Effect of Applied Voltages on Corn Stover Biomethanation and Microbial Community Characteristics in a Microbial Electrolytic Cell-Assisted Anaerobic Digestion System
Abstract
1. Introduction
2. Materials and Methods
2.1. Feedstock and Inoculum
2.2. Experimental Set-Up and Design
2.3. Analytical Methods
2.4. Microbial Community Analysis
2.5. Statistical Analysis
3. Results and Discussion
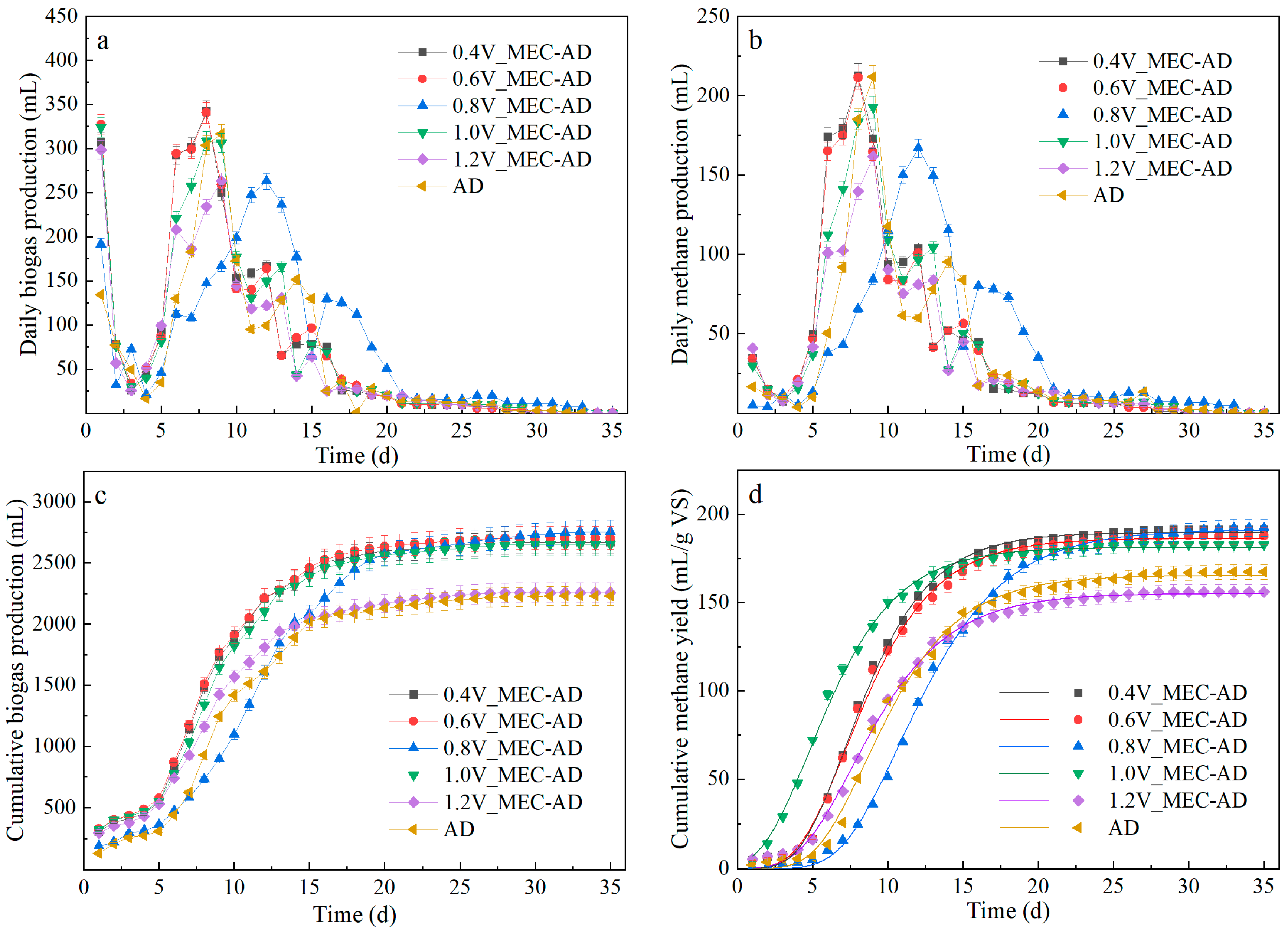
3.1. Biogas and Biomethane Production
3.1.1. Daily Biogas Production
3.1.2. Accumulative Biogas Yield
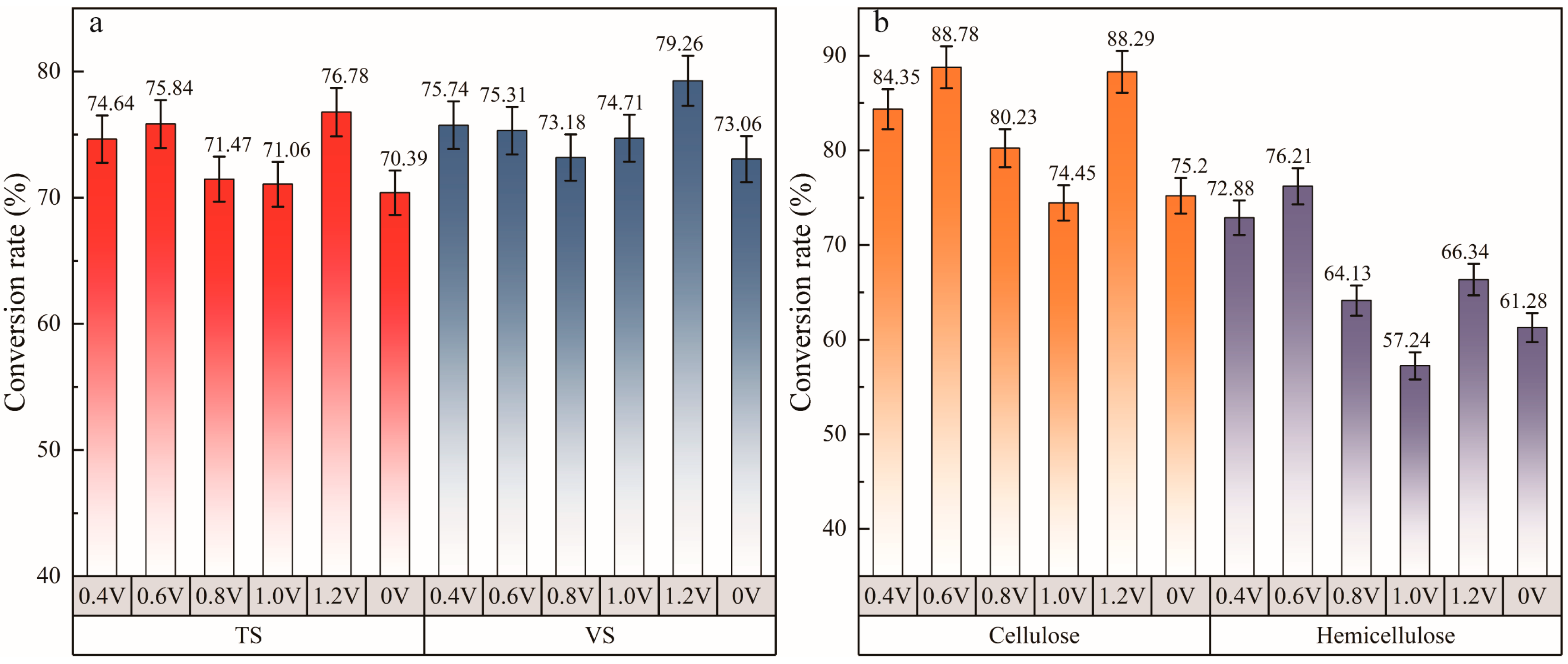
3.2. Substance Conversion
3.3. Bio-Electrochemical Characteristics
3.3.1. Variation in Current
3.3.2. Correlation Between Current and Biogas Production
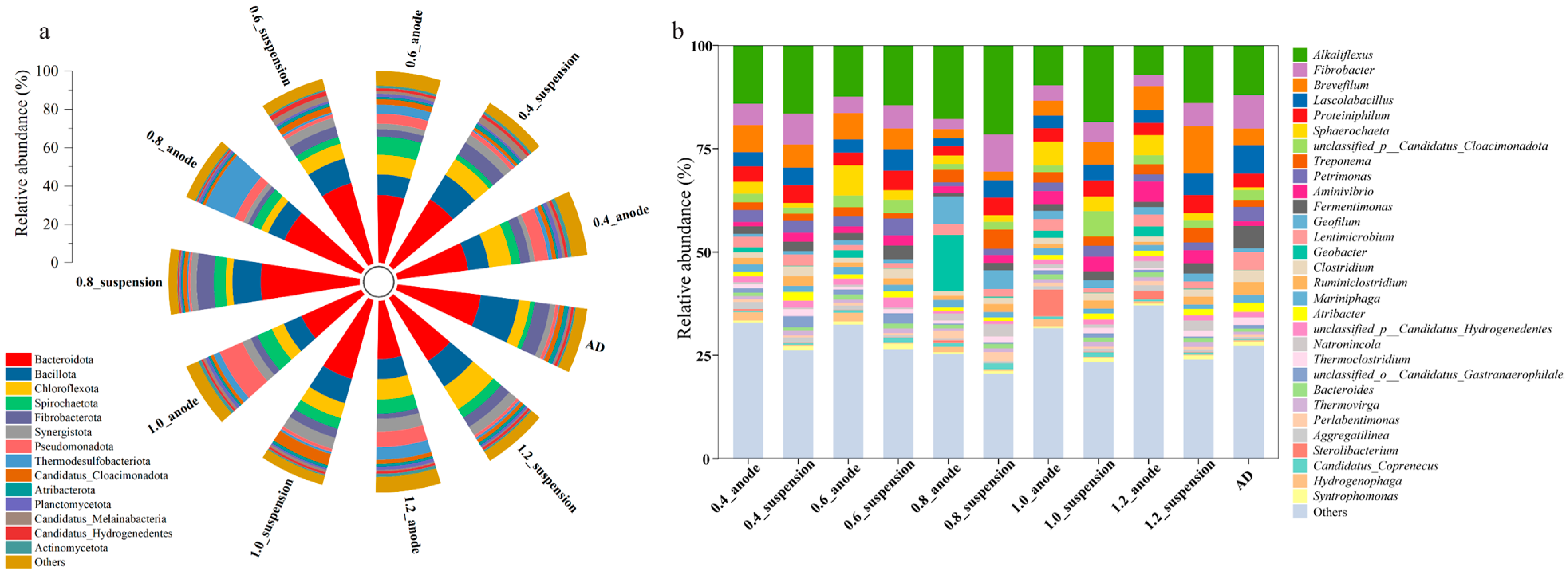
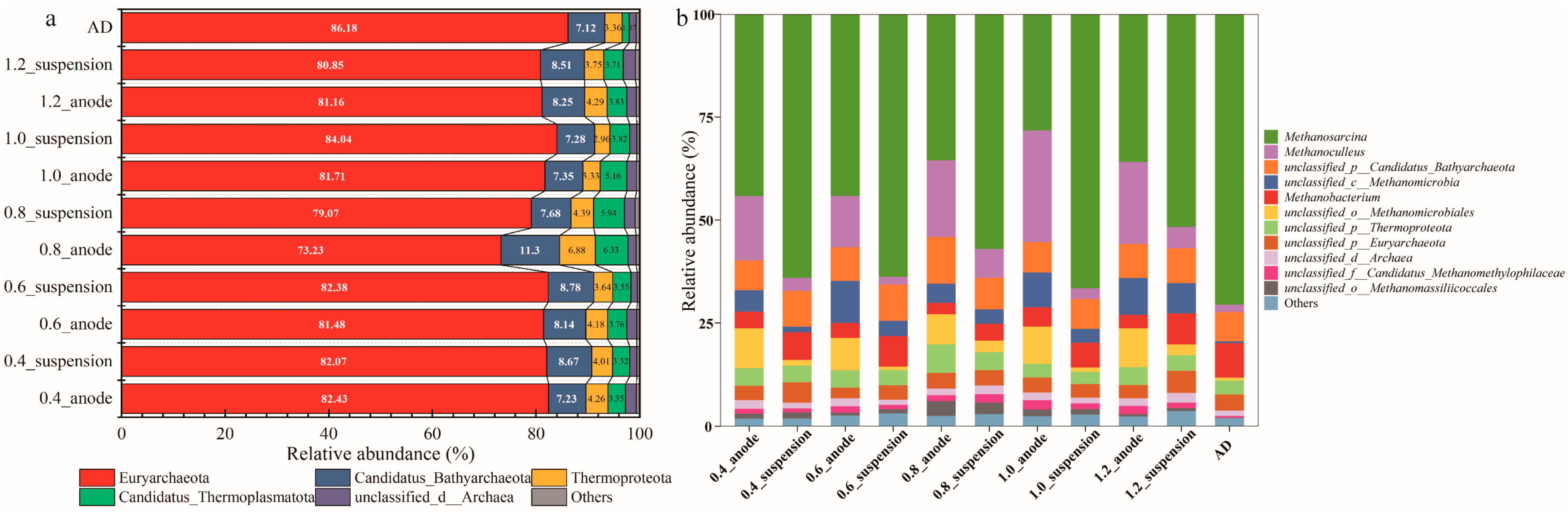
3.4. Microbiological Community Characteristics
3.4.1. Microbial Community Structure
3.4.2. Microbial Correlation Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peng, C.; Wang, T.; Feng, Y.; Fan, X.; Niu, J.; Wang, J.; Gao, W.; Zhou, Y.; Hu, W.; Zhang, Q. Enhanced Hydrolysis and Methane Yield of Temperature-Phased Dewatered Sludge Anaerobic Digestion by Microbial Electrolysis Cell. Bioresour. Technol. 2024, 400, 130682. [Google Scholar] [CrossRef] [PubMed]
- Zabed, H.M.; Akter, S.; Yun, J.; Zhang, G.; Zhao, M.; Mofijur, M.; Awasthi, M.K.; Kalam, M.A.; Ragauskas, A.; Qi, X. Towards the Sustainable Conversion of Corn Stover into Bioenergy and Bioproducts through Biochemical Route: Technical, Economic and Strategic Perspectives. J. Clean. Prod. 2023, 400, 136699. [Google Scholar] [CrossRef]
- Ao, T.-J.; Liu, C.-G.; Sun, Z.-Y.; Zhao, X.-Q.; Tang, Y.-Q.; Bai, F.-W. Anaerobic Digestion Integrated with Microbial Electrolysis Cell to Enhance Biogas Production and Upgrading in Situ. Biotechnol. Adv. 2024, 73, 108372. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, W.; Hou, J.; Qi, X.; Ye, M.; Jiang, N.; Meng, F.; Xi, B.; Li, M. Biogas Upgrading Performance and Underlying Mechanism in Microbial Electrolysis Cell and Anaerobic Digestion Integrated System. Bioresour. Technol. 2024, 400, 130683. [Google Scholar] [CrossRef]
