Abstract
Tea saponin is a kind of natural non-ionic surfactant. Saponins were extracted from tea seed cake using an ultrasonic-assisted enzymatic method. The optimization of the tea saponin extraction procedure was conducted by using the response surface method to increase the yield. Study results indicated that the maximum yield of tea saponin was 69.81 mg/g under the optimum conditions of an enzyme concentration of 0.67%, a solvent-to-material ratio of 16.82 mL/g, an extraction temperature of 58.14 °C and an extraction time of 1.89 h. The surface activity experimental study results indicated that the critical micelle concentration of tea saponin was 0.5 g/L at 30 °C, and the lowest surface tension was 39.61 mN/m. The surface tension and CMC of tea saponin remained basically unchanged between 30 °C to 60 °C. When the pH of the solution was slightly acidic, the surface tension of tea saponin decreased significantly, while the CMC remained almost unchanged. Tea saponin has good salt and hard water resistance, and its surface tension decreases to a certain extent in both saltwater and hard water. The foam volume of tea saponin can reach 490 mL, with a half-life of 2350 s, and the foam is relatively stable. The combination of tea saponin and other surfactants has a certain synergistic effect. The critical micelle concentration of its complex system with the natural surfactant rhamnolipids can increase by 69.23%, and the surface tension can be reduced to a minimum of 22.56 mN/m. Moreover, by using the proposed method, the foam performance and stability of the system have been improved to a certain extent. This has significant practical significance for fully utilizing and developing waste camellia dried cake resources.
1. Overview
Camellia oleifera is a significant woody oil-producing crop. Tea oil is favored by people who pursue health because of its high unsaturated fatty acid content, which also promotes the development of tea oil industry [1]. The by-product of Camellia oleifera is a large amount of seed meal. China produces more than 690,000 tons of waste residue every year, and most of the Camellia oleifera meal is burned as fuel or exported at a low price, which pollutes the environment and wastes resources [2]. In the past, tea saponin from Camellia oleifera production was frequently discarded or utilized as oil cake and applied as a low-value chemical fertilizer in the traditional oil refining process [3]. It turns out that tea saponin is a valuable ingredient, with good emulsification and biological activity, which can be very useful for foaming, emulsification, dispersion, and wetting things. It can also fight inflammation, bacteria, cancer, and other biological problems, among other things [4]. It has been utilized extensively in food, pharmaceuticals, insecticides, and various other industries [5]. Recent research on the extraction of Camellia saponins has advanced significantly through many methods, including water extraction, organic solvent extraction, microwave-assisted extraction, bio-extraction, and subcritical water extraction [6]. An extraction rate of 74.21% was attained for Camellia saponins under supercritical water conditions. Tang et al. employed a specialized deep eutectic solvent as an eco-friendly medium to extract tea saponins from tea seed cakes, with a maximum extraction efficiency of 23.22% [7]. The water extraction and biological extraction procedures were eco-friendly, but the tea saponin obtained had more impurities and lower purity [8]. Microwave-assisted extraction and subcritical water extraction are excellent extraction techniques, but they require specific equipment [9]. At the same time, whether these techniques can be industrialized still needs further process optimization and related research. In addition, the maintenance and operation of the equipment require professional knowledge and skills, increasing the complexity and cost of operations [10].
The optimization of tea saponin extraction from Camellia oleifera seed meal is a critical aspect of sustainable resource utilization [11]. The purity of saponin extracted using traditional organic solvents is higher, but compared with water extraction, due to the large amount of solvent required, there are some problems in recovery and reuse [12]. This research explores the innovative use of the combination of ultrasonic-assisted extraction and enzymes to provide a more environmentally friendly method by using the synergistic effect of ultrasound and enzymes [13]. The investigation incorporates response surface methodology (RSM) for efficient parameter optimization, enhancing the extraction efficiency of tea saponins [14]. This research seeks to contribute to the high-value utilization of Camellia oleifera seed meal through the efficient extraction of beneficial tea saponins [15]. The key objective of this work is to explore the utilization of enzymes to enhance the extraction of saponin from Camellia oleifera using ultrasonic waves [16]. The disruptive power of ultrasonic waves is combined with the specificity of enzymes to break down plant cell walls. By leveraging the disruptive force of ultrasonic waves and the specificity of enzymatic action, plant cell walls can be more effectively broken down, thereby facilitating the release of saponins into the extraction medium [17]. These steps include ultrasonic enzyme soaking, centrifugation, impurity removal, solvent extraction, and purification [18]. The enzymes have high efficiency and specificity, which can change the permeability of the plant cell wall and facilitate the dissolution of active ingredients [19]. The ultrasonic waves create micro-turbulences and cavitation that disrupt the cell structure, making it more permeable and allowing the enzymes to access and break down complex cell wall components more efficiently [20]. Due to their synergistic effect, saponin yields are augmented, and their bioactive qualities are maintained. This minimizes degradation and the loss of crucial chemicals during the extraction process [21]. The principle of the water extraction method is that tea saponin can dissolve in hot water, and hot water can be used as an extraction solvent [22]. It entails using water as a solvent to dissolve and extract soluble materials, isolating chemicals like saponins by utilizing water’s polarity [23]. Camellia oleifera seed cakes were first soaked in a water enzyme solution [24,25,26]. The process of extracting saponin can be made more efficient by applying a small amount of heat, which can improve solubility and diffusion rates [27]. Plant cell walls are targeted and broken down by biological agents, like enzymes, in biological extraction processes, which release the desired chemicals [28]. This technique breaks down connections in the plant matrix by utilizing the selectivity and strength of enzymes [29]. This makes it easier to extract bioactive compounds [30]. The experimental approach for optimizing the extraction of saponins from Camellia oleifera begins with an ultrasonic enzyme-soaking extraction, where ultrasonic waves create micro-vibrations and cavitation in the solution, aiding the enzyme’s penetration into plant cells to facilitate the release of saponins into the aqueous medium [31]. Centrifugation then separates the solid biomass from the liquid extract while optimizing the solid-to-liquid ratio to maximize saponin recovery [32]. Chitosan is then added to the extract to bind and precipitate impurities, which are removed via subsequent centrifugation [33]. The clarified filtrate undergoes concentration to increase the saponin concentration in the solution. Utilizing 90% ethanol as a solvent allows for the selective extraction of saponins, further refining the process [34]. The solution is then dried in an oven to obtain crude saponins. Extraction with saturated n-butanol solution is a common method used for the purification of tea saponin in the laboratory [35]. The integrity and bioactivity of the saponins are maintained by freeze-drying the n-butanol layer that has been enhanced with saponins [36]. In this work, the extraction conditions were optimized using Box–Behnken Design (BBD) to more effectively extract a significant proportion of tea saponins. The extraction method, surface activity and foam properties of tea saponin can provide a theoretical basis for the further development and utilization of tea saponin. It has been found that tea saponin has excellent foaming, emulsifying and wetting properties, making it widely used in daily chemical applications and the field of petroleum development [37,38,39,40].
2. Materials and Methods
2.1. Materials
The tea saponin standard sample (98%), rhamnolipid, and commercial enzymes were purchased from Solarbio Co., Ltd. (Shanghai, China). Analytical reagents used were anhydrous alcohol, vanillin, sulfuric acid, acetic acid, sodium chloride, n-butyl alcohol, petroleum ether (60–90 °C), calcium chloride, magnesium chloride hexahydrate, and sodium chloride. The acetonitrile and phosphoric acid used for HPLC analysis were guaranteed reagents. All reagents and pharmaceuticals were procured from Shanghai National Medicine Co., Ltd. (Shanghai, China).
2.2. Instruments and Apparatus
The following apparatus was used: a KM615C ultrasonic cleaner (Guangzhou Kemeng Instrument Technology Co., Ltd., Guangzhou, China, 2022), a Rose-Miles foam apparatus (Shanghai Anting Instrument Co., Ltd., Shanghai, China, 2021), a Fourier infrared spectrometer (IS50, Thermofisher Scientific, Waltham, MA, USA, 2014), a KZK-25 electric vacuum drying oven (Shanghai Jinghong Instrument Co., Ltd., Shanghai, China, 2021), a RE52A rotary evaporator (Shanghai Yarong Instrument Equipment Co., Ltd., Shanghai, China, 2020), a K100 surface tension meter (Bruker Technology Co., Ltd., Berlin, Germany, 2019), a Scientz-10N freeze dryer (Zhejiang Xinzhi Equipment Co., Ltd., Ningbo, China, 2021), and an Agilent 1100 HPLC system with Eclipse Plus C18 column (250 mm × 4.6 mm, 5 μm) (Agilent Technology Co., Ltd., Santa Clara, CA, USA, 2018).
2.3. Pre-Treatment of Camellia Oleifera Cakes
The tea seed cakes used in the study were collected from Qingtian Oil and Fat Company (Ganzhou, Jiangxi Province, China). First, the tea seed cakes were ground into powder and then sieved through an 80-mesh filter. The cake power was then subjected to a defatting process using petroleum ether at 80 °C for 5 h. Subsequent to defatting, the obtained powder was subjected to drying in an oven at 60 °C for a period of 5 h. The obtained tea seed powder was then preserved in a glass desiccator for subsequent study.
2.4. Ultrasonic Combined with Enzyme Extraction
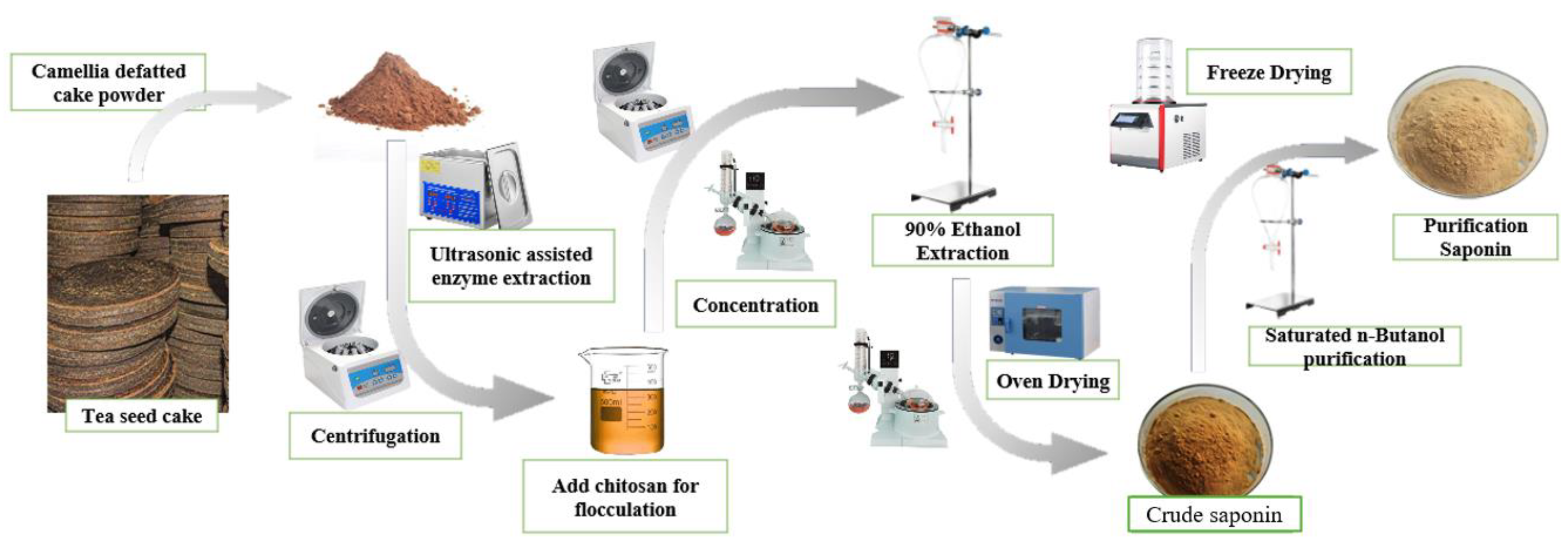
A total of 40 g of processed tea seed powder was immersed in 400 mL of distilled water, with the pH adjusted to 4.5 through the addition of acetic acid. Subsequently, commercial cellulase was included and the mixture was left to soak for four hours. The solutions were subsequently removed twice and placed in an ultrasonic cleaner at 50 °C for 60–180 min. The extract solution was obtained through centrifugation and subsequently concentrated using a rotary evaporator at 65 °C under vacuum conditions. The concentrated liquid was transferred into a separating funnel, a suitable volume of 90% ethanol was added for extraction three times, the upper extraction layer was collected, and the three extraction portions were amalgamated. The utilization of 90% ethanol facilitates the aggregation and sedimentation of impurities like proteins and polysaccharides, which is conducive to improving the purity of saponin in the extraction solution. The ethanol extraction solution was concentrated and evaporated under vacuum at 60 °C for 3 h using a rotary evaporator. Thus, the crude saponin was acquired (Figure 1).
