Fe3+ Reducing Power as the Most Common Assay for Understanding the Biological Functions of Antioxidants
Abstract
1. Introduction
1.1. Reactive Oxygen Species (ROS)
1.2. Oxidative Stress
1.3. The Importance of Iron in Biological Systems
2. Antioxidants
3. Antioxidant Evaluation Methods
4. Chemical Reduction Pathways
5. Biological Sources of Reducing Power
5.1. NADH
5.2. NADPH
5.3. Glutathione (GSH)
5.4. Ascorbic Acid (Vitamin C)
6. Biochemical Mechanisms of Fe3+ Reduction
6.1. Non-Enzymatic Reduction
6.1.1. Organic Reducing Agents
6.1.2. Inorganic Reducing Agents
6.1.3. Photochemical Reduction
6.2. Enzymatic Reduction
6.3. Microbial Mediators
7. Procedure for Determining Reducing Power in Antioxidants
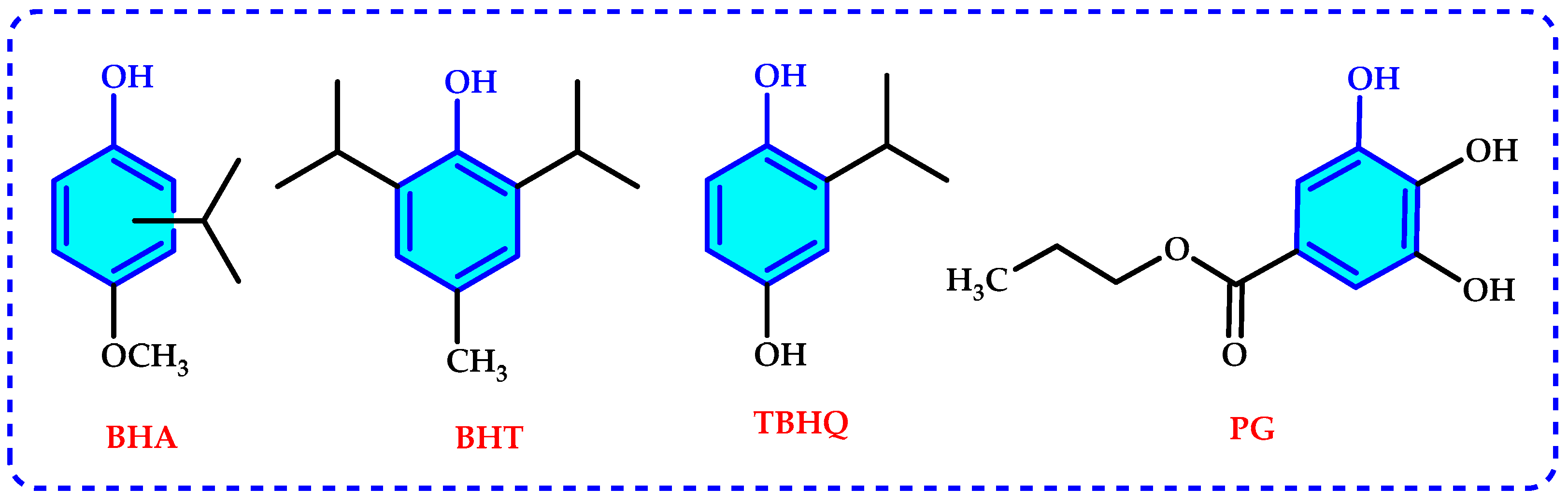
8. Reducing Power of Various Antioxidants
9. Advantages of the Fe3+ Reducing Ability Assay
10. Limitations of the Fe3+ Reducing Ability Assay
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gulcin, I. Antioxidants and antioxidant methods—An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Akyuz, M. The determination of antidiabetic, anticholinesterase and antioxidant properties of ethanol and water extracts of blackberry (Rubus fruticosus L.) fruits at different maturity stages. S. Afr. J. Bot. 2022, 151, 1035–1048. [Google Scholar] [CrossRef]
- Akyuz, M.; Yabo-Dambagi, L.; Kilic, T.; Cakir, A. Antidiabetic, neuroprotective and antioxidant potentials of different parts of Pistacia terebinthus fruits. S. Afr. J. Bot. 2022, 147, 443–456. [Google Scholar] [CrossRef]
- Buldurun, K.; Turan, N.; Mahmoudi, G.; Bursal, E. Half-sandwich ruthenium(II)(η6-p-cymene) complexes: Syntheses, characterization, transfer hydrogenation reactions, antioxidant and enzyme inhibitory activities. J. Mol. Struct. 2022, 1262, 133075. [Google Scholar] [CrossRef]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Fransen, M.; Lismont, C.; Walton, P. The peroxisome-mitochondria connection: How and why? Int. J. Mol. Sci. 2012, 13, 5860–5878. [Google Scholar] [CrossRef]
- Azzam, E.I.; Jay-Gerin, J.P.; Pain, D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012, 327, 48–60. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, K. 8-hydroxy-2′-deoxyguanosine as a biomarker of oxidative DNA damage in carcinogenesis: Measurement methods and applications. Mol. Cell. Biochem. 2013, 356, 435–456. [Google Scholar]
- Pelicano, H.; Carney, D.; Huang, P. ROS stress in cancer cells and therapeutic implications. Drug Resist. Updates 2004, 7, 97–110. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.; Ding, A. Reactive oxygen and nitrogen species in immunity. Nat. Rev. Immunol. 2010, 10, 349–361. [Google Scholar]
- Sena, L.A.; Chandel, N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 2012, 48, 158–167. [Google Scholar] [CrossRef]
- Yin, H.; Xu, L.; Porter, N.A. Free radical lipid peroxidation: Mechanisms and analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Mirza, B. Phytochemical analysis and comprehensive evaluation of anti-microbial and antioxidant properties of 61 medicinal plant species. Arab. J. Chem. 2018, 11, 1223–1235. [Google Scholar] [CrossRef]
- Stadtman, E.R.; Levine, R.L. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 2003, 25, 207–218. [Google Scholar] [CrossRef]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef]
- Kavaz Yuksel, A.; Dikici, E.; Yüksel, M.; Isık, M.; Tozoglu, F.; Koksal, E. Phytochemicals analysis and some bioactive properties of Erica manipuliflora Salisb. (EMS); antibacterial, antiradical and anti-lipid peroxidation. Iran. J. Pharm. Res. 2021, 20, 422–434. [Google Scholar]
- Ayguni, R.B.; Zengini, G.; Yıldıztugayi, E.; Jugreeti, S.; Yılmazi, M.A.; Mahomoodally, F.M. Chemical characterization, anti-oxidant and anti-enzymatic properties of extracts from two Silene species: A focus on different plant parts and extraction methods. Process Biochem. 2022, 116, 206–213. [Google Scholar] [CrossRef]
- Zhang, L.; Miras-Moreno, B.; Yildiztugay, E.; Ozfidan-Konakci, C.; Arikan, B.; Elbasan, F.; Ak, G.; Rouphael, Y.; Zengin, G.; Lucini, L. Metabolomics and physiological insights into the ability of exogenously applied chlorogenic acid and hesperidin to modulate salt stress in lettuce distinctively. Molecules 2021, 26, 6291. [Google Scholar] [CrossRef] [PubMed]
- Ionita, P. The chemistry of DPPH· free radical and congeners. Int. J. Mol. Sci. 2021, 22, 1545. [Google Scholar] [CrossRef] [PubMed]
- Avsar, O.K.; Kasbolat, S.; Ak, G.; Nilofar; Caprioli, G.; Santanatoglia, A.; Uysal, A.; Uba, A.I.; Ponniya, S.K.M.; Paksoy, M.Y.; et al. Integrating chemical analysis with in vitro, in silico, and network pharmacology to discover potential functional compounds from Marrubium astracanicum subsp. macrodon. J. Mol. Liq. 2024, 398, 124204. [Google Scholar] [CrossRef]
