Abstract
Waste paper, with its high cellulose and hemicellulose content, represents a promising bioresource for producing fermentable sugars in biorefining processes. In this study, five types of waste paper were analyzed for cellulose content, and tissue paper (TP), exhibiting the highest cellulose content, was selected for hydrothermal pretreatment. Optimal pretreatment conditions were determined through single-factor experiments: 160 °C, water as the solvent, and a retention time of 50 min, corresponding to a severity factor (SF) of 3.47. Under these conditions, the reducing sugar yield from pretreated TP reached 0.61 g sugar/g paper, a 38.64% increase compared to untreated TP. The enhancement was attributed to lignin solubilization, disruption of crystalline cellulose regions, and increased specific surface area. These findings demonstrate the effectiveness of hydrothermal pretreatment in improving the enzymatic digestibility of waste paper for biorefining applications.
1. Introduction
The sugar platform has emerged as a central pillar in the development of renewable biofuels and value-added bioproducts, enabling the conversion of carbohydrate-rich biomass into a wide spectrum of chemicals, fuels, and materials [1]. Traditionally, commercial sugar feedstocks have been derived from edible crops such as sugarcane, corn, and starch-based materials. However, increasing concerns about food security, land use conflicts, and environmental sustainability have driven the shift toward second-generation feedstocks—non-food lignocellulosic biomass and industrial/agricultural residues—as alternative sugar sources [2]. This shift aims to ensure a stable and sustainable sugar supply chain without competing with food production.
Globally, the transition from food-based to waste-based sugar production has become a key strategy in achieving the dual goals of waste valorization and carbon neutrality. The depletion of fossil resources, coupled with growing greenhouse gas (GHG) emissions from conventional fuel and chemical production, has reinforced the urgency for biorefinery concepts that can efficiently utilize underexploited biomass [3]. According to the International Energy Agency (IEA), sustainable biomass has the potential to meet up to 27% of global transport fuel demand by 2050, provided that effective conversion technologies are employed. Central to this vision is the efficient release of fermentable sugars from lignocellulosic residues, which serve as essential intermediates for fermentation-based bioprocessing [4].
Among various lignocellulosic wastes, waste paper has attracted attention due to its high carbohydrate content and abundant availability. It constitutes over 35% of total lignocellulosic waste in municipal solid waste streams, with more than 400 million tons generated annually worldwide [5]. Although recycling efforts have mitigated some of its environmental burden, only 50–65% of waste paper is effectively recycled [6]. Moreover, when pulp fibers become too short for reuse, they are rejected from recycling systems and often end up in landfills or incinerators. These non-recyclable fractions typically contain 60–70% cellulose, 10–20% hemicellulose, and 5–10% lignin, making them promising substrates for sugar release and bioconversion [7].
In particular, tissue paper waste represents a significant proportion of non-recyclable fractions due to its low fiber strength and contamination with food residues, oils, or hygiene-related materials [8]. Despite being rich in cellulose, tissue paper is often neglected in bioresource valorization studies due to difficulties in collection, characterization, and standardized processing. However, its physicochemical simplicity (i.e., high cellulose content and low lignin levels) and relatively homogeneous structure compared to agricultural residues offer unique advantages for enzymatic processing, provided that effective pretreatment is applied [9,10].
The enzymatic hydrolysis of waste paper offers a valuable strategy for valorizing this underutilized resource. Enzymatic digestion, driven by cellulases and hemicellulases, releases monosaccharides that serve as direct substrates for microbial fermentation to produce ethanol, organic acids, biosurfactants, and other bio-based chemicals [11]. However, the tight interactions between cellulose, hemicellulose, and residual lignin in paper fibers result in a recalcitrant structure that limits enzymatic digestibility. To overcome this barrier, effective pretreatment is essential to disrupt the compact lignocellulosic matrix, enhance enzyme accessibility, and promote the release of fermentable sugars.
Pretreatment technologies for lignocellulosic materials have evolved significantly, ranging from mechanical comminution and steam explosion to chemical treatments using acids, alkalis, solvents, and oxidative agents [12]. More recently, integrated physicochemical approaches have been developed to combine the benefits of thermal and chemical disruption. Hydrothermal pretreatment (HTP), also known as autohydrolysis or aqueous thermal treatment, has gained particular attention due to its environmental and operational advantages.
HTP operates by heating biomass with water (or aqueous solutions) at temperatures typically between 140 and 220 °C under saturated pressure conditions. The absence of added chemicals minimizes environmental discharge and simplifies downstream processing [13]. During HTP, the breakdown of hemicellulose into soluble oligosaccharides and organic acids (e.g., acetic acid) naturally lowers the system pH and facilitates autohydrolysis of glycosidic linkages. Lignin undergoes partial depolymerization, while cellulose crystallinity may be altered, improving its susceptibility to enzymatic cleavage. Importantly, HTP avoids the formation of heavy inhibitors such as furfural or hydroxymethylfurfural (HMF), which often hinder microbial growth during fermentation [14].
Compared to acid or alkali pretreatments, which often leave behind inhibitory residues and corrode equipment, HTP avoids the use of corrosive chemicals and minimizes environmental pollution [15]. Furthermore, life cycle assessment (LCA) studies have identified HTP as a favorable option due to its lower environmental footprint, reduced chemical consumption, and compatibility with industrial-scale equipment. For instance, Cheng et al. demonstrated that HTP-based systems could achieve comparable sugar yields with fewer downstream washing and detoxification steps [16].
In practice, the effectiveness of HTP can be further enhanced by combining it with mild chemical agents. Acid-assisted HTP (e.g., with diluted sulfuric or acetic acid) increases hemicellulose solubilization and cellulose deconstruction. Alkali-assisted HTP improves lignin removal and fiber swelling. Solvent-assisted HTP using ionic liquids (ILs) or deep eutectic solvents (DESs) has also gained interest in selectively disrupting hydrogen bonding in cellulose-rich matrices. For example, Zhu et al. reported that combining ILs with HTP improved glucose yield by over 40% compared to HTP alone [17]. Meanwhile, ammonia fiber expansion (AFEX)–HTP systems have demonstrated selective deacetylation and delignification effects while maintaining polysaccharide integrity [18].
Despite these advances, most studies on integrated hydrothermal systems have focused on agricultural residues (e.g., corn stover, wheat straw, and bagasse) or woody biomass (e.g., poplar and eucalyptus), with limited attention to municipal waste-derived substrates like waste paper. Moreover, comparative studies on the performance of different solvents during HTP of paper waste are lacking, especially for non-recyclable tissue paper [19]. Key questions regarding solvent selection, process optimization, and the mechanistic pathways of fiber disruption remain largely unexplored [20,21].
In this study, we systematically evaluate the hydrothermal pretreatment of various types of non-recyclable waste paper using different solvents. The novelty of this work lies in its focus on tissue paper, a largely overlooked but abundant lignocellulosic feedstock. By optimizing reaction conditions and elucidating the structural changes induced by pretreatment, we aim to improve the enzymatic hydrolysis performance of waste paper and enhance its potential as a sustainable sugar source for biofuel production. The findings contribute to both fundamental understanding and applied innovation in the context of waste-to-bioenergy technologies. Furthermore, by expanding the scope of sugar-based feedstocks to include municipal solid waste fractions, this work supports the development of more resilient and decentralized biorefinery systems.
2. Materials and Methods
2.1. Materials
Five types of waste paper—office paper, cardboard, tissue paper, newspaper, and kraft paper—were collected from a university campus in Beijing, China. Commercial cellulase (Sinopharm Chemical Reagent Co. Ltd., Shanghai, China; activity: 145 FPU/g) and β-glucosidase (Sinopharm Chemical Reagent Co. Ltd., Shanghai, China; activity: 250 IU/g) were obtained from Sigma-Aldrich (St. Louis, MO, USA) and used for enzymatic saccharification. The deep eutectic solvent (DES) was prepared by mixing choline chloride and lactic acid at a molar ratio of 1:2 and stirring at 80 °C until a homogeneous liquid was formed. The DNS reagent was prepared according to the standard dinitrosalicylic acid method for quantifying reducing sugars [22].
2.2. Basic Quantitative Compositional Analysis
To determine the most suitable type of waste paper for enzymatic hydrolysis, a basic compositional analysis was conducted. The cellulose, hemicellulose, and lignin fractions of the five paper types were analyzed using a Fiber analyzer (ANKOM220, Macedon, NY, USA).
Dry Matter Analysis: The dry matter content was determined by drying the samples in an oven at 105 °C until a constant weight was reached, following the ASTM D2974-1987 standard [4].
Ash Content Analysis: Ash content was measured by incinerating the samples in a muffle furnace at 575 °C, based on the ASTM E1755-2007 method [17]. The residual mass was weighed after complete combustion [17].
2.3. Material Characterization
X-ray Diffraction (XRD): The crystallinity of waste paper samples was analyzed using an X-ray diffractometer (Rigaku SmartLab, Japan) equipped with Cu-Kα radiation (λ = 1.5406 Å). The samples were finely ground and placed onto a glass sample holder. Scanning was conducted in reflection mode over a 2θ range of 5–60° at a scanning speed of 2°/min. The crystallinity index (CrI) was calculated using the peak deconvolution method by separating crystalline and amorphous regions within the diffraction pattern.
