Investigation of Complex ZnO-Porous Silicon Structures with Different Dimensions Obtained by Low-Temperature Synthesis †
Abstract
1. Introduction
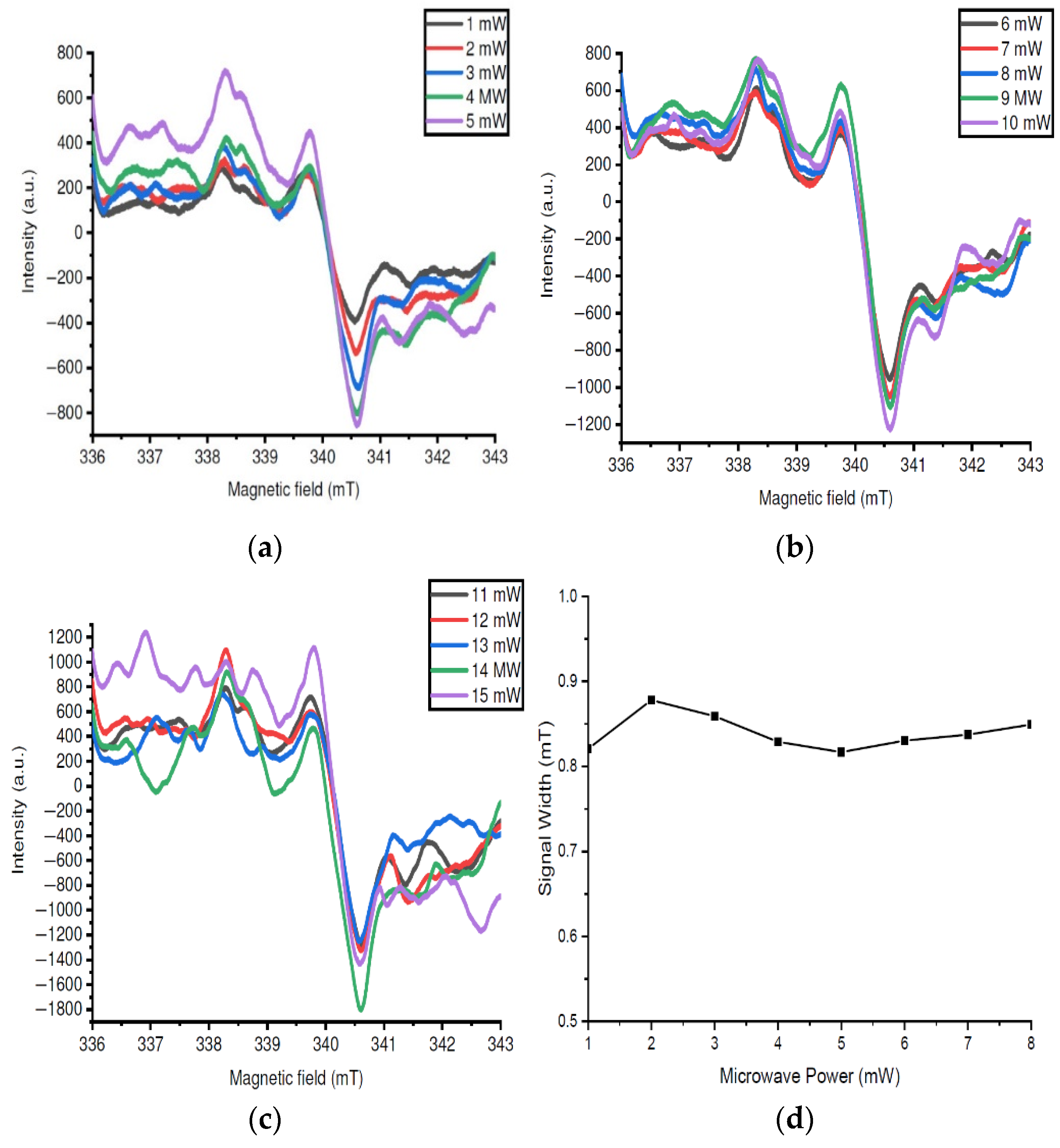
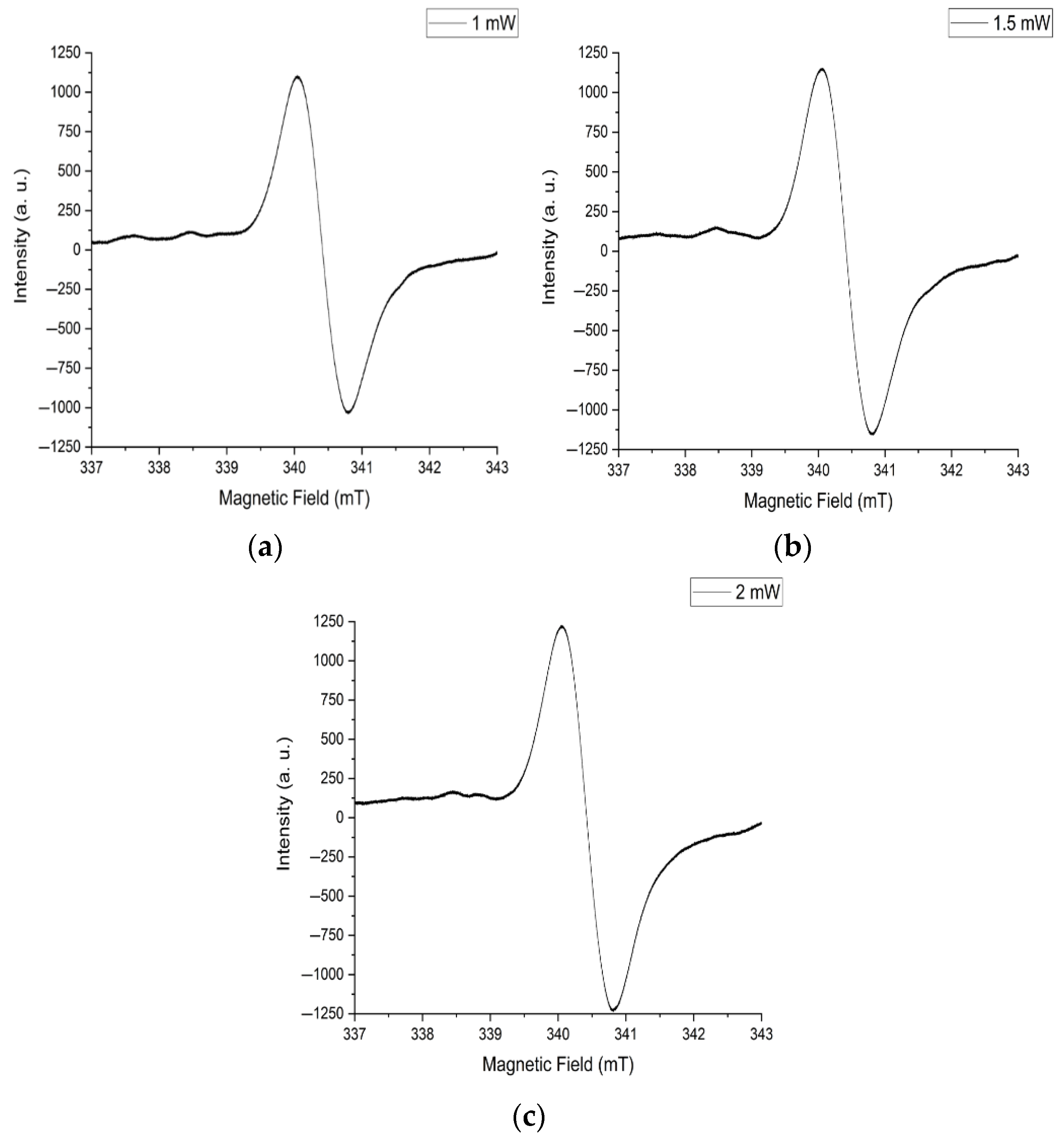
- The stability of the EPR signal when subjected to a constant increase in microwave radiation power has shown a free energy exchange between homogeneous spins and the crystal lattice for structures with a high degree of organization under external excitation.
