Research Progress of Surfactant Demulsifier
Abstract
1. Introduction
2. Basic Concepts and Classification of Surfactants
2.1. Anionic Demulsifier
2.2. Nonionic Demulsifier
2.3. Cationic Demulsifier
2.4. Natural Demulsifier
2.5. Metal Demulsifier
2.6. Summary of Demulsifiers
3. Study on the Mechanism of Surfactants as Demulsifiers
3.1. Demulsification Mechanism of W/O Emulsion
3.1.1. Substitution Mechanism
- The efficient interfacial adsorption of demulsifiers on the oil–water interface is beneficial to the demulsification performance improvement: During the initial stage of demulsification, demulsifier molecules rapidly migrate to the oil–water interface and adsorb firmly onto it due to their superior surface activity. This critical step establishes a foundational framework for subsequent disruption and destabilization of the interfacial film.
- Deep interference and destruction of the oil–water interfacial film: Upon successful adsorption at the interface, demulsifier molecules initiate their primary function—profound disruption and destabilization of the original oil–water interfacial film. By substituting or weakening the stabilizing molecules comprising the interfacial film, demulsifiers compromise the film’s structural integrity, thereby diminishing its capacity to resist oil droplet coalescence.
- Significant reduction in interfacial tension: As the interfacial film progressively breaks down, the interfacial tension between oil and water undergoes a marked decrease. This decrease directly facilitates the coalescence of oil droplets, as reduced interfacial tension lowers the energy barrier required for droplet merging. Consequently, this thermodynamic advantage accelerates the demulsification and phase separation processes of the emulsion.
3.1.2. Bridge Replacement Mechanism
3.1.3. Flocculation Aggregation Mechanism
3.1.4. Competitive Adsorption Mechanism
3.1.5. Derjaguin–Landau–Verwey–Overbeek Theory
3.2. Demulsification Mechanism of O/W Emulsion
3.2.1. Anti-Phase Transformation Mechanism
3.2.2. Mechanism of Neutralizing Interface Charges
3.2.3. Mechanism of Counter-Ion Action
3.2.4. Wetting and Solubilization Mechanism
3.3. Summary of Demulsification Mechanism
4. Prospect
- Advancing Research on Demulsification and Stabilization Mechanisms: Given the differences in the composition and characteristics of emulsions in various oil fields, it is urgent to explore the underlying mechanisms of demulsification and stabilization. Molecular dynamics simulation technology is used to analyze the micro behavior of complex lotion and oil–water interfaces. In combination with the characteristics of lotion and the actual needs of the site, multitechnology integration strategy is adopted to screen and optimize the types of surfactants to achieve more efficient and accurate demulsification effect.
- Optimization of surfactant compounding technology: The surfactant compounding technology has shown great potential in improving the demulsification performance and has become an important direction in the research and development of demulsifiers. However, the current compounding rules are not clear, and the optimal ratio between different emulsifiers needs to be verified through extensive experiments. Therefore, in the future, we should focus on the optimization research of binary and multivariate compound systems and improve the synergistic effect and practical application efficiency of compound emulsifiers by finely adjusting the ratio.
