Abstract
The last decades have offered new challenges to researchers worldwide through the problems our planet is facing both in the environmental protection field and the need to replace fossil fuels with new environmentally friendly alternatives. Bioenergy, as a form of renewable energy, is an acceptable option from all points of view, and biofuels, due to their biological origin, have the ability to satisfy the new needs of humanity. As they release non-polluting combustion products into the atmosphere, biofuels have already been adopted as additives in traditional liquid fuels, intended mainly for the internal combustion engines of automobiles. The current work proposes an extension of the biofuel application in combustion processes specific to industrial furnaces. This technical concern has not been found in the literature, except for the achievements of the research team involved in this work, who performed the previous investigations. A 51.5 kW burner was designed to operate with glycerin originating from the triglycerides of plants and animals, mixed with ethanol, an alcohol produced by the chemical industry recently used as an additive in gasoline for automobile engines. Industrial oxygen was chosen as the oxidizing agent necessary for the liquid mixture combustion, allowing us to obtain much higher flame temperatures compared with the usual combustion processes using air. Mixing glycerin with ethanol in an 8.8 ratio allowed for growing flame stability, also accentuated by creating swirl currents in the flame through the speed regime of fluids at the exit from the burner body. Results were excellent in both the flame stability and low level of polluting emissions.
1. Introduction
Traditional combustion methods using fossil fuels have been the main method of converting the chemical energy contained in these fuels into heat since ancient times. This conversion is achieved by initiating the exothermic oxidation processes of the energetically active components of fossil fuels (mainly carbon). These combustion processes are the key to most industrial manufacturing processes based on the technological necessity of reaching high temperatures.
The excessive increase in these emissions has brought humanity to the brink of possible natural disasters, caused by the damage to the protective ozone layer [1]. Therefore, reducing the carbon footprint throughout all economic activities at the global level has become an important challenge of the current times.
According to Chen et al. [2], presently, the challenge of achieving carbon neutrality is very acute. Given that only less than 5% of countries worldwide have achieved zero carbon emissions, in 2021, the 26th Summit of the United Nations Climate Change Conference in Glasgow, UK, established a set of measures to control carbon emissions in order to obtain neutrality.
In the past decades, renewable power, including bioenergy, has come to constitute an important alternative for reducing the emissions of pollutants in the atmosphere and preserving the current natural energy resources that are in sharp decline.
Biological mass as a provenance of renewable energy has the particularity of being able to be turned directly into biofuel, unlike other known sources of renewable energy. The most well-known types of liquid biofuel used, particularly in transport, are ethanol and biodiesel, constituting the basis of biofuel technology [3].
Ethanol (C2H5OH) is prepared from some vegetable sources (biomass). In most cases, ethanol is extracted from the sugar and starch existing in plants. Recent research refers to the possibility of obtaining ethanol from cellulose and hemicellulose, which are available in high quantities in plant mass [4,5].
Mixed with gasoline, ethanol has the ability to increase its octane number and reduce the emission of carbon monoxide, which is responsible for the creation of smog [6]. According to the literature [7], in road transport, the most frequently used proportion of ethanol is 10%. Furthermore, there are known liquid fuels of this type in which the proportion of ethanol can even reach 85% [8].
The accentuated increase in crude diesel production has influenced the increase in the demand for glycerin [9,10]. As a result, at the end of the 1990s, the world glycerin market reached a very high level, with the supply of this biological product being much greater than the demand (about six times) [11]. Under these conditions, the glycerin price significantly dropped, and the need for new ways to capitalize it appeared [12].
Considering the problems related to the continuous deterioration of the environmental quality at the global level, the International Energy Agency is mainly focused on energy security, environmental protection, and economic development. The significant reduction in CO2 emissions in the atmosphere and the use of environmentally friendly alternatives are the main elements of environmental protection.