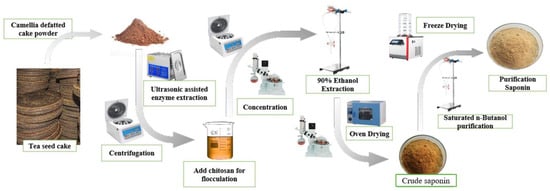
Figure 1.
Extraction and purification process of tea saponin.
2.5. Determination of Saponin
A chromatographic column with Eclipse Plus C18 as the packing and an adjustable wavelength UV detector were used to determine tea saponins in the sample using Agilent 1100 high-performance liquid chromatography. The detection wavelength was 215 nm, the flow rate was 1 mL/min and the column temperature was 30 °C. Gradient elution was performed using an acetonitrile-water system, with phase A as 0.05% phosphoric acid aqueous solution and phase B as acetonitrile. The elution conditions were as follows: the mobile phase B volume fraction was maintained at 20% between 0 and 5 min, increased from 20% to 100% between 5 and 10 min, and returned from 100% to 20% between 10 and 15 min.
The standard saponin sample was accurately weighed and dissolved in 1:1 acetonitrile and 0.05% phosphoric acid aqueous solution. It was then diluted into 0, 0.2, 0.4, 0.6, 0.8 and 1.0 mg/L solutions. The prepared tea saponin standard solution was injected according to the above conditions, and the standard curve was drawn with the peak area as the vertical coordinate and the mass concentration as the horizontal coordinate. The regression equation of the standard curve is y = 62.451x − 0.8245, R2 = 0.9992. The concentration and purity of saponin in the sample solution can be ascertained using the standard curve, allowing for the subsequent calculation of saponin yield.
2.6. FT-IR Analysis
The saponin was finely ground in an onyx mortar to produce a translucent disc on a tablet press. Spectral data were obtained by scanning the spectrum between 400 and 4000 cm−1 using a FT-IR spectrometer (IS50, Thermofisher Scientific, Waltham, MA, USA, 2014).
2.7. Single Factor Experiment
The influence of extraction temperature (°C), extraction time (min), liquid-to-material ratio (mL/g), and enzyme concentration (%) on the extraction efficiency of tea saponin was examined using a single-factor test.
2.8. Experimental Design of RSM
Response surface methodology (RSM) was utilized to assess the impact of the extraction parameters and to optimize the extraction process. Box–Behnken experiments with four numerical factors at three levels were devised. The four factors identified to affect the extraction efficiency of tea saponins were extraction temperature (A, 50, 55, 60 °C), extraction time (B, 90, 120, 150 min), solvent-to-material ratio (C, 5:1, 10:1, 15:1 mL/g) and enzyme concentration (D, 0.4, 0.6, 0.8%), as determined from the single-factor experiments (Table 1). The yield of tea saponins served as the response variable (Y).

Table 1.
The optimal response variables for the extraction of tea saponin.
2.9. Purification of Tea Saponin
We dissolved a certain amount of crude saponin in hot water and then added three times the volume of water-saturated n-butanol solution for extraction twice. We combined the upper layers and concentrated them before freeze-drying to obtain purified saponin. After purification with a water-saturated n-butanol solution, the purity of saponin can be increased from over 30% to around 80%.
2.10. Measurement of Surface Tension and Experiment of Influencing Factors
The hanging plate method was used to measure the surface tension and CMC of a series of saponins with different concentrations at 30 °C.
Separate tests were conducted on the effects of temperature, pH value, salinity, and water hardness on the surface tension and CMC of tea saponin.
2.11. Foam Properties of Saponin
Different concentrations of saponin aqueous solutions were prepared and the Rose-Miles determination method was used to measure the volume and the half-life of the foam. The Rose-Miles determination method was carried out in accordance with GB/T 7462-94 [41].
2.12. Synergistic Effect of Saponin and Rhamnolipid Compounding System
Rhamnolipid is also a kind of green biosurfactant. As an anionic surfactant, it can play a synergistic role when used in combination with saponin. A series of tea saponin and rhamnolipid complex solutions with a mass fraction (X1) of 0% and gradient concentrations of 1–100% were prepared, and the surface tension and CMC of the complex solutions were measured, respectively. The foam properties and half-life of the composite system were also determined using the Rose-Miles method.
The theoretical value of CMC was calculated using the Clint ideal solution rule equation. The synergistic coefficient Y was used to evaluate the synergistic effect of the compound system. When the efficiency coefficient Y is larger, it means that the efficiency of the compounding system is better, and vice versa. Y can be calculated using the following formula:
In the formula, X1 and X2 are the mass fractions of saponin and rhamnolipid, respectively, and CMC1 and CMC2 are the actual critical micelle concentrations of saponin and rhamnolipid, respectively.
3. Results and Discussion
3.1. Single-Factor Experimental Analysis
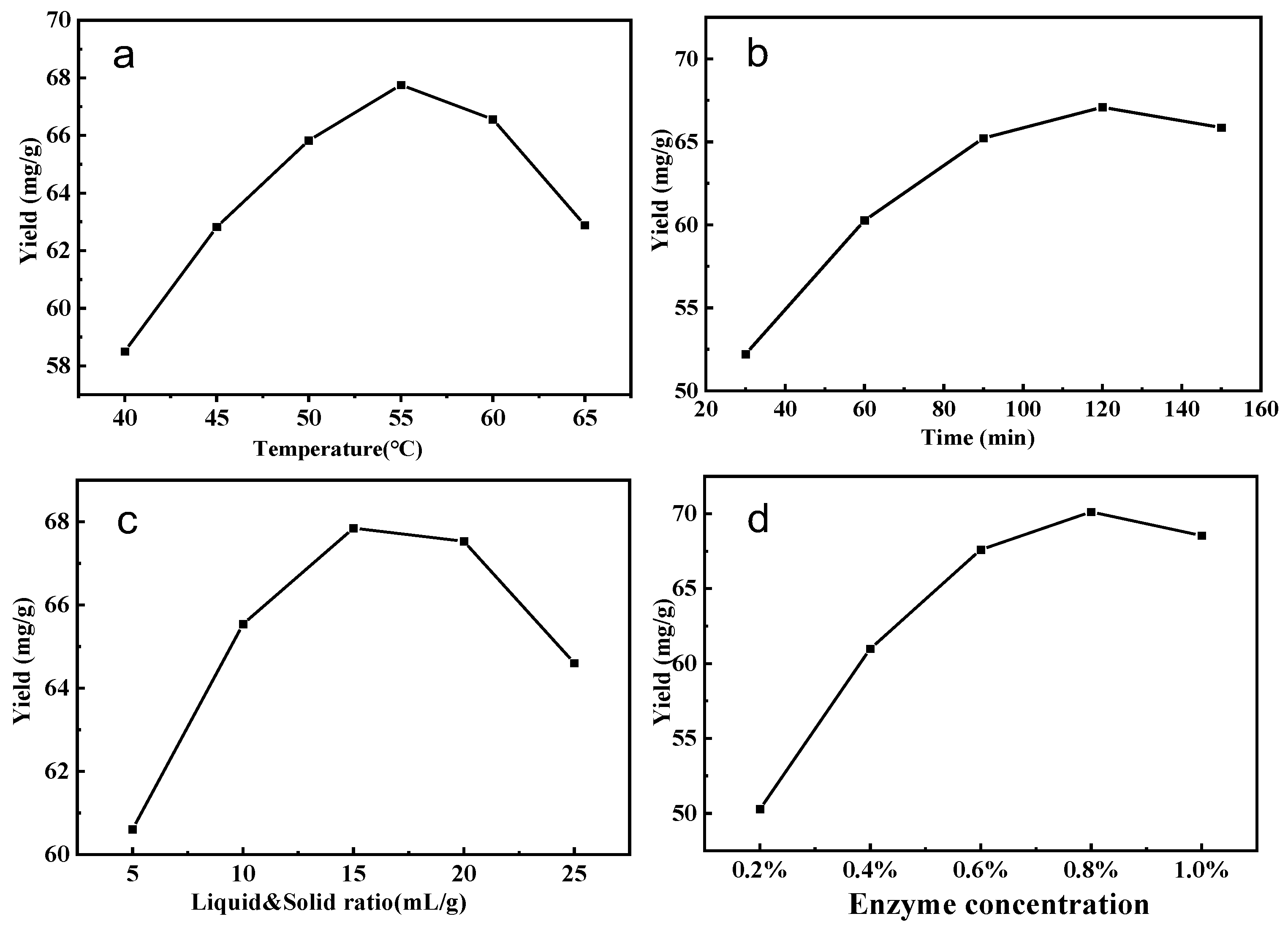
The influence of temperature on saponin production is illustrated in Figure 2a. Obviously, high temperature increased the diffusion rate, and the yield increased with increasing temperature, up to 55 °C. However, when the temperature exceeded 60 °C, cellulase activity began to decline. Too low or too high a temperature can lead to a decrease in or inactivation of cellulase activity. Consequently, 55 °C is regarded as the optimum temperature.
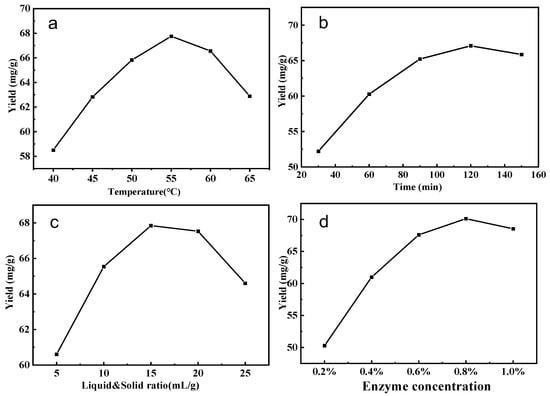
Figure 2.
Effect of extraction conditions on the yield of tea saponin (a–d).
Figure 2b examines the impact of varying enzymatic hydrolysis durations on the extraction efficiency of tea saponin. As time progresses, the solubility of saponin can be enhanced within a specific range. Nonetheless, as the duration of enzymolysis extended, the extraction rate of saponin initially rose and subsequently declined. At an enzymolysis duration of 120 min, the saponin extraction rate attained its peak. Consequently, to minimize experimental duration and energy expenditure, the best enzymatic hydrolysis duration was determined to be 120 min.
Figure 2c illustrates the impact of the liquid-to-solid ratio on the extraction rate of tea saponin. In the preliminary phase, an increase in the liquid-to-solid ratio resulted in a considerable enhancement of saponin yield. When the liquid-to-solid ratio surpasses 1:15, the yield significantly diminishes, suggesting that a liquid-to-solid ratio of 1:15 represents the optimal condition.
