Preparation and Application of Multifunctional Chitosan–Polyvinyl Alcohol–Nanosilver–Chrysanthemum Extract Composite Gel
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CTS/PVA/Ag/CHR Gel
2.3. Characterization of CTS/PVA/Ag/CHR Gel
2.4. Bacteriostatic Test
2.5. Effect of Ag+ and CTS Concentration on CTS/PVA/Ag/CHR Gel Preparation and Gel Preparation Optimization
2.6. Effect of CTS/PVA/Ag/CHR Gel Dose and Reaction Time on Antimicrobial Properties
2.7. Reusability and Stability of CTS/PVA/Ag/CHR Gels
2.8. Milk Preservation Test
2.9. Cytotoxicity Test
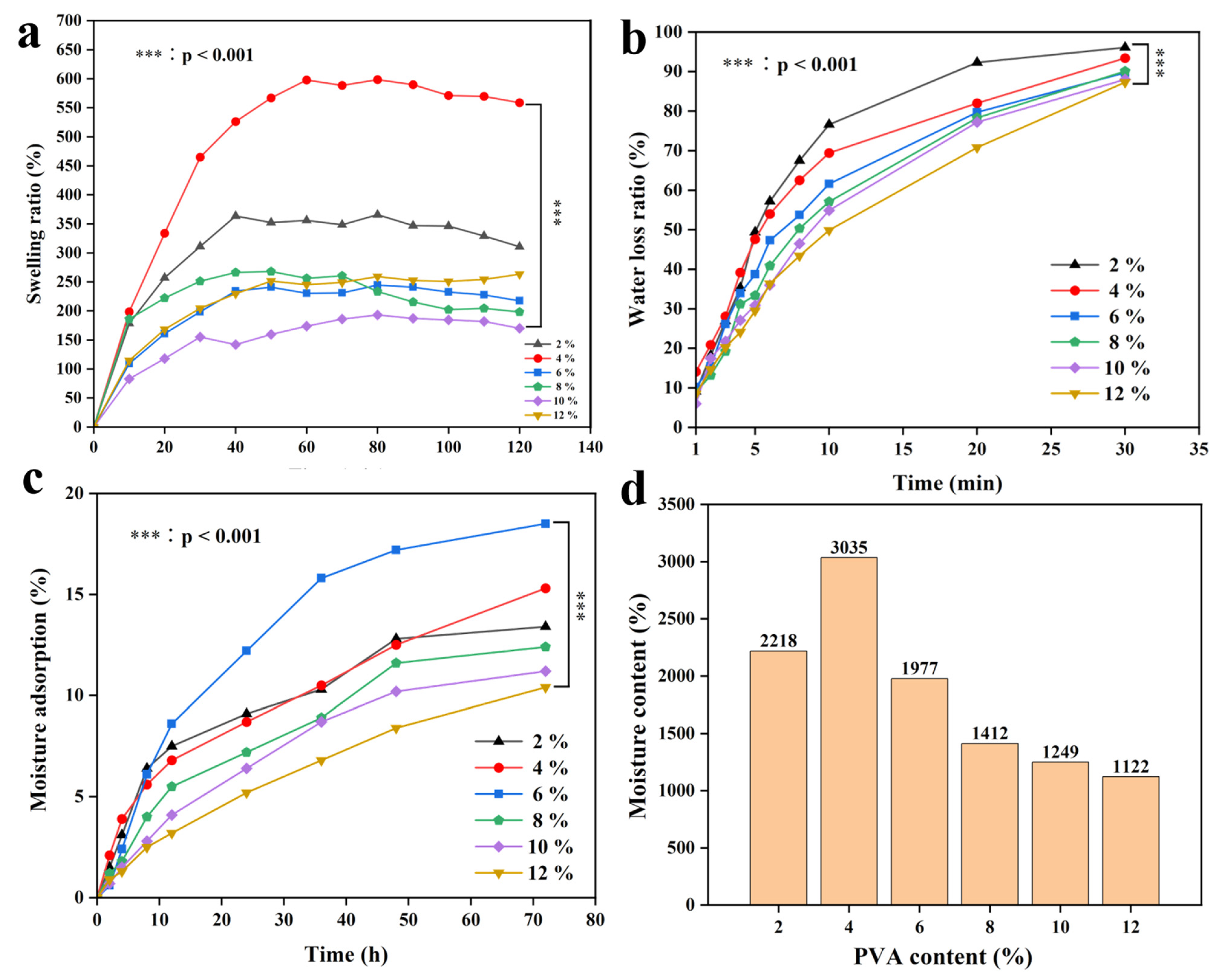
2.10. Swelling Ratio, Water Loss, Moisture Adsorption, and Moisture Content of CTS/PVA/Ag/CHR Gels
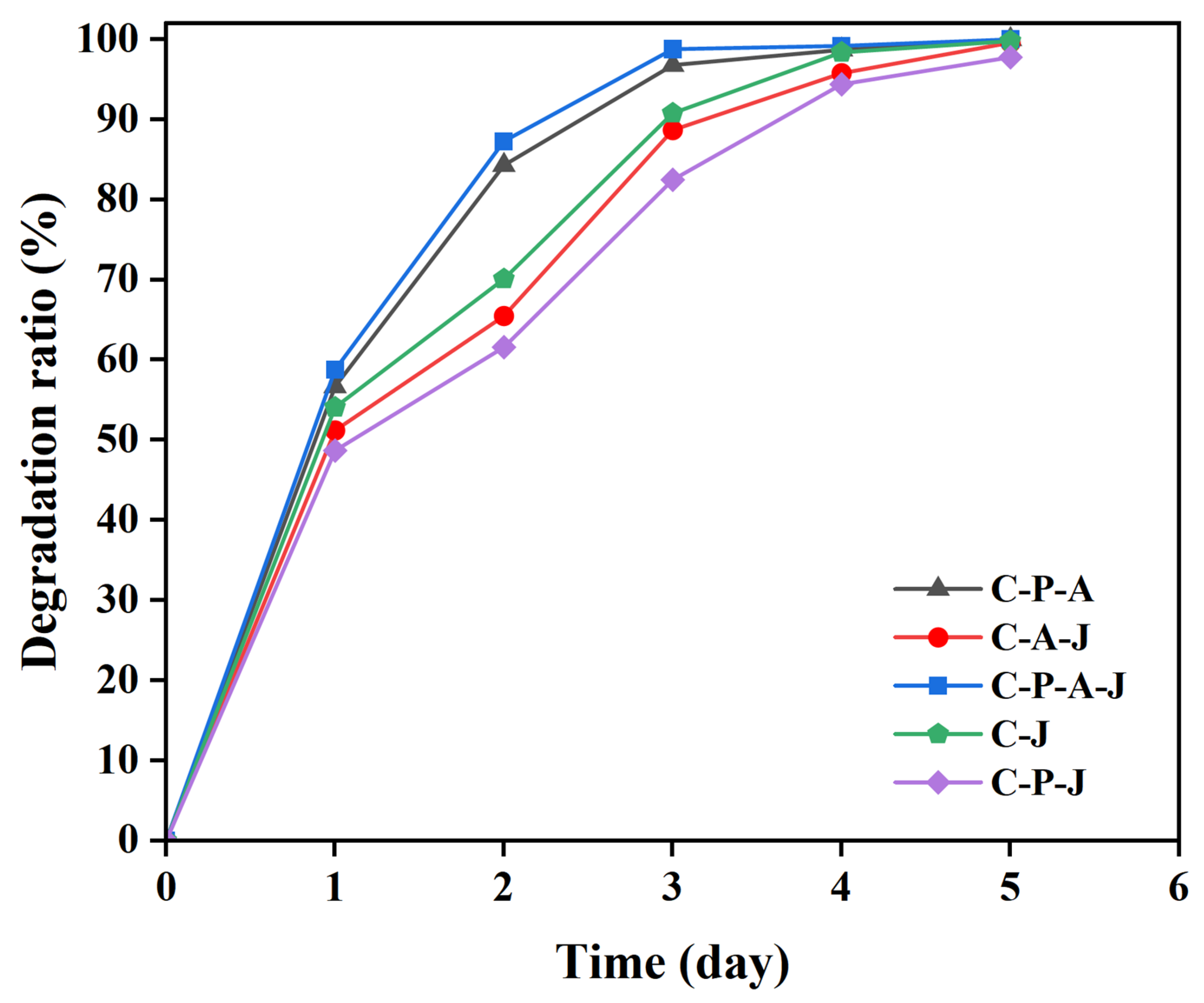
2.11. Degradation Characteristics of CTS/PVA/Ag/CHR Gels
2.12. Adsorption of Dyes onto CTS/PVA/Ag/CHR Gels
2.13. Statistical Analysis
3. Results and Discussion
3.1. Characterization of CTS/PVA/Ag/CHR Gels
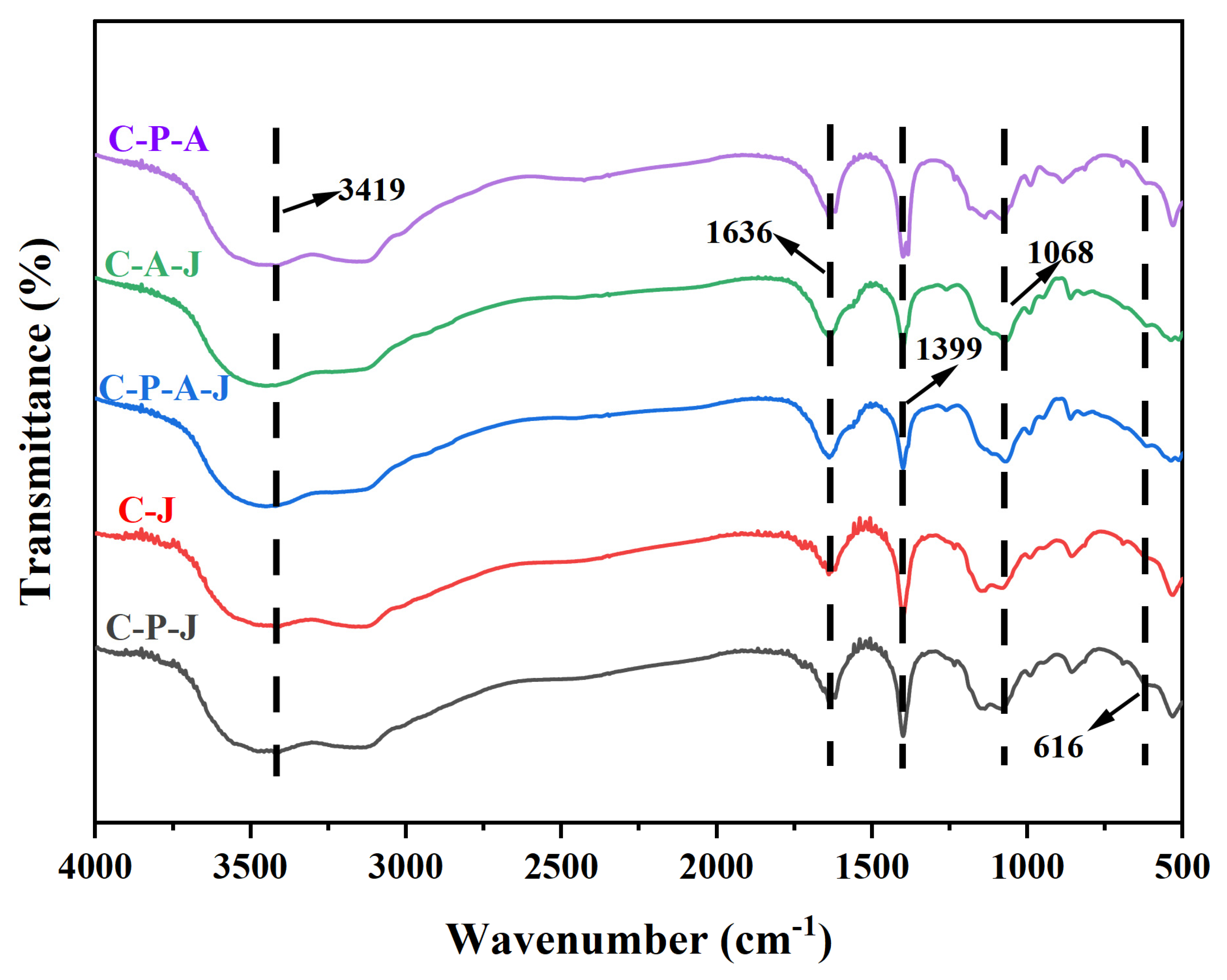
3.1.1. Fourier-Transform Infrared (FT-IR) Spectrometry Analysis
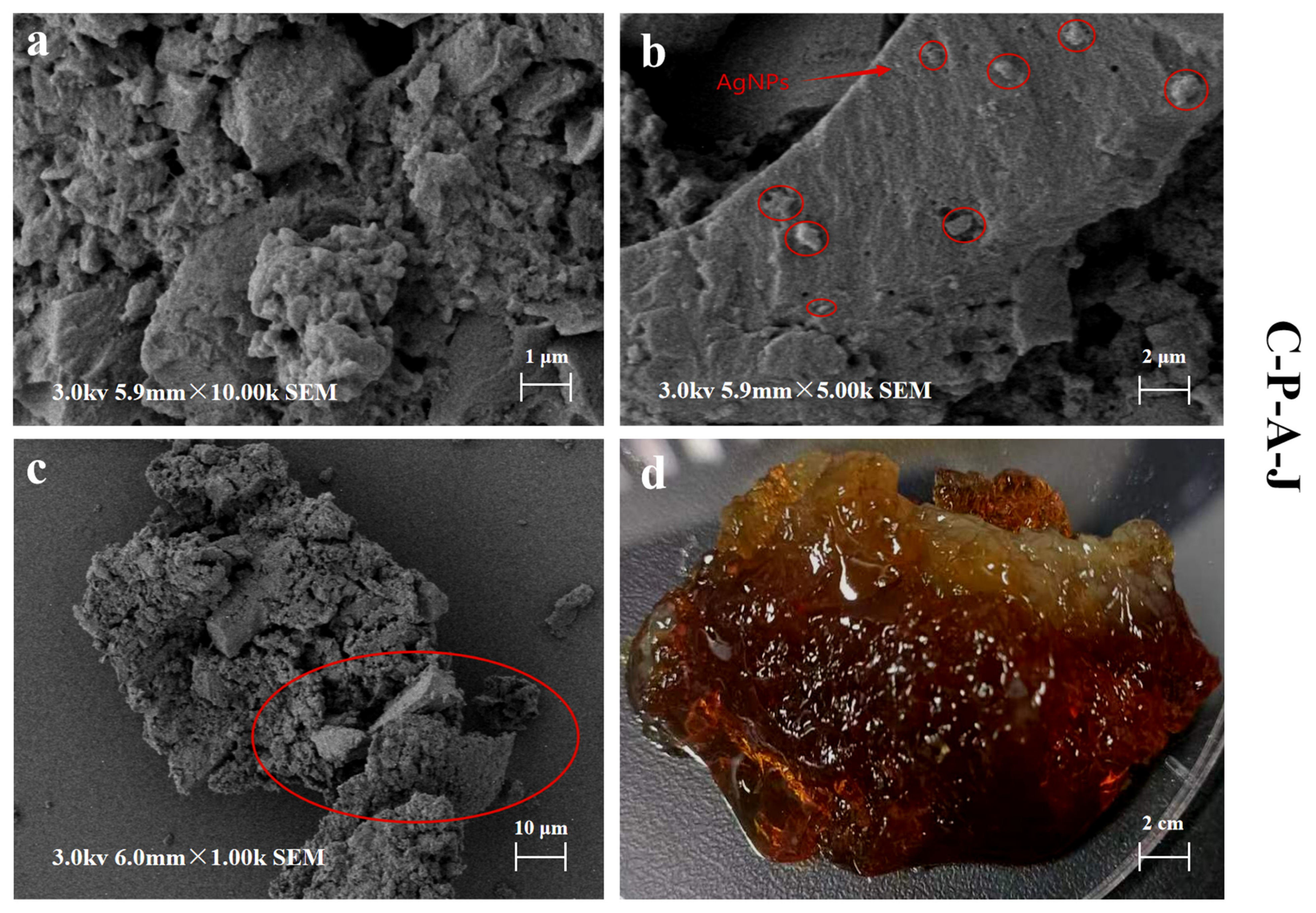
3.1.2. SEM Analysis
3.1.3. XRD Analysis
3.2. Antibacterial Characteristics
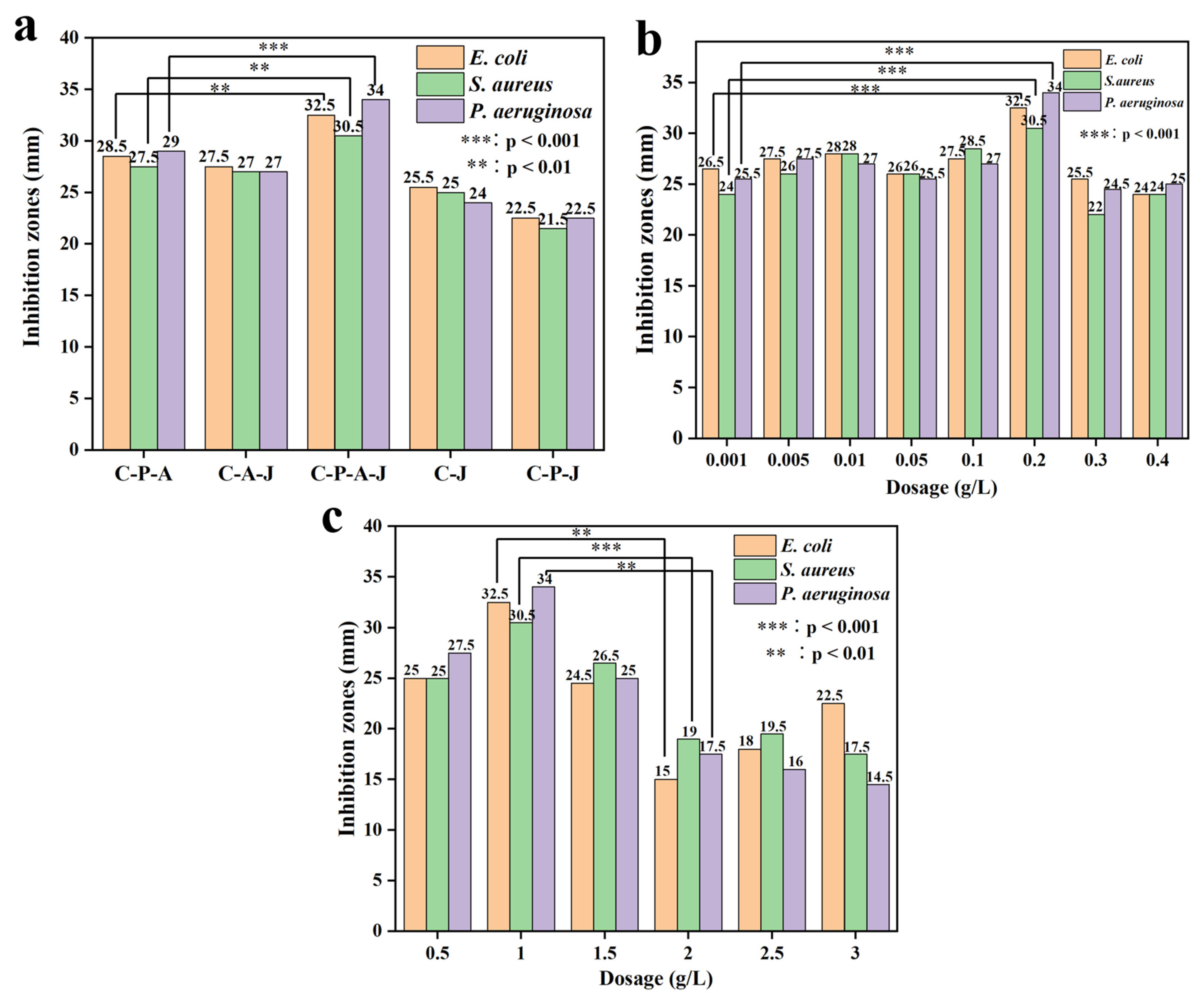
3.2.1. Enhancement of Gel Methods of Preparation and the Impact of CTS and Ag+ Dose on Antibacterial Efficacy in CTS/PVA/Ag/CHR Gels
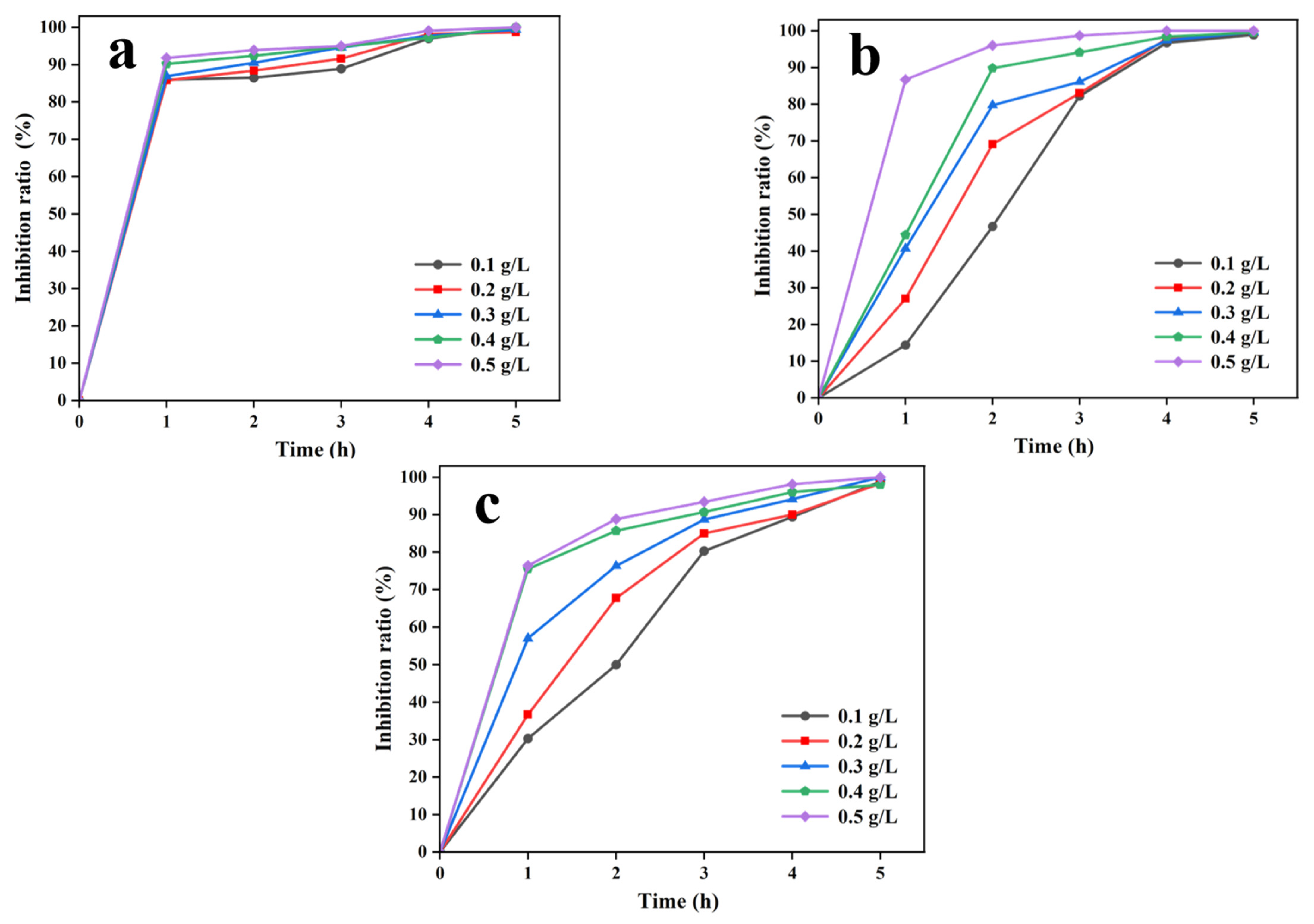
3.2.2. Impact of Incubation Duration and CTS/PVA/Ag/CHR Gel Dosage
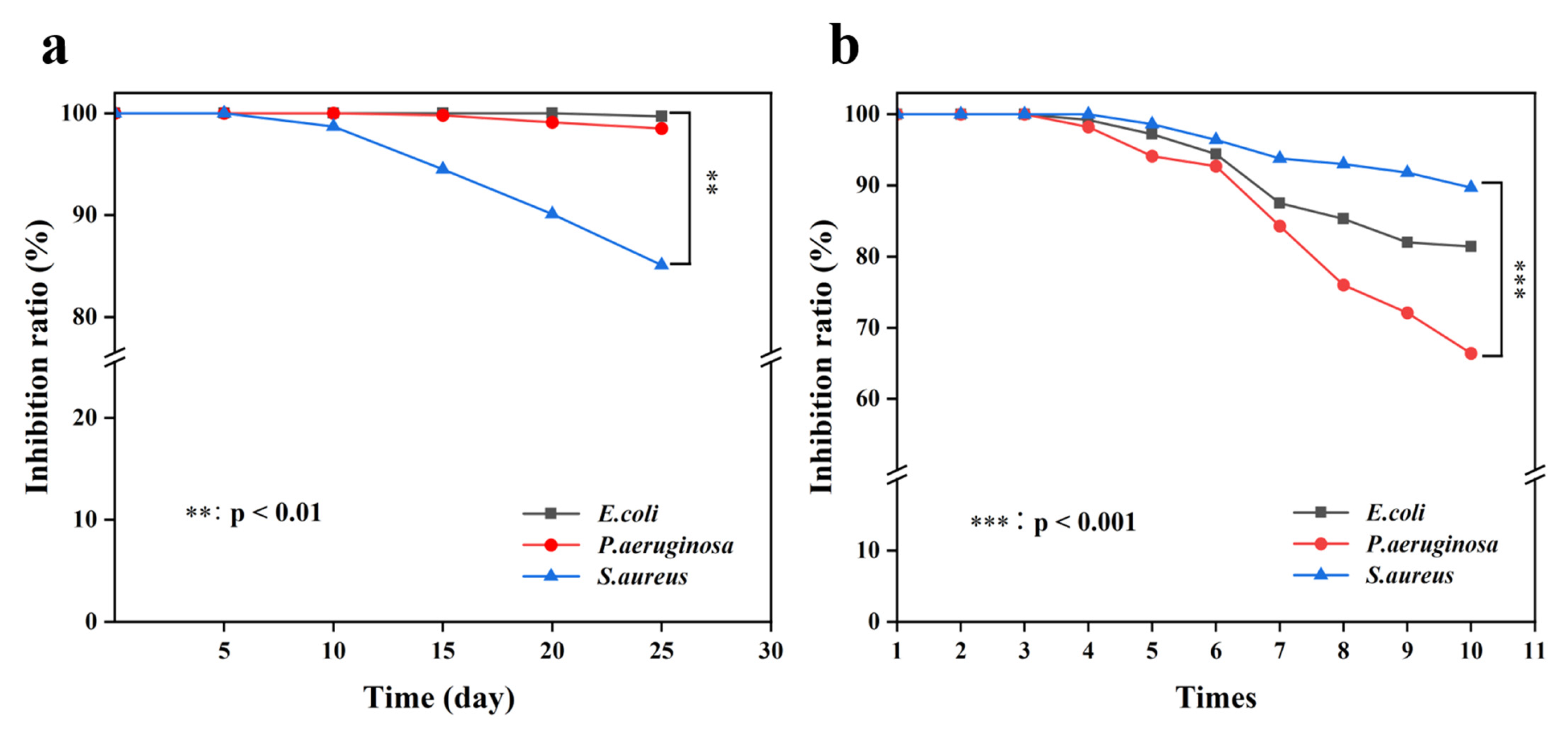
3.2.3. Stability and Reusability of CTS/PVA/Ag/CHR Gels
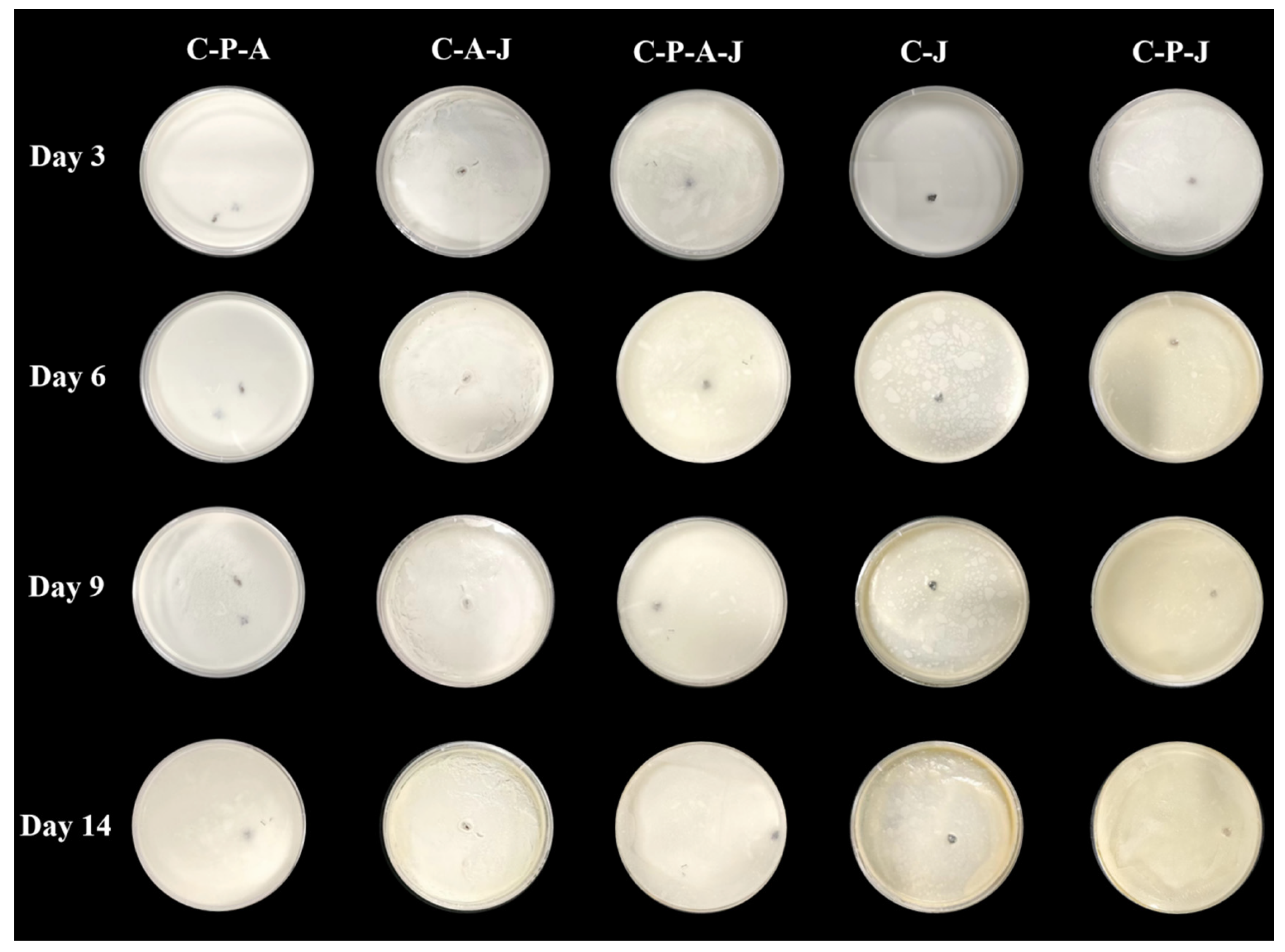
3.3. Preservation Applications of Complex Gels
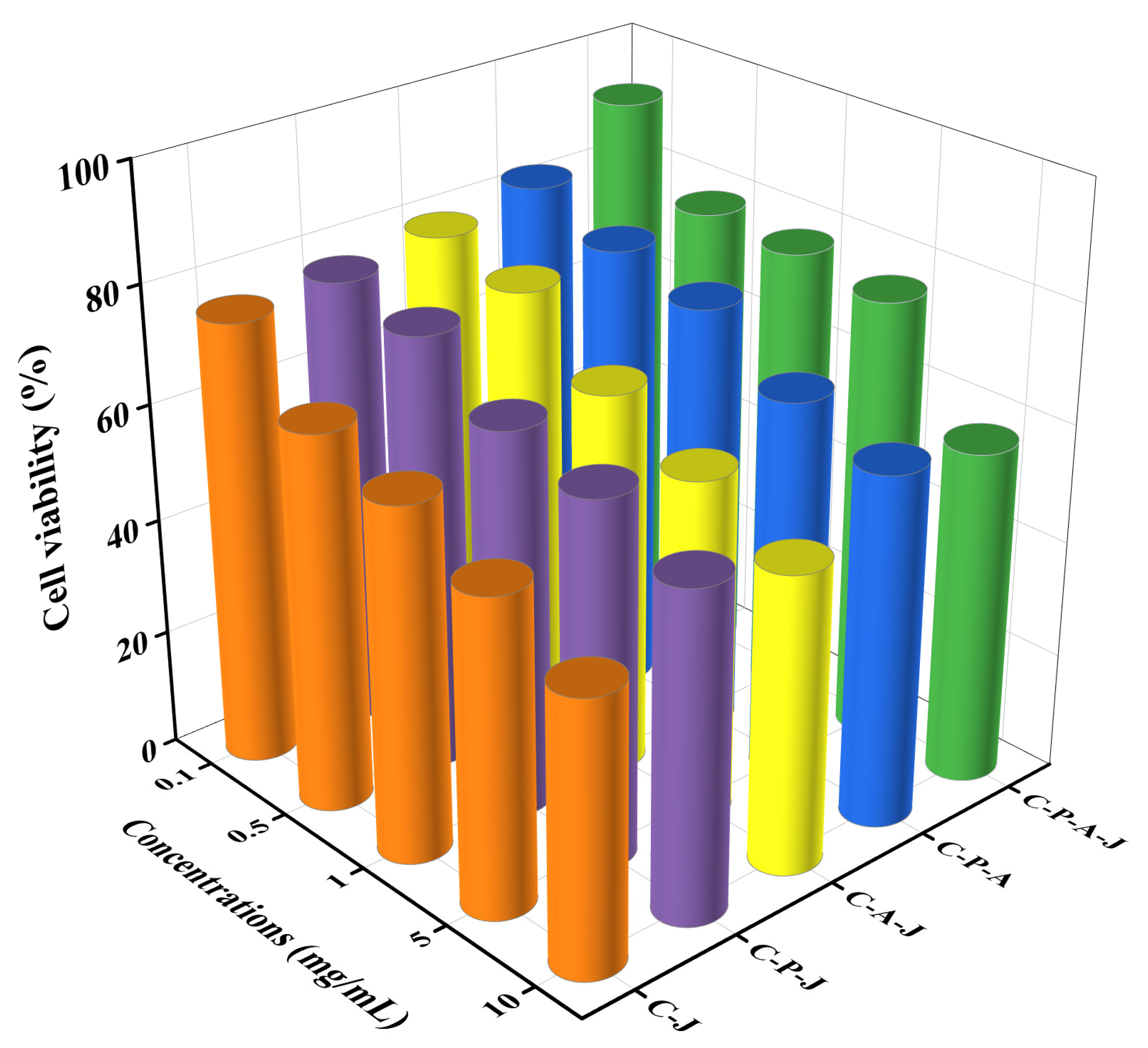
3.4. Cytotoxicity of Complex Gels
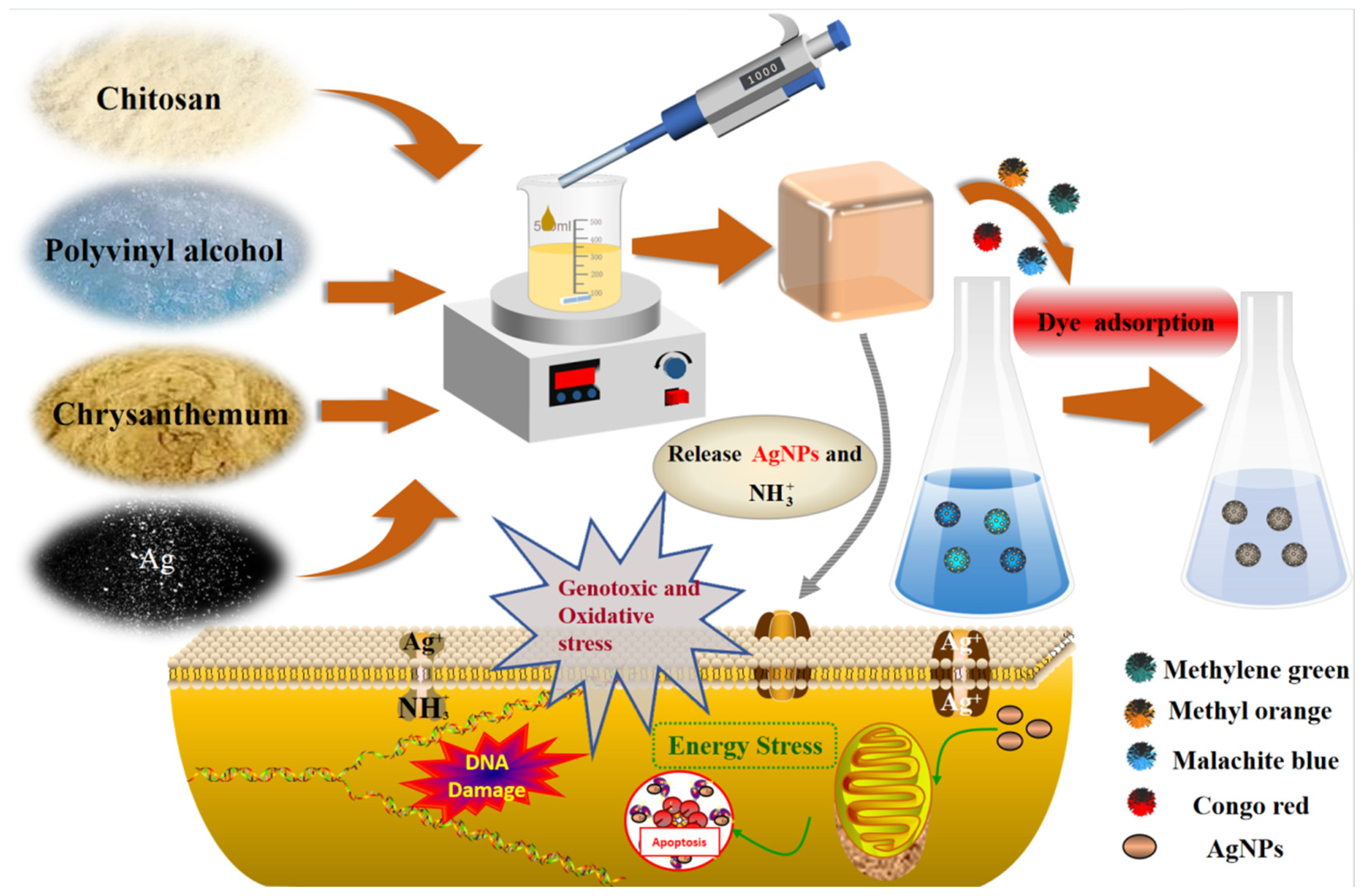
3.5. Synergistic Antimicrobial Mechanism of CTS/PVA/Ag/CHR Gel
3.6. Swelling Ratio, Moisture Content, and Moisture Adsorption of CTS/PVA/Ag/CHR Gel
3.7. CTS/PVA/Ag/CHR Gel Degradation
3.8. CTS/PVA/Ag/CHR Gel Dye Adsorption Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, F.; Liao, S.; Li, W.; Jiang, C.; Wei, Q.; Xia, X.; Wang, Q. Amphiphilic sodium alginate-polylysine hydrogel with high antibacterial efficiency in a wide pH range. Carbohydr. Polym. 2023, 299, 120195. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Jiang, B.; Chen, J.; Jin, Z. Blend-modification of soy protein/lauric acid edible films using polysaccharides. Food Chem. 2014, 151, 1–6. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, T.T.; Huang, M.; Zhang, M.; He, Y.C. Preparation of dandelion flower extract-based polyvinyl alcohol-chitosan-dandelion-CuNPs composite gel for efficient bacteriostatic and dye adsorption. In. J. Biol. Macromol. 2024, 281, 136512. [Google Scholar] [CrossRef] [PubMed]
