Extraction and Characterization of High-Value Compounds from Yarrowia lipolytica W29 Using Sequential Hydrolysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Sequential Hydrolysis to Cell Wall Disruption
2.3. Extraction of Mannoprotein
2.4. Characterization of Mannoprotein
2.4.1. Proximal Composition
2.4.2. Surface Tension (ST) and Emulsification Index (EI)
2.4.3. Thermogravimetric Analysis (TGA)
2.4.4. Fourier Transform Infrared Spectroscopy (FTIR)
2.4.5. Optical Microscopy Analysis
2.4.6. Ultrafiltration Separation
2.4.7. Determination of Total Protein and Carbohydrate Content
2.4.8. Determination of Total Glucan, β-Glucan, and α-Glucan
2.4.9. Technological Features
2.4.10. Antioxidant Activity
2.4.11. Statistical Analysis
3. Results
3.1. Characterization of Mannoproteins
3.2. Characterization of Crude Biomass and the Food Ingredient
3.2.1. Chemical Composition of Crude Biomass and the Food Ingredients
3.2.2. Technological Properties of Crude Biomass and the Food Ingredient
3.2.3. Structural Characterization of Yarrowia lipolytica W29 Biomass and Food Ingredients
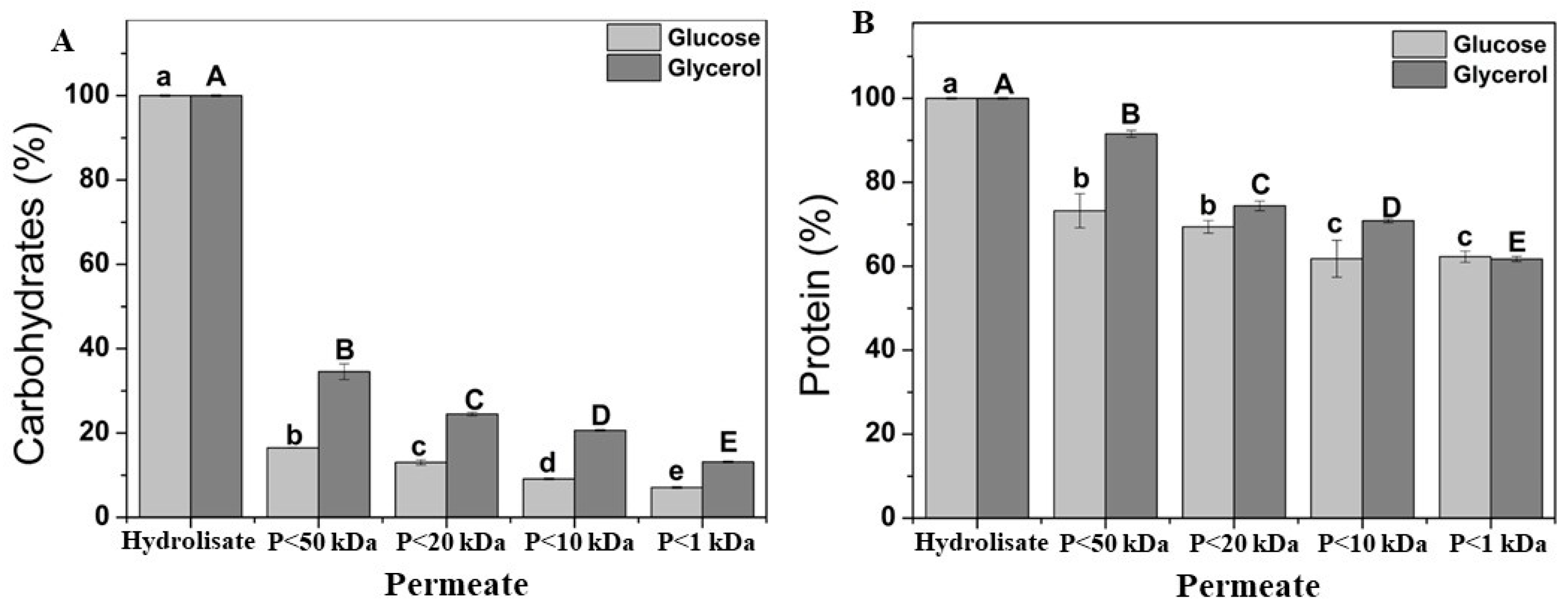
3.3. Ultrafiltration of Acid Hydrolysate
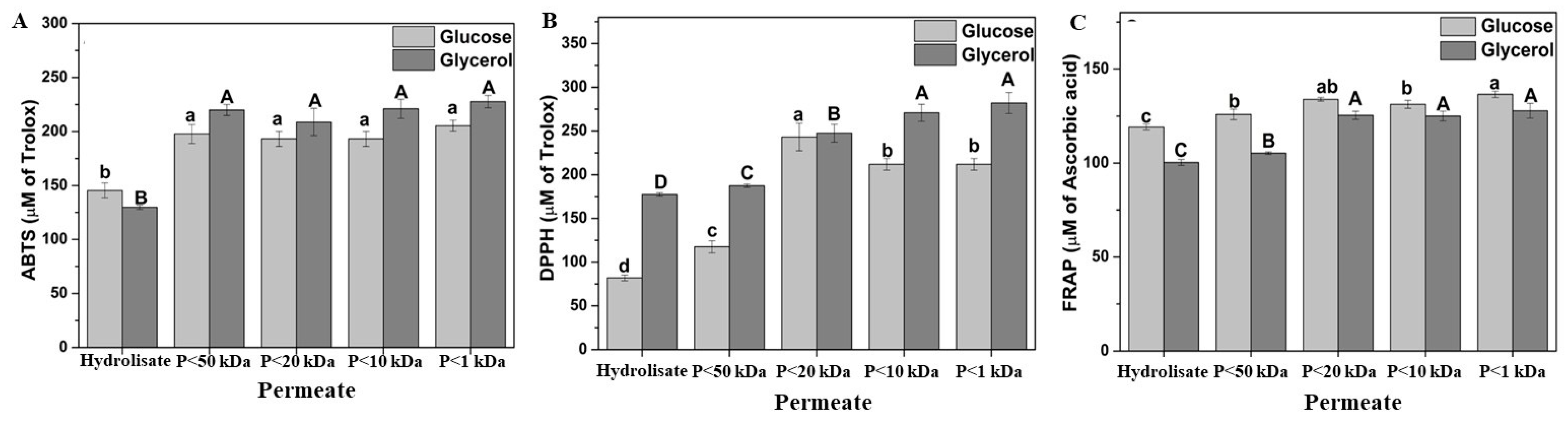
3.4. Antioxidant Property of the Acid Hydrolysate
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Martin, G.J.O.; Chan, S. Future Production of Yeast Biomass for Sustainable Proteins: A Critical Review. Sustain. Food Technol. 2024, 2, 1592–1609. [Google Scholar] [CrossRef]
- Wani, A.K.; Akhtar, N.; Sher, F.; Navarrete, A.A.; Américo-Pinheiro, J.H.P. Microbial Adaptation to Different Environmental Conditions: Molecular Perspective of Evolved Genetic and Cellular Systems. Arch. Microbiol. 2022, 204, 144. [Google Scholar] [CrossRef] [PubMed]
- Berzosa, A.; Marín-Sánchez, J.; Delso, C.; Sanz, J.; Álvarez, I.; Sánchez-Gimeno, C.; Raso, J. Sequential Extraction Optimization of Compounds of Interest from Spent Brewer’s Yeast Biomass Treated by Pulsed Electric Fields. Innov. Food Sci. Emerg. Technol. 2024, 94, 103705. [Google Scholar] [CrossRef]
- Yu, S.; Sun, J.; Shi, Y.; Wang, Q.; Wu, J.; Liu, J. Nanocellulose from various biomass wastes: Its preparation and potential usages towards the high value-added products. Environ. Sci. Ecotechnol. 2021, 5, 100077. [Google Scholar] [CrossRef]