- Promotion of the development of green demulsification technology: In response to the global trend of green environmental protection and low-carbon economy, developing environmentally friendly demulsifiers has become a key focus of future research. This type of demulsifier should have characteristics such as high mineralization resistance and acid and alkali resistance while ensuring good compatibility to reduce negative impacts on the environment. In addition, exploring multitechnology integration strategies, such as combining physical, chemical, and biological methods, to further enhance demulsification efficiency and sustainability is the key to promoting the green transformation of the petroleum industry.
- Promotion of the development of emerging innovative technologies: In current research, switchable materials have received extensive attention from scholars. At the basic research level, current studies on switchable materials mostly focus on their static properties before and after switching, while the switching process is actually dynamic. Future research should focus on exploring the kinetics of the switching behavior to clarify its switching speed, which is crucial for improving practical application efficiency. In terms of the development of new materials, although certain progress has been made in switchable materials, further optimization is still needed to overcome existing limitations. For switchable interfacial-active materials, efforts should be made to solve the problem of irreversible adsorption on solid surfaces. By rationally designing the charge characteristics of surfactants, the negative impacts of adsorption on reducing the oil–water interfacial tension and switching performance can be minimized. At the same time, new methods such as vapor-phase treatment should be explored to regulate surface adsorption. However, before practical application, these new methods need to be fully verified conceptually.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, Y.; Song, X.; Yang, X.; Wang, C.; Wu, X.; Wang, Y.; Xiang, W.; Zhao, S.; Liu, H. Optimization of chitosan-based demulsifiers via interfacial displacement: A molecular dynamics and principal component analysis approach. Sep. Purif. Technol. 2025, 365, 132693. [Google Scholar] [CrossRef]
- Lv, X.; Zhang, J.; Zhao, Y.; Liu, Y.; Xu, J.; Ma, Q.; Song, S.; Zhou, S. Experimental study of the growth kinetics of natural gas hydrates in an oil–water emulsion system. ACS Omega 2021, 7, 599–616. [Google Scholar] [CrossRef]
- Shen, L.; Ai, G.; Liu, H.; Zhu, L.; Lai, L.; Yan, X.; Yu, W.; Mi, Y. Synthesis and demulsification performance of a novel low-temperature demulsifier based on trimethyl citrate. J. Hazard. Mater. 2024, 472, 134543. [Google Scholar] [CrossRef]
- Bao, Y.; Lee, W.J.; Guan, C.; Liang, Y.N.; Lim, T.-T.; Hu, X. Highly efficient activation of peroxymonosulfate by bismuth oxybromide for sulfamethoxazole degradation under ambient conditions: Synthesis, performance, kinetics and mechanisms. Sep. Purif. Technol. 2021, 276, 119203. [Google Scholar] [CrossRef]
- Sadighian, H.; Mohamadnia, Z.; Ahmadi, E. Demulsification of crude oil emulsions using versatile and eco-friendly demulsifiers based on cellulose decorated with imidazolium-bearing triazole moiety. Langmuir 2023, 39, 9627–9637. [Google Scholar] [CrossRef]
