Preparing and Characterizing Nano Relative Permeability Improver for Low-Permeability Reservoirs
Abstract
1. Introduction
2. Preparation of Nano Relative Permeability Improver
2.1. Single Factor Experimental Analysis
2.2. Two-Factor Experimental Analysis
3. Research on the Performance of Nano Relative Permeability Improver
3.1. Study on Rheological Properties
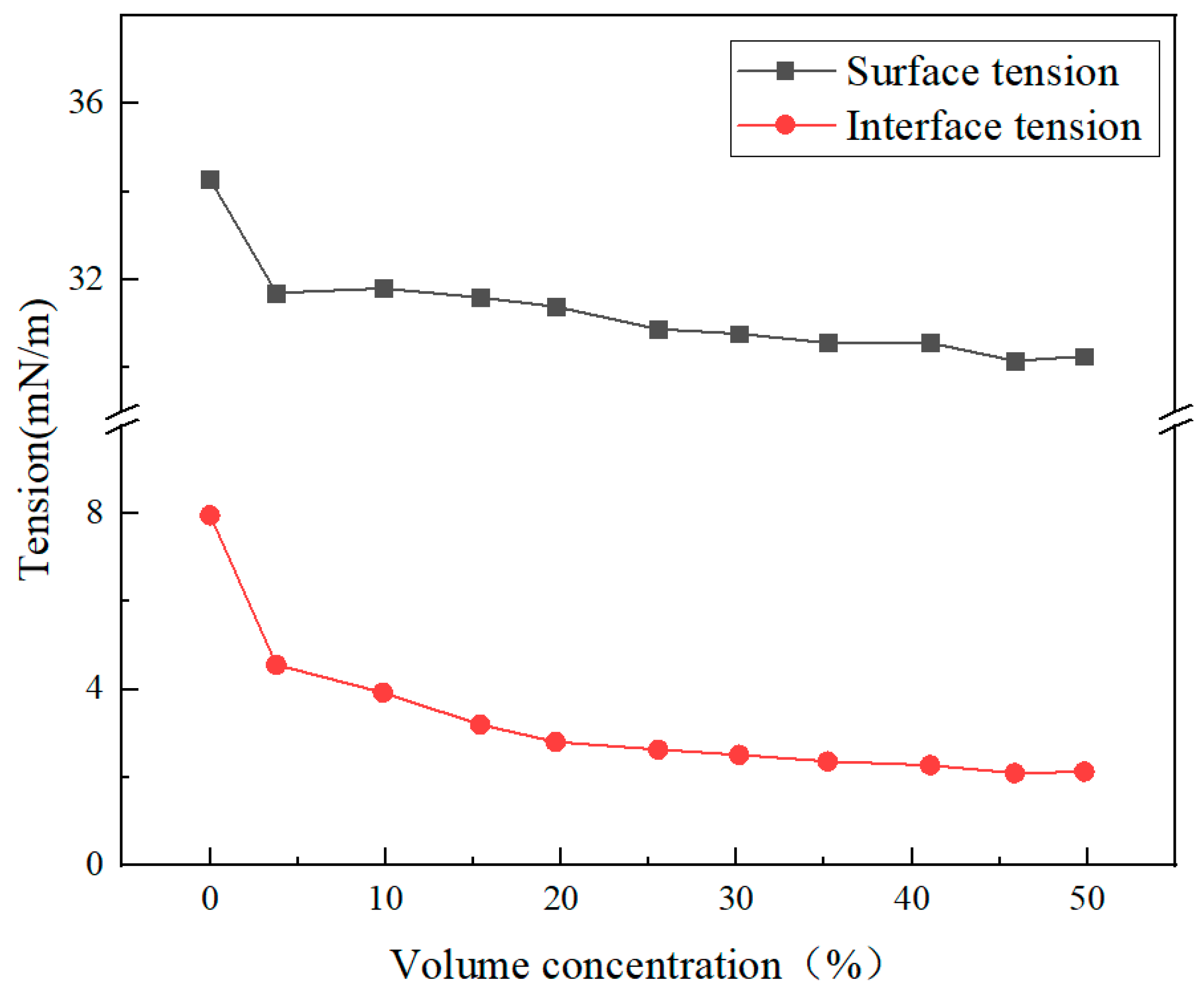
3.2. Interface Performance Research
3.3. Wetting Performance Study
3.4. Stability Performance Study
- (1)
- Temperature resistance performance
- (2)
- Shear resistance performance
- (3)
- Acid alkali resistance
- (4)
- Salt resistance performance
4. Relative Permeability Improvement Test
5. Conclusions
- (1)
- This article successfully developed a nano relative permeability improver suitable for low-permeability oil and gas reservoirs. The optimized formula includes a nano SiO2 content of 5.1%, a surfactant Span-80 content of 33%, a co-surfactant isobutanol content of 18%, and an additive NaCl content of 2%. After testing, the median particle size of the nano relative permeability improver is only 4.2 nm.
