Abstract
Carvedilol (CARV) is a nonselective beta and alpha-1 adrenoceptor antagonist commonly indicated for chronic heart failure and hypertension. Its clinical potential is limited by its low aqueous solubility, resulting in poor bioavailability. Encapsulation of CARV by cyclodextrins (CDs) was performed to exceed its solubility-related barriers. This study examines the impact of the CD type and ethanol, as a co-solvent used in the preparation step, on the complexation of CARV with two β-CD derivatives. The inclusion complexes (ICs) were prepared employing the kneading method and investigated using different analytical techniques, including thermoanalytical methods, powder X-ray diffractometry (PXRD), universal attenuated total reflectance Fourier transform infrared (UATR-FTIR) spectroscopy, UV spectroscopy and saturation solubility studies. The binary products of CARV with heptakis(2,6-di-O-methyl)-β-cyclodextrin (DM-β-CD) and randomly methylated β-cyclodextrin (RM-β-CD) exhibit different thermal behavior, different FTIR spectral and diffractometric profiles from those of the parent compounds, emphasizing the interaction between the components and the IC formation. CARV solubility increased 1.78 to 3.32 times as a result of drug complexation with CDs. Analytical data indicate a significant influence of both solvent systems and CD type on the IC solubility, highlighting the CARV/DM-β-CD IC as a promising entity for further research to obtain new formulations containing CARV with improved bioavailability.
1. Introduction
In the pharmaceutical sciences, solubility is a crucial and challenging subject in drug discovery and development research. Several factors, including solubility, dissolution rate, drug permeability and drug metabolism before reaching the systemic bloodstream, account for this. Among these factors, low water solubility and low permeability are the main causes of low bioavailability of drugs [1,2,3,4]. It has been estimated that approximately 40% of marketed pharmaceuticals and between 70 and 90% of drugs in development are poorly soluble, leading to poor bioavailability, reduced therapeutic effects and increased doses. Consequently, solubility must be a consideration in the development and production of pharmaceuticals [4]. Therefore, optimizing the solubility of poorly water-soluble drugs is vital to obtain a bioavailable and therapeutically effective product [2].
Cyclodextrins (CDs) represent cyclic oligosaccharides constituted of (α-1,4)-linked α-D-glucopyranose units, post-processed following starch conversion by specific bacteria, such as Bacillus macerans. The geometrical organization of CDs indicates that they possess a hydrophobic interior, which enables the entrapment of hydrophobic molecules, and a hydrophilic exterior, which results in enhanced solubility [5,6,7]. Structurally, both the primary and secondary hydroxyl groups, localized on the narrower, respectively the wider, side of the CD ring, provide the hydrophilic exterior behavior of CDs, ensuring water solubility. In addition, the methylene groups and anomeric oxygen atoms contribute to the hydrophobic cavity that assures environmental safety. The mentioned elements provide CDs the ability to interact with molecules characterized by low water solubility. CDs exhibit a strongly negative Log P o/w, as a result of their hydrophilic outer surface as well as an abundance of hydrogen bond donor acceptors. Notwithstanding their stability under alkaline conditions, CDs show sensitivity toward acid hydrolysis in aqueous solutions at low pH levels, causing the opening of the ring and the subsequent genesis of diverse linear oligosaccharides and glucose units. Around pH 12, the hydroxyl groups connected to the ring eventually begin to be deprotonated [8,9,10].
Research has been devoted extensively to the investigation of CDs in recent decades, emphasizing their large-scale potential use in the pharmaceutical, biomedical, food, cosmetics, textile and chemical industries, agricultural production, as well as for analytical aims [6,10,11]. The pharmaceutical industry has been embracing CDs based on their properties as complexing agents in order to increase the solubility, stability and bioavailability of active ingredients characterized as having low water solubility [7,12]. Furthermore, from the perspective that CDs minimize the quantity of free drug in solution, another important application of CDs is to promote the conversion of liquid oils and drugs into microcrystalline or amorphous powders, with the aim of diminishing or removing unpleasant odors or tastes and preventing drug–drug or drug–additive interactions encountered in a formulation [13].
Alpha (α-CD), beta (β-CD) and gamma (γ-CD) CDs, which contain 6, 7 and 8 glucopyranose units, respectively, appear to be the most widespread CDs. β-CD has acquired pharmaceutical relevance due to its economic convenience and internal cavity size appropriate for a large number of molecules with biological activity, although it has limited solubility in water [10,14]. To overcome this limitation, derivatives of β-CD have been successfully designed, including methylated β-CDs, such as heptakis(2,6-di-O-methyl)-β-cyclodextrin (DM-β-CD) and randomly methylated β-cyclodextrin (RM-β-CD) (Figure 1a), which possess enhanced water solubility and encapsulation abilities [15,16].
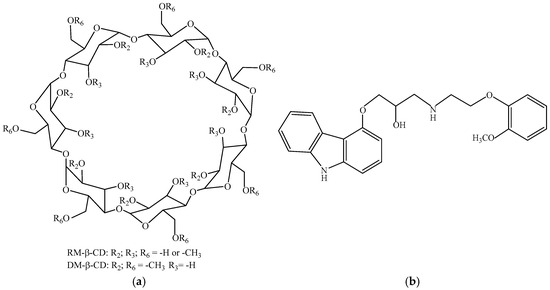
Figure 1.
Chemical structures of DM-β-CD and RM-β-CD (a) and CARV (b).
Carvedilol (CARV), (1-(9H-carbazol-4-yloxy)-3-[2-(2-(2-methoxyphenoxy)ethylamino]propan-2-ol) (Figure 1b), is a carbazole derivative known as an adrenergic antagonist notable for its nonselective beta and alpha-1 receptor blocking effects and also possessing vasodilator and Ca2+ channel blocking activity [17,18]. It is used to treat congestive heart failure, left ventricular dysfunction following myocardial infarction, angina [18] and hypertension [19]. CARV also exhibits antioxidant and cardioprotective properties [20], antimicrobial activity, especially against Gram-positive bacteria [21,22], anti-inflammatory, anti-tumor and anti-angiogenic properties [23], and shows the ability to protect cochlear cultures from aminoglycoside-induced damage [24]. Recent studies highlight its potential to be repurposed for skin cancer chemoprevention [25,26]. One of the serious drawbacks of CARV entails the administration of a high oral dose to reach the therapeutic action as a result of its slow dissolution rate caused by low aqueous solubility, explaining weak absorption in the gastrointestinal tract. CARV displays high membrane permeability, justifying its classification in class II according to the Biopharmaceutical Classification System [27,28]. Moreover, CARV exhibits polymorphism, which may affect its solubility, dissolution rate and therefore its bioavailability [29,30].
Work was undertaken in an attempt to overcome the issue of CARV solubility via a series of approaches, exemplified by physical and chemical adjustments to the preparation, including transforming the crystalline species to an amorphous one and reducing the number of particles in order to enhance effective surface area, porosity as well as wettability [28,31]. Additionally, some methods have been aimed at decreasing the hydrophobicity of CARV by creating microemulsions [32], carboxylate carbon microparticles [33], lipid nanoparticles [32], self-emulsifying and self-microemulsifying systems [34], cyclodextrin complexes [35,36] and solid polymer dispersions [37].
CDs are capable of establishing inclusion complexes (ICs) with drug substances through entrapment of the molecule, or a lipophilic moiety of the molecule, by the central cavity. During the formation of the complex, effects such as the formation or breakage of covalent bonds are not present, allowing the drug molecules in the complex to maintain rapid balance with the free molecules in solution. Several factors, including the release of high-enthalpy water molecules from the cavity, hydrogen bond formation, Van der Waals interactions and charge transfer, determine the development of the complex [8,10].
The solvent serves as a medium and plays an active role in a large number of chemical reactions. Both inorganic and coordination chemistry traditionally accept water as the main solvent. However, for today’s solution chemistry, the solvent is a critical element, being a medium but at the same time having unique chemical properties. In the chemical process, both complexation and solvation are involved, as a result of the transfer of electron pairs and the subsequent creation of donor–acceptor bonds following the bonding of atomic–molecular particles. However, in recent studies, the combination of co-solvent and CD has gained considerable scientific interest. The co-solvent facilitates better solubilization of the reactants, further aiding the complexation process, simultaneously minimizing water–water interactions and instead eliminating non-polar solutions [38]. In the literature, there are studies investigating the inclusion complex formation of native CDs and their derivatives in mixed solvents, such as water–alcohol [39,40,41,42].
The concomitant presence of a co-solvent and CDs may exert either a synergistic or agonistic effect on both the solubility of the drug substance and the complexation efficiency, depending on the dominant promoting or destabilizing effect. The literature suggests that only β-CD and hydroxypropyl-β-cyclodextrin (HP-β-CD) have been widely studied for evaluating co-solubilization using co-solvents, but the impact of CD type in relation to co-solvent influence remains a less addressed topic [42].
The purpose of the current study was to obtain, characterize and compare the host–guest ICs of CARV with two β-CD derivatives and to investigate the manner how ethanol, used as a co-solvent in the preparation step, as well as CD type, impacts the physicochemical properties of the ICs in the solid state. Encapsulation of the drug substance with CDs, which consisted of DM-β-CD and RM-β-CD, was performed in order to overcome the limited solubility of CARV, which was predicted to provide an optimized biopharmaceutical profile. The ICs were formulated by the kneading method and analyzed by various analytical techniques, including thermoanalytical methods (TG-thermogravimetry/DTG-derivative thermogravimetry), powder X-ray diffractometry (PXRD), universal attenuated total reflectance Fourier transform infrared (UATR-FTIR) spectroscopy, UV spectroscopy and saturation solubility studies.
2. Materials and Methods
2.1. Materials
CARV used in this study was obtained from Sigma-Aldrich, St. Louis, MI, USA, with a purity in conformity with the quality standard of the European Pharmacopoeia (CAS 72956-09-3, MDL number: MFCD00864692). The CDs, randomly methylated β-cyclodextrin (DS~12) (CY-2004.1) and heptakis(2,6-di-O-methyl)-β-cyclodextrin (CYL-4668), were obtained from Cyclolab R&L Ltd. (Budapest, Hungary). The substances were used as received. For the present study, all chemicals and reagents used were of analytical grade.
2.2. Preparation of the Solid Binary Systems
To achieve the formation of ICs of CARV with DM-β-CD and RM-β-CD based on a molar ratio of 1:1 of CARV/CD, the kneading method was applied. In order to obtain CARV/DM-β-CD ICs in the absence of ethanol (ICD1), weighed quantities of 0.1406 g CARV and 0.4608 g DM-β-CD were used, and for the CARV/RM-β-CD IC in the absence of ethanol (ICR1), weighed quantities of 0.1414 g CARV and 0.4585 g RM-β-CD were used, for which the product mixture was triturated vigorously in an agate mortar with distilled water. A similar approach was used to obtain CARV/DM-β-CD and CARV/RM-β-CD ICs in the presence of ethanol (ICD2 and ICR2) and the weighed parent quantities were 0.1412 g CARV with 0.4623 g DM-β-CD, and 0.1414 g CARV with 0.4585 g RM-β-CD, respectively. The mixture was ground energetically in an agate mortar using a distilled water/ethanol absolute (1:1, m/m) as the solvent. Further, the paste was kneaded for 45 min, with the necessary quantity of solvent added throughout the kneading process to preserve the consistency of the paste; the products were then dried at room temperature, followed by oven drying at 40 °C for 24 h. The samples were subsequently pulverized and passed through a 75 µm sieve.
Physical mixtures (PMs) of CARV with DM-β-CD (DPM) and RM-β-CD (RPM) were the result of direct mixing of the ingredients according to their molar ratio, as in the kneaded products (KPs), using an agate mortar for 10 min by stirring slightly with a spatula. Afterwards, the product was passed through a 75 µm sieve.
2.3. Determination of the Stability Constant and Binding Ratio
The values of the stability constants and the binding ratio of CARV to CDs were assayed by UV-Vis spectrophotometry associated with the Benesi–Hildebrand equation. A stock solution composed of CARV and a distilled water/ethanol absolute (1:1, m/m) mixture was obtained. Increasing concentrations of each CD (from 0 to 4 × 10−3 M) were added in a slow manner under magnetic stirring and reacted for 12 h. The spectrophotometric method was applied by measuring the absorbance of CARV in the presence of increasing concentrations of CDs and also in the absence of CDs. The same procedure was applied to the stock solution composed of CARV and distilled water. The stability constant value and inclusion ratio were determined following Formula (1) to determine an inclusion ratio of 1:1, and Formula (2) for an inclusion ratio of 1:2 [43]:
where Δε represents the change in molar attenuation coefficient, ΔA defines change in absorbance and K is the stability constant.