- Wei, L.; Chao, M.; Dai, X.; Jia, X.; Geng, X.; Guo, H. Synthesis and Characterization of a Novel Multibranched Block Polyether Demulsifier by Polymerization. ACS Omega 2021, 6, 10454–10461. [Google Scholar] [CrossRef]
- Alvarado, J.G.; Delgado-Linares, J.G.; Forgiarini, A.M.; Salager, J.-L. Breaking of water-in-crude oil emulsions. 8. demulsifier performance at optimum formulation is significantly improved by a small aromatic content of the oil. Energy Fuels 2019, 33, 1928–1936. [Google Scholar] [CrossRef]
- Song, Z.; Pan, W.; Wang, S.; Lv, X.; Zhao, X.; Sun, J.; Mao, Y.; Wang, X.; Wang, W. Microwave demulsification characteristics and product analysis of oily sludge. Energy Sources Part A Recovery Util. Environ. Eff. 2021, 47, 9720–9739. [Google Scholar] [CrossRef]
- Zhou, L.; Lai, Y.; Shao, Z.; Jian, Y.; Zhuang, W.-Q. Keystone bacteria in a thiosulfate-driven autotrophic denitrification microbial community. Chem. Eng. J. 2023, 470, 144321. [Google Scholar] [CrossRef]
- Hosseinpour, S.; Götz, V.; Peukert, W. Effect of surfactants on the molecular structure of the buried oil/water interface. Angew. Chem. Int. Ed. 2021, 60, 25143–25150. [Google Scholar] [CrossRef]
- Ahmad, S.; Ayoub, M.H.; Khan, A.M.; Waseem, A.; Yasir, M.; Khan, M.S.; Bajwa, T.M.; Shaikh, A.J. Diverse comparative studies for preferential binding of graphene oxide and transition metal oxide nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2022, 647, 129057. [Google Scholar] [CrossRef]
- Pathan, T.; Girase, M.; Patel, K.; Pillai, S.A.; Pathan, S.; Parekh, P.; Kuperkar, K.; Patel, V.I. Synergistic self-assembly in a surface-active ionic liquid and an anionic surfactant mixed system: A comprehensive physicochemical analysis. Ind. Eng. Chem. Res. 2024, 63, 9688–9700. [Google Scholar] [CrossRef]
- Horstmann, R.; Hecht, L.; Kloth, S.; Vogel, M. Structural and dynamical properties of liquids in confinements: A review of molecular dynamics simulation studies. Langmuir 2022, 38, 6506–6522. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, N.; Xia, S. Research progress and development trend of heavy oil emulsifying viscosity reducer: A review. Pet. Sci. Technol. 2021, 39, 550–563. [Google Scholar] [CrossRef]
- Mohy-ud-Din, K.; Raza, S.A. Corrigendum to “Role of board indexes on corporate social responsibility (CSR) and shareholders’ wealth”. J. Clean. Prod. 2023, 416, 137944. [Google Scholar] [CrossRef]
- Muhpidah; Hambali, E.; Suryani, A.; Kartika, I. Palm oil anionic surfactants based emulsion breaker (Case study of emulsions breaker at Semanggi Field production wells). In IOP Conference Series: Earth and Environmental Science, Proceedings of the International Conference on Biomass: Technology, Application, and Sustainable Development, Bogor, Indonesia, 10–11 October 2016; IOP Publishing: Bristol, UK, 2017; Volume 65, p. 12033. [Google Scholar]
- Jabbari, M.; Izadmanesh, Y.; Ghavidel, H. Synthesis of ionic liquids as novel emulsifier and demulsifiers. J. Mol. Liq. 2019, 293, 111512. [Google Scholar] [CrossRef]
- Hashem, H.; Kikhavani, T.; Moradkhani, M. Experimental study and machine learning modeling of water removal efficiency from crude oil using demulsifier. Sci. Rep. 2024, 14, 9187. [Google Scholar] [CrossRef]
- Dhandhi, Y.; Kumar Saw, R.; Singh, R.; Naiya, T.K. Application of a novel surface-active green demulsifier for demulsification of field crude oil emulsion. Sep. Sci. Technol. 2023, 58, 1654–1678. [Google Scholar] [CrossRef]