- The isotropy of the properties of the PMCs responsible for the central EPR signal is due to the equally probable flow of charges in different directions along the axis of the nanowires.
2. Materials and Methods
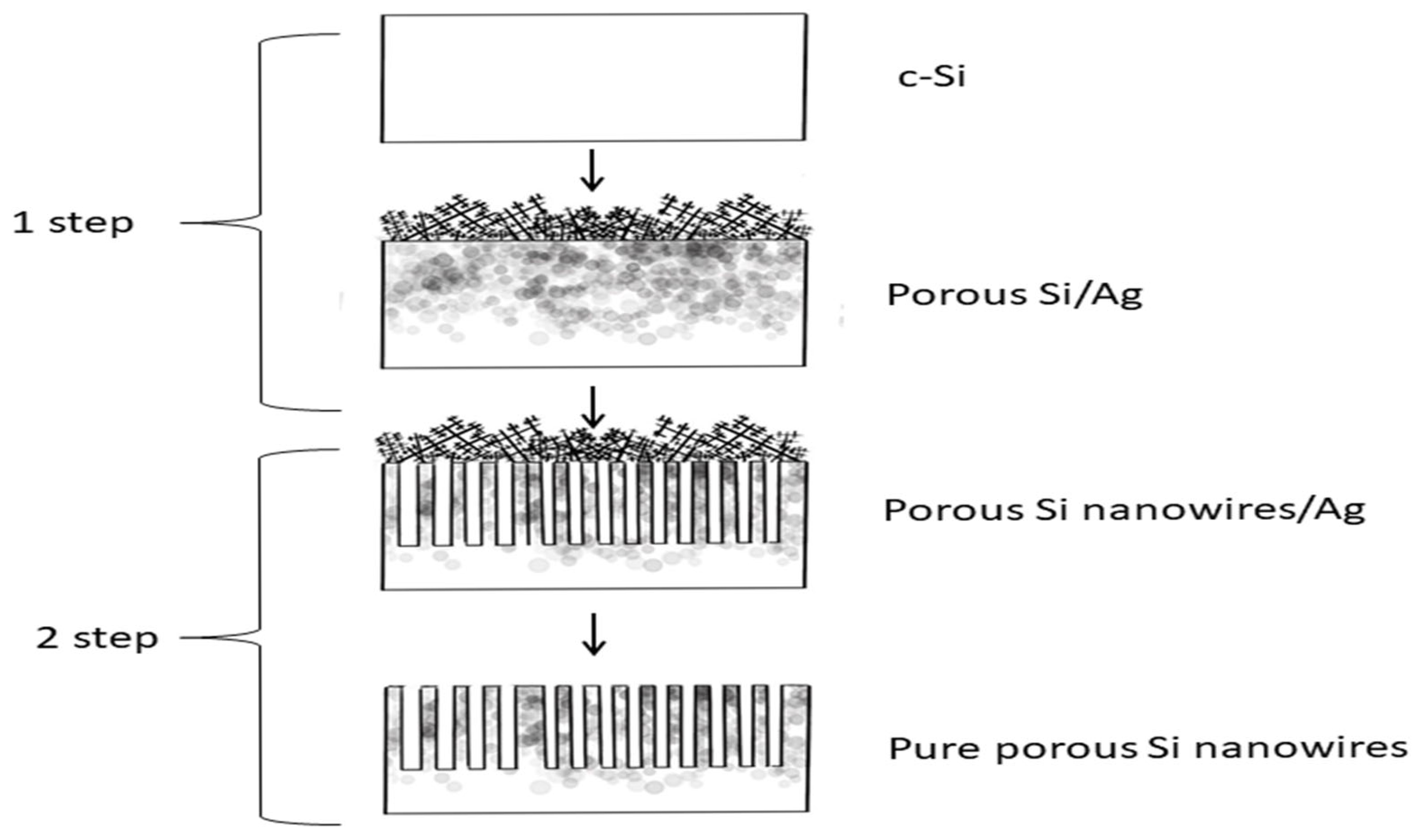
2.1. Synthesis and Transformation of the Studied Structures
+ 2CH3COOH + 6HCHO + 4NH3 + C16H33N(CH3)3Br
2.2. Instrumentation
3. Results
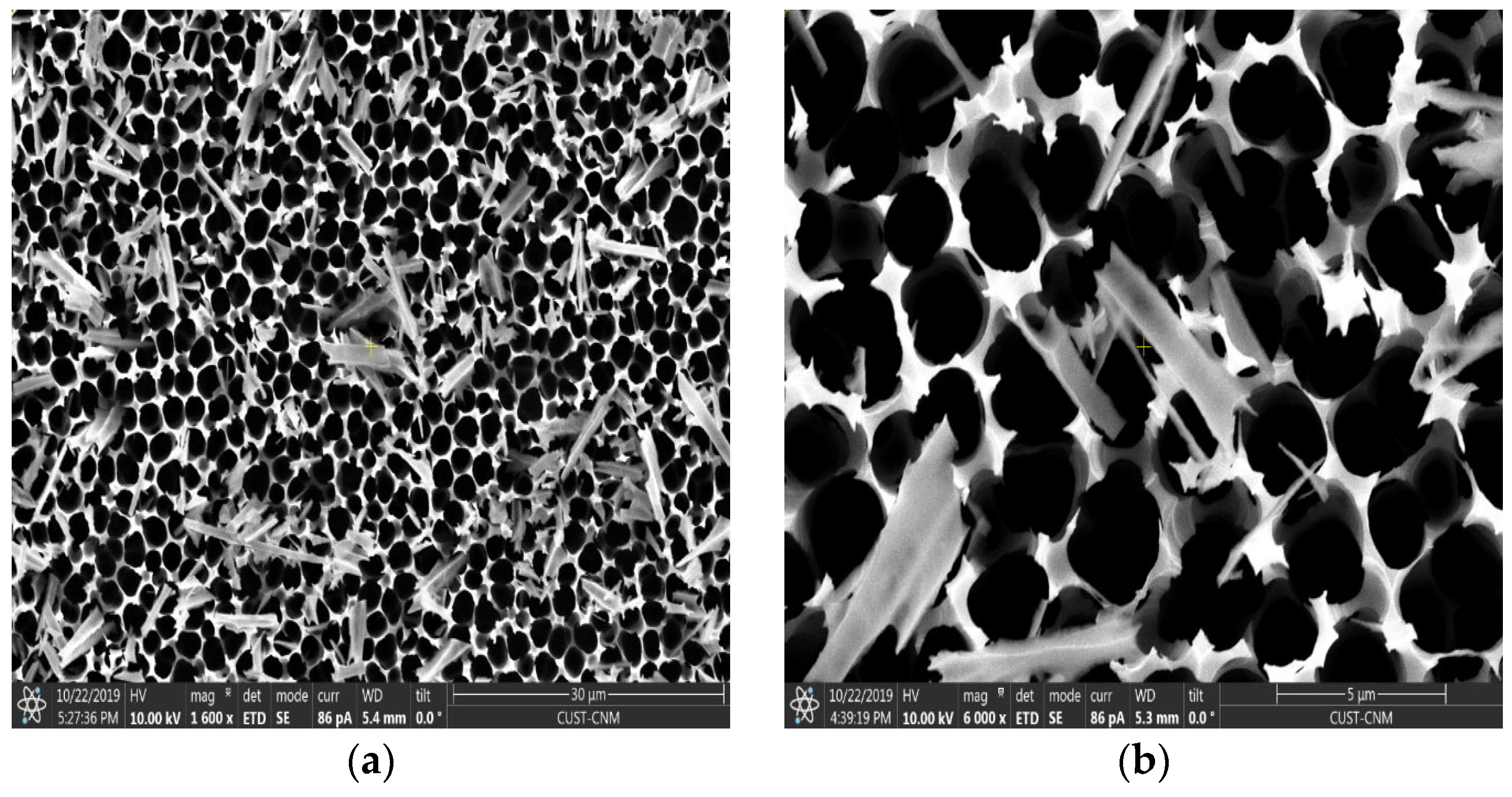
3.1. Study of Surface Morphology of Samples
3.2. Investigation of the Sample Structure by XRD and Raman Spectroscopy Methods
3.3. Photoluminescence Studies
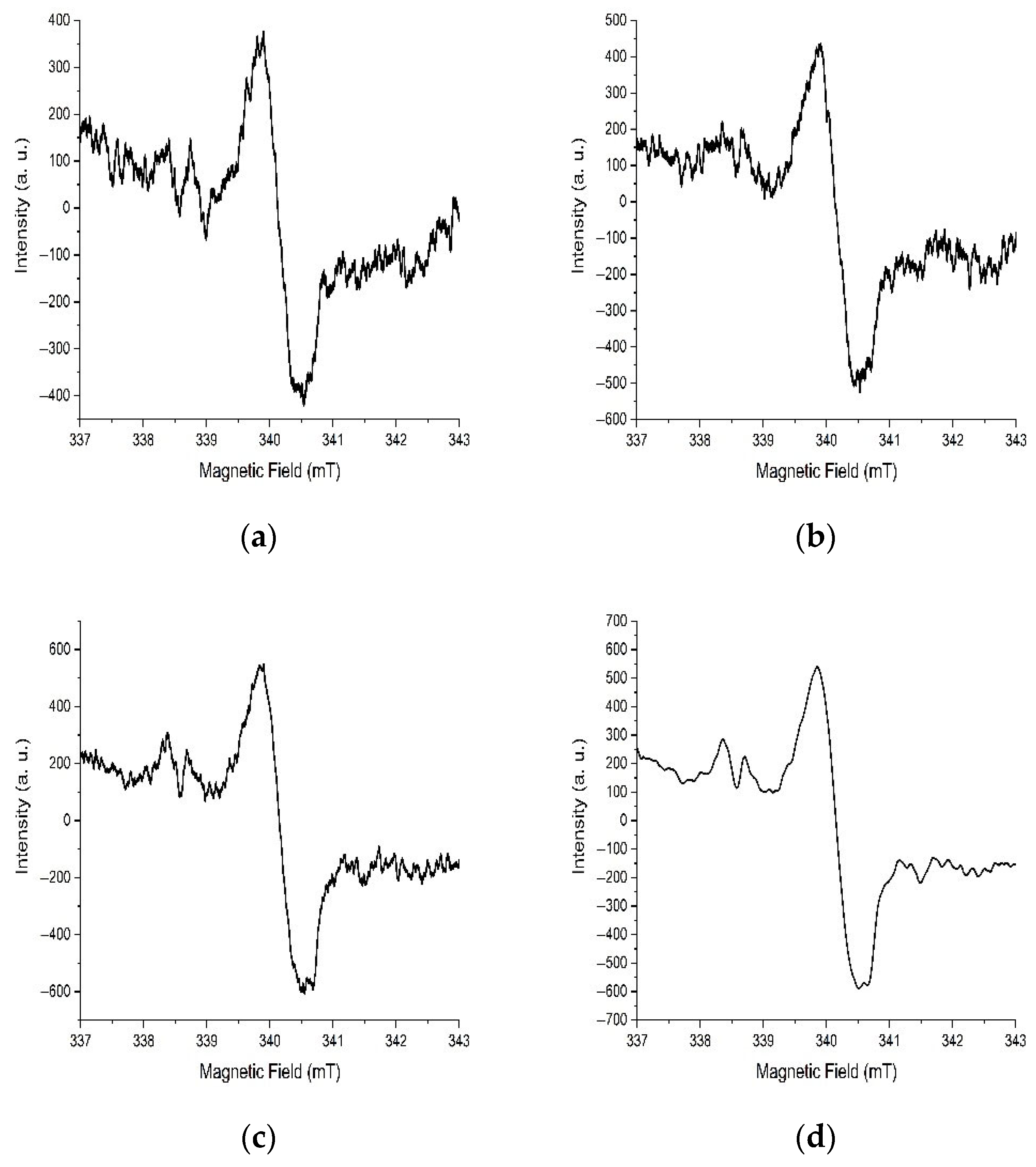
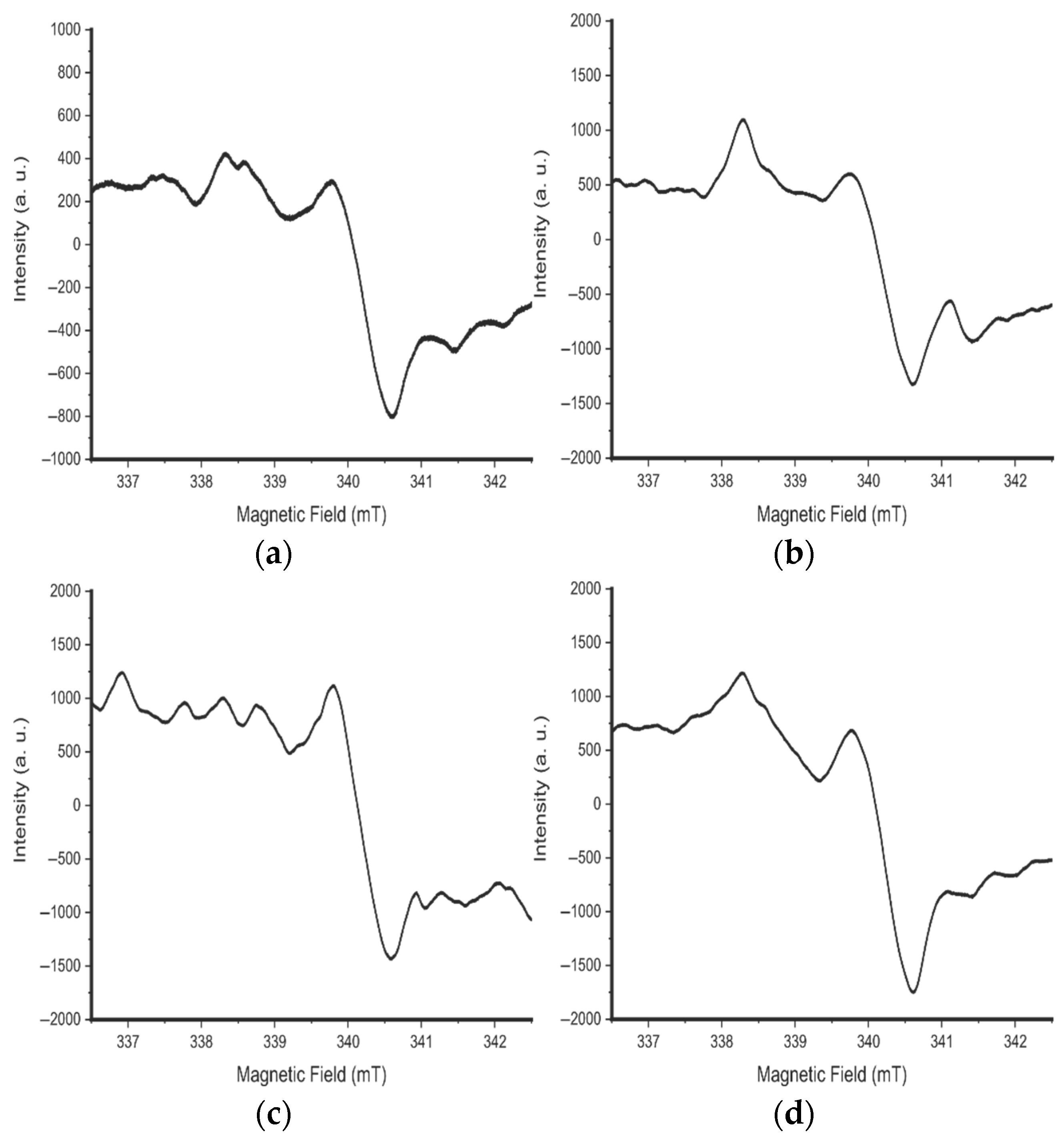
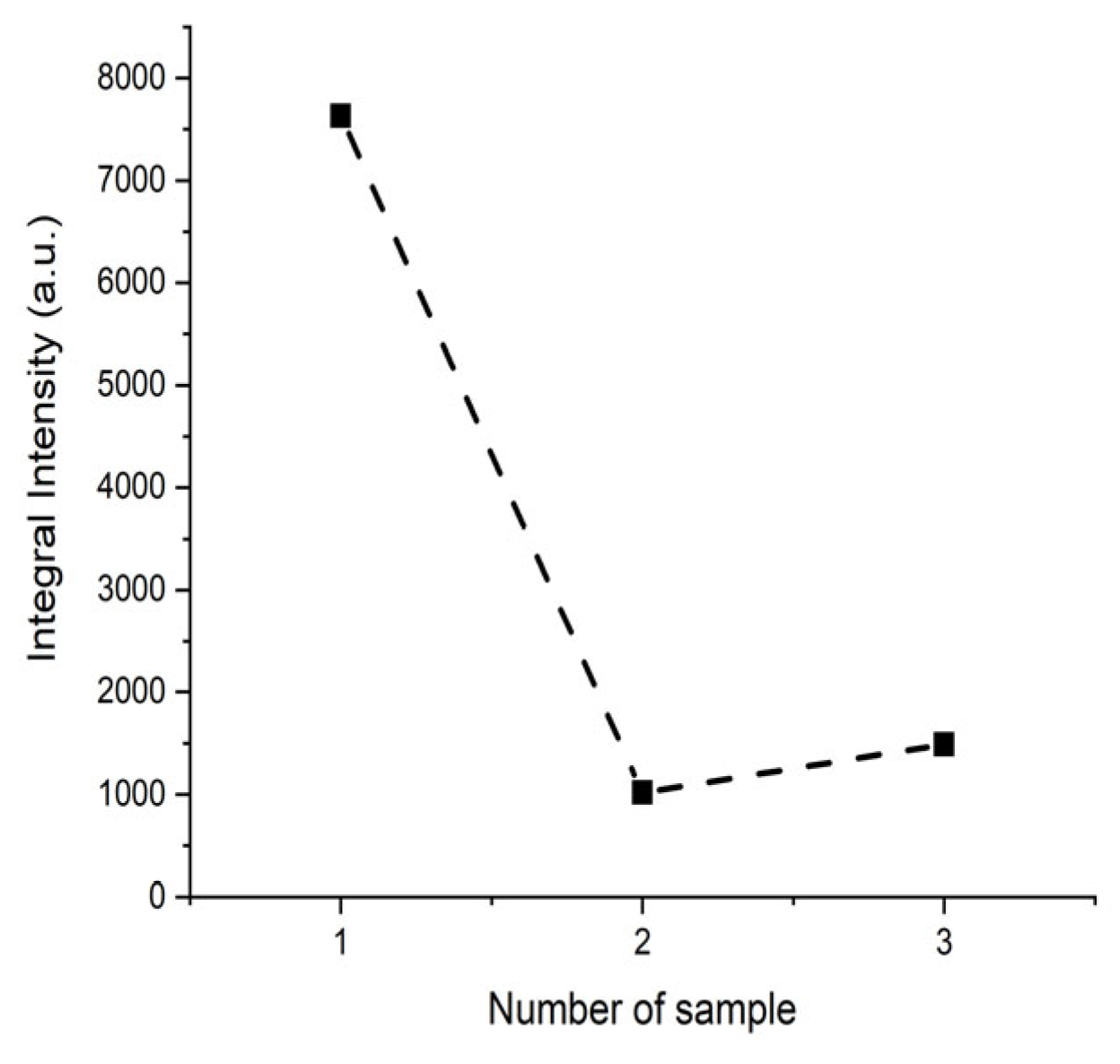
3.4. EPR Studies of Samples After Synthesis
3.5. EPR Studies of Samples After Annealing at 200 °C
3.6. EPR Studies of Samples After Annealing at 300 °C
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Begunov, M.; Gagarina, A.; Zhapakov, R.; Murzalinov, D.; Seredavina, T.; Novikova, K.; Kemelbekova, A.; Spivak, Y.; Moshnikov, V.; Dmitriyeva, E. An Investigation of the Synthesis of a Hybrid Homogeneous Structure of ZnO/Si Nanowires with Stable Energy Properties. Eng. Proc. 2024, 67, 28. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Rheima, A.; Abbas, Z.; Faryad, M.; Kadhim, M.; Altimari, U.; Dawood, A.; Al-Bayati, A.; Abed, Z.; Radhi, R.; et al. Nanowires Properties and Applications: A Review Study. S. Afr. J. Chem. Eng. 2023, 46, 286–311. [Google Scholar] [CrossRef]
- Abdulgafour, H.I.; Hassan, Z.; Yam, F.K.; Jawad, M.J. Growth of ZnO nanowires without catalyst on porous silicon. AIP Conf. Proc. 2011, 1341, 18–20. [Google Scholar] [CrossRef]
- Sherstnev, A.I.; Chubenko, E.B.; Redko, S.V.; Petrovich, V.A.; Pilipenko, V.A.; Bondarenko, V.P. Formation of nanocomposite materials based on porous silicon and zinc oxide by electrochemical method. Rep. Belarusian State Univ. Inform. Radioelectron. 2016, 95, 82–88. [Google Scholar]
- Stehr, J.E.; Chen, S.L.; Chen, W.M.; Cai, L.; Shen, S.; Buyanova, I.A. Identification of a Nitrogen-related acceptor in ZnO nanowires. Nanoscale 2019, 11, 10921–10926. [Google Scholar] [CrossRef]
- Dey, S.; Mohanty, D.; Divya, N.; Bakshi, V.; Mohanty, A.; Rath, D.; Das, S.; Mondal, A.; Roy, S.; Sabui, R. A Critical Review on Zinc Oxide Nanoparticles: Synthesis, Properties and Biomedical Applications. Intell. Pharm. 2024, 3, 53–70. [Google Scholar] [CrossRef]
