Downstream Processes in a Microalgae Biorefinery: Cascaded Enzymatic Hydrolysis and Pulsed Electric Field as Green Solution
Abstract
:1. Introduction
2. Microalgae and Bioactive Intracellular Compounds
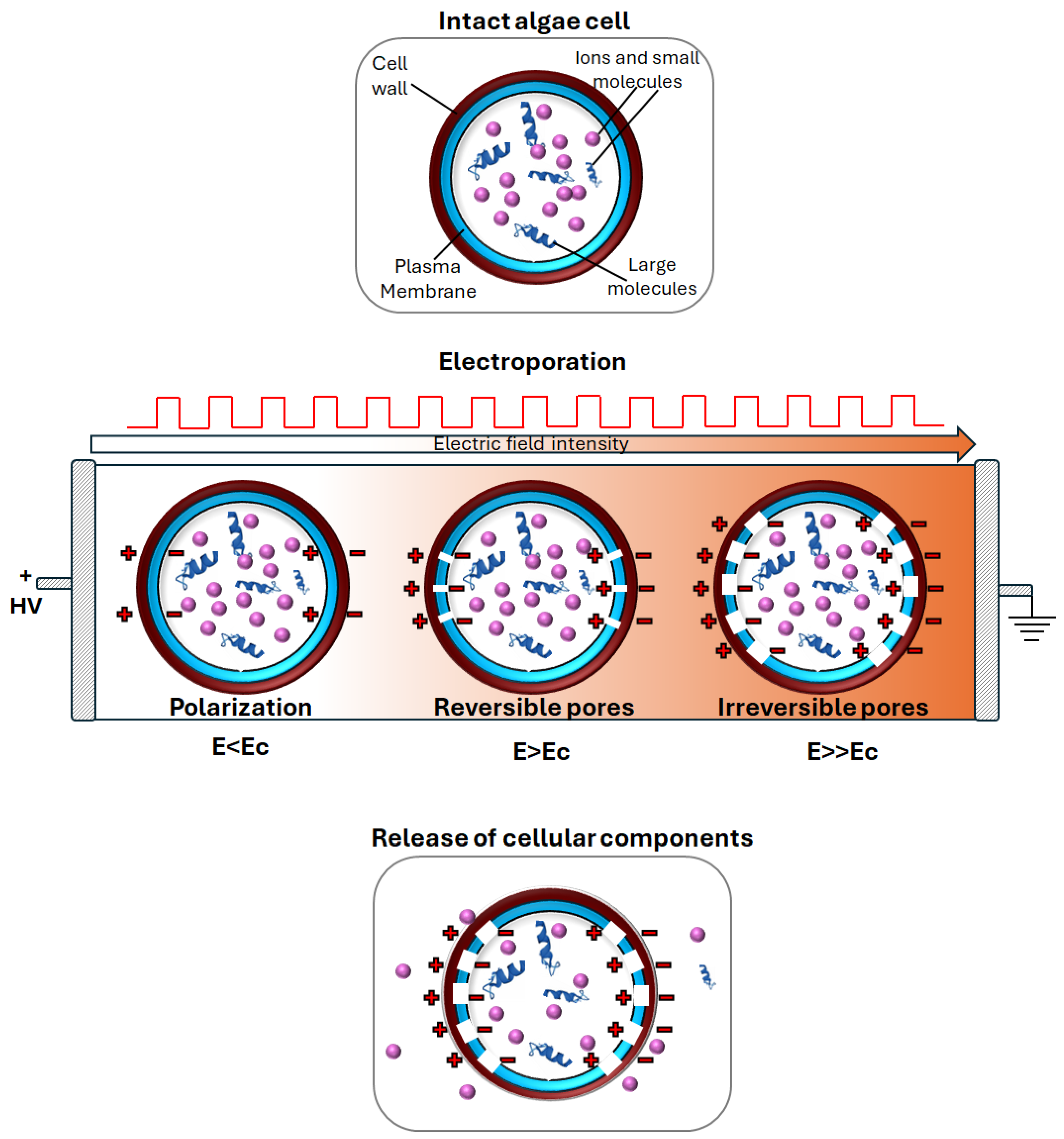
3. Pulsed Electric Field (PEF) Technology
3.1. Overview Pulsed Electric Fields (PEF) Technology
3.2. Effect of PEF Processing Conditions on the Extractability of Valuable Compounds
3.3. Limitations and Challenges of Electroporation Process
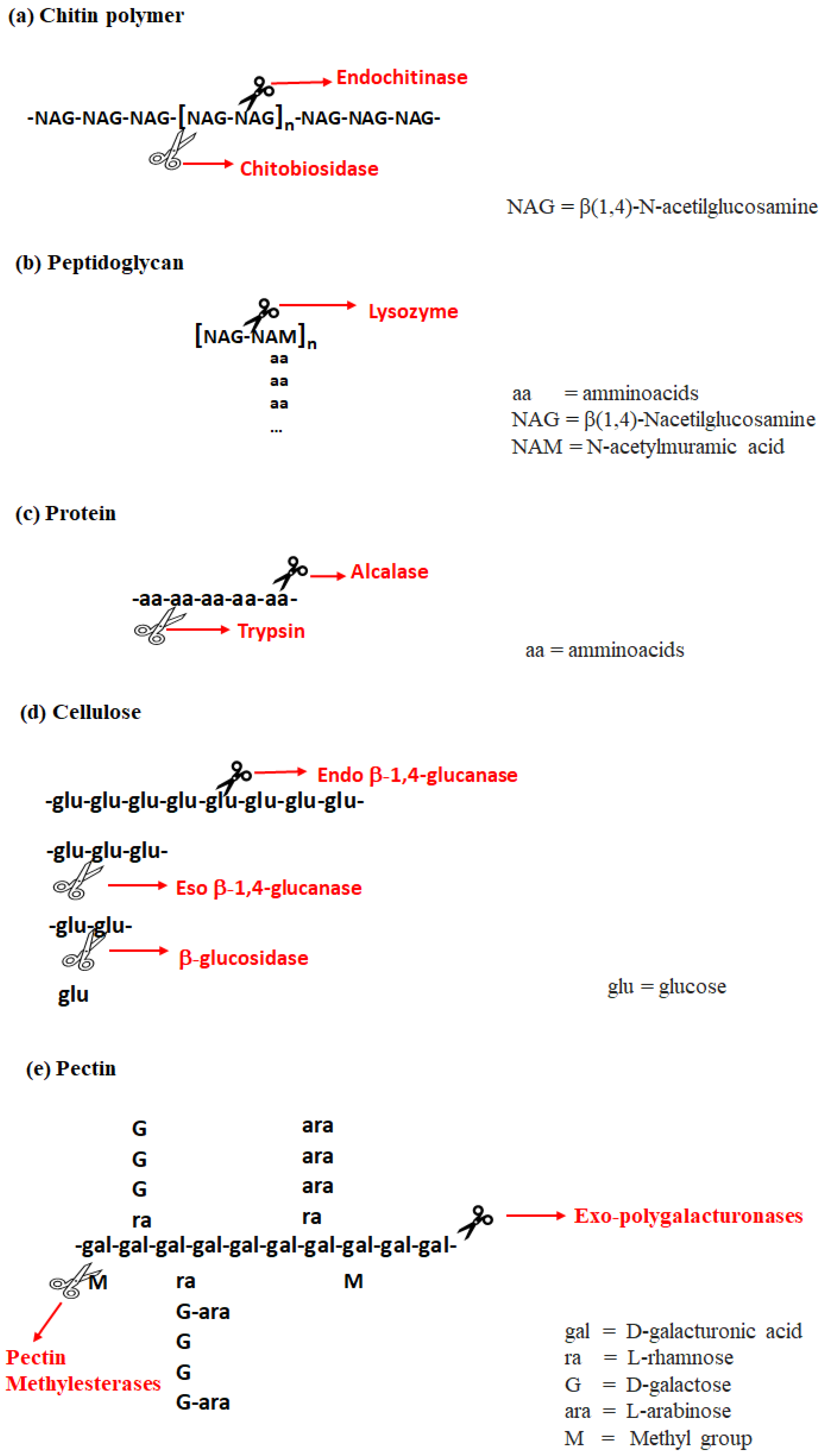
4. Enzymatic Hydrolysis (EH) Technology
4.1. Overview of Enzymatic Treatment
