1. Introduction
Foodborne diseases pose a significant threat to global health and food safety. Contaminated food containing bacteria, viruses, parasites, or chemicals can cause more than 200 types of disease. At a global scale, roughly 600 million people fall ill annually as a result of contaminated food consumption, with more than 420,000 cases being fatal. Children under the age of five and individuals living in low-income countries are particularly vulnerable in this regard. Such diseases can occur at any stage of the food chain, from production to consumption. Globalization, migration, and climate change represent factors exacerbating this issue [
1]. Foods are susceptible to spoilage caused by microbial, chemical, or physical processes, which may impact their nutritional quality, appearance, texture, and overall edibility [
2]. Food spoilage is a major concern as it results in economic losses and can also threaten consumer health [
3]. Furthermore, approximately 1.3 billion tons—nearly 30% of global food production—is lost annually due to microbial spoilage, significantly affecting economic resources and global food availability [
4,
5]. In recent decades, ensuring food safety and extending its shelf life have become important issues for the food industry [
6]. Poultry meat is one of the most popular sources of protein, but its high moisture content and nutritional value make it vulnerable to microbiological spoilage and the development of pathogens, such as Salmonella and Listeria monocytogenes [
7]. These microorganisms not only reduce the shelf life of products, but also pose a serious threat to consumer health.
Poultry meat is an important source of protein globally; however, its high moisture and nutrient contents make it vulnerable to microbial spoilage and foodborne pathogens, such as
Salmonella and
Listeria, resulting in economic losses and potential public health hazards [
7]. Poultry slaughter and processing can inadvertently facilitate the spread of gastrointestinal tract-associated microorganisms between carcasses and lead to the contamination of processing surfaces and the surrounding environment, particularly during the defeathering and evisceration stages [
8,
9]. These microorganisms compromise the quality of poultry meat, shorten its shelf life, and may cause foodborne outbreaks of campylobacteriosis and salmonellosis if the meat is not properly handled by consumers [
10].
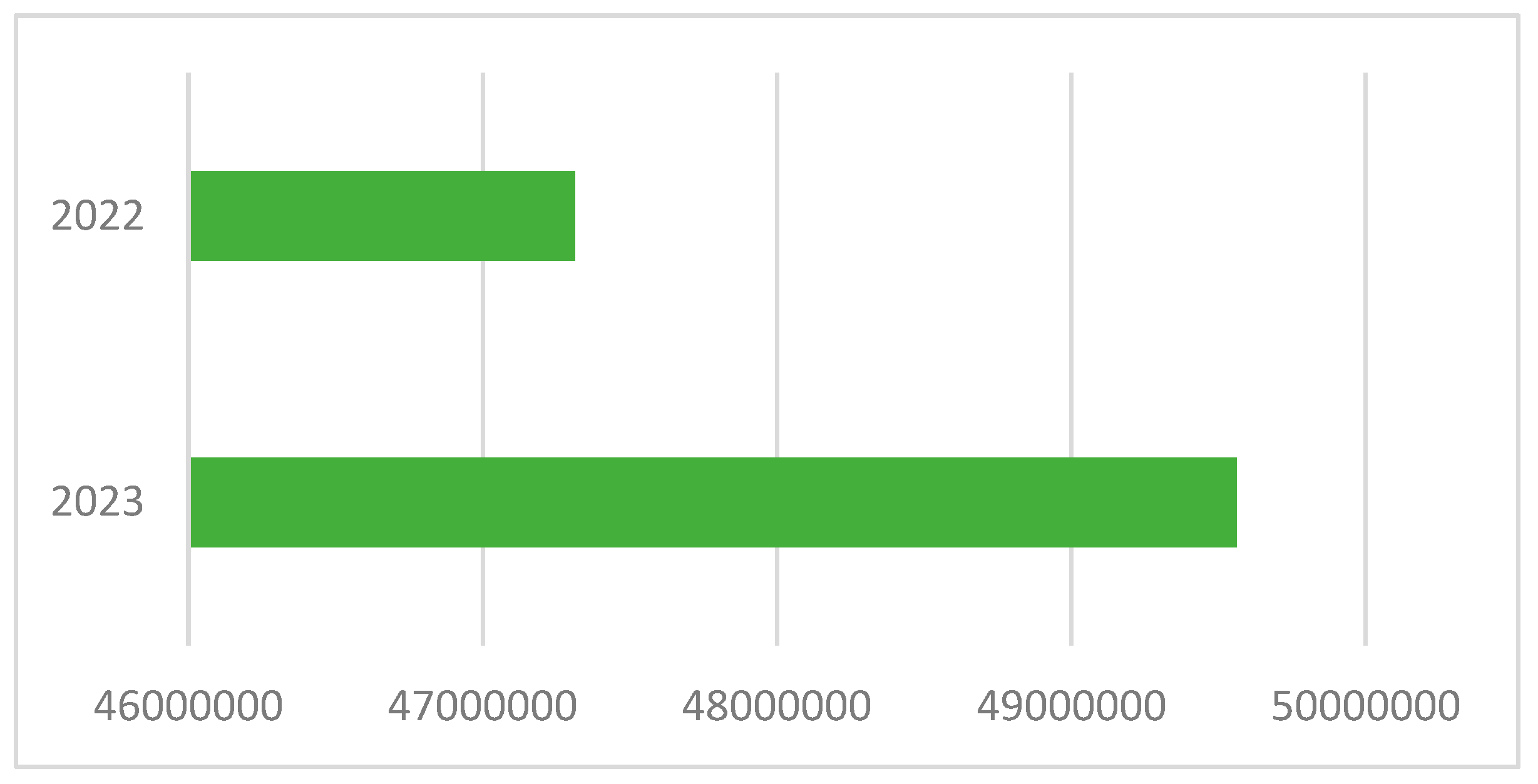
The growth in the consumption of poultry meat in Kazakhstan is evidenced by statistical data—an increase in domestic production, coupled with an increase in imports and a decrease in exports abroad. In recent years, the state of poultry farming in the country has begun to stabilize, and annual increases in the number of poultry [
11] and the volume of poultry production can be observed (
Figure 1).
In 2024, the domestic consumption of poultry meat in Kazakhstan amounted to 409.3 thousand tons, of which 121 thousand tons (almost 30%) [
12] was derived from the country’s main suppliers: Russia (65.5 thousand tons), the USA (48.1 thousand tons), Ukraine, and Belarus. Concurrently, exports of domestic poultry meat reached 38 thousand tons in this same year, an increase of 34.2% year-on-year. It is expected that, by 2027, Kazakhstan will cover the needs of the domestic market through the implementation of 29 poultry projects, with a total capacity of 210 thousand tons [
13].
