Abstract
Botanical extracts are being considered for integration into disease management programs to control plant pathogenic fungi and oomycetes. A promising extract with potential is the essential oil of Lippia graveolens. However, its extraction process has not been optimized. Since optimizing process conditions can impact fungicidal and/or oomyceticidal effects, this research implemented time, temperature, and solid/liquid ratio conditions to maximize the fungicidal and oomyceticidal effects. The effectiveness was evaluated through parameters of mycelial growth inhibition, spore germination inhibition, minimum inhibitory and fungicidal/oomyceticidal concentration for the fungi Gilbertella persicaria, Agroathelia rolfsii, and Colletotrichum gloeosporioides, as well as the oomycete Phytophthora capsici. Optimal conditions were as follows: time: 46.27 min; temperature: 329.34 °C; and solid/liquid ratio: 80.35 g/L. In general, the optimized treatment was more effective in A. rolfsii ≥ P. capsica > G. persicaria ≥ C. gloeosporioides in all assays. These results demonstrate the fungicidal and oomiceticidal effects of L. graveolens essential oil, with potential for commercial product formulation.
Keywords:
essential oil; Lippia graveolens; optimization; fungicidal; oomyceticidal; plant pathogens 1. Introduction
The demographic explosion has led to an increase in food demand and production, bringing with it the problem of pests affecting crops. One of the main causes of these losses is the attack of plant pathogens, causing diseases that drastically affect the plant. Approximately 10 to 30 % of crop production is lost due to pre- and post-harvest diseases [1]. Among the plant pathogens with the greatest impact on crop losses are fungi, oomycetes, viruses, bacteria, and nematodes. Fungi and oomycetes are among the groups causing the greatest severity and incidence of diseases worldwide. These can cause crop losses of up to 100 %, therefore, their management must be carried out under the best conditions [2].
To control plant pathogenic fungi and oomycetes, the most implemented method, due to its high effectiveness, is the use of chemical products; however, due to poor handling in applications, resistance occurs in these fungi and oomycetes. In addition to this problem, there are significant environmental and health impacts due to the excessive use of these products [3,4]. An alternative is the application of botanical extracts from various plant matrices, as they contain biomolecules capable of controlling or eradicating fungi and other plant pathogens, with the advantage of presenting little or no environmental impact and minimizing the risk of resistance development [5,6].
Among the numerous available extracts, the use of Mexican oregano essential oil (Lippia graveolens) stands out, as multiple studies have demonstrated its fungicidal potential due to the presence of molecules in its composition, such as carvacrol and thymol, which are the main constituents of the essential oil. However, the extraction conditions that maximize its fungicidal and oomyceticidal effects have not yet been optimized. Since extraction conditions differ, such as time, temperature, and solid/liquid ratio, the chemical profile of the essential oil is altered accordingly [7,8,9].
Additionally, the effect depends on the type of fungus or oomycete being targeted for control, so the extraction conditions that maximize the fungicidal effects on different phyla of plant pathogenic fungi (Mucoromycota, Basidiomycota, Ascomycota) must be considered, as well as its oomyceticidal effects on oomycetes from the Oomycota phylum. Therefore, the objective of this research was to optimize the extraction of Mexican oregano essential oil (L. graveolens) and its fungicidal and oomyceticidal effects in vitro against four plant pathogens.
2. Materials and Methods
2.1. Raw Material
Mexican oregano leaves grown in pots under greenhouse conditions were used. Fresh leaves were collected from plants at the onset of fruit production and then dried in an oven (Excalibur 3900B) at 37 °C for 24 h. After complete moisture removal, the leaves were ground and sieved to obtain a particle size of less than 425 µm.
2.2. Plant Pathogens
Representative plant pathogenic fungi from different important phyla were used: Colletotrichum gloeosporioides (Ascomycota) blueberry isolate, Agroathelia rolfsii (Basidiomycota) sesame plant isolate, and Gilbertella persicaria (Mucoromycota) papaya isolate, as well as the oomycete Phytophthora capsici (Oomycota) bell pepper plant isolate.