The Climate Change Summit, including solutions for the future, was scheduled to take place in Bucharest (Romania) from 15 to 17 October 2024. Renewable energy projects are essential to decarbonize economic activities. Climate changes, determined by the growing concentration of greenhouse gases in the atmosphere, are mainly due to the use of fossil fuels, which are currently recognized worldwide as a planetary menace. This recognition was recorded with global consensus at the Summit of the International Energy Agency in 2019 in Paris (France) [13] by the world’s nations.
An excellent alternative for diminishing greenhouse gas emissions (mainly CO2) is the use of biofuels, especially in internal combustion engines in the transport domain. As these are derived from vegetable mass, the burning of liquid biofuels occurs under advantageous ecological conditions [14,15,16].
Although the share of biofuels in the use of total renewable energy was very small until recently, the biofuel market has registered significant increases in recent years due to the increased concern regarding climate change, energy security, and the need for sustainable alternatives to fossil fuels [17,18].
The market is projected to continue growing with an annual growth rate of 5.7% [19]. One of the solutions to the forecasted increase includes environmental regulations in many countries worldwide, thus promoting the use of biofuels to reduce dangerous emissions. In addition, the technological development of biofuels is reflected in the accelerated growth of the market for these products [19].
The main areas of the world where the biofuel market (including ethanol, biodiesel, and other advanced biofuels) is intensively exhibited are North America, Europe, and the Asia-Pacific region [19].
Unlike the above-mentioned applications of biofuels in internal combustion engines, the current paper addresses the use of biofuels in oxy-combustion thermal processes in industrial furnaces. Our approach represents a concern that has not been encountered in the literature, except for the work published in 2022 by Paunescu et al. [20] (i.e., which included some authors of the present paper).
The above-mentioned article tested the use of glycerin as a polyol compound together with ethanol as an alcohol. The combustion of this liquid mixture atomized with water was performed by the addition of industrial oxygen. The glycerin/ethanol mass ratio had the value of 8.1, while the biofuel mix/oxygen flow ratio was 0.41 kg·(m3N)−1.
The effect of using these proportions in the content of CO2, CO, NO, and NO2 in the residual gases resulting from the combustion process showed the existence of extremely low contents (0.06–0.13 vol. % CO2, zero vol. % CO, less than 199 mg·(m3N)−1 NO, and under 210 mg·(m3N)−1 NO2).
As stated in [21], “Liquid biofuels like biodiesel and bioethanol are crucial in the transition to low-carbon and high-energy alternatives to fossil fuels”. One significant by-product of biodiesel production is glycerol, which accounts for about 10% of the total conversion output. While waste glycerol poses challenges due to its impurities and contaminants, it also holds potential as a metabolic resource for essential cellular components in microorganisms. Crude glycerol production is reviewed, highlighting relevance in current biodiesel technologies and its biochemical composition. To efficiently utilize waste glycerol, co-valorization with low-cost substrates through biocircular platforms using various microorganisms or insects for second- and third-generation oxy-biofuels has been explored. Among these, the black soldier fly larvae have demonstrated higher competitiveness for lipid contents (35–43%), making them a promising organism for recycling waste glycerol into biodiesel production, alongside microalgae and oleaginous yeast. The microbial biodiesel productivity from oleaginous yeast is notably higher (3546 kg ha−1 y−1) than soybean biodiesel (562 kg ha−1 y−1), while microalgal biodiesel productivity surpasses palm biodiesel by more than 25 times.
The objective of the paper was to continue the research on the combustion of biofuels for applications in oxy-combustion thermal processes, aiming to improve the performances previously obtained.
2. Methods and Materials
2.1. Methods
In the case of burning biofuels, the flame stability in operation depends on their concentration in aqueous solution. Thus, glycerin has a lower volatility in comparison with water, and ethanol has a higher volatility. This is why, in the case of using glycerin, water is predominantly transformed into vapor, while in the case of using ethanol, this biofuel is predominantly turned into vapor.
The operational stability of the flame with low vaporization compared with water is difficult to obtain under the conditions of using large amounts of water to atomize the liquid fuel. Increasing the flame stability of a biofuel with low volatility (e.g., glycerin) is possible by adding small amounts of ethanol. According to Yi and Axelbaum [17], satisfactory experimental results were obtained in the case of utilizing an aqueous solution of glycerin with a 30% concentration and the addition of 10% ethanol.