2.4. Encapsulation Efficiency and Loading Efficiency Analysis
An accurate amount of IC CARV/CD (0.0104 g ICD1, 0.0101 g ICD2, 0.0102 g ICR1 and 0.0103 g ICR2) was measured and stirred with 5 mL ethanol in a 10 mL volumetric flask. Subsequently, the solvent was added progressively until the mark was achieved, followed by sonication of the solutions for 10 min. Samples were subjected to filtration through a 0.45 µm nylon disk filter and suitably diluted for drug substance measurement employing UV spectrophotometric measurements.
Encapsulation efficiency (EE) and loading efficiency (LE) were quantified using Equations (3) and (4) [44,45,46]:
where WE stands for the quantity of encapsulated CARV, WT indicates the total amount of CARV that was added at the incipient preparation step of each IC and WI is refers to the mass of each IC.
2.5. Thermal Analysis
The thermal behavior of CARV, CDs, PMs and ICs obtained was investigated by TG and DTG measurements performed on the NETZSCH TG 209F1 Libra (Selb, Germany). For dynamic assessment, samples with masses of approximately 3–4 mg were heated in an air atmosphere with a flow rate of 100 mL min−1 at a temperature range of 30–450 °C, under a heating rate of 10 °C min−1, in alumina pans.
2.6. Powder X-Ray Diffraction
The PXRD pattern of pure substances (CARV, CDs) and ICs, as well as of PMs, was recorded at ambient temperature using a Bruker D8 Advance powder X-ray powder diffractometer (Karlsruhe, Germany). The PXRD data were gathered rover the angular range 2θ = 3–45°, using CuK radiation (40 kV, 40 mA) and a Ni filter.
2.7. FTIR Spectroscopy
The UATR-FTIR spectra of the CARV, CDs and binary systems, respectively, were carried out using a Shimadzu IRTracer-100 FT-IR (Kyoto, Japan) spectrometer equipped with ATR to assess the interaction of the drug substance with CDs. Data were collected from solid samples, in the wavenumber range of 4000–400 cm−1, were generated after 16 co-added acquisitions at a resolution of 2 cm−1.
2.8. Solubility Profile After Complexation
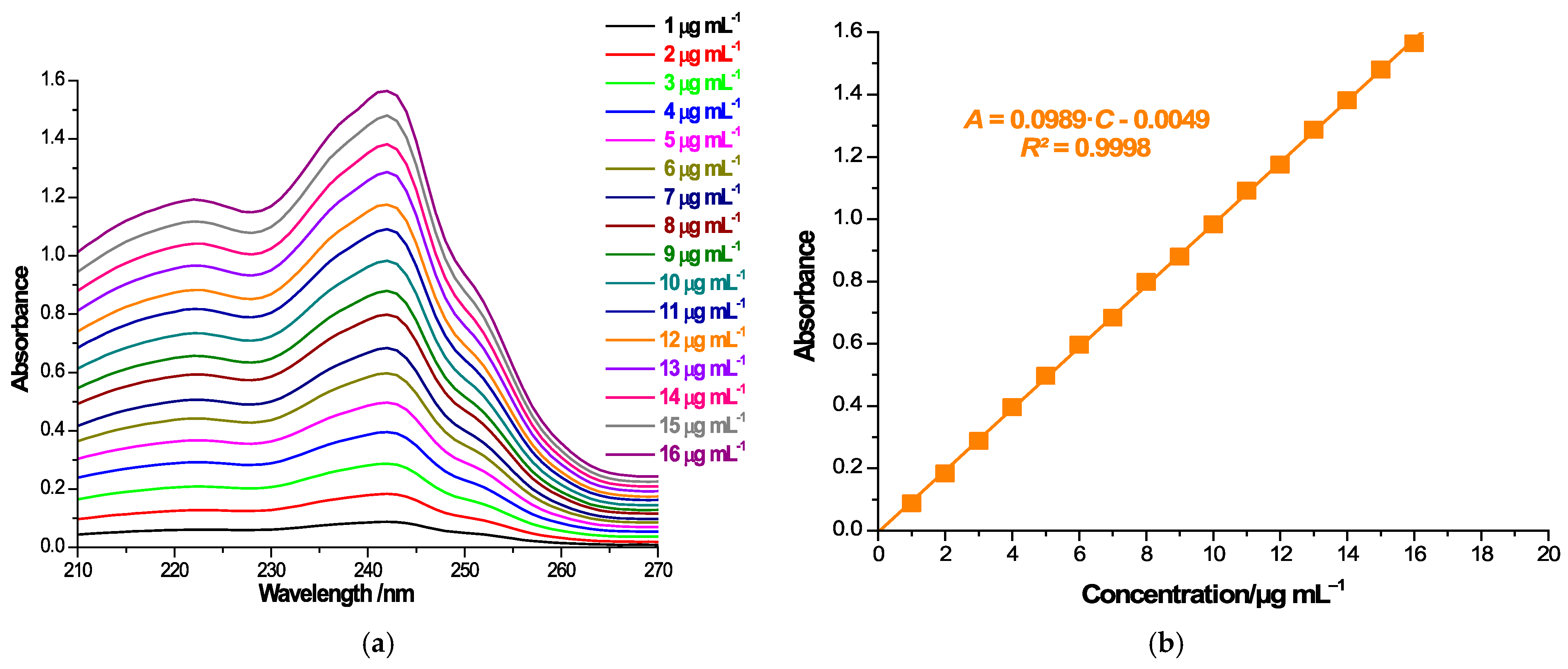
As an assessment of the manner in which CD complexation impacts the solubility of CARV, we used the shake-flask method. Therefore, a surplus quantity of CARV, CARV/CD PMs and KPs was introduced into 4 mL of 0.1 M phosphate buffer, pH 6.8, to reach saturation conditions. Once stirring was completed for 24 h at room temperature, the samples were filtered by a 0.45 µm nylon disk filter and properly diluted, and further assayed for UV spectrophotometric measurements at 242 nm. CARV quantification was performed using calibration curve. For this purpose, a set of CARV samples was prepared in 0.1 M phosphate buffer over the concentration interval of 1–16 µg mL−1 and measured using a Jena Analytik Specord 250 Plus (Jena, Germany) double-beam spectrophotometer at a 200–400 nm spectral range to achieve the wavelength of maximum absorbance. All solution absorbance values were reported at the wavelength of maximum absorbance of 242 nm. The equation of the calibration curve was generated with the absorbance values (A) of the CARV solutions plotted as a function of their concentration (C): A = 0.0989∙C (µg mL−1) − 0.0049. To determine if the differences between the solubility profiles of the ICs obtained are statistically significant, the MedCalc® Statistical Software version 22.016 (MedCalc Software Ltd., Ostend, Belgium) was used.
3. Results and Discussion
3.1. Determination of the Stability Constant and Binding Ratio
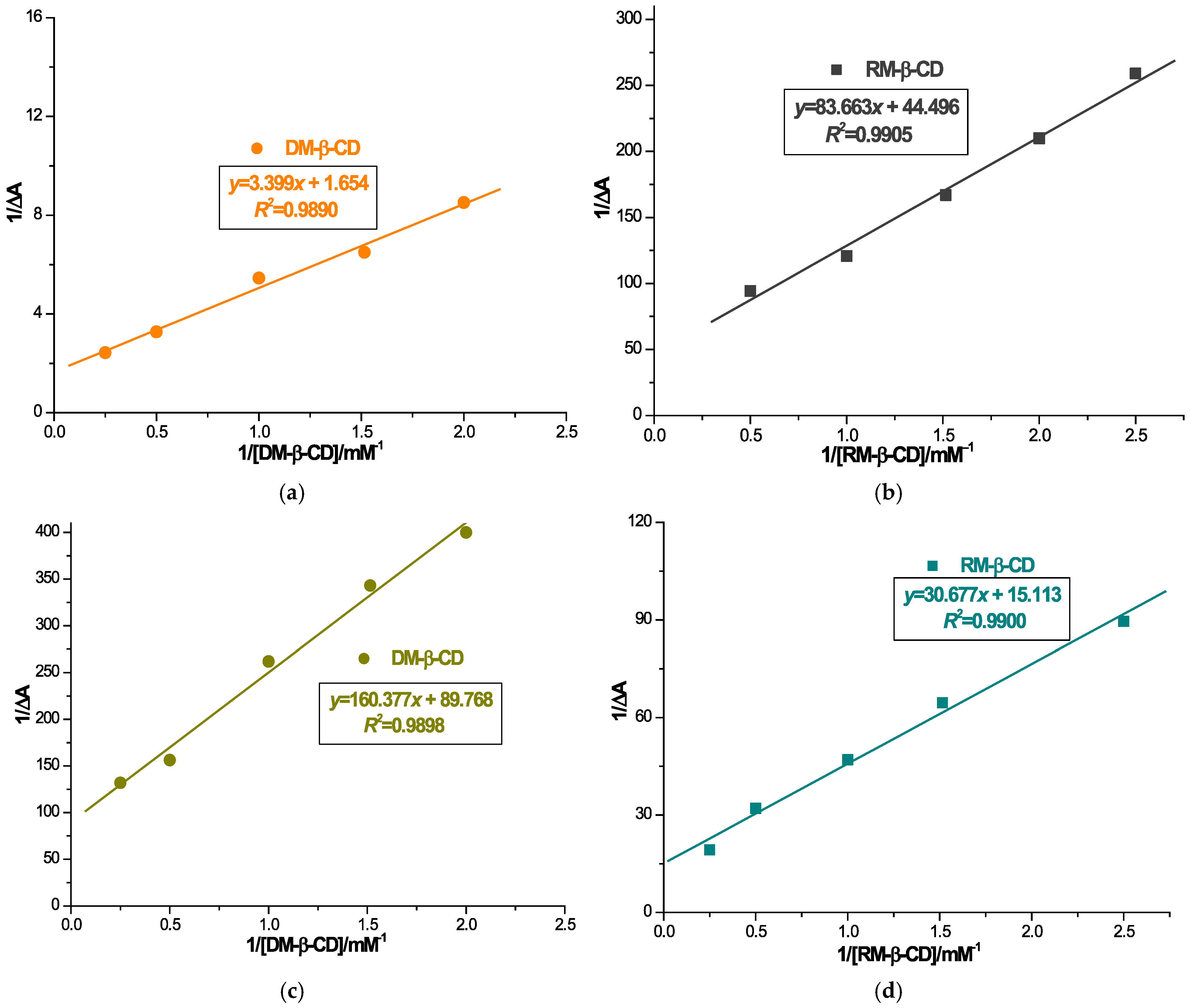
The binding ratio of CARV to DM-β-CD and of CARV to RM-β-CD was determined by inverse double curve plotting 1/ΔA vs. 1/[CD] and 1/[CD]2 using the Benesi–Hildebrand equation. Based on Table 1, the fitting correlation coefficient of the first-order Benesi plot is higher than that of the second-order fitting correlation coefficient (Figure S1). Hence, this brings us to the conclusion that the stoichiometry of the inclusion complex is 1:1. Results indicated that CARV formed ICs with both CDs in a 1:1 ratio.

Table 1.
Fitting correlation coefficients of 1/ΔA vs. 1/[CD] and 1/[CD]2.
Binding constant values were assessed employing the Benesi–Hildebrand Equation (1) as a ratio of the intercept to the slope of the straight line utilizing the Benesi–Hildebrand double reciprocal diagram (Figure 2). The stability constant values were assessed to be 486.614 ± 3.958 M−1 for ICD1, 559.731 ± 5.541 M−1 for ICD2, 531.848 ± 4.137 M−1 for ICR1 and 492.649 ± 4.015 M−1 for ICR2 (as shown in Table 2). Analysis of the stability constants values suggests a potential competitive interaction of alcohol with CARV to occupy the RM-β-CD cavity, whereas in the case of DM-β-CD, ethanol shows an effect promoting the interaction of CARV with CD. The determination of the stability constant values provides insights into the binding strength between the guest and CD. Values in the range of 100–5000 M−1 are considered optimal for the formation of an IC, favorably influencing bioavailability [47]. Results of the stability constants of both CARV ICs support the suitable stability of the ICs, as well as their feasibility in enhancing CARV solubility.
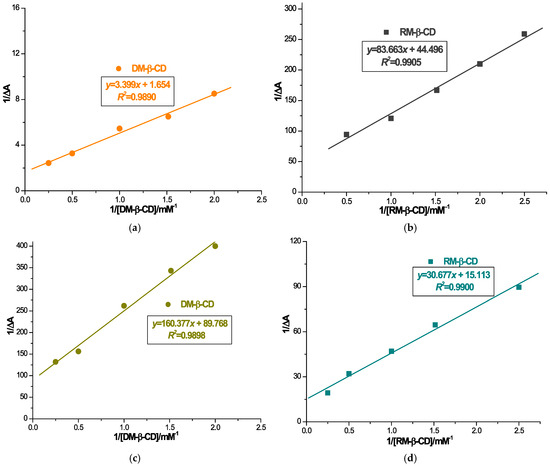
Figure 2.