2.3. Essential Oil Extraction
Mexican oregano essential oil (MOEO) was extracted by hydrodistillation using a Clevenger apparatus, starting with the pulverized plant material in contact with water, heating the mixture until it reaches the boiling point (100 °C) within the flask, using as a heat source the electric resistance of a CIMAREC+ 18 × 18 cm (ThermoScientific, Waltham, MA, USA) heating plate, causing the water vapor and essential oil mixture to evaporate, which then condenses, allowing the essential oil to be separated from the water. As indicated in the experimental design, the amounts of plant material varied (16.14 to 133.86 g/L) and were added to a 1 L round-bottom flask with 400 mL of water. The mixture was heated while stirring at various indicated temperatures on the heating plate (temperature variable) (235.91 to 404.09 °C) and times (2.96 to 87.04 min), according to the experimental design. After the specified time, the essential oil obtained was separated by decantation and stored in refrigeration at 4 °C in the dark until use [10]. The yield percentage was calculated using the following formula:
2.4. Gas Chromatography-Mass Spectrometry (GC/MS)
Chromatographic analysis was performed on an Agilent 7890B gas chromatography system coupled with an ion trap mass spectrometer (IT-240), an FID detector, and an Agilent Technologies CombiPal 80 autosampler. High-purity helium was used as the carrier gas at a 1 mL/min flow rate. The chromatographic column employed was a TG-WAXMS 30 m × 0.25 mm × 0.25 μm. The inlet pressure was set at 9.37 psi. The column temperature was initially maintained at 40 °C for 1 min and then increased at a rate of 10 °C/min up to 280 °C, where it was held for 6 min. A sample volume of 1 µL to 0.025 µL of essential oil/mL of hexane from the 20 runs of the experimental design was injected. The major components of interest in the MOEO (thymol and carvacrol, Sigma Aldrich, St. Louis, MO, USA) were examined using a calibration curve on the GC-FID system. The results were expressed as the percentage composition of carvacrol and thymol.
2.5. In Vitro Fungicidal and Oomyceticidal Activity Assays
To evaluate the fungicidal and oomyceticidal potential of the MOEO, the following methods were employed: mycelial growth inhibition (MGI), spore germination inhibition (SGI), minimum inhibitory concentration (MIC), and minimum fungicidal concentration (MFC).
2.5.1. MGI
For the MGI method, the agar incorporation technique was followed as described by Sharma et al. [11]. Different concentrations of MOEO (1, 10, 25, 50, 100, 250, and 500 µL/L) were added to potato dextrose agar (PDA, Bioxon, Kowale, Poland) medium at 50 °C and allowed to solidify in sterile 90 mm Petri dishes. Subsequently, a 5 mm diameter mycelial plug of the pathogen was placed at the central point of each Petri dish. The plates were then incubated at 28 °C in darkness for varying durations, depending on the plant pathogen, until the control completely covered the Petri dish surface. After the incubation period, mycelial growth was measured, and the percentage of MGI was calculated using the following formula:
Once the % MGI was calculated, a linear regression of concentration versus % MGI was performed to determine the effective concentration that inhibits 50% of mycelial growth (EC50).
2.5.2. SGI
The microdilution technique was followed for the spore germination inhibition method [12]. Different MOEO concentrations (ranging from 20 to 1000 µL/L) were used, employing a 96-well microplate. Each treatment received 180 µL of potato dextrose broth and 20 µL of extract to achieve the final concentration, as indicated in the experimental design, followed by 50 µL of a spore suspension at 106 spores/mL. The microplates were incubated for 6 to 24 h (depending on the germination rate of each evaluated species) at 28 °C in darkness. The number of germinated spores was examined from three 10 µL aliquots from each well using an optical microscope at 400× magnification, where the presence of a germ tube at least twice the size of the spore was visible. The percentage of SGI was calculated using the following formula:
Once the % SGI was calculated, a linear regression of concentration versus % SGI was performed to determine the effective concentration that inhibits 50% of spore germination (EC50).
2.5.3. MIC and MFC
The sequence of the microdilution method proposed by Stupar et al. [13] was followed with slight modifications due to different microorganisms and extracts, which caused variations in incubation time and culture medium conditions. After 24 h of incubation in the SGI assay, three 10 µL aliquots from each well were examined under an optical microscope at 400× magnification to determine the lowest concentration at which no spore germination was observed. This was additionally confirmed by the absence of turbidity in the liquid medium and the lack of mycelial growth observed directly in the wells of the microplates at 40× magnification. The MIC and MFC are expressed in µL of MOEO/L.