Scanning Electron Microscopy (SEM): Surface morphologies of the raw and pretreated samples were observed using a field emission scanning electron microscope (Hitachi SU8010, Japan). Samples were air-dried, fixed on aluminum stubs using carbon tape, and sputter-coated with a thin layer of gold (approximately 5 nm) using a magnetron sputtering instrument. Images were captured at accelerating voltages of 5–10 kV and magnifications of 1000× and 10,000×.
Energy Dispersive X-ray Spectroscopy (EDS): Elemental analysis of the waste paper surface was performed using an EDS system (Oxford X-MaxN, UK) attached to the SEM. The analysis was conducted under high vacuum mode at an accelerating voltage of 15 kV. Spectra and elemental mapping were obtained for both untreated and pretreated samples to compare changes in elemental distribution.
3D Excitation–Emission Matrix (3DEEM) Spectroscopy: The hydrolysate samples were analyzed using a fluorescence spectrophotometer (Hitachi F-7100, Japan) to detect the presence of fluorescent compounds such as phenolics and furans. Measurements were conducted at room temperature. Excitation wavelengths ranged from 200 to 400 nm in 5 nm increments, and emission wavelengths were recorded from 250 to 550 nm. The slit widths for excitation and emission were both set at 5 nm. The resulting EEM spectra were used to qualitatively evaluate the presence of potential inhibitory compounds in the hydrolysates.
2.4. Chemical Composition and Sugar Analysis
The reducing sugar content in the hydrolysates was quantified using the dinitrosalicylic acid (DNS) spectrophotometric method. Absorbance was measured at 540 nm using a UV–Vis spectrophotometer (TU-1810, Persee, China). The reducing sugar yield was calculated using the following formula:
where Q is the sugar yield (g/g), q is the measured sugar concentration (mg/mL), d is the dilution factor, V is the total hydrolysate volume (L), v is the sample volume analyzed (mL), and R is the initial dry mass of waste paper (g).
The severity factor (SF), expressed as log R0, was calculated to quantify the combined effects of temperature and residence time during hydrothermal pretreatment. It is defined as follows:
where t is the reaction time (min) and T is the reaction temperature (°C).
Glucose and xylose concentrations in the hydrolysates were determined using high-performance liquid chromatography (HPLC, Agilent 1260 Infinity II, Santa Clara, CA, USA) equipped with a refractive index detector (RID) and a Shodex SH1011 column (300 mm × 8.0 mm I.D., Showa Denko, Japan). The mobile phase was a 5 mmol/L H2SO4 solution, operated at a flow rate of 0.6 mL/min. The column temperature was maintained at 60 °C, and the injection volume was 10 μL. Prior to injection, hydrolysate samples were filtered through a 0.22 μm PTFE membrane filter to remove particulate matter. Each analysis was conducted in triplicate to ensure accuracy and reproducibility.
2.5. Hydrothermal Pretreatment of Waste Paper
Waste paper was first shredded into fine particles using a mechanical grinder. For each pretreatment batch, 2 g of shredded paper was mixed with 70 mL of solvent and loaded into a 100 mL Teflon-lined stainless-steel autoclave. Three treatment groups were prepared based on the pretreatment solvent used: distilled water (Group A), 1% NaOH solution (Group B), and a deep eutectic solvent (DES) composed of choline chloride and lactic acid at a 1:2 molar ratio (Group C). A control group (Group D) received no pretreatment. All autoclaves were heated to 160 °C and maintained at that temperature for 50 min. After cooling to room temperature, the mixtures were vacuum filtered and thoroughly washed with deionized water until the pH of the filtrate was neutral. The treated solids were then dried at 60 °C for subsequent enzymatic hydrolysis and characterization [17].
2.6. Enzymatic Hydrolysis
Hydrolysis experiments were conducted using 2 g (dry weight) of untreated or pretreated waste paper at a biomass loading of 4% (w/v) [19]. Samples were incubated in 50 mL of 0.05 M citrate buffer (pH 4.8) at 50 °C with shaking at 100 rpm for 72 h. Enzymes were loaded at 50 FPU/g for cellulase and 25 FPU/g for β-glucosidase. Supernatants were withdrawn at 6, 12, 24, 48, and 72 h for sugar analysis. Each enzymatic hydrolysis was performed in triplicate to ensure reproducibility [23].
3. Results and Discussion
3.1. Basic Quantitative Compositional Analysis of the Material
3.1.1. Analysis of Raw Material Components
Cellulose was considered to represent the glucose content, while hemicellulose was associated with the xylose content in the subsequent enzymatic hydrolysis. The reducing sugar solution obtained during the saccharification of waste paper was used as the basis for producing liquid biofuels [23]. The analysis of raw cellulose, hemicellulose, and lignin from five types of waste paper is presented in Table 1. Tissue paper (TP) exhibited the highest cellulose content among all waste paper types at 59.6 ± 2.98%, followed by newspaper and kraft paper, with contents of 51.47 ± 2.57% and 49.45 ± 2.47%, respectively. These results were consistent with previously reported values by Nishimura [7], Wang [9], Annamalai [10], and other researchers. The high cellulose content in discarded TP (59.6 ± 2.98%) was considered more favorable for biomass conversion than that of maize stover (30.3%), rice straw (35.1%), and wheat straw (42.4%) [24]. An additional advantage of waste TP was its classification as non-recyclable waste, which could offer notable environmental and economic benefits if appropriately valorized. Therefore, TP was selected in this study as the raw material for subsequent hydrolysis.

Table 1.
Quantitative compositional analysis of the tested types of waste paper.
3.1.2. Effect of Different Hydrothermal Solvent Pretreatments
To evaluate the most effective solvents for hydrothermal pretreatment of tissue paper (TP), three representative solvents—acidic, neutral, and alkaline (DES, purified water, and NaOH)—were selected based on variations in their chemical compositions. The primary role of these solvents in hydrothermal systems was to facilitate structural disruption of lignocellulosic biomass by elevating the concentration of ionized species (H+ and OH−), which catalyze the cleavage of lignin and hemicellulose fractions [25].
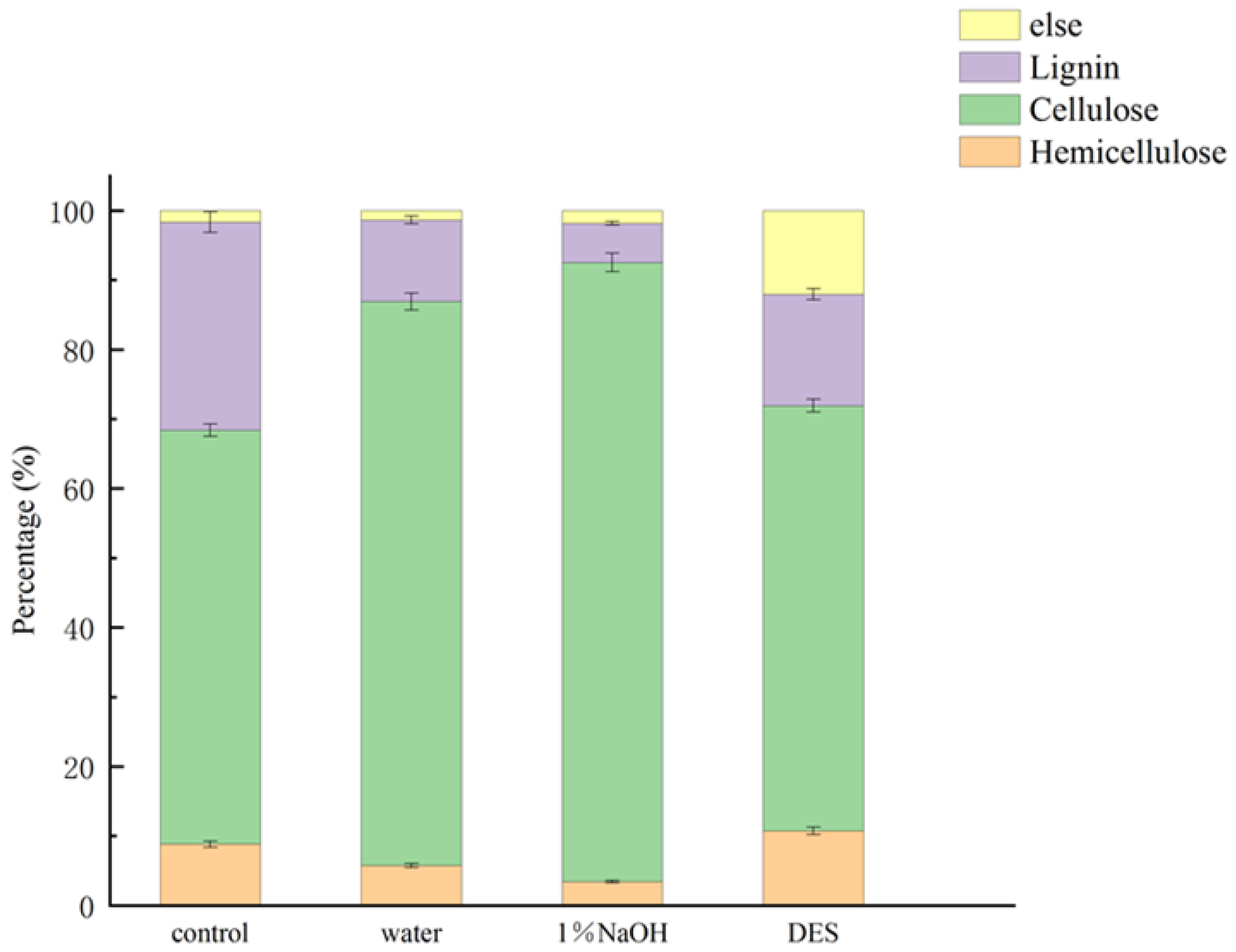
Figure 1 presents the compositional changes in TP after treatment. The cellulose content increased under all pretreatment conditions. The order of effectiveness was DES < water < 1% NaOH. Treatment with 1% NaOH increased cellulose content to 94.0 ± 4.62%, compared to 68.42 ± 3.42% in the control group—a 40.22% improvement. Concurrently, lignin content was reduced to 5.66 ± 0.28% from 29.93 ± 1.50%, indicating an 81.09% reduction. These improvements were attributed to the alkaline hydrolysis of lignin–carbohydrate ester linkages and the saponification of hemicellulose–lignin structures [26]. The effective removal of lignin and hemicellulose increased the internal surface area and porosity of the biomass [27]. Hydrothermal pretreatment with pure water also enhanced cellulose enrichment, reaching 92.67 ± 4.35%, a 35.44% increase. Lignin content decreased to 11.73 ± 0.59%, equivalent to a 60.81% reduction. The ionization of water at high temperature and pressure produced sufficient H+ and OH− ions to disrupt the lignocellulose matrix. DES treatment led to a more moderate increase in cellulose content (71.92 ± 3.60%) and a lignin reduction of 46.37%. The comparatively lower efficiency of DES was likely due to excessive ionic strength under hydrothermal conditions, which may have caused partial degradation of cellulose and hemicellulose. Despite this, prior studies showed that acidic DES could enhance xylose release from hemicellulose and assist in the breakdown of lignin [28,29].
