Abstract
Yeast biomass, a by-product of various industrial processes, is a sustainable source of food ingredients. Despite its nutritional richness, studies on Yarrowia lipolytica W29 biomass for high-value compound production using low-cost substrates like glycerol and glucose remain limited. These substrates enhance productivity and modulate cell wall composition. Extracting these compounds is complex but can be optimized through sequential hydrolysis, including autolysis and acid hydrolysis. In this study, mannoprotein exhibited a 60% emulsification index, 40 mN m−1 surface tension for both substrates, and thermal stability with a mass retention above 30%. Acid hydrolysis yielded bioactive peptides (<1 kDa) with the highest antioxidant activity: 220 µM Trolox (ABTS), 270 µM Trolox (DPPH), and 125 µM ascorbic acid (FRAP). The raw biomass and feed ingredient (dry residue) provided 100% and 90% of the daily protein intake, respectively, with a β-glucan content of 17%. Glycerol and glucose resulted in similar high-value compounds, highlighting glycerol as a cost-effective carbon source. Thus, sequential hydrolysis is an effective strategy for extracting compounds from Y. lipolytica W29 biomass, offering a promising alternative for industrial applications due to its high nutritional value and functional properties.
1. Introduction
Yeasts are unicellular microorganisms involved in several industrial processes, whether in producing fermented foods, alternative sources of proteins with high biological value or in producing high-value-added compounds such as carotenoids, proteins and organic acids [1,2]. However, all these industrial processes generate large amounts of yeast biomass, representing an environmental challenge and high management costs for industries. For example, approximately 1.9 billion hectoliters of beer were produced worldwide in 2022, generating 2.85 and 5.70 million tons of yeast biomass from beer production [3]. Nevertheless, this by-product is typically used as animal feed. Biomass is one of the leading renewable resources used as an option for producing a range of high-value-added products and generating energy, thus helping promote sustainable development and the application of bioeconomy in several areas [4].
Yeast biomass is recognized as a rich source of proteins (45–60%), carbohydrates (70%), lipids (6%), and other high-value compounds, including β-glucans, chitin, mannoproteins, B vitamins, and antioxidants such as glutathione. Specifically, the yeast cell wall is abundant in carbohydrates such as glucans, chitin, and mannans, which are linked to proteins [5,6]. Glucans are polymers composed of glucose units with β-1,3 and β-1,6 linkages, forming both short and long chains. These polymers can be utilized as food additives due to their emulsifying, thickening, water retention, and prebiotic properties [7,8]. Conversely, chitin is a linear homopolymer of N-acetylglucosamine linked via β-1,4 bonds, exhibiting different conformations such as long, thin microfibrils and short, thick rodlets [9,10]. This homopolymer is typically deacetylated to produce chitosan, an industrially significant derivative valued for its excellent technological properties, including its antimicrobial activity and its application as a polymeric material in the production of food films [11].
Mannans, a group of polysaccharides composed mainly of mannose residues, play a crucial role in the structural integrity and functionality of the yeast cell wall. These carbohydrate polymers are normally found as part of mannoproteins, which are covalently linked to cell wall proteins, contributing to cell wall porosity, adhesion properties and immunomodulatory activities. Mannans form complex branched structures that influence cell wall stability and interactions with the environment. In addition, yeast mannans have been studied for their potential prebiotic effects, as they can selectively promote the growth of beneficial intestinal microbiota. Their bioactive properties also make them valuable in the food, pharmaceutical, and cosmetics industries, which apply to them for their emulsifying, immunostimulatory, and anti-inflammatory effects [12,13].
Mannoproteins are considered natural functional ingredients, consisting of hydrophilic mannose polymers covalently linked to a central protein chain. These polymers possess excellent technological properties, such as the ability to form and stabilize foams, enhance protein and organic acid (e.g., tartaric acid) stability, reduce astringency, and act as flavor enhancers. Additionally, they serve as effective substitutes for commercial emulsifiers and exhibit functional properties by stimulating the growth of beneficial microorganisms [3,11]. In yeasts, proteins are present either as glycosylated proteins within the cell wall or as enzymes involved in metabolic processes [14,15]. Due to their high nutritional value—enriched with essential amino acids and bioactive peptides with antimicrobial and antioxidant properties—yeast proteins are emerging as a novel alternative protein source [16].
Given this context, the oleaginous yeast Yarrowia lipolytica W29 is a genetically modified strain derived from multiple Yarrowia lipolytica strains originally isolated from wastewater in France [17]. This unconventional yeast exhibits accelerated growth, adapts to a wide range of aeration conditions, pH levels, and temperatures, and tolerates various inhibitory compounds [18]. Additionally, industrial processes based on this yeast are recognized as generally safe (GRAS) by the Food and Drug Administration (FDA) of the United States of America [19].
The growing interest in Yarrowia lipolytica is primarily due to its ability to utilize a variety of low-cost carbon sources, including vegetable oils, fatty acids derived from food waste, glycerol, and sugars such as xylose and glucose, for the production of industrially valuable metabolites. These include lipids, terpenoids, enzymes, and proteins [20,21]. However, the extraction of cell wall components from Y. lipolytica has been less extensively studied compared to other yeasts, such as Saccharomyces cerevisiae, for which extensive research has been conducted on the extraction of mannoproteins, β-glucans, bioactive peptides, nucleic acids, and other valuable compounds [22].
Various extraction methods, including ball milling, autolysis, enzymatic hydrolysis, acid–alkaline hydrolysis, high-pressure homogenization, ultrasound, and pulsed electric fields, have been employed to extract compounds from yeast biomass. However, these methods present several limitations, such as the extraction of single components, high economic costs, low efficiency in cell wall disruption, and reduced extract purity [7,23,24]. Sequential hydrolysis has been proposed as a viable alternative for extracting multiple compounds, as it enhances cell wall disruption and improves overall efficiency [22].
Autolysis is a mild and cost-effective method that utilizes endogenous enzymes to break down the yeast cell wall. However, incorporating a second hydrolysis step can further enhance cell wall disruption and improve the release of target compounds [11]. Acid hydrolysis is considered a promising and effective alternative, as it cleaves the glycosidic bonds of yeast cell wall components while simultaneously releasing their constituents [25].
Following extraction, β-glucans and mannoproteins remain as a mixture, necessitating additional purification. Ultrafiltration, a membrane filtration technology, is widely used for the separation and purification of bioactive proteins and peptides from yeast hydrolysates. This technique functions as a selective barrier, preventing certain molecular and ionic compounds from passing through the membrane [26]. The process operates at low temperatures, requires minimal energy, eliminates the need for solvents or additives, and preserves the biological properties of the extracted compounds. Due to its classification as a green technology, membrane filtration has been extensively applied in the food industry for the development of novel ingredients [27].
This study aims to evaluate the effectiveness of sequential hydrolysis (autolysis followed by acid hydrolysis) in extracting high-value-added compounds, particularly mannoproteins and bioactive peptides, from the cell wall of Yarrowia lipolytica W29. Furthermore, the yeast biomass and the residual material obtained after hydrolysis will be comprehensively characterized based on surface tension, emulsification index, protein and carbohydrate content, glucan composition (total glucans, α-glucans, and β-glucans), technological properties, antioxidant activity, thermogravimetric behavior, Fourier transform infrared (FTIR) spectroscopy, and optical properties.
2. Materials and Methods
2.1. Microorganisms
The Yarrowia lipolytica W29 strain used in this study was originally isolated from a wastewater treatment plant in Paris, France, and preserved in a stock solution containing 30 wt% glycerol at −80 °C. For propagation, the yeast was cultivated in a YPD medium (2.0% peptone [Oxoid], 2.0% glucose [Vetec, purity ≥ 98 wt%], and 1.0% yeast extract [Sigma-Aldrich]) under controlled conditions of 28 °C, 160 rpm, for 72 h. Following this initial cultivation period, 1 g L−1 of cells was inoculated into shake flasks containing fresh YPD medium and incubated at 28 °C, 250 rpm, for 48 h [25]. After cultivation, the yeast biomass was harvested, washed with distilled water, and centrifuged at 400 rpm for 5 min at 20 °C. The biomass was then dried in an oven at 60 °C for 18 h. To ensure uniformity, the particle size was standardized to 0.5 mm before storage in tubes at 25 °C. The overall process yielded 4 g of biomass per 1000 mL of YPD-fermented broth.
2.2. Sequential Hydrolysis to Cell Wall Disruption
The sequential hydrolysis process used to disrupt the cell wall of Yarrowia lipolytica W29 was performed in two phases: autolysis and acid hydrolysis.
Autolysis occurred by adding distilled water to the dry biomass (15%, w/v) under the following conditions: pH 6, 60 °C, 120 rpm of agitation, for 24 h. Then, the suspended material was centrifuged under the following conditions: 20 °C for 5 min, and the supernatant was collected for mannoprotein extraction. The precipitated fraction was then subjected to a second hydrolysis step [28].
Acid hydrolysis was performed using the same biomass concentration (15% w/v), with the pH adjusted to 2 by adding sulfuric acid (Isofar, purity ≥ 99 wt%). The reaction was carried out at 60 °C with agitation at 120 rpm for 1 h. After hydrolysis, the suspension was centrifuged (20 °C, 5 min), and the resulting supernatant was processed by ultrafiltration. The remaining precipitate was dried at 60 °C for 18 h, followed by particle size standardization to 0.5 mm [29]. Finally, the dried biomass was characterized as a food ingredient. The process yield was determined according to Equation (1).
where is the weight of dried biomass and is the weight of dried biomass after acid hydrolysis.
2.3. Extraction of Mannoprotein
Extraction was performed by adding ethanol (Synth, purity ≥ 96 wt%) to the supernatant obtained from the autolysis process, followed by incubation at 4 °C for 18 h. The precipitate obtained in this process was then dried at 60 °C for 18 h. The dried material was then weighed and the extraction yield calculated according to Equation (2). Finally, the dried mannoprotein was dissolved in deionized water at a final concentration of 30 mg mL−1 for further characterization.
where is the weight of dried mannoprotein and is the weight of dried yeast.
2.4. Characterization of Mannoprotein
2.4.1. Proximal Composition
The proximate composition of the crude biomass and the food ingredient extracted from the cell wall of Y. lipolytica W29, cultivated in glucose and glycerol, was analyzed according to the methodology described by AOAC International [30]. Moisture, ash, protein (conversion factor 5.8), lipid, and total carbohydrate contents were determined. The caloric value was calculated using conversion factors of 9 kcal g−1 for lipids, 4 kcal g−1 for proteins, and 4 kcal g−1 for carbohydrates, with results expressed in kcal 100 g−1.
2.4.2. Surface Tension (ST) and Emulsification Index (EI)
The surface tension (ST) was assessed using a K 100 Tensiometer (Kruss), using the ring method at room temperature [7]. To calculate the emulsification index (EI), 1 mL of hexadecane (Sigma-Aldrich) was added to the samples. Then, the samples were homogenized in a vortex for 2 min and remained at rest for 24 h. The EI was calculated based on Equation (3).
where is the emulsified layer height and is the total height of the liquid column.