- Schwan, A.M.; Chwatal, S.; Hendler, C.; Kopp, D.; Lackner, J.M.; Kaindl, R.; Tscherner, M.; Zirkl, M.; Angerer, P.; Friessnegger, B.; et al. Morphology-controlled atmospheric pressure plasma synthesis of zinc oxide nanoparticles for piezoelectric sensors. Appl. Nanosci. 2023, 13, 6421–6432. [Google Scholar] [CrossRef]
- Erdem, E.; Justeau, C.; Poulin-Vittrant, G. Investigation of Intrinsic and Extrinsic Defect Centers of ZnO Nanowires for Nano-Generators. LE Stud. Multidiscip. J. 2018, 61–64. [Google Scholar] [CrossRef]
- Stehr, J.E.; Chen, W.M.; Reddy, N.K.; Tu, C.W.; Buyanova, I.A. Efficient nitrogen incorporation in ZnO nanowires. Sci. Rep. 2015, 5, 13406. [Google Scholar] [CrossRef]
- Hsu, H.C.; Cheng, C.S.; Chang, C.C.; Yang, S.; Chang, C.S.; Hsieh, W.F. Orientation-enhanced growth and optical properties of ZnO nanowires grown on porous silicon substrates. Nanotechnology 2005, 16, 297. [Google Scholar] [CrossRef]
- Ryabko, A.A.; Nalimova, S.S.; Permyakov, N.V.; Bobkov, A.A.; Maksimov, A.I.; Kondratev, V.M.; Kotlyar, K.P.; Ovezov, M.K.; Komolov, A.S.; Lazneva, E.F.; et al. Architectonics of Zinc Oxide Nanorod Coatings for Adsorption Gas Sensors. Tech. Phys. 2024, 69, 2103–2110. [Google Scholar] [CrossRef]
- Yang, J.J.; Pickett, M.D.; Li, X.; Ohlberg, D.A.; Stewart, D.R.; Williams, R.S. Memristive switching mechanism for metal/oxide/metal nanodevices. Nat. Nanotechnol. 2008, 3, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Sawa, A. Resistive switching in transition metal oxides. Mater. Today 2008, 11, 28–36. [Google Scholar] [CrossRef]
