Abstract
Process mineralogy is an important technique to evaluate the economic value of ore, and it also has an important guiding role in flotation. Copper–cobalt sulfide ore, a significant source of copper and cobalt metals, is abundant in the Democratic Republic of the Congo (DRC). In this paper, DRC copper–cobalt sulfide ore is employed to validate process mineralogy guidance for flotation, thereby enhancing Cu-Co recovery. Process mineralogy results indicate that the economically valuable metals in copper–cobalt sulfide ore are Cu and Co. Cu is predominantly deposited in chalcopyrite, bornite, chalcocite, and carrollite, while Co is primarily found in carrollite. However, a part of the chalcopyrite and carrollite is closely embedded with other minerals, which complicates mineral dissociation and poses challenges for the efficient recovery of Cu and Co. Guided by process mineralogy results, conditional, open-circuit, and locked-cycle experiments were conducted to explore the feasibility of flotation recovery for Cu and Co. The results show that through flotation, the grade of Cu/Co can be increased from 1.27%/0.56% to 24.43%/9.78%, and the recovery of Cu/Co reached 94.47%/86.35%, which is significantly better than conventional flotation without the guidance of process mineralogy. This case is of great significance for process-mineralogy-guided flotation for the efficient recovery of Cu-Co in the DRC.
1. Introduction
Copper is a critical metal that plays an important role in the development of societies [1,2]. Due to its good ductility, electrical conductivity, thermal conductivity, and ease of recycling, copper is widely used in many fields such as electrical, renewable energy, and machinery manufacturing fields [3], and its production and consumption are third to steel and aluminum [4]. Meanwhile, cobalt has the advantages of having a high melting point, low thermal and electrical conductivity, and strong ferromagnetism properties, which makes it play an important role in producing the heat-resistant alloys, cemented carbide, anti-corrosion alloys, and magnetic alloy industries [5,6]. Especially in recent years, the demand for cobalt has increased rapidly with the development of batteries and energy storage technology [7].
Most of the produced copper and cobalt are derived from the mining industry. Copper–cobalt oxide ore is easy to extract directly by hydrometallurgy [8]. However, copper–cobalt sulfide ore is a difficult processing target and it is a challenge to achieve satisfactory results by direct hydrometallurgy [5]. Flotation is a common method that is used to improve the grade of sulfide ore at a low cost, which has a significant impact on reducing the cost for subsequent metallurgy. A number of studies have been reported on copper sulfide flotation including process mineralogy, flotation processes, and flotation reagents [9,10].
The mineralogical property of the ore plays an important role in improving the flotation process. Tijsseling [11] employed QEMSCAN (Quantitative Evaluation of Minerals by SCANning electron microscopy) to investigate the mineralogy in sediment-hosted copper–cobalt deposits in the Democratic Republic of the Congo (DRC). The study revealed that bornite, chalcopyrite, chalcocite, and carrollite were the main copper-bearing sulfide minerals, while carrollite was the only cobalt-bearing mineral. They studied the effect of mineralogy on flotation performance using ore samples from the same deposit and developed a process recovery prediction model based on mineralogical and flotation performance data. Meanwhile, a simple and practical recovery model based on mineral liberation characteristics was also developed, further extending the application of process mineralogy in the process design of the Kamoa project [12].
The slurry potential has a significant effect on the flotation of non-ferrous metal sulfides [13], and slurry aeration is a commonly used method in industry to control the slurry potential [14]. Aeration was used to assist HAP (high alkaline process) flotation in the copper sulfide ore at the Dongguashan concentrator, and the copper recovery was increased from 79.8% to 87.7% on the industrial scale [15]. Moimane [16] investigated the critical degree of the surface oxidation of minerals in copper sulfide flotation. It was found that the degree of chalcocite oxidation (1.74–4.85) was lower than that of chalcopyrite oxidation (11.7), indicating that chalcocite flotation was more sensitive to surface oxidation. In addition, reducing the oxidation in flotation may have a negative impact on the flotation of complex Cu-Zn sulfide ores [17].
The research on copper sulfide flotation reagents focused on collectors. In addition to conventional xanthate reagents, new collectors including heterocyclic reagents and phosphates have also been reported [18,19,20,21,22]. Recently, the research on sulfide flotation inhibitors including sodium phytate and xanthan gum has gradually increased [23,24]. In addition, the effect of unconventional agents including anionic polyacrylamide, n-dodecane, and residual xanthate on sulfide flotation has also been reported [25,26].
The DRC is rich in copper–cobalt sulfide deposits, and most of the world’s cobalt comes from copper–cobalt sulfide ore [27,28]. Tijsseling [29] researched the process mineralogy of the mixed-oxide/sulfide copper–cobalt minerals in the DRC, and the flotation effects of xanthate, phosphorodithioate, dithiophosphate, thiocarbamate, and a blend type of collector were studied. It was found that through a two-stage rougher–scavenger flotation process with dithiophosphate as the collector, 94% of the carrollite, more than 90% of the copper sulfides, and 70% of the copper oxide minerals can be recovered. Significant differences in the recovery rates of copper and cobalt were observed. Copper recovery varied between 70% and 83%, while cobalt recovery ranged from 38% to 48%. In addition, Dehaine [30] investigated the flotation of a copper–cobalt sulfide ore and found that ore mineralogy can effectively guide the flotation process. Carrollite, a copper–cobalt sulfide mineral, exhibited good floatability but had a lower recovery rate compared to other copper sulfides. The particle size of carrollite was crucial for cobalt recovery, and increasing the collector dosage could raise the upper limit of the particle size for effective recovery.
In summary, process mineralogy plays a significant guiding role in flotation. In this paper, a case study of a DRC copper–cobalt sulfide ore was conducted. Guided by process mineralogy, experiments on rougher flotation, cleaner flotation, scavenger flotation, and locked-cycle tests were carried out, yielding favorable flotation results for the recovery of Cu–Co. And, the result will be of great significance for the efficient utilization of copper and cobalt sulfide ore processing.
2. Materials and Methods
2.1. Materials
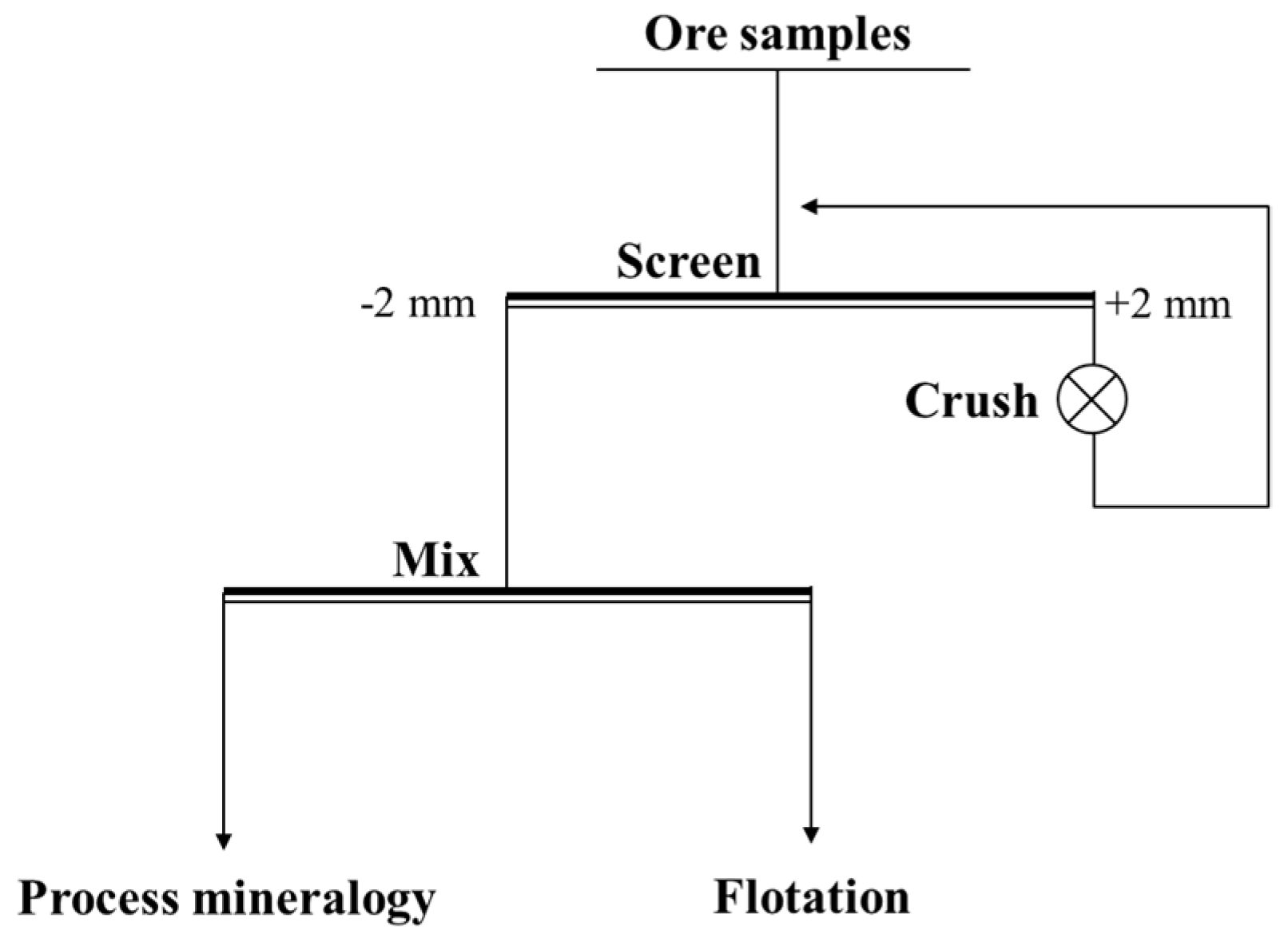
The copper–cobalt sulfide ore samples were from a drill core sample, which was taken from a large copper–cobalt sulfide deposit in the southeastern DRC. Some of the representative bulk samples were cut, smoothed, and polished into light sheets for mineralogical microscopy measurement. The rest of the samples were crushed, screened, and mixed according to Figure 1. A portion of the sample was used for process mineralogy analysis, and a portion for flotation experiments. The samples were stored in a dry environment.
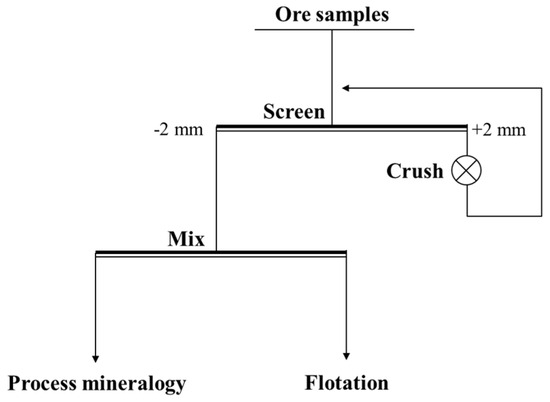
Figure 1.
The process of ore sample treatment.
2.2. Process Mineralogy
The chemical composition of the sample was determined by multi-component chemical analysis, the mineral composition was determined by X-ray diffraction (XRD, D8 advance, Bruker Corporation) analysis, and the embedding relationship of the minerals was determined by mineralogical microscope (XTL-2400, Shanghai Precision Instrument Co., LTD., Shanghai, China).
The samples for multi-component chemical analysis and XRD measurement were milled to −0.074 mm, and the samples for mineralogical microscope measurement were light sheets with a smooth surface.
For XRD analysis, the wavelength of the X-ray was 0.15406 nm. The scanning interval was set between 10° and 70°, with a scanning rate of 0.02° per second. And, the software Jade 6 was employed for the processing and analysis of XRD data. For the mineralogical microscope, the testing mode employed was the reflected light and orthogonal polarization mode.
2.3. Flotation
The flotation experiment was completed by the XFD-II flotation machine (1.5 L, Jitan machinery Co., Ltd., Changchun, China). Firstly, 500 g of ore samples and 500 g of water were sequentially put into a laboratory ball mill (XMQ, Wuhan exploration machinery Co., Ltd., Wuhan, China) for grinding. Then, the fine samples were fed into the flotation machine. The speed of the flotation machine was controlled at 2000 rpm, and the slurry concentration was controlled at 33%. Afterwards, a certain amount of the flotation reagents including the regulator, collector, and frother were added sequentially. The flotation machine was aerated at 200 L/h in the flotation process and the flotation time was fixed at 5 min. After flotation, the concentrate, middling, and tailings were filtered, dried, weighed, and analyzed.
The media used for grinding and regrinding was steel balls, and the water used in grinding, flotation, and reagent preparation was deionized water. For flotation reagents, CaO was used as a pH regulator, which increased the pH of the slurry, and had a good inhibitory effect on pyrite. NaHS was used as a regulator to improve the recovery of copper and cobalt. Sodium isopropyl xanthate (SIPX) and isopropyl ethyl thiamine (Z-200) were used as collectors. SIPX is one of the most commonly used sulfide collectors, and Z-200 has a good effect in separating copper sulfide minerals and pyrite. Pine oil (PO) was used as a frother. Carboxymethyl cellulose (CMC) was used as an inhibitor in the cleaner flotation because it can inhibit gangue minerals such as dolomite, chlorite, and talc in copper–cobalt sulfide ores, thus upgrading the final concentrate grade.