Based on data from the Ministry of Health of Kazakhstan, almost 10% of imported poultry meat does not meet sanitary standards, indicating the presence of microbiological threats. In addition, poultry meat produced in Kazakhstan is characterized by freshness, minimal logistical burden, and, in some cases, a more transparent system of sanitary control at enterprises. However, Kazakhstani raw materials are also susceptible to microbiological contamination if appropriate slaughtering, cooling, and storage conditions are not implemented [
14].
Traditional preservation methods—such as chemical additives, thermal processing, and refrigeration—are effective to some extent, but often compromise the nutritional quality and sensory properties of the meat or demand high energy consumption. In this context, food irradiation has emerged as a promising non-thermal technology that improves food safety, extends shelf life, and helps to preserve nutritional value [
15]. Food irradiation is a non-thermal preservation technique in which food or agricultural products are exposed to specific doses of non-ionizing radiation (e.g., visible light, infra-red, and radio waves) or ionizing radiation (e.g., gamma rays, X-rays, accelerated electron beams, and UV-C (100–280 nm)), which exerts ionizing effects on biological materials [
16]. This process enhances hygiene and safety while also extending storage life and improving the distribution of these products [
17,
18]. Irradiation has a minimal impact on the flavor, color, taste, nutritional value, and other sensory attributes of food [
15]. Food irradiation involves exposing food products to controlled doses of ionizing radiation to eliminate or significantly reduce microbial contamination [
19].
One promising method for ensuring the safety and extending the shelf life of meat products is electron beam irradiation (E-beam) [
20]. Electron irradiation is the process of exposing food products to a stream of high-energy electrons, which facilitates the effective elimination of microorganisms while also preserving most of the physical and organoleptic characteristics of the product. In contrast with gamma irradiation, electron irradiation possesses a lower penetration depth [
21], which enables more precise control of the dose and consequently the quality of the processed product. A cathode (composed of tungsten, for example) is electrically excited in a vacuum system and will emit electrons that will be picked up and subjected to a potential difference in an electron gun [
22]. The electrons are thus accelerated to an energy proportional to the voltage used and then shaped to form a beam that will be directed toward the product to be treated [
23].
The use of electron irradiation is being actively studied in the food industry because it effectively eliminates pathogenic microorganisms, improves safety, and extends the shelf life of products without significantly affecting their organoleptic properties. As noted in a previous study [
5], this method is especially useful for processing products that cannot be subjected to high heat treatment, such as poultry. At a scientific level, irradiation acts primarily by breaking microbial DNA or disrupting cellular integrity through oxidative processes induced by free radicals. This process leads to a dramatic reduction in pathogenic and spoilage microorganisms without it being necessary to apply elevated temperatures. With these advantages, irradiation effectively preserves the fresh qualities of meat products and reduces reliance on chemical preservatives [
24].
In one study [
25], dried yak meat was treated with electron beam irradiation at doses of 2, 5, 7, and 9 kGy, with the results demonstrating that the total number of aerobic bacteria and E. coli decreased with increasing irradiation dose.
Consumer acceptance remains one of the key barriers to the widespread adoption of food irradiation. Research indicates that public understanding plays a critical role in shaping acceptance; the provision of scientifically accurate information and clear labeling—such as the use of the Radura symbol—can greatly enhance consumer perception and willingness to adopt irradiated products [
26]. Therefore, effective communication strategies that convey the safety, benefits, and transparency of the irradiation process are essential for building public trust and facilitating broader market integration [
27].
In addition, one of the key advantages of the electron beam treatment of poultry meat is the increase in product shelf life. As noted in one study [
27], irradiation doses of 2–4 kGy can extend the shelf life of chilled meat to 2–3 weeks without a significant deterioration in quality. The use of vacuum packaging in combination with irradiation doses up to 6–8 kGy can extend shelf life to 4–5 weeks while simultaneously maintaining microbiological safety and organoleptic properties at a satisfactory level [
28,
29].
Irradiated products are more expensive as a result of the additional handling and packaging required, the cost of the irradiation process, and post-irradiation sampling for pathogen testing. However, poor production practices and hygiene, cross-contamination from slaughter to cutting, and improper storage can increase the risk of fresh meat contamination, re-contamination, and spoilage. These problems pose a threat to human health and result in economic losses of USD 218 billion for the food industry per year [
30,
31]. Radiation treatment is an economically justified and necessary mechanism to prevent the spoilage of products as a result of these issues, as demonstrated by the data presented in [
32]. Poultry meat is one of the three main types of food treated with ionizing radiation in the European Union.
Electron irradiation as a method of poultry meat processing could provide a universal solution for both imported and domestic raw materials. However, in this study, we focus on Kazakhstani poultry meat as a strategically important processing object.
Regarding the impact on the production environment and personnel, industrial electron accelerators are a source of ionizing radiation. As such, they are installed in shelters (bunkers). The level of ionizing radiation in the workplace is continuously monitored by means of a radiation-monitoring system. The use of such a system ensures safety in the workplace. Radiolysis of air produces ozone (O
3), which is unstable and ultimately converts into oxygen (O
2). During the construction phase of the accelerator, the level of ozone produced by the accelerator is assessed, and exhaust ventilation and measures are provided to protect personnel from exposure to ozone. Personnel are prohibited from entering any area where ozone concentrations may be above safe limits. Freshening centers are designed so that ozone does not migrate into areas in which people are present [
33].
In light of the above findings, the objective of this study was to develop an appropriate dose of irradiation for electron beam treatment to sterilize chilled poultry meat. Our aim was to identify whether it would be possible to extend its shelf life and to determine the optimal physicochemical, sensory, and microbiological indicators of poultry meat.
Through this study, we make an important scientific contribution, given the limited scientific research on modern methods of food preservation, despite the rapid development of the poultry sector in Kazakhstan. The results of this study provide specific scientific data on the effective radiation dose that, in addition to destroying pathogenic microorganisms, enables the preservation of the nutritional value of the product, extends its shelf life, and ensures safety during transportation and storage. The results of this study can serve as a basis for the development of regional protocols aimed at food safety, consistent with international and national (Kazakhstani) standards.
2. Materials and Methods
2.1. Sample
Fresh poultry meat samples (n = 30) were collected immediately after slaughter from a processing plant in the Almaty region, Kazakhstan. Samples were selected from the breast and thigh muscles to ensure homogeneity. After slaughter, the meat obtained was cooled to a core temperature of −1–+4 °C within 1 h and packaged.
