Phytochemical Composition and Evaluation of Antimicrobial Activities of Five Salvia Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Samples
2.2. Extraction of Essential Oil
2.2.1. GC-MS Analysis
2.2.2. GC Analysis
2.3. Identification of Components
2.4. Preparation of Methanol Extract
2.5. LC-MS/MS Analysis
2.6. Antimicrobial Evaluation
3. Results
3.1. Essential Oil
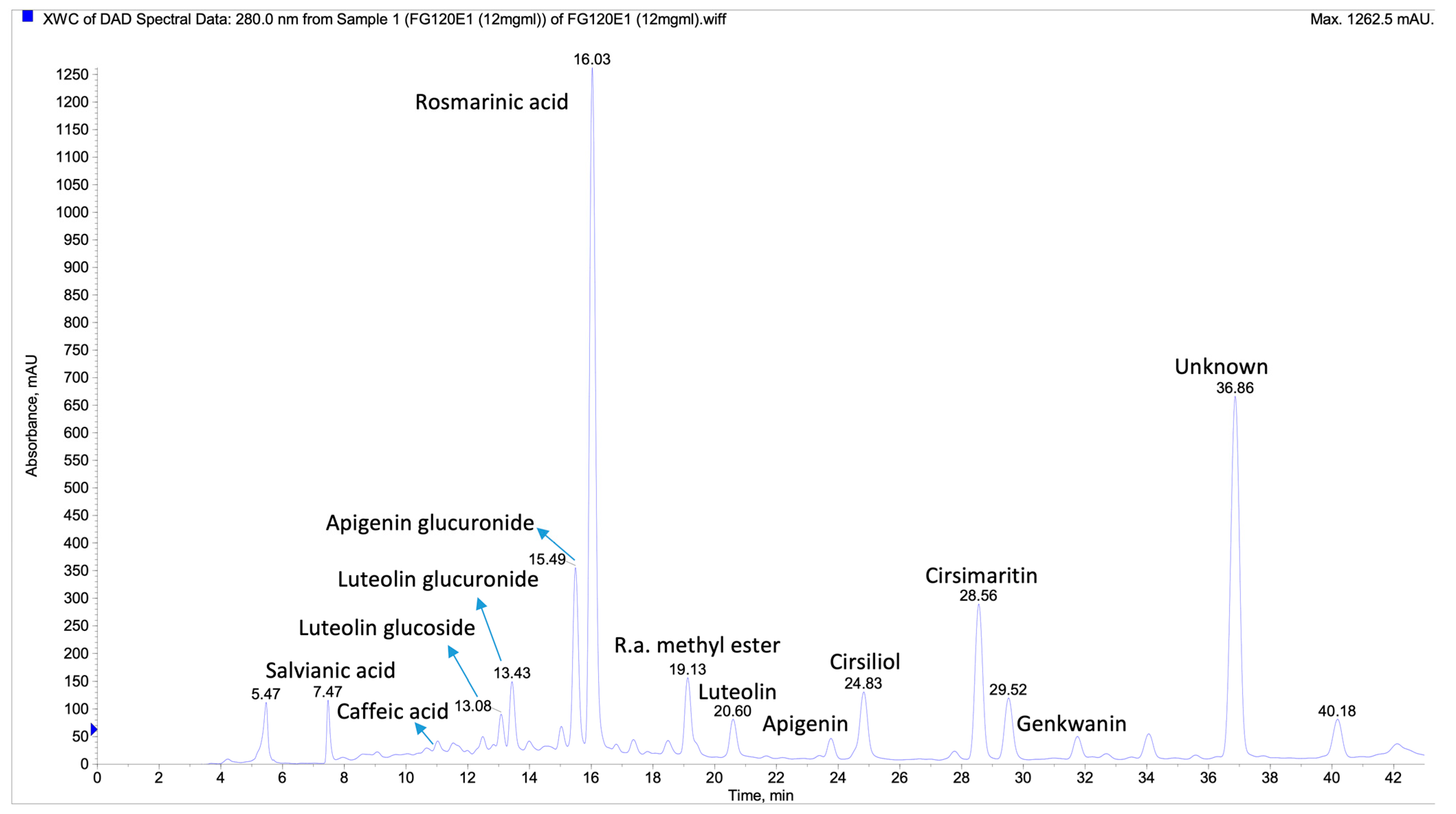
3.2. LC-MS/MS Analysis of the Extracts
3.3. Antimicrobial Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- WFO The World Flora Online. Available online: https://www.worldfloraonline.org/search?query=Salvia (accessed on 7 June 2023).
- Bizim Bitkiler. Available online: https://www.bizimbitkiler.org.tr/v2/filtre.php?familya=Lamiaceae&taxon=&taxonKriter=0&epitetType=0&epitet=&epitetKriter=0&authorType=0&author=&authorKriter=0&endemik=evet&sayfa=1&trkAdi=&trkKriter=0 (accessed on 7 June 2023).