- (2)
- The rheological properties of the nano relative permeability improver are jointly affected by concentration and shear rate. The viscosity of high-concentration systems is significantly enhanced under low-shear conditions. At the same time, when the shear rate is greater than 10 s−1 in systems with different concentrations, the viscosity should be less than 30 mPs·s to ensure its injectability, making it suitable for oilfield permeability enhancement applications.
- (3)
- This improver has excellent wetting properties (contact angle between 50 and 84°), low surface tension (30–35 mN/m), and interfacial tension (3–8 mN/m), and gradually enhances wetting properties that tend to stabilize with increasing concentration, indicating that both tension and interfacial tension decrease, which can reduce capillary resistance and enhance fluid fluidity.
- (4)
- The temperature resistance, shear resistance, and acid–base resistance tests of the improver show that its stability meets the complex working conditions of low-permeability reservoirs. This research achievement provides reliable technical support for the efficient development of low-permeability oil and gas reservoirs.
- (5)
- Building on these laboratory successes, future work will focus on long-term stability studies under dynamic flow conditions (>6 months), detailed environmental impact assessments and cost optimization, and pilot-scale validation in representative well conditions to evaluate field performance.
Funding
Data Availability Statement
Conflicts of Interest
References
- Hosseini, E.; Hosseini, N.; Sarmadivaleh, M. Wettability modification effects on relative permeability end-points: Comparative analysis of surfactant agents for enhanced oil recovery. Pet. Res. 2024, 9, 206–218. [Google Scholar] [CrossRef]
- Xie, K.; Mei, J.; Cao, W.; Cao, B.; Yao, L.; Zhang, B.; Wang, H.; Guo, K.; Wu, Z.; Yan, K.; et al. Improving oil mechanism of polymer gel fracturing fluid based on filtration displacement. J. Pet. Sci. Eng. 2022, 218, 111030. [Google Scholar] [CrossRef]
- Li, J.; Wen, M.; Jiang, Z.; Xian, L.; Liu, J.; Chen, J. Development and characterization of a surfactant responsive to redox conditions for gas recovery in foam drainage. Sci. Rep. 2025, 15, 511. [Google Scholar] [CrossRef] [PubMed]
- Keykhosravi, A.; Vanani, M.B.; Aghayari, C. TiO2 nanoparticle-induced Xanthan Gum Polymer for EOR: Assessing the underlying mechanisms in oil-wet carbonates. J. Pet. Sci. Eng. 2021, 204, 108756. [Google Scholar] [CrossRef]
- Khattab, H.; Gawish, A.A.; Hamdy, A.; Gomaa, S.; El-Hoshoudy, A.N. Assessment of a novel xanthan gum-based composite for oil recovery improvement at reservoir conditions; Assisted with simulation and economic studies. J. Polym. Environ. 2024, 32, 3363–3391. [Google Scholar] [CrossRef]
