Recent Trends in the Use of Electrode Materials for Microbial Fuel Cells Accentuating the Potential of Photosynthetic Cyanobacteria and Microalgae: A Review
Abstract
1. Introduction
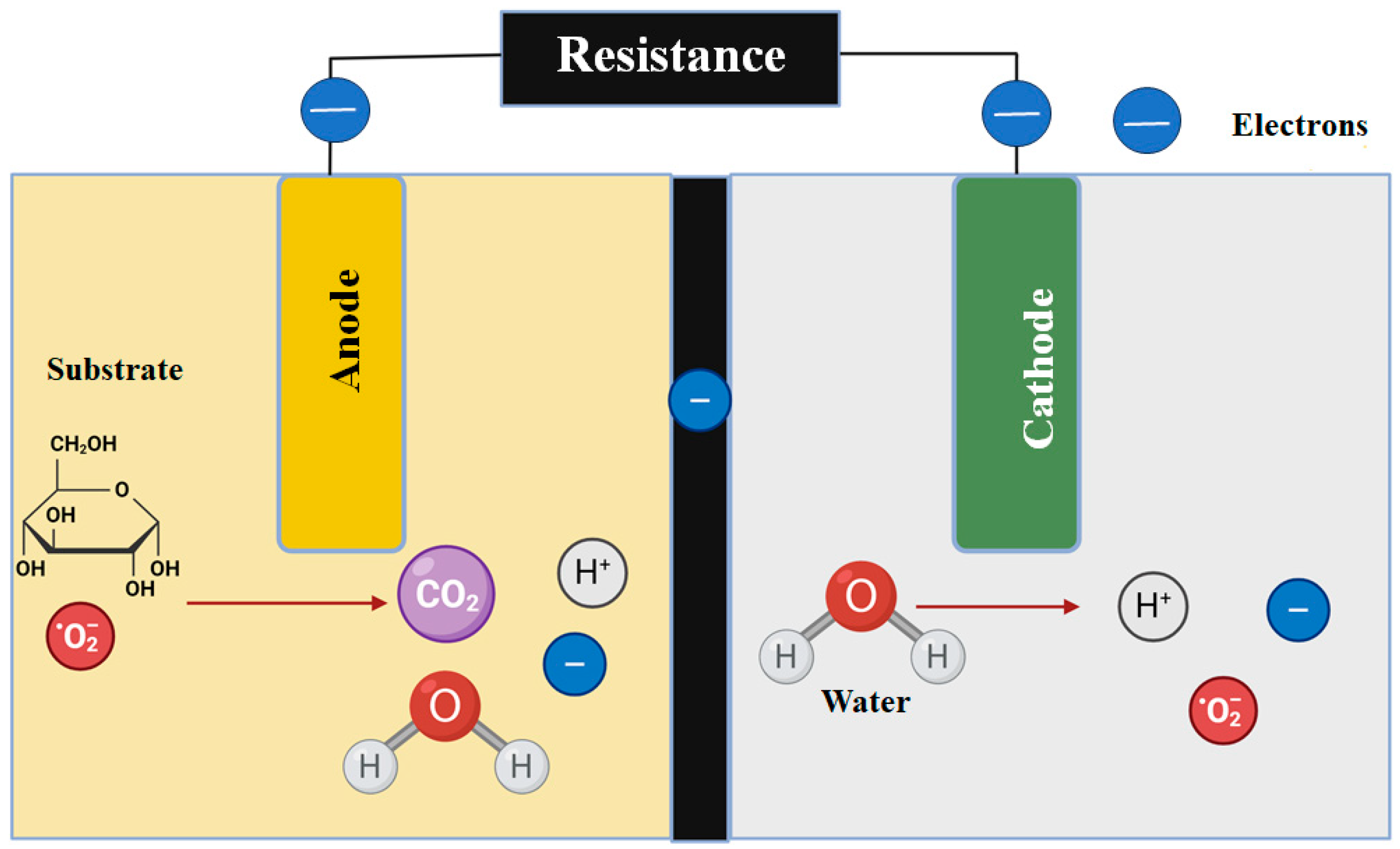
2. MFCs and Their Variations
2.1. Microbial Fuel Cells with a Single Compartment
2.2. Double-Chambered Microbial Fuel Cells
2.3. Continuous Mode (CFP-MFC)
2.4. MFCs Using a Flat Plate (FP-MFC)
2.5. Sediment Microbial Fuel Cell
2.6. Stacking of Microbial Fuel Cells
2.7. Upward Flow Fuel Cells
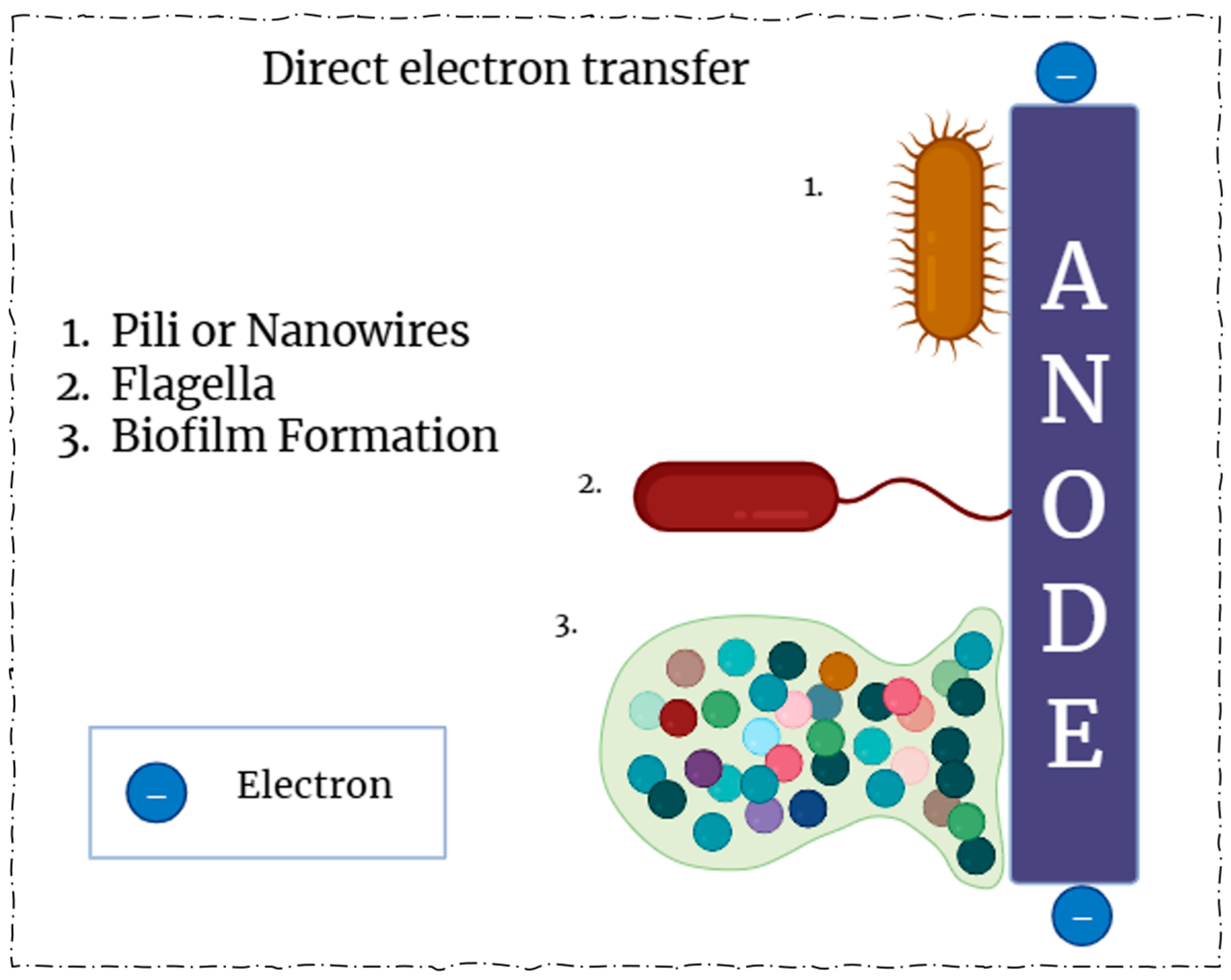
3. Methods of Electron Transfer
3.1. Direct Electron Transfer (DET)
3.2. Indirect or Mediated Electron Transfer (I)
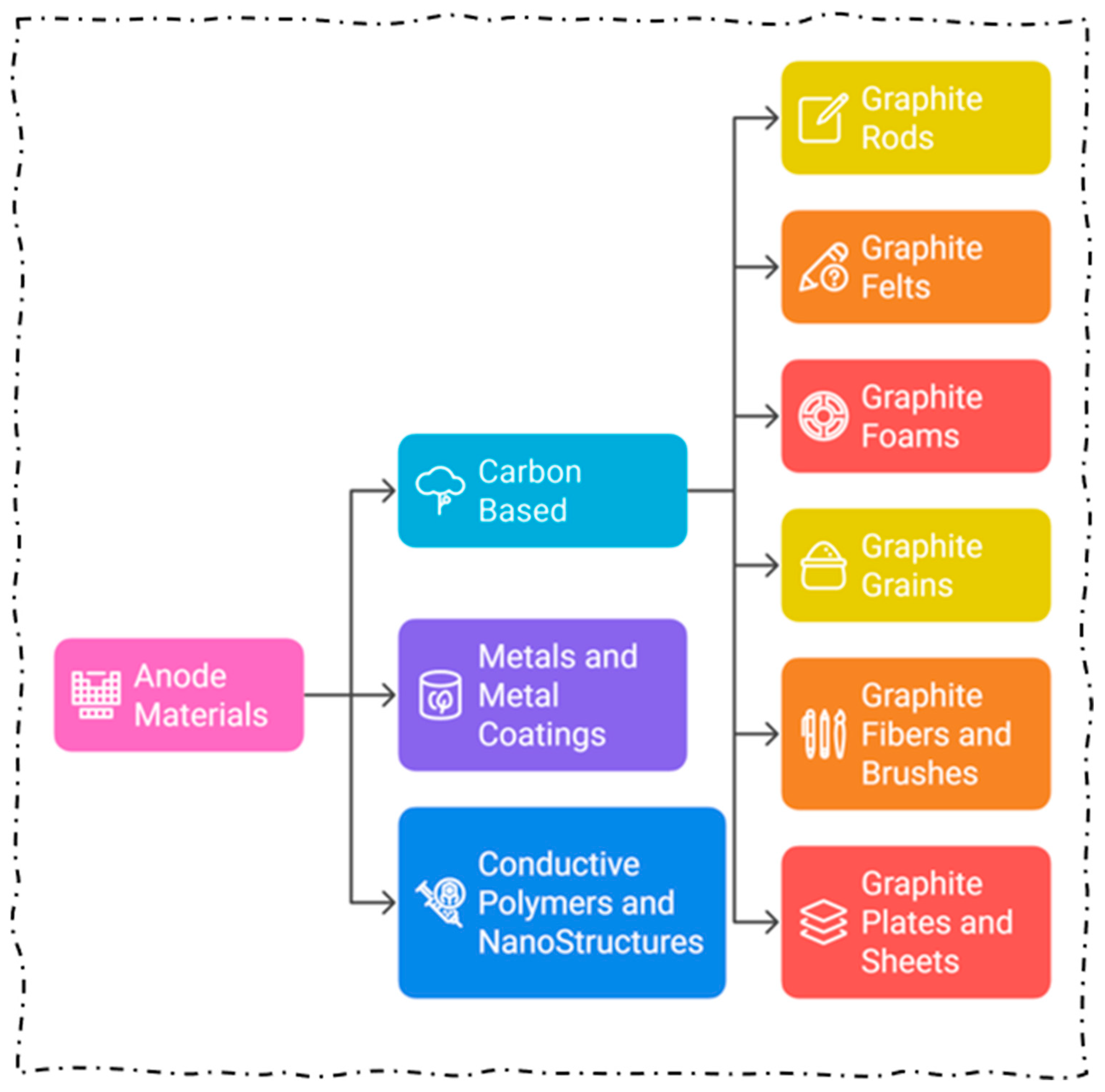
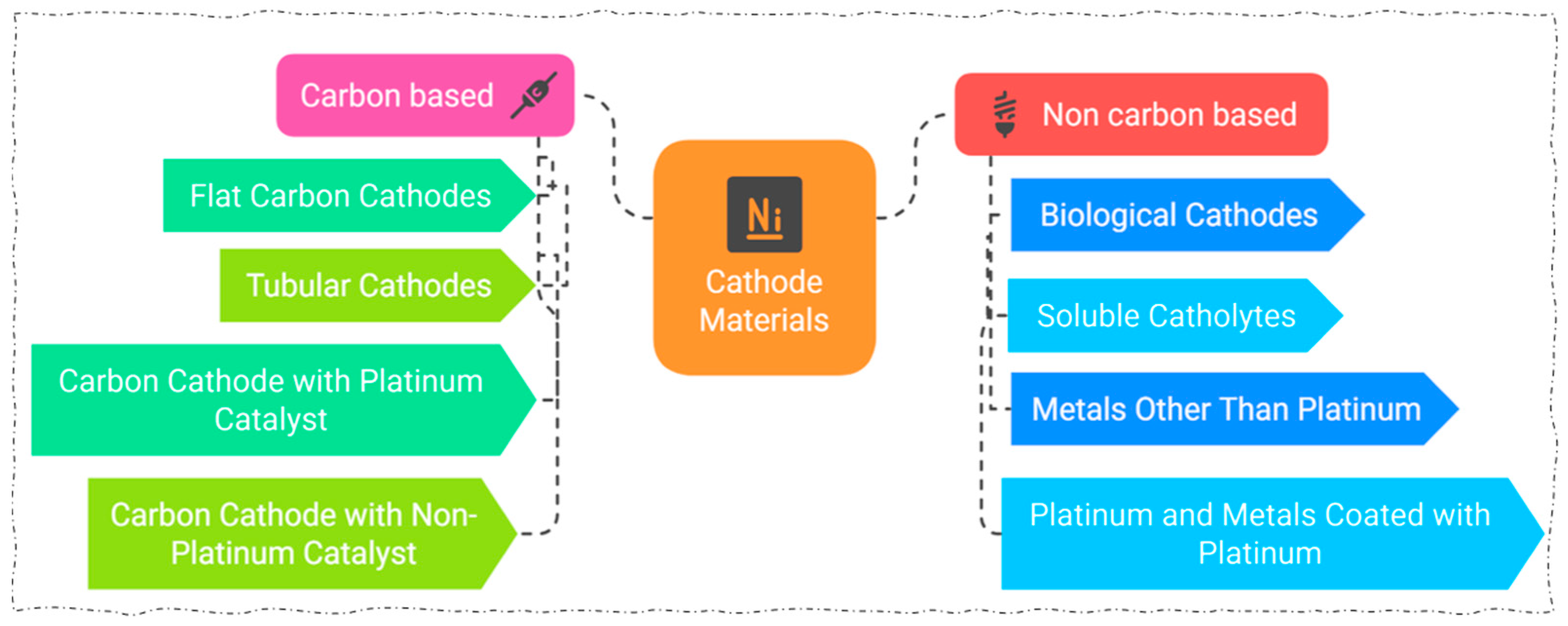
4. Design of Electrodes
4.1. Oxide Electrodes
4.2. Metallic Electrodes
4.3. Pt-/CNT Electrode
4.4. Graphene Electrodes
4.5. Natural Electrodes
4.6. Advancement of Electrodes
5. Material Fabrication
5.1. Nickel Metallic Thin
5.2. Nanocoated Electrodes
5.3. Granular Activated Carbon (GAC)
5.4. Immobilization of the Electrode
5.5. Electrodeposition Using MnO2
5.6. Pyrolysis
5.7. Hummer’s Approach
5.8. Surface–Bacteria Interaction
5.9. Interactions Using Cyclic Voltagrams
6. Photosynthetic Microbial Fuel Cells
6.1. Whole-Cell Fabrication
6.2. Plant Microbial Fuel Cells
6.3. Cyanobacteria and Microalgae Use in Microbial Fuel Cells
7. Electrogenic Bacteria Use in Microbial Fuel Cells
Fusion of Photosynthetic and Non-Photosynthetic Organisms
8. The Microbe’s Genetic Modification
9. Synthetic Biology Approach to Improved Energy Harvest
10. Practical and Technical Challenges to Implementing Microbial Fuel Cells on a Large Scale
11. Comparative Analysis of Power Generation in Different MFCs
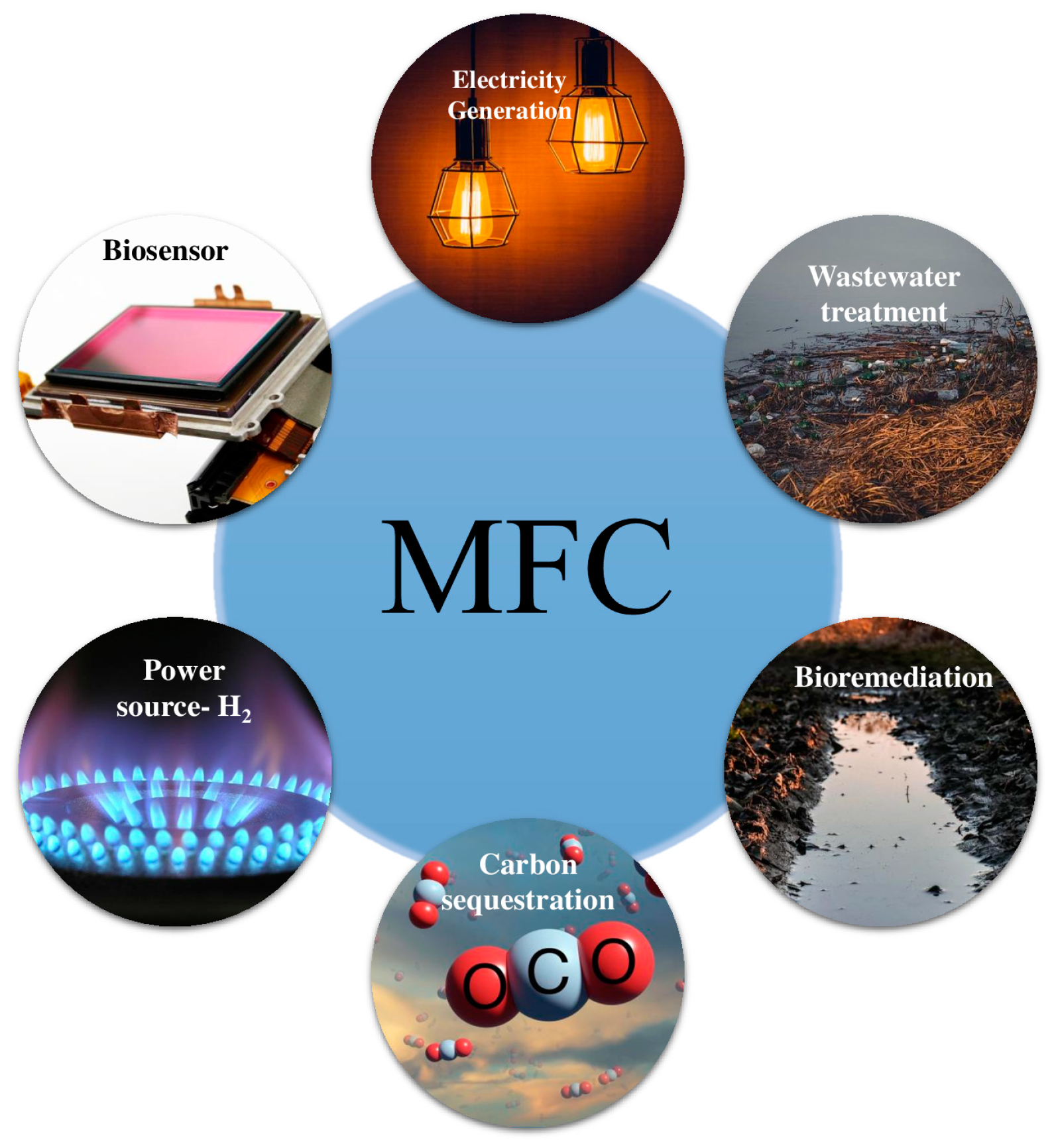
12. Applications for MFCs
13. Future Perspectives
14. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MFCs | Microbial fuel cells |
| PEM | Proton exchange membrane |
| FP-MFCs | Flat-plate microbial fuel cells |
| BM-MFCs | Batch mode microbial fuel cells |
| SCM-MFCs | Semi-continuous microbial fuel cells |
| CM-MFCs | Continuous mode microbial fuel cells |
| PD | Power density |
| ECE | Energy conversion efficiency |
| DET | Direct electron transfer |
| TEAs | Terminal electron acceptors |
| EET | Extracellular electron transfer |
| MET | Mediated electron transfer |
| CNTs | Carbon nanotubes |
| CF | Carbon felt |
| GF | Graphite/graphene felt |
| GP | Graphite/graphene plate |
| BPEC | Biophoto electrochemical cell |
| COD | Chemical oxygen demand |
| Ni@Fb | Nickel-coated carbon felt |
| Ni@Gp | Nickel-coated graphite plate |
| mV | Milli volt |
| mg | Milligram |
| mW/m2 | Milliwatts per square meter |
| cms−1 | Centimeter per second |
| mA·cm−2 | Milliampere per square centimeter |
| W/m3 | Watts per cubic meter |
| LSCV | Low scan rate cyclic voltammetry |
| EIS | Electron impedance spectroscopy |
| ESEM | Environmental scanning electron microscopy |
| PEDOT/PSS | Poly(3,4-ethylenedioxythiophene) (PEDOT) and polystyrene sulfonate (PSS) |
| TCO | Transparent conductive oxides |
| KOH | Potassium hydroxide |
| AP-VPP | Atmospheric pressure–vapor phase polymerization |
| EDOT | 3,4-ethylenedioxythiophene |
| NiSO4·6H2O | Nickel sulfate |
| H3BO3 | Boric acid |
| GAC | Granular acitivated carbon |
| YPD | Yeast peptone dextrose |
| GNWs | Graphene nanowalls |
| [Fe(CN)6]3 | Ferricyanide |
| DCBQ | 2,6-Dichloro-1,4-benzoquinone |
| NADH | Nicotinamide-adenine dinucleotide |
References
- Mehdizadeh Allaf, M.; Peerhossaini, H. Cyanobacteria: Model Microorganisms and Beyond. Microorganisms 2022, 10, 696. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Baracaldo, P.; Bianchini, G.; Wilson, J.D.; Knoll, A.H. Cyanobacteria and Biogeochemical Cycles through Earth History. Trends Microbiol. 2022, 30, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Lourembam, J.; Haobam, B.; Singh, K.B.; Verma, S.; Rajan, J.P. The Molecular Insights of Cyanobacterial Bioremediations of Heavy Metals: The Current and the Future Challenges. Front. Microbiol. 2024, 15, 1450992. [Google Scholar] [CrossRef] [PubMed]
- Guevara, G.; Solorzano, J.S.E.; Ramírez, M.V.; Rusu, A.; Llorens, J.M.N. Characterizing A21: Natural Cyanobacteria-Based Consortium with Potential for Steroid Bioremediation in Wastewater Treatment. Int. J. Mol. Sci. 2024, 25, 13018. [Google Scholar] [CrossRef]
- Zahra, Z.; Choo, D.H.; Lee, H.; Parveen, A. Cyanobacteria: Review of Current Potentials and Applications. Environments 2020, 7, 13. [Google Scholar] [CrossRef]
- Antolini, E. Composite Materials for Polymer Electrolyte Membrane Microbial Fuel Cells. Biosens. Bioelectron. 2015, 69, 54–70. [Google Scholar] [CrossRef]
- Kumar, S.S.; Kumar, V.; Kumar, R.; Malyan, S.K.; Pugazhendhi, A. Microbial Fuel Cells as a Sustainable Platform Technology for Bioenergy, Biosensing, Environmental Monitoring, and Other Low Power Device Applications. Fuel 2019, 255, 115682. [Google Scholar] [CrossRef]
- Pandya, R.S.; Kaur, T.; Bhattacharya, R.; Bose, D.; Saraf, D. Harnessing Microorganisms for Bioenergy with Microbial Fuel Cells: Powering the Future. Water-Energy Nexus 2024, 7, 1–12. [Google Scholar] [CrossRef]
- Khan, M.J.; Singh, N.; Mishra, S.; Ahirwar, A.; Bast, F.; Varjani, S.; Schoefs, B.; Marchand, J.; Rajendran, K.; Banu, J.R.; et al. Impact of Light on Microalgal Photosynthetic Microbial Fuel Cells and Removal of Pollutants by Nanoadsorbent Biopolymers: Updates, Challenges and Innovations. Chemosphere 2022, 288, 132589. [Google Scholar] [CrossRef]
- Shlosberg, Y.; Schuster, G.; Adir, N. Harnessing Photosynthesis to Produce Electricity Using Cyanobacteria, Green Algae, Seaweeds and Plants. Front. Plant Sci. 2022, 13, 955843. [Google Scholar] [CrossRef]
