Abstract
Ozone (O3), a strong oxidizing agent, has found widespread applications since its structure was confirmed by Schubbe in 1839. It can be produced through ultraviolet radiation, electrochemical methods, or dielectric barrier discharge (DBD), with DBD being the most efficient for large-scale production due to its high stability. Ozone is widely used in environmental management, particularly in water treatment, air pollution control, and soil remediation. In water treatment, ozone effectively removes microorganisms and contaminants without generating secondary pollutants. In air pollution control, it degrades organic compounds in industrial waste and neutralizes toxic gases in automobile exhausts. Ozone also breaks down persistent pollutants like polycyclic aromatic hydrocarbons in soil, improving soil quality. However, challenges remain related to ozone’s stability and high production costs. Beyond environmental uses, ozone is critical in industries and medicine. It helps remove pathogens and heavy metals in wastewater treatment, extends shelf life and deactivates mycotoxins in food processing, and shows promise in medical fields like orthopedics and cancer therapy. In the power industry, ozone plays a key role in water treatment and air purification. Overall, ozone technology offers significant potential for both environmental and industrial applications.
1. Introduction
Ozone (O3) is an allotrope of oxygen (O2) composed of three oxygen atoms. It has a distinctive fishy smell and is highly oxidizing [1]. In 1839, German scientist J.D.C. von Schubein (Schonbein) first identified ozone at the Natural Science Congress in Basel, characterizing it as a molecule consisting of one oxygen molecule and one additional oxygen atom. Due to its unstable chemical properties, Schonbein described it as reactive oxygen. In 1840, he formally submitted a report to the Munich Academy of Sciences in Germany, announcing the discovery of ozone. Since then, the properties and functions of ozone have gradually been elucidated through scientific research.
After the efficacy of ozone in disinfection, sterilization, water treatment, and bleaching was recognized, it began to be adopted in industrial applications. In 1868, de Gebeth was the first to apply ozone industrially, using it to oxidize coal tar mixtures to produce products suitable for use in coatings and paints [2]. By 1873, ozone was being used in Europe for sugar refining and linen bleaching [3]. In 1902, the world’s first large-scale water treatment facility utilizing ozone was built in Paderborn, Germany. In 1937, the first commercial swimming pool treated with ozone was opened in the United States, and since then, ozone has become the designated water disinfection method for Olympic aquatic competitions. Following World War II, ozone was employed in Europe, the United States, and Japan for preserving fruits and vegetables throughout storage, manufacturing, and transportation [2]. Ozone also found applications in the medical field. During World War II, Japan used ozone for physical therapy, while Russia utilized ozonated air in athletic facilities. Today, ozone has numerous medical applications, including air disinfection in hospital wards and operating theatres, disinfection of medical instruments using ozonated water, and dental treatments for sterilization. Additionally, it is used for gynecological treatments, and ozone microbubble water is employed for its bactericidal activity against pathogens in the mouth and upper respiratory tract, as well as for disinfecting contaminated medical equipment [4]. In the 1960s and 1970s, ozone technology began to be used for treating domestic sewage in the United States, which led to the establishment of an independent ozone technology industry [5]. The International Ozone Association (IOA), founded in 1973 in Canada, has played a key role in advancing ozone technology. The association hosts an international conference every two years to facilitate the exchange of research and advancements. Developed countries have also established regional IOA organizations to foster academic collaboration. In 1982, ozone began to be widely used to sterilize bottled water, and today, it is employed by nearly all manufacturers of mineral and purified water. Ozone applications can be classified into four main categories: water treatment, chemical oxidation, food processing and preservation, and medical treatment. Each of these fields has seen significant advancements in research and equipment development, contributing to the widespread adoption of ozone technology over the past century.
In China, research into and application of ozone technology started relatively late. It was not until the mid-1970s that China began research and development in this field. In the 1990s, the promotion of ozone disinfection technology for mineral water, purified water, and pharmaceutical air sterilization, along with the introduction of small household ozone generators, helped advance China’s ozone industry. After 2000, significant breakthroughs were made in the development of large-scale industrial ozone equipment, including the successful production of intermediate frequency ozone generators with capacities ranging from 3 to 120 kg/h, raising China’s ozone technology to the international level.
Ozone (O3) is a pale blue gas with a characteristic fishy odor. It has a higher relative molecular weight than air, is slightly soluble in water, and is more soluble in liquid nitrogen and alkaline solutions. Due to its inherently unstable nature, ozone exists in a transient state, consisting of a V-shaped molecule composed of three oxygen atoms. In its liquid form, ozone decomposes slowly at room temperature and rapidly at elevated temperatures, producing oxygen. It is highly reactive and can explode under impact or friction. The presence of a highly reactive oxygen atom makes ozone an excellent oxidant with a high oxidation potential, making it an ideal oxidizing agent that does not lead to secondary pollution. Ozone’s strong oxidizing ability also causes most pigments to fade, which is why it is often used as a bleaching agent.
Ozone is an extremely powerful oxidizing and bactericidal agent, and is one of the strongest oxidants found in nature. In water, its redox potential is second only to that of fluorine, with a value of 2.07 eV. After participating in redox reactions, ozone reverts back to oxygen, making it an efficient oxidant without producing secondary pollution [6]. As a strong oxidant, ozone has the following notable characteristics: (1) it can be used for selective oxidation, resulting in a high yield of the desired product [7]; (2) it has a low oxidation temperature and strong oxidation potential at normal pressure, which is advantageous for the oxidation of sensitive substances [8]; (3) it has a rapid reaction rate and can perform quantitative oxidation [9]; and (4) it is easy to use and manufacture [10].
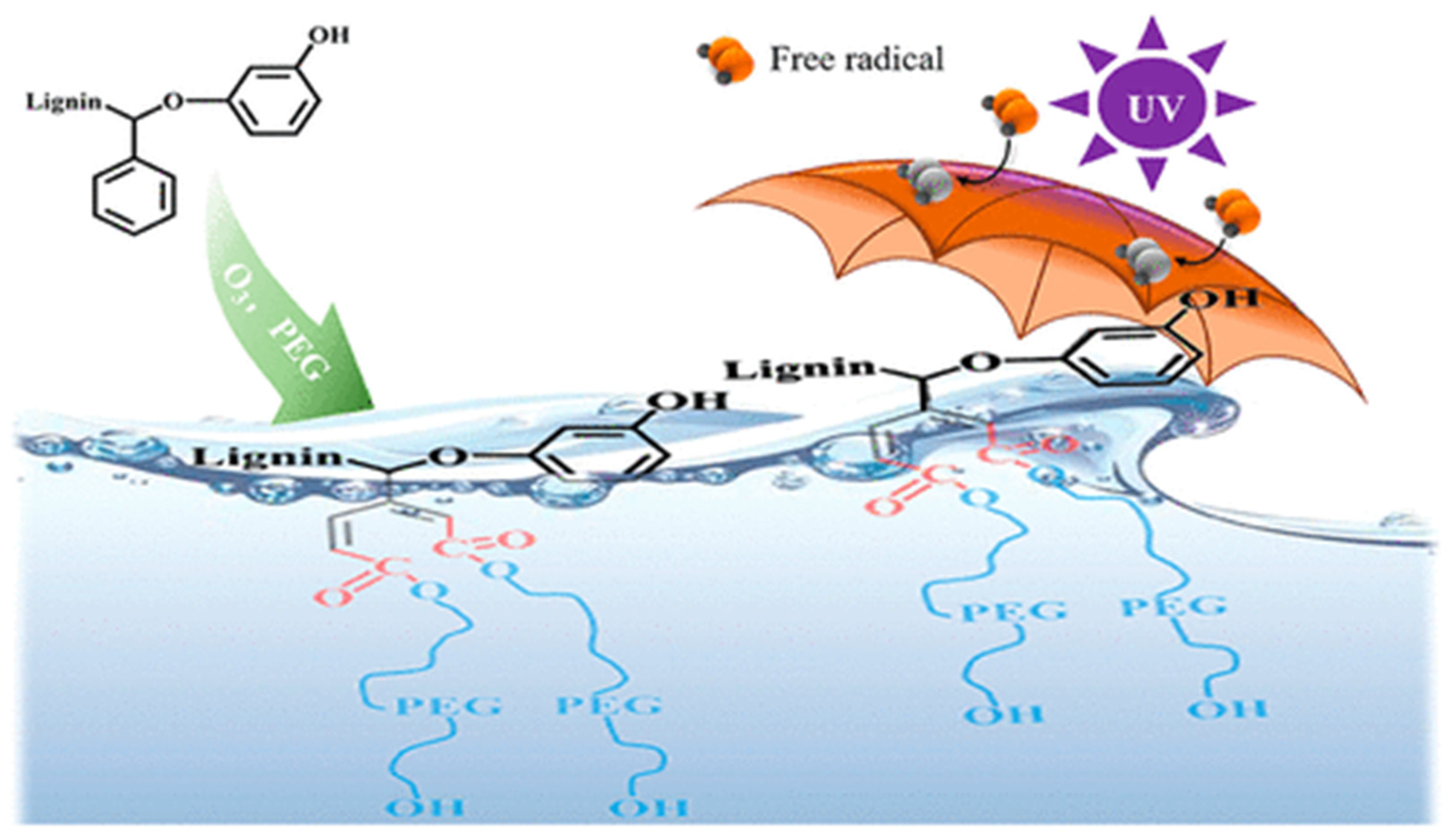
Ozone is also widely employed as a bleaching agent. In the pulp and paper industry, ozone effectively removes lignin, thereby increasing the whiteness of pulp [11]. In 1976, at the International Pulp Bleaching Conference, Krausler, Kraus, and Reich proposed a mechanism for ozone’s reaction with lignin and lignin model compounds. They suggested that when O3 reacts with lignin, it opens the aromatic ring at C3 and C4. As illustrated in Figure 1, a schematic diagram of the preparation of highly active lignin by ozone oxidation, this results in a highly polar structure that inhibits the chromogenic group’s ability to impart color. Ozone can also react with unsaturated double bonds in lignin, producing hydrogen peroxide, which further aids in bleaching. Ozone bleaching is highly efficient and environmentally friendly, indicating a promising future for its use in sustainable pulp bleaching processes.
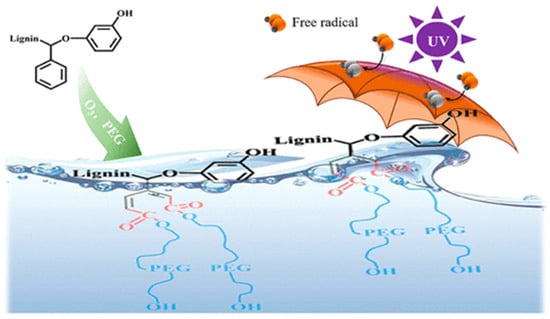
Figure 1.
Schematic diagram of preparation of highly active lignin by ozone oxidation [1].
The primary aim of this review is to provide a comprehensive overview of the advancements in ozone technology, focusing on its applications in environmental management, industrial processes, and medical treatment. By synthesizing the latest research, this review not only highlights the versatile applications of ozone in various fields but also critically examines the challenges and limitations associated with its widespread adoption. This paper is novel in its approach to integrating a wide range of applications of ozone, from water and air treatment to its emerging role in medical therapies, which have not been comprehensively addressed in previous literature reviews. The review also offers a discussion on the future directions of ozone technology, particularly in relation to the cost-effectiveness, scalability, and technological advancements needed to improve its practical applications.
2. Method of Ozone Preparation
Due to its physical properties, ozone is highly prone to decomposition, which makes it challenging to store and limits its application and widespread adoption. As a result, ozone is typically generated and used on-site. Currently, there are three primary methods for generating ozone: ultraviolet irradiation, electrolysis, and dielectric barrier discharge. The advantages and disadvantages of different methods have been organized in Table 1.

Table 1.
Comparation of ozone generation methods.
