Three-Dimensional Carbon Nanotube-Coated Copper Mesh as a Current Collector for Graphite Anodes in High-Performance Lithium-Ion Batteries
Abstract
1. Introduction
2. Experimental
2.1. Sample Preparation
2.2. Characterization and Electrochemical Testing
3. Results and Discussion
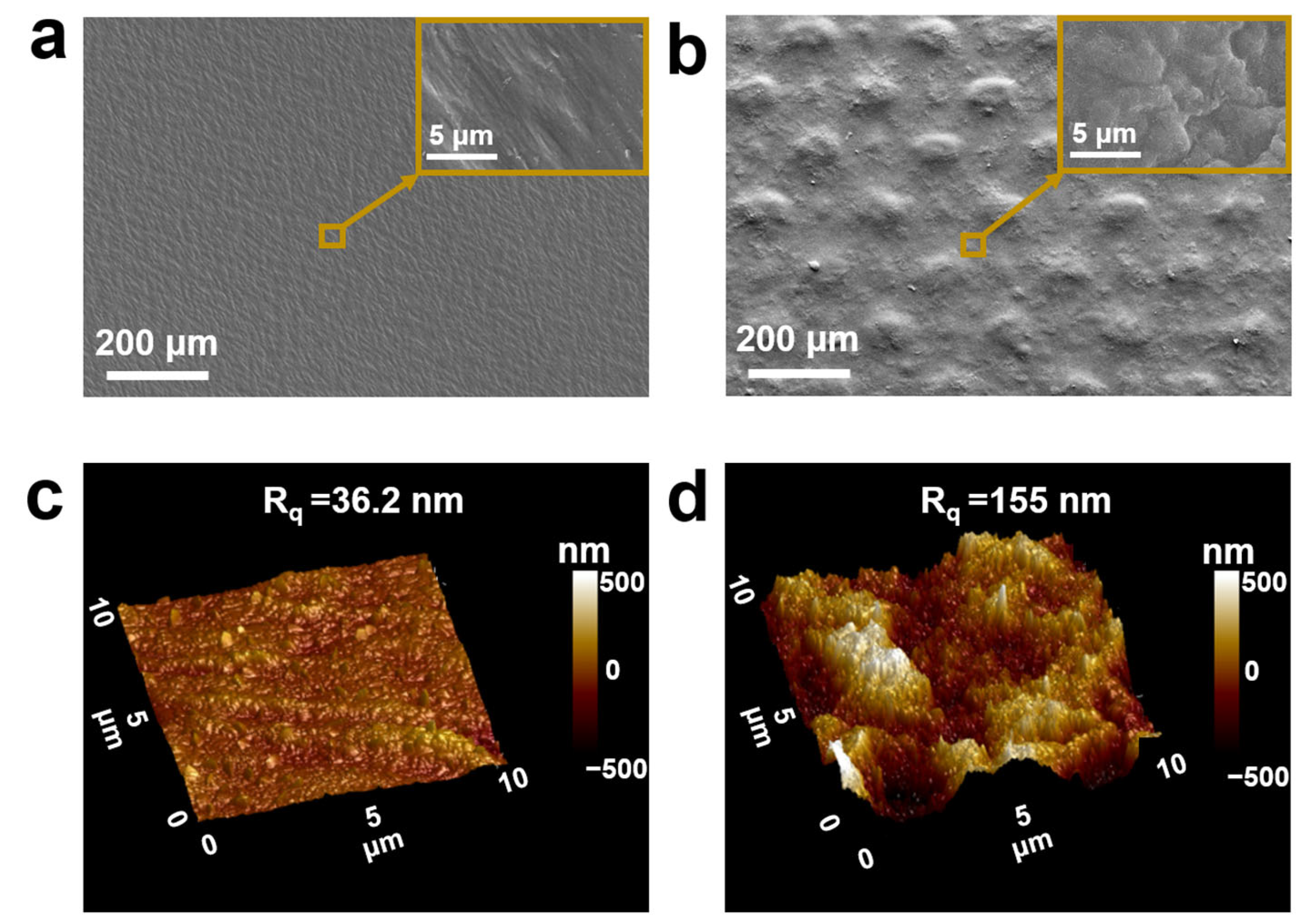
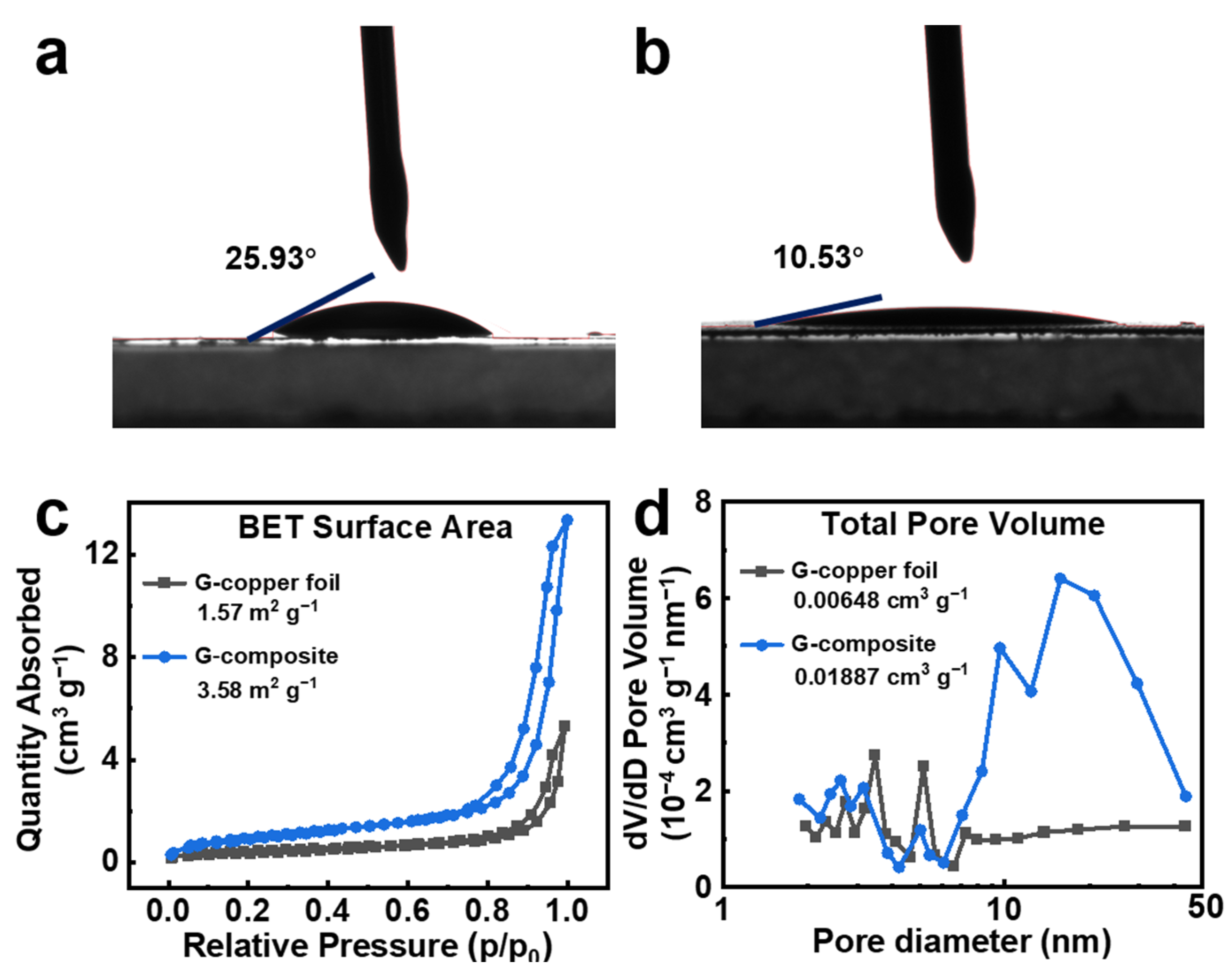
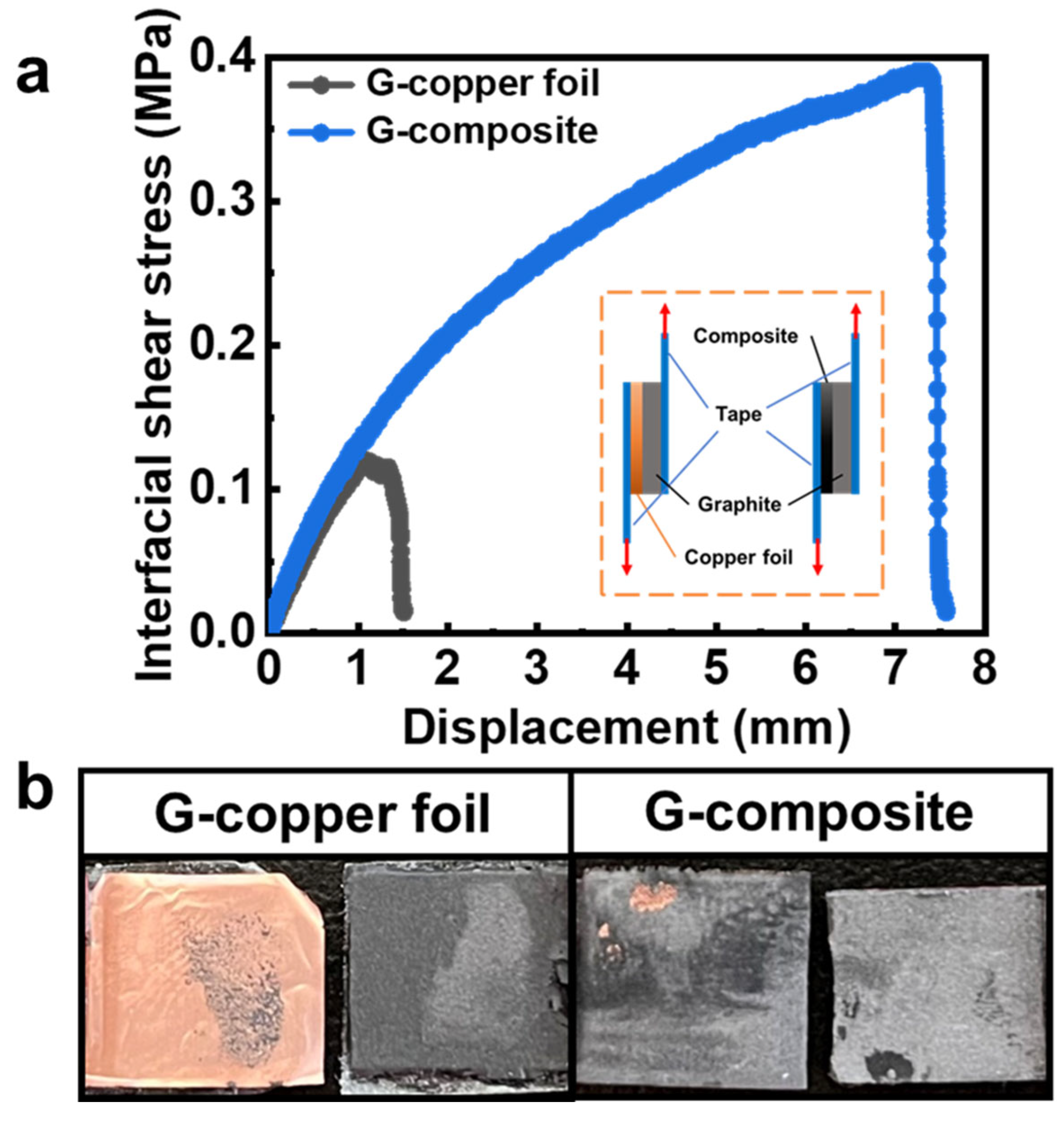
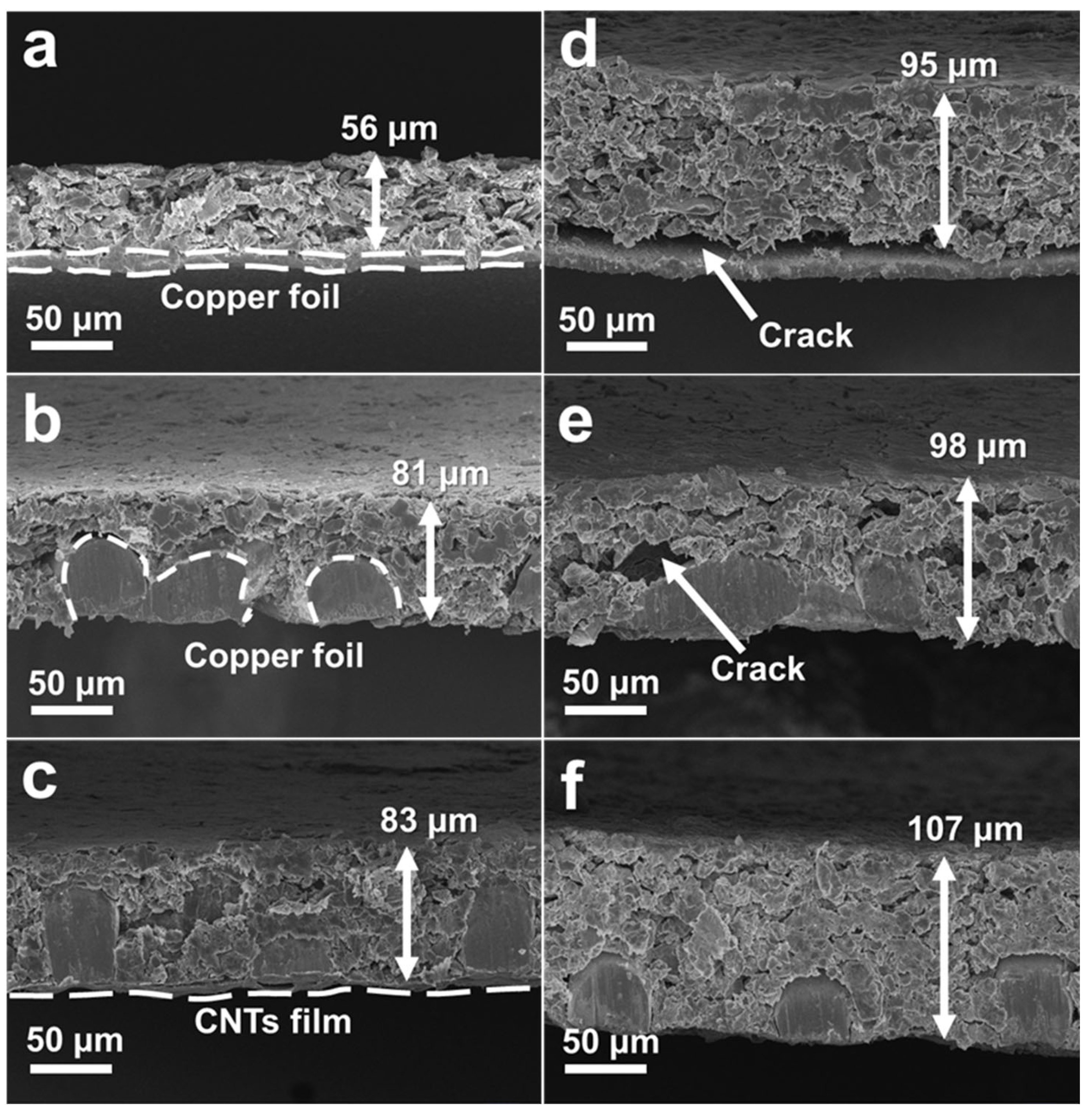
3.1. Characterization of the Current Collectors and Electrodes
3.2. Electrochemical Testing of the Electrodes
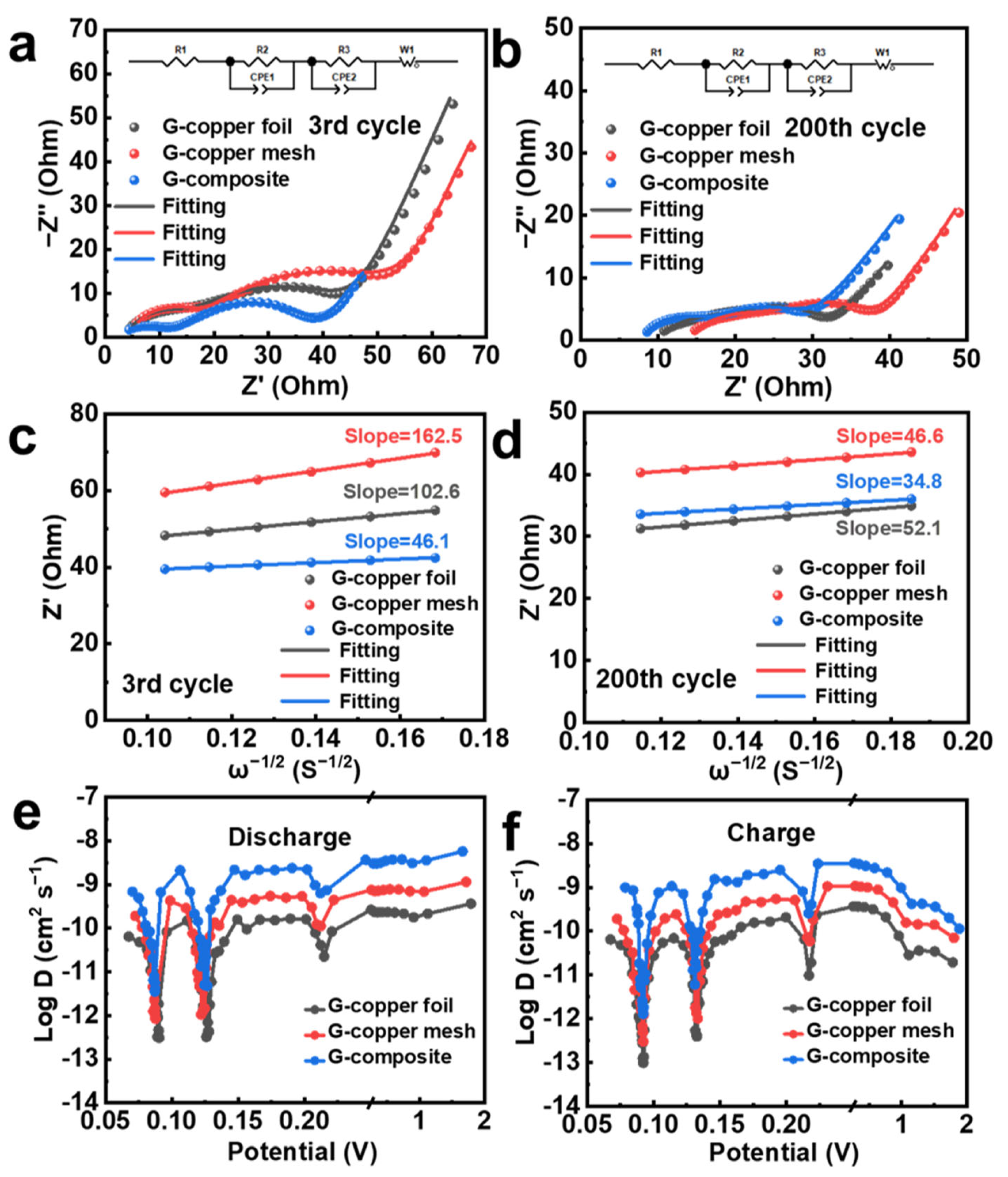
3.3. Structural Characterization of the Electrodes Before and After Cycling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lai, X.; Yi, W.; Li, H.; Han, X.; Feng, X.; Li, S.; Zhou, L.; Zheng, Y. Online internal short circuit detection method considering equalization electric quantity for lithium-ion battery pack in electric vehicles. Int. J. Energy Res. 2021, 45, 7326–7340. [Google Scholar] [CrossRef]
- Mesbahi, T.; Bartholomeüs, P.; Rizoug, N.; Sadoun, R.; Khenfri, F.; Le Moigne, P. Advanced model of hybrid energy storage system integrating lithium-ion battery and supercapacitor for electric vehicle applications. IEEE Trans. Ind. Electron. 2021, 68, 3962–3972. [Google Scholar] [CrossRef]
- Zhu, P.; Gastol, D.; Marshall, J.; Sommerville, R.; Goodship, V.; Kendrick, E. A review of current collectors for lithium-ion batteries. J. Power Sources 2021, 485, 229321. [Google Scholar] [CrossRef]
- Acebedo, B.; Morant-Miñana, M.; Gonzalo, E.; Ruiz de Larramendi, I.; Villaverde, A.; Rikarte, J.; Fallarino, L. Current status and future perspective on lithium metal anode production methods. Adv. Energy Mater. 2023, 13, 2203744. [Google Scholar] [CrossRef]