- Schneider, K.; Thorne, R.J.; Cameron, P.J. An Investigation of Anode and Cathode Materials in Photomicrobial Fuel Cells. Philosophical Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150080. [Google Scholar] [CrossRef]
- Luo, S.; Berges, J.A.; He, Z.; Young, E.B. Algal-Microbial Community Collaboration for Energy Recovery and Nutrient Remediation from Wastewater in Integrated Photobioelectrochemical Systems. Algal Res. 2017, 24, 527–539. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, L.; Zularisam, A.W. Microbial Fuel Cells: Types and Applications. In Waste Biomass Management—A Holistic Approach; Springer International Publishing: Cham, Switzerland, 2017; pp. 367–384. [Google Scholar]
- Wang, J.; Ren, K.; Zhu, Y.; Huang, J.; Liu, S. A Review of Recent Advances in Microbial Fuel Cells: Preparation, Operation, and Application. BioTech 2022, 11, 44. [Google Scholar] [CrossRef]
- Borole, A.P.; Reguera, G.; Ringeisen, B.; Wang, Z.-W.; Feng, Y.; Kim, B.H. Electroactive Biofilms: Current Status and Future Research Needs. Energy Environ. Sci. 2011, 4, 4813. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, L.; Wahid, Z.A.; Din, M.F.M. Exoelectrogens in Microbial Fuel Cells toward Bioelectricity Generation: A Review. Int. J. Energy Res. 2015, 39, 1048–1067. [Google Scholar] [CrossRef]
- Naha, A.; Debroy, R.; Sharma, D.; Shah, M.P.; Nath, S. Microbial Fuel Cell: A State-of-the-Art and Revolutionizing Technology for Efficient Energy Recovery. Clean. Circ. Bioeconomy 2023, 5, 100050. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhou, S.; Tang, J. In Situ Investigation of Cathode and Local Biofilm Microenvironments Reveals Important Roles of OH− and Oxygen Transport in Microbial Fuel Cells. Environ. Sci. Technol. 2013, 47, 4911–4917. [Google Scholar] [CrossRef]
- Tiwari, S.; Koreti, D.; Kosre, A.; Mahish, P.K.; Jadhav, S.K.; Chandrawanshi, N.K. Fungal Microbial Fuel Cells, an Opportunity for Energy Sources. In Energy; Wiley: New York, NY, USA, 2021; pp. 250–273. [Google Scholar]
- Gorby, Y.A.; Yanina, S.; McLean, J.S.; Rosso, K.M.; Moyles, D.; Dohnalkova, A.; Beveridge, T.J.; Chang, I.S.; Kim, B.H.; Kim, K.S.; et al. Electrically Conductive Bacterial Nanowires Produced by Shewanella Oneidensis Strain MR-1 and Other Microorganisms. Proc. Natl. Acad. Sci. USA 2006, 103, 11358–11363. [Google Scholar] [CrossRef]
- Pasupuleti, S.B.; Srikanth, S.; Mohan, S.V.; Pant, D. Continuous Mode Operation of Microbial Fuel Cell (MFC) Stack with Dual Gas Diffusion Cathode Design for the Treatment of Dark Fermentation Effluent. Int. J. Hydrogen Energy 2015, 40, 12424–12435. [Google Scholar] [CrossRef]
- Kazemi, S.; Mohseni, M.; Fatih, K. A Systematic Study of Separators in Air-Breathing Flat-Plate Microbial Fuel Cells—Part 1: Structure, Properties, and Performance Correlations. Energies 2016, 9, 78. [Google Scholar] [CrossRef]
- Blossfeld, S.; Perriguey, J.; Sterckeman, T.; Morel, J.-L.; Lösch, R. Rhizosphere pH Dynamics in Trace-Metal-Contaminated Soils, Monitored with Planar pH Optodes. Plant Soil 2010, 330, 173–184. [Google Scholar] [CrossRef]
- Hirano, A.; Hon-Nami, K.; Kunito, S.; Hada, M.; Ogushi, Y. Temperature Effect on Continuous Gasification of Microalgal Biomass: Theoretical Yield of Methanol Production and Its Energy Balance. Catal. Today 1998, 45, 399–404. [Google Scholar] [CrossRef]
- Abbas, S.Z.; Rafatullah, M.; Khan, M.A.; Siddiqui, M.R. Bioremediation and Electricity Generation by Using Open and Closed Sediment Microbial Fuel Cells. Front. Microbiol. 2019, 9, 3348. [Google Scholar] [CrossRef]
- Zabihallahpoor, A.; Rahimnejad, M.; Talebnia, F. Sediment Microbial Fuel Cells as a New Source of Renewable and Sustainable Energy: Present Status and Future Prospects. RSC Adv. 2015, 5, 94171–94183. [Google Scholar] [CrossRef]
- Abbas, S.Z.; Rafatullah, M.; Ismail, N.; Syakir, M.I. A Review on Sediment Microbial Fuel Cells as a New Source of Sustainable Energy and Heavy Metal Remediation: Mechanisms and Future Prospective. Int. J. Energy Res. 2017, 41, 1242–1264. [Google Scholar] [CrossRef]
- Hong, S.W.; Chang, I.S.; Choi, Y.S.; Chung, T.H. Experimental Evaluation of Influential Factors for Electricity Harvesting from Sediment Using Microbial Fuel Cell. Bioresour. Technol. 2009, 100, 3029–3035. [Google Scholar] [CrossRef]
- Aelterman, P.; Rabaey, K.; Pham, H.T.; Boon, N.; Verstraete, W. Continuous Electricity Generation at High Voltages and Currents Using Stacked Microbial Fuel Cells. Environ. Sci. Technol. 2006, 40, 3388–3394. [Google Scholar] [CrossRef]
- Prasad, J.; Tripathi, R.K. Scale-up and Control the Voltage of Sediment Microbial Fuel Cell for Charging a Cell Phone. Biosens. Bioelectron. 2021, 172, 112767. [Google Scholar] [CrossRef]
- Veerubhotla, R.; Nag, S.; Das, D. Internet of Things Temperature Sensor Powered by Bacterial Fuel Cells on Paper. J. Power Sources 2019, 438, 226947. [Google Scholar] [CrossRef]
- Walter, X.A.; Gajda, I.; Forbes, S.; Winfield, J.; Greenman, J.; Ieropoulos, I. Scaling-up of a Novel, Simplified MFC Stack Based on a Self-Stratifying Urine Column. Biotechnol. Biofuels 2016, 9, 93. [Google Scholar] [CrossRef]
- Jang, J.K.; Pham, T.H.; Chang, I.S.; Kang, K.H.; Moon, H.; Cho, K.S.; Kim, B.H. Construction and Operation of a Novel Mediator- and Membrane-Less Microbial Fuel Cell. Process Biochem. 2004, 39, 1007–1012. [Google Scholar] [CrossRef]
- He, Z.; Wagner, N.; Minteer, S.D.; Angenent, L.T. An Upflow Microbial Fuel Cell with an Interior Cathode: Assessment of the Internal Resistance by Impedance Spectroscopy. Environ. Sci. Technol. 2006, 40, 5212–5217. [Google Scholar] [CrossRef]
- Cao, Y.; Mu, H.; Liu, W.; Zhang, R.; Guo, J.; Xian, M.; Liu, H. Electricigens in the Anode of Microbial Fuel Cells: Pure Cultures versus Mixed Communities. Microb. Cell Factories 2019, 18, 39. [Google Scholar] [CrossRef] [PubMed]
