1. Introduction
Green chemistry refers to the design of products and processes that avoid the use of toxic and harmful chemicals. Green chemistry can be applied across the life cycle of a chemical product, including its design, manufacture, use, and ultimate disposal. It is also known as “sustainable chemistry”, and it prevents pollution at the molecular level. Essentially, green chemistry is a philosophy that covers all areas of chemistry, not just a single discipline. It applies innovative scientific solutions to real-world environmental problems and, as such, results in source reduction because it prevents the generation of pollution. Moreover, it reduces the negative impacts of chemical products and processes on human health and the environment, and it lessens, and sometimes even eliminates, hazardous elements from existing products and processes [
1,
2,
3,
4].
One of the most important solvents in green chemistry is water. It is non-toxic, non-flammable, inexpensive, and readily available. Water is a protic and polar solvent and has been the solvent for various biological reactions for millions of years [
5].
Using water in organic reactions as a green solvent has many advantages: cost, safety, synthetic efficiency, simple operation, environmental benefits, and potential for new synthetic methodologies [
6].
At first glance, water appears to be a poor solvent for organic transformations due to the low solubility of organic compounds in water, which have long been considered contaminants. However, it is now well understood that the unique structure and physicochemical properties of water lead to certain interactions such as polarity, hydrogen bonding, hydrophobic effect, and trans-phase interactions that can greatly affect the reaction process [
7,
8].
Water is a relatively redox-inert molecule. In addition to being difficult to oxidize, it is supportive of a variety of chemical and electrochemical oxidants as well as transition metal catalysts. Hence, it could be a useful solvent for the oxidation and reduction of organic compounds [
9].
The use of natural and sustainable raw materials is important in green chemistry. The essential oil obtained from the Cinnamomum cassia plant is a natural, sustainable, and easily obtainable raw material.
1.1. Cinnamon (Cinnamomum cassia) and Cinnamon cassia Oil
As one of the earliest spices known to humankind,
Cinnamomum cassia has been used to maintain physical health and support emotional well-being for thousands of years. Known for its calming properties,
Cinnamomum cassia has a strong “sweet” scent and is considered a “warming” oil that helps promote the healthy function of the immune system. Given its warming properties and spiciness, it tends to be widely used in winter.
Cinnamomum cassia is also known as the Earth element. Traditional Chinese Medicine associates the Earth element with the spleen, which is correlated with Yin, and the stomach, which is correlated with Yang. Thus, it can be useful in treating patients with Qi deficiency in the stomach or spleen [
10].
Around 250 species of the Cinnamon genus have been identified worldwide.
Cinnamomum cassia contains certain primary components such as cinnamaldehyde and
trans-cinnamaldehyde, which are found in the essential oil of the bark and contribute to its fragrance and various biological activities [
11].
Cinnamomum cassia and the essential oil of the bark are reported to have anti-inflammatory, antioxidant, anticancer, antifungal, antipyretic, antimicrobial, antiangiogenic and larvicidal, and antihepatoma properties [
12].
The bark of
Cinnamomum cassia is not only known as a spice and sweetener but is also considered a traditional medicine around the world. It is commonly used to treat amenorrhea, rheumatoid arthritis, heart palpitations, diarrhea, and gastrointestinal neurosis [
13].
1.2. Benzaldehyde
The simplest aromatic aldehyde, benzaldehyde, occurs naturally as the glycoside amygdalin. Benzaldehyde is an aromatic flavor compound with a distinctive bitter almond taste and odor. Among the natural flavors, benzaldehyde is the second-largest produced flavor after vanillin in the world. It is widely used in food, beverages, cosmetics, and pharmaceuticals. Usually, natural benzaldehyde can be produced by the alkaline hydrolysis of cinnamon oil or amygdalin. Several synthetic processes based on the conversion of natural cinnamon oil were reported, such as the ozonization process, near-critical water hydrolysis process, reactive distillation process, oxidation by hydrogen peroxide, toxic phase transfer, or surfactants catalytic process [
14,
15,
16,
17,
18,
19].
1.3. Benzoic Acid and Benzyl Alcohol
Benzyl alcohol, benzoic acid, and their salts (i.e., sodium benzoate) are used as odor components, pesticides, pH adjusters, preservatives, solvents, and viscosity-reducing substances. In 2001, the Cosmetic Ingredient Review (CIR) published a final report on the safety assessment of benzyl alcohol, benzoic acid, and sodium benzoate and concluded that they are safe for use in cosmetic formulations in concentrations of up to 5%. Benzyl alcohol is considered safe for use in hair dyes in concentrations of up to 10% [
20].
According to the Select Committee of the US Food and Drug Administration’s (FDA) Generally Recognized as Safe (GRAS) substances, benzoic acid (21 CFR [Code of Federal Regulations] 184.1021) and sodium benzoate (21 CFR 184.17333) are classified as GRAS food substances. Benzyl alcohol is used as an FDA-approved diluent in mixtures with color additives for external pharmacological use. Benzoic acid is also used as an acne and fungus preventive, in oral care, and as a skin protector [
20].
Benzyl alcohol can be used as an active ingredient in over-the-counter (OTC) drug preparations. Additionally, benzyl alcohol is an effective local anesthetic. Subjects complained of less pain after receiving intramuscular injections of various medications containing benzyl alcohol [
21].
Benzoic acid is usually synthesized through the oxidation reaction of benzaldehyde using reagents such as potassium permanganate [
22,
23]. Benzyl alcohol is usually synthesized through the reduction reaction of benzaldehyde using reagents such as sodium borohydride [
24,
25,
26].
