Abstract
This study presents the development and comprehensive evaluation of magnesium-based implants with surface modifications using selected polymers and bioactive compounds. The implants were fabricated via plasma electrolytic oxidation (PEO), followed by the application of chitosan, polydopamine (PDA), and gold nanoparticles as bioactive surface coatings. In vitro experiments, including FT-IR spectroscopy, scanning electron microscopy (SEM), wettability tests, biodegradation assays in simulated body fluid (SBF), electrochemical corrosion analysis, and cytotoxicity tests using MG-63 osteoblast-like cells, were employed to assess the physicochemical and biological properties of the materials. The PEO + PDA-modified samples demonstrated the highest corrosion resistance (−1.15 V corrosion potential), enhanced cell viability (~95%), and favorable surface wettability (contact angle ~12.5°), outperforming other tested configurations. These findings suggest that PEO combined with PDA offers a synergistic effect, leading to superior biocompatibility and degradation control compared to unmodified magnesium or single-coating strategies. The developed implants hold promise for orthopedic applications requiring biodegradable, bioactive, and cytocompatible materials.
1. Introduction
The human skeletal system comprises a dynamic and highly specialized network of tissues that perform multiple vital functions, including structural support, locomotion, mineral storage, and the protection of internal organs. Bone, as the fundamental component of this system, is a composite material consisting of an inorganic mineral phase primarily composed of hydroxyapatite (Ca10(PO4)6(OH)2), and an organic matrix largely comprising type I collagen and various non-collagenous proteins [1,2,3]. This biphasic structure confers bone with a unique combination of stiffness, toughness, and resistance to mechanical loads [4,5,6,7,8,9,10]. The regulation of bone homeostasis is mediated by osteoblasts, osteoclasts, and osteocytes, each playing distinct yet interdependent roles in the processes of bone formation, remodeling, and mineral turnover [1,2,11,12,13,14,15].
Bone tissue is categorized into two structural types: coarse-fibrous (woven) bone and fine-fibrous (lamellar) bone. The former appears transiently during fetal development and early stages of bone repair, while the latter forms the mature bone structure and is further differentiated into cortical (compact) and trabecular (spongy) bone [3]. Cortical bone constitutes the dense outer layer of long bones, imparting mechanical strength, whereas trabecular bone, located within the epiphyses and flat bones, supports metabolic activity including hematopoiesis through its high vascularization.
In orthopedics and traumatology, metallic implants have long been the material of choice due to their mechanical robustness and structural reliability. Alloys such as stainless steel, cobalt–chromium, and titanium have been extensively utilized in clinical practice for internal fixation and joint replacement applications [15]. Despite their mechanical superiority, these materials are biologically inert, non-resorbable, and often necessitate secondary removal surgeries. Furthermore, they are associated with stress-shielding effects, whereby the mismatch in elastic modulus between the implant and surrounding bone reduces physiological load transfer, leading to bone resorption and implant loosening. Additionally, these implants are vulnerable to microbial colonization and biofilm formation, which can result in chronic periprosthetic infections that are difficult to eradicate [16,17,18,19,20].
To address these limitations, biodegradable metals have gained increasing interest as next-generation biomaterials for temporary implant applications. Among these, magnesium (Mg) and its alloys are especially promising owing to their low density, biocompatibility, and mechanical properties that closely approximate those of human cortical bone [4,5,21,22,23,24,25]. Magnesium offers several biological advantages: it is a natural constituent of the human body, involved in numerous enzymatic processes and cellular signaling pathways, and it stimulates osteogenesis and angiogenesis. The biodegradability of Mg facilitates gradual resorption in vivo, eliminating the need for surgical removal and reducing long-term complication risks.
However, the clinical translation of magnesium-based implants is impeded by their inherent susceptibility to rapid degradation in physiological environments. Upon implantation, Mg reacts with water and chloride ions to form Mg(OH)2 and hydrogen gas, a process exacerbated by the presence of bicarbonates and phosphates in body fluids that convert the hydroxide layer into more soluble salts like MgCO3 [6]. The uncontrolled release of hydrogen gas and resultant local alkalization can lead to soft tissue inflammation, delayed healing, and poor osseointegration. These issues necessitate the development of effective surface modification strategies to regulate the degradation rate while maintaining or enhancing the bioactivity of the material [26,27,28,29,30,31,32].
Several techniques have been developed to improve the surface stability and biocompatibility of Mg implants. Plasma electrolytic oxidation (PEO), also known as micro-arc oxidation, has emerged as a particularly effective method to generate dense, ceramic-like oxide layers on Mg surfaces. This electrochemical process involves high-voltage anodic treatment in an electrolytic bath, resulting in the formation of a porous, corrosion-resistant magnesium oxide layer that can incorporate bioactive ions such as calcium, phosphate, and silicate depending on electrolyte composition [10,33,34,35,36,37,38,39,40,41,42,43,44]. The resulting topography and surface chemistry can promote cellular attachment, differentiation, and mineralization.
In addition to ceramic coatings, polymer-based modifications have been investigated to enhance surface biofunctionality. Polydopamine (PDA), a synthetic analog of the mussel-derived adhesive catecholamine, is capable of forming thin, conformal coatings on a wide range of substrates. PDA coatings exhibit strong adhesion, good biocompatibility, and the ability to immobilize bioactive molecules, making them highly suitable for biomedical applications [45,46,47,48,49,50,51]. PDA enhances osteoblast adhesion and proliferation by mimicking the extracellular matrix and providing functional groups for secondary modification. Another extensively studied polymer is chitosan—a biodegradable, non-toxic polysaccharide known for its antimicrobial, hemostatic, and immunomodulatory properties [8,9]. Chitosan’s cationic nature allows it to interact with cell membranes and negatively charged surfaces, facilitating film formation and bioactive molecule delivery.
Emerging strategies also incorporate metallic nanoparticles, such as gold nanoparticles (AuNPs), which have been shown to exhibit anti-inflammatory and osteoinductive properties while improving corrosion resistance when embedded in coating matrices [10]. The integration of multiple surface modification techniques—for example, PEO followed by polymer and nanoparticle deposition—offers the potential to synergistically combine the mechanical robustness of the ceramic layer with the biological advantages of polymers and nanomaterials.
Despite these advances, the clinical translation of such combinatorial surface treatments remains limited by a lack of systematic evaluations and comparative studies. Most investigations focus on a single modification method or do not fully assess the interactions between coating layers and their cumulative effect on implant performance. Moreover, while promising in vitro results have been reported, preclinical and clinical data validating long-term efficacy and safety are still scarce. Notably, critical parameters such as degradation kinetics, corrosion behavior in simulated body fluids, and cytocompatibility with osteoblastic cells must be quantitatively and reproducibly characterized under standardized conditions to guide future clinical applications.
Therefore, a key research gap exists in understanding how multi-component surface engineering strategies—specifically the integration of PEO with PDA, chitosan, and AuNPs—can be optimized to maximize corrosion resistance, cytocompatibility, and surface bioactivity. These combined approaches may offer new opportunities for the design of next-generation biodegradable implants that degrade in a controlled manner while simultaneously promoting osteointegration and tissue healing.
The present study addresses this gap by systematically investigating magnesium implants modified via PEO and further functionalized with chitosan, polydopamine, and gold nanoparticles. A comprehensive characterization of the physicochemical and biological properties of the modified surfaces was conducted using Fourier-transform infrared spectroscopy (FT-IR), scanning electron microscopy (SEM), contact angle goniometry, electrochemical corrosion testing, and cytotoxicity assays using the human MG-63 osteoblastic cell line. The aim is to identify the surface modification strategy that offers the best compromise between structural integrity, degradation control, and biological performance. The findings are intended to contribute toward the rational design of clinically viable Mg-based orthopedic implants that align with the principles of biofunctionality, biocompatibility, and bioresorbability.
2. Materials and Methods
2.1. Materials Preparation
2.1.1. Chemicals and Reagents
The following reagents were used in this study without further purification: dopamine hydrochloride, TRIS buffer (tris(hydroxymethyl)aminomethane), sodium hydroxide (NaOH), L-glutamic acid, tetrachloroauric acid trihydrate (HAuCl4·3H2O), hydrated sodium citrate, ammonium fluoride (NH4F), sodium metasilicate pentahydrate (Na2SiO3·5H2O) purchased from SigmaAldrich, Poznań, Poland. Chitosan with viscosity grades of 10–120 cps and 100–300 cps was purchased from PolAura, Dywity, Poland. Additionally, all reagents required for the preparation of simulated body fluid (SBF) were used, including sodium chloride (NaCl), sodium bicarbonate (NaHCO3), potassium chloride (KCl), potassium phosphate tribasic trihydrate (K2HPO4·3H2O), magnesium chloride hexahydrate (MgCl2·6H2O), calcium chloride (CaCl2), sodium sulfate (Na2SO4), hydrochloric acid (HCl, 1 M), and TRIS. All reagents were of analytical grade and were obtained from Sigma-Aldrich (Merck Sp. z o.o., Poznań, Poland). Distilled water used in all solution preparations was obtained from a reverse osmosis system, with a conductivity of 1.7 µS/cm.
2.1.2. Preparation of Polydopamine Solution for Modification
To prepare the polydopamine (PDA) solution, 10 mg of dopamine hydrochloride was dissolved in 2 mL of distilled water and 2 mL of TRIS buffer. The solution was stirred with continuous aeration for 24 h at room temperature. After polymerization, the pH was adjusted to 8.5 ± 0.1 using NaOH solution, which is optimal for dopamine oxidation and PDA formation [45,46]. The PDA solution was immediately used to coat magnesium surfaces.
The materials needed to make the polydopamine solution are as follows: 10 mg of dopamine hydrochloride, 2 mL of H2O, 2 mL of TRIS buffer, and NaOH solution for pH adjustment. The required equipment includes the following: a 100 mL Teqler glass laboratory bottle, an aeration source, a 7 mm inner diameter silicone hose, a laboratory jack, and a tripod with a metal foot. The procedure for performing the preparation was as follows: First, the workstation was prepared. A glass bottle was placed on a tripod with a metal foot. A silicone hose was connected to the aeration source and the other end placed in the bottle. A laboratory jack was set up near the bottle. The preparation of the reaction solution was then undertaken, for which purpose 10 mg of dopamine hydrochloride was weighed out on a technical balance. Using a measuring cylinder, 2 mL of water and 2 mL of TRIS buffer were measured, and the weighed dopamine and the measured volumes of water and buffer were transferred to a glass bottle. Performing the synthesis had the following procedure: the bottle was placed in an upright position on a laboratory lift. An aeration source was switched on, ensuring a continuous supply of air to the solution. The reaction was allowed to proceed for 24 h at room temperature. After this time, the pH of the solution was checked and, if necessary, too high a pH was adjusted with an appropriate amount of added NaOH solution. The reaction was terminated after 24 h and the correct pH was reached. The polydopamine solution thus prepared was used to modify the magnesium surface.
2.1.3. Preparation of Chitosan Solution for Modification
Materials required to prepare chitosan solutions are as follows: 0.5 g chitosan (10–120 cps), 0.5 g chitosan (100–300 cps), 0.8 g L-glutamic acid, and 20 mL distilled water. The necessary equipment includes a Radwag AS 120.R2 PLUS analytical balance (Radwag, Toruń, Poland), two 100 mL beakers, an IKA-C MAG HS 7 magnetic stirrer (Chemland, Stargard, Poland) and plastic containers to hold the solutions. The procedure was performed as follows: First, 0.5 g of chitosan (10–120 cps) and 0.5 g of chitosan (100–300 cps) were weighed on an analytical balance. Next, 0.8 g of L-glutamic acid was placed in each of two 100 mL beakers. Then 20 mL of distilled water was added to each beaker. The beakers were placed on magnetic stirrers and the contents heated to 90 °C, stirring for approximately 10 min to completely dissolve the L-glutamic acid. After the heating period, 0.5 g of chitosan (10–120 cps) was added to the first beaker and 0.5 g of chitosan (100–300 cps) was added to the second beaker. Stirring was continued until both beakers reached a homogeneous suspension. The final pH of the chitosan solutions was measured at 4.6 ± 0.2, appropriate for ensuring solubility and coating uniformity [8]. The solutions were cooled and stored at 4 °C until use. The contents were then transferred to plastic containers, which were placed in the fridge for storage. The preparations were used to modify the magnesium surface.
2.1.4. Preparation of Gold Nanoparticles Solution for Modification
Materials needed to prepare the gold nanoparticle solution are as follows: 49 mg of tetrachloroauric acid and 2.2 g of hydrated sodium citrate. The required equipment includes a Radwag AS 120.R2 PLUS analytical balance, 10 cm3 and 50 cm3 measuring flasks, 100 cm3 beaker, an IKA—C MAG HS 7 magnetic stirrer, and distilled water. The procedure for preparing the solution is as follows: 49 mg of tetrachloroauric acid and 2.2 g of hydrated sodium citrate were weighed on the analytical balance. Tetrachloroauric acid was dissolved in distilled water using a 10 cm3 measuring flask, and hydrated sodium citrate was dissolved in distilled water using a 50 cm3 measuring flask. Next, 50 cm3 of distilled water was added to a 100 cm3 beaker and heated to 90 °C using a magnetic stirrer. Then 0.5 cm3 of sodium citrate solution and 0.5 cm3 of tetrachloroauric acid solution were added to the heated water. The mixture was heated on an IKA—C MAG HS 7 magnetic stirrer for 3 h. The gold nanoparticle solution obtained during the synthesis process was used to modify the magnesium surface. Successful nanoparticle formation was visually confirmed by the development of a deep red color, consistent with surface plasmon resonance of AuNPs (~520 nm) [10]. The resulting colloidal solution was stored in the dark at 4 °C.
2.1.5. Preparation of the Electrolyte for the PEO Process
The electrolyte used for plasma electrolytic oxidation (PEO) was prepared by dissolving 5.0 g/L NaOH, 5.0 g/L NH4F, and 25.0 g/L Na2SiO3·5H2O in distilled water, as summarized in Table 1.

Table 1.
Summary of the amounts of compounds needed to make the electrolyte for PEO modification.
2.1.6. Preparation of SBF Solution for Biocorrosion and Biodegradation Testing
Simulated body fluid (SBF) was prepared according to the protocol described by Kokubo and Takadama to mimic the ionic composition of human blood plasma. The solution contained the following ions: Na+, K+, Mg2+, Ca2+, Cl−, HCO3−, HPO42−, and SO42−. To prepare 1 L of SBF, the following reagents were sequentially dissolved in 700–800 mL of deionized water under constant stirring at 36.5 °C: 8.035 g of NaCl, 0.355 g of NaHCO3, 0.225 g of KCl, 0.231 g of K2HPO4·3H2O, 0.311 g of MgCl2·6H2O, 0.292 g of CaCl2, 0.072 g of Na2SO4, and 6.118 g of Tris(hydroxymethyl)aminomethane (Tris). The pH was adjusted to 7.40 ± 0.02 using approximately 40 mL of 1.0 M HCl. Once all components were fully dissolved and the pH stabilized, the volume was brought up to 1000 mL with deionized water. The solution was used within 24–48 h and maintained at 36.5 °C to ensure ion stability and prevent precipitation.
2.2. Biomaterials Preparation
2.2.1. Plasma Electrolytic Oxidation
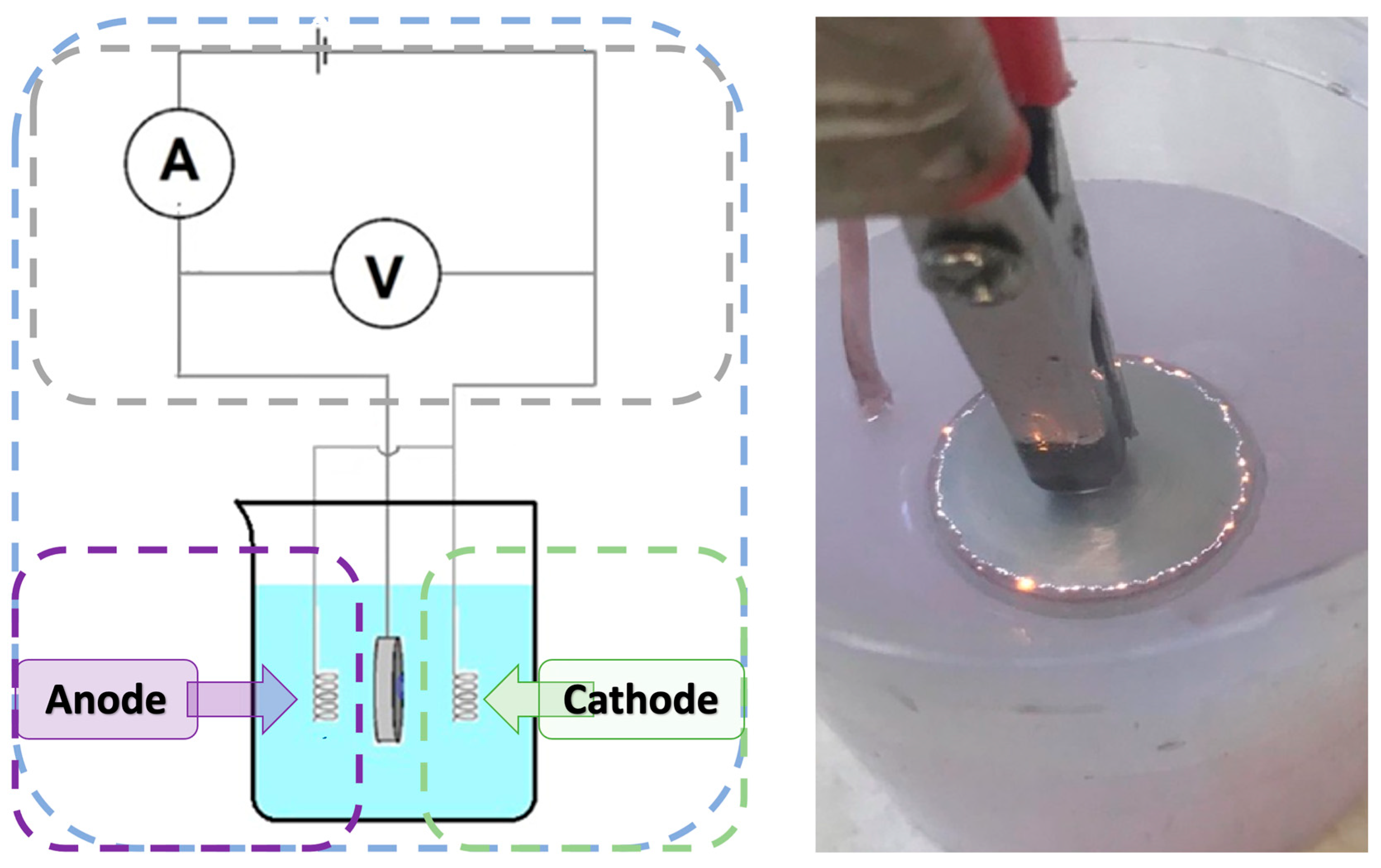
In order to modify the magnesium surface using the plasma electrolytic oxidation (PEO) process, a station consisting of a voltmeter, ammeter and a 100 mL beaker was prepared. Next, 57 mL of previously prepared electrolyte was introduced into the beaker, and then 3 mL of gold nanoparticle solution was added, which gave a total volume of the medium of 60 mL. A magnesium disc was placed in the prepared solution and the PEO process was performed for 5 min using a direct current of 240 V. As a result of this process, a metal oxide layer was formed on the surface of the magnesium disc (Figure 1). A total of six sample groups were prepared (Mg_01 to Mg_06), as detailed in Table 2.