2.4.3. Thermogravimetric Analysis (TGA)
The thermal stability of the extracted mannoproteins was evaluated using thermogravimetric analysis (TGA). TGA was conducted using a Shimadzu TGA-50 analyzer, applying a nitrogen flow rate of 100 mL min−1. The samples were subjected to a controlled heating rate of 10 °C min−1 within a temperature range of 25–500 °C to evaluate their thermal stability.
2.4.4. Fourier Transform Infrared Spectroscopy (FTIR)
The chemical characteristics of Yarrowia lipolytica W29 before and after cell disruption by sequential hydrolysis were analyzed using an FTIR spectrophotometer. Lyophilized yeast biomass and extracted mannoproteins were assessed to identify structural and compositional changes. Analyses were performed with an IRTracer-100 FTIR spectrophotometer (Shimadzu, Tokyo, Japan), operating in the wavenumber range (4000–400 cm−1) with a resolution of 4 cm−1.
2.4.5. Optical Microscopy Analysis
Optical microscopy (Nikon-Nikon Eclipse E200, Tokyo, Japan, ×100 resolution) was used to analyze the cell morphology of the crude yeast biomass and feed ingredients of Y. lipolytica W29.
2.4.6. Ultrafiltration Separation
Ultrafiltration was performed in a 300 mL stirred dead-end cell using the NADIR® membranes (Frings of Brazil, Piracicaba, São Paulo) of different molecular weight cutoffs (50 kDa UH050 P, 20 kDa UP020 P, 10 kDa UP010 P, and 1 kDa NP010 P) sequentially to produce different permeate fractions (P < 50 kDa, P < 20 kDa and P < 1 kDa). The system operated under a pressure of 5 bars and a temperature of 25 °C. The retentate and permeate were collected separately to determine carbohydrate, protein, and antioxidant activity [17].
2.4.7. Determination of Total Protein and Carbohydrate Content
Total protein content was determined using the Lowry method, with absorbance measured at 750 nm. A standard curve was generated using bovine serum albumin (BSA) as the protein standard. A 30 mg mL−1 solution was prepared to assess mannoprotein content. Total carbohydrate content was quantified using the phenol–sulfuric acid method described by Dubois et al. [31]. In this procedure, 10 mg of the supernatant was mixed with 270 μL of distilled water, 270 μL of 5 wt% phenol solution, and 1460 μL of 98 wt% sulfuric acid. The mixture was allowed to stand for 30 min at room temperature to ensure complete reaction. Absorbance measurements were recorded at 490 nm using a spectrophotometer to quantify total sugar content.
2.4.8. Determination of Total Glucan, β-Glucan, and α-Glucan
Total glucan, α-glucan and β-glucan contents were determined using the β-glucan Assay Kit (K-YBGL, Megazyme, Wicklow, Irland). This enzymatic kit is a quantitative method based on acid hydrolysis used to determine the content of total glucans, α-glucan and β-glucan, i.e., soluble fibers in yeast preparations [32]. The analysis was performed following all the recommendations proposed by the manufacturer.
2.4.9. Technological Features
The water holding capacity (WHC) and water solubility index (WSI) were assessed following the method of Berloto et al. [33], with modifications. A 5 mL aliquot of distilled water (5% w/v) was added to the samples (yeast biomass and feed ingredients), and the mixture was homogenized in a vortex at 200 rpm for 30 min at 25 °C. The suspension was then centrifuged at 2057× g for 25 min at 25 °C. The supernatant was collected and dried in an oven at 70 °C for 18 h. WHC was determined using Equation (4), while WSI was calculated using Equation (5).
Oil binding capacity (OBC) was determined by adding 5 mL of vegetable oil (5%, w/v) to yeast biomass and feed ingredients. After homogenization (25 °C, 200 rpm and 30 min) and centrifugation (2.057× g, 25 °C and 25 min), the supernatant (oil dispersion) was collected, and the OBC was calculated using Equation (6).
2.4.10. Antioxidant Activity
The antioxidant activity of the samples (after acid hydrolysis and fractionated by ultrafiltration) was determined by three colorimetric methods: ABTS radical scavenging, DPPH radical scavenging, and ferric reducing power (FRAP). The ABTS method was performed according to the methodology of Rufino et al. [34]. The absorbance was measured at 734 nm, and the results are expressed in μM of Trolox equivalent. The 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging method was determined using a methanolic DPPH solution and a reaction time of 40 min protected from light. Afterward, the absorbance was read at 515 nm, and the results were expressed in μM of Trolox equivalent. The ferric-reducing activity power was determined using the Benzie and Strain [35] FRAP reagent preparation method. The absorbance was measured at 593 nm, and the results are expressed in μM of ascorbic acid equivalent.
2.4.11. Statistical Analysis
All analyses were performed in triplicate with the mean and standard deviation calculation. Data processing was performed using Statistic 8.0 Software (Statsoft®), which used analysis of variance (ANOVA) and Tukey’s test at 5% significance.
3. Results
3.1. Characterization of Mannoproteins
Yarrowia lipolytica, a non-conventional yeast, is gaining attention for its ability to produce mannoproteins from low-cost substrates like glycerol, an abundant by-product of biodiesel production. Thus, Table 1 presents the characterization of mannoproteins extracted from Y. lipolytica W29 cells grown in glucose- and glycerol-based media. Glucose resulted in a higher mannoprotein yield (13.15%) than glycerol (11.71%), indicating a significant influence of the carbon source (p < 0.05). Despite its lower yield, glycerol remains an attractive, low-cost substrate for sustainable production. Yeast cell walls contain ~20–25% mannoproteins, and glucose-based extraction recovered over 50% of the total mannoproteins. Interestingly, glycerol yielded higher carbohydrate (13.47 g L−1) and protein (30.35 g L−1) content than glucose (10.26 g L−1 and 26.46 g L−1, respectively). This may be attributed to glycerol stimulating β(1,3)-glucan synthase, responsible for yeast cell wall polysaccharide synthesis [36]. Additionally, mannoproteins feature carbohydrate side chains linked to asparagine residues and disulfide bridges in the outer layer, contributing to polymer content and bioactivity [37]. It is important to emphasize that by using glycerol as a carbon source the polymer content in the yeast cell wall can increase, resulting in biomass as a more efficient source of biologically active polysaccharides.

Table 1.
Characterization of mannoprotein from Y. lipolytica W29 grown in glucose and glycerol.
Contrasting findings were reported by Silva et al. [23], who observed higher mannoprotein yields from Y. lipolytica IMUFRJ 50682 when cultured in glycerol (10.13%) compared to glucose (7.70%). Differences in yeast strain, genetic modification, and isolation source may explain these variations [29]. Y. lipolytica W29, isolated from a wastewater treatment plant, exhibits distinct metabolic behavior from IMUFRJ 50682, isolated from marine environments.
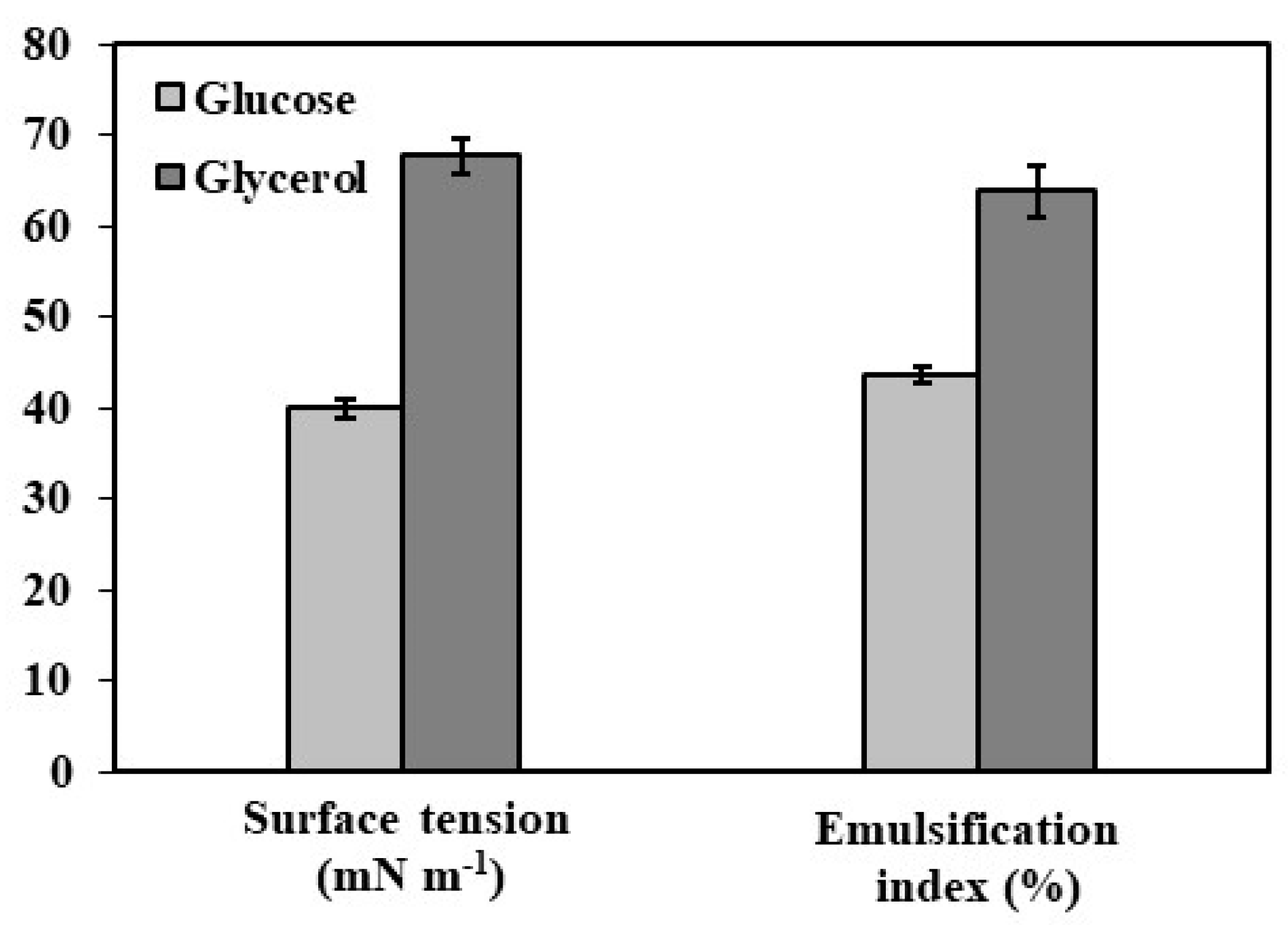
Mannoproteins’ emulsification properties are closely linked to their protein and carbohydrate content. Higher protein levels enhance amphiphilicity and emulsification, while carbohydrates contribute to emulsion stability through protein interactions [38,39]. Glucose-derived mannoproteins exhibited a 67.8% emulsification index and 26.46 nN m−1 surface tension, whereas glycerol-derived mannoproteins showed 63.89% emulsification and 30.35 nN m−1 surface tension (Figure 1). The lower surface tension indicates higher hydrophobicity, influencing biosurfactant potential [22]. Extracellular biosurfactant extraction from Y. lipolytica is influenced by medium composition, yeast strain, agitation, and process duration [18,29,38]. Silva et al. [23] reported superior emulsifying properties in mannoproteins from Y. lipolytica IMUFRJ 50682 grown in glucose (44.50 nN m−1, EI 37.2%). Additionally, biosurfactants from glucose-grown cultures exhibited stability across a pH range of 3.0–9.0 [29].