4.2. Effect of EH Processing Conditions on the Extractability of Valuable Compounds
4.3. Limitations and Challenges of Enzymatic Hydrolysis
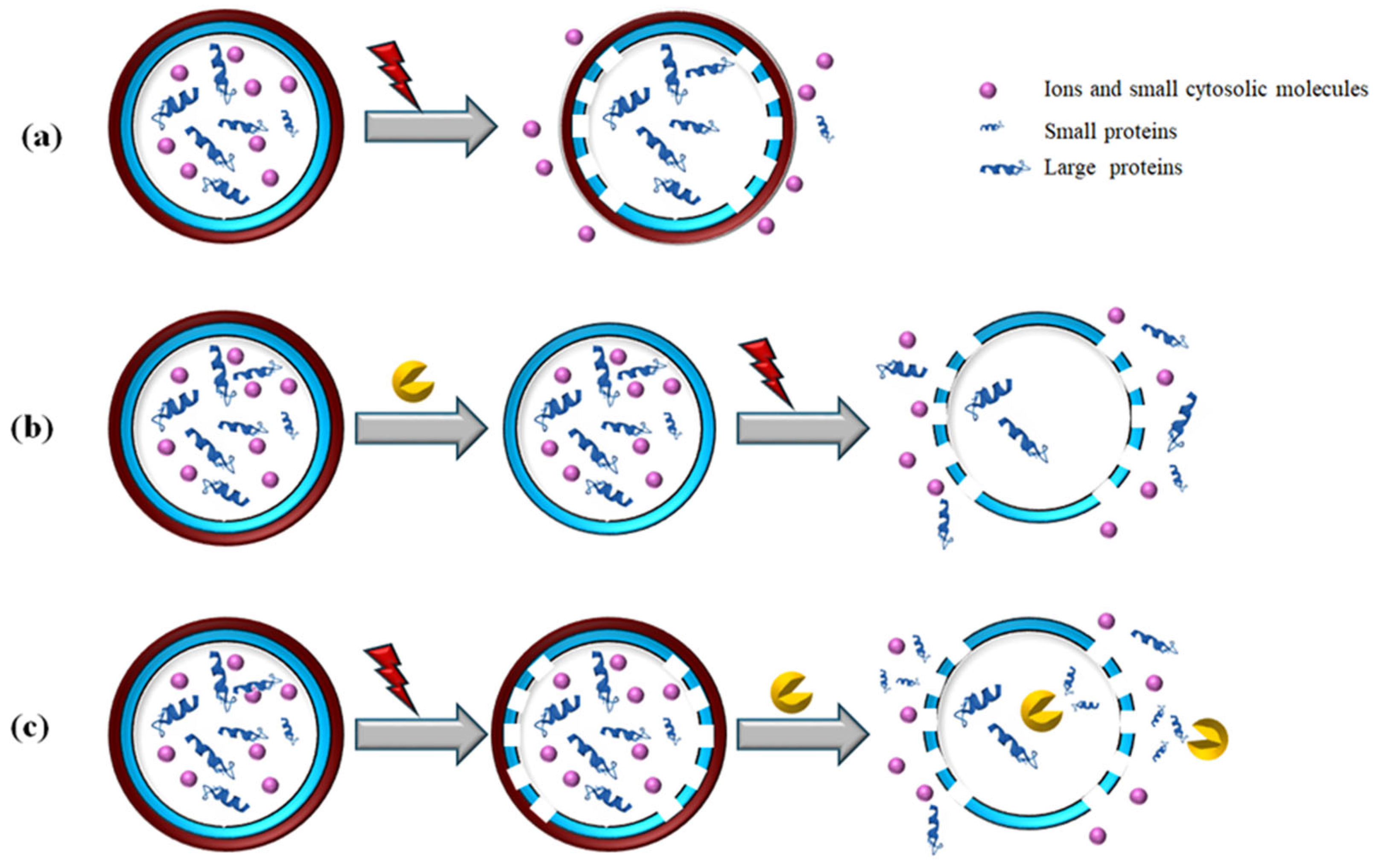
5. Cascaded PEF and Enzymatic Hydrolysis (EH): Mechanism, Synergies, and Benefits
6. Conclusions and Future Direction
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Starckx, S. A Place in the Sun—Algae is the Crop of the Future, According to Researchers in Geel, Flanders Today. 2012. Available online: https://www.flanderstoday.eu/current-affairs/place-sun (accessed on 27 October 2024).
