Room-Temperature Complete Oxidation of Formaldehyde over Lactic Acid-Modified HZSM-5-Supported Pt Catalyst
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Catalyst Synthesis
2.3. Catalyst Characterization
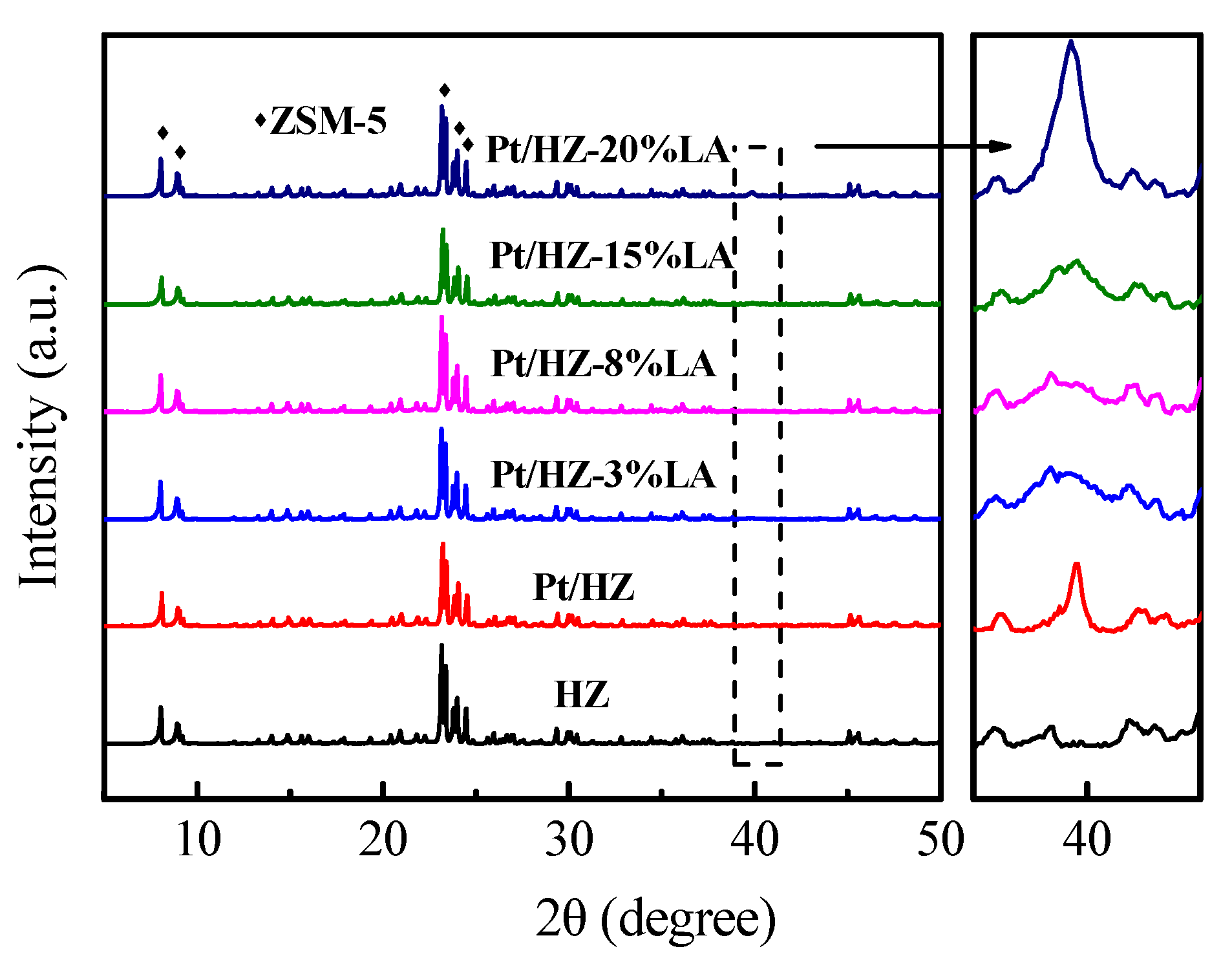
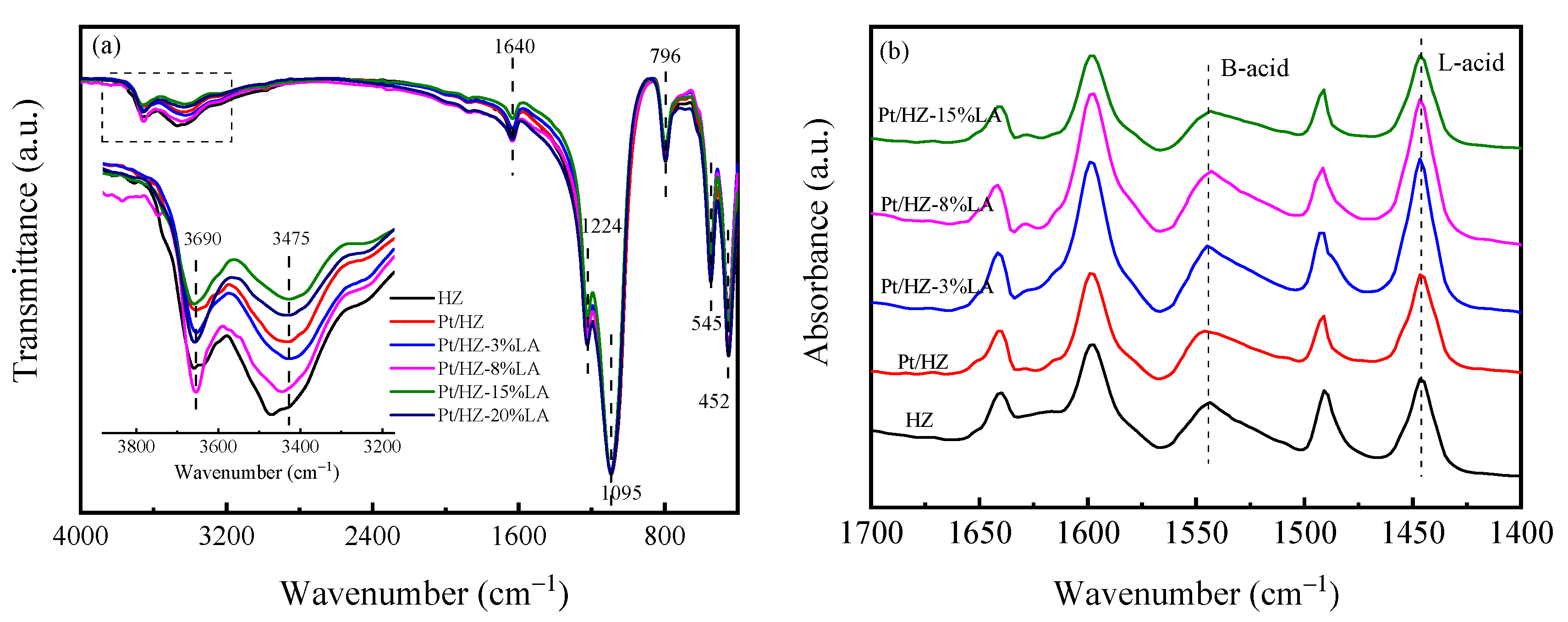
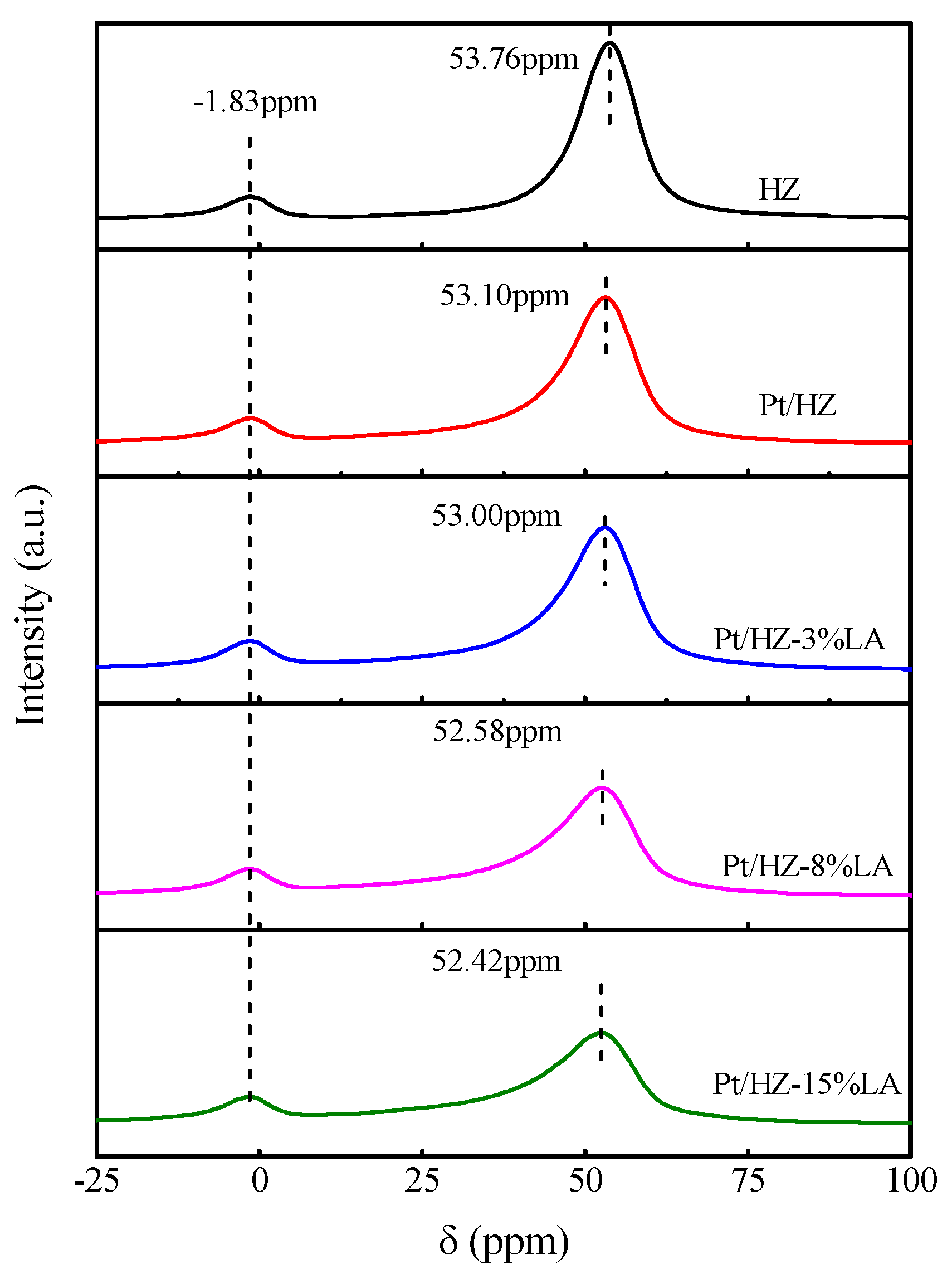
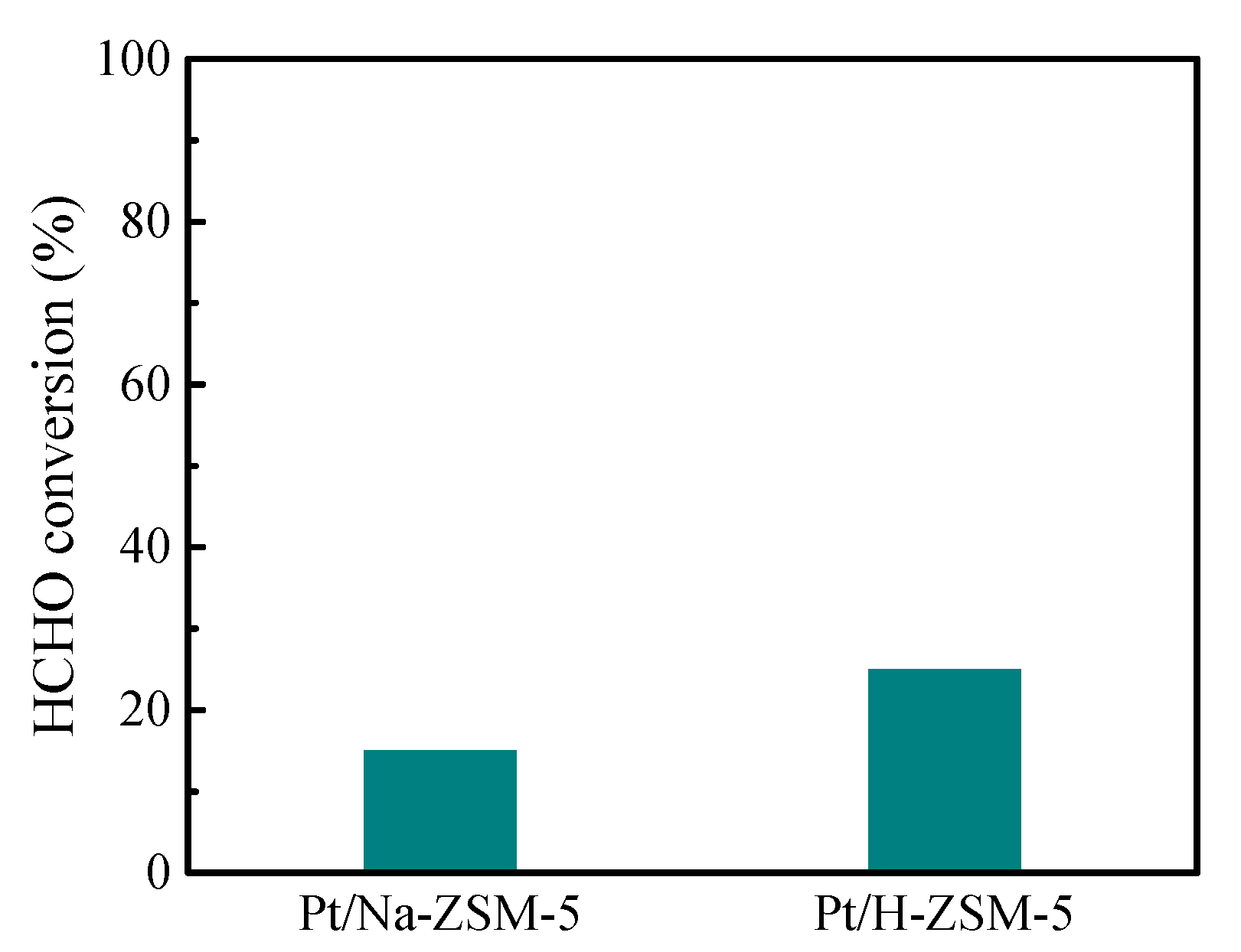
3. Results
3.1. Catalyst Characterization
3.2. Catalytic Oxidation of HCHO
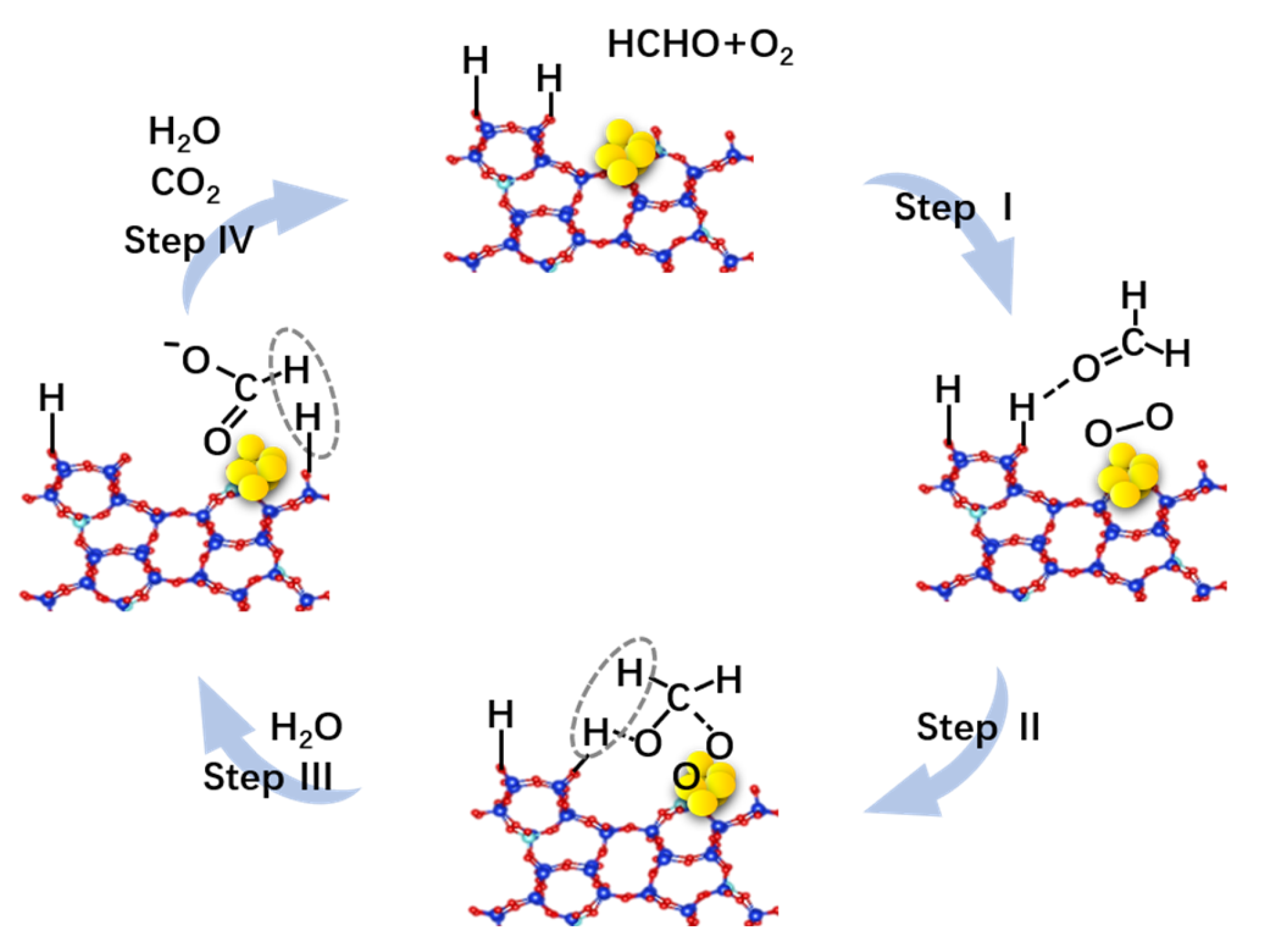
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Salthammer, T.; Mentese, S.; Marutzky, R. Formaldehyde in the Indoor Environment. Chem. Rev. 2010, 110, 2536–2572. [Google Scholar] [CrossRef] [PubMed]
- Hakim, M.; Broza, Y.Y.; Barash, O.; Peled, N.; Phillips, M.; Amann, A.; Haick, H. Volatile Organic Compounds of Lung Cancer and Possible Biochemical Pathways. Chem. Rev. 2012, 112, 5949–5966. [Google Scholar] [CrossRef] [PubMed]
- Salthammer, T. Formaldehyde in the Ambient Atmosphere: From an Indoor Pollutant to an Outdoor Pollutant? Angew. Chem. Int. Ed. 2013, 52, 3320–3327. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.Y.; Qiao, Q.; Li, J.H.; Hao, J.M. Progress in research on catalysts for catalytic oxidation of formaldehyde. Chin. J. Catal. 2016, 37, 102–122. [Google Scholar] [CrossRef]
- Bellat, J.P.; Bezverkhyy, I.; Weber, G.; Royer, S.; Averlant, R.; Giraudon, J.M.; Lamonier, J.F. Capture of formaldehyde by adsorption on nanoporous materials. J. Hazard. Mater. 2015, 300, 711–717. [Google Scholar] [CrossRef]
- Pei, J.J.; Zhang, J.S. On the performance and mechanisms of formaldehyde removal by chemi-sorbents. Chem. Eng. J. 2011, 167, 59–66. [Google Scholar] [CrossRef]