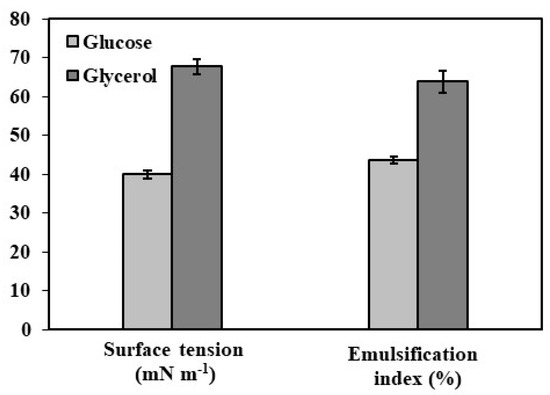
Figure 1.
Surface tension and emulsification index analysis of mannoprotein in 30 mg ml−1 from Y. lipolytica W29 grown in glucose (■) and glycerol (■).
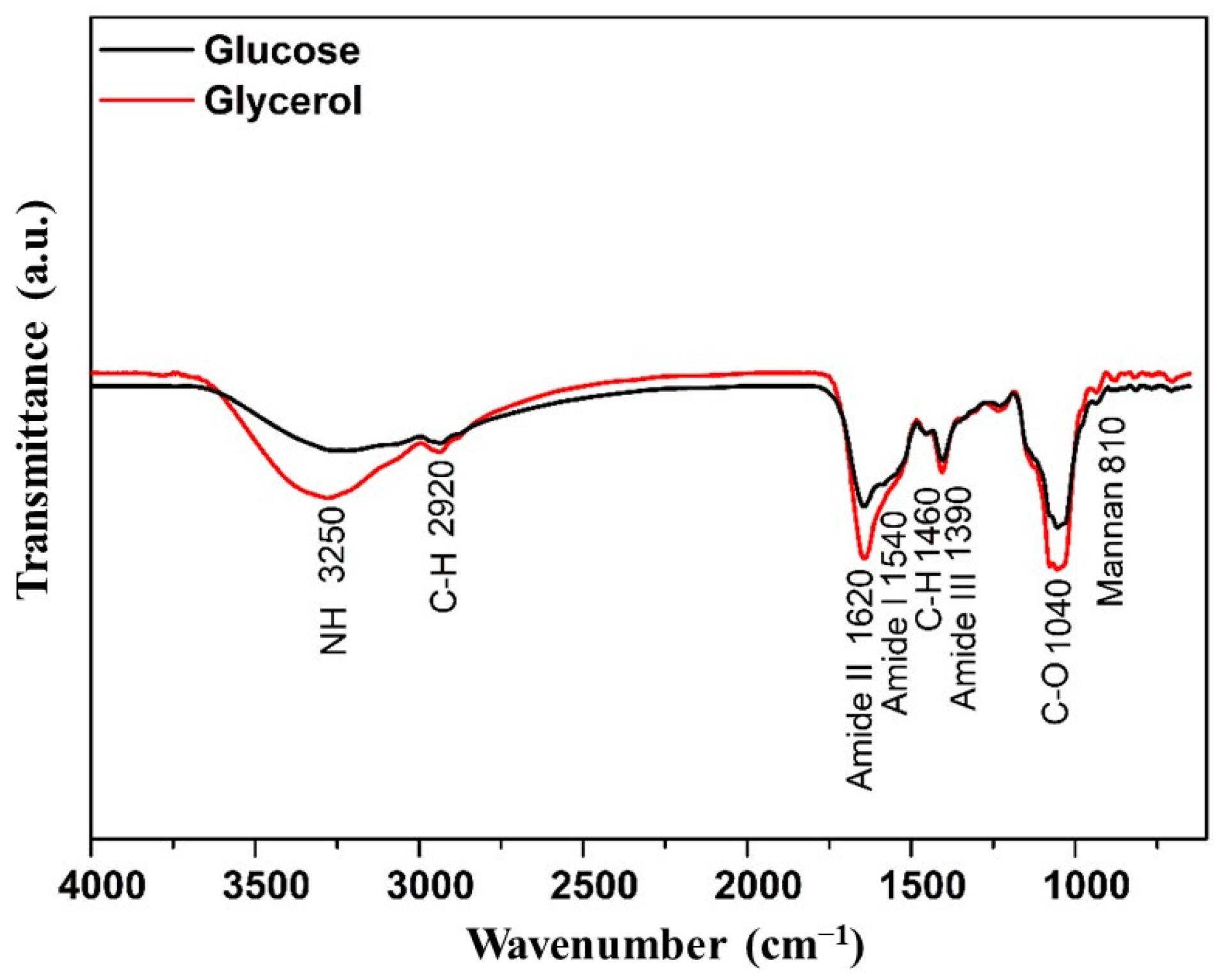
Figure 2 illustrates a small variation between the two mannoproteins obtained following the autolysis. The primary distinction appears at the 3250 cm−1 peaks, corresponding to the asymmetric stretching of the -NH functional group, and at 1620 cm−1, associated with amide II. Another notable difference pertains to the saccharide components of mannoproteins, reflected in the peaks at 1040 cm−1 (C-O stretching vibrations) and 1460 cm−1 (C-H bending vibrations). The increased absorption at these peaks correlates with the protein and carbohydrate content, which was more pronounced in the mannoprotein extracted from fermentation using glycerol as a substrate. Additionally, both mannoproteins exhibited the characteristic mannoprotein absorption band at 820 cm−1, indicative of mannan. Li et al. [40] highlighted the most intense absorption peak at 1624 cm−1, attributed to β-sheet structures, suggesting that its secondary structure comprised 6% aggregated β-strands, 24% β-sheet, 58% β-turns, and 20% random coil for mannoprotein derived from Saccharomyces cerevisiae.
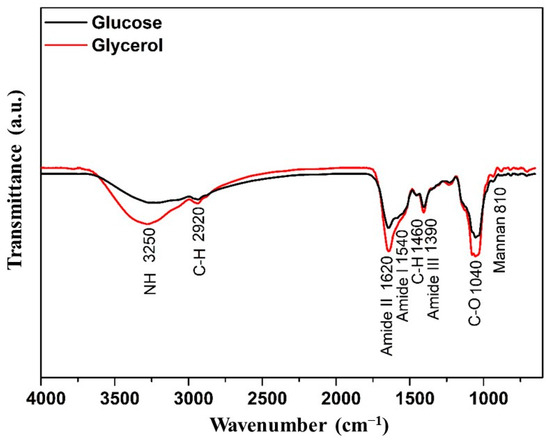
Figure 2.
FTIR of the mannoprotein obtained by the autolysis of crude biomass from the Yarrowia lipolytica W29 growth in glucose (-) and glycerol (-).
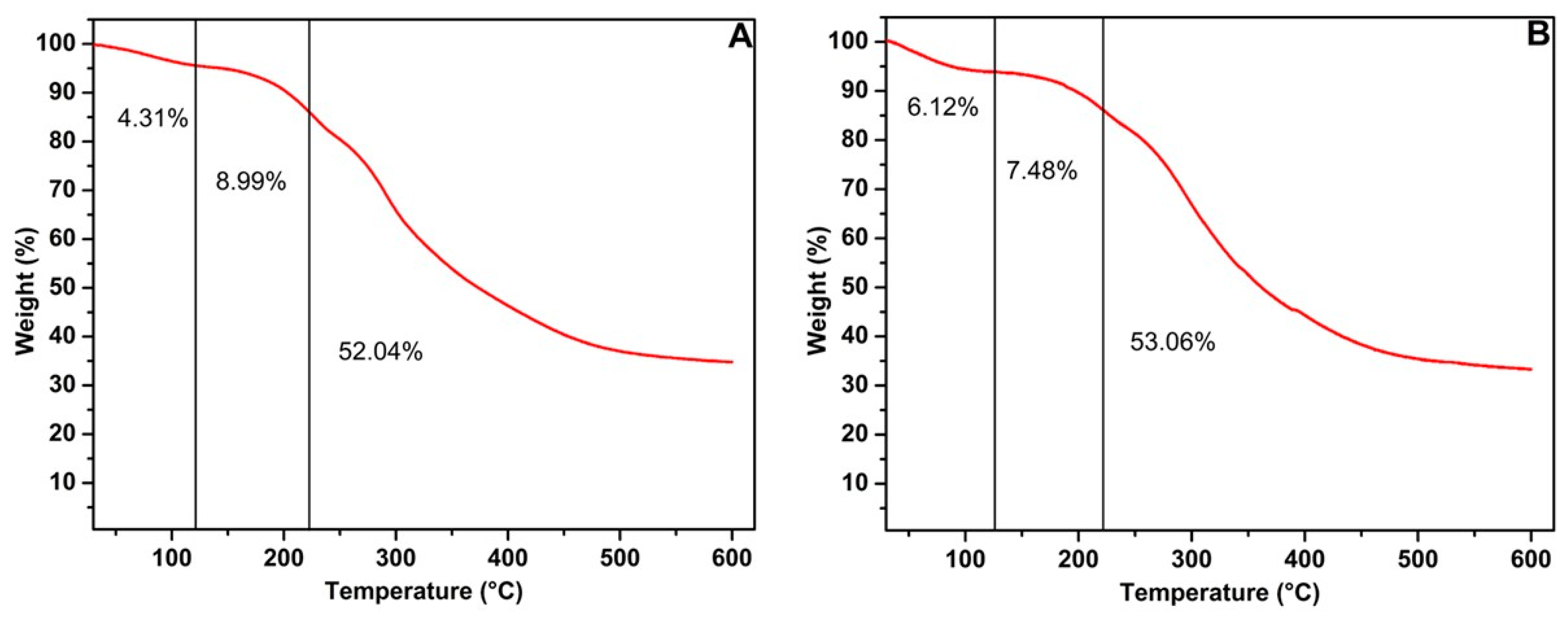
Figure 3 illustrates the thermal stability of mannoproteins, in which the degradation patterns of both mannoproteins followed a similar trend, corresponding to their protein and carbohydrate composition. The results indicate that an initial weight reduction, ranging between 4% and 6%, occurred at 120 °C likely due to the evaporation of adsorbed or structural water and the decomposition of low-molecular-weight molecules. The subsequent mass reduction, between 7% and 9%, was observed at 220 °C, marking the onset of partial protein degradation [41]. The final mass decline, between 52% and 53%, took place between 220 °C and 600 °C, corresponding to the extensive breakdown of proteins and other organic compounds. After this stage, approximately 30% of the mannoprotein’s residual mass remained. In contrast, Silva et al. [23] observed that mannoprotein from IMUFRJ 50682 strain exhibited greater thermal stability when derived from yeast cultured with glycerol as the carbon source, retaining 40% of its residual mass. In this case, the yeast appears to preferentially utilize glycerol over glucose as a carbon source, corroborating other literature studies [29]. Furthermore, glycerol-based fermentation influences the structural attributes of the yeast cell wall, modifying its polysaccharide composition. For instance, employing glycerol as a carbon source alters the concentrations of β-glucan and mannoprotein, thereby increasing the dry cell wall mass in S. cerevisiae R9 brewer’s yeast [42].