The flotation yield can be calculated by Equation (1).
where γ is the flotation yield, m is the weight of the product being calculated, and m0 is the total weight of all products.
γ = m/m0,
The flotation recovery can be calculated by Equation (2).
where ξ is the flotation recovery, γ is the flotation yield, β is the grade of the product being calculated, and α is the grade of the raw ore.
ξ = γ ∗ β/α,
2.4. The Flowchart of the Research
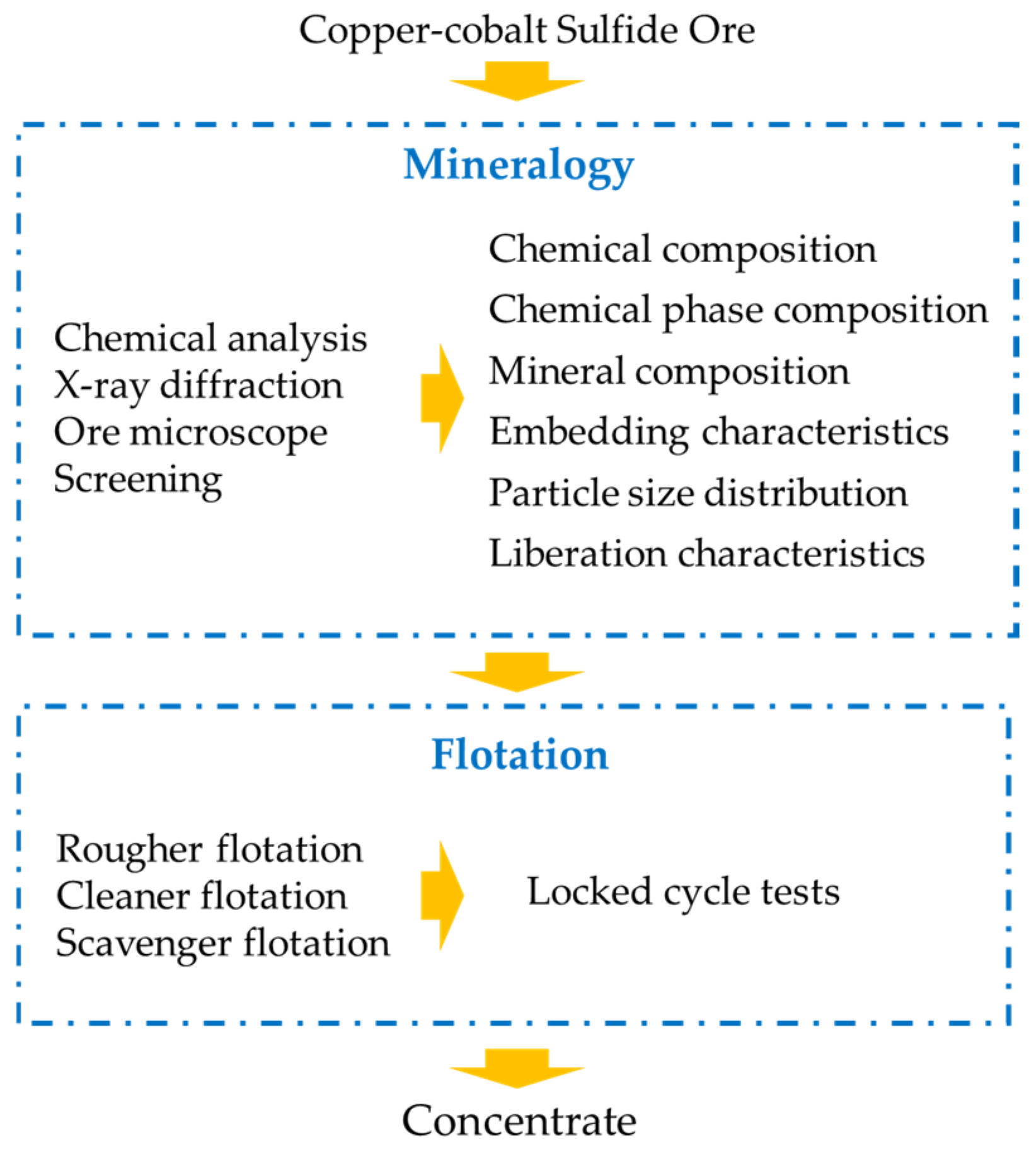
The flowchart of the research is shown in Figure 2. First, the process mineralogy of copper–cobalt sulfide ore was investigated, including the chemical composition, mineral composition, embedding characteristics, particle size distribution, and liberation characteristics. Subsequently, guided by process mineralogy, rougher flotation, cleaner flotation, and scavenger flotation were conducted in sequence. Finally, the final flotation indices were confirmed through the locked-cycle tests.
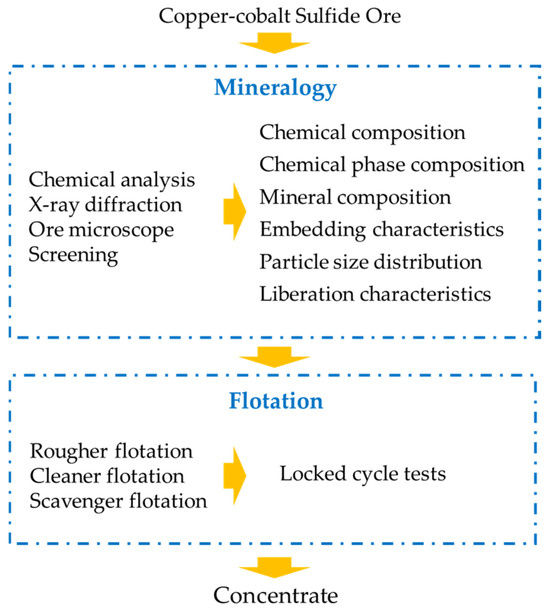
Figure 2.
The flowchart of the research.
3. Results and Discussion
3.1. Chemical and Mineral Composition of Copper–Cobalt Sulfide Ore
3.1.1. Chemical Composition
The chemical composition of copper–cobalt sulfide ore is shown in Table 1. The Cu content and Co content were 1.26% and 0.58%, respectively, and had a high economic utilization value and were the key metals to be recovered. The contents of other valuable metal elements including Pb, Zn, and Mo were low, and they do not have an economic utilization value. In addition, the contents of the precious metals Au and Ag were very low, which were also difficult to be comprehensively utilized.

Table 1.
The chemical composition of copper–cobalt sulfide ore.
3.1.2. Chemical Phase Composition
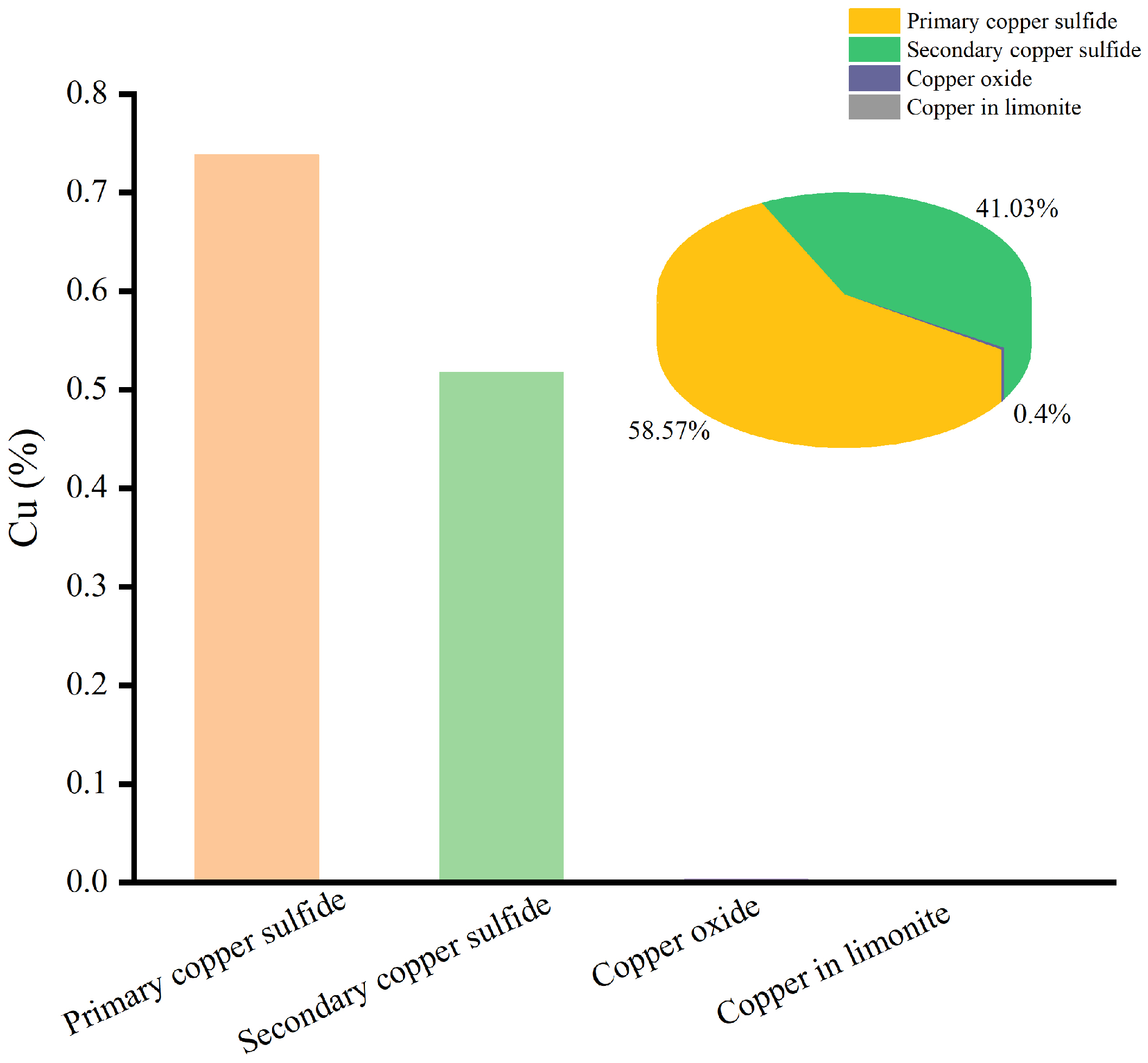
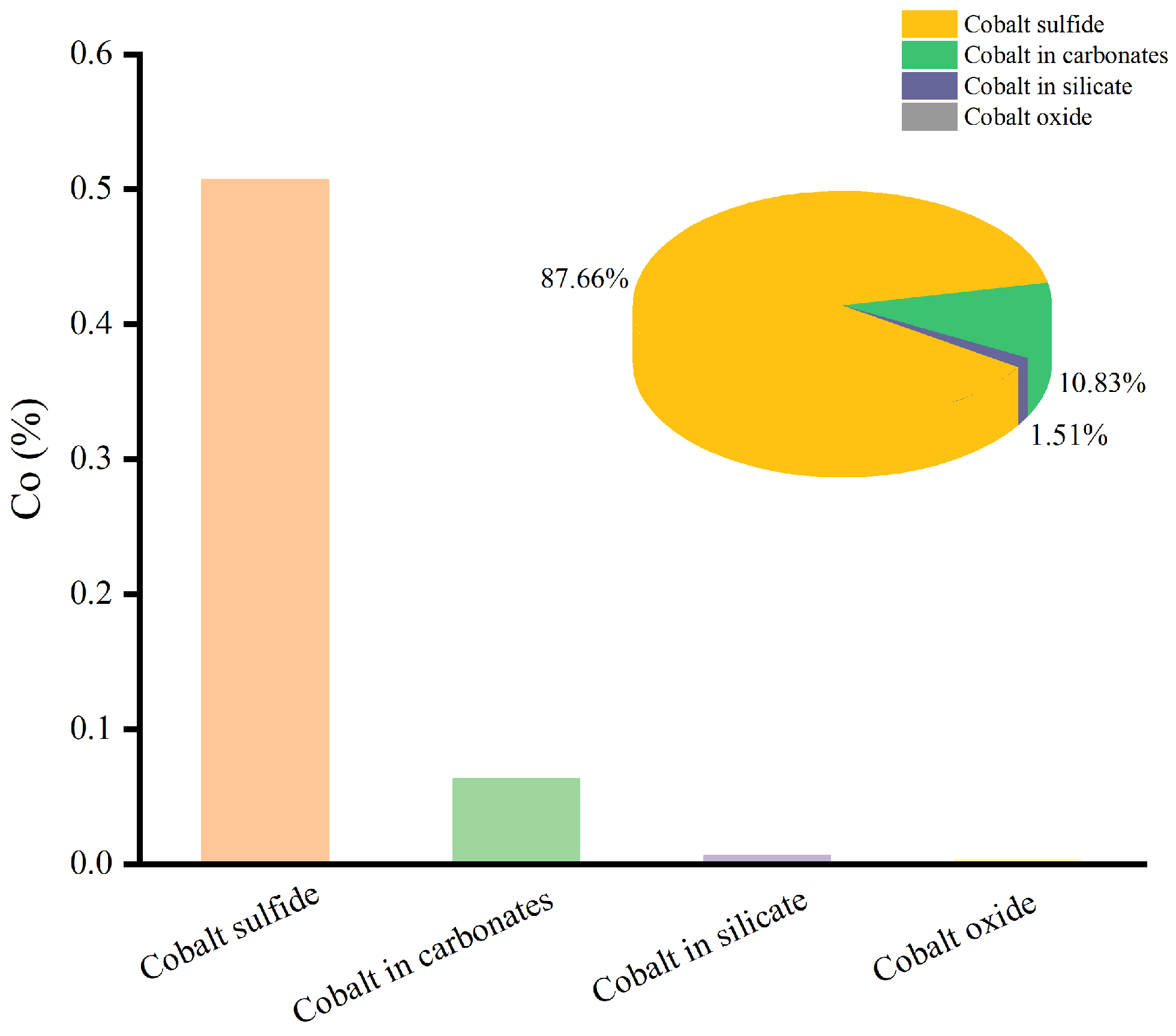
Since Cu and Co were metals with economic value, the chemical phase analysis of Cu and Co was carried out, and the results are shown in Figure 3 and Figure 4.