Once the MIC was identified, the MFC was determined by resubmitting a 10 µL aliquot to three Petri dishes containing PDA with added lactic acid for each concentration tested. After 24 h of incubation, the presence or absence of pathogen growth was observed, with the MFC being the minimum concentration at which no growth was observed.
2.6. Optimization
Process optimization was conducted using a central composite rotatable design of the response surface methodology. The process variables for the extraction of MOEO were as follows: time (min), temperature (temperature of the heating source, °C), and solid/liquid ratio (S/L in g/L), with response variables being the yield (%), % MGI (at 60 µL/L), % SGI (at 70 µL/L), MIC, and MFC. The aim was to find an optimal region where all response variables that fit the model would maximize the fungicidal and oomyceticidal effects by maximizing the % MGI and % SGI and minimizing the MIC and MFC.
2.7. Statistical Analysis
The data for all evaluated response variables were examined using the statistical package Minitab 19 (Minitab Inc, State College, PA, USA). The three-factor optimization design resulted in 20 runs and was evaluated using ANOVA, ensuring the overall model significance (p < 0.05), a minimum R2 determination coefficient of 0.7, and no lack of fit (p > 0.05). After the final model prediction, the optimal process variable conditions were replicated five times to conduct all response variable assays involved in the final prediction. Five data points were obtained, which were subjected to a Student’s t-test to verify if there was a significant difference. If the model is adequate and reproducible, there should be no significant difference between experimental data and optimization prediction. Once optimal conditions were obtained, each experiment was performed in triplicate and evaluated by mean comparison (among each phytopathogen) using Tukey’s method (p < 0.05), expressing results as the mean ± standard deviation.
3. Results and Discussion
3.1. Experimental Prediction Models
All data obtained from the central composite experimental design are presented in Table 1. Here, we observe all the response variables considered initially in search of the best effect. However, some variables could not be fitted to the model due to non-significance (R2 less than 0.7) or lack of fit, therefore, Table 2 presents all response variables considered for the prediction analysis to find the optimal process conditions.

Table 1.
Experimental data from the central composite design (n = 20) with all initially considered response variables.

Table 2.
Experimental data from the central composite design (n = 20) with response variables successfully fitted to the model.
For the variables that were successfully adjusted, their ANOVA tables, regression equation in uncoded units, and summary model were obtained. For those that were not successfully adjusted, only their ANOVA table was obtained, which demonstrates that a good fit was not achieved for these variables; therefore, they are discarded in the optimization model (Tables S1–S14).
While the yield and all the % MGI variables were successfully adjusted, the % SGI for P. capsici and none of the MIC variables could be adjusted. Only the MFC for G. persicaria was fitted. Contour and surface plots (time vs. temperature, time vs. solid/liquid ratio, and temperature vs. solid/liquid ratio) were generated with these variables.