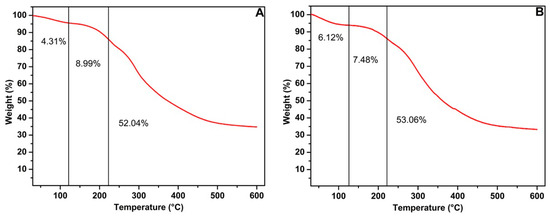
Figure 3.
TGA of the mannoprotein obtained by the autolysis of crude biomass from the Yarrowia lipolytica W29 growth in glucose (A) and glycerol (B).
3.2. Characterization of Crude Biomass and the Food Ingredient
3.2.1. Chemical Composition of Crude Biomass and the Food Ingredients
The chemical composition of raw biomass and food ingredients is described in Table 2. The moisture content of raw biomass and food ingredients was 4.65% and 5.56%, respectively, for Yarrowia lipolytica cultivated in glucose, whereas for yeast grown in glycerol, the values were 4.55% and 4.27%, respectively. The highest moisture content was observed in the food ingredient in the medium containing glucose, which corresponded to the lowest total soluble solids value (94.44%). Moisture regulation is crucial for maintaining the quality and safety of food ingredients and extending shelf life, as excessive water content can alter food consistency and promote microbial growth.

Table 2.
Proximate analysis of using glucose and glycerol as substrates for the Y. lipolytica W29 grown, along with its derived food ingredient.
The ash content of the raw biomass was higher (5.76–5.78%) than that of the food ingredients (1.68–1.84%). The ash content in Y. lipolytica was 3.60% of dry matter, corresponding to 0.48% of dry matter of calcium and 0.21% of phosphorus [14]. A higher ash content was observed in Y. lipolytica grown in crude glycerol, approximately 5%, due to the high content of impurities (soaps, organic impurities and unused reagents). In addition, potassium (0.78%) and sodium (0.44%) were considered the main minerals of Y. lipolytica S5, while smaller amounts of calcium (0.07%), magnesium (0.11%), copper (0.0002%), iron (0.005%), and zinc (0.011%) were present [43].
The highest carbohydrate content was observed in the food ingredient obtained from both glucose- and glycerol-based substrates, ranging between 46% and 48%. The primary carbohydrates in the yeast cell wall are polysaccharides, predominantly glucans (α-glucan and β-glucan), which contribute to cell wall integrity and constitute 30–60% of the dry weight. These polysaccharides are recognized for their functional properties, including antioxidants, prebiotic, and immunomodulatory activities [7,8]. Total glucan content was higher in raw biomass and food ingredients derived from yeast cultivated in glycerol (21.66% and 16.78%, respectively) compared to glucose (19.12% and 16.16%). The highest β-glucan content was detected in raw biomass grown in glycerol, whereas the lowest concentration was found in the corresponding food ingredient. In contrast, α-glucan content was the highest in both raw biomass and food ingredients obtained from glycerol-based cultivation. For the IMUFRJ 50682 strain, α-glucan content was greater in glucose-grown yeast (2.30%), while β-glucan levels were higher when cultured in glycerol (19.31%) [23]. Similarly, in Saccharomyces cerevisiae var. boulardii, cultivation in glycerol resulted in a higher total glucan content, particularly β-glucan (36%) [43]. The protein (53%) and lipid (7%) content were highest in raw biomass grown in glycerol, whereas food ingredients from both substrates contained approximately 43% protein and 3% lipid. The caloric value ranged between 384.80 and 391.77 kcal for the samples analyzed. Compared to Y. lipolytica IMUFRJ 50682 (carbohydrates: 38–32%, protein: 45–48%, lipids: 5–9% for glucose and glycerol, respectively), the W29 strain exhibited a lower carbohydrate content (30%) and higher protein content (50–53%) for both glucose- and glycerol-based growth conditions.
The lipid content ranged from 6% to 7% for the W29 strain, while the CBS 7504 strain showed similar values with 7% lipids [44]. However, the IMUFRJ 50682 strain accumulated 9% lipids when using glycerol as a carbon source [23]. An interesting observation is that Das et al. [45] highlighted that increasing the carbon-nitrogen ratio (C/N) by adjusting the concentrations of glycerol and ammonium sulphate led to the lipid accumulation of 4 g L−1 in the IMUFRJ 50682 strain, with oleic acid (46.8%), palmitoleic acid (18%), and palmitic acid (13.5%) being the primary fatty acids. Juszczyk et al. [43] observed a 16% lipid content for the S6 strain when grown in glycerol under nitrogen-limited conditions with oleic, linoleic, palmitoleic, and palmitic acids, the main fatty acids produced.
Given this, it is clear that oleic acid is the predominant fatty acid in Y. lipolytica, although palmitoleic, stearic and linoleic acids are also present. Fatty acids play essential physiological roles and serve as key nutraceutical components, aiding in the prevention of inflammation, depression, arthritis, osteoporosis, diabetes, and cancer [18]. Furthermore, long-chain fatty acids, such as stearyl palmitate and acetyl stearate, are valuable as plasticizers, lubricants, and additives in food packaging materials, with their use regulated by the U.S. Food and Drug Administration (FDA) [46].
Y. lipolytica W29 exhibits varying lipid accumulation behavior depending on the substrate. When cultivated in lignocellulosic biomass hydrolysate, lipid turnover occurred within 72 h, reaching 28% (w/w). In contrast, when grown in glucose, lipid accumulation peaked at 35% (w/w) at 72 h before declining to 15% (w/w) after 120 h, likely due to carbon source depletion in the medium. The safety and nutritional value of Y. lipolytica biomass have been recognized by the European Food Safety Authority (EFSA) Panel on Nutrition, Novel Foods, and Food Allergens (NDA), which has approved its use in dietary supplements at doses of up to 3 g/day for children aged 3 to 10 years and up to 6 g/day for individuals older than 10 years. Y. lipolytica W29 biomass provides 100% of the recommended protein intake for adults, while its processed feed ingredient supplies approximately 90% [47].
3.2.2. Technological Properties of Crude Biomass and the Food Ingredient
The technological properties of Yarrowia lipolytica W29 in its raw biomass form and as a feed ingredient (dry residue after sequential hydrolysis) are displayed in Table 3. As can be observed, the process yield was higher for yeast grown on glucose (47.70%) compared to glycerol (42.82%), indicating that sequential hydrolysis was more effective in releasing cell wall components when glucose was used as the carbon source. Additionally, the substrate composition directly influenced the yeast cell wall structure. Growth in glucose resulted in a cell wall predominantly composed of carbohydrates (70%), proteins (15%), and a lipid fraction (5%). Similar findings were reported for Y. lipolytica IMUFRJ 50682, where biomass grown on glucose achieved a process yield of 55.58% [23].

Table 3.
Technological properties of Yarrowia lipolytica W29 biomass in media containing glucose and glycerol.
Regarding technological properties, the raw biomass grown in glucose exhibited a higher water holding capacity (WHC) (6.87%) compared to biomass cultivated in glycerol (4.87%). However, for both feed ingredients (approximately 5%), no significant difference (p ≤ 0.05) in WHC was observed. The decrease in WHC has been associated with the surface availability of hydrophobic and hydrophilic groups, as sequential hydrolysis reduces protein content, leading to fewer hydrophilic groups, such as amino acids, on the yeast surface [48].
The water solubility index (WSI) was higher for raw biomass (25%) than for the feed ingredient (10%), which may be attributed to the extraction of proteins and carbohydrates from the cell wall. Nutrient-rich food powders with low WSI values are suitable for applications in baked goods such as cakes, cookies, and other bakery products that do not require immediate solubility in water at room temperature [49]. Compared to Y. lipolytica IMUFRJ 50682, WSI was higher for raw biomass when the yeast was grown in glucose (29.12%). The oil holding capacity (OHC) ranged from 2.68% to 3.40%, indicating that both raw biomass and feed ingredient powders exhibited a similar ability to absorb oil. This property is primarily influenced by the cell surface characteristics, which are expected to be more hydrophilic than hydrophobic [50].