- Qin, G.H.; Zhang, Y.; Ke, X.B.; Tong, X.L.; Sun, Z.; Liang, M.; Xue, S. Photocatalytic reduction of carbon dioxide to formic acid, formaldehyde, and methanol using dye-sensitized TiO2 film. Appl. Catal. B Environ. 2013, 129, 599–605. [Google Scholar] [CrossRef]
- Zhu, X.B.; Gao, X.; Qin, R.; Zeng, Y.X.; Qu, R.Y.; Zheng, C.H.; Tu, X. Plasma-catalytic removal of formaldehyde over Cu–Ce catalysts in a dielectric barrier discharge reactor. Appl. Catal. B Environ. 2015, 170–171, 293–300. [Google Scholar] [CrossRef]
- Chen, F.; Wang, F.; Li, Q.; Cao, C.Y.; Zhang, X.; Ma, H.; Guo, Y. Effect of support (Degussa P25 TiO2, anatase TiO2, γ-Al2O3, and AlOOH) of Pt-based catalysts on the formaldehyde oxidation at room temperature. Catal. Commun. 2017, 99, 39–42. [Google Scholar] [CrossRef]
- Wang, C.Y.; Li, Y.B.; Zhang, C.B.; Chen, X.Y.; Liu, C.L.; Weng, W.Z.; Shan, W.P.; He, H. A simple strategy to improve Pd dispersion and enhance Pd/TiO2 catalytic activity for formaldehyde oxidation: The roles of surface defects. Appl. Catal. B Environ. 2021, 282, 119540–119547. [Google Scholar] [CrossRef]
- Ye, J.W.; Yu, Y.; Fan, J.J.; Cheng, B.; Yu, J.G.; Ho, W.K. Room-temperature formaldehyde catalytic decomposition. Environ. Sci. Nano 2020, 7, 3655–3709. [Google Scholar] [CrossRef]
- Li, L.C.; Li, L.; Wang, L.; Zhao, X.J.; Hua, Z.L.; Chen, Y.Y.; Li, X.B.; Gu, X.L. Enhanced catalytic decomposition of formaldehyde in low temperature and dry environment over silicate-decorated titania supported sodium-stabilized platinum catalyst. Appl. Catal. B Environ. 2020, 277, 119216. [Google Scholar] [CrossRef]