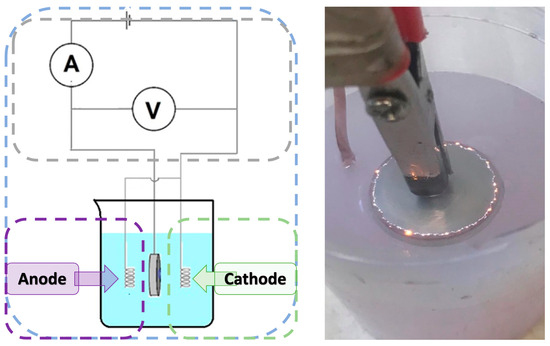
Figure 1.
The schematic diagram of the PEO process and photographic representation of micro-discharges during the PEO process.

Table 2.
List of samples and description of prepared magnesium-based implants.
2.2.2. Surface Modification of Magnesium Samples Using Biocompatible Polymers
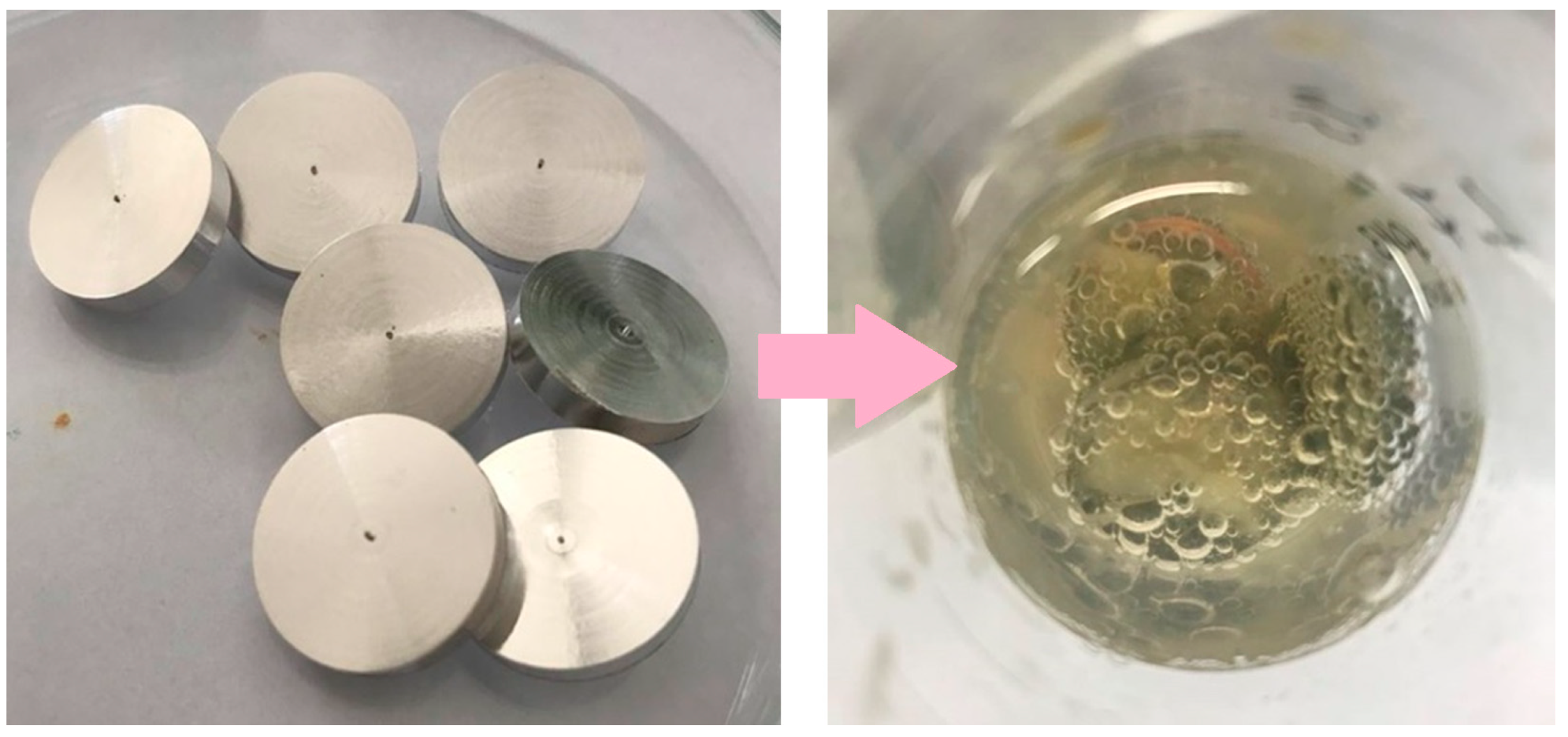
To modify the surface of magnesium samples, several stages were used to cover the metal with coatings. Initially, a magnesium rod was machined using a Vevor lathe to obtain 3 mm thick magnesium discs. Then, the surface of these discs was smoothed and degreased, and they were left to dry for 2 h. The discs prepared in this way are shown in Figure 1. The next stage was to apply coatings to the discs by placing them in chitosan suspensions and a polydopamine solution after which they were left for 48 h (Figure 2). Similarly, the coatings were applied to the discs after the plasma electrolytic oxidation (PEO) process.
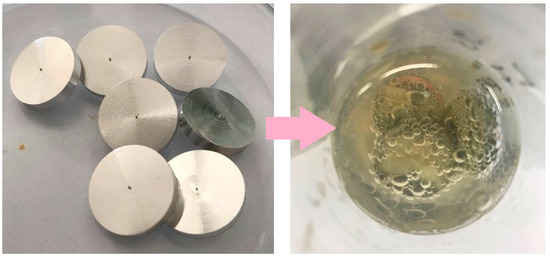
Figure 2.
Image shows 3 mm thick magnesium discs prepared for modification, and a photographic representation of the immersion method involving the surface modification of a magnesium disc placed in a chitosan suspension (100–300 cps).
2.2.3. Prepared Biomaterials
In this study, six magnesium implants were prepared. The name and description are given in Table 2.
2.3. Analysis Assessment Method
Coating formation was confirmed using a stereoscopic microscope under 100× magnification, which enabled the macroscopic visualization of surface changes on the prepared implant samples. This facilitated the assessment of surface morphology, coating uniformity, and the presence of visible defects such as cracks or delamination. Observations were conducted for all samples (n = 3 per group).
2.3.1. Chemical Structure Study
The studies to determine the chemical structure of the synthesized coating compounds were performed using a Thermo Nicolet Nexus 470 FT-IR spectrometer. This device is equipped with a laser as a radiation source (Nicolet 470 Nexus optics), a DTGS detector, and a Smart Orbit attachment with a diamond ATR crystal. The Omnic 6.1 software was used to operate the device. For the analysis, 1 cm3 was taken from each solution, applied to a polystyrene plate, and left to evaporate. After drying, the obtained powder samples were placed in the device, where the measurements were taken.
2.3.2. Surface Morphology and Composition Studies
To investigate the morphology and composition of the coatings applied to magnesium implants, a high-resolution scanning electron microscope (Apreo 2 S LoVac, Thermo Fisher Scientific, Waltham, MA, USA) was utilized. This instrument was equipped with energy-dispersive X-ray spectroscopy (EDS) detectors: UltraDry (Thermo Fisher Scientific) and Octane Elect (EDAX Ametek GmbH, China, Beijing). The analyses were conducted at the Faculty Research Laboratory of the Cracow University of Technology, within the Faculty of Chemical Engineering and Technology. Due to the vacuum conditions required by the microscope, proper sample preparation was essential. Segments were cut from the modified magnesium implants using a Dremel rotary tool, which operates at 130 W and 230 V. These segments were then affixed to double-sided carbon tape. The prepared samples were placed into the microscope for examination. At least three samples per group (n = 3) were analyzed.
2.3.3. Biodegradation Study
Magnesium samples (n = 3) with applied coatings were placed in 100 mL vessels, each filled with 60 mL of simulated body fluid (SBF). The samples were incubated for 336 h (14 days) at 37 °C to perform the biodegradation test. In parallel, the same procedure was applied to control samples with oxide coatings obtained via the PEO method, as well as to those with additional surface modifications.
2.3.4. Wettability Assessment
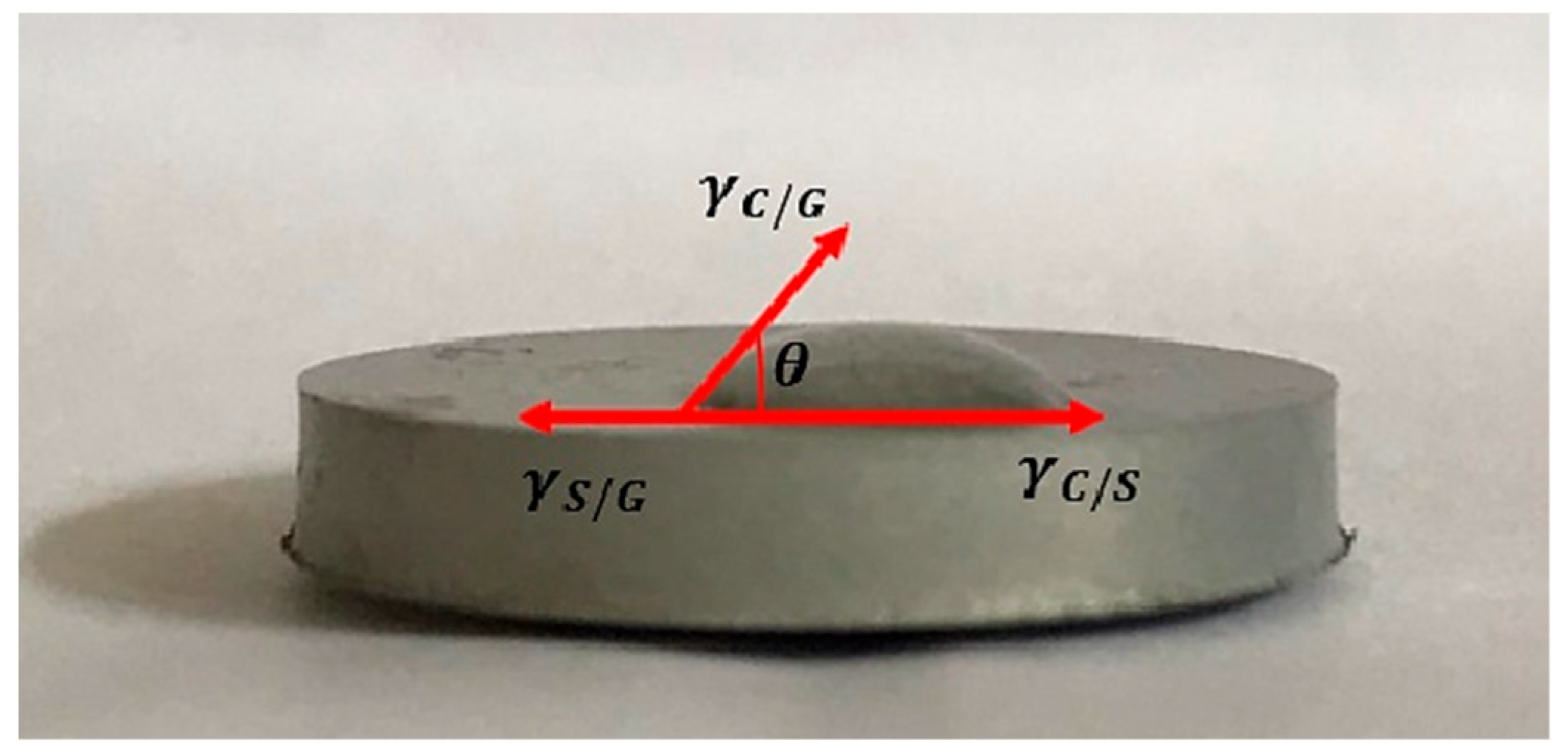
To test the surface wettability, first the implants with the coatings applied were prepared (n = 3). They were thoroughly dried by leaving them in the air for 7 h. Then the test stand was set up, ensuring that the implant was on a perfectly flat surface, which was achieved using the Survgeo H35 circular vial. Water drops were applied to the prepared implant and the results were documented. Figure 3 shows an example of a sample with a method for determining the wetting angle.
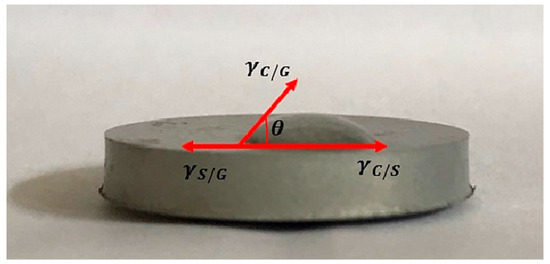
Figure 3.
Example graphic of conducting a wetting angle test of a magnesium implant.
2.3.5. Biocorrosion Studies
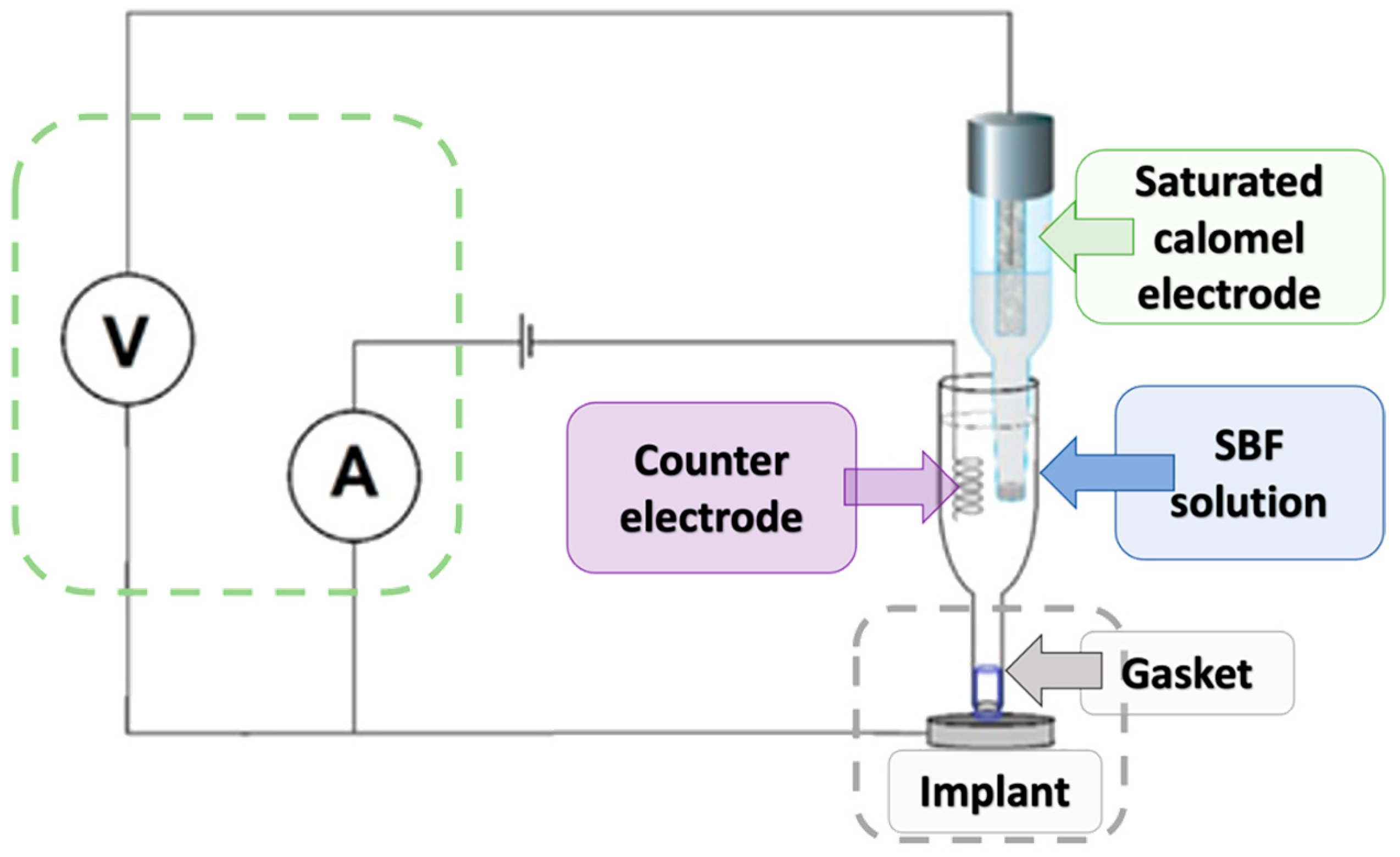
To investigate the corrosion behavior of the tested magnesium-based coatings, a standard three-electrode electrochemical cell was used (Figure 4). The setup consisted of a working electrode (the magnesium implant), a saturated calomel electrode (SCE) serving as the reference electrode, and a platinum wire as the counter electrode. The electrodes were immersed in simulated body fluid (SBF), which mimics the ionic composition of human plasma. The working electrode (implant, n = 3) was placed in a dedicated holder with a sealing gasket to ensure that only a defined surface area was exposed to the solution. The reference electrode was positioned close to the implant surface to minimize ohmic drop, while the counter electrode was placed on the opposite side to complete the circuit. All electrodes were connected to a potentiostat/galvanostat, which controlled the applied potential and recorded the resulting current. The measurement system also included a voltmeter and ammeter to monitor real-time voltage and current values. Potentiodynamic polarization tests were conducted by scanning the potential in the range from −1200 mV to +400/600 mV vs. SCE.