- Günerken, E.; D’Hondt, E.; Eppink, M.H.M.; Garcia-Gonzales, L.; Elst, K.; Wijffels, R.H. Cell disruption for microalgae biorefineries. Biotechnol. Adv. 2015, 33, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Nelson, D.R.; Yi, Z.; Xu, M.; Khraiwesh, B.; Jijakli, K.; Chaiboonchoe, A.; Alzahmi, A.; Al-Khairy, D.; Brynjolfsson, S.; et al. Bioactive Compounds From Microalgae: Current Development and Prospects. Stud. Nat. Prod. Chem. 2017, 54, 199–225. [Google Scholar]
- Moshood, T.D.; Nawanir, G.; Mahmud, F. Microalgae biofuels production: A systematic review on socioeconomic prospects of microalgae biofuels and policy implications. Environ. Chall 2021, 5, 100207. [Google Scholar] [CrossRef]
- Carullo, D.; Abera, B.D.; Casazza, A.A.; Donsì, F.; Perego, P.; Ferrari, G.; Pataro, G. Effect of Pulsed Electric Fields and High-Pressure Homogenization on the aqueous extraction of intracellular compounds from the microalgae Chlorella vulgaris. Algal Res. 2018, 31, 60–69. [Google Scholar] [CrossRef]
- Carullo, D.; Donsì, F.; Ferrari, G.; Pataro, G. Extraction improvement of water-soluble compounds from Arthrospira platensis through the combination of high-shear homogenization and pulsed electric fields. Algal Res. 2021, 57, 102341. [Google Scholar] [CrossRef]
- Carullo, D.; Abera, B.D.; Scognamiglio, M.; Donsì, F.; Ferrari, G.; Pataro, G. Application of pulsed electric fields and high-pressure homogenization in biorefinery cascade of C. vulgaris microalgae. Foods 2022, 11, 471. [Google Scholar] [CrossRef]
- Garcia, E.S.; van Leeuwen, J.; Safi, C.; Sijtsma, L.; Eppink, M.H.M.; Wijffels, R.H.; van den Berg, C. Selective and energy efficient extraction of functional proteins from microalgae for food applications. Bioresour. Technol. 2018, 268, 197–203. [Google Scholar] [CrossRef]
- Pataro, G.; Carullo, D.; Ferrari, G. PEF-assisted Supercritical CO2 Extraction of Pigments from Microalgae Nannochloropsis oceanica in a Continuous Flow System. Chem. Eng. Trans. 2019, 74, 97–102. [Google Scholar] [CrossRef]
- Vanthoor-Koopmans, M.; Wijffels, R.H.; Barbosa, M.J.; Eppink, M.H.H. Biorefinery of microalgae for food and fuel. Bioresour. Technol. 2013, 135, 142–149. [Google Scholar] [CrossRef]
- Zhang, R.; Gu, X.; Xu, G.; Fu, X. Improving the lipid extraction yield from Chlorella based on the controllable electroporation of cell membrane by pulsed electric field. Bioresour. Technol 2021, 330, 124933. [Google Scholar] [CrossRef]
- Kokkali, M.; Martí-Quijal, F.J.; Taroncher, M.; Ruiz, M.J.; Kousoulaki, K.; Barba, F.J. Improved Extraction Efficiency of An-tioxidant Bioactive Compounds from Tetraselmis chuii and Phaedoactylum tricornutum Using Pulsed Electric Fields. Molecules 2020, 25, 3921. [Google Scholar] [CrossRef] [PubMed]
- Radakovits, R.; Jinkerson, R.E.; Darzins, A.; Posewitz, M.C. Genetic Engineering of Algae for Enhanced Biofuel Production. Eukaryot. Cell 2010, 9, 486–501. [Google Scholar] [CrossRef] [PubMed]
- Postma, P.R.; ‘t Lam, G.P.; Barbosa, M.J.; Wijffels, R.H.; Eppink, M.H.M.; Olivieri, G. Microalgal Biorefinery for Bulk and High-Value Products: Product Extraction Within Cell Disintegration. In Handbook of Electroporation, 1st ed.; Miklavcic, D., Ed.; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 2205–2224. [Google Scholar]
- de Jesus Raposo, M.F.; de Morais, R.M.S.C.; de Morais, A.M.M.B. Health applications of bioactive compounds from marine microalgae. Life Sci. 2013, 15, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Carbone, D.A.; Pellone, P.; Lubritto, C.; Ciniglia, C. Evaluation of Microalgae Antiviral Activity and Their Bioactive Compounds. Antibiotics 2021, 10, 746. [Google Scholar] [CrossRef]
- Donsì, F.; Ferrari, G.; Pataro, G. Emerging Technologies for the Clean Recovery of Antioxidants from Microalgae. In Microalgae; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 173–205. [Google Scholar]
- Poojary, M.M.; Barba, F.J.; Aliakbarian, B.; Donsì, F.; Pataro, G.; Dias, D.A.; Juliano, P. Innovative Alternative Technologies to Extract Carotenoids from Microalgae and Seaweeds. Mar. Drugs 2016, 14, 214. [Google Scholar] [CrossRef] [PubMed]
- Postma, P.R.; Pataro, G.; Capitoli, M.; Barbosa, M.J.; Wijffels, R.H.; Eppink, M.H.M.; Olivieri, G.; Ferrari, G. Selective extraction of intracellular components from the microalga Chlorella vulgaris by combined pulsed electric field–temperature treatment. Bioresour. Technol. 2016, 203, 80–88. [Google Scholar] [CrossRef]
- Luengo, E.; Condón-Abanto, S.; Álvarez, I.; Raso, J. Effect of pulsed electric field treatments on permeabilization and extraction of pigments from Chlorella vulgaris. J. Membr. Biol. 2014, 247, 1269–1277. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hosano, N.; Hosano, H. Recovering Microalgal Bioresources: A Review of Cell Disruption Methods and Extraction Technologies. Molecules 2022, 27, 2786. [Google Scholar] [CrossRef]
- Safi, C.; Ursu, A.V.; Laroche, C.; Zebib, B.; Merah, O.; Pontalier, P.Y.; Vaca-Garcia, C. Aqueous extraction of proteins from microalgae: Effect of different cell disruption methods. Algal Res. 2014, 3, 61–65. [Google Scholar] [CrossRef]
- Parniakov, O.; Apicella, E.; Koubaa, M.; Barba, F.J.; Grimi, N.; Lebovka, N.; Pataro, G.; Ferrari, G.; Vorobiev, E. Ultrasound-assisted green solvent extraction of high-added value compounds from microalgae Nannochloropsis spp. Bioresour. Technol. 2015, 198, 262–267. [Google Scholar] [CrossRef]
- Golberg, A.; Sack, M.; Teissie, J.; Pataro, G.; Pliquett, U.; Saulis, G.; Stefan, T.; Miklavcic, D.; Vorobiev, E.; Frey, W. Energy-efficient biomass processing with pulsed electric fields for bioeconomy and sustainable development. Biotechnol. Biofuels 2016, 9, 94. [Google Scholar] [CrossRef] [PubMed]