Figure 2d illustrates the impact of cellulase concentration on the extraction yield of tea saponin. As enzyme concentration increased, saponin yield began to climb; however, when the enzyme concentration rose above 0.6%, the yield increased gradually, and the saponin content attained a specific equilibrium. The saponin content was mostly unaffected by the increase in enzyme concentration. From the standpoint of resource optimization, a 0.6% enzyme concentration was identified as the optimal reaction condition.
3.2. RSM (Box–Behnken) Optimization
In this study, a central composite design was conducted with factors and levels of four independent variables, resulting in a total of 29 experimental settings. The experimental design and the response surface results are shown in Table 2.

Table 2.
The experimental design and response surface results.
In each treatment, the highest yield of tea saponin, 69.63 mg/g, was attained using a temperature of 60 °C, a duration of 120 min, a solvent-to-material ratio of 15:1 (mL/g), and an extraction enzyme concentration of 0.8%. The lowest yield of tea saponin, 60.21 mg/g, was attained using a temperature of 55 °C, a duration of 90 min, a solvent-to-material ratio of 15:1 (mL/g), and an extraction enzyme concentration of 0.4%. The following regression equation can be used to express the second-order polynomial equation:
Y = 68.19 + 1.81A + 0.95B + 0.66C + 3.15D + 0.33AB + 0.50AC − 0.24AD + 0.31BC + 0.35BD − 0.04CD − 0.05A2 − 1.76B2 − 1.72C2 − 2.03D2
Design-Expert software was used to determine the variance in significance of the regression equation, and the results are displayed in Table 3.

Table 3.
ANOVA for response surface quadratic model.
The quadratic regression model is very significant, as indicated by a low p-value (p < 0.001). The analysis of variance revealed an R2 of 0.9337, and the exceptionally low variable parameters (C.V. = 1.63%) supported the experimental data’s high dependability.
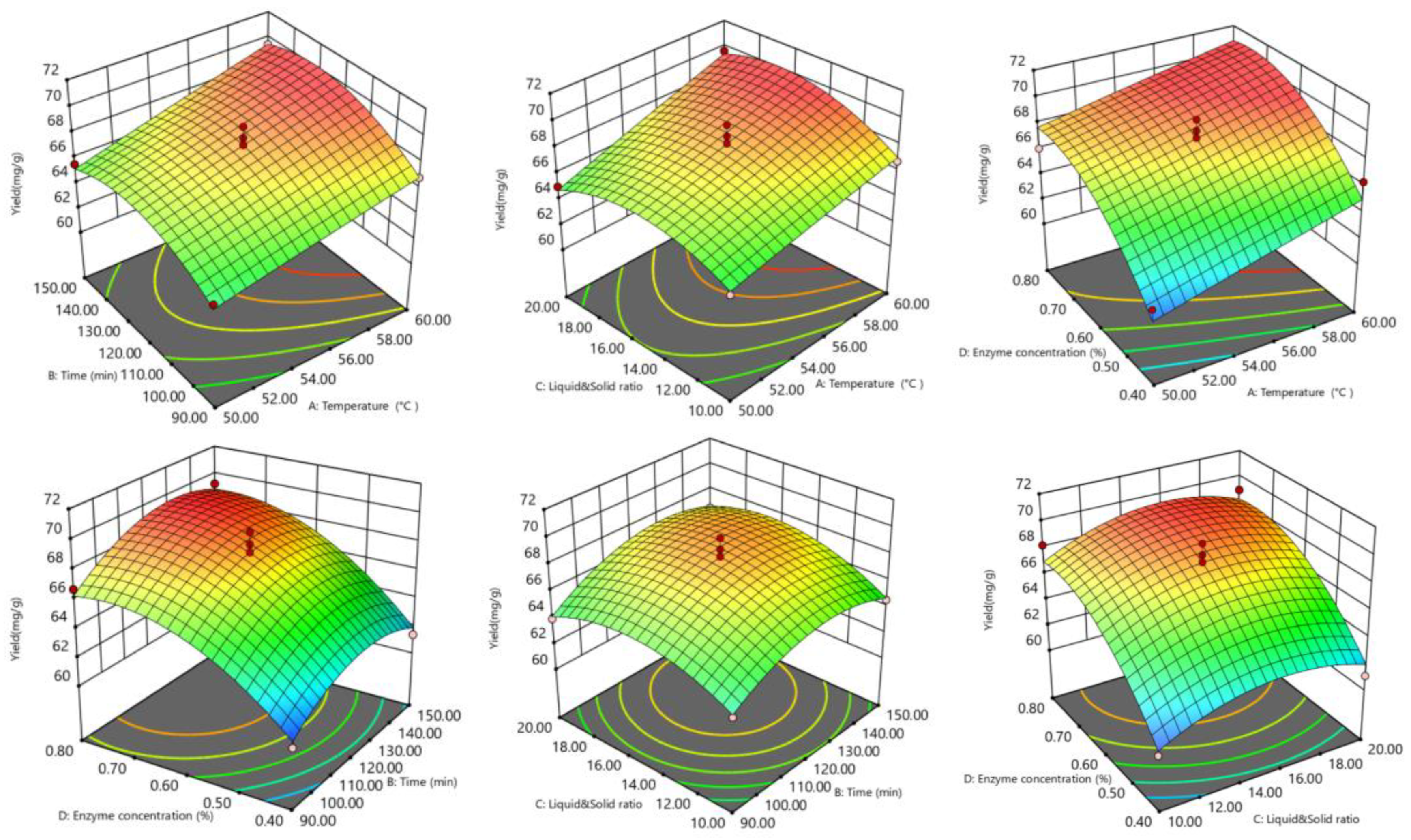
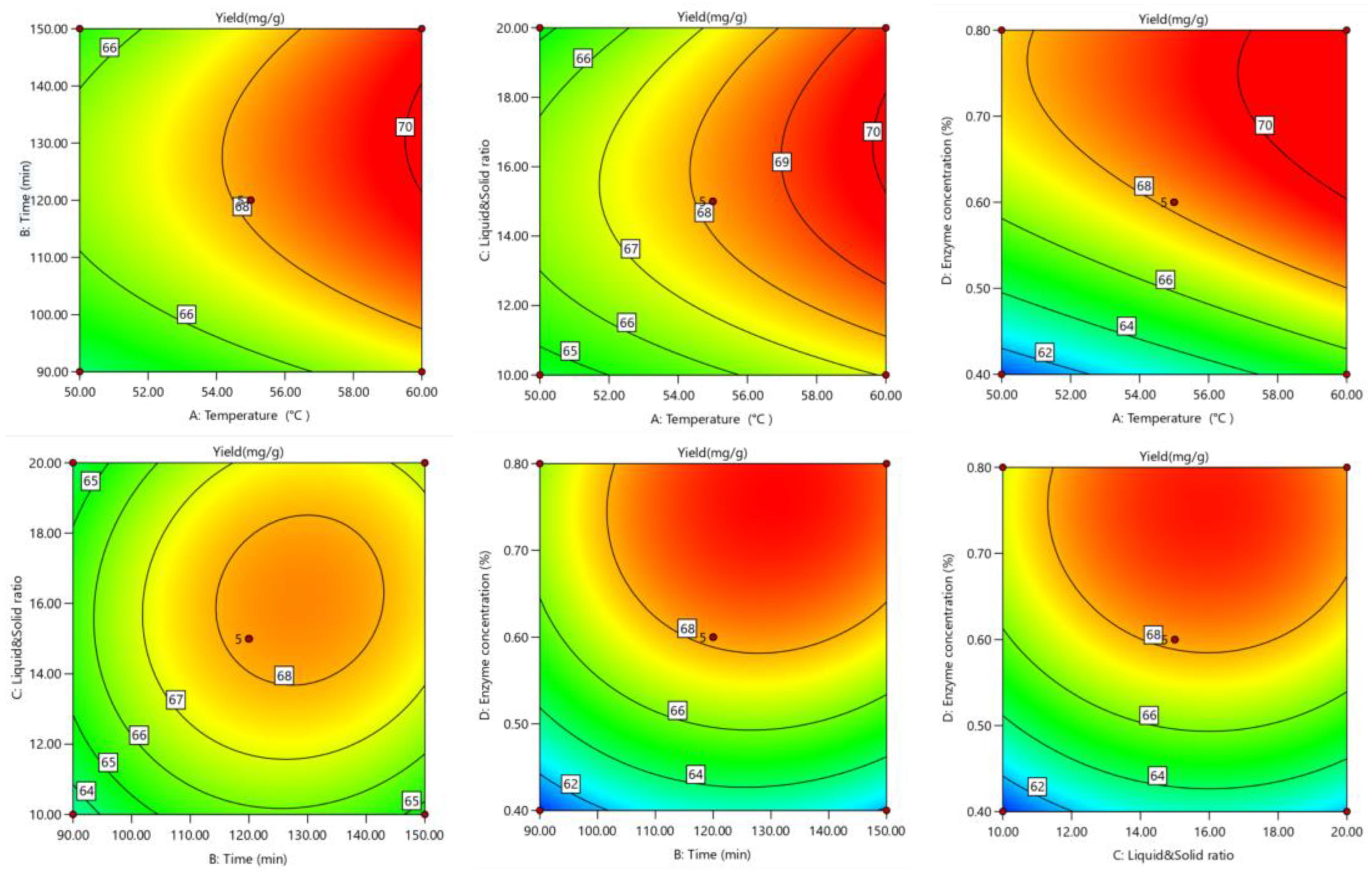
The response surface and contour lines showed how the interaction of factors affected tea saponin yield. The yield trend (Figure 3 and Figure 4) showed that the interaction effect of each element was not significant. The optimal extraction conditions were 58.14 °C, 1.89 h, 0.67%, and 16.82 mL/g resulting in a tea saponin yield of 69.81 mg/g.
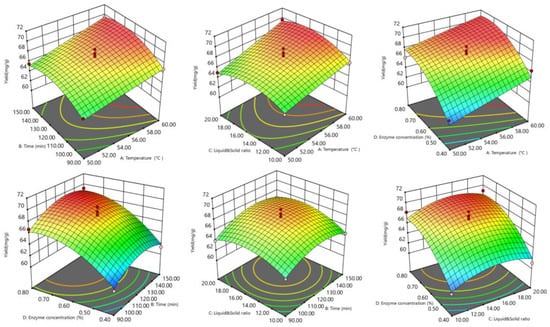
Figure 3.
Optimized response surfaces for saponin response surface extraction.
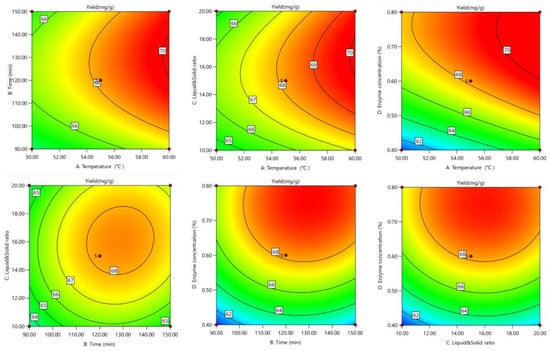
Figure 4.
Optimized contour lines for saponin response surface extraction.