Generally, the basic constructive features of the achieved and tested burner in reference [20] were unchanged and maintained according to the scheme in Figure 1. One of the important elements of obtaining the stability of flame development remained the recirculation of biofuel and oxygen jets in the exit area from the burner by creating turbulence on the metallic frontal surface of the combustion equipment.
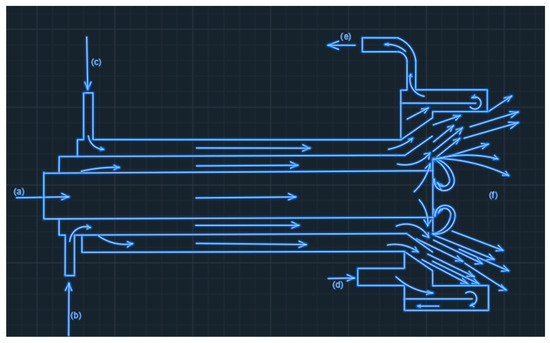
Figure 1.
Constructive and operational scheme of the oxy-biofuel burner. a—glycerin and ethanol mix; b—water for atomizing; c—oxygen; d—cold water entrance; e—water exit; f—propagated flame.
The method of swirling the flame by creating turbulence currents in the flame propagation is previously known from the literature [18,22,23], being especially applied to low calorific value gaseous fuels.
According to the scheme presented above, the supply of the liquid mixture of biofuels is carried out through a central pipe provided at the front end, with a metal plate that completely closes this end of the pipe. The output of the biofuel is made through several radial orifices placed near the closing plate.
The radial fuel jets meet the atomizing water flow, which circulates through an annular space between the fuel supply pipe and the oxygen pipe. The contact between the radial jets and the annular water jet, distributed with a high rate, finely sprays this mixture that is, at the same time, divergently oriented under an angle of 45 degrees to the axis of the burner.
The external annular jet for oxygen, also divergently oriented under the same value of the angle, is distributed in the area of the combustion chamber (intensively cooled with water). Due to the high speed of the water-atomized biofuel jets, a high enough depression is created in the area of the outer surface of metal plate, causing their partial recirculation.
Additionally, in the space between the divergent slope for the orientation of the oxygen circulation at the exit from the burner body and the cylindrical surface of the combustion chamber, conditions are created for the formation of recirculation currents of atomized biofuel and oxygen jets. The two zones in the burner head where the recirculation currents are produced play a major role in the functional stabilization process of the flame.
By design, energy fluids are supplied on concentric routes. Of these, the outer jet of oxygen is the fastest. Due to its superior speed, the annular jet of oxygen sucks the atomized oxy-fuel jet, thus ensuring an adequate mixture between them and excellent conditions for the combustion process to take place.
The combustion process is the result of the oxidation reactions of glycerin (1) and ethanol (2), both of which are characterized by intense heat release. The fact that we decided to use industrial oxygen instead of air or oxygen-enriched air allowed for the total elimination of nitrogen, which is an important inert component of air. Nitrogen does not participate in chemical reactions, and its negative role in combustion processes is to cool the combustion gases, lowering the process temperature [24].
The oxidation reactions of glycerin and ethanol are as follows:
C3H8O3 + 3.5O2 → 3CO2 + 4H2O
C2H5OH + 3O2 = 2CO2 + 3H2O
A burner testing stand (Figure 2) at the Romanian Metallurgical Research Institute SA was utilized to investigate the main thermal characteristics of the oxy-biofuel burner.
Figure 2.
Partial image of the burner testing stand.
2.2. Materials
As above-mentioned, glycerin and ethanol (0.7–1.3 E/l, [25]) were chosen as biofuels for this experiment. Industrial oxygen was adopted as the oxidant product able to supply the pure oxygen necessary for the thermal oxidation (i.e., burning) of the two fuels. Water, as an atomizing agent for the liquid biofuel mix, was used as a necessary component of the process.