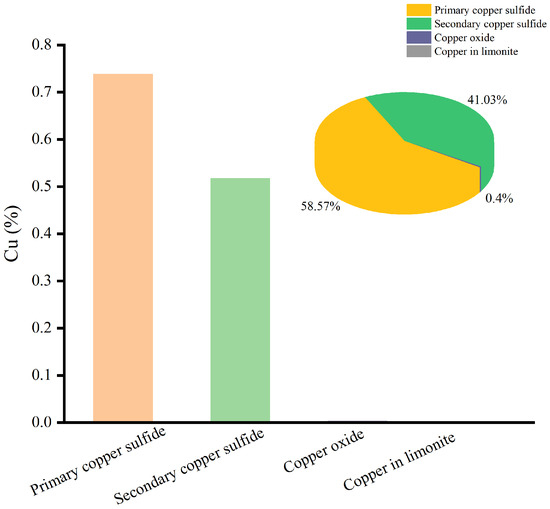
Figure 3.
The chemical phase analysis of Cu.
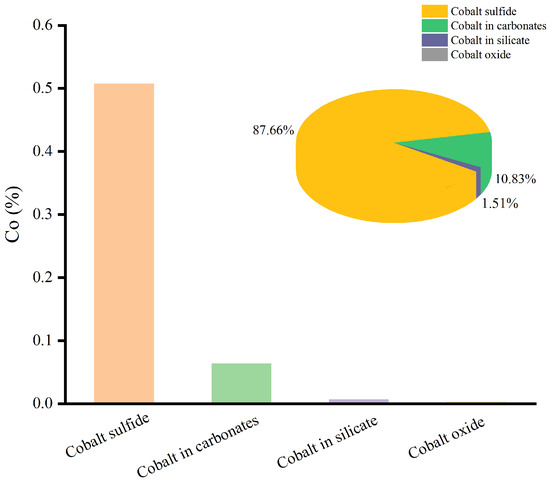
Figure 4.
The chemical phase analysis of Co.
The results of Figure 3 show that most of the copper was found in the primary copper sulfide and secondary copper sulfide minerals, with contents of 58.57% and 41.03%, respectively. Meanwhile, only a small amount of copper occurred in copper oxide and limonite.
According to Figure 4, Co was primarily deposited in cobalt sulfide, accounting for 87.66% of the total Co. In addition, 10.83% of the Co was deposited in carbonates, while small amounts of Co were present in cobalt oxide and silicate.
Therefore, the valuable metals of Cu and Co were mostly found in sulfide minerals, and the ore sample was a typical copper–cobalt sulfide ore.
3.1.3. Mineral Composition
The mineral composition of the ore is shown in Table 2.

Table 2.
The mineral composition of copper–cobalt sulfide ore (%).
The major rock-forming minerals in the ore were dolomite, quartz, chlorite, and talc, with contents of 47.83%, 33.87%, 6.38%, and 2.92%, respectively. Chalcopyrite and carrollite were the major copper-bearing minerals; however, there were small amounts of bornite and chalcocite, and trace amounts of malachite. Similarly, carrollite was the predominant cobalt-bearing mineral, and small amounts of cobalt occurred in spherocobaltite and heterogenite.
In addition, hematite, limonite, ilmenite, pyrite, galena, and sphalerite were present in the ore, which had no utilization value due to their low content.
3.2. Embedding Characteristics of Key Cu/Co-Bearing Minerals
According to Table 2, chalcopyrite was the key Cu-bearing mineral and carrollite was the key Co-bearing mineral in the ore. To further guide the flotation process, the embedding characteristics of chalcopyrite and carrollite were analyzed by a mineralogical micro-scope.
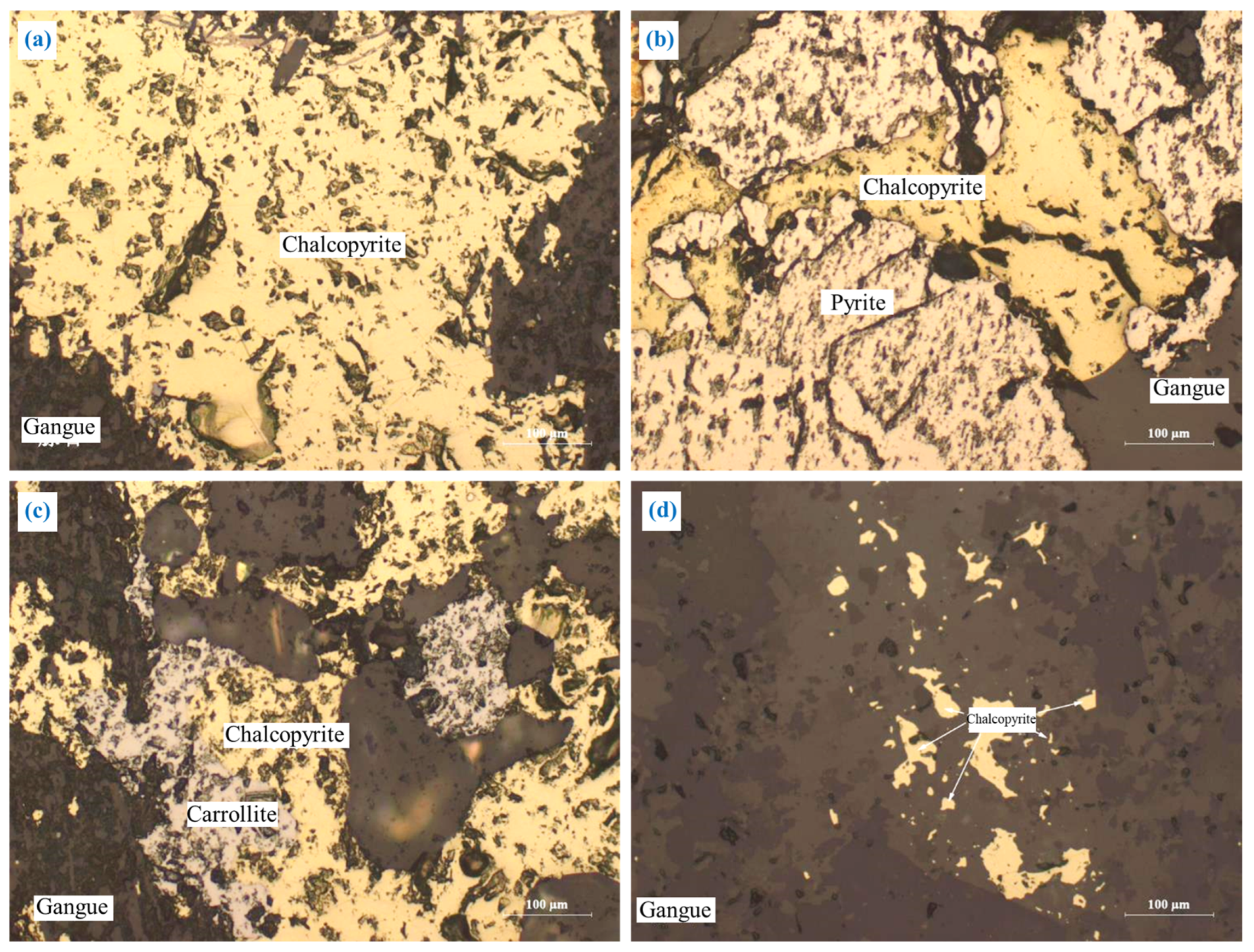
3.2.1. Chalcopyrite (CuFeS2)
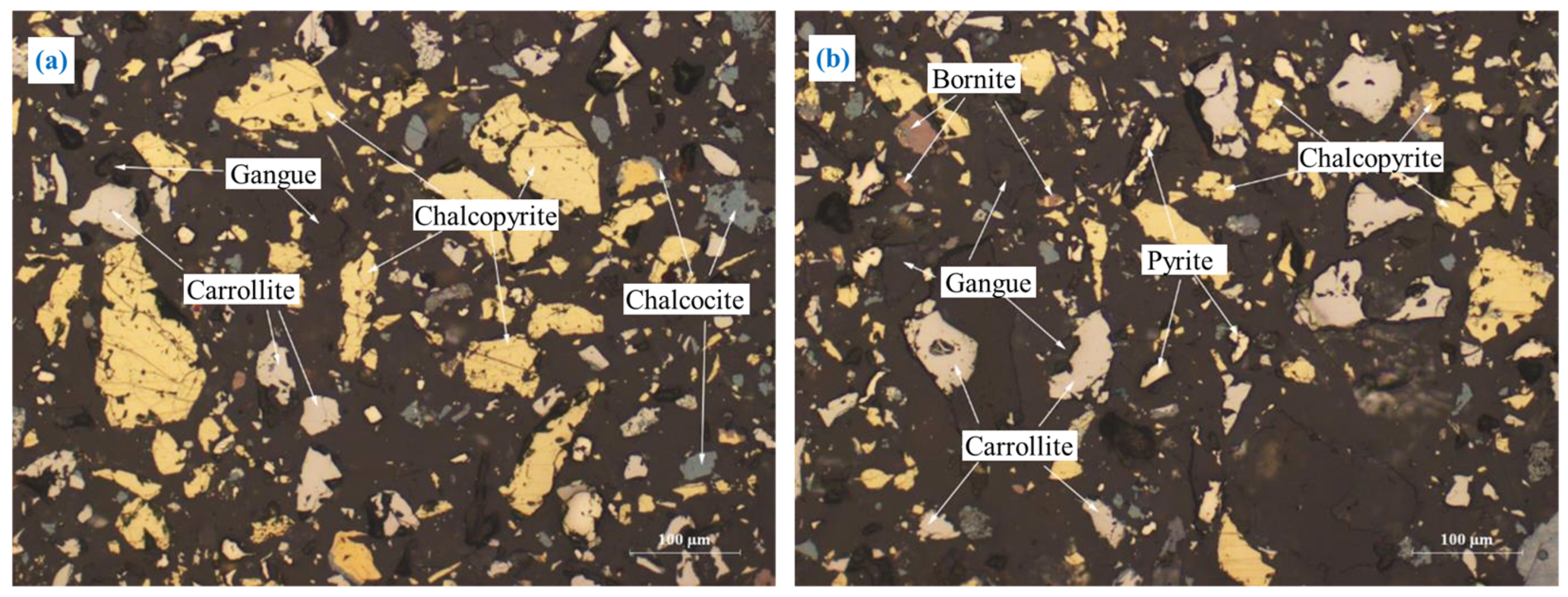
The embedding characteristics of chalcopyrite are shown in Figure 5. Chalcopyrite was primarily embedded in granular form (Figure 5a). A portion of chalcopyrite was closely interlaid with pyrite and carrollite in irregular shapes (Figure 5b,c), and a small amount of chalcopyrite was embedded in gangue in a fine-grained irregular shape (Figure 5d). In addition, most of the chalcopyrite particles were coarse-grained, and the embedded grain size was concentrated at 0.05–0.9 mm.
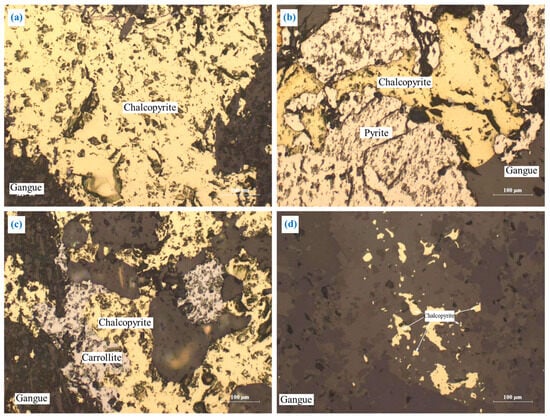
Figure 5.
The embedding characteristics of chalcopyrite: (a) granular form; (b) closely interlaid with pyrite in irregular shapes; (c) closely interlaid with carrollite in irregular shapes; (d) fine-grained irregular shapes.
This embedding characteristic is conducive to the monomer dissociation and flotation separation of chalcopyrite. However, the close intercalation relationship between chalcopyrite and pyrite or carrollite will affect the monomer dissociation and efficient separation of the chalcopyrite.