Benesi–Hildebrand linear plot for 1/ΔA vs. 1/[DM-β-CD] in distilled water (a); 1/ΔA vs. 1/[RM-β-CD] in distilled water (b); 1/ΔA vs. 1/[DM-β-CD] in distilled water/ethanol absolute (1:1, m/m) (c); 1/ΔA vs. 1/[RM-β-CD] in distilled water/ethanol absolute (1:1, m/m) (d).

Table 2.
Binding constants of ICs.
3.2. Encapsulation Efficiency and Loading Efficiency Analysis
EE is expressed as the percentage by mass of encapsulated CARV in relation to the total quantity of drug that was added to the inclusion complex, and LE defines the mass ratio of entrapped CARV to the mass of the IC. Determination of the content of CARV in the CARV/CD ICs was performed by spectrophotometric evaluation employing the calibration curve. The EE and LE values were calculated as 82.66 ± 3.06% and 19.60 ± 1.56% for ICD1, 92.43 ± 3.18% and 21.00 ± 1.61% for ICD2, 88.51 ± 3.24% and 21.88 ± 1.59% for ICR1 and 88.36 ± 2.23% and 20.67 ± 1.84% for ICR2, respectively.
3.3. Thermal Analysis
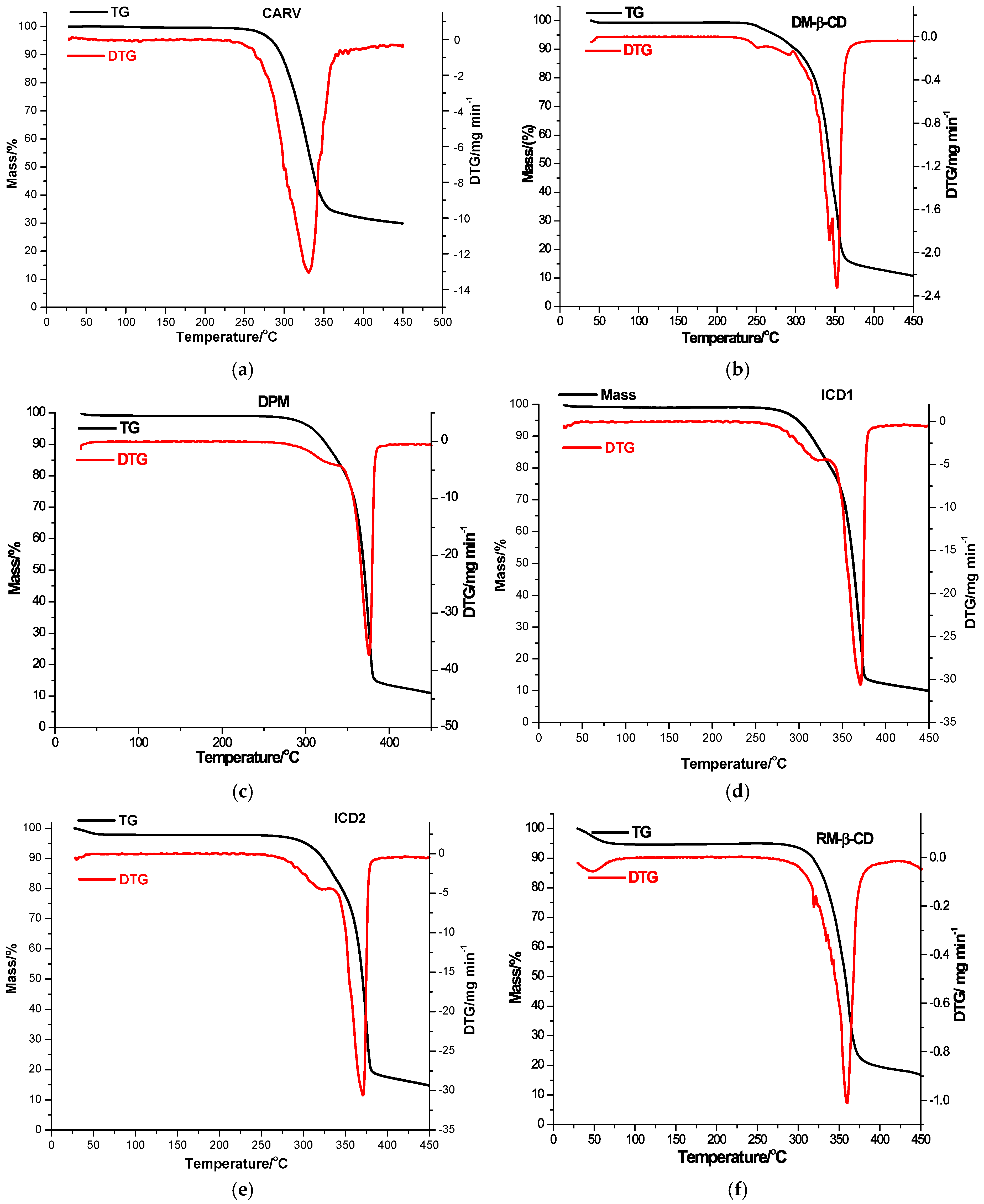
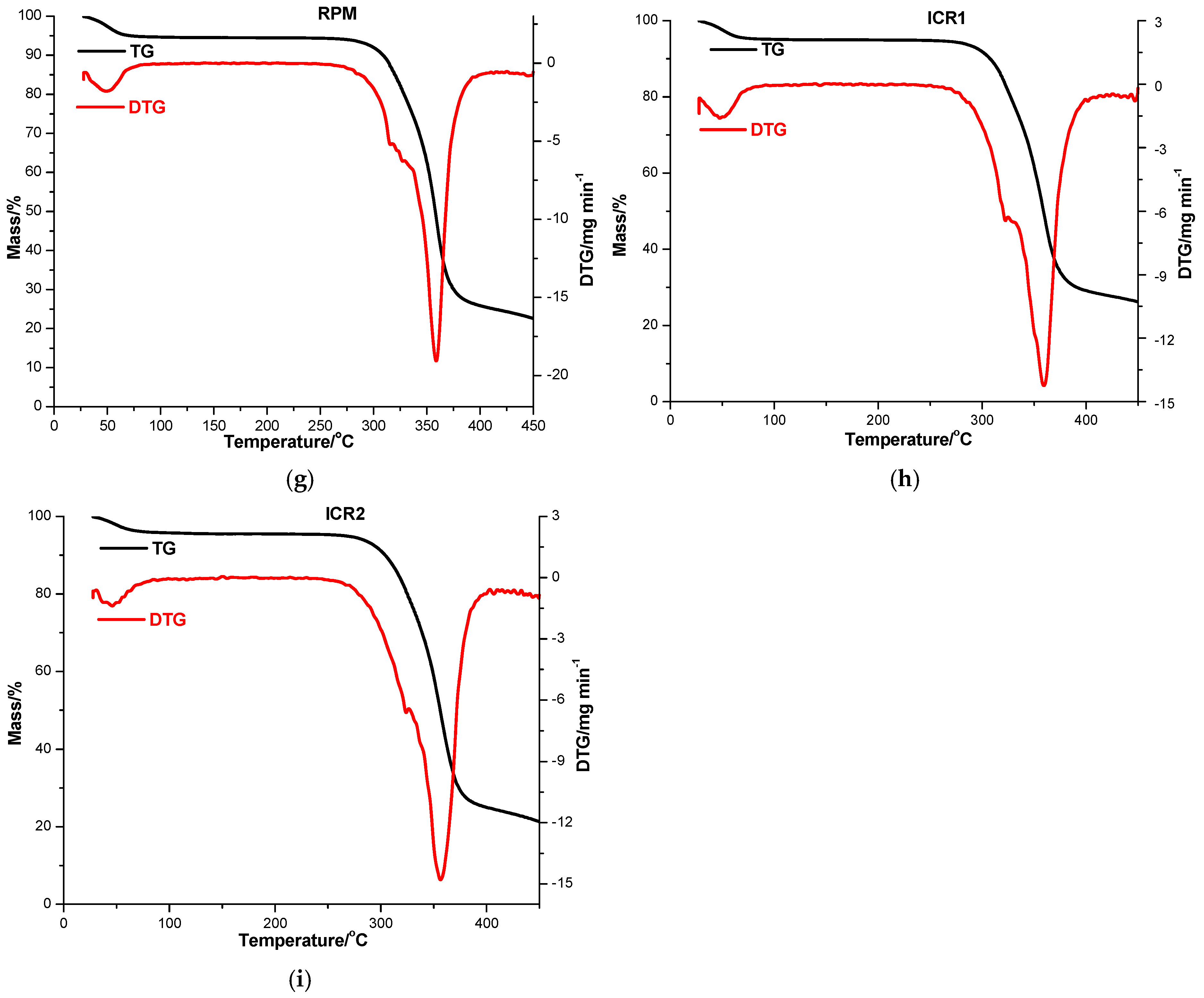
The thermal behavior of parent substances, PMs (DPM and RPM) and KPs (ICD1 and ICD2 for DM-β-CD; ICR1 and ICR2 for RM-β-CD) was explored in order to exploit the interaction between CARV and CDs in the solid state using TG and DTG in an air atmosphere, as illustrated in Figure 3 and presented in Table 3.
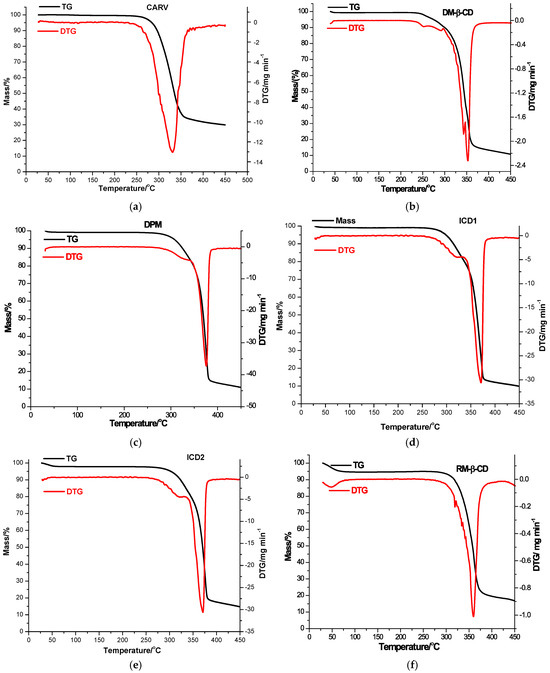
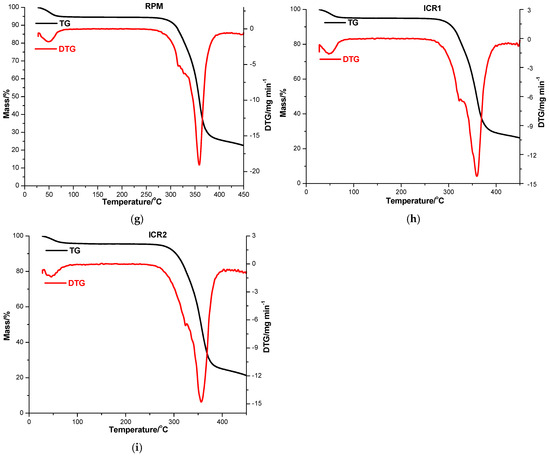
Figure 3.
TG/DTG thermoanalytical curves of CARV (a), DM-β-CD (b), DPM (c), ICD1 (d), ICD2 (e), RM-β-CD (f), RPM (g), ICR1 (h) and ICR2 (i) in an air atmosphere.

Table 3.
The thermoanalytical data for CARV, CDs, PMs and ICs with DM-β-CD and RM-β-CD in an air atmosphere with a heating rate of 10 °C min−1.
The TG/DTG curves of CARV showed remarkable thermal stability, characterized by a one-step decomposition beginning at approximately 220 °C, with a continuous mass loss in the temperature interval of 220–450 °C (Figure 3a, DTGpeak at 331 °C), leading to a residual mass of 29.88% (Table 3).
According to the characteristic thermal profile (as shown in Figure 3b), DM-β-CD is also distinguished by a notable thermal stability, with decomposition initiated around 226 °C. The DTG curve outlines two peaks belonging to the two decomposition stages associated with thermo-oxidation processes. While the first one is observed in the temperature range 226−347 °C with DTGpeak at 343 °C (Δm = 56.26%), the second appears in the range 347−450 °C with DTGpeak at 353 °C (Δm = 32.14%), and the loss of mass persists to a residual mass of 10.77% at 450 °C [48].