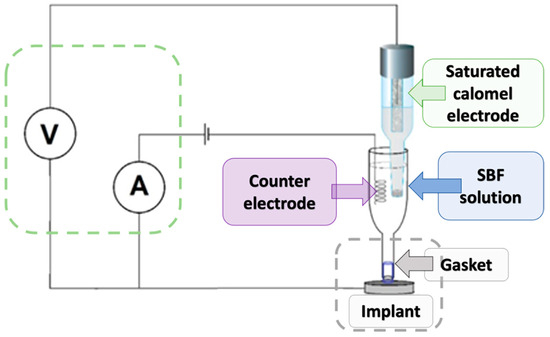
Figure 4.
Schematic diagram of the measuring apparatus for biocorrosion testing.
2.3.6. Cytotoxicity Assessment
Cytotoxicity was assessed using the human osteoblastic cell line MG-63 under direct contact conditions. Samples were sterilized with 70% ethanol and UV light in a laminar flow cabinet, before being transferred to polystyrene Petri dishes for cell culture. The samples were then plated with the cell suspension and complete DMEM culture medium supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic/antimycotic was introduced. After a 48 h incubation, a qualitative assessment of cytotoxicity was performed. A quantitative analysis was performed using the XTT assay (n = 5), which allowed the percentage of metabolically active cells to be determined. A standard control cell culture was used as a reference. The XTT assay was performed using a Microplate Reader RT-6900, supplied by DRG MedTek Sp. z o.o., Warsaw, Poland, according to the manufacturer’s protocol (Roche). Cell culture observations were performed using an inverted optical microscope from DeltaOptics, Planeta Oczu, Zielona Góra, Poland.
All experiments were conducted in triplicate unless stated otherwise. Data are presented as mean ± standard deviation (SD) values. Statistical comparisons were performed using a one-way ANOVA followed by Tukey’s post hoc test. A p-value of <0.05 was considered statistically significant. The analysis was performed using GraphPad Prism v9.0 (GraphPad Software, San Diego, CA, USA).
3. Results and Discussion
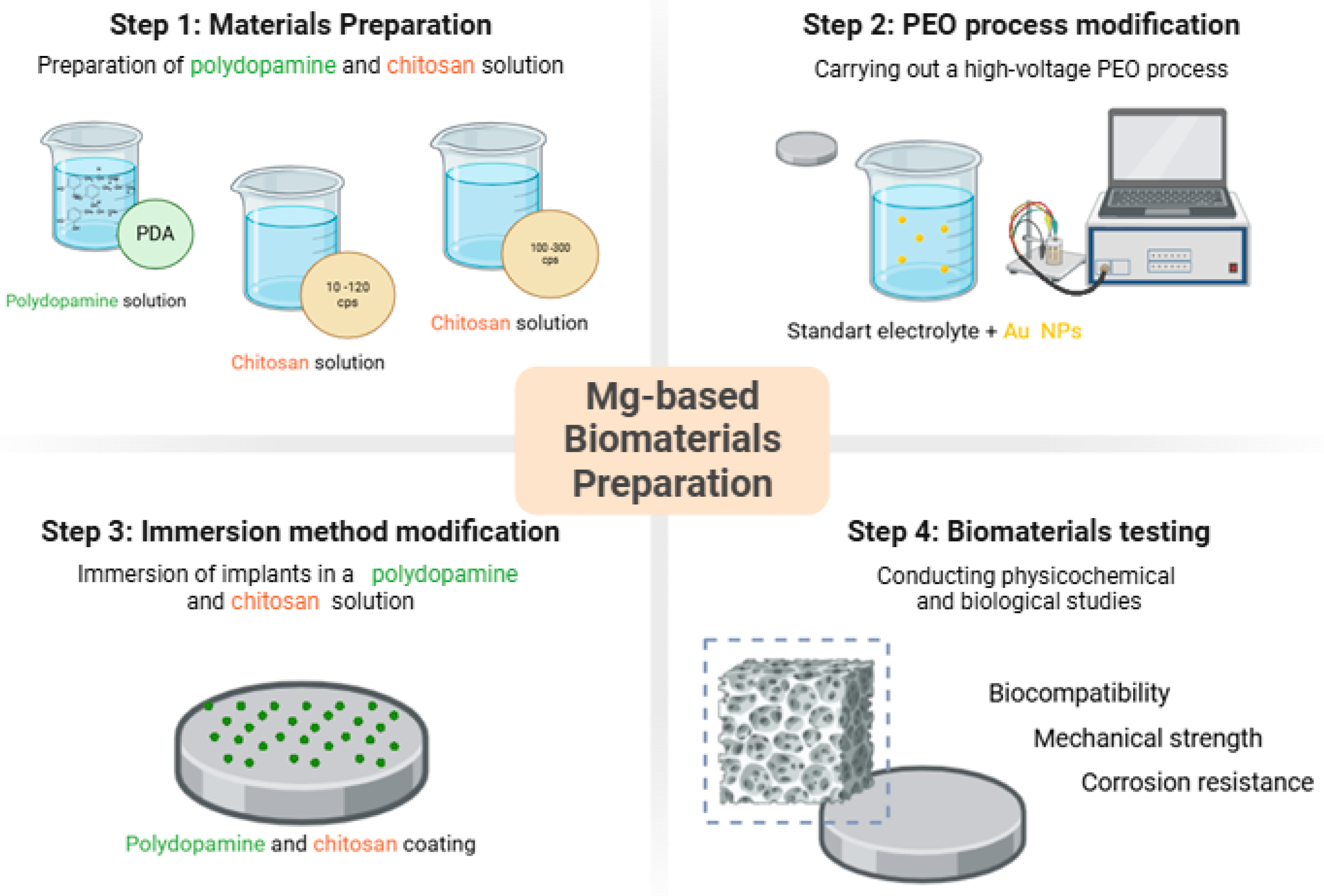
As a result of a series of tests, six different types of samples with advanced coatings have been developed. The general scheme of the article is given in Figure 5.
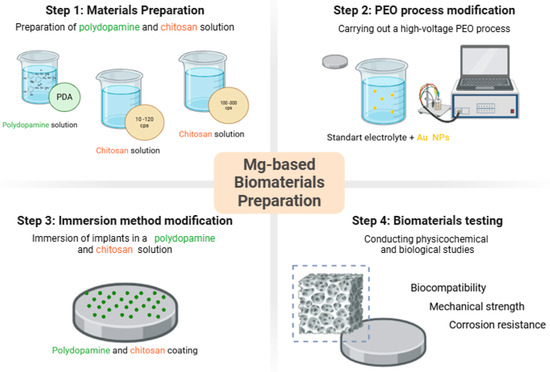
Figure 5.
General approach to magnesium-based biomaterials obtainment.
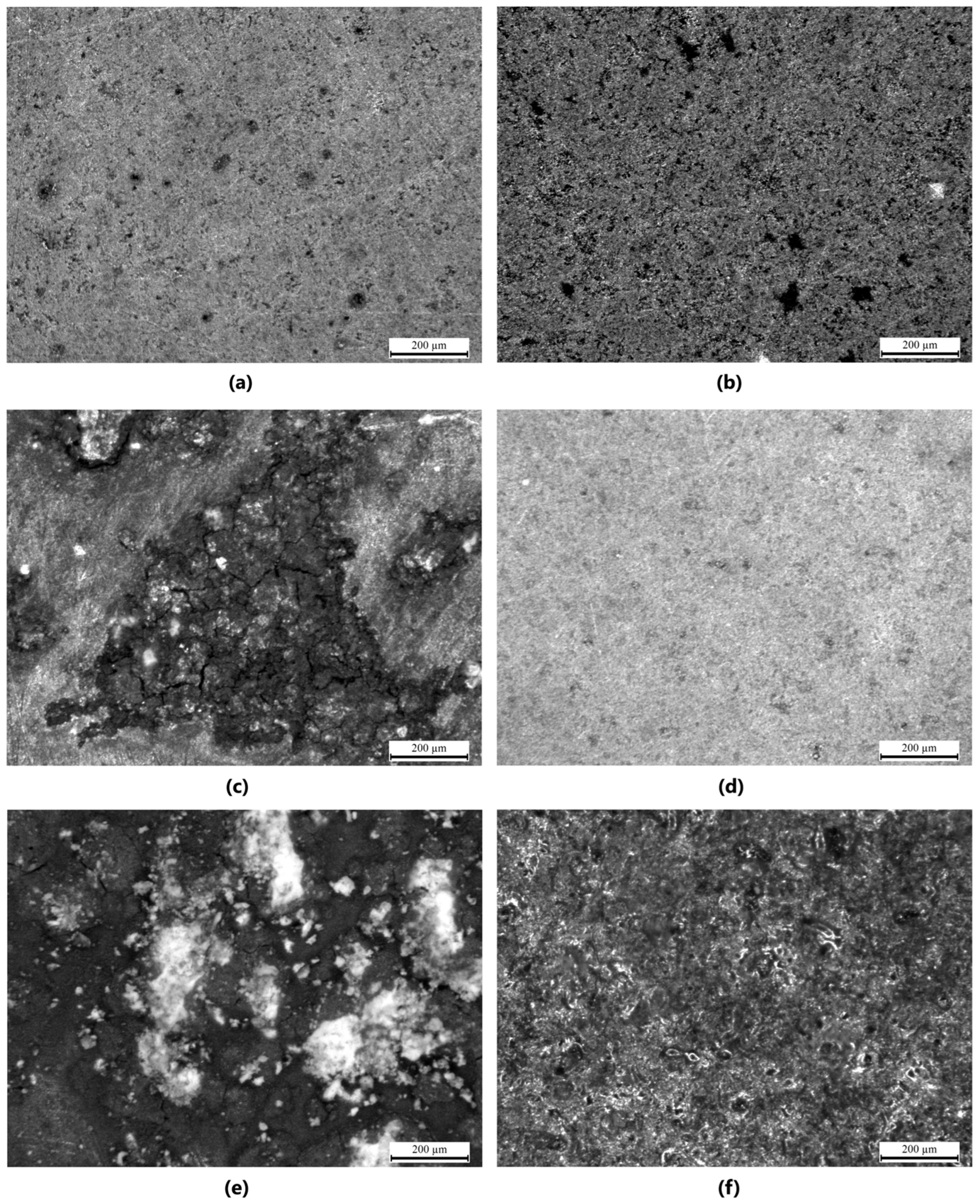
Stereoscopic microscope images provided qualitative insights into the surface morphology of the modified magnesium samples. For all groups (n = 3 per sample type), coatings were examined for visual homogeneity, aggregation, and coverage. Mg_01 and Mg_02 demonstrated relatively smooth and continuous layers; however, slight variations in thickness were observed at the periphery. Polydopamine-coated samples (Mg_03 and Mg_06) exhibited a granular surface texture indicative of PDA aggregation, as previously reported [45]. To enable quantitative comparisons, images were further processed using ImageJ software (NIH, Bethesda, MD, USA, Fiji version 2.16.0–available as of June 2023, based on ImageJ 1.54p) to estimate surface porosity. Porosity was calculated as the percentage of dark pixel area relative to total sample area under threshold segmentation. PDA-modified samples (Mg_03 and Mg_06) showed porosity values of ~49.2% ± 2.1% and 51.8% ± 1.6%, respectively, which were significantly higher (p < 0.05, ANOVA) than chitosan-coated counterparts (Mg_01: 34.7% ± 2.9%, Mg_02: 39.1% ± 1.8%). PEO-treated samples exhibited more interconnected and stable porosity due to plasma discharge-induced microchannels, favoring polymer infiltration.
The results obtained from the stereoscopic microscope images of the samples provide a detailed visual representation of the surface modifications and coatings applied to the pure magnesium discs. The samples were subjected to different coating methods and polymer formulations, with the objective of enhancing surface properties such as corrosion resistance and biocompatibility. For Mg_01, the magnesium disc was coated with chitosan solution with a viscosity range of 10–120 cps. The surface exhibited a smooth and homogeneous coating with minimal aggregation of the chitosan material. The coating adhered well to the magnesium surface, though a slight unevenness in thickness was observed at specific points, which could be attributed to the viscosity variation during the coating process. In Mg_02, the magnesium disc was modified with both chitosan (viscosity range of 100–300 cps) and polydopamine coating. The images revealed a more pronounced surface texture with visible features resulting from the polydopamine coating. The chitosan layer was more uniformly applied compared to Mg_01, and the polydopamine layer appeared to form small, granular aggregates over the chitosan base, which may contribute to additional surface roughness, potentially enhancing the adhesion of the coating to biological tissues. Mg_03 displayed a magnesium disc modified solely with a polydopamine coating. The surface was characterized by a thicker layer of polydopamine compared to Mg_02, with noticeable clusters forming at certain regions. The coating was more porous and uneven in distribution, suggesting that the polydopamine solution was applied at a higher concentration or with a different viscosity, which led to less uniformity in coverage across the surface. For the samples subjected to the plasma electrolytic oxidation (PEO) process, such as Mg_04, the magnesium disc showed a more roughened surface due to the formation of oxide layers typical of the PEO process. This modification significantly increased the surface area, which is beneficial for coating adhesion. When coated with chitosan (10–120 cps), the surface appeared to have a more adherent and homogeneous coating compared to Mg_01, with the rough surface providing better anchorage for the chitosan material. However, some areas still exhibited slight inconsistencies in coating thickness. In Mg_05, the PEO-modified magnesium disc was coated with chitosan (100–300 cps). The stereoscopic microscope images revealed a more textured surface compared to Mg_04, as the higher viscosity of the chitosan solution resulted in a thicker, more robust coating. This thicker coating appeared to cover more of the surface irregularities from the PEO treatment, suggesting improved coating stability and protection against corrosion. Finally, Mg_06 exhibited a PEO-modified magnesium disc with a polydopamine coating. The surface showed distinct, prominent polydopamine clusters, similarly to Mg_03, but the underlying oxide layer from the PEO treatment was more visible through the coating, indicating that the polydopamine did not fully cover the surface. The resulting rough and porous surface structure might enhance interactions with biological materials, although the unevenness could affect the uniformity of performance in certain applications (Figure 6).
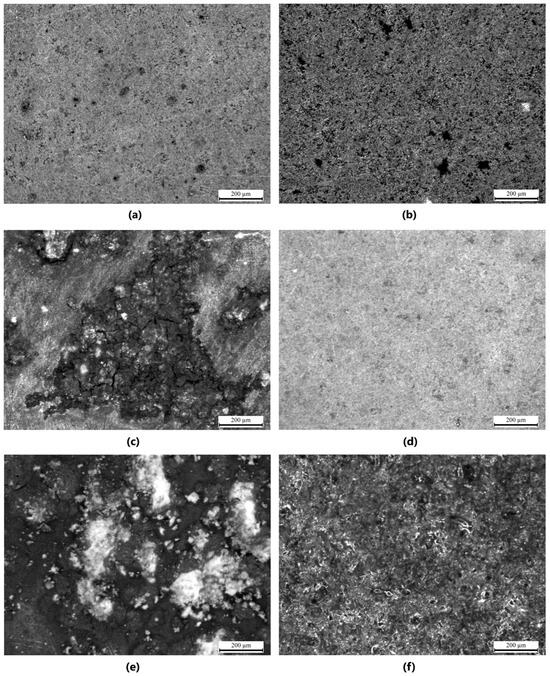
Figure 6.