2.1. Ultraviolet Radiation
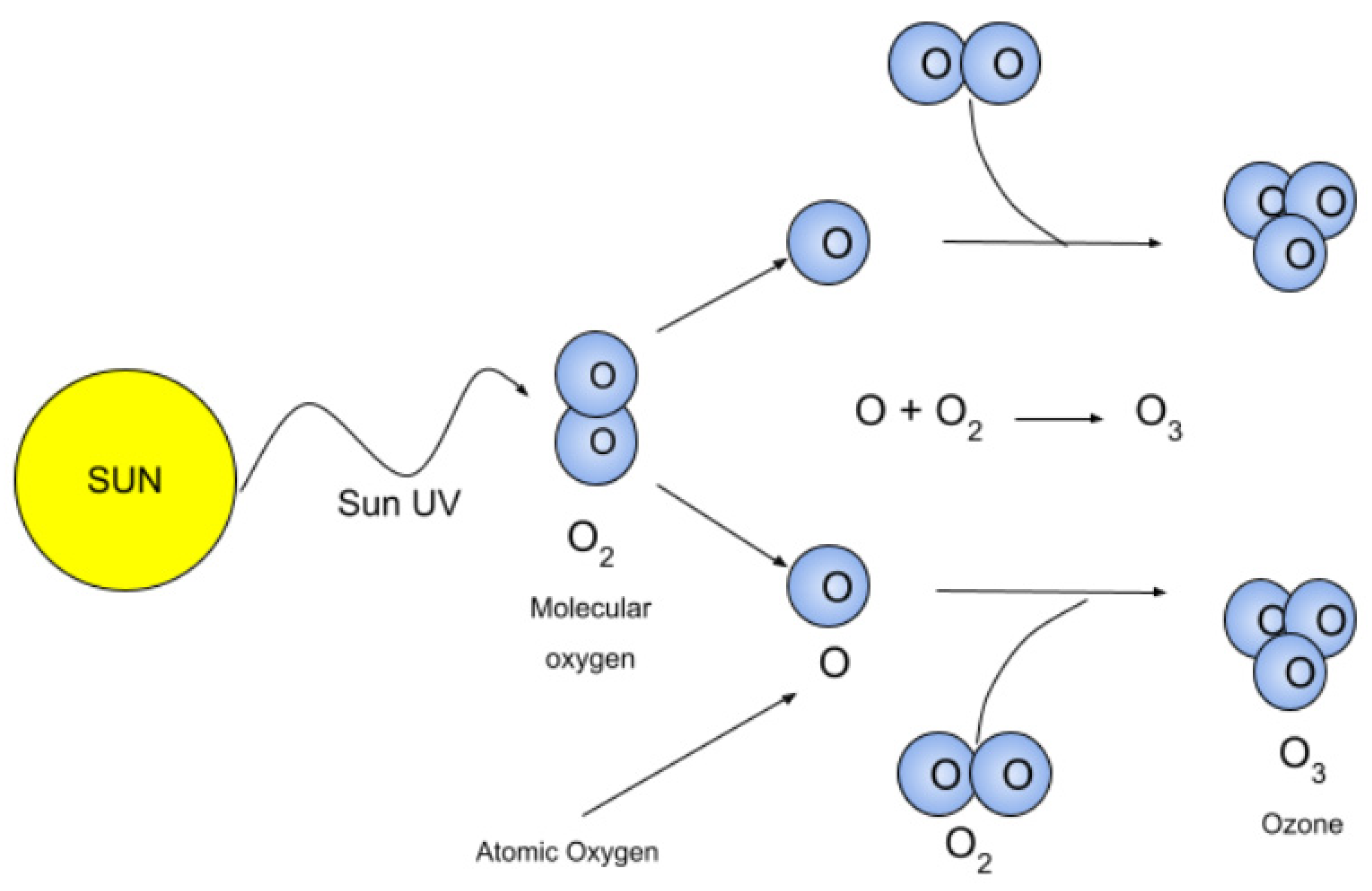
Ultraviolet light interacts with oxygen molecules, dissociating them into individual oxygen atoms. These oxygen atoms then combine with O2 molecules to form ozone (O3). For effective photolysis of O2 molecules, the radiation energy must be sufficient to dissociate O2 (i.e., with a wavelength λ < 242 nm), but must not be high enough to cause ionization (λ > 103 nm) [2].
In the stratosphere, under solar radiation, these reactions proceed continuously, resulting in both the continuous formation and destruction of ozone. Researchers have also attempted to produce ozone using UV radiation; however, the UV method has the significant drawback of low production efficiency. If all the UV energy is used to generate ozone, the production efficiency can be as low as 130 g/kWh [2,3]. Due to the low conversion efficiency of electrical energy into optical energy, the actual ozone production efficiency is usually less than 1 g/kWh [4]. The advantages of the UV irradiation method include good reproducibility and low humidity requirements, while the disadvantages include the low concentration of ozone produced, high power consumption, and strict radiation protection requirements. Consequently, the UV method is only suitable for a limited number of low-concentration applications, and its ozone yield is insufficient for large-scale production.
The process of generating ozone using external ultraviolet (UV) radiation involves using a UV light source to split oxygen (O2) molecules. The high-energy radiation emitted by the UV light source dissociates the oxygen molecules into individual oxygen atoms. These free oxygen atoms then recombine with other oxygen molecules to form ozone (O3). This process is typically carried out in specialized reactors to maximize ozone production and increase reaction efficiency, as illustrated in Figure 2.
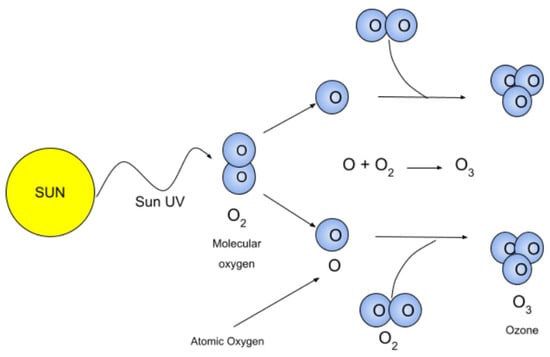
Figure 2.
A diagram showing the use of ultraviolet radiation to generate ozone, taken from [5].
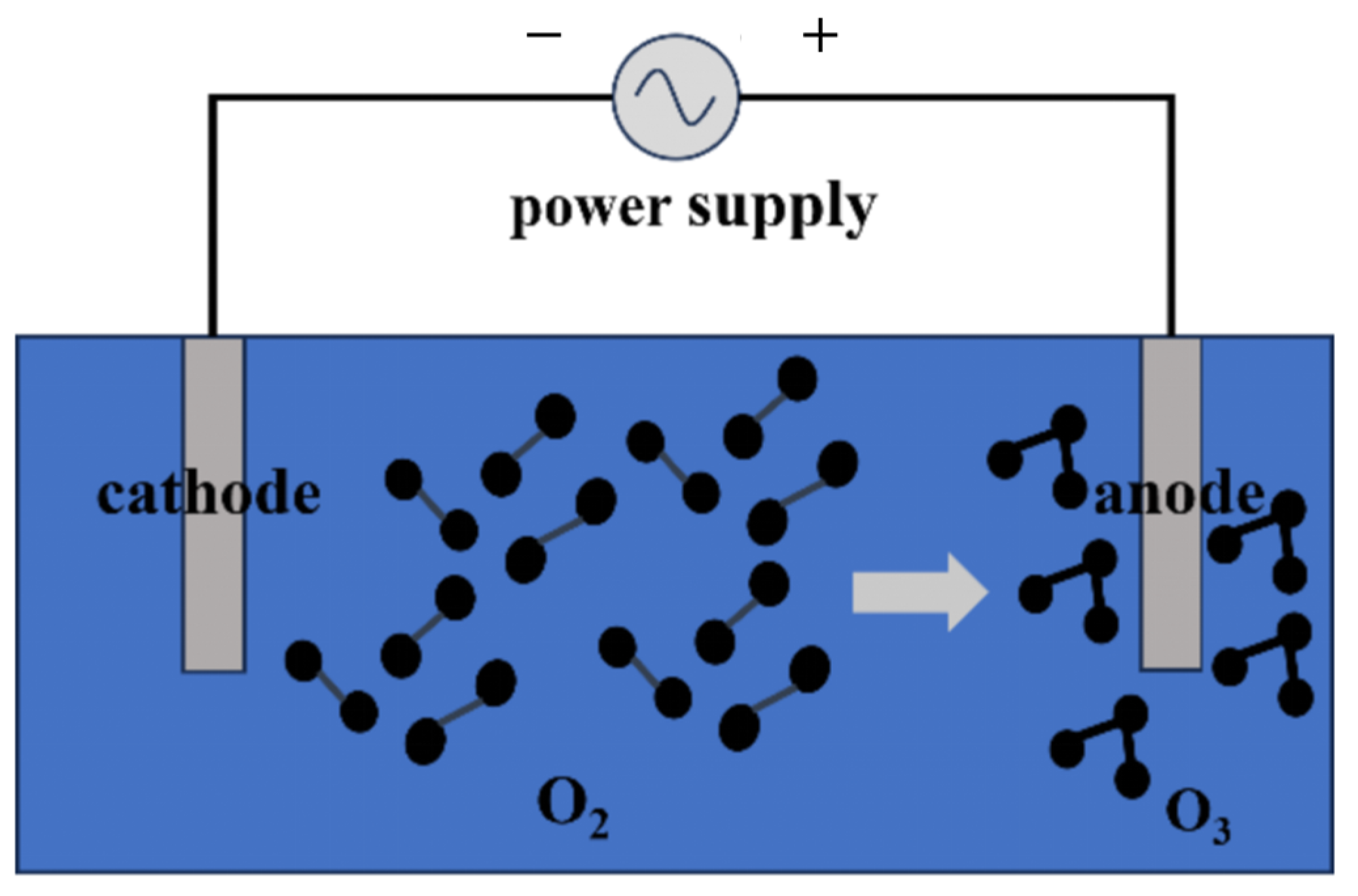
2.2. Electrochemical Ozone Production
Electrochemical ozone generation is a highly efficient and environmentally friendly method of producing ozone. This method uses an electric current to drive oxygen molecules in an electrolytic cell to generate ozone. Electrochemical ozone generation allows for precise control of the ozone production process and has significant practical applications across various fields, including water treatment and air purification. The main reaction involved in this process is as follows:
It is known that the electrochemical production of ozone occurs as a result of the oxidation of water on the surface of the anode of an electrochemical cell [6]. In this process, oxygen molecules, under the influence of an electric current, decompose to form ozone. The main factors affecting ozone production include the reaction temperature, anode type and composition, current density, electrolyte composition, and pH. Different anode materials (e.g., platinum, titanium, and electrodes coated with conductive materials) have a significant effect on the efficiency and yield of the electrochemical reaction. The current density determines the rate of ozone production, while the electrolyte composition and pH influence the electrochemical environment and overall reaction efficiency. A significant advantage of the electrochemical method is that it allows for ozone production in a closed system, enabling effective control of the reaction conditions so that external factors such as temperature and humidity have minimal impact on ozone production. The advantages of this method include the long equipment lifespan, high concentration of ozone produced, simplicity in equipment design, and ease of operation and maintenance. Additionally, because the reactions occur in a stable system, the electrochemical method can maintain consistent ozone production and quality over extended periods (Figure 3).
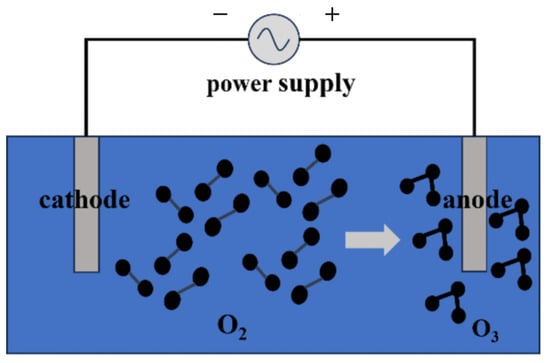
Figure 3.
Schematic diagram of electrochemical method for generating ozone.
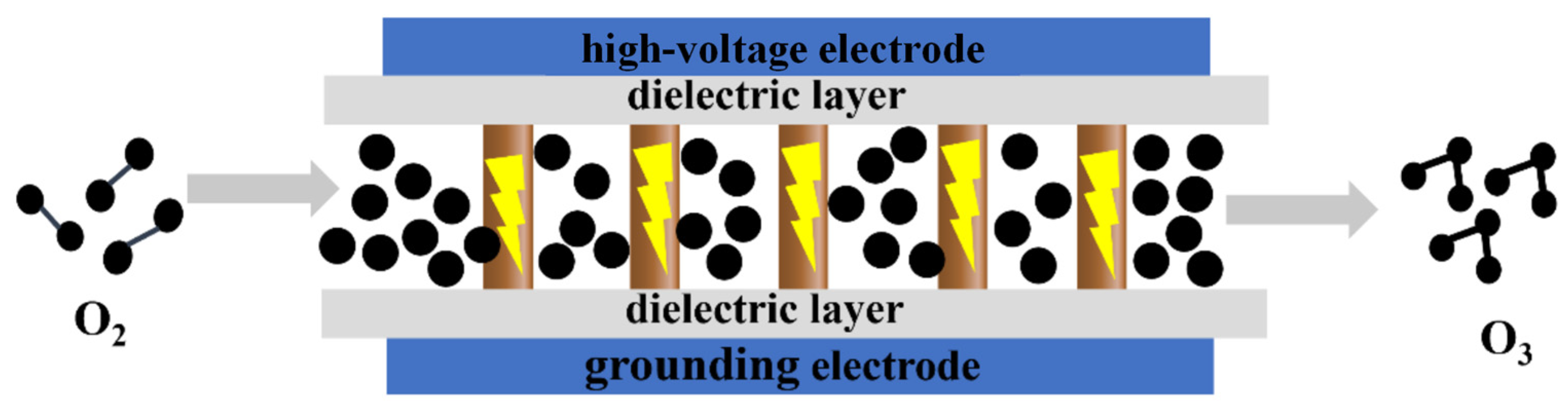
2.3. Dielectric Barrier Discharge
At present, there are several methods for producing ozone, including the ultraviolet (UV) oxidation method, electrochemical method, and dielectric barrier discharge method. The UV oxidation method involves irradiating oxygen with high-energy UV light to produce ozone. This approach has the advantage of being simple and suitable for small-scale applications. The electrochemical method generates ozone through an electrochemical reaction, with the advantage of producing a high concentration of ozone and working stably in a closed system. However, it has certain drawbacks, such as relatively low ozone production and the need for frequent electrode replacement. The dielectric barrier discharge method offers high ozone generation efficiency, simple equipment, and good stability. This method involves passing a dielectric barrier gas through a high-voltage alternating electric field, which results in corona discharge. The high-energy particles in the corona decompose oxygen molecules into oxygen atoms, which then collide and polymerize to form ozone molecules [7].
Among the components involved, M primarily refers to O2, O3, and N2. The oxygen atom in reactions (5) and (6) is generated through the excitation and dissociation of oxygen molecules by high-energy electrons produced during the blocked discharge of the medium in reaction (4).
The key factors influencing dielectric barrier discharge include the applied voltage and frequency [8,9,10], electrode material [10,11], gas pressure [15], gas composition [16], reactor structure [17,18], and other parameters. Recently, Gou et al. modified the electrode material by using a water flow as the ground electrode, replacing the traditional metal electrode. This approach resulted in a significantly improved ozone generation efficiency compared to that of commercial ozone generators. The ozone concentration reached approximately 13,000 ppm, with efficiency stabilized at around 200 g/kWh during a 400-min stability test [11]. Additionally, Kefeng Shang et al. introduced a volume–surface hybrid dielectric barrier discharge (V-SDBD) by affixing another grounded electrode to the dielectric surface. This configuration demonstrated superior ozone production and benzene decomposition efficiency compared to those of traditional volume dielectric barrier discharge (VDBD) and surface dielectric barrier discharge (SDBD) methods [18]. The dielectric barrier discharge method offers several advantages over UV irradiation and electrolysis, including a high ozone concentration, high yield, and excellent stability. As a result, it has become the focus of extensive research, with more and more studies being conducted on ozone production using this method (Figure 4).