Considerable changes in the thermal behavior of the binary products of CARV with DM-β-CD are noted. DPM exhibits a slight mass loss between 30 and 55 °C (Δm = 0.78%), assigned to CD water removal; no mass loss is then observed up to 242 °C when the decomposition attributed to thermo-oxidation processes begins, occurring in two stages based on its thermal profile (illustrated in Figure 3c). The first stage of decomposition takes place in the temperature range of 242−342 °C (DTGpeak at 323 °C, Δm = 16.24%), while the second one is noted between 342−450 °C (DTGpeak at 376 °C, Δm = 72.00%), with a residual mass value of 10.98% at 450 °C. The ICD1 thermal pattern (Figure 3d) shows an initial small mass loss of 0.72% between 30 and 55 °C attributed to DM-β-CD dehydration, followed by a stability stage from 55 to 250 °C. Thermal decomposition in two stages begins over this temperature value, together with an important mass loss up to a residual mass of 9.89% at 450 °C (DTGpeaks at 322 and 371 °C). Regarding the ICD2 thermal pattern (shown in Figure 3e), it reveals an initial mass loss between 30 and 64 °C (Δm = 2.09%), followed by a stability phase in the range of 64–257 °C. Thermal decomposition is initiated above this temperature value, associated with mass loss up to a residual mass of 14.77% at 450 °C (DTGpeaks at 322 and 371 °C).
The thermal analysis of RM-β-CD indicates a mass loss observed in the temperature range of 30−100 °C (DTGpeak at 48 °C; Δm = 5.36%), as a result of CD crystallization water loss (Figure 3f). Following dehydration, a steady equilibrium phase is reached between 100 and 262 °C. At higher temperatures, degradation progresses from 262 to 450 °C, accompanied by mass loss (DTGpeak at 360 °C, Δm = 77.86%) [49].
Remarkable differences are noticed in the thermal curves of the binary compounds of CARV and RM-β-CD. A mass loss (Δm = 5.22%) is noticed as a result of CD dehydration in the TG/DTG curves of RPM between 30 and 84 °C, with DTGpeak at 49 °C, as shown in Figure 3g. Between 84 and 250 °C, a stability phase is exhibited in the TG curves. At higher temperature values decomposition begins with progressive mass loss up to a residual mass of 22.60%. ICR1 presents a small mass loss between 30 and 84 °C (Δm = 4.76%, DTGpeak at 48 °C), attributable to CD dehydration, followed by a stability stage in the temperature range of 84−256 °C (as illustrated in Figure 3h). Decomposition starts around 256 °C with gradual mass loss, leading to a residual mass of 26.20% (DTGpeak at 359 °C with a shoulder at 324 °C). Regarding the ICR2 thermal pattern (Figure 3i), it reveals an initial mass loss of 4.21% between 30 and 96 °C (DTGpeak at 46 °C), followed by no mass loss in the range of 96−250 °C. Thermal decomposition is promoted over this temperature value, accompanied by continuous mass loss process until a residual mass of 21.32% at 450 °C (DTGpeak at 356 °C).
Following the determination of the theoretical residual mass of the binary compounds of CARV with CDs, the values of 15.24% and 19.87% were obtained when using DM-β-CD and RM-β-CD, respectively. The results of thermal analysis indicate smaller values of experimental residual mass than the theoretical ones for all the binary products of CARV with DM-β-CD (Table 3) and suggest a higher thermal stability in the case of ICD2 compared to ICD1. Differences between the values of experimental and theoretical mass residues are also noticed for RM-β-CD, the experimental being higher than the theoretical ones for RPM, ICR1 and ICR2 (Table 3); in this case, ICR1 possesses a higher thermal stability than ICR2. These differences, alongside the different thermal profiles of the binary entities compared with those of the individual substances, provide evidence for the existence of an interaction between CARV and CDs, supporting the formation of the ICs. The data also suggest an interaction, even in the case of PMs.
3.4. FTIR Spectroscopy
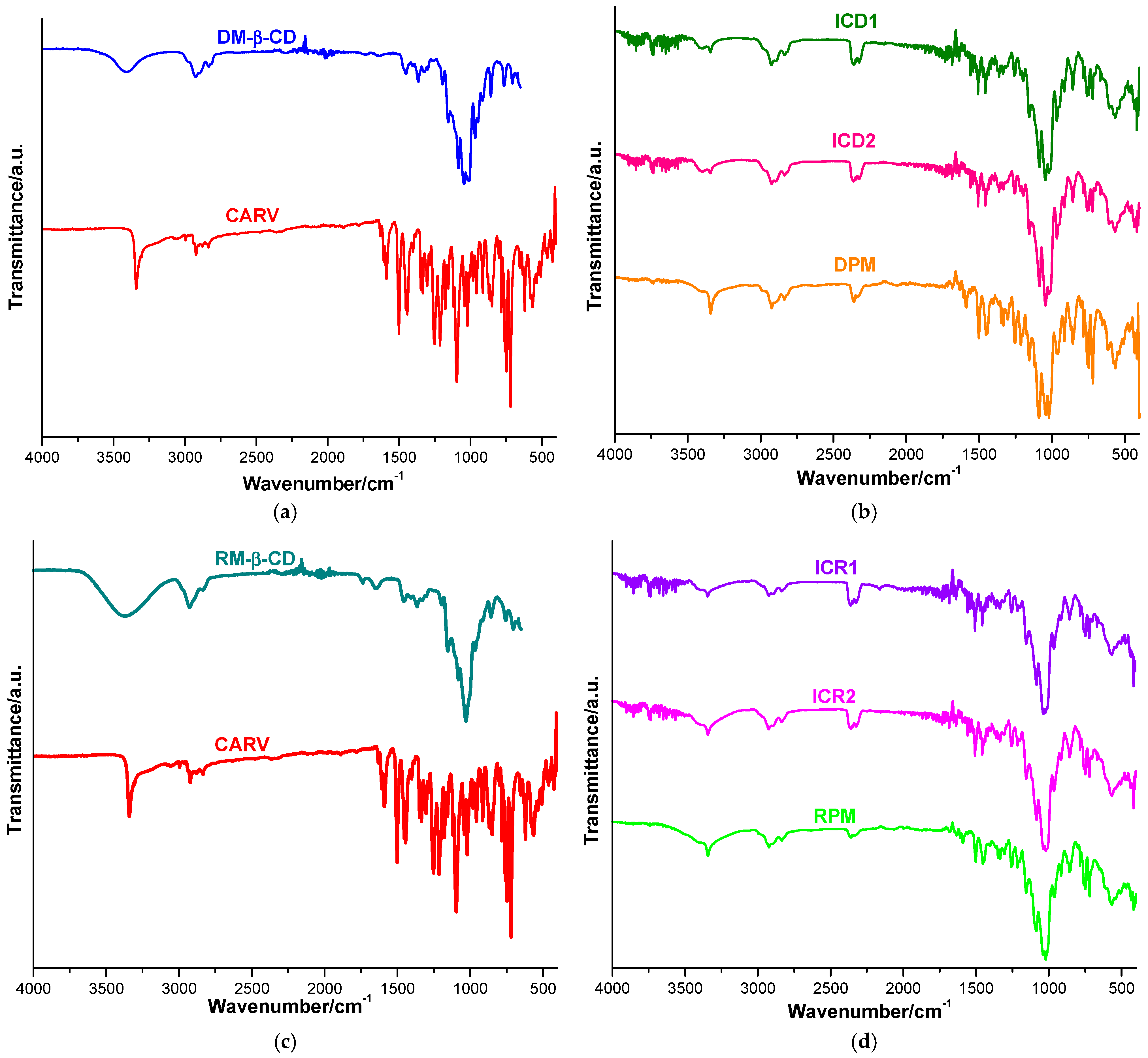
The UATR-FTIR spectra of the CARV, CDs, PMs and KPs are outlined in Figure 4 and were performed in order to assess the host–guest interaction by studying absorption modifications of functional groups of parent compounds (as shown in Table 4).
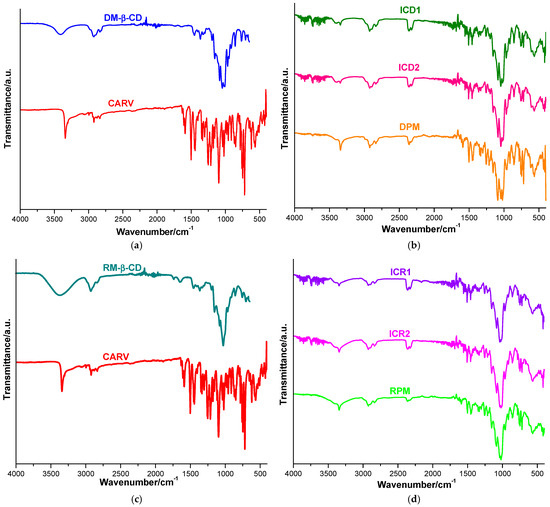
Figure 4.
UATR-FTIR spectra of CARV and DM-β-CD (a); ICD1, ICD2 and DPM (b); CARV and RM-β-CD (c) and ICR1, ICR2 and RPM (d).

Table 4.
FTIR characteristic bands of CARV, CDs, PMs and ICs.
Characteristic absorption bands are detected in the UATR-FTIR spectrum of CARV (shown in Figure 4a) at 3342 and 3304 cm−1, assigned to O–H and N–H stretching vibrations; at 2923 cm−1, attributed to aliphatic C–H stretching vibration; at 1252 cm−1, corresponding to C–N stretching vibration; and at 1213 cm−1, attributed to C–O stretching vibration. The C=C stretching vibrations of the phenyl ring arise at 1607, 1502 and 1453 cm−1. Other CARV characteristic bands are identified at 1348 and 1286 cm−1, assigned to C–C stretching vibrations; at 1303 cm−1, attributed to CH2 wagging; and 1223 cm−1, corresponding to CH2 twisting. The CARV bands from 1177, 1156, 1118 and 1097 cm−1 correspond to C–H in-plane bending vibrations, while those from 914, 870, 858 and 850 cm−1 are attributed to C–H out-of-plane bending vibrations. CCC in-plane bending vibrations appear at 784, 759 and 746 cm−1; C–OH bending vibration arises at 728 cm−1; and CCO in-plane bending vibration is observed at 620 cm−1. The CARV band from 1588 is assigned to N–H bending vibration [31,37,50,51,52].
The FTIR spectrum of DM-β-CD (Figure 4a) shows a broad absorption band at 3408 cm−1, assigned to the O-H stretching vibration of nonmethylated hydroxyl groups, followed by other distinctive bands at 2925 cm−1, attributed to the C–H stretching vibration of CH2; at 2835 cm−1, correlating to the stretching vibration of methyl groups; and at 1365 cm−1, underlying the C–H bending of CH2. In addition, the absorption bands at 1156 and 1045 cm−1 were then assigned to the C–O stretching vibration and the C–O–C stretching vibration [15,52].
The FTIR spectrum of DPM (illustrated in Figure 4b) reveals the presence of CARV characteristic bands, either at the same wavenumbers as in the pure drug or slightly shifted to different wavenumbers. Characteristic bands assigned to N–H stretching vibration (3342 cm−1), aliphatic C–H stretching vibration (2923 cm−1), N–H bending vibration (1588 cm−1), the aromatic C=C stretching vibrations (1502 cm−1 and 1453 cm−1), the Car–N stretching vibration (1252 cm−1) and the C–O stretching vibration (1213 cm−1) can be observed in the DPM spectra. The data also revealed differences in terms of band position in the DPM FTIR spectrum, compared to those of the pure drug. The CARV characteristic band corresponding to the C–H in-plane bending vibration from 1097 cm−1 is displaced to 1088 cm−1 in the DPM spectrum.
The CARV characteristic band of N–H stretching vibration from 3342 cm−1 is attenuated and displaced to 3343 cm−1 and 3345 cm−1 in the ICD1 and ICD2 spectra; the band attributed to aliphatic C–H stretching vibration is shifted from 2923 cm−1 in the pure CARV spectrum to 2924 cm−1 in both the ICD1 and ICD2 spectra. Additionally, the band assigned to N–H bending vibration is displaced from 1588 cm−1 in the pure CARV spectrum to 1560 cm−1 in ICD2 and disappears in ICD1; the aromatic C=C stretching vibrations bands from 1502 cm−1 and 1453 cm−1 in pure CARV are attenuated and displaced at 1507 cm−1 and 1457 cm−1 in both the ICD1 and ICD2 spectra. Moreover, the band attributed to the Car–N stretching vibration (1252 cm−1) is smoothed and shifted to 1258 cm−1 (ICD1) and 1256 cm−1 (ICD2); the band correlated with the C–O stretching vibration (1213 cm−1) is displaced to 1216 cm−1 in both the ICD1 and ICD2 spectra; and the CARV characteristic bands from 1177 and 1041 cm−1 are displaced to 1197 and 1047 cm−1 in the ICD1 spectrum and to 1198 and 1045 cm−1 in the ICD2 spectrum, respectively. Furthermore, the characteristic CARV bands from 1286, 1348, 1334, 1118 and 870 cm−1 are no longer present in either the ICD1 or ICD2 spectra. Collectively, these data support the hypothesis of the existence of an interaction between CARV and DM-β-CD, thus supporting the formation of IC.