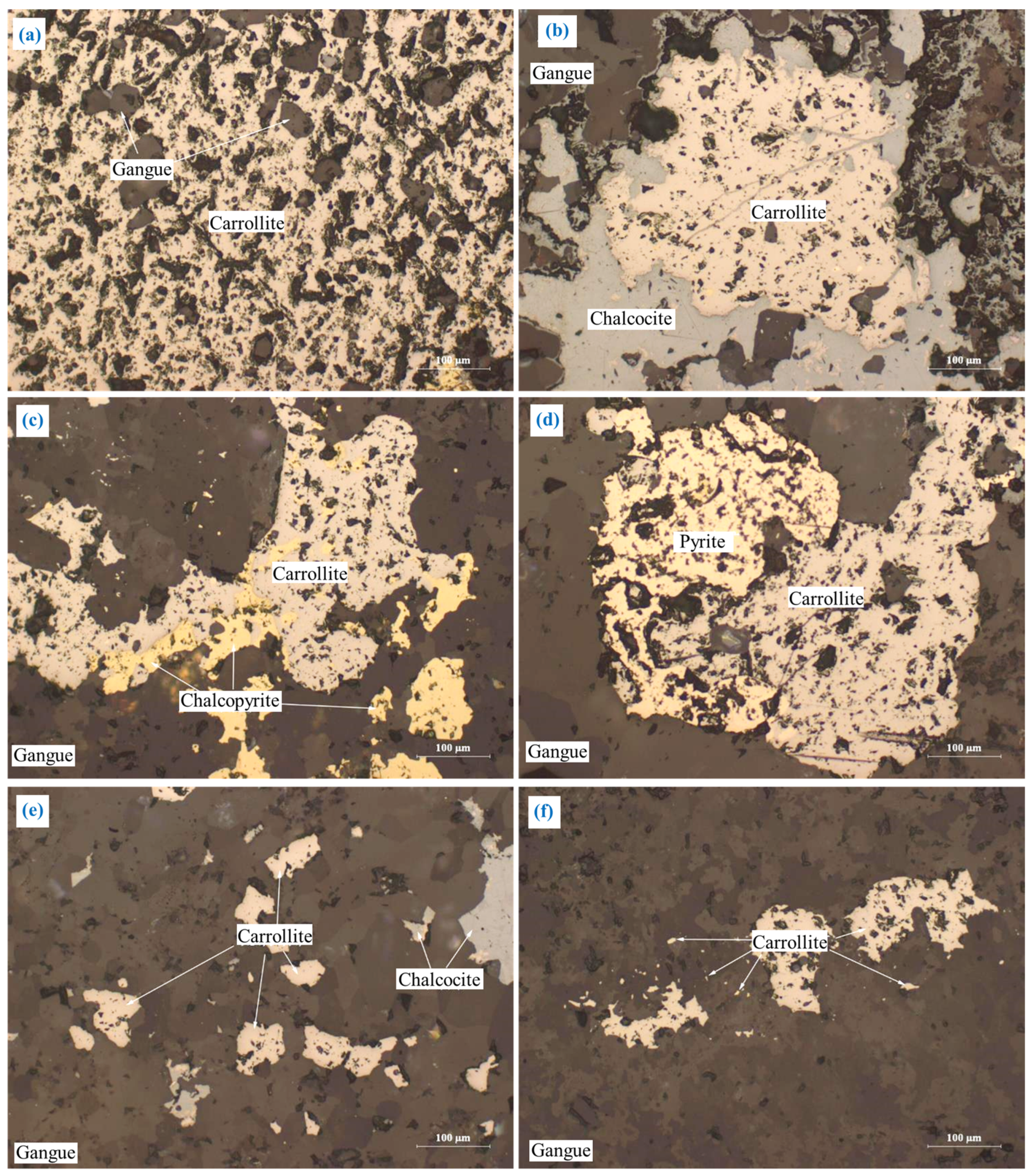
3.2.2. Carrollite (CuCo2S4)
The embedding characteristics of carrollite are relatively complex, as shown in Figure 6. Major carrollite deposits were embedded in the granular form (Figure 6a). Part of carrollite was irregularly interbedded with chalcocite, chalcopyrite, and pyrite (Figure 6b–d). A small amount of carrollite was embedded in the form of fine-grained, semi-automorphic crystals (Figure 6e). A trace amount of carrollite was embedded in gangue in the form of micro- and fine-grained dots (Figure 6f).
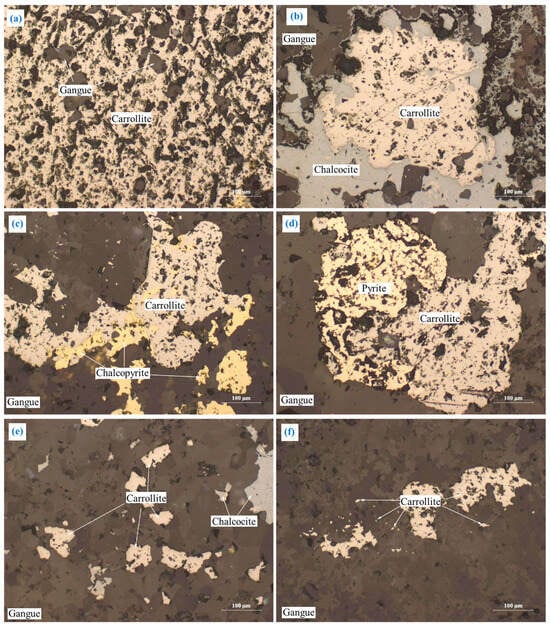
Figure 6.
The embedding characteristics of carrollite: (a) granular form; (b) irregularly interbedded with chalcocite; (c) irregularly interbedded with chalcopyrite; (d) irregularly interbedded with pyrite; (e) fine-grained; (f) micro- and fine-grained dots.
Carrollite has the close relationship with chalcopyrite, chalcocite, and pyrite (Figure 6b–e). It is difficult for carrollite to completely dissociate from chalcopyrite, chalcocite, and pyrite during the grinding process, which in turn affects the flotation of carrollite.
3.3. Particle Size Distribution and the Liberation Characteristics of Sulfides
The particle size distribution characteristics of sulfide minerals (chalcopyrite, chalcocite, bornite, carrollite, and pyrite) were determined by the line segment method, and the results are shown in Table 3. This shows that the particle size of sulfide minerals was coarse, with a distribution rate of 73.58% at +0.074 mm and only 2.93% at −0.010 mm.

Table 3.
The particle size distribution characteristics of sulfides.
The liberation characteristics of sulfides under different grinding fineness values were determined by the line segment method, and the results are shown in Table 4. The liberation degree of the sulfide minerals was 81.61% when the proportion of particles smaller than 0.074 mm accounted for 70%. This value increased to 91.05% when the proportion of particles smaller than 0.074 mm reached 80%. The unliberated part was mostly connected with gangue minerals, and a small amount existed in the form of gangue mineral inclusions.

Table 4.
The liberation characteristics of sulfides (line segment method).
3.4. Flotation Behavior of Copper–Cobalt Sulfide Ore
In the flotation process of sulfide minerals, a variety of flotation reagents were usually added, including regulators, collectors, and frothers. Regulators were used to modulate the chemical environment of the slurry, or to enhance the interaction of the collector with the target mineral surface, or to reduce the floatability of gangue minerals. Collectors were used to improve the floatability of target minerals through surface adsorption. Frothers were used to provide a gas–liquid–solid three-phase interface suitable for the separation of target minerals and gangue minerals. When they were used wisely, the separation of target minerals and gangue minerals could be effectively achieved.