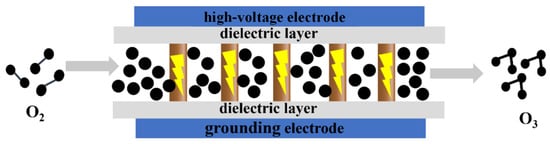
Figure 4.
Schematic diagram of ozone generation using the medium barrier discharge method.
2.4. Other Ozone Generation Methods
While ultraviolet irradiation, electrolysis, and dielectric barrier discharge remain the most widely adopted and proven methods for ozone production, several other techniques, including corona discharge, pulsed power technology, and silent discharge plasma, provide promising alternatives or enhancements for specific applications. These methods, while not as widely used as the primary three, offer advantages in particular scenarios, such as improved efficiency, scalability, or temperature sensitivity. They are best suited for specialized uses where the primary methods may face limitations.
2.4.1. Corona Discharge
The corona discharge method is one of the most commonly used techniques for industrial-scale ozone production. It involves applying a high-voltage electrical discharge to oxygen or air, which splits the oxygen molecules into individual atoms. These atoms then recombine with other oxygen molecules to form ozone. This method is highly efficient and cost-effective, especially for large-scale production. It is widely used in applications such as municipal water treatment, air purification, and industrial ozone applications due to its scalability and relatively low operating costs [7].
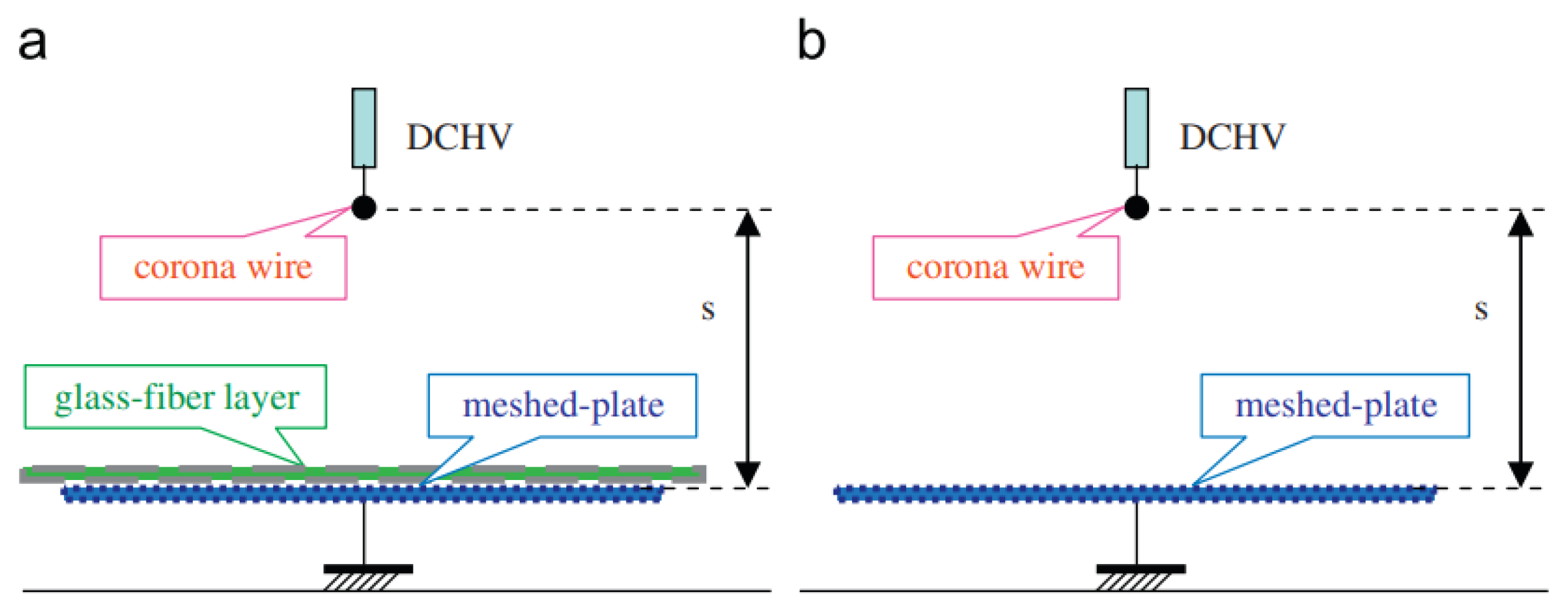
In recent years, innovations in electrode design and electrical configurations have significantly improved the efficiency and output of corona discharge ozone generators. Advanced dielectric barrier coatings and modified electrode geometries have been explored to enhance the ozone yield while minimizing energy consumption [8]. Jung et al. studied the ozone generation characteristics in a corona discharge system with a glass-fiber layer and found that the addition of the glass-fiber layer significantly enhanced the ozone output (Figure 5) [12].
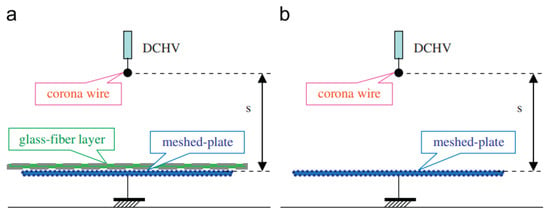
Figure 5.
Schematic configuration of the proposed discharge system with glass-fiber layer: (a) wire-plate system with glass-fiber layer, (b) conventional wire-plate system [12].
2.4.2. Pulsed Power Technology
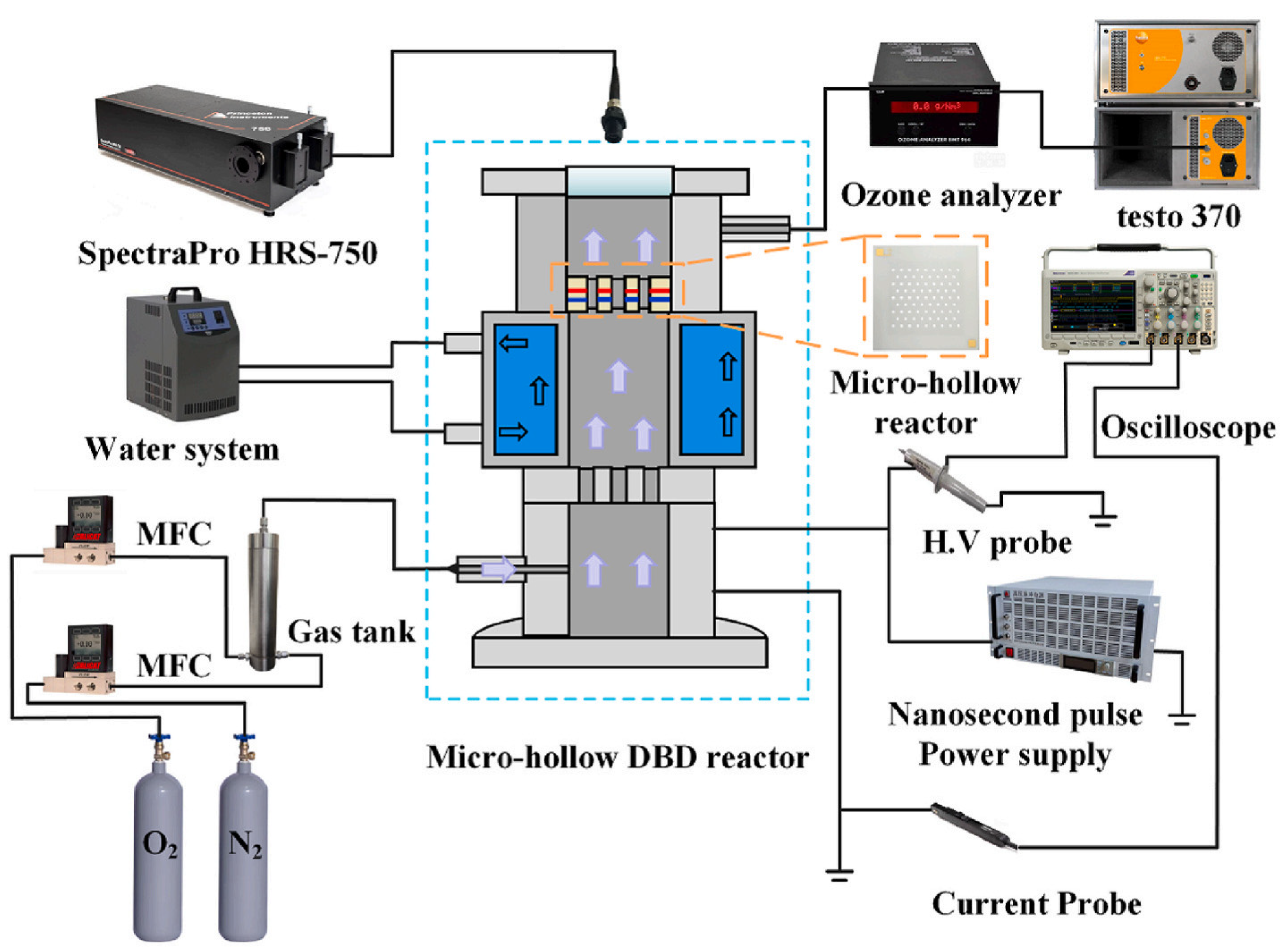
Pulsed power technology involves delivering high-energy electrical pulses into a gas, typically oxygen or air, to create a plasma state, which leads to ozone formation. This method offers the advantage of high energy efficiency and allows for precise control over ozone generation. Pulsed power technology is particularly beneficial in applications where rapid variation in ozone concentration is required, such as advanced oxidation processes (AOPs) for wastewater treatment or air purification. While still under development, it shows promise for various industrial and environmental applications, especially where flexible, on-demand ozone production is needed [19]. Jin et al. investigated ozone production and nitrogen oxide formation in a micro-hollow surface dielectric barrier discharge reactor driven by nanosecond pulses at atmospheric pressure for the first time (Figure 6) [13].
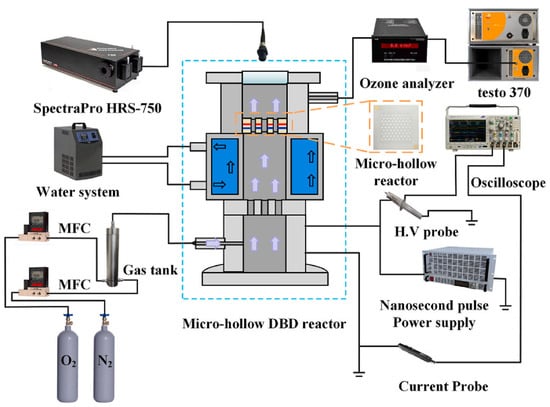
Figure 6.
A schematic of pulsed experimental system [13].
2.4.3. Silent Discharge Plasma
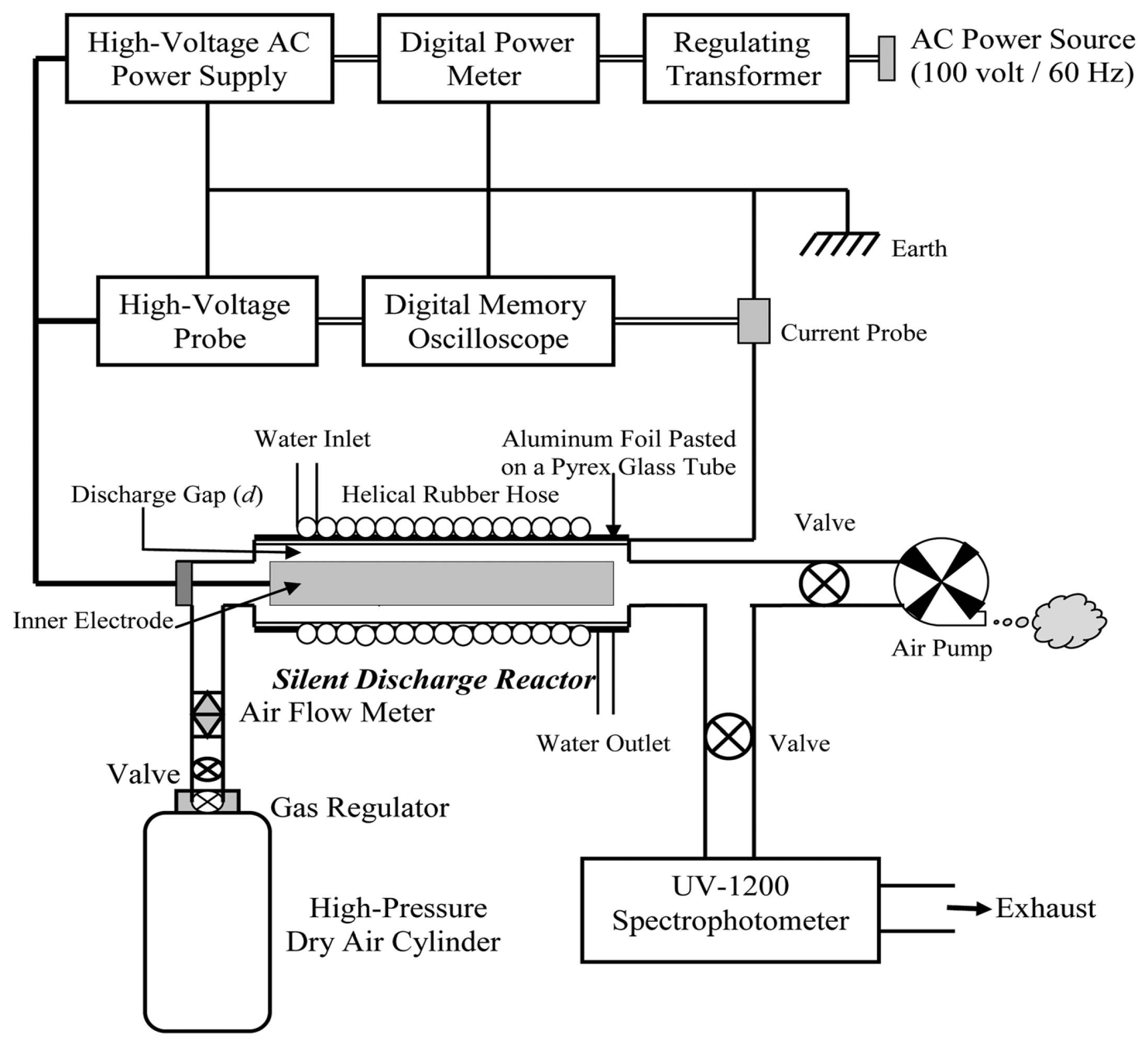
Silent discharge plasma, also known as cold plasma, generates ozone through a non-thermal plasma state, without visible sparks or audible noise. The process occurs under low temperatures, which makes this method ideal for applications involving temperature-sensitive materials. Silent discharge plasma is increasingly used in air and water treatment, food processing, and sterilization, as it offers the advantage of ozone production at low temperatures without generating excessive heat. Additionally, it selectively degrades pollutants and minimizes the formation of unwanted by-products, making it suitable for eco-friendly and efficient applications [20]. Ashraf Yehia derived two equations from the experimental setup shown in Figure 7 to predict ozone generation in dry air within a silent discharge reactor [14].