3.2.3. Structural Characterization of Yarrowia lipolytica W29 Biomass and Food Ingredients
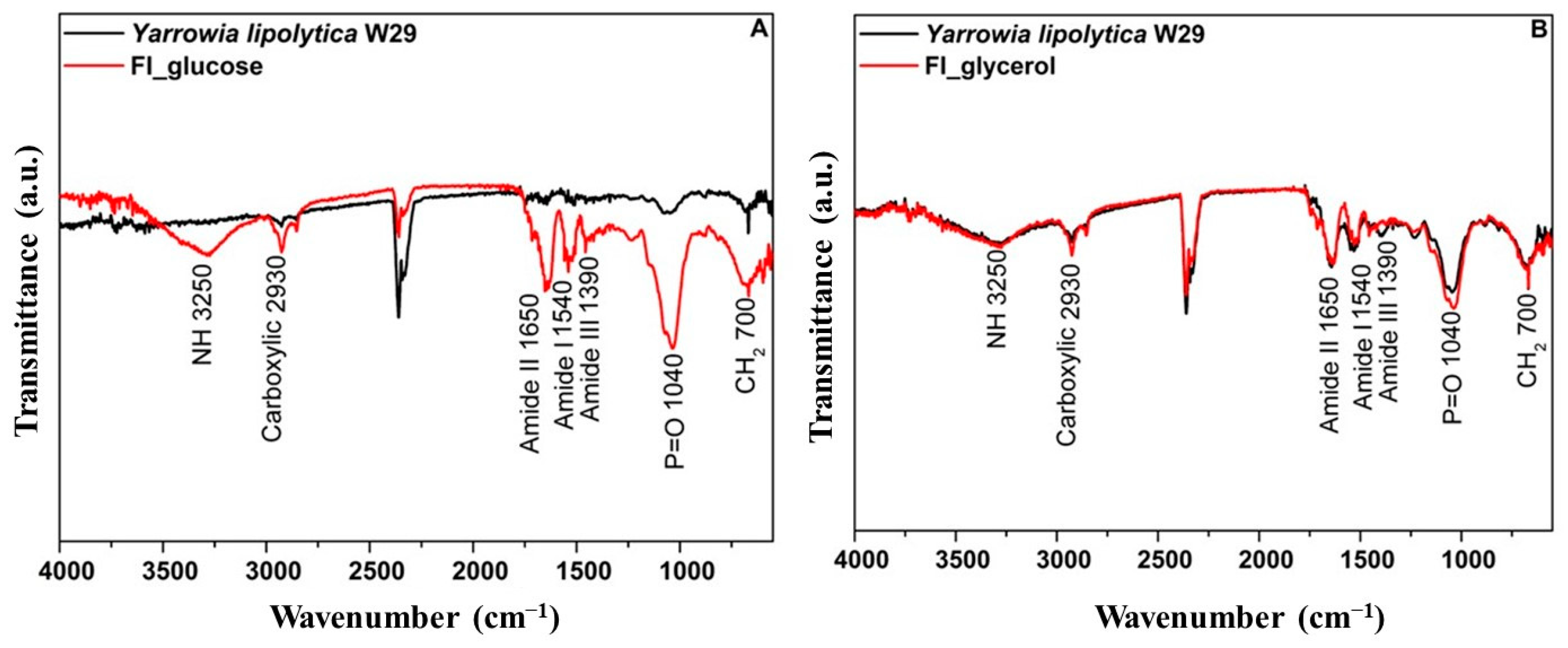
The FTIR spectra are shown in Figure 4 and reveal multiple peaks for crude biomass, highlighting the intricate composition of the yeast cell surface. It is important to emphasize that Yarrowia lipolytica is primarily characterized by absorption peaks at 3250 cm−1, corresponding to the asymmetric stretching of the -NH group; 1620 cm−1, 1540 cm−1, and 1390 cm−1, associated with amide II, amide I, and amide III, respectively; and 1040 cm−1, corresponding to the C-O vibrational modes [51,52]. Comparing the crude biomass to its respective food ingredients, variations in band intensity were observed across different spectral regions. These differences were more pronounced in the food ingredient derived from biomass cultivated in glucose, particularly at 3250 cm−1 (-NH asymmetric stretching), as well as 1620 cm−1, 1540 cm−1, and 1390 cm−1 (amide II and amide III, respectively). Additionally, notable changes were detected at 1040 cm−1 (C-O vibration) and 700 cm−1 (CH2).
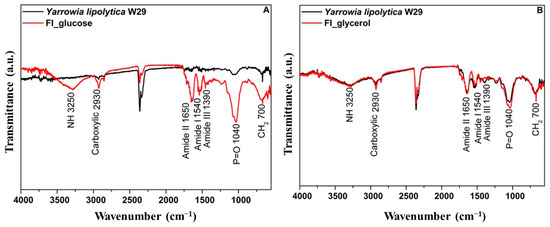
Figure 4.
FTIR of the Y. lipolytica W29 crude biomass (-) and the food ingredient (-) cultivated in glucose (A) or glycerol (B).
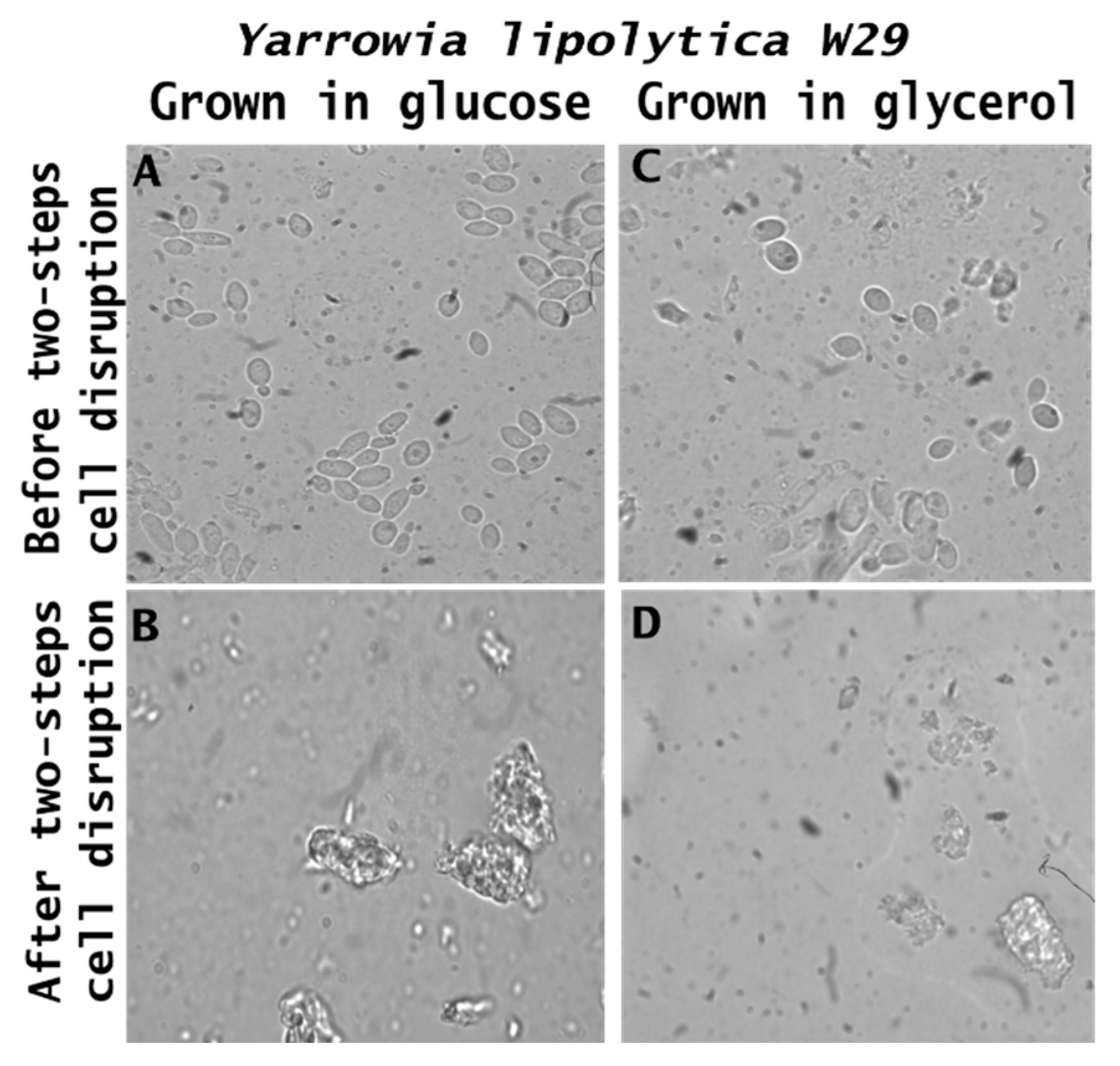
Morphological transformations in the raw biomass and its respective feed ingredients are presented in Figure 5. From microscopic analysis, it is possible to observe that the yeast cells exhibit an intact morphology, maintaining an oval shape with a smooth surface, a characteristic commonly reported in Y. lipolytica species (Figure 5A,C). However, following cell wall disruption through sequential hydrolysis (autolysis and acid hydrolysis) (Figure 5B,D), the cells display an irregular shape, a reduction in size, and a significant increase in surface roughness. Despite these morphological changes, cell wall degradation facilitates the release of key constituents, including proteins, carbohydrates, lipids, and glucans, forming the feed ingredient, which differs in composition and properties from the raw biomass.
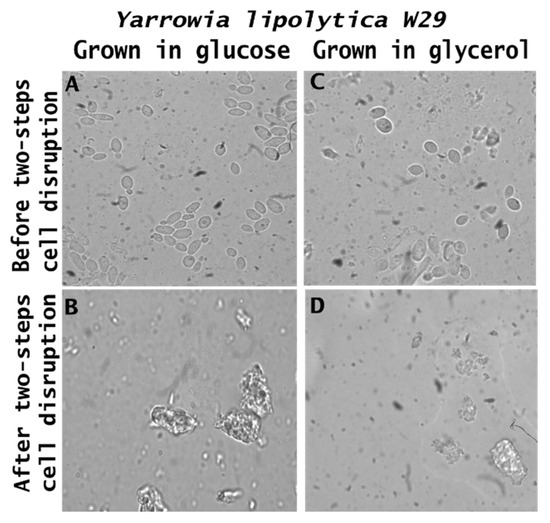
Figure 5.
Microscopy of the crude biomass of Yarrowia lipolytica W29 and the food ingredient cultivated in glucose (A,B) and glycerol (C,D).
Similar structural alterations were observed in Y. lipolytica IMUFRJ 50682 grown on glucose and glycerol [23]. In a previous study using the ACA-DC 50109 strain, three distinct cell morphologies were identified during growth in glycerol. The exponential phase was characterized by a mixture of short true mycelia, pseudomycelia, and some yeast cells. In the early stationary phase, also referred to as the lipogenic phase, only large yeast cells containing lipid globules were observed. The late stationary phase was dominated by shorter yeast cells [53]. It is important to note that, depending on growth conditions and genetic background, Y. lipolytica strains can exhibit a variety of morphologies, ranging from convoluted and opaque to smooth and glossy [54].