Therefore, guided by process mineralogy, it is highly meaningful to enhance the value of copper–cobalt sulfide ore through the flotation recovery of Cu-Co. This can be effectively achieved by optimizing flotation conditions and processes.
3.4.1. Rougher Flotation
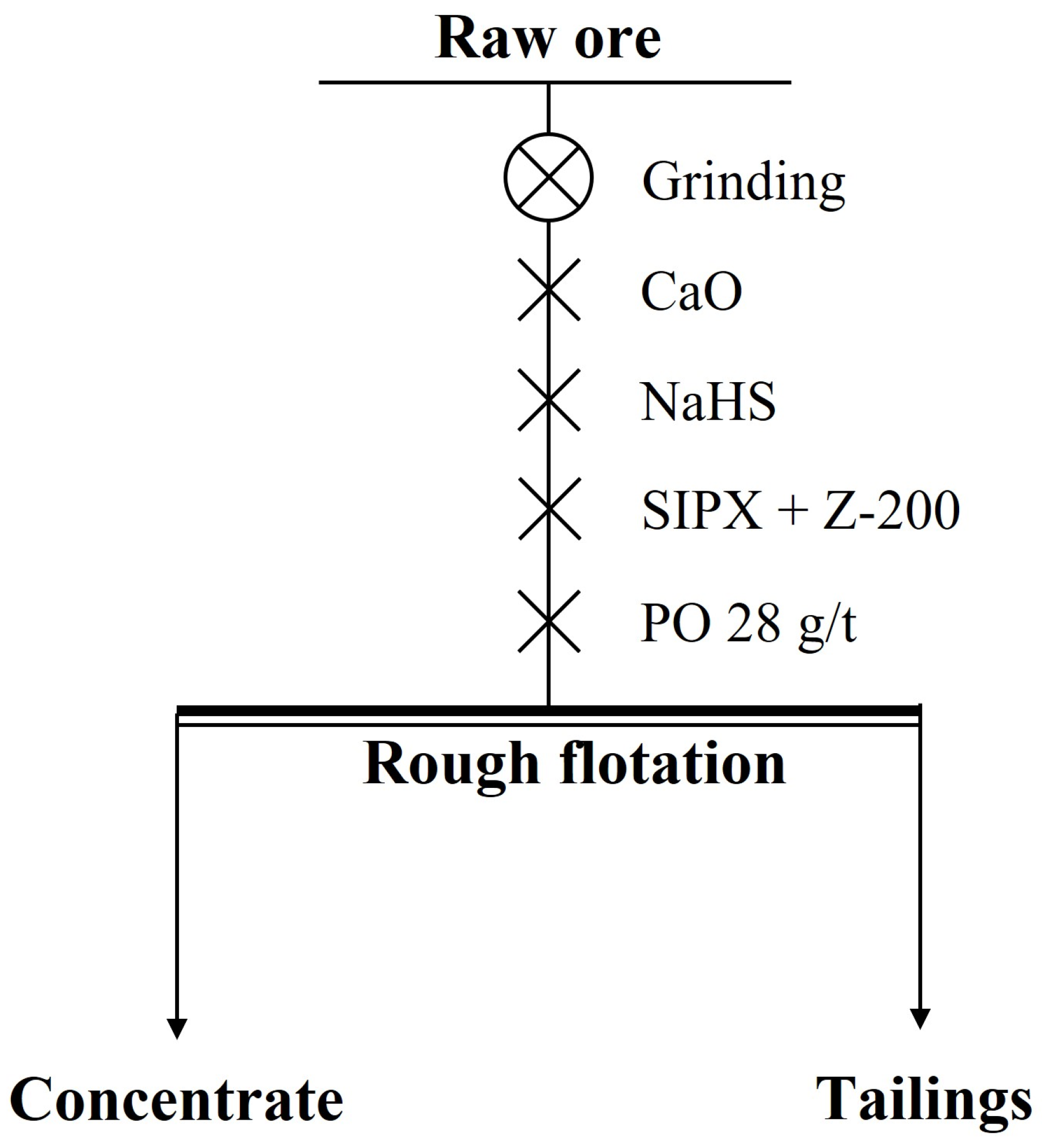
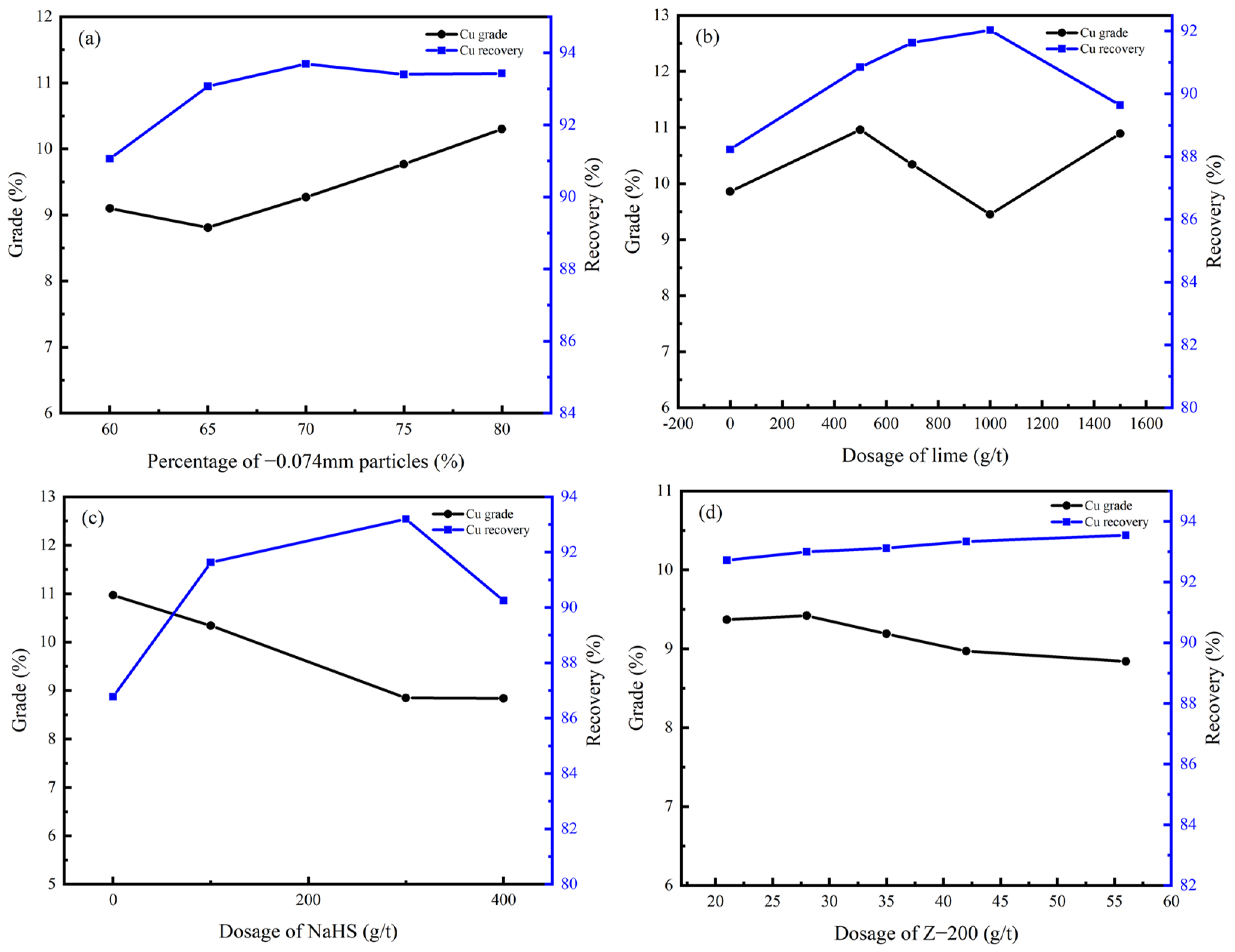
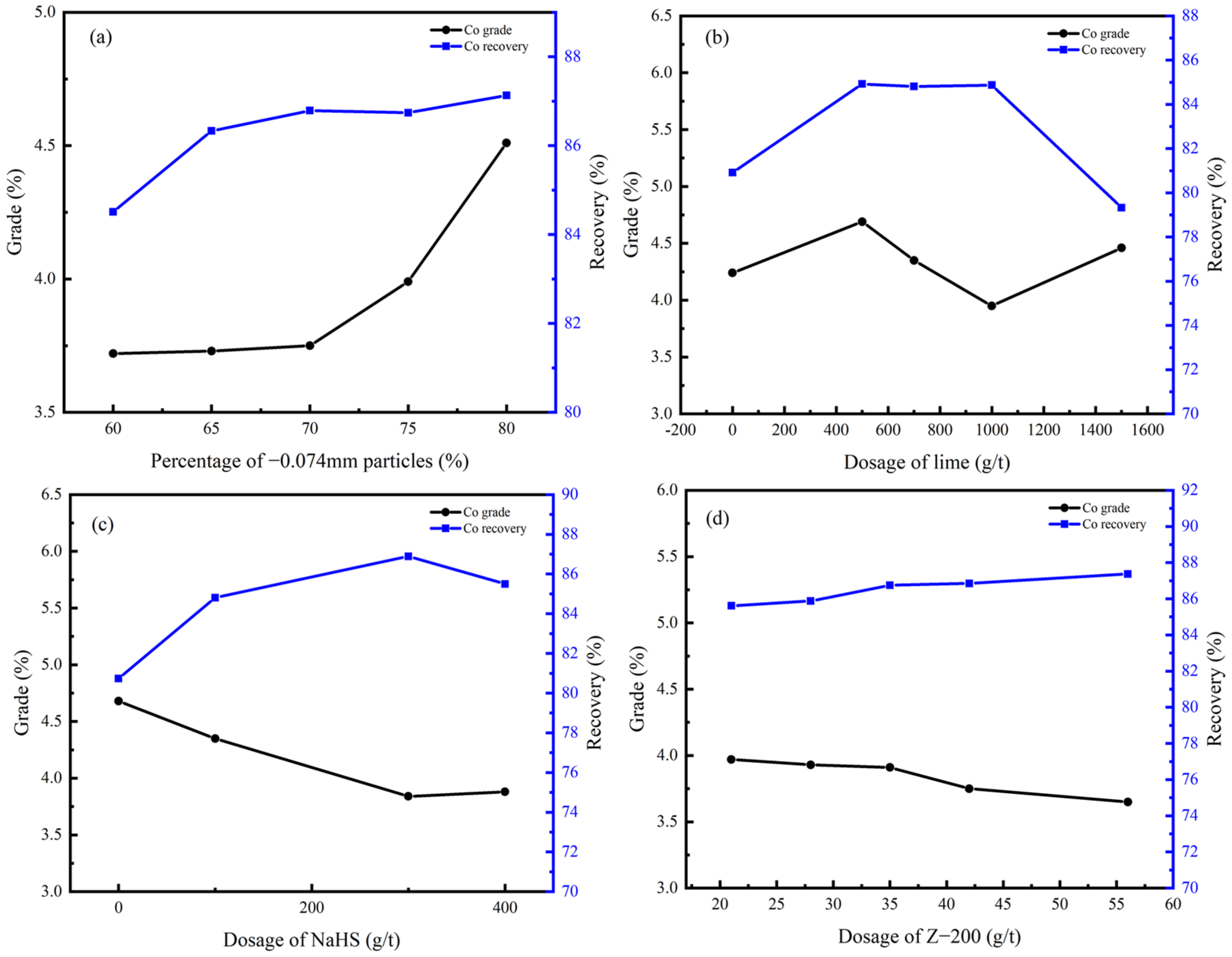
In the rougher flotation, the effects of grinding fineness and the dosage of CaO, NaHS, and Z-200 were researched. The experimental conditions and the process of the rougher flotation are shown in Table 5 and Figure 7, and the results are shown in Figure 8 and Figure 9.

Table 5.
The experimental conditions in the rougher flotation.
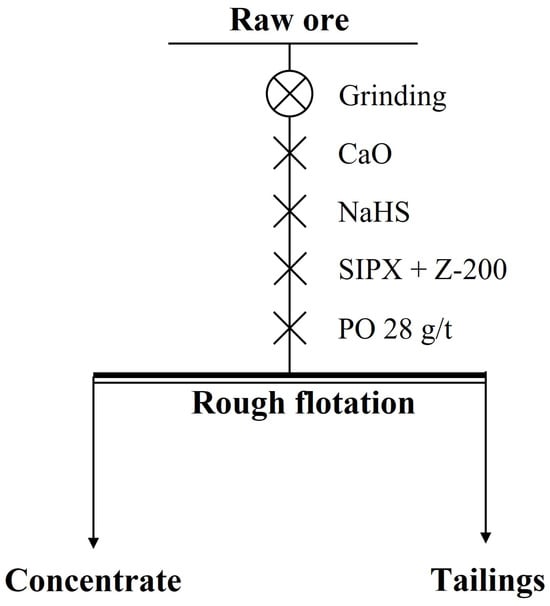
Figure 7.
The process of the rougher flotation.
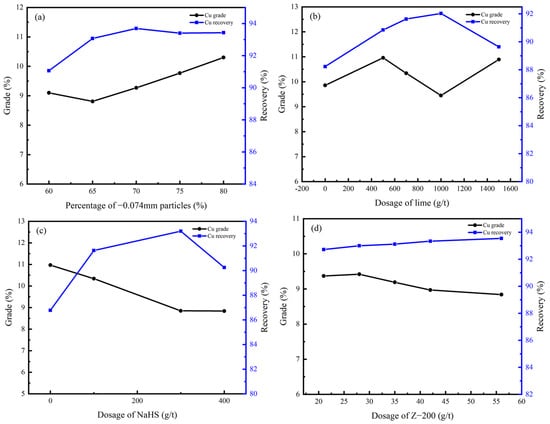
Figure 8.
Cu grade and recovery of rougher flotation concentrate under different conditions: (a) grinding fineness; (b) dosage of CaO; (c) dosage of NaHS; (d) dosage of Z-200.
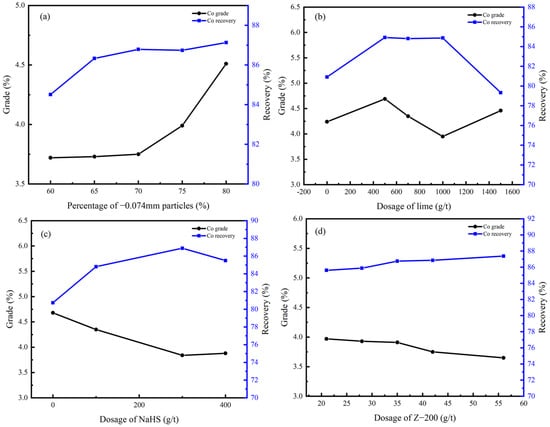
Figure 9.
Co grade and recovery of rougher flotation concentrate under different conditions: (a) grinding fineness; (b) dosage of CaO; (c) dosage of NaHS; (d) dosage of Z-200.
According to Figure 8 and Figure 9, the percentage of −0.074 mm particles should be controlled at 80%; the optimal dosage of CaO and NaHS conditioner was 500 g/t and 300g/t, respectively; and the optimal dosage of the collector Z-200 was 35 g/t.
Under optimal conditions, the Cu grade of copper–cobalt sulfide ore can be increased from 1.28% to 9.27%, and the Co grade can be increased from 0.56% to 3.75% through a rougher flotation, with recoveries of 93.69% and 86.79%, respectively.
In order to further improve the grade and recovery of Cu/Co, the mineralogical characterization of the rougher tailings and rougher concentrate were studied, and the results are shown in Figure 10 and Figure 11, respectively.

Figure 10.
(a,b) The embedding characteristics of rougher tailings.
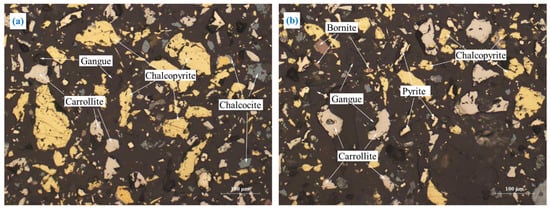
Figure 11.
(a,b) The embedding characteristics of rougher concentrate.
Figure 10 shows that there was only a small amount of fine-grained sulfide minerals such as carrollite and chalcocite, and the majority of the minerals were gangue minerals. Combined with the chemical phase analysis of Co in Figure 4, it can be seen that Co was mostly lost to carbonates in the gangue that was difficult to recover by flotation. However, small amounts of Cu and Co were lost to fine-grained sulfide minerals.
Figure 11 shows that a certain amount of gangue and pyrite were mixed in the rougher concentrate, which affected the concentrate grade. In addition, it was worth noting that some of the target minerals and gangue minerals were tightly interbedded, making it difficult to improve the grade by direct flotation. Therefore, the cleaner flotation and regrinding of rougher concentrate were required. In addition, CMC was added as an inhibitor in the cleaner flotation to improve the grade of the final concentrate.