- Reguera, G.; McCarthy, K.D.; Mehta, T.; Nicoll, J.S.; Tuominen, M.T.; Lovley, D.R. Extracellular Electron Transfer via Microbial Nanowires. Nature 2005, 435, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Busalmen, J.P.; Esteve-Núñez, A.; Berná, A.; Feliu, J.M. C-Type Cytochromes Wire Electricity-Producing Bacteria to Electrodes. Angew. Chem. Int. Ed. 2008, 47, 4874–4877. [Google Scholar] [CrossRef]
- Liu, J.; Chakraborty, S.; Hosseinzadeh, P.; Yu, Y.; Tian, S.; Petrik, I.; Bhagi, A.; Lu, Y. Metalloproteins Containing Cytochrome, Iron–Sulfur, or Copper Redox Centers. Chem. Rev. 2014, 114, 4366–4469. [Google Scholar] [CrossRef]
- Aiyer, K.S. How Does Electron Transfer Occur in Microbial Fuel Cells? World J. Microbiol. Biotechnol. 2020, 36, 19. [Google Scholar] [CrossRef]
- Reguera, G. Microbial Nanowires and Electroactive Biofilms. FEMS Microbiol. Ecol. 2018, 94, fiy086. [Google Scholar] [CrossRef]
- Fengbin, W.; Yangqi, G.J.; Patrick, O.B.; Sophia, M.Y.; Sibel, E.Y.; Vishok, S.; Cong, S.; Dennis, V.; Nicole, L.I.; Allon, I.H.; et al. Structure of Microbial Nanowires Reveals Stacked Hemes that Transport Electrons over Micrometers. Cell 2019. [Google Scholar] [CrossRef]
- Sure, S.; Ackland, M.L.; Torriero, A.A.J.; Adholeya, A.; Kochar, M. Microbial Nanowires: An Electrifying Tale. Microbiology 2016, 162, 2017–2028. [Google Scholar] [CrossRef]
- Zhao, F.; Slade, R.C.T.; Varcoe, J.R. Techniques for the Study and Development of Microbial Fuel Cells: An Electrochemical Perspective. Chem. Soc. Rev. 2009, 38, 1926. [Google Scholar] [CrossRef] [PubMed]
- Malvankar, N.S.; Lovley, D.R. Microbial Nanowires for Bioenergy Applications. Curr. Opin. Biotechnol. 2014, 27, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Malvankar, N.S.; Lovley, D.R. Microbial nanowires: A new paradigm for biological electron transfer and bioelectronics. ChemSusChem 2012, 5, 1039–1046. [Google Scholar] [CrossRef]
- Bjerg, J.T.; Boschker, H.T.S.; Larsen, S.; Berry, D.; Schmid, M.; Millo, D.; Tataru, P.; Meysman, F.J.R.; Wagner, M.; Nielsen, L.P.; et al. Long-Distance Electron Transport in Individual, Living Cable Bacteria. Proc. Natl. Acad. Sci. USA 2018, 115, 5786–5791. [Google Scholar] [CrossRef] [PubMed]
- Meysman, F.J.R. Cable Bacteria Take a New Breath Using Long-Distance Electricity. Trends Microbiol. 2018, 26, 411–422. [Google Scholar] [CrossRef]
- Winaikij, P.; Sreearunothai, P.; Sombatmankhong, K. Probing Mechanisms for Microbial Extracellular Electron Transfer (EET) Using Electrochemical and Microscopic Characterisations. Solid State Ion. 2018, 320, 283–291. [Google Scholar] [CrossRef]
- Debabov, V.G. Electricity from Microorganisms. Microbiology 2008, 77, 123–131. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, P.; Bilal, M.; Wang, W.; Hu, H.; Zhang, X. Enhanced Biosynthesis of Phenazine-1-Carboxamide by Engineered Pseudomonas Chlororaphis HT66. Microb. Cell Factories 2018, 17, 117. [Google Scholar] [CrossRef]
- Dantas, P.V.; Peres, S.; Campos-Takaki, G.M.; Rotta, C.E.L. Utilization of Raw Glycerol for Pyocyanin Production from Pseudomonas Aeruginosa in Half-Microbial Fuel Cells: Evaluation of Two Electrochemical Approaches. J. Electrochem. Soc. 2013, 160, G142–G148. [Google Scholar] [CrossRef]
- Park, D.H.; Laivenieks, M.; Guettler, M.V.; Jain, M.K.; Zeikus, J.G. Microbial Utilization of Electrically Reduced Neutral Red as the Sole Electron Donor for Growth and Metabolite Production. Appl. Environ. Microbiol. 1999, 65, 2912–2917. [Google Scholar] [CrossRef]
- Thygesen, A.; Poulsen, F.W.; Min, B.; Angelidaki, I.; Thomsen, A.B. The Effect of Different Substrates and Humic Acid on Power Generation in Microbial Fuel Cell Operation. Bioresour. Technol. 2009, 100, 1186–1191. [Google Scholar] [CrossRef]
- Zhang, E.; Cai, Y.; Luo, Y.; Piao, Z. Riboflavin-Shuttled Extracellular Electron Transfer from Enterococcus Faecalis to Electrodes in Microbial Fuel Cells. Can. J. Microbiol. 2014, 60, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Ieropoulos, I.A.; Greenman, J.; Melhuish, C.; Hart, J. Comparative Study of Three Types of Microbial Fuel Cell. Enzym. Microb. Technol. 2005, 37, 238–245. [Google Scholar] [CrossRef]
- Longatte, G.; Buriez, O.; Labbé, E.; Guille-Collignon, M.; Lemaître, F. Electrochemical Behavior of Quinones Classically Used for Bioenergetical Applications: Considerations and Insights about the Anodic Side. ChemElectroChem 2024, 11, e202300542. [Google Scholar] [CrossRef]
- Chen, X.; Lawrence, J.M.; Wey, L.T.; Schertel, L.; Jing, Q.; Vignolini, S.; Howe, C.J.; Kar-Narayan, S.; Zhang, J.Z. 3D-Printed Hierarchical Pillar Array Electrodes for High-Performance Semi-Artificial Photosynthesis. Nat. Mater. 2022, 21, 811–818. [Google Scholar] [CrossRef]
- Ludwig, K.A.; Langhals, N.B.; Joseph, M.D.; Richardson-Burns, S.M.; Hendricks, J.L.; Kipke, D.R. Poly(3,4-Ethylenedioxythiophene) (PEDOT) Polymer Coatings Facilitate Smaller Neural Recording Electrodes. J. Neural Eng. 2011, 8, 014001. [Google Scholar] [CrossRef] [PubMed]
- You, S.-J.; Ren, N.-Q.; Zhao, Q.-L.; Wang, J.-Y.; Yang, F.-L. Power Generation and Electrochemical Analysis of Biocathode Microbial Fuel Cell Using Graphite Fibre Brush as Cathode Material. Fuel Cells 2009, 9, 588–596. [Google Scholar] [CrossRef]
- Borsje, C.; Liu, D.; Sleutels, T.H.J.A.; Buisman, C.J.N.; ter Heijne, A. Performance of Single Carbon Granules as Perspective for Larger Scale Capacitive Bioanodes. J. Power Sources 2016, 325, 690–696. [Google Scholar] [CrossRef]