- Tripathi, A.; Kumar, S.; Jadhav, G.S.; Jadhav, D.A.; Ghangrekar, M.M.; Surampalli, R.Y. Electromethanogenic Reactor for Biogas Production Using Agricultural and Livestock Waste and Its Comparative Analysis with Biogas Plant: A Mini-Review. Biomass Bioenergy 2024, 185, 107246. [Google Scholar] [CrossRef]
- Wang, X.-T.; Zhao, L.; Chen, C.; Chen, K.-Y.; Yang, H.; Xu, X.-J.; Zhou, X.; Liu, W.-Z.; Xing, D.-F.; Ren, N.-Q.; et al. Microbial Electrolysis Cells (MEC) Accelerated Methane Production from the Enhanced Hydrolysis and Acidogenesis of Raw Waste Activated Sludge. Chem. Eng. J. 2021, 413, 127472. [Google Scholar] [CrossRef]
- Logan, B.E.; Rabaey, K. Conversion of Wastes into Bioelectricity and Chemicals by Using Microbial Electrochemical Technologies. Science 2012, 337, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Xing, D.; Call, D.F.; Logan, B.E. Direct Biological Conversion of Electrical Current into Methane by Electromethanogenesis. Environ. Sci. Technol. 2009, 43, 3953–3958. [Google Scholar] [CrossRef]
- Baek, G.; Saikaly, P.E.; Logan, B.E. Addition of a Carbon Fiber Brush Improves Anaerobic Digestion Compared to External Voltage Application. Water Res. 2021, 188, 116575. [Google Scholar] [CrossRef]
- Wang, W.; Lee, D.-J.; Lei, Z. Integrating Anaerobic Digestion with Microbial Electrolysis Cell for Performance Enhancement: A Review. Bioresour. Technol. 2022, 344, 126321. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Q.; Liu, Y.; Zhu, J.; Sun, F.; Cui, M.-H.; Liu, H.; Zhang, T.C.; Chen, C. Enhancing Degradation of Organic Matter in Microbial Electrolytic Cells Coupled with Anaerobic Digestion (MEC-AD) Systems by Carbon-Based Materials. Sci. Total Environ. 2023, 900, 165805. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Yu, Q.; Sun, C.; Wang, Z.; Jin, Z.; Zhu, Y.; Zhao, Z.; Zhang, Y. Additional Electric Field Alleviates Acidity Suppression in Anaerobic Digestion of Kitchen Wastes via Enriching Electro-Active Methanogens in Cathodic Biofilms. Water Res. 2022, 212, 118118. [Google Scholar] [CrossRef]