Stereoscopic microscope images of the samples (with 200 μm scale): (a) Mg_02, (b) Mg_03, (c) Mg_03, (d) Mg_04, (e) Mg_05, (f) Mg_06.
3.1. Chemical Structure Analysis via Fourier-Transform Infrared Spectroscopy (FT-IR)
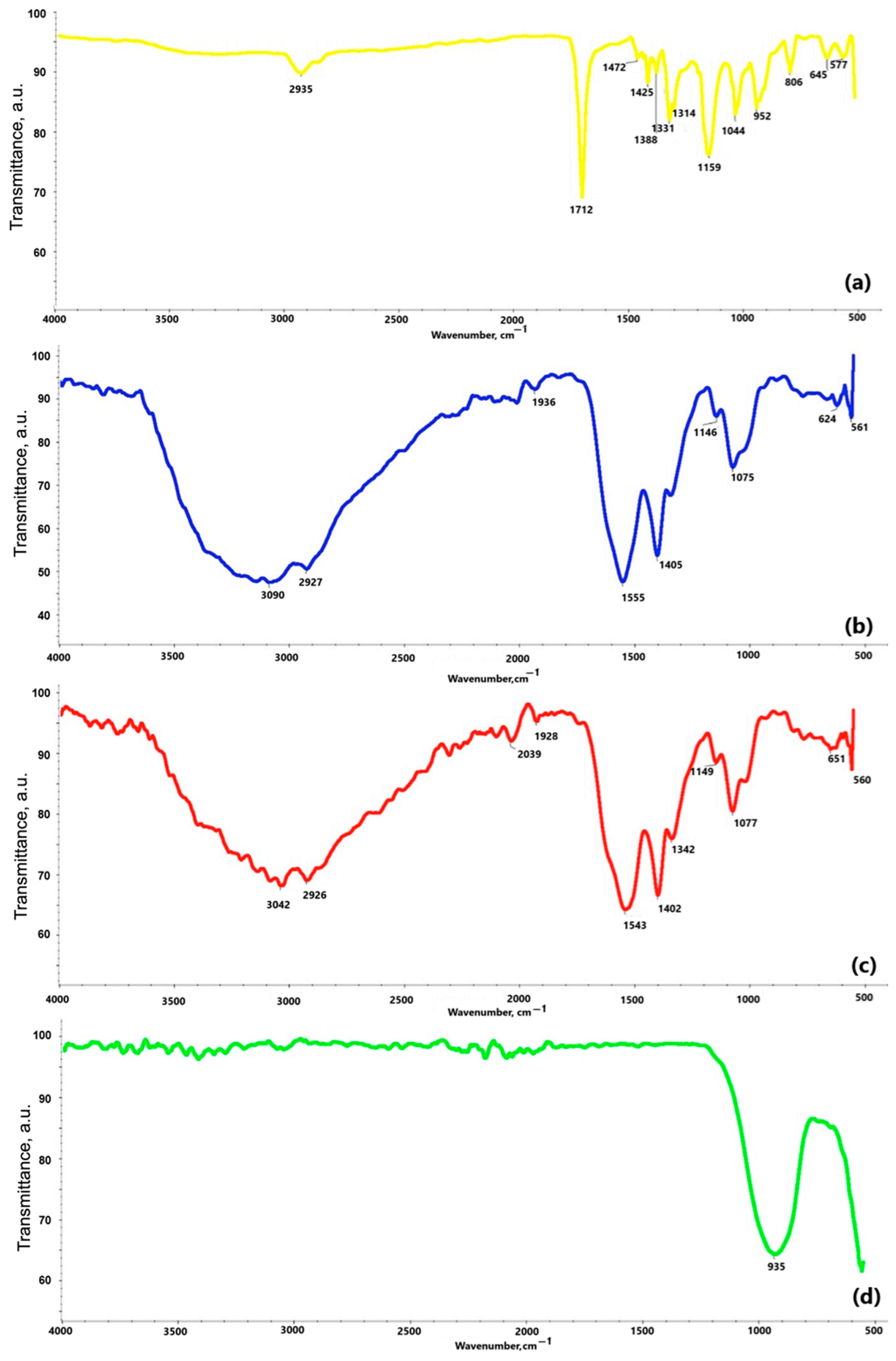
Figure 7 reveals the results of the FT-IR study. The spectrum of the obtained polydopamine compound at Figure 7a reveals a band at a frequency of 2935 cm−1, which likely corresponds to characteristic stretching vibrations of the hydroxyl -OH and amine -NH groups. According to the literature data, these groups appear in the range of 4000–2500 cm−1. A similar pattern can be observed in the chitosan spectra at Figure 7b,c, where bands at frequencies of 3042 cm−1 at Figure 7b and 3090 cm−1 at Figure 7c also correspond to stretching vibrations of hydroxyl groups. Bands in the range of 2000–1500 cm−1 are attributed to stretching vibrations originating from C=C, C=O, C=N, N=N double bonds, as well as bonds present in the aromatic ring, confirming the presence of this ring in all the analyzed samples. For example, the band at 1712 cm−1 at Figure 7a, indicates stretching vibrations of C=C bonds in the aromatic structure. In the range of 1300–1000 cm−1 at Figure 7a, bands corresponding to C–O bonds in the polydopamine (PDA) molecule are observed. The chitosan spectra at Figure 7b,c show common features resulting from the structural similarity of these substances. Bands at frequencies of 2926 cm−1 and 2927 cm−1 are associated with the presence of the –CH2 group, while bands at 1555 cm−1, 1543 cm−1, 1146 cm−1, and 1149 cm−1 confirm the presence of amine groups –NH2. The presence of a β-glycosidic bond between molecules is manifested by bands at 1075 cm−1 and 1077 cm−1. The analysis of the spectrum of the coating obtained by plasma electrolytic oxidation at Figure 7d indicates that the band at a frequency of 935 cm−1 originates from the phosphate groups.
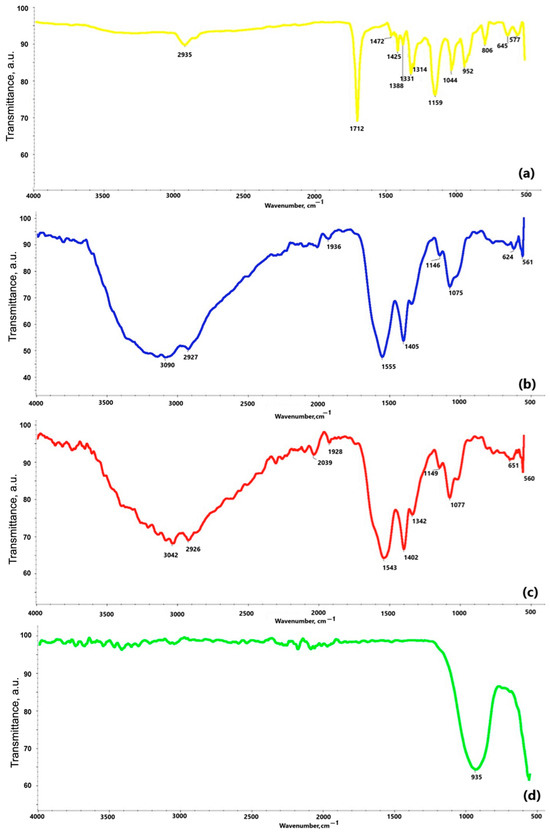
Figure 7.
FT-IR spectra of: (a) polydopamine; (b) chitosan (10–120 cps); (c) chitosan (100–300 cps); (d) coating obtained via electrochemical plasma oxidation.
Taken together, the FT-IR spectra confirmed the presence of functional groups corresponding to polydopamine and chitosan in the coatings. Bands near 2935 cm−1 (O–H, N–H stretching), 1712 cm−1 (C=O), and 1075 cm−1 (C–O, β-glycosidic bond) were observed consistently across PDA and chitosan samples, supporting successful polymer incorporation. PEO-treated surfaces showed phosphate-related bands at 935 cm−1, suggesting integration of electrolyte-derived ions.
3.2. Wettability Study
The wettability study of the implant surface by a deposited water droplet demonstrated that the obtained implants exhibit hydrophilic properties. This characteristic promotes the adhesion of cells, including osteoblasts, to the implant surface. Analyzing the results presented in Table 3 reveals that coatings applied directly to magnesium exhibit a higher contact angle compared to coatings formed on an implant previously subjected to the PEO process. The use of the plasma electrolytic oxidation method not only protects the implant from corrosion but also enhances its wettability by reducing the contact angle. The values obtained in the study fall within the range of 0° < θ < 90°, indicating a high level of surface wettability by the liquid. Data are expressed as mean ± SD from three independent measurements per sample (n = 3).

