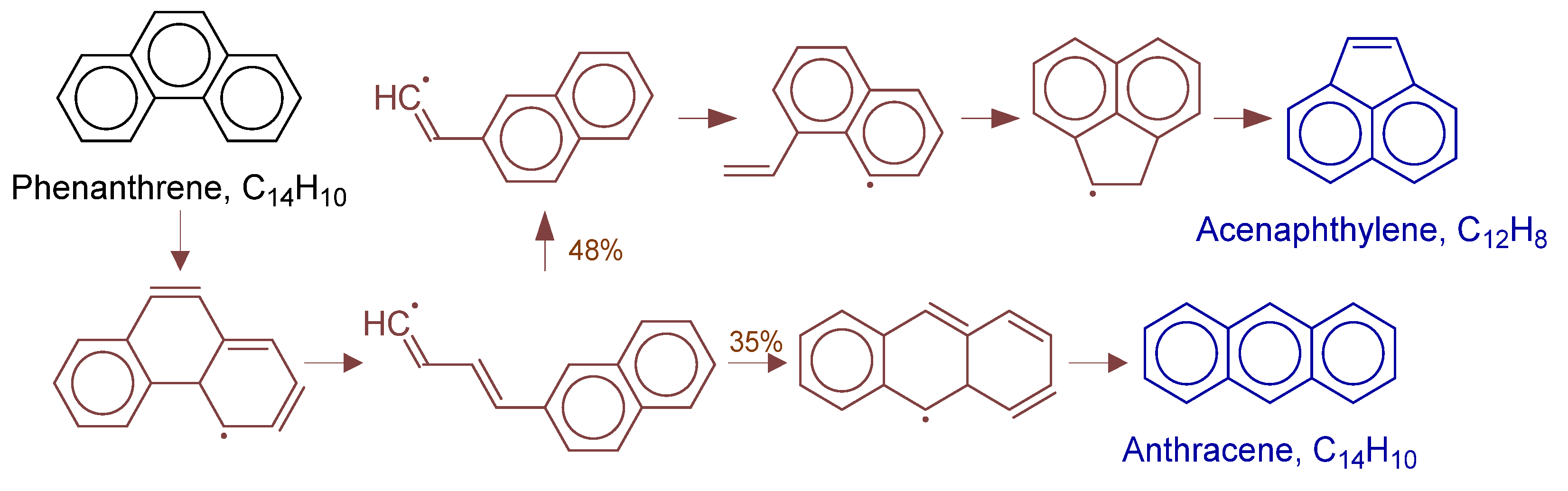
Primary formation pathways of acenaphthylene and anthracene formation.
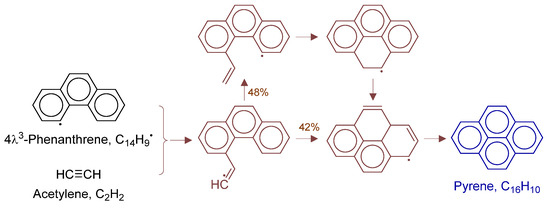
Figure 19.