- Zhao, Q.; Yuan, H.; Wang, H.; Li, X. Enhancing Biomethane Production from Corn Stover via Anaerobic Digestion Incorporated with Microbial Electrolysis Cell. Chin. J. Chem. Eng. 2025, in press. [Google Scholar] [CrossRef]
- Zhu, G.; Feng, Q.; Wang, K.; Song, Y.-C.; Zhou, Y.; Zhou, Q. Investigating the Performance of Different Applied Voltages on Lignite Biomethanation in Microbial Electrolytic Cell Coupled Anaerobic Digestion. Int. J. Hydrogen Energy 2024, 52, 147–159. [Google Scholar] [CrossRef]
- Zou, L.; Wang, C.; Zhao, X.; Wu, K.; Liang, C.; Yin, F.; Yang, B.; Liu, J.; Yang, H.; Zhang, W. Enhanced Anaerobic Digestion of Swine Manure via a Coupled Microbial Electrolysis Cell. Bioresour. Technol. 2021, 340, 125619. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Q.; Li, M.; Sun, F.; Liu, H.; Cui, M.; Chen, C. The Microbial Electrolysis Cell Combined with Anaerobic Digestion for High Salinity Landfill Leachate Treatment: Operation Parameter Optimization, Microbial Analysis and Degradation Pathways. J. Water Process Eng. 2024, 65, 105795. [Google Scholar] [CrossRef]
- Ao, T.-J.; Zhao, X.-Q.; Mehmood, M.A.; Wang, N.; Zhu, H.; Liu, C.-G.; Bai, F.-W. A Double-Chamber Microbial Electrolysis Cell Improved the Anaerobic Digestion Efficiency and Elucidated the Underlying Bio-Electrochemical Mechanism. Chem. Eng. J. 2023, 471, 144228. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, Y.; Ranjan Dhar, B. A Multifaceted Screening of Applied Voltages for Electro-Assisted Anaerobic Digestion of Blackwater: Significance of Temperature, Hydrolysis/Acidogenesis, Electrode Corrosion, and Energy Efficiencies. Bioresour. Technol. 2022, 360, 127533. [Google Scholar] [CrossRef]
- Korai, R.M.; Li, X. Effect of Ultrasonic Assisted KOH Pretreatment on Physiochemical Characteristic and Anaerobic Digestion Performance of Wheat Straw. Chin. J. Chem. Eng. 2020, 28, 2409–2416. [Google Scholar] [CrossRef]
- Li, Z.; Wachemo, A.C.; Yuan, H.; Korai, R.M.; Li, X. Improving Methane Content and Yield from Rice Straw by Adding Extra Hydrogen into a Two-Stage Anaerobic Digestion System. Int. J. Hydrogen Energy 2020, 45, 3739–3749. [Google Scholar] [CrossRef]