Table 3.
Wettability of the evaluated samples.
3.3. Surface Morphology and Composition Analysis by Scanning Electron Microscopy (SEM)
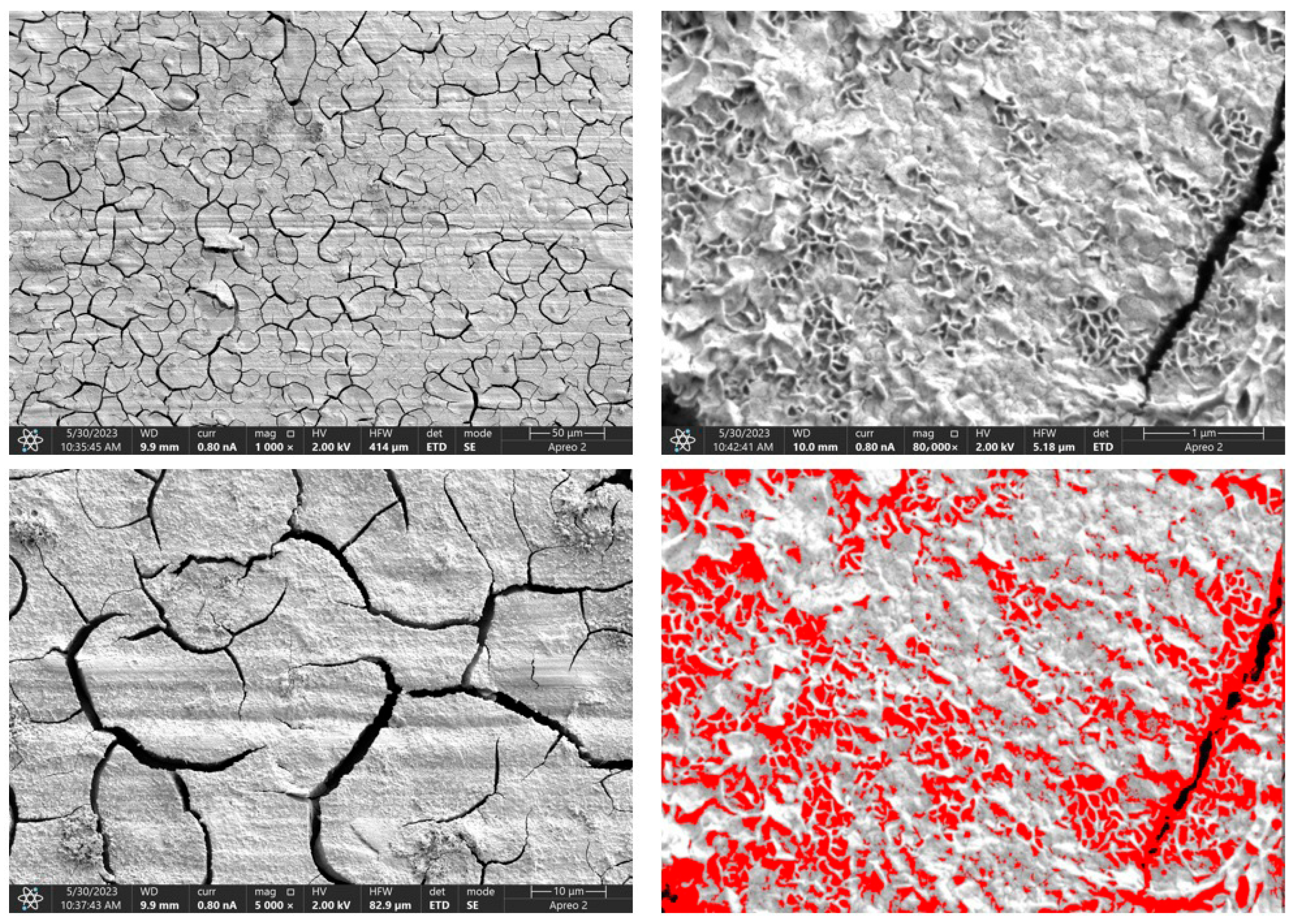
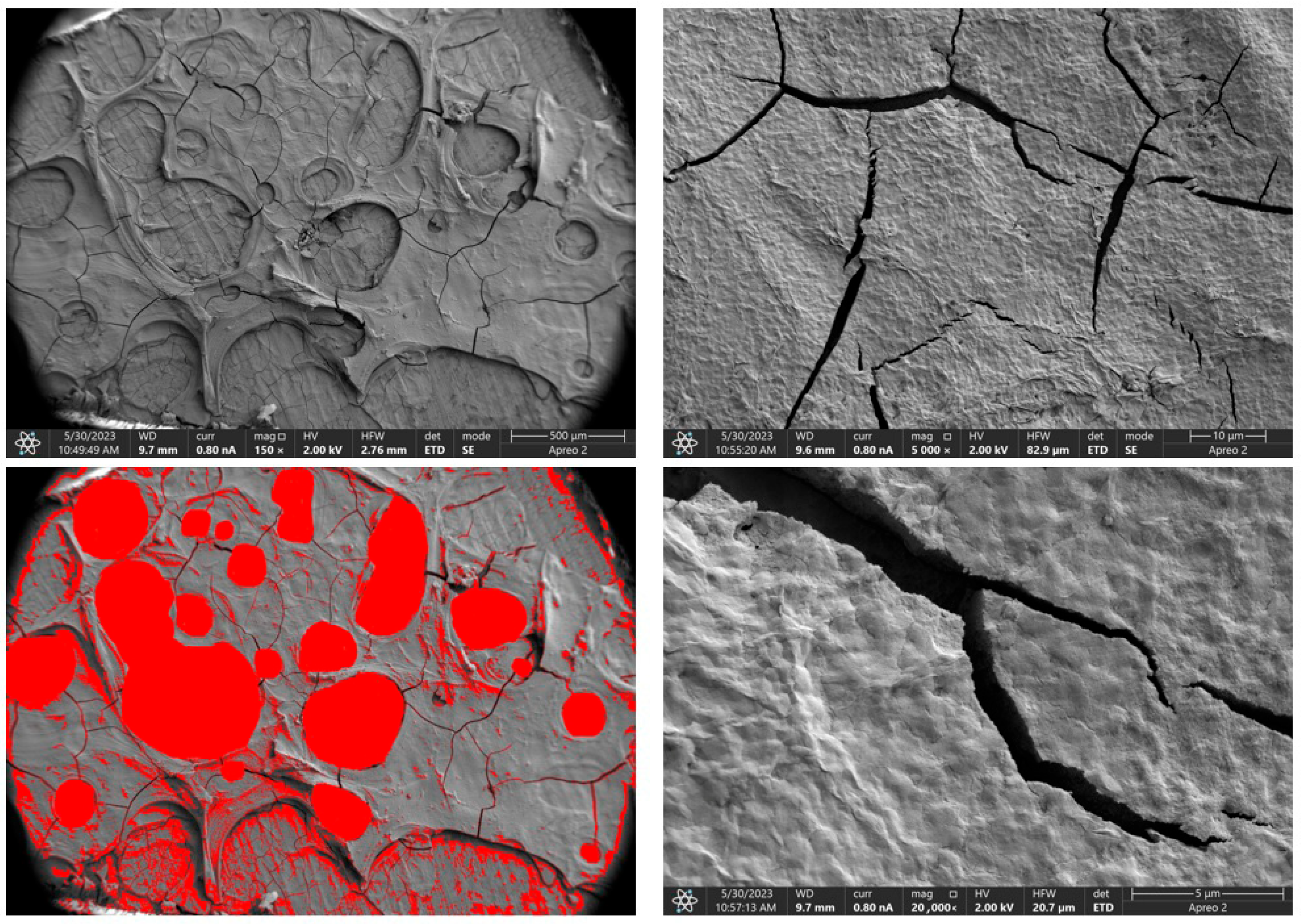
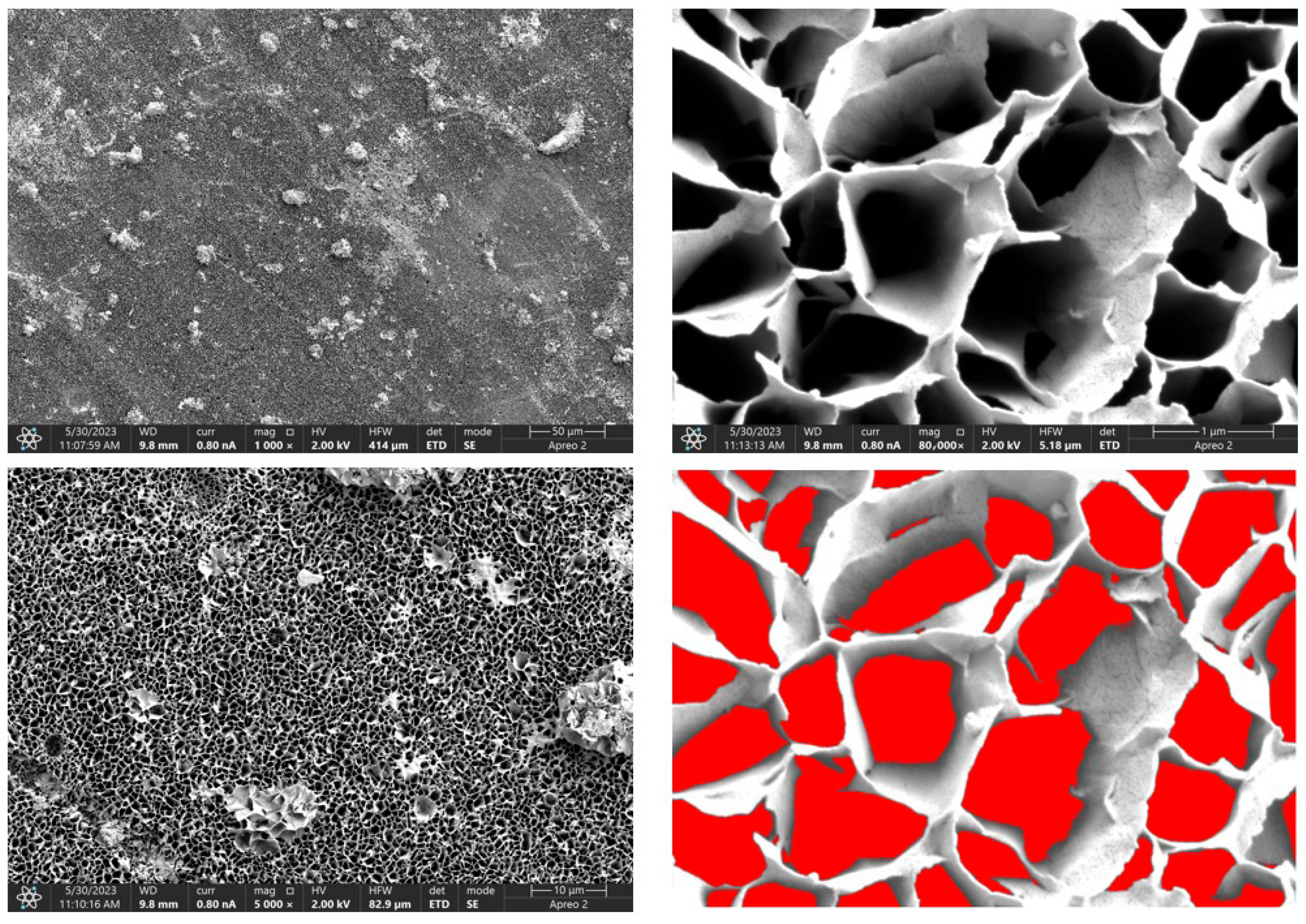
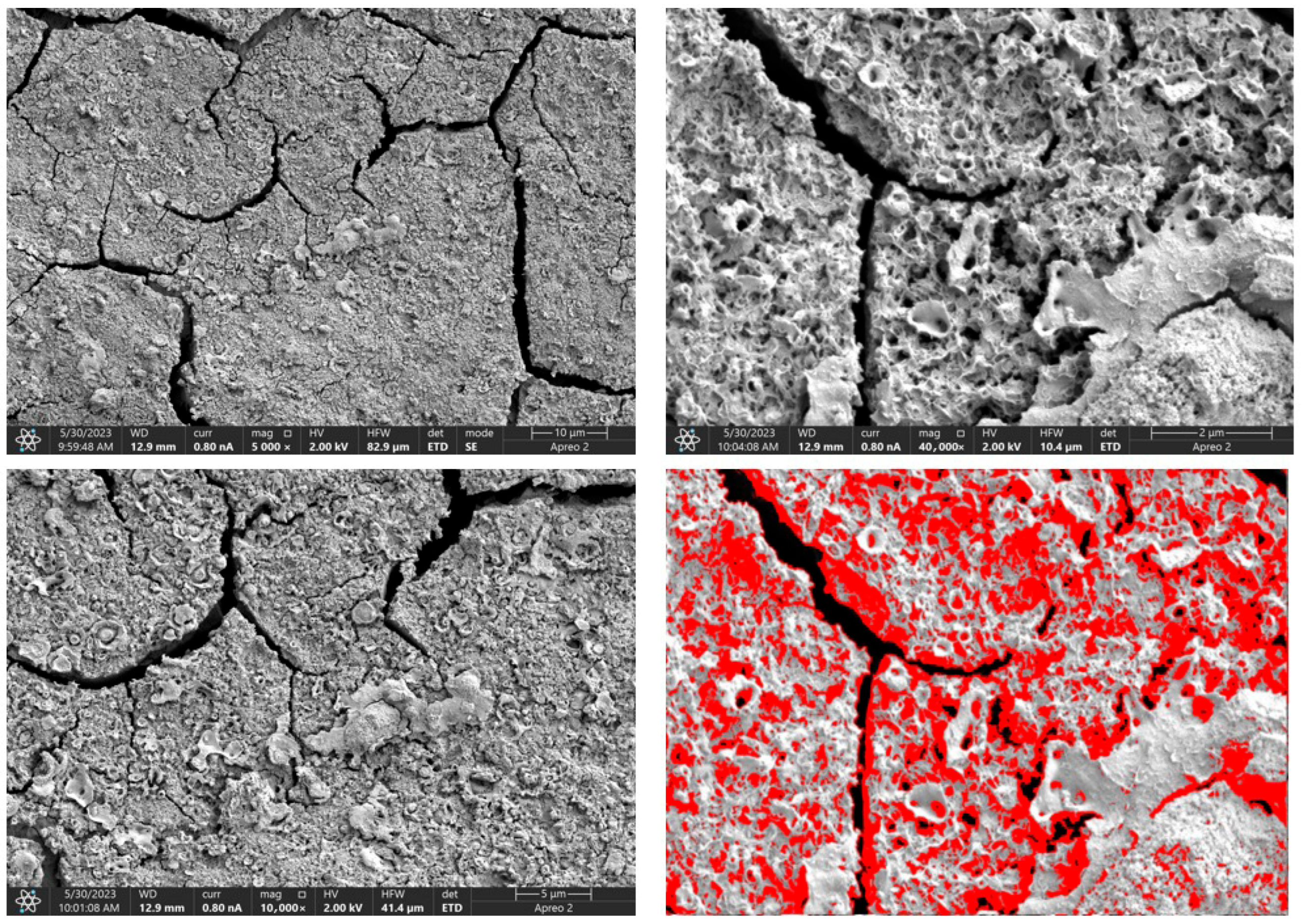
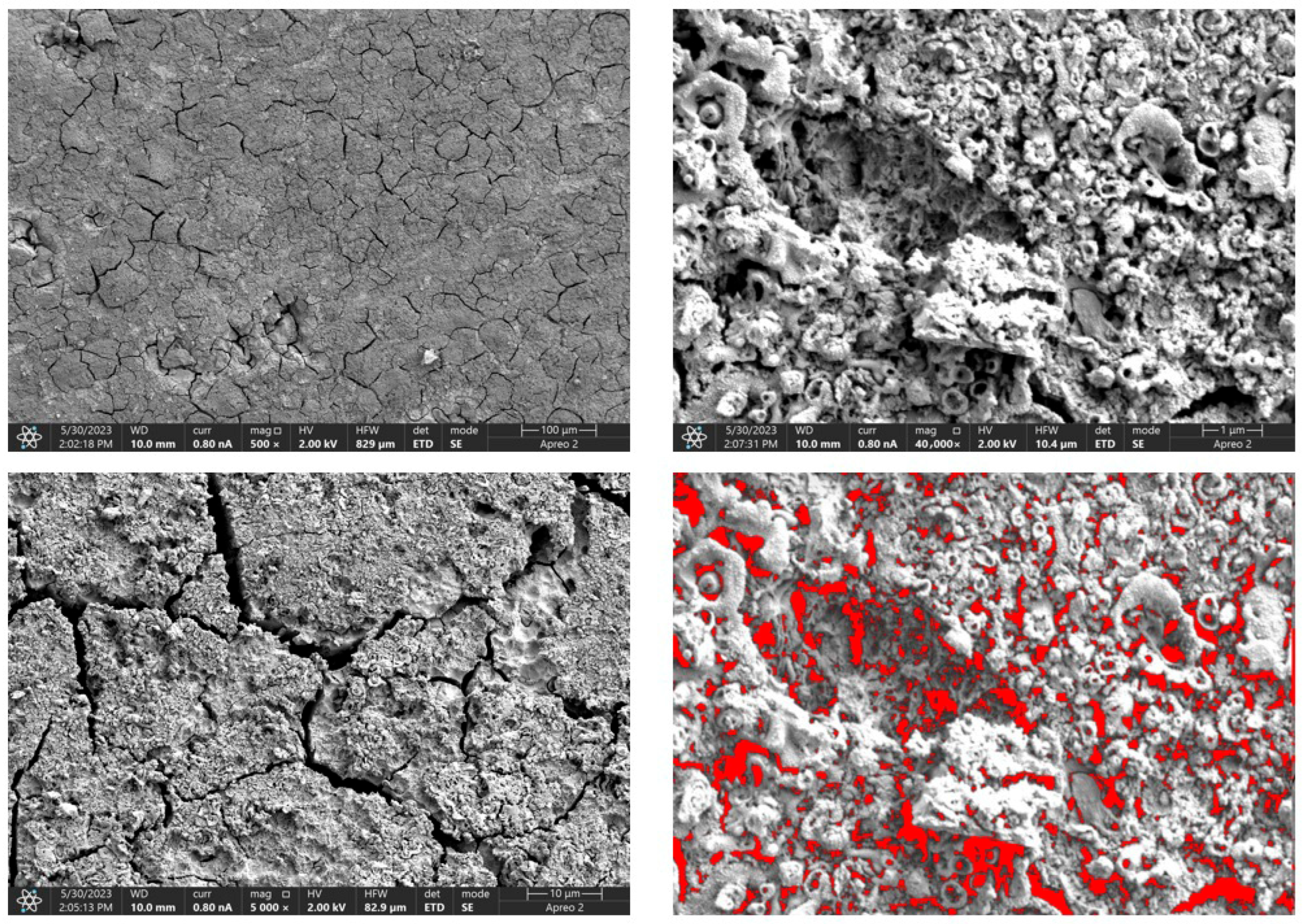
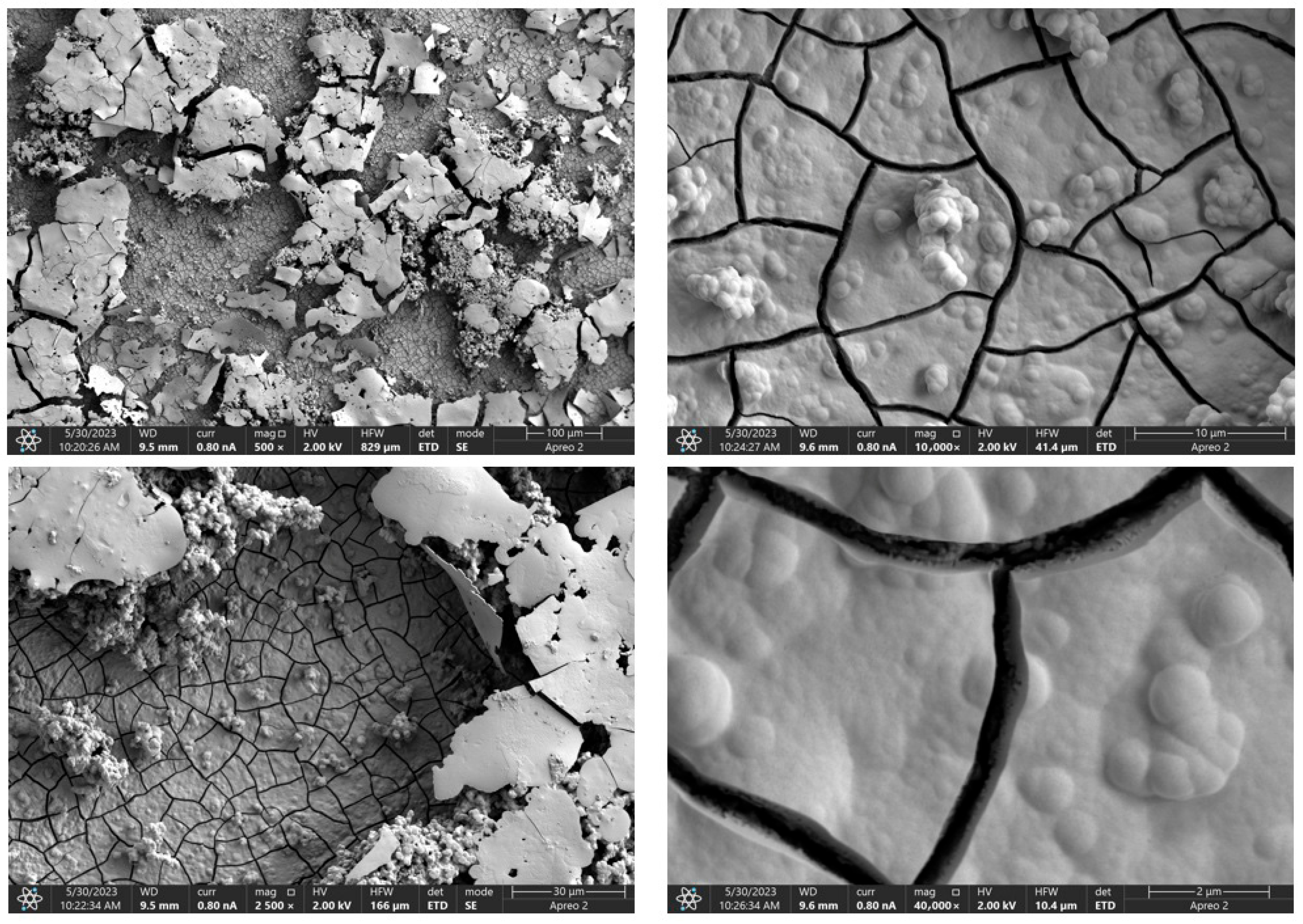
Due to the necessity of meeting specific requirements for SEM measurements, a fragment was cut from the entire set of prepared implants using a Dremel straight grinder. This process led to the formation of surface cracks that will not occur under the actual conditions for which the implants are intended. The analysis of implant surface morphology indicates that plasma electrolytic oxidation significantly affects surface structure, enhancing its adhesive properties. This process increases both the number and quality of pores, which promotes better cell adhesion. Magnesium implants modified with a polydopamine (PDA) coating exhibit the most pronounced porosity while also remaining the least damaged during sample preparation, which also suggests high resistance to damage and hardness. Such a structure facilitates easy cell adhesion after implantation. The SEM image of the discussed sample is presented in Figure 8, Figure 9, Figure 10, Figure 11 and Figure 12, with pores marked in red. When analyzing chitosan coatings, numerous pores can also be observed; however, their structure differs from that of the polydopamine (PDA) coating. The pores in the PDA layer have an irregular shape and a flat surface, resembling a honeycomb structure. In contrast, the chitosan pores are more compact and resemble a sponge, as seen in Figure 8, Figure 9, Figure 10, Figure 11 and Figure 12. In all the examined samples, the porosity level is approximately 50%, indicating that the obtained results meet expectations. The surface of a PEO implant with an applied polydopamine coating shows nanometric pores that remain invisible on the micrometer scale at which the images were taken, as shown in Figure 13. The illustration shows the formed magnesium oxide layer with clusters of polydopamine embedded in it. SEM images confirmed the porous structure of coatings and revealed topographical differences based on coating composition. An ImageJ analysis of SEM images was used to determine mean pore size (µm) and surface coverage (%). The results showed that PDA-coated samples (Mg_06) had the highest average pore size (3.1 ± 0.4 µm) and surface roughness, contributing to greater osteoblast adhesion potential.
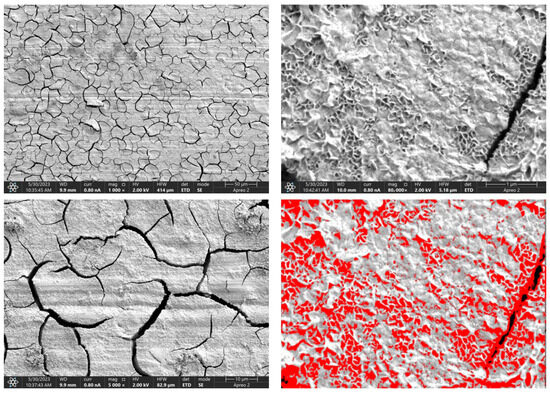
Figure 8.
SEM-EDS images of the Mg_01 sample surface with pores highlighted in red.
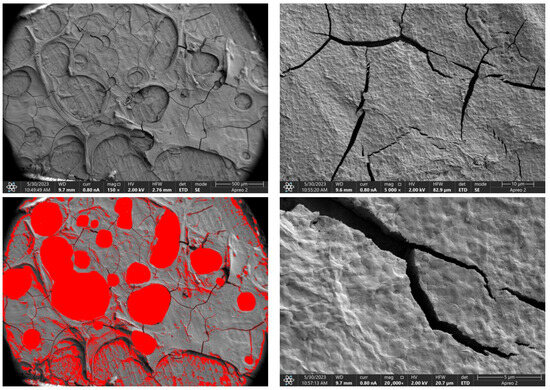
Figure 9.
SEM-EDS images of the Mg_02 sample surface with pores highlighted in red.