Only water was used in this study, as a previous study determined the effects of benzoic acid synthesized from
Cinnamomum cassia by green chemistry method on gene expressions related to autism development in the case of valproic acid toxicity. Acetylcholinesterase, lipid peroxidation, nitric oxide, sialic acid, glutathione (GSH)-S-transferase, catalase, and superoxide dismutase activities are determined spectrophotometrically. Autism-related genes eif4b, adsl, and shank3a expressions are determined by RT-PCR [
27].
1.4. Cinnamaldehyde, Cinnamyl Alcohol, and Cinnamic Acid
trans-Cinnamaldehyde is recognized as GRAS by the FDA and the Flavoring and Extract Manufacturers Association (FEMA). Furthermore, it has been granted A status (i.e., can be used in foods) by the Council of Europe.
trans-Cinnamaldehyde can be viewed as a safe food and flavor additive with a pleasant taste and odor, and it has found many commercial uses in food. Cinnamaldehyde has also been incorporated into edible antimicrobial films prepared from food items such as fruits and vegetables, inactivating foodborne pathogens either through direct contact or through vapors released from films in sealed containers [
28].
Cinnamyl alcohol, cinnamic aldehyde, and cinnamic acid are phenylpropanoid compounds commonly found in the volatile oil of barks of species in the genus Cinnamon. Cinnamyl alcohol and its derivatives present important pharmacological properties such as anti-inflammatory activities, neurological disorders, antidiabetic activity, antimicrobial activity, anticancer activity, cardiovascular diseases, cholesterol- and lipid-lowering effects, and advanced glycation end products (AGEs) [
29,
30].
1.5. Phenylpropanol (3-Phenyl-1-propanol)
Phenylpropanol gives a pleasant hyacinth scent and apricot-like taste and is widely used as a fragrance ingredient in food, beverages, and cosmetics with the approval of the FDA (21 CFR 172.515) [
31].
Phenylpropanol is a fragrance component used in many compounds. It can be found in fragrances used in decorative cosmetics, fragrances, shampoos, toilet soaps, and other toiletries, as well as in non-cosmetic products such as household cleaners and detergents. It is a colorless, slightly oily liquid with a warm and mellow, balsamic–floral, sweet odor and moderate strength. It also shows a broad spectrum of antimicrobial activity that helps with the design of self-preserving cosmetic products. A 100% natural phenylpropanol is produced starting from
Cinnamon cassia oil, which is traditionally obtained by steam distillation from the leaves and branches of the Chinese cinnamon tree
Cinnamomum cassia [
32].
The goal of this work was the green synthesis of five important organic compounds, which are used in cosmetics, pharmaceutical, and food industries (
Figure 1). Commercially available, sustainable, natural
Cinnamon cassia oil was used in these syntheses. Water was used as the solvent in all reactions except one (phenylpropanol). The target products were produced with high yield without the formation of by-products. There were no toxicological or accident-causing hazards in any of the reactions. These properties make it suitable for industrial-scale production.
2. Materials, Methods, and Results
2.1. Materials and Instruments
The NMR spectra were recorded on a Varian Gemini spectrometer (Agilent Technologies Inc., Santa Clara, CA, USA) [400 MHz (1H) and 100 MHz (13C)]. NMR spectroscopy is a method frequently applied to understand molecular physics, crystals, non-crystalline materials, and organic molecules in solution. Our study used NMR to determine the molecular structure of the compounds we obtained, and the spectra were recorded at 25 °C using CDCl3 or DMSO-d6 as a solvent. Chemical shifts are indicated as δ values (ppm) against tetramethylsilane (TMS), which is the internal standard.
EI mass spectra were obtained with an Agilent GC7890B-MSD5977B machine (Agilent Technologies Inc., Santa Clara, CA, USA) and Agilent MassHunter Software 12.0 (Agilent Technologies Inc., Santa Clara, CA, USA). An excellent method to assess and confirm the purity of raw materials is GC-MS. All the compounds synthesized were purity- and assay-tested using GC-MS analysis applying HP-5 ms Ultra Inert as the parameter for the column, and dimensions 30 m × 250 µm × 0.25 µm. The other programs applied were as follows: Beginning: 60 °C, Final Temperature: 260 °C, Temperature Increase Rate: 3 °C per minute, Analysis Duration: 66.6 min; Inlet Temperature 250 °C; MS Detector Temperature: 230 °C; Helium Flow Rate: 1.1 mL/minute; Split Ratio: 20:1; Sample Preparation: 5 mg of sample dissolved in 1.5 mL of acetone. The volume of injection was 1 µL.
Analytical standards: Benzaldehyde Merck (MERCK KGAA, Darmstadt, Germany) Supelco 09143; cinnamaldehyde Merck Supelco 06536; benzoic acid Merck Supelco 47849; benzyl alcohol Merck Supelco 08421; cinnamyl alcohol Merck Supelco 93066; 3-phenyl-1-propanol Merck 140856; cinnamic acid Merck Supelco 97013.
2.2. GC-MS Analysis of Cinnamon cassia Oil [Supplier: Frey Lau Company (Frey + Lau GmbH, Henstedt-Ulzburg, Germany) or Sensient Company (Sensient Flavors & Extracts Group, Hoffman Estates, IL, USA)]
78–79% cinnamaldehyde; 10–12% 2-methoxycinnamaldehyde; 2–3.5% cinnamyl acetate; 1.7–2.2% coumarin; 0.7–0.8% benzaldehyde.