- Polat Kose, L. Evaluation of antioxidant capacity, anticholinergic and antidiabetic activities, and phenolic ingredients of Asphodelus aestivus by LC-MS/MS. Chem. Biodivers. 2024, 21, e202301965. [Google Scholar] [CrossRef]
- Yuldasheva, N.; Acikyildiz, N.; Akyuz, M.; Yabo-Dambagi, L.; Aydin, T.; Cakir, A.; Kazaz, C. The Synthesis of Schiff bases and new secondary amine derivatives of p-vanillin and evaluation of their neuroprotective, antidiabetic, antidepressant and antioxidant potentials. J. Mol. Struct. 2022, 1270, 133883. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, R. Oxidative Stress and Antioxidants: Their Role in Human Diseases; Nova: New York, NY, USA, 2009; pp. 9–10. [Google Scholar]
- Christodoulou, M.C.; Orellana Palacios, J.C.; Hesami, G.; Jafarzadeh, S.; Lorenzo, J.M.; Domínguez, R.; Moreno, A.; Hadidi, M. Spectrophotometric methods for measurement of antioxidant activity in food and pharmaceuticals. Antioxidants 2022, 11, 2213. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, M.A.; Zengin, G.; Alp-Turgut, F.N.; Elbasan, F.; Ozfidan-Konakci, C.; Arikan, B.; Yildiztugay, E.; Zhang, L.; Lucini, L. Glutamate, humic acids and their combination modulate the phenolic profile, antioxidant traits, and enzyme-inhibition properties in lettuce. Plants 2023, 12, 1822. [Google Scholar] [CrossRef]
- Kedare, S.B.; Sing, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93–109. [Google Scholar] [CrossRef]
- Aktumsek, A.; Zengin, G.; Guler, G.O.; Cakmak, Y.S.; Duran, A. Assessment of the antioxidant potential and fatty acid composition of four Centaurea L. taxa from Turkey. Food Chem. 2013, 141, 91–97. [Google Scholar] [CrossRef]
- Lourenco, S.C.; Moldao-Martins, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef]
- Uysal, S.; Cvetanovic, A.; Zengin, G.; Durovic, S.; Zekovic, Z.; Aktumsek, A. Effects of orange leaves extraction conditions on antioxidant and phenolic content: Optimization using response surface methodology. Anal. Lett. 2017, 51, 1505–1519. [Google Scholar] [CrossRef]
- Angelini, P.; Venanzoni, R.; Flores, G.A.; Tirillini, B.; Orlando, G.; Recinella, L.; Chiavaroli, A.; Brunetti, L.; Leone, S.; Di Simone, S.C.; et al. Evaluation of antioxidant, antimicrobial and tyrosinase inhibitory activities of extracts from Tricholosporum goniospermum, an edible wild mushroom. Antibiotics 2020, 9, 513. [Google Scholar] [CrossRef] [PubMed]
- Mamadalieva, R.; Khujaev, V.; Zengin, G. Chemical profiling and biological activities of the roots of Allochrusa gypsophiloides. Nat. Prod. Res. 2024, 1–11. [Google Scholar] [CrossRef]
- Zengin, G.; Cakmak, Y.S.; Guler, G.O.; Aktumsek, A. In vitro antioxidant capacities and fatty acid compositions of three Centaurea species collected from Central Anatolia region of Turkey. Food Chem. Toxicol. 2010, 48, 2638–2641. [Google Scholar] [CrossRef] [PubMed]
- Fenton, H.J.H. Oxidation of tartaric acid in the presence of iron. J. Chem. Soc. Trans. 1984, 65, 899–910. [Google Scholar] [CrossRef]
- Haber, F.; Weiss, J. The catalytic decomposition of hydrogen peroxide by iron salts. Proc. R. Soc. A 1934, 147, 332–351. [Google Scholar]
- Mahomoodally, M.F.; Zengin, G.; Aumeeruddy, M.Z.; Sezgin, M.; Aktumsek, A. Phytochemical profile and antioxidant properties of two Brassicaceae species: Cardaria draba subsp. draba and Descurainia sophia. Biocatal. Agric. Biotechnol. 2018, 16, 453–458. [Google Scholar] [CrossRef]
- Zengin, G.; Uysal, A.; Gunes, E.; Aktumsek, A. Survey of phytochemical composition and biological effects of three extracts from a wild plant (Cotoneaster nummularia Fisch et Mey.): A potential source for functional food ingredients and drug formulations. PLoS ONE 2014, 9, e113527. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Piluzza, G.; Bullitta, S. Correlations between phenolic content and antioxidant properties in twenty-four plant species of traditional ethnoveterinary use in the Mediterranean area. Pharm. Biol. 2011, 49, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, R.; Zengin, G.; Guler, G.O.; Aktumsek, A. Bioactive constituents of Lathyrus czeczottianus and ethyl acetate and water extracts and their biological activities: An endemic plant to Turkey. S. Afr. J. Bot. 2021, 143, 306–311. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Introductory remarks. In Oxidative Stress; Academic Press: Cambridge, MA, USA, 1985; pp. 1–8. [Google Scholar]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.; Cui, R.; Zhu, L.; Han, J.; Sun, J.; Xu, Q.; Karrar, E.; Zhang, H.; Jin, Q.; et al. Effect of different processing practices on physicochemical parameters, phenolic compounds, and antioxidant properties of chia seed oils. J. Am. Oil Chem. Soc. 2024, 101, 225–236. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Akyuz, M.; Yabo-Dambagi, L.; Simitcioglu, B.; Karagoz, I.D.; Aydin, T.; Gokdemir, G.; Cakir, A.; Kazaz, C. The chemical composition and antidiabetic, neuroprotective and cytotoxic activities of soft hulls (Mesocarp) of pistachio (Pistacia vera) fruits. J. Chem. Soc. Pak. 2023, 45, 551–567. [Google Scholar]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Ergün, A.I.; Topal, M. Determination of the antioxidant capacity of puerarin and its effect on cholinesterases by in vitro and in silico methods. ChemistrySelect 2023, 8, e202302751. [Google Scholar] [CrossRef]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef]
- Tousif, M.; Abbas, Z.; Nazir, M.; Saleem, M.; Tauseef, S.; Hassan, A.; Ali, S.; Ahmed, M.; Khan, J.; Zengin, G.; et al. Secondary metabolic profiling, antioxidant potential, enzyme inhibitory activities and in silico and ADME studies: A multifunctional approach to reveal medicinal and industrial potential of Tanacetum falconeri. BMC Complement. Med. Ther. 2024, 24, 167. [Google Scholar] [CrossRef]
- Jendoubi, O.; Majdoub, S.; AL-Hmadi, H.B.; El Mokni, R.; Angeloni, S.; Mustafa, A.M.; Caprioli, G.; Zengin, G.; Maggi, F.; Hammami, S. Antioxidant potential, enzyme inhibitory activity and HPLC-MS/MS phenolics profiles in Salvia aegyptiaca L. (Lamiaceae, Nepetoideae, Mentheae) growing in Tunisia. Fitoterapia 2025, 82, 106473. [Google Scholar] [CrossRef]
- Zengin, G.; Arslan, E.; Toyran, K.; Arslan, A.; Koygun, G. Screening for antioxidant effects and chemical profiles of different extracts of Rheum ribes parts. Chem. Biodivers. 2025, 202403240. [Google Scholar] [CrossRef] [PubMed]
- Madamanchi, N.R.; Runge, M.S. Redox signaling in cardiovascular health and disease. Free Radical Biol. Med. 2007, 43, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Yildirim, M.A.; Sevinc, B.; Paydas, S.; Karaselek, M.A.; Duran, T.; Kuccukturk, S.; Vatansev, H.; Cetiz, M.V.; Caprioli, G.; Acquaticci, L.; et al. Exploring the anticancer potential of Dianthus orientalis in pancreatic cancer: A molecular and cellular study. Food Biosci. 2025, 66, 106183. [Google Scholar] [CrossRef]
- Ahmed, S.; Zengin, G.; Fernández-Ochoa, A.; de la Luz Cádiz-Gurrea, M.D.; Leyva-Jiménez, F.J.; Elkiran, O.; Cakilcioglu, U.; Akgul, B.H.; Pereira, C.G.; Custódio, L. Exploration of UHPLC-ESI-QTOF-MS profiles and the neuroprotective, antidiabetic, antioxidant and cytotoxic effects of extracts from Achillea maritima (L.) Ehrend. & YPGuo (Asteraceae) collected in Türkiye. Ital. J. Food Sci. 2025, 81, 66. [Google Scholar]
- Erdogan, M.K.; Toy, Y.; Gundogdu, R.; Gecibesler, I.H.; Sever, A.; Yapar, Y.; Behcet, L.; Zengin, G. Assessment of cytotoxic, apoptotic, enzyme inhibitory, and antioxidant properties, and phytochemical characterization of ethanolic extract from Cionura erecta. Foods 2025, 65, 106082. [Google Scholar] [CrossRef]
- Stumm, W.; Sulzberger, B. The cycling of iron in natural environments: Considerations based on laboratory studies of heterogeneous redox processes. Geochim. Cosmochim. Acta 1992, 56, 3233–3257. [Google Scholar] [CrossRef]
- Nersezashvili, M.; Berashvili, D.; Jokhadze, M.; Metreveli, M.; Swiatek, L.; Salwa, K.; Pecio, L.; Wojtanowski, K.K.; Skiba, A.; Korona-Glowniak, I.; et al. Seseli foliosum (Somm. et Levier) Manden.-A comprehensive phytochemical and biological evaluation. Molecules 2025, 30, 725. [Google Scholar] [CrossRef]
- Artunç, T.; Menzek, A.; Taslimi, P.; Gulçin, İ.; Kazaz, C.; Şahin, E. Synthesis and antioxidant activities of phenol derivatives from 1,6-bis(dimethoxyphenyl)hexane-1,6-dione. Bioorg. Chem. 2020, 100, 103884. [Google Scholar] [CrossRef]
- Lovley, D.R. Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol. Rev. 1991, 55, 259–287. [Google Scholar] [CrossRef] [PubMed]
- Karagecili, H.; Yılmaz, M.A.; Ertürk, A.; Kızıltaş, H.; Güven, L.; Alwasel, S.H.; Gulcin, I. Comprehensive metabolite profiling of Berdav propolis using LC-MS/MS: Determination of antioxidant, anticholinergic, antiglaucoma, and antidiabetic effects. Molecules 2023, 28, 1739. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Dong, H.; Reguera, G.; Beyenal, H.; Lu, A.; Liu, J.; Yu, H.Q.; Fredrickson, J.K. Extracellular electron transfer mechanisms between microorganisms and minerals. Nat. Rev. Microbiol. 2012, 10, 751–762. [Google Scholar] [CrossRef]
- Aly, S.H.; Uba, A.I.; Nilofar, N.; Majrashi, T.A.; El Hassab, M.A.; Eldehna, W.M.; Zengin, G.; Eldahshan, O.A. Chemical composition and biological activity of lemongrass volatile oil and n-Hexane extract: GC/MS analysis, in vitro and molecular modelling studies. PLoS ONE 2025, 22, e0319147. [Google Scholar] [CrossRef] [PubMed]