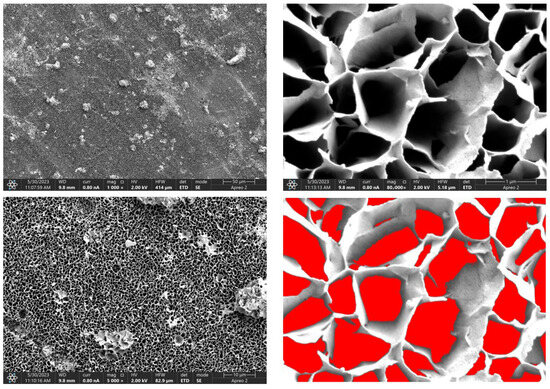
Figure 10.
SEM-EDS images of the Mg_03 sample surface with pores highlighted in red.
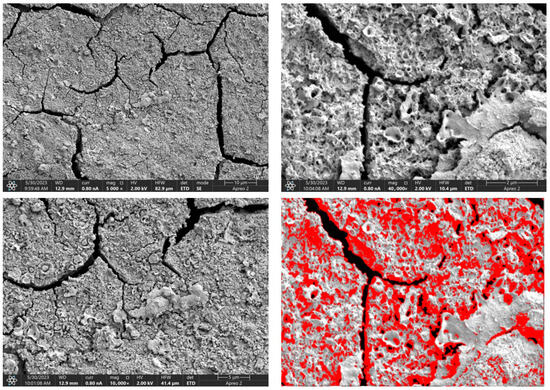
Figure 11.
SEM-EDS images of the Mg_04 sample surface with pores highlighted in red.
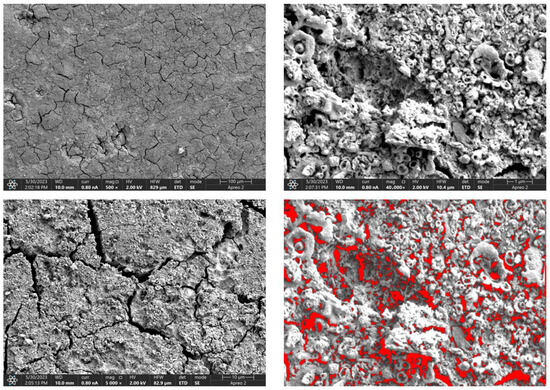
Figure 12.
SEM-EDS images of the Mg_05 sample surface with pores highlighted in red.
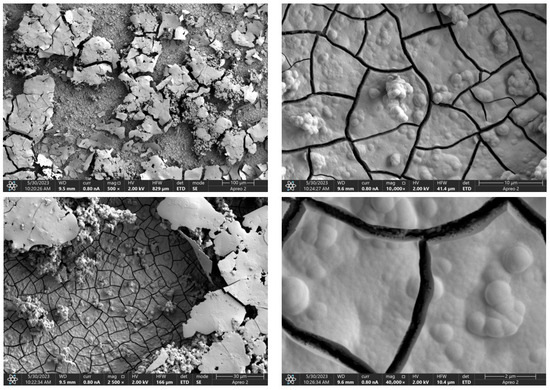
Figure 13.
SEM-EDS images of the Mg_06 sample surface.
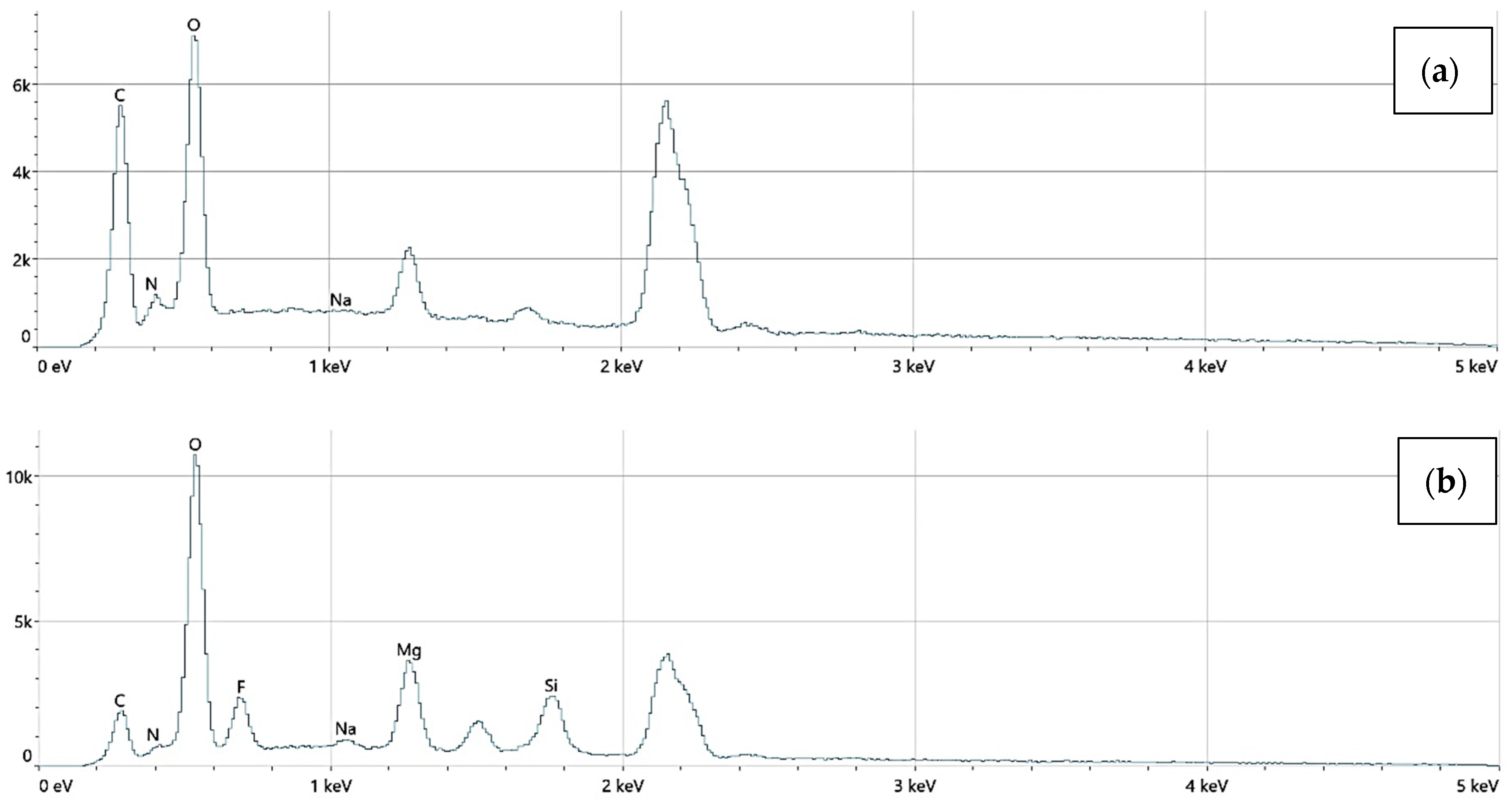
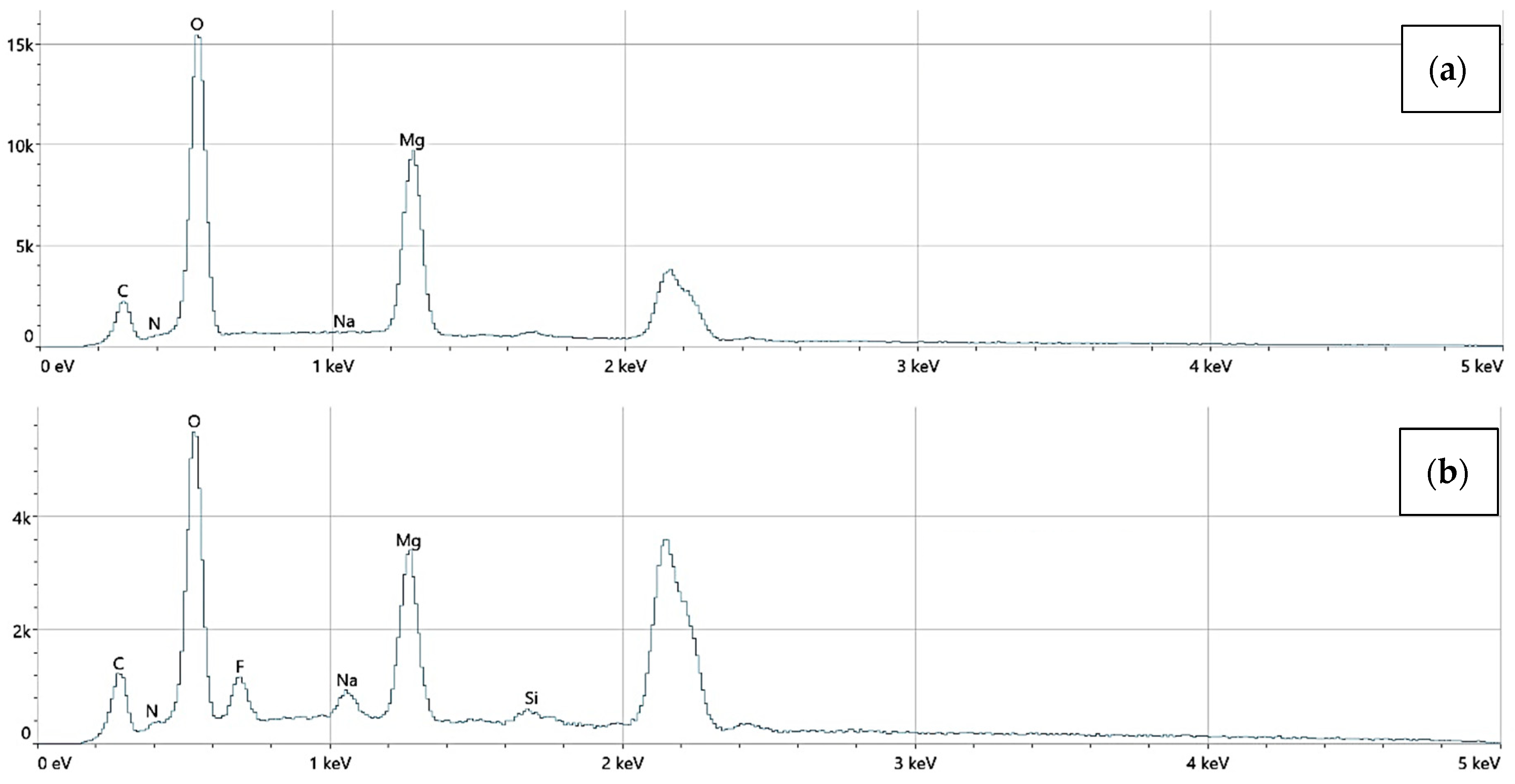
An EDS analysis confirmed the presence of elements including Mg, O, C, N, Si, and Au. No toxic elements were detected in any sample. Mg_06 samples exhibited trace gold signals, verifying the incorporation of AuNPs.
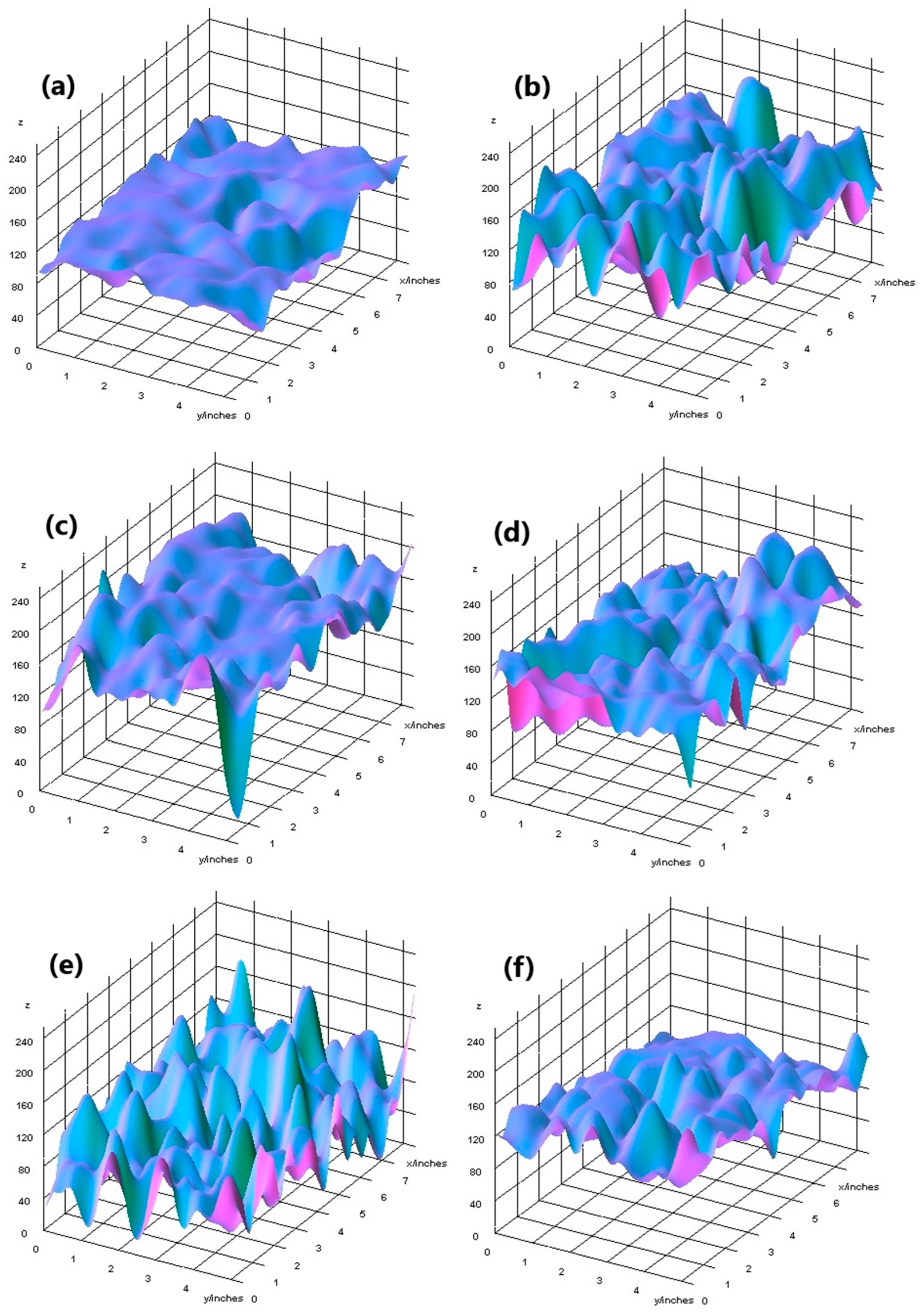
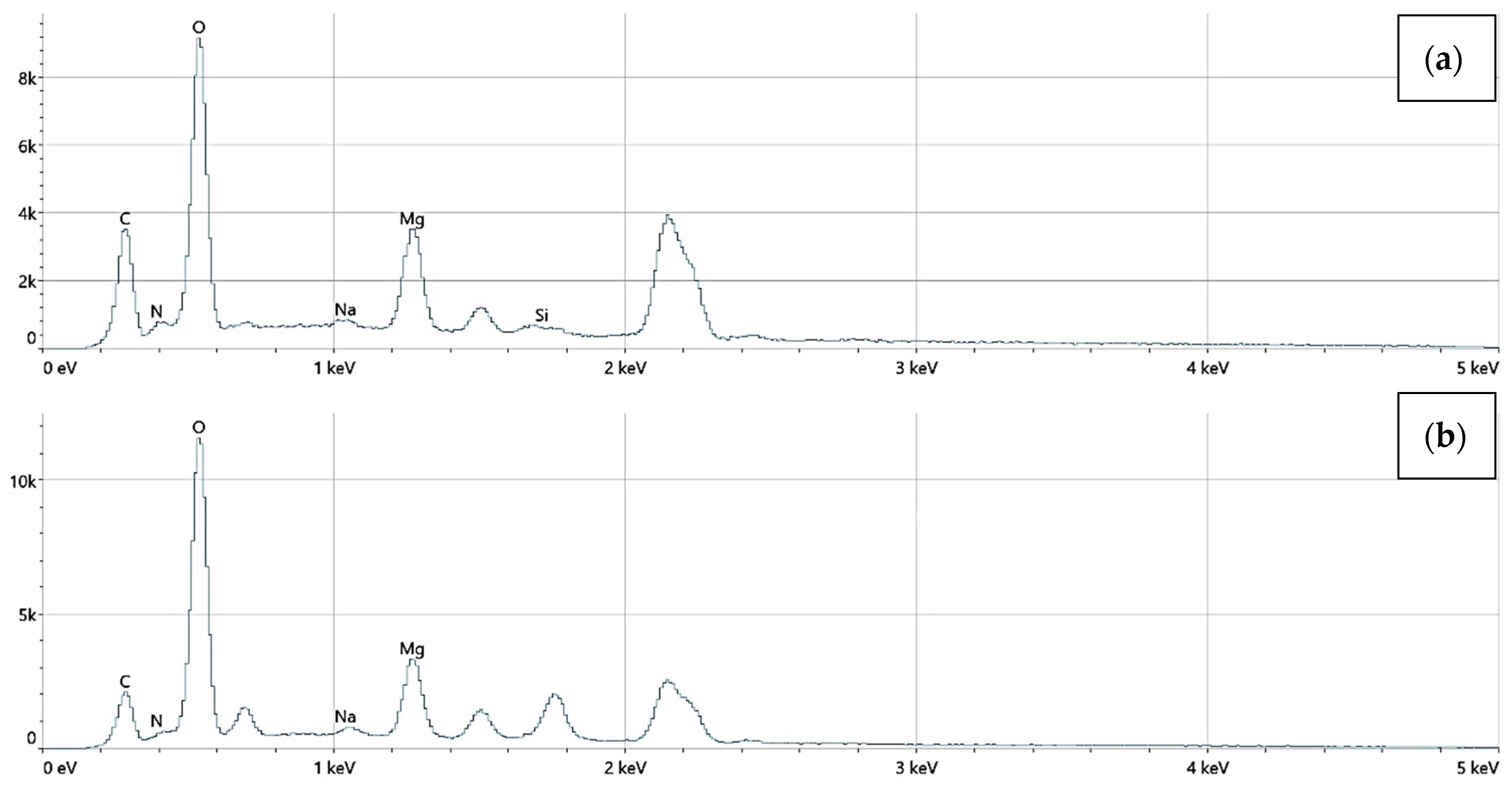
The elemental surface composition analysis showed that all coatings contain elements essential for the human body, which are safe and non-toxic. Below, in Figure 14, the topography of all the prepared samples is presented, along with the description in Table 4, which lists the elements found on the surface of the implants. Figure 15, Figure 16 and Figure 17 also show the quantitative elemental composition, confirming, through the EDS system, the presence of biocompatible elements that promote implant integration into the human body.