- Su, X.R.; Xue, Y.Y.; Li, Q. Advances in the research of antibacterial properties of silver ion and its application in wound treatment. Zhonghua Shao Shang Za Zhi = Zhonghua Shaoshang Zazhi = Chin. J. Burn. 2018, 34, 183–186. [Google Scholar]
- Fuhrmann, P.L.; Powell, J.; Rousseau, D. Structure and rheology of oil-continuous capillary suspensions containing water-swellable cellulose beads and fibres. Food Hydrocoll. 2023, 139, 108503. [Google Scholar] [CrossRef]
- Kumaraswamy, S.; Patil, S.L.; Mallaiah, S.H. In vitro biocompatibility evaluation of radiolytically synthesized silver/polyvinyl hydrogel nanocomposites for wound dressing applications. J. Bioact. Compat. Polym. 2020, 35, 435–450. [Google Scholar] [CrossRef]
- Yang, Z.; Li, K.R.; Zhang, Y.Y.; Hu, J.L.; Li, T.Y.; Weng, Z.X.; Wu, L.X. Antibiotic Silver Particles Coated Graphene Oxide/polyurethane Nanocomposites Foams and Its Mechanical Properties. Chin. J. Struct. Chem. 2022, 41, 2203125–2203131. [Google Scholar]
- Qin, Z.; Zheng, Y.; Wang, Y.; Du, T.; Li, C.; Wang, X.; Jiang, H. Versatile roles of silver in Ag-based nanoalloys for antibacterial applications. Coord. Chem. Rev. 2021, 449, 214218. [Google Scholar] [CrossRef]
- Sassi, A.B.; Harzallah-Skhiri, F.; Bourgougnon, N.; Aouni, M. Antimicrobial activities of four Tunisian Chrysanthemum species. Indian. J. Med. Res. 2008, 127, 183–192. [Google Scholar] [PubMed]
- Ma, G.; Qian, B.; Yang, J.; Hu, C.; Nie, J. Synthesis and properties of photosensitive chitosan derivatives. Int. J. Biol. Macromol. 2010, 46, 558–561. [Google Scholar] [CrossRef]
- Yang, S.F.; Wen, Y.; Yi, P.; Xiao, K.; Dong, C.F. Effects of chitosan inhibitor on the electrochemical corrosion behavior of 2205 duplex stainless steel. Int. J. Miner. Metall. Mater. 2017, 24, 1260–1266. [Google Scholar] [CrossRef]
- Yang, D.; Gong, L.; Li, Q.; Fan, B.; Ma, C.L.; He, Y.C. Preparation of a biobased polyelectrolyte complex from chitosan and sodium carboxymethyl cellulose and its antibacterial characteristics. Int. J. Biol. Macromol. 2023, 227, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Lu, F.; Zhou, G.; Yu, K.; Lu, B.; Xiao, Y.; Dai, F.; Wu, D.; Lan, G. Silver Inlaid with Gold Nanoparticle/Chitosan Wound Dressing Enhances Antibacterial Activity and Porosity, and Promotes Wound Healing. Biomacromolecules 2017, 18, 3766–3775. [Google Scholar] [CrossRef] [PubMed]