- Chen, X.Y.; Wang, H.H.; Chen, M.; Qin, X.X.; He, H.; Zhang, C.B. Co-function mechanism of multiple active sites over Ag/TiO2 for formaldehyde oxidation. Appl. Catal. B Environ. 2021, 282, 119543. [Google Scholar] [CrossRef]
- Liu, X.F.; Wang, C.Y.; Li, Y.B.; He, H. Acid pretreatment of support promotes Pd/SiO2 activity for formaldehyde oxidation at room temperature. Catal. Sci. Technol. 2022, 12, 6540–6547. [Google Scholar] [CrossRef]
- Wang, C.Y.; Li, Y.B.; Zheng, L.R.; Zhang, C.B.; Wang, Y.; Shan, W.P.; Liu, F.D.; He, H. A Nonoxide Catalyst System Study: Alkali Metal-Promoted Pt/AC Catalyst for Formaldehyde Oxidation at Ambient Temperature. ACS Catal. 2021, 11, 456–465. [Google Scholar] [CrossRef]
- Li, Y.B.; Zhang, C.B.; He, H.; Zhang, J.H.; Chen, M. Influence of alkali metals on Pd/TiO2 catalysts for catalytic oxidation of formaldehyde at room temperature. Catal. Sci. Technol. 2016, 6, 2289–2295. [Google Scholar] [CrossRef]
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z.P. Recent Advances in the Catalytic Oxidation of Volatile Organic Compounds: A Review Based on Pollutant Sorts and Sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef]
- Tian, H.Y.; Wang, J.; Yu, S.Z.; Zhou, K.B. Support Morphology-Dependent Catalytic Activity of Pd/CeO2 for Formaldehyde Oxidation. Environ. Sci. Technol. 2015, 49, 8675–8682. [Google Scholar]
- Li, Y.B.; Zhang, C.B.; Ma, J.Z.; Chen, M.; Deng, H.; He, H. High temperature reduction dramatically promotes Pd/TiO2 catalyst for ambient formaldehyde oxidation. Appl. Catal. B Environ. 2017, 217, 560–569. [Google Scholar] [CrossRef]