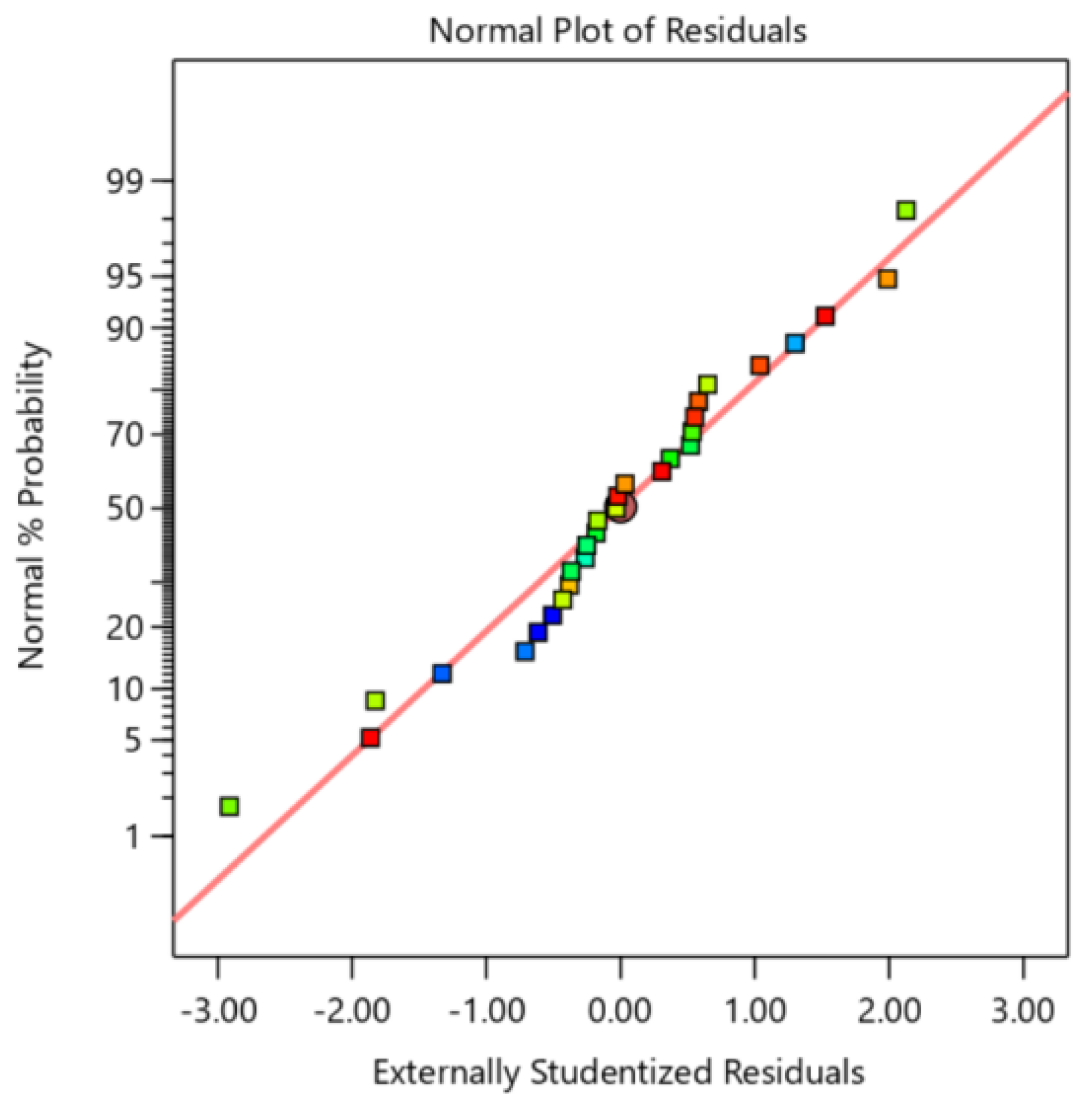
The red line in the Figure 5 delineates the linear trajectory that residuals from an ideal normal distribution are expected to follow. As illustrated, the data points, encompassing residuals from distinct groups denoted by blue, green, and red squares, are approximately distributed along this red line. This distribution pattern demonstrates that the majority of residuals adhere to a normal distribution, suggesting that the model’s fitting efficacy to the data is generally acceptable. Consequently, the parameter estimation and statistical inference results exhibit a reasonable degree of reliability.
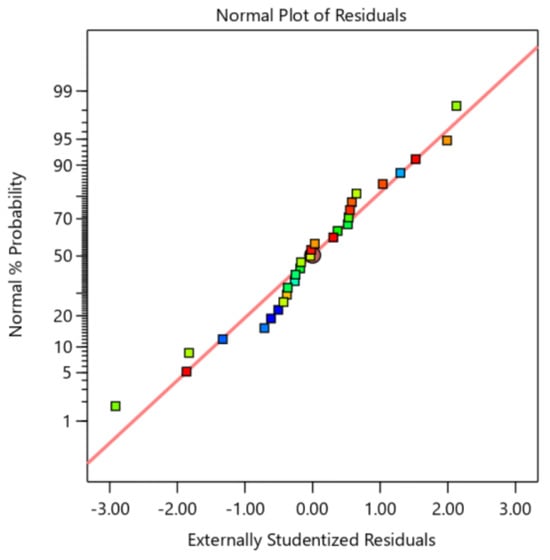
Figure 5.
Residual normalization technique for the extraction of saponin response surfaces.
3.3. Analysis of Infrared Spectra
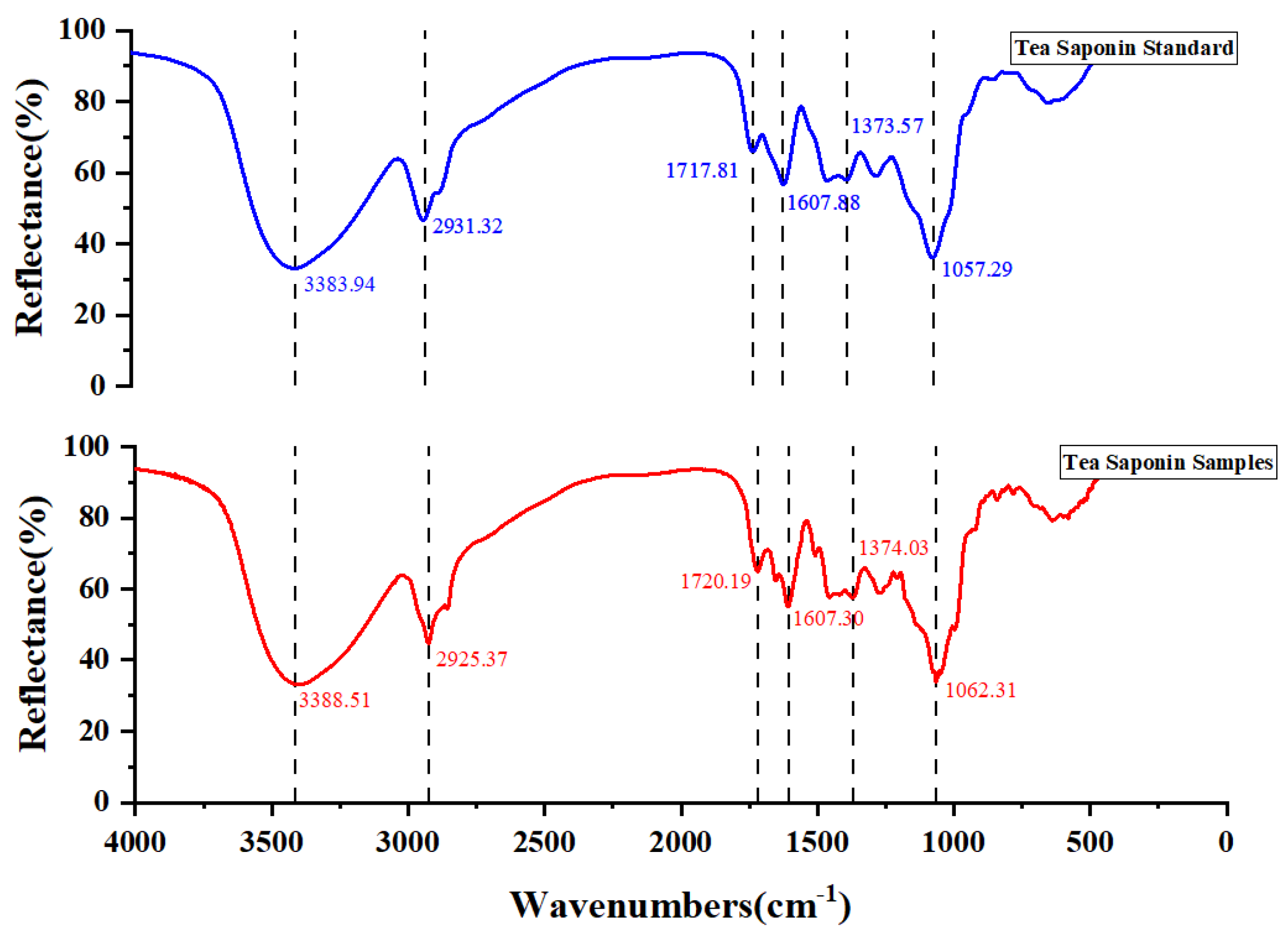
The FT-IR spectra of standard tea saponin and extracted tea saponin samples are shown in Figure 6. The O-H stretching vibration band at 3383.94 cm−1 and 3388.51 cm−1 were obviously broadened. The absorption peak near 2917–2931 cm−1 is usually related to the stretching vibration of C-H, resulting from the C-H bond of the alkyl chain in the structure of tea saponin. The C-H stretching vibrational peak of the standard saponin was observed near 2930 cm−1 and the absorption peak of the corresponding tea saponin samples was at 2925.37 cm−1. From 2500 cm−1 to 1900 cm−1, there was no characteristic absorption, which means that there were no triple or double bonds. The absorption peaks from 1717 cm−1 to 1607 cm−1 suggested the existence of a carbonyl group (C=O). The peaks near 1373 cm−1 were due to the symmetric and anti-symmetric bending vibrations of the methyl group (-CH3). The peaks at 1057.29 cm−1 and 1062.31 cm−1 were due to the C-O-C stretching vibration. The chemical structure of tea saponin contains the hydroxyl group (-OH), carbonyl group (C=O), glucoside bond (C-O-C) and other characteristic functional groups, which show unique absorption peaks in the infrared spectrum. The infrared spectrum analysis showed that the structure of the standard tea saponins was basically the same as the purified tea saponin samples.
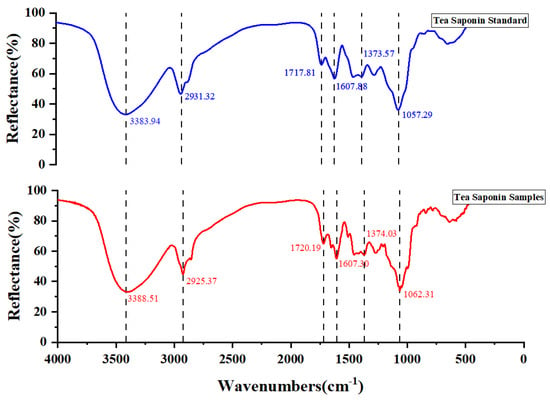
Figure 6.
Comparison of infrared spectral profiles of standard tea saponin and extracted tea saponin samples.