- ‘t Lam, G.P.; Postma, P.R.; Fernandes, D.A.; Timmermans, R.A.H.; Vermue, M.H.; Barbosa, M.J.; Eppink, M.H.M.; Wijffels, R.H.; Olivieri, G. Pulsed electric field for protein release of the microalgae Chlorella vulgaris and Neochloris oleoabundans. Algal Res. 2017, 24, 181–187. [Google Scholar] [CrossRef]
- Papachristou, I.; Akaberi, S.; Silve, A.; Navarro-Lopez, R.; Wustner, R.; Leber, K.; Nazarova, N.; Muller, G.; Frey, W. Analysis of the lipid extraction performance in a cascade process for Scenedesmus almeriensis biorefinery. Biotechnol. Biofuels 2021, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for Biodiesel Production and Other Applications: A Review. Ren. Sust. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Martínez, J.M.; Delso, C.; Álvarez, I.; Raso, J. Pulsed electric field-assisted extraction of valuable compounds from microorganisms. Comp. Rev. Food Sci. Food Saf. 2020, 19, 530–552. [Google Scholar] [CrossRef] [PubMed]
- D’Hondt, E.; Martin-Juarez, J.; Bolado, S.; Kasperoviciene, J.; Koreiviene, J.; Sulcius, S.; Bastiaens, L. Cell disruption technologies. In Microalgae-Based Biofuels and Bioproducts; Muñoz, R., Gonzalez-Fernandez, C., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 133–154. [Google Scholar]
- Chan, W.S.; Kwok, A.C.M.; Wong, J.T.Y. Knockdown of Dinoflagellate Cellulose Synthase CesA1 Resulted in Malformed Intracellular Cellulosic Thecal Plates and Severely Impeded Cyst-to-Swarmer Transition. Front. Microbiol. 2019, 10, 546. [Google Scholar] [CrossRef]
- Hoef-Emden, K.; Melkonian, M. Revision of the Genus Cryptomonas (Cryptophyceae): A combination of molecular phylogeny and morphology provides insights into a long-hidden dimorphism. Protist 2003, 154, 371–409. [Google Scholar] [CrossRef]
- Walker, J.M.; Marzec, B.; Lee, R.B.Y.; Vodrazkova, K.; Day, S.J.; Tang, C.C.; Rickaby, R.E.M.; Nudelman, F. Polymorph Selectivity of Coccolith-Associated Polysaccharides from Gephyrocapsa Oceanica on Calcium Carbonate Formation in Vitro. Adv. Funct. Mater. 2018, 29, 1807168. [Google Scholar] [CrossRef]
- De Tommasi, E.; Gielis, J.; Rogato, A. Diatom Frustule Morphogenesis and Function: A Multidisciplinary Survey. Mar. Genom. 2017, 35, 1–18. [Google Scholar] [CrossRef]
- Okuda, K. Structure and phylogeny of cell coverings. J. Plant Res. 2002, 115, 283–288. [Google Scholar] [CrossRef]
- Rawindran, H.; Khoo, K.S.; Satpati, G.G.; Maity, S.; Chandran, K.; Lim, J.W.; Tong, W.Y.; Setiabudi, H.D.; Yunus, N.M. Composition of carbohydrate, protein and lipid derived from microalgae using thermally pretreated solid waste. J. Sci. Food Agric. 2024. [Google Scholar] [CrossRef] [PubMed]
- Chini Zittelli, G.; Lauceri, R.; Faraloni, C.; Silva Benavides, A.M.; Torzillo, G. Valuable pigments from microalgae: Phycobiliproteins, primary carotenoids, and fucoxanthin. Photochem. Photobiol. Sci. 2020, 22, 1733–1789. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, M.F. Biofuels from algae for sustainable development. Appl. Energy 2011, 88, 3473–3480. [Google Scholar] [CrossRef]
- Di Lena, G.; Casini, I.; Lucarini, M.; Lombardi-Boccia, G. Carotenoid profiling of five microalgae species from large-scale production. Food Res. Int. 2019, 120, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.L.; Langellotti, A.L.; Oliviero, M.; Baselice, M.; Sacchi, R.; Masi, P. Valorization of second cheese whey through cultivation of extremophile microalga Galdieria sulphuraria. AIMS Environ. Sci. 2021, 8, 435–448. [Google Scholar] [CrossRef]
- Tsvetanova, F.; Yankov, D. Bioactive compounds from red microalgae with therapeutic and nutritional value. Microorganisms 2022, 10, 2290. [Google Scholar] [CrossRef]
- Natesungnoen, M.; Pongrakhananon, V.; Lindblad, P.; Jantaro, S. Overexpressing carotenoid biosynthetic genes in Synechocystis sp. PCC 6803 improved intracellular pigments and antioxidant activity, which can decrease the viability and proliferation of lung cancer cells in vitro. Int. J. Mol. Sci. 2023, 24, 9370. [Google Scholar] [CrossRef]
- Gentscheva, G.; Nikolova, K.; Panayotova, V.; Peycheva, K.; Makedonski, L.; Slavov, P.; Radusheva, P.; Petrova, P.; Yotkovska, I. Application of Arthrospira platensis for Medicinal Purposes and the Food Industry: A Review of the Literature. Life 2023, 13, 845. [Google Scholar] [CrossRef]
- Geada, P.; Rodrigues, R.; Loureiro, L.; Pereira, R.; Fernandes, B.; Teixeira, J.A.; Vasconcelos, V.; Vicente, A.A. Electrotechnologies applied to microalgal biotechnology–Applications, techniques and future trends. Renew. Sustain. Energy Rev. 2018, 94, 656–668. [Google Scholar] [CrossRef]
- da Silva Ferreira, V.; Sant’Anna, C. Impact of culture conditions on the chlorophyll content of microalgae for biotechnological applications. World J. Microbiol. Biotechnol. 2017, 33, 20. [Google Scholar] [CrossRef]
- Memije-Lazaro, I.N.; Blas-Valdivia, V.; Franco-Colín, M.; Cano-Europa, E. Arthrospira maxima (Spirulina) and C-phycocyanin prevent the progression of chronic kidney disease and its cardiovascular complications. J. Funct. Foods 2018, 43, 37–43. [Google Scholar] [CrossRef]
- Fernández-Rojas, B.; Hernández-Juárez, J.; Pedraza-Chaverri, J. Nutraceutical properties of phycocyanin. J. Funct. Foods. 2014, 11, 375–392. [Google Scholar] [CrossRef]
- Kang, K.; Park, Y.; Hye, J.H.; Seong, H.K.; Jeong, G.L.; Shin, H.C. Antioxidative properties of brown algae polyphenolics and their perspectives as chemopreventive agents against vascular risk factors. Arch. Pharm. Res. 2003, 26, 286–293. [Google Scholar] [CrossRef]
- Ibañez, E.; Herrero, M.; Mendiola, J.A.; Castro-Puyana, M. Extraction and characterization of bioactive compounds with health benefits from marine resources: Macro and micro algae, cyanobacteria, and invertebrates. In Marine Bioactive Compounds: Sources, Characterization and Applications; Springer: Boston, MA, USA, 2012; pp. 55–98. [Google Scholar]