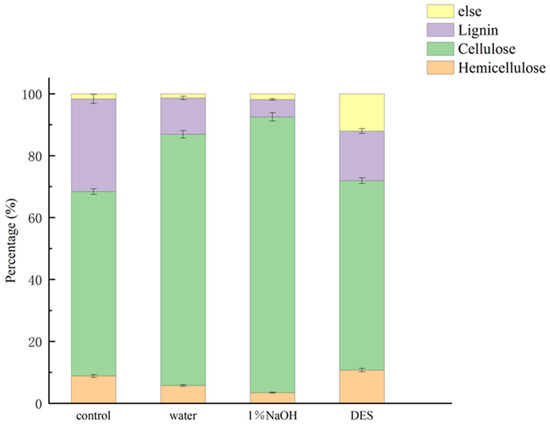
Figure 1.
Percentage of material composition of TP after different pretreatments.
To further explore the impact of each solvent on the formation of fermentation inhibitors, 3DEEM fluorescence analysis was performed on the hydrolysates (Figure 1). Peaks observed at EX/EM = 340–380/340–380 nm indicated the presence of fulvic acid-like substances and phenolics, typically derived from lignin degradation. Among all treatments, DES-treated hydrolysate exhibited the strongest fluorescence intensity, suggesting higher levels of soluble lignin compounds. The NaOH-treated group also showed notable phenolic peaks, whereas the water-treated group exhibited the lowest intensity. Importantly, no detectable fluorescence signal was observed at EX/EM = 400/500 nm, which corresponds to furfural, indicating that degradation of cellulose and hemicellulose into furan compounds was minimal under these pretreatment conditions. This aligns with findings by Annamalai and Rocha, who also reported the absence of furfural and HMF in similarly pretreated lignocellulosic substrates [27,28].
In summary, hydrothermal pretreatment using 1% NaOH, water, and DES (in descending order of efficiency) resulted in effective cellulose enrichment and lignin removal, with varying degrees of inhibitor formation. The reaction was driven by the catalytic action of ionized water under high-temperature conditions, leading to cleavage of hemicellulose–lignin structures and the release of acetyl groups, thus enhancing delignification [30]. Given that lignin severely inhibits enzymatic saccharification, its solubilization during pretreatment was critical for improving enzyme accessibility [13]. This was consistent with previous studies on chili pepper and maize stover, where lignin content was substantially reduced and inhibitor profiles altered following hydrothermal processing [31,32]. While compositional analysis revealed major structural changes, the combined inhibitor data highlighted the differential chemical environments created by each solvent. This emphasizes the importance of integrated structural and chemical assessment in evaluating hydrothermal pretreatment mechanisms.
3.2. Characterization of TP
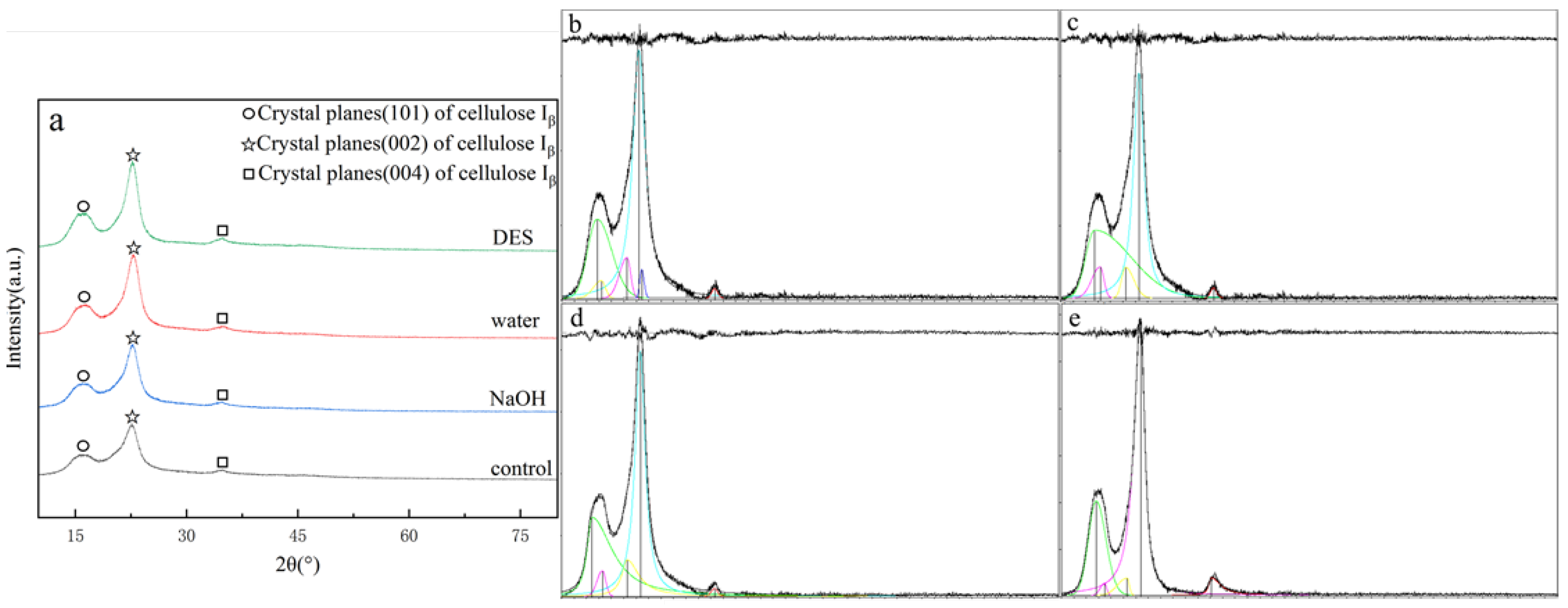
The X-ray diffraction (XRD) patterns of tissue paper (TP), both before and after hydrothermal treatment with various solvents, are shown in Figure 2a. The diffraction peaks in the 2θ range of 16–17° were attributed to the (101) crystalline plane of cellulose I_β, representing the amorphous region of cellulose [6], while the peaks in the 2θ range of 22–23° were associated with the (002) crystalline plane of cellulose I_β, indicating the crystalline region of cellulose [33]. The peaks at 2θ between 34° and 35° corresponded to the (004) plane, further confirming the presence of cellulose I [34]. Although the general diffraction patterns after hydrothermal treatment remained similar, variations in peak intensities and areas were observed. As illustrated in Figure 2b–e, the crystallinity index (CrI) of cellulose in the samples was calculated using the peak deconvolution method (PDM) with Jade 6.5 (MDI, Newton Square, PA, USA) [35]. The CrI of untreated TP was measured at 47.92%. After hydrothermal pretreatment, it was reduced to 33.7% with water, 34.27% with 1% NaOH, and 29.28% with DES. The observed reductions in CrI indicated that the crystalline structure of the tissue paper was disrupted. Hydrothermal pretreatment had a more pronounced effect on the crystalline regions of cellulose compared to the amorphous structure [10]. Hydrothermal conditions resulted in swelling and hydrolytic degradation, which led to a lower-order structure [36]. The destruction of the crystalline structure of cellulose, lignin, and hemicellulose effectively increased biomass porosity, enhancing enzyme accessibility to cellulose. Rahmani et al. demonstrated that hydrothermal pretreatment reduced biomass crystallinity and polymerization while increasing surface area [37]. Numerous studies have shown that reducing the crystallinity index of cellulosic biomass enhances biomass resource conversion efficiency [38]. These crystallinity changes helped explain the total cellulose enrichment mechanism discussed in Section 3.1.2, suggesting that crystalline disruption was accompanied by lignin solubilization.