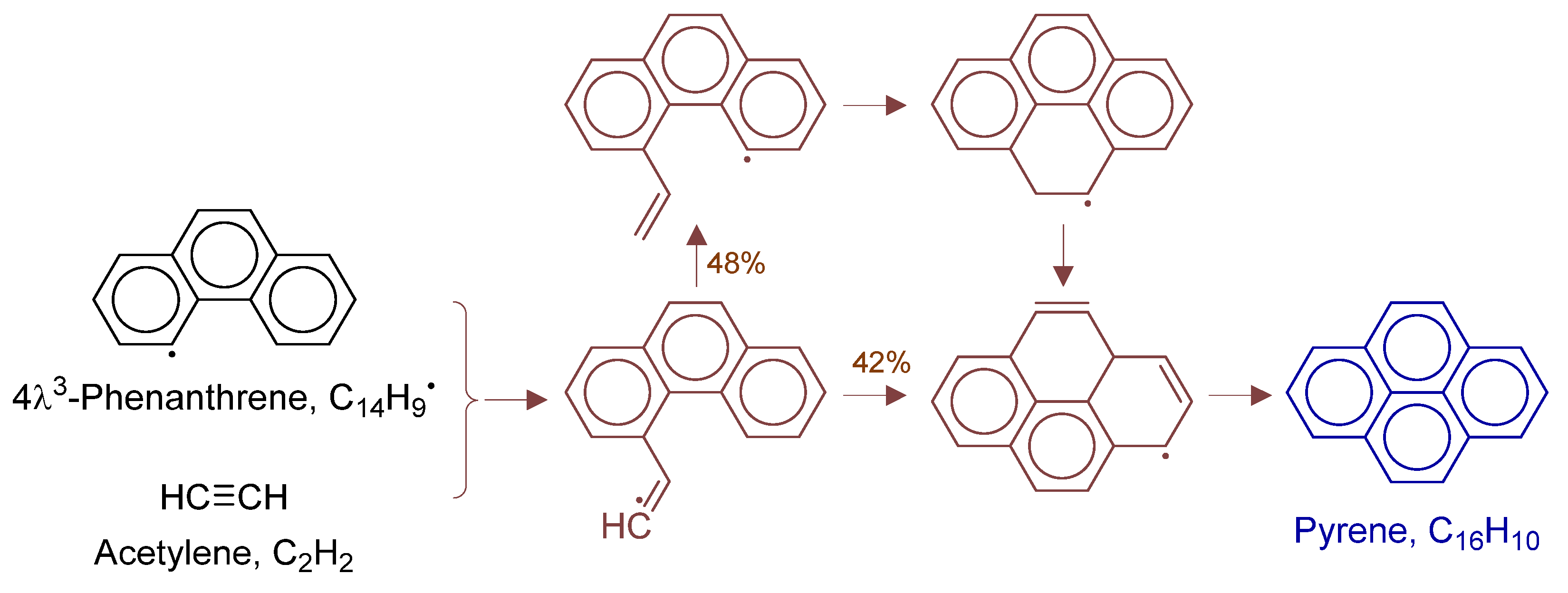
Primary formation pathways of pyrene formation.
Figure 12 systematically displays the reaction network of C0-C4 hydrocarbons in POX of natural gas, with kinetic parameters from authoritative databases including FFCM-1 [9], Burke H2/O2 in N2 [30], 2005-Senosiain-OH-C2H2 [31], and 2003-Miller-Propargyl-Recomb-High-P [32]. C2H6 forms mainly via CH3 recombination (2CH3(+M) ⇌ C2H6(+M)) and consumes through sequential dehydrogenation. C2H4 formation is controlled by C2H6 dehydrogenation (H + C2H4(+M) ⇌ C2H5(+M)), while consumption occurs via H-abstraction/β-scission. Ethylene (C2H4) reacts with methyl radicals (CH3·) to form propylene (C3H6), with hydroxyl radicals (OH·) to produce formaldehyde (CH2O), and undergoes hydrogen abstraction by methyl to form ethyl radicals (C2H5·). C2H2 formation is governed by: (1) C2H4 dehydrogenation (H + C2H2(+M) ⇌ C2H3(+M)), and (2) isomerization (H2CC(+M) ⇌ C2H2(+M)). Acetylene (C2H2) is consumed via methyl radical addition forming propyne (HCCCH3). Formaldehyde (CH2O) reacts with H· to form CO, ultimately oxidizing to CO2. Propyne dehydrogenation forms propargyl radical (C3H3), which combines with methyl to yield 1,2-butadiene. 1,3-butadiene formation is controlled by 1,2-butadiene isomerization.
Figure 13 details benzene and toluene formation pathways in POX of natural gas, with kinetics from 2003-Miller-Propargyl-Recomb-High-P [32] and kislovB [33] databases. Benzene forms via C3H3 radical recombination, first generating (2E)-hex-2-en-5-yn-2-yl intermediate that isomerizes to methyl-substituted 5-membered ring, followed by H-transfer and ring expansion. Toluene forms via two competing paths: (a) CH3·abstraction of benzene H forming phenyl radical + CH3· recombination, and (b) CH3· addition to benzene yielding 2-methylphenyl radical followed by H elimination.
Figure 14 details indene formation pathways in POX of natural gas, with kinetics from kislovB [33] database. The process initiates with H-abstraction from toluene’s methyl group, forming reactive benzyl radical, followed by C2H2 addition and 5-membered ring closure to 9-H-indenyl radical, which finally yields indene via β-H elimination.