3.4. Measurement of Surface Tension at Different Temperature
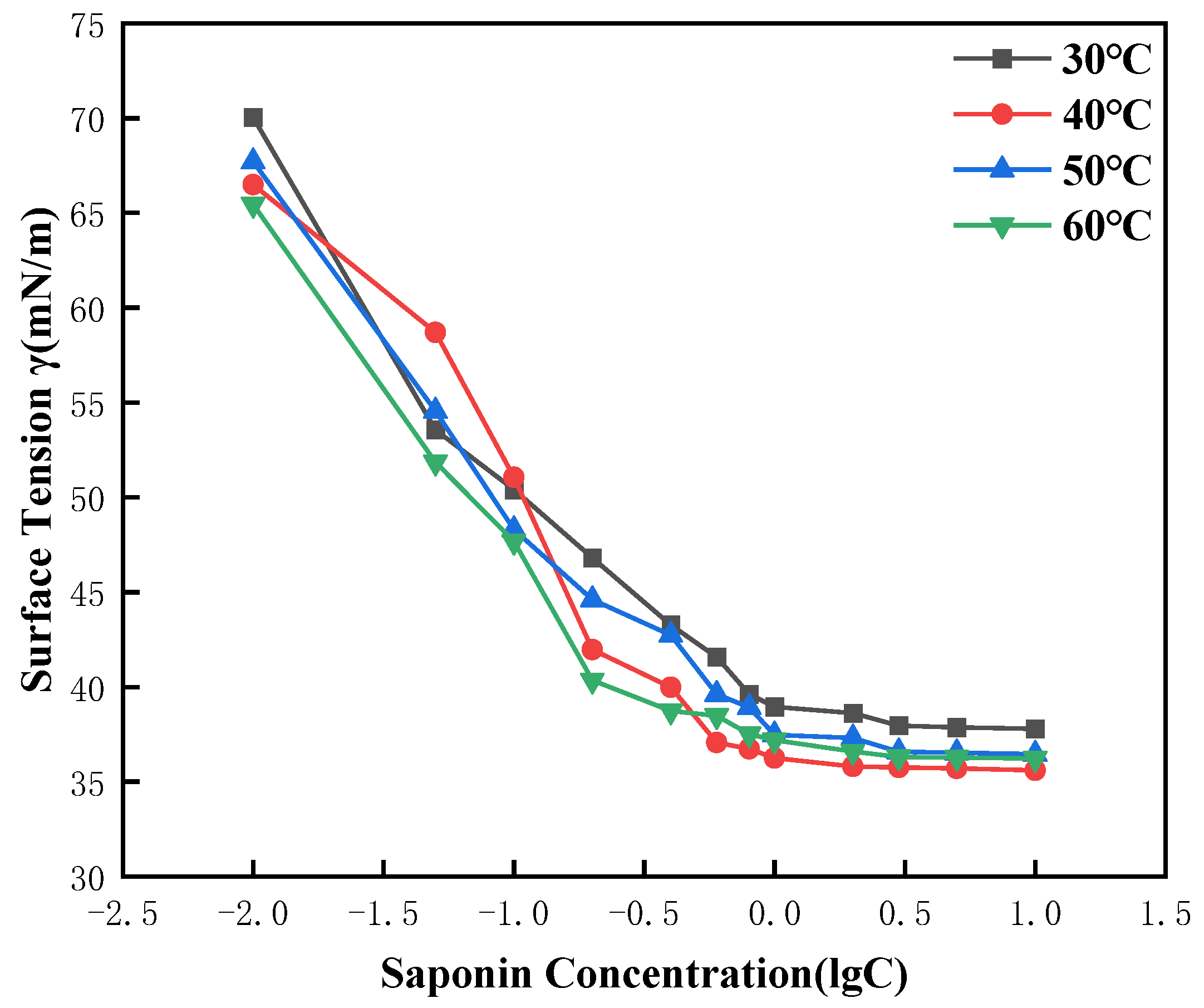
The surface tension of saponin solutions over a concentration range from 0.01 to 10 g/L was investigated as a function of temperature from 30 °C to 60 °C. Results showed a clear trend: Surface tension decreased slightly as temperature increased, suggesting that thermal energy increases the mobility of saponin molecules and decreases intermolecular forces at the air–liquid interface. This increase in molecular motion permits saponin molecules to more effectively disperse over the surface so that the surface tension is lowered. In the range of 30 °C to 60 °C, CMC values basically remained unchanged, indicating that saponin has good surface activity in room-temperature water (Figure 7).
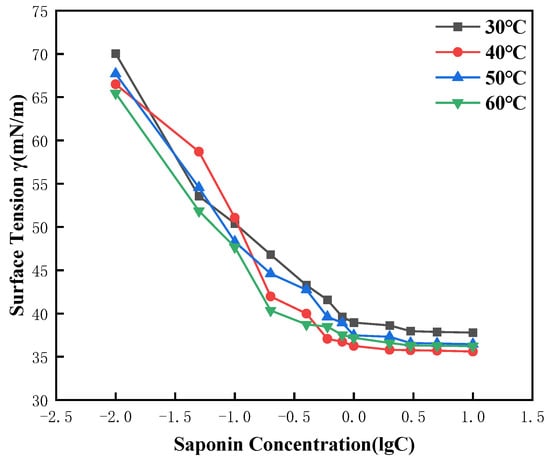
Figure 7.
Effect of temperature on the surface tension of saponin.
3.5. Effect of pH on Surface Tension
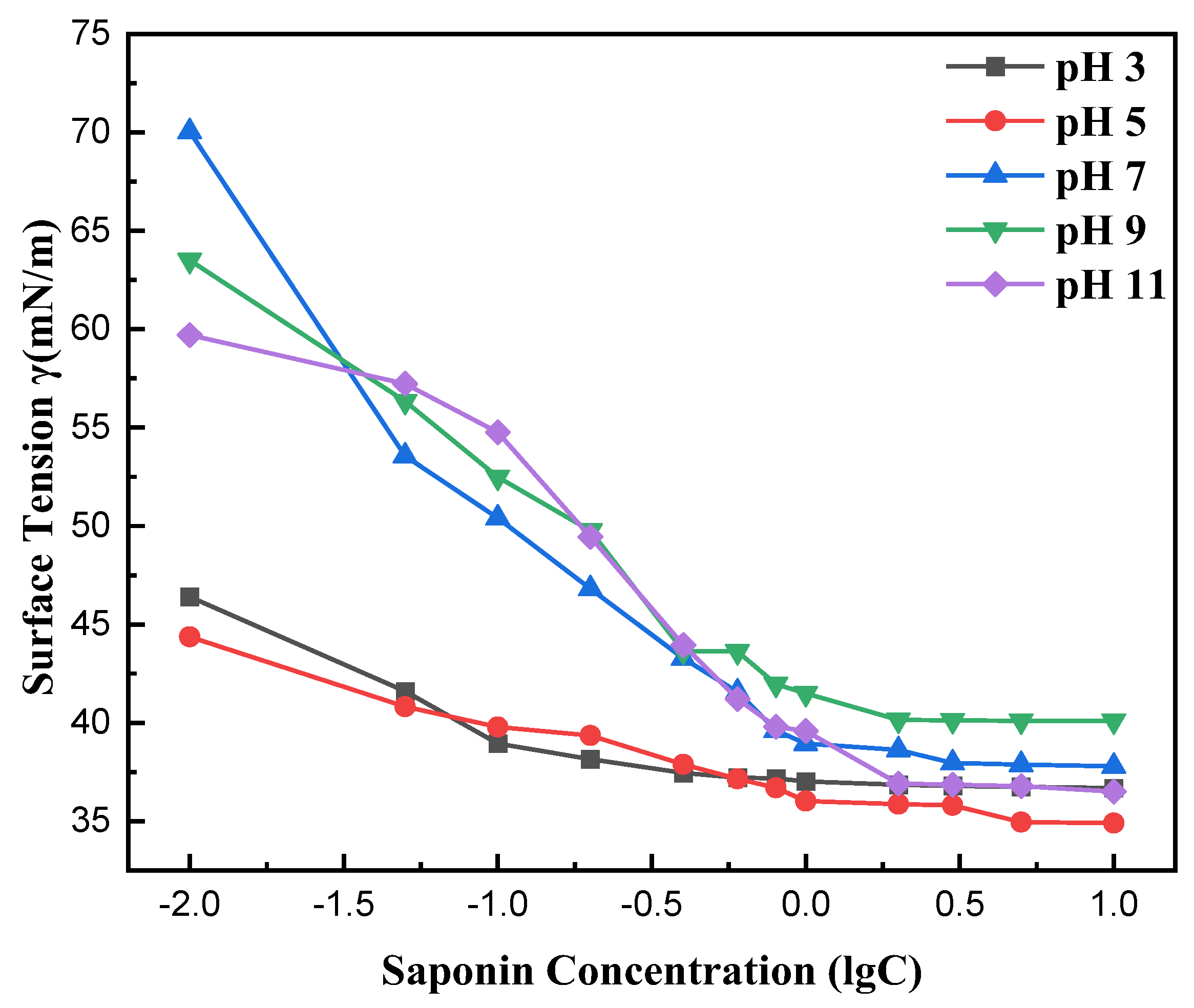
Figure 8 illustrates the effect of pH on the surface tension of saponin aqueous solutions and its correlation with mass concentration. The results indicate that saponin solutions exhibited the lowest surface tension at pH 3, suggesting optimal molecular interaction at the air–liquid interface under acidic conditions. This may result from protonation, which enhances saponin solubility and optimizes molecular alignment at the surface, hence lowering surface tension. Conversely, surface tension escalated when pH approached alkaline levels, which can be attributed to the deprotonation and ionization that impede molecular orientation and adhesion strength.
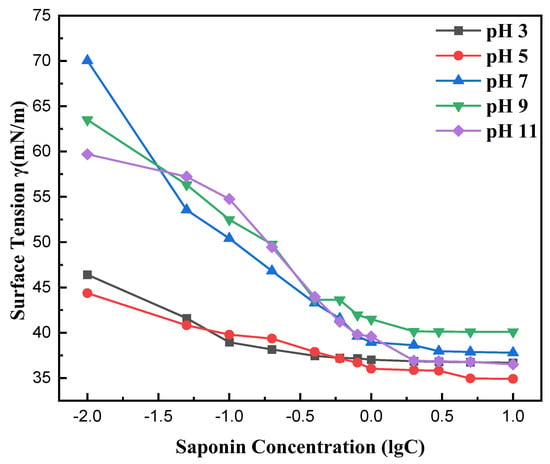
Figure 8.
Effect of pH value on the surface tension of saponin.
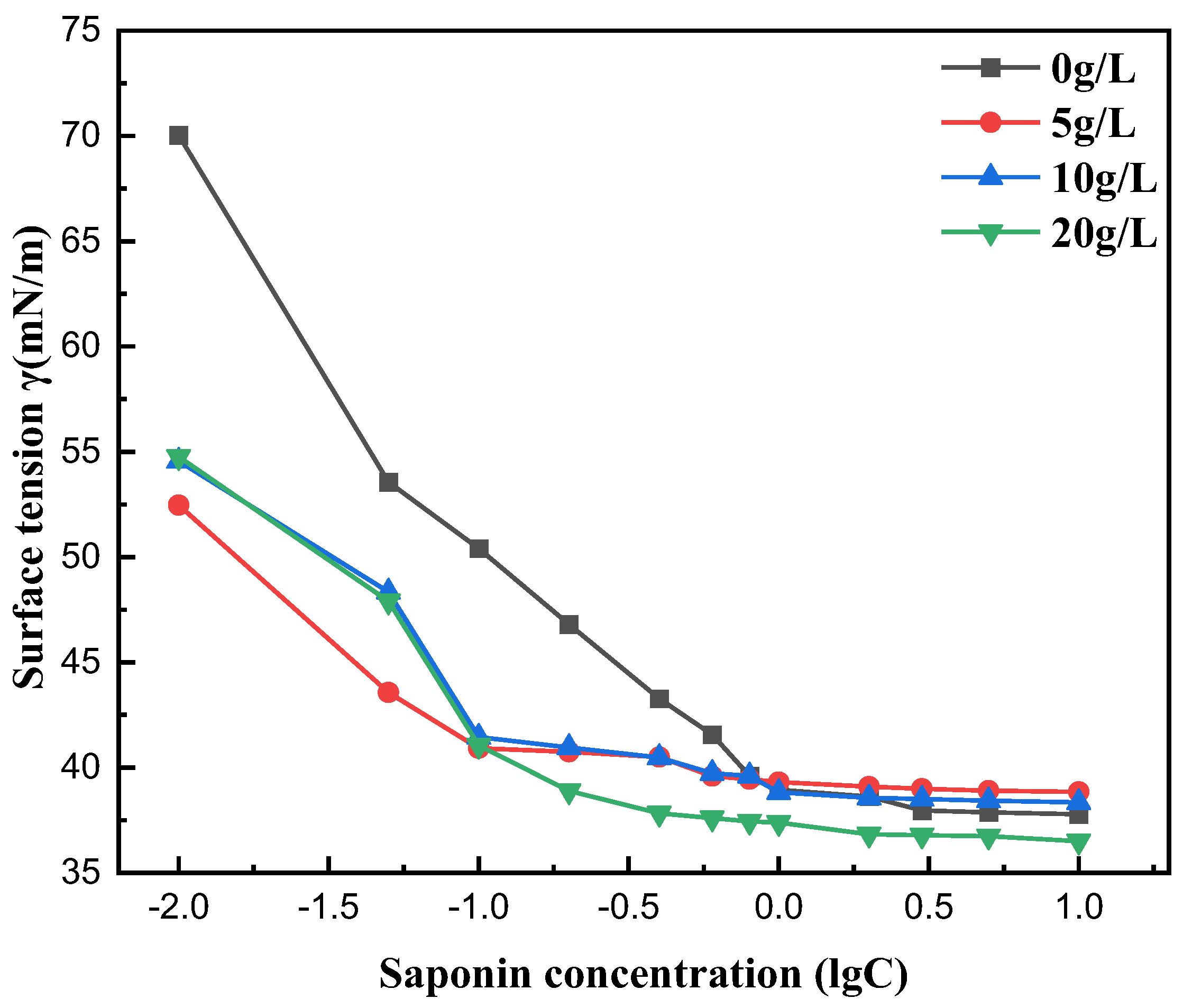
3.6. Effect of Salt Ion Concentration on Surface Tension
Measurements of surface tension were taken for the saponin solutions in saline environments, including NaCl solutions with different concentrations (5, 10, 20 g/L). The surface tension and CMC of the tea saponin solutions diminished as the content of NaCl increased. This indicates that tea saponin has good salt tolerance. The inorganic chloride ions, with high hydration properties, may augment the hydrophobic effect of the surfactant, facilitating the formation of a dense adsorption film at the interface and promoting micelle formation in the solution, hence decreasing the CMC (Figure 9).