2.3. Green Synthesis of Natural Benzaldehyde and Cinnamaldehyde
These natural compounds were obtained using a procedure similar to that previously reported in the patent. About 15.10 g of Cinnamon cassia oil (purchased from a commercial supplier) was added to 100 mL of a 3% aqueous sodium carbonate solution. Nitrogen gas was passed over the reaction vessel to help prevent oxidation of the formed benzaldehyde. The reaction mixture was heated to reflux for 7 h (100 °C). The resulting material contained 70% benzaldehyde (boiling point: 178 °C at 760 mmHg) and 30% cinnamaldehyde (boiling point: 248 °C at 760 mmHg) according to GC-MS analysis (benzaldehyde: RT 6.09 min; cinnamaldehyde: RT 18.3 min). Benzaldehyde and cinnamaldehyde were purified by distillation.
2.4. Green Synthesis of Natural Benzoic Acid
Green organic synthesis techniques were applied during the synthesis of natural benzoic acid using natural benzaldehyde from Cinnamon cassia oil. Only water was used as the solvent in the reaction.
A purple colored solution was prepared by dissolving 12 g (0.104 mmol) of potassium permanganate in 200 g of water. A cloudy colored solution was prepared by mixing 10 g (0.094 mmol) of natural benzaldehyde in 25 g of water. A purple permanganate solution was added to this solution. Heating was applied for 45 min with slow stirring at a temperature of approximately 90 °C. When the reaction was completed, it was cooled, and the brown solid (manganese dioxide) formed was filtered through a 15-micron paper filter; 37% hydrochloric acid was added to the transparent colorless solution, and the pH of the solution was adjusted to 1.5. The white benzoic acid crystals formed are filtered through a paper filter and washed with cool water. Vacuuming first allowed the crystals to slightly dry. White crystals were transferred to a dry beaker and dried in an oven set at 40 °C for 1 week (Yield: 90–95%) [
27].
Characterization of Natural Benzoic Acid
Appearance: white powder; Melting point: 121–122 °C; Solubility in water: 0.2 g/100 mL (25 °C); pH (0.2% in water): 3.5; Assay (GC-MS): 100%, RT 15.67 min; EI-MS (m/e): 122 [M]+, 105, 77, 51; 1H-NMR (CDCl3, 400 MHz) δ: 7.5 (t, 2H), 7.6 (t, 1H), 8.15 (dd, 2H), 12.3 (br.s, COOH), 13C-NMR (CDCl3, 100 MHz) δ: 172.8 (COOH), 134.0, 130.4, 129.5, 128.6.
2.5. Green Synthesis of Natural Benzyl Alcohol
About 5.3 g (0.05 mmol) of natural benzaldehyde was added to 26.5 g of water and mixed for 5–10 min to create a homogeneous mixture; 0.58 g (0.015 mmol) of sodium borohydride was slowly added to the mixed benzaldehyde solution. Since the reaction produced heat and gas, the addition should be made very slowly. If the temperature of the reaction solution exceeds 50 °C, it must be cooled. After the addition, mixing was continued for 1 h. The reaction solution was then cooled to ambient temperature, and the mixing was stopped. It was kept at ambient temperature for 10–12 h to ensure separation of the water phase (bottom) and organic phase (upper). The organic phase was mixed with anhydrous sodium sulfate and dried. When its appearance became clear, it was filtered through a paper filter, and the sodium sulfate was removed. It was purified by distillation at 204 °C (Yield: 90–95%).
Characterization of Natural Benzyl Alcohol
Appearance: transparent colorless liquid; Odor: fruity–mild–sweet; Density: 1.044 g/cm3 (25 °C), Boiling Point: 204 °C at 760 mmHg; Solubility in water: 4.29 g/100 mL (25 °C); Assay (GC-MS): 100%, RT 8.39 min; EI-MS (m/e): 108 [M]+, 91, 79, 63, 51; 1H-NMR (CDCl3, 400 MHz) δ: 2.4 (br.s, OH), 4.6 (s, CH2, 2H), 7.3–7.4 (m, Ar, 5H), 13C-NMR (CDCl3, 100 MHz) δ: 140.9 (C-1), 128.6, 127.7, 127.1, 65.2.
2.6. Green Synthesis of Natural Cinnamyl Alcohol
About 13.2 g (0.1 mmol) of natural cinnamaldehyde was added to 66 g of water and mixed for 5–10 min to create a homogeneous mixture; 1.43 g (0.038 mmol) of sodium borohydride was slowly added to the cinnamaldehyde solution. Since the reaction produces heat and gas, the addition should be made very slowly. If the temperature of the reaction solution exceeds 50 °C, it must be cooled. After the addition, mixing was continued for 1 h. The reaction solution was then cooled to ambient temperature, and the mixing was stopped. It was kept at ambient temperature for 10–12 h to ensure separation of the water phase (bottom) and organic phase (upper). The organic phase was mixed with anhydrous sodium sulfate and dried. When its appearance became clear, it was filtered through a paper filter, and the sodium sulfate was removed. It is purified by distillation at 250 °C (Yield: 90–95%).
Characterization of Natural Cinnamyl Alcohol
Appearance: transparent light yellow liquid; Odor: sweet–balsamic–floral; Density: 1.044 g/cm3 (25 °C), Boiling Point: 250 °C at 760 mmHg; Solubility in water 2.54 g/1000 mL (25 °C); Assay (GC-MS): 99.6%, RT 20.15 min; EI-MS (m/e): 134 [M]+, 115, 103, 92, 78; 1H-NMR (CDCl3, 400 MHz) δ: 2.6 (br.s, OH), 4.28 (dd, CH2, 2H), 6.33 (dt, CH), 6.63 (dt, CH), 7.2–7.6 (m, Ar, 5H); 13C-NMR (CDCl3, 100 MHz) δ: 136.7 (C-C=C), 131.2 (C-C=C), 128.7 (C-C=C), 128.5 (2C), 127.8, 126.6 (2C), 63.8 (CH-OH).