- Pan, C.; Chen, S.; Huang, Y.; Wang, L.; Luo, J.; Fu, X. A facile method to fabricate lightweight copper coated polyimide film current collectors for lithium-ion batteries. J. Power Sources 2022, 528, 231207. [Google Scholar] [CrossRef]
- Jeong, H.; Jang, J.; Jo, C. A review on current collector coating methods for next generation batteries. Chem. Eng. J. 2022, 446, 136860. [Google Scholar] [CrossRef]
- Ye, Y.; Chou, L.; Liu, Y.; Wang, H.; Lee, H.; Huang, W.; Wan, J.; Liu, K.; Zhou, G.; Yang, Y.; et al. Ultralight and fire-extinguishing current collectors for high-energy and high-safety lithium-ion batteries. Nat. Energy 2020, 5, 786–793. [Google Scholar] [CrossRef]
- Yehezkel, S.; Auinat, M.; Sezin, N.; Starosvetsky, D.; Ein-Eli, Y. Distinct copper electrodeposited carbon nanotubes (CNT) tissues as anode current collectors in Li-ion battery. Electrochim. Acta 2017, 229, 404–414. [Google Scholar] [CrossRef]
- Wang, H.; Watkins, T.; Simunovic, S.; Bingham, P.; Allu, S.; Turner, J. Fragmentation of copper current collectors in Li-ion batteries during spherical indentation. J. Power Sources 2017, 364, 432–436. [Google Scholar] [CrossRef]
- Fu, A.; Wang, C.; Peng, J.; Su, M.; Pei, F.; Cui, J.; Fang, X.; Li, J.; Zheng, N. Lithiophilic and antioxidative copper current collectors for highly stable lithium metal batteries. Adv. Funct. Mater. 2021, 31, 2009805. [Google Scholar]
- Jeon, H.; Cho, I.; Jo, H.; Kim, K.; Ryou, M.; Lee, Y. Highly rough copper current collector: Improving adhesion property between a silicon electrode and current collector for flexible lithium-ion batteries. RSC Adv. 2017, 7, 35681–35686. [Google Scholar]