- Voelker, B.M.; Sulzberger, B. Effects of fulvic acid on Fe(II) oxidation pathways in natural waters. Environ. Sci. Technol. 1996, 30, 1106–1114. [Google Scholar] [CrossRef]
- Güven, L.; Ertürk, A.; Demirkaya Miloğlu, F.; Alwasel, S.; Gulcin, I. Screening of antiglaucoma, antidiabetic, anti-Alzheimer, and antioxidant activities of Astragalus alopecurus Pall-Analysis of phenolics profiles by LC-MS/MS. Pharmaceuticals 2023, 16, 659. [Google Scholar] [CrossRef]
- Li, X. Microbial reduction of Fe³⁺ and its environmental implications. Environ. Sci. Technol. 2012, 46, 276–283. [Google Scholar]
- Zengin, G.; Yagi, S.; Cziáky, Z.; Jeko, J.; Yildiztugay, E.; Maugeri, A.; Russo, C.; Cetiz, M.V.; Navarra, M. Exploring the chemical constituents and biological activities of leaf and twig extracts of two Amygdalus species from Turkey’s flora: Cell-free, in vitro and molecular docking approaches. Food Biosci. 2025, 64, 105869. [Google Scholar] [CrossRef]
- Lovley, D.R.; Coates, J.D. Bioremediation of metal contamination. Curr. Opin. Biotechnol. 1997, 8, 285–289. [Google Scholar] [CrossRef]
- Conrad, M.E.; Umbreit, J.N.; Andrews, N.C. Disorders of iron metabolism. N. Engl. J. Med. 2000, 342, 1293–1294. [Google Scholar]
- Ibrahimova, N.; Çete, S.; Anakök, D.A.; Özmen, Ü.Ö.; Gündüzalp, A.B.; Öztürk, A.; Aydemir, I. Synthesis of new sulfa drugs containing FDA-approved sulfa pyridine: Evaluation of cholinesterase inhibition, antimicrobial, antibiofilm, anticancer, and antioxidant activities, along with theoretical calculation and molecular docking study. J. Mol. Struct. 2025, 1335, 142013. [Google Scholar] [CrossRef]
- Bsharat, I.; Abdalla, L.; Sawafta, A.; Abu-Reidah, I.M.; Al-Nuri, M.A. Synthesis, characterization, anticancer, antibacterial and antioxidant activities of novel thymol ester compound. J. Mol. Struct. 2025, 1334, 141771. [Google Scholar] [CrossRef]
- Uysal, S.; Loncar, B.; Kljakic, A.C.; Zengin, G. Optimization of ultrasound-assisted extraction from Olea europaea leaves and analysis of their antioxidant and enzyme inhibition activities. Food Biosci. 2025, 63, 105798. [Google Scholar] [CrossRef]
- Zhang, C.Z.; Ding, W.H.; Mamattursun, A.; Ma, X.Y.; Qi, S.W.; Wu, Y.K.; Zhang, J.; Ma, X.L. Optimization of enzyme-ultrasound assisted extraction from mulberries anthocyanins based on response surface methodology and deep neural networks and analysis of in vitro antioxidant activities. Food Chem. 2025, 478, 143597. [Google Scholar] [CrossRef] [PubMed]
- Shantabi, L.; Jagetia, G.C.; Ali, M.A.; Singh, T.T.; Devi, S.V. Antioxidant potential of Croton caudatus leaf extract in vitro. Transl. Med. Biotechnol. 2014, 2, 1–15. [Google Scholar]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, A.; Bursal, E. Synthesis, characterization, biological evaluation, ADMET, and molecular docking studies of novel chalcone-sulfonate hybrid compounds as potential antioxidant and antiobesity activities. J. Mol. Struct. 2025, 1332, 141638. [Google Scholar] [CrossRef]
- Turan, N.; Buldurun, K.; Bursal, E.; Mahmoudi, G. Pd(II)-Schiff base complexes: Synthesis, characterization, Suzuki-Miyaura and Mizoroki-Heck cross-coupling reactions, enzyme inhibition and antioxidant activities. J. Organometal. Chem. 2022, 970, 122370. [Google Scholar] [CrossRef]
- Cınar, E. Investigation of docking, antioxidant, and anti-Alzheimer activities of newly Schiff bases containing aryl sulfonate group. J. Mol. Struct. 2025, 1332, 141632. [Google Scholar] [CrossRef]
- Kim, S.H.; Jang, J.S.; Kim, E.H.; Lee, W.H.; Yu, Y.H.; Kim, H.J.; Huh, C.K. Physicochemical fermentation characteristics and changes in antioxidant activity of mealworms (Tenebrio molitor) during fermentation with lactic acid bacteria: Application and selection of commercial lactic acid bacteria starters. Appl. Food Res. 2025, 5, 100811. [Google Scholar] [CrossRef]
- Alavi, F.; Ciftci, O.N. Boosting curcumin bioavailability with egg white protein aerogels: A sustainable supercritical CO2-based approach for enhanced bioaccessibility, antioxidant activity, and anti-inflammatory effects. J. Enzym. Inhib. Med. Chem. 2025, 11, 100532. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Revisiting the polar paradox theory: A critical overview. J. Agric. Food Chem. 2011, 59, 3499–3504. [Google Scholar] [CrossRef]
- Vergara-Castro, M.; Soto-Maldonado, C.; Zúñiga-Hansen, M.E.; Arancibia-Díaz, A.; Fuentes, P.; Altamirano, C. Chemical and biological antioxidant activity evaluation of extracts from berries pomaces. Appl. Food Res. 2025, 5, 100781. [Google Scholar] [CrossRef]