- Zhu, X.F.; Yu, J.G.; Jiang, C.J.; Cheng, B. Catalytic decomposition and mechanism of formaldehyde over Pt–Al2O3 molecular sieves at room temperature. Phys. Chem. Chem. Phys. 2017, 19, 6957–6963. [Google Scholar] [CrossRef]
- Zhang, C.B.; He, H.; Tanaka, K.I. Perfect catalytic oxidation of formaldehyde over a Pt/TiO2 catalyst at room temperature. Catal. Commun. 2005, 6, 211–214. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Wang, F.G. Room temperature HCHO oxidation over the Pt/CeO2 catalysts with different oxygen mobilities by changing ceria shapes. Appl. Catal. A Gen. 2022, 630, 118469. [Google Scholar] [CrossRef]
- Sun, D.; Wageh, S.; Al-Ghamdi, A.A.; Le, Y.; Yu, J.G.; Jiang, C.J. Pt/C@MnO2 composite hierarchical hollow microspheres for catalytic formaldehyde decomposition at room temperature. Appl. Surf. Sci. 2019, 466, 301–308. [Google Scholar] [CrossRef]
- Hua, Q.Q.; Liu, K.J.; Ye, J.W.; Ming, L.; Xu, J.S.; Cao, S.W. Hierarchical Pt/NiCo2O4 nanosheets decorated carbon nanofibers for room-temperature catalytic formaldehyde oxidation. Appl. Surf. Sci. 2023, 623, 157012. [Google Scholar] [CrossRef]
- Zhang, Z.L.; He, G.Z.; Li, Y.B.; Zhang, C.B.; Ma, J.Z.; He, H. Effect of Hydroxyl Groups on Metal Anchoring and Formaldehyde Oxidation Performance of Pt/Al2O3. Environ. Sci. Technol. 2022, 56, 10916–10924. [Google Scholar] [CrossRef]
- Qi, L.F.; Cheng, B.C.; Yu, J.G.; Ho, W. High-surface area mesoporous Pt/TiO2 hollow chains for efficient formaldehyde decomposition at ambient temperature. J. Hazard. Mater. 2016, 301, 522–530. [Google Scholar] [CrossRef]