- Jung, D.; Lee, C.; Park, S.; Oh, E. Characterization of electric double-layer capacitors with carbon nanotubes directly synthesized on a copper plate as a current collector. Korean J. Met. Mater. 2011, 49, 419–424. [Google Scholar]
- Luan, J.; Zhang, Q.; Yuan, H.; Sun, D.; Peng, Z.; Tang, Y.; Ji, X.; Wang, H. Plasma-Strengthened lithiophilicity of copper oxide nanosheet-decorated Cu foil for stable lithium metal anode. Adv. Sci. 2019, 6, 1901433. [Google Scholar]
- Li, Q.; Sun, X.; Zhao, W.; Hou, X.; Zhang, Y.; Zhao, F.; Li, X.; Mei, X. Processing of a large-scale microporous group on copper foil current collectors for lithium batteries using femtosecond laser. Adv. Eng. Mater. 2020, 22, 2000710. [Google Scholar]
- Xiao, Z.; Chen, J.; Liu, J.; Liang, T.; Xu, Y.; Zhu, C.; Zhong, S. Microcrystalline copper foil as a high-performance collector for lithium-ion batteries. J. Power Sources 2019, 438, 226973. [Google Scholar]
- Wotango, A.; Su, W.; Leggesse, E.; Haregewoin, A.; Lin, M.; Zegeye, T.; Cheng, J.; Hwang, B. Improved interfacial properties of MCMB electrode by 1-(trimethylsilyl) imidazole as new electrolyte additive to suppress LiPF6 decomposition. ACS Appl. Mater. Interfaces 2017, 9, 2410–2420. [Google Scholar]
- Chae, S.; Choi, S.; Kim, N.; Sung, J.; Cho, P. Integration of graphite and silicon anodes for the commercialization of high-energy lithium-ion batteries. Angew. Chem. Int. Ed. 2020, 59, 110–135. [Google Scholar]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The success story of graphite as a lithium-ion anode material–fundamentals, remaining challenges, and recent developments including silicon (oxide) composites. Sustain. Energy Fuels 2020, 4, 5387–5416. [Google Scholar]