- Xu, Z.; Yuan, H.; Li, X. Anaerobic Bioconversion Efficiency of Rice Straw in Continuously Stirred Tank Reactor Systems Applying Longer Hydraulic Retention Time and Higher Load: One-Stage vs. Two-Stage. Bioresour. Technol. 2021, 321, 124206. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wang, H.; Liu, R.; Yuan, H.; Li, X. Enhancing Biomethane Yield and Metabolic Pathways in Kitchen Waste Anaerobic Digestion Through Microbial Electrolysis Cell Integration. Energies 2025, 18, 1629. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, Q.; Li, Y.; Mazarji, M.; Feng, L.; Pan, J. Deciphering the Impact of Lignin on Anaerobic Digestion: Focus on Inhibition Mechanisms and Methods for Alleviating Inhibition. ACS Omega 2024, 9, 44033–44041. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, K.O.; Ahmed, N.A.; Ogunkunle, O. Optimization of Biogas Yield from Lignocellulosic Materials with Different Pretreatment Methods: A Review. Biotechnol. Biofuels 2021, 14, 159. [Google Scholar] [CrossRef]
- Vu, M.T.; Noori, M.T.; Min, B. Magnetite/Zeolite Nanocomposite-Modified Cathode for Enhancing Methane Generation in Microbial Electrochemical Systems. Chem. Eng. J. 2020, 393, 124613. [Google Scholar] [CrossRef]
- Shen, R.; Zhao, L.; Yao, Z.; Feng, J.; Jing, Y.; Watson, J. Efficient Treatment of Wood Vinegar via Microbial Electrolysis Cell with the Anode of Different Pyrolysis Biochars. Front. Energy Res. 2020, 8, 216. [Google Scholar] [CrossRef]
- Choi, J.-M.; Lee, C.-Y. Bioelectrochemical Enhancement of Methane Production in Anaerobic Digestion of Food Waste. Int. J. Hydrogen Energy 2019, 44, 2081–2090. [Google Scholar] [CrossRef]
- Mortezaei, Y.; Demirer, G.N.; Williams, M.R. Fate of Intracellular and Extracellular Antibiotic Resistance Genes in Sewage Sludge by Full-Scale Anaerobic Digestion. Sci. Total Environ. 2024, 951, 175760. [Google Scholar] [CrossRef]
- Czatzkowska, M.; Wolak, I.; Harnisz, M.; Korzeniewska, E. Microbial Diversity and Biosafety Judgment of Digestates Derived from Different Biogas Plants for Agricultural Applications. J. Environ. Manag. 2024, 371, 123329. [Google Scholar] [CrossRef]