- Reis, S.F.; Fernandes, P.A.R.; Martins, V.J.; Gonçalves, S.; Ferreira, L.P.; Gaspar, V.M.; Figueira, D.; Castelo-Branco, D.; Mano, J.F.; Coimbra, M.A.; et al. Brewer’s Spent Yeast Cell Wall Polysaccharides as Vegan and Clean Label Additives for Mayonnaise Formulation. Molecules 2023, 28, 3540. [Google Scholar] [CrossRef]
- Pereyra, C.M.; Gil, S.; Cristofolini, A.; Bonci, M.; Makita, M.; Monge, M.P.; Montenegro, M.A.; Cavaglieri, L.R. The Production of Yeast Cell Wall Using an Agroindustrial Waste Influences the Wall Thickness and Is Implicated on the Aflatoxin B1 Adsorption Process. Food Res. Int. 2018, 111, 306–313. [Google Scholar] [CrossRef]
- Gautério, G.V.; Silvério, S.I.D.C.; Egea, M.B.; Lemes, A.C. β-Glucan from Brewer’s Spent Yeast as a Techno-Functional Food Ingredient. Front. Food Sci. Technol. 2022, 2, 1074505. [Google Scholar] [CrossRef]
- Luo, J.; Chen, D.; Mao, X.; He, J.; Yu, B.; Cheng, L.; Zeng, D. Purified β-Glucans of Different Molecular Weights Enhance Growth Performance of LPS-Challenged Piglets via Improved Gut Barrier Function and Microbiota. Animals 2019, 9, 602. [Google Scholar] [CrossRef]
- Afroz, M.M.; Kashem, M.N.H.; Piash, K.M.P.S.; Islam, N. Saccharomyces cerevisiae as an Untapped Source of Fungal Chitosan for Antimicrobial Action. Appl. Biochem. Biotechnol. 2021, 193, 3765–3786. [Google Scholar] [CrossRef]
- Huq, T.; Khan, A.; Brown, D.; Dhayagude, N.; He, Z.; Ni, Y. Sources, Production and Commercial Applications of Fungal Chitosan: A Review. J. Bioresour. Bioprod. 2022, 7, 85–98. [Google Scholar] [CrossRef]
- Martínez, J.M.; Cebrián, G.; Álvarez, I.; Raso, J. Release of Mannoproteins during Saccharomyces cerevisiae Autolysis Induced by Pulsed Electric Field. Front. Microbiol. 2016, 7, 1435. [Google Scholar] [CrossRef]
- Faustino, M.; Durão, J.; Pereira, C.F.; Pintado, M.E.; Carvalho, A.P. Mannans and mannan oligosaccharides (MOS) from Saccharomyces cerevisiae–A sustainable source of functional ingredients. Carbohydr. Polym. 2021, 272, 118467. [Google Scholar] [PubMed]
- Singh, S.; Singh, G.; Arya, S.K. Mannans: An overview of properties and applications in food products. Int. J. Biol. Macromol. 2018, 119, 79–95. [Google Scholar] [PubMed]
- Michalik, B.; Biel, W.; Lubowicki, R.; Jacyno, E. Chemical Composition and Biological Value of Proteins of the Yeast Yarrowia lipolytica Growing on Industrial Glycerol. Can. J. Anim. Sci. 2014, 94, 99–104. [Google Scholar] [CrossRef]