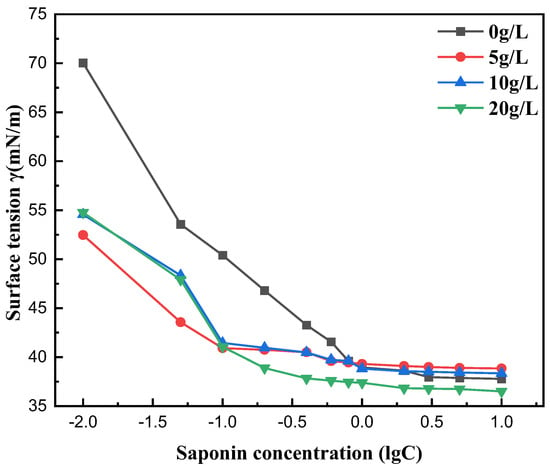
Figure 9.
Effect of salt ion concentration on surface tension.
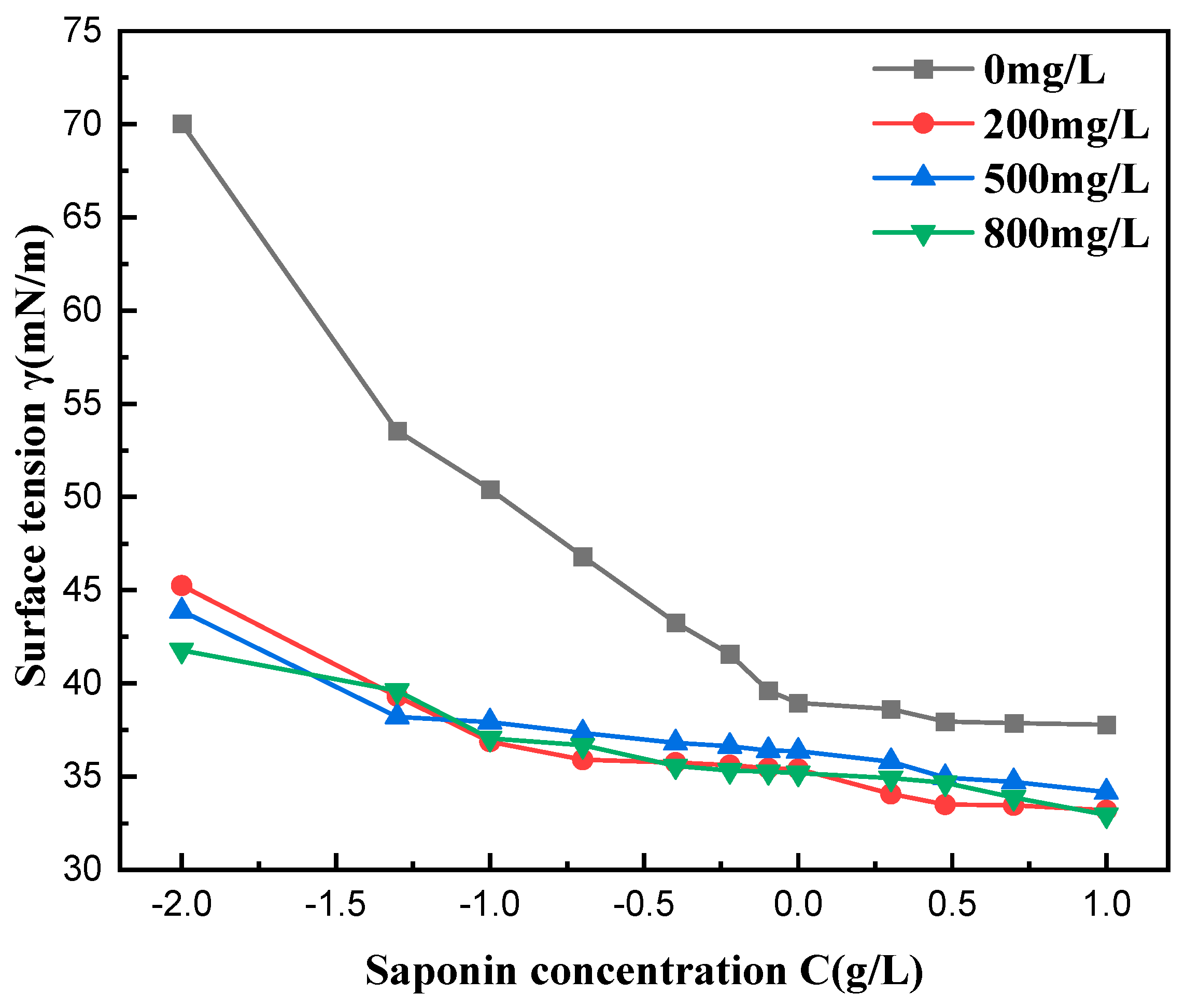
3.7. Effect of Water Hardness on Surface Tension
Figure 10 shows the impact of water hardness on the surface tension of saponin aqueous solutions and its correlation with mass concentration. Results indicate that an increase in water hardness results in a small but steady reduction in surface tension, more so in solutions with higher levels of calcium and magnesium. It is hypothesized that divalent ions, such as Ca2+ and Mg2+, facilitate the packing of saponin molecules at the air–liquid interface, enhancing its surface and active properties in hard water environments. At the same time, the CMC value of the solutions did not change significantly with the increase in water hardness, which indicates that the ions have little effect on the hydrophilic part of the surfactant, meaning that the micelle is still formed normally. The results showed that saponin had good resistance to hard water.
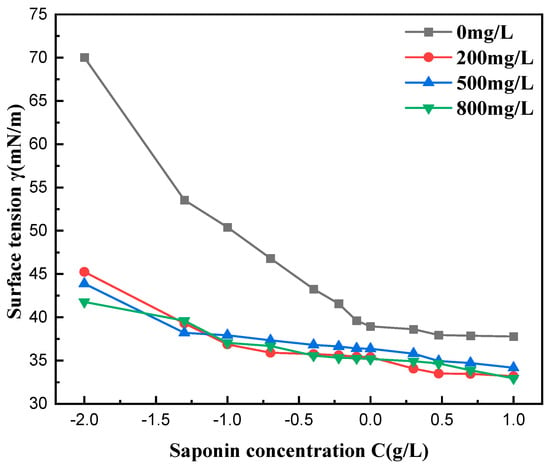
Figure 10.
Effect of water hardness on surface tension.
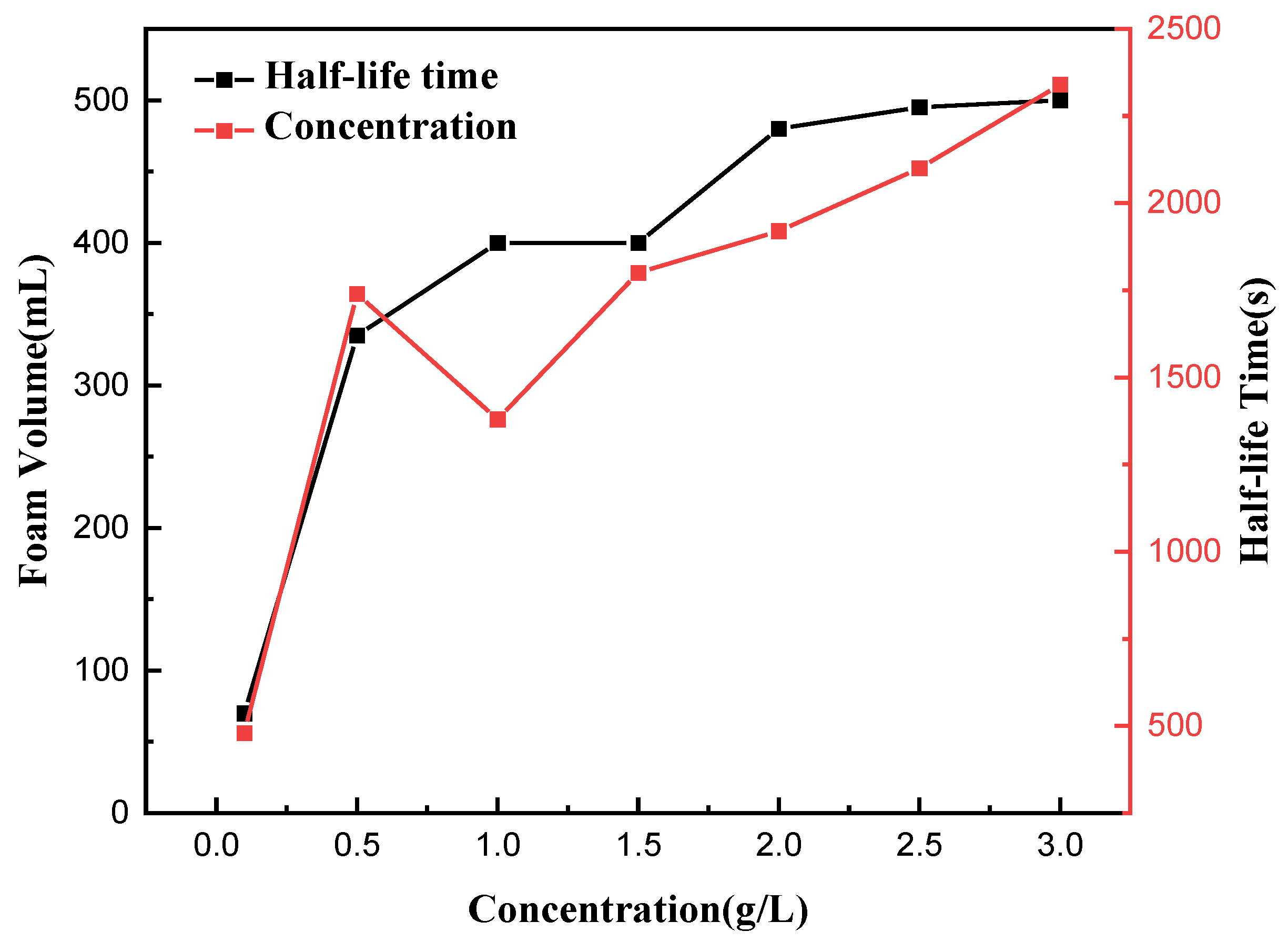
3.8. Foam Properties of Saponin
The foaming volume and foam half-life served as metrics to examine the influence of concentration on the foam characteristics of tea saponin, shown in Figure 11. It illustrates that when the concentration of saponins escalated from 0.1 g/L to 3 g/L, the foam volume of the saponin solution exhibited an upward trend. The half-life duration saw a minor reduction at a concentration of 1 g/L, thereafter, rising with further increases in concentration. At a concentration of 3 g/L, the foaming volume measured 490 mL, and the half-life was approximately 2350 s, suggesting that saponins are inclined to generate enduring foam. This stability is especially crucial for applications necessitating resilient foams.