- Halliwell, B. Oxidative stress, nutrition and health. Experimental strategies for optimization of nutritional antioxidant intake in humans. Free Radic. Res. 1996, 25, 57–74. [Google Scholar] [CrossRef]
- Wang, D.F.; Yan, Z.H.; Ren, L.K.; Jiang, Y.; Zhou, K.; Li, X.P.; Cui, F.C.; Li, T.T.; Li, J.R. Carbon dots as new antioxidants: Synthesis, activity, mechanism and application in the food industry. Food Chem. 2025, 425, 143377. [Google Scholar] [CrossRef] [PubMed]
- Grigore-Gurgu, L.; Dumitrascu, L.; Aprodu, I. Aromatic herbs as a source of bioactive compounds: An overview of their antioxidant capacity, antimicrobial activity, and major applications. Molecules 2025, 30, 1304. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Damasceno, R.O.S.; Pinheiro, J.L.S.; da Silva, L.D.; Rodrigues, L.H.M.; Emídio, J.J.; Lima, T.C.; de Sousa, D.P. Phytochemistry and anti-inflammatory and antioxidant activities of Cinnamomum osmophloeum and its bioactive constituents: A review. Plants 2025, 14, 564. [Google Scholar] [CrossRef] [PubMed]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Comp. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Liu, X.F.; Huang, L.F.; Zhang, X.W.; Xu, X.F. Polysaccharides with antioxidant activity: Extraction, beneficial roles, biological mechanisms, structure-function relationships, and future perspectives: A review. Int. J. Biol. Macromol. 2018, 21, 374–384. [Google Scholar] [CrossRef]
- Shahidi, F.; Wanasundara, P.D. Phenolic antioxidants. Crit. Rev. Food Sci. Nutr. 1992, 32, 67–103. [Google Scholar] [CrossRef]
- Guedes, L.J.L.; Tavares, V.B.; Carneiro, S.R.; Neves, L.M.T. The effect of physical activity on markers of oxidative and antioxidant stress in cancer patients: A systematic review and meta-analysis. BMC Cancer 2025, 25, 74. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J. New insights into aspirin’s anticancer activity: The predominant role of its iron-chelating antioxidant metabolites. Antioxidants 2025, 14, 29. [Google Scholar] [CrossRef]
- Zaldivar-Ortega, A.K.; Cenobio-Galindo, A.D.; Morfin, N.; Aguirre-alvarez, G.; Campos-Montiel, R.G.; Esturau-Escofet, N.; Garduño-García, A.; Angeles-Hernandez, J.C. The physicochemical parameters, phenolic content, and antioxidant activity of honey from stingless bees and Apis mellifera: A systematic review and meta-analysis. Antioxidants 2025, 13, 1539. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Schoonen, M.A. The role of sulfur in the abiotic formation of hydroxyl radicals and Fe²⁺/Fe³⁺ cycling. Geochim. Cosmochim. Acta 2000, 64, 817–836. [Google Scholar]
- Morgan, B.; Lahav, O. The effect of pH on the kinetics of spontaneous Fe(II) oxidation by O₂ in aqueous solution. Chemosphere 2007, 68, 2080–2084. [Google Scholar] [CrossRef] [PubMed]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences, and Uses; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Kraemer, S.M. Iron oxide dissolution and solubility in the presence of siderophores. Aquat. Sci. 2004, 66, 3–18. [Google Scholar] [CrossRef]
- Nicholls, D.G.; Ferguson, S.J. Bioenergetics 4; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Sies, H. Glutathione and its role in cellular functions. Free Radical Biol. Med. 1999, 27, 916–921. [Google Scholar] [CrossRef]
- Jones, D.P. Redox potential of GSH/GSSG couple: Assay and biological significance. Methods Enzymol. 2006, 378, 229–246. [Google Scholar]
- Apak, R.; Calokerinos, A.; Gorinstein, S.; Segundo, M.A.; Hibbert, D.B.; Gülçin, İ.; Demirci Çekiç, S.; Güçlü, K.; Özyürek, M.; Esin Çelik, S.; et al. Methods to evaluate the scavenging activity of antioxidants toward reactive oxygen and nitrogen species. Pure Appl. Chem. 2022, 94, 87–144. [Google Scholar] [CrossRef]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef] [PubMed]
- Stryer, L.; Berg, J.M.; Tymoczko, J.L.; Gatto, G.J., Jr. Biochemistry; W.H. Freeman & Company: New York, NY, USA, 2019. [Google Scholar]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell; Garland Science: New York, NY, USA, 2014. [Google Scholar]
- Imai, S.; Guarente, L. NAD⁺ and sirtuins in aging and disease. Trends Cell Biol. 2014, 24, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.J.; Miranda-Massari, J.R.; Mora, E.M. Advances in clinical use of NADH. Altern. Med. Rev. 2017, 22, 14–27. [Google Scholar]
- Noctor, G.; Foyer, C.H. Ascorbate and Glutathione: Keeping active oxygen under control. Annu. Rev. Plant Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef] [PubMed]
- Krebs, H.A.; Kornberg, H.L. Energy transformations in living matter. Proc. R. Soc. (Lond.) B 1957, 147, 536–558. [Google Scholar]
- Lambeth, J.D. Nox enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004, 4, 181–189. [Google Scholar] [CrossRef]