2.7. Green Synthesis of Natural Phenylpropanol (3-Phenyl-1-propanol)
About 13.2 g (0.1 mmol) of natural cinnamaldehyde was added to 50 g of natural ethanol and mixed for 5–10 min to create a homogeneous mixture; 1.4–1.6 g (approximately 0.04 mmol) of lithium aluminum hydride was slowly added to the mixed cinnamaldehyde solution. Since the reaction produces heat and gas, the addition should be made very slowly. If the temperature of the reaction solution exceeds 50 °C, it must be cooled. After the addition, mixing was continued for 1 h. The reaction solution was then cooled to ambient temperature. The resulting material contained ~60% phenylpropanol (Boiling Point: 220 °C at 760 mmHg) and ~40% cinnamyl alcohol (Boiling Point: 250 °C at 760 mmHg) according to GC-MS analysis. Phenylpropanol was purified by distillation (Yield: 60–65%).
Characterization of Natural Phenylpropanol (3-Phenyl-1-propanol)
Appearance: transparent colorless liquid; Odor: sweet–balsamic–floral; Density: 1.001 g/cm3 (25 °C), Boiling Point: 220 °C at 760 mmHg; Assay (GC-MS): 99.6%, RT 16.3 min; EI-MS (m/e): 136 [M]+, 117, 105, 91, 77, 65, 51; 1H-NMR (CDCl3, 400 MHz) δ: 1.70 (s, OH, 1H), 1.90 (dt, CH2, 2H), 2.70 (t, Ar-CH2, 2H), 3.70 (t, HO-CH2, 2H), 7.25 (m, Ar, 5H); 13C-NMR (CDCl3, 100 MHz) δ: 141.9, 128.5, 128.5, 126.0, 62.3 (HO-CH2), 34.3 (CH2), 32.2 (CH2).
2.8. Green Synthesis of Natural Cinnamic Acid
This natural compound was obtained using a procedure similar to that previously reported in the literature [
33]. About 10.3 g (0.078 mmol) of natural cinnamaldehyde was added to 60 g of water and mixed for 5–10 min to create a homogeneous mixture in a closed reaction vessel. The container was filled with oxygen and sealed with an oxygen-filled balloon. Then, the reaction mixture was heated to 37 °C and kept in a shaking incubator (160 rpm) for 24 h. After the reaction was completed, the pH of the mixture was 10. The reaction mixture was cooled to −20 °C, then kept in a freeze dryer until all the water was removed. Then, 50 mL of water was added to the resulting powder; 37% hydrochloric acid was added to the transparent colorless solution, and the pH of the solution was adjusted to a range of 3–4. The white cinnamic acid crystals formed were filtered through a paper filter and washed with some water. Vacuuming first allowed the crystals to dry slightly. The white crystals were transferred to a dry beaker and dried in an oven set at 40 °C for 1 week (Yield: 75%).
Characterization of Natural Cinnamic Acid
Appearance: white powder; Melting point: 133–135 °C; Solubility in water: 0.04 g/100 mL (25 °C); pH (0.2% in water): 3.5; Assay (GC-MS): 99.5%, RT 26.71 min; EI-MS (m/e): 147 [M]+, 131, 120, 103, 91, 77, 51; 1H-NMR (DMSO-d6, 400 MHz) δ: 12.5 (s, COOH, 1H), 7.7 (m, 2H), 7.6 (d, 1H), 7.4 (m, 3H), 6.55 (d, CH-C=C, 1H, J= 16 Hz), 13C-NMR (DMSO-d6, 100 MHz) δ: 167.7, 143.8, 134.3, 130.3, 129.0, 128.2, 119.5.
3. Discussion
3.1. Green Synthesis
Various reaction conditions were applied to find suitable green chemistry procedures using mild solvents for the formation of all organic compounds from
Cinnamon cassia oil. Equivalents (molar) of oxidation reagents to starting materials were optimized (
Table 1,
Table 2,
Table 3 and
Table 4).
The highest yields (75 and 90%) of the oxidation reactions were achieved using water as a solvent and potassium permanganate as an oxidation reagent. In the synthesis of cinnamic acid, the use of oxidants (potassium permanganate, hydrogen peroxide, sodium hypochlorite) led to the decomposition of cinnamic acid and the formation of benzoic acid and acetic acid. Therefore, the synthesis of cinnamic acid was carried out in an oxygen gas environment without using an oxidant. The use of organic solvents (ethanol, acetone, isopropyl alcohol) instead of water in all oxidation reactions caused the yield to decrease significantly (<50%) and made it difficult to crystallize organic acids (
Table 1 and
Table 2).
To optimize the oxidation reactions, 4 different oxidants, 6 different equimolar concentrations, 4 different solvents, and 6 different reaction times were tested (
Table 1).
The highest yields (60–90%) of the reduction reactions were achieved using Sodium borohydride–Water and Lithium aluminum hydride–Ethanol. To obtain phenylpropanol, lithium aluminum hydride was used as a stronger reduction reagent compared to sodium borohydride for the reduction of both the aldehyde group and the alkene group of cinnamon aldehyde. In the reduction reactions, the use of solvents such as acetone and isopropyl alcohol both reduced the yields (<50%) and made purification steps of the final product difficult (
Table 3 and
Table 4).
There are no references in the literature to the use of water as a solvent in reduction reactions involving sodium borohydride; such reactions are generally carried out using anhydrous organic solvents. In this study, experiments were conducted using organic solvents, and although the reduction was successful, it was found to cause a decrease in yield during the purification process. In this study, reduction was carried out with high yield in an aqueous medium in the presence of sodium borohydride. The reducing agent lithium aluminum hydride reacts violently with water, making it unsafe to use. Therefore, reduction was carried out with moderate yield using ethanol, one of the green solvents.