2.2. Radiation Treatment
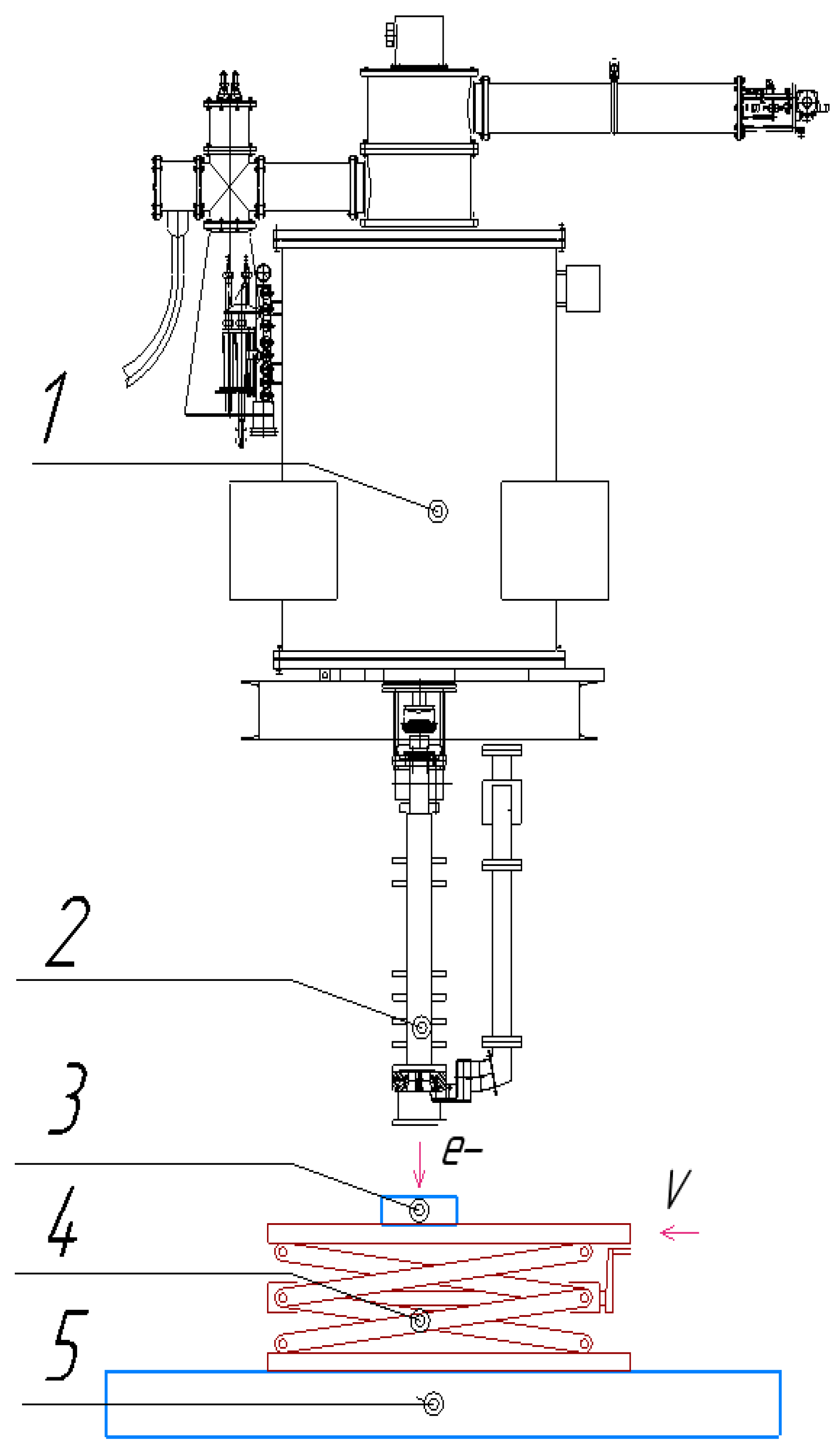
The samples were irradiated using an industrial electron accelerator, ILU-10 (Institute of Nuclear Physics SB RAS, Novosibirsk, Russia), with an accelerated electron energy of 2.5 to 5 MeV, and a maximum beam current of up to 10 mA was used. An automated roller conveyor equipped with specialized scissor-type lifting tables with a speed control range of 2–8 cm/s was used to transport the samples to the irradiation zone. The ILU-10 accelerator consisted of the following main components (
Figure 2) [
34].
2.3. Experimental Procedure
Samples were <5 cm3/m2/24 vacuum-sealed (40–60 mm Hg, <1% residual O2) in irradiation-compliant polyethylene bags (20 × 25 cm, 80 μm; O2 transmission h at 4 °C).
The obtained model systems were irradiated in the ILU-10 industrial electron accelerator at the Institute of Nuclear Physics of the Ministry of Energy of the Republic of Kazakhstan. Electron beam treatment was applied sequentially from two opposite sides of each sample to ensure uniform dose distribution throughout the product volume. Control samples were sham-irradiated (0 kGy). The samples were irradiated at four predetermined doses commonly used in food processing: 2 kGy, 4 kGy, 6 kGy, and 8 kGy. The doses of 2, 4, 6, and 8 kGy were selected based on international guidelines for food safety. Of note, 2 kGy is commonly used for surface decontamination and short-term storage; 4–6 kGy are considered pasteurization levels, effectively reducing major pathogens such as Salmonella and Listeria; and 8 kGy approaches sterilization and is used in cases requiring maximum microbial reduction. These doses align with Codex Alimentarius, FAO/WHO/IAEA, and FDA recommendations. Based on standards from the WHO/FAO/IAEA Joint Study Group, foods irradiated at doses above 10 kGy are considered safe and nutritionally adequate when processed under good manufacturing practice. Irradiation not only ensures microbiological safety, but also supports the development of shelf-stable foods for vulnerable groups such as immunocompromised patients and astronauts [
35,
36,
37].
This study consisted of a set of interrelated stages. In the initial stage, an investigation was conducted into the effectiveness of using accelerated electron beam treatment for food products to reduce microbiological contamination. Based on the implementation of this stage, the optimal irradiation modes were selected to ensure complete suppression of the selected groups of organisms. The primary focus was on optimizing the absorbed dose to ensure microbiological safety. At this stage, the ratio of the absorbed dose to the change in the main microbiological indicators of various types of meat was estimated.
2.4. Irradiation Parameters for ILU-10 Accelerator
The absorbed dose and its uniform distribution were carefully controlled during irradiation. Key factors such as electron energy, sample geometry, density, conveyor speed, and scanning width were monitored. The irradiation parameters are summarized in
Table 1.