Figure 15 details the reaction pathway network of naphthalene formation in non-catalytic partial oxidation of natural gas. The kinetic parameters were obtained from C6H5-C4H4-Mebel [35], First-to-Second-Aromatic-Ring/2017-Mebel-C6H5-C4H4-highP [35], Aromatics-high-pressure/C10H11-3 [36], Aromatics-high-pressure/C10H9-2 [35], and Aromatics-high-pressure/C10H9-3 [35]. The analysis indicated that naphthalene formation initiated with hydrogen abstraction from benzene to form phenyl radical, followed by addition reaction with vinylacetylene. Two competitive pathways were identified: (i) Pathway (a) directly formed naphthalene via intramolecular cyclization of but-3-en-1-ynylbenzene intermediate; (ii) Pathway (b) underwent multiple steps including dehydrogenation of the triple bond in but-3-en-1-ynylbenzene, followed by hydrogen transfer, 1,3-cycloaddition, and β-scission to form naphthyl radical, which finally yielded naphthalene through β-scission of C-H bond.
Figure 16 details the reaction network of phenylacetylene formation in POX of natural gas, with kinetic parameters from Aromatics-high-pressure/C9H9-1 [34] database. Analysis showed phenylacetylene formation initiated with benzene dehydrogenation to phenyl radical, followed by propyne addition forming (1E)-1-phenylprop-2-en-1-yl radical intermediate. The intermediate underwent two competitive pathways: (a) direct 1,2-H shift, and (b) isomerization followed by H-transfer. Both pathways yielded (1E)-1-phenylprop-1-en-3-yl radical, which cyclized to phenylacetylene via intramolecular C-C bond formation.
Figure 17 details phenanthrene formation pathways in POX of natural gas, with kinetic parameters from Aromatics-high-pressure/C14H11-2 [39]. Analysis revealed phenanthrene formation via phenylacetylene through: (i) selective phenyl radical attack on the triple bond; (ii) 1,4-H transfer -forming trans-isomer; then, (iii) four-membered ring transition state isomerization to cis-configuration; and (iv) intramolecular cyclization forming the new six-membered ring.
Figure 18 details anthracene and acenaphthene formation pathways in POX of natural gas, with kinetic parameters from Aromatics-high-pressure/C14H11-1 [38]. Analysis revealed phenanthrene activation leads to C-C cleavage forming key (3E)-4-(naphthalen-2-yl)buta-1,3-dien-1-yl radical intermediate. The intermediate converts via: (a) intramolecular cyclization to anthracene precursor followed by hydrogenation, or (b) vinyl elimination forming 2-(naphthalen-2-yl)vinyl radical that yields acenaphthene through H-transfer and cyclization.
Figure 19 details pyrene formation pathways in POX of natural gas, with kinetic parameters from Aromatics-high-pressure/C16H11 [42]. Path (a) involves: (i) phenanthrenyl radical + C2H2 electrophilic addition, (ii) intramolecular cyclization to tetracyclic C16H11, and (iii) H-elimination/decomposition. Path (b) undergoes: (i) H-transfer in phenanthrenyl-C2H2 adduct, followed by (ii) ring closure and unimolecular decomposition. Pyrene forms through two competing pathways.
5. Conclusions
This work established an efficient framework for modeling and optimization of POX of natural gas processes through the proposed HI-Mechanism. The comprehensive mechanism contained 1112 species and 106,877 reactions, providing complete C1-C16 coverage including light hydrocarbon oxidation and PAHs formation pathways. Product-directed simplification via Ansys Workbench yielded an optimized mechanism (397 species, 5135 reactions) balancing computational efficiency and accuracy. Experimental validation confirmed the accuracy of the HI-Mechanism in predicting shock tube ignition delays and major species concentrations. Chemkin-Pro microkinetic analyses enabled pathway quantification through production rates, key reaction identification via sensitivity analysis, and complete CH4-to-PAHs transformation mapping with temperature dependence.
Mechanistic analysis based on the HI-Mechanism reveals that light hydrocarbons (1100–1500 K) and aromatic hydrocarbons (1300–1700 K) are generated in distinct temperature ranges, demonstrating significant potential for process optimization. Furthermore, key operating parameters such as the oxygen-to-alkane ratio and residence time significantly influence the selectivity of target products. The mechanistic model established in this study provides a reliable theoretical foundation for process optimization, and the systematic optimization results will be discussed in detail in a subsequent dedicated publication.