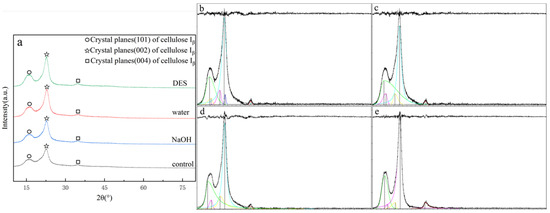
Figure 2.
XRD patterns (a) and jade images (b–e) of tissue paper with different hydrothermal solvents: (b) control, (c) water, (d) NaOH, and (e) DES.
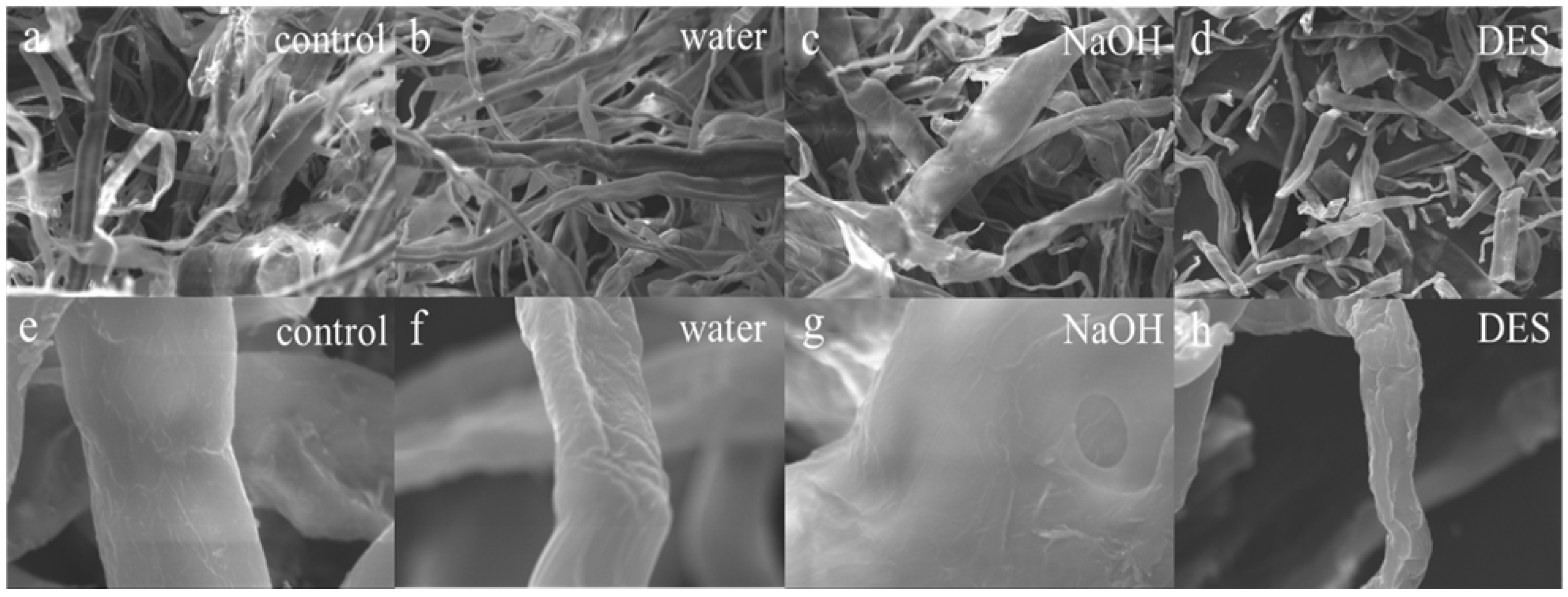
The scanning electron microscope (SEM) provided a more intuitive observation of the surface microstructural changes in the material. Figure 3a–h shows the micromorphological alterations in fiber surfaces before and after treatment with different solvents. At a magnification of 1.00 K, as shown in Figure 3a–d, the pretreated samples (Figure 3b–d) exhibited cellulose breakage, shorter lengths, and a staggered arrangement compared to the untreated samples (Figure 3a). Such disruption was expected to enhance cellulose biodegradability and facilitate ethanol production. At a higher magnification of 10.00 K, Figure 3e–h reveals that the unpretreated sample (Figure 3e) had smooth, intact fibers, which posed a rate-limiting barrier for subsequent hydrolysis. Samples treated with solvents (Figure 3f–h) exhibit varying levels of surface collapse and roughness. Figure 3g, using NaOH as a solvent, exhibits surface voids indicative of lignin removal and hemicellulose breakdown. Conversely, DES-treated samples (Figure 3h) demonstrated severe structural disintegration, consistent with excessive degradation of biomass components. The breakdown of these cavities and fibers increased biomass porosity, thereby exposing more cellulose to enzymatic attack by microorganisms. This observation also explained why the crystallinity after DES treatment was the lowest among all solvents. A similar trend was observed by Song in lignocellulosic biomass after hydrothermal pretreatment [39]. Tang documented fiber collapse in wheatgrass after surfactant-assisted hydrothermal pretreatment [40]. Hydrothermal pretreatment, conducted under high temperature and pressure, resulted not only in destructive effects from ionized ions but also in increased vapor pressure, which elevated the internal pressure within the container, leading to various degrees of surface folds and consequently increasing the specific surface area of the material. SEM results confirmed that TP fiber morphology was substantially altered following hydrothermal treatment with various solvents, mainly due to cellulose modification and hemicellulose hydrolysis. It was evident that hydrothermal pretreatment had a more significant impact on the innate barrier structure for the decay of lignocellulose, establishing favorable conditions for the transformation of cellulose in discarded tissue paper.
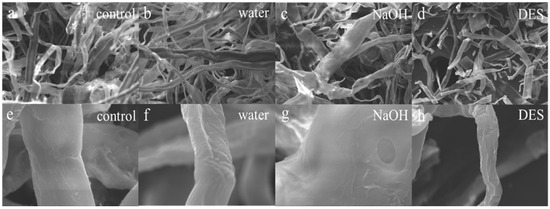
Figure 3.
SEM images (a–h) of tissue paper with different hydrothermal solvents: (a–d) Mag = 1.00 KX and (e–h) Mag = 10.00 KX.
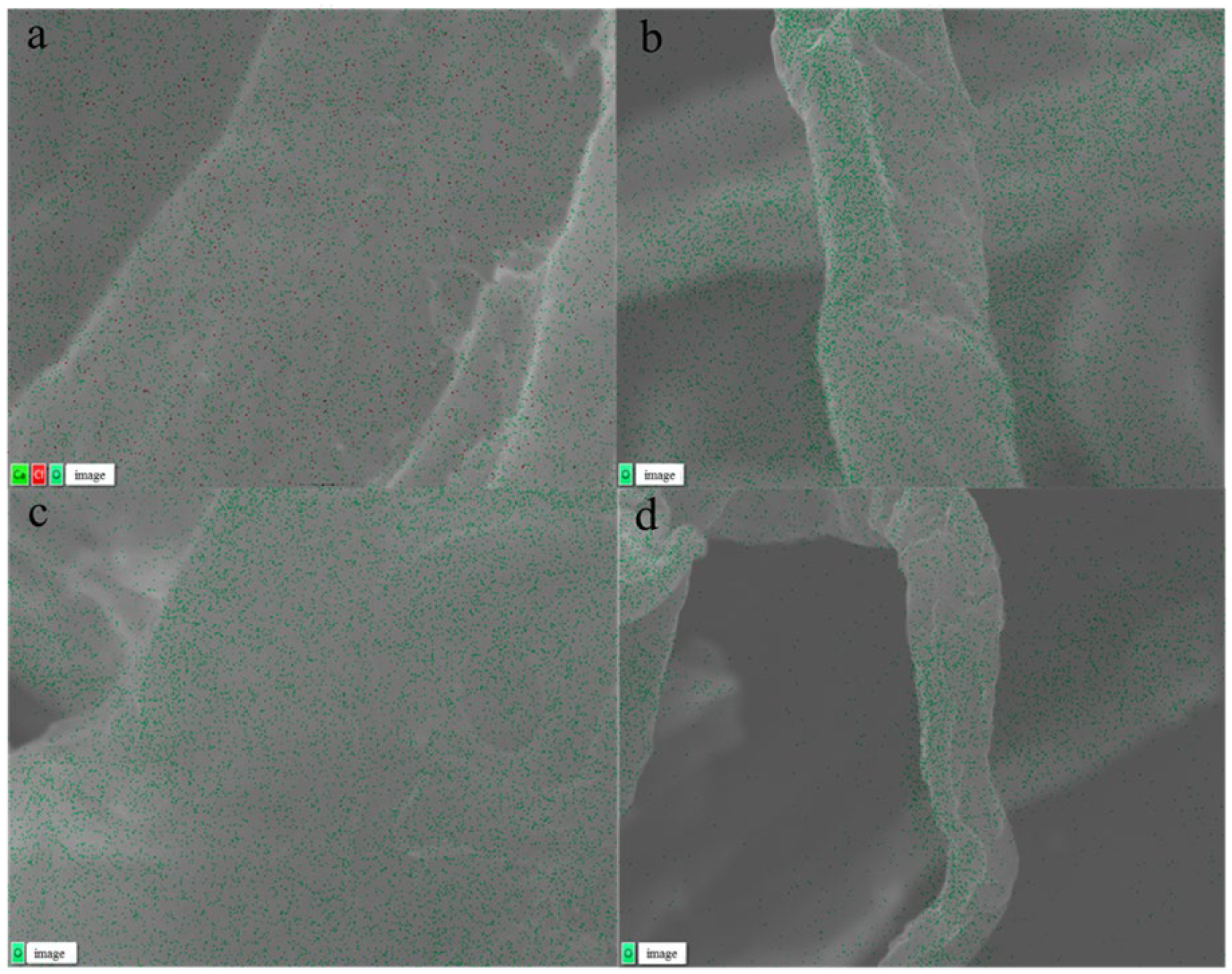
EDS was employed for microzone compositional analysis, quantitative analysis, and elemental distribution analysis [41]. Figure 4a–d presents the EDS findings for hydrothermal pretreatment using various solvents. The untreated tissue paper (TP), depicted in Figure 4a, exhibited the presence of calcium (Ca) and chlorine (Cl) elements on its surface, along with oxygen (O) elements representative of cellulose, hemicellulose, and lignin. The presence of Ca and Cl indicated the additive components in waste tissue paper, as calcium carbonate (CaCO3) and other additives such as fillers, glues, and pigments (FGPs) are commonly used in the papermaking process to enhance surface finish and printing properties [42]. The absence of elemental Ca and Cl on the surface of TP after hydrothermal pretreatment (Figure 4b–d) indicated that these additive components were effectively removed during the washing step following pretreatment. Most of the CaCO3 and other additives likely reacted with H+ and OH− ions to form soluble salts, which were subsequently removed during the washing step. The removal of these mineral deposits enhanced enzyme accessibility to the cellulose matrix, as calcium ions and insoluble residues can interfere with enzyme binding and reduce hydrolysis efficiency. By eliminating these barriers, the enzyme was able to make more complete and effective contact with cellulose during hydrolysis [43]. The EDS analysis results confirmed that hydrothermal pretreatment with different solvents effectively removed surface additives, thereby improving cellulose accessibility in the biomass.