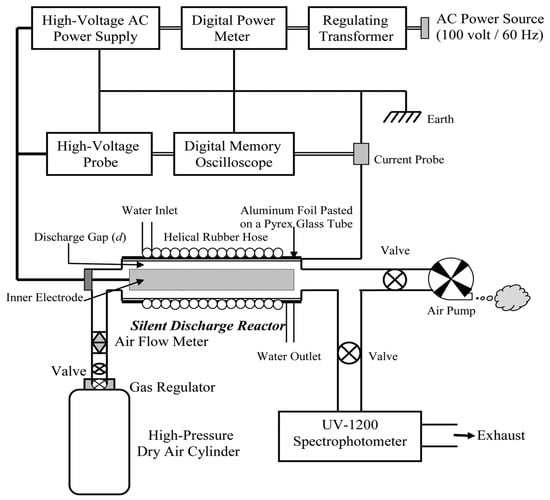
Figure 7.
Schematic diagram of the experimental setup [14].
3. Application of Ozone
3.1. Ozone in Environmental Management
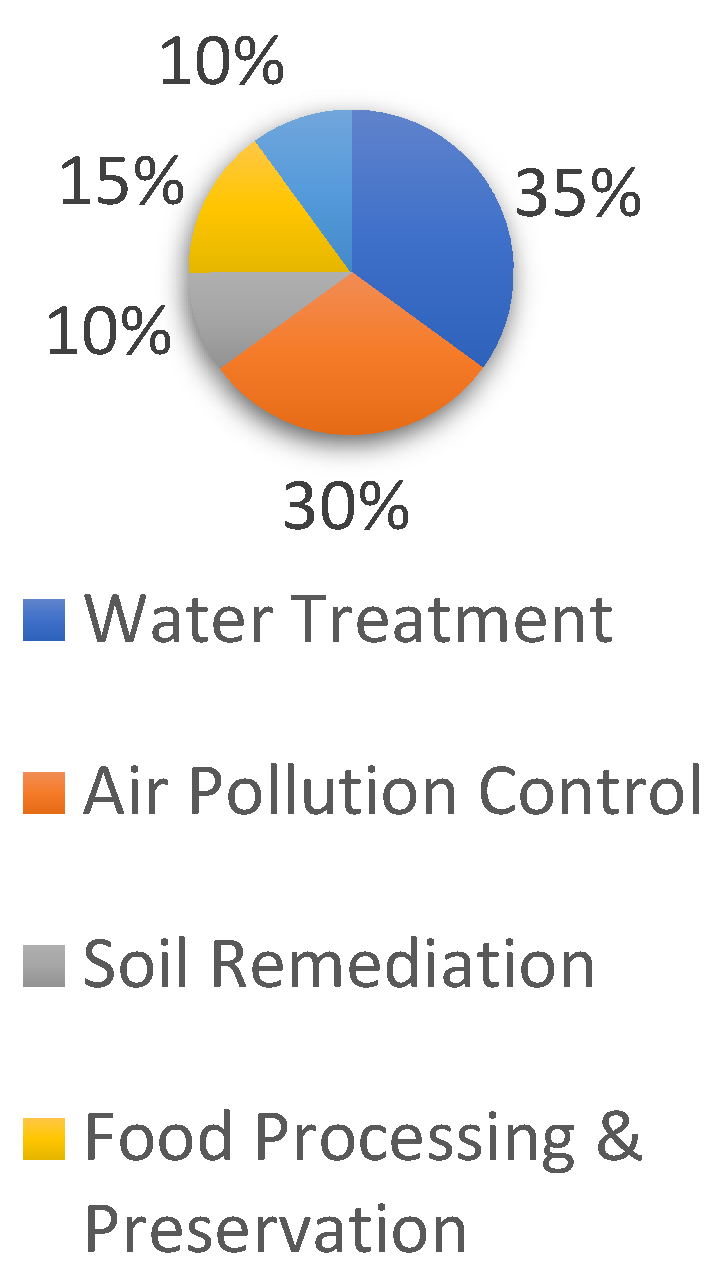
Before ozone became widely adopted in environmental management, chlorine was the most commonly used disinfectant. However, due to ozone’s superior oxidizing properties and its environmental benefits, it gradually replaced chlorine, which produces harmful by-products detrimental to human health. Additionally, because ozone is relatively easy to generate, it has found broad applications in water treatment, air pollution control, soil remediation, and other pollution control processes (Figure 8).
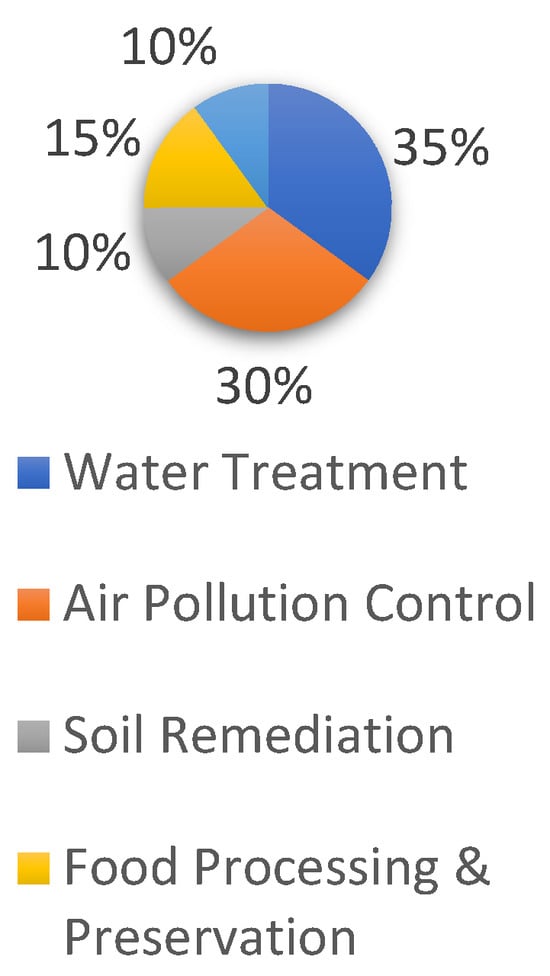
Figure 8.
Popularity of ozone applications.
3.1.1. Water Treatment
The strong oxidizing properties of ozone, its effective purifying ability, and the absence of secondary contamination allow it to play a significant role in water treatment. The use of ozone in water treatment was first initiated in France [21], and, today, it is widely employed for the disinfection of drinking water as well as in wastewater treatment. In addition to its disinfection capabilities, ozone is also effective in controlling the color and taste of water [22], making it a popular choice in municipal wastewater treatment.
Ozone has demonstrated strong efficacy in disinfecting and removing fungi and bacteria present in water. Studies have shown that a concentration of 1.5 mg/L of ozone can eliminate 99% of Candida albicans, while a dose of 60 mg/L can remove 78% of Aspergillus flavus [23]. Moreover, ozone effectively removes chlorine-resistant bacteria by damaging both their cellular structure and genetic material [24]. For example, after ozone treatment, the DNA content of Bacillus cereus spores sharply decreases, although some target gene fragments are retained. Additionally, the spore structure undergoes significant changes, leading to the release of intracellular substances.
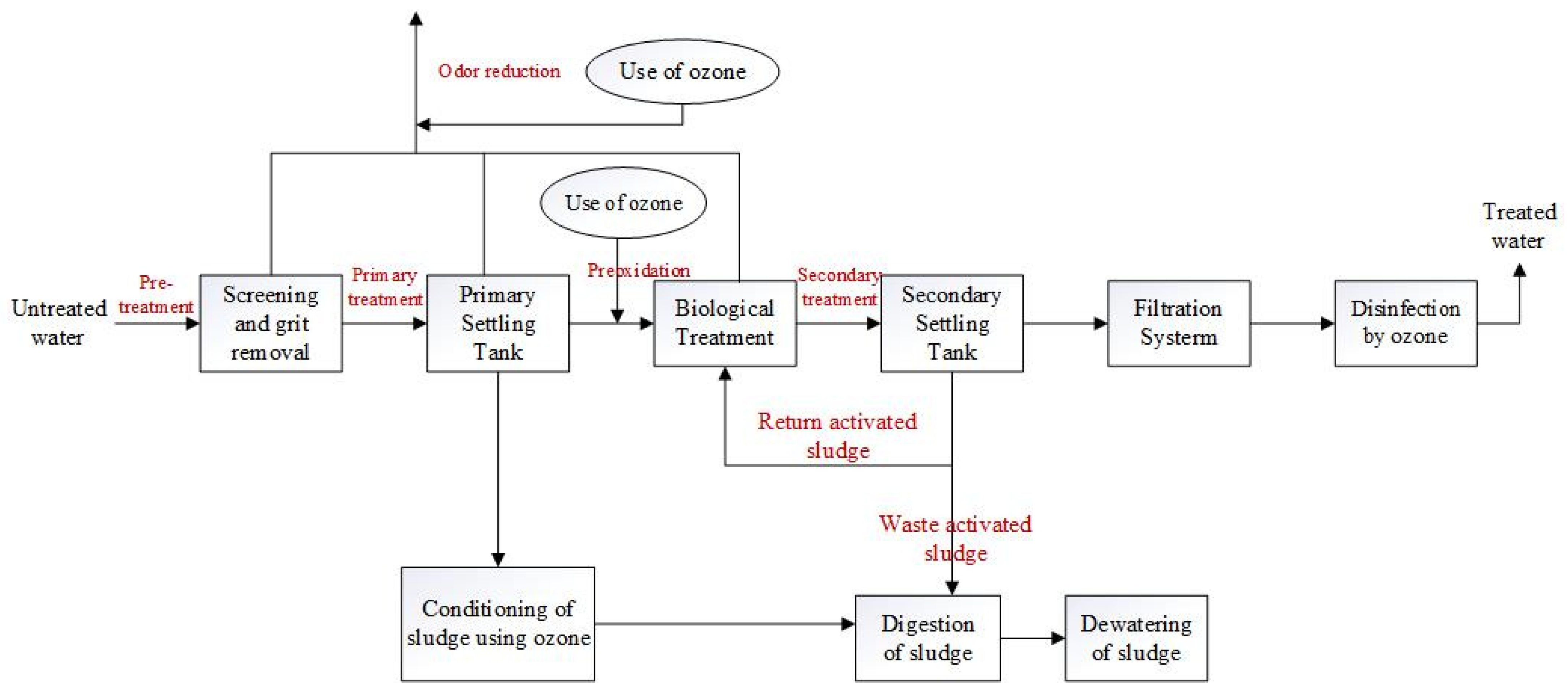
The efficiency of ozone in wastewater treatment is also notable: it disinfects more rapidly than chlorine and does not produce carcinogenic by-products. Pharmaceuticals are consumed in large quantities worldwide, and these pharmaceutical compounds ultimately reach wastewater treatment facilities after being excreted from the human body. Several studies have demonstrated that ozonation can effectively remove most pharmaceutical and personal care products (PPCPs) from secondary effluents within 10–15 min. Further research is required to optimize the ozonation process, particularly to minimize the ozone dosage while ensuring the simultaneous degradation of PPCPs [25]. Hollender et al. investigated the removal of PPCPs from secondary effluents using ozone, successfully removing approximately 55 micropollutants with various functional groups from municipal wastewater. Compounds with activated aromatic components, amine groups, or double bonds, such as sulfamethizole and carbamazepine, exhibited high second-order reaction rate constants with ozone (>104 M−1s−1) and were found to have concentrations below the detection limit of the O3/DOC (dissolved organic carbon) ratio, which was only 0.47. In contrast, other compounds, such as the antidepressant venlafaxine (amine), had an O3/DOC ratio of 0.62, indicating effective elimination [26]. Figure 9 shows the ozone treatment process of urban sewage.
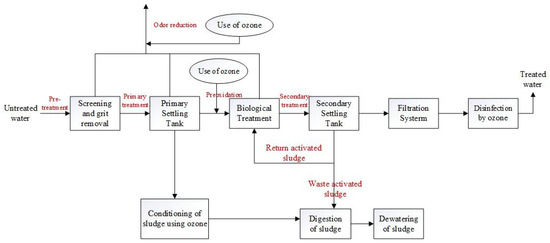
Figure 9.