RM-β-CD’s spectral behavior displays a broad absorption band characteristic of the O–H stretching vibration of nonmethylated hydroxyl groups in the spectral region 3600–3100 cm−1 and a broad region under 1500 cm−1, exhibiting distinct peaks corresponding to the CD ring (as shown in Figure 4c) [48,53,54].
The FTIR spectrum of RPM (Figure 4d) shows the existence of CARV characteristic bands attributed to N–H stretching vibration (3342 cm−1), aliphatic C–H stretching vibration (displaced to 2924 cm−1), N–H bending vibration (shifted to 1591 cm−1), aromatic C=C stretching vibrations (1608, 1502 and 1453 cm−1), C–N aromatic stretching vibration (shifted to 1256 cm−1) and C–O stretching vibration (shifted to 1214 cm−1).
Differences are noticed in the FTIR spectra of the binary products ICR1 and ICR2 when compared with the spectra of the parent compounds (presented in Figure 4d) regarding intensity, as well as spectral band positions. The CARV characteristic band assigned to the N–H bending vibration is displaced from 1588 cm−1 in the pure CARV spectrum to 1559 cm−1 in ICR1, as well as in ICR2; the aromatic C=C stretching vibration bands from 1502 cm−1 and 1453 cm−1 are attenuated and displaced to 1507 cm−1 and 1457 cm−1 in both ICR1 and ICR2 spectra; the band attributed to C–N aromatic stretching vibration (1252 cm−1) is smoothed and shifted to 1256 cm−1 in both ICR1 and ICR2 spectra; and the band correlated with the C–O stretching vibration (1213 cm−1) is displaced to 1216 cm−1 in both ICR1 and ICR2 spectra. In addition, the characteristic CARV bands from 1334, 1177, 1118 and 870 cm−1 are no longer reported in ICR1 or ICR2 spectra. Furthermore, a new band without a correspondent in the spectra of the parent compounds is displayed at 1540 cm−1 in both ICR1 and ICR2 spectra, but not in the RPM spectrum.
The results of FTIR investigations indicate a decrease in the intensity of the characteristic CARV bands, accompanied by a small shift to other wavenumbers of some CARV characteristic bands in the binary systems. In addition, in the spectra of KPs, the disappearance of several bands is detected, followed by a larger shift toward different wavenumbers of the CARV bands compared to PMs. Moreover, in all binary systems, two new spectral bands, which cannot be assigned to either CARV or CDs, were revealed; these bands are noticed at 2359, 2324 cm−1 (DPM), 2363, 2322 cm−1 (ICD1), 2364, 2322 cm−1 (ICD2), 2363, 2324 (RPM), 2360, 2323 (ICR1) and 2359, 2324 (ICR2). Hence, our data highlight the interaction between CARV and DM-β-CD and RM-β-CD in the binary products achieved by the kneading method, as well as in PMs, weaker in PMs, supporting the outcomes of thermal methods.
3.5. Powder X-Ray Diffraction
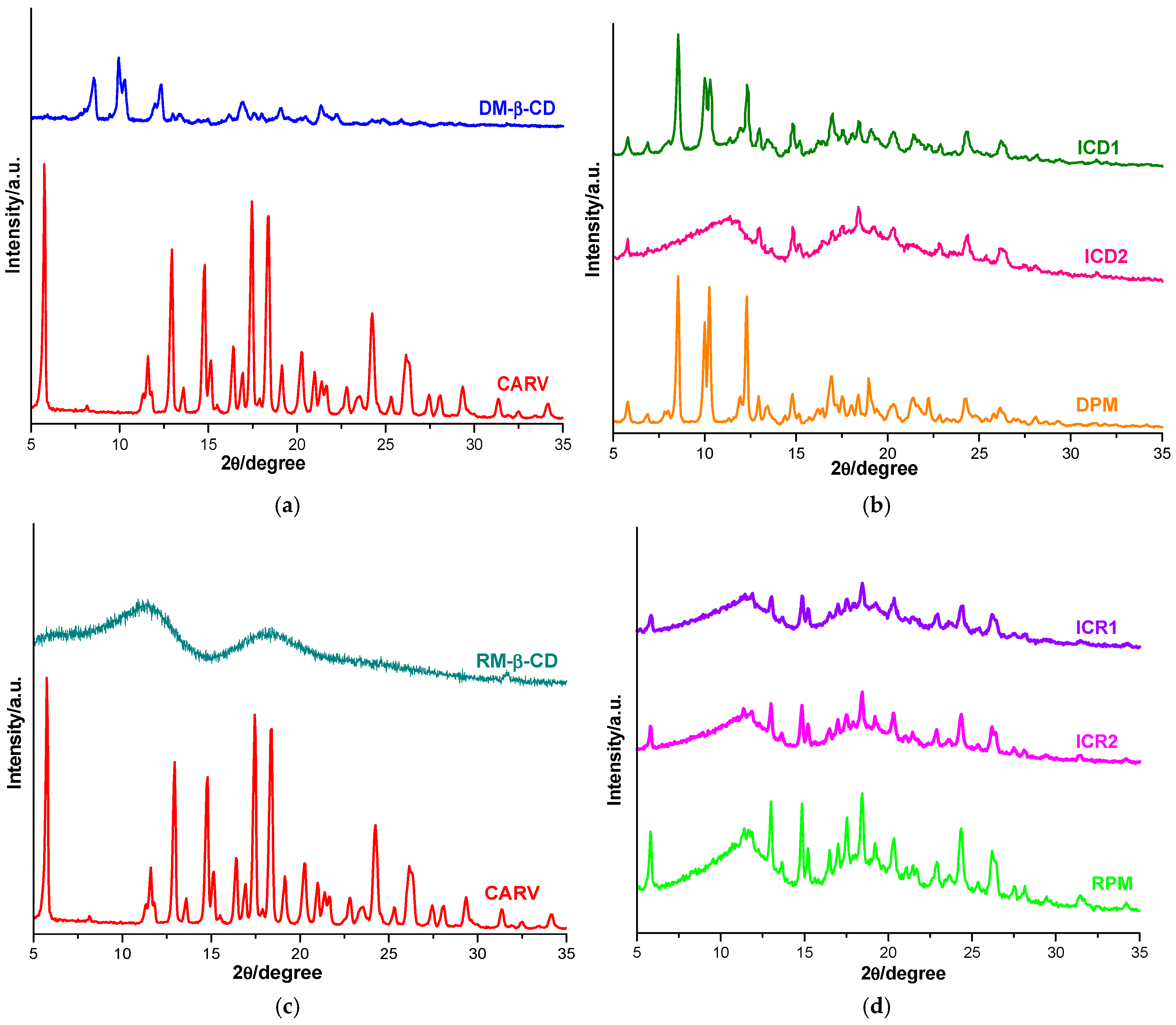
The diffraction profiles of the CARV, CDs, PMs and ICs are presented in Figure 5.
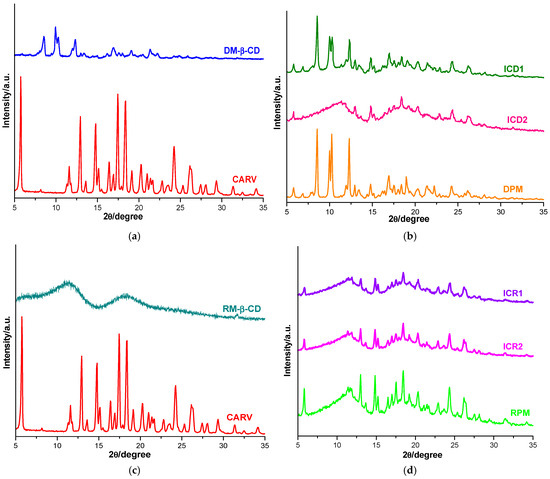
Figure 5.
Diffraction profiles of CARV and DM-β-CD (a); ICD1, ICD2 and DPM (b); CARV and RM-β-CD (c); ICR1, ICR2 and RPM (d).
The CARV diffractometric profile displays characteristic reflections of polymorph II [29]. Specifically, it reveals the presence of sharp characteristic reflections at 2θ angles of 5.75, 12.95, 14.80, 17.45 and 18.40, together with other characteristic peaks at 11.60, 13.60, 15.15, 16.40, 16.95, 19.15, 20.25, 21.00, 21.40, 21.65, 22.80, 23.55, 24.25, 25.30, 26.15, 27.45, 28.10, 29.35, 31.35, 32.50 and 34.15 2θ, confirming the crystalline nature of CARV. The DM-β-CD PXRD spectrum presents characteristic diffraction peaks at 8.54, 9.95, 10.29, 12.31, 16.97, 19.04 and 21.35 2θ, highlighting its crystalline structure [47,54].
The diffraction pattern of DPM indicates a noticeable decreasing in intensity of the characteristic CARV peaks, while DM-β-CD reflections at 2θ angles of 8.54, 9.95, 10.29, 12.31 and 16.97 increased their intensity, and the CD peak at 19.04 2θ is shifted to 18.95 2θ and shows higher intensity. In addition, the CARV peak at 2θ angle of 21.00 has disappeared, and new peaks are observed at 6.90, 18.95 and 22.25 2θ. A higher reduction in the intensity of CARV peaks is detected in the PXRD spectrum of both ICD1 and ICD2, with the intensity reduction effect being more pronounced in ICD2. Furthermore, the absence of CARV crystalline reflections at 21.00, 29.35 and 31.35 2θ and a new peak at 6.90 2θ are noticed in the ICD1 diffraction profile. The diffractometric pattern of ICD2 reveals a broad peak with many diffused and undefined peaks in the range of 6–12.85 2θ angles, together with the disappearance of DM-β-CD reflections at 8.54, 9.95, 10.29, and 12.31 2θ, and the CARV peak from 11.60 2θ, suggesting an amorphization process for ICD2. Moreover, CARV characteristic reflections from 2θ angles of 21.00, 23.55 and 29.35 were no longer present in the PXRD spectrum of ICD2, and a new peak at 15.60 2θ is also shown. These findings outline a significant reduction in CARV crystallinity in all binary systems, with the highest reduction in ICD2, offering evidence for an interaction between CARV and DM-β-CD.
Regarding the RM-β-CD diffractogram, two broad peaks, along with multiple undefined peaks characterized by low intensity, are revealed, suggesting its amorphous nature [48,54].
An appreciable reduction in the intensity of the characteristic CARV reflections is exhibited by the RPM diffractometric profile, together with the disappearance of the CARV peak at 32.50 2θ and a slight shift of several peaks to other 2θ angles. The PXRD patterns of ICR1 and ICR2 reveal a significant decrease in the intensity of CARV peaks, greater for ICR1 than ICR2, and the absence of CARV crystalline reflections at 32.50 2θ for both ICR1 and ICR2. In addition, in the ICR1 PXRD spectrum, CARV characteristic peaks at 2θ angles of 21.00, 21.40, 21.65 and 29.35 were no longer present, and CARV reflections from 5.75, 12.95, 16.40, 20.25, 22.80, 25.30, 27.45 and 31.35 were shifted to other 2θ values (5.85, 13.05, 16.50, 20.35, 22.95, 25.45, 27.55 and 31.55). This behavior testifies to the formation of the ICs when the kneading method was employed.
These data support the formation of new compounds with a remarkable reduction in CARV crystallinity. This decrease in crystallinity, due to its interaction with DM-β-CD and RM-β-CD, is expected to lead to a higher solubility. In both binary systems analyzed, the interaction is weaker in the case of the PM system. These observations are also supported by the results obtained from thermal analysis and FTIR spectroscopy.