The Hierarchical-Integrated Mechanism construction method addressed the balance challenge among mechanism size, computational efficiency and prediction accuracy in complex reaction systems, with its “full-range construction—target-directed simplification—systematic validation” framework providing methodological significance. In engineering practice, the model directly guides industrial reactor design and process optimization, Future work could develop a multi-scale coupled model incorporating soot sub-models. This approach would significantly enhance particulate formation prediction while maintaining the core gas-phase reaction prediction accuracy and computational efficiency, thereby providing more comprehensive solutions for industrial reactor design and optimization. Meanwhile, mechanism-based intelligent control systems can enhance operational efficiency, advancing intelligent and precise natural gas conversion technologies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr13072287/s1, Supplementary Material S1: Chemkin file (PDF); Supplementary Material S2: Species dictionary file (PDF).
Author Contributions
Conceptualization, W.Y. and H.D.; methodology, H.Y.; software, W.L. and Q.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (NO. 21276039), Dalian University of Technology (DUT21RC(3)084) and the National Natural Science Foundation of China (NO. 52204208).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to express their sincere gratitude to all participants for their valuable contributions.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
| POX | non-catalytic partial oxidation |
| PAHs | polycyclic aromatic hydrocarbons |
| HI-Mechanism | Hierarchical-Integrated Mechanism |
| RMG | Reaction Mechanism Generator |
| DRG | directed relation graph |
| DRG-EP | DRG with error propagation |
| DRG-PFA | path flux analysis |
| FSSA | full species sensitivity analysis |
| RCMs | rapid compression machines |
References
- Adler, T.B.; Knizia, G.; Werner, H.J. A simple and efficient CCSD(T)-F12 approximation. J. Chem. Phys. 2007, 127, 22. [Google Scholar] [CrossRef] [PubMed]
- Raimi, D.; Campbell, E.; Newell, R.G.; Prest, B.; Villanueva, S.; Wingenroth, J. Global Energy Outlook 2022: Turning Points and Tension in the Energy Transition; Resources for the Future: Washington, DC, USA, 2022; Available online: https://www.rff.org/publications/reports/global-energy-outlook-2022/ (accessed on 10 June 2025).
- Rizvi, S.K.A.; Naqvi, B.; Boubaker, S.; Mirza, N. The power play of natural gas and crude oil in the move towards the financialization of the energy market. Energy Econ. 2022, 112, 106131. [Google Scholar] [CrossRef]
- Makaryan, I.A.A.; Salgansky, E.A.A.; Arutyunov, V.S.S.; Sedov, I.V.V. Non-Catalytic Partial Oxidation of Hydrocarbon Gases to Syngas and Hydrogen: A Systematic Review. Energies 2023, 16, 2916. [Google Scholar] [CrossRef]
- Cohen, K.; Blanchard, J., Jr.; Rodriguez, P.; Kelly, K.; Dorman, J.A.; Dooley, K.M. Non-Catalytic Direct Partial Oxidation of Methane to Methanol in a Wall-Coated Microreactor. Chem. Eng. J. 2024, 482, 149049. [Google Scholar] [CrossRef]
- Norinaga, K.; Yatabe, H.; Matsuoka, M.; Hayashi, J.-i. Application of an Existing Detailed Chemical Kinetic Model to a Practical System of Hot Coke Oven Gas Reforming by Noncatalytic Partial Oxidation. Ind. Eng. Chem. Res. 2010, 49, 10565–10571. [Google Scholar] [CrossRef]