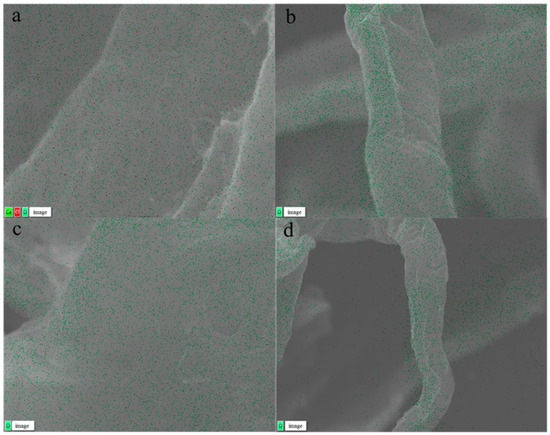
Figure 4.
EDS images (a–d) of tissue paper with different hydrothermal solvents: (a) control, (b) water, (c) NaOH, and (d) DES.
3.3. 3DEEM Analysis of Pretreatment Hydrolysate
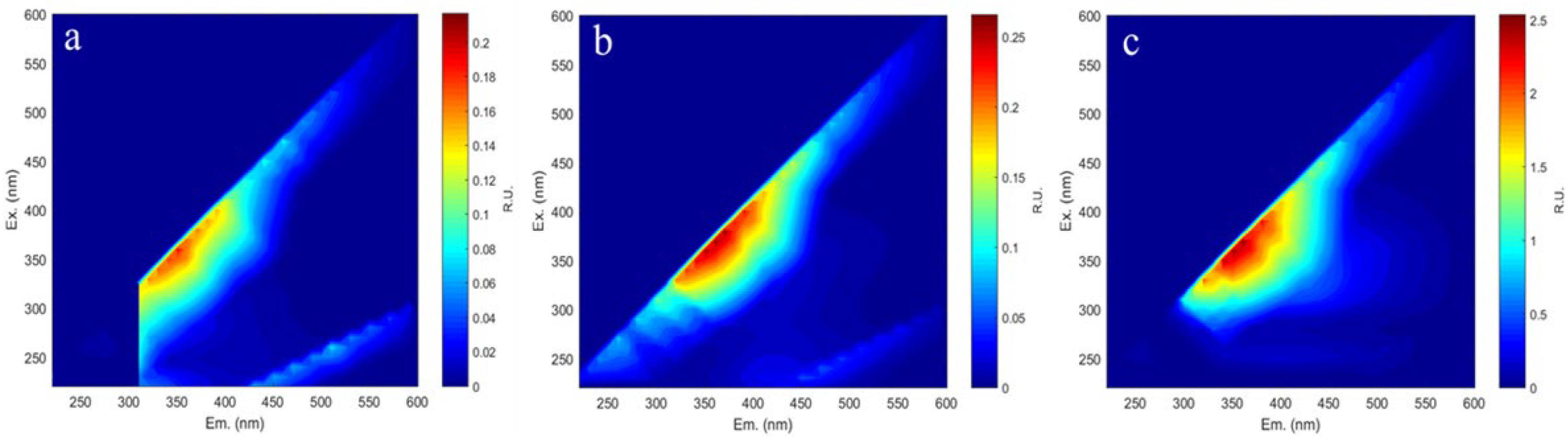
Hydrothermal pretreatment efficiently exposed cellulose to biodegradation by eliminating portions of the hemicellulose and lignin fractions. However, the degradation of these components resulted in the formation of compounds that were difficult to degrade. For example, pentose dehydration from hemicellulose was known to generate furfural, while cellulose-derived glucose was degraded to hydroxymethylfurfural (HMF). Additionally, lignin degradation produces phenolics, which are detrimental to yeast cells during subsequent bioethanol fermentation [44]. The inhibitors formed in hydrolysates derived from hydrothermally pretreated waste tissue paper with different solvents were analyzed via 3D excitation–emission matrix (3DEEM) fluorescence spectroscopy, as presented in Figure 5a–c.
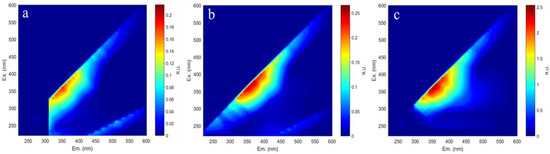
Figure 5.
3DEEM images (a–c) of tissue paper hydrolysate with different hydrothermal solvents: (a) water, (b) NaOH, and (c) DES.
The hydrolysates obtained from water, 1% NaOH, and DES pretreatments showed typical fluorescence peaks at excitation/emission (EX/EM) wavelengths of 340–380 nm/340–380 nm. Generally, the fluorescence peaks in this range are attributed to Class II (Ex around 310–360 nm, Em around 370–450 nm) and are identified as fulvic acid-like fluorescence, associated with the carbonyl and carboxyl groups of dissolved substances [45]. According to Shi, phenolic characteristics were typically represented by fluorescence peaks within the EX/EM range of 300–400 nm [46]. The intensity of these peaks varied, with the highest observed in the hydrolysate using DES as a solvent, followed by 1% NaOH and pure water. This trend correlated with the relative degree of lignin solubilization. Importantly, no fluorescence peak was detected at EX/EM = 400/500 nm, the spectral position typically associated with furfural [3]. This finding implies that furfural was not present at detectable levels in the hydrolysate post-pre-treatment, indicating that the degradation of cellulose and hemicellulose was less severe under the milder hydrothermal treatment conditions. Similarly, Annamalai reported the absence of detectable furfural and HMF in hydrolysates from untreated and pretreated office paper, though phenolics were present due to lignin degradation [47]. Similarly, Rocha reported that furfural and 5-HMF were not detected in office paper pretreated with 1% H2SO4 [48]. Only phenolic compounds originating from lignin were identified, while no degradation products of cellulose (HMF) or hemicellulose (furfural) were found. This observation indicated that hydrothermal pretreatment primarily facilitated the solubilization of lignin, with minimal decomposition of cellulose and hemicellulose. This also elucidated why the lignin content in the materials decreased to varying degrees after pretreatment, resulting in an increased ratio of cellulose to hemicellulose. The analysis of the hydrolysate provided mechanistic insight into hydrothermal pretreatment, revealing that lignin was depolymerized into soluble phenolics, which disrupted the structural integrity of the biomass.
3.4. Effect of Various Hydrothermal Conditions on the Enzymatic Properties of TP
A prosperous pretreatment method needs to generate extremely digestible solids, with the availability of lignocellulosic biomass being essential in establishing the effectiveness of transforming it into bioproducts [49]. The yield of reducing sugars obtained from enzymatic hydrolysis was used as a precise indicator of pretreatment effectiveness. As a key product of lignocellulosic biomass resource utilization, the level of yield has an impact on the production of high-value end-products such as bioethanol, depending on its output rate. Mechanistic analysis revealed that hydrothermal pretreatment enhanced the hydrolysis degree of lignocellulosic biomass, altered its elemental composition, disrupted its surface structure, and modified its lignocellulosic fraction content.