- Nicaud, J.M. Yarrowia lipolytica . Yeast 2012, 29, 409–418. [Google Scholar]
- Łukaszewicz, M.; Leszczyński, P.; Jabłoński, S.J.; Kawa-Rygielska, J. Potential Applications of Yeast Biomass Derived from Small-Scale Breweries. Appl. Sci. 2024, 14, 2529. [Google Scholar] [CrossRef]
- da Silva, L.V.; Coelho, M.A.Z.; Amaral, P.F.F.; Fickers, P. A Novel Osmotic Pressure Strategy to Improve Erythritol Production by Yarrowia lipolytica from Glycerol. Bioprocess Biosyst. Eng. 2018, 41, 1883–1886. [Google Scholar] [CrossRef]
- Buarque, F.S.; Ribeiro, B.D.; Freire, M.G.; Coelho, M.A.Z.; Pereira, M.M. Assessing the Role of Deep Eutectic Solvents in Yarrowia lipolytica Inhibition. J. Biotechnol. 2025, 398, 1–10. [Google Scholar] [CrossRef]
- Dias, B.; Lopes, M.; Fernandes, H.; Marques, S.; Gírio, F.; Belo, I. Biomass and microbial lipids production by Yarrowia lipolytica W29 from eucalyptus bark hydrolysate. Renew. Energy 2024, 224, 120173. [Google Scholar] [CrossRef]
- Pereira, A.S.; Belo, I.; Lopes, M. Enhancing Microbial Lipids Synthesis for Biodiesel Production by Y. lipolytica W29 from Volatile Fatty Acids: Two-Stage Batch Strategies. Appl. Sci. 2022, 12, 8614. [Google Scholar] [CrossRef]
- Gessler, N.N.; Ivanova, N.O.; Kokoreva, A.S.; Klein, O.I.; Isakova, E.P.; Deryabina, Y.I. The Physiological Adaptation Features of the Poly-Extremophilic Yeast Yarrowia lipolytica W29 During Long-Term Cultivation. Appl. Biochem. Microbiol. 2022, 58, 771–779. [Google Scholar]
- Marson, G.V.; Lacour, S.; Hubinger, M.D.; Belleville, M.P. Serial Fractionation of Spent Brewer’s Yeast Protein Hydrolysate by Ultrafiltration: A Peptide-Rich Product with Low RNA Content. J. Food Eng. 2022, 312, 110737. [Google Scholar] [CrossRef]
- da Silva, R.M.; Ribeiro, B.D.; Lemes, A.C.; Coelho, M.A.Z. Recovery of High-Value Compounds from Yarrowia lipolytica IMUFRJ 50682 Using Autolysis and Acid Hydrolysis. Processes 2024, 12, 1132. [Google Scholar] [CrossRef]
- Berzosa, A.; Delso, C.; Sanz, J.; Sánchez-Gimeno, C.; Raso, J. Sequential extraction of compounds of interest from yeast biomass assisted by pulsed electric fields. Front. Bioeng. Biotechnol. 2023, 11, 1197710. [Google Scholar]