- Martinez-Gomez, J.; Napoles-Rivera, F.; Ponce-Ortega, J.M.; El-Halwagi, M.M. Optimization of the production of syngas from shale gas with economic and safety considerations. Appl. Therm. Eng. 2017, 110, 678–685. [Google Scholar] [CrossRef]
- Nourbakhsh, H.; Shahrouzi, J.R.; Zamaniyan, A.; Ebrahimi, H.; Nasr, M.R.J. A thermodynamic analysis of biogas partial oxidation to synthesis gas with emphasis on soot formation. Int. J. Hydrogen Energy 2018, 43, 15703–15719. [Google Scholar] [CrossRef]
- Osswald, P.; Zinsmeister, J.; Kathrotia, T.; Alves-Fortunato, M.; Burger, V.; van der Westhuizen, R.; Viljoen, C.; Lehto, K.; Sallinen, R.; Sandberg, K.; et al. Combustion kinetics of alternative jet fuels, Part-I: Experimental flow reactor study. Fuel 2021, 302, 120735. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, B. Visualization of ignition modes in methane-based mixture induced by shock wave focusing. Combust. Flame 2023, 247, 112491. [Google Scholar] [CrossRef]
- Sun, Z.; Xu, J.; Su, S.; Qing, M.; Wang, L.; Cui, X.; Mostafa, M.E.; Zhang, C.; Hu, S.; Wang, Y.; et al. Formation and reduction of NO from the oxidation of NH3/CH4 with high concentration of H2O. Fuel 2019, 247, 19–25. [Google Scholar] [CrossRef]
- Garten, B.; Hunger, F.; Messig, D.; Stelzner, B.; Trimis, D.; Hasse, C. Detailed radiation modeling of a partial-oxidation flame. Int. J. Therm. Sci. 2015, 87, 68–84. [Google Scholar] [CrossRef]
- Koehler, M.; Osswald, P.; Xu, H.; Kathrotia, T.; Hasse, C.; Riedel, U. Speciation data for fuel-rich methane oxy-combustion and reforming under prototypical partial oxidation conditions. Chem. Eng. Sci. 2016, 139, 249–260. [Google Scholar] [CrossRef]
- Di, Q.; Dai, L.; Wang, Y.; Zanobetti, A.; Choirat, C.; Schwartz, J.D.; Dominici, F. Association of Short-term Exposure to Air Pollution With Mortality in Older Adults. Jama-J. Am. Med. Assoc. 2017, 318, 2446–2456. [Google Scholar] [CrossRef] [PubMed]
- Shindell, D.; Kuylenstierna, J.C.I.; Vignati, E.; van Dingenen, R.; Amann, M.; Klimont, Z.; Anenberg, S.C.; Muller, N.; Janssens-Maenhout, G.; Raes, F.; et al. Simultaneously Mitigating Near-Term Climate Change and Improving Human Health and Food Security. Science 2012, 335, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.P. The GRI-Mech 3.0 Mechanism. Available online: http://combustion.berkeley.edu/gri-mech (accessed on 10 June 2025).
- Donato, N.; Aul, C.; Petersen, E.; Zinner, C.; Curran, H.; Bourque, G. Ignition and Oxidation of 50/50 Butane Isomer Blends. J. Eng. Gas. Turbines Power-Trans. ASME 2010, 132, 9. [Google Scholar] [CrossRef]
- Healy, D.; Kalitan, D.M.; Aul, C.J.; Petersen, E.L.; Bourque, G.; Curran, H.J. Oxidation of C1-C5 Alkane Quinternary Natural Gas Mixtures at High Pressures. Energy Fuels 2010, 24, 1521–1528. [Google Scholar] [CrossRef]
- Healy, D.; Donato, N.S.; Aul, C.J.; Petersen, E.L.; Zinner, C.M.; Bourque, G.; Curran, H.J. Isobutane ignition delay time measurements at high pressure and detailed chemical kinetic simulations. Combust. Flame 2010, 157, 1540–1551. [Google Scholar] [CrossRef]
- Smith, G.P.; Tao, Y.; Wang, H. Available online: https://web.stanford.edu/group/haiwanglab/FFCM1 (accessed on 10 June 2025).