3.3. Ultrafiltration of Acid Hydrolysate
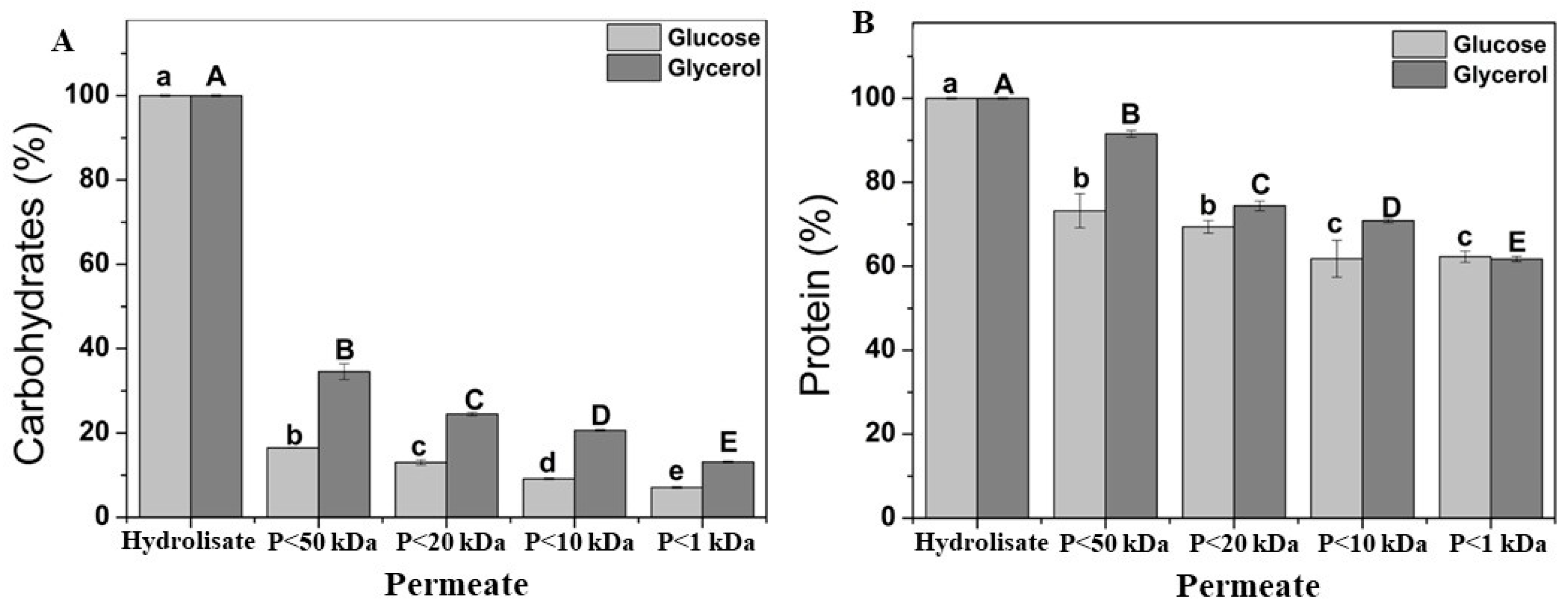
The acid hydrolysis process, following autolysis, facilitates the rupture of the Yarrowia lipolytica W29 cell wall, enabling the release of high-value compounds such as carbohydrates and proteins from the residual biomass. As shown in Figure 6A, more than 75% of the carbohydrates extracted from the cell wall of Y. lipolytica W29 grown in glucose were retained due to their molecular weights exceeding 50 kDa, whereas over 60% were retained after cultivation in glycerol. A previous study on Y. lipolytica IMUFRJ 50682 attributed carbohydrate retention to the varying molecular weights of cell wall polysaccharides, including β-glucan (300–2000 kDa) and chitin (1000 kDa) [23]. In addition to the molecular weight cutoff, polysaccharide conformation (e.g., packed sphere, random coil, rigid rod) can influence ultrafiltration permeation. Eder et al. [55] reported significant differences in the recovery of pullulan and scleroglucan polysaccharides with values of 1 and 71%, respectively, using 10 kDa polyethersulfone membranes. These variations stem from their distinct glycosidic bonding patterns, which determine their conformational clusters. This behavior can be due to the pullulan, a water-soluble linear (1 → 4;1 → 6)-α-D-glucan, adopts a random coil conformation in aqueous solutions, whereas scleroglucan, a water-soluble (1 → 3;1 → 6)-β-D-glucan, exhibits a rigid rod-like structure.
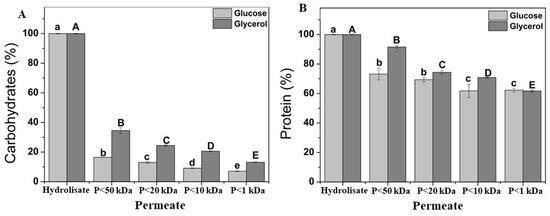
Figure 6.
Soluble fraction from fractional acid hydrolysis by ultrafiltration of Yarrowia lipolytica biomass grown on glucose (light grey bars) and glycerol (dark grey bars). Carbohydrate (A); protein (B). “P” refers to the permeate fraction. According to Tukey’s test, different upper/lower case letters denote differences (p ≤ 0.05) between the mean values.
Figure 6B shows that 60% of the protein in the acid hydrolysate has a molecular weight of less than 1 kDa. Yeast hydrolysate consists of peptides of varying sizes (both low and high in molecular weight) resulting from the action of endogenous proteolytic enzymes, which cleave peptide bonds. Ultrafiltration, a filtration technique utilizing membranes of different pore sizes, can be applied to selectively obtain low-molecular-weight peptides, thereby enhancing their functional properties. The yeast hydrolysate obtained after ultrafiltration demonstrated greater heat stability, likely due to its high degree of hydrolysis and the presence of short peptides. These peptides reduce the secondary structure, minimizing chemical changes upon heating [56]. A two-stage ultrafiltration process using 10 kDa and 5 kDa membranes effectively separated high-molecular-weight peptides, yielding 77.49% of peptides smaller than 300 Da, compared to 75.45% and 75.07% obtained using single 10 kDa and 5 kDa membranes, respectively. Low-molecular-weight peptides exhibit advantages such as high antioxidant activity, structural stability, easy absorption, and low toxicity [57]. This behavior corroborates with the ultrafiltration of soluble proteins after the cultivation of mono- (Kluyveromyces marxianus) and mixed (K. marxianus and Saccharomyces cerevisiae) cultures using 10 kDa and 1 kDa membranes resulted in protein recoveries of 84% and 92%, respectively [54]. Additionally, ultrafiltration for invertase recovery from baker’s yeast vinasse demonstrated that a 30 kDa membrane retained a significantly higher protein concentration (15 mg g−1) compared to a 2 kDa membrane (7 mg g−1) [58].
3.4. Antioxidant Property of the Acid Hydrolysate
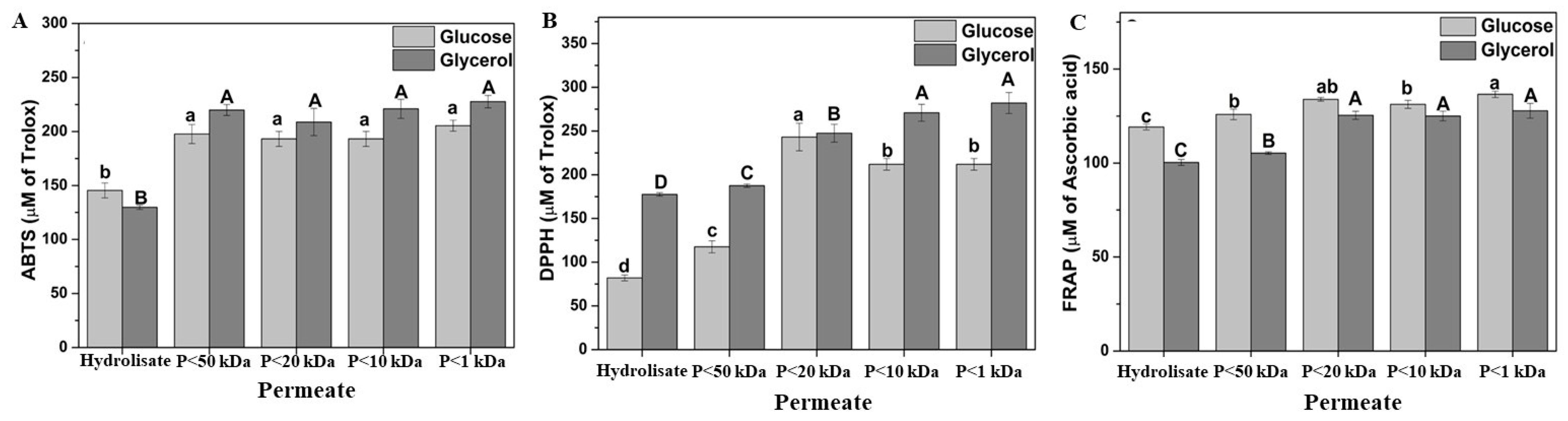
Three different methodologies were employed to evaluate antioxidant activity: ABTS, DPPH, and FRAP. Each method operates through a distinct mechanism, enabling the assessment of various antioxidant compounds [59]. As shown in Figure 7, all three methodologies demonstrated increased antioxidant activity with decreasing membrane size. The permeate fraction with a molecular weight of 1 kDa exhibited the highest antioxidant activity, with values of 220 µM Trolox in ABTS, 270 µM Trolox in DPPH, and 125 µM ascorbic acid in FRAP. The enzymatic hydrolysis of S. cerevisiae facilitated the release of cell wall components, enhancing antioxidant activity to 12 µmol TE g−1 in FRAP and 35 µmol TE g−1 in DPPH [22].
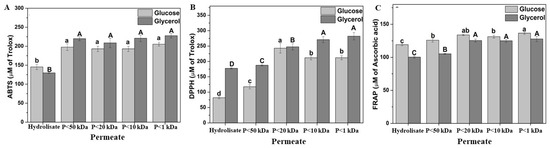
Figure 7.
Antioxidant activity of the soluble fraction of acid hydrolysis fractioned by ultrafiltration from the Yarrowia lipolytica W29 biomass grown in glucose (light grey bars) and glycerol (dark grey bars). (A) ABTS; (B) DPPH; (C) FRAP. “P” refers to the permeate fraction. According to Tukey’s test, different upper/lower case letters denote differences (p ≤ 0.05) between the mean values.
The increase in antioxidant activity may be attributed not only to the lower carbohydrate content in each permeate fraction (<50 kDa), but also the presence of different peptide fractions. Studies have demonstrated that low-molecular-weight peptides exhibit stronger antioxidant properties than high-molecular-weight peptides [60]. The peptide fractions (2–10 kDa) of S. cerevisiae ISA 1028 displayed significant antioxidant potential, with 81.03% DPPH radical scavenging activity and FRAP values of 1042.50 µM TE mL−1, which were attributed to their amino acid composition, sequence, and hydrophobic amino acid content [54]. Serial fractionation of brewer’s yeast hydrolysate using 15, 8, and 1 kDa molecular cutoff membranes resulted in a final composition consisting of 90% peptides smaller than 1 kDa, establishing it as an innovative protein-rich ingredient with a low RNA content [22]. Among the 20 essential amino acids, 7, i.e., tryptophan, methionine, histidine, lysine, cysteine, arginine, and tyrosine, exhibit antioxidant activity [61]. This property is linked to their capacity to transfer hydrogen and scavenge lipid peroxyl radicals. Additionally, amino acids containing aromatic residues can donate protons to unpaired radicals, thereby enhancing the antioxidant properties of peptides. Y. lipolytica contains essential amino acids such as histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, and valine in viable amounts [45].
The functionality of peptides obtained through the enzymatic hydrolysis of spent brewer’s yeast demonstrated that neutrase hydrolysate exhibited the highest DPPH radical scavenging activity at 116.9 μM TE g−1, followed by trypsin hydrolysate at 102.8 μM TE g−1. Furthermore, low-molecular-weight peptides (<3 kDa) from bromelain hydrolysate showed the highest antioxidant activity at 50.06 μM TE g−1, suggesting the presence of hydrophobic amino acids such as alanine, proline, valine, methionine, isoleucine, leucine, phenylalanine, and tryptophan in the peptide sequences [62]. These findings highlight the potential of bioactive peptides extracted from Y. lipolytica W29 cultivated in glycerol as a promising alternative for applications in nutraceuticals, cosmetics, and functional foods.