- Zoolfakar, A.S.; Kadir, R.A.; Rani, R.A.; Balendhran, S.; Liu, X.; Kats, E.; Bhargava, S.K.; Bhaskaran, M.; Sriram, S.; Zhuiykov, S.; et al. Engineering electrodeposited ZnO films and their memristive switching performance. Phys. Chem. Chem. Phys. 2013, 15, 10376–10384. [Google Scholar] [CrossRef]
- Liu, L.; Chen, B.; Gao, B.; Zhang, F.; Chen, Y.; Liu, X.; Kang, J. Engineering oxide resistive switching materials for memristive device application. Appl. Phys. A 2011, 102, 991–996. [Google Scholar] [CrossRef]
- Martínez, L.; Ocampo, O.; Kumar, Y.; Agarwal, V. ZnO-porous silicon nanocomposite for possible memristive device fabrication. Nanoscale Res. Lett. 2014, 9, 437. [Google Scholar] [CrossRef]
- Gagarina, A.Y.; Bogoslovskaya, L.S.; Spivak, Y.M.; Novikova, K.N.; Kuznetsov, A.; Moshnikov, V.A. Synthesis of arrays nanostructured porous silicon wires in electron conductivity type silicon with crystallographic orientation (111). Tech. Phys. 2023, 68, 254. [Google Scholar] [CrossRef]
- Sekar, K.; Doineau, R.; Mayarambakam, S.; Schmaltz, B.; Poulin-Vittrant, G. Control of ZnO nanowires growth in flexible perovskite solar cells: A mini-review. Heliyon 2014, 10, e24706. [Google Scholar] [CrossRef]