In order to optimize the reduction reactions, two different reductants, five different equimolar concentrations, four different solvents, and five different reaction times were tested (
Table 3). Optimum conditions are indicated in bold.
Comparison of the Reported Methods
According to previous studies [
34,
35,
36], the use of H
2O
2, which is highly flammable, is considered dangerous and unsuitable for scale-up. In the work of Zheng et al., the reaction is carried out in an organic solvent (acetone), which is not compatible with green chemistry principles [
23]. In another research, hazardous and toxic organic solvents such as carbon tetrachloride, dichloromethane, and diethyl ether were used during the purification steps [
37]. Finally, in Hao et al.’s study, the reduction reactions are conducted over extended periods and under extreme reaction conditions [
38] (
Table 5).
3.2. Scale Up
The scale-up quantities are 5 kg for both natural benzoic acid and natural benzyl alcohol. A decrease in yield has been observed during the scale-up of benzoic acid, primarily due to the washing process with water during filtration, which caused a portion of the benzoic acid to dissolve in water and be lost. This is one of the drawbacks of the filter press device. Additionally, Environmental factor (
E Factor) calculations for the scale-up production of benzoic acid and benzyl alcohol molecules were performed according to the relevant literature [
39]. Larger scale-up sizes will be attempted in the future (10–100 kg).
3.2.1. Scale Up of Natural Benzoic Acid (5 kg)
Purple permanganate solution (8.2 kg of potassium permanganate in 164 kg of water) was added to a cloudy colored solution (5 kg of natural benzaldehyde in 15 kg of water). Heating was applied for 30–45 min with slow stirring at a temperature of 90–100 °C. When the reaction was completed, it was cooled, and the brown solid (manganese dioxide) formed was filtered through a 15-micron filter using a filter press; 3.5–4.0 kg of 37% hydrochloric acid was added to the transparent colorless solution (pH 1.5). The solution with white benzoic acid crystals was filtered through a 15-micron filter using another filter press. After filtration, it was washed with pure water and filtered again. After all the filtration processes were completed, air was applied for a while to ensure that the crystals dried thoroughly. White crystals were transferred to an oven set at 40 °C for 1.5–2 weeks (Yield: 3.75 kg, 65%; E factor: 3.45).
3.2.2. Scale Up of Natural Benzyl Alcohol (5 kg)
About 5.4 kg of sodium borohydride was slowly added to the mixing benzaldehyde solution (5 kg of natural benzaldehyde in 25 kg of water). After the addition, mixing was continued for 1 h. The reaction solution was then cooled to ambient temperature, and the mixing was stopped. It was kept at ambient temperature for 12 h to ensure separation of the water phase (bottom) and organic phase (upper). The organic phase was mixed with 1 kg of anhydrous sodium sulfate and dried. When its appearance became clear, it was filtered through a 15-micron filter, and the sodium sulfate was removed using a filter press. It was purified by distillation at 200–205 °C (Yield: 4.8 kg, 96%; E factor: 1.37).
3.3. Green Analytical Chemistry Evaluations
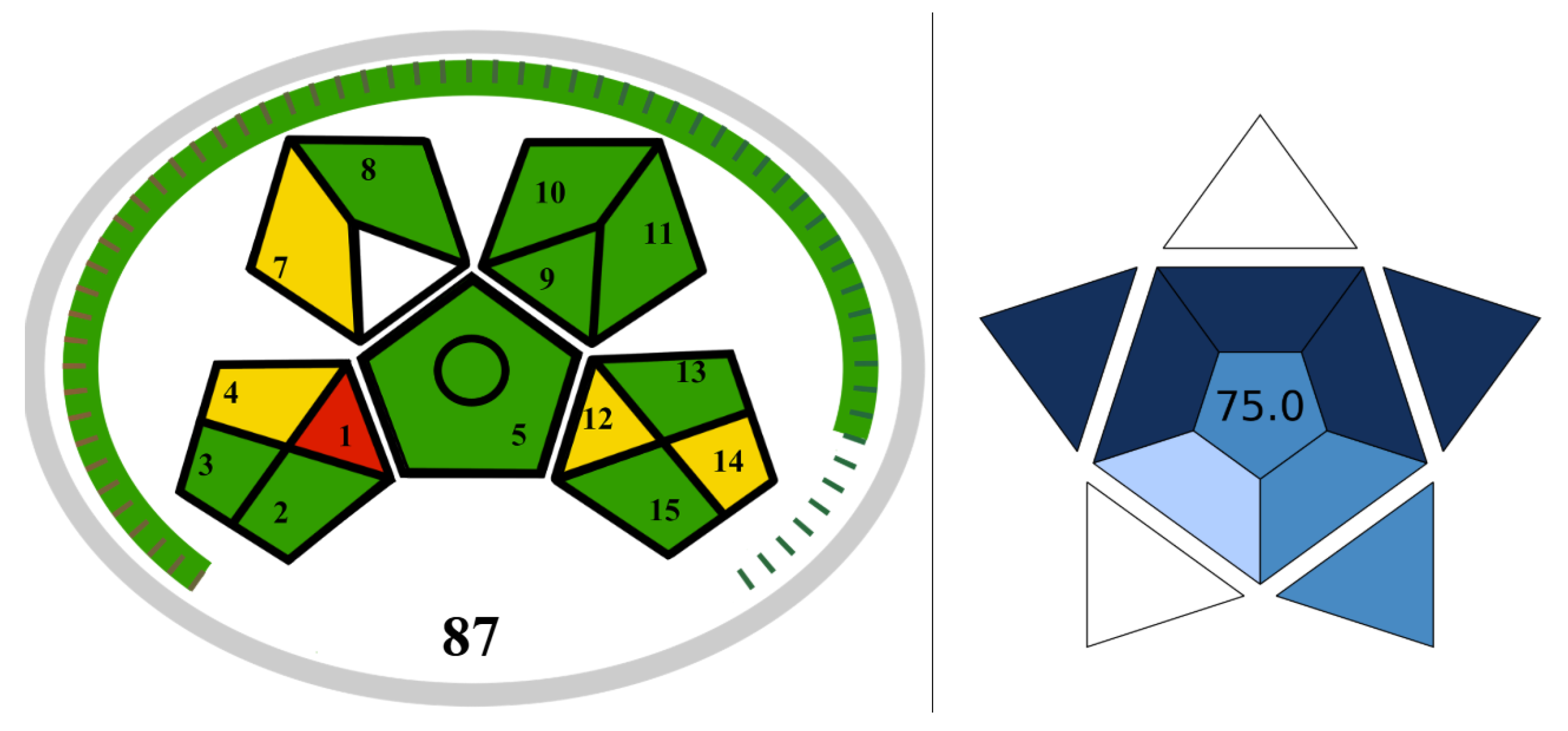
The purity and identification tests of the five synthesized target molecules were carried out using the GC-MS analysis method. Three volatile molecules (alcohols) and two solid molecules (acids) were identified by comparison with analytical standards, allowing for the calculation of their purity and assay percentages. Sample preparation for all five molecules was straightforward, and autosamplers were used for the analysis. A small amount of non-toxic acetone was used as the solvent. The only disadvantage is the high energy consumption due to the column temperature in the GC-MS instrument. Based on all these criteria, the Modified Green Analytical Procedure Index (MoGAPI) and Blue Applicability Grade Index (BAGI) scores for the GC-MS analyses of the five molecules are presented in