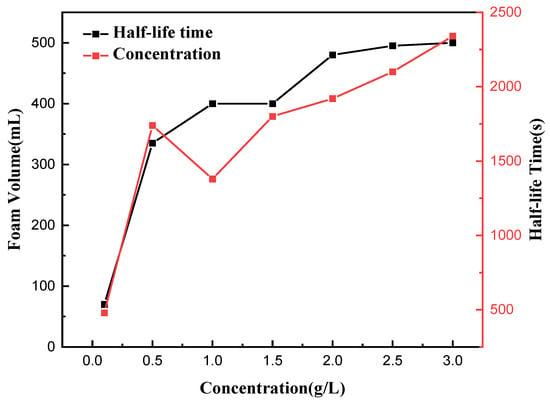
Figure 11.
Determination of foam properties of saponin solution at different concentrations.
3.9. Composite Effects of Camellia Oleifera Saponin and Rhamnolipid Surfactants
The compound system of tea saponin and rhamnolipid was constructed with different mass fraction ratios, and its surface tension is shown in Table 4. It can be seen from the data in Table 4 that the critical micelle concentrations and corresponding surface tensions of saponin and rhamnolipid are 0.5 g/L, 39.61 mN/m and 0.8 g/L, 25.29 mN/m, respectively. For the multi-component mixed solution system, the CMC theoretical value of the mixed solution can be calculated using the mass percentage according to the Clint binary system ideal solution rule equation.

Table 4.
Synergistic effect of the combination system of saponin and rhamnolipid.
It can be seen from the data in Table 4 that the CMC of the combination system of oleifera saponin and rhamnolipid gradually decreases with the increase in the proportion of oleifera saponin in the mixed system. By comparing the experimental results of the mixed system with the theoretical calculated values, it was found that Y is positive. This shows that no matter the ratio of the two systems, they have a synergistic effect. When the mass fraction of saponin X1 is 50%, the Y value reaches a maximum value of 69.23%, which indicates that the synergistic effect of the saponin and rhamnolipid complex system is the strongest. The CMC of the hybrid system was reduced to 0.2 g/L. In conclusion, the combination of saponin and rhamnolipid has a good synergistic effect. This is because, for anionic surfactants such as rhamnolipid, the addition of non-ionic surfactants not only strengthens the mutual attraction between the hydrophobic tails of the surfactants but also delays the negative electric repulsion between the ionized head groups, and the resulting mixed micelles have enhanced aggregation ability at the air–water interface. The surfactants are also more closely aligned with each other, resulting in a further enhancement of the micelle formation ability of the compound system, resulting in a synergistic effect.
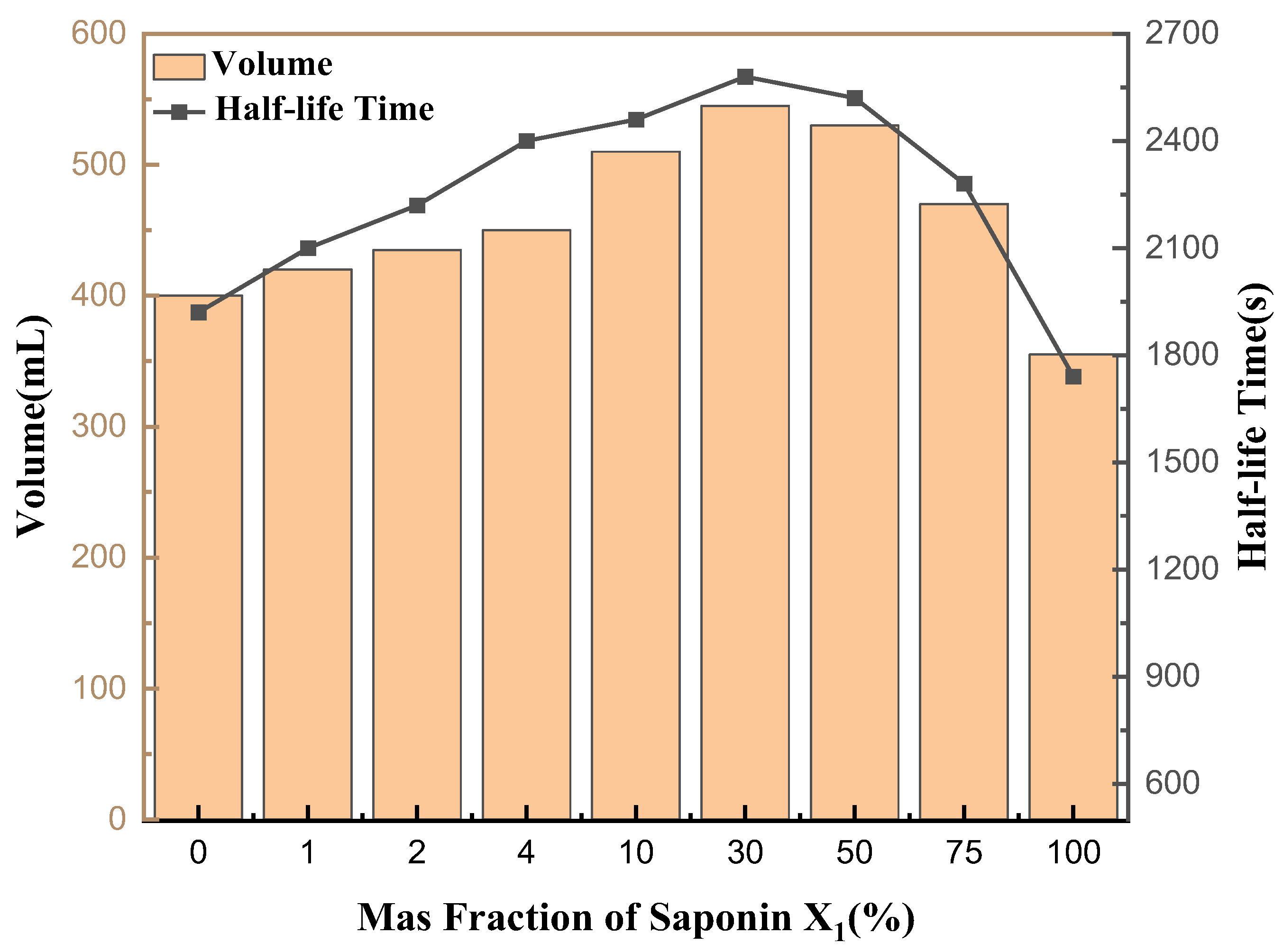
3.10. Determination of Foam Properties of Saponin Solution at Different Concentrations
As shown in Figure 12, the foam stability and foamability of the mixed solution of saponins and rhamnolipids at different ratios were determined to evaluate the foam stability of saponins. When the saponin concentration was 30%, the foaming volume and half-life time reached their maximum values, which were 545 mL and 2580 s, respectively. When the saponin concentration was 100% and 0%, the foaming force and half-life value of pure saponin solution and pure rhamnolipid solution were much lower than 30%. It can be inferred that the mixture of biosurfactants has a synergistic effect on foaming capacity and foam stability compared with a single biosurfactant and synthetic surfactant. Therefore, a mixture of rhamnolipids and saponins may, to some extent, serve as a potential alternative in detergents, cosmetics and the oil industry.
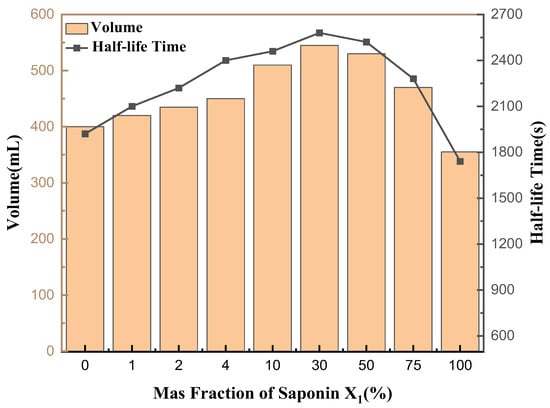
Figure 12.
Determination of foam properties of saponin and rhamnolipids solution at different concentrations (1:1).
4. Conclusions
The utilization of ultrasonic waves in conjunction with cellulase enhances the yield of tea saponin extraction. The response surface method was employed to improve extraction circumstances based on a single factor, resulting in an ideal quadratic polynomial mathematical model. The findings indicate that the maximum yield of tea saponin was 69.81 mg/g under the following optimal circumstances: 0.67% enzyme concentration, a solvent-to-material ratio of 16.82 mL/g, an extraction temperature of 58.14 °C, and an extraction duration of 1.89 h. Tea saponin is a natural non-ionic surfactant with excellent surface activity. The critical concentration of micelles at 30 °C is 0.5 g/L, and the lowest surface tension is 39.61 mN/m. The surface tension and CMC of tea saponin remained basically unchanged between 30 and 60 °C. When the pH of the solution is slightly acidic, the surface tension of tea saponin significantly decreases, while the CMC remains basically unchanged. Tea saponin has good salt and hard water resistance, and its surface tension is reduced to a certain extent in both saltwater and hard water. Tea saponin has strong foaming ability and stable foam. When the concentration of saponin solution was 3.0 g/L, the volume of foam was 490 mL, and the half-life was 2350 s. The combination of tea saponin and other surfactants has a certain synergistic effect. The critical micelle concentration of the composite system with natural surfactant rhamnolipids can be increased by 69.23%, and the surface tension can be reduced to a minimum of 22.56 mN/m. The foam performance and stability of the system were improved to a certain extent.
Author Contributions
Writing original draft, investigation, conceptualization, methodology, supervision, validation, funding acquisition, N.Z.; writing review and editing, software, investigation, data curation, Z.M.S.E.; investigation, visualization, supervision, writing review and editing, L.T. Test and analysis, W.S., W.L. (Wenxin Li) and W.L. (Wenlong Lu). All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Open Fund of Engineering Research Center of Development and Management for Low to Ultra-Low Permeability Oil & Gas Reservoirs in West China, Ministry of Education. (NO. KFJJ-XB-2023-1).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank the anonymous reviewers for their constructive and valuable opinions.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to affect the work reported in this paper.