- Lim, J.W.; Chiam, J.A.; Wang, J.-Y. Microbial Community Structure Reveals How Microaeration Improves Fermentation during Anaerobic Co-Digestion of Brown Water and Food Waste. Bioresour. Technol. 2014, 171, 132–138. [Google Scholar] [CrossRef]
- Zhu, R.; Wang, D.-H.; Zheng, Y.; Zou, H.; Fu, S.-F. Understanding the Mechanisms behind Micro-Aeration to Enhance Anaerobic Digestion of Corn Straw. Fuel 2022, 318, 123604. [Google Scholar] [CrossRef]
- Demergasso, C.; Neilson, J.W.; Tebes-Cayo, C.; Véliz, R.; Ayma, D.; Laubitz, D.; Barberán, A.; Chong-Díaz, G.; Maier, R.M. Hyperarid Soil Microbial Community Response to Simulated Rainfall. Front. Microbiol. 2023, 14, 1202266. [Google Scholar] [CrossRef]
- Yang, B.; Xu, H.; Liu, Y.; Li, F.; Song, X.; Wang, Z.; Sand, W. Role of GAC-MnO2 Catalyst for Triggering the Extracellular Electron Transfer and Boosting CH4 Production in Syntrophic Methanogenesis. Chem. Eng. J. 2020, 383, 123211. [Google Scholar] [CrossRef]
- Lobo, S.A.L.; Warren, M.J.; Saraiva, L.M. Chapter Seven—Sulfate-Reducing Bacteria Reveal a New Branch of Tetrapyrrole Metabolism. In Advances in Microbial Physiology; Poole, R.K., Ed.; Advances in Bacterial Respiratory Physiology; Academic Press: Cambridge, MA, USA, 2012; Volume 61, pp. 267–295. [Google Scholar]
- Jiao, Y.; Yuan, Y.; He, C.; Liu, L.; Pan, X.; Li, P. Enrichment Culture Combined with Microbial Electrochemical Enhanced Low-Temperature Anaerobic Digestion of Cow Dung. Bioresour. Technol. 2022, 360, 127636. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R.; Holmes, D.E. Electromicrobiology: The Ecophysiology of Phylogenetically Diverse Electroactive Microorganisms. Nat. Rev. Microbiol. 2022, 20, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.E.; Shrestha, P.M.; Walker, D.J.F.; Dang, Y.; Nevin, K.P.; Woodard, T.L.; Lovley, D.R. Metatranscriptomic Evidence for Direct Interspecies Electron Transfer between Geobacter and Methanothrix Species in Methanogenic Rice Paddy Soils. Appl. Environ. Microbiol. 2017, 83, e00223-17. [Google Scholar] [CrossRef]