Figure 2. These scores align with the principles of green analytical chemistry [
40,
41].
3.4. Green Synthesis Evaluations with the Scoring in ComplexMoGAPI
This study was evaluated using the scoring system in ComplexMoGAPI, as proposed by Mansour et al. [
42]. Our study falls within the green color point in terms of yield for both benzoic acid and benzyl alcohol synthesis. Regarding reaction time, it also qualifies as a green color point since the reactions are completed within one hour or less. In terms of temperature, it is at the green color point in reduction, but it is at the yellow color point since it is heated to 90–100 °C in oxidation. It is a completely green color point in terms of the toxicity of the reagents and solvents used. It is at the green color point because simple crystallization and distillation methods are used for the ease of the purification step. The purity of the target molecules obtained is 99% and above, which is green in color. The prevalence and supply of the instruments used in the synthesis are in green color point because of the common setup. Aqueous non-toxic waste in the synthesis of benzoic acid and benzyl alcohol is in green color point since non-toxic sodium sulfate and water are formed.
The starting material (Cinnamon cassia oil) used is sustainable and readily available in the world. The starting material, reagents, and all synthesized molecules are stable and can be easily stored under room conditions.
4. Conclusions
Green chemistry is not a new branch of science, but rather a new philosophical approach that can lead to significant developments in the fields of chemistry, chemical industry, and environmental protection.
Water is the greenest solvent when considering the principles of green chemistry. Performing the reaction in water instead of an organic solvent reduces the environmental negative impact of the synthetic toxic solvent. It also improves many other parameters, such as atomic efficiency, yield, processing, and purification processes. Water is probably the greenest solvent in view of its price, availability, safety, and environmental effects.
In green chemistry techniques, starting materials used in terms of atom efficiency are very important. Versatile, natural sustainable starting materials should be used in the synthesis of target compounds. Cinnamomum cassia is an important natural starting material to synthesize natural carboxylic acids and alcohol compounds for use in cosmetics, pharmaceutical, and food industries. Benzoic acid, benzyl alcohol, cinnamyl alcohol, phenylpropanol, and cinnamic acid were synthesized from Cinnamon cassia oil using green chemistry methods. Water was used as the solvent in all reactions except phenylpropanol.
Although oxidation and reduction reactions often use hazardous starting materials, solvents, and catalysts, five compounds of this research were synthesized in high yields (60–90%) using one natural starting material, low-cost reagents, and mild solvents in all syntheses. The uses of a single starting material, water as a solvent, and low-cost oxidation and reduction reagents are all in line with green chemistry techniques. The novelty of this work was the green synthesis of five important organic compounds that are used in cosmetics, pharmaceutical, and food industries. In these syntheses, commercially available, sustainable, natural cinnamon oil was used as a starting material.
Optimum circumstances were established using various reaction conditions and catalysts throughout the research. The structures of all synthesized compounds were analyzed by
1H-NMR,
13C-NMR, and GC-MS spectral methods in the
Supplementary Materials.
Supplementary Materials
The following supporting information can be downloaded at
https://www.mdpi.com/article/10.3390/pr13072002/s1, Figures S1–S5: Chromatogram and MS spectra for
Cinnamon cassia oil, natural Benzaldehyde, and natural Cinnamaldehyde; Figures S6–S21: Chromatogram and MS spectra and NMR spectra for natural Benzoic acid, natural Benzyl alcohol, natural Cinnamyl alcohol, natural Phenylpropanol, and natural Cinnamic acid.