- Gussenov, I.S.; Mukhametgazy, N.; Shakhvorostov, A.V.; Kudaibergenov, S.E. Comparative study of oil recovery using amphoteric terpolymer and hydrolyzed polyacrylamide. Polymers 2022, 14, 3095. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, L.; Wan, X.; Tang, Y.; Liu, Q.; Li, W.; Liao, J. Synthesis and Characterization of a Temperature-Sensitive Microcapsule Gelling Agent for High-Temperature Acid Release. ACS Omega 2024, 9, 20849–20858. [Google Scholar] [CrossRef]
- Wang, L.; Li, Z.; Adenutsi, C.D.; Wang, C. An experimental study of the effect of three metallic oxide nanoparticles on oil-water relative permeability curves derived from the JBN and extended JBN methods. J. Pet. Sci. Eng. 2020, 192, 107257. [Google Scholar] [CrossRef]
- Li, J.; Wen, M.; Jiang, Z.; Gao, S.; Xiao, X.; Xiang, C.; Tao, J. Formulation and characterization of surfactants with antibacterial and corrosion-inhibiting properties for enhancing shale gas drainage and production. Sci. Rep. 2025, 15, 2376. [Google Scholar] [CrossRef]
- Nazir, I.; Shahzadi, I.; Jalil, A.; Bernkop-Schnürch, A. Hydrophobic H-bond pairing: A novel approach to improve membrane permeability. Int. J. Pharm. 2020, 573, 118863. [Google Scholar] [CrossRef]
- Pogodin, S.; Werner, M.; Sommer, J.U.; Baulin, V.A. Nanoparticle-induced permeability of lipid membranes. Acs Nano 2012, 6, 10555–10561. [Google Scholar] [CrossRef]
- Roustaei, A. An evaluation of spontaneous imbibition of water into oil-wet carbonate reservoir cores using nanofluid. Petrophysics 2014, 55, 31–37. [Google Scholar]
- Al-Shargabi, M.; Davoodi, S.; Wood, D.A.; Rukavishnikov, V.S.; Minaev, K.M. Carbon dioxide applications for enhanced oil recovery assisted by nanoparticles: Recent developments. ACS Omega 2022, 7, 9984–9994. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jiang, Z.; Pu, H.; Ding, Y.; Du, J.; Wang, Y.; Shan, Y. Effect of the SiO2 nanoparticle size on application of enhanced oil recovery in medium-permeability formations. Energy Fuels 2023, 37, 5143–5153. [Google Scholar] [CrossRef]
- Doutsi, M.; Vlachou, M.C.; Koukiotis, C.; Kostoglou, M.; Karapantsios, T.D. Experimental Investigation of Stability of Emulsions Produced by Catastrophic Phase Inversion Using Non-Ionic Surfactants. Colloids Interfaces 2025, 9, 6. [Google Scholar] [CrossRef]
- Zhou, M.; Yuan, P.; Xu, X.; Yang, J. Construction of a Covalent Crosslinked Membrane Exhibiting Superhydrophilicity and Underwater Superoleophobicity for the Efficient Separation of High-Viscosity Oil–Water Emulsion Under Gravity. Molecules 2025, 30, 1840. [Google Scholar] [CrossRef]
- Tang, S.; Yang, X.; Wang, C.; Wang, C. Effects of Polyphenols on the Structure, Interfacial Properties, and Emulsion Stability of Pea Protein: Different Polyphenol Structures and Concentrations. Molecules 2025, 30, 1674. [Google Scholar] [CrossRef]
- Sun, J.; Wang, L.; Chen, H.; Yin, G. Enhancing Biodegradable Packaging: The Role of Tea Polyphenols in Soybean Oil Body Emulsion Films. Coatings 2025, 15, 162. [Google Scholar] [CrossRef]