- Kot, A.M.; Gientka, I.; Bzducha-Wróbel, A.; Błażejak, S.; Kurcz, A. Comparison of Simple and Rapid Cell Wall Disruption Methods for Improving Lipid Extraction from Yeast Cells. J. Microbiol. Methods 2020, 176, 105999. [Google Scholar] [CrossRef]
- Alavi, F.; Ciftci, O.N. Purification and Fractionation of Bioactive Peptides through Membrane Filtration: A Critical and Application Review. Trends Food Sci. Technol. 2023, 131, 118–128. [Google Scholar]
- Park, Y.; Kim, J.H.; Lee, H.S.; Jung, E.Y.; Lee, H.; Noh, D.O.; Suh, H.J. Thermal Stability of Yeast Hydrolysate as a Novel Anti-Obesity Material. Food Chem. 2013, 136, 316–321. [Google Scholar] [CrossRef]
- Takalloo, Z.; Nikkhah, M.; Nemati, R.; Jalilian, N.; Sajedi, R.H. Autolysis, plasmolysis and enzymatic hydrolysis of baker’s yeast (Saccharomyces cerevisiae): A comparative study. World J. Microbiol. Biotechnol. 2020, 36, 68. [Google Scholar]
- Gonçalves, F.A.G.; Colen, G.; Takahashi, J.A. Yarrowia lipolytica and its multiple applications in the biotechnological industry. Sci. World J. 2014, 2014, 476207. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar]
- McCleary, B.V.; Draga, A. Measurement of β-Glucan in Mushrooms and Mycelial Products. J. AOAC Int. 2016, 99, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Bertolo, A.P.; Biz, A.P.; Kempka, A.P.; Rigo, E.; Cavalheiro, D. Yeast (Saccharomyces cerevisiae): Evaluation of Cellular Disruption Processes, Chemical Composition, Functional Properties and Digestibility. J. Food Sci. Technol. 2019, 56, 3697–3706. [Google Scholar] [CrossRef] [PubMed]
- Maria do Socorro, M.R.; Alves, R.E.; de Brito, E.S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive Compounds and Antioxidant Capacities of 18 Non-Traditional Tropical Fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar]
- Bzducha-Wróbel, A.; Błażejak, S.; Kieliszek, M.; Pobiega, K.; Falana, K.; Janowicz, M. Modification of the Cell Wall Structure of Saccharomyces cerevisiae Strains during Cultivation on Waste Potato Juice Water and Glycerol towards Biosynthesis of Functional Polysaccharides. J. Biotechnol. 2018, 281, 1–10. [Google Scholar] [CrossRef]
- Klis, F.M.; Mol, P.; Hellingwerf, K.; Brul, S. Dynamics of Cell Wall Structure in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2002, 26, 239–256. [Google Scholar] [CrossRef]