- Raso, J.; Frey, W.; Ferrari, G.; Pataro, G.; Knorr, D.; Teissie, J.; Miklavčič, D. Recommendations guidelines on the key information to be reported in studies of application of PEF technology in food and biotechnological processes. Innov. Food Sci. Emer. Technol. 2016, 37, 312–321. [Google Scholar] [CrossRef]
- Kotnik, T.; Kramar, P.; Pucihar, G.; Miklavčič, D.; Tarek, M. Cell membrane electroporation-Part 1: The phenomenon. IEEE Electr. Insul. 2012, 28, 14–23. [Google Scholar] [CrossRef]
- Carullo, D.; Pataro, G.; Donsì, F.; Ferrari, G. Pulsed Electric Fields-Assisted Extraction of Valuable Compounds From Arthrospira Platensis: Effect of Pulse Polarity and Mild Heating. Front. Bioeng. Biotechnol. 2020, 8, 551272. [Google Scholar] [CrossRef]
- Käferböck, A.; Smetana, S.; de Vos, R.; Schwarz, C.; Toepfl, S.; Parniakov, O. Sustainable extraction of valuable components from Spirulina assisted by pulsed electric fields technology. Algal Res. 2020, 48, 101914. [Google Scholar] [CrossRef]
- ‘t Lam, G.P.; van der Kolk, J.A.; Chordia, A.; Vermue, M.H.; Olivieri, G.; Eppink, M.H.M.; Wijffels, R.H. Mild and Selective Protein Release of Cell Wall Deficient Microalgae with Pulsed Electric Field. ACS Sustain. Chem. Eng. 2017, 5, 6046–6053. [Google Scholar] [CrossRef]
- Han, S.F.; Jin, W.; Yang, Q.; Abomohra, A.E.F.; Zhou, X.; Tu, R.; Chen, C.; Xie, G.Y.; Wang, Q. Application of pulsed electric field pretreatment for enhancing lipid extraction from Chlorella pyrenoidosa grown in wastewater. Renew. Energy 2019, 133, 233–239. [Google Scholar] [CrossRef]
- Martínez, J.M.; Luengo, E.; Saldaña, G.; Álvarez, I.; Raso, J. C-phycocyanin extraction assisted by pulsed electric field from Artrosphira platensis. Food Res. Int. 2017, 99, 1042–1047. [Google Scholar] [CrossRef]
- Phong, W.N.; Show, P.L.; Ling, T.C.; Juan, J.C.; Ng, E.-P.; Chang, J.-S. Mild cell disruption methods for bio-functional proteins recovery from microalgae—Recent developments and future perspectives. Algal Res. 2018, 31, 506–516. [Google Scholar] [CrossRef]
- Akaberi, S.; Gusbeth, C.; Silve, A.; Senthilnathan, D.S.; Navarro-López, E.; Molina-Grima, E.; Müller, G.; Frey, W. Effect of pulsed electric field treatment on enzymatic hydrolysis of proteins of Scenedesmus almeriensis. Algal Res. 2019, 43, 101656. [Google Scholar] [CrossRef]
- Töpfl, S. Pulsed Electric Fields PEF for Permeabilization of Cell Membranes in Food and Bioprocessing—Applications, Process and Equipment Design and Cost Analysis. Ph.D. Thesis, University of Technology, Berlin, Germany, 2006. [Google Scholar]
- Luengo, E.; Martínez, J.M.; Coustets, M.; Álvarez, I.; Teissié, J.; Rols, M.P.; Raso, J. A comparative study on the effects of millisecond-and microsecond-pulsed electric field treatments on the permeabilization and extraction of pigments from Chlorella vulgaris. J. Membr. Biol. 2015, 248, 883–891. [Google Scholar] [CrossRef]
- Luengo, E.; Martínez, J.M.; Bordetas, A.; Álvarez, I.; Raso, J. Influence of the treatment medium temperature on lutein extraction assisted by pulsed electric fields from Chlorella vulgaris. Innov. Food Sci. Emerg. Technol. 2015, 29, 15–22. [Google Scholar] [CrossRef]
- Pataro, G.; Goettel, M.; Straessner, R.; Gusbeth, C.; Ferrari, G.; Frey, W. Effect of PEF treatment on extraction of valuable compounds from microalgae C. vulgaris. Chem. Eng. Trans. 2017, 57, 67–72. [Google Scholar] [CrossRef]
- Jaeschke, D.P.; Mercali, G.D.; Marczak, L.D.F.; Müller, G.; Frey, W.; Gusbeth, C. Extraction of valuable compounds from Arthrospira platensis using pulsed electric field treatment. Bioresour. Technol. 2019, 283, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Parniakov, O.; Barba, F.J.; Grimi, N.; Marchal, L.; Jubeau, S.; Lebovka, N.; Vorobiev, E. Pulsed electric field assisted extraction of nutritionally valuable compounds from microalgae Nannochloropsis spp. using the binary mixture of organic solvents and water. Innov. Food Sci. Emerg. Technol. 2015, 27, 79–85. [Google Scholar] [CrossRef]
- Parniakov, O.; Barba, F.J.; Grimi, N.; Marchal, L.; Jubeau, S.; Lebovka, N.; Vorobiev, E. Pulsed electric field and pH assisted selective extraction of intracellular components from microalgae nannochloropsis. Algal Res. 2015, 8, 128–134. [Google Scholar] [CrossRef]
- Grimi, N.; Dubois, A.; Marchal, L.; Jubeau, S.; Lebovka, N.I.; Vorobiev, E. Selective extraction from microalgae Nannochloropsis sp. using different methods of cell disruption. Bioresour. Technol. 2014, 153, 254–259. [Google Scholar] [CrossRef]
- Martínez, J.M.; Delso, C.; Álvarez, I.; Raso, J. Pulsed electric field permeabilization and extraction of phycoerythrin from Porphyridium cruentum. Algal Res. 2019, 37, 51–56. [Google Scholar] [CrossRef]
- Coustets, M.; Joubert-Durigneux, V.; Hérault, J.; Schoefs, B.; Blanckaert, V.; Garnier, J.P.; Teissié, J. Optimization of protein electroextraction from microalgae by a flow process. Bioelectrochemistry 2015, 103, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Zbinden, M.D.A.; Sturm, B.S.; Nord, R.D.; Carey, W.J.; Moore, D.; Shinogle, H.; Stagg-Williams, S.M. Pulsed electric field (PEF) as an intensification pretreatment for greener solvent lipid extraction from microalgae. Biotechnol. Bioeng. 2013, 110, 1605–1615. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.S.; Parameswaran, P.; Li, A.; Baez, M.; Rittmann, B.E. Effects of pulsed electric field treatment on enhancing lipid recovery from the microalga, Scenedesmus. Bioresour. Technol. 2014, 173, 457–461. [Google Scholar] [CrossRef]
- Goettel, M.; Eing, C.; Gusbeth, C.; Straessner, R.; Frey, W. Pulsed electric field assisted extraction of intracellular valuables from microalgae. Algal Res. 2013, 2, 401–408. [Google Scholar] [CrossRef]