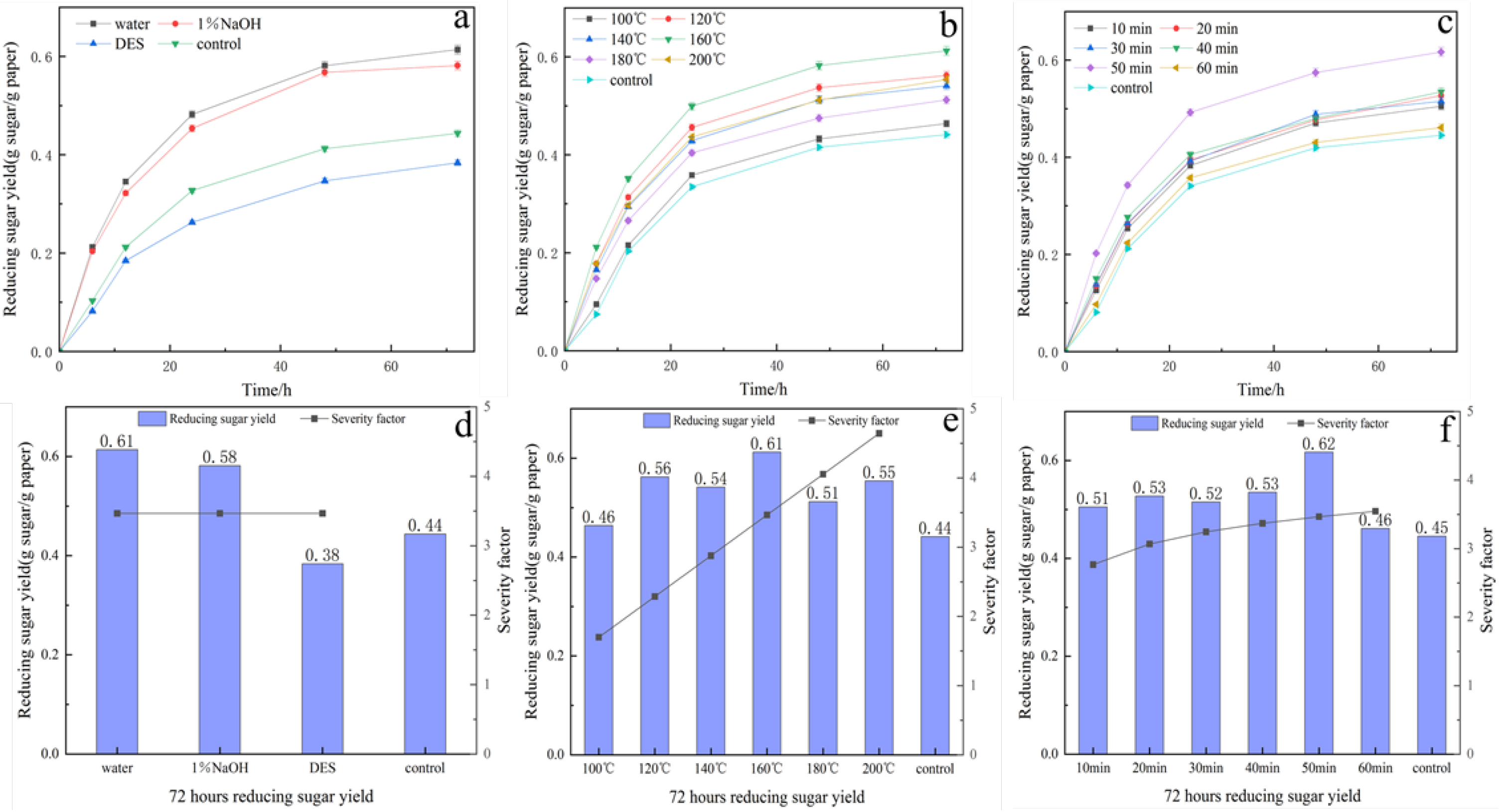
The hydrothermal results for various solvents are presented in Figure 6a,d. Hydrothermal temperature was fixed at 160 °C, hydrothermal time was 50 min, and the calculation value of SF was 3.47. Water-based hydrothermal pretreatment resulted in the highest enzymatic accessibility of the biomass. The resulting enzyme hydrolysis yield of the pretreated waste tissue paper using water, 1% NaOH, and DES was 0.61 g sugar/g paper, 0.58 g sugar/g paper, and 0.38 g, respectively, while those in the control group was 0.44 g sugar/g paper. Organic acid-based DES disrupts the hydrogen bonding network between lignocellulosic components, while selectively solubilizing specific components of the feedstock. However, it has the highest pKa of all tested solvents and ionizes the largest amount of hydrogen ions to attack lignocellulose during pretreatment [50]. The presence of reactive compounds during ionic liquid pretreatment was also known to inhibit hydrolytic enzymes [51]. This accounts for the 13.64% decrease in enzymatic hydrolysis results for tissue paper pretreated with hydrothermal-assisted organic acid-based DES compared to the blank control group. Meanwhile, hydrothermal pretreatment using 1% NaOH improved the results by 31.82%, compared to the control group. This improvement was attributed to NaOH’s ability to cleave the α,β-aryl ether bonds between hemicellulose and lignin, leading to lignin disintegration and hemicellulose depolymerization [32]. The enzyme hydrolysis yield from the hydrothermal pretreatment with 1% NaOH as solvent was lower than that of the hydrothermal pretreatment with pure water as solvent due to the partial absence of hemicellulose in the TP. Chen et al. achieved a glucose yield of 48.8% using hydrothermal pretreatment at 180 °C for 0.5 h [52]. Dubey et al. conducted diverse pre-treatments on corn stover [33]. The findings indicate that higher sugar yields can be achieved for waste tissue paper through pre-treatment with pure water at appropriate temperatures without the addition of solvents or acidic and alkaline substances. This approach is deemed more economically and practically viable compared to the expense of other chemicals.
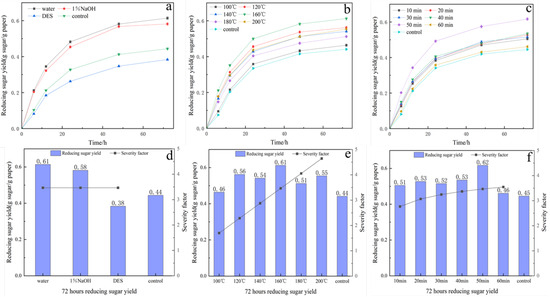
Figure 6.
Enzyme hydrolysis images (a–f) of tissue paper with different hydrothermal pretreatment conditions: (a,d) solvent, (b,e) temperature, and (c,f) time.
Subsequently, the impact of hydrothermal temperature and duration on the enzymatic yield was examined with optimized hydrothermal solvents. How to regulate the effect of the hydrothermal pretreatment process effectively is crucial for the technology’s application. The primary factors affecting hydrothermal pretreatment were identified as temperature and time. These ions act as autocatalysts, forming acids and bases that disrupt the cross-linking structure of the lignocellulosic biomass, resulting in open and porous matrices. This mechanism increased the porosity and accessibility of cellulose for enzymatic saccharification [19]. The physical properties of water, including viscosity, diffusivity, polarity, dielectric constant, and density, were altered under high-temperature conditions. Specifically, reduced water viscosity resulted in higher molecular mobility and more efficient mass transfer, thereby promoting chemical reactions [53]. The results displayed in Figure 6b,e demonstrate that the greatest enzymatic hydrolysis yield of 0.61 g sugar/g paper was attained at a temperature of 160 °C. The experimental findings indicated that excessive or insufficient hydrothermal temperatures had an adverse effect on the liberation of reducing sugars from lignocellulosic biomass. Lower hydrothermal temperatures do not completely destroy the cellulose structure, and only a fraction of the hemicellulose undergoes hydrolytic degradation [54]. The slowly degradable fraction of the hemifiber degrades only after the fast-degrading fraction becomes stable, yet excessive hemicellulose degradation was observed at high hydrothermal temperatures [55]. The influence of hydrothermal temperature on pretreatment can be assessed by the change in pressure, which fluctuates with the hydrothermal temperature. This is consistent with the ideal gas equation of state, where pressure varies proportionally to the hydrothermal temperature and pressure, with the volume remaining constant. The consequence of high pressure on lignocellulosic biomass is demonstrated in its delignification [15]. Therefore, it was established that the optimal reaction temperature is 160 °C.
Reaction time was identified as another key factor affecting pretreatment efficiency. The results of the experiment can be seen in Figure 6c,f. The maximum enzymatic hydrolysis yield achieved was 0.61 g sugar/g paper. This was achieved after hydrothermal heating for 50 min at 160 °C. The findings revealed that both insufficient and excessive treatment durations hindered the release of reducing sugars. A brief pretreatment time is insufficient for breaking down the lignocellulosic biomass structure; however, cellulose, hemicellulose, and lignin are degraded to different extents as the treatment time extends, leading to the rise in reducing sugars and overall sugars in the hydrolysate. The data confirmed that the optimal enzymatic yield was obtained after 50 min of hydrothermal treatment.