- Alshoaibi, A. The Influence of Annealing Temperature on the Microstructure and Electrical Properties of Sputtered ZnO Thin Films. Inorganics 2024, 12, 236. [Google Scholar] [CrossRef]
- Uthirakumar, P.; Hong, C.-H. Effect of annealing temperature and pH on morphology and optical property of highly dispersible ZnO nanoparticles. Mater. Charact. 2009, 60, 1305–1310. [Google Scholar] [CrossRef]
- Murzalinov, D.; Zhapakov, R. The evolution of defect formation during the deposition of thin layers of ZnO on the surface of hierarchical porous silicon and subsequent multi-stage annealing. In Proceedings of the 3rd International Electronic Conference on Processes, Virtual, 29–31 May 2024; MDPI: Basel, Switzerland, 2024. [Google Scholar]
- Murzalinov, D.; Seredavina, T.; Kemelbekova, A.; Spivak, Y.; Moshnikov, V.; Mukhamedshina, D.; Fedosimova, A. Investigation of Surface Nanoclusters and Paramagnetic Centers of ZnO/Por-Si Structures as the Basis of Sensory Properties. Processes 2023, 11, 3332. [Google Scholar] [CrossRef]
- Rodnyi, P.A.; Chernenko, K.A.; Venevtsev, I.D. Mechanisms of ZnO Luminescence in the Visible Spectral Region. Opt. Spectrosc. 2018, 125, 372–378. [Google Scholar] [CrossRef]