3.6. Solubility Profile After Complexation
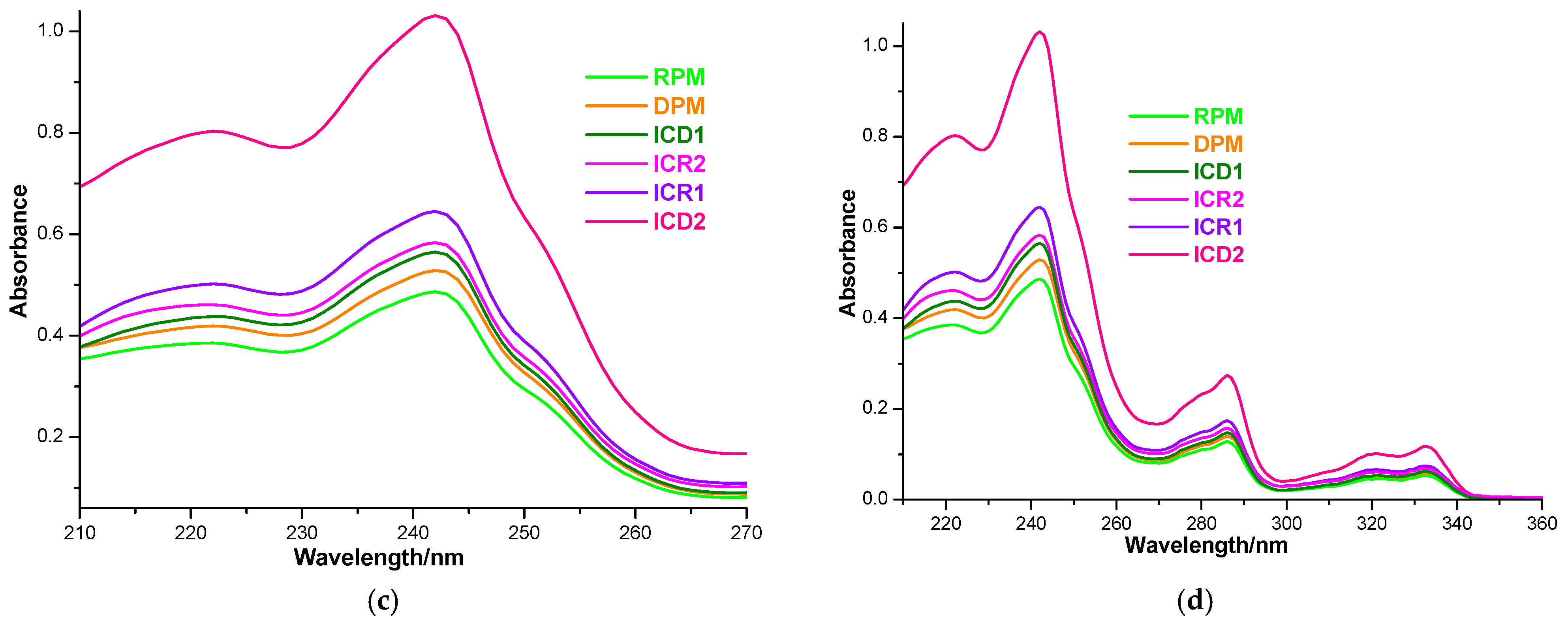
Drug solubility in host–guest binary systems obtained by both kneading and physical mixing was investigated by the shake-flask method [48,55,56]. UV spectroscopic measurements and the calibration curve at 242 nm (Figure 6a,b) were employed for the quantification of CARV in a saturated solution (Figure 6c,d).
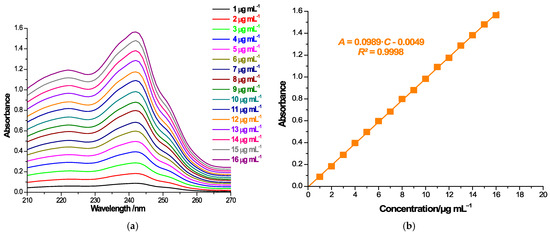
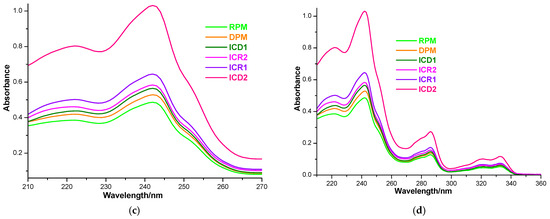
Figure 6.
Absorption spectra of CARV solutions of 1–16 µg mL−1 in 0.1 M phosphate buffer (pH 6.8) in the spectral range 210–270 (a); CARV calibration curve at 242 nm (b); absorption spectra of a 1:10 dilution of saturated samples’ solutions in the spectral range 210–270 nm (c) and 210–360 nm (d).
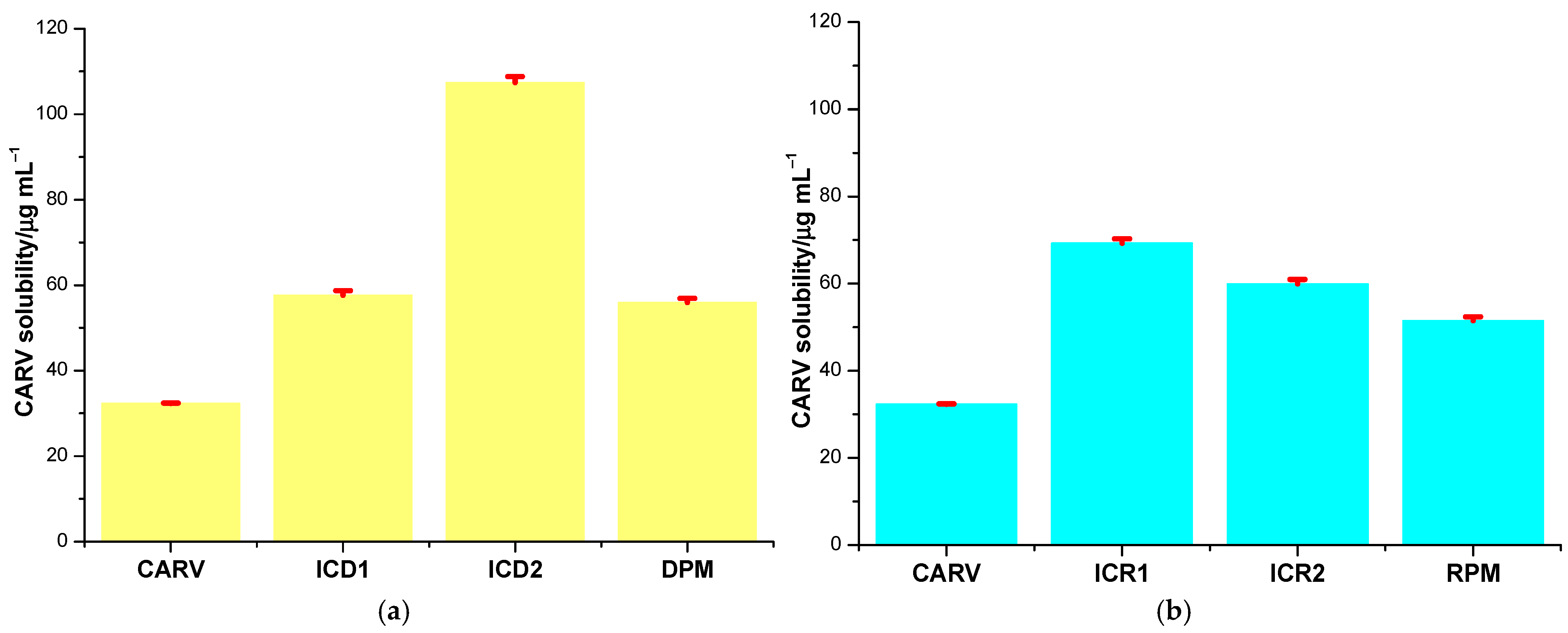
CARV solubility in host–guest systems obtained with DM-β-CD through the kneading method is 57.616 ± 1.115 µg mL−1 (ICD1) and 107.413 ± 1.384 µg mL−1 (ICD2). Considering the solubility of CARV in the binary products with RM-β-CD, ICR1 and ICR2, the obtained values are 69.293 ± 1.012 µg mL−1 and 59.963 ± 0.985 µg mL−1, respectively. Saturation solubility studies revealed information regarding the increase in solubility due to the formation of ICs with the two methylated CDs. According to the data, an increase in CARV solubility (presented in Figure 7) was observed in both DM-β-CD and RM-β-CD ICs as follows: 1.78-fold in ICD1, 3.32-fold in ICD2, 2.14-fold in ICR1 and 1.85-fold in ICR2 compared to pure CARV (32.325 ± 0.021 µg mL−1). The solubility-increasing capacity was revealed to be higher for DM-β-CD compared to RM-β-CD when ethanol was used as a co-solvent in the preparation of IC. In the case of ICs containing RM-β-CD, the presence of ethanol led to a lower solubility increase compared to using only water as a solvent.
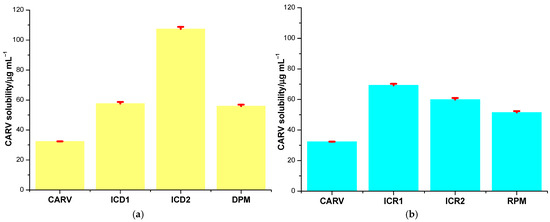
Figure 7.
CARV solubility in the binary systems with DM-β-CD (a) and with RM-β-CD (b).
Solubility of CARV was additionally determined for DPM and RPM (Figure 7). Reported data were 55.948 ± 0.972 µg mL−1 for DPM and 51.522 ± 0.869 µg mL−1 for RPM. In DPM, the solubility of CARV increased 1.73-fold, and in RPM, 1.59-fold. Therefore, our findings indicate a weaker drug–CD interaction in PM products than in ICs, lending evidence to confirm the results of thermal and spectroscopic techniques.
In the case of the ICs and PM containing DM-β-CD, the ANOVA test revealed statistically significant differences (p < 0.001) between the solubility of CARV, ICD1, ICD2 and DPM. The use of the Tukey–Kramer test for all pairwise comparisons revealed statistically significant differences (p < 0.05) between the comparison of CARV and both ICs and PM, and the comparison of ICD2 with ICD1 and DPM, while the comparison of ICD1 with DPM did not show any statistically significant difference (p > 0.05). Regarding the ICs with RM-β-CD, the ANOVA test showed that the differences in solubility for CARV, ICR1, ICR2 and RPM are statistically significant (p < 0.001). Additionally, the Tukey–Kramer test indicated statistically significant differences (p < 0.05) between the active pharmaceutical ingredient and the three binary systems, and between the ICs obtained.
Favorable effects on increasing the solubility of CARV have been reported in the literature in the presence of natural CDs and derivative CDs. Among the β-CD derivatives, only HP-β-CD was investigated using the saturation solubility studies of its IC with CARV. Zoghbi et al. reported that inclusion complexation of CARV with HP-β-CD results in an increase in CARV solubility in phosphate buffer (pH 6.8) from 0.027 mg L−1 (pure CARV) to 0.201 mg L−1 [57]. Compared with the results of our study, HP-β-CD shows a solubilizing potency higher than DM-β-CD and RM-β-CD.
The ethanol absolute/water system was chosen in the preparation of the ICs using the kneading method to achieve an optimal complexation efficiency, the use of co-solvents being an approach employed to enhance complexation efficiency [9,58,59]. Using ethanol as a co-solvent may have favorable effects both by increasing the apparent solubility of CARV [29] and by hindering the tendency of CDs to form aggregates, which can alter their binding affinity [9,58]. In contrast, ethanol can also compete with drug molecules for the cavity of CDs, exhibiting a destabilizing effect on the drug/CD ICs that leads to a weaker interaction between drugs and CDs and reduced CD solubilizing effect on the drugs [60]. Hence, the co-solvent impact on CD complexation efficiency depends on its dominant promoting or destabilizing effect on IC formation. Results of the present study on the solubility of CARV post-complexation corroborated with the values of the stability constants of the complexes and with the results of the EE and LE analysis suggest a potential competitive interaction of ethanol with CARV for the RM-β-CD cavity, whereas, in the case of DM-β-CD, ethanol shows an effect promoting the interaction of CARV with CD.
On the other hand, the solubilizing capacity of CDs for different drug substances is strongly influenced by both their complexing ability and their intrinsic water solubility. The substitution of the primary hydroxyl groups with methyl groups increases the CD binding potential, while the methylation of the secondary hydroxyl groups decreases it, with the solubilizing capacity being the consequence of these opposite effects [48]. Both DM-β-CD and RM-β-CD contain methyl groups as substituents at both the primary and secondary positions. The findings of solubility studies for PMs and ICs obtained using ethanol as a co-solvent emphasized a superior encapsulation ability for DM-β-CD. Similar results were previously reported with aripiprazole and olmesartan medoxomil as guest molecules [47,54]. The complexation of olmesartan with RM-β-CD and DM-β-CD resulted in an increase in drug solubility of 1.78-fold and 1.83-fold, respectively [47,61], and the host–guest interaction of aripiprazole with CDs led to an 11.36-fold increase in drug solubility with RM-β-CD and 37.87-fold in the presence of DM-β-CD [52,54], when the kneading method was employed and ethanol was used as a co-solvent. The results of this study on CARV solubility are consistent with those conducted on olmesartan and aripiprazole, emphasizing a greater solubilizing ability of DM-β-CD compared to RM-β-CD when ethanol was used as a co-solvent in the preparation of ICs.