- Wang, Q.D. Skeletal Mechanism Generation for High-Temperature Combustion of H2/CO/C1-C4 Hydrocarbons. Energy Fuels 2013, 27, 4021–4030. [Google Scholar] [CrossRef]
- Blanquart, G.; Pepiot-Desjardins, P.; Pitsch, H. Chemical mechanism for high temperature combustion of engine relevant fuels with emphasis on soot precursors. Combust. Flame 2009, 156, 588–607. [Google Scholar] [CrossRef]
- Skjoth-Rasmussen, M.S.; Glarborg, P.; Ostberg, M.; Johannessen, J.T.; Livbjerg, H.; Jensen, A.D.; Christensen, T.S. Formation of polycyclic aromatic hydrocarbons and soot in fuel-rich oxidation of methane in a laminar flow reactor. Combust. Flame 2004, 136, 91–128. [Google Scholar] [CrossRef]
- Narayanaswamy, K.; Blanquart, G.; Pitsch, H. A consistent chemical mechanism for oxidation of substituted aromatic species. Combust. Flame 2010, 157, 1879–1898. [Google Scholar] [CrossRef]
- Slavinskaya, N.A.; Frank, P. A modelling study of aromatic soot precursors formation in laminar methane and ethene flames. Combust. Flame 2009, 156, 1705–1722. [Google Scholar] [CrossRef]
- Savchenko, V.I.; Zimin, Y.S.; Busillo, E.; Nikitin, A.V.; Sedov, I.V.; Arutyunov, V.S. Equilibrium Composition of Products Formed by Non-catalytic Conversion of Hydrocarbons. Pet. Chem. 2022, 62, 515–525. [Google Scholar] [CrossRef]
- Voloshchuk, Y.; Richter, A. Reduced order modeling and large-scale validation for non-catalytic partial oxidation of natural gas. Chem. Eng. Sci. 2022, 255, 117620. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Y.; Chen, T.; Yu, X.; Wang, J.; Wang, T. Simulations of methane partial oxidation by CFD coupled with detailed chemistry at industrial operating conditions. Chem. Eng. Sci. 2016, 142, 126–136. [Google Scholar] [CrossRef]
- Gao, C.W.; Allen, J.W.; Green, W.H.; West, R.H. Reaction Mechanism Generator: Automatic construction of chemical kinetic mechanisms. Comput. Phys. Commun. 2016, 203, 212–225. [Google Scholar] [CrossRef]
- Burke, M.P.; Chaos, M.; Ju, Y.G.; Dryer, F.L.; Klippenstein, S.J. Comprehensive H2/O2 kinetic model for high-pressure combustion. Int. J. Chem. Kinet. 2012, 44, 444–474. [Google Scholar] [CrossRef]
- Senosiain, J.P.; Klippenstein, S.J.; Miller, J.A. The reaction of acetylene with hydroxyl radicals. J. Phys. Chem. A 2005, 109, 6045. [Google Scholar] [CrossRef]
- Miller, J.A.; Klippenstein, S.J. The recombination of propargyl radicals and other reactions on a C6H6 potential. J. Phys. Chem. A 2003, 107, 7783–7799. [Google Scholar] [CrossRef]
- Kislov, V.V.; Mebel, A.M. Ab initio G3-type/statistical theory study of the formation of indene in combustion flames. I. Pathways involving benzene and phenyl radical. J. Phys. Chem. A 2007, 111, 3922–3931. [Google Scholar] [CrossRef]
- Mebel, A.M.; Georgievskii, Y.; Jasper, A.W.; Klippenstein, S.J. Pressure-dependent rate constants for PAH growth: Formation of indene and its conversion to naphthalene. Faraday Discuss. 2016, 195, 637–670. [Google Scholar] [CrossRef]
- Mebel, A.M.; Landera, A.; Kaiser, R.I. Formation Mechanisms of Naphthalene and Indene: From the Interstellar Medium to Combustion Flames. J. Phys. Chem. A 2017, 121, 901–926. [Google Scholar] [CrossRef]
- Vervust, A.J.; Djokic, M.R.; Merchant, S.S.; Carstensen, H.H.; Long, A.E.; Marin, G.B.; Green, W.H.; Van Geem, K.M. Detailed Experimental and Kinetic Modeling Study of Cyclopentadiene Pyrolysis in the Presence of Ethene. Energy Fuels 2018, 32, 3920–3934. [Google Scholar] [CrossRef]
- Long, A.E.; Merchant, S.S.; Vandeputte, A.G.; Carstensen, H.H.; Vervust, A.J.; Marin, G.B.; Van Geem, K.M.; Green, W.H. Pressure dependent kinetic analysis of pathways to naphthalene from cyclopentadienyl recombination. Combust. Flame 2018, 187, 247–256. [Google Scholar] [CrossRef]