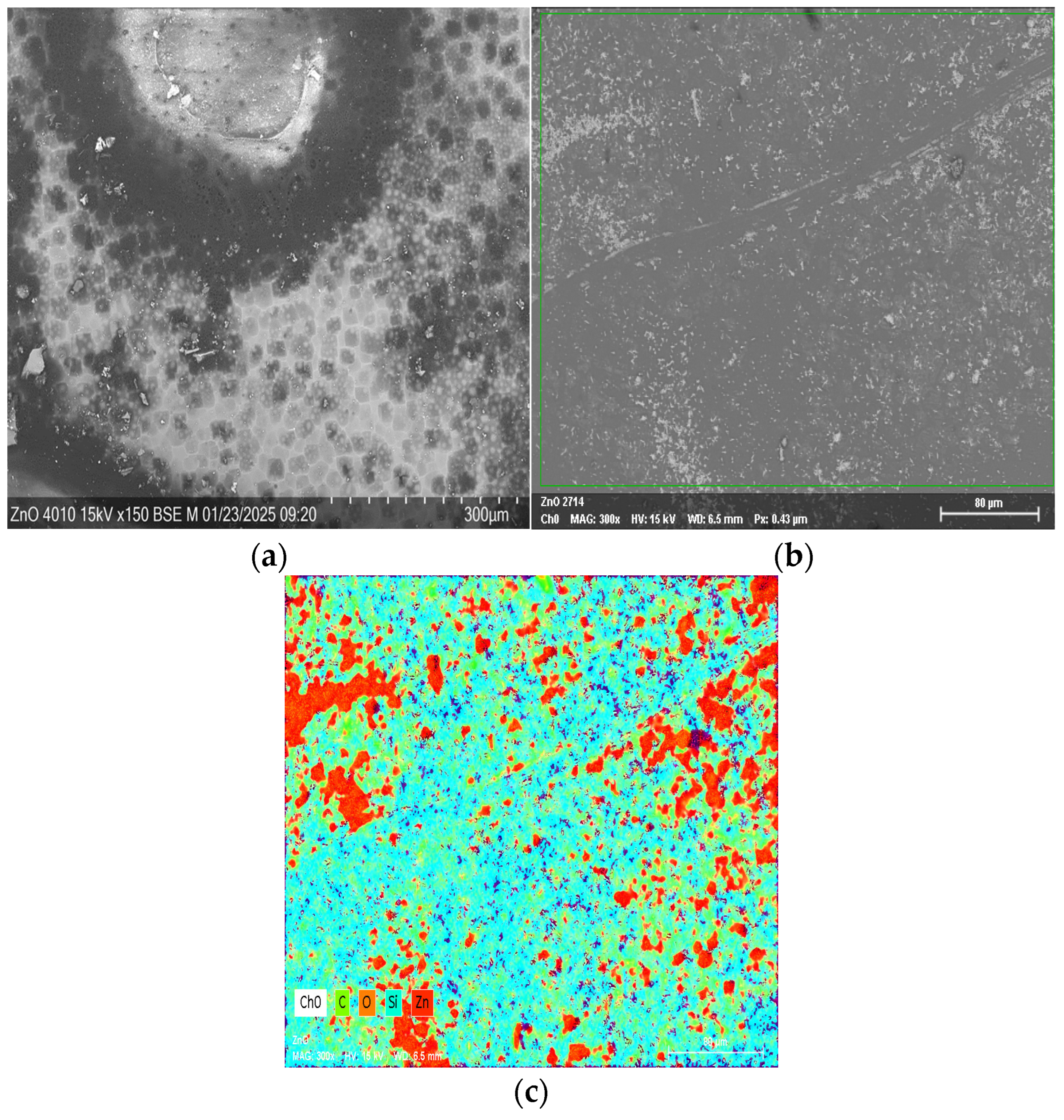

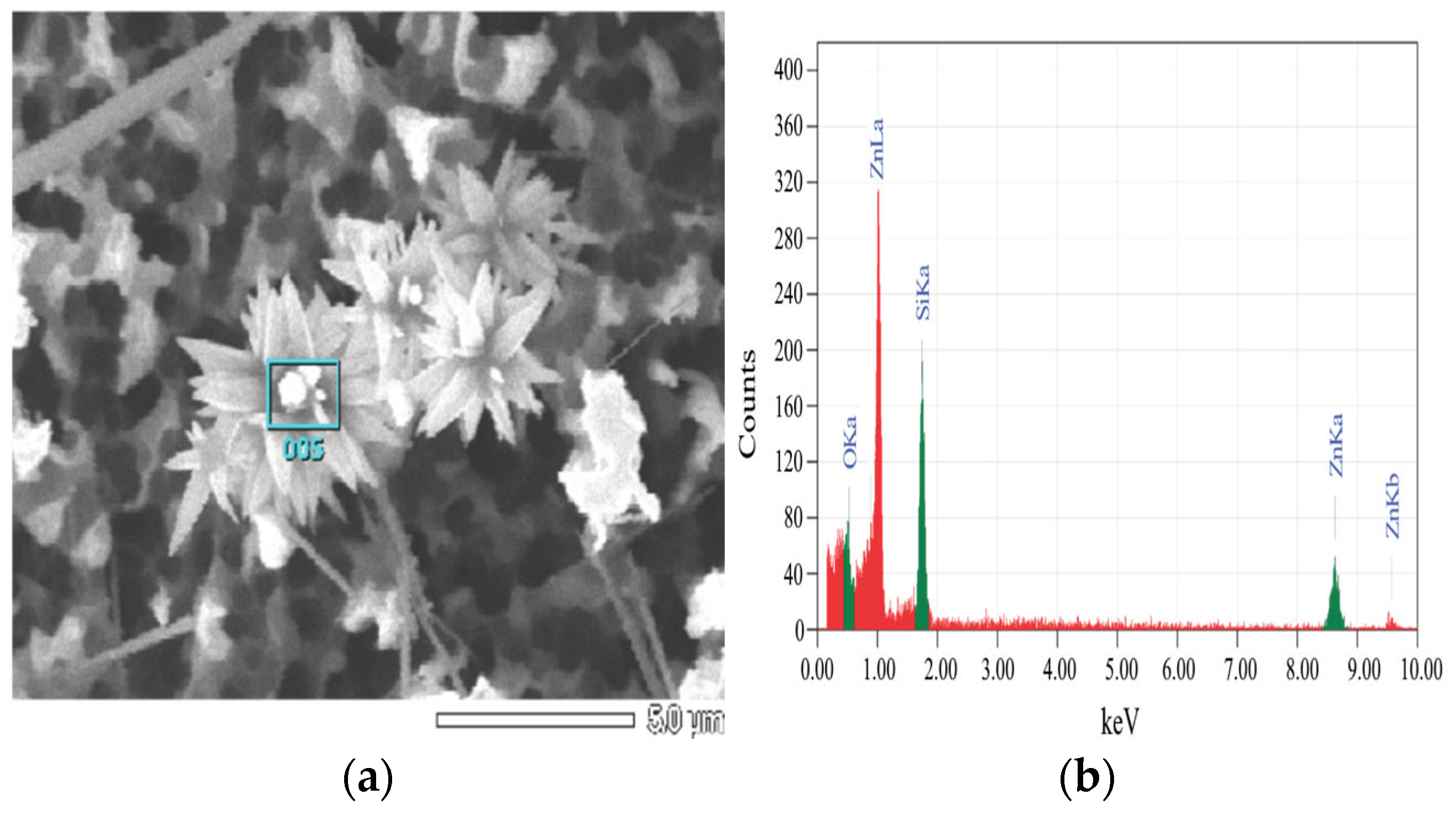
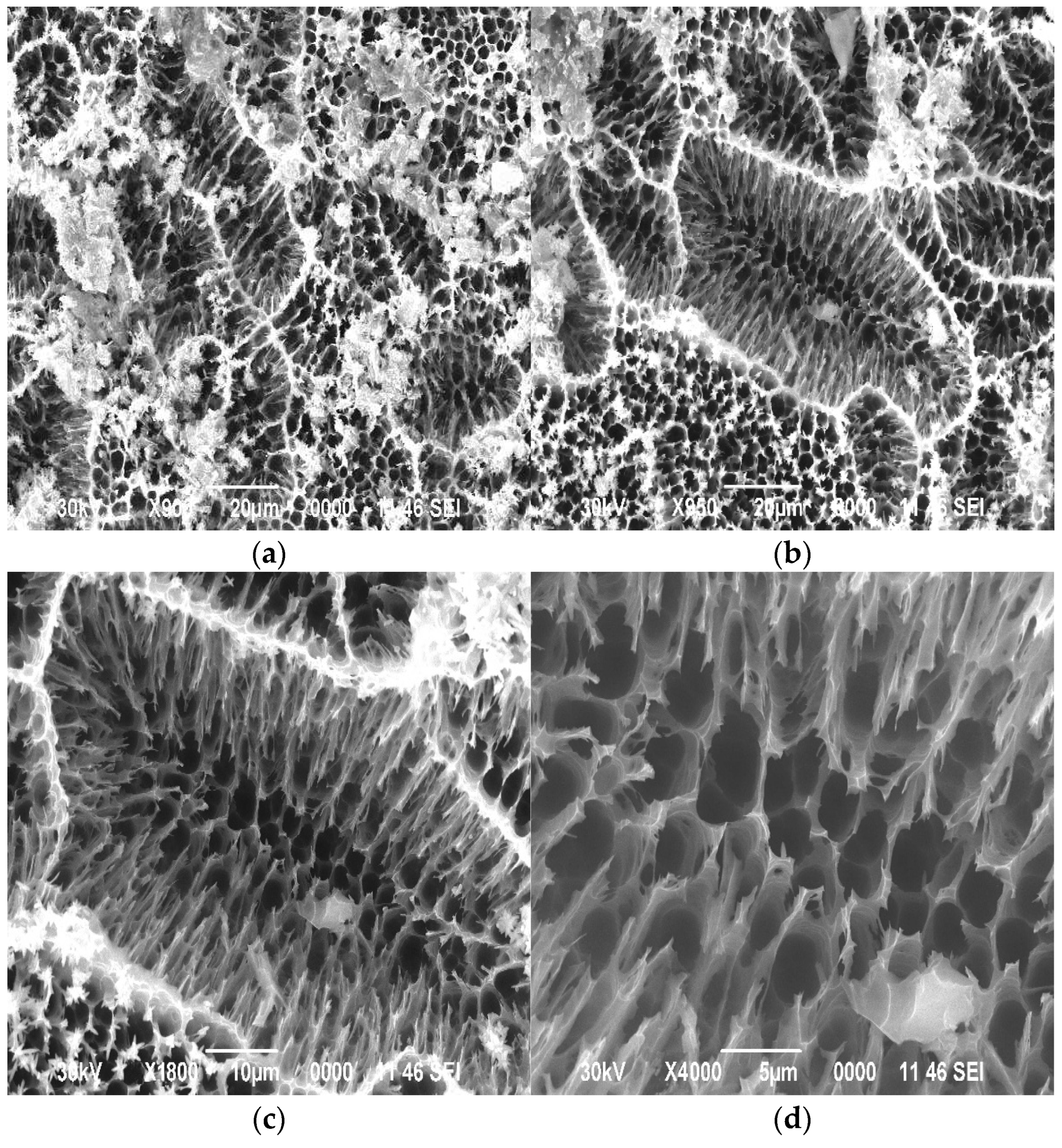

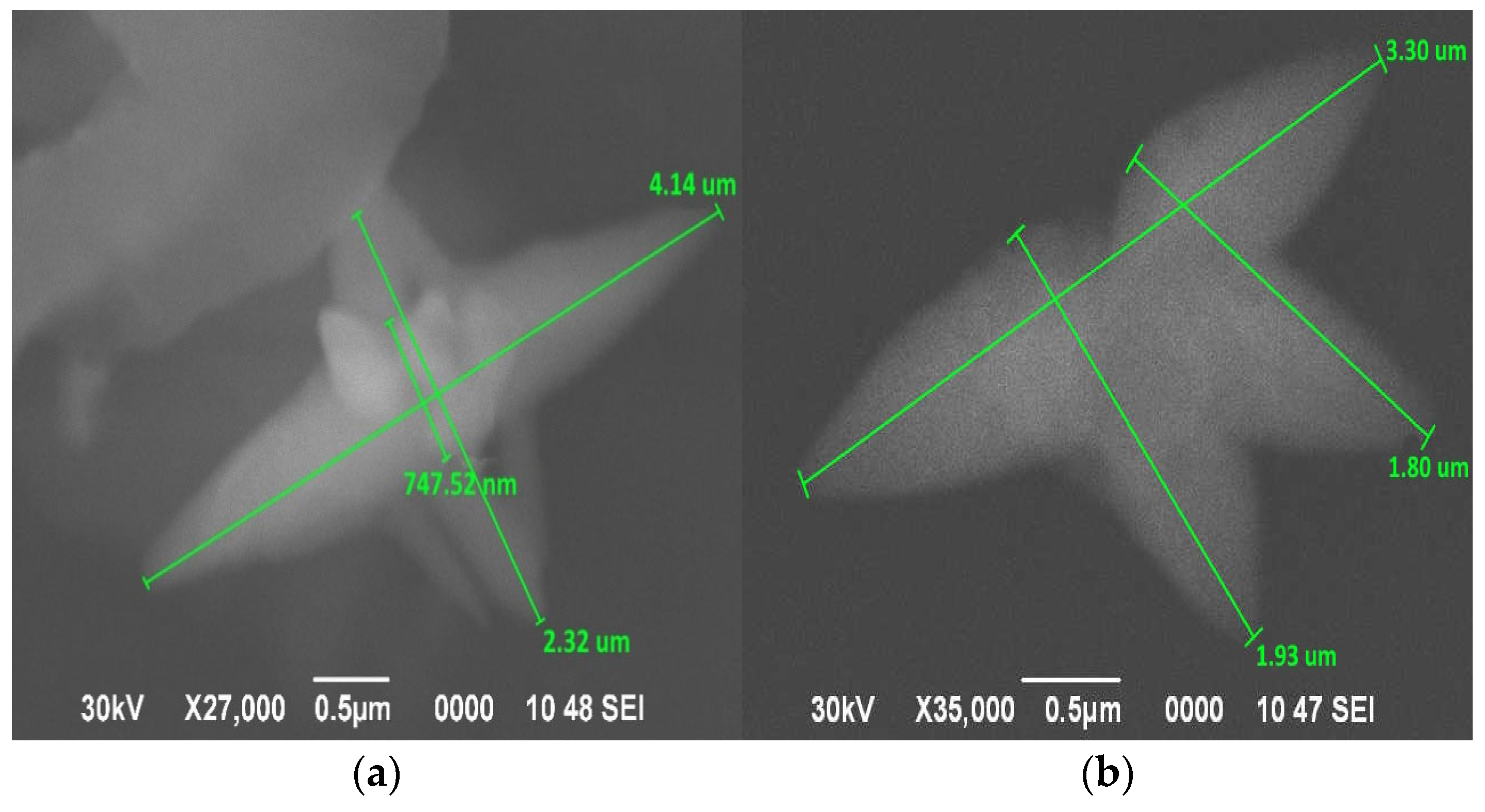


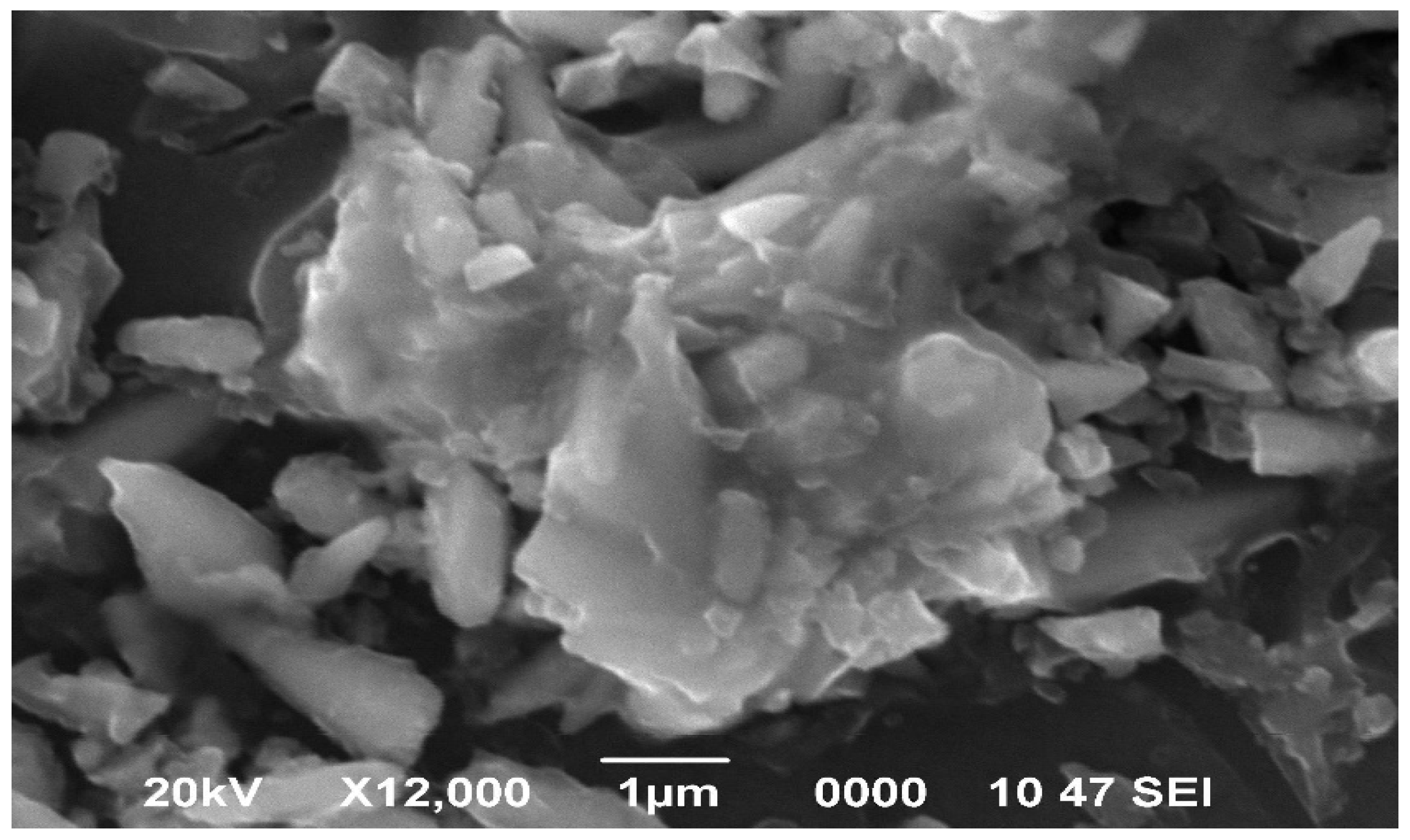






| Element | At. No. | Netto | Mass [%] | Mass Norm. [%] | Atom [%] | Abs. Error [%] (1 sigma) | Rel. Error [%] (1 sigma) |
|---|---|---|---|---|---|---|---|
| C | 6 | 45,518 | 26.87 | 26.87 | 45.68 | 1.48 | 5.51 |
| O | 8 | 32,345 | 6.24 | 6.24 | 7.96 | 0.34 | 5.45 |
| Si | 14 | 2,539,355 | 61.45 | 61.45 | 44.67 | 1.70 | 2.77 |
| Zn | 30 | 14,243 | 5.44 | 5.44 | 1.70 | 0.16 | 2.96 |
| Sum | 100.00 | 100.00 | 100.00 |
| Element | (kEV) | Mass% | Error% | At% | K |
|---|---|---|---|---|---|
| O K | 0.525 | 2.48 | 0.13 | 7.77 | 2.7645 |
| Si K | 1.739 | 17.10 | 0.18 | 30.53 | 12.0252 |
| Zn K | 8.630 | 80.42 | 2.45 | 61.70 | 85.2104 |
| Total | 100.00 | 100.00 |
| Element | (kEV) | Mass% | Error% | At% | K |
|---|---|---|---|---|---|
| O K | - | - | - | - | - |
| Si K | 1.739 | 9.41 | 0.29 | 19.48 | 4.7032 |
| Zn K | 8.630 | 90.59 | 1.26 | 80.52 | 95.2968 |