- Yang, Y.; Wu, S.; Zhang, Y.; Liu, C.; Wei, X.; Luo, D.; Lin, Z. Towards efficient binders for silicon based lithium-ion battery anodes. Chem. Eng. J. 2021, 406, 126807. [Google Scholar]
- Han, C.M. Advances in carbon coatings for current collectors in lithium-ion battery applications: Focus on three-dimensional carbon nanowalls. Coatings 2025, 15, 86. [Google Scholar] [CrossRef]
- Zhang, J.; Chan, L.; Gao, T.; Wang, Q.; Zeng, S.; Bian, H.; Lee, C.; Xu, Z.; Li, Y.; Lu, J. Bulk monolithic electrodes enabled by surface mechanical attrition treatment-facilitated dealloying. J. Mater. Chem. A 2016, 4, 15057–15063. [Google Scholar]
- Gnana Kumar, G.; Chung, S.H.; Raj Kumar, T.; Manthiram, A. A 3D graphene-carbon nanotube-Ni hierarchical architecture as a polysulfide trap for lithium-sulfur batteries. ACS Appl. Mater. Interfaces 2018, 10, 20627–20634. [Google Scholar] [PubMed]
- Jiang, J.; Nie, P.; Ding, B.; Wu, W.; Chang, Z.; Wu, Y.; Dou, H.; Zhang, X. Effect of graphene modified Cu current collector on the performance of Li4Ti5O12 anode for lithium-ion batteries. ACS Appl. Mater. Interfaces 2016, 8, 30926–30932. [Google Scholar] [PubMed]
- Chen, J.; Yang, J.; Cheng, M. Induced nanoscale roughness of current collectors enhances lithium-ion battery performances. J. Power Sources 2019, 430, 169–174. [Google Scholar]
- Kang, S.; Xie, H.; Zhang, W.; Zhang, J.; Ma, Z.; Wang, R.; Wua, X. Improve the overall performances of lithium-ion batteries by a facile method of modifying the surface of Cu current collector with carbon. Electrochim. Acta 2015, 176, 604–609. [Google Scholar]