- Sivararnan, A.; Ganti, S.S.; Nguyen, H.X.; Birk, G.; Wieber, A.; Lubda, D.; Banga, A.K. Development and evaluation of a polyvinyl alcohol based topical gel. J. Drug Deliv. Sci. Technol. 2017, 39, 210–216. [Google Scholar] [CrossRef]
- Ingle, J.; Patel, U.D. Electrochemical reduction of nitrate in the presence of silver-coated polyvinyl alcohol beads as a spatially suspended catalyst. J. Water Process Eng. 2022, 49, 103082. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.; Zhao, Z.; Shi, X.; Zhang, R.; He, Y.; Zhang, H.; Wang, W. Magnesium-doped nano-hydroxyapatite/polyvinyl alcohol/chitosan composite hydrogel: Preparation, characterization. Int. J. Nanomed. 2024, 19, 651–671. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, R.; Espinosa-Andrews, H.; Velasquillo-Martinez, C.; Garcia-Carvajal, Z.Y. Composite hydrogels based on gelatin, chitosan and polyvinyl alcohol to biomedical applications: A review. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 1–20. [Google Scholar] [CrossRef]
- Kuila, S.B.; Ray, S.K. Dehydration of dioxane by pervaporation using filled blend membranes of polyvinyl alcohol and sodium alginate. Carbohydr. Polym. 2014, 101, 1154–1165. [Google Scholar] [CrossRef]
- Lewis, K.; Lee, R.E.; Broetz-Oesterhelt, H.; Hiller, S.; Rodnina, M.V.; Schneider, T.; Weingarth, M.; Wohlgemuth, I. Sophisticated natural products as antibiotics. Nature 2024, 632, 39–49. [Google Scholar] [CrossRef]
- Tuyen, N.V.; Ryu, J.H.; Yae, J.B.; Kim, H.G.; Hong, S.W.; Ahn, D.H. Nitrogen removal performance of anammox process with PVA–SA gel bead crosslinked with sodium sulfate as a biomass carrier. J. Ind. Eng. Chem. 2018, 67, 326–332. [Google Scholar] [CrossRef]
- Wang, S.W.; Dong, X.X.; Liu, M.T.; Ren, D.D.; Wang, T.T.; Chen, C.; Leng, Y.T.; Li, Q. High strength polyvinyl alcohol/polyacrylic acid-based hydrogels with multiple networks. Polym.-Plast. Technol. Mater. 2021, 60, 392–404. [Google Scholar] [CrossRef]
- Kim, Y.J.; Min, J. Property modulation of the alginate-based hydrogel via semi-interpenetrating polymer network (semi-IPN) with poly(vinyl alcohol). Int. J. Biol. Macromol. 2021, 193, 1068–1077. [Google Scholar] [CrossRef] [PubMed]
- Nagarpita, M.V.; Roy, P.; Shruthi, S.B.; Sailaja, R.R.N. Synthesis and swelling characteristics of chitosan and CMC grafted sodium acrylate-co-acrylamide using modified nanoclay and examining its efficacy for removal of dyes. Int. J. Biol. Macromol. 2017, 102, 1226–1240. [Google Scholar] [CrossRef]