| Total | 100.00 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhapakov, R.; Murzalinov, D.; Begunov, M.; Seredavina, T.; Gagarina, A.; Spivak, Y.; Moshnikov, V.; Dmitriyeva, E.A.; Osipov, P.; Kemelbekova, A. Investigation of Complex ZnO-Porous Silicon Structures with Different Dimensions Obtained by Low-Temperature Synthesis. Processes 2025, 13, 2099. https://doi.org/10.3390/pr13072099
Zhapakov R, Murzalinov D, Begunov M, Seredavina T, Gagarina A, Spivak Y, Moshnikov V, Dmitriyeva EA, Osipov P, Kemelbekova A. Investigation of Complex ZnO-Porous Silicon Structures with Different Dimensions Obtained by Low-Temperature Synthesis. Processes. 2025; 13(7):2099. https://doi.org/10.3390/pr13072099
Chicago/Turabian StyleZhapakov, Rashid, Danatbek Murzalinov, Mikhail Begunov, Tatyana Seredavina, Alena Gagarina, Yulia Spivak, Vyacheslav Moshnikov, Elena A. Dmitriyeva, Petr Osipov, and Ainagul Kemelbekova. 2025. "Investigation of Complex ZnO-Porous Silicon Structures with Different Dimensions Obtained by Low-Temperature Synthesis" Processes 13, no. 7: 2099. https://doi.org/10.3390/pr13072099
APA StyleZhapakov, R., Murzalinov, D., Begunov, M., Seredavina, T., Gagarina, A., Spivak, Y., Moshnikov, V., Dmitriyeva, E. A., Osipov, P., & Kemelbekova, A. (2025). Investigation of Complex ZnO-Porous Silicon Structures with Different Dimensions Obtained by Low-Temperature Synthesis. Processes, 13(7), 2099. https://doi.org/10.3390/pr13072099