2.5. Determination of Physico-Chemical Indicators
In order to assess the effects of electron beam irradiation on meat quality, a range of physicochemical indicators were evaluated, including moisture, protein, fat, and ash content. These indicators provide important information on nutritional value and shelf life stability changes in meat structure during storage. Analyses were conducted on days 1, 5, and 14 after treatment to monitor dynamic changes over time. This systematic monitoring aids in determining the optimal radiation dose for safety and quality assurance during storage and marketing.
Of note, 2.0 g cold poultry meat samples were cut into pieces and added to 20 mL of ultrapure water for homogenization and centrifugation, and the pH value of the supernatant was measured using a pH meter (Mettler Toledo, Greifensee, Switzerland) and recorded [
38] (ISO 2917:1999).
Moisture content was determined by oven-drying ~5 g of minced meat at 105 ± 2 °C for 16–18 h until a constant weight was achieved. After cooling in a desiccator, the samples were reweighed. Moisture content (%) was calculated based on weight loss during drying [
39].
Protein content was measured using the Kjeldahl method. Roughly 1–2 g of meat was digested with sulfuric acid, potassium sulfate, and copper sulfate at 350–400 °C to convert nitrogen into ammonium sulfate. After neutralization with sodium hydroxide, ammonia was distilled into boric acid and titrated with standard sulfuric acid. The nitrogen content was calculated and multiplied by 6.25 to estimate the total protein content (%) [
40].
The protein content was calculated by Formula (1):
where:
V1, V2V1, and V2 = acid volumes for sample/control;
KK = acid concentration correction;
mm = sample mass (g);
6.25 = nitrogen-to-protein factor.
Ash content was determined by incinerating approximately 2–5 g of the dried meat sample in a muffle furnace at 550 ± 25 °C until a constant weight was achieved. The remaining inorganic residue was weighed and expressed as a percentage of the original sample weight.
The fat content in the meat was measured using the Soxhlet extraction method (GOST 23042-2015, Russia [
41]). A 2–5 g dried sample was extracted with petroleum ether for 4–6 h (6–10 cycles). After solvent evaporation, the remaining fat was dried at 105 °C to a constant weight and expressed as a percentage of the original sample [
41].
The fat content was calculated using Formula (2):
where:
All analyses were performed in triplicate for each sample group. The results are ex-pressed as mean ± standard deviation.
2.6. Determination of Microbiological Indicators
The microbiological quality of the poultry meat samples was assessed according to the current laboratory and food safety regulations of the Republic of Kazakhstan, including Sanitary Rules No. CH 3.3.2524-09 “Sanitary and Epidemiological Requirements for Food Safety” and the relevant GOST (State Standard) methods harmonized with ISO standards [
42]. The following microbiological indicators were determined: total mesophilic aerobic and facultative anaerobic microorganisms (TMAFAMs) using GOST 10444.15-94, which corresponds to standard enumeration methods [
43], and molds (Microscopic Fungi), determined according to GOST 10444.12-2013, which specifies colony-counting techniques for enumerating molds in food products [
44]. A 10 g sample of poultry meat was homogenized in sterile saline and serially diluted tenfold. For enumeration, 0.2 cm
3 of the appropriate dilution was applied onto Petritest™ (Saratov, Russia) culture matrices. Total mesophilic aerobic and facultative anaerobic microorganisms (TMAFAMs) were incubated at 36 ± 1 °C for 24 h. Molds were incubated at 24 ± 1 °C for 120 h. Colonies were counted within valid ranges (the detection limits were 10 CFUs for TMAFAMs and 5 CFUs for molds) and multiplied by the dilution factor to calculate the final result as colony-forming units per gram (CFUs/g) of sample. All measurements were performed in triplicate to ensure reproducibility and accuracy.
Samples were collected aseptically and transported to the laboratory under refrigerated conditions (0–4 °C). Microbiological analyses were conducted on day 1 (post-treatment) and subsequently on days 3, 5, 7, 9, 11, and 14 of storage. All microbiological analyses were performed in triplicate. The results are expressed as colony-forming units per gram (CFUs/g) of sample.
2.7. Sensory Evaluation
Sensory analysis was conducted based on ISO 13299:2016 using a trained panel (n = 8) [
45]. Samples were evaluated on day 1 (post-treatment) and subsequently on days 5 and 14 of storage (0 ± 2 °C). Prior to evaluation, all meat samples were cooked to an internal temperature of 75 °C using standardized grilling procedures. The cooked samples were then uniformly cut into 2 cm
3 cubes and presented to the panelists in odorless containers at room temperature under controlled D65 lighting conditions to ensure consistent visual assessment. Panelists rated appearance, odor, texture, and taste using a 5-point hedonic scale (5 = excellent; 1 = unacceptable) [
46]. Between evaluations, water was provided for palate cleansing. All sessions were conducted in a controlled sensory laboratory environment.
2.8. Statistical Analysis
To determine the differences in the mean values between groups of irradiation doses and days of storage, data were subjected to analysis of variance (ANOVA) using the statistical package SPSS for Windows (Ver.27.0, SPSS Inc., Chicago, IL, USA, 2019). The calculated mean values were compared using Tukey’s multiple range test with significance defined at p ≤ 0.05. In addition, to investigate the influence of irradiation dose and storage time on poultry meat parameters, in addition to assessing the interaction between these factors, a two-factor analysis of variance was performed via SPSS. The mathematical modeling of the influence of various factors was performed using STATISTICA for Windows (Ver.12.5, StatSoft Inc., Tulsa, Ok, USA, 2014). Microsoft Excel (version 2016) was used to process the experimental data from the microbiological studies and construct graphs.