- Wu, Y.; Ni, Z.; Shi, Q.; Dong, M.; Hiromasa, K.; Gu, Y.-C.; Cong, B. Constituents from Salvia Species and Their Biological Activities. Chem. Rev. 2012, 112, 5967–6026. [Google Scholar] [CrossRef] [PubMed]
- Demirpolat, A. Essential Oil Composition Analysis, Antimicrobial Activities, and Biosystematic Studies on Six Species of Salvia. Life 2023, 13, 634. [Google Scholar] [CrossRef]
- Hayta, S.; Korkmaz, M. Composition of the essential oil of two Salvia taxa (Salvia sclarea and Salvia verticillata subsp. verticillata) from Turkey. Nat. Sci. Discov. 2015, 5, 62–67. [Google Scholar] [CrossRef][Green Version]
- Kunduhoğlu, B.; Keleş, M.S.; Çelik, H. Antimicrobial and anticholinesterase activities of the essential oils isolated from Salvia dicroantha Stapf., Salvia verticillata L. subsp. amasiaca (Freyn and Bornm.) Bornm. and Salvia wiedemannii Boiss. J. Med. Plants Res. 2011, 5, 2268–2273. [Google Scholar]
- Golparvar, A.R.; Mohammadi, S. Chemical composition and antimicrobial activity of essential oil of Salvia officinalis L. and Salvia virgata Jacq. J. Herb. Drugs 2017, 8, 71–78. [Google Scholar] [CrossRef]
- Mathew, J.; Thoppil, J.E. Chemical composition and mosquito larvicidal activities of Salvia essential oils. Pharm. Biol. 2011, 49, 385–392. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abu-Irmaileh, B.E.; Afifi, F.U. Herbal medicine in Jordan with special emphasis on commonly used herbs. J. Ethnopharmacol. 2003, 89, 193–197. [Google Scholar] [CrossRef]
- Mozaffarian, V. A Dictionary of Iranian Plant Names: Latin, English, Persian; Farhang Moaser Publishers: Tehran, Iran, 1996. [Google Scholar]
- Baytop, T. Türkiye’de Bitkiler ile Tedavi: Geçmişte ve Bugün; Nobel Tıp Kitabevi: İstanbul, Turkey, 1999. [Google Scholar]
- Kintzios, S.E. Sage: The Genus Salvia; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Perry, E.K.; Pickering, A.T.; Wang, W.W.; Houghton, P.J.; Perry, N.S. Medicinal plants and Alzheimer’s disease: From ethnobotany to phytotherapy. J. Pharm. Pharmacol. 2000, 52, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Ulubelen, A. Biological activities of Turkish Labiatae species. Pure Appl. Chem. 2000, 72, 1393–1398. [Google Scholar]
- Smith, J.; Rodriguez, L.; Patel, K.; Zhang, M. Phytochemical analysis and bioactivity of Salvia albimaculata essential oil. Nat. Prod. Res. 2018, 32, 895–902. [Google Scholar]
- Johnson, R.; Thompson, A.; Williams, B.; Kim, D. Camphor and borneol as major constituents of Salvia blepharochlaena essential oil with antifungal potential. Phytother. Res. 2020, 34, 1234–1241. [Google Scholar]
- Ahmed, M.; Gonzalez, L.; Brown, C.; Martin, S. Chemical composition and antifungal activity of Salvia palaestina essential oil. J. Essent. Oil Res. 2017, 29, 245–252. [Google Scholar]
- Khan, S.; Rivera, J.; Adams, T.; Mitchell, P. Thymol and carvacrol in Salvia cryptantha essential oil: Antimicrobial and anticandidal effects. Mycopathologia 2019, 184, 567–576. [Google Scholar]
- Brown, T.; Lee, K.; Nelson, H.; Wang, J. Antioxidant and chemical properties of Salvia albimaculata essential oil. Antioxidants 2019, 8, 102. [Google Scholar]
- Lee, H.; Gonzalez, R.; Carter, M.; Thompson, J. Antibacterial properties of Salvia blepharochlaena essential oil: A chemical and biological study. J. Appl. Microbiol. 2021, 131, 978–987. [Google Scholar]
- White, D.; Anderson, P.; Lewis, R.; Green, S. Traditional uses and bioactive compounds of Salvia palaestina essential oil. Ethnopharmacol. J. 2020, 12, 214–221. [Google Scholar]
- Green, P.; Mitchell, T.; Parker, B.; Hernandez, L. Chemical composition and antifungal properties of Salvia cryptantha essential oil. Phytomedicine 2018, 45, 85–92. [Google Scholar]
- Karpiński, T.M.; Ożarowski, M.; Seremak-Mrozikiewicz, A.; Wolski, H.; Adamczak, A. Plant preparations and compounds with activities against biofilms formed by Candida spp. J. Fungi 2021, 7, 360. [Google Scholar] [CrossRef]
- Bardaweel, S.K.; Darwish, R.M.; Alzweiri, M.; Al-Hiari, Y. Synergism and efficacy of some naturally occurring D-amino acids against clinically relevant bacterial and fungal species. Jord. J. Pharm. Sci. 2014, 7, 1–10. [Google Scholar]
- Cui, H.; Wang, Y. Antimicrobial activity and mechanisms of Salvia sclarea essential oil. Bot. Stud. 2015, 56, 16. [Google Scholar] [CrossRef]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data 2011, 40, 1–47. [Google Scholar] [CrossRef]
- Pubchem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/ (accessed on 11 April 2025).