- Velidandi, A.; Pabbathi, N.P.P.; Dahariya, S.; Baadhe, R.R. Green synthesis of novel Ag–Cu and Ag–Znbimetallic nanoparticles and their in vitro biological, eco-toxicity and catalytic studies. Nano-Struct. Nano-Objects 2021, 26, 100687. [Google Scholar] [CrossRef]
- Chen, Q.; Ma, J.; Hu, Y.; Fei, P. Multifunctional antibacterial films with silver nanoparticles reduced in situ by lemon juice. Food Chem. 2021, 365, 130517. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Tian, C.; Song, G.; Pan, Q.; Wang, Z.; Shi, L.; Qiao, Y.; Fu, H. A green route to synthesize novel Ag/C antibacterial agent. Mater. Res. Bull. 2012, 47, 458–463. [Google Scholar] [CrossRef]
- Zhang, Z.; He, Y. Synthesis and characteristics of a fish scale-based biochar–nanosilver antibacterial material. Processes 2023, 11, 1992. [Google Scholar] [CrossRef]
- Afshari, M.J.; Sabzi, M.; Jiang, L.; Behshad, Y.; Zanjanijam, A.R.; Mahdavinia, G.R.; Ahmadi, M. Incorporation of dynamicboronate links and Ag nanoparticles into PVA hydrogels for pH-Regulated and prolonged release of methotrexate. J. Drug Deliv. Sci. Technol. 2021, 63, 102502. [Google Scholar] [CrossRef]
- Bahrami, A.; Mokarram, R.R.; Khiabani, M.S.; Ghanbarzadeh, B.; Salehi, R. Physico-mechanical and antimicrobial properties of tragacanth/hydroxypropyl methylcellulose/beeswax edible films reinforced with silver nanoparticles. Int. J. Biol. Macromol. 2019, 129, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Salam, A.; Pawlak, J.J.; Venditti, R.A.; El-tahlawy, K. Synthesis and Characterization of Starch Citrate-Chitosan Foam with Superior Water and Saline Absorbance Properties. Biomacromolecules 2010, 11, 1453–1459. [Google Scholar] [CrossRef]
- Zhao, T.; Li, X.; Gong, Y.; Guo, Y.; Quan, F.; Shi, Q. Study on polysaccharide polyelectrolyte complex and fabrication of alginate/chitosan derivative composite fibers. Int. J. Biol. Macromol. 2021, 184, 181–187. [Google Scholar] [CrossRef]
- Huang, Z.H.; Sun, X.Y.; Li, Y.; Ge, W.; Wang, J.D. Adsorption behaviors of chitosan and the analysis of FTIR spectra. Spectrosc. Spectr. Anal. 2005, 25, 698–700. [Google Scholar]