Schematic diagram of ozone treatment of urban sewage [27].
Furthermore, the use of ozone oxidation for textile wastewater purification offers promising potential for reuse, providing both direct environmental and economic benefits. Research conducted by Tecnotessile showed that an ozone dosage of 30 g/m3 is sufficient to achieve the effective decolorization of textile wastewater, with a contact time of approximately 60 min. Increasing the dosage beyond this level did not yield additional improvements in decolorization. However, when the contact time is reduced to 30 min, an optimal dosage of 40 g/m3 is required [28]. Ferrero [29] also reported that the removal of chemical oxygen demand (COD) and detergents became effective when the ozone dosage exceeded 50 mg/L.
Overall, the application of ozone in water treatment has vast potential and promising prospects. With ongoing technological advancements and reductions in cost, ozone is expected to be utilized in more fields, playing an increasingly important role in the protection and sustainable use of water resources. At the same time, it is crucial to continue exploring and researching innovative water treatment technologies to address the escalating issue of water pollution and safeguard our water resources and ecological environment.
3.1.2. Air Pollution Control
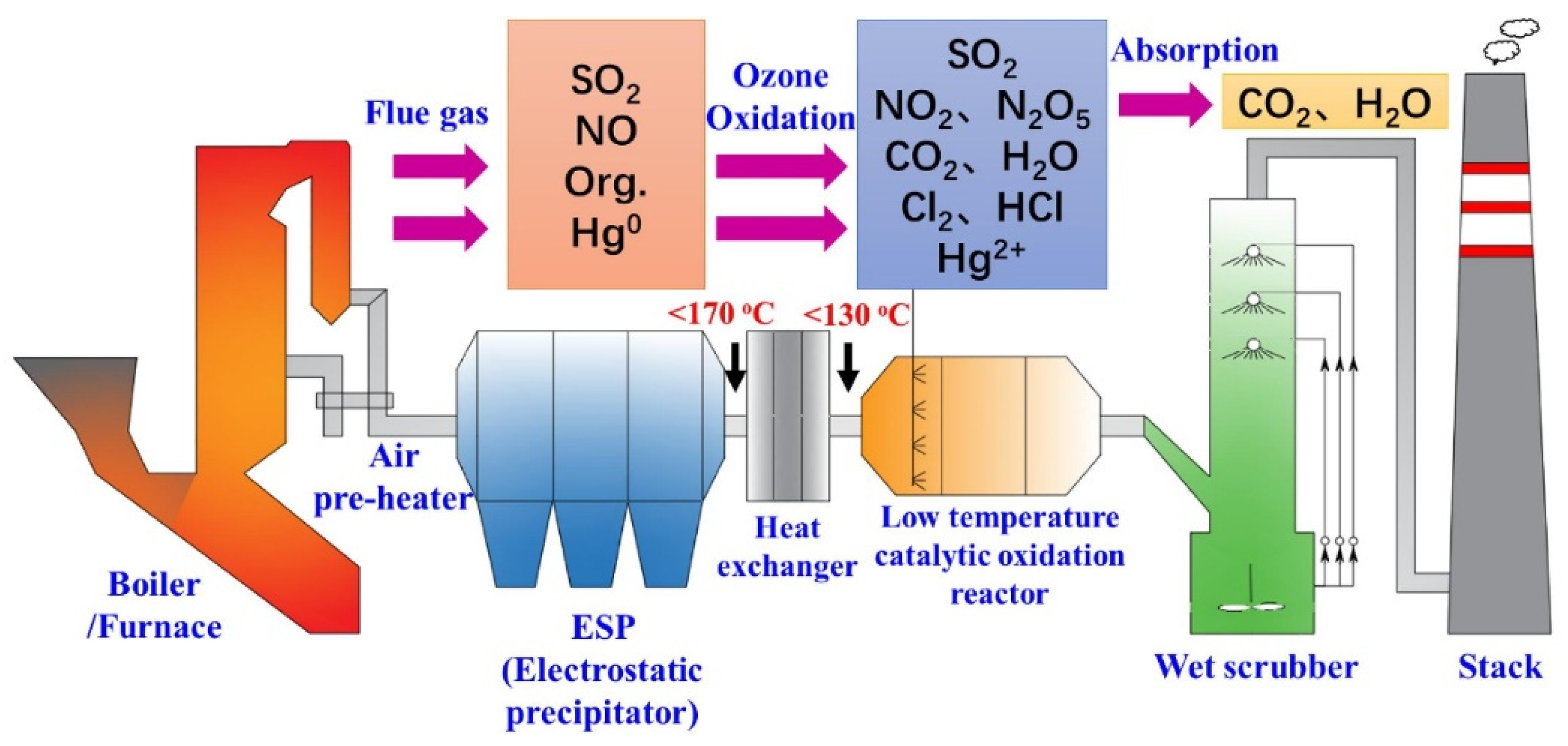
First and foremost, the application of ozone in atmospheric pollution control is primarily focused on the treatment of industrial waste gases. Industrial emissions often contain significant amounts of organic compounds and pollutants. If these substances are released directly into the atmosphere, they can cause substantial environmental damage. Ozone treatment technology offers an effective solution by oxidizing and decomposing these pollutants, transforming them into harmless substances, thus purifying the waste gases. Given that ozone oxidation of industrial gases generates non-toxic products such as oxygen, the following process steps can be implemented to render the emissions harmless: First, the industrial waste gases are captured and passed through a water filter. Next, the sulfuric and nitrous acids produced by the ozone treatment are completely and quantitatively converted into sulfuric and nitric acids. Finally, the resulting acids are neutralized into non-harmful products [30]. Ozone oxidation has the capability to simultaneously remove multiple pollutants, including SO2, NOx, organic pollutants, and mercury. A schematic diagram of the technical system is shown in Figure 10. Typically, after passing through the air preheater and dust control device, the flue gas temperature can be reduced to 200 °C, or in most cases, even below 170 °C. At these lower temperatures, ozone exhibits a high oxidation potential, which is favorable for the oxidation reactions. Additionally, the relatively clean conditions of the flue gas help to alleviate end-of-pipe treatment challenges, such as dust clogging and catalyst deactivation. Studies have shown that the optimal oxidation temperatures for NO and organic pollutants are below 130 °C. To ensure proper oxidation temperatures for odor removal, excess heat can be exchanged through a heat exchanger, and the recovered heat can be used for on-site heating via heat recovery technology. The ozone generated by medium barrier discharge can be effectively injected into an oxidation reactor, where it undergoes advanced oxidation with a small amount of ozone, aided by a catalyst. Under various ozone injection and reaction conditions, pollutants such as NO, organic compounds, and Hg0 are oxidized into NO2, N2O5, CO2, H2O, Cl2, HCl, Hg2+, and other substances. These highly soluble oxidized pollutants are then directed to the absorption section, typically a wet scrubber, where they are removed along with SO2. In summary, the ozone oxidation–absorption process has been well established and is a promising method for industrial gas purification [20].
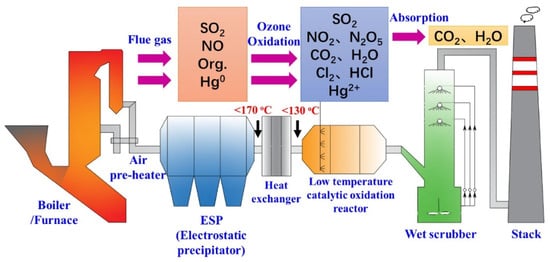
Figure 10.
Schematic diagram of ozone removing pollutants, cited from [20].
Secondly, ozone also plays a significant role in the treatment of vehicle exhaust. Vehicle emissions contain harmful gases such as nitrogen oxides (NOx) and sulfur dioxide (SO2), which are major contributors to air pollution. Ozone treatment technology reacts ozone with carbon monoxide (CO), nitrogen oxides (NOx), and unburned hydrocarbons (HC) in the exhaust, converting them into harmless carbon dioxide (CO2), nitrogen (N2), and water vapor (H2O) before they are released into the atmosphere. This process effectively reduces the atmospheric pollution caused by vehicle exhaust. Compared to the direct use of plasma technology, the addition of ozone leads to a higher conversion efficiency of NOx into NO2 under the same specific input energy (SIE) [31].
However, despite its significant advantages in atmospheric pollution control, the application of ozone faces certain challenges and limitations. For example, the concentration of ozone and treatment duration must be precisely controlled to prevent any unnecessary harm to the environment and human health. Additionally, the capital investment and operational costs associated with ozone treatment systems are important factors that must be considered.
3.1.3. Soil Remediation and Pollution Control
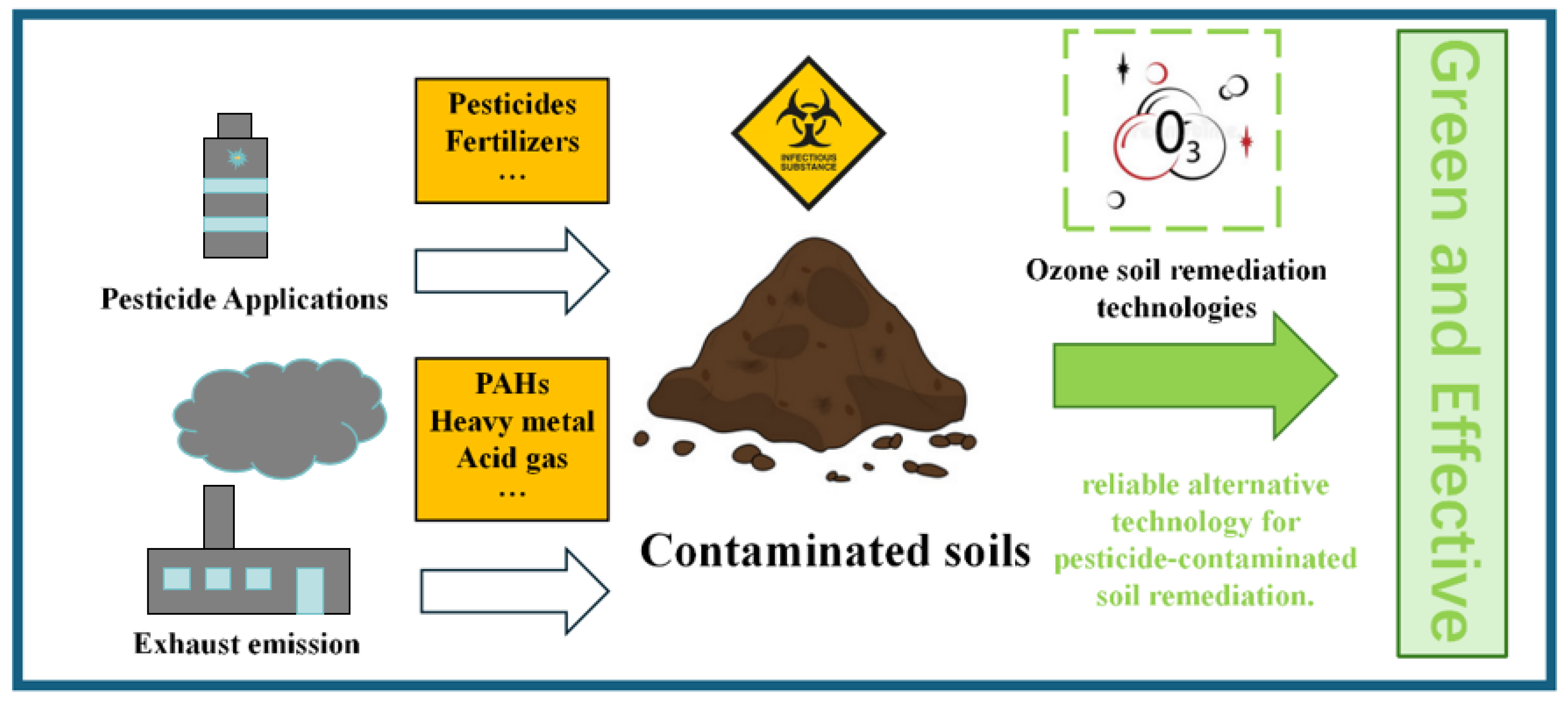
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous organic compounds primarily originating from the incomplete combustion of petroleum products and fossil fuels. Due to their persistence and significant environmental and health risks, PAHs are classified as priority pollutants in many countries. Given the persistence of PAHs in soil and their potential hazards, effective remediation methods are essential. Among various remediation technologies, ozone soil remediation has gained considerable attention due to its unique advantages. Ozone’s strong oxidizing properties allow it to effectively degrade PAHs, particularly those pollutants that are resistant to biodegradation. This ozone-based remediation method involves directly injecting ozone into the contaminated soil, ensuring targeted and efficient treatment. Consequently, ozonation has emerged as an attractive option for remediating PAH-contaminated soil, offering effective solutions for environmental matrices contaminated with recalcitrant pollutants [32]. Ozone soil remediation technology is an innovative approach that utilizes the strong oxidizing power of ozone to effectively treat contaminated soils, removing organic pollutants, heavy metals, and other harmful substances. By injecting ozone directly into the contaminated area, this method allows for quick reactions with refractory organic pollutants, significantly improving the soil quality [33]. Numerous studies have demonstrated the successful application of ozone and advanced oxidation processes in treating various forms of soil contamination [34].
In recent years, ozone soil remediation technology has seen further development and application. Among chemical methods, the ozone oxidation process has gained increasing attention due to its ability to rapidly degrade pollutants. Liu et al. [35] explored a coupled process of soil washing and ozone oxidation to remediate artificially contaminated soil. This combined approach not only enhanced remediation effectiveness but also expanded the range of applications for ozone soil remediation technology.