Several studies have also shown that the increase in saccharification yield of pretreated substrates is a result of the removal of hemicellulose, enhancing fiber porosity and increasing enzyme accessibility to cellulose fractions [56,57]. However, overly harsh hydrothermal conditions were found to cause partial hydrolysis of holocellulose and damage to the lignocellulosic matrix, which led to increased energy consumption and reduced overall efficiency. Therefore, it is necessary to investigate optimal hydrothermal pretreatment conditions that can facilitate a high degree of enzyme accessibility whilst minimizing the loss of whole cellulose. The ideal hydrothermal pretreatment condition for TP was established through one-way optimization experiments, identifying pure water as solvent, and a temperature of 160 °C maintained for 50 min, with an optimum SF of 3.47. Under these conditions, the reducing sugar yield after 72 h of enzymatic hydrolysis reached 0.61 g sugar/g paper, which represented a 38.64% improvement compared to the untreated TP.
4. Conclusions
In this study, the cellulose, hemicellulose, and lignin compositions of five types of waste paper were analyzed. Tissue paper (TP), which exhibited the highest total cellulose content (68.42%), was selected for further investigation. Hydrothermal pretreatment using water as a solvent was applied to enhance cellulose accessibility for enzymatic hydrolysis. After pretreatment, the total cellulose content in TP increased to 92.67%, while the crystallinity decreased from 47.9% to 33.6%. Following 72 h of enzymatic hydrolysis, the reducing sugar yield reached 0.61 g sugar/g paper, representing a 38.64% improvement compared to untreated TP. Mechanistic analysis indicated that hydrothermal pretreatment facilitated biomass conversion through high-temperature water ionization and high-pressure structural disruption, which collectively enhanced lignin degradation and its transformation into soluble phenolic compounds. These effects improved enzymatic accessibility and saccharification efficiency. Overall, this study demonstrated that hydrothermal pretreatment is a clean, effective, and scalable strategy for valorizing non-recyclable tissue paper waste. The findings provide theoretical and technical support for integrating underutilized lignocellulosic materials into biorefinery pathways aimed at bioethanol or biochemical production.
Author Contributions
Methodology, S.W.; Formal analysis, J.S.; Investigation, Y.L.; Resources, X.Y.; Data curation, P.L.; Writing—original draft, J.Y.; Project administration, D.Q.; Funding acquisition, H.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Key & D Program of China (2022YFE0105700), Key R&D Program of Xinjiang Uygur Autonomous Region (2022B02021), and Ordos Science and Technology Major Project (ZD20232319).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
Yong Liang, Shuang Wu and Juncheng Song were employed by the Shougang Environmental Industry Co., Ltd., and Xiaobin Yang was employed by the Beijing Shougang Ecological Technology Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Zhang, J.; Rentizelas, A.; Zhang, X.; Li, J. Sustainable production of lignocellulosic bioethanol towards zero waste biorefinery. Sustain. Energy Technol. Assess. 2022, 53, 102627. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, A.; Sivakumar, N.; Verma, J.P. Deconstruction of lignocellulosic biomass for bioethanol production: Recent advances and future prospects. Fuel 2022, 327, 125109. [Google Scholar] [CrossRef]
- Dharmaraja, J.; Shobana, S.; Arvindnarayan, S.; Francis, R.R.; Jeyakumar, R.B.; Saratale, R.; Veeramuthu, A.K.; Bhatia, S.K.; Kumar, V.; Kumar, G. Lignocellulosic biomass conversion via greener pretreatment methods towards biorefinery applications. Bioresour. Technol. 2022, 369, 128328. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.-L.; Zhu, Y.-H.; Su, H.-Y.; Sun, G.-T.; Kang, F.-R.; Zhu, M.-Q. Life cycle assessment and techno-economic analysis of fuel ethanol production via bio-oil fermentation based on a centralized-distribution model. Renew. Sustain. Energy Rev. 2022, 167, 112714. [Google Scholar] [CrossRef]
- Annamalai, N.; Sivakumar, N.; Oleskowicz-Popiel, P. Enhanced production of microbial lipids from waste office paper by the oleaginous yeast Cryptococcus curvatus. Fuel 2018, 217, 420–426. [Google Scholar] [CrossRef]
- Wang, B.; Li, K.; Nan, D.-H.; Feng, S.-Y.; Hu, B.; Wang, T.-P.; Lu, Q. Enhanced production of levoglucosenone from pretreatment assisted catalytic pyrolysis of waste paper. J. Anal. Appl. Pyrolysis 2022, 165, 105567. [Google Scholar] [CrossRef]
- Nishimura, H.; Tan, L.; Sun, Z.Y.; Tang, Y.Q.; Kida, K.; Morimura, S. Efficient production of ethanol from waste paper and the biochemical methane potential of stillage eluted from ethanol fermentation. Waste Manag. 2016, 48, 644–651. [Google Scholar] [CrossRef]
- Kumar, V.; Pathak, P.; Bhardwaj, N.K. Waste paper: An underutilized but promising source for nanocellulose mining. Waste Manag. 2020, 102, 281–303. [Google Scholar] [CrossRef]
- Wang, L.; Sharifzadeh, M.; Templer, R.; Murphy, R.J. Technology performance and economic feasibility of bioethanol production from various waste papers. Energy Environ. Sci. 2012, 5, 5717–5730. [Google Scholar] [CrossRef]
- Annamalai, N.; Al Battashi, H.; Anu, S.N.; Al Azkawi, A.; Al Bahry, S.; Sivakumar, N. Enhanced Bioethanol Production from Waste Paper Through Separate Hydrolysis and Fermentation. Waste Biomass Valorization 2018, 11, 121–131. [Google Scholar] [CrossRef]
- Meenakshisundaram, S.; Fayeulle, A.; Leonard, E.; Ceballos, C.; Pauss, A. Fiber degradation and carbohydrate production by combined biological and chemical/physicochemical pretreatment methods of lignocellulosic biomass—A review. Bioresour. Technol. 2021, 331, 125053. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K.; Ahuja, V.; Chandel, N.; Gurav, R.; Bhatia, R.K.; Govarthanan, M.; Tyagi, V.K.; Kumar, V.; Pugazendhi, A.; Banu, J.R. Advances in algal biomass pretreatment and its valorisation into biochemical and bioenergy by the microbial processes. Bioresour. Technol. 2022, 358, 127437. [Google Scholar]
- Singh, S.; Jaiswal, D.K.; Sivakumar, N.; Verma, J.P. Developing Efficient Thermophilic Cellulose Degrading Consortium for Glucose Production From Different Agro-Residues. Front. Energy Res. 2019, 7, 61. [Google Scholar] [CrossRef]
- Yan, W.; Xu, H.; Lu, D.; Zhou, Y. Effects of sludge thermal hydrolysis pretreatment on anaerobic digestion and downstream processes: Mechanism, challenges and solutions. Bioresour. Technol. 2022, 344, 126248. [Google Scholar] [CrossRef]
- Mikulski, D.; Klosowski, G. High-pressure microwave-assisted pretreatment of softwood, hardwood and non-wood biomass using different solvents in the production of cellulosic ethanol. Biotechnol. Biofuels Bioprod. 2023, 16, 19. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, Y.; Li, Y.; Zhang, Y.; Liu, T.; Wang, Y.; Sharpton, T.J.; Zhu, W. Progressive colonization of bacteria and degradation of rice straw in the rumen by Illumina sequencing. Front. Microbiol. 2017, 8, 2165. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, H.; Jiao, N.; Xu, G.; Xu, Y. Fractionation of poplar using hydrothermal and acid hydrotropic pretreatments for co-producing xylooligosaccharides, fermentable sugars, and lignin nanoparticles. Ind. Crops Prod. 2022, 181, 114853. [Google Scholar] [CrossRef]
- Wu, R.; Liu, W.; Li, L.; Ren, Q.; Jiang, C.; Hou, Q. Combination of hydrothermal and chemi-mechanical pretreatments to enhance enzymatic hydrolysis of poplar branches and insights on cellulase adsorption. Bioresour. Technol. 2021, 342, 126024. [Google Scholar] [CrossRef]
- Soares, L.A.; Solano, M.G.; Lindeboom, R.E.F.; van Lier, J.B.; Silva, E.L.; Varesche, M.B.A. Valorization of sugarcane bagasse through biofuel and value-added soluble metabolites production: Optimization of alkaline hydrothermal pretreatment. Biomass Bioenergy 2022, 165, 106564. [Google Scholar] [CrossRef]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent trends in the pretreatment of lignocellulosic biomass for value-added products. Front. Energy Res. 2018, 6, 141. [Google Scholar] [CrossRef]
- Ma, H.; Fu, P.; Zhao, J.; Lin, X.; Wu, W.; Yu, Z.; Xia, C.; Wang, Q.; Gao, M.; Zhou, J. Pretreatment of wheat straw lignocelluloses by deep eutectic solvent for lignin extraction. Molecules 2022, 27, 7955. [Google Scholar] [CrossRef] [PubMed]