Glycerin is naturally found in the triglycerides of plants and animals. The method of obtaining glycerin as a by-product includes several available processes, of which the most frequently used is hydrolysis [26]. Glycerin obtained from triglycerides is intensively used worldwide at an extremely advantageous price of up to USD 0.05 cents per kilogram [8].
Ethanol belongs to the alcohol class and is an important product of the chemical industry. Pure ethanol is a flammable liquid made using fermentation or hydration techniques of some chemical materials [27]. It is utilized as a solvent, in the synthesis processes of other organic chemical products as well as an additive in gasoline for diesel motor vehicles.
Both chemical products above-mentioned were commercially purchased and bottled. The mixture of the two liquids was previously carried out in a tank for the purpose of the experiment. The burner supply with this mixture was performed through an adequate hose with the help of a pump. The water from the industrial circuit was injected with a pressure pump into the burner body for fine atomization of the fuel mixture, using a hose for water transport.
The industrial oxygen adopted for biofuel combustion was provided by a small internal factory of the Metallurgical Research Institute, being taken over through a suitable flexible connection from the general distribution pipe.
2.3. Methods for Determining the Technical Performances of the Burner
The main tools utilized to determine the hourly flows of energy fluids were as follows: the Tec fluid flowmeter series DP 65 for glycerin and ethanol, LZQ-7 oxygen flow meter 3–30 LPM for oxygen, and the flow meter rotameter LS 32–600 for water. Measuring the flame temperature was conducted with a Pyrovar radiation pyrometer. The analysis of the chemical composition of the gas emission (especially CO, NO, and NO2) was carried out with a TESTO 350 analyzer.
The main technical specifications of the TESTO 350 analyzer are the follows:
- CO measuring range (H2 compensated): 0 to +10,000 ppm;
- CO low measuring range (H2 compensated): 0 to 500 ppm;
- NO measuring range: 0 to +4000 ppm;
- NO low measuring range: 0 to 300 ppm;
- NO2 measuring range: 0 to 500 ppm;
- SO2 measuring range: 0 to 50 00 ppm;
- Efficiency measuring range (Eta): 0 to +120%;
- Flue gas loss measuring range: 0 to +99.9%;
- Flue gas dew point measuring range: 0 to +99.9 °Ctd;
- H2S measuring range: 0 to 300 ppm;
- CO2 measuring range (infrared): 0 to +50 vol.%;
- Flow velocity measuring range: 0 to +40 m/s;
- Methane measuring range: 100 to 40,000 ppm;
- Propane measuring range: 100 to 21,000 ppm;
- Butane measuring range: 100 to 18,000 ppm.
3. Results and Discussion
3.1. Results
The constructive principle of the burner previously designed and tested by the team of Romanian researchers [20] was generally maintained in the current work. The nominal thermal power had the value of 51.5 kW.
The main difference between the nominal parameters of the improved burner and those of the reference burner was the new ratio between the nominal flow values of glycerin (10.10 kg·h−1) and ethanol (1.15 kg·h−1), which increased to 8.8 compared with 8.1. Therefore, the ratio growing (by about 8%) solution was adopted by slightly improving the glycerin share compared with ethanol.
In order not to lose the functional stability of the flame, we chose solutions for growing the recirculation volume of jets at the exit from the burner body by adopting higher speeds of fluids involved in the combustion process, in accordance with the functional principle of swirl burners [18,22].
The nominal technical parameters of the burner designed in this work are presented in Table 1.

Table 1.
Nominal parameters of the burner.
During the burner testing, eight thermal regimes with values included between the maximum and minimum limits were tried. The biofuel mix flow had values in the range of 6.08–11.33 m3N·h−1, the oxygen flow fell within the limits of 7.80–11.94 m3N·h−1, and the atomizing water flow had values between 47.40 and 82.71 kg·h−1.
The pollutant composition in waste gases (CO, NO, and NO2) after leaving the combustion chamber of the burner, temperature, and size of the flame for each of the tried thermal regimes are presented in Table 2.