- Webook. Available online: https://webbook.nist.gov/chemistry/ (accessed on 11 April 2025).
- The Pherobase 2024. Available online: http://www.pherobase.com/database/kovats/kovats-detailsulcatone.php (accessed on 11 April 2025).
- Wiley, R. Mass Spectral Library, 11th ed.; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar]
- Hochmuth, D.H. MassFinder 4.0; Hochmuth Scientific Consulting: Hamburg, Germany, 2008. [Google Scholar]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 2nd ed.; Approved Standard; CLSI Document M7-A7; CLSI: Wayne, PA, USA, 2006. [Google Scholar]
- NCCLS. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 2nd ed.; Approved Standard; NCCLS Document M27-A2; NCCLS: Wayne, PA, USA, 2002. [Google Scholar]
- Zengin, G.; Mollica, A.; Locatelli, M.; Stefanucci, A.; Novellino, E.; Macedonio, G.; Menghini, L.; Recinella, L.; Leone, S.; Brunetti, L.; et al. Chemical composition and biological activities of extracts from three Salvia species: S. blepharochlaena, S. euphratica var. leiocalycina, and S. verticillata subsp. amasiaca. Ind. Crops Prod. 2018, 111, 11–21. [Google Scholar] [CrossRef]
- Gulsoy Toplan, G.; Aydin, A.; Calis, I.; Saracoglu, I.; Goger, F.; Demirci, B.; Demirci, F.; Baser, K.H.C. Composition and biological activities of Salvia veneris Hedge growing in Cyprus. Ind. Crops Prod. 2017, 97, 41–48. [Google Scholar] [CrossRef]
- Miski, M.; Mabry, T.J.; Ohtani, K.; Kasai, R.; Tanaka, O. Antibacterial activity studies of flavonoids from Salvia palaestina. J. Nat. Prod. 1983, 46, 874–875. [Google Scholar] [CrossRef] [PubMed]
- Gürbüz, P.; Demirezer, L.Ö.; Kuruüzüm-Uz, A.; Güvenalp, Z.; Kazaz, C.; Secen, H.; Sonmez, U.; Demirtas, I.; Yigit, D.; Goren, A.C.; et al. In vitro biological activity of Salvia fruticosa Mill. infusion against amyloid β-peptide-induced toxicity and inhibition of GSK-3β, CK-1δ, and BACE-1 enzymes relevant to Alzheimer’s disease. Saudi Pharm. J. 2021, 29, 236–243. [Google Scholar] [CrossRef]
- Karatoprak, G.Ş.; Yildirim, A.B.; Yilmaz, M.A.; Celep, F.; Demirtas, I.; Goren, A.C. Phytochemical profile, antioxidant, antiproliferative, and enzyme inhibition-docking analyses of Salvia ekimiana Celep & Doğan. S. Afr. J. Bot. 2022, 146, 36–47. [Google Scholar] [PubMed]
- Marder, M.; Viola, H.; Wasowski, C.; Wolfman, C.; Waterman, P.; Medina, J.H.; Paladini, A.C. Cirsiliol and caffeic acid ethyl ester, isolated from Salvia guaranitica, are competitive ligands for the central benzodiazepine receptors. Phytomedicine 1996, 3, 29–31. [Google Scholar] [CrossRef]
- Al-Qudah, M.A.; Al-Ramamneh, E.A.; Abu Zarga, M.H.; Hafez, S.; Abu Orabi, S.T. Flavonoid and phenolic compounds from Salvia palaestina L. growing wild in Jordan and their antioxidant activities. Phytochemistry 2014, 99, 115–120. [Google Scholar] [CrossRef]
- Göğer, F.; Demirci, B.; Gören, A.C.; Demirci, F. Phenolic compounds determination and antioxidant activity of Teucrium cavernarum. Eskisehir Tech. Univ. Sci. Technol. J. C Life Sci. Biotechnol. 2019, 8, 229–237. [Google Scholar] [CrossRef]
- Kang, Y.-J.; Jung, U.J.; Lee, M.K.; Kim, H.J.; Jeong, T.S.; Choi, M.S. Eupatilin, isolated from Artemisia princeps Pampanini, enhances hepatic glucose metabolism and pancreatic β-cell function in type 2 diabetic mice. Diabetes Res. Clin. Pract. 2008, 82, 25–32. [Google Scholar] [CrossRef]