Ozone offers broad potential for soil remediation. Ozone oxidation can break down organic pollutants and toxic compounds in the soil, purify the soil environment, and even improve the soil structure, thereby promoting plant growth and supporting ecosystem restoration. However, when applying ozone for soil remediation, factors such as its environmental stability, by-product generation, and operational costs must be carefully considered. Therefore, combining ozone technology with other remediation methods, such as bioremediation and chemical remediation, can enhance efficiency and reduce costs. The integrated use of diverse technologies allows for a more comprehensive and effective approach to soil remediation (Figure 11).
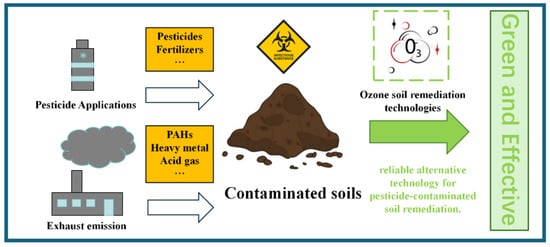
Figure 11.
Ozone remediation of soil contaminants.
3.2. Ozone in Manufacturing
3.2.1. Industrial Wastewater Treatment
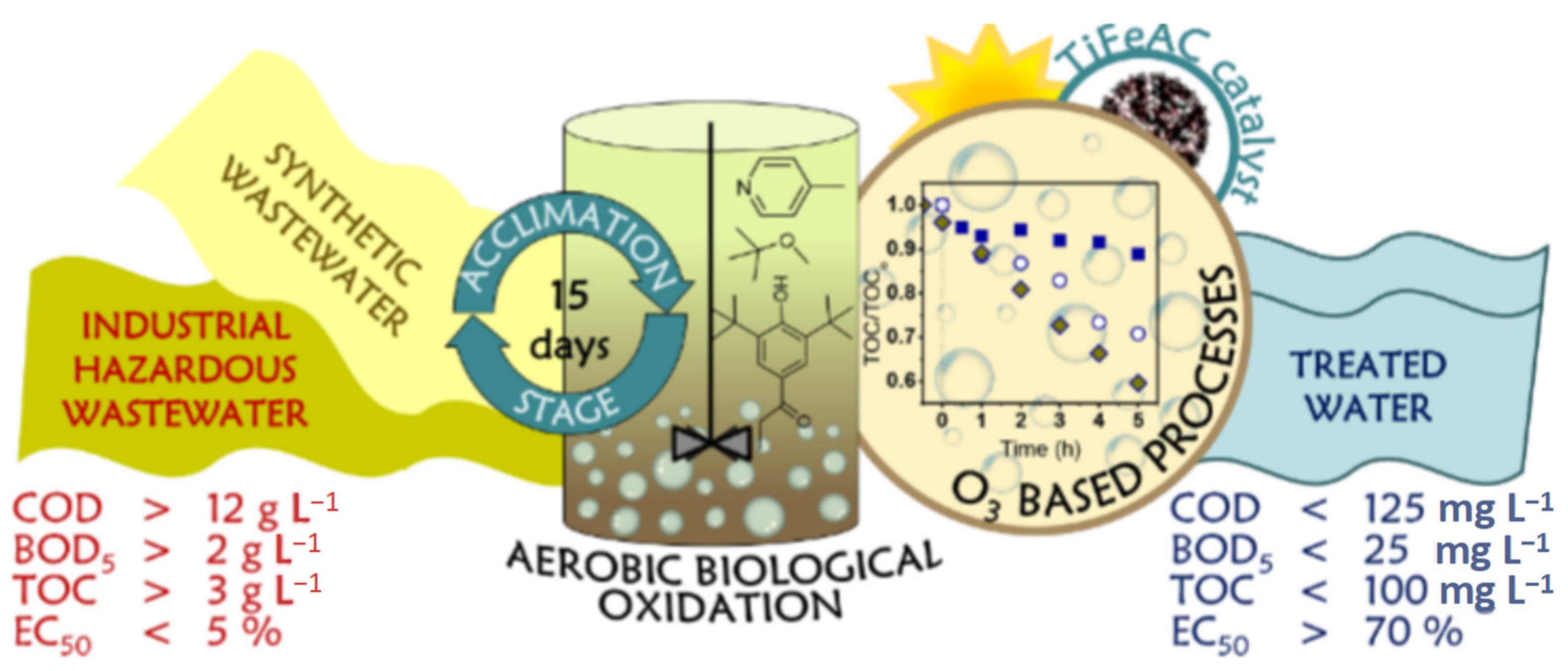
With the rapid pace of industrialization, wastewater treatment has become an increasingly critical issue, particularly for wastewater containing complex pollutants and hazardous substances, such as cyanide-containing effluents, coking wastewater, printing and dyeing wastewater, and municipal wastewater. These types of wastewater not only pose a significant environmental threat but can also cause long-term damage to human health. In this context, ozone treatment technology has garnered widespread attention due to its unique advantages. Ozone is widely utilized in wastewater treatment, primarily due to its excellent reliability and reactivity. As a broad-spectrum disinfectant and a strong oxidant, ozone possesses powerful oxidative properties that allow it to effectively remove a wide range of pollutants. It can efficiently degrade harmful substances such as pathogens, persistent organic pollutants, pesticides, and heavy metals found in wastewater. The application of ozone in treating industrial wastewater—such as cyanide-containing effluents, coking wastewater, printing and dyeing wastewater, as well as municipal wastewater—deserves further exploration, particularly in the context of drinking water disinfection and pre- and post-treatment processes [33]. Ozone’s strong oxidizing ability allows it to act as both a disinfectant and an advanced oxidant in wastewater treatment. As illustrated in Figure 12, an advanced oxidation process (AOP), which combines aerobic biodegradation and ozone treatment through a sequential batch reactor (SBR), has been shown to achieve a pollutant removal rate of 50% when applied to industrial wastewater with high organic loads (TOC > 3 g/L, COD > 12 g/L, BOD5 > 2 g/L) [36].
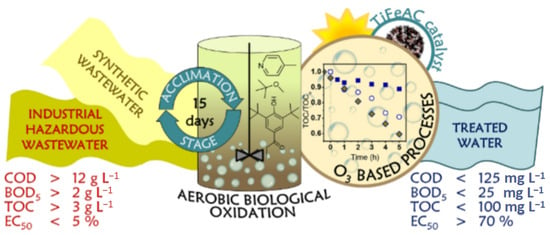
Figure 12.
Schematic diagram of wastewater treatment [36].
Every day, large volumes of wastewater from various industries and facilities worldwide continue to contain harmful substances such as pathogens, persistent pollutants, pesticides, and heavy metals like arsenic, chromium, copper, boron, and nickel. These pollutants pose significant risks to both human health and the environment. A typical example is the molasses brewing industry, which generates wastewater with high concentrations of degradable organic matter and dissolved salts. For heavily polluting industries such as molasses brewing, ozone treatment technology has demonstrated considerable potential. P. Asaithambi and colleagues conducted a comparative study on the pollutant removal efficiency of three different treatment methods: ozone oxidation, electrocoagulation, and ozone-assisted electrocoagulation. They found that ozone-assisted electrocoagulation was the most effective process for removing pollutants [37]. In addition to this, A.M. Chávez et al. proposed a combined process of aerobic biological degradation (SBR) and ozone-based advanced oxidation processes (AOPs). This process uses SBR to treat industrial and municipal wastewater (e.g., MIW wastewater) by biodegrading the water with activated sludge. By combining biodegradation with ozone oxidation, this method more effectively removes harmful substances and achieves superior wastewater treatment. This combined approach has shown great promise as an efficient treatment solution [36].
As technology continues to advance, future developments in ozone treatment will focus on enhancing efficiency, safety, and environmental sustainability. Ozone treatment is expected to become increasingly widespread and popular in the field of industrial wastewater treatment.
3.2.2. Food Processing and Preservation
With the improvement of living standards and growing awareness of health among consumers, food processing and preservation technologies are receiving increasing attention. Ozone, as a highly effective oxidant, holds significant potential in the field of food processing and preservation. This paper provides a comprehensive review of the application of ozone in food processing, focusing on its role in inactivating mycotoxins and preserving fruits and vegetables. The aim is to offer insights that can guide the further development of ozone technology in this area.
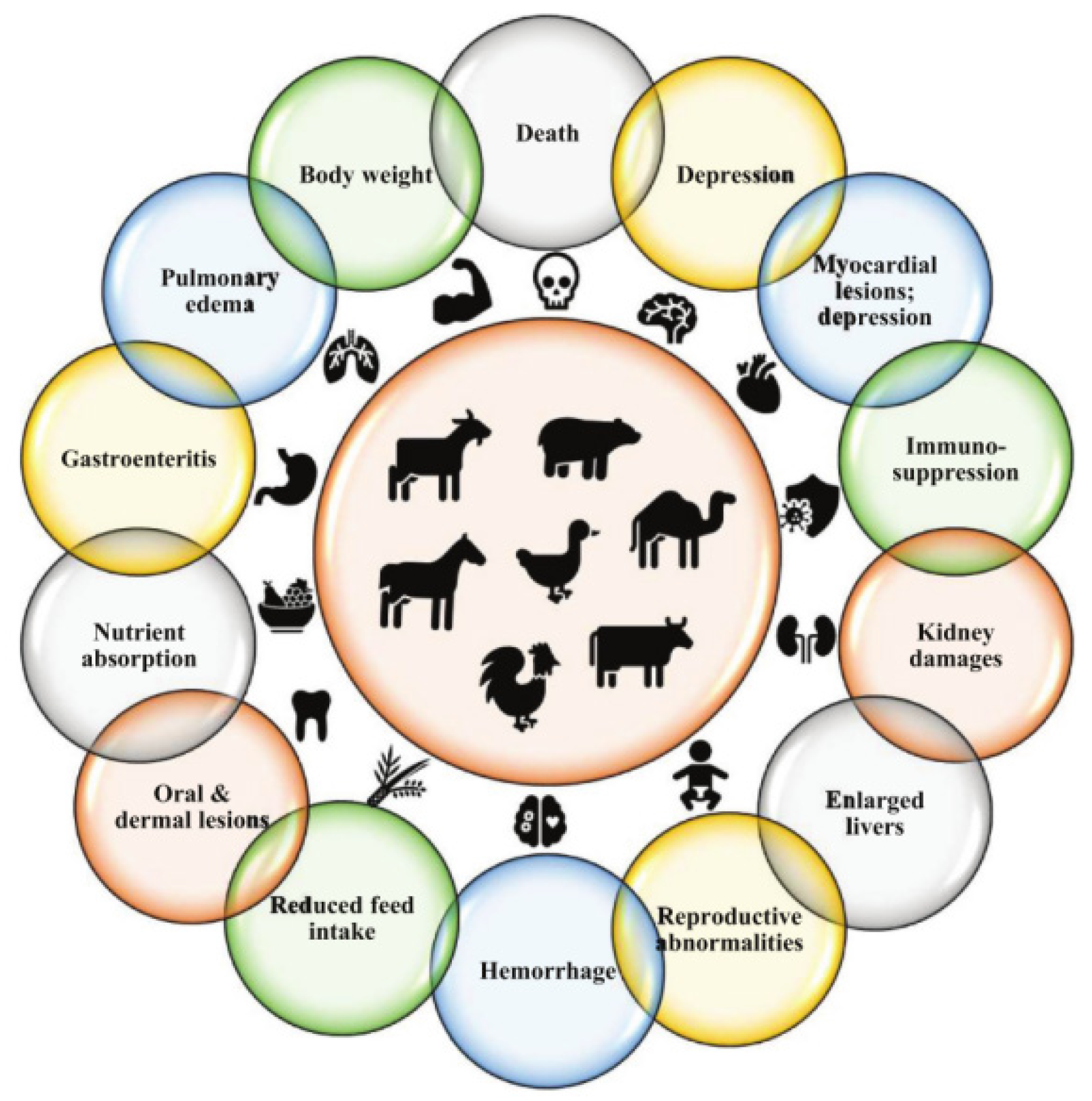
Mycotoxins are toxic metabolites produced by fungi, which pose serious health risks. As shown in Figure 13, these toxins are present in various foods worldwide [38] and are commonly found in food and feed products. The genera Aspergillus, Fusarium, and Penicillium are the primary producers of mycotoxins. Aspergillus, in particular, is known for producing nephrotoxins (e.g., ochratoxin A, OTA) and carcinogens (e.g., aflatoxins, AFs), and is prevalent in many food products. Studies have demonstrated that ozone gas can effectively inactivate Fusarium and Aspergillus, making ozone treatment a safe and environmentally friendly technology for food preservation and contamination control [39]. Fruits and vegetables, while essential for a healthy diet, are highly perishable and prone to spoilage after harvest, compromising both their quality and safety. Research by Elodie Sarron et al. has shown that ozone treatment can prevent and control microbial growth on vegetables, preserving their appearance, sensory qualities, and nutritional properties. Ozone can be applied in both the liquid and gas phases: in the liquid phase, it can disinfect processed water and vegetables, while in the gas phase, it can help disinfect and preserve vegetables during storage [40]. The versatility of ozone makes it a promising food processing agent. Its rapid decomposition and low residual effect make it an attractive alternative to traditional preservatives used in food storage. Ozone technology is gradually replacing conventional hygiene and fumigation methods, such as chlorine, steam or hot water treatments, and insecticides like phosphine, aluminum phosphide, and methyl bromide (fumigation) [41]. This shift reflects ozone’s growing appeal as a green, safe, and effective solution in the food preservation industry.
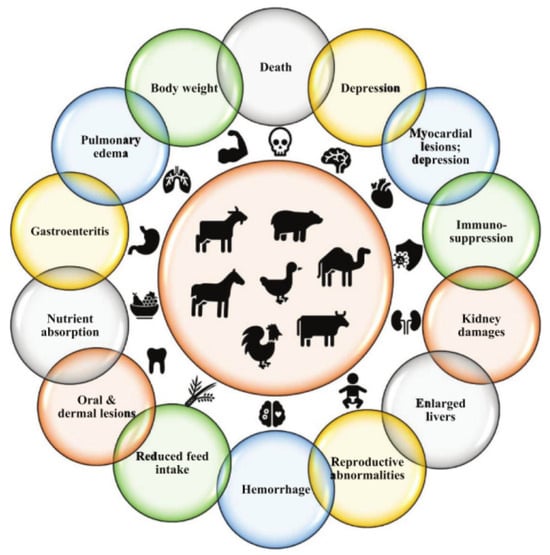
Figure 13.