- Liu, F.; Liu, X.P.; Shen, J.; Bahi, A.; Zhang, S.Y.; Wan, L.; Ko, F. The role of oxygen vacancies on Pt/NaInO2 catalyst in improving formaldehyde oxidation at ambient condition. Chem. Eng. J. 2020, 395, 125131. [Google Scholar] [CrossRef]
- Dong, C.Y.; Li, Y.L.; Cheng, D.Y.; Zhang, M.T.; Liu, J.J.; Wang, Y.G.; Xiao, D.Q.; Ma, D. Supported Metal Clusters: Fabrication and Application in Heterogeneous Catalysis. ACS Catal. 2020, 10, 11011–11045. [Google Scholar] [CrossRef]
- Sui, X.L.; Zhang, L.; Li, J.J.; Doyle-Davis, K.; Li, R.Y.; Wang, Z.B.; Sun, X.L. Advanced Support Materials and Interactions for Atomically Dispersed Noble-Metal Catalysts: From Support Effects to Design Strategies. Adv. Energy Mater. 2022, 12, 2102556. [Google Scholar] [CrossRef]
- Wang, F.; Qi, G.Q.; Zhang, C.Q.; Ren, H.C.; Ma, H.; Guo, Y. Na-promoted Pt/Al2O3 activity stability for the complete oxidation of HCHO at room temperature. Catal. Commun. 2020, 139, 105713. [Google Scholar] [CrossRef]
- Ding, J.J.; Chen, J.H.; Rui, Z.B.; Liu, Y.L.; Lv, P.T.; Liu, X.K.; Li, H.Q.; Ji, H.B. Synchronous pore structure and surface hydroxyl groups amelioration as an efficient route for promoting HCHO oxidation over Pt/ZSM-5. Catal. Today 2018, 316, 107–113. [Google Scholar] [CrossRef]
- Ding, J.J.; Rui, Z.B.; Lyu, P.T.; Liu, Y.L.; Liu, X.K.; Ji, H.B. Enhanced formaldehyde oxidation performance over Pt/ZSM-5 through a facile nickel cation modification. Appl. Surf. Sci. 2018, 457, 670–675. [Google Scholar] [CrossRef]
- Shahami, M.; Dooley, K.M.; Shantz, D.F. Steam-assisted crystallized Fe-ZSM-5 materials and their unprecedented activity in benzene hydroxylation to phenol using hydrogen peroxide. J. Catal. 2018, 368, 354–364. [Google Scholar] [CrossRef]