- Pacifico, S.; D’Abrosca, B.; Scognamiglio, M.; Gallicchio, M.; Potenza, N.; Piccolella, S. A polyphenol complex from Thymus vulgaris L. plants cultivated in the Campania Region (Italy): New perspectives against neuroblastoma. J. Funct. Foods 2016, 20, 253–266. [Google Scholar] [CrossRef]
- Kontogianni, V.G.; Tomic, G.; Nikolic, I.; Nerantzaki, A.A.; Sayyad, N.; Stosic-Grujicic, S.; Stojanovic, I.; Gerothanassis, I.P.; Tzakos, A.G. Phytochemical profile of Rosmarinus officinalis and Salvia officinalis extracts and correlation to their antioxidant and anti-proliferative activity. Food Chem. 2013, 136, 120–129. [Google Scholar] [CrossRef] [PubMed]
- de Rijke, E.; Out, P.; Niessen, W.M.; Ariese, F.; Gooijer, C.; Brinkman, U.A. Analytical separation and detection methods for flavonoids. J. Chromatogr. A 2006, 1112, 31–63. [Google Scholar] [CrossRef]
- Bozkurt, M.; Kaya, A.; Yılmaz, H.; Şahin, H.; Demirci, B.; Baser, K.H.C. Chemical characterization of Salvia albimaculata essential oil. Phytochem. Rev. 2017, 16, 625–638. [Google Scholar]
- Sellami, I.H.; Maamouri, E.; Chahed, T.; Wannes, W.A.; Kchouk, M.E.; Marzouk, B. Antioxidant and antimicrobial activities of Salvia essential oils. Food Chem. 2009, 120, 671–678. [Google Scholar]
- Kaya, A.; Demirci, B.; Başer, K.H.C.; Kürkçüoğlu, M. The volatile composition of Salvia blepharochlaena. Nat. Prod. Res. 2015, 29, 1155–1162. [Google Scholar]
- Abu-Darwish, M.S.; Cabral, C.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Al-Bdour, T.H.; Salgueiro, L. Essential oil composition and biological activity of Salvia palaestina. J. Ethnopharmacol. 2014, 155, 1000–1006. [Google Scholar]
- Demirci, B.; Tabanca, N.; Başer, K.H.C. Chemical diversity of Salvia virgata essential oil. Ind. Crops Prod. 2013, 50, 500–506. [Google Scholar]
- Dudai, N.; Putievsky, E.; Lerner, A.; Ravid, U.; Palevitch, D.; Lewinsohn, E. The ethnobotanical and pharmacological significance of Salvia essential oils. J. Ethnopharmacol. 2005, 98, 201–208. [Google Scholar]
- Tumen, I.; Baser, K.H.C.; Kirimer, N.; Demirci, B.; Kürkçüoğlu, M. Chemical composition of Salvia cryptantha essential oil. Nat. Prod. Commun. 2012, 7, 1365–1370. [Google Scholar]
- Wang, W.; Li, N.; Luo, M.; Zu, Y.; Efferth, T. The pharmacological effects of borneol in Salvia species. Nat. Prod. Res. 2007, 21, 678–686. [Google Scholar]
- Chokheli, V.A.; Butenko, E.V.; Pokudina, I.O.; Firsova, I.M.; Azarov, A.S.; Belyaev, M.O.; Tsymerskaia, C.A.; Sushkova, S.N. Analysis of Phytoextracts of Medicinal Plants for Antimicrobial Activity Against Pathogenic Strains of Microorganisms. bioRxiv 2025. preprint. [Google Scholar] [CrossRef]
- Kowalska-Krochmal, B.; Dudek-Wicher, R. The Minimum Inhibitory Concentration of Antibiotics: Methods, Interpretation, Clinical Relevance. Pathogens 2021, 10, 165. [Google Scholar] [CrossRef] [PubMed]
- Aparecida Purgato, G.; Soares Píccolo, M.; Aparecida Scatamburlo Moreira, M.; Ramos Pizziolo, V.; Diaz-Muñoz, G.; Rossi, C.C.; Alves Nogueira Diaz, M. Isolation and identification of antimicrobial multicyclic terpenoids from the medicinal plant Salvia officinalis and development of a formulation against clinical Staphylococcus aureus strains. Lett. Appl. Microbiol. 2024, 77, ovae077. [Google Scholar]