- Lekshmi, G.S.; Bazaka, K.; Ramakrishna, S.; Kumaravel, V. Microbial Electrosynthesis: Carbonaceous Electrode Materials for CO2 Conversion. Mater. Horiz. 2023, 10, 292–312. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Umar, K. Biomass-Derived Composite Anode Electrode: Synthesis, Characterizations, and Application in Microbial Fuel Cells (MFCs). J. Environ. Chem. Eng. 2021, 9, 106111. [Google Scholar] [CrossRef]
- Koventhan, C.; Pandiyarajan, S.; Chen, S.M.; Selvan, C.S. Novel design of perovskite-structured neodymium cobalt oxide nanoparticle-embedded graphene oxide nanocomposites as efficient active materials of energy storage devices. ACS Appl. Mater. Interfaces 2023, 15, 44876–44886. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Ye, D.; Zhu, X.; Liao, Q.; Zhang, B. Tubular Bamboo Charcoal for Anode in Microbial Fuel Cells. J. Power Sources 2014, 272, 277–282. [Google Scholar] [CrossRef]
- Cereda, A.; Hitchcock, A.; Symes, M.D.; Cronin, L.; Bibby, T.S.; Jones, A.K. A Bioelectrochemical Approach to Characterize Extracellular Electron Transfer by Synechocystis Sp. PCC6803. PLoS ONE 2014, 9, e91484. [Google Scholar] [CrossRef]
- Thepsuparungsikul, N.; Phonthamachai, N.; Ng, H.Y. Multi-Walled Carbon Nanotubes as Electrode Material for Microbial Fuel Cells. Water Sci. Technol. 2012, 65, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Wen, G.-Y.; Wu, X.-S.; Zou, L. l-Cysteine Tailored Porous Graphene Aerogel for Enhanced Power Generation in Microbial Fuel Cells. RSC Adv. 2015, 5, 58921–58927. [Google Scholar] [CrossRef]
- Najafabadi, A.T.; Ng, N.; Gyenge, E. Electrochemically Exfoliated Graphene Anodes with Enhanced Biocurrent Production in Single-Chamber Air-Breathing Microbial Fuel Cells. Biosens. Bioelectron. 2016, 81, 103–110. [Google Scholar] [CrossRef]
- Liu, J.; Qiao, Y.; Guo, C.X.; Lim, S.; Song, H.; Li, C.M. Graphene/Carbon Cloth Anode for High-Performance Mediatorless Microbial Fuel Cells. Bioresour. Technol. 2012, 114, 275–280. [Google Scholar] [CrossRef]
- Jatoi, A.S.; Baloch, A.G.; Jadhav, A.; Nizamuddin, S.; Aziz, S.; Soomro, S.A.; Nazir, I.; Abro, M.; Baloch, H.A.; Ahmed, J.; et al. Improving fermentation industry sludge treatment as well as energy production with constructed dual chamber microbial fuel cell. SN Appl. Sci. 2020, 2, 9. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Y.; Shi, F.; Zhang, J.; Zhu, J.-J. High Biocurrent Generation in Shewanella-Inoculated Microbial Fuel Cells Using Ionic Liquid Functionalized Graphene Nanosheets as an Anode. Chem. Commun. 2013, 49, 6668. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhou, S.; Zhao, B.; Zhuang, L.; Wang, Y. Microbially-Reduced Graphene Scaffolds to Facilitate Extracellular Electron Transfer in Microbial Fuel Cells. Bioresour. Technol. 2012, 116, 453–458. [Google Scholar] [CrossRef]
- Yang, W.; Kim, K.-Y.; Saikaly, P.E.; Logan, B.E. The Impact of New Cathode Materials Relative to Baseline Performance of Microbial Fuel Cells All with the Same Architecture and Solution Chemistry. Energy Environ. Sci. 2017, 10, 1025–1033. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, S.; Feng, Y.; Merrill, M.D.; Saito, T.; Logan, B.E. Use of Carbon Mesh Anodes and the Effect of Different Pretreatment Methods on Power Production in Microbial Fuel Cells. Environ. Sci. Technol. 2009, 43, 6870–6874. [Google Scholar] [CrossRef]
- Tabish, A.N.; Farhat, I.; Irshad, M.; Hussain, M.A.; Usman, M.; Chaudhary, T.N.; Fouad, Y.; Raza, S.; Ashraf, W.M.; Krzywanski, J. Electrochemical Insight into the Use of Microbial Fuel Cells for Bioelectricity Generation and Wastewater Treatment. Energies 2023, 16, 2760. [Google Scholar] [CrossRef]
- Ghangrekar, M.M.; Shinde, V.B. Simultaneous Sewage Treatment and Electricity Generation in Membrane-Less Microbial Fuel Cell. Water Sci. Technol. 2008, 58, 37–43. [Google Scholar] [CrossRef]
- Huang, J.; Yang, P.; Guo, Y.; Zhang, K. Electricity Generation during Wastewater Treatment: An Approach Using an AFB-MFC for Alcohol Distillery Wastewater. Desalination 2011, 276, 373–378. [Google Scholar] [CrossRef]
- Sakdaronnarong, C.K.; Thanosawan, S.; Chaithong, S.; Sinbuathong, N.; Jeraputra, C. Electricity Production from Ethanol Stillage in Two-Compartment MFC. Fuel 2013, 107, 382–386. [Google Scholar] [CrossRef]
- Tremouli, A.; Antonopoulou, G.; Bebelis, S.; Lyberatos, G. Operation and Characterization of a Microbial Fuel Cell Fed with Pretreated Cheese Whey at Different Organic Loads. Bioresour. Technol. 2013, 131, 380–389. [Google Scholar] [CrossRef]
- Dongmei, M.; Zong, H.J.; Chyi, H.L.; Dandan, Z. Electricity Generation from swine wastewater in Microbial Fuel cell: Hydraulic reaction time effect. Hydrog. Energy 2016. [Google Scholar] [CrossRef]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial Fuel Cells: From Fundamentals to Applications. A Review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef]
- Dumas, C.; Mollica, A.; Féron, D.; Basséguy, R.; Etcheverry, L.; Bergel, A. Marine Microbial Fuel Cell: Use of Stainless Steel Electrodes as Anode and Cathode Materials. Electrochim. Acta 2007, 53, 468–473. [Google Scholar] [CrossRef]
- Dominguez, B.X.; Navarro, Á.S.G.; Carrera, F.C. Electrochemical Evaluation of Ti/TiO2-polyaniline Anodes for Microbial Fuel Cells using Hypersaline Microbial Consortia for Synthetic-wastewater Treatment. J. New Mater. Electrochem. Syst. 2010, 13, 1–6. [Google Scholar]
- Chen, J.; Deng, F.; Hu, Y.; Sun, J.; Yang, Y. Antibacterial Activity of Graphene-Modified Anode on Shewanella Oneidensis MR-1 Biofilm in Microbial Fuel Cell. J. Power Sources 2015, 290, 80–86. [Google Scholar] [CrossRef]
- Kirubaharan, C.J.; Santhakumar, K.; kumar, G.G.; Senthilkumar, N.; Jang, J.-H. Nitrogen Doped Graphene Sheets as Metal Free Anode Catalysts for the High Performance Microbial Fuel Cells. Int. J. Hydrogen Energy 2015, 40, 13061–13070. [Google Scholar] [CrossRef]