3.1.1. Yield (%)
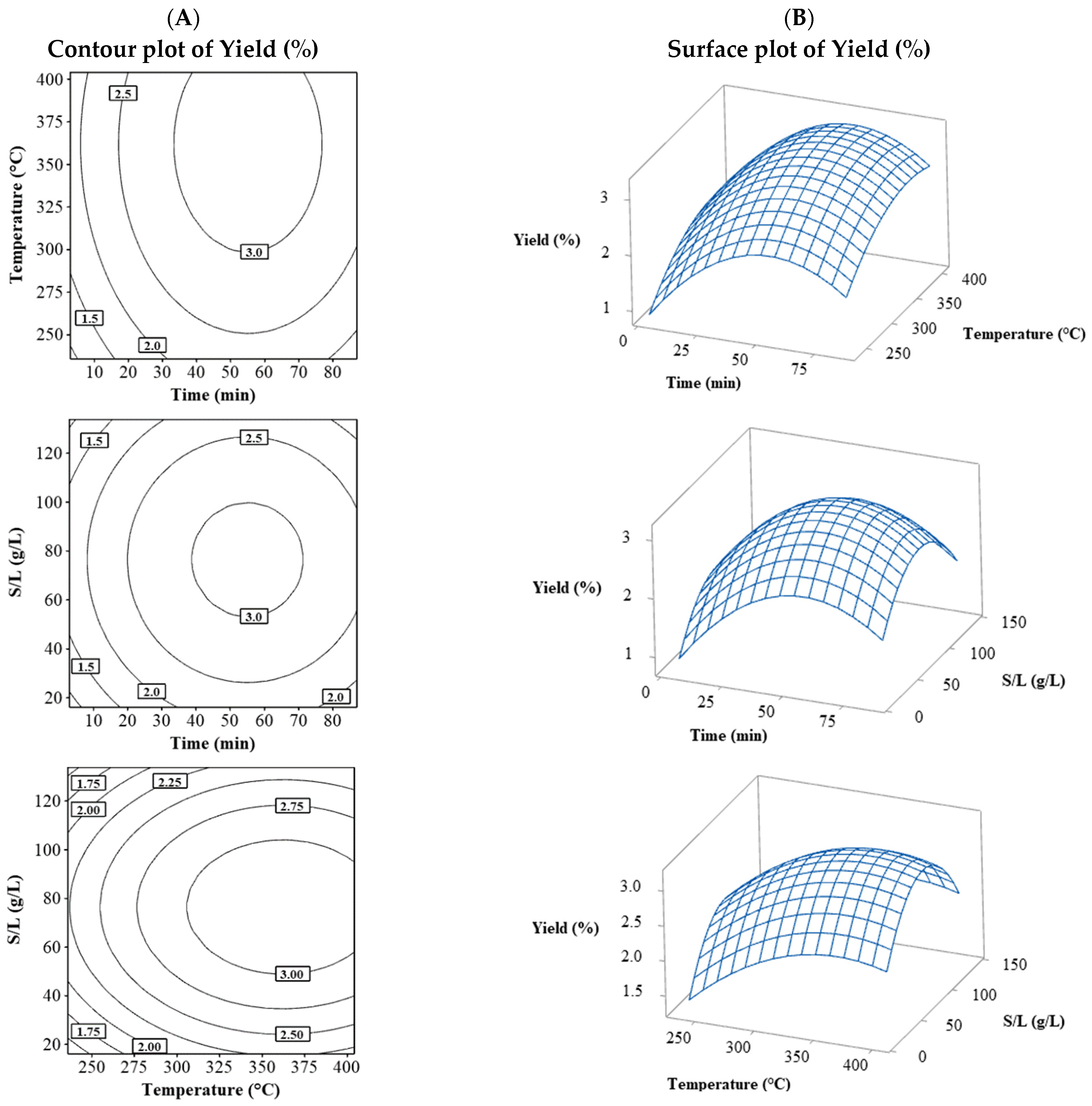
As shown in Table 2, yield values ranged from 1.53% to 3.23%. Figure 1 shows the combination of the three contour plots (Figure 1A) and surface plots (Figure 1B) for the yield variable. The yield behavior for the three combinations of variables was very similar, with a peak forming near the central values. As the levels of variables increased, the yield reached a maximum; however, as the levels continued to rise, the yield began to decrease, indicating the optimal point was achieved with a yield above 3%.
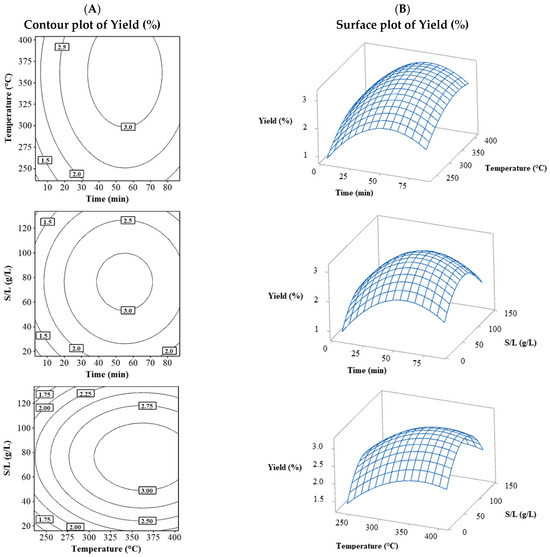
Figure 1.