3. Results and Discussion
3.1. Physicochemical Analysis
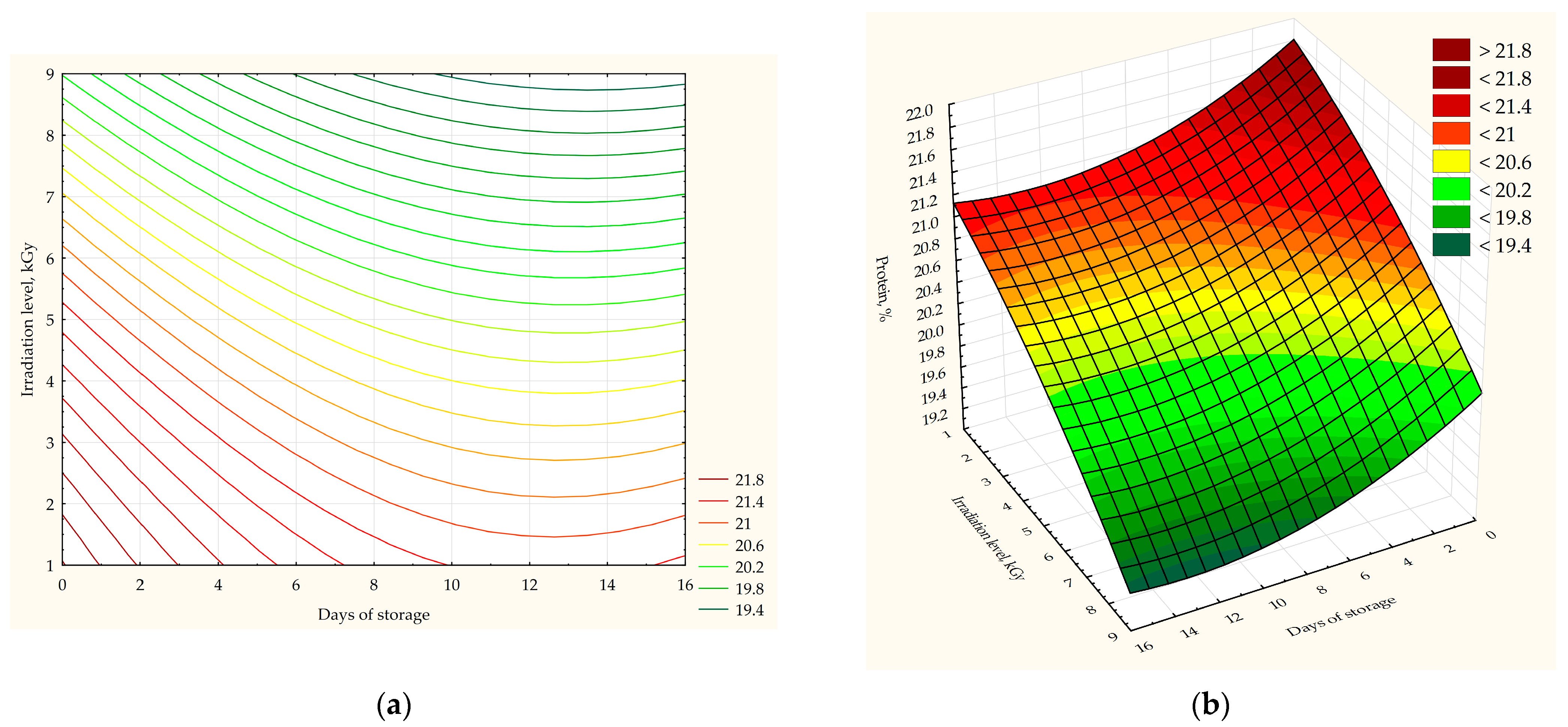
The results of this study on the influence of irradiation factors and storage duration on the physicochemical quality indicators of poultry meat are illustrated in
Table 2. The results presented in
Figure 3,
Figure 4,
Figure 5 and
Figure 6, which detail the relationship between the influence of irradiation levels and storage duration on the acidity (
Figure 3), humidity (
Figure 4), and fat (
Figure 5), protein (
Figure 6), and ash (
Figure 7) contents determined using mathematical modeling. Each figure includes the corresponding equation.
A statistically significant difference was found between samples in terms of acidity as the radiation dose and storage time increased (F = 54.036, p = 0.0001 for the first day, F = 112.042, p = 0.0001 for the fifth day, and F = 142.667, p = 0.0001 for the fourteenth day, respectively). Following irradiation, the acidity of the experimental samples decreased compared with that of the control sample (by 2.71% for 2 kGy, 3.51% for 4 kGy, 4.31% for 6 kGy, and 5.1% for 8 kGy). After 5 days of storage, the acidity of the irradiated samples decreased by 2.91%, 5.34%, 7.77%, and 9.39% for treatments at 2, 4, 6, and 8 kGy, respectively, compared with the control.
The results of this study demonstrated that, at irradiation doses of 2 and 4 kGy, the pH of the meat remained stable at 5.8–6.1 during the first 5 days of storage (1.64% and 3.31% lower compared with the moment of irradiation for 2 and 4 kGy, respectively). In samples subjected to irradiation doses of 6 and 8 kGy, a decrease in pH to 5.2–5.5 was observed on the fourteenth day. One mechanism of pH change under the influence of electron irradiation is the dose-dependent formation of acidic products as a result of water radiolysis and the destruction of organic substances [
47]. Hashim et al. noted, in their study, that the pH of gamma-irradiated meat and poultry significantly decreases during storage under the influence of several factors, such as active oxidation of proteins and fats as a result of decomposition, which leads to the occurrence of rancidity reactions [
27]. In light of these findings, pH dynamics based on dose and time can be used as a reliable indicator of quality control and the intensity of radiation treatment. In addition, storage temperature is an important factor in this regard. In the absence of freezing control, the pH of the irradiated samples decreased. However, when frozen, acidity indicators may remain unchanged [
48]. In one study, a preliminary study was conducted to evaluate avian influenza virus inactivation in contaminated poultry products using electron beam irradiation [
48].
Increasing the radiation dose significantly impacted the moisture content in poultry meat throughout the storage period. On average, after irradiation, the experimental samples lost moisture at rates of 1.48% for 2 kGy, 1.52% for 4 kGy, 2.76% for 6 kGy, and 3.3% for 8 kGy, respectively, compared with the control sample (F = 67.697;
p = 0.0001). On the fifth day of storage, the irradiated samples, when compared with the control, dried out by 0.19%, 1.04%, 1.38%, and 2.16% for 2, 4, 6, and 8 kGy, respectively (F = 17.251;
p = 0.0001). The moisture content in the non-irradiated poultry meat samples also decreased by 2.92% by the fifth day, after which they were discarded from further analysis due to spoilage. On the fourteenth day, a significant difference was observed between the experimental samples (F = 37.532;
p = 0.0001). The greatest change in moisture content was observed in poultry meat samples irradiated at a dose of 8 kGy (5.37% moisture loss over 14 days). Sadakuzzaman et al. explained that the decrease in moisture content is dependent on the decrease in metabolic activity, in addition to the decrease in the amount of extracellular fluid in the meat [
49].