References
- Zhang, F.; Zhu, F.; Chen, B.; Su, E.; Chen, Y.; Cao, F. Composition, bioactive substances, extraction technologies and the influences on characteristics of Camellia oleifera oil: A review. Food Res. Int. 2022, 156, 111159. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, Y.; Gao, Y.; Jiang, Z.; Zhao, Z.; Zeng, W.; Xie, M.; Liu, S.; Liu, R.; Chao, Y.; et al. Valorization of Camellia oleifera oil processing byproducts to value-added chemicals and biobased materials: A critical review. Green Energy Environ. 2024, 9, 28–53. [Google Scholar] [CrossRef]
- Lei-Ting, M.; Ya-Hui, Q.; Zhen-Dong, S.; Qiang, Z.; Feng, Y. Extraction of tea saponin and surface performance of its solutions. J. Tianjin Polytech. Univ. 2016, 35, 32–36. [Google Scholar] [CrossRef]
- Rai, S.; Acharya-Siwakoti, E.; Kafle, A.; Devkota, H.P.; Bhattarai, A. Plant-Derived Saponins: A Review of Their Surfactant Properties and Applications. Sci 2021, 3, 44. [Google Scholar] [CrossRef]
- Qin, P.; Shen, J.; Wei, J.; Chen, Y. A critical review of the bioactive ingredients and biological functions of Camellia oleifera oil. Curr. Res. Food Sci. 2024, 8, 100753. [Google Scholar] [CrossRef]
- An, L.; Wang, G.; Jia, H.; Liu, C.; Sui, W.; Si, C. Fractionation of Enzymatic Hydrolysis Lignin by Sequential Extraction for Enhancing Antioxidant Performance. Int. J. Biol. Macromol. 2017, 99, 674–681. [Google Scholar] [CrossRef]
- Wu, H.; Li, C.; Li, Z.; Liu, R.; Zhang, A.; Xiao, Z.; Ma, L.; Li, J.; Deng, S. Simultaneous extraction of oil and tea saponin from Camellia oleifera Abel. seeds under subcritical water conditions. Fuel Process. Technol. 2018, 174, 88–94. [Google Scholar] [CrossRef]
- Guo, J.; Zhao, N.; Zhao, Y.; Jin, H.; Sun, G.; Yu, J.; Zhang, H.; Shao, J.; Yu, M.; Yang, D.; et al. The Extraction Using Deep Eutectic Solvents and Evaluation of Tea Saponin. Biology 2024, 13, 438. [Google Scholar] [CrossRef]
- López-Salazar, H.; Camacho-Díaz, B.H.; Arenas Ocampo, M.L.; Jiménez-Aparicio, A.R. Microwave-Assisted Extraction of Functional Compounds from Plants: A Review. BioResources 2023, 18, 6614–6638. [Google Scholar] [CrossRef]
- Javid, M.; Haleem, A.; Singh, R.P.; Suman, R.; Gonzalez, E.S. Understanding the Adoption of Industry 4.0 Technologies in Improving Environmental Sustainability. Sustain. Oper. Comput. 2022, 3, 203–217. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, Z.; Yan, X.; Xie, J.; Yu, Q.; Chen, Y. Extraction Optimization of Tea Saponins from Camellia Oleifera Seed Meal with Deep Eutectic Solvents: Composition Identification and Properties Evaluation. Food Chem. 2023, 403, 136681. [Google Scholar] [CrossRef] [PubMed]
- Dessie, W.; Luo, X.; Wang, M.; Liao, Y.; Li, Z.; Khan, M.R.; Qin, Z. Enhancing the valorization efficiency of Camellia oil extraction wastes through sequential green acid pretreatment and solid-state fermentation-based enzymatic hydrolysis. Ind. Crops Prod. 2024, 217, 118893. [Google Scholar] [CrossRef]
- Singh, L.; Singh, B.; Balodi, S.; Kewlani, P.; Bhatt, I.D. Synergistic effects of enzyme-based ultrasonic-assisted extraction of phenolic compounds from Rhododendron arboreum and evaluation of thermal kinetic stability. J. Appl. Res. Med. Aromat. Plants 2022, 31, 100395. [Google Scholar] [CrossRef]
- Cano-Lamadrid, M.; Martínez-Zamora, L.; Mozaffari, L.; Bueso, M.C.; Kessler, M.; Artés-Hernández, F. Response Surface Methodology to Optimize the Extraction of Carotenoids from Horticultural By-Products—A Systematic Review. Foods 2023, 12, 4456. [Google Scholar] [CrossRef]
- Li, G.; Li, M.; Yan, Z.; Zhu, Q.; Cai, J.; Wang, S.; Yuan, Y.; Chen, Y.; Deng, S. Extraction of Oils and Phytochemicals from Camellia Oleifera Seeds: Trends, Challenges, and Innovations. Processes 2022, 10, 1849. [Google Scholar] [CrossRef]
- Guo, X.; Qiao, Q.; Jin, Y.; Lei, H.; Guo, K.; Zhao, Z.; Li, P.; Liu, A.; Sun, R. Optimization of ultrasound-assisted extraction of two saponins from Paris polyphylla var. yunnanensis leaves using response surface methodology. Front. Sustain. Food Syst. 2024, 8, 1424285. [Google Scholar] [CrossRef]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H.; et al. A comprehensive review of ultrasonic assisted extraction (UAE) for bioactive components: Principles, advantages, equipment, and combined technologies. Ultrason. Sonochem. 2023, 101, 106646. [Google Scholar] [CrossRef]
- Mahato, N.; Sinha, M.; Sharma, K.; Koteswararao, R.; Cho, M.H. Modern Extraction and Purification Techniques for Obtaining High Purity Food-Grade Bioactive Compounds and Value-Added Co-Products from Citrus Wastes. Foods 2019, 8, 523. [Google Scholar] [CrossRef]
- Yolandani; Ma, H.; Li, Y.; Liu, D.; Zhou, H.; Liu, X.; Wan, Y.; Zhao, X. Ultrasound-assisted limited enzymatic hydrolysis of high concentrated soy protein isolate: Alterations on the functional properties and its relation with hydrophobicity and molecular weight. Ultrason. Sonochem. 2023, 95, 106414. [Google Scholar] [CrossRef]
- Carreira-Casais, A.; Otero, P.; Garcia-Perez, P.; Garcia-Oliveira, P.; Pereira, A.G.; Carpena, M.; Soria-Lopez, A.; Simal-Gandara, J.; Prieto, M.A. Benefits and Drawbacks of Ultrasound-Assisted Extraction for the Recovery of Bioactive Compounds from Marine Algae. Int. J. Environ. Res. Public Health 2021, 18, 9153. [Google Scholar] [CrossRef]
- Bui, T.D.; Do, L.T.K.; Dang, T.N.D.; Pham, V.D.; Hoang, V.C. Recovery of Saponins, Phenolic Compounds, and Antioxidant Capacity from Curculigo Orchioides Gaertn Rhizomes by Different Extraction Methods. Appl. Sci. 2024, 14, 7535. [Google Scholar] [CrossRef]
- Castro-Puyana, M.; Marina, M.L.; Plaza, M. Water as green extraction solvent: Principles and reasons for its use. Curr. Opin. Green Sustain. Chem. 2017, 5, 31–36. [Google Scholar] [CrossRef]
- Lajoie, L.; Fabiano-Tixier, A.-S.; Chemat, F. Water as Green Solvent: Methods of Solubilization and Extraction of Natural Products—Past, Present and Future Solutions. Pharmaceuticals 2022, 15, 1507. [Google Scholar] [CrossRef]
- Plattes, M.; Koehler, C.; Galle, T. Purely ultrasonic enzyme extraction from activated sludge in an ultrasonic cleaning bath. MethodsX 2017, 4, 214–217. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, S.; Wan, F.; Zhou, Y.; Wang, Z.; Fan, G.; Wang, P.; Luo, H.; Liao, S.; He, L.; et al. Quantitative Analysis of Camellia oleifera Saponins and Aqueous Two-Phase Extraction and Separation. Molecules 2023, 28, 2132. [Google Scholar] [CrossRef]
- Cao, J.; Wu, G.; Wang, L.; Cao, F.; Jiang, Y.; Zhao, L. Oriented Deep Eutectic Solvents as Efficient Approach for Selective Extraction of Bioactive Saponins from Husks of Xanthoceras sorbifolia Bunge. Antioxidants 2022, 11, 736. [Google Scholar] [CrossRef]
- Holland, C.; Ryden, P.; Edwards, C.H.; Grundy, M.M.-L. Plant Cell Walls: Impact on Nutrient Bioaccessibility and Digestibility. Foods 2020, 9, 201. [Google Scholar] [CrossRef]
- Alba, A.T.; Allaño-González, M.J.; Palma, M.; Barbero, G.F.; Carrera, C. Enhancing Efficiency of Enzymatic-Assisted Extraction Method for Evaluating Bioactive Compound Analysis in Mulberry: An Optimization Approach. Agronomy 2023, 13, 2548. [Google Scholar] [CrossRef]
- Hu, J.-L.; Nie, S.-P.; Huang, D.-F.; Li, C.; Xie, M.-Y. Extraction of saponin from Camellia oleifera cake and evaluation of its antioxidant activity. Int. J. Food Sci. Technol. 2012, 47, 1676–1687. [Google Scholar] [CrossRef]
- Mujahid, R.; Riaz, A.; Insyani, R.; Kim, J. A Centrifugation-First Approach for Recovering High-Yield Bio-Oil with High Caloric Values in Biomass Liquefaction: A Case Study of Sewage Sludge. Fuel 2020, 262, 116628. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Hu, Y.; Cui, X.; Zhang, Z.; Chen, L.; Zhang, Y.; Wang, C.; Yang, X.; Qu, Y.; Xiong, Y. Optimisation of Ethanol-Reflux Extraction of Saponins from Steamed Panax notoginseng by Response Surface Methodology and Evaluation of Hematopoiesis Effect. Molecules 2018, 23, 1206. [Google Scholar] [CrossRef]
- Rai, S.; Kafle, A.; Devkota, H.P.; Bhattarai, A. Characterization of Saponins from the Leaves and Stem Bark of Jatropha curcas L. for Surface-Active Properties. Heliyon 2023, 9, e15807. [Google Scholar] [CrossRef]
- Coşkun, N.; Saritas, S.; Jaouhari, Y.; Bordiga, M.; Karav, S. The Impact of Freeze Drying on Bioactivity and Physical Properties of Food Products. Appl. Sci. 2024, 14, 9183. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, Y.; Tao, L.; Zhang, X.; Hao, F.; Zhao, S.; Han, L.; Bai, C. Recent Advances in Separation and Analysis of Saponins in Natural Products. Separations 2022, 9, 163. [Google Scholar] [CrossRef]
- Son, H.L.; Phuong, V.N.L.; Minh, T.V. Application of Response Surface Methodology for Optimizing the Extraction Conditions of Total Saponins from Polyscias fruticosa (L.) Harms Planted in Southern Vietnam. Asian J. Biotechnol. Bioresour. Technol. 2023, 9, 20–30. [Google Scholar] [CrossRef]
- Zhang, N.; Si, S.; Tao, L.; Li, J.; Li, J.; Du, H. Preparation and Performance Evaluation of Environmental-Friendly, Temperature-Resistant and Salt-Resistant Tea Saponin Foaming Agent. Energy Sources Part A Recovery Util. Environ. Eff. 2023, 45, 1564–1585. [Google Scholar] [CrossRef]
- Xie, J.; Huang, W.; Wu, X. Effect of Tea Saponin on the Foaming Properties of Pea Protein. Food Funct. 2023, 14, 4339–4353. [Google Scholar] [CrossRef]
- Saleh, Z.S.; Hossain, M.M. A study of the separation of proteins from multicomponent mixtures by a semi-batch foaming process. Chem. Eng. Process. Process Intensif. 2001, 40, 371–378. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Yang, C.-H.; Chang, M.-S.; Chou, Y.-P.; Huang, Y.-C. Foam Properties and Detergent Abilities of the Saponins from Camellia Oleifera. Int. J. Mol. Sci. 2010, 11, 4417–4425. [Google Scholar] [CrossRef]
- GB/T 7462-94; Foaming Power Determination of Surfactant Improvement of Ross-Miles Method. State Administration of Quality Supervision and Inspection: Beijing, China; China Standard Publisher: Beijing, China, 1994.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).