- Schmidt, F.; Lohe, M.R.; Büchner, B.; Giordanino, F.; Bonino, F.; Kaskel, S. Improved catalytic performance of hierarchical ZSM-5 synthesized by desilication with surfactants. Microporous Mesoporous Mater. 2013, 165, 148–157. [Google Scholar] [CrossRef]
- Bahari, M.B.; Jalil, A.A.; Mamat, C.R.; Arifin, M.A.; Hassan, N.S.; Alhassan, M.; Sawal, M.H.; Izzudin, N.M.; Hatta, A.H.; Aziz, M.A.; et al. Optimizing hydrogen-driven n-pentane isomerization over Pt-doped fibrous ZSM-5. Mol. Catal. 2024, 557, 113951. [Google Scholar] [CrossRef]
- Spennati, E.; Iturrate, M.; Bogni, S.; Cosso, A.; Millini, R.; Riani, P.; Busca, G.; Garbarino, G. Ethanol conversion to hydrocarbons over Sn-doped H-ZSM-5 zeolite catalysts. Microporous Mesoporous Mater. 2024, 379, 113284. [Google Scholar] [CrossRef]
- Wu, M.; Ye, J.W.; Cheng, B.; Yu, J.G.; Zhang, L.Y. Pt/NiCo2O4 nanowire arrays on Ni foam as catalysts for efficient formaldehyde oxidation at room temperature. Appl. Surf. Sci. 2023, 608, 155183. [Google Scholar] [CrossRef]
- Yu, L.Y.; Ji, J.; Dong, T.; Liu, B.Y.; Li, K.; Huang, H.B. Dual function catalysis of Pd/desilicated-ZSM-5 for formaldehyde oxidation at ambient temperature. Appl. Catal. B Environ. Energy 2025, 365, 124981. [Google Scholar] [CrossRef]
- Yalcin, B.K.; Ipek, B. Fluoride-free synthesis of mesoporous [Al]-[B]-ZSM-5 using cetyltrimethylammonium bromide and methanol-to-olefin activity with high propene selectivity. Appl. Catal. A Gen. 2021, 610, 117915. [Google Scholar] [CrossRef]