- Yu, H.; Yang, H.; Yu, T.; Jin, Z.; Chen, Z. Enhanced interfacial bonding for boosting the performance of lithium-ion batteries through an etched ordered checkerboard patterns on current collectors. J. Energy Storage 2024, 84, 110919. [Google Scholar]
- Kim, H.; Choi, W. Graphene modified copper current collector for enhanced electrochemical performance of Li-ion battery. Scr. Mater. 2018, 146, 100–104. [Google Scholar]
- Wen, S.; Li, Z.; Zou, C.; Zhong, W.; Wang, C.; Chen, J.; Zhong, S. Improved performances of lithium-ion batteries by conductive polymer modified copper current collector. New J. Chem. 2021, 45, 10541–10548. [Google Scholar]
- Ventrapragada, L.K.; Creager, S.E.; Rao, A.M. Carbon nanotubes coated paper as current collectors for secondary Li-ion batteries. Nanotechnol. Rev. 2019, 8, 18–23. [Google Scholar]
- Jin, S.; Jiang, Y.; Ji, H.; Yu, Y. Advanced 3D current collectors for lithium-based batteries. Adv. Mater. 2018, 30, 1802014. [Google Scholar]
- Chu, H.; Tuan, H. High-performance lithium-ion batteries with 1.5 μm thin copper nanowire foil as a current collector. J. Power Sources 2017, 346, 40–48. [Google Scholar]
- Huang, Z.; Zhang, C.; Lv, W.; Zhou, G.; Zhang, Y.; Deng, Y.; Wu, H.; Kang, F.; Yang, Q. Realizing stable lithium deposition by in situ grown Cu2S nanowires inside commercial Cu foam for lithium metal anodes. J. Mater. Chem. A 2019, 7, 727–732. [Google Scholar]
- Fang, R.; Chen, K.; Yin, L.; Sun, Z.; Li, F.; Cheng, H. The regulating role of carbon nanotubes and graphene in lithium-ion and lithium-sulfur batteries. Adv. Mater. 2019, 31, 1800863. [Google Scholar]
- Pushnitsa, K.; Kosenko, A.; Chernyavsky, V.; Pavlovskii, A.; Novikov, P.; Popovich, A. Copper-coated graphite felt as current collector for Li-ion batteries. Coatings 2022, 12, 1321. [Google Scholar] [CrossRef]