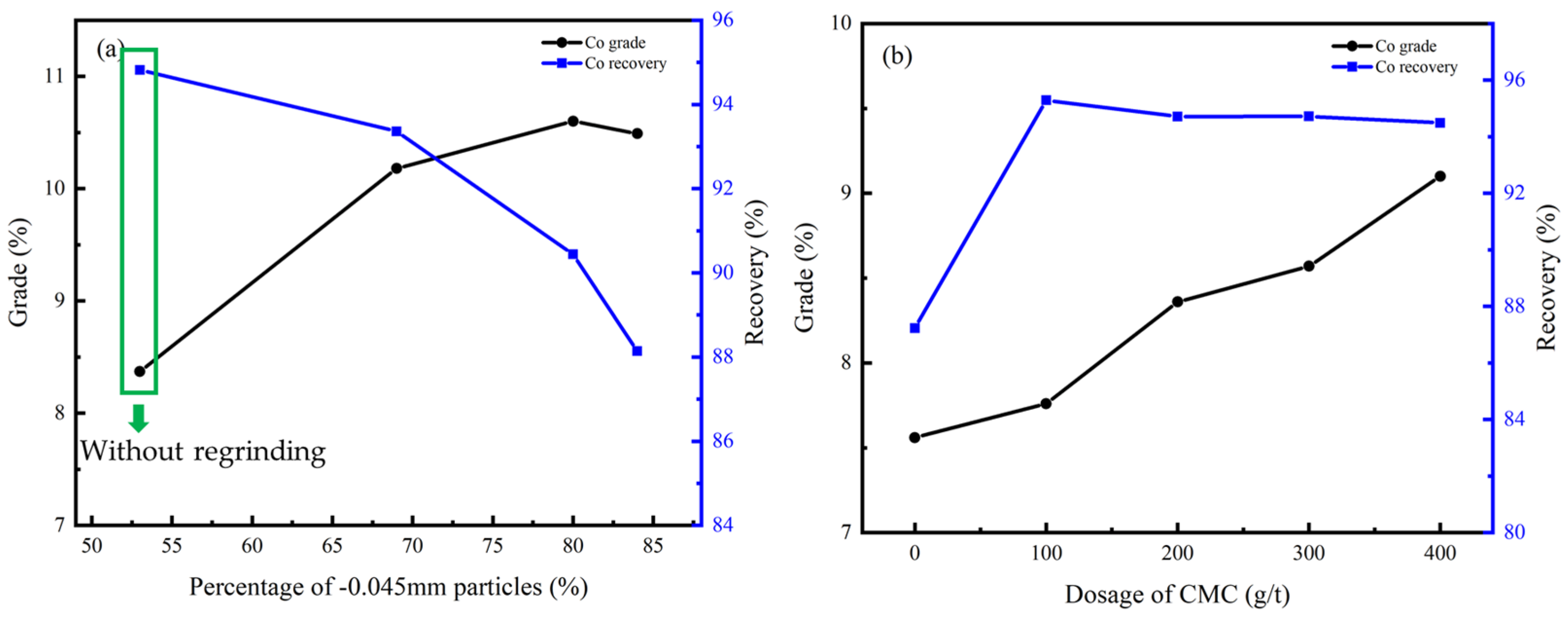
3.4.2. Cleaner Flotation
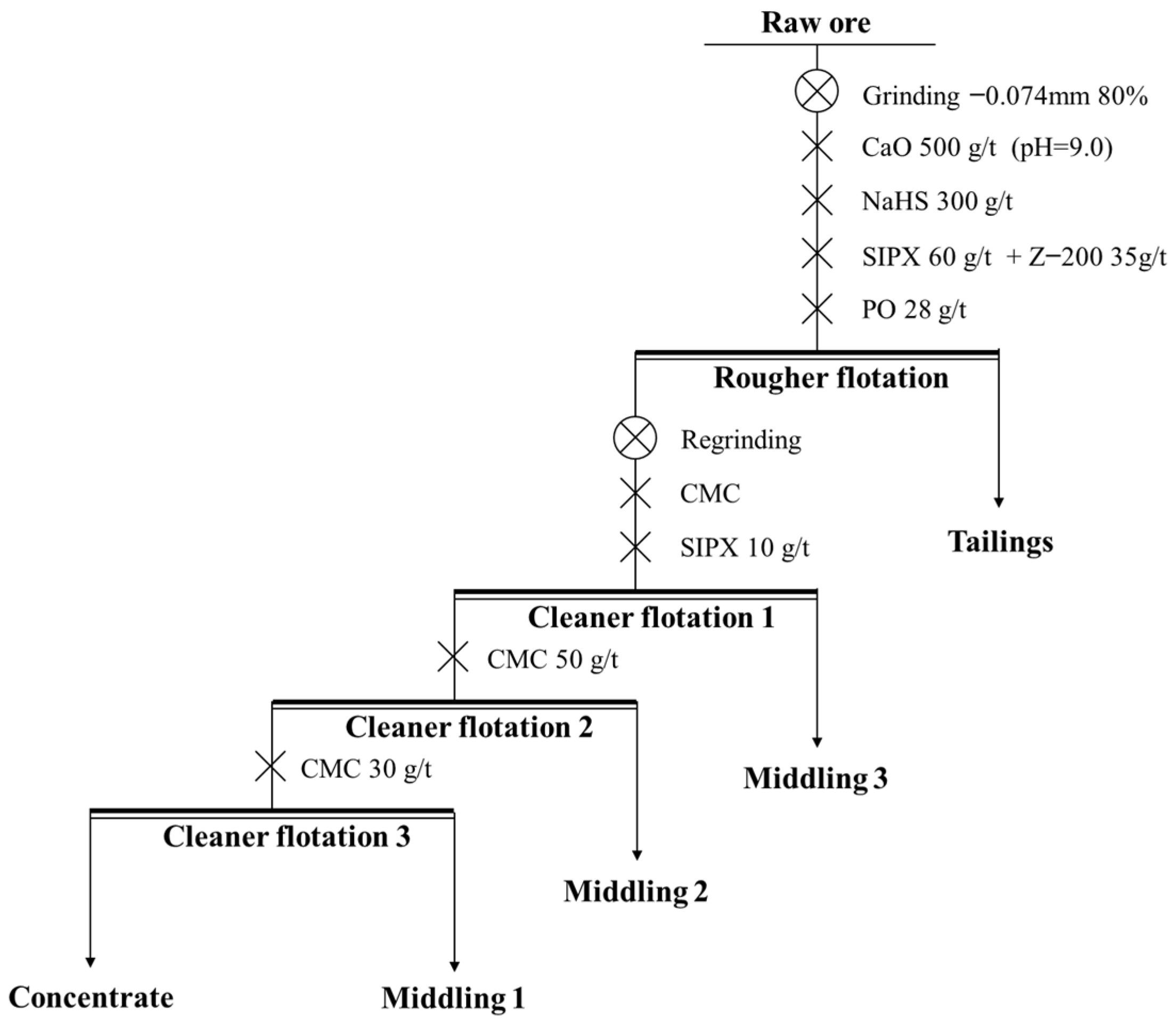
The process and conditions of the cleaner flotation are shown in Figure 12, and the influence of experimental conditions on cleaner flotation are shown in Figure 13 and Figure 14.
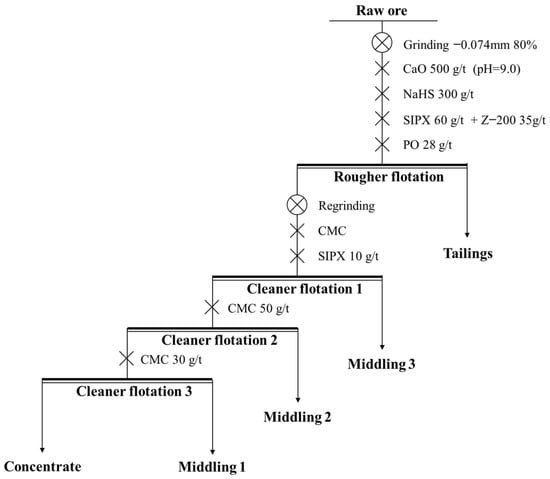
Figure 12.
The process and conditions of the cleaner flotation.
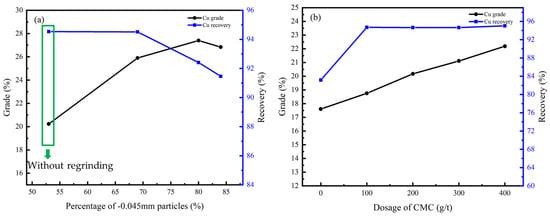
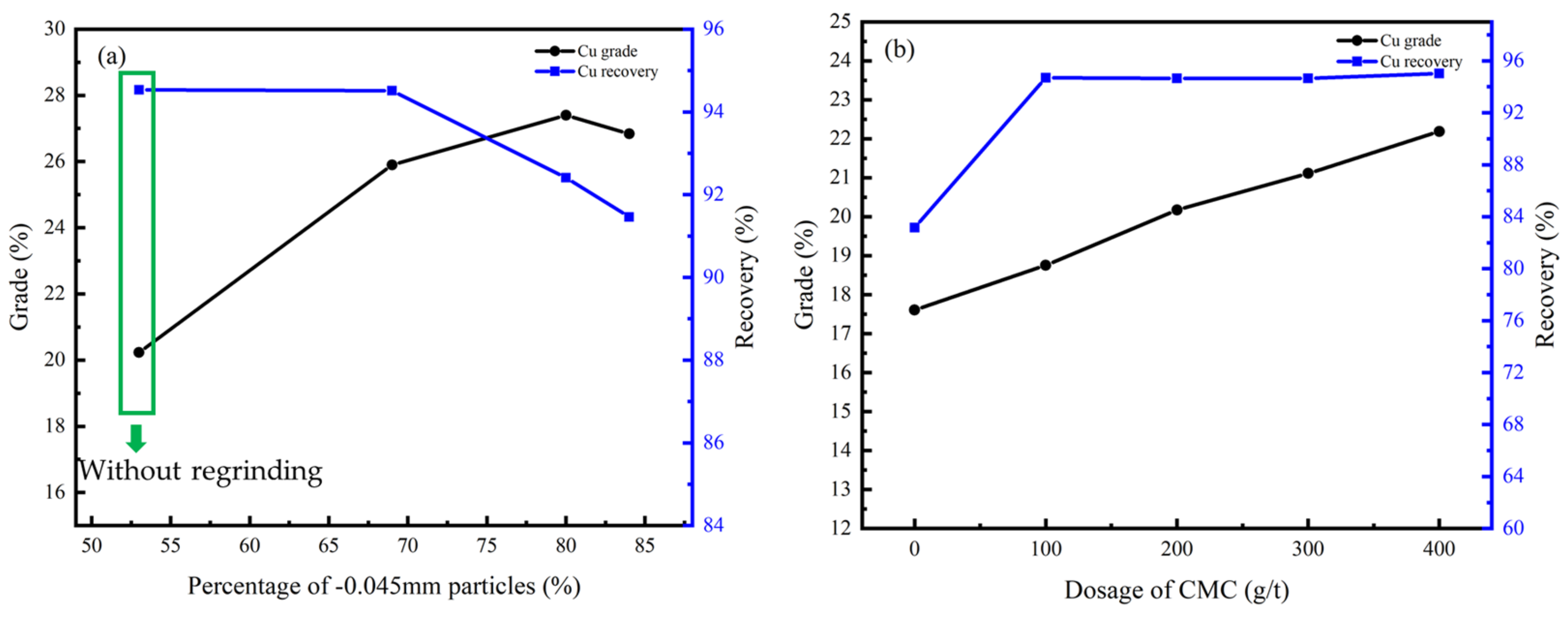
Figure 13.
Cu grade and recovery of cleaner flotation concentrate under different conditions: (a) regrinding fineness; (b) dosage of CMC.
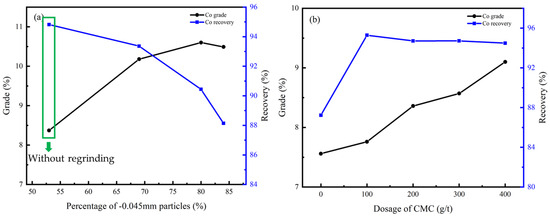
Figure 14.
Co grade and recovery of cleaner flotation concentrate under different conditions: (a) regrinding fineness; (b) dosage of CMC.
According to Figure 13a and Figure 14a, the flotation effect can be significantly improved by regrinding, and the content of particles less than −0.045 mm should be controlled at 70%. CMC was effective as an inhibitor due to its ability to inhibit gangue minerals including dolomite, chlorite, and talc, and the optimal dosage was 400 g/t according to Figure 13b and Figure 14b.
Under this condition, the Cu grade of the concentrate can be improved from 9.27% to 27.40% while the Co grade can be improved from 3.75% to 10.60%, and the recovery of the cleaner flotation 1–3 reached 92.41% and 90.44%, respectively.
3.4.3. Scavenger Flotation
The results of rougher flotation and cleaner flotation show that the copper–cobalt sulfide minerals can be enriched by flotation. However, small amounts of Cu and Co were lost to fine-grained sulfide minerals according to Figure 10. Therefore, the scavenger flotation was conducted to further define the flotation process and improve the grade and recovery of the final flotation concentrate.
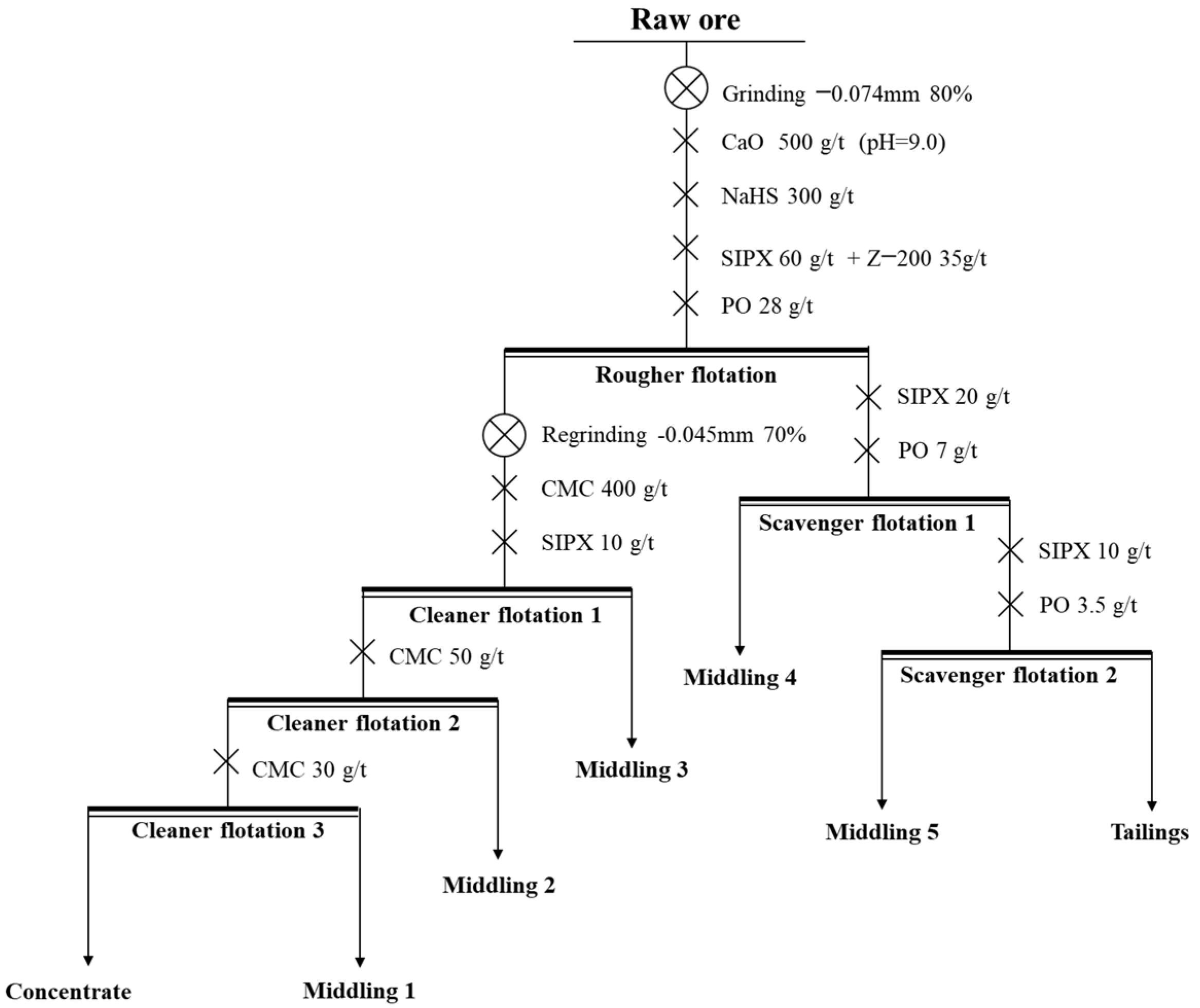
The process and conditions of the experiment are shown in Figure 15, and the result is shown in Table 6.