- Pareek, A.; Sravan, J.S.; Mohan, S.V. Fabrication of Three-Dimensional Graphene Anode for Augmenting Performance in Microbial Fuel Cells. Carbon Resour. Convers. 2019, 2, 134–140. [Google Scholar] [CrossRef]
- Lv, Z.; Chen, Y.; Wei, H.; Li, F.; Hu, Y.; Wei, C.; Feng, C. One-Step Electrosynthesis of Polypyrrole/Graphene Oxide Composites for Microbial Fuel Cell Application. Electrochim. Acta 2013, 111, 366–373. [Google Scholar] [CrossRef]
- Zhao, C.; Gai, P.; Song, R.; Zhang, J.; Zhu, J.-J. Graphene/Au Composites as an Anode Modifier for Improving Electricity Generation in Shewanella-Inoculated Microbial Fuel Cells. Anal. Methods 2015, 7, 4640–4644. [Google Scholar] [CrossRef]
- Dewan, A.; Beyenal, H.; Lewandowski, Z. Scaling up Microbial Fuel Cells. Environ. Sci. Technol. 2008, 42, 7643–7648. [Google Scholar] [CrossRef]
- Jiang, D.; Li, B. Novel Electrode Materials to Enhance the Bacterial Adhesion and Increase the Power Generation in Microbial Fuel Cells (MFCs). Water Sci. Technol. 2009, 59, 557–563. [Google Scholar] [CrossRef]
- Fangzhou, D.; Zhenglong, L.; Shaoqiang, Y.; Beizhen, X.; Hong, L. Electricity Generation Directly Using Human Feces Wastewater for Life Support System. Acta Astronaut. 2011, 68, 1537–1547. [Google Scholar] [CrossRef]
- Karthikeyan, R.; Krishnaraj, N.; Selvam, A.; Wong, J.W.-C.; Lee, P.K.H.; Leung, M.K.H.; Berchmans, S. Effect of Composites Based Nickel Foam Anode in Microbial Fuel Cell Using Acetobacter Aceti and Gluconobacter Roseus as a Biocatalysts. Bioresour. Technol. 2016, 217, 113–120. [Google Scholar] [CrossRef]
- Seghiouer, A.; Chevalet, J.; Barhoun, A.; Lantelme, F. Electrochemical Oxidation of Nickel in Alkaline Solutions: A Voltammetric Study and Modelling. J. Electroanal. Chem. 1998, 442, 113–123. [Google Scholar] [CrossRef]
- Caizán-Juanarena, L.; Servin-Balderas, I.; Chen, X.; Buisman, C.J.N.; ter Heijne, A. Electrochemical and Microbiological Characterization of Single Carbon Granules in a Multi-Anode Microbial Fuel Cell. J. Power Sources 2019, 435, 126514. [Google Scholar] [CrossRef]
- Bailey, M.J.; Biely, P.; Poutanen, K. Interlaboratory Testing of Methods for Assay of Xylanase Activity. J. Biotechnol. 1992, 23, 257–270. [Google Scholar] [CrossRef]
- Fan, Z.; Chen, J.; Zhang, B.; Sun, F.; Liu, B.; Kuang, Y. Electrochemically Induced Deposition Method to Prepare γ-MnO2/Multi-Walled Carbon Nanotube Composites as Electrode Material in Supercapacitors. Mater. Res. Bull. 2008, 43, 2085–2091. [Google Scholar] [CrossRef]
- Yang, W.; Li, J.; Fu, Q.; Zhang, L.; Zhu, X.; Liao, Q. A Simple Method for Preparing a Binder-Free Paper-Based Air Cathode for Microbial Fuel Cells. Bioresour. Technol. 2017, 241, 325–331. [Google Scholar] [CrossRef]
- Zaaba, N.I.; Foo, K.L.; Hashim, U.; Tan, S.J.; Liu, W.W.; Voon, C.H. Synthesis of graphene oxide using modified hummers method: Solvent influence. Procedia Eng. 2017, 184, 469–477. [Google Scholar] [CrossRef]
- Dumas, C.; Mollica, A.; Féron, D.; Basseguy, R.; Etcheverry, L.; Bergel, A. Checking Graphite and Stainless Anodes with an Experimental Model of Marine Microbial Fuel Cell. Bioresour. Technol. 2008, 99, 8887–8894. [Google Scholar] [CrossRef]
- Tanvir, R.U.; Zhang, J.; Canter, T.; Chen, D.; Lu, J.; Hu, Z. Harnessing Solar Energy Using Phototrophic Microorganisms: A Sustainable Pathway to Bioenergy, Biomaterials, and Environmental Solutions. Renew. Sustain. Energy Rev. 2021, 146, 111181. [Google Scholar] [CrossRef]
- Jadhav, D.A.; Mungray, A.K.; Mathuriya, A.S.; Chendake, A.D.; Gunaseelan, K.; Gajalakshmi, S. Application Niche of Microbial Fuel Cell as a Bio-Energy Source for Sustainable Development. In Bioelectrochemical Systems; Springer: Singapore, 2020; pp. 21–42. [Google Scholar]
- Nikkanen, L.; Hubacek, M.; Allahverdiyeva, Y. 10 Photosynthetic Microorganisms as Biocatalysts. In Photosynthesis; De Gruyter: Berlin, Germany, 2021; pp. 257–278. [Google Scholar]
- Strik, D.P.B.T.B.; Terlouw, H.; Hamelers, H.V.M.; Buisman, C.J.N. Renewable Sustainable Biocatalyzed Electricity Production in a Photosynthetic Algal Microbial Fuel Cell (PAMFC). Appl. Microbiol. Biotechnol. 2008, 81, 659–668. [Google Scholar] [CrossRef]
- Voloshin, R.A.; Bozieva, A.M.; Bruce, B.D.; Allakhverdiev, S.I. Photosynthetic Microbial Fuel Cells: Practical Applications of Electron Transfer Chains. Russ. Chem. Rev. 2023, 92, RCR5073. [Google Scholar] [CrossRef]
- Thirumurthy, M.A.; Hitchcock, A.; Cereda, A.; Liu, J.; Chavez, M.S.; Doss, B.L.; Ros, R.; El-Naggar, M.Y.; Heap, J.T.; Bibby, T.S.; et al. Type IV Pili-Independent Photocurrent Production by the Cyanobacterium Synechocystis Sp. PCC 6803. Front. Microbiol. 2020, 11, 1344. [Google Scholar] [CrossRef] [PubMed]
- Elshobary, M.E.; Zabed, H.M.; Yun, J.; Zhang, G.; Qi, X. Recent Insights into Microalgae-Assisted Microbial Fuel Cells for Generating Sustainable Bioelectricity. Int. J. Hydrogen Energy 2021, 46, 3135–3159. [Google Scholar] [CrossRef]
- Kannan, N.; Donnellan, P. Algae-Assisted Microbial Fuel Cells: A Practical Overview. Bioresour. Technol. Rep. 2021, 15, 100747. [Google Scholar] [CrossRef]
- Zimina, T.M.; Mandrik, I.V.; Pudova, A.V.; Gataullin, A.O.; Snarskaya, D.D. Biophotovoltaic Energy Sources Based on Cyanobacteria. Nanobiotechnology Rep. 2023, 18, S156–S164. [Google Scholar] [CrossRef]
- Verseux, C.; Heinicke, C.; Ramalho, T.P.; Determann, J.; Duckhorn, M.; Smagin, M.; Avila, M.A. Low-Pressure, N2/CO2 Atmosphere Is Suitable for Cyanobacterium-Based Life-Support Systems on Mars. Front. Microbiol. 2021, 12. [Google Scholar] [CrossRef]
- Barber, J. Photosynthetic Energy Conversion: Natural and Artificial. Chem. Soc. Rev. 2009, 38, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Choi, Y. Cofactor Engineering in Cyanobacteria to Overcome Imbalance between NADPH and NADH: A Mini Review. Front. Chem. Sci. Eng. 2017, 11, 66–71. [Google Scholar] [CrossRef]