Author Contributions
Conceptualization, G.Ö., A.B. and M.K.G.; methodology, G.Ö.; investigation, G.Ö.; writing—original draft preparation, G.Ö. and A.B.; writing—review and editing, G.Ö., A.B. and M.K.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Acknowledgments
The authors are thankful to GLOHE Company (Istanbul, Türkiye) for its kind contributions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sheldon, R.A. Fundamentals of green chemistry: Efficiency in reaction design. Chem. Soc. Rev. 2012, 41, 1437–1451. [Google Scholar] [CrossRef] [PubMed]
- Linthorst, A.J. An Overview: Origins and development of green chemistry. Found. Chem. 2010, 12, 55–68. [Google Scholar] [CrossRef]
- Kharissova, O.V.; Kharisov, B.I.; Oliva González, C.M.; Méndez, Y.P.; López, I. Greener synthesis of chemical compounds and materials. R. Soc. Open Sci. 2019, 6, 191378. [Google Scholar] [CrossRef] [PubMed]
- Li, C.J.; Trost, B.M. Green chemistry for chemical synthesis. Proc. Natl. Acad. Sci. USA 2008, 105, 13197–13202. [Google Scholar] [CrossRef]
- Shanab, K.; Neudorfer, C.; Schirmer, E.; Spreitzera, H. Green Solvents in Organic Synthesis: An Overview. Cur. Org. Chem. 2013, 17, 1179–1187. [Google Scholar] [CrossRef]
- Deligeorgiev, T.; Gadjev, N.; Vasilev, A.; Kaloyanova, S.; Vaquero, J.J.; Alvarez-Builla, J. Green Chemistry in Organic Synthesis. Mini-Rev. Org. Chem. 2010, 7, 44–53. [Google Scholar]
- Simon, M.O.; Li, C.J. Green chemistry oriented organic synthesis in water. Chem. Soc. Rev. 2012, 41, 1415–1427. [Google Scholar] [CrossRef]
- Zhou, F.; Hearne, Z.; Li, C.J. Water-the greenest solvent overall. Curr. Opin. Green Sustain. Chem. 2019, 18, 118–123. [Google Scholar] [CrossRef]
- Chanda, A.; Fokin, V.V. Organic Synthesis “On Water”. Chem. Rev. 2009, 109, 725–748. [Google Scholar] [CrossRef]
- Wu, H.Z.; Fang, Z.Q.; Cheng, P.J. World Century Compendium To Tcm—Volume 1: Fundamentals Of Traditional Chinese Medicine; World Century Publishing Corporation: Hackensack, NJ, USA, 2013; pp. 55–82. [Google Scholar]
- Kumar, S.; Kumari, R.; Mishra, S. Pharmacological properties and their medicinal uses of Cinnamomum: A review. J. Pharm. Pharmacol. 2019, 71, 1735–1761. [Google Scholar] [CrossRef]
- Zaidi, S.F.; Aziz, M.; Muhammad, J.S.; Kadowaki, M. Diverse pharmacological properties of Cinnamomum cassia: A review. Pak. J. Pharm. Sci. 2015, 28, 1433–1438. [Google Scholar] [PubMed]
- Brodowska, K.M.; Brodowska, A.J.; Śmigielski, K.; Chruścińska, E.L. Antioxidant profile of essential oils and extracts of cinnamon bark (Cinnamomum cassia). Eur. J. Biol. Res. 2016, 6, 310–316. [Google Scholar] [CrossRef]
- Li, H.; Meng, Y.; Shu, C.; Li, X.; Kiss, A.A.; Gao, X. Innovative Reactive Distillation Process for the Sustainable Synthesis of Natural Benzaldehyde. ACS Sustain. Chem. Eng. 2018, 6, 14114–14124. [Google Scholar] [CrossRef]
- Pittet, A.O.; Muralidhara, R.; Liberman, A.L. Process for Preparing Natural Benzaldehyde and Acetaldehyde, Natural Benzaldehyde and Acetaldehyde Compositions, Products Produced Thereby and Organoleptic Utilities Thereof. U.S. Patent 4,727,058, 29 June 1987. [Google Scholar]
- Buck, K.T.; Boeing, A.J.; Dolfini, J.E. Method of Producing Benzaldehyde. U.S. Patent 4,673,766, 25 April 1986. [Google Scholar]
- Wiener, C.; Pittet, A.O. Process for Preparing Natural Benzaldehyde and Acetaldehyde, Natural Benzaldehyde and Acetaldehyde Compositions, Products Produced Thereby and Organoleptic Utilities Thereof. U.S. Patent 4,617,419, 14 October 1986. [Google Scholar]
- Chen, H.; Ji, H.; Zhou, X.; Wang, L. Green synthesis of natural benzaldehyde from cinnamon oil catalyzed by hydroxypropyl-b-cyclodextrin. Tetrahedron 2010, 66, 9888–9893. [Google Scholar] [CrossRef]
- Nie, Y.; An, Y.; Peng, C.; Ya, X. Highly Selective Oxidation of Cinnamaldehyde to Benzaldehyde by Hydrogen Peroxide in mild conditions. Appl. Mech. Mater. 2014, 522–524, 458–464. [Google Scholar] [CrossRef]
- Johnson, W., Jr.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Benzyl Alcohol, Benzoic Acid and its Salts, and Benzyl Benzoate. Int. J. Toxicol. 2017, 36, 5S–30S. [Google Scholar] [CrossRef]
- Nair, B. Final Report on the Safety Assessment of Benzyl Alcohol, Benzoic Acid, and Sodium Benzoate. Int. J. Toxicol. 2001, 20, 23–50. [Google Scholar] [CrossRef]
- Barros, T.G.; Williamson, J.S.; Antunes, O.A.C.; Muri, E.M.F. Hydroxamic Acids Designed as Inhibitors of Urease. Lett. Drug Des. Discov. 2009, 6, 186–192. [Google Scholar] [CrossRef]