A preliminary technical and economic analysis shows that the cost of the raw materials used in biotechnological processes can have a direct impact on the economic directive and competitiveness of the final products. The average price of 1 kg of glycerol is approximately USD 0.47, while 1 kg of glucose costs around USD 4.30. This significant difference shows that glycerol is around 89% cheaper than glucose, thus becoming an economically viable alternative for the production of biocomposites, especially when looking to reduce operating costs without compromising the efficiency of the process. The use of glycerol as a carbon source in fermentation processes can be even more advantageous due to its availability and sustainability. As a by-product of the biodiesel industry, glycerol has a low cost due to its high production volume and lower demand compared to conventional sugars such as glucose. In addition, its use contributes to the recovery of industrial waste, promoting a circular economic approach that reduces environmental impacts and improves the sustainability of the process.
Another crucial point in this analysis is the added value of the final product. The mannoprotein market is highly valued, with 1 kg being sold for approximately USD 545. This high market value highlights the economic relevance of extracting and purifying mannoprotein from alternative, low-cost sources, such as yeast biomass grown in glycerol-based media. The combination of a low-cost substrate and a highly valued bioproduct improves the profitability of the process, increasing its attractiveness for industrial applications in the food, pharmaceutical, and cosmetics sectors. Therefore, the choice of glycerol as a fermentation substrate not only reduces the costs of biomass and biocomposites production, as well as aligning with global trends in sustainable production and resource optimization, reinforcing its viability for large-scale industrial applications.
4. Conclusions
This study demonstrated that glycerol is an effective and cost-efficient alternative to glucose as a carbon source for Y. lipolytica W29 cultivation. Yeast biomass proved to be a valuable source of high-value compounds, including proteins, carbohydrates, mannoproteins, and glucans. The mannoprotein obtained after autolysis exhibited excellent emulsification properties, with a surface tension of approximately 40 mN m−1 and an emulsification index of 60%. Additionally, its thermal stability, retaining more than 30% of its mass, suggests potential applications in various industrial processes. Both the raw biomass and the feed ingredient (dry residue after sequential hydrolysis) demonstrated high nutritional value, meeting 100% and 90% of the daily protein intake requirements, respectively, while also containing 17% β-glucan. Furthermore, bioactive peptides present in the acid hydrolysate exhibited the highest antioxidant activity for peptides smaller than 1 kDa, with values of 220 µM Trolox in ABTS, 270 µM Trolox in DPPH, and 125 µM ascorbic acid in FRAP. Overall, Y. lipolytica W29 biomass and its extracted products represent promising alternatives for diverse industrial applications due to their high nutritional content and functional and technological properties. Future studies on process optimization are necessary to enhance process efficiency and maximize extraction yields.
Author Contributions
Conceptualization: B.D.R., A.C.L. and M.A.Z.C.; methodology: R.M.d.S., C.N.d.S. and C.S.d.F.-J.; validation: F.S.B. and B.D.R.; formal analysis: R.M.d.S. and F.S.B.; investigation: R.M.d.S. and C.N.d.S.; data curation: A.C.L. and M.A.Z.C.; writing—original draft preparation: C.N.d.S. and F.S.B.; writing—review and editing: F.S.B. and B.D.R.; supervision: A.C.L. and M.A.Z.C.; project administration: M.A.Z.C.; funding acquisition: M.A.Z.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financed by national funds through the National Council for Scientific and Technological Development (CNPq); Coordination of Improvement of Higher-Level Personnel (CAPES); Foundation for Research Support and Technological Innovation of the State of Rio de Janeiro (FAPERJ); Buarque, F.S. acknowledge the scholarship grant from FAPERJ: E-26/204.344/2021.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Martin, G.J.O.; Chan, S. Future Production of Yeast Biomass for Sustainable Proteins: A Critical Review. Sustain. Food Technol. 2024, 2, 1592–1609. [Google Scholar] [CrossRef]
- Wani, A.K.; Akhtar, N.; Sher, F.; Navarrete, A.A.; Américo-Pinheiro, J.H.P. Microbial Adaptation to Different Environmental Conditions: Molecular Perspective of Evolved Genetic and Cellular Systems. Arch. Microbiol. 2022, 204, 144. [Google Scholar] [CrossRef] [PubMed]
- Berzosa, A.; Marín-Sánchez, J.; Delso, C.; Sanz, J.; Álvarez, I.; Sánchez-Gimeno, C.; Raso, J. Sequential Extraction Optimization of Compounds of Interest from Spent Brewer’s Yeast Biomass Treated by Pulsed Electric Fields. Innov. Food Sci. Emerg. Technol. 2024, 94, 103705. [Google Scholar] [CrossRef]
- Yu, S.; Sun, J.; Shi, Y.; Wang, Q.; Wu, J.; Liu, J. Nanocellulose from various biomass wastes: Its preparation and potential usages towards the high value-added products. Environ. Sci. Ecotechnol. 2021, 5, 100077. [Google Scholar] [CrossRef]
- Reis, S.F.; Fernandes, P.A.R.; Martins, V.J.; Gonçalves, S.; Ferreira, L.P.; Gaspar, V.M.; Figueira, D.; Castelo-Branco, D.; Mano, J.F.; Coimbra, M.A.; et al. Brewer’s Spent Yeast Cell Wall Polysaccharides as Vegan and Clean Label Additives for Mayonnaise Formulation. Molecules 2023, 28, 3540. [Google Scholar] [CrossRef]
- Pereyra, C.M.; Gil, S.; Cristofolini, A.; Bonci, M.; Makita, M.; Monge, M.P.; Montenegro, M.A.; Cavaglieri, L.R. The Production of Yeast Cell Wall Using an Agroindustrial Waste Influences the Wall Thickness and Is Implicated on the Aflatoxin B1 Adsorption Process. Food Res. Int. 2018, 111, 306–313. [Google Scholar] [CrossRef]
- Gautério, G.V.; Silvério, S.I.D.C.; Egea, M.B.; Lemes, A.C. β-Glucan from Brewer’s Spent Yeast as a Techno-Functional Food Ingredient. Front. Food Sci. Technol. 2022, 2, 1074505. [Google Scholar] [CrossRef]
- Luo, J.; Chen, D.; Mao, X.; He, J.; Yu, B.; Cheng, L.; Zeng, D. Purified β-Glucans of Different Molecular Weights Enhance Growth Performance of LPS-Challenged Piglets via Improved Gut Barrier Function and Microbiota. Animals 2019, 9, 602. [Google Scholar] [CrossRef]
- Afroz, M.M.; Kashem, M.N.H.; Piash, K.M.P.S.; Islam, N. Saccharomyces cerevisiae as an Untapped Source of Fungal Chitosan for Antimicrobial Action. Appl. Biochem. Biotechnol. 2021, 193, 3765–3786. [Google Scholar] [CrossRef]
- Huq, T.; Khan, A.; Brown, D.; Dhayagude, N.; He, Z.; Ni, Y. Sources, Production and Commercial Applications of Fungal Chitosan: A Review. J. Bioresour. Bioprod. 2022, 7, 85–98. [Google Scholar] [CrossRef]
- Martínez, J.M.; Cebrián, G.; Álvarez, I.; Raso, J. Release of Mannoproteins during Saccharomyces cerevisiae Autolysis Induced by Pulsed Electric Field. Front. Microbiol. 2016, 7, 1435. [Google Scholar] [CrossRef]
- Faustino, M.; Durão, J.; Pereira, C.F.; Pintado, M.E.; Carvalho, A.P. Mannans and mannan oligosaccharides (MOS) from Saccharomyces cerevisiae–A sustainable source of functional ingredients. Carbohydr. Polym. 2021, 272, 118467. [Google Scholar] [PubMed]
- Singh, S.; Singh, G.; Arya, S.K. Mannans: An overview of properties and applications in food products. Int. J. Biol. Macromol. 2018, 119, 79–95. [Google Scholar] [PubMed]
- Michalik, B.; Biel, W.; Lubowicki, R.; Jacyno, E. Chemical Composition and Biological Value of Proteins of the Yeast Yarrowia lipolytica Growing on Industrial Glycerol. Can. J. Anim. Sci. 2014, 94, 99–104. [Google Scholar] [CrossRef]
- Nicaud, J.M. Yarrowia lipolytica . Yeast 2012, 29, 409–418. [Google Scholar]
- Łukaszewicz, M.; Leszczyński, P.; Jabłoński, S.J.; Kawa-Rygielska, J. Potential Applications of Yeast Biomass Derived from Small-Scale Breweries. Appl. Sci. 2024, 14, 2529. [Google Scholar] [CrossRef]
- da Silva, L.V.; Coelho, M.A.Z.; Amaral, P.F.F.; Fickers, P. A Novel Osmotic Pressure Strategy to Improve Erythritol Production by Yarrowia lipolytica from Glycerol. Bioprocess Biosyst. Eng. 2018, 41, 1883–1886. [Google Scholar] [CrossRef]
- Buarque, F.S.; Ribeiro, B.D.; Freire, M.G.; Coelho, M.A.Z.; Pereira, M.M. Assessing the Role of Deep Eutectic Solvents in Yarrowia lipolytica Inhibition. J. Biotechnol. 2025, 398, 1–10. [Google Scholar] [CrossRef]
- Dias, B.; Lopes, M.; Fernandes, H.; Marques, S.; Gírio, F.; Belo, I. Biomass and microbial lipids production by Yarrowia lipolytica W29 from eucalyptus bark hydrolysate. Renew. Energy 2024, 224, 120173. [Google Scholar] [CrossRef]
- Pereira, A.S.; Belo, I.; Lopes, M. Enhancing Microbial Lipids Synthesis for Biodiesel Production by Y. lipolytica W29 from Volatile Fatty Acids: Two-Stage Batch Strategies. Appl. Sci. 2022, 12, 8614. [Google Scholar] [CrossRef]
- Gessler, N.N.; Ivanova, N.O.; Kokoreva, A.S.; Klein, O.I.; Isakova, E.P.; Deryabina, Y.I. The Physiological Adaptation Features of the Poly-Extremophilic Yeast Yarrowia lipolytica W29 During Long-Term Cultivation. Appl. Biochem. Microbiol. 2022, 58, 771–779. [Google Scholar]