A significant difference in fat content was found between each storage interval at different irradiation levels (F = 7.441;
p = 0.024 for 2 kGy; F = 6.573;
p = 0.031 for 4 kGy; F = 6.963;
p = 0.027 for 6 kGy; and F = 9.402;
p = 0.014 for 8 kGy). The amounts of fat in the samples following irradiation, when compared with the control, were 0.36%, 1.79%, 3.21%, and 5% lower as the radiation dose increased and showed no statistical difference between the groups (F = 0.193;
p = 0.937). On average, the fat content of the control samples decreased by 9.3% on day 5; in comparison, the irradiated samples exhibited a greater decrease in fat content (9.68% for 2 kGy; 10.18% for 4 kGy; 11.07% for 6 kGy; and 11.65% for 8 kGy). In addition, the irradiated samples exhibited greater fat loss compared with the control (0.79% for 2 kGy; 2.76% for 4 kGy; 5.12% for 6 kGy; and 7.48% for 8 kGy); however, these differences were not statistically significant (F = 0.687;
p = 0.617). On day 14 of storage, on average, the treated samples had lost 25.6% of their fat content compared with the irradiated samples (24.37% for 2 kGy; 25.09% for 4 kGy; 25.09% for 6 kGy; and 27.82% for 8 kGy). No statistically significant differences were observed among the irradiated samples (F = 0.230;
p = 0.873). The authors of [
50] reported that, at doses of 3 and 7 kGy, a decrease in the polyunsaturated fatty acid content was observed compared with the untreated samples. This decrease was associated with the oxidation of PUFAs induced by free radicals formed as a result of irradiation.
Significant changes were observed in the protein content of the control and irradiated poultry meat samples. The amount of protein in the irradiated samples, when compared with the control samples, decreased by 2.5%, 3.58%, 5.49%, and 7.49% at irradiation levels of 2, 4, 6, and 8 kGy, respectively (F = 22.560;
p = 0.0001). A significant difference was noted for each irradiation level on different storage days (F = 4.748;
p = 0.058 for 2 kGy; F = 11.543;
p = 0.009 for 4 kGy; F = 9.572;
p = 0.014 for 6 kGy; and F = 7.765;
p = 0.022 for 8 kGy). In addition, on the fifth day of storage, a significant decrease in protein content was observed compared with the control samples (1.53% less for 2 kGy; 2.88% for 4 kGy; 5.29% for 6 kGy; and 7.93% for 8 kGy; F = 50.957;
p = 0.0001). After 14 days of storage, the irradiated samples exhibited statistically significant differences based on the irradiation dose (F = 15.962;
p = 0.001). The most promising results for the protein content in poultry meat were demonstrated by samples irradiated with doses of 2 kGy and 4 kGy, which lost the least amount of protein during storage. In one study [
51], it was found that irradiation at doses of 1, 2, and 3.5 kGy led to a significant decrease in protein content in chilled broiler meat from 0.1% to 0.4% over a period of 14 days when stored at −20 °C.
The ash content in the irradiated and non-irradiated samples decreased with the irradiation dose and with increasing storage time. Significant differences were observed between the sample groups at each storage interval (F = 16.475;
p = 0.0001 for control and experimental samples after irradiation; F = 11.103;
p = 0.001 for the fifth day; and F = 10.741;
p = 0.004 for the fourteenth day). The ash content of the test samples after irradiation was lower than that of the control samples when treated with 6 and 8 kGy (16.85% and 25.54% lower, respectively) and 12.5% higher in samples treated with 2 kGy. The ash content of meat irradiated at 4 kGy was 1.09% higher than that of the control sample at the time of irradiation; however, on day 5, 2.4% loss of ash content was observed in a similar manner. A significant effect on ash content was observed between the groups of results by storage day for each radiation dose (F = 9.213;
p = 0.015 for 2 kGy; F = 9.077;
p = 0.015 for 4 kGy; F = 13.779;
p = 0.006 for 6 kGy; and F = 19.47;
p = 0.002 for 8 kGy), which is evident when comparing the results of the samples on the fifth day of storage with the results obtained after irradiation (9.66% less for 2 kGy; 12.37% less for 4 kGy; 9.8% less for 6 kGy; and 8.76% less for 8 kGy). A tendency for the ash content to decrease was also observed on the fourteenth day of storage (27.54% less for 2 kGy; 26.88% less for 4 kGy; 32.68% less for 6 kGy; and 32.85% less for 8 kGy). In one study [
52], a decrease in the ash content in meat samples subjected to gamma irradiation at doses of 1.5, 2, and 4 kGy was recorded at approximately 1–4% during a 60-day period of frozen storage.
Based on our study results, sterilization of poultry meat using electron beam irradiation in the dose range from 2 to 4 kGy aids in maintaining quality indicators during storage for up to 5–6 days without significant losses.
3.2. Microbiological Analysis
In another study [
53], the authors found that 1, 3, and 5 kGy gamma-irradiated poultry meat packed in trays exhibited shelf lives ranging from 7 days to more than 2 weeks. The irradiation of poultry thighs and breasts with 1, 2, and 3 kGy reduced the number of aerobic bacteria, but did not eliminate the organisms [
54]. In one study [
55], total wing numbers were reduced by gamma irradiation at 1.4 kGy from 104 to 44 CFUs/cm
2. Viable CFUs were found on wings inoculated with 1000 or 10,000 CFUs/cm
2 at 1.8 kGy, but not on wings irradiated with 2.7 kGy or higher. When chicken breasts were irradiated with 2.5 kGy gamma rays, the number of aerobic bacteria decreased by roughly two log cycles, reaching a level of 106 CFUs/g roughly 19 days after death [
56].
Through microbiological analysis of the treated meat, we found a significant decrease in the total number of microorganisms with increasing radiation dose. The results presented in
Figure 7 illustrate changes in the number of mesophilic aerobic and facultative anaerobic microorganisms (MAFAMs) in poultry meat based on the radiation dose. Microbiological indicators were determined in poultry meat samples after irradiation with 2, 4, 6, and 8 kGy, after 1, 3, 5, 7, 9, 11, and 14 days of meat storage at a temperature of −1 °C to +4 °C, as shown in
Figure 8.
In
Figure 8, we present the microbiological indicators of poultry meat subjected to different doses of electron beam irradiation (2, 4, 6, and 8 kGy) and stored at temperatures ranging from −1 °C to +4 °C over a period of 14 days. The values represent the microbial load measured in log
10 CFUs/g, with each data point corresponding to the mean value obtained at specific time intervals, as shown in the figure. This finding indicates that increasing irradiation doses resulted in a progressive reduction in microbial counts immediately after treatment.
Over the 14-day storage period, microbial counts generally increased in all samples, reflecting microbial proliferation over time. However, the extent of growth was markedly influenced by the irradiation dose. In the control (non-irradiated), the microbial load increased from 3.39 on day 1 to 4.50 on day 14, indicating steady microbial growth during storage and exceeding the hygiene standards of EAEU TR 051/2021 by 3.2 times [
57].