- Altuwaijri, N.; Almutairi, S.M.; Almujayri, R.S.; Elbehairi, S.E.A.I.; Elbehairi, S.E.A.A. Enhancing antibacterial activity through green synthesis of silver nanoparticles with Salvia officinalis extracts. Int. J. Adv. Appl. Sci. 2024, 11, 19–23. [Google Scholar] [CrossRef]
| Species | Collection Sites | Coordinate | Voucher Number |
|---|---|---|---|
| S. absconditiflora | Türkiye, Niğde, Meydan Plateau, July 2015 | 37°26′18″ N 34°35′16″ E | YBK1606 |
| S. blepharochlaena | Türkiye, Nevşehir, 1 km to Avanos, jipsy area May 2014 | 38°43′03″ N 34°53′54″ E | ESSE 15003 |
| S. albimaculata | Türkiye, Niğde, Maden village July 2015 | 37°28′20″ N 34°35′14″ E | ESSE 15079 |
| S. palaestina | Türkiye, Urfa, Göbeklitepe, May 2014 | 37°13′20″ N 38°55′05″ E | YBK1612 |
| S. virgata | Türkiye, İçel, Çamlıyayla road, May 2014 | 37°38′51″ N 34°44′36″ E | ESSE 15002 |
| RRI a | RRI b | Compound | Sa % | Sb % | Sab % | Sp % | Sv % | IM |
|---|---|---|---|---|---|---|---|---|
| 1008–1039 c | 1032 | α-Pinene | 12.0 | 3.7 | 4.8 | - | 9.0 | tR, MS |
| 1012–1039 c | 1035 | α-Thujene | 0.7 | 0.3 | - | - | 0.8 | MS |
| 1043–1086 c | 1076 | Camphene | 0.8 | 4.6 | 4.2 | - | 3.5 | tR, MS |
| 1085–1130 c | 1118 | β-Pinene | 2.1 | 7.3 | 11.0 | - | 8.0 | tR, MS |
| 1098–1140 c | 1132 | Sabinene | 13.5 | 0.8 | - | - | 0.4 | tR, MS |
| 1140–1175 c | 1174 | Myrcene | 1.2 | 1.4 | 2.0 | 0.4 | 1.2 | tR, MS |
| 1148–1186 c | 1176 | α-Phellandrene | - | 0.1 | 0.1 | - | 0.5 | tR, MS |
| 1137–1183 d | 1183 | Pseudolimonene | 0.4 | - | - | - | - | MS |
| 1154–1195 c | 1188 | α-Terpinene | 0.9 | - | - | - | 0.2 | tR, MS |
| 1178–1219 c | 1203 | Limonene | 1.4 | 2.4 | 6.2 | 0.1 | 2.0 | tR, MS |
| 1186–1231 c | 1213 | 1,8-Cineole | 1.7 | 2.0 | 30.2 | - | - | tR, MS |
| 1188–1233 c | 1218 | β-Phellandrene | - | - | - | - | 2.0 | tR, MS |
| 1211–1251 c | 1246 | (Z)-β-Ocimene | <0.1 | <0.1 | 0.1 | 0.9 | MS | |
| 1222–1266 c | 1255 | γ-Terpinene | 3.4 | 0.1 | 0.3 | - | 0.7 | tR, MS |
| 1232–1267 c | 1266 | (E)-β-Ocimene | 0.1 | 0.4 | - | 0.1 | 0.2 | MS |
| 1246–1291 c | 1280 | p-Cymene | 3.8 | 0.5 | 2.8 | - | 1.5 | tR, MS |
| 1280–1312 d | 1285 | Isoamyl isovalerate | - | - | - | 0.4 | - | MS |
| 1261–1300 c | 1290 | Terpinolene | 0.5 | <0.1 | - | 0.3 | tR, MS | |
| 1286–1334 d | 1299 | 2-Methylbutyl isovalerate | - | - | - | 0.9 | - | MS |
| 1411–1465 c | 1452 | 1-Octen-3-ol | - | 0.3 | - | - | 0.3 | MS |
| 1425–1478 c | 1474 | cis-Sabinene hydrate | 1.2 | 0.4 | - | - | 6.0 | MS |
| 1462–1522 c | 1497 | α-Copaene | 0.8 | 10.1 | - | 1.2 | - | tR, MS |
| 1500 e | 1499 | α-Campholene aldehyde | 0.2 | - | - | - | - | tR, MS |
| 1481–1537 c | 1532 | Camphor | 1.0 | 28.5 | 8.7 | - | 14.6 | tR, MS |
| 1496–1546 c | 1535 | β-Bourbonene | 0.9 | - | - | 0.3 | - | MS |
| 1507–1564 c | 1553 | Linalool | 2.3 | 0.3 | 0.8 | 26.5 | - | tR, MS |
| 1526–1565 c | 1556 | trans-Sabinene hydrate | 0.8 | 0.3 | - | - | 1.7 | MS |
| 1532–1570 c | 1565 | Linalyl acetate | 2.3 | - | - | 20.2 | - | tR, MS |
| 1560–1590 c | 1568 | trans-α-Bergamotene | - | - | - | 0.3 | - | MS |
| 1557–1625 c | 1571 | trans-p-Menth-2-en-1-ol | 0.4 | - | - | - | 0.2 | MS |
| 1545–1590 c | 1586 | Pinocarvone | 0.3 | 0.1 | - | - | - | tR, MS |