4. Conclusions
This study was performed with the aim of investigating the influence of the type of CD and ethanol, as a co-solvent employed in the formulation step on the complexation of CARV with two β-CD derivatives in the solid state. ICs were obtained following the kneading method and were studied on the basis of various analytical techniques comprising thermoanalytical methods, PXRD, ATR-FTIR spectroscopy, UV spectrophotometry and saturation solubility studies. Data determined by the Bensi–Hildebrand method showed that CARV formed ICs with both CDs in a 1:1 ratio, with stability constants in the range 100–5000 M−1, found to be well-suited for the formation of an IC positively affecting its bioavailability. Thermoanalytical methods reveal a distinct thermal behavior of the binary compounds in contrast to the parent substances and provide evidence for the existence of an interaction between CARV and CDs, supporting the formation of the ICs, as well as highlighting a potential interaction in the PMs. The outcomes of the FTIR investigations suggest a reduction in the intensity of the CARV characteristic bands along with the disappearance and shift to other wavenumbers of some CARV characteristic bands in binary systems, emphasizing the interaction between the drug substance and both CDs. The PXRD data support the formation of new compounds with a marked decrease in CARV crystallinity, predicted to have a higher solubility due to the interaction between CARV and DM-β-CD, as well as CARV and RM-β-CD, in both binary systems, PMs and ICs, with weaker results in the PMs. The solubility of CARV increased by 1.78 to 3.32-fold as a consequence of drug complexation with CDs. Our results indicate a remarkable influence of both the solvent system and CD type on the solubility of ICs, underlining the CARV/DM-β-CD IC obtained with ethanol as a co-solvent as a valuable potential candidate for further investigation to achieve new CARV-containing formulations characterized by improved bioavailability.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr13041141/s1, Figure S1. Benesi–Hildebrand linear plot for 1/ΔA vs. 1/[DM-β-CD]2 in distilled water (a); 1/ΔA vs. 1/[RM-β-CD]2 in distilled water (b); 1/ΔA vs. 1/[DM-β-CD]2 in distilled water:ethanol absolute (1:1, m/m) (c); 1/ΔA vs. 1/[RM-β-CD]2 in distilled water:ethanol absolute (1:1, m/m) (d).
Author Contributions
Conceptualization, E.-T.N., A.R., A.L. and L.S.; methodology, E.-T.N., C.T., A.L., I.L. and L.S.; software, C.M., G.R. and S.S.; validation, S.S., I.M., L.B. and I.L.; formal analysis, E.-T.N., C.T., C.M., G.R. and I.L.; investigation, E.-T.N., A.R., S.S., I.M. and L.B.; resources, E.-T.N., C.M., G.R., A.L. and L.S.; data curation, A.R., S.S., L.B. and I.L.; writing—original draft preparation, E.-T.N., A.R., C.T., I.M. and S.S.; writing—review and editing, I.L., A.L. and L.S.; visualization, C.M., G.R. and A.L.; supervision, L.S.; project administration, E.-T.N. All authors have read and agreed to the published version of the manuscript.
Funding
We would like to acknowledge “Victor Babes” University of Medicine and Pharmacy Timisoara for their support in covering the costs of publication for this research paper.
Data Availability Statement
Data is contained within the article or Supplementary Material.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CARV | Carvedilol |
| CD | Cyclodextrin |
| IC | Inclusion complex |
| UV | Ultraviolet |
| DM-β-CD | Heptakis(2,6-di-O-methyl)-β-cyclodextrin |
| RM-β-CD | Randomly methylated β-cyclodextrin |
| α-CD | Alpha cyclodextrin |
| β-CD | Beta cyclodextrin |
| γ-CD | Gamma cyclodextrin |
| HP-β-CD | Hydroxypropyl-β-cyclodextrin |
| TG | Thermogravimetry |
| DTG | Derivative thermogravimetry |
| PXRD | Powder X-ray diffractometry |
| UATR-FTIR | Universal-attenuated total reflectance Fourier transform infrared |
| DS | Degree of substitution |
| ICD1 | CARV/DM-β-CD inclusion complex prepared with distilled water (without ethanol) |
| ICR1 | CARV/RM-β-CD inclusion complex prepared with distilled water (without ethanol) |
| ICD2 | CARV/DM-β-CD inclusion complex prepared with distilled water/ethanol |
| ICR2 | CARV/RM-β-CD inclusion complex prepared with distilled water/ethanol |
| PM | Physical mixture |
| KP | Kneaded product |
| A | Absorbance |
| C | Concentration |
| DPM | Physical mixture of CARV with DM-β-CD |
| RPM | Physical mixture of CARV with RM-β-CD |
References
- Zarghampour, A.; Jouyban, K.; Jouyban-Gharamaleki, V.; Jouyban, A.; Rahimpour, E. A Description on the Shake-Flask and Laser Monitoring-Based Techniques for Determination of the Drug’s Solubility. Pharm. Sci. 2024, 30, 274–278. [Google Scholar] [CrossRef]
- Nyamba, I.; Sombié, C.B.; Yabré, M.; Zimé-Diawara, H.; Yaméogo, J.; Ouédraogo, S.; Lechanteur, A.; Semdé, R.; Evrard, B. Pharmaceutical Approaches for Enhancing Solubility and Oral Bioavailability of Poorly Soluble Drugs. Eur. J. Pharm. Biopharm. 2024, 204, 114513. [Google Scholar] [CrossRef]
- Zhuo, Y.; Zhao, Y.G.; Zhang, Y. Enhancing Drug Solubility, Bioavailability, and Targeted Therapeutic Applications Through Magnetic Nanoparticles. Molecules 2024, 29, 4854. [Google Scholar] [CrossRef]
- Kumari, L.; Choudhari, Y.; Patel, P.; Gupta, G.D.; Singh, D.; Rosenholm, J.M.; Bansal, K.K.; Kurmi, B. Das Advancement in Solubilization Approaches: A Step Towards Bioavailability Enhancement of Poorly Soluble Drugs. Life 2023, 13, 1099. [Google Scholar] [CrossRef] [PubMed]
- Mura, P. Analytical Techniques for Characterization of Cyclodextrin Complexes in the Solid State: A Review. J. Pharm. Biomed. Anal. 2015, 113, 226–238. [Google Scholar] [CrossRef]
- Nicolaescu, O.E.; Belu, I.; Mocanu, A.G.; Manda, V.C.; Rău, G.; Pîrvu, A.S.; Ionescu, C.; Ciulu-Costinescu, F.; Popescu, M.; Ciocîlteu, M.V. Cyclodextrins: Enhancing Drug Delivery, Solubility and Bioavailability for Modern Therapeutics. Pharmaceutics 2025, 17, 288. [Google Scholar] [CrossRef]
- Hădărugă, N.G.; Bandur, G.N.; David, I.; Hădărugă, D.I. A Review on Thermal Analyses of Cyclodextrins and Cyclodextrin Complexes. Environ. Chem. Lett. 2019, 17, 349–373. [Google Scholar] [CrossRef]
- Chandra, S.; Sangeetha, S.; Kavibharathi, S.; Nandhini, B.; Suresh, R.; Sanjeevkumar, C. Approach to enhance the solubility of carvedilol using β-CD complexation. World J. Pharm. Res. 2015, 10, 163. [Google Scholar] [CrossRef]
- Poulson, B.G.; Alsulami, Q.A.; Sharfalddin, A.; El Agammy, E.F.; Mouffouk, F.; Emwas, A.H.; Jaremko, L.; Jaremko, M. Cyclodextrins: Structural, Chemical, and Physical Properties, and Applications. Polysaccharides 2022, 3, 1. [Google Scholar] [CrossRef]
- Kou, X.; Xu, X.; Gao, N.; Zhang, Y.; Huang, X.; Chen, F.; Ke, Q.; Meng, Q. Supramolecular Chemistry in Cyclodextrin Inclusion Complexes: The Formation Rules of Terpenes/β-Cyclodextrin Inclusion Complexes. Food Hydrocoll. 2024, 157, 110441. [Google Scholar] [CrossRef]
- Zhou, J.; Jia, J.; He, J.; Li, J.; Cai, J. Cyclodextrin Inclusion Complexes and Their Application in Food Safety Analysis: Recent Developments and Future Prospects. Foods 2022, 11, 3871. [Google Scholar] [CrossRef]
- Ahad, A.; Jardan, Y.A.B.; Raish, M.; Al-Mohizea, A.M.; Al-Jenoobi, F.I. Ternary Inclusion Complex of Sinapic Acid with Hydroxypropyl-β-Cyclodextrin and Hydrophilic Polymer Prepared by Microwave Technology. Processes 2022, 10, 2637. [Google Scholar] [CrossRef]
- Sbârcea, L.; Udrescu, L.; Ledeţi, I.; Szabadai, Z.; Fuliaş, A.; Sbârcea, C. β-Cyclodextrin Inclusion Complexes of Lisinopril and Zofenopril: Physicochemical Characterization and Compatibility Study of Lisinopril-β-Cyclodextrin with Lactose. J. Therm. Anal. Calorim. 2016, 123, 2377–2390. [Google Scholar] [CrossRef]
- Arruda, T.R.; Silva, R.R.A.; Marques, C.S.; e Moraes, A.R.F.; Bernardes, P.C.; de Oliveira, T.V.; de Oliveira, S.O.; Muranyi, P.; Soares, N.d.F.F. β-Cyclodextrin versus Hydroxypropyl-β-Cyclodextrin: Is Inclusion Complexation a Suitable Alternative to Improve the Properties of Hop-Derived β-Acids? Food Hydrocoll. 2024, 149, 109622. [Google Scholar] [CrossRef]
- Li, H.; Chang, S.L.; Chang, T.R.; You, Y.; Wang, X.D.; Wang, L.W.; Yuan, X.F.; Tan, M.H.; Wang, P.D.; Xu, P.W.; et al. Inclusion Complexes of Cannabidiol with β-Cyclodextrin and Its Derivative: Physicochemical Properties, Water Solubility, and Antioxidant Activity. J. Mol. Liq. 2021, 334, 116070. [Google Scholar] [CrossRef]
- Shinde, B.; Patil, D.; Nandre, V.; Gautam, M.; Doshi, P.; Gairola, S. Development and Validation of a Spectrophotometric Method for Quantification of Residual Cyclodextrin (DIMEB; Heptakis) in Pertussis Antigens. Biologicals 2023, 81, 101663. [Google Scholar] [CrossRef]
- Wang, M.; Bai, J.; Chen, W.N.; Ching, C.B. Metabolomic Profiling of Cellular Responses to Carvedilol Enantiomers in Vascular Smooth Muscle Cells. PLoS ONE 2010, 5, e15441. [Google Scholar] [CrossRef]
- Zawadzka, K.; Bernat, P.; Felczak, A.; Lisowska, K. Microbial Detoxification of Carvedilol, a Β-Adrenergic Antagonist, by the Filamentous Fungus Cunninghamella Echinulata. Chemosphere 2017, 183, 18–26. [Google Scholar] [CrossRef]
- Loftsson, T.; Vogensen, S.B.; Desbos, C.; Jansook, P. Carvedilol: Solubilization and Cyclodextrin Complexation: A Technical Note. AAPS PharmSciTech 2008, 9, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Ruffolo, R.R.; Feuerstein, G.Z. Carvedilol. In Comprehensive Medicinal Chemistry II; Elsevier: Amsterdam, The Netherlands, 2007; pp. 137–147. [Google Scholar] [CrossRef]
- Zawadzka, K.; Bernat, P.; Felczak, A.; Różalska, S.; Lisowska, K. Antibacterial Activity of High Concentrations of Carvedilol against Gram-Positive and Gram-Negative Bacteria. Int. J. Antimicrob. Agents 2018, 51, 458–467. [Google Scholar] [CrossRef]
- Zawadzka, K.; Felczak, A.; Głowacka, I.E.; Piotrowska, D.G.; Lisowska, K.; Janga, C. Evaluation of the Antimicrobial Potential and Toxicity of a Newly Synthesised 4-(4-(Benzylamino)Butoxy)-9H-Carbazole. Int. J. Mol. Sci. 2021, 22, 12796. [Google Scholar] [CrossRef] [PubMed]