At a 2 kGy irradiation dose, counts increased from 3.18 to 4.05, demonstrating moderate microbial proliferation. At a 4 kGy irradiation dose, counts increased from 3.04 to 4.06, with microbial growth similar to the 2 kGy group, also exceeding the hygienic standards of EAEU TR 051/2021 by 1.1 times. At a 6 kGy irradiation dose, counts increased from 2.90 to 3.99, reflecting notable suppression of microbial growth compared with lower doses. Lastly, at an 8 kGy irradiation dose, counts increased from 2.70 to 3.91, also demonstrating a significant inhibitory effect on microbial proliferation.
Our data clearly demonstrate that irradiation at doses of 4 kGy and above effectively reduces the initial microbial load and suppresses microbial growth during storage. The higher doses (6 and 8 kGy) maintained lower microbial counts throughout the storage period compared with the control and lower doses, indicating enhanced microbiological safety and potential extension of shelf life.
Electron beam irradiation, particularly at doses of 6 and 8 kGy, significantly diminished the microbial load of poultry meat immediately after treatment and effectively inhibits microbial proliferation during refrigerated storage. These findings support the application of irradiation as a viable method for improving the microbiological safety and extending the shelf life of poultry meat under refrigerated conditions.
Neither Salmonella, L. Monocytogenes, nor coliform bacteria (coliform bacteria) were detected, and the amount of mold did not exceed the established norm for 11 days. From the fourteenth day, the mold in the control sample exceeded the norm of 12 CFUs/g. According to the above, our results demonstrate that poultry sterilized via radiation at doses of 2, 4, 6, and 8 kGy maintain microbiological safety indicators in a chilled state for up to 14 days.
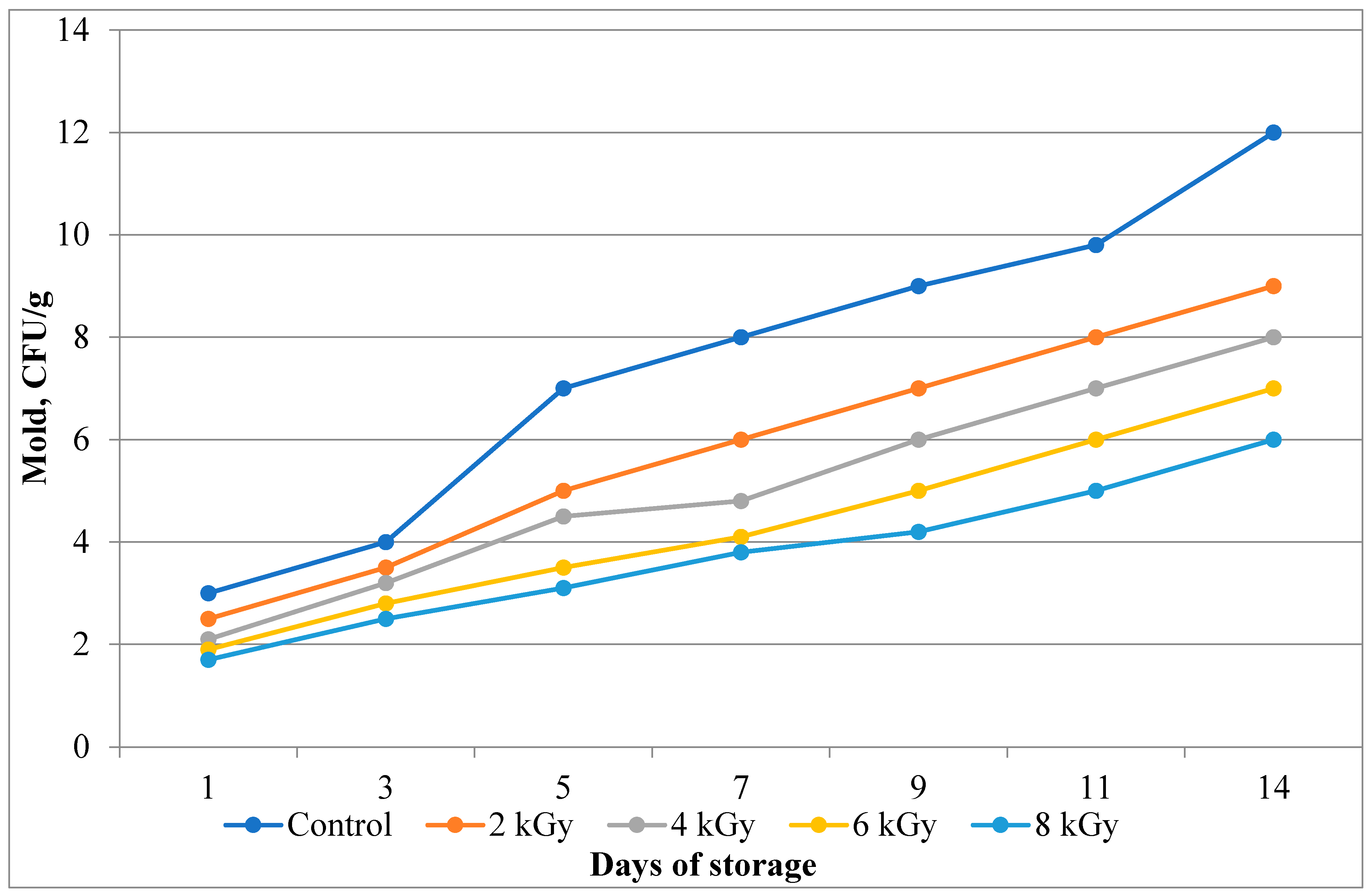
In a similar vein, the analysis of the fungal content of the processed meat, presented in
Figure 9, demonstrates a significant reduction in their number as the radiation dose increases. The data confirm that electron irradiation effectively destroys fungal spores, preventing their further development during storage.