- Mohamed, S.N.; Jayabalan, T.; Muthukumar, K. Simultaneous Bioenergy Generation and Carbon Dioxide Sequestration from Food Wastewater Using Algae Microbial Fuel Cell. Energy Sources Part A Recovery Util. Environ. Eff. 2023, 45, 2913–2921. [Google Scholar] [CrossRef]
- Yang, Z.; Pei, H.; Hou, Q.; Jiang, L.; Zhang, L.; Nie, C. Algal Biofilm-Assisted Microbial Fuel Cell to Enhance Domestic Wastewater Treatment: Nutrient, Organics Removal and Bioenergy Production. Chem. Eng. J. 2018, 332, 277–285. [Google Scholar] [CrossRef]
- Kakarla, R.; Min, B. Photoautotrophic Microalgae Scenedesmus Obliquus Attached on a Cathode as Oxygen Producers for Microbial Fuel Cell (MFC) Operation. Int. J. Hydrogen Energy 2014, 39, 10275–10283. [Google Scholar] [CrossRef]
- Lakshmidevi, R.; Gandhi, N.N.; Muthukumar, K. Carbon Neutral Electricity Production from Municipal Solid Waste Landfill Leachate Using Algal-Assisted Microbial Fuel Cell. Appl. Biochem. Biotechnol. 2020, 191, 852–866. [Google Scholar] [CrossRef] [PubMed]
- Walter, X.A.; Greenman, J.; Taylor, B.; Ieropoulos, I.A. Microbial Fuel Cells Continuously Fuelled by Untreated Fresh Algal Biomass. Algal Res. 2015, 11, 103–107. [Google Scholar] [CrossRef]
- Jadhav, D.A.; Jain, S.C.; Ghangrekar, M.M. Simultaneous Wastewater Treatment, Algal Biomass Production and Electricity Generation in Clayware Microbial Carbon Capture Cells. Appl. Biochem. Biotechnol. 2017, 183, 1076–1092. [Google Scholar] [CrossRef] [PubMed]
- Pathirana, K.; Imanthi, A.; Idroos, F.S.; Pathmalal, M.M. Biotechnological Performance of a Cyanobacteria Based Microbial Fuel Cell. Songklanakarin J. Sci. Technol. 2023, 45, 470–475. [Google Scholar]
- Bhadra, S.; Nayak, S.; Sevda, S. Simultaneous Organic Wastewater Treatment and Bioelectricity Production in a Dual Chamber Microbial Fuel Cell with Scenedesmus Obliquus Biocathode. Energy Convers. Manag. 2024, 316, 118849. [Google Scholar] [CrossRef]
- Pudova, A.V.; Mandrik, I.V.; Kolesova, A.D.; Zimina, T.M.; Snarskaya, D.D. Miniature BioFuel Cell for Photogeneration of Electricity Based on Cyanobacteria. In Proceedings of the 2020 IEEE Conference of Russian Young Researchers in Electrical and Electronic Engineering (EIConRus), Petersburg and Moscow, Russia, 27–30 January 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 1571–1574. [Google Scholar]
- Pudova, A.V.; Mandrik, I.V.; Kolesova, A.D.; Zimina, T.M.; Snarskaya, D.D. Output Power of Miniature Bio Fuel Cell versus Anode and Cathode Material Type. In Proceedings of the 2021 IEEE Conference of Russian Young Researchers in Electrical and Electronic Engineering (ElConRus), Petersburg and Moscow, Russia, 27–30 January 2020; IEEE: Piscataway, NJ, USA, 2021; pp. 1808–1813. [Google Scholar]
- Lin, C.-C.; Wei, C.-H.; Chen, C.-I.; Shieh, C.-J.; Liu, Y.-C. Characteristics of the Photosynthesis Microbial Fuel Cell with a Spirulina Platensis Biofilm. Bioresour. Technol. 2013, 135, 640–643. [Google Scholar] [CrossRef]
- Kondaveeti, S.; Choi, K.S.; Kakarla, R.; Min, B. Microalgae Scenedesmus Obliquus as Renewable Biomass Feedstock for Electricity Generation in Microbial Fuel Cells (MFCs). Front. Environ. Sci. Eng. 2014, 8, 784–791. [Google Scholar] [CrossRef]
- Xu, C.; Poon, K.; Choi, M.M.F.; Wang, R. Using Live Algae at the Anode of a Microbial Fuel Cell to Generate Electricity. Environ. Sci. Pollut. Res. 2015, 22, 15621–15635. [Google Scholar] [CrossRef]
- Liu, T.; Rao, L.; Yuan, Y.; Zhuang, L. Bioelectricity Generation in a Microbial Fuel Cell with a Self-Sustainable Photocathode. Sci. World J. 2015, 2015, 864568. [Google Scholar] [CrossRef]
- González del Campo, A.; Cañizares, P.; Rodrigo, M.A.; Fernández, F.J.; Lobato, J. Microbial fuel cell with an algae-assisted cathode: A preliminary assessment. J. Power Sources 2013, 242, 638–645. [Google Scholar] [CrossRef]
- Wang, D.-B.; Song, T.-S.; Guo, T.; Zeng, Q.; Xie, J. Electricity Generation from Sediment Microbial Fuel Cells with Algae-Assisted Cathodes. Int. J. Hydrogen Energy 2014, 39, 13224–13230. [Google Scholar] [CrossRef]
- Zhang, Y.; Noori, J.S.; Angelidaki, I. Simultaneous Organic Carbon, Nutrients Removal and Energy Production in a Photomicrobial Fuel Cell (PFC). Energy Environ. Sci. 2011, 4, 4340. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Lay, C.-H.; Chia, S.R.; Chew, K.W.; Show, P.L.; Hsieh, P.-H.; Chen, C.-C. Economic Potential of Bioremediation Using Immobilized Microalgae-Based Microbial Fuel Cells. Clean Technol. Environ. Policy 2021, 23, 2251–2264. [Google Scholar] [CrossRef]
- Zamanpour, M.K.; Kariminia, H.-R.; Vosoughi, M. Electricity Generation, Desalination and Microalgae Cultivation in a Biocathode-Microbial Desalination Cell. J. Environ. Chem. Eng. 2017, 5, 843–848. [Google Scholar] [CrossRef]
- Yadav, G.; Sharma, I.; Ghangrekar, M.; Sen, R. A Live Bio-Cathode to Enhance Power Output Steered by Bacteria-Microalgae Synergistic Metabolism in Microbial Fuel Cell. J. Power Sources 2020, 449, 227560. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Z.; Zheng, Y.; Xiao, Y.; Yang, Z.; Zhao, F. Light Intensity Affects the Performance of Photo Microbial Fuel Cells with Desmodesmus Sp. A8 as Cathodic Microorganism. Appl. Energy 2014, 116, 86–90. [Google Scholar] [CrossRef]
- Commault, A.S.; Laczka, O.; Siboni, N.; Tamburic, B.; Crosswell, J.R.; Seymour, J.R.; Ralph, P.J. Electricity and Biomass Production in a Bacteria-Chlorella Based Microbial Fuel Cell Treating Wastewater. J. Power Sources 2017, 356, 299–309. [Google Scholar] [CrossRef]