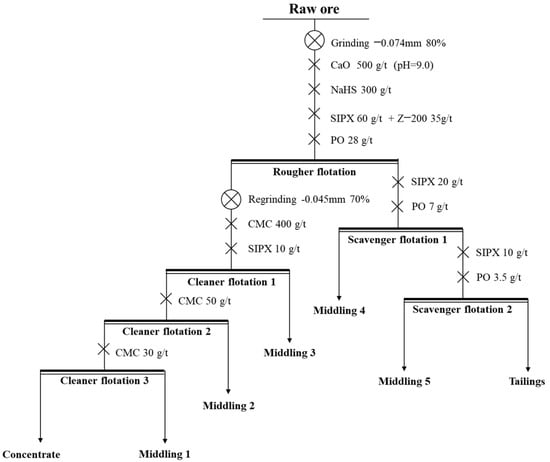
Figure 15.
The process and conditions of the open-circuit experiment.

Table 6.
The result of the open-circuit experiment (%).
Through the open-circuit process of “one rougher flotation, three cleaner flotations, and two scavenger flotations”, the Cu and Co of copper–cobalt sulfide ore were significantly enriched. The grade of Cu can be increased from 1.29% to 27.23%, and the grade of Co can be increased from 0.57% to 10.88%. However, the recovery of the concentrate was relatively low, and the locked-cycle tests were needed to improve the recovery of concentrate.
3.4.4. Locked-Cycle Tests
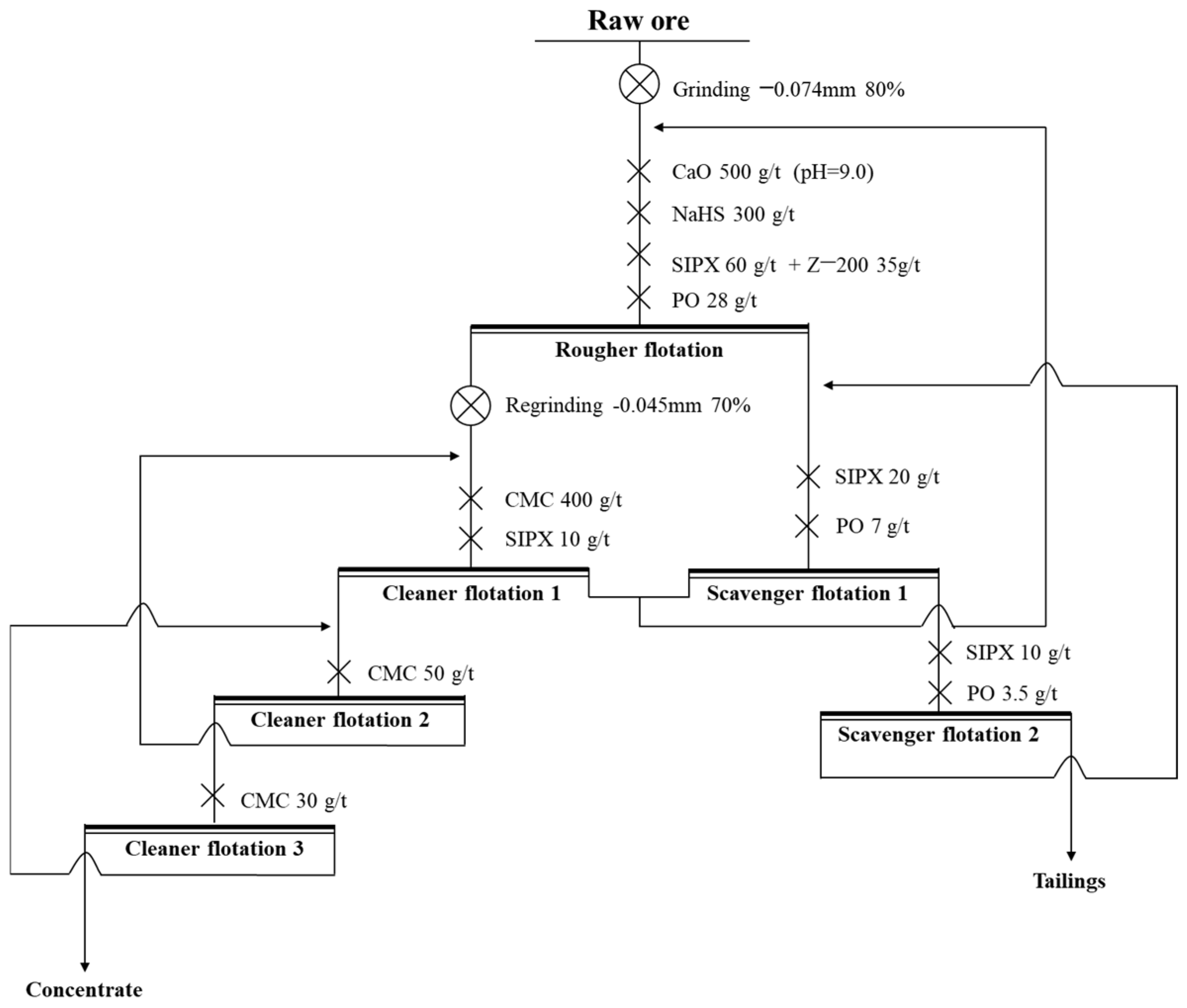
To investigate the influence of middling on the flotation process to simulate the actual flotation process, the locked-cycle tests were conducted. The procedure of the locked-cycle tests was as follows: (1) The first cycle was the same as the open-circuit experiment. (2) From the second cycle, the middling obtained from the first cycle was added to the set position according to Figure 16. (3) When the yield and grade of concentrate and tailings obtained in the last three cycles were balanced, the locked-cycle tests were completed. And, the final result was taken as the average of the last three cycles.
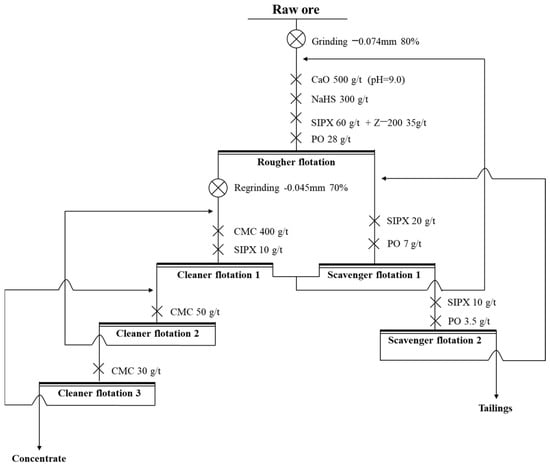
Figure 16.
The process and conditions of the locked-cycle tests.
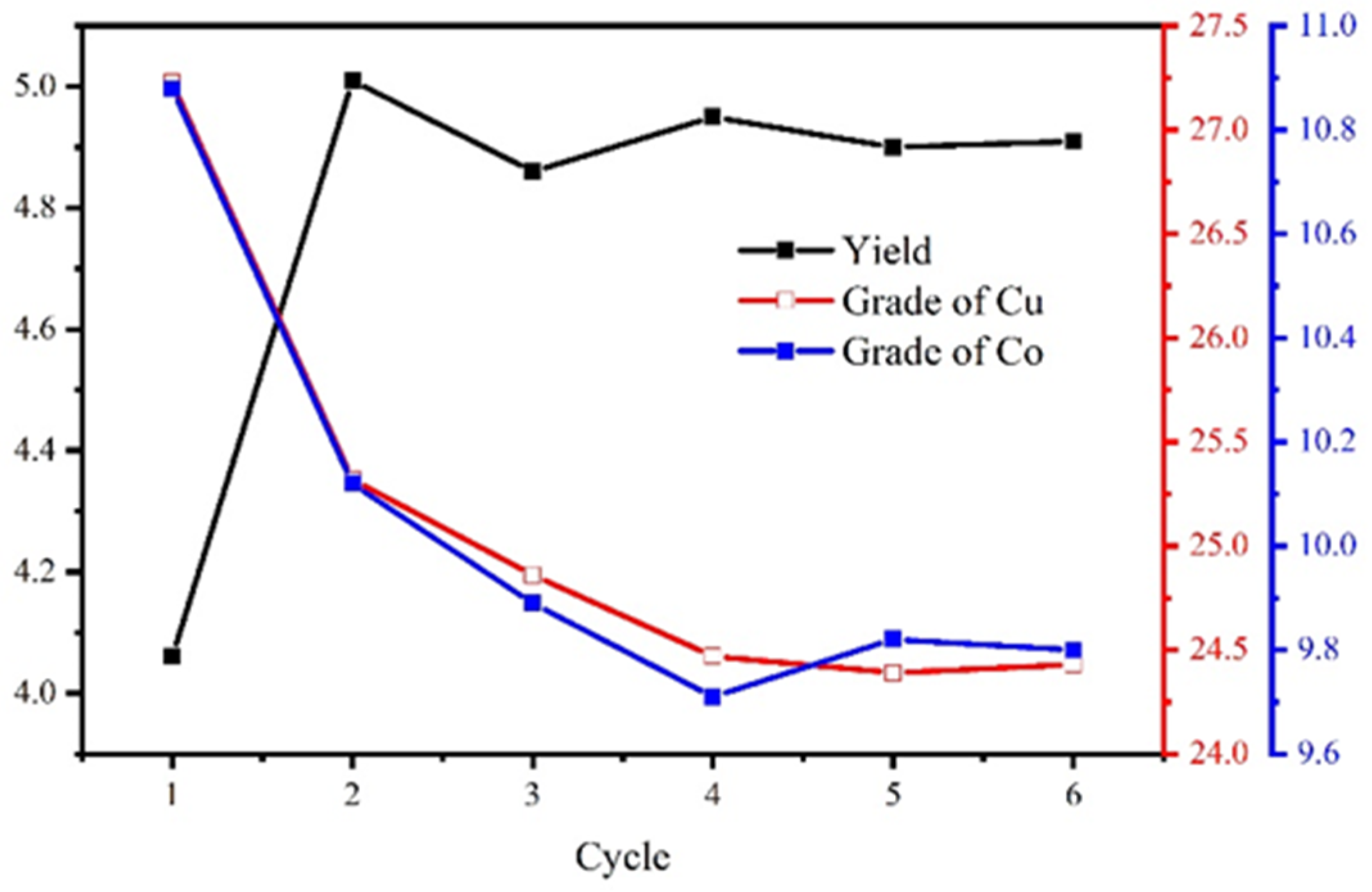
The process and conditions of the locked-cycle tests are shown in Figure 16, and the result is shown in Figure 17 and Table 7.
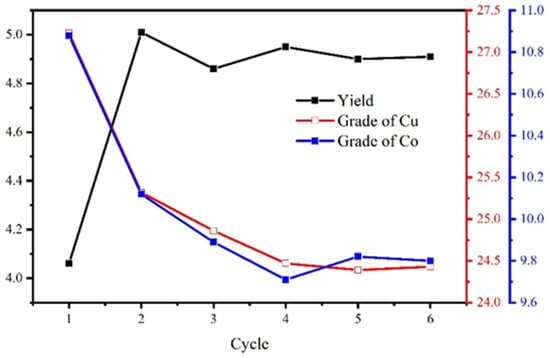
Figure 17.
The locked-cycle stability curve of the concentrate.

Table 7.
The result of the locked-cycle tests (%).
The results show that the locked-cycle flotation process of “one rougher flotation, three cleaner flotations, and two scavenger flotations” is effective. For the copper–cobalt sulfide ore with a Cu grade of 1.27% and a Co grade of 0.56%, the Cu/Co grades can be increased to 24.43%/9.78%, and the recovery of Cu/Co reached 94.47%/86.35%. This satisfied the flotation target.