- Marson, G.V.; Lacour, S.; Hubinger, M.D.; Belleville, M.P. Serial Fractionation of Spent Brewer’s Yeast Protein Hydrolysate by Ultrafiltration: A Peptide-Rich Product with Low RNA Content. J. Food Eng. 2022, 312, 110737. [Google Scholar] [CrossRef]
- da Silva, R.M.; Ribeiro, B.D.; Lemes, A.C.; Coelho, M.A.Z. Recovery of High-Value Compounds from Yarrowia lipolytica IMUFRJ 50682 Using Autolysis and Acid Hydrolysis. Processes 2024, 12, 1132. [Google Scholar] [CrossRef]
- Berzosa, A.; Delso, C.; Sanz, J.; Sánchez-Gimeno, C.; Raso, J. Sequential extraction of compounds of interest from yeast biomass assisted by pulsed electric fields. Front. Bioeng. Biotechnol. 2023, 11, 1197710. [Google Scholar]
- Kot, A.M.; Gientka, I.; Bzducha-Wróbel, A.; Błażejak, S.; Kurcz, A. Comparison of Simple and Rapid Cell Wall Disruption Methods for Improving Lipid Extraction from Yeast Cells. J. Microbiol. Methods 2020, 176, 105999. [Google Scholar] [CrossRef]
- Alavi, F.; Ciftci, O.N. Purification and Fractionation of Bioactive Peptides through Membrane Filtration: A Critical and Application Review. Trends Food Sci. Technol. 2023, 131, 118–128. [Google Scholar]
- Park, Y.; Kim, J.H.; Lee, H.S.; Jung, E.Y.; Lee, H.; Noh, D.O.; Suh, H.J. Thermal Stability of Yeast Hydrolysate as a Novel Anti-Obesity Material. Food Chem. 2013, 136, 316–321. [Google Scholar] [CrossRef]
- Takalloo, Z.; Nikkhah, M.; Nemati, R.; Jalilian, N.; Sajedi, R.H. Autolysis, plasmolysis and enzymatic hydrolysis of baker’s yeast (Saccharomyces cerevisiae): A comparative study. World J. Microbiol. Biotechnol. 2020, 36, 68. [Google Scholar]
- Gonçalves, F.A.G.; Colen, G.; Takahashi, J.A. Yarrowia lipolytica and its multiple applications in the biotechnological industry. Sci. World J. 2014, 2014, 476207. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar]
- McCleary, B.V.; Draga, A. Measurement of β-Glucan in Mushrooms and Mycelial Products. J. AOAC Int. 2016, 99, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Bertolo, A.P.; Biz, A.P.; Kempka, A.P.; Rigo, E.; Cavalheiro, D. Yeast (Saccharomyces cerevisiae): Evaluation of Cellular Disruption Processes, Chemical Composition, Functional Properties and Digestibility. J. Food Sci. Technol. 2019, 56, 3697–3706. [Google Scholar] [CrossRef] [PubMed]
- Maria do Socorro, M.R.; Alves, R.E.; de Brito, E.S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive Compounds and Antioxidant Capacities of 18 Non-Traditional Tropical Fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar]
- Bzducha-Wróbel, A.; Błażejak, S.; Kieliszek, M.; Pobiega, K.; Falana, K.; Janowicz, M. Modification of the Cell Wall Structure of Saccharomyces cerevisiae Strains during Cultivation on Waste Potato Juice Water and Glycerol towards Biosynthesis of Functional Polysaccharides. J. Biotechnol. 2018, 281, 1–10. [Google Scholar] [CrossRef]
- Klis, F.M.; Mol, P.; Hellingwerf, K.; Brul, S. Dynamics of Cell Wall Structure in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2002, 26, 239–256. [Google Scholar] [CrossRef]
- Ereifej, K.I.; Rababah, T.M.; Al-Rababah, M.A. Quality Attributes of Halva by Utilization of Proteins, Non-Hydrogenated Palm Oil, Emulsifiers, Gum Arabic, Sucrose, and Calcium Chloride. Int. J. Food Prop. 2005, 8, 415–422. [Google Scholar] [CrossRef]
- Herceg, Z.; Režek, A.; Lelas, V.; Krešić, G.; Franetović, M. Effect of Carbohydrates on the Emulsifying, Foaming and Freezing Properties of Whey Protein Suspensions. J. Food Eng. 2007, 79, 279–286. [Google Scholar] [CrossRef]
- Li, J.; Karboune, S.; Sedman, J.; Ismail, A. Characterization of the Structural Properties of Mannoproteins Isolated from Selected Yeast-Based Products upon the Enzymatic Treatment. LWT 2020, 131, 109596. [Google Scholar] [CrossRef]
- Cottet, C.; Ramirez-Tapias, Y.A.; Delgado, J.F.; De la Osa, O.; Salvay, A.G.; Peltzer, M.A. Biobased Materials from Microbial Biomass and Its Derivatives. Materials 2020, 13, 1263. [Google Scholar] [CrossRef]
- Bzducha-Wróbel, A.; Kieliszek, M.; Błażejak, S. Chemical Composition of the Cell Wall of Probiotic and Brewer’s Yeast in Response to Cultivation Medium with Glycerol as a Carbon Source. Eur. Food Res. Technol. 2013, 237, 489–499. [Google Scholar] [CrossRef]
- Juszczyk, P.; Tomaszewska, L.; Kita, A.; Rymowicz, W. Biomass Production by Novel Strains of Yarrowia lipolytica Using Raw Glycerol, Derived from Biodiesel Production. Bioresour. Technol. 2013, 137, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Vidakovic, A.; Singh, A.; Dicksved, J.; Schnürer, A.; Lundh, T. Yarrowia lipolytica Yeast as a Dietary Supplement for Rainbow Trout (Oncorhynchus mykiss): Effects on Gut Microbiota, Health and Immunity. Aquaculture 2024, 590, 741065. [Google Scholar] [CrossRef]
- Das, S.; Zarur Coelho, M.A.; Amaral, P.F.F.; Sil, J. Development of Nutrient Media to Increase the Accumulation of Lipids without Genetic Modification of a Lipogenic Microorganism. RSC Adv. 2017, 7, 38149–38154. [Google Scholar] [CrossRef]
- Barani pour, S.; Jahanbin Sardroodi, J.; Rastkar Ebrahimzadeh, A.; Pazuki, G. Investigation the Effect of Water Addition on Intermolecular Interactions of Fatty Acids-Based Deep Eutectic Solvents by Molecular Dynamics Simulations. Sci. Rep. 2023, 13, 7433. [Google Scholar] [CrossRef]
- Jach, M.E.; Malm, A. Yarrowia lipolytica as an Alternative and Valuable Source of Nutritional and Bioactive Compounds for Humans. Molecules 2022, 27, 2300. [Google Scholar] [CrossRef]
- Fu, W.; Zhao, G.; Liu, J. Effect of Preparation Methods on Physiochemical and Functional Properties of Yeast β-Glucan. LWT 2022, 160, 113284. [Google Scholar] [CrossRef]
- de Carvalho, W.T.; Soares Júnior, M.S.; Caliari, M.; da Silva, F.A.; de Oliveira Ribeiro, K. Physicochemical and Functional Characteristics of Residual Pulp of Potato. Food Sci. Technol. 2016, 36, 570–576. [Google Scholar] [CrossRef]
- He, C.A.; Qi, J.R.; Liao, J.S.; Song, Y.T.; Wu, C.L. Excellent Hydration Properties and Oil Holding Capacity of Citrus Fiber: Effects of Component Variation and Microstructure. Food Hydrocoll. 2023, 144, 108988. [Google Scholar] [CrossRef]
- Sales, J.C.S.; Botelho, A.M.; Carvalho, A.S.S.; Giudicelli, L.; de Castro, A.M.; Ribeiro, B.D.; Amaral, P.F.F.; Coelho, M.A.Z. Evaluation of Yarrowia lipolytica Potential for the Biodegradation of Poly (Ethylene Terephthalate) (PET) from Mooring Lines of Oil & Gas Offshore Platforms. Clean. Chem. Eng. 2023, 7, 100109. [Google Scholar] [CrossRef]
- Bankar, A.V.; Kumar, A.R.; Zinjarde, S.S. Removal of Chromium (VI) Ions from Aqueous Solution by Adsorption onto Two Marine Isolates of Yarrowia lipolytica. J. Hazard. Mater. 2009, 170, 487–494. [Google Scholar] [CrossRef]
- Makri, A.; Fakas, S.; Aggelis, G. Metabolic Activities of Biotechnological Interest in Yarrowia lipolytica Grown on Glycerol in Repeated Batch Cultures. Bioresour. Technol. 2010, 101, 2351–2358. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Su, L.; Liu, Q.; Zhu, Y.; Dai, Z.; Wang, Q. Dissecting carbon metabolism of Yarrowia lipolytica type strain W29 using genome-scale metabolic modelling. Comput. Struct. Biotechnol. J. 2022, 20, 2503–2511. [Google Scholar] [PubMed]
- Eder, S.; Zueblin, P.; Diener, M.; Peydayesh, M.; Boulos, S.; Mezzenga, R.; Nyström, L. Effect of Polysaccharide Conformation on Ultrafiltration Separation Performance. Carbohydr. Polym. 2021, 260, 117830. [Google Scholar] [CrossRef]
- Marson, G.V.; Pereira, D.T.V.; da Costa Machado, M.T.; Di Luccio, M.; Martinez, J.; Belleville, M.P.; Hubinger, M.D. Ultrafiltration performance of spent brewer’s yeast protein hydrolysate: Impact of pH and membrane material on fouling. J. Food Eng. 2021, 302, 110569. [Google Scholar] [CrossRef]
- Md Saleh, N.I.; Wan Ab Karim Ghani, W.A.; Mustapa Kamal, S.M.; Harun, R. Performance of Single and Two-Stage Cross-Flow Ultrafiltration Membrane in Fractionation of Peptide from Microalgae Protein Hydrolysate (Nannochloropsis gaditana). Processes 2021, 9, 610. [Google Scholar] [CrossRef]
- Yadav, J.S.S.; Yan, S.; Ajila, C.M.; Bezawada, J.; Tyagi, R.D.; Surampalli, R.Y. Food-Grade Single-Cell Protein Production, Characterization and Ultrafiltration Recovery of Residual Fermented Whey Proteins from Whey. Food Bioprod. Process. 2016, 99, 156–165. [Google Scholar] [CrossRef]
- Vukušić, J.L.; Millenautzki, T.; Sedaghati, M.; Schallenberg, M.; Müller, P.; Hof, J.; Mösche, M.; Barbe, S. Fractionation of Baker’s Yeast Vinasse via Ultrafiltration: Assessment of Feasibility. Int. J. Food Sci. Technol. 2019, 54, 1794–1803. [Google Scholar] [CrossRef]
- Gómez-Urios, C.; Viñas-Ospino, A.; Puchades-Colera, P.; Blesa, J.; López-Malo, D.; Frígola, A.; Esteve, M.J. Choline Chloride-Based Natural Deep Eutectic Solvents for the Extraction and Stability of Phenolic Compounds, Ascorbic Acid, and Antioxidant Capacity from Citrus Sinensis Peel. LWT 2023, 177, 114595. [Google Scholar] [CrossRef]
- Xu, N.; Chen, G.; Liu, H. Antioxidative Categorization of Twenty Amino Acids Based on Experimental Evaluation. Molecules 2017, 22, 2066. [Google Scholar] [CrossRef]
- Dumitrașcu, L.; Lanciu Dorofte, A.; Grigore-Gurgu, L.; Aprodu, I. Proteases as Tools for Modulating the Antioxidant Activity and Functionality of the Spent Brewer’s Yeast Proteins. Molecules 2023, 28, 3763. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).