- Hajighasemi, F.; Gaeini, A. Sensitivity of Human Leukemic Cells to Carvedilol. J. Basic Clin. Pathophysiol. 2018, 7, 37–42. [Google Scholar] [CrossRef]
- O’Reilly, M.; Kirkwood, N.K.; Kenyon, E.J.; Huckvale, R.; Cantillon, D.M.; Waddell, S.J.; Ward, S.E.; Richardson, G.P.; Kros, C.J.; Derudas, M. Design, Synthesis, and Biological Evaluation of a New Series of Carvedilol Derivatives That Protect Sensory Hair Cells from Aminoglycoside-Induced Damage by Blocking the Mechanoelectrical Transducer Channel. J. Med. Chem. 2019, 62, 5312–5329. [Google Scholar] [CrossRef]
- Abdullah Shamim, M.; Yeung, S.; Shahid, A.; Chen, M.; Wang, J.; Desai, P.; Parsa, C.; Orlando, R.; Meyskens, F.L.; Kelly, K.M.; et al. Topical Carvedilol Delivery Prevents UV-Induced Skin Cancer with Negligible Systemic Absorption. Int. J. Pharm. 2022, 611, 121302. [Google Scholar] [CrossRef]
- Huang, K.M.; Liang, S.; Yeung, S.; Oiyemhonlan, E.; Cleveland, K.H.; Parsa, C.; Orlando, R.; Meyskens, F.L.; Andresen, B.T.; Huang, Y. Topically Applied Carvedilol Attenuates Solar Ultraviolet Radiation Induced Skin Carcinogenesis. Cancer Prev. Res. 2017, 10, 598–606. [Google Scholar] [CrossRef]
- Prieto, C.; Evtoski, Z.; Pardo-Figuerez, M.; Lagaron, J.M. Bioavailability Enhancement of Nanostructured Microparticles of Carvedilol. J. Drug Deliv. Sci. Technol. 2021, 66, 102780. [Google Scholar] [CrossRef]
- Sharapova, A.V.; Ol’khovich, M.V.; Blokhina, S.V. Thermodynamic Consideration of Dissolution and Distribution Behavior of Carvedilol in Pharmaceutical Significant Media. J. Chem. Thermodyn. 2024, 190, 107207. [Google Scholar] [CrossRef]
- Beattie, K.; Phadke, G.; Novakovic, J. Carvedilol. In Profiles of Drug Substances, Excipients and Related Methodology; Academic Press Inc.: Cambridge, MA, USA, 2013; Volume 38, pp. 113–157. [Google Scholar]
- Deris Prado, L.; Vinícius, H.; Rocha, A.; Lamounier, J.A.; Resende, C.; Ferreira, G.B.; Rangel, A.M.; Teixeira, F. An insight into carvedilol solid forms: Effect of supramolecular interactions on the dissolution profiles. CrystEngComm 2014, 16, 3168–3179. [Google Scholar] [CrossRef]
- Sopyan, I.; Sari, W.A.; Megantara, S.; Rusdiana, T. Solubility Enhancement of Carvedilol by Multicomponent Crystal Approach Using Glycine and Arginine as Coformers. Pharmacia 2023, 70, 1479–1486. [Google Scholar] [CrossRef]
- Hosny, K.M.; El-Say, K.M.; Alkhalidi, H.M. Quality by Design Approach to Screen the Formulation and Process Variables Influencing the Characteristics of Carvedilol Solid Lipid Nanoparticles. J. Drug Deliv. Sci. Technol. 2018, 45, 168–176. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhi, Z.; Li, X.; Gao, J.; Song, Y. Carboxylated Mesoporous Carbon Microparticles as New Approach to Improve the Oral Bioavailability of Poorly Water-Soluble Carvedilol. Int. J. Pharm. 2013, 454, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Sun, P.; Nie, S.; Pan, W. Preparation and Evaluation of SEDDS and SMEDDS Containing Carvedilol. Drug Dev. Ind. Pharm. 2005, 31, 785–794. [Google Scholar] [CrossRef]
- Sharma, A.; Jain, C.P. Carvedilol-β-Cyclodextrin Systems: Preparation, Characterization and In Vitro Evaluation. Dhaka Univ. J. Pharm. Sci. 2013, 12, 51–58. [Google Scholar] [CrossRef]
- Rigaud, S.; Mathiron, D.; Moufawad, T.; Landy, D.; Djedaini-Pilard, F.; Marçon, F. Cyclodextrin Complexation as a Way of Increasing the Aqueous Solubility and Stability of Carvedilol. Pharmaceutics 2021, 13, 1746. [Google Scholar] [CrossRef]
- Yuvaraja, K.; Khanam, J. Enhancement of Carvedilol Solubility by Solid Dispersion Technique Using Cyclodextrins, Water Soluble Polymers and Hydroxyl Acid. J. Pharm. Biomed. Anal. 2014, 96, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.L.; Usacheva, T.R.; Kuz’mina, I.A.; Nguyen, T.N.; Thai, H.; Volkova, M.A.; Le, H.K.; Nguyen, T.D.; Volynkin, V.A.; Tran, D.L. Effect of Cyclodextrin Types and Reagents Solvation on the Stability of Complexes Between B-Cyclodextrins and Rutin in Water-Ethanol Solvents. J. Mol. Liq. 2020, 318, 114308. [Google Scholar] [CrossRef]
- Li, P.; Zhao, L.; Yalkowsky, S.H. Combined Effect of Cosolvent and Cyclodextrin on Solubilization of Nonpolar Drugs. J. Pharm. Sci. 1999, 88, 1107–1111. [Google Scholar] [CrossRef]
- Crestani De Miranda, J.; Elyan, T.; Martins, A.; Veiga, F.; Gomes Ferraz, H. Cyclodextrins and Ternary Complexes: Technology to Improve Solubility of Poorly Soluble Drugs. Braz. J. Pharm. Sci. 2011, 47, 665–681. [Google Scholar] [CrossRef]
- Chatjigakis, A.K.; Donz6, C.; Coleman, A.W.; Cardot, P. Solubility Behavior of @-Cyciodextrin In WaterKosolvent Mixtures. Amerlcan Chem. Soc. 1992, 64, 1632–1634. [Google Scholar]
- Charumanee, S.; Okonogi, S.; Sirithunyalug, J.; Wolschann, P.; Viernstein, H. Effect of Cyclodextrin Types and Co-Solvent on Solubility of a Poorly Water Soluble Drug. Sci. Pharm. 2016, 84, 694–704. [Google Scholar] [CrossRef]
- Kou, X.; Zhang, Y.; Su, D.; Wang, H.; Huang, X.; Niu, Y.; Ke, Q.; Xiao, Z.; Meng, Q. Study on Host-Guest Interaction of Aroma Compounds/γ-Cyclodextrin Inclusion Complexes. LWT 2023, 178, 114589. [Google Scholar] [CrossRef]
- Xiao, Z.; Yu, P.; Sun, P.; Kang, Y.; Niu, Y.; She, Y.; Zhao, D. Inclusion Complexes of β-Cyclodextrin with Isomeric Ester Aroma Compounds: Preparation, Characterization, Mechanism Study, and Controlled Release. Carbohydr. Polym. 2024, 333, 121977. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Li, S.; Wang, S.; Zhang, B.; Huang, Q. Encapsulation of Menthol into Cyclodextrin Metal-Organic Frameworks: Preparation, Structure Characterization and Evaluation of Complexing Capacity. Food Chem. 2021, 338. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Wang, C.; Yue, J.; Deng, Y.; Jiao, S.; Zhao, Y.; Zhou, J.; Cao, T. Optimization and Characterization of 1,8-Cineole/Hydroxypropyl-β-Cyclodextrin Inclusion Complex and Study of Its Release Kinetics. Food Hydrocoll. 2021, 110, 106159. [Google Scholar] [CrossRef]
- Man, D.E.; Nițu, E.T.; Temereancă, C.; Sbârcea, L.; Ledeți, A.; Ivan, D.; Ridichie, A.; Andor, M.; Jîjie, A.R.; Barvinschi, P.; et al. Host–Guest Complexation of Olmesartan Medoxomil by Heptakis(2,6-Di-O-Methyl)-β-Cyclodextrin: Compatibility Study with Excipients. Pharmaceutics 2024, 16, 1557. [Google Scholar] [CrossRef]
- Sbârcea, L.; Tănase, I.M.; Ledeți, A.; Cîrcioban, D.; Vlase, G.; Barvinschi, P.; Miclău, M.; Văruţ, R.M.; Suciu, O.; Ledeți, I. Risperidone/Randomly Methylated β-Cyclodextrin Inclusion Complex—Compatibility Study with Pharmaceutical Excipients. Molecules 2021, 26, 1690. [Google Scholar] [CrossRef]
- Savic-Gajic, I.; Savic, I.M.; Nikolic, V.D.; Nikolic, L.B.; Popsavin, M.M.; Kapor, A.J. Study of the Solubility, Photostability and Structure of Inclusion Complexes of Carvedilol with β-Cyclodextrin and (2-Hydroxypropyl)-β-Cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2016, 86, 7–17. [Google Scholar] [CrossRef]
- Ramanjaneyulu, G.S.; Kumar, I.V.S.; Rao, K.N.; Kishore, J.V.V.K. Process for the Preparation of Carvedilol Form II. U.S. Patent 8,058,453 B2, 1 November 2011. [Google Scholar]
- Jagannathan, L.; Meenakshi, R.; Gunasekaran, S.; Srinivasan, S. FT-IR, FT-Raman and UV-Vis Spectra and Quantum Chemical Investigation of Carvedilol. Mol. Simul. 2010, 36, 283–290. [Google Scholar] [CrossRef]
- Tănase, I.M.; Sbârcea, L.; Ledeţi, A.; Barvinschi, P.; Cîrcioban, D.; Vlase, G.; Văruţ, R.M.; Ledeţi, I. Compatibility Studies with Pharmaceutical Excipients for Aripiprazole–Heptakis (2,6-Di-O-Methyl)-β-Cyclodextrin Supramolecular Adduct. J. Therm. Anal. Calorim. 2020, 142, 1963–1976. [Google Scholar] [CrossRef]
- Circioban, D.; Ledeţi, I.; Vlase, G.; Ledeţi, A.; Axente, C.; Vlase, T.; Dehelean, C. Kinetics of Heterogeneous-Induced Degradation for Artesunate and Artemether. J. Therm. Anal. Calorim. 2018, 134, 749–756. [Google Scholar] [CrossRef]
- Tănase, I.M.; Sbârcea, L.; Ledeți, A.; Vlase, G.; Barvinschi, P.; Văruţ, R.M.; Dragomirescu, A.; Axente, C.; Ledeți, I. Physicochemical Characterization and Molecular Modeling Study of Host–Guest Systems of Aripiprazole and Functionalized Cyclodextrins. J. Therm. Anal. Calorim. 2020, 141, 1027–1039. [Google Scholar] [CrossRef]
- Sbârcea, L.; Tănase, I.M.; Ledeți, A.; Cîrcioban, D.; Vlase, G.; Barvinschi, P.; Miclău, M.; Văruţ, R.M.; Trandafirescu, C.; Ledeți, I. Encapsulation of Risperidone by Methylated β-Cyclodextrins: Physicochemical and Molecular Modeling Studies. Molecules 2020, 25, 5694. [Google Scholar] [CrossRef] [PubMed]
- Detrich, Á.; Dömötör, K.J.; Katona, M.T.; Markovits, I.; Vargáné Láng, J. Polymorphic Forms of Bisoprolol Fumarate: Preparation and Characterization. J. Therm. Anal. Calorim. 2019, 135, 3043–3055. [Google Scholar] [CrossRef]
- Zoghbi, A.; Geng, T.; Wang, B. Dual Activity of Hydroxypropyl-β-Cyclodextrin and Water-Soluble Carriers on the Solubility of Carvedilol. AAPS PharmSciTech 2017, 18, 2927–2935. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek-Klinowska, M.; Łudzik, K.; Jażdżewska, M.; Jóźwiak, M.; Hornowski, T.; Bilski, P. Characterization of Behavior of the Inclusion Complex between Methyl-β-Cyclodextrin and Nimodipine in Water-Ethanol Mixed Media. J. Mol. Liq. 2024, 395. [Google Scholar] [CrossRef]
- Jambhekar, S.S.; Breen, P. Cyclodextrins in Pharmaceutical Formulations II: Solubilization, Binding Constant, and Complexation Efficiency. Drug Discov. Today 2016, 21, 363–368. [Google Scholar] [CrossRef]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef]
- Andor, M.; Temereancă, C.; Sbârcea, L.; Ledeți, A.; Man, D.E.; Mornoș, C.; Ridichie, A.; Cîrcioban, D.; Vlase, G.; Barvinschi, P.; et al. Host–Guest Interaction Study of Olmesartan Medoxomil with β-Cyclodextrin Derivatives. Molecules 2024, 29, 2209. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).