- Ibrahim, A.Z.; El Sayed, H.; Mahmoud, M.A.; Al-Abyadh, M.; El Nagy, H. Optimizing dehydration and viscosity reduction in Egyptian heavy crude oil emulsions using eco-friendly demulsifiers. Fuel 2025, 387, 134244. [Google Scholar] [CrossRef]
- Xu, H.; Li, Z.; Wang, C.; Wang, Z.; Yu, R.; Tan, Y. Synthesis and application of amphiphilic copolymer as demulsifier for super heavy oil emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2023, 669, 131498. [Google Scholar] [CrossRef]
- Adewunmi, A.A.; Kamal, M.S.; Hussain, S.M.S. Nonionic Demulsifier for Smart Demulsification of Crude Oil Emulsion at Room and Moderate Temperatures. ACS Omega 2024, 9, 48405–48415. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Li, C.; Jia, W.; Maeda, N.; Zhang, X.; Xiao, H.; Yang, F.; Zhang, Y. Adsorption behavior of non-ionic demulsifiers at the oil/water interface stabilized by asphaltenes: Experiments, adsorption kinetics, and mechanisms. Sep. Purif. Technol. 2025, 355, 129703. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; Sharaky, A.; Noor El-din, M.R.; Hussein, K.M. Destabilization of gas condensate oil-water emulsion by dissolved air flotation using new Non Ionic Surfactants. Tenside Surfactants Deterg. 2015, 52, 88–98. [Google Scholar] [CrossRef]
- Atta, A.M.; Abdullah, M.M.; Al-Lohedan, H.A.; Ezzat, A.O. Demulsification of heavy crude oil using new nonionic cardanol surfactants. J. Mol. Liq. 2018, 252, 311–320. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, L.; Chao, M.; Jia, X.; Liu, C.; Shi, L. Synthesis and study of a new type of nonanionic demulsifier for chemical flooding emulsion demulsification. ACS Omega 2021, 6, 17709–17719. [Google Scholar] [CrossRef]
- Zhang, X.; He, C.; Zhou, J.; Tian, Y.; He, L.; Sui, H.; Li, X. Demulsification of water-in-heavy oil emulsions by oxygen-enriched non-ionic demulsifier: Synthesis, characterization and mechanisms. Fuel 2023, 338, 127274. [Google Scholar] [CrossRef]
- Atta, A.M.; Al-Lohedan, H.A.; Ezzat, A.O. Synthesis and application of geminal dicationic ionic liquids and poly (ionic liquids) combined imidazolium and pyridinium cations as demulsifiers for petroleum crude oil saline water emulsions. J. Mol. Liq. 2021, 325, 115264. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Z.; Zhang, H.; Wang, Q.; Tang, Y.; Qu, Q.; Shen, L.; Mi, Y.; Yan, X. An ionic liquid demulsifier with double cationic centers and multiple hydrophobic chains. J. Mol. Liq. 2023, 374, 121265. [Google Scholar] [CrossRef]
- Shu, G.; Bu, K.; Zhao, B.; Zheng, S. Evaluation of newly developed reverse demulsifiers and cationic polyacrylamide flocculants for efficient treatment of oily produced water. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125646. [Google Scholar] [CrossRef]
- Mi, Y.; Shen, L.; Huang, X.; Yu, Y.; Zhang, Z.; Ding, Y.; Chen, L.; Zhao, Y.; Tang, Y.; Qu, Q. Synthesis of an efficient demulsifier derived from cotton cellulose and its demulsification performance in oily wastewater. Int. J. Biol. Macromol. 2025, 296, 139839. [Google Scholar] [CrossRef]
- Huang, Y.; Qiao, C.; Li, Z.; Zeng, H. Mechanistic insights into the role of branched polyethylenimine in breaking Asphaltene-Stabilized Oil-in-Water emulsions: Temperature effects. Sep. Purif. Technol. 2025, 362, 131913. [Google Scholar] [CrossRef]