- Ionita, M.; Pandele, M.A.; Iovu, H. Sodium alginate/graphene oxide composite films with enhanced thermal and mechanical properties. Carbohydr. Polym. 2013, 94, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Koneru, A.; Dharmalingam, K.; Anandalakshmi, R. Cellulose based nanocomposite hydrogel films consisting of sodium carboxymethylcellulose–grapefruit seed extract nanoparticles for potential wound healing applications. Int. J. Biol. Macromol. 2020, 148, 833–842. [Google Scholar] [CrossRef]
- Quadrado, R.F.N.; Gohlke, G.; Oliboni, R.S.; Smaniotto, A.; Fajardo, A.R. Hybrid hydrogels containing one-step biosynthesized silver nanoparticles: Preparation, characterization and catalytic application. J. Ind. Eng. Chem. 2019, 79, 326–337. [Google Scholar] [CrossRef]
- Cheng, Y.; Far, B.F.; Jahanbakhshi, M.; Bahrami, S.; Tamimi, P.; Sedaghat, M.; Ghazizadeha, E. Exploring the potential of a polyvinyl alcohol/chitosan-based nanofibrous matrix for erythromycin delivery: Fabrication, in vitro and in vivo evaluation. RSC Adv. 2023, 13, 18450–18460. [Google Scholar] [CrossRef]
- Rahaman, M.S.; Hasnine, S.M.M.; Ahmed, T.; Sultana, S.; Bhuiyan, M.A.Q.; Manir, M.S.; Ullah, N.; Sen, S.K.; Hossain, M.N.; Hossain, M.S.; et al. Radiation crosslinked polyvinyl alcohol/polyvinyl pyrrolidone/acrylic acid hydrogels: Swelling, crosslinking and dye adsorption study. Iran. Polym. J. 2021, 30, 1101–1116. [Google Scholar] [CrossRef]
- Spagnol, C.; Fragal, E.H.; Pereira, A.G.B.; Nakamura, C.V.; Muniz, E.C.; Follmann, H.D.M.; Silva, R.; Rubira, A.F. Cellulose nanowhiskers decorated with silver nanoparticles as an additive to antibacterial polymers membranes fabricated by electrospinning. J. Colloid. Interface Sci. 2018, 531, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Malik, M.M.; Qureshi, M.S. Study of synergistic effects of antibiotics and triangular shaped silver nanoparticles, synthesized using UV-light irradiation, on S. aureus and P. aeruginosa. Mater. Today Proc. 2019, 18, 920–927. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Y.; Zhang, J.; Liu, J.; Wang, A.; Ding, L. Recent development of copper- based nanozymes for biomedical applications. Adv. Healthc. Mater. 2024, 13, 2302023. [Google Scholar] [CrossRef] [PubMed]
- Zeng, A.R.; Wang, Y.T.; Li, D.J.; Guo, J.D.; Chen, Q.W. Preparation and antibacterial properties of polycaprolactone/quaternized chitosan blends. Chin. J. Chem. Eng. 2021, 32, 462–471. [Google Scholar] [CrossRef]
- Sun, N.; Wang, T.; Yan, X. Self-assembled supermolecular hydrogel based on hydroxyethyl cellulose: Formation, in vitro release and bacteriostasis application. Carbohydr. Polym. 2017, 172, 49–59. [Google Scholar] [CrossRef]