Effects of mycotoxins on animal health [38].
Although ozone offers promising applications in food processing and preservation, several challenges remain. First, the generation and utilization of ozone require specialized equipment and technical expertise, placing significant demands on both the technological capabilities and management skills of the enterprise. Second, the concentration and exposure time of ozone must be carefully controlled to prevent any adverse effects on food quality and safety.
In conclusion, ozone holds substantial potential in the field of food processing and preservation. With ongoing research and technological advancements, ozone technology is expected to provide safer and more effective preservation solutions, aligning with consumer demand for healthy, high-quality food products.
3.3. Ozone in Medical Treatment
Ozone, a gas with unique chemical properties, has gained widespread application in the medical field in recent years. Its remarkable bactericidal, anti-inflammatory, antioxidant, analgesic, and immunomodulatory effects have made it a valuable therapeutic agent in various medical areas, including orthopedics, pain management, and cancer treatment.
In orthopedics and pain medicine, ozone is particularly effective due to its anti-inflammatory and analgesic properties. For chronic pain conditions such as neck, shoulder, back, and leg pain, ozone therapy has been shown to significantly reduce patient discomfort. Additionally, ozone has demonstrated effectiveness in the acute and recovery stages of cerebral hemorrhage, improving cerebral oxygenation and promoting patient recovery through multiple mechanisms [42]. Minimally invasive oxygen–ozone (O2-O3) therapy, which leverages the biochemical effects of O2-O3 mixtures, is commonly employed to treat musculoskeletal pain. Current data suggest that minimally invasive O2-O3 therapy for musculoskeletal neuropathic pain may be both beneficial and relatively safe [43]. Ozone therapy has long been used for its ability to inactivate a wide range of viruses and tumors, and it is increasingly being utilized in the treatment of musculoskeletal disorders, including rheumatoid arthritis, lumbar facet joint syndrome, subacromial bursitis, and carpal tunnel syndrome [42].
Cancer, one of the leading causes of death globally, is typically treated with radiotherapy and chemotherapy. However, these treatments can induce oxidative stress and chemotherapy-induced peripheral neuropathy (CIPN), damaging healthy tissue. While options for preventing or managing these side effects remain limited, ozone therapy has shown promise by inducing controlled oxidative stress, which triggers adaptive antioxidant responses in healthy tissues, potentially mitigating some of the damaging effects of cancer treatments [44,45].
Recent advancements have also highlighted ozone’s potential in cancer therapy. Studies have demonstrated that medical ozone can inhibit the growth of more than 90% of lung, breast, and uterine tumor cells. Furthermore, ozone can induce tumor cell death through the rapid generation of reactive oxygen species, laying the groundwork for its potential use in immune checkpoint blockade therapy. Bernardino Clavo and colleagues have reviewed the most significant studies on ozone as an adjuvant in cancer therapy, finding that ozone improves hemoglobin dissociation curves, increases 2,3-diphosphoglycerate levels, enhances local blood flow, and alleviates tumor hypoxia, all of which support its potential benefits in cancer treatment [46]. Ozone therapy has demonstrated a wide range of therapeutic effects, from pathogen disinfection to cancer treatment and pain management. In clinical practice, ozone is typically used adjunctively, often in combination with other treatments to enhance or complement their therapeutic mechanisms. Although its use as a standalone treatment remains controversial, ozone’s effectiveness when combined with other therapies is widely recognized [47].
In conclusion, the application of ozone in medicine holds vast potential. With continued research into its properties and therapeutic efficacy, it is expected that ozone will play an increasingly important role in various medical fields, contributing significantly to advancements in human health (Table 2).

Table 2.
Key findings on ozone applications.
4. Ozone in the Power Industry
Ozone (O3), a potent oxidizing agent, has demonstrated significant potential in the power industry, particularly in areas such as water treatment, air purification, and equipment maintenance. Its unique chemical properties enable ozone to play a crucial role in enhancing the power production efficiency, ensuring the smooth operation of equipment, and contributing to environmental protection.
4.1. Ozone in Water Treatment
In the power industry, particularly in thermal and nuclear power plants, water treatment plays a crucial role in ensuring the stable operation of power generation. Given the significant volume of water required in the power production process, wastewater treatment and cooling water management are of paramount importance. While wastewater treatment is addressed in Section 3.2.1, the focus here shifts to the ozone treatment of cooling water.
Cooling water treatment is a key application of ozone technology in the power sector, as cooling systems often encounter issues with algae and bacterial growth. Chlorination has traditionally been used to prevent biological fouling in cooling water systems. However, chlorination has notable drawbacks, including health risks, acute toxicity, and the potential to form harmful chlorinated organic compounds [48]. As a result, there is increasing interest in alternative methods for cooling water treatment. Ozone has shown significant promise as an effective solution for controlling water quality in cooling systems. It offers several advantages, including (1) providing effective corrosion control for most alloys, (2) removing existing scale and preventing its recurrence, and (3) damaging or inactivating microorganisms that may cause biocorrosion or biopollution [49]. Furthermore, with ongoing advancements in this technology, a novel cooling water treatment approach, the Integrated Ozone Treatment Cooling System (IOTCS), has been proposed. This system combines pinch analysis with mathematical programming to optimize heat exchanger configurations, maximizing water and energy savings while minimizing the overall costs [50].
4.2. Effect of Ozone Air Purification
Maintaining good air quality during power generation is essential, particularly in the context of plant maintenance and servicing. The combustion of coal and other fossil fuels in conventional thermal power plants results in a range of emissions, including carbon dioxide, sulfur oxides, nitrogen oxides, particulate matter, and heavy metals. These emissions not only contribute to environmental pollution but also pose significant risks to human health.
Today, the efficiency of exhaust gas purification has been enhanced through the use of specialized ozone-saturated solutions [51]. Ozone can effectively improve the working environment by eliminating odors, volatile organic compounds (VOCs), and microorganisms from the air. This is especially beneficial in enclosed or semi-enclosed workspaces, where ozone’s bactericidal properties can help reduce the transmission risk of pathogenic microorganisms, thereby protecting worker health.
4.3. Application of Ozone in Equipment Maintenance and Protection
The use of ozone in plant maintenance should not be overlooked. Over extended periods of operation, equipment in the power industry can be significantly affected by various pollutants, leading to a decline in performance. Currently, oxidants such as potassium permanganate, nitric acid, and hydrogen peroxide, along with complexing agents like oxalic acid, are used to decontaminate primary circuit equipment in nuclear power plants. However, the high concentrations of reagents, the multi-stage nature of the process, and the need for multiple treatment cycles to achieve the desired effectiveness with conventional techniques result in large volumes of waste. Ershov et al. have demonstrated that concentrated ozone is an effective and environmentally friendly technology for decontaminating nuclear power plant equipment [52].
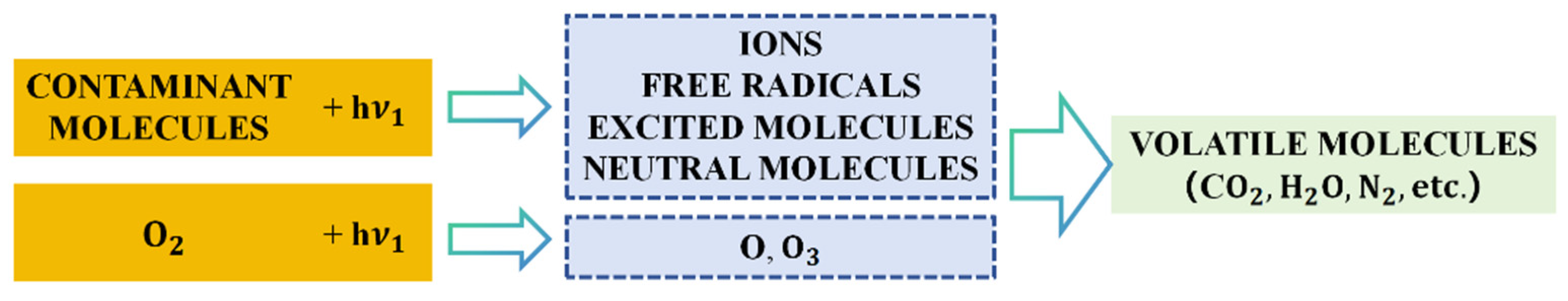
Ultraviolet (UV) ozone surface cleaning methods are also well established for treating surfaces to remove contaminants. This process involves photo-oxidative decomposition, where contaminants are excited or dissociated by short-wavelength UV light. UV light at 245.4 nm decomposes oxygen, producing oxygen atoms and ozone. Ozone then absorbs longer-wavelength radiation, breaking down into oxygen atoms as well. The excited pollutant molecules and free radicals generated from pollutant dissociation react with atomic oxygen to form simpler, volatile molecules, which are subsequently removed. UV ozone is also used for cleaning alumina substrates during the processing of thin-film hybrid circuits [53]. The schematic diagram is shown in Figure 14, as quoted from [53].

Figure 14.
Simplified schematic representation of UV/ozone cleaning process cited by [53].
This highlights that ozone can serve as an effective cleaning agent to remove oil, dust, and other pollutants from equipment surfaces, playing a crucial role in maintaining equipment cleanliness and hygiene. Additionally, ozone treatment of insulating materials is particularly valuable. It has been shown that the resistance and service life of insulating materials treated with ozone can be significantly improved, thereby reducing equipment failures and downtime, while enhancing the overall reliability of power production.
5. Conclusions and Outlooks
The origin of ozone (O3) can be traced back to 1839, when Schube first confirmed that it is formed through the combination of oxygen molecules (O2) and oxygen atoms (O). Ozone is widely utilized across various industries and applications, including water treatment, bleaching, food preservation, and medicine, primarily due to its strong oxidizing and sterilizing properties. Ozone can be produced through three main methods: ultraviolet radiation, electrochemistry, and dielectric barrier discharge. The ultraviolet method is less efficient and is typically used for small-scale production. The electrochemical method, on the other hand, generates high and stable ozone concentrations in a closed system, though the output is generally low.
In the realm of environmental management, ozone has found broad application in several key areas, including water treatment, air pollution control, and soil remediation. In water treatment, ozone’s powerful oxidizing properties and its ability to avoid secondary pollution make it highly effective at removing harmful microorganisms and pollutants, thereby improving water quality. In air pollution control, ozone plays an important role in decomposing organic matter and pollutants found in industrial waste gases. It can also convert harmful gases in automobile exhaust into harmless substances. Finally, ozone has proven effective in soil remediation, where its strong oxidizing properties degrade refractory pollutants, such as polycyclic aromatic hydrocarbons (PAHs), improving the soil environment. Despite its wide-ranging potential, ozone technology faces challenges related to stability and cost.
Beyond environmental applications, ozone has significant uses in industry and medicine. In wastewater treatment, ozone serves as a potent oxidant, efficiently removing pathogens, persistent pollutants, and heavy metals. It is commonly employed in processes such as the sequential batch activated sludge process and ozonated advanced oxidation processes, particularly in the treatment of wastewater containing cyanide or coking residues. In food processing, ozone is used to preserve food by eliminating contaminants and extending the shelf life. In the medical field, ozone has shown considerable promise, particularly in orthopedics, pain management, and cancer treatment. Its anti-inflammatory and antioxidant effects have contributed to improved treatment outcomes, with particularly encouraging results in adjuvant therapy.
Despite its advantages as a powerful oxidizing agent, several limitations must be considered for its widespread adoption and further development. One major challenge is the technical limitations associated with ozone production methods. The efficiency of ozone generation, particularly using ultraviolet radiation, remains low, and the high energy consumption involved in some production techniques makes them less cost-effective. Additionally, the storage and transportation of ozone present significant difficulties due to its unstable nature. From an economic standpoint, the high cost of ozone generation equipment and the need for regular maintenance contribute to the overall expense of ozone-based applications. While ozone has proven effective in small-scale operations, the scalability of its use in large industrial systems remains a barrier. Furthermore, regulatory challenges surrounding the safe use of ozone, particularly in air and water treatment, are often complicated by safety concerns and varying regulations across different regions. These factors may hinder the broader implementation of ozone technology in certain sectors.
Given these challenges, there are promising future directions for ozone technology. Future research could focus on advancing production methods to improve efficiency and reduce energy consumption. Developing more sustainable and cost-effective ozone generators could make large-scale applications more feasible. Additionally, exploring the potential of hybrid technologies, which combine ozone with other treatment methods, may increase its effectiveness while reducing costs. The role of ozone in emerging fields, such as cancer therapy and advanced medical treatments, shows great promise and warrants further exploration. Additionally, there is a need for comprehensive studies on the environmental impact and potential by-products of ozone-based processes to ensure their sustainability in the long term. Overall, further research is crucial to overcome the existing limitations and unlock the full potential of ozone technology, especially in large-scale and economically viable applications.
Author Contributions
Conceptualization, K.Z. and J.L.; methodology, H.L. and L.D.; software, K.Z.; validation, J.L. and H.L.; formal analysis, X.Z.; investigation, Z.L. and L.D.; resources, X.Z.; data curation, X.Z.; writing—original draft preparation, C.J.; writing—review and editing, X.Z.; visualization, Z.L.; supervision, K.Z.; project administration, J.L.; funding acquisition, L.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the Science and Technology Project of State Grid Corporation of China (5400-202419199A-1-1-ZN).