- Chang, D.C. Cell fusion and cell poration by pulsed radio-frequency electric fields. In Electroporation and Electrofusion in Cell Biology; Springer: Boston, MA, USA, 1989; pp. 215–227. [Google Scholar]
- Qin, B.L.; Zhang, Q.; Barbosa-Canovas, G.V.; Swanson, B.G.; Pedrow, P.D. Inactivation of microorganisms by pulsed electric fields of different voltage waveforms. IEEE Trans. Dielectr. Elect. Insul. 1994, 1, 1047–1057. [Google Scholar]
- Beveridge, J.R.; MacGregor, S.J.; Marsili, L.; Anderson, J.G.; Rowan, N.J.; Farish, O. Comparison of the effectiveness of biphase and monophase rectangular pulses for the inactivation of micro-organisms using pulsed electric fields. IEEE Trans. Plasma Sci. 2002, 30, 1525–1531. [Google Scholar] [CrossRef]
- Evrendilek, G.A.; Zhang, Q.H. Effects of pulse polarity and pulse delaying time on pulsed electric fields-induced pasteurization of E. coli O157: H7. J. Food Eng. 2005, 68, 271–276. [Google Scholar] [CrossRef]
- Steinbruch, E.; Wise, J.; Levkov, K.; Chemodanov, A.; Israel, Á.; Livney, Y.D.; Golberg, A. Enzymatic cell wall degradation combined with pulsed electric fields increases yields of water-soluble-protein extraction from the green marine macroalga Ulva sp. Innov. Food Sci. Emerg. Technol. 2023, 84, 103231. [Google Scholar] [CrossRef]
- Safi, C.; Olivieri, G.; Campos, R.P.; Engelen-Smit, N.; Mulder, W.J.; Van den Broek, L.A.M.; Sijtsma, L. Biorefinery of microalgal soluble proteins by sequential processing and membrane filtration. Bioresour. Technol. 2017, 225, 151–158. [Google Scholar] [CrossRef]
- Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry, 4th ed.; Recording for the Blind & Dyslexic: New York, NY, USA, 2004. [Google Scholar]
- Demuez, M.; Mahdy, A.; Tomás-Pejó, E.; González-Fernández, C.; Ballesteros, M. Enzymatic cell disruption of microalgae biomass in biorefinery processes. Biotechnol. Bioeng. 2015, 112, 1955–1966. [Google Scholar] [CrossRef]
- Lau, N.S.; Furusawa, G. Polysaccharide degradation in Cellvibrionaceae: Genomic insights of the novel chitin-degrading marine bacterium, strain KSP-S5-2, and its chitinolytic activity. Sci. Total Environ. 2024, 912, 169134. [Google Scholar] [CrossRef] [PubMed]
- Astafyeva, Y.; Gurschke, M.; Streit, W.R.; Krohn, I. Interplay between the microalgae Micrasterias radians and its symbiont Dyadobacter sp. HH091. Front Microbiol. 2022, 13, 1006609. [Google Scholar] [CrossRef]
- Flores-Fernández, C.N.; Cárdenas-Fernández, M.; Lye, G.J.; Ward, J.M. Synergistic action of thermophilic pectinases for pectin bioconversion into D-galacturonic acid. Enzyme Microb. Technol. 2022, 160, 110071. [Google Scholar] [CrossRef]
- Scherer, D.; Krust, D.; Frey, W.; Mueller, G.; Nick, P.; Gusbeth, C. Pulsed electric field (PEF)-assisted protein recovery from Chlorella vulgaris is mediated by an enzymatic process after cell death. Algal Res. 2019, 41, 101536. [Google Scholar] [CrossRef]
- Harun, R.; Danquah, M.K. Enzymatic hydrolysis of microalgal biomass for bioethanol production. Chem. Eng. J. 2011, 168, 1079–1084. [Google Scholar] [CrossRef]
- Al-Zuhair, S.; Ashraf, S.; Hisaindee, S.; Darmaki, N.A.; Battah, S.; Svistunenko, D.; Reeder, B.; Stanway, G.; Chaudhary, A. Enzymatic pre-treatment of microalgae cells for enhanced extraction of proteins. Eng. Life Sci. 2017, 17, 175–185. [Google Scholar] [CrossRef]
- Coelho, D.; Lopes, P.A.; Cardoso, V.; Ponte, P.; Brás, J.; Madeira, M.S.; Alfaia, C.M.; Bandarra, N.M.; Gerken, H.G.; Fontes, C.M.G.A.; et al. Novel combination of feed enzymes to improve the degradation of Chlorella vulgaris recalcitrant cell wall. Sci. Rep. 2019, 9, 5382. [Google Scholar] [CrossRef]
- Koruyucu, A.; Peest, T.; Korzin, E.; Gröninger, L.; Patricia; Brück, T.; Weuster-Botz, D. Cell Disruption and Hydrolysis of Microchloropsis salina Biomass as a Feedstock for Fermentation. Appl. Sci. 2024, 14, 9667. [Google Scholar] [CrossRef]
- Zhang, J.; He, Y.; Luo, M.; Chen, F. Utilization of enzymatic cell disruption hydrolysate of Chlorella pyrenoidosa as potential carbon source in algae mixotrophic cultivation. Algal Res. 2020, 45, 101730. [Google Scholar] [CrossRef]
- Rojo, E.M.; Filipigh, A.A.; Bolado, S. Assisted-enzymatic hydrolysis vs chemical hydrolysis for fractional valorization of microalgae biomass. Process Saf. Environ. Prot. 2023, 174, 276–285. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, X.; Wang, Z.; Sun, Y.; Zhu, S.; Li, L.; Lv, P. Optimization of enzymatic hydrolysis for effective lipid extraction from microalgae Scenedesmus sp. Renew. Energy 2018, 125, 1049–1057. [Google Scholar] [CrossRef]
- Cho, H.S.; Oh, Y.K.; Park, S.C.; Lee, J.W.; Park, J.Y. Effects of enzymatic hydrolysis on lipid extraction from Chlorella vulgaris. Renew. Energy 2013, 54, 156–160. [Google Scholar] [CrossRef]
- Shokrkar, H.; Keighobadi, A. Effect of fluid hydrodynamic situations on enzymatic hydrolysis of mixed microalgae: Experimental study and simulation. Energy 2022, 241, 122804. [Google Scholar] [CrossRef]
- de Farias Silva, C.E.; Meneghello, D.; de Souza Ab-ud, A.K.; Bertucco, A. Pretreatment of microalgal biomass to improve the enzymatic hydrolysis of carbohydrates by ultrasonication: Yield vs. energy consumption. J. King Saud Univ. Sci. 2020, 32, 606–613. [Google Scholar] [CrossRef]
- Lyu, X.; Gonzalez, R.; Horton, A.; Li, T. Immobilization of Enzymes by Polymeric Materials. Catalysts 2021, 11, 1211. [Google Scholar] [CrossRef]
- Maribu, I.; Blikra, M.J.; Eilertsen, K.E.; Elvevold, K. Protein enrichment of the red macroalga Palmaria palmata using pulsed electric field and enzymatic processing. J. Appl. Phycol. 2024, 36, 3665–3673. [Google Scholar] [CrossRef]

| Class | Major Compounds | Reference |
|---|---|---|
| Dinophyceae | Cellulose | [30] |
| Cryptophyceae | Proteins | [31] |
| Haptophyceae | Calcium carbonate | [32] |