- Kislov, V.V.; Sadovnikov, A.I.; Mebel, A.M. Formation Mechanism of Polycyclic Aromatic Hydrocarbons beyond the Second Aromatic Ring. J. Phys. Chem. A 2013, 117, 4794–4816. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Iparraguirre, J.; Klopper, W. Density functional theory study of the formation of naphthalene and phenanthrene from reactions of phenyl with vinyl- and phenylacetylene. J. Chem. Theory Comput. 2007, 3, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Frenklach, M.; Singh, R.I.; Mebel, A.M. On the low-temperature limit of HACA. Proc. Combust. Inst. 2019, 37, 969–976. [Google Scholar] [CrossRef]
- Richter, H.; Howard, J.B. Formation of polycyclic aromatic hydrocarbons and their growth to soot—A review of chemical reaction pathways. Prog. Energy Combust. Sci. 2000, 26, 565–608. [Google Scholar] [CrossRef]
- Zhao, L.; Kaiser, R.I.; Xu, B.; Ablikim, U.; Ahmed, M.; Joshi, D.; Veber, G.; Fischer, F.R.; Mebel, A.M. Pyrene synthesis in circumstellar envelopes and its role in the formation of 2D nanostructures. Nat. Astron. 2018, 2, 413–419. [Google Scholar] [CrossRef]
- Ribeiro, J.M.; Mebel, A.M. Reaction mechanism and product branching ratios of the CH + C3H4 reactions: A theoretical study. Phys. Chem. Chem. Phys. 2017, 19, 14543–14554. [Google Scholar] [CrossRef]
- Hahn, D.K.; Klippenstein, S.J.; Miller, J.A. A theoretical analysis of the reaction between propargyl and molecular oxygen. Faraday Discuss. 2001, 119, 79–100. [Google Scholar] [CrossRef] [PubMed]
- Harding, L.B.; Klippenstein, S.J.; Georgievskii, Y. On the combination reactions of hydrogen atoms with resonance-stabilized hydrocarbon radicals. J. Phys. Chem. A 2007, 111, 3789–3801. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Yang, B.; Zhang, F. Initiation mechanism of 1,3-butadiene combustion and its effect on soot precursors. Combust. Flame 2017, 184, 167–175. [Google Scholar] [CrossRef]
- Kislov, V.V.; Singh, R.I.; Edwards, D.E.; Mebel, A.M.; Frenklach, M. Rate coefficients and product branching ratios for the oxidation of phenyl and naphthyl radicals: A theoretical RRKM-ME study. Proc. Combust. Inst. 2015, 35, 1861–1869. [Google Scholar] [CrossRef]
- Narendrapurapu, B.S.; Simmonett, A.C.; Schaefer, H.F.; Miller, J.A.; Klippenstein, S.J. Combustion Chemistry: Important Features of the C3H5 Potential Energy Surface, Including Allyl Radical, Propargyl+H2, Allene+H, and Eight Transition States. J. Phys. Chem. A 2011, 115, 14209–14214. [Google Scholar] [CrossRef]
- Klippenstein, S.J.; Miller, J.A. The addition of hydrogen atoms to diacetylene and the heats of formation of i-C4H3 and n-C4H3. J. Phys. Chem. A 2005, 109, 4285–4295. [Google Scholar] [CrossRef]
- Petersson, G.A.; Malick, D.K.; Wilson, W.G.; Ochterski, J.W.; Montgomery, J.A.; Frisch, M.J. Calibration and comparison of the Gaussian-2, complete basis set, and density functional methods for computational thermochemistry. J. Chem. Phys. 1998, 109, 10570–10579. [Google Scholar] [CrossRef]
- Knizia, G.; Adler, T.B.; Werner, H.J. Simplified CCSD(T)-F12 methods: Theory and benchmarks. J. Chem. Phys. 2009, 130, 20. [Google Scholar] [CrossRef]
- Pfeiffer, F.; Rauhut, G.; Feller, D.; Peterson, K.A. Anharmonic zero point vibrational energies: Tipping the scales in accurate thermochemistry calculations? J. Chem. Phys. 2013, 138, 10. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.L.; Ning, H.B.; Shi, J.C.; Wang, H.Y.; Luo, S.N. Chemical kinetics of H-abstractions from dimethyl amine by H, CH3, OH, and HO2 radicals with multi-structural torsional anharmonicity. Phys. Chem. Chem. Phys. 2019, 21, 12685–12696. [Google Scholar] [CrossRef]