- Qu, Q.; Tong, M.; Hu, J.; Xie, F.; Yan, X.; Lai, L.; Yu, W.; Mi, Y. Preparation of a low-temperature demulsifier derived from natural cottonseed oil. Fuel 2024, 373, 132305. [Google Scholar] [CrossRef]
- Pal, B.; Kumar, R.; Naiya, T.K. Demulsification of crude oil-water emulsion using naturally formulated demulsifier. Pet. Sci. Technol. 2021, 39, 1027–1042. [Google Scholar] [CrossRef]
- Ye, F.; Zhang, Z.; Ao, Y.; Li, B.; Chen, L.; Shen, L.; Feng, X.; Yang, Y.; Yuan, H.; Mi, Y. Demulsification of water-in-crude oil emulsion driven by a carbonaceous demulsifier from natural rice husks. Chemosphere 2022, 288, 132656. [Google Scholar] [CrossRef]
- Leontyeva, A.; Al Fadhli, K.H.K.; Farhan, W.H. Mathematical Modeling of the Separation Process of Oil-Water Emulsions Using a Demulsifier of Natural Origin. In IOP Conference Series: Earth and Environmental Science, Proceedings of the International Science and Technology Conference "Earth Science", Vladivostok, Russia, 8–10 December 2020; IOP Publishing: Bristol, UK, 2021; Volume 666, p. 52045. [Google Scholar]
- Adewunmi, A.A.; Kamal, M.S.; Solling, T.I.; Salami, B.A. Palm oil fuel ash (POFA) as a demulsifier for crude oil emulsions: Performance and mechanism. J. Pet. Sci. Eng. 2019, 183, 106430. [Google Scholar] [CrossRef]
- Wang, R.; Feng, Y.; Zhong, Y.; Zou, Y.; Yang, M.; Liu, Y.; Zhou, Y. Enhancing demulsification performance for oil–water separation through encapsulating ionic liquids in the pore of MIL-100 (Fe). Langmuir 2021, 37, 8232–8239. [Google Scholar] [CrossRef]
- Azizi, N.; Bashipour, F. Demulsification of water-in-oil emulsions applying Fe3O4 magnetic nanoparticles for demulsifier modification: Experimental optimization via response surface methodology. J. Pet. Sci. Eng. 2022, 216, 110806. [Google Scholar] [CrossRef]
- Zhou, J.; Sui, H.; Ma, J.; Li, X.; Al-Shiaani, N.H.; He, L. Fast demulsification of oil-water emulsions at room temperature by functionalized magnetic nanoparticles. Sep. Purif. Technol. 2021, 274, 118967. [Google Scholar] [CrossRef]
- Liu, Y.; He, H.; Zhang, T.-J.; Zhang, T.C.; Wang, Y.; Yuan, S. A biomimetic beetle-like membrane with superoleophilic SiO2-induced oil coalescence on superhydrophilic CuC2O4 nanosheet arrays for effective O/W emulsion separation. J. Hazard. Mater. 2023, 451, 131142. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, Q.; Cui, C.; Wu, Y.; Xie, Y.; Wang, H. Aromatic poly (amino acids) as an effective low-temperature demulsifier for treating crude oil-in-water emulsions. J. Hazard. Mater. 2024, 472, 134608. [Google Scholar] [CrossRef]
- Ding, Y.; Li, H.; Jia, J.; Ouyang, S.; Jiang, W.; Chen, L.; Zhao, Y.; Zhang, Z.; Mi, Y. Synthesis of a demulsifier for treating crude oil-in-water emulsion through a straightforward low-temperature process. J. Mol. Liq. 2024, 410, 125635. [Google Scholar] [CrossRef]
- Qu, Q.; Hu, Y.; Xiong, J.; Ding, Y.; Tang, Y.; Chen, L.; Zhao, Y.; Mi, Y. Two-step synthesis of ionic liquid demulsifiers for demulsification of water-in-oil emulsion. Sep. Purif. Technol. 2025, 357, 130210. [Google Scholar] [CrossRef]
- Amiri, Z.; Shekarriz, M.; Halladj, R.; Rashidi, A. Sustainable nanodemulsifiers for enhanced demulsification of water and saline in crude oil emulsions: Synthesis and application. J. Ind. Eng. Chem. 2024, 138, 440–450. [Google Scholar] [CrossRef]