- Guengerich, F.P. Common and uncommon cytochrome P450 reactions related to metabolism and chemical toxicity. Chem. Res. Toxicol. 2001, 14, 611–650. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef]
- Meister, A.; Anderson, M.E. Glutathione. Ann. Rev. Biochem. 1983, 52, 711–760. [Google Scholar] [CrossRef]
- Hayes, J.D.; Flanagan, J.U.; Jowsey, I.R. Glutathione transferases. Ann. Rev. Pharmacol. Toxicol. 2005, 45, 51–88. [Google Scholar] [CrossRef]
- Dröge, W.; Breitkreutz, R. Glutathione and immune function. Proc. Nutr. Soc. 2000, 59, 595–600. [Google Scholar] [CrossRef]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta 2013, 1830, 3143–3153. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Frei, B. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am. J. Clin. Nutr. 1999, 69, 1086–1107. [Google Scholar] [CrossRef] [PubMed]
- Englard, S.; Seifter, S. The biochemical functions of ascorbic acid. Annu. Rev. Nutr. 1986, 6, 365–406. [Google Scholar] [CrossRef]
- Hemilä, H.; Chalker, E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst. Rev. 2013, 2013, CD000980. [Google Scholar] [CrossRef]
- Roychoudhury, A.N.; Merrett, G.L. Redox pathways in a petroleum contaminated shallow sandy aquifer: Iron and sulfate reductions. Sci. Total Environ. 2006, 366, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Buerge, I.J.; Hug, S.J. Influence of organic ligands on chromium(VI) reduction by iron(II). Environ. Sci. Technol. 1998, 32, 2092–2099. [Google Scholar] [CrossRef]
- Elmagirbi, A.; Sulistyarti, H.; Atikah, A. Study of ascorbic acid as iron(III) reducing agent for spectrophotometric iron speciation. J. Pure Appl. Chem. Res. 2012, 1, 11–17. [Google Scholar] [CrossRef]
- Nealson, K.H.; Saffarini, D. Iron and manganese in anaerobic respiration: Environmental significance, physiology, and regulation. Annu. Rev. Microbiol. 1994, 48, 311–343. [Google Scholar] [CrossRef]
- Weber, K.A.; Achenbach, L.A.; Coates, J.D. Microorganisms pumping iron: Anaerobic microbial iron oxidation and reduction. Nat. Rev. Microbiol. 2006, 4, 752–764. [Google Scholar] [CrossRef]
- Rickard, D.; Luther, G.W. Chemistry of iron sulfides. Chem. Rev. 2007, 107, 514–562. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Dong, Y.; Thompson, A. Electron transfer, atom exchange, and transformation of iron minerals in soils: The influence of soil organic matter. Environ Sci Technol. 2023, 57, 10696–10707. [Google Scholar] [CrossRef] [PubMed]
- Barbeau, K.; Rue, E.L.; Bruland, K.W.; Butler, A. Photochemical cycling of iron in the ocean environment: Implications for iron bioavailability. Science 2006, 314, 1096–1100. [Google Scholar]
- Morris, J.J.; Rose, A.L.; Lu, Z. Reactive oxygen species in the world ocean and their impacts on marine ecosystems. Redox Biol. 2022, 52, 102285. [Google Scholar] [CrossRef]
- Voelker, B.M.; Morel, F.M.M.; Sulzberger, B. Iron redox cycling in surface waters: Effects of humic substances and light. Environ. Sci. Technol. 1997, 31, 1004–1011. [Google Scholar] [CrossRef]
- Coursolle, D.; Gralnick, J.A. Modularity of the Mtr respiratory pathway of Shewanella oneidensis strain MR-1. Mol. Microbiol. 2010, 77, 995–1008. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. Dissimilatory metal reduction: From early life to bioremediation. ASM News 2002, 68, 231–237. [Google Scholar]
- Lovley, D.R. Dissimilatory metal reduction. Annu. Rev. Microbiol. 1993, 47, 263–290. [Google Scholar] [CrossRef]
- Roden, E.E. Geochemical and microbiological controls on dissimilatory iron reduction. Comptes Rendus Geosci. 2006, 338, 456–467. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reactions: Antioxidative activities of product of browning reaction prepared from glucosamine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Zhuang, X.C.; Shi, W.; Shen, T.; Cheng, X.Y.; Wan, Q.L.; Fan, M.X.; Hu, D.B. Research updates and advances on flavonoids derived from dandelion and their antioxidant activities. Antioxidants 2025, 13, 1449. [Google Scholar] [CrossRef]
- Yusoff, M.H.M.; Shafie, M.H. A review of in vitro antioxidant and antidiabetic polysaccharides: Extraction methods, physicochemical and structure-activity relationships. Int. J. Biol. Macromol. 2003, 83, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Lopez, P.L.; Guerberoff Enemark, G.K.; Grosso, N.R.; Olmedo, R.H. Antioxidant effectiveness between mechanisms of “Chain breaking antioxidant” and “Termination enhancing antioxidant” in a lipid model with essential oils. Food Biosci. 2024, 57, 103498. [Google Scholar] [CrossRef]
- Lopez, P.L.; Juncos, N.S.; Grosso, N.R.; Olmedo, R.H. Determination in the efficiency of the use of essential oils of oregano and hop as antioxidants in deep frying processes. Europ. J. Lip. Sci. Technol. 2023, 126, 2300145. [Google Scholar] [CrossRef]