- Achinas, S.; Achinas, V.; Euverink, G.J.W. A Technological Overview of Biogas Production from Biowaste. Engineering 2017, 3, 299–307. [Google Scholar] [CrossRef]
- Ajay, C.M.; Mohan, S.; Dinesha, P.; Rosen, M.A. Review of Impact of Nanoparticle Additives on Anaerobic Digestion and Methane Generation. Fuel 2020, 277, 118234. [Google Scholar] [CrossRef]
- Duc, L.V.; Miyagawa, Y.; Inoue, D.; Ike, M. Identification of Key Steps and Associated Microbial Populations for Efficient Anaerobic Digestion under High Ammonium or Salinity Conditions. Bioresour. Technol. 2022, 360, 127571. [Google Scholar] [CrossRef]
- Basak, B.; Patil, S.M.; Kumar, R.; Ahn, Y.; Ha, G.-S.; Park, Y.-K.; Ali Khan, M.; Jin Chung, W.; Woong Chang, S.; Jeon, B.-H. Syntrophic Bacteria- and Methanosarcina-Rich Acclimatized Microbiota with Better Carbohydrate Metabolism Enhances Biomethanation of Fractionated Lignocellulosic Biocomponents. Bioresour. Technol. 2022, 360, 127602. [Google Scholar] [CrossRef]
- Zheng, D.; Wang, H.-Z.; Gou, M.; Nobu, M.K.; Narihiro, T.; Hu, B.; Nie, Y.; Tang, Y.-Q. Identification of Novel Potential Acetate-Oxidizing Bacteria in Thermophilic Methanogenic Chemostats by DNA Stable Isotope Probing. Appl. Microbiol. Biotechnol. 2019, 103, 8631–8645. [Google Scholar] [CrossRef]
- Xie, Z.; Meng, X.; Ding, H.; Cao, Q.; Chen, Y.; Liu, X.; Li, D. The Synergistic Effect of Rumen Cellulolytic Bacteria and Activated Carbon on Thermophilic Digestion of Cornstalk. Bioresour. Technol. 2021, 338, 125566. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-T.; Zhang, Y.-F.; Wang, B.; Wang, S.; Xing, X.; Xu, X.-J.; Liu, W.-Z.; Ren, N.-Q.; Lee, D.-J.; Chen, C. Enhancement of Methane Production from Waste Activated Sludge Using Hybrid Microbial Electrolysis Cells-Anaerobic Digestion (MEC-AD) Process—A Review. Bioresour. Technol. 2022, 346, 126641. [Google Scholar] [CrossRef]
- Xing, T.; Wang, Z.; Zhen, F.; Liu, H.; Wo, D.; Li, L.; Guo, Y.; Kong, X.; Sun, Y. Initial pH-Driven Production of Volatile Fatty Acid from Hybrid Pennisetum. Bioresour. Technol. 2022, 347, 126426. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Liu, M.; Fang, T.; Ma, H.; Beadham, I.; Ruan, W.; Wang, S.; Zhang, X.; Zhang, C. Enhancement of Anaerobic Digestion of Rice Straw by Amino Acid-Derived Ionic Liquid. Bioresour. Technol. 