- Chen, G.; He, L.; Zhang, P.; Zhang, J.; Mei, X.; Wang, D.; Zhang, Y.; Ren, X.; Chen, Z. Encapsulation of green tea polyphenol nanospheres in PVA/alginate hydrogel for promoting wound healing of diabetic rats by regulating PI3K/AKT pathway. Mater. Sci. Eng. C-Mater. Biol. Appl. 2020, 110, 110686. [Google Scholar] [CrossRef] [PubMed]
- Troïa, T.; Siad, J.; Di Giorgio, C.; Brunel, J.M. Design and synthesis of new polyamine quinoline antibiotic enhancers to fight resistant gram-negative P. aeruginosa bacteria. Eur. J. Med. Chem. Rep. 2022, 5, 100054. [Google Scholar] [CrossRef]
- Kumar, M.; Kaushik, D.; Kumar, A.; Gupta, P.; Proestos, C.; Oz, E.; Orhan, E.; Kaur, J.; Khan, M.R.; Elobeid, T.; et al. Green synthesis of copper nanoparticles from Nigella sativa seed extract and evaluation of their antibacterial and antiobesity activity. Int. J. Food Sci. Technol. 2023, 58, 2883–2892. [Google Scholar] [CrossRef]
- Gasti, T.; Hiremani, V.D.; Kesti, S.S.; Vanjeri, V.N.; Goudar, N.; Masti, S.P.; Thimmappa, S.C.; Chougale, R.B. Physicochemical and antibacterial evaluation of poly (vinyl alcohol)/guar gum/silver nanocomposite films for food packaging applications. J. Polym. Environ. 2021, 29, 3347–3363. [Google Scholar] [CrossRef]
- Bugatti, V.; Zuppardi, F.; Viscusi, G.; Gorrasi, G. Active Packaging Based on Coupled Nylon/PE Pouches Filled with Active Nano-Hybrid: Effect on the Shelf Life of Fresh Milk. Nanomaterials 2021, 11, 1881. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Koirala, B.; Hernandez, Y.; Zimmerman, M.; Park, S.; Perlin, D.S.; Brady, S.F. A naturally inspired antibiotic to target multidrug-resistant pathogens. Nature 2022, 601, 606. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Fan, B.; Yan, P.; Liu, C. Research on the antibacterial mechanism of medical nanosilver latex composite materials. Front. Mater. 2024, 11, 1349870. [Google Scholar] [CrossRef]
- Vityazev, F.V.; Khramova, D.S.; Saveliev, N.Y.; Ipatova, E.A.; Burkov, A.A.; Beloserov, V.S.; Belyi, V.A.; Kononov, L.O.; Martinson, E.A.; Litvinets, S.G.; et al. Pectin–glycerol gel beads: Preparation, characterization and swelling behaviour. Carbohydr. Polym. 2020, 238, 116166. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Kelly, A.L.; Miao, S. The impact of pH on mechanical properties, storage stability and digestion of alginate-based and soy protein isolate-stabilized emulsion gel beads with encapsulated lycopene. Food Chem. 2022, 372, 131262. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liang, J.; Sun, L.; Li, G.; Temmink, H.; Rijnaarts, H.H.M. Granule-based immobilization and activity enhancement of anammox biomass via PVA/CS and PVA/CS/Fe gel beads. Bioresour. Technol. 2020, 309, 123448. [Google Scholar] [CrossRef]