- Ereifej, K.I.; Rababah, T.M.; Al-Rababah, M.A. Quality Attributes of Halva by Utilization of Proteins, Non-Hydrogenated Palm Oil, Emulsifiers, Gum Arabic, Sucrose, and Calcium Chloride. Int. J. Food Prop. 2005, 8, 415–422. [Google Scholar] [CrossRef]
- Herceg, Z.; Režek, A.; Lelas, V.; Krešić, G.; Franetović, M. Effect of Carbohydrates on the Emulsifying, Foaming and Freezing Properties of Whey Protein Suspensions. J. Food Eng. 2007, 79, 279–286. [Google Scholar] [CrossRef]
- Li, J.; Karboune, S.; Sedman, J.; Ismail, A. Characterization of the Structural Properties of Mannoproteins Isolated from Selected Yeast-Based Products upon the Enzymatic Treatment. LWT 2020, 131, 109596. [Google Scholar] [CrossRef]
- Cottet, C.; Ramirez-Tapias, Y.A.; Delgado, J.F.; De la Osa, O.; Salvay, A.G.; Peltzer, M.A. Biobased Materials from Microbial Biomass and Its Derivatives. Materials 2020, 13, 1263. [Google Scholar] [CrossRef]
- Bzducha-Wróbel, A.; Kieliszek, M.; Błażejak, S. Chemical Composition of the Cell Wall of Probiotic and Brewer’s Yeast in Response to Cultivation Medium with Glycerol as a Carbon Source. Eur. Food Res. Technol. 2013, 237, 489–499. [Google Scholar] [CrossRef]
- Juszczyk, P.; Tomaszewska, L.; Kita, A.; Rymowicz, W. Biomass Production by Novel Strains of Yarrowia lipolytica Using Raw Glycerol, Derived from Biodiesel Production. Bioresour. Technol. 2013, 137, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Vidakovic, A.; Singh, A.; Dicksved, J.; Schnürer, A.; Lundh, T. Yarrowia lipolytica Yeast as a Dietary Supplement for Rainbow Trout (Oncorhynchus mykiss): Effects on Gut Microbiota, Health and Immunity. Aquaculture 2024, 590, 741065. [Google Scholar] [CrossRef]
- Das, S.; Zarur Coelho, M.A.; Amaral, P.F.F.; Sil, J. Development of Nutrient Media to Increase the Accumulation of Lipids without Genetic Modification of a Lipogenic Microorganism. RSC Adv. 2017, 7, 38149–38154. [Google Scholar] [CrossRef]
- Barani pour, S.; Jahanbin Sardroodi, J.; Rastkar Ebrahimzadeh, A.; Pazuki, G. Investigation the Effect of Water Addition on Intermolecular Interactions of Fatty Acids-Based Deep Eutectic Solvents by Molecular Dynamics Simulations. Sci. Rep. 2023, 13, 7433. [Google Scholar] [CrossRef]
- Jach, M.E.; Malm, A. Yarrowia lipolytica as an Alternative and Valuable Source of Nutritional and Bioactive Compounds for Humans. Molecules 2022, 27, 2300. [Google Scholar] [CrossRef]