Contour (A) and surface (B) plots for the Yield (%) combining the process variables (temperature, time, and S/L).
The yield in this optimization design primarily exhibited behavior near the upper-central levels of time, temperature, and solid/liquid ratio (Figure 1). This demonstrated that as the factor values increased, a maximum point was reached; however, the extraction presented a physical limit concerning time (yield remained constant as the value increased) [7]. Regarding the solid/liquid ratio and temperature, the distribution was crucial, as a low solid saturation in the liquid did not achieve a significant extraction amount, similar to excessive saturation. In the latter, the effect is due to low mass transfer that occurs when saturating a solution [8,14]. Meanwhile, temperature can increase yield up to an optimal point and decrease if it is too high, as increased heat transfer lowers viscosity and benefits mass transfer. Still, if it is too high, it can lead to yield reduction mainly due to possible degradation or chemical changes in molecules from excessive heat [15].
3.1.2. MGI (%)
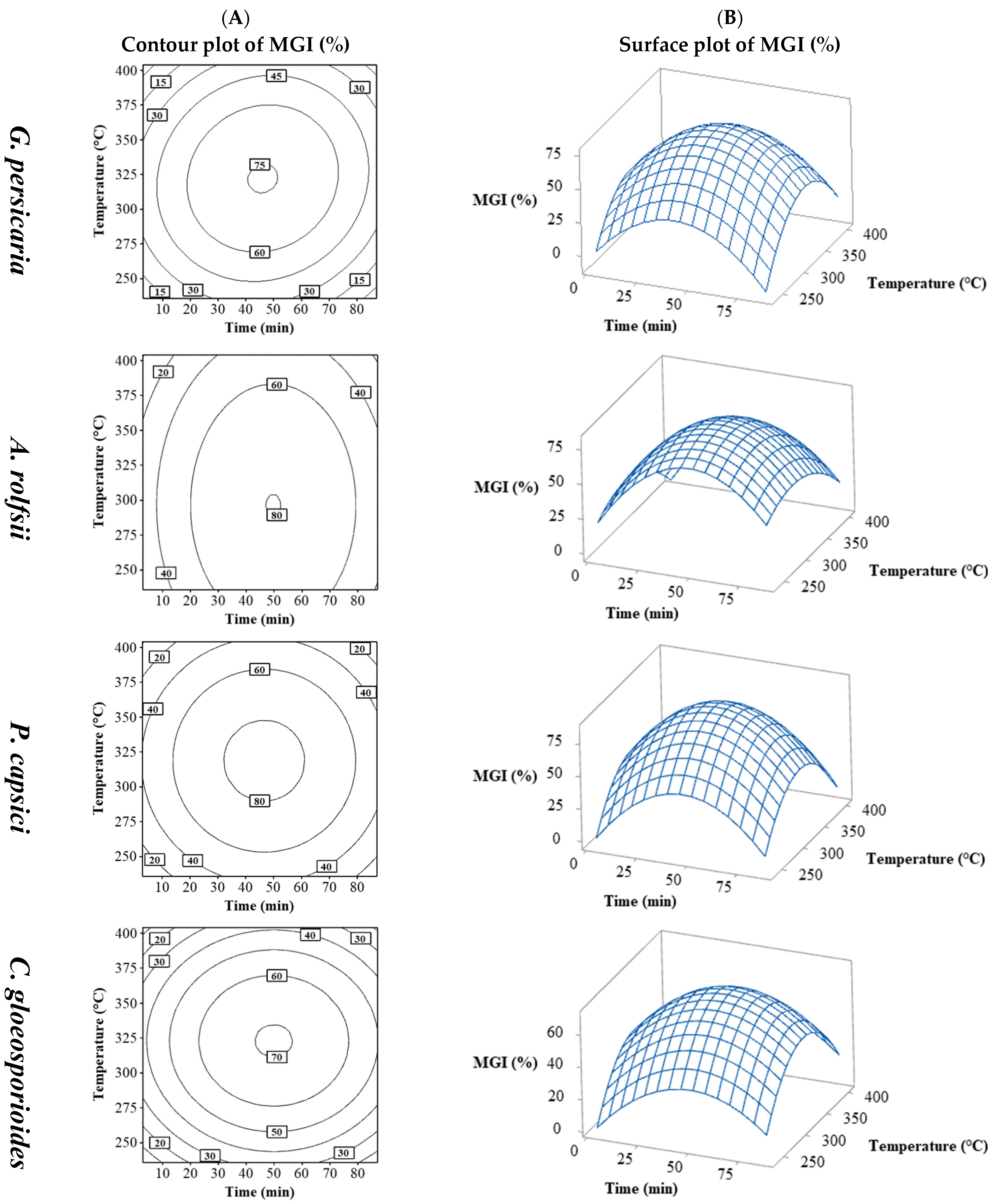
The % MGI values varied according to the phytopathogen species: G. persicaria (18.9 to 85%), A. rolfsii (28.89 to 83.33%), P. capsici (6.67 to 92.22%), and C. gloeosporioides (21.11 to 81.11%) (Table 2).