- Bayout, A.; Cammarano, C.; Costa, I.M.; Veryasov, G.; Sachse, A.; Hulea, V. Highly para-selective alkylation of toluene by methyl mercaptan over silylated ZSM-5 zeolite. J. Catal. 2024, 440, 115828. [Google Scholar] [CrossRef]
- Ayub, A.; Mahadi, A.H.; Alim, M.A.S.; Bahruji, H. The effect of Zn:Ti ratios on PdZn/ZnO–TiO2 catalysts for CO2 hydrogenation to methanol. Mol. Catal. 2025, 570, 114703. [Google Scholar] [CrossRef]
- Kumar, A.; Kanchan, D.R.; Banerjee, A.; Singh, B.P.; Srivastava, R. Synergistic Effect of Ni Exsolution and Oxygen Vacancies in NiAl2O4 for Catalytic Transfer Hydrodeoxygenation of Furfural to 2-Methylfuran. ACS Catal. 2025, 15, 8239–8258. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Mori, K.; Kuwahara, Y.; Kobayashi, H.; Yamashita, H. Defect Engineering of Pt/TiO2–x Photocatalysts via Reduction Treatment Assisted by Hydrogen Spillover. ACS Appl. Mater. Interfaces 2021, 13, 48669–48678. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, G.; Woo, M.H.; Kwak, G.; Kim, S.K. Control of Hierarchical Structure and Framework-Al Distribution of ZSM-5 via Adjusting Crystallization Temperature and Their Effects on Methanol Conversion. ACS Catal. 2019, 9, 2880–2892. [Google Scholar] [CrossRef]
- Gardner, D.W.; Huo, J.J.; Hoff, T.C.; Johnson, R.L.; Shanks, B.H.; Tessonnier, J.P. Insights into the Hydrothermal Stability of ZSM-5 under Relevant Biomass Conversion Reaction Conditions. ACS Catal. 2015, 5, 4418–4422. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Li, Y.B.; Peng, Y.X.; Chen, F.; Wang, L.; Zhang, C.B.; Meng, X.J.; He, H.; Xiao, F.S. Complete oxidation of formaldehyde at room temperature over an Al-rich Beta zeolite supported platinum catalyst. Appl. Catal. B Environ. 2017, 219, 200–208. [Google Scholar] [CrossRef]