- Fu, W.; Zhao, G.; Liu, J. Effect of Preparation Methods on Physiochemical and Functional Properties of Yeast β-Glucan. LWT 2022, 160, 113284. [Google Scholar] [CrossRef]
- de Carvalho, W.T.; Soares Júnior, M.S.; Caliari, M.; da Silva, F.A.; de Oliveira Ribeiro, K. Physicochemical and Functional Characteristics of Residual Pulp of Potato. Food Sci. Technol. 2016, 36, 570–576. [Google Scholar] [CrossRef]
- He, C.A.; Qi, J.R.; Liao, J.S.; Song, Y.T.; Wu, C.L. Excellent Hydration Properties and Oil Holding Capacity of Citrus Fiber: Effects of Component Variation and Microstructure. Food Hydrocoll. 2023, 144, 108988. [Google Scholar] [CrossRef]
- Sales, J.C.S.; Botelho, A.M.; Carvalho, A.S.S.; Giudicelli, L.; de Castro, A.M.; Ribeiro, B.D.; Amaral, P.F.F.; Coelho, M.A.Z. Evaluation of Yarrowia lipolytica Potential for the Biodegradation of Poly (Ethylene Terephthalate) (PET) from Mooring Lines of Oil & Gas Offshore Platforms. Clean. Chem. Eng. 2023, 7, 100109. [Google Scholar] [CrossRef]
- Bankar, A.V.; Kumar, A.R.; Zinjarde, S.S. Removal of Chromium (VI) Ions from Aqueous Solution by Adsorption onto Two Marine Isolates of Yarrowia lipolytica. J. Hazard. Mater. 2009, 170, 487–494. [Google Scholar] [CrossRef]
- Makri, A.; Fakas, S.; Aggelis, G. Metabolic Activities of Biotechnological Interest in Yarrowia lipolytica Grown on Glycerol in Repeated Batch Cultures. Bioresour. Technol. 2010, 101, 2351–2358. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Su, L.; Liu, Q.; Zhu, Y.; Dai, Z.; Wang, Q. Dissecting carbon metabolism of Yarrowia lipolytica type strain W29 using genome-scale metabolic modelling. Comput. Struct. Biotechnol. J. 2022, 20, 2503–2511. [Google Scholar] [PubMed]
- Eder, S.; Zueblin, P.; Diener, M.; Peydayesh, M.; Boulos, S.; Mezzenga, R.; Nyström, L. Effect of Polysaccharide Conformation on Ultrafiltration Separation Performance. Carbohydr. Polym. 2021, 260, 117830. [Google Scholar] [CrossRef]
- Marson, G.V.; Pereira, D.T.V.; da Costa Machado, M.T.; Di Luccio, M.; Martinez, J.; Belleville, M.P.; Hubinger, M.D. Ultrafiltration performance of spent brewer’s yeast protein hydrolysate: Impact of pH and membrane material on fouling. J. Food Eng. 2021, 302, 110569. [Google Scholar] [CrossRef]