- Yu, X.; Wang, J.; Ye, F. Cyclodextrin decorated with quaternary ammonium salt and hydrophobic chain as green demulsifier for the demulsification of oily wastewater. J. Mol. Liq. 2024, 406, 125100. [Google Scholar] [CrossRef]
- Farooq, U.; Patil, A.; Panjwani, B.; Simonsen, G. Review on application of nanotechnology for asphaltene adsorption, crude oil demulsification, and produced water treatment. Energy Fuels 2021, 35, 19191–19210. [Google Scholar] [CrossRef]
- Yao, M.; Ju, Z.; Ran, Z.; Chen, T.; Pan, H. Enhancing the efficiency of novel PCNF demulsifier for oil-contaminated wastewater treatment through numerical simulation optimization of demulsification mechanism. J. Water Process Eng. 2025, 69, 106626. [Google Scholar] [CrossRef]
- Zolfaghari, R.; Fakhru’l-Razi, A.; Abdullah, L.C.; Elnashaie, S.S.; Pendashteh, A. Demulsification techniques of water-in-oil and oil-in-water emulsions in petroleum industry. Sep. Purif. Technol. 2016, 170, 377–407. [Google Scholar] [CrossRef]
- Zhang, M.; Kang, W.; Yang, H.; Zhou, B.; Li, Z.; He, Y.; Yurievich, K.G.; Viktorovich, L.A. De-emulsification performance and mechanism of β-CD reverse demulsifier for amphiphilic polymer oil in water (O/W) emulsion. J. Mol. Liq. 2021, 342, 117441. [Google Scholar] [CrossRef]
- Yuan, S.; Wang, Z.; Yuan, S. Understanding the Chemical Demulsification Mechanism of Oil/Water Emulsion by Polyether Polymers. Ind. Eng. Chem. Res. 2024, 63, 12680–12687. [Google Scholar] [CrossRef]
- Fajun, Z.; Zhexi, T.; Zhongqi, Y.; Hongzhi, S.; Yanping, W.; Yufei, Z. Research status and analysis of stabilization mechanisms and demulsification methods of heavy oil emulsions. Energy Sci. Eng. 2020, 8, 4158–4177. [Google Scholar] [CrossRef]
- Ma, J.; Yao, M.; Yang, Y.; Zhang, X. Comprehensive review on stability and demulsification of unconventional heavy oil-water emulsions. J. Mol. Liq. 2022, 350, 118510. [Google Scholar] [CrossRef]
- Hernandez-Rodriguez, G.; Tenorio-Garcia, E.; Ettelaie, R.; Lishchuk, S.V.; Harbottle, D.; Murray, B.S.; Sarkar, A. Demulsification of Pickering emulsions: Advances in understanding mechanisms to applications. Soft Matter 2024, 20, 7344–7356. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.-H.; Su, C.-H.; Hu, C.-C.; Yeh, K.-H.; Lin, W.-C. Localized surface plasmon resonance-based colorimetric assay featuring thiol-capped Au nanoparticles combined with a mobile application for on-site parathion organophosphate pesticide detection. Langmuir 2022, 38, 838–848. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, S.; Chen, H.; He, L.; Ni, Y.; Liu, S.; Chen, Z.; Tian, Y. Comprehensive review of stabilising factors, demulsification methods, and chemical demulsifiers of oil-water emulsions. Sep. Purif. Technol. 2024, 358, 130206. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, J.; Lin, X.; Yang, Z.; Wang, L.; Lu, H.; Zhang, Z. Realizing the release of drag reducers through weakly acidic-induced demulsification of inverse polymer emulsion. Colloids Surf. A Physicochem. Eng. Asp. 2025, 709, 136099. [Google Scholar] [CrossRef]
- Tang, L.; Wang, T.; Xu, Y.; He, X.; Yan, A.; Zhang, Z.; Li, Y.; Chen, G. Research and Application Progress of Crude Oil Demulsification Technology. Processes 2024, 12, 2292. [Google Scholar] [CrossRef]
- Bai, X.; Liu, Y.; Kang, Y. Demulsification performance of oil-in-water emulsions utilizing bidirectional pulse electric field and fiber media. Sep. Purif. Technol. 2025, 367, 132923. [Google Scholar] [CrossRef]