- Neethu, B.; Bhowmick, G.D.; Ghangrekar, M.M. Enhancement of Bioelectricity Generation and Algal Productivity in Microbial Carbon-Capture Cell Using Low Cost Coconut Shell as Membrane Separator. Biochem. Eng. J. 2018, 133, 205–213. [Google Scholar] [CrossRef]
- Das, S.; Das, S.; Ghangrekar, M.M. Quorum-Sensing Mediated Signals: A Promising Multi-Functional Modulators for Separately Enhancing Algal Yield and Power Generation in Microbial Fuel Cell. Bioresour. Technol. 2019, 294, 122138. [Google Scholar] [CrossRef]
- Hadiyanto, H.; Christwardana, M.; da Costa, C. Electrogenic and Biomass Production Capabilities of a Microalgae–Microbial Fuel Cell (MMFC) System Using Tapioca Wastewater and Spirulina Platensis for COD Reduction. Energy Sources Part A Recovery Util. Environ. Eff. 2023, 45, 3409–3420. [Google Scholar] [CrossRef]
- Mitra, P.; Hill, G.A. Continuous Microbial Fuel Cell Using a Photoautotrophic Cathode and a Fermentative Anode. Can. J. Chem. Eng. 2012, 90, 1006–1010. [Google Scholar] [CrossRef]
- Röling, W.F.M. The Family Geobacteraceae. In The Prokaryotes; Springer: Berlin/Heidelberg, Germany, 2014; pp. 157–172. [Google Scholar]
- Jiang, Y.; Zeng, R.J. Bidirectional Extracellular Electron Transfers of Electrode-Biofilm: Mechanism and Application. Bioresour. Technol. 2019, 271, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Tamirat, A.G.; Guan, X.; Liu, J.; Luo, J.; Xia, Y. Redox Mediators as Charge Agents for Changing Electrochemical Reactions. Chem. Soc. Rev. 2020, 49, 7454–7478. [Google Scholar] [CrossRef]
- Kar, M.L.; Greg, W.; Mohamed, Y.E.; Yuri, G.; Gordan, S.; Woon, M.L.; Jun, Y. Shewenella oneidensis MR-1 Bacterial Nanowires Exhibit p-Type, Tunable Electronic Behavior. Nono Lett. 2013, 13, 2407–2411. [Google Scholar] [CrossRef]
- Wenzel, T.; Härtter, D.; Bombelli, P.; Howe, C.J.; Steiner, U. Porous Translucent Electrodes Enhance Current Generation from Photosynthetic Biofilms. Nat. Commun. 2018, 9, 1299. [Google Scholar] [CrossRef]
- Wey, L.T.; Yewale, R.; Hautala, E.; Hannonen, J.; Katavisto, K.; Kvarnström, C.; Allahverdiyeva, Y.; Damlin, P. Optoelectronic Enhancement of Photocurrent by Cyanobacteria on Sustainable AP-VPP-Fabricated PEDOT Electrodes. Electrochim. Acta 2024, 475, 143597. [Google Scholar] [CrossRef]
- Kusama, S.; Kojima, S.; Kimura, K.; Shimakawa, G.; Miyake, C.; Tanaka, K.; Okumura, Y.; Nakanishi, S. Order-of-Magnitude Enhancement in Photocurrent Generation of Synechocystis Sp. PCC 6803 by Outer Membrane Deprivation. Nat. Commun. 2022, 13, 3067. [Google Scholar] [CrossRef]
- Govindasamy, R.; Gayathiri, E.; Sankar, S.; Venkidasamy, B.; Prakash, P.; Rekha, K.; Savaner, V.; Pari, A.; Thirumalaivasan, N.; Thiruvengadam, M. Emerging Trends of Nanotechnology and Genetic Engineering in Cyanobacteria to Optimize Production for Future Applications. Life 2022, 12, 2013. [Google Scholar] [CrossRef]
- Flynn, J.M.; Ross, D.E.; Hunt, K.A.; Bond, D.R.; Gralnick, J.A. Enabling Unbalanced Fermentations by Using Engineered Electrode-Interfaced Bacteria. mBio 2010, 1, 10–1128. [Google Scholar] [CrossRef]
- Liu, J.; Yong, Y.-C.; Song, H.; Li, C.M. Activation Enhancement of Citric Acid Cycle to Promote Bioelectrocatalytic Activity of arcA Knockout Escherichia Coli Toward High-Performance Microbial Fuel Cell. ACS Catal. 2012, 2, 1749–1752. [Google Scholar] [CrossRef]
- Han, S.; Gao, X.; Ying, H.; Zhou, C.C. NADH Gene Manipulation for Advancing Bioelectricity in Clostridium Ljungdahlii Microbial Fuel Cells. Green Chem. 2016, 18, 2473–2478. [Google Scholar] [CrossRef]
- Dong, F.; Simoska, O.; Gaffney, E.; Minteer, S.D. Applying Synthetic Biology Strategies to Bioelectrochemical Systems. Electrochem. Sci. Adv. 2022, 2, e2100197. [Google Scholar] [CrossRef]
- Mustakeem. Electrode Materials for Microbial Fuel Cells: Nanomaterial Approach. Mater. Renew. Sustain. Energy 2015, 4, 22. [Google Scholar] [CrossRef]
- Narayanasamy, S.; Jayaprakash, J. Application of Carbon-Polymer Based Composite Electrodes for Microbial Fuel Cells. Rev. Environ. Sci. Bio/Technol. 2020, 19, 595–620. [Google Scholar] [CrossRef]
- Jafary, T.; Ghasemi, M.; Alam, J.; Aljlil, S.A.; Yusup, S. Carbon-Based Polymer Nanocomposites as Electrodes for Microbial Fuel Cells. In Carbon-Based Polymer Nanocomposites for Environmental and Energy Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 361–390. [Google Scholar]
- Luo, H.-P.; Liu, G.-L.; Zhang, R.-D.; Jin, S. Comparison of Power Generation in Microbial Fuel Cells of Two Different Structures. Huan Jing Ke Xue = Huanjing Kexue 2009, 30, 621–624. [Google Scholar]
- Ismail, Z.Z.; Jaeel, A.J. Sustainable Power Generation in Continuous Flow Microbial Fuel Cell Treating Actual Wastewater: Influence of Biocatalyst Type on Electricity Production. Sci. World J. 2013, 2013, 713515. [Google Scholar] [CrossRef]
- Choudhury, P.; Uday, U.S.P.; Bandyopadhyay, T.K.; Ray, R.N.; Bhunia, B. Performance Improvement of Microbial Fuel Cell (MFC) Using Suitable Electrode and Bioengineered Organisms: A Review. Bioengineered 2017, 8, 471–487. [Google Scholar] [CrossRef]
- Jayashree, C.; Sweta, S.; Arulazhagan, P.; Yeom, I.T.; Iqbal, M.I.I.; Banu, J.R. Electricity Generation from Retting Wastewater Consisting of Recalcitrant Compounds Using Continuous Upflow Microbial Fuel Cell. Biotechnol. Bioprocess Eng. 2015, 20, 753–759. [Google Scholar] [CrossRef]
- Shah, S.; Venkatramanan, V.; Prasad, R. Microbial Fuel Cell: Sustainable Green Technology for Bioelectricity Generation and Wastewater Treatment. In Sustainable Green Technologies for Environmental Management; Springer: Singapore, 2019; pp. 199–218. [Google Scholar]
- Mohan, S.V.; Srikanth, S.; Chiranjeevi, P.V.; Arora, S.; Chandra, R. Algal biocathode for in situ terminal electron acceptor (TEA) production: Synergetic association of bacteria-microalgae metabolism for the functioning of biofuel cell. Bioresour. Technol. 2014, 166, 566–574. [Google Scholar] [CrossRef]