Table 2.
Operational parameters of the oxy-biofuel burner.
According to the data in Table 2, the conditions of the biofuel combustion process using industrial oxygen led to much higher flame temperatures compared with the combustion processes that used air not enriched in oxygen. On the other hand, the influence of increasing the temperature of the combustion process on the formation of nitrogen oxides (NO and NO2) is known.
Despite the very high temperature of the flame (1790–1860 °C), compensated for by the formation of recirculation currents during the propagation of the flame, the amounts of NO and NO2 emitted into the atmosphere were relatively low, with maximums of 181 and 197 mg·m3N−1, respectively, which are accepted by the existing regulations regarding the limits of these emissions [28].
It is also remarkable that no traces of CO were found in any of the tested thermal regimes.
The flame corresponding to the biofuel mix combustion process using industrial oxygen had excellent functional stability across the entire minimum–maximum operating range. The strongly radiant aspect of the flame in all of the tried thermal regimes is highlighted in the images in Figure 3 (referring to the nominal regime—no. 2).
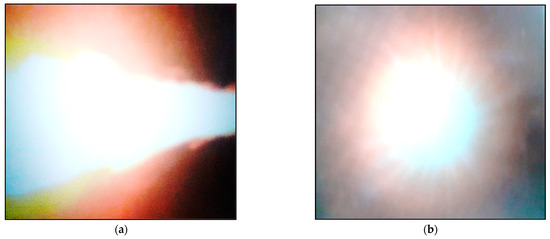
Figure 3.
Images of the flame propagation: (a) longitudinal image; (b) frontal image.
The picture shows a longitudinal image of the flame propagation (a) as well as a frontal image (b).
3.2. Discussion
The literature [29,30] considers, based on previous experimental results, that burning glycerin as the only fuel is not possible due to its high level of cohesion and self-ignition capability as well as the release of emissions with a high danger degree to health.
Instead, mixed in relatively small proportions with products of the chemical industry belonging to the alcohol class (such as methanol, butanol, ethanol), it was found that glycerin can become a biofuel with radically improved properties. The three mentioned products are usually used as solvents and intermediate chemicals for the manufacture of a large number of other products, but also as additives for the internal combustion engines in vehicles, ships, etc.
The authors of the current paper had previously carried out tests combining glycerin with ethanol, with the mass ratio between the two liquids being 8.1 [20]. As a result, the stability of the thermal intensified flame by using oxygen was significantly improved. The total and free glycerin content in the biofuel was below the limits allowed by international purity standards, with a final purity (UPS grade) of 99.7%.
The experiment described in the current paper continued further investigations in the field of oxy-biofuel burners by using the constructive principle of the previously tested combustion equipment, but by first modifying the glycerin/ethanol mass ratio to 8.8 as well as some functional elements (slightly higher speed of fluids in combustion zone) that were able to increase the flame swirling.
Testing under the experimental conditions of the modifications made to the burner showed their viability, leading to improved performances in the functional stability of the flame, its high radiant character, the increase in the flame temperature up to 1850 °C (at the nominal regime) and 1860 °C (at the maximum regime) as well as the decrease in the emission values of nitrogen oxides (between 154 and 182 mg·m3N−1 for NO and 169–197 mg·m3N−1 for NO2) compared with the results obtained in the first stage of the research [20].
The transition from applications of biofuel combustion in internal combustion engines (at an advanced stage) to testing their combustion for industrial thermal processes at high temperature constitutes the main original element of the work, and the experimental results are promising.
4. Conclusions
An oxy-biofuel burner intended for thermal processes at high temperature in industrial furnaces was designed, fabricated, and experimentally tested, representing a forward step compared with the current knowledge of applying biofuel combustion in internal combustion engines, which was the objective of the research presented in the paper.
The research was based on the fact that atomized liquid biofuel is not a viable option for combustion processes when used alone, without mixing with an alcohol (methanol, butanol or ethanol), due to the poor functional stability of the flame. In the current work, glycerin as a biofuel was used in a mixture with ethanol (in mass ratio 8.8) and subjected to combustion in the presence of industrial oxygen for the thermal intensification of the flame.