- Liu, R.; Gao, J.; Liu, Y.; Zhang, W.; Wu, T.; Li, Y. Superhydrophilic and mixed-charge sponge: Revealing the mechanism of three-dimensional demulsification materials for oil-in-water emulsions under charge effect. J. Clean. Prod. 2024, 449, 141694. [Google Scholar] [CrossRef]
- Wang, K.; Bi, X.; Xiao, P.; Jiang, M.; Tian, R.; Zhao, P.; Fang, W.; Liu, B. Polar component networks transformation regulated by bidirectional pulsed electric field for rapid demulsification of O/W emulsion. J. Water Process Eng. 2025, 71, 107300. [Google Scholar] [CrossRef]
- Balaj, R.V.; Xue, W.; Bayati, P.; Mallory, S.; Zarzar, L.D. Dynamic Partitioning of Surfactants into Nonequilibrium Emulsion Droplets. J. Am. Chem. Soc. 2024, 146, 26340–26350. [Google Scholar] [CrossRef]
- Chen, Y.; Gui, X. Synthesis and demulsification performance of tea polyphenol amine resin-based triblock polyether demulsifier. J. Polym. Res. 2025, 32, 54. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, Y.; Yang, D.; Yang, W.; Yan, B.; Zhang, L. Thermally regulated flocculation-coalescence process by temperature-responsive cationic polymeric surfactant for enhanced crude oil-water separation. J. Hazard. Mater. 2025, 481, 136491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, T.; Chen, Q.; Xiao, B.; Xian, X.; Ye, Z. The influence and mechanism exploration of hydration environment on the stability of natural clay crude oil emulsion. Front. Energy Res. 2024, 12, 1362462. [Google Scholar] [CrossRef]
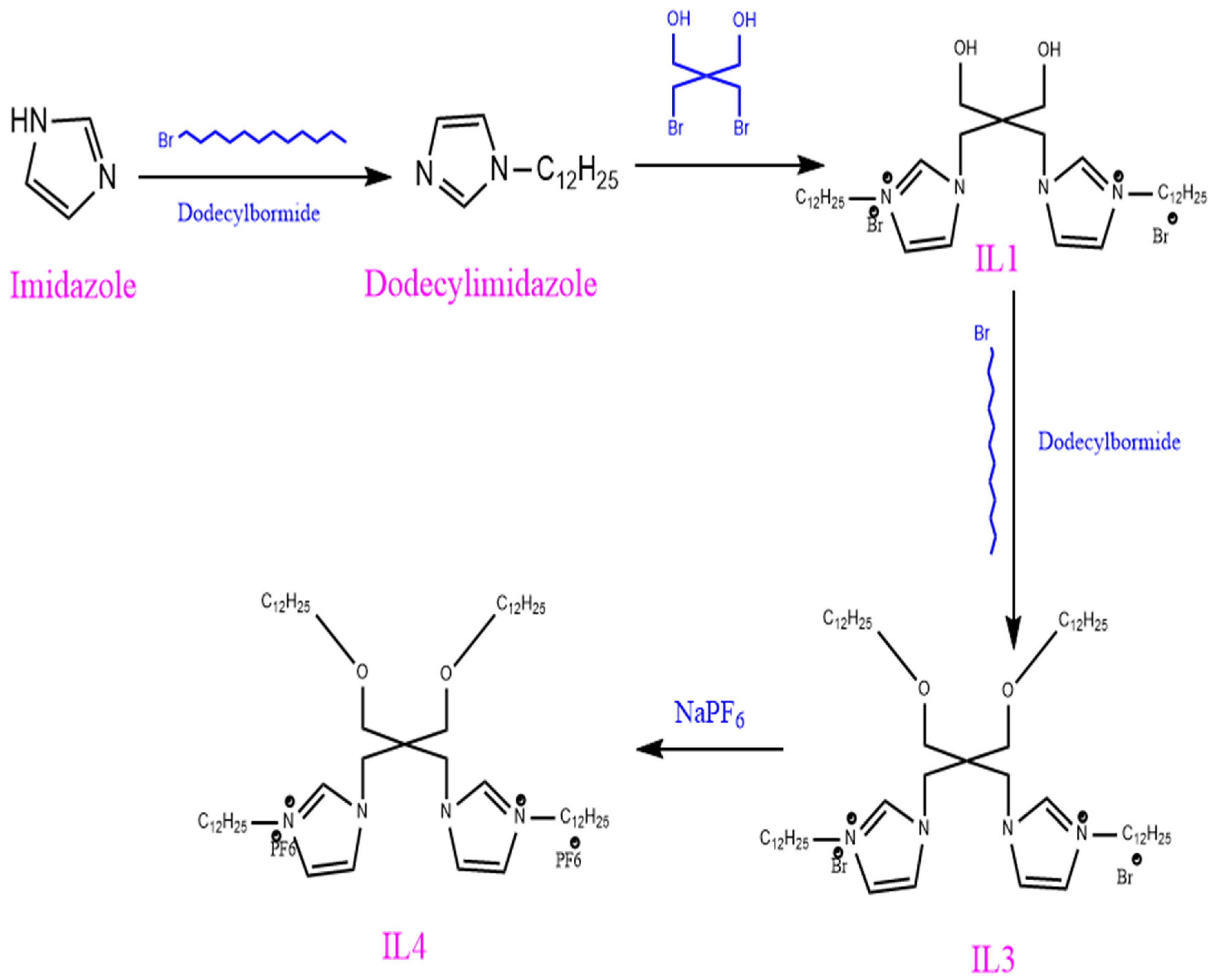
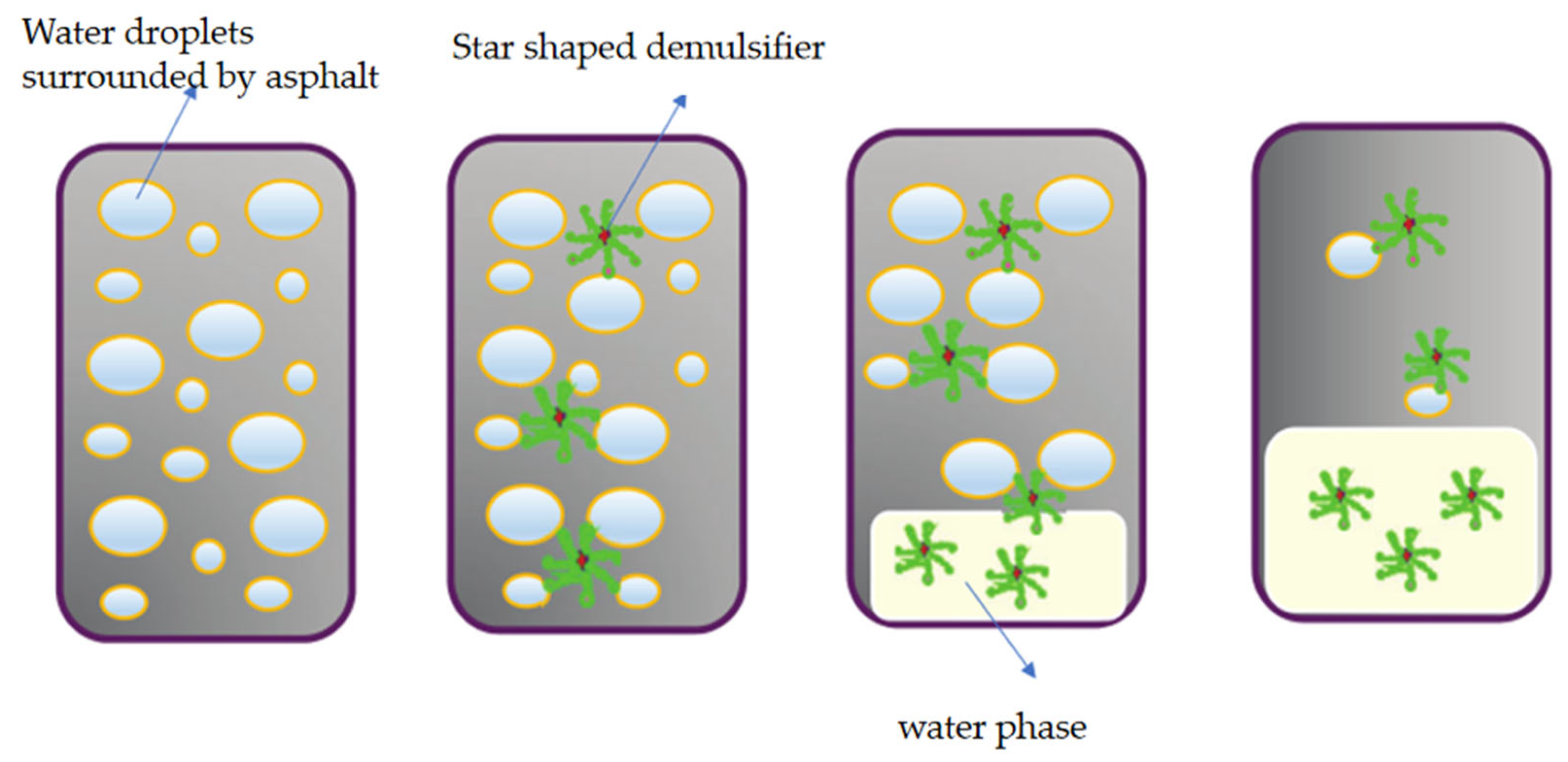

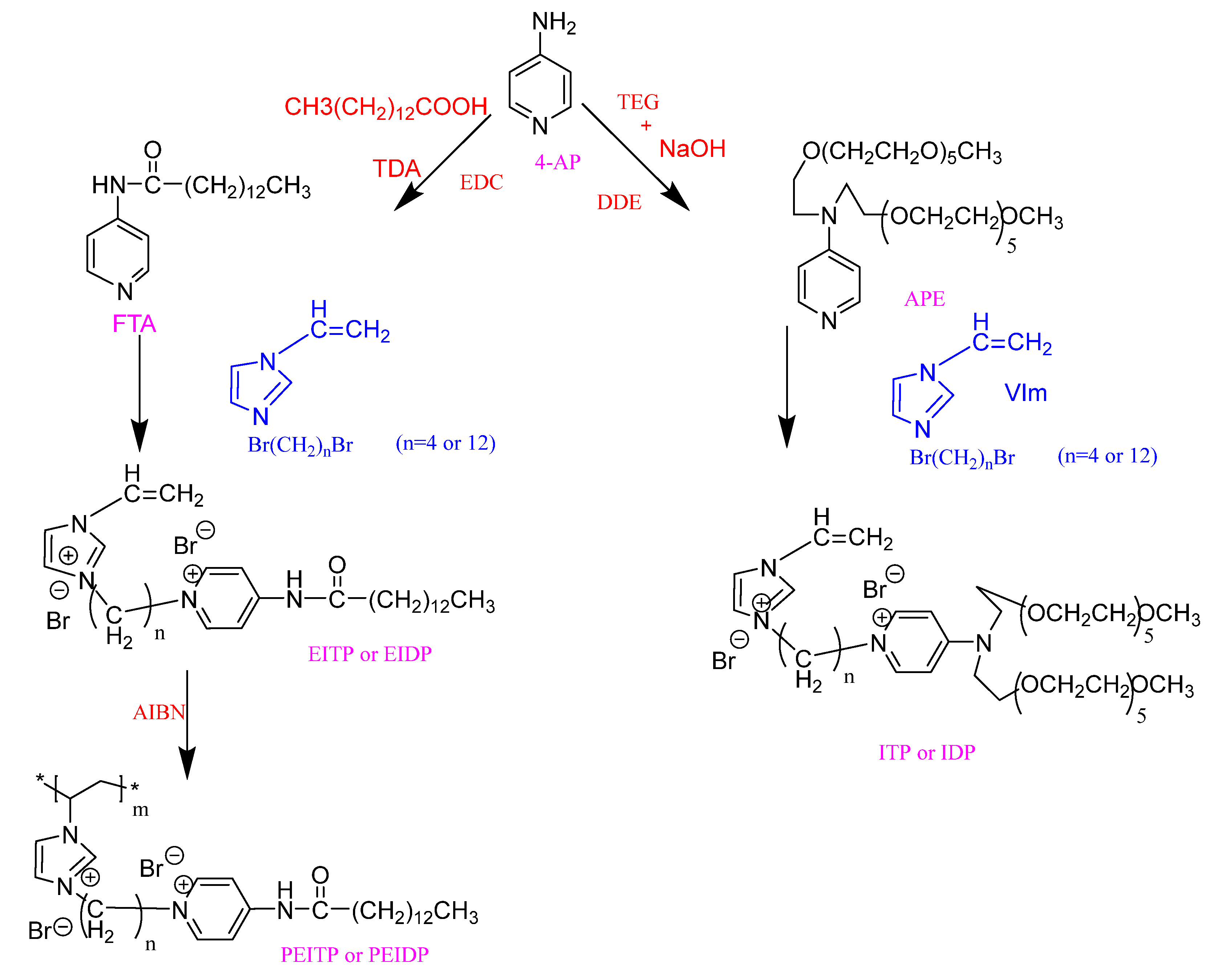


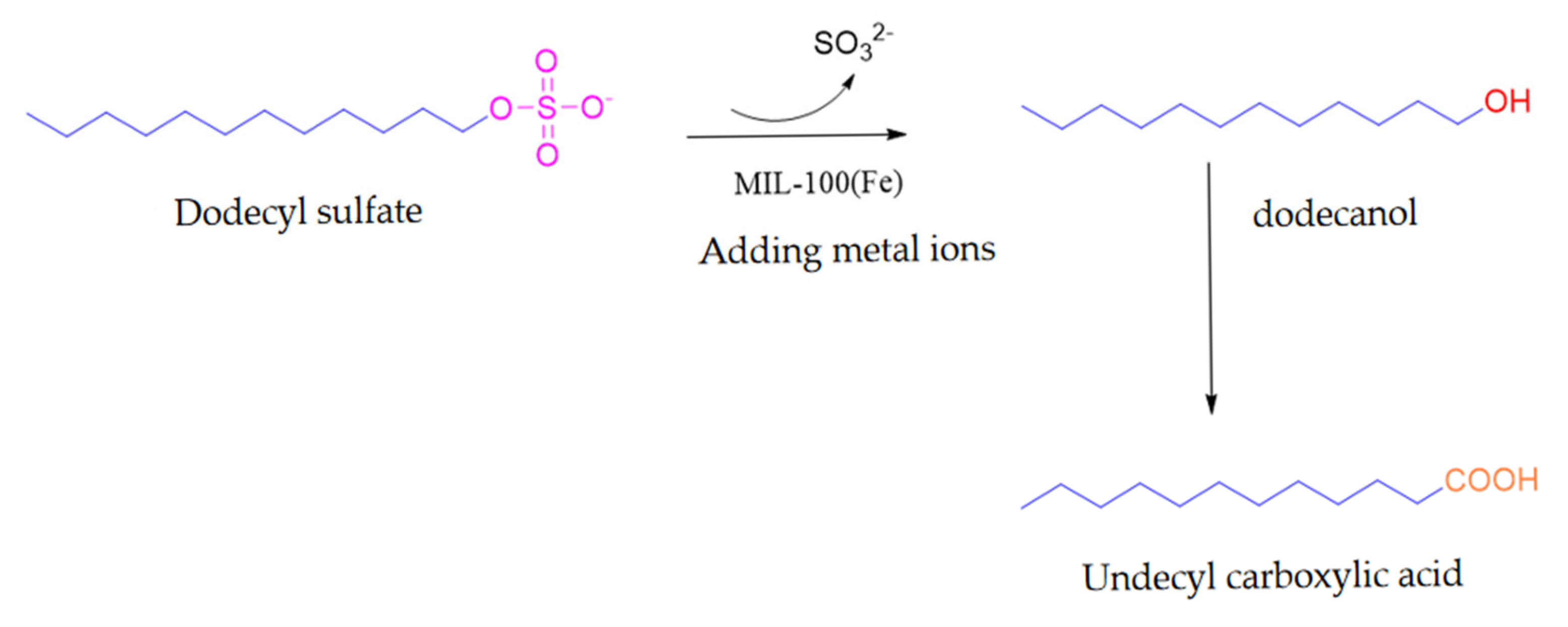
| Demulsifier | Demulsification Conditions | Demulsification Rate/% | CMC (mg/L) | Hydrophilic-Lipophilic Balance Value (HLB) | Cloud Point (°C) | Average Droplet Size (μm) | Interfacial Viscosity (mPa s) | Advantage | Disadvantage |
|---|---|---|---|---|---|---|---|---|---|
| Anionic demulsifier | 60 °C, 100 mg L−1 | 98.60 | 0.2–5.0 | 8–18 | 5–15 | 10–30 | Good effect and low cost | Poor adaptability | |
| 70 °C, 100 mg L−1 | 100 | ||||||||
| Non-ionic demulsifier | 60 °C, 400 mg L−1 | 94.7 | 2.0–50.0 | 10–16 | 40–100 | 2–5 | 5–15 | Strong adaptability and high efficiency | Large dosage, difficult to degrade |
| Room temperature, 400 mg L−1 | 92.6 | ||||||||
| Cationic demulsifier | 60 °C, 200 mg L−1 | 100 | 0.5–10.0 | 12–20 | 8–20 | 15–35 | Low cost, salt and acid alkali resistance | Long demulsification time | |
| Room temperature, 200 mg L−1 | 98.9 | ||||||||
| Natural demulsifier | 70 °C, 200 mg L−1 | 98.5 | 3.0–100.0 | 4–12 | 30–80 | 15–30 | 20–40 | Naturally degradable, with minimal harm | Non-recyclable |
| 60 °C, 200 mg L−1 | 94.6 | ||||||||
| Metal type demulsifier | Room temperature, 200 mg L−1 | 95.6 | 0.1–5.0 | 6–14 | 3–8 | 8–20 | High efficiency and recyclability | Incomplete recycling poses a safety hazard | |
| 40 °C, 200 mg L−1 | 97.8 |
| Demulsifier | Cost | Validity (Demulsification Rate) | Biodegradability | Suitability | Environmental Toxicity (EC50, mg/L) | Regulatory Compliance (REACH/EPA) |
|---|---|---|---|---|---|---|
| Anionic demulsifier | Low | Tall | General (partially refractory) | Poor (specific emulsion) | 12.5 | Partially restricted (REACH Annex XIV) |
| Non-ionic demulsifier | Medium to high (large dosage) | Tall | Poor (difficult to degrade) | Strong (widely applicable) | 8.3 | Meet EPA standards |