2023, 380, 129076. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Nguyen, D.; Lam, T.Y.C.; Zhuang, H.; Shrestha, S.; Raskin, L.; Khanal, S.K.; Lee, P.-H. Synergistic Association between Cytochrome Bd-Encoded Proteiniphilum and Reactive Oxygen Species (ROS)-Scavenging Methanogens in Microaerobic-Anaerobic Digestion of Lignocellulosic Biomass. Water Res. 2021, 190, 116721. [Google Scholar] [CrossRef]
- Amin, F.R.; Khalid, H.; El-Mashad, H.M.; Chen, C.; Liu, G.; Zhang, R. Functions of Bacteria and Archaea Participating in the Bioconversion of Organic Waste for Methane Production. Sci. Total Environ. 2021, 763, 143007. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Cheng, J.; Li, H.; Yue, L.; Xia, R.; Zhou, J. Electron Transfer from Geobacter Sulfurreducens to Mixed Methanogens Improved Methane Production with Feedstock Gases of H2 and CO2. Bioresour. Technol. 2022, 347, 126680. [Google Scholar] [CrossRef]
- Pierson, L.S.; Pierson, E.A. Metabolism and Function of Phenazines in Bacteria: Impacts on the Behavior of Bacteria in the Environment and Biotechnological Processes. Appl. Microbiol. Biotechnol. 2010, 86, 1659–1670. [Google Scholar] [CrossRef]
- Li, X.; Chu, S.; Wang, P.; Li, K.; Su, Y.; Wu, D.; Xie, B. Potential of Biogas Residue Biochar Modified by Ferric Chloride for the Enhancement of Anaerobic Digestion of Food Waste. Bioresour. Technol. 2022, 360, 127530. [Google Scholar] [CrossRef]


| Total Solids (TS, %) b | Volatile Solids (VS, %) b | Cellulose (%) c | Hemicellulose (%) c | Lignin (%) c | pH | |
|---|---|---|---|---|---|---|
| Original inoculum | 7.78 ± 0.30 | 5.23 ± 0.14 | 3.65 ± 0.19 | 9.84 ± 0.45 | 7.04 ± 0.28 | 7.68 |
| 0.4 V-electrically acclimated inoculum | 8.52 ± 0.38 | 5.48 ± 0.20 | 3.84 ± 0.10 | 7.76 ± 0.21 | 7.67 ± 0.12 | 7.86 |
| 0.6 V-electrically acclimated inoculum | 8.58 ± 0.42 | 5.51 ± 0.21 | 7.63 ± 0.20 | 9.57 ± 0.35 | 8.01 ± 0.10 | 7.75 |
| 0.8 V-electrically acclimated inoculum | 7.98 ± 0.23 | 5.23 ± 0.36 | 2.95 ± 0.17 | 7.49 ± 0.25 | 6.42 ± 0.29 | 8.03 |