| 1588–1610 c | 1588 | Bornyl formate | 0.3 | - | - | - | - | MS |
| 1547–1589 c | 1589 | β-Ylangene | - | - | - | <0.1 | - | MS |
| 1549–1597 c | 1591 | Bornyl acetate | 0.4 | 6.4 | - | 1.0 | tR, MS | |
| 1565–1608 c | 1600 | β-Elemene | - | - | - | <0.1 | - | tR, MS |
| 1610 | Calarene | 0.5 | - | - | - | - | MS | |
| 1564–1630 c | 1611 | Terpinen-4-ol | 4.1 | 0.5 | 1.1 | - | 2.9 | tR, MS |
| 1569–1632 c | 1612 | β-Caryophyllene | 2.5 | <0.1 | 5.5 | 7.8 | 3.0 | tR, MS |
| 1597–1648 c | 1648 | Myrtenal | 0.4 | 0.1 | - | - | - | MS |
| 1624–1668 c | 1661 | Alloaromadendrene | - | <0.1 | - | - | - | MS |
| 1647–1668 c | 1663 | cis-Verbenol | 0.3 | - | - | - | MS | |
| 1643–1671 c | 1670 | trans-Pinocarveol | 4.5 | 0.1 | - | - | 0.4 | tR, MS |
| 1655–1687 c | 1682 | δ-Terpineol | - | - | 0.7 | - | - | MS |
| 1643–1671 c | 1683 | trans-Verbenol | 2.1 | <0.1 | - | - | 0.2 | MS |
| 1635–1675 c | 1684 | Isoborneol | - | - | - | - | 0.3 | MS |
| 1637–1689 c | 1687 | α-Humulene | - | - | - | 0.4 | - | tR, MS |
| 1644–1690 c | 1690 | Cryptone | 2.4 | - | - | - | - | MS |
| 1655–1714 c | 1704 | γ-Muurolene | - | - | 0.3 | - | - | MS |
| 1659–1724 c | 1706 | α-Terpineol | 1.5 | - | 1.8 | 2.3 | 3.5 | tR, MS |
| 1672–1718 c | 1709 | α-Terpinyl acetate | - | - | 1.2 | - | - | tR, MS |
| 1653–1728 c | 1719 | Borneol | 7.2 | 10.6 | 5.1 | - | 19.5 | tR, MS |
| 1676–1726 c | 1726 | Germacrene D | 1.9 | - | - | 2.3 | 0.8 | MS |
| 1693–1740 c | 1733 | Neryl acetate | - | - | - | 1.0 | - | tR, MS |
| 1687–1770 c | 1744 | Phellandral | 0.4 | - | - | - | - | MS |
| 1699–1751 c | 1751 | Carvone | 0.3 | - | - | - | - | tR, MS |
| 1692–1757 c | 1755 | Bicyclogermacrene | 0.4 | - | - | 0.1 | 0.2 | MS |
| 1668–1771 c | 1758 | cis-Piperitol | 0.3 | 0.3 | - | - | - | MS |
| 1728–1772 c | 1765 | Geranyl acetate | 0.4 | - | - | 2.3 | - | tR, MS |
| 1722–1774 c | 1773 | δ-Cadinene | 0.4 | 2.8 | 0.1 | - | - | MS |
| 1735–1782 c | 1776 | γ-Cadinene | 0.7 | 1.4 | <0.1 | - | - | MS |
| 1743–1788 c | 1786 | ar-Curcumene | 0.6 | 0.1 | - | - | - | MS |
| 1782–1805 d | 1793 | α-Campholene alcohol | - | - | 1.1 | - | - | MS |
| 1747–1805 c | 1802 | Cumin aldehyde | 1.2 | - | - | - | - | tR, MS |
| 1743–1808 c | 1804 | Myrtenol | 0.1 | 0.1 | - | - | 0.2 | MS |
| 1734–1803 c | 1807 | α-Cadinene | - | 0.1 | - | - | - | MS |
| 1752–1832 c | 1808 | Nerol | - | - | - | 0.5 | - | tR, MS |
| 1788–1825 c | 1815 | 2-Tridecanone | - | 0.2 | - | - | - | MS |
| 1784–1851 c | 1838 | 2-Phenylethyl acetate | 0.5 | - | - | - | - | MS |
| 1800–1853 c | 1853 | cis-Calamenene | - | 1.4 | - | - | - | MS |
| 1795–1865 c | 1857 | Geraniol | 0.3 | - | - | 1.6 | - | tR, MS |
| 1813–1865 c | 1864 | p-Cymen-8-ol | 0.3 | - | - | - | - | tR, MS |
| 1820–1873 c | 1868 | (E)-Geranyl acetone | 0.1 | 0.1 | - | - | - | MS |
| 1868–1900 d | 1893 | Dodecyl acetate | - | 0.1 | - | - | - | MS |
| 1854–1928 c | 1900 | epi-Cubebol | 0.2 | - | - | 0.3 | - | MS |
| 1884–1964 c | 1957 | Cubebol | 1.0 | 0.2 | - | 0.1 | - | MS |
| 1924–1980 c | 1973 | 1-Dodecanol | - | 0.1 | - | - | - | MS |
| 1959–2003 d | 2001 | Isocaryophyllene oxide | - | - | - | 0.3 | 0.3 | MS |
| 1936–2023 c | 2008 | Caryophyllene oxide | 3.0 | 0.9 | 2.2 | 4.6 | 4.7 | tR, MS |
| 1995–2055 c | 2050 | (E)-Nerolidol | - | 0.3 | - | - | - | MS |