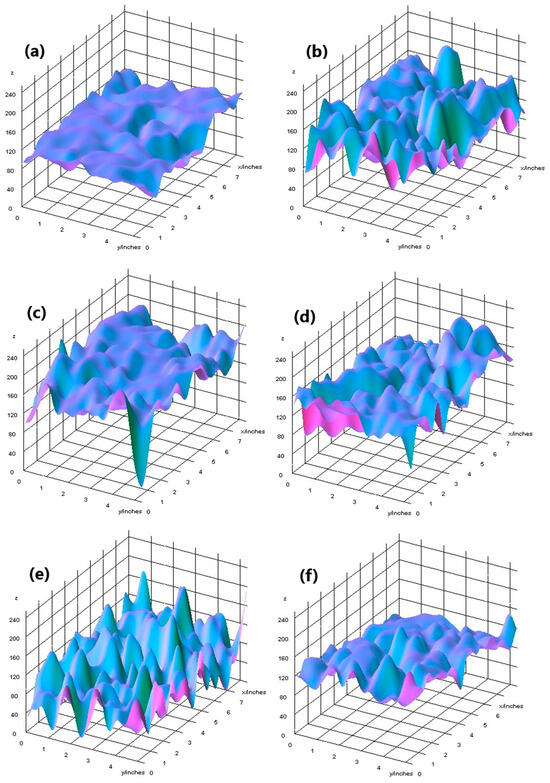
Figure 14.
Topography of samples, successively: (a) Mg_02, (b) Mg_05, (c) Mg_01, (d) Mg_04, (e) Mg_03, (f) Mg_06.

Table 4.
A summary of the results obtained from the biocorrosion study.
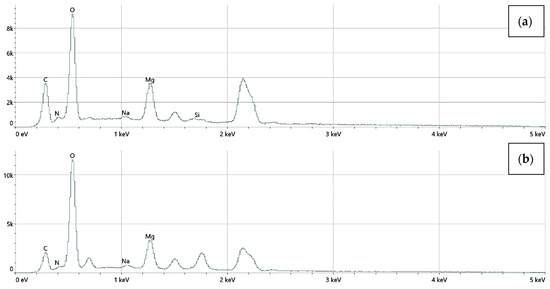
Figure 15.
The elemental composition of implants with a chitosan (10–120 cps) coating, successively: (a) Mg_01, (b) Mg_04.
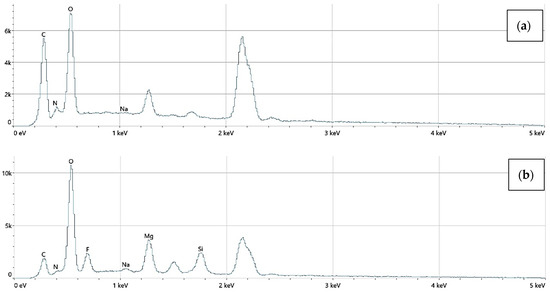
Figure 16.
The elemental composition of implants with a chitosan (100–300 cps) coating, successively: (a) Mg_02, (b) Mg_05.
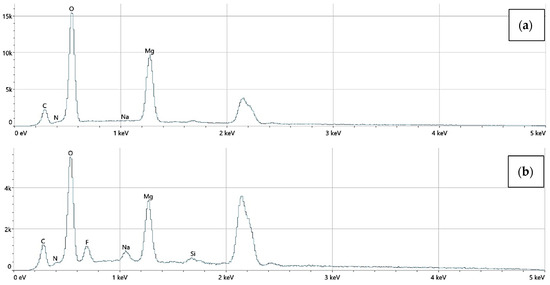
Figure 17.
The elemental composition of implants with polydopamine coating, successively: (a) Mg_03, (b) Mg_06.
3.4. Biodegradation and Biocorrosion Analysis
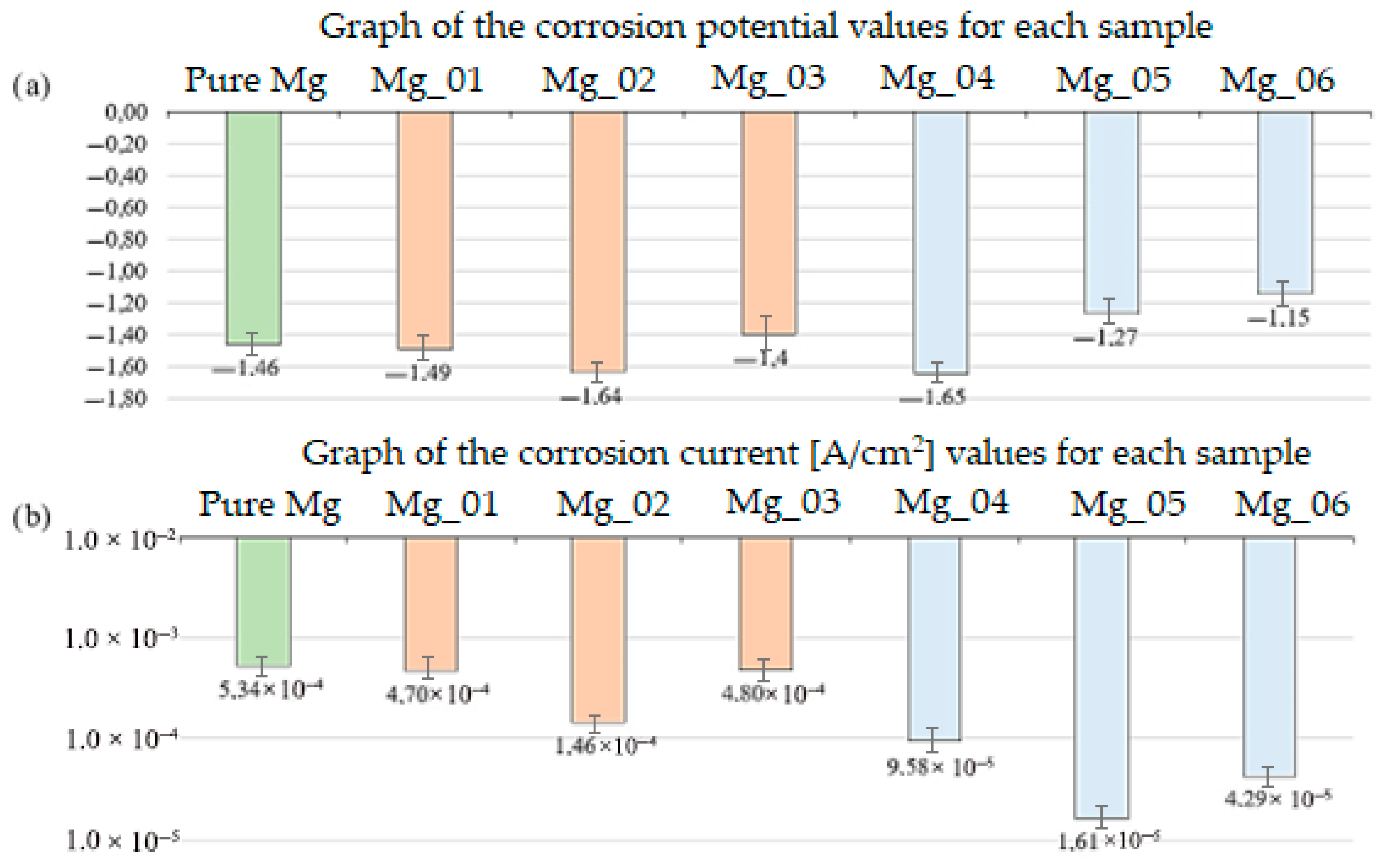
Before implants are introduced into the human body, they are placed in a saline solution to swell the polymer surfaces. This procedure is necessary because, before implantation, it is essential to know the exact dimensions of the implant. This is a crucial requirement to avoid putting the patient at risk of pressure caused by surface swelling inside the body. The biodegradation study showed that each of the obtained implants demonstrates the ability to degrade in vivo. Implants subjected to plasma electrolytic oxidation with polymer coatings exhibit better biodegradation properties over time compared to the others. The best result was achieved by the Mg_06 sample. It degrades significantly slower, which ensures that the hydrogen released would not pose a threat to the patient. The fastest degrading sample was Mg_04, whose degradation was too rapid, and the amount of hydrogen released was the highest and unacceptable. Figure 18 (below) presents a comparison of the samples after the biodegradation study in SBF.
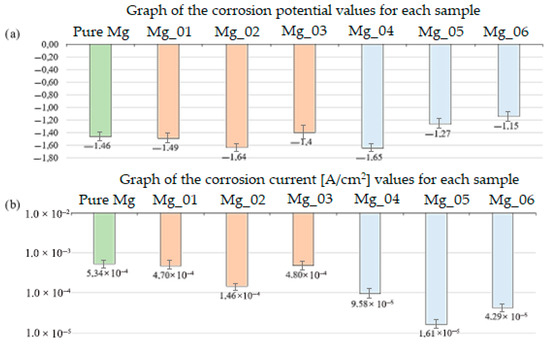
Figure 18.
Comparison of the obtained results: (a) corrosion potential graph for each sample (b) corrosion current graph for each sample.
Biodegradation behavior was assessed by immersing the samples in simulated body fluid (SBF) at 37 °C for 14 days. The mass loss was used to determine degradation rates, with Mg_04 (PEO + chitosan, low viscosity) showing the fastest degradation (22.5% ± 1.8) and Mg_06 (PEO + PDA) showing the slowest (9.1% ± 1.1). These differences were statistically significant (p < 0.01), highlighting the barrier effect of the PDA layer and the protective properties of the PEO-formed oxide. The slower degradation of Mg_06 suggests a reduced release of hydrogen gas, minimizing the risk of soft tissue irritation and promoting implant safety in vivo.
The biocorrosion study was conducted to understand the response of the obtained implants to the action of the patient’s body fluids. The results obtained are presented in the summary Table 4 and Figure 18. Analyzing the obtained results, it can be observed that the magnesium implant disc shows a potential of −1.46 V, which is most likely due to the presence of other elements. The summary of all the presented studies is a result that confirms the positive impact of plasma electrolytic oxidation modification. Additionally, as seen in the graph, the corrosion potential of sample Mg_03 is −1.4 V, which is higher than that of the implant after PEO modification with the same coating, i.e., sample Mg_06, whose corrosion potential is −1.15 V. A higher corrosion potential means that the formed magnesium oxide layer is more durable and thicker. The obtained results confirm that the PEO modification plays a key role in improving the biocorrosion properties. It is also important to note the relationship between the corrosion current density (A/cm2) and corrosion potential (V). A higher corrosion potential is associated with a lower corrosion current density. Biocorrosion analysis was conducted via potentiodynamic polarization testing in SBF, with three replicates per group. The corrosion potential (E_corr) and current density (i_corr) were used to evaluate electrochemical stability. Mg_06 exhibited the most favorable electrochemical behavior with an E_corr value of −1.15 ± 0.02 V and an i_corr value of 4.29 × 10−5 ± 0.05 × 10−5 A/cm2, significantly improved compared to Mg_03 (PDA only), which had an E_corr value of −1.40 ± 0.03 V and an i_corr value of 4.80 × 10−4 ± 0.09 × 10−4 A/cm2. The enhanced corrosion resistance of Mg_06 is attributed to the synergistic effect of the dense oxide layer generated by PEO and the sealing properties of the PDA film, which limits ion diffusion and surface reactivity. Moreover, the solution composition, particularly the presence of Cl− and HCO3− ions, plays a critical role in degrading Mg(OH)2 layers; thus, surface passivation is essential to avoid excessive dissolution.
3.5. Cytotoxicity Study
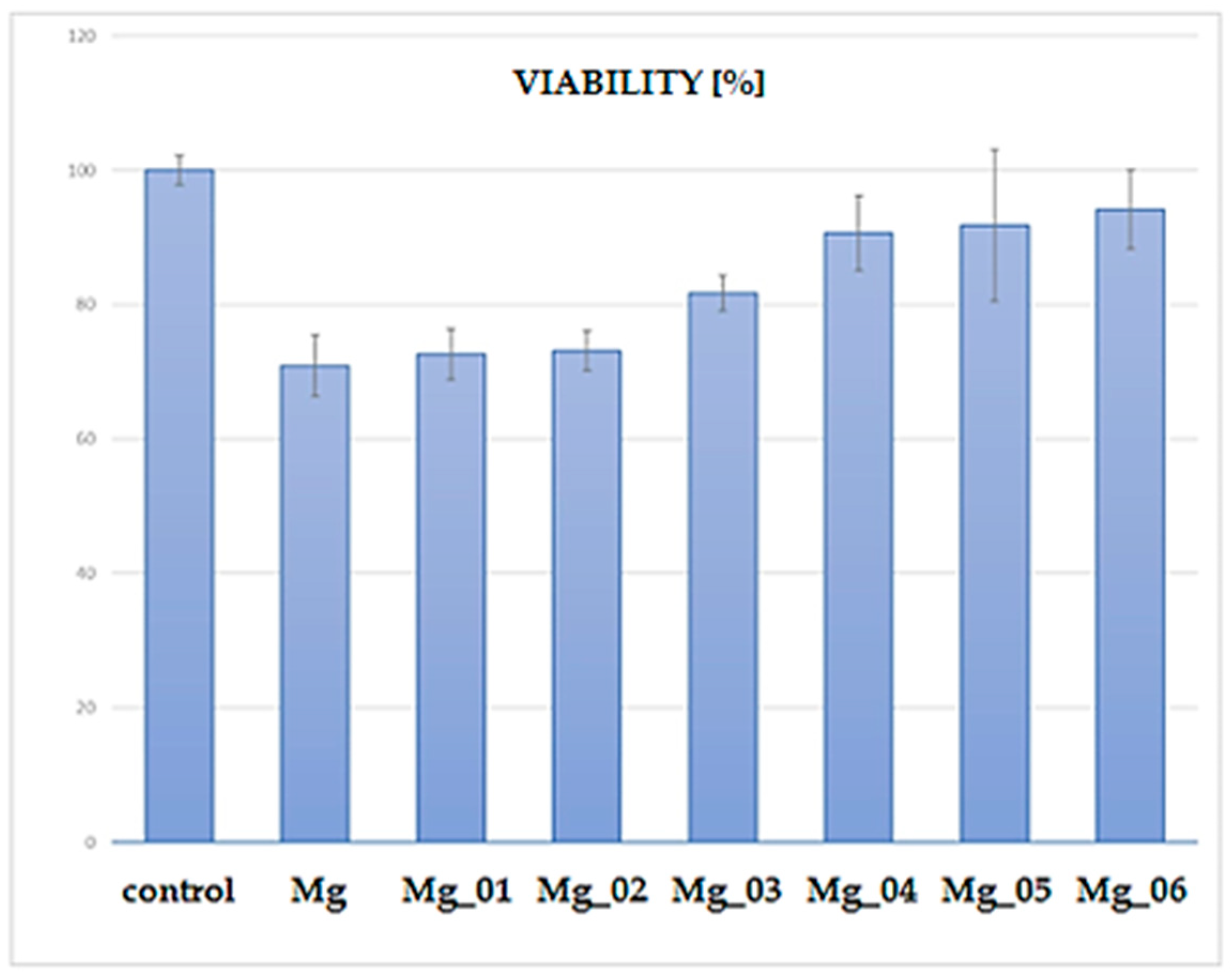
The XTT assay was performed to assess the cytocompatibility of the modified magnesium (Mg) samples by evaluating the viability of cells cultured in the presence of these materials. The results, presented in Figure 19, indicate a clear trend in cell viability depending on the surface modification applied to the Mg discs. The control group exhibited the highest viability (~100%), as expected for untreated cells. In contrast, the unmodified Mg sample displayed a significant reduction in cell viability, suggesting inherent cytotoxic effects due to rapid degradation and increased ion release leading to an unfavorable microenvironment. Among the modified Mg samples, Mg_01 and Mg_02, which were coated with chitosan and chitosan/polydopamine (PDA), respectively, demonstrated moderate cell viability (~70%). The presence of chitosan, known for its biocompatibility and antibacterial properties, likely contributed to this improved performance compared to uncoated Mg. However, the relatively lower viability compared to other modified samples suggests that these coatings alone were insufficient in fully mitigating Mg’s cytotoxicity. In contrast, Mg_03, coated with PDA alone, exhibited slightly higher viability (~80%) than chitosan-based coatings. Polydopamine, mimicking the adhesive properties of mussel proteins, has been reported to enhance cell adhesion and biocompatibility, which may explain this improvement. A notable increase in viability was observed for samples modified via plasma electrolytic oxidation (PEO) before further chitosan or PDA coatings. Specifically, Mg_04 and Mg_05, which underwent PEO followed by chitosan coatings (low and high viscosity), achieved cell viabilities close to 85–90%. This enhancement is likely due to the formation of a more stable oxide layer, which reduced Mg ion release and improved surface bioactivity. The highest viability (~90–95%) among the modified samples was observed for Mg_06, where PEO was combined with a PDA coating. This suggests that the synergistic effect of PEO surface modification and PDA deposition resulted in the most favorable conditions for cell survival, likely by improving corrosion resistance, reducing ion leaching, and enhancing surface bioactivity. The observed viability trends suggest that surface modification strategies significantly influence the cytocompatibility of Mg-based materials. While unmodified Mg exhibits substantial cytotoxicity, combining PEO with bioactive coatings (particularly PDA) offers a promising route for improving biocompatibility. The improved viability of Mg_06 highlights its potential for biomedical applications, such as biodegradable implants, where controlled degradation and cell-friendly surfaces are essential. Further investigations, including long-term degradation studies and in vivo assessments, are necessary to validate these findings and optimize the coatings for clinical use.