On the other hand, the method, known for more than 50 years of favoring the stability of the flame by swirling it and applied in burners operating with gaseous fuels, was also surpassed in the case of burning biofuels.
The experimental results confirmed the viability of the adopted solution by obtaining excellent stability of the flame in operation, increasing its temperature, and achieving a low level of nitrogen oxide emissions (NO and NO2).
Author Contributions
Conceptualization, A.I. and L.P.; methodology, L.P.; validation, N.C., formal analysis, A.S.; investigation, I.L.C. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by the University Politehnica of Bucharest—PubArt Programme supporting scientific articles and communications publication.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Andres, R.J.; Boden, T.A.; Bréon, F.M.; Ciais, P.; Davis, S.; Erickson, D.; Gregg, J.S.; Jacobson, A.; Marland, G.; Miller, J.; et al. A synthesis of carbon dioxide emissions from fossil-fuel combustion. Biogeosciences 2012, 9, 1845–1871. [Google Scholar] [CrossRef]
- Chen, L.; Msigwa, G.; Yang, M.; Osman, A.I.; Fawzy, S.; Rooney, D.W.; Yap, P. Strategies to achieve a carbon neutral society: A review. Environ. Chem. Lett. 2022, 20, 2277–2319. [Google Scholar] [CrossRef] [PubMed]
- Biofuel Basics. Office of Energy Efficiency & Renewable Energy—US Department of Energy. 2023. Available online: https://www.energy.gov/eere/bioenergy/biofuel-basics (accessed on 23 February 2025).
- Sharmila, S.; Jeyanthi, L.R.; Ankush, S.D.R.; Kowsalya, E. Extraction of bioethanol from plant leaves. Pharm. Lett. 2016, 8, 97–99. [Google Scholar]
- Chandrakart, P.; Bisaria, V.S. Simultaneous bioconversion of cellulose and hemicellulose to ethanol. Crit. Rev. Biotechnol. 1998, 18, 295–331. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.; Ko, Y.; Huang, C.; Chiang, H. Effects of blending ethanol and gasoline on the performance of motorcycle catalysts and airborne pollutant emissions. Aerosol Air Qual. Res. 2019, 19, 539. [Google Scholar] [CrossRef]
- U.S. Department of Energy’s Office of Energy Efficiency and Renewable Energy. Alternative Fuels Data Center. Ethanol Fuel Basics. 2023. Available online: https://afdc.energy.gov/fuels/ethanol-fuel-basics (accessed on 15 February 2025).
- Kohse-Höinghaus, K.; Osswald, P.; Cool, T.A.; Kasper, T.; Hansen, N.; Qi, F.; Westbrook, C.K.; Westmoreland, P.R. Biofuel combustion chemistry: From ethanol to biodiesel. Angew. Chem. Int. Ed. 2010, 49, 3572–3579. [Google Scholar] [CrossRef] [PubMed]
- Nicol, R.W.; Marchand, K.; Lubitz, W.D. Bioconversion of crude glycerol by fungi. Appl. Microbiol. Biotechnol. 2012, 93, 1865–1875. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture. US Renewable Diesel Production Growth Drastically Impacts Global Feedstock Trade; International Agricultural Trade Report; US Department of Agriculture: Washington, DC, USA, 2024. [Google Scholar]
- Christoph, R.; Schmidt, B.; Steinberner, U.; Dilla, W.; Karinen, R. Glycerol. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; Volume 17, pp. 67–82. [Google Scholar]
- Cornejo, A.; Barrio, I.; Campoy, M.; Lazaro, J.; Navarrete, B. Oxygenated fuel additives from glycerol valorization. Main production pathways and effects on fuel properties and engine performance: A critical review. Renew. Sustain. Energy Rev. 2017, 79, 1400–1413. [Google Scholar] [CrossRef]