- Jiang, G.; Jiang, N.; Zheng, N.; Chen, X.; Mao, J.; Ding, G.; Li, Y.; Sun, F.; Li, Y. MOF-derived porous Co3O4-NC nanoflake arrays on carbon fiber cloth as stable hosts for dendrite-free Li metal anodes. Energy Storage Mater. 2019, 23, 181–189. [Google Scholar]
- Zhou, J.; Xie, M.; Wu, F.; Wei, G.; Mei, Y.; Huang, R.; Tan, G.; Li, L.; Chen, R. Toward uniform Li plating/stripping by optimizing Li-ion transport and nucleation of engineered graphene aerogel. Chem. Eng. J. 2022, 427, 130967. [Google Scholar]
- Wang, K.; Luo, S.; Wu, Y.; He, X.; Zhao, F.; Wang, J.; Jiang, K.; Fan, S. Super-aligned carbon nanotube films as current collectors for lightweight and flexible lithium-ion batteries. Adv. Funct. Mater. 2013, 23, 846–853. [Google Scholar]
- Liao, A.; Zhu, W.; Chen, J.; Zhang, X.; Wang, C. Vertically aligned single-crystalline ultra-thin CuO nanosheets: Low-temperature fabrication, growth mechanism, and excellent field emission. J. Alloys Compd. 2014, 609, 253–261. [Google Scholar]
- Wen, Y.; Shao, L.; Zhao, P.; Wang, B.; Cao, G.; Yang, Y. Carbon coated stainless steel mesh as a low-cost and corrosion-resistant current collector for aqueous rechargeable batteries. J. Mater. Chem. A 2017, 5, 15752–15758. [Google Scholar]
- Wen, L.; Liang, J.; Liu, C.; Chen, J.; Huang, Q.; Luo, H.; Li, F. Li4Ti5O12 on graphene for high-rate lithium-ion batteries. J. Electrochem. Soc. 2016, 163, A2951–A2955. [Google Scholar]
- Jiang, Z.; Yang, D.; Li, C.; Chen, J.; Sui, Z.; Tian, Q. Bi2O3/Bi quasi-nanospheres in-situ embedded in N-doped porous carbon and reinforced via Bi-O-C bonding towards improved lithium storage performance. J. Alloys Compd. 2025, 1010, 177647. [Google Scholar] [CrossRef]