| Cationic demulsifier | Low | Polar altitude | General (partially degradable) | Medium (acid, alkali and salt resistance) | 5.1 | Need to declare (REACH) |
| Natural demulsifier | Medium (raw material restriction) | Tall | Excellent (naturally degradable) | Medium (to be optimized) | >100 | Full compliance (EPA/REACH) |
| Metal type demulsifier | Medium to high (recovery cost) | Polar altitude | Poor (partially recyclable) | Strong (resistant to complex conditions) | 2.4 | Limit (EPA heavy metal limit) |
| Demulsifier | Experimental Dose (ppm) | Industry Routine Dose (ppm) | Evaluate |
|---|---|---|---|
| Anionic demulsifier | 100–500 | 50–300 | The dosage of LA1 (500 ppm, 100%) is on the high side, which is high in efficiency, but may increase the cost. Need to optimize the compound to reduce the dosage. |
| Non-ionic demulsifier | 400–1500 | 200–800 | Star demulsifier (1500 ppm, 98%) far exceeds the industrial economic consumption (usually <1000 ppm), so its practicability is limited. |
| Cationic demulsifier | 200–500 | 100–400 | The dosage of MDBr-IL (500 ppm, 95.24%) is reasonable, but the demulsification time is long (180 min), so it is necessary to balance the efficiency and time cost. |
| Natural demulsifier | 200–2000 | 100–1000 | The dosage of DEMLOCS (2000 ppm, 88%) is too high, so it is necessary to optimize the extraction process of raw materials to reduce the dosage. |
| Metal type demulsifier | 200–300 | 50–200 | Feomnps (300 ppm, 97.83%) is close to the industrial standard, but incomplete recovery may increase the long-term cost. |
| Mechanism | Core of Action | Suitable Emulsion Type | Typical Demulsifier Types | Key Indicators |
|---|---|---|---|---|
| Displacement replacement | Interfacial molecular substitution | W/O; O/W | Anionic, nonionic type | Decreased range of interfacial tension, demulsification time |
| Bridge replacement | Polymer-bridged aggregated water droplets | W/O | Non-ionic (such as star polyether) | Flocculation size, sedimentation rate |
| Flocculation aggregation | Charge neutralization or adsorption coalescence | O/W | Cationic type, metal type | Zeta potential change, oil droplet coalescence efficiency |
| Competitive adsorption | Competitive adsorption of interfacial film | W/O | Cationic (complex branched structure) | Interfacial membrane strength, demulsifier adsorption capacity |
| Inverse phase change type | Emulsion type conversion | O/W→W/O | Reversed demulsifier | Emulsion type transition speed, separation thoroughness |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, L.; Wang, T.; Xu, Y.; Li, Y.; He, X.; Yan, A.; Tao, P.; Chen, G. Research Progress of Surfactant Demulsifier. Processes 2025, 13, 2087. https://doi.org/10.3390/pr13072087
Tang L, Wang T, Xu Y, Li Y, He X, Yan A, Tao P, Chen G. Research Progress of Surfactant Demulsifier. Processes. 2025; 13(7):2087. https://doi.org/10.3390/pr13072087
Chicago/Turabian StyleTang, Longhao, Tingyi Wang, Yingbiao Xu, Yongfei Li, Xinyi He, Aobo Yan, Peng Tao, and Gang Chen. 2025. "Research Progress of Surfactant Demulsifier" Processes 13, no. 7: 2087. https://doi.org/10.3390/pr13072087
APA StyleTang, L., Wang, T., Xu, Y., Li, Y., He, X., Yan, A., Tao, P., & Chen, G. (2025). Research Progress of Surfactant Demulsifier. Processes, 13(7), 2087. https://doi.org/10.3390/pr13072087