There was significant variation in % MGI, influenced more by extraction conditions than by the plant pathogen, with similar ranges across species but noticeable differences within the same range. Figure 2 shows the contour plots (Figure 2A) and surface plots (Figure 2B) of time vs. temperature for the four plant pathogens. The contour and surface plots resulting from the combination of the process variables temperature vs. S/L and S/L vs. time can be found in the Supplementary Material (Figures S10–S13).
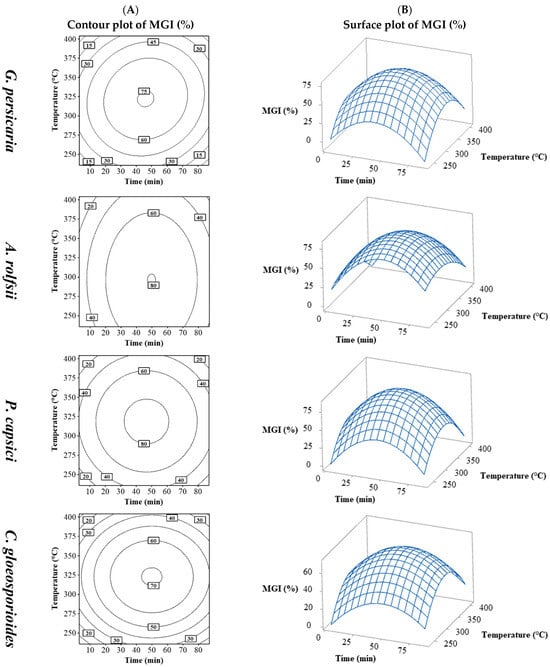
Figure 2.
Contour (A) and surface (B) plots for MGI (%) in G. persicaria, A. rolfsii, P. capsici, and C. gloeosporioides combining process variables (temperature and time).
The pattern observed was similar, with a peak % MGI at increasing times and temperatures, followed by a decrease beyond those levels, indicating maximum inhibition near the central values for all the plant pathogens. C. gloeosporioides was the least sensitive (around 70%), followed by G. persicaria (approximately 75%), with P. capsici and A. rolfsii (around 80%) being the most sensitive.