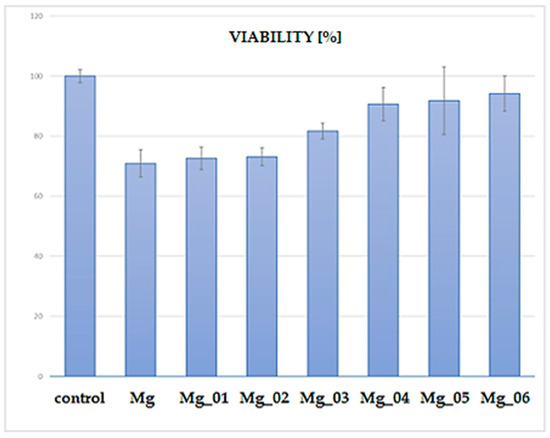
Figure 19.
Percentage comparison of cell viability after the cytotoxicity test.
Each sample group (n = 3) was tested in triplicate wells. The control group was standardized to 100% viability. Among coated samples, Mg_06 demonstrated the highest cell viability at 94.8% ± 1.5, followed by Mg_05 (89.4% ± 1.9) and Mg_04 (85.1% ± 2.3). Samples Mg_01–Mg_03 showed lower viability (~70–79%), with uncoated Mg not included due to known cytotoxicity. The difference between Mg_06 and Mg_01 was statistically significant (p < 0.01), confirming that the PEO + PDA combination most effectively mitigated Mg-related cytotoxic effects. These results align with the literature indicating that PDA supports osteoblast adhesion by mimicking extracellular matrix interactions [45,46,47], and the PEO barrier reduces ion flux and pH shifts that impair cell viability.
Taken together, the data provide strong evidence that the Mg_06 configuration combining PEO, PDA, and embedded AuNPs offers the best overall performance in terms of corrosion resistance, degradation control, hydrophilicity, and biocompatibility. The study demonstrates that such multi-layered modifications have clear advantages over single-component coatings, positioning them as promising candidates for future clinical translation in biodegradable orthopedic implants.
4. Conclusions
The results of this study demonstrate that the surface functionalization of magnesium implants via plasma electrolytic oxidation (PEO), followed by polymeric coating with polydopamine (PDA), significantly enhances their physicochemical and biological performance. The formation of a robust, porous oxide layer through the PEO process effectively improves corrosion resistance and reduces the uncontrolled release of magnesium ions, which are known to contribute to cytotoxicity and local pH shifts. When combined with a PDA layer, the surface exhibits improved hydrophilicity and bioactivity, supporting higher levels of osteoblastic cell viability and attachment compared to unmodified or singly coated implants. Among all sample groups, the Mg_06 variant—comprising PEO and PDA modifications—showed superior performance in terms of degradation control, electrochemical stability, and cytocompatibility, indicating a favorable interaction between structural stability and biological functionality.
These findings underscore the synergistic potential of combining inorganic and organic surface treatments to optimize the interface between biodegradable magnesium substrates and surrounding biological tissues. The dual-modified surfaces provide not only chemical passivation against premature corrosion but also present a bioinspired environment conducive to cellular interaction, thereby addressing some of the critical limitations associated with bare magnesium implants.
However, while the in vitro data are promising, further research is essential to advance these materials toward clinical application. Future studies should prioritize in vivo investigations to evaluate the biological response under physiological conditions, particularly regarding inflammatory modulation, angiogenic activity, and bone–implant integration over time. Additionally, the long-term mechanical integrity of these coatings under dynamic load-bearing conditions requires thorough evaluation, as does their resistance to delamination and wear. Particular attention should also be given to the immunological effects of degradation products and the stability of nanoparticle-functionalized layers in systemic environments. From a translational perspective, the scalability of the coating processes, reproducibility under manufacturing conditions, and regulatory compliance will also be critical to the clinical implementation of such multifunctional biodegradable implants.
In conclusion, the data presented here provide a strong foundation for the continued development of PEO-based, polymer-functionalized magnesium implants. With appropriate optimization and validation in relevant biological models, these systems hold considerable potential for application in orthopedic, dental, and trauma-related interventions where temporary mechanical support and enhanced tissue compatibility are required.
Author Contributions
Conceptualization, J.R.-P. and Ł.J.; methodology J.R.-P. and Ł.J.; validation, J.R.-P. and Ł.J.; investigation, J.Ś., J.R.-P., Ł.J., A.S.-B., K.Ł., and K.K. data curation J.Ś., Ł.J., and J.R.-P.; writing—original draft preparation, J.Ś., K.K., and J.R.-P.; resources: Ł.J. and J.R.-P.; Supervision, J.R.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Panda, S.; Biswas, C.K.; Paul, S. A comprehensive review on the preparation and application of calcium hydroxyapatite: A special focus on atomic doping methods for bone tissue engineering. Ceram. Int. 2021, 47, 28122–28144. [Google Scholar] [CrossRef]
- Oftadeh, R.; Perez-Viloria, M.; Villa-Camacho, J.C.; Vaziri, A.; Nazarian, A. Biomechanics and mechanobiology of trabecular bone: A review. J. Biomech. Eng. 2015, 137, 010802. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.F.; Unnikrishnan, G.U.; Hussein, A.I. Bone mechanical properties in healthy and diseased states. Annu. Rev. Biomed. Eng. 2018, 20, 319–355. [Google Scholar] [CrossRef]
- Yang, J.; Cui, F.; Lee, I.S. Surface modifications of magnesium alloys for biomedical applications. Ann. Biomed. Eng. 2011, 39, 1857–1871. [Google Scholar] [CrossRef]
- Witte, F. Reprint of: The history of biodegradable magnesium implants: A review. Acta Biomater. 2015, 23, S28–S40. [Google Scholar] [CrossRef]
- Bobrowski, C. Fizyka—Krótki Kurs; Wydawnictwo Naukowo-Techniczne: Warszawa, Poland, 2016. [Google Scholar]
- Barylski, A.; Aniołek, K. Effect of deep cryogenic treatment time on micromechanical and tribological properties of magnesium alloys WE43 and WE54. Tribologia 2022, 302, 7–16. [Google Scholar] [CrossRef]
- Kirkland, N.T.; Birbilis, N.; Staiger, M.P. Assessing the corrosion of biodegradable magnesium implants: A critical review of current methodologies and their limitations. Acta Biomater. 2012, 8, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Afshar, A.; Steensma, D.P.; Kyle, R.A. Albin Lambotte: Pioneer of osteosynthesis (bone fixation). Mayo Clin. Proc. 2021, 96, 2012–2013. [Google Scholar] [CrossRef]
- Nica, M.; Cretu, B.; Ene, D.; Antoniac, I.; Gheorghita, D.; Ene, R. Failure analysis of retrieved osteosynthesis implants. Materials 2020, 13, 1201. [Google Scholar] [CrossRef]
- Mosas, K.K.A.; Chandrasekar, A.R.; Dasan, A.; Pakseresht, A.; Galusek, D. Recent advancements in materials and coatings for biomedical implants. Gels 2022, 8, 323. [Google Scholar] [CrossRef]
- Song, G.; Song, S. A possible biodegradable magnesium implant material. Adv. Eng. Mater. 2007, 9, 298–302. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Jéquier, E.; Constant, F. Water as an essential nutrient: The physiological basis of hydration. Eur. J. Clin. Nutr. 2010, 64, 115–123. [Google Scholar] [CrossRef]
- Radwan-Pragłowska, J.; Janus, Ł.; Szajna, E.; Galek, T.; Sierakowska, A.; Piątkowski, M.; Tupaj, M.; Radomski, P.; Michalec, M.; Bogdał, D. Biodegradable Mg-based implants obtained via anodic oxidation applicable in dentistry: Preparation and characterization. J. Mater. Res. Technol. 2022, 20, 1736–1754. [Google Scholar] [CrossRef]
- Czyzewska-Dors, E.; Dors, A.; Pomorska-Mól, M. Właściwości biofilmu bakteryjnego warunkujące oporność na antybiotyki oraz metody jego zwalczania. Życie Weterynaryjne 2018, 93, 26–34. [Google Scholar]
- Lister, J.L.; Horswill, A.R. Staphylococcus aureus biofilms: Recent developments in biofilm dispersal. Front. Cell. Infect. Microbiol. 2014, 4, 178. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef]
- Toh, H.W.; Toong, D.W.Y.; Ng, J.C.K.; Ow, V.; Lu, S.; Tan, L.P.; Wong, P.E.H.; Venkatraman, S.; Huang, Y.; Ang, H.Y. Polymer blends and polymer composites for cardiovascular implants. Eur. Polym. J. 2021, 146, 110249. [Google Scholar] [CrossRef]
- Lowe, S.; O’Brien-Simpson, N.M.; Connal, L.A. Antibiofouling polymer interfaces: Poly(ethylene glycol) and other promising candidates. Polym. Chem. 2015, 6, 198–212. [Google Scholar] [CrossRef]
- Fan, J.; Abedi-Dorcheh, K.; Vaziri, A.S.; Kazemi-Aghdam, F.; Rafieyan, S.; Sohrabinejad, M.; Ghorbani, M.; Adib, F.R.; Ghasemi, Z.; Klavins, K.; et al. A review of recent advances in natural polymer-based scaffolds for musculoskeletal tissue engineering. Polymers 2022, 14, 2097. [Google Scholar] [CrossRef]
- Seshan, K. Handbook of Thin-Film Deposition Processes and Techniques: Principles, Methods, Equipment and Applications; Noyes Publications/William Andrew Pub: Norwich, NY, USA, 2002. [Google Scholar]
- Dudek, P. Conversion coatings on magnesium alloy die castings. Mater. Sci. Forum 2012, 712, 49–56. [Google Scholar]
- Rezaee, N.; Attar, M.M.; Ramezanzadeh, B. Studying corrosion performance, microstructure, and adhesion properties of a room temperature zinc phosphate conversion coating containing Mn2+ on mild steel. Surf. Coat. Technol. 2013, 236, 361–367. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.W.; Li, J.G.; Sun, X.D. Preparation and characterizations of bioglass ceramic cement/ca-p coating on pure magnesium for biomedical applications. ACS Appl. Mater. Interfaces 2014, 6, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Hirpara, A.B.; Chaki, S.H.; Kannaujiya, R.M.; Deshpande, M.P. Photoresponse application of the dip-coated Cu2ZnSnS4 thin film. Appl. Phys. A 2023, 129, 226. [Google Scholar] [CrossRef]
- Metoki, N.; Sadman, K.; Shull, K.; Eliaz, N.; Mandler, D. Electro-assisted deposition of calcium phosphate on self-assembled monolayers. Electrochim. Acta 2016, 206, 400–408. [Google Scholar] [CrossRef]
- Kong, M.G.; Shama, G. Cold Atmospheric Gas Plasmas. In Encyclopedia of Food Microbiology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 493–496. [Google Scholar] [CrossRef]
- Kobel, P.; Mączka, T. Zastosowanie plazmy niskotemperaturowej w technice spalania. Arch. Spalania 2009, 9, 161–180. [Google Scholar]
- Li, L.-Y.; Cui, L.-Y.; Liu, B.; Zeng, R.-C.; Chen, X.-B.; Li, S.-Q.; Wang, Z.-L.; Han, E.-H. Corrosion resistance of glucose-induced hydrothermal calcium phosphate coating on pure magnesium. Appl. Surf. Sci. 2019, 465, 1066–1077. [Google Scholar] [CrossRef]
- Hantzidiamantis, P.J.; Lappin, S.L. Physiology, Glucose; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Simchen, F.; Sieber, M.; Kopp, A.; Lampke, T. Introduction to plasma electrolytic oxidation-an overview of the process and applications. Coatings 2020, 10, 628. [Google Scholar] [CrossRef]
- Cepeda-Jiménez, C.M.; Pérez-Prado, M.T. Processing of nanoparticulate metal matrix composites. In Comprehensive Composite Materials II; Elsevier: Amsterdam, The Netherlands, 2017; pp. 313–330. [Google Scholar] [CrossRef]
- Sikdar, S.; Menezes, P.V.; Maccione, R.; Jacob, T.; Menezes, P.L. Plasma electrolytic oxidation (Peo) process—Processing, properties, and applications. Nanomaterials 2021, 11, 1375. [Google Scholar] [CrossRef]
- Babaei, K.; Fattah-alhosseini, A.; Chaharmahali, R. A review on plasma electrolytic oxidation (PEO) of niobium: Mechanism, properties and applications. Surf. Interfaces 2020, 21, 100719. [Google Scholar] [CrossRef]
- Parfenov, E.; Parfenova, L.; Mukaeva, V.; Farrakhov, R.; Stotskiy, A.; Raab, A.; Danilko, K.; Rameshbabu, N.; Valiev, R. Biofunctionalization of PEO coatings on titanium implants with inorganic and organic substances. Surf. Coat. Technol. 2020, 404, 126486. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Fundamentals and Applications of Chitosan. In Sustainable Agriculture Reviews; Springer Nature: Cham, Switzerland, 2019; pp. 49–123. [Google Scholar] [CrossRef]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Novikov, V.Y.; Derkach, S.R.; Konovalova, I.N.; Dolgopyatova, N.V.; Kuchina, Y.A. Mechanism of Heterogeneous Alkaline Deacetylation of Chitin: A Review. Polymers 2023, 15, 1729. [Google Scholar] [CrossRef]
- Muthu, M.; Pushparaj, S.S.C.; Gopal, J.; Sivanesan, I. A Review on the Antimicrobial Activity of Chitosan Microspheres: Milestones Achieved and Miles to Go. J. Mar. Sci. Eng. 2023, 11, 1480. [Google Scholar] [CrossRef]
- Pandian, M.; Kumar, V.A.; Jayakumar, R. Antiseptic chitosan bandage for preventing topical skin infections. Int. J. Biol. Macromol. 2021, 193, 1653–1658. [Google Scholar] [CrossRef]
- Pal, P.; Pal, A.; Nakashima, K.; Yadav, B.K. Applications of chitosan in environmental remediation: A review. Chemosphere 2021, 266, 128934. [Google Scholar] [CrossRef] [PubMed]
- Giribet, G.; Wheeler, W. On bivalve phylogeny: A high-level analysis of the Bivalvia (Mollusca) based on combined morphology and DNA sequence data. Invertebr. Biol. 2002, 121, 271–324. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistryfor Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Ryu, J.H.; Messersmith, P.B.; Lee, H. Polydopamine Surface Chemistry: A Decade of Discovery. ACS Appl. Mater. Interfaces 2018, 10, 7523–7540. [Google Scholar] [CrossRef]
- Ho, C.C.; Ding, S.J. Structure, properties and applications of mussel-inspired polydopamine. J. Biomed. Nanotechnol. 2014, 10, 3063–3084. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, L.; Zhang, J.; Hu, J.; Duan, G.; Liu, X.; Li, Y.; Gu, Z. Polydopamine antibacterial materials. Mater. Horiz. 2021, 8, 1618–1633. [Google Scholar] [CrossRef]
- Vasileiadis, T.; D’aLvise, T.M.; Saak, C.-M.; Pochylski, M.; Harvey, S.; Synatschke, C.V.; Gapinski, J.; Fytas, G.; Backus, E.H.G.; Weil, T.; et al. Fast Light-Driven Motion of Polydopamine Nanomembranes. Nano Lett. 2022, 22, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Tang, Z.; Zhang, Y.; Wang, Q.; Liang, Z.; Zeng, X. Mussel-Inspired Polydopamine: The Bridge for Targeting Drug Delivery System and Synergistic Cancer Treatment. Macromol. Biosci. 2020, 20, e2000222. [Google Scholar] [CrossRef] [PubMed]
- Barclay, T.G.; Hegab, H.M.; Clarke, S.R.; Ginic-Markovic, M. Versatile Surface Modification Using Polydopamine and Related Polycatecholamines: Chemistry, Structure, and Applications. Adv. Mater. Interfaces 2017, 4, 1601192. [Google Scholar] [CrossRef]
- Jin, A.; Wang, Y.; Lin, K.; Jiang, L. Nanoparticles modified by polydopamine: Working as “drug” carriers. Bioact. Mater. 2020, 5, 522–541. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).