- Md Saleh, N.I.; Wan Ab Karim Ghani, W.A.; Mustapa Kamal, S.M.; Harun, R. Performance of Single and Two-Stage Cross-Flow Ultrafiltration Membrane in Fractionation of Peptide from Microalgae Protein Hydrolysate (Nannochloropsis gaditana). Processes 2021, 9, 610. [Google Scholar] [CrossRef]
- Yadav, J.S.S.; Yan, S.; Ajila, C.M.; Bezawada, J.; Tyagi, R.D.; Surampalli, R.Y. Food-Grade Single-Cell Protein Production, Characterization and Ultrafiltration Recovery of Residual Fermented Whey Proteins from Whey. Food Bioprod. Process. 2016, 99, 156–165. [Google Scholar] [CrossRef]
- Vukušić, J.L.; Millenautzki, T.; Sedaghati, M.; Schallenberg, M.; Müller, P.; Hof, J.; Mösche, M.; Barbe, S. Fractionation of Baker’s Yeast Vinasse via Ultrafiltration: Assessment of Feasibility. Int. J. Food Sci. Technol. 2019, 54, 1794–1803. [Google Scholar] [CrossRef]
- Gómez-Urios, C.; Viñas-Ospino, A.; Puchades-Colera, P.; Blesa, J.; López-Malo, D.; Frígola, A.; Esteve, M.J. Choline Chloride-Based Natural Deep Eutectic Solvents for the Extraction and Stability of Phenolic Compounds, Ascorbic Acid, and Antioxidant Capacity from Citrus Sinensis Peel. LWT 2023, 177, 114595. [Google Scholar] [CrossRef]
- Xu, N.; Chen, G.; Liu, H. Antioxidative Categorization of Twenty Amino Acids Based on Experimental Evaluation. Molecules 2017, 22, 2066. [Google Scholar] [CrossRef]
- Dumitrașcu, L.; Lanciu Dorofte, A.; Grigore-Gurgu, L.; Aprodu, I. Proteases as Tools for Modulating the Antioxidant Activity and Functionality of the Spent Brewer’s Yeast Proteins. Molecules 2023, 28, 3763. [Google Scholar] [CrossRef]
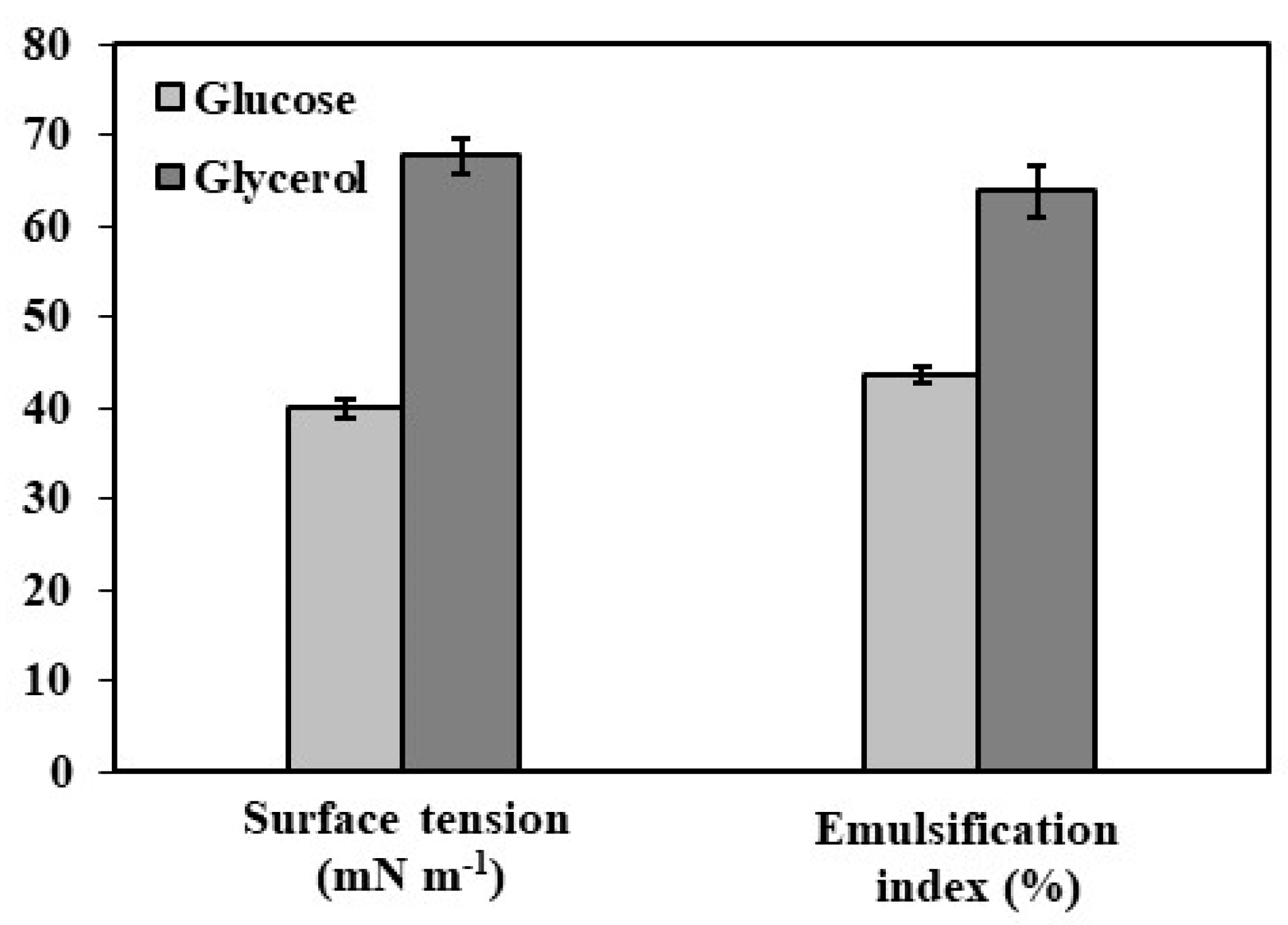
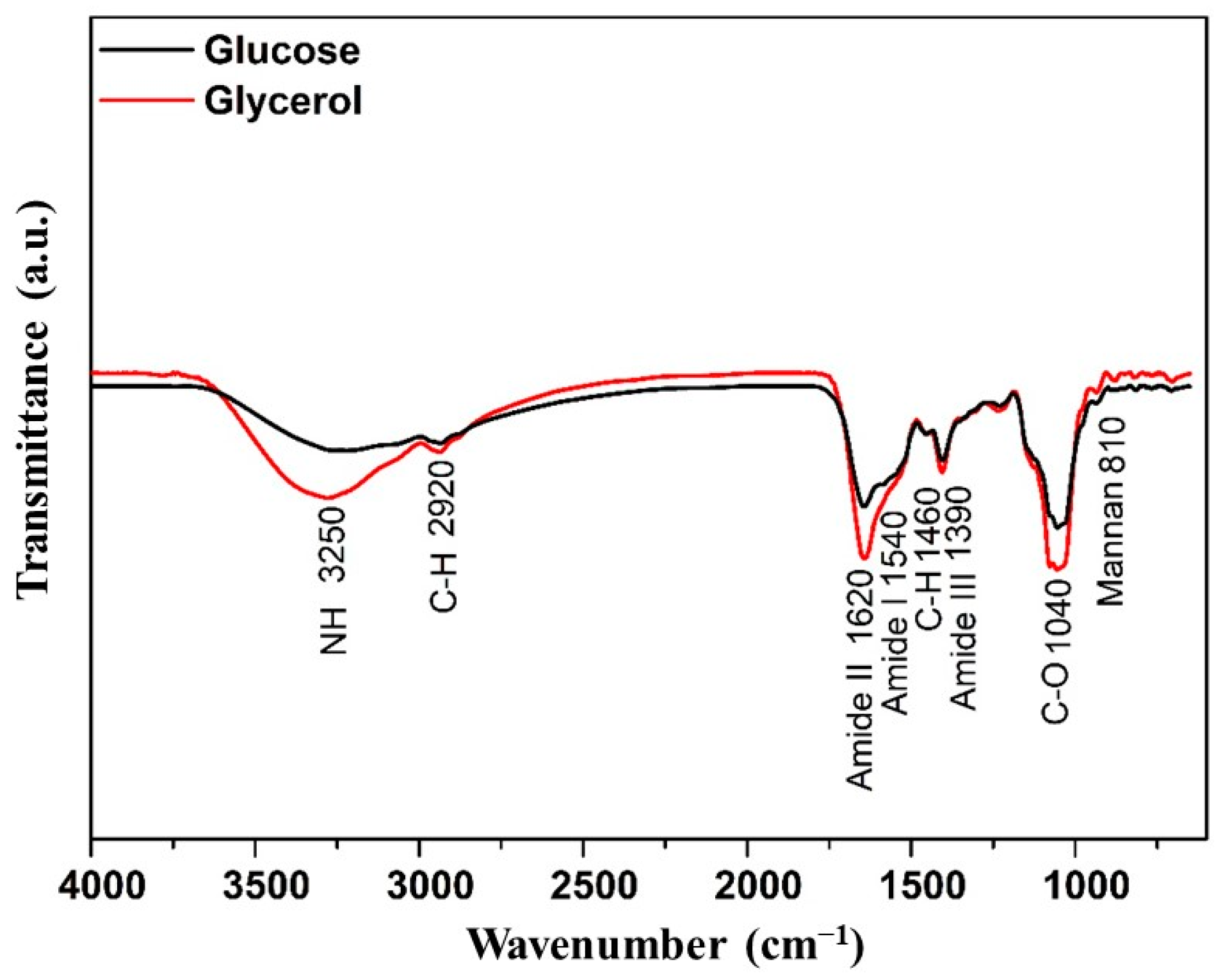
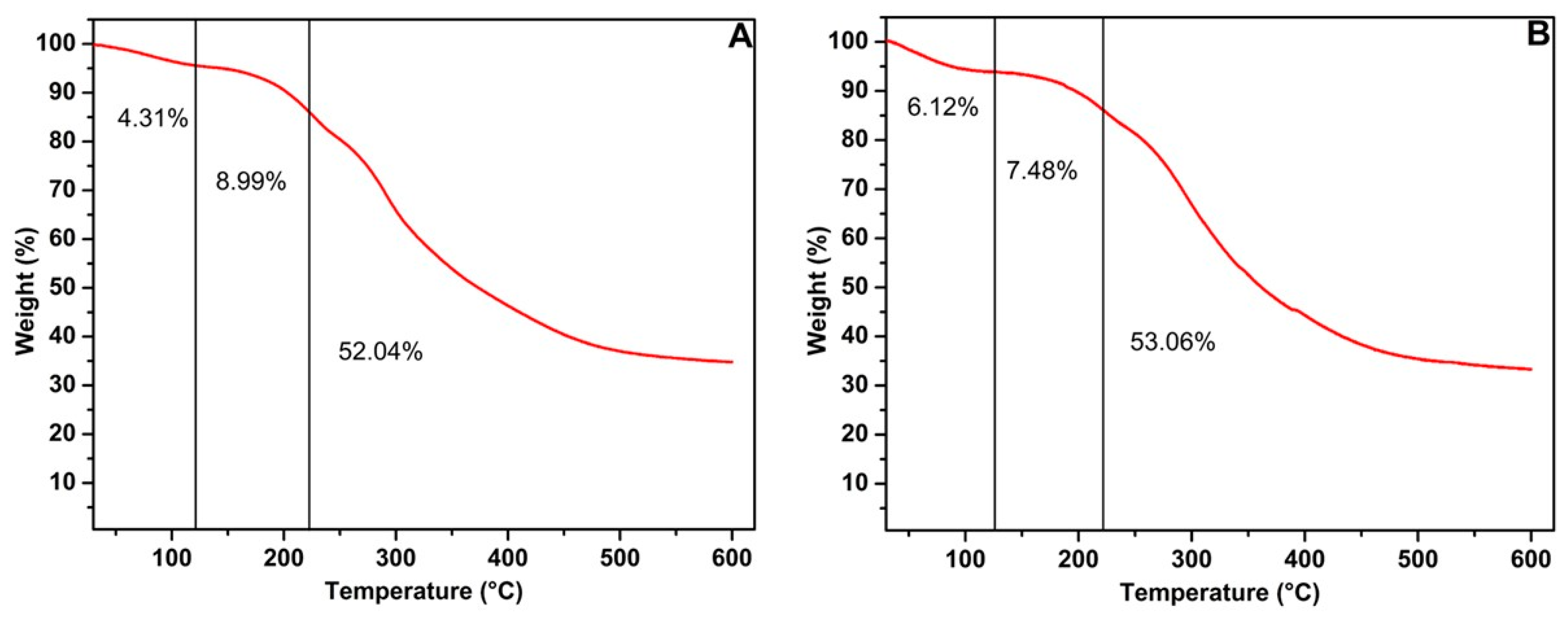
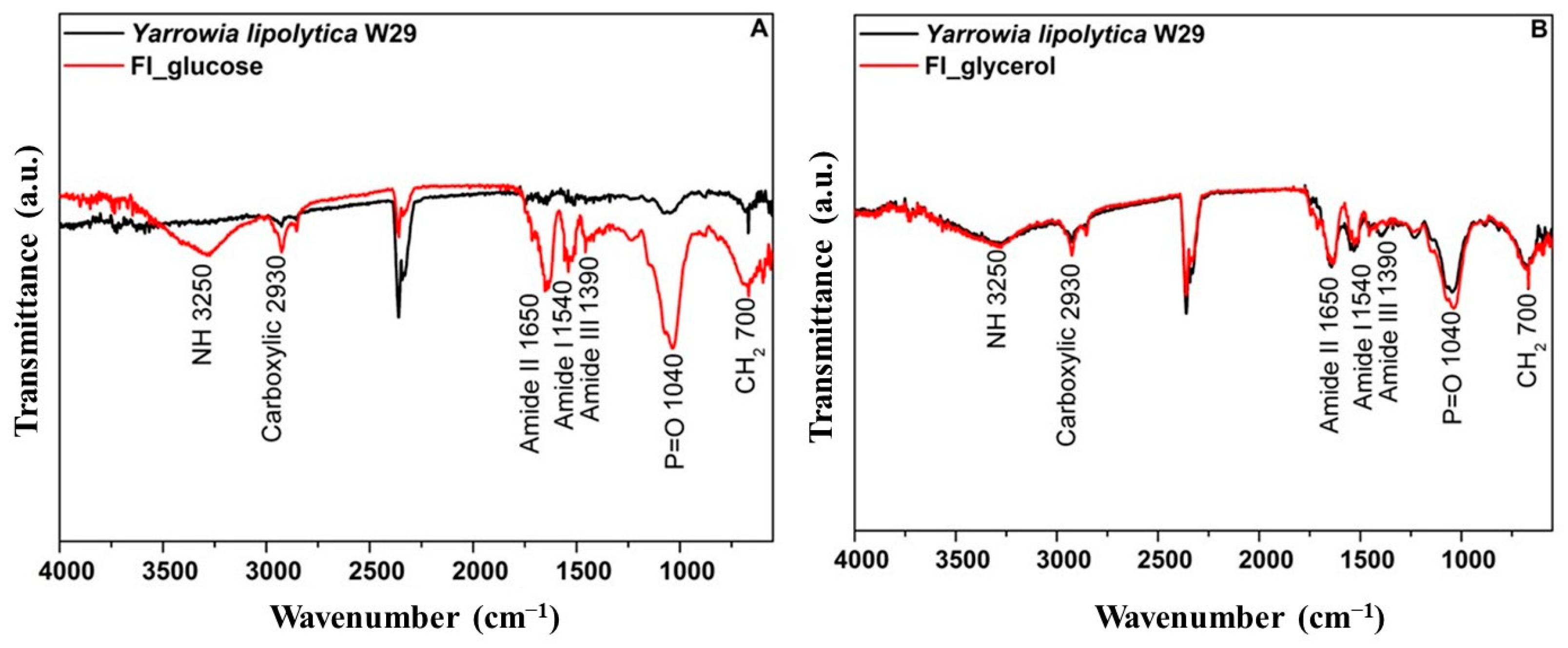

| Parameter | Glucose | Glycerol |
|---|---|---|
| Extraction Yield (%) | 13.15 ± 0.22 a | 11.71 ± 0.29 b |
| Total Carbohydrate (g L−1) | 10.26 ± 0.05 b | 13.47 ± 0.09 a |
| Total Protein (g L−1) | 26.46 ± 0.85 b | 30.35 ± 1.80 a |
| Parameter | Glucose | Glycerol | ||
|---|---|---|---|---|
| Biomass (%) | Food Ingredient (%) | Biomass (%) | Food Ingredient (%) | |
| Moisture | 4.65 ± 0.11 b | 5.56 ± 0.36 a | 4.55 ± 0.34 bc | 4.27 ± 0.09 c |
| Total soluble solids | 95.35 ± 0.11 ab | 94.44 ± 0.36 c | 95.45 ± 0.34 ab | 95.73 ± 0.09 a |