- Chen, J.; Cheng, Y.; Zhang, Q.; Luo, C.; Li, H.; Wu, Y.; Zhang, H.; Wang, X.; Liu, H.; He, X.; et al. Designing and understanding the superior potassium storage performance of nitrogen/phosphorus Co-doped hollow porous bowl-like carbon anodes. Adv. Funct. Mater. 2021, 31, 2007158. [Google Scholar] [CrossRef]
- Mae, T.; Kaneko, K.; Sakurai, H.; Noda, S. A stable full cell having high energy density realized by using a three-dimensional current collector of carbon nanotubes and partial prelithiation of silicon monoxide. Carbon 2024, 218, 118663. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Ren, M.; Lei, B.; Hou, Y.; Meng, W.; Zhao, D. 3D CNTs networks enable core-shell structured Si@Ni nanoparticle anodes with enhanced reversible capacity and cyclic performance for lithium ion batteries. Int. J. Hydrogen Energy 2021, 46, 16179–16187. [Google Scholar] [CrossRef]
- Park, S.; Copic, D.; Zhao, T.; Rutkowska, A.; Wen, B.; Sanders, K.; He, R.; Kim, H.; Volder, M. 3D porous Cu-composites for stable Li-metal battery anodes. ACS Nano 2023, 17, 14658–14666. [Google Scholar] [CrossRef]
- Itagaki, M.; Honda, K.; Hoshi, Y.; Shitanda, I. In-situ EIS to determine impedance spectra of lithium-ion rechargeable batteries during charge and discharge cycle. J. Electroanal. Chem. 2015, 737, 78–84. [Google Scholar] [CrossRef]
- Lv, C.; Tong, Z.; Zhou, S.; Pan, S.; Liao, H.; Zhou, Y.; Li, J. Spontaneous local redox reaction to passivate CNTs as lightweight current collector for high energy density lithium-ion batteries. J. Energy Chem. 2023, 80, 553–561. [Google Scholar] [CrossRef]