| Sample | SBET (m2/g) [a] | Dp (nm) [b] | Vp (cm3/g) [c] | SiO2/Al2O3 (%) [d] | Pt Dispersion (%) | Osur/Ototal (%) |
|---|---|---|---|---|---|---|
| HZ | 396 | 0.6 | 0.17 | 20.2 | - | - |
| Pt/HZ | 396 | 0.6 | 0.17 | 20.1 | 12.3 | 46.5 |
| Pt/HZ-3% LA | 375 | 0.6 | 0.16 | 20.4 | 13.2 | 50.7 |
| Pt/HZ-8% LA | 371 | 0.6 | 0.16 | 20.4 | 35.6 | 59.9 |
| Pt/HZ-15% LA | 374 | 0.6 | 0.17 | 20.7 | 21.7 | 46.4 |
| Pt/HZ-20% LA | 375 | 0.6 | 0.17 | 20.7 | 6.8 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Wang, S.; Li, X.; Du, Y.; Hu, J.; Jiang, S.; Guo, Y. Room-Temperature Complete Oxidation of Formaldehyde over Lactic Acid-Modified HZSM-5-Supported Pt Catalyst. Processes 2025, 13, 1440. https://doi.org/10.3390/pr13051440
Zhang T, Wang S, Li X, Du Y, Hu J, Jiang S, Guo Y. Room-Temperature Complete Oxidation of Formaldehyde over Lactic Acid-Modified HZSM-5-Supported Pt Catalyst. Processes. 2025; 13(5):1440. https://doi.org/10.3390/pr13051440
Chicago/Turabian StyleZhang, Tongtong, Sijia Wang, Xingyuan Li, Yupeng Du, Jiajun Hu, Shi Jiang, and Yu Guo. 2025. "Room-Temperature Complete Oxidation of Formaldehyde over Lactic Acid-Modified HZSM-5-Supported Pt Catalyst" Processes 13, no. 5: 1440. https://doi.org/10.3390/pr13051440
APA StyleZhang, T., Wang, S., Li, X., Du, Y., Hu, J., Jiang, S., & Guo, Y. (2025). Room-Temperature Complete Oxidation of Formaldehyde over Lactic Acid-Modified HZSM-5-Supported Pt Catalyst. Processes, 13(5), 1440. https://doi.org/10.3390/pr13051440