| 1.0 V-electrically acclimated inoculum | 9.10 ± 0.25 | 5.79 ± 0.25 | 1.01 ± 0.09 | 2.61 ± 0.11 | 3.31 ± 0.16 | 7.82 |
| 1.2 V-electrically acclimated inoculum | 8.94 ± 0.42 | 5.65 ± 0.17 | 4.74 ± 0.24 | 9.77 ± 0.22 | 8.23 ± 0.30 | 7.84 |
| Corn stover | 94.03 ± 0.53 | 87.38 ± 0.42 | 39.18 ± 0.35 | 25.56 ± 0.42 | 3.56 ± 0.29 | / |
| Modified Gompertz Model | |||||
|---|---|---|---|---|---|
| Reactors | H (mL CH4/g VS) | p (mL CH4/g VS) | a (d) | λ (mL CH4/(g VS·d)) | R2 |
| MEC-AD_0.4 V | 191.18 ± 4.78 | 189.64 ± 0.73 | 4.16 ± 0.11 | 22.62 ± 0.54 | 0.998 |
| MEC-AD_0.6 V | 187.74 ± 4.69 | 186.35 ± 0.81 | 4.03 ± 0.12 | 21.11 ± 0.54 | 0.998 |
| MEC-AD_0.8 V | 192.40 ± 4.81 | 191.21 ± 0.77 | 6.93 ± 0.10 | 18.14 ± 0.34 | 0.999 |
| MEC-AD_1.0 V | 182.75 ± 4.57 | 181.23 ± 0.54 | 1.59 ± 0.09 | 20.66 ± 0.40 | 0.998 |
| MEC-AD_1.2 V | 156.11 ± 3.90 | 155.12 ± 0.64 | 4.08 ± 0.12 | 16.03 ± 0.36 | 0.998 |
| AD | 167.33 ± 4.18 | 165.35 ± 0.99 | 5.18 ± 0.16 | 17.86 ± 0.59 | 0.996 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Yuan, H.; Li, X. Effect of Applied Voltages on Corn Stover Biomethanation and Microbial Community Characteristics in a Microbial Electrolytic Cell-Assisted Anaerobic Digestion System. Processes 2025, 13, 1271. https://doi.org/10.3390/pr13051271
Zhao Q, Yuan H, Li X. Effect of Applied Voltages on Corn Stover Biomethanation and Microbial Community Characteristics in a Microbial Electrolytic Cell-Assisted Anaerobic Digestion System. Processes. 2025; 13(5):1271. https://doi.org/10.3390/pr13051271
Chicago/Turabian StyleZhao, Qing, Hairong Yuan, and Xiujin Li. 2025. "Effect of Applied Voltages on Corn Stover Biomethanation and Microbial Community Characteristics in a Microbial Electrolytic Cell-Assisted Anaerobic Digestion System" Processes 13, no. 5: 1271. https://doi.org/10.3390/pr13051271
APA StyleZhao, Q., Yuan, H., & Li, X. (2025). Effect of Applied Voltages on Corn Stover Biomethanation and Microbial Community Characteristics in a Microbial Electrolytic Cell-Assisted Anaerobic Digestion System. Processes, 13(5), 1271. https://doi.org/10.3390/pr13051271