- Gulcin, I. Comparison of in vitro antioxidant and antiradical activities of L-Tyrosine and L-Dopa. Amino Acids 2007, 32, 431–438. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidant properties of resveratrol: A structure-activity insight. Innov. Food Sci. Emerg. 2010, 11, 210–218. [Google Scholar] [CrossRef]
- Durmaz, L.; Erturk, A.; Akyuz, M.; Polat Kose, L.; Uc, E.M.; Bingol, Z.; Sağlamtas, R.; Alwasel, S.; Gulcin, I. Screening of carbonic anhydrase, acetylcholinesterase, butyrylcholinesterase and α-glycosidase enzymes inhibition effects and antioxidant activity of coumestrol. Molecules 2022, 27, 3091. [Google Scholar] [CrossRef]
- Durmaz, L.; Kiziltas, H.; Guven, L.; Karagecili, H.; Alwasel, S.; Gulcin, I. Antioxidant, antidiabetic, anticholinergic, and antiglaucoma effects of magnofluorine. Molecules 2022, 27, 5902. [Google Scholar] [CrossRef]
- Topal, F.; Topal, M.; Gocer, H.; Kalın, P.; Kocyigit, U.M.; Gulcin, I.; Alwasel, S.H. Antioxidant activity of taxifolin: An activity-structure relationship. J. Enzym. Inhib. Med. Chem. 2016, 31, 674–683. [Google Scholar] [CrossRef]
- Topal, M.; Gocer, H.; Topal, F.; Kalin, P.; Polat Kose, P.; Gulcin, I.; Cakmak, K.C.; Kucuk, M.; Durmaz, L.; Goren, A.C.; et al. Antioxidant, antiradical and anticholinergic properties of cynarin purified from the illyrian thistle (Onopordum illyricum L.). J. Enzym. Inhib. Med. Chem. 2016, 31, 266–275. [Google Scholar] [CrossRef]
- Huyut, Z.; Beydemir, S.; Gulcin, I. Antioxidant and antiradical properties of some flavonoids and phenolic compounds. Biochem. Res. Int. 2017, 2017, 7616791. [Google Scholar] [CrossRef]
- Durmaz, L.; Karagecili, H.; Gulcin, I. Evaluation of carbonic anhydrase, acetylcholinesterase, butyrylcholinesterase and α-glycosidase inhibition effects and antioxidant activity of baicalin hydrate. Life 2023, 13, 2136. [Google Scholar] [CrossRef] [PubMed]
- Durmaz, L.; Gulcin, I.; Taslimi, P.; Tüzün, B. Isofraxidin: Antioxidant, anti-carbonic anhydrase, anti-cholinesterase, anti-diabetic, and in silico properties. ChemistrySelect 2023, 8, e202300170. [Google Scholar] [CrossRef]
- Durmaz, L.; Kiziltas, H.; Karageçili, H.; Alwasel, S.; Gulcin, I. Potential antioxidant, anticholinergic, antidiabetic and antiglaucoma activities and molecular docking of spiraeoside as a secondary metabolite of onion (Allium cepa). Saudi Pharm. J. 2023, 31, 101760. [Google Scholar] [CrossRef]
- Ozaslan, M.S.; Saglamtas, R.; Demir, Y.; Genç, Y.; Saraçoğlu, İ.; Gulcin, I. Isolation of some phenolic compounds from Plantago subulata L. and determination of its antidiabetic, anticholinesterase, antiepileptic and antioxidant activity. Chem. Biodivers. 2022, 19, e202200280. [Google Scholar] [CrossRef] [PubMed]
- Durmaz, L.; Karageçili, H.; Erturk, A.; Ozden, E.M.; Taslimi, P.; Alwasel, S.; Gulcin, I. Hamamelitannin’s antioxidant effect and its inhibition capability on α-glycosidase, carbonic anhydrase, acetylcholinesterase, and butyrylcholinesterase enzymes. Processes 2024, 12, 2341. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 2006, 217, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, I.; Huyut, Z.; Elmastas, M.; Aboul-Enein, H.Y. Radical scavenging and antioxidant activity of tannic acid. Arab. J. Chem. 2010, 3, 43–53. [Google Scholar] [CrossRef]
- Gulcin, I.; Tel, A.Z.; Goren, A.C.; Taslimi, P.; Alwasel, S. Sage (Salvia pilifera): Determination its polyphenol contents, anticholinergic, antidiabetic and antioxidant activities. J. Food Meas. Charact. 2019, 13, 2062–2074. [Google Scholar] [CrossRef]
- Koksal, E.; Bursal, E.; Gulcin, I.; Korkmaz, M.; Caglayan, C.; Goren, A.C.; Alwasel, S.H. Antioxidant activity and poly-810 phenol content of Turkish thyme (Thymus vulgaris) monitored by LC-MS/MS. Int. J. Food Proper. 2017, 20, 514–525. [Google Scholar] [CrossRef]
- Bursal, E.; Koksal, E.; Gulcin, I.; Bilsel, G.; Goren, A.C. Antioxidant activity and polyphenol content of cherry stem (Cerasus avium L.) determined by LC-MS/MS. Food Res. Int. 2013, 51, 66–74. [Google Scholar] [CrossRef]
- Polat Kose, L.; Gulcin, I.; Goren, A.C.; Namiesnik, J.; Martinez-Ayala, A.L.; Gorinstein, S. LC-MS/MS analysis, antioxidant and anticholinergic properties of galanga (Alpinia officinarum Hance) rhizomes. Ind. Crops Prod. 2015, 74, 712–721. [Google Scholar] [CrossRef]
- Tohma, H.; Gulcin, I.; Bursal, E.; Goren, A.C.; Alwasel, S.H.; Koksal, E. Antioxidant activity and phenolic compounds of ginger (Zingiber officinale Rosc.) determined by HPLC-MS/MS. J. Food Meas. Charact. 2017, 11, 556–566. [Google Scholar] [CrossRef]