| 2016–2043 c | 2037 | Salvial-4(14)-en-1-one | - | - | - | - | 0.2 | MS |
| 2003–2071 c | 2071 | Humulene epoxide-II | - | 0.3 | - | - | 0.2 | MS |
| 2068–2115 d | 2113 | Cumin alcohol | 0.7 | - | - | - | - | tR, MS |
| 2074–2150 c | 2144 | Spathulenol | 2.0 | 5.9 | <0.1 | 1.7 | 3.4 | MS |
| 2168 d | 2161 | Muurola-4,10(14)-dien-1-ol | - | 1.1 | - | - | - | MS |
| 2100–2198 c | 2186 | Eugenol | 0.2 | - | - | - | - | tR, MS |
| 2136–2198 e | 2187 | T-Cadinol | 0.7 | - | - | - | - | MS |
| 2110–2196 c | 2192 | Nonanoic acid | - | - | - | 0.1 | - | tR, MS |
| 2241–2247 d | 2247 | trans-α-Bergamotol | - | 0.2 | - | - | - | MS |
| 2180–2255 c | 2255 | α-Cadinol | 4.4 | - | <0.1 | 0.4 | - | MS |
| 2196–2272 c | 2257 | β-Eudesmol | - | 0.2 | - | - | - | tR, MS |
| 2262, 2269 d | 2269 | Guaia-6,10(14)-dien-4β-ol | - | - | <0.1 | - | - | MS |
| 2231, 2278 d | 2278 | Torilenol | - | - | - | 0.5 | 0.9 | MS |
| 2287 d | 2287 | 8,13-Epoxy-15,16-dinor-labd-12-ene | - | - | - | 0.5 | - | MS |
| 2312 | 9-Geranyl-p-cymene | - | - | - | 0.8 | - | MS | |
| 2369 | Eudesma-4(15),7-dien-4β-ol | - | - | - | - | 0.7 | MS | |
| 2391, 2396 d | 2380 | 8α,13-Oxy-14-en-epilabdane | - | - | - | 0.2 | - | MS |
| 2392 | Caryophylla-2(12),6-dien-5β-ol (=Caryophyllenol II) | 1.0 | - | - | - | <0.1 | MS | |
| 2634–2719 c | 2670 | Tetradecanoic acid | - | 0.2 | - | - | - | tR, MS |
| 2370–2628 d | 2679 | Manool | - | - | - | 0.5 | - | MS |
| 2735 | Labda-7,14 dien-13-ol | - | - | - | 0.3 | - | MS | |
| 2900 f | 2900 | Nonacosane | - | 0.2 | - | - | - | tR, MS |
| 2862–2945 c | 2931 | Hexadecanoic acid | - | 0.6 | - | 15.0 | - | tR, MS |
| Monoterpene Hydrocarbons | 40.2 | 21.2 | 31.4 | 0.5 | 29.8 | |||
| Oxygenated Monoterpenes | 35.9 | 43.8 | 57.1 | 54.6 | 51.9 | |||
| Sesquiterpene Hydrocarbons | 8.7 | 15.9 | 5.9 | 12.4 | 4.0 | |||
| Oxygenated Sesquiterpenes | 12.3 | 9.1 | 2.2 | 7.9 | 10.4 | |||
| Fatty acids | - | 0.8 | - | 15.1 | - | |||
| Diterpenes | - | - | - | 2.3 | - | |||
| Others | 2.9 | 0.9 | 1.3 | 0.3 | ||||
| Oil Yields (%) | 0.25 | 0.2 | 0.2 | 0.26 | 0.66 | |||
| Total | 100 | 91.7 | 96.6 | 94.1 | 96.4 |
| No | Rt | [M-H] m/z | Fragments | Identification | Plant | Refs |
|---|---|---|---|---|---|---|
| 1 | 7.7 | 196.9 | 178.8, 134.9 | Salvianic acid | Spv | [34,35] |
| 2 | 11.6 | 178.9 | 134.9 | Caffeic acid | Spbv | [36,37] |
| 3 | 13.3/19.4 | 446.7 | 284.9, 150.8, 133.0 | Luteolin glucoside | Spbvaab | [35,36] |
| 4 | 13.6/20.1 | 460.6 | 285, 150.9, 133.0 | Luteolin glucuronide | Spab | [35,36] |
| 5 | 15.1 | 592.7 | 472.6, 382.7, 352.7 | Vicenin 2 | Sab | [34] |
| 6 | 15.2/21.4 | 430.7 | 267.7, 150.8 | Apigenin glucoside | Spbvaab | [38] |
| 7 | 15.6 | 445 | 269, 175, 113 | Apigenin glucuronide | Sp | [36,37] |
| 8 | 16.2/23.1 | 358.8 | 197.1, 179.2, 161.0, 134.7 | Rosmarinic acid * | pbvaab | [38] |
| 9 | 19.2 | 372.7 | 354.7, 310.9, 196.9, 178.9, 175.0, 161.0, 135.0 | Rosmarinic acid methyl ester | pv | [38] |
| 10 | 20.7/27.4 | 284.7 | 150.9, 133.0 | Luteolin | pbvaab | [35] |
| 11 | 23.9/31.0 | 268.8 | 151.0, 116.9 | Apigenin | pbab | [34,38] |
| 12 | 24.9 | 329.8 | 313.7, 298.7, 284.7, 151.0 | Cirsiliol | p | [35,39] |
| 13 | 24.9 | 716.5 | 358.8, 338.8, 310.8, 294.9 | Similar to Salvianolic acid | a | [34] |