- IEA. World Energy Outlook. Report Extract Data; The International Energy Agency (IEA): Paris, France, 2019. [Google Scholar]
- Agarwal, A.K. Biofuels (alcohols and biodiesel) applications as fuels for internal combustion engines. Prog. Energy Combust. Sci. 2007, 33, 233–271. [Google Scholar] [CrossRef]
- Caliskan, H.; Yildiz, I.; Mori, K. Chapter 10, Biofuels combustion in internal combustion engines. In Advances in Biofuels Production, Optimization and Applications; Elsevier: Amsterdam, Netherlands, 2024; pp. 185–205. [Google Scholar]
- Ramos, J.L.; Valdivia, M.; Garcia-Lorente, F.; Segura, A. Benefits and perspectives on the use of biofuels. Microb. Biotechnol. 2016, 9, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Yi, F.; Axelbaum, R.L. Oxy-combustion of low-volatility liquid fuel with high water content. Energy Fuels 2015, 29, 1137–1142. [Google Scholar] [CrossRef]
- Mihaescu, L.; Cristea, E.D.; Panoiu, N. Swirl Burners: Theory, Construction, Use, 1st ed.; Technical Publishing House: Bucharest, Romania, 1986. [Google Scholar]
- ReportLinker. Global Biofuel Market Overview, October 2024–2028. 2024. Available online: https://www.reportlinker.com/market-report/Bioenergy/132956/Biofuel?term=biofuel%20outlook (accessed on 10 February 2025).
- Paunescu, L.; Surugiu, G.; Volceanov, E. Experimentally use of glycerol as a biofuel in oxy-combustion thermal process. Nonconvent. Technol. Rev. 2022, 26, 22–27. [Google Scholar]
- Elsayed, M.; Eraky, M.; Osman, A.I.; Wang, J.; Farghali, M.; Rashwan, A.; Yacoub, I.; Hanelt, D.; Abomohra, A. Sustainable valorization of waste glycerol into bioethanol and biodiesel through biocircular approaches: A review. Environ. Chem. Lett. 2024, 22, 609–634. [Google Scholar] [CrossRef]
- Froud, D.Y.; Syred, N. Characterisation of industrial swirl burners for efficient combustion of low calorific value gases. In Proceedings of the Institute of Energy Conference Held, London, UK, 4–5 December 1995. [Google Scholar]
- Basu, P.; Kefa, C.; Jestin, L. Swirl burners. In Boilers and Burners; Mechanical Engineering Series; Springer: Boston, MA, USA, 2000; pp. 212–242. [Google Scholar]
- Çengel, Y.A.; Boles, M.A.; Kanoglu, M. Thermodynamics: An Engineering Approach, 10th ed.; McGraw Hill: New York, NY, USA, 2024; ISBN 9781266664489. [Google Scholar]
- eMAG. Available online: https://www.emag.ro/search/etanol (accessed on 5 February 2025).
- Pei, S.K.; Mohamed, K.A.; Dand, A.W.; Mohd, W. Conversion of crude and pure glycerol into derivates: A feasibility evaluation. Renew. Sustain. Energy Rev. 2016, 63, 533–555. [Google Scholar] [CrossRef]
- Britannica. Ethanol—Chemical Compound. Available online: https://www.britannica.com/science/ethanol (accessed on 15 August 2022).
- Moron, W.; Rybak, W. NOx and SO₂ emissions of coals, biomass and their blends under different oxy-fuel atmospheres. Atmos. Environ. 2015, 116, 65–71. [Google Scholar] [CrossRef]
- Bohon, M.D.; Metzger, B.A.; Linak, W.P.; King, C.J.; Roberts, W.L. Glycerol combustion and emissions. Proc. Combust. Inst. 2011, 33, 2717–2724. [Google Scholar] [CrossRef]
- Stainmetz, S.A.; Herrington, J.S.; Winterrowd, C.K.; Roberts, W.L.; Wendt, J.O.L. Crude glycerol combustion: Particulate, acrolein, and other volatile organic emissions. Proc. Combust. Inst. 2012, 34, 2749–2757. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).