- Gulcin, I.; Kaya, R.; Goren, A.C.; Akıncıoglu, H.; Topal, M.; Bingol, Z.; Cetin Cakmak, K.; Ozturk Sarikaya, S.B.; Durmaz, L.; Alwasel, S. Anticholinergic, antidiabetic and antioxidant activities of cinnamon (Cinnamomum verum) bark extracts: Polyphenol contents analysis by LC-MS/MS. Int. J. Food Proper. 2019, 22, 1511–1526. [Google Scholar] [CrossRef]
- Gulcin, I.; Goren, A.C.; Taslimi, P.; Akyuz, B.; Tuzun, B. Anticholinergic, antidiabetic and antioxidant activities of Anatolian pennyroyal (Mentha pulegium) -Analysis of its polyphenol contents by LC-MS/MS. Biocat. Agric. Biotechnol. 2020, 23, 101441. [Google Scholar] [CrossRef]
- Polat Kose, L.; Bingol, Z.; Kaya, R.; Goren, A.C.; Akincioglu, H.; Durmaz, L.; Koksal, E.; Alwasel, S.; Gulcin, I. Anticholinergic and antioxidant activities of avocado (Folium perseae) leaves—Phytochemical content by LC-MS/MS analysis. Int. J. Food Prop. 2020, 23, 878–893. [Google Scholar] [CrossRef]
- Kızıltas, H.; Bingol, Z.; Goren, A.C.; Polat Kose, L.; Durmaz, L.; Topal, F.; Alwasel, S.H.; Gulcin, I. LC-HRMS profiling, antidiabetic, anticholinergic and antioxidant activities of aerial parts of kınkor (Ferulago stelleta). Molecules 2021, 26, 2469. [Google Scholar] [CrossRef]
- Karagecili, H.; Izol, E.; Kireçci, E.; Gulcin, I. Determination of antioxidant, anti-Alzheimer, antidiabetic, antiglaucoma and antimicrobial effects of zivzik pomegranate (Punica granatum)-A chemical profiling by LC-MS/MS. Life 2023, 13, 735. [Google Scholar] [CrossRef]
| Reactive Oxygen Species | Non-Free-Radical Species | ||
|---|---|---|---|
| Hydroxyl radical | HO· | Hydrogen peroxide | H2O2 |
| Superoxide radical | O2·− | Singlet oxygen | 1O2 |
| Hydroperoxyl radical | HOO· | Ozone | O3 |
| Lipid radical | L· | Lipid hydroperoxide | LOOH |
| Lipid peroxyl radical | LOO· | Hypochlorous acid | HOCl |
| Peroxyl radical | ROO· | Peroxynitrite | ONOO− |
| Lipid alkoxyl radical | LO· | Dinitrogen trioxide | N2O3 |
| Nitrogen dioxide radical | NO2· | Nitrous acid | HNO2 |
| Nitric oxide radical | NO· | Nitryl chloride | NO2Cl |
| Nitrosyl cation | NO+ | Nitroxyl anion | NO− |
| Thiyl radical | RS· | Peroxynitrous acid | ONOOH |
| Protein radical | P· | Nitrous oxide | N2O |
| Antioxidants | Absorbance (nm) | Concentration (µg/mL) | References |
|---|---|---|---|
| L-Dopa | 2.443 | 20 | [142] |
| L-Tyrosine | 0.388 | 20 | [142] |
| Resveratrol | 2.156 | 30 | [143] |
| Coumestrol | 0.739 | 30 | [144] |
| Magnofluorine | 0.966 | 30 | [145] |
| Taxifoline | 2.847 | 30 | [146] |
| Cynarine | 3.613 | 30 | [147] |
| Pelargonin | 2.064 | 30 | [148] |
| Silychristin | 1.181 | 30 | [148] |
| Callistephin | 2.328 | 30 | [148] |
| Oenin | 2.351 | 30 | [148] |
| Malvin | 2.189 | 30 | [148] |
| Baicalin | 1.249 | 30 | [149] |
| Isofraxidin | 0.547 | 30 | [150] |
| Spiraeoside | 1.012 | 30 | [151] |
| Acteoside | 1.387 | 30 | [152] |
| Isoacteoside | 1.682 | 30 | [152] |
| Echinacoside | 0.820 | 30 | [152] |
| Arenarioside | 0.544 | 30 | [152] |
| Hamamelitannin | 1.030 | 30 | [153] |
| Caffeic acid | 2.769 | 20 | [154] |
| Tannic acid | 2.737 | 45 | [155] |
| Antioxidant Plants | Absorbance (nm) | Concentration (µg/mL) | References | |
|---|---|---|---|---|
| Water Extract | Ethanol Extract | |||
| Sage (Salvia pilifera) | 1.636 | 1.762 | 30 | [156] |
| Thyme (Thymus vulgaris) | 2.020 | 1.889 | 30 | [157] |
| Cherry stem (Cerasus avium) | 0.926 | 1.517 | 30 | [158] |
| Galanga (Alpinia officinarum) | 1.332 | 1.976 | 30 | [159] |
| Ginger (Zingiber offcinale) | 0.332 | 1.122 | 30 | [160] |
| Cinnamon (Cinnamomum verum) | 0.719 | 0.886 | 30 | [161] |
| Pennyroyal (Mentha pulegium) | 1.433 | 1.362 | 30 | [162] |
| Avocado (Folium perseae) | 0.401 | 0.606 | 30 | [163] |
| Kınkor (Ferulago stellata) | 0.456 | 0.643 | 30 | [164] |
| Pomegranate (Punica granatum) | 1.278 | 1.219 | 30 | [165] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gulcin, İ.; Alwasel, S.H. Fe3+ Reducing Power as the Most Common Assay for Understanding the Biological Functions of Antioxidants. Processes 2025, 13, 1296. https://doi.org/10.3390/pr13051296
Gulcin İ, Alwasel SH. Fe3+ Reducing Power as the Most Common Assay for Understanding the Biological Functions of Antioxidants. Processes. 2025; 13(5):1296. https://doi.org/10.3390/pr13051296
Chicago/Turabian StyleGulcin, İlhami, and Saleh H. Alwasel. 2025. "Fe3+ Reducing Power as the Most Common Assay for Understanding the Biological Functions of Antioxidants" Processes 13, no. 5: 1296. https://doi.org/10.3390/pr13051296
APA StyleGulcin, İ., & Alwasel, S. H. (2025). Fe3+ Reducing Power as the Most Common Assay for Understanding the Biological Functions of Antioxidants. Processes, 13(5), 1296. https://doi.org/10.3390/pr13051296