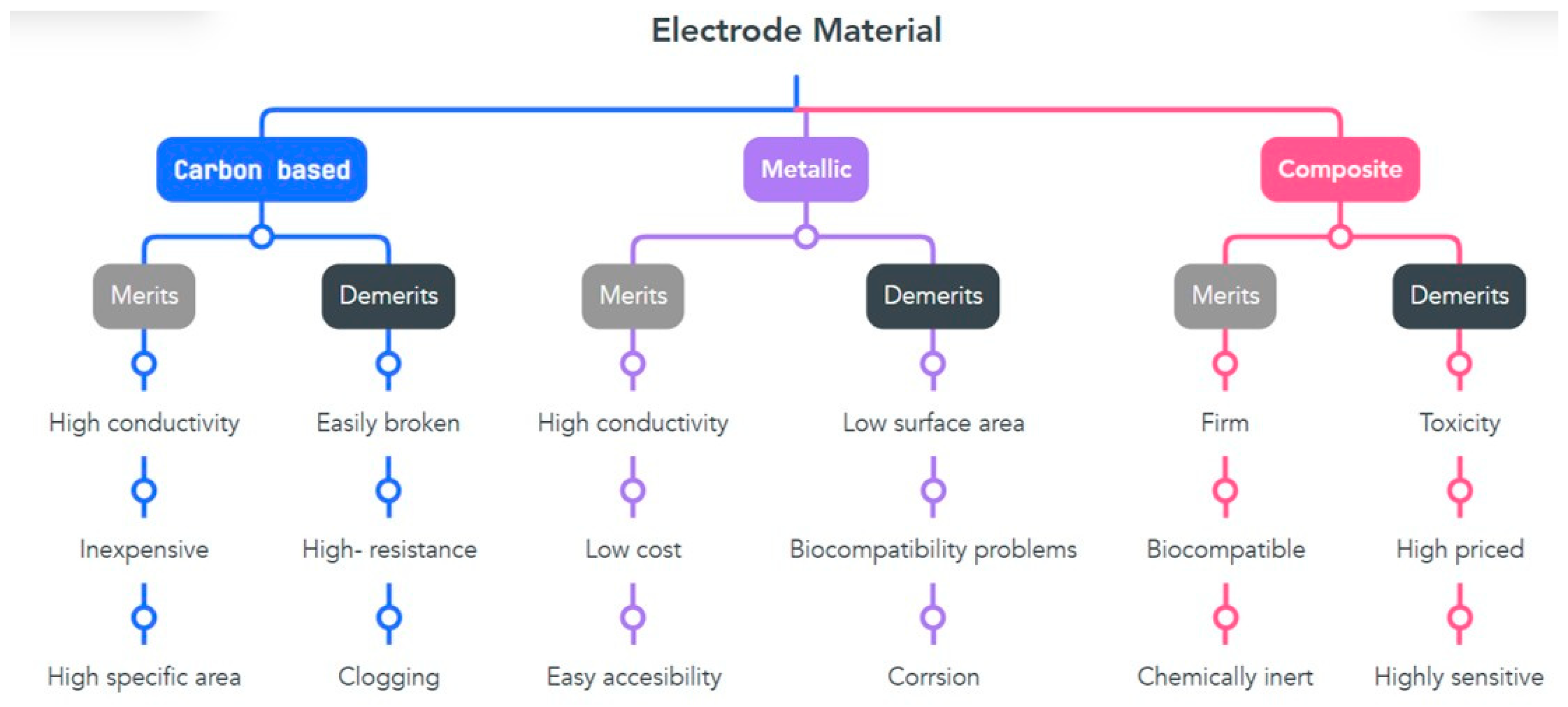
| Type of Material | Anode | Cathode | Power Density | Reference |
|---|---|---|---|---|
| Carbon-based material | Carbon cloth | Carbon cloth | 679.7 mW/m2 | [67] |
| Graphene | Carbon cloth | 2850 mW/m2 | [68] | |
| Graphene coating on carbon cloth | Carbon cloth | 52.5 mW/m2 | [69] | |
| Graphene nanosheet coating on carbon paper | Carbon cloth | 610 mW/m2 | [70] | |
| Graphene oxide | Carbon paper | 102 mW/m2 | [71] | |
| Glassy carbon | Carbon cloth | 1905 mW/m2 | [72] | |
| Carbon felt | Carbon fiber felt | 784 mW/m2 | [73] | |
| Carbon mesh | Carbon mesh | 893 mW/m2 | [74] | |
| Activated carbon | Activated carbon | 36.39 mW/m2 | [75] | |
| Graphite rod | Graphite rod | 6.73 mW/m2 | [76] | |
| Carbon fiber paper | Carbon fiber paper | 124 mW/m2 | [77] | |
| Graphite rod | Carbon cloth | 93 mW/m2 | [78] | |
| Carbon paper | Carbon cloth | 46 mW/m2 | [79] | |
| Carbon cloth | Carbon cloth | 13 mW/m2 | [80] | |
| Graphite plate | Graphite fiber brush | 68.4 W/m3 | [81] | |
| Metal | Stainless steel | Stainless steel | 23 mW/m2 | [82] |
| Metal and metal oxide | Titanium/titanium dioxide | Platinum mesh | 2317 mW/m3 | [83] |
| Composite | Polyaniline networks applied to graphene nanoribbons coated on carbon paper | Carbon paper | 856 mW/m2 | [84] |
| N-doped graphene nanosheets on carbon cloth | Carbon cloth | 1008 mW/m2 | [85] | |
| Graphene powder/polytetrafluoroethylene on carbon cloth | Carbon cloth | 0.329 mW/m2 | [86] | |
| Polypyrrole/graphene oxide | Carbon felt | 1326 mW/m2 | [87] | |
| Graphene/Au composite | Carbon paper | 508 mW/m2 | [88] | |
| Graphite plates | Platinum meshes | 1410 mW/m2 | [89] | |
| Polypyrrole coating on carbon cloth | Granular activated carbon | 5 W/m3 | [90] | |
| Carbon paper | Platinum-coated carbon paper | 70.8 mW/m2 | [91] |
| Organism | Electrode Material | Power Density | References |
|---|---|---|---|
| Calothrix | Polypyrrole/carbon fabric | 6 mW/m2 | [108] |
| Nostoc | Polypyrrole/carbon fabric | 1.2 mW/m2 | [108] |
| Pseudanabaena limnetica | Stainless steel | 1.2 × 10−7 mW/m2 | [109] |
| Synechococcus sp. PCC 6803 | Indium tin oxide/polyethylene terephthalate | 10 mW/m2 | [110] |
| Synechocystis |
| 3.770 mW/m2 0.630 mW/m2 | [111] |
| Synechococcus | Carbon fiber | 10.3 mW/m2 | [108] |
| Oscillatoria sp. | Graphite plate | 32.5 ± 0.5 mW/m2 | [112] |
| Scenedesmus sp. | Graphite plate | 28.5 ± 0.3 mW/m2 | [112] |
| Scenedesmus quadricauda SDEC-8 | Carbon cloth cathode with titanium | 0.094 kWh per m3 | [113] |
| Scenedesmus obliquus | Platinum-coated carbon paper | 153 mW/m2 | [114] |
| Synechococcus | Graphite electrodes | 0.0956 W/m2 | [115] |
| Synechococcus leopoliensis | Black acrylic as the cathode and carbon fiber veil as the anode | 42,500 mW/m3 | [116] |
| Anabaena ambigua | Carbon felt | 63.84 mW/m2 | [117] |
| Chroococcus sp. | Carbon cloth | 467.55 mW/m2 | [118] |
| Scenedesmus obliquus | Graphite rod | 1.94 mW/m2 | [119] |
| Synechococcus | Carbon felt | 183 mW/m2 | [120] |
| Synechococcus | Carbon felt | 10 mW/m2 | [121] |
| Spirulina platensis | Gold mesh as an anode and a graphite carbon cloth as a cathode | 10 mW/m2 | [122] |
| Scenedesmus obliquus | Carbon paper | 102 mW/m2 | [123] |
| Chlorella pyrenoidosa | Graphite/carbon electrodes | 30.15 mW/m2 | [124] |
| Chlorella vulgaris | The anode was made of carbon fiber brushes and the cathode was carbon felt (Pt catalyst) | 187 mW/m2 | [125] |
| Chlorella vulgaris | Carbon cloths with 10% Teflon | 13.5 mW/m2 | [126] |
| Chlorella vulgaris | Carbon nanotube | 38 mW/m2 | [127] |
| Chlorella vulgaris | Carbon paper | 68 ± 5 mW/m2 | [128] |
| Chlorella sp. G29-5 | Carbon cloth | 505.6 mW/m2 | [129] |
| Chlorella vulgaris | Graphite sheet as the anode and a stainless-steel grid as the cathode | 19.8 mW/m2 | [130] |
| Chlorella sp. | Stainless steel mesh as the anode and carbon felt as the cathode | 54.48 mW/m2 | [131] |
| Desmodesmus sp. A8 | Graphite felt | 99.09 mW/m2 | [132] |
| Chlorella vulgaris | Graphite plate | 34.2 mW/m2 | [133] |
| Chlorella sorokiniana | Carbon felt | 213 mW/m2 | [134] |
| Chlorella sorokiniana | Carbon felt | 24.09 mW/m2 | [135] |
| Spirulina platensis | Graphite rod | 14.47 ± 0.7 mW/m2 | [136] |
| Chlorella vulgaris | Carbon graphite | 0.6 mW/m2 | [137] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramesh, P.; Gupta, R.; Koventhan, C.; Muralitharan, G.; Lo, A.-Y.; Huang, Y.-J.; Ramasamy, S. Recent Trends in the Use of Electrode Materials for Microbial Fuel Cells Accentuating the Potential of Photosynthetic Cyanobacteria and Microalgae: A Review. Processes 2025, 13, 1348. https://doi.org/10.3390/pr13051348
Ramesh P, Gupta R, Koventhan C, Muralitharan G, Lo A-Y, Huang Y-J, Ramasamy S. Recent Trends in the Use of Electrode Materials for Microbial Fuel Cells Accentuating the Potential of Photosynthetic Cyanobacteria and Microalgae: A Review. Processes. 2025; 13(5):1348. https://doi.org/10.3390/pr13051348
Chicago/Turabian StyleRamesh, Ponnusamy, Rishika Gupta, Chelliah Koventhan, Gangatharan Muralitharan, An-Ya Lo, Yi-Jen Huang, and Saravanan Ramasamy. 2025. "Recent Trends in the Use of Electrode Materials for Microbial Fuel Cells Accentuating the Potential of Photosynthetic Cyanobacteria and Microalgae: A Review" Processes 13, no. 5: 1348. https://doi.org/10.3390/pr13051348
APA StyleRamesh, P., Gupta, R., Koventhan, C., Muralitharan, G., Lo, A.-Y., Huang, Y.-J., & Ramasamy, S. (2025). Recent Trends in the Use of Electrode Materials for Microbial Fuel Cells Accentuating the Potential of Photosynthetic Cyanobacteria and Microalgae: A Review. Processes, 13(5), 1348. https://doi.org/10.3390/pr13051348