- Lu, T.F.; Law, C.K. On the applicability of directed relation graphs to the reduction of reaction mechanisms. Combust. Flame 2006, 146, 472–483. [Google Scholar] [CrossRef]
- Wu, Y.C.; Liu, Y.F.; Lu, T.F. A linearized error propagation method for skeletal mechanism reduction. Combust. Flame 2020, 211, 303–311. [Google Scholar] [CrossRef]
- Liu, Z.T.; Yang, L.P.; Song, E.Z.; Wang, J.Q.; Zare, A.; Bodisco, T.A.; Brown, R.J. Development of a reduced multi-component combustion mechanism for a diesel/natural gas dual fuel engine by cross-reaction analysis. Fuel 2021, 293, 19. [Google Scholar] [CrossRef]
- Pepiot-Desjardins, P.; Pitsch, H. An efficient error-propagation-based reduction method for large chemical kinetic mechanisms. Combust. Flame 2008, 154, 67–81. [Google Scholar] [CrossRef]
- Stagni, A.; Frassoldati, A.; Cuoci, A.; Faravelli, T.; Ranzi, E. Skeletal mechanism reduction through species-targeted sensitivity analysis. Combust. Flame 2016, 163, 382–393. [Google Scholar] [CrossRef]
- Li, H.Y.; Zhang, H.W.; Chen, Z. Effects of endothermic chain-branching reaction on spherical flame initiation and propagation. Combust. Theory Model. 2019, 23, 496–514. [Google Scholar] [CrossRef]
- Petersen, E.L.; Hall, J.M.; Smith, S.D.; de Vries, J.; Amadio, A.R.; Crofton, M.W. Ignition of lean methane-based fuel blends at gas turbine pressures. J. Eng. Gas. Turbines Power-Trans. ASME 2007, 129, 937–944. [Google Scholar] [CrossRef]
- Rickard, M.J.A.; Hall, J.M.; Petersen, E.L. Effect of silane addition on acetylene ignition behind reflected shock waves. Proc. Combust. Inst. 2005, 30, 1915–1923. [Google Scholar] [CrossRef]
- Seery, D.J.; Bowman, C.T. An experimental and analytical study of methane oxidation behind shock waves. Combust. Flame 1970, 14, 37–47. [Google Scholar] [CrossRef]
- Healy, D.; Donato, N.S.; Aul, C.J.; Petersen, E.L.; Zinner, C.M.; Bourque, G.; Curran, H.J. n-Butane: Ignition delay measurements at high pressure and detailed chemical kinetic simulations. Combust. Flame 2010, 157, 1526–1539. [Google Scholar] [CrossRef]
- Healy, D.; Kopp, M.M.; Polley, N.L.; Petersen, E.L.; Bourque, G.; Curran, H.J. Methane/n-Butane Ignition Delay Measurements at High Pressure and Detailed Chemical Kinetic Simulations. Energy Fuels 2010, 24, 1617–1627. [Google Scholar] [CrossRef]
- Zhang, F.; Huang, C.; Xie, B.; Wu, X. Revisiting the chemical kinetics of CH3 + O2 and its impact on methane ignition. Combust. Flame 2019, 200, 125–134. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, X.; Wei, L.; Zhang, J.; Tang, C.; Huang, Z. Experimental and modeling study on auto-ignition characteristics of methane/hydrogen blends under engine relevant pressure. Int. J. Hydrogen Energy 2012, 37, 19168–19176. [Google Scholar] [CrossRef]
- Konnov, A.A.; Zhu, J.N.; Bromly, J.H.; Zhang, D.K. Noncatalytic partial oxidation of methane into syngas over a wide temperature range. Combust. Sci. Technol. 2004, 176, 1093–1116. [Google Scholar] [CrossRef]
- Pässler, P.; Hefner, W.; Buckl, K.; Meinass, H.; Meiswinkel, A.; Wernicke, H.J.; Ebersberg, G.; Müller, R.; Bässler, J.; Behringer, H. Acetylene; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000. [Google Scholar]
- Jasper, A.W.; Miller, J.A. Collisional Energy Transfer in Unimolecular Reactions: Direct Classical Trajectories for CH4 ⇆ CH3 + H in Helium. J. Phys. Chem. A 2009, 113, 5612–5619. [Google Scholar] [CrossRef]
- Barnes, R.W.; Pratt, G.L.; Wood, S.W. Pressure dependence of methane dissociation. J. Chem. Soc.-Faraday Trans. Ii 1989, 85, 229–238. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).