- Xu, R.; Liao, L.; Liang, W.; Wang, H.; Zhou, Q.; Liu, W.; Chen, M.; Fang, B.; Wu, D.; Jin, H.; et al. Fast Removing Ligands from Platinum-Based Nanocatalysts by a Square-Wave Potential Strategy. Angew. Chem. Int. Ed. Engl. 2025, 5, e202509746. [Google Scholar]
- Cheng, T.; Yang, Z.; Yan, W.; Huang, B.; Wu, J.; Yang, E.; Liu, H.; Zhao, K. Impact of the nano-SiO2 particle size on oil recovery dynamics: Stability, interfacial tension, and viscosity reduction. Energy Fuels 2024, 38, 15160–15171. [Google Scholar] [CrossRef]
- Mousavi, M.A.; Hassanajili, S.; Rahimpour, M.R. Synthesis of fluorinated nano-silica and its application in wettability alteration near-wellbore region in gas condensate reservoirs. Appl. Surf. Sci. 2013, 273, 205–214. [Google Scholar] [CrossRef]
- Feng, R.; Dong, J.; Dang, L. Numerical simulation and evaluation of residual oil saturation in waterflooded reservoirs. Fuel 2025, 384, 134018. [Google Scholar]
- Wang, P.; Li, S.; Li, Y.; Feng, X.; Xie, C.; Wan, Z.; Wang, M.; Zhou, H.; Ma, P. Experimental research on rheological and mechanical properties of nano silica sol grout. J. Sol-Gel Sci. Technol. 2019, 91, 178–188. [Google Scholar] [CrossRef]
- Viades-Trejo, J.; Gracia-Fadrique, J. Spinning drop method: From Young–Laplace to Vonnegut. Colloids Surf. A Physicochem. Eng. Asp. 2007, 302, 549–552. [Google Scholar] [CrossRef]
- Sun, L.; Jiang, Z.; Yuan, B.; Zhi, Y.; Li, J.; Wu, A. Ultralight and superhydrophobic perfluorooctyltrimethoxysilane modified biomass carbonaceous aerogel for oil-spill remediation. Chem. Eng. Res. Des. 2021, 174, 71–78. [Google Scholar] [CrossRef]
- Wang, S.; Gu, Z.; Guo, P.; Zhao, W. Comparative laboratory wettability study of sandstone, Tuff, and shale using 12-MHz NMR T1-T2 fluid typing: Insight of shale. SPE J. 2024, 29, 4781–4803. [Google Scholar] [CrossRef]














| Property | This Work | Nanofluid [13] | Polymer-Based Improver [6] |
|---|---|---|---|
| Particle size (nm) | 4.2 | 64~316 | Not Applicable (N.A.) |
| Interfacial tension (mN/m) | 3–8 | 8–9.5 | N.A. |
| Temperature tolerance (°C) | ≤90 | ≤60 | ≤60 |
| Salinity tolerance (mg/L) | ≤60,000 | ≤50,000 | ≤300,000 |
| viscosity (mPa·s) | 7.5–23.6 | 0.84–1.14 | 3.25–26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B. Preparing and Characterizing Nano Relative Permeability Improver for Low-Permeability Reservoirs. Processes 2025, 13, 2071. https://doi.org/10.3390/pr13072071
Li B. Preparing and Characterizing Nano Relative Permeability Improver for Low-Permeability Reservoirs. Processes. 2025; 13(7):2071. https://doi.org/10.3390/pr13072071
Chicago/Turabian StyleLi, Bo. 2025. "Preparing and Characterizing Nano Relative Permeability Improver for Low-Permeability Reservoirs" Processes 13, no. 7: 2071. https://doi.org/10.3390/pr13072071
APA StyleLi, B. (2025). Preparing and Characterizing Nano Relative Permeability Improver for Low-Permeability Reservoirs. Processes, 13(7), 2071. https://doi.org/10.3390/pr13072071