However, it is worth noting that only a Cu-Co concentrate can be obtained via flotation. Although it has been reported that chalcopyrite and carrollite can be separated by flotation [31], the high content of carrollite in the concentrate in this study, coupled with the fact that carrollite contains both Cu and Co metals, makes it difficult to separate Cu and Co by flotation alone. This separation can only be achieved in subsequent metallurgical processes [32].
3.5. Process-Mineralogy-Guided Flotation for Cu-Co Recovery
The results of this case study show that process mineralogy does provide important guidance for the flotation recovery of Cu-Co from copper–cobalt sulfide ore in the DRC. In particular, flotation can be designed and optimized based on the embedding characteristics of the raw ore and flotation products to further improve the recovery efficiency of Cu-Co.
In addition to this study, there are numerous reports on the flotation of Cu-Co ores from the DRC guided by process mineralogy. Dehaine [30], guided primarily by QEMSCAN-based process mineralogy, investigated the flotation behavior of copper–cobalt sulfide ores from the DRC and achieved satisfactory flotation results. Tijsseling [11], by averaging the mineralogical characteristics, employed Multiple Linear Regression (MLR) to develop predictive models for flotation performance parameters based on process mineralogy, thereby providing a quantifiable basis for guiding the flotation of Cu-Co ores from the DRC through process mineralogy. Additionally, under the guidance of process mineralogy, sulfide–oxide mixed ores with Cu-Co can be efficiently separated by potential control and appropriate collector selection. This expands the role of process mineralogy in flotation guidance and offers a new way to effectively use the DRC’s Cu-Co resources [29].
However, it is worth noting that the case study is confined to the copper–cobalt sulfide ore in the DRC, and the final product is the flotation concentrates containing both Cu and Co metals. Therefore, the separation of copper-bearing and cobalt-bearing minerals, as well as the separation of Cu and Co metals in carrollite, remains an important direction for future research.
4. Conclusions
A case study of process-mineralogy-guided flotation for Cu-Co recovery from DRC copper–cobalt sulfide ore is reported.
The results of process mineralogy shows that the grades of Cu/Co were about 1.26%/0.58%. Cu existed in chalcopyrite, bornite, chalcocite, and carrollite, while Co primarily existed in carrollite. The particle size distribution of sulfide minerals showed a rate of 73.58% for particles larger than 0.074 mm and only 2.93% for particles smaller than 0.010 mm. However, the embedding characteristics of copper–cobalt sulfide ore were complex, which brought difficulties to the separation of Cu-Co from the gangue minerals.
Under the guidance of the process mineralogy of copper–cobalt sulfide ore and the flotation products, the optimized flotation conditions and the flotation process of “one rougher flotation, three cleaner flotations, and two scavenger flotations” were adopted. The Cu/Co grades in the copper–cobalt sulfide ore were increased from 1.27%/0.56% to 24.43%/9.78%, and the recoveries were 94.47% and 86.35%, respectively. These results show an outstanding guiding role of process mineralogy in flotation, and may have important significance for the efficient and comprehensive extraction of copper–cobalt sulfide ore.
Author Contributions
Conceptualization, Y.S.; methodology, H.B.; software, W.L.; validation, X.L. and T.C.; investigation, Y.S.; resources, H.B.; data curation, C.F.; writing—original draft preparation, Y.S.; writing—review and editing, H.B. and C.F.; visualization, Y.Y.; supervision, J.W.; funding acquisition, J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Plan of China (No. 2022YFC2105300), National Natural Science Foundation of China (No. 52274288 and 52004086), and China Postdoctoral Science Foundation (No. 2024T171049 and 2021M703620).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
Authors Yuchen Shi, Hongfei Ba, Wei Liu, Yiquan Yang, Xinyu Liu and Tianhao Chen were employed by Norin Mining Limited. The remaining authors declare that the research was coducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Fang, C.; Wang, J.; Qiu, G. Advancements on Chemical-biological Dissolution Mechanism and Leaching Kinetics of Chalcocite. Trans. Nonferrous Met. Soc. China 2024, 34, 283–297. [Google Scholar] [CrossRef]
- Fang, C.; Cai, T.; Yu, S.; Gao, L.; Huang, L.; Peng, H.; Qiu, G.; Wang, J. How Ferric Salt Enhances the First-Stage Acidic Leaching of Chalcocite: Performance of intermediate Crystallite. JOM 2022, 74, 1969–1977. [Google Scholar] [CrossRef]
- Moreno-Leiva, S. Renewable Energy in Copper Production: A Review on Systems Design and Methodological Approaches. J. Clean. Prod. 2020, 246, 118978. [Google Scholar] [CrossRef]
- Jena, S.; Tripathy, S.; Mandre, N.; Venugopal, R.; Farrokhpay, S. Sustainable Use of Copper Resources: Beneficiation of Low-Grade Copper Ores. Minerals 2022, 12, 545. [Google Scholar] [CrossRef]
- Zheng, C.; Jiang, K.; Cao, Z.; Wang, H.; Liu, S.; Waters, K.E.; Ma, H. Pressure Leaching Behaviors of Copper-Cobalt Sulfide Concentrate from Congo. Sep. Purif. Technol. 2023, 309, 123010. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, F.; Zhao, Z.; Liu, X.; Chen, X.; Li, J.; He, L. Kinetics Study on Cobalt Leaching from Cobalt-Bearing Ternary Sulfide in Sulfuric Acid Solution under Atmospheric Pressure. Trans. Nonferrous Met. Soc. China 2024, 34, 1669–1680. [Google Scholar] [CrossRef]
- Crundwell, F.K.; du Preez, N.B.; Knights, B.D.H. Production of Cobalt from Copper-Cobalt Ores on the African Copperbelt—An Overview. Miner. Eng. 2020, 156, 106450. [Google Scholar] [CrossRef]
- Zuo, Y.; Zhang, W.; Che, J.; Feng, S.; Chen, Y.; Wang, C. Efficient Extraction of Cobalt and Copper: Leveraging Redox Chemistry in Oxide and Sulfide Copper-Cobalt Ores. Sep. Purif. Technol. 2025, 354, 128671. [Google Scholar] [CrossRef]
- Chang, Z.; Niu, S.; Shen, Z.; Zou, L.; Wang, H. Latest Advances and Progress in the Microbubble Flotation of Fine Minerals: Microbubble Preparation, Equipment, and Applications. Int. J. Miner. Metall. Mater. 2023, 30, 1244–1260. [Google Scholar] [CrossRef]
- Turysbekov, D.; Tussupbayev, N.; Kenzhaliev, B.; Narbekova, S.; Semushkina, L. The Effect of Novel Submicronic Solid Activators on Sphalerite Flotability. Minerals 2024, 14, 243. [Google Scholar] [CrossRef]
- Tijsseling, L.T.; Dehaine, Q.; Rollinson, G.K.; Glass, H.J. Mineralogical Prediction of Flotation Performance for a Sediment-Hosted Copper-Cobalt Sulphide Ore. Minerals 2020, 10, 474. [Google Scholar] [CrossRef]
- Whiteman, E.; Lotter, N.O.; Amos, S.R. Process mineralogy as a predictive tool for flowsheet design to advance the Kamoa project. Miner. Eng. 2016, 96–97, 185–193. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, G.; Liu, J.; Pan, G.; Chen, Y.; Wang, M. Studies on the Surface Oxidation and Its Role in the Flotation of Mixed Cu-Ni Sulfide Ore. Powder Technol. 2021, 381, 576–584. [Google Scholar] [CrossRef]
- Wang, C.; Liu, R.; Zhai, Q.; Dong, W.; Xie, Z.; Sun, W.; Hu, W. Prospects of Pulp Aeration for the Cleaner Production of Pyrrhotite-Rich Type Copper Sulfide Ore: Mechanism and Application. J. Clean. Prod. 2023, 406, 136921. [Google Scholar] [CrossRef]
- Wang, C.; Liu, R.; Zhai, Q.; Xie, Z.; Sun, W.; Li, P.; Wang, Z. Exploring the Effect of Pulp Aeration and Lime-Aid Grinding on Pyrrhotite-Rich Type Copper Sulfide Ore Flotation Separation. Sep. Purif. Technol. 2023, 311, 123268. [Google Scholar] [CrossRef]
- Moimane, T.; Plackowski, C.; Peng, Y. The Critical Degree of Mineral Surface Oxidation in Copper Sulphide Flotation. Miner. Eng. 2020, 145, 106075. [Google Scholar] [CrossRef]
- Ozcelik, S.; Ekmekci, Z. Reducing Negative Effects of Oxidation on Flotation of Complex Cu-Zn Sulfide Ores. Minerals 2022, 12, 1016. [Google Scholar] [CrossRef]
- Lavrinenko, A.A.; Gol’berg, G.Y. Heterocyclic Reagents in Flotation of Sulfide Ore: A Review. J. Min. Sci. 2022, 58, 430–437. [Google Scholar] [CrossRef]
- Zhao, K.; Yan, W.; Wang, X.; Wang, Z.; Gao, Z.; Wang, C.; He, W. Effect of a Novel Phosphate on the Flotation of Serpentine-Containing Copper-Nickel Sulfide Ore. Miner. Eng. 2020, 150, 106276. [Google Scholar] [CrossRef]
- Zhao, J.; Godirilwe, L.L.; Haga, K.; Yamada, M.; Shibayama, A. Flotation Behavior and Surface Analytical Study of Synthesized (Octylthio) Aniline and Bis(Octylthio)Benzene as Novel Collectors on Sulfide Minerals. Miner. Eng. 2023, 204, 108422. [Google Scholar] [CrossRef]
- Deng, R.; Duan, W.; Wang, Y.; Hu, Y. Effect of the Foaming Performance of Ammonium Dibutyl Dithio-Phosphate on the Flotation of Slime-Containing Copper Sulfide Ore. Physicochem. Probl. Miner. Process. 2022, 58, 153492. [Google Scholar]
- Xu, B.; Wu, J.; Dong, Z.; Jiang, T.; Li, Q.; Yang, Y. Flotation Performance, Structure-Activity Relationship and Adsorption Mechanism of a Newly-Synthesized Collector for Copper Sulfide Minerals in Gacun Polymetallic Ore. Appl. Surf. Sci. 2021, 551, 149420. [Google Scholar] [CrossRef]
- Xie, J.; Zhao, K.; Sun, L.; Li, Y.; Hu, C.; Wang, Z.; Gao, Z. Upgrading of Talc-Bearing Copper-Nickel Sulfide Ore by Froth Flotation Using Sodium Phytate as Depressant. Colloid Surf. A-Physicochem. Eng. Asp. 2024, 687, 133561. [Google Scholar] [CrossRef]
- Yan, H.; Yang, B.; Zeng, M.; Huang, P.; Teng, A. Selective Flotation of Cu-Mo Sulfides Using Xanthan Gum as a Novel Depressant. Miner. Eng. 2020, 156, 106486. [Google Scholar] [CrossRef]
- Bakalarz, A.; Gloy, G.; Luszczkiewicz, A. Flotation of Sulfide Components of Copper Ore in the Presence of N-Dodecane. Miner. Process Extr. Metall. Rev. 2015, 36, 103–111. [Google Scholar] [CrossRef]
- Jimenez, G.; Cabrera, P.; Rodriguez, A.; Cuervo, C.; Gutierrez, L. The Effect of an Anionic Polyacrylamide on the Flotation of Chalcopyrite, Enargite, and Bornite. Minerals 2024, 14, 634. [Google Scholar] [CrossRef]
- Mbuya, B.; Mulaba-Bafubiandi, A.F. Predicting Optimized Dissolution of Selected African Copperbelt Copper-Cobalt-Bearing Ores by Means of Neural Network Prediction and Response Surface Methodology Modeling. Process Integr. Optim. Sustain. 2023, 7, 583–597. [Google Scholar] [CrossRef]
- Shengo, M.L.; Kime, M.-B.; Mambwe, M.P.; Nyembo, T.K. A Review of the Beneficiation of Copper-Cobalt-Bearing Minerals in the Democratic Republic of Congo. J. Sustain. Min. 2019, 18, 226–246. [Google Scholar] [CrossRef]
- Tijsseling, L.T.; Dehaine, Q.; Rollinson, G.K.; Glass, H.J. Flotation of mixed oxide sulphide copper-cobalt minerals using xanthate, dithiophosphate, thiocarbamate and blended collectors. Miner. Eng. 2019, 138, 246–256. [Google Scholar] [CrossRef]
- Dehaine, Q.; Tijsseling, L.T.; Rollinson, G.K.; Glass, H.J. Flotation of a copper-cobalt sulphide ore: Quantitative insights into the role of mineralogy. Miner. Eng. 2024, 218, 108958. [Google Scholar] [CrossRef]
- Zeng, G.S.; Wang, M.T.; Zhang, G.F.; Gao, Y.Q. Selective depression and adsorption of a novel eco-friendly depressant on the flotation separation of chalcopyrite from carrollite. Colloid Surf. A-Physicochem. Eng. Asp. 2024, 695, 134290. [Google Scholar] [CrossRef]
- Yang, H.Y.; Liu, W.; Chen, G.B.; Liu, Y.Y.; Tong, L.L.; Jin, Z.N.; Liu, Z.L. Function of microorganism and reaction pathway for carrollite dissolution during bioleaching. Trans. Nonferrous Met. Soc. China 2015, 25, 2718–2724. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).