- Hu, S.; Hsieh, Y.-L. Silver nanoparticle synthesis using lignin as reducing and capping agents: A kinetic and mechanistic study. Int. J. Biol. Macromol. 2016, 82, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, W.; Chen, G.; Li, X.; Wang, Y.; Xiong, J.; Wei, L. Preparation and characterization of polyvinyl alcohol-chitosan/cerium hydrogel with significant antibacterial activity. Starch–Stärke 2021, 73, 2000253. [Google Scholar] [CrossRef]
- Nan, N.; DeVallance, D.B.; Xie, X.F.; Wang, J.X. The effect of bio-carbon addition on the electrical, mechanical, and thermal properties of polyvinyl alcohol/biochar composites. J. Compos. Mater. 2016, 50, 1161–1168. [Google Scholar] [CrossRef]
- Li, K.; Zhang, B.; Yang, Z.; Jiang, X.; Li, X. Degradation behaviors of silicone gel encapsulation material with moisture intrusion. Polym. Degrad. Stab. 2022, 206, 110197. [Google Scholar] [CrossRef]
- An, N.; Li, K.; Wang, Y.; Shen, W.; Huang, X.; Xu, S.; Wu, L.; Huang, H. Biodegradable bio-film based on Cordyceps militaris and metal-organic frameworks for fruit preservation. Int. J. Biol. Macromol. 2024, 262, 130095. [Google Scholar] [CrossRef]
- Liu, B.T.; Li, X.; Wu, Y.H.; Shi, Y. Removal of organic pollutants and antibacterial properties of modified chitosan and their composites. Desalination Water Treat. 2022, 276, 70–82. [Google Scholar] [CrossRef]
- Gonçalves, J.O.; da Silva, K.A.; Rios, E.C.; Crispim, M.M.; Dotto, G.L.; Pinto, L.A.D. Single and Binary Adsorption of Food Dyes on Chitosan/Activated Carbon Hydrogels. Chem. Eng. Technol. 2019, 42, 454–464. [Google Scholar] [CrossRef]
- Ren, L.; Xu, J.; Zhang, Y.; Zhou, J.; Chen, D.; Chang, Z. Preparation and characterization of porous chitosan microspheres and adsorption performance for hexavalent chromium. Int. J. Biol. Macromol. 2019, 135, 898–906. [Google Scholar] [CrossRef]
- Chen, T.; Wen, X.; Li, X.; He, J.; Yan, B.; Fang, Z.; Zhao, L.; Liu, Z.; Han, L. Single/co-adsorption and mechanism of methylene blue and lead by beta-cyclodextrin modified magnetic alginate/biochar. Bioresour. Technol. 2023, 381, 129130. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhu, H.; Luo, Y. Chitosan-based oral colon-specific delivery systems for polyphenols: Recent advances and emerging trends. J. Mater. Chem. B 2022, 10, 7328–7348. [Google Scholar] [CrossRef] [PubMed]