- Ghose, T. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Brummer, V.; Jurena, T.; Hlavacek, V.; Omelkova, J.; Bebar, L.; Gabriel, P.; Stehlik, P. Enzymatic hydrolysis of pretreated waste paper--source of raw material for production of liquid biofuels. Bioresour. Technol. 2014, 152, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Zhou, T.; Wang, Y.; Cao, X.; Wu, S.; Zhao, M.; Wang, H.; Xu, M.; Zheng, B.; Zheng, J.; et al. Pretreatment of wheat straw leads to structural changes and improved enzymatic hydrolysis. Sci. Rep. 2018, 8, 1321. [Google Scholar] [CrossRef]
- Chen, W.-H.; Nižetić, S.; Sirohi, R.; Huang, Z.; Luque, R.; Papadopoulos, A.M.; Sakthivel, R.; Phuong Nguyen, X.; Tuan Hoang, A. Liquid hot water as sustainable biomass pretreatment technique for bioenergy production: A review. Bioresour. Technol. 2022, 344, 126207. [Google Scholar] [CrossRef] [PubMed]
- Gandam, P.K.; Chinta, M.L.; Pabbathi, N.P.P.; Baadhe, R.R.; Sharma, M.; Thakur, V.K.; Sharma, G.D.; Ranjitha, J.; Gupta, V.K. Second-generation bioethanol production from corncob—A comprehensive review on pretreatment and bioconversion strategies, including techno-economic and lifecycle perspective. Ind. Crops Prod. 2022, 186, 115245. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: A review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef]
- Lyu, Q.; Dar, R.A.; Baganz, F.; Smoliński, A.; Rasmey, A.-H.M.; Liu, R.; Zhang, L. Effects of Lignocellulosic Biomass-Derived Hydrolysate Inhibitors on Cell Growth and Lipid Production During Microbial Fermentation of Oleaginous Microorganisms—A Review. Fermentation 2025, 11, 121. [Google Scholar] [CrossRef]
- Ponnusamy, V.K.; Nguyen, D.D.; Dharmaraja, J.; Shobana, S.; Banu, J.R.; Saratale, R.G.; Chang, S.W.; Kumar, G. A review on lignin structure, pretreatments, fermentation reactions and biorefinery potential. Bioresour. Technol. 2019, 271, 462–472. [Google Scholar] [CrossRef]
- Dai, L.; Wang, Y.; Liu, Y.; Ruan, R.; He, C.; Duan, D.; Zhao, Y.; Yu, Z.; Jiang, L.; Wu, Q. Bridging the relationship between hydrothermal pretreatment and co-pyrolysis: Effect of hydrothermal pretreatment on aromatic production. Energy Convers. Manag. 2019, 180, 36–43. [Google Scholar] [CrossRef]
- Sindhu, R.; Binod, P.; Mathew, A.K.; Abraham, A.; Pandey, A.; Gnansounou, E.; Castro, G.E. An effective surfactant-assisted hydrothermal pretreatment strategy for bioethanol production from chili post-harvest residue by separate hydrolysis and fermentation. Bioprocess Biosyst. Eng. 2018, 41, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Niu, Z.; Yu, T.; Wu, R.; Wang, G. Effect of pH value on hydrolysis performance of corn stover during hydrothermal pretreatment. China Pulp Pap. 2020, 39, 30–36. [Google Scholar]
- Dubey, A.K.; Gupta, P.K.; Garg, N.; Naithani, S. Bioethanol production from waste paper acid pretreated hydrolyzate with xylose fermenting Pichia stipitis. Carbohydr. Polym. 2012, 88, 825–829. [Google Scholar] [CrossRef]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Wichaphian, A.; Yasan, P.; Pathom-Aree, W.; Lumyong, S.; Suwannarach, N.; Kumla, J.; Chaipoot, S.; Hoijang, S.; Krasian, T.; Worajittiphon, P.; et al. From agricultural waste to active films: Enhanced crystallinity of spent mushroom substrate-derived cellulose via deep eutectic solvent-based microwave-assisted pretreatment and its application in reinforcing CMC-based composite films. J. Agric. Food Res. 2025, 20, 101759. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Zhang, L.; Zhang, R.; Liu, G.; Cheng, G. Understanding changes in cellulose crystalline structure of lignocellulosic biomass during ionic liquid pretreatment by XRD. Bioresour. Technol. 2014, 151, 402–405. [Google Scholar] [CrossRef]
- Rahmani, A.M.; Tyagi, V.K.; Ahmed, B.; Kazmi, A.A.; Ojha, C.S.P.; Singh, R. Critical insights into anaerobic co-digestion of wheat straw with food waste and cattle manure: Synergistic effects on biogas yield and kinetic modeling. Environ. Res. 2022, 212, 113382. [Google Scholar] [CrossRef]
- Dobele, G.; Zhurinsh, A.; Volperts, A.; Jurkjane, V.; Pomilovskis, R.; Meile, K. Study of levoglucosenone obtained in analytical pyrolysis and screw-type reactor, separation and distillation. Wood Sci. Technol. 2020, 54, 383–400. [Google Scholar] [CrossRef]
- Song, X.; Zhao, C.; Wang, C.; Shen, P.; Chen, Z. Research on the mechanism of hydrothermal pretreatment of corn stover and anaerobic digestion characteristics. China Biogas 2021, 39, 12–18. [Google Scholar]
- Tang, W.; Huang, C.; Ling, Z.; He, Y.-C. Enhancing cellulosic digestibility of wheat straw by adding sodium lignosulfonate and sodium hydroxide to hydrothermal pretreatment. Bioresour. Technol. 2023, 379, 129058. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Romero, E.; Ragel, C.; LeGeros, R. XRD, SEM-EDS, and FTIR studies of in vitro growth of an apatite-like layer on sol-gel glasses. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. 1999, 44, 416–421. [Google Scholar] [CrossRef]
- Yu, Q.; Zhu, Y.; Bian, S.; Chen, L.; Zhuang, X.; Zhang, Z.; Wang, W.; Yuan, Z.; Hu, J.; Chen, J. Structural characteristics of corncob and eucalyptus contributed to sugar release during hydrothermal pretreatment and enzymatic hydrolysis. Cellulose 2017, 24, 4899–4909. [Google Scholar] [CrossRef]
- Yun, Z.; Ji, Z.; Li, S. Calcium homeostasis and its dysregulation in ischemia-reperfusion injury: A study. MEDS Basic Med. 2024, 2, 1–6. [Google Scholar]
- Yu, X.; Zheng, Y.; Dorgan, K.M.; Chen, S. Oil production by oleaginous yeasts using the hydrolysate from pretreatment of wheat straw with dilute sulfuric acid. Bioresour. Technol. 2011, 102, 6134–6140. [Google Scholar] [CrossRef]
- Fu, P. Dissolved Organic Matter and Its Interaction with Metal Ions in the Aqueous Environment—A Fluorescence Spectroscopy Study. Master’s Thesis, Graduate School of the Chinese Academy of Sciences (Institute of Geochemistry), Guiyang, China, 2004. [Google Scholar]
- Shi, F. A study on the open set identification method of organic pollutants in water based on three-dimensional fluorescence spectroscopy, characteristics. China Biogas 2021, 39, 12–18. [Google Scholar]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef]
- Rocha, J.; Alencar, B.; Mota, H.; Gouveia, E. Enzymatic hydrolysis of waste office paper for ethanol production by Spathaspora passalidarum. Cellul. Chem. Technol. 2016, 50, 243–246. [Google Scholar]
- Tang, Z.; Wu, C.; Tang, W.; Huang, M.; Ma, C.; He, Y.-C. Enhancing enzymatic saccharification of sunflower straw through optimal tartaric acid hydrothermal pretreatment. Bioresour. Technol. 2023, 385, 129279. [Google Scholar] [CrossRef]
- Tang, Z.; Wu, C.; Tang, W.; Ma, C.; He, Y.-C. A novel cetyltrimethylammonium bromide-based deep eutectic solvent pretreatment of rice husk to efficiently enhance its enzymatic hydrolysis. Bioresour. Technol. 2023, 376, 128806. [Google Scholar] [CrossRef]
- Araújo, D.; Vilarinho, M.; Machado, A. Effect of combined dilute-alkaline and green pretreatments on corncob fractionation: Pretreated biomass characterization and regenerated cellulose film production. Ind. Crops Prod. 2019, 141, 111785. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Sun, S.; Cao, X.; Sun, R. Co-production of oligosaccharides and fermentable sugar from wheat straw by hydrothermal pretreatment combined with alkaline ethanol extraction. Ind. Crops Prod. 2018, 111, 78–85. [Google Scholar] [CrossRef]
- Sarker, T.R.; Pattnaik, F.; Nanda, S.; Dalai, A.K.; Meda, V.; Naik, S. Hydrothermal pretreatment technologies for lignocellulosic biomass: A review of steam explosion and subcritical water hydrolysis. Chemosphere 2021, 284, 131372. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, M.; Oliva, M.; Téllez-Luis, S.J.; Ramírez, J.A. Hydrolysis of sorghum straw using phosphoric acid: Evaluation of furfural production. Bioresour. Technol. 2007, 98, 3053–3060. [Google Scholar] [CrossRef] [PubMed]
- Borrega, M.; Nieminen, K.; Sixta, H. Degradation kinetics of the main carbohydrates in birch wood during hot water extraction in a batch reactor at elevated temperatures. Bioresour. Technol. 2011, 102, 10724–10732. [Google Scholar] [CrossRef]
- Jeoh, T.; Ishizawa, C.I.; Davis, M.F.; Himmel, M.E.; Adney, W.S.; Johnson, D.K. Cellulase digestibility of pretreated biomass is limited by cellulose accessibility. Biotechnol. Bioeng. 2007, 98, 112–122. [Google Scholar] [CrossRef]
- ASTM E1755-2007; Standard Test Method for Ash in Biomass. ASTM: West Conshohocken, PA, USA, 2007.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).