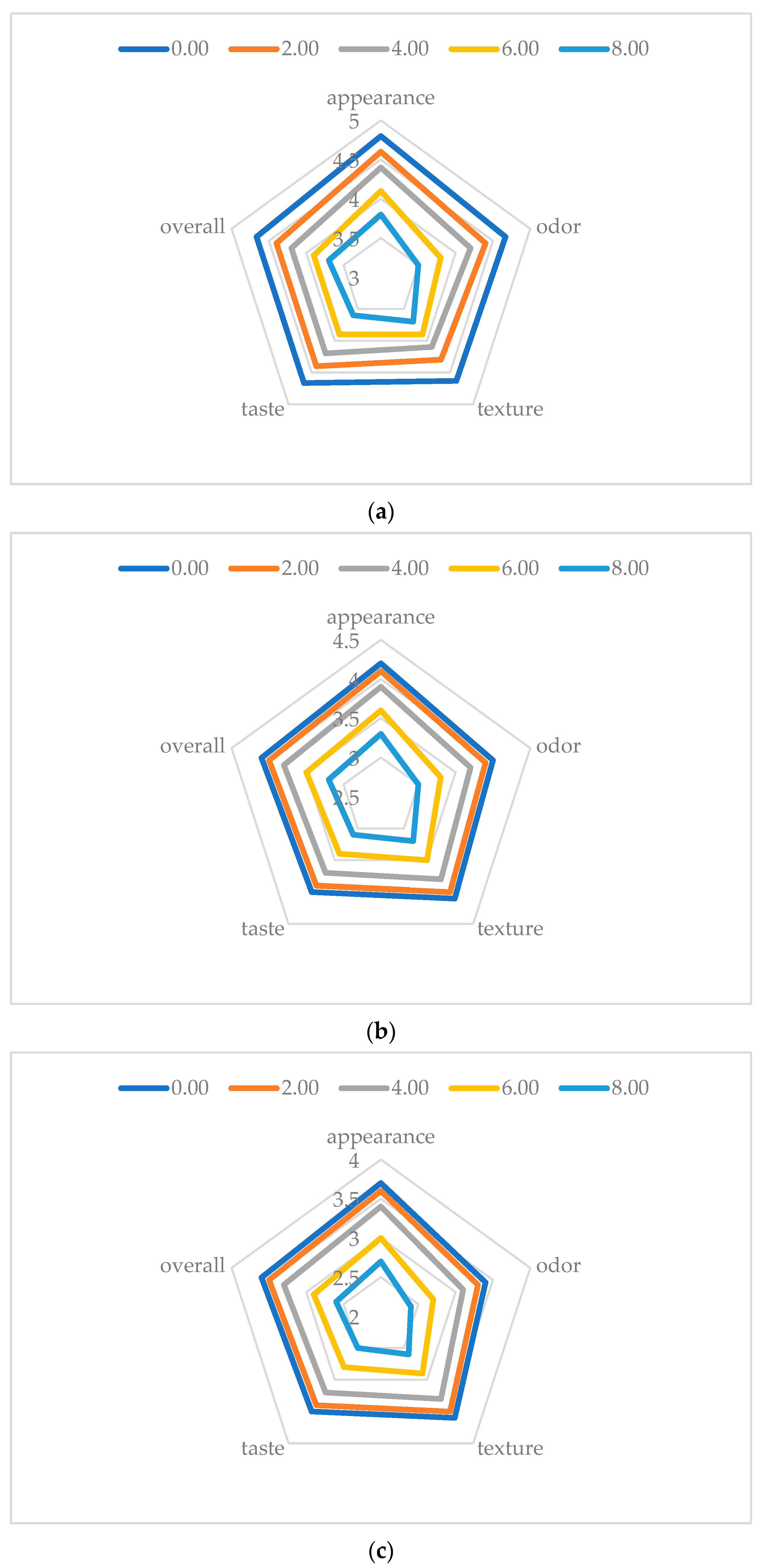
3.3. Sensory Evaluation Results
The sensory attributes of poultry meat—namely appearance, odor, texture, taste, and overall acceptability—were significantly impacted by both irradiation dose and storage duration (
p < 0.001 for all attributes, two-way ANOVA), as shown in
Figure 10.
Irradiation dose had a significant and measurable impact on the retention of sensory quality. Non-irradiated control samples (0 kGy) consistently exhibited the highest sensory scores throughout the storage period, with overall acceptability declining from 4.7 ± 0.1 on day 1 to 3.7 ± 0.1 on day 14. A 2 kGy dose resulted in a slight, but statistically significant, reduction (overall: 4.4 ± 0.1 on day 1; F = 140.8, p < 0.001 vs. control). In contrast, higher doses (≥4 kGy) led to progressively greater deterioration in sensory attributes. The 8 kGy treatment group showed the most pronounced decline, with an initial overall acceptability of 3.3 ± 0.1 on day 1 (F = 133.2, p < 0.001).
Storage duration had a significant linear effect on sensory decline (p < 0.001 for all attributes). In the control group, overall acceptability decreased by 12.8% by day 5; in comparison, overall acceptability in the irradiated groups exhibited a decrease between 14.6% and 20.4%. By day 14, in the 8 kGy group, loss of acceptability reached 29.8% compared with the control, which was mainly due to severe deterioration in odor and texture (F = 53.1, p < 0.001 for both).
These findings align with those presented in [
27], the authors of which reported stable sensory quality in gamma-irradiated chicken products at doses ≤ 3 kGy. However, our study results demonstrate that electron-beam irradiation offers superior preservation of juiciness at equivalent doses, likely due to reduced oxidative damage [
50]. Notably, the 4 kGy threshold identified in the present study exceeds the 2.5 kGy required for shelf life extension in beef [
5], underscoring poultry’s heightened susceptibility to irradiation-induced oxidation [
51].
On a practical level, doses up to 2 kGy are optimal for preserving sensory quality during short-term storage (≤5 days); in contrast, higher doses (4–8 kGy) may necessitate antioxidants or advanced packaging to minimize quality loss.
4. Conclusions
This study evaluated the characteristics of chilled poultry meat after treatment with electron beams, and a significant extension of the period of realization was achieved. Based on local normative documents, the shelf life of chilled poultry meat ranges from 48 h [
58] to 3 days; following treatment with electron beams, however, it is possible to increase this period twice, i.e., by 6 days, without compromising quality indicators.
The limitations of the application of electron beam treatment include lipid oxidation (LPOD), protein oxidation (PNOD), and physicochemical and organoleptic changes that limit its application at high doses.
Radiation treatment of food products is a relatively inexpensive, easily controlled method that does not require the use of chemicals and does not alter the organoleptic properties of irradiated products [
59,
60]. However, the large-scale promotion of electron beam irradiation in the food industry faces challenges for several reasons: low consumer awareness [
61], radiophobia, and differences in the regulatory framework of states.
The most effective and scientifically valid methods are combined methods—for example, chilled meat treatment plus vacuum packaging or chilled meat treatment with antimicrobial packaging, which provide not only an increase in shelf life, but also preservation of the organoleptic and nutritional properties of poultry meat. Food products are irradiated with doses not exceeding 10 kGy, which does not increase the temperature of the food, leaves no harmful residues, and can be applied to packaged food, thus limiting the likelihood of contamination or re-contamination. The main effect of temperature on products may be due to interaction with the environment. However, this issue can be easily resolved by controlling and maintaining the temperature in the exposure area through refrigeration and ventilation systems. The temperature of food products can be monitored either online, using temperature sensors, or using temperature indicators. Through further prospective research by our team, we aim to study combined measures to extend the shelf life of chilled meat.