3.1.3. SGI (%)
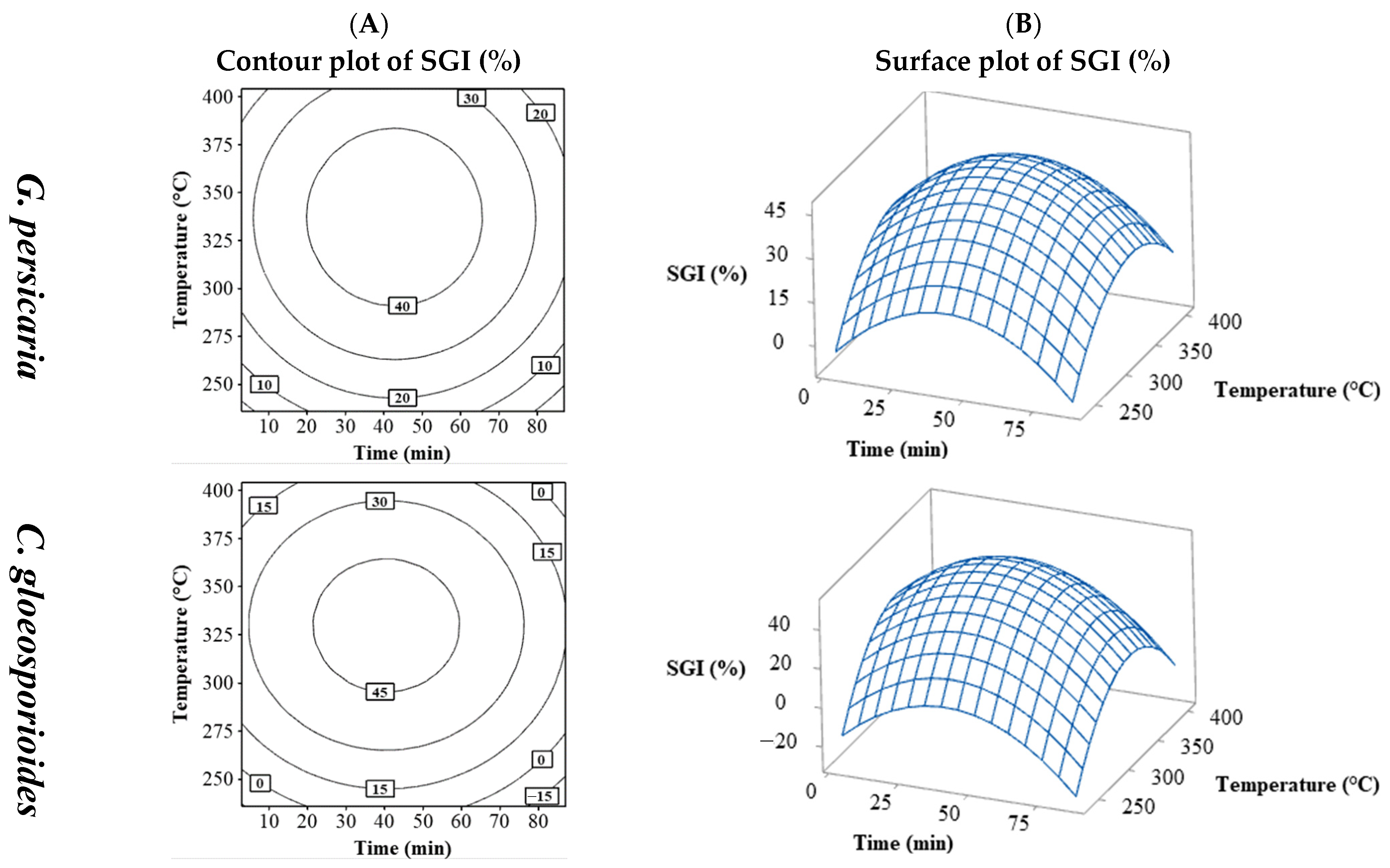
Similar to % MGI, % SGI showed slight variation among plant pathogens but was significantly affected by extraction conditions, with some treatments causing minor spore germination inhibition and others considerably higher.
The SGI values (%) varied by pathogen species, with only those for G. persicaria (11.59 to 53.63%) and C. gloeosporioides (4.83 to 62.08%) being adjustable (Table 2). Figure 3 shows the SGI for G. persicaria and C. gloeosporioides with their contour plots (Figure 3A) and surface plots (Figure 3B) of time vs. temperature. The contour and surface plots resulting from the combination of the process variables temperature vs. S/L and S/L vs. time can be found in the Supplementary Material (Figures S14 and S15). Both fungi followed a similar pattern to % MGI, with % SGI maximized near central values of time and temperature and decreasing with further increases. The optimal % SGI was found in the central region, higher for C. gloeosporioides (around 45%) compared to G. persicaria (not exceeding 40%).