| α-glucan | 2.05 ± 0.00 c | 1.96 ± 0.05 d | 2.96 ± 0.01 a | 2.76 ± 0.03 b |
| β-glucan | 17.07 ± 0.11 b | 16.16 ± 0.31 c | 18.70 ± 0.04 a | 14.02 ± 0.69 d |
| Total glucan | 19.12 ± 0.12 b | 16.16 ± 0.31 c | 21.66 ± 0.05 a | 16.78 ± 0.67 c |
| Ash | 5.76 ± 0.24 a | 1.68 ± 0.07 c | 5.79 ± 0.12 a | 1.84 ± 0.04 b |
| Carbohydrate | 32.35 ± 0.15 c | 46.68 ± 0.07 b | 28.50 ± 0.38 d | 48.00± 0.38 a |
| Protein | 50.83 ± 0.65 b | 43.33 ± 0.18 c | 53.66 ± 0.11 a | 42.64 ± 0.38 d |
| Lipid | 6.42 ± 0.36 b | 2.75 ± 0.17 d | 7.50 ± 0.31 a | 3.25 ± 0.12 c |
| Caloric value (kcal) | 390.45 | 384.80 | 396.16 | 391.77 |
| Parameter | Glucose | Glycerol | ||
|---|---|---|---|---|
| Biomass (%) | Food Ingredient (%) | Biomass (%) | Food Ingredient (%) | |
| Process yield | 47.70 ± 1.87 a | - | 42.82 ± 1.74 b | - |
| WHC | 6.87 ± 0.26 a | 5.59 ± 0.41 b | 4.78 ± 0.18 c | 5.35 ± 0.18 b |
| WSI | 24.64 ± 0.34 b | 9.93 ± 0.52 b | 25.50 ± 0.02 a | 10.60 ± 0.50 b |
| OHC | 3.03 ± 0.02 c | 3.26 ± 0.07 b | 2.68 ± 0.02 d | 3.40 ± 0.02 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, R.M.; da Silva, C.N.; de Faria-Júnior, C.S.; Buarque, F.S.; Ribeiro, B.D.; Lemes, A.C.; Coelho, M.A.Z. Extraction and Characterization of High-Value Compounds from Yarrowia lipolytica W29 Using Sequential Hydrolysis. Processes 2025, 13, 615. https://doi.org/10.3390/pr13030615
da Silva RM, da Silva CN, de Faria-Júnior CS, Buarque FS, Ribeiro BD, Lemes AC, Coelho MAZ. Extraction and Characterization of High-Value Compounds from Yarrowia lipolytica W29 Using Sequential Hydrolysis. Processes. 2025; 13(3):615. https://doi.org/10.3390/pr13030615
Chicago/Turabian Styleda Silva, Rhonyele Maciel, Cristiane Nunes da Silva, Célio Santos de Faria-Júnior, Filipe Smith Buarque, Bernardo Dias Ribeiro, Ailton Cesar Lemes, and Maria Alice Zarur Coelho. 2025. "Extraction and Characterization of High-Value Compounds from Yarrowia lipolytica W29 Using Sequential Hydrolysis" Processes 13, no. 3: 615. https://doi.org/10.3390/pr13030615
APA Styleda Silva, R. M., da Silva, C. N., de Faria-Júnior, C. S., Buarque, F. S., Ribeiro, B. D., Lemes, A. C., & Coelho, M. A. Z. (2025). Extraction and Characterization of High-Value Compounds from Yarrowia lipolytica W29 Using Sequential Hydrolysis. Processes, 13(3), 615. https://doi.org/10.3390/pr13030615