- Ayutthaya, N.M.N.; Ummartyotin, S.; Inprasit, T.; Pisitsak, P. Genipin-crosslinked water hyacinth/chitosan sponges as green adsorbents for reactive dye removal. Fibers Polym. 2023, 25, 13–25. [Google Scholar] [CrossRef]
- Kunwar, S.; Roy, A.; Bhusal, U.; Gacem, A.; Abdullah, M.M.S.; Sharma, P.; Yadav, K.K.; Rustagi, S.; Chatterjee, N.; Deshwal, V.K.; et al. Bio-fabrication of Cu/Ag/Zn nanoparticles and their antioxidant and dye degradation activities. Catalysts 2023, 13, 891. [Google Scholar] [CrossRef]
- Chen, Z.; Ni, Y.Z.; Zhang, W.W.; Wang, Z.Y.; Ma, R.J.; Jia, Z.H.; Chen, K.L. Fabrication of multiple protective fabric based on ZnO/chitosan composite microcapsules via thiol-ene click modification. Cellulose 2023, 30, 8023–8036. [Google Scholar] [CrossRef]
- He, Q.; Zhang, H.; Ma, M.; He, Y.; Jia, J.; Hu, Q.; Gong, Y. Critical assessment of protozoa contamination and control measures in mass culture of the diatom Phaeodactylum tricornutum. Bioresour. Technol. 2022, 359, 127460. [Google Scholar] [CrossRef] [PubMed]

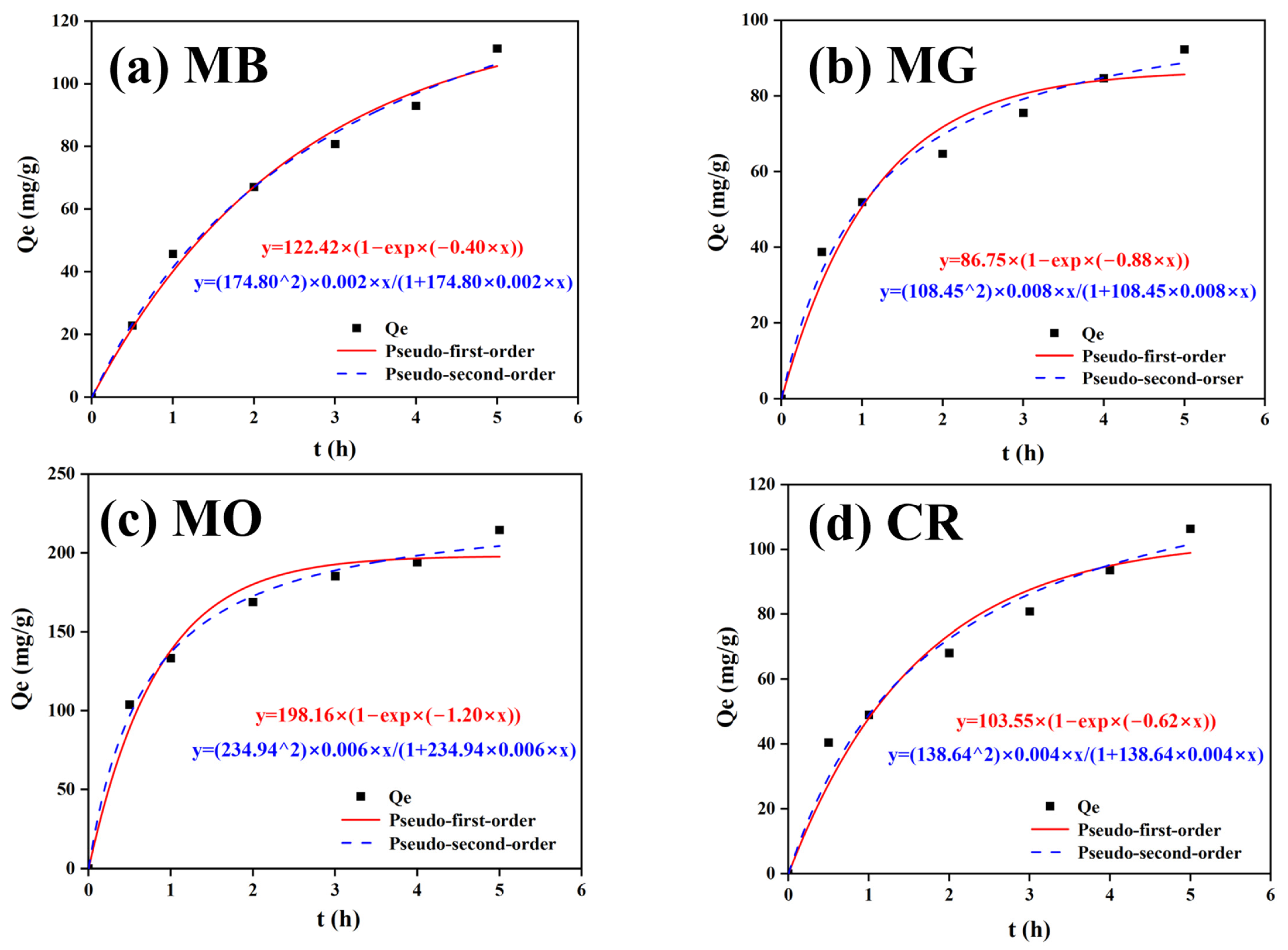
| Model of Pseudo-First Order | Model of Pseudo-Second Order | ||||||
|---|---|---|---|---|---|---|---|
| Qe exp (mg/g) | Qe cal (mg/g) | k1 (min−1) | R2 | Qe cal (mg/g) | k2 (g/mg·min) | R2 | |
| MB | 111.28 | 122.42 | 0.40 | 0.99 | 174.80 | 0.002 | 0.99 |
| MG | 92.26 | 86.75 | 0.88 | 0.99 | 108.45 | 0.008 | 0.99 |
| MO | 214.52 | 198.16 | 1.20 | 0.98 | 234.94 | 0.006 | 0.99 |
| CR | 106.38 | 103.55 | 0.62 | 0.96 | 138.64 | 0.004 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, K.; He, Y. Preparation and Application of Multifunctional Chitosan–Polyvinyl Alcohol–Nanosilver–Chrysanthemum Extract Composite Gel. Processes 2025, 13, 517. https://doi.org/10.3390/pr13020517
Shen K, He Y. Preparation and Application of Multifunctional Chitosan–Polyvinyl Alcohol–Nanosilver–Chrysanthemum Extract Composite Gel. Processes. 2025; 13(2):517. https://doi.org/10.3390/pr13020517
Chicago/Turabian StyleShen, Kejian, and Yucai He. 2025. "Preparation and Application of Multifunctional Chitosan–Polyvinyl Alcohol–Nanosilver–Chrysanthemum Extract Composite Gel" Processes 13, no. 2: 517. https://doi.org/10.3390/pr13020517
APA StyleShen, K., & He, Y. (2025). Preparation and Application of Multifunctional Chitosan–Polyvinyl Alcohol–Nanosilver–Chrysanthemum Extract Composite Gel. Processes, 13(2), 517. https://doi.org/10.3390/pr13020517