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
Authors Kang Zhang, Hongkun Lv and Liwei Ding were employed by State Grid Zhejiang Electric Power Research Institute. Author Jianzheng Liu was employed by Daya Bay Nuclear Power Operations and Management Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Shi, C.; Zhang, S.; Wang, W.; Linhardt, R.J.; Ragauskas, A.J. Preparation of highly reactive lignin by ozone oxidation: Application as surfactants with antioxidant and anti-UV properties. ACS Sustain. Chem. Eng. 2019, 8, 22–28. [Google Scholar]
- Eliasson, B.; Kogelschatz, U. Ozone Generation with Narrow–Band UV Radiation. Ozone Sci. Eng. 1991, 13, 365–373. [Google Scholar] [CrossRef]
- Claus, H. Ozone generation by ultraviolet lamps. Photochem. Photobiol. 2021, 97, 471–476. [Google Scholar]
- Peng, Z.; Day, D.A.; Symonds, G.A.; Jenks, O.J.; Stark, H.; Handschy, A.V.; de Gouw, J.A.; Jimenez, J.L. Significant Production of Ozone from Germicidal UV Lights at 222 nm. Environ. Sci. Technol. Lett. 2023, 10, 668–674. [Google Scholar] [CrossRef]
- Meher, P.; Deshmukh, N.; Mashalkar, A.; Kumar, D. Ozone (O3) generation and its applications: A review. AIP Conf. Proc. 2023, 2764, 070011. [Google Scholar]
- Thanos, J.C.; Wabner, D.W. Elektrochemische abscheidung von sauerstoff und ozon an bleidioxid und platin-anoden in wässrigen elektrolyten mit 18O-markiertem wasser. Electrochim. Acta 1985, 30, 753–756. [Google Scholar]
- Kogelschatz, U.; Eliasson, B.; Hirth, M. Ozone Generation from Oxygen and Air: Discharge Physics and Reaction Mechanisms. Ozone Sci. Eng. 1988, 10, 367–377. [Google Scholar] [CrossRef]
- Gadkari, S.; Gu, S. Numerical investigation of co-axial DBD: Influence of relative permittivity of the dielectric barrier, applied voltage amplitude, and frequency. Phys. Plasmas 2017, 24, 053517. [Google Scholar]
- Valdivia-Barrientos, R.; Pacheco-Sotelo, J.; Pacheco-Pacheco, M.; Benítez-Read, J.S.; López-Callejas, R. Analysis and electrical modelling of a cylindrical DBD configuration at different operating frequencies. Plasma Sources Sci. Technol. 2006, 15, 237. [Google Scholar]
- Jahanmiri, A.; Rahimpour, M.; Shirazi, M.M.; Hooshmand, N.; Taghvaei, H. Naphtha cracking through a pulsed DBD plasma reactor: Effect of applied voltage, pulse repetition frequency and electrode material. Chem. Eng. J. 2012, 191, 416–425. [Google Scholar]
- Gou, X.; Yuan, D.; Wang, L.; Xie, L.; Wei, L.; Zhang, G. Enhancing ozone production in dielectric barrier discharge utilizing water as electrode. Vacuum 2023, 212, 112047. [Google Scholar]
- Jung, J.-S.; Moon, J.-D. Corona discharge and ozone generation characteristics of a wire-plate discharge system with a glass-fiber layer. J. Electrost. 2008, 66, 335–341. [Google Scholar]
- Jin, C.; Lin, F.; Peng, B.; Wei, L.; Ling, Z.; Zeng, X.; Yuan, D.J.V. Nanosecond Pulsed Multi-hollow Surface Dielectric Barrier Discharge for Ozone Production. Vacuum 2025, 238, 114252. [Google Scholar]
- Yehia, A. Assessment of ozone generation in dry air fed silent discharge reactors. Phys. Plasmas 2012, 19, 023503. [Google Scholar]
- Yuan, D.; Wang, Z.; Ding, C.; He, Y.; Whiddon, R.; Cen, K. Ozone production in parallel multichannel dielectric barrier discharge from oxygen and air: The influence of gas pressure. J. Phys. D Appl. Phys. 2016, 49, 455203. [Google Scholar]
- Wei, L.; Yuan, D.; Zhang, Y.; Hu, Z.; Tan, Z.; Dong, G.; Tao, S. An analysis of the effect of inert gases on ozone generation using dielectric barrier discharge in oxygen. Eur. Phys. J. D 2014, 68, 17. [Google Scholar]
- Liang Chen, H.; Ming Lee, H.; Been Chang, M. Enhancement of energy yield for ozone production via packed-bed reactors. Ozone Sci. Eng. 2006, 28, 111–118. [Google Scholar]
- Shang, K.; Wang, M.; Peng, B.; Li, J.; Lu, N.; Jiang, N.; Wu, Y. Characterization of a novel volume-surface DBD reactor: Discharge characteristics, ozone production and benzene degradation. J. Phys. D Appl. Phys. 2019, 53, 065201. [Google Scholar]
- Banaschik, R.; Lukes, P.; Jablonowski, H.; Hammer, M.U.; Weltmann, K.-D.; Kolb, J.F. Potential of pulsed corona discharges generated in water for the degradation of persistent pharmaceutical residues. Water Res. 2015, 84, 127–135. [Google Scholar]
- Lin, F.; Wang, Z.; Zhang, Z.; He, Y.; Zhu, Y.; Shao, J.; Yuan, D.; Chen, G.; Cen, K. Flue gas treatment with ozone oxidation: An overview on NOx, organic pollutants, and mercury. Chem. Eng. J. 2020, 382, 123030. [Google Scholar]
- Glaze, W.H. Drinking-water treatment with ozone. Environ. Sci. Technol. 1987, 21, 224–230. [Google Scholar]
- Rahman, M.; Jasim, S.; Yanful, E.; Ndiongue, S.; Borikar, D. Advanced oxidation treatment of drinking water: Part II. Turbidity, particles and organics removal from Lake Huron water. Ozone Sci. Eng. 2010, 32, 295–304. [Google Scholar]
- Rojas-Valencia, M. Research on ozone application as disinfectant and action mechanisms on wastewater microorganisms. Virus 2011, 3, 263–271. [Google Scholar]
- Ding, W.; Jin, W.; Cao, S.; Zhou, X.; Wang, C.; Jiang, Q.; Huang, H.; Tu, R.; Han, S.-F.; Wang, Q. Ozone disinfection of chlorine-resistant bacteria in drinking water. Water Res. 2019, 160, 339–349. [Google Scholar]
- Paucar, N.E.; Kim, I.; Tanaka, H.; Sato, C. Ozone treatment process for the removal of pharmaceuticals and personal care products in wastewater. Ozone Sci. Eng. 2019, 41, 3–16. [Google Scholar]
- Hollender, J.; Zimmermann, S.G.; Koepke, S.; Krauss, M.; McArdell, C.S.; Ort, C.; Singer, H.; Von Gunten, U.; Siegrist, H. Elimination of organic micropollutants in a municipal wastewater treatment plant upgraded with a full-scale post-ozonation followed by sand filtration. Environ. Sci. Technol. 2009, 43, 7862–7869. [Google Scholar]
- Tripathi, S.; Hussain, T. Water and wastewater treatment through ozone-based technologies. In Development in Wastewater Treatment Research and Processes; Elsevier: Amsterdam, The Netherlands, 2022; pp. 139–172. [Google Scholar]
- Ciardelli, G.; Ranieri, N. The treatment and reuse of wastewater in the textile industry by means of ozonation and electroflocculation. Water Res. 2001, 35, 567–572. [Google Scholar]
- Ferrero, F.; Nano, A. L’ozono in tintoria ci può dare una mano Ozone can help in dyeing. Riv. Tecnol. Tessili 1995, 8, 67–72. [Google Scholar]
- Parisheva, Z.; Demirev, A. The effect of ozone on harmful, oxidizable substances in industrial waste gases. Environ. Prot. Eng. 1995, 20, 137–144. [Google Scholar]
- Apeksha, M.; Rajanikanth, B. Plasma/adsorbent system for NOx treatment in diesel exhaust: A case study on solid industrial wastes. Int. J. Environ. Sci. Technol. 2019, 16, 2973–2988. [Google Scholar]
- Russo, L.; Rizzo, L.; Belgiorno, V. PAHs contaminated soils remediation by ozone oxidation. Desalination Water Treat. 2010, 23, 161–172. [Google Scholar]
- Wang, T.C.; Qu, G.; Li, J.; Lu, N. Transport characteristics of gas phase ozone in soil during soil remediation by pulsed discharge plasma. Vacuum 2014, 101, 86–91. [Google Scholar]
- Pawłat, J.; Stryczewska, H.D.; Ebihara, K. Sterilization techniques for soil remediation and agriculture based on ozone and AOP. J. Adv. Oxid. Technol. 2010, 13, 138–145. [Google Scholar]
- Liu, J. Soil remediation using soil washing followed by ozone oxidation. J. Ind. Eng. Chem. 2018, 65, 31–34. [Google Scholar]
- Chávez, A.; Gimeno, O.; Rey, A.; Pliego, G.; Oropesa, A.; Álvarez, P.; Beltrán, F. Treatment of highly polluted industrial wastewater by means of sequential aerobic biological oxidation-ozone based AOPs. Chem. Eng. J. 2019, 361, 89–98. [Google Scholar]
- Asaithambi, P.; Aziz, A.R.A.; Daud, W.M.A.B.W. Integrated ozone—Electrocoagulation process for the removal of pollutant from industrial effluent: Optimization through response surface methodology. Chem. Eng. Process. Process Intensif. 2016, 105, 92–102. [Google Scholar]
- Sujayasree, O.; Chaitanya, A.; Bhoite, R.; Pandiselvam, R.; Kothakota, A.; Gavahian, M.; Mousavi Khaneghah, A. Ozone: An advanced oxidation technology to enhance sustainable food consumption through mycotoxin degradation. Ozone Sci. Eng. 2022, 44, 17–37. [Google Scholar]
- Afsah-Hejri, L.; Hajeb, P.; Ehsani, R.J. Application of ozone for degradation of mycotoxins in food: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1777–1808. [Google Scholar]
- Sarron, E.; Gadonna-Widehem, P.; Aussenac, T. Ozone treatments for preserving fresh vegetables quality: A critical review. Foods 2021, 10, 605. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Subhashini, S.; Banuu Priya, E.; Kothakota, A.; Ramesh, S.; Shahir, S. Ozone based food preservation: A promising green technology for enhanced food safety. Ozone Sci. Eng. 2019, 41, 17–34. [Google Scholar]
- Seyam, O.; Smith, N.L.; Reid, I.; Gandhi, J.; Jiang, W.; Khan, S.A. Clinical utility of ozone therapy for musculoskeletal disorders. Med. Gas Res. 2018, 8, 103–110. [Google Scholar] [PubMed]
- Jandura, J.; Vajda, M.; Cech, M.; Ryska, P. Oxygen–Ozone Therapy of Musculoskeletal Neck Pain: A Review. J. Pers. Med. 2024, 14, 326. [Google Scholar] [CrossRef]
- Clavo, B.; Martínez-Sánchez, G.; Rodríguez-Esparragón, F.; Rodríguez-Abreu, D.; Galván, S.; Aguiar-Bujanda, D.; Díaz-Garrido, J.A.; Cañas, S.; Torres-Mata, L.B.; Fabelo, H. Modulation by ozone therapy of oxidative stress in chemotherapy-induced peripheral neuropathy: The background for a randomized clinical trial. Int. J. Mol. Sci. 2021, 22, 2802. [Google Scholar] [CrossRef] [PubMed]
- Clavo, B.; Rodríguez-Esparragón, F.; Rodríguez-Abreu, D.; Martínez-Sánchez, G.; Llontop, P.; Aguiar-Bujanda, D.; Fernández-Pérez, L.; Santana-Rodríguez, N. Modulation of oxidative stress by ozone therapy in the prevention and treatment of chemotherapy-induced toxicity: Review and prospects. Antioxidants 2019, 8, 588. [Google Scholar] [CrossRef] [PubMed]
- Clavo, B.; Santana-Rodriguez, N.; Llontop, P.; Gutierrez, D.; Suárez, G.; Lopez, L.; Rovira, G.; Martinez-Sanchez, G.; Gonzalez, E.; Jorge, I.J. Ozone therapy as adjuvant for cancer treatment: Is further research warranted? Evid. Based Complement. Altern. Med. 2018, 2018, 7931849. [Google Scholar]
- Smith, A.J.; Oertle, J.; Warren, D.; Prato, D. Ozone therapy: A critical physiological and diverse clinical evaluation with regard to immune modulation, anti-infectious properties, anti-cancer potential, and impact on anti-oxidant enzymes. Open J. Mol. Integr. Physiol. 2015, 5, 37–48. [Google Scholar]
- Wellauer, R.; Oldani, M. Cooling water treatment with ozone. Ozone Sci. Eng. 1990, 12, 243–253. [Google Scholar]
- You, S.H.; Tseng, D.H.; Guo, G.L.; Lee, S.Y. Mechanism and effects of ozone treatment for cooling water system. J. Environ. Sci. Health Part A 2000, 35, 87–107. [Google Scholar]
- Ataei, A.; Gharaie, M.; Parand, R.; Panjeshahi, E. Application of ozone treatment and pinch technology in cooling water systems design for water and energy conservation. Int. J. Energy Res. 2010, 34, 494–506. [Google Scholar]
- Sydykova, G.; Umbetova, S.; Baimakhanova, Z.; Abieva, G.; Kurmanbayev, G. Modern Applications of Ozone Technology. Evergreen 2023, 10, 2308–2316. [Google Scholar]
- Ershov, B.; Seliverstov, A.; Basiev, A.; Basiev, A.; Korchagin, Y.P. Application of concentrated ozone for decontamination of equipment in a nuclear power plant. At. Energy 2009, 107, 89–94. [Google Scholar]
- Vig, J.R. UV/ozone cleaning of surfaces. J. Vac. Sci. Technol. A Vac. Surf. Film. 1985, 3, 1027–1034. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).