| Bacillariophyceae | Silica | [33] |
| Chlorophyceae | Cellulose | [34] |
| Charophyceae | Cellulose | [34] |
| Rhodophyceae | Cellulose agar sulphated polysaccharides | [22] |
| Microalgae | Biochemical Composition (% Dry Cell Weight) | Pigments (mg g−1 DW) | References | |||||
|---|---|---|---|---|---|---|---|---|
| Carbohydrate | Protein | Lipid | Violaxanthin | Zeaxanthin | Lutein | β-Carotene | ||
| Nannochloropsi oceanica | 12.4 | 36.4 | 27.8 | 30.2 | [35] | |||
| [36] | ||||||||
| Chlorella vulgaris | 14 | 55 | 18 | 3.7 | [37] | |||
| [36] | ||||||||
| Scenedesmus sp. | 10–52 | 8–56 | 2–40 | 7.4–42.0 | [37] | |||
| [36] | ||||||||
| Dunaliella salina | 32 | 57 | 6 | 30–130 | [37] | |||
| [36] | ||||||||
| Porphyridium Cruentum | 49 | 33 | 12 | 1 | 0.5 | [37] | ||
| [38] | ||||||||
| Galdieria sulphuraria | 45 | 30 | 8 | 0.4 | [39] | |||
| [40] | ||||||||
| Synechococcus sp. | 35 | 35 | 0.5 | 0.4 * | 0.8 * | [35] | ||
| [41] | ||||||||
| Arthrospira platensis | 11 | 56 | 6 | 0.02–2.3 | [37] | |||
| [42] | ||||||||
| Microalgae | Operating Conditions | Extraction Conditions | Improved Recovery of Target Products | Reference |
|---|---|---|---|---|
| Chlorella vulgaris | 15 kV/cm, 100 kJ/kg dw | NA | Enhanced carotenoid recovery (+525% compared to conventional ball milling) | [58] |
| 10–25 kV/cm, 0.6–93 kJ/L of culture | 96% ethanol, 20 °C, 1 h | Enhanced carotenoid recovery (up to 1.04 mg/g dw) | [20] | |
| 10–25 kV/cm, 9–150 kJ/L of culture | 96% ethanol, 20 °C, 1 h | Enhanced carotenoid recovery (up to 1.58 mg/L) | [59] | |
| 25 kV/cm, 61 kJ/kg, 10–40 °C, | 96% ethanol, 20 °C, 1 h | Enhanced lutein recovery (up to 0.753 mg/g dw) | [60] | |
| 27 kV/cm, 100 kJ/kg | Water, 25 °C for 1 h | Enhanced recovery of proteins (20 times) and carbohydrates (2.7 times) in comparison to untreated | [61] | |
| 17 kV/cm, 100 kJ/kg, 25–55 °C | Recovery: 25–39% carbohydrates and 3–5% proteins | [19] | ||
| 20 kV/cm, 100 kJ/kg, 25 °C | Water, 25 °C for 1 h | Recovery: 35.8% of total carbohydrates, and 5.2% of total proteins | [5] | |
| 20 kV/cm and 100 kJ/kg-mono/bipolar pulses, temperature (35 °C) | Water, 1 h at 25 °C | Recovery: 74% of total carbohydrates, 37% of total water-soluble proteins, and 74% of total C-Phycocyanin, Bipolar pulses less effective than monopolar pulses | [51] | |
| 10 kV/cm, 100 kJ/kg, 25 °C | 10% ethanol, 1 h at 25 °C | Total carotenes and chlorophyll a were 1.6 and 1.4 times greater than untreated | [9] | |
| 20 kV/cm, 100 kJ/kg, 25 °C | I step: Carbohydrates and protein extraction in water, 1 h at 25 °C II step: Lipid extraction in ethyl acetate, 3 h, 25 °C | Recovery: 4.9% of total proteins, 24.3% of total carbohydrates, 46.7% of total lipids | [7] | |
| 25 kV/cm 93 kJ/kg | Distilled water, at 20 °C for up to 420 min | Total C-phycocyanin content extraction after 6 h | [55] | |
| Arthrospiraplatensis | 20 kV/cm and 100 kJ/kg-mono/bipolar pulses, pulse delay (1–20 µs), 35 °C | Water, 25 °C, 1 h | Recovery: 73.8% of total carbohydrates, 37.4% of total water-soluble proteins, and 73.7% of total C-Phycocyanin. Bipolar pulses were less effective than monopolar pulses | [6] |
| 20 kV/cm, 100 kJ/kg, 25 °C | Water, 25 °C, 3 h | Enhanced recovery of proteins, (14.1 times), carbohydrates (20 times), and C-Phycocyanin (130 times) in comparison to untreated | [6] | |
| 40 kV/cm, 56 J/mL, 25 °C | Sodium-phosphate buffer (pH 7.2), 25 °C, 6 h | Enhanced recovery of phycocyanin (up to 85.2 mg/g dw) and proteins (48.4 mg/g dw) | [62] | |
| Arthrospira maxima | 25 kV/cm, 100 kJ/kg | Water, 21 °C, 2 h | Enhanced recovery of phycocyanin (2.5 times) in comparison to untreated | [52] |
| Rhodotorula glutinous | 15 kV/cm, 150 µs | I step: Citrate phosphate buffer (pH 8), 24 h, 25 °C II step: ethanol, 1 h, 25 °C | Enhanced recovery of carotenoids (up to 375 µg/g dw) | [55] |
| Nannochloropsis spp. | 20 kV/cm 96 kJ/kg | I step: water, 10 min 20 °C II step: pure water and binary mixtures with 30%, 50%, and 100% DMSO or ethanol in water, 240 min, 20 °C | Efficient extraction of proteins in water in first step and improved extraction in dimethyl sulfoxide/ethanol of pigments in the second step | [63] |
| 20 kV/cm, 0.01–6 ms treatment time, 1–600 pulses. | 1% (w/w) in distilled water, 3 h, 50 °C, pH = 8.5–11 | Enhanced carotenoid recovery (up to 0.2 mg/g dw) | [64] | |
| 20 kV/cm 13.3–53 kJ/kg | Water | Water-soluble proteins Recovery: 5% Protein extraction: 5% after PEF versus 91% after HPH (150 MPa, 6 passes). Pigment Extraction: Negligible after PEF treatment | [65] | |
| Porphyridium cruentum | 8 kV/cm 15 kJ/kg | Citrate-phosphate McIlvaine buffer, 25 °C. | Total content of β-phycoerythrin (32 mg/g dw) extracted after 24 h | [66] |
| Chlamydomonas reinhardtii | 15 kV/cm 12 kJ/kg | Water, 1 h | Proteins Recovery 70% | [53] |
| Haematococcus pluvialis | 3 kV/cm 8 kJ/kg, bipolar electric pulses of 2 ms | Phosphate buffer (pH = 7), 20 °C for 24 h | Proteins Release 8 times greater than from untreated | [67] |
| Ankistrodesmus falcatus | 45 kV/cm 42 kJ/kg | Ethyl acetate, for 24 h | Lipids 130% extraction with respect to untreated | [68] |
| Scenedesmus spp. | 30 kV/cm 216 kJ/kg | chloroform:methanol:water = 1:2:0.8, room temperature for 3 h | Enhanced recovery of crude lipid and fatty acid methyl ester (3.1 times) in comparison to untreated | [69] |
| Scenedesmus almeriensis | 40 kV/cm, 1.5 MJ/kg dw | Ethanol:hexane (1:0.41 vol/vol), 24 h | PEF-treatment promoted extraction with almost 70% of total lipids extracted against 43% from untreated biomass. | [26] |
| Microalgae/Macroalgae | Enzymatic Hydrolysis | Results | Reference |
|---|---|---|---|
| Ulva sp. | 2% cellulase Onozuka R-10 | Protein extraction yield (%) = 9.72 | [75] |
| Chlorella vulgaris | Protease inhibitor (PI) and 0.1 M NaOH for 24 h. | Protein extraction efficiency (%dbm) = 13.5 | [82] |