- Yang, D.; Li, C.; Jiang, Z.; Chen, J.; Chen, M.; Tian, Q. Preparation of porous carbon-coated SnO2 nanoplates and their improved lithium storage. Chem. Phys. Lett. 2024, 857, 141737. [Google Scholar] [CrossRef]
- Yu, W.; Liu, C.; Hou, P.; Zhang, L.; Shan, X.; Li, F.; Cheng, H. Lithiation of silicon nanoparticles confined in carbon nanotubes. ACS Nano 2015, 9, 5063–5071. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Jin, S.; Meng, J.; Sun, T.; Chen, C.; Fu, D.; Zhong, Y.; Dmytro, S.; Zhang, Q.; Ma, Q. Three-Dimensional Carbon Nanotube-Coated Copper Mesh as a Current Collector for Graphite Anodes in High-Performance Lithium-Ion Batteries. Processes 2025, 13, 964. https://doi.org/10.3390/pr13040964
Wang F, Jin S, Meng J, Sun T, Chen C, Fu D, Zhong Y, Dmytro S, Zhang Q, Ma Q. Three-Dimensional Carbon Nanotube-Coated Copper Mesh as a Current Collector for Graphite Anodes in High-Performance Lithium-Ion Batteries. Processes. 2025; 13(4):964. https://doi.org/10.3390/pr13040964
Chicago/Turabian StyleWang, Fangrui, Shan Jin, Junxia Meng, Tiankai Sun, Chaohui Chen, Dehao Fu, Yingxiang Zhong, Sydorov Dmytro, Qian Zhang, and Quanxin Ma. 2025. "Three-Dimensional Carbon Nanotube-Coated Copper Mesh as a Current Collector for Graphite Anodes in High-Performance Lithium-Ion Batteries" Processes 13, no. 4: 964. https://doi.org/10.3390/pr13040964
APA StyleWang, F., Jin, S., Meng, J., Sun, T., Chen, C., Fu, D., Zhong, Y., Dmytro, S., Zhang, Q., & Ma, Q. (2025). Three-Dimensional Carbon Nanotube-Coated Copper Mesh as a Current Collector for Graphite Anodes in High-Performance Lithium-Ion Batteries. Processes, 13(4), 964. https://doi.org/10.3390/pr13040964