- Zheng, M.Y.; Wei, Y.S.; Fan, G.; Huang, Y. Organic Solvent Controlling the Oxidativity of Potassium Permanganate. Asian J. Chem. 2012, 24, 161–164. [Google Scholar]
- Mirtaghizadeh, M.; Setamdideh, D. Ultrasonic-Promoted Selective Reduction of Aldehydes vs. ketones by NaBH4/PhCO2Na/H2O. Orient. J. Chem. 2016, 32, 1539–1543. [Google Scholar] [CrossRef]
- Firdaus, M.; Handayani, N.; Marfu’ah, L.T. Reduction of Aldehydes Using Sodium Borohydride under Ultrasonic Irradiation. Indones. J. Chem. 2016, 16, 229–232. [Google Scholar] [CrossRef]
- Ward, D.E.; Rhee, C.K. Chemoselective reductions with sodium borohydride. Can. J. Chem. 1989, 67, 1206–1211. [Google Scholar] [CrossRef]
- Cansız, D.; Özokan, G.; Bilginer, A.; Işıkoğlu, S.; Mızrak, Z.; Ünal, İ.; Beler, M.; Alturfan, A.A.; Emekli-Alturfan, E. Effects of benzoic acid synthesized from Cinnamomum cassia by green chemistry on valproic acid-induced neurotoxicity in zebrafish embryos. Toxicol. Mech. Methods 2024, 34, 833–843. [Google Scholar] [CrossRef]
- Doyle, A.A.; Stephens, J.C. A review of cinnamaldehyde and its derivatives as antibacterial agents. Fitoterapia 2019, 139, 104405. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.B.; Nunes de Andrade, H.H.; Felipe, C.F.B.; Nóbrega de Almeida, R. Pharmacological Studies on Cinnamic Alcohol and Its Derivatives. Rev. Bras. Farmacogn. 2021, 31, 16–23. [Google Scholar] [CrossRef]
- Rao, P.V.; Gan, S.H. Cinnamon: A Multifaceted Medicinal Plant. Evid.-Based Complement. Altern. Med. 2014, 4, 642942. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, X.; Lei, D.; Qiao, B.; Zhao, G.R. Metabolic engineering of Escherichia coli for de novo production of 3-phenylpropanol via retrobiosynthesis approach. Microb. Cell Fact. 2021, 20, 121. [Google Scholar] [CrossRef]
- Bhatia, S.P.; Wellington, G.A.; Cocchiara, J.; Lalko, J.; Letizia, C.S.; Api, A.M. Fragrance material review on 3-phenyl-1-propanol. Food Chem. Toxicol. 2011, 49, S246–S251. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, Y.; Cai, H.; He, S.; Shan, Q.; Zhao, H.; Chena, Y.; Wang, B. Catalyst-free aerobic oxidation of aldehydes into acids in water under mild conditions. Green Chem. 2017, 19, 5708–5713. [Google Scholar] [CrossRef]
- Sancineto, L.; Tidei, C.; Bagnoli, L.; Marini, F.; Lenardão, E.; Santi, C. Selenium Catalyzed Oxidation of Aldehydes: Green Synthesis of Carboxylic Acids and Esters. Molecules 2015, 20, 10496–10510. [Google Scholar] [CrossRef]
- Huang, Q.Y.; Dong, B.B.; Zheng, X.C. Oxidation of benzaldehyde to benzoic acid with aqueous hydrogen peroxide over dodecatungstophosphoric acid. Adv. Mater. Res. 2013, 781, 219–222. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Kim, N.; Lee, J.C. Oxidation of aldehydes to carboxylic acids with hydrogen peroxide and PTSA catalyzed by β-cyclodextrin. Bull. Korean Chem. Soc. 2014, 35, 3366–3368. [Google Scholar] [CrossRef]
- Zeynizadeh, B.; Behyar, T. Fast and efficient method for reduction of carbonyl compounds with NaBH4/Wet SiO2 under solvent free condition. J. Braz. Chem. Soc. 2005, 16, 1200–1209. [Google Scholar] [CrossRef]
- Hao, Y.; Pischetola, C.; Cárdenas-Lizana, F.; Keane, M.A. Selective liquid phase hydrogenation of benzaldehyde to benzyl alcohol over alumina supported gold. Catal. Lett. 2020, 150, 881–887. [Google Scholar] [CrossRef]
- Hwu, J.R.; Gupta, N.K.; Panja, A.; Chang, C.H.; Huang, W.C.; Tsai, Y.R.; Chung, K.S.; Tan, K.T.; Lin, C.C.; Hwang, K.C.; et al. Sustainable Domino C− N/C− C Bond Formation with Outstanding E Factors and High Volume Productivity. Eur. J. Org. Chem. 2024, 27, e202400658. [Google Scholar] [CrossRef]
- Mansour, F.R.; Płotka-Wasylka, J.; Locatelli, M. Modified GAPI (MoGAPI) Tool and Software for the Assessment of Method Greenness: Case Studies and Applications. Analytica 2024, 5, 451–457. [Google Scholar] [CrossRef]
- Manousi, N.; Wojnowski, W.; Płotka-Wasylka, J.; Samanidou, V. Blue Applicability Grade Index (BAGI) and Software: A New Tool for the Evaluation of Method Practicality. Green Chem. 2023, 25, 7598–7604. [Google Scholar] [CrossRef]
- Mansour, F.R.; Omer, K.M.; Płotka-Wasylka, J. A Total Scoring System and Software for Complex Modified GAPI (ComplexMoGAPI) Application in the Assessment of Method Greenness. Green Anal. Chem. 2024, 10, 100126. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).