| Chlorococcum sp. | Cellulase obtained from Trichoderma reesei, ATCC 26921 40 °C, and a substrate concentration of 10 g/L of microalgal biomass | Glucose yield (%) 64.2 | [83] |
| Chlorella | Cellulase from Trichoderma longibrachiatum 1 U/mg | Protein yield (mg/mg) = 0.7 | [84] |
| Chlorella vulgaris | Carbohydrate-Active enzymes (CAZymes) 20 mg/L 37 °C | Oligosaccharides 2 mmol/microalgae | [85] |
| Microchloropsis salina | 5.9% cellulase mixture, and 0.12% mannanase | Saccharification efficiency (%) = 25 | [86] |
| Chlorella pyrenoidosa | Cellulase derived from Trichoderma viride 15,000 U/g, | protein (%) = 32.30–42.16 (dry cell weight) lipid (%) = 16.9–23.7 DCW | [87] |
| Scenedesmus almeriensis | Protamex (endo-protease, consisting of a mixture of Alcalase and Neutrase) 1:100 w/wdry biomass of Protamex | Protein solubilization yield (%) = 20 Carbohydrate solubilization yield (%) = 40 | [88] |
| Protamex + Celluclast 1:100 w/dry biomass of Protamex and 10 FPU/carbohydrate of Celluclast 1.5 L | Protein solubilization yield (%) = 30 Carbohydrate solubilization yield (%) = 40 | ||
| Microalgae | Enzymatic hydrolysis and extraction procedure | Results | Reference |
| Scenedesmus almeriensis | Enzymatic Hydrolysis 3 h at 50 °C Alcalase 2.5 L and Flavorzyme 1000 L 3% (vol/w) Organic solvent 24 h ethanol:hexane (1:0.41 vol/vol) | Lipid yield (% CDW) = 16 | [26] |
| Nannochloropsis gaditana | Enzymatic hydrolysis 4 h 5% (v/w) Alcalase per dry matter. pH = 8 T = 50 °C Ultrafiltration/Diafiltration Membrane cutoff = 300 kDa Trans membrane pressure = 2.07 bar Filtration area = 50 cm2 | Protein yield (%) = 24.8 | [76] |
| Scenedesmus sp. | Enzymatic hydrolysis cellulase, lysozyme, protease, and pectinase 200 IU/g of each enzyme solvent extraction chloroform: methanol = 1:1 v/v | Lipid recovery from dry biomass (%) = 86.4% | [89] |
| Chlorella vulgaris | Enzymatic hydrolysis Cellulases pH 4.8 and 50 °C solvent extraction hexane, methanol, and chloroform | Lipid extraction yield increased from 29.2% to 73.1%, depending on the organic solvents used, compared to extraction without hydrolysis | [90] |
| Advantages | Challenges |
|---|---|
| High specificity and selectivity | High enzyme cost |
| No by-products formation | Reaction time |
| Green process (no chemicals) | Enzyme recovery and separation |
| Reusability of enzymes | Loss of enzymatic activity |
| Low energy demand |
| Microalgae/Macroalgae | Cascaded Combination | Procedure | Results | Reference |
|---|---|---|---|---|
| C. reinharditii | E + PEF | Wild type C. reinharditii (cc-124) Cell wall deficient C. reinharditii (cc-400) PEF E = 7.5 kV/cm; 2 kWh/kgdw | Protein yield from C. reinharditii (cc-400): 31% (3 times higher in comparison to wild type, and similar to bead beating) | [53] |
| Ulva sp. | E + PEF | EH 2% cellulase Onozuka R-10, precooled (4 °C) deionized water with 1% NaCl, 2 mM 2-[N-Morpholino] ethanesulfonic acid (MES), 0.5% dextran sulfate, pH 6, 25 °C for 120 min PEF E = 1 kV/cm, 30 pulses of 30 µs pulse width duration; Aqueous extraction 30 °C, 120 rpm, 60 min | Recovery yield of protein: E: 9.7% PEF: 10.8% E+PEF: 19.6% (+182% higher than single PEF) | [75] |
| Chlorella vulgaris | PEF + EH | PEF E = 40 kV/cm; WT = 150 kJ/kg; Biomass concentration: 2.5–12.5 mg/mL; Tinitial = 21 °C; Tmax = 38 °C; Incubation after PEF: for up to 24 h EH Protease inhibitor (PI) and 0.1 M NaOH for 24 h. | Enhanced proteins release at the lowest biomass concentration and 30 °C. Protease inhibition impaired protein release. | [82] |
| Palmaria palmata | PEF + EH | PEF E = 0.5 kV/cm; 134.6 kJ/kg; 20 °C EH Enzyme: Depol 793 (mixture of β-glucanase, pectin lyase, and cellulase). Incubation at 40 °C for 1 h at 50 rpm. | Protein content in supernatant on dry basis: 11% (after EH) 10% (after PEF) 9% (after PEF+EH) Protein content in pellet on dry basis: 42% (after EH) 22% (after PEF) 40% (after PEF+EH) | [94] |
| Scenedesmus almeriensis | PEF + EH | PEF E = 40 kV/cm; WT = 75–150 kJ/kg; Pulses duration = 1 ms EH 3 h incubation at 50 °C using 3% enzymes (vol/w). Alcalase and Flavourzyme pH = 8, 0.1 M NaOH. | Degree of Hydrolysis (%) = 50.6 | [57] |
| PEF + EH | PEF E = 40 kV/cm; WT = 1.5 MJ/kgdw EH 3 h incubation at 50 °C, 3% enzymes Alcalase and Flavourzyme. pH = 8 0.1 M NaOH Extraction 24 h extraction with ethanol:hexane (1:0.41 vol/vol) | Lipid yield: 16% (EH) 17% (PEF+EH) 11% (HPH+EH). PEF-EH had the highest degree of hydrolysis | [26] | |
| PEF + EH | PEF E = 40 kV/cm; WT = 1.5 MJ/kgdw; Pulses duration = 1 ms EH 3 h incubation at 50 °C using 3% enzymes (vol/w). Alcalase and Flavourzyme pH = 8 0.1 M NaOH. | Degree of Hydrolysis (%) = 57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pataro, G.; Eslami, E.; Pignataro, F.; Procentese, A. Downstream Processes in a Microalgae Biorefinery: Cascaded Enzymatic Hydrolysis and Pulsed Electric Field as Green Solution. Processes 2025, 13, 1629. https://doi.org/10.3390/pr13061629
Pataro G, Eslami E, Pignataro F, Procentese A. Downstream Processes in a Microalgae Biorefinery: Cascaded Enzymatic Hydrolysis and Pulsed Electric Field as Green Solution. Processes. 2025; 13(6):1629. https://doi.org/10.3390/pr13061629
Chicago/Turabian StylePataro, Gianpiero, Elham Eslami, Francesco Pignataro, and Alessandra Procentese. 2025. "Downstream Processes in a Microalgae Biorefinery: Cascaded Enzymatic Hydrolysis and Pulsed Electric Field as Green Solution" Processes 13, no. 6: 1629. https://doi.org/10.3390/pr13061629
APA StylePataro, G., Eslami, E., Pignataro, F., & Procentese, A. (2025). Downstream Processes in a Microalgae Biorefinery: Cascaded Enzymatic Hydrolysis and Pulsed Electric Field as Green Solution. Processes, 13(6), 1629. https://doi.org/10.3390/pr13061629