| 14 | 28.0 | 298.8 | 283.9, 254.9, 150.8, 132.8 | Diosmetin/Chrysoeriol | p | [35,36,40] |
| 15 | 28.6 | 313.7 | 298.7, 283.8, 269.8, 255.8, 151.0 | Cirsimaritin | pbv | [36,41] |
| 16 | 29.5 | 343.8 | 328.7, 313.7, 298.6, 285.7 | Eupatilin | pb | [36,42,43] |
| 17 | 31.9 | 282.3 | 268.0, 238.8, 210.8, 116.9 | Genkwanin | pb | [36,44,45] |
| 18 | 35.2 | 312.8 | 256.9 | Unknown | v | |
| 19 | 36.1 | 312.7 | 297.8, 282.7, 254.6 | Dihydroxy dimethoxyflavone | a | |
| 20 | 37.9 | 292.8 | 274.9, 231.0, 170.9 | Unknown | pbv | |
| 21 | 38.4 | 315 | 259 | Unknown | v |
| mo | Sa | Sb | Sab | Sp | Sv | S1 | S2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EO | MeOH | EO | MeOH | EO | MeOH | EO | MeOH | EO | MeOH | |||
| C. albicans | 1000 | 1000 | 250 | 500 | 1000 | 1000 | 500 | 250 | 500 | 500 | 0.25 | 0.5 |
| C. utilis | 250 | 500 | 31.25 | 125 | 250 | 500 | 125 | 125 | 62.5 | 250 | 0.5 | 1 |
| C. tropicalis | 500 | 500 | 250 | 500 | 1000 | 1000 | 500 | 500 | 500 | 500 | 0.25 | 0.5 |
| C. albicans | 500 | 1000 | 250 | 500 | 500 | 500 | 1000 | 500 | 500 | 500 | 0.25 | 1 |
| C. parapsilosis | 250 | 1000 | 250 | 500 | 250 | 500 | 250 | 500 | 250 | 500 | 0.125 | 2 |
| C. krusei | 500 | 250 | 125 | 250 | 500 | 250 | 250 | 500 | 250 | 500 | 1 | 1 |
| mo | Sa | Sb | Sab | Sp | Sv | S3 | S4 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EO | MeOH | EO | MeOH | EO | MeOH | EO | MeOH | EO | MeOH | |||
| B. cereus | 125 | 500 | 500 | 62.5 | 1000 | 1000 | 125 | 62.5 | 500 | 125 | 1 | 4 |
| B. subtilis | 125 | 500 | 500 | 125 | 1000 | 1000 | 125 | 250 | 500 | 250 | 1 | 2 |
| S. marcescens | 1000 | 1000 | 2000 | 1000 | >2000 | 2000 | 2000 | 1000 | 2000 | 2000 | 16 | 8 |
| E. coli | 1000 | 2000 | 2000 | 1000 | >2000 | 1000 | 2000 | 1000 | 2000 | 2000 | 1 | 4 |
| S. typhimurium | 1000 | 2000 | 2000 | 1000 | 2000 | 1000 | 2000 | 2000 | 2000 | 2000 | 1 | 2 |
| S. aureus | 125 | 1000 | 500 | 125 | 1000 | 1000 | 125 | 250 | 500 | 125 | 1 | 4 |
| E. coli O157:H7 | 1000 | 1000 | 1000 | 2000 | 2000 | 1000 | 1000 | 1000 | 1000 | 2000 | 1 | 2 |
| P. aeruginosa | 1000 | 1000 | 2000 | 2000 | 2000 | 1000 | 2000 | 1000 | 2000 | 1000 | 16 | 32 |
| L. monocytogenes | 125 | 1000 | 500 | 500 | 1000 | 2000 | 500 | 250 | 1000 | 500 | 1 | 2 |
| S. epidermidis | 1000 | 500 | 250 | 500 | 2000 | 500 | 500 | 125 | 500 | 125 | 1 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Köse, Y.B.; İşcan, G.; Göger, F.; Demirci, B. Phytochemical Composition and Evaluation of Antimicrobial Activities of Five Salvia Species. Processes 2025, 13, 2011. https://doi.org/10.3390/pr13072011
Köse YB, İşcan G, Göger F, Demirci B. Phytochemical Composition and Evaluation of Antimicrobial Activities of Five Salvia Species. Processes. 2025; 13(7):2011. https://doi.org/10.3390/pr13072011
Chicago/Turabian StyleKöse, Yavuz Bülent, Gökalp İşcan, Fatih Göger, and Betül Demirci. 2025. "Phytochemical Composition and Evaluation of Antimicrobial Activities of Five Salvia Species" Processes 13, no. 7: 2011. https://doi.org/10.3390/pr13072011
APA StyleKöse, Y. B., İşcan, G., Göger, F., & Demirci, B. (2025). Phytochemical Composition and Evaluation of Antimicrobial Activities of Five Salvia Species. Processes, 13(7), 2011. https://doi.org/10.3390/pr13072011