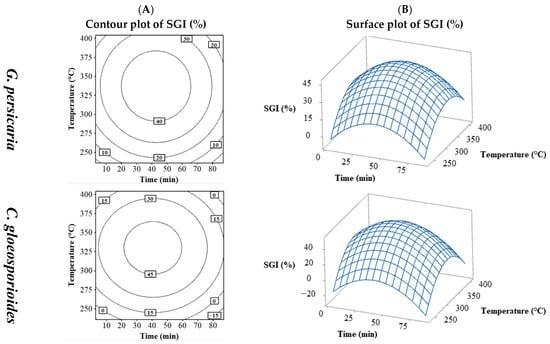
Figure 3.
Contour (A) and surface (B) plots for the SGI (%) of G. persicaria and C. gloeosporioides combining the process variables (temperature and time).
3.1.4. MFC
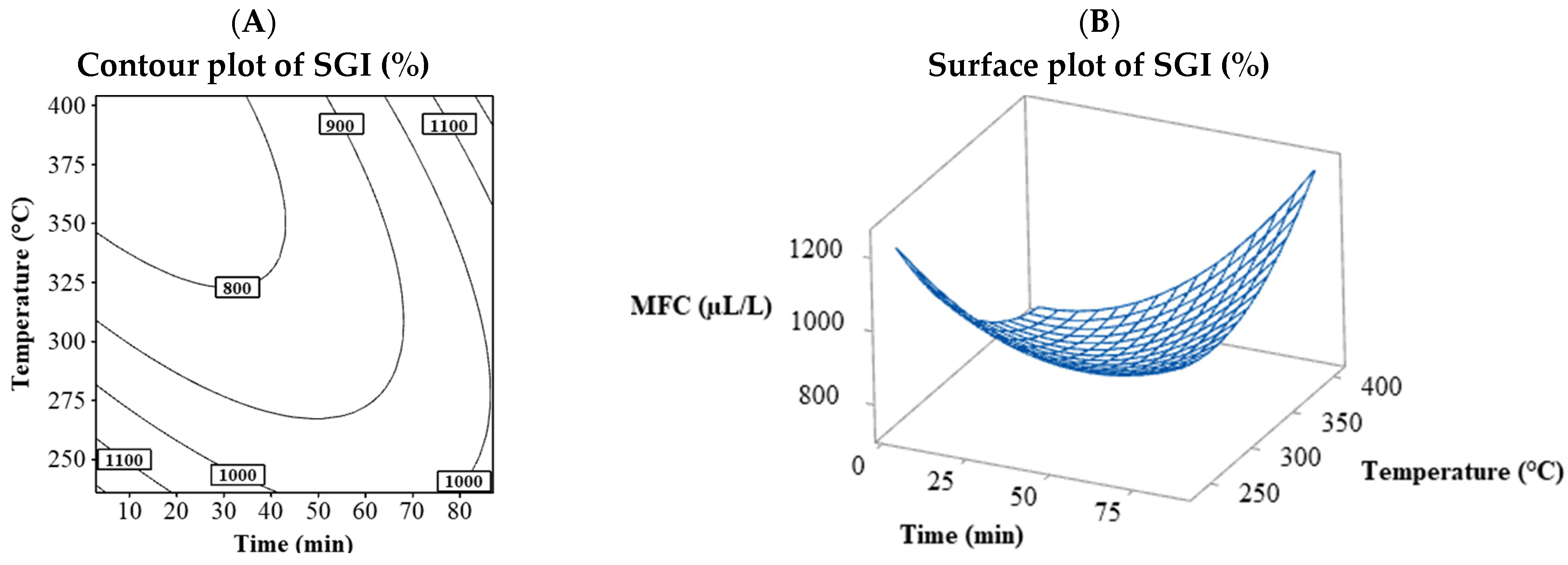
Table 2 shows that no MIC values were fitted to the model; however, only one MFC value was successfully adjusted, corresponding to G. persicaria, with values ranging from 750 to 1200 µL/L. Figure 4 shows the MFC behavior for G. persicaria, with its contour plot (Figure 4A) and surface plot (Figure 4B) plotting time vs. temperature. The contour and surface plots resulting from the combination of the process variables temperature vs S/L and S/L vs time can be found in the Supplementary Material (Figure S16). The behavior differed from the % MGI and % SGI, with the lowest MFC found between 325 and 400 °C and times below 45 min, indicating higher temperatures and shorter times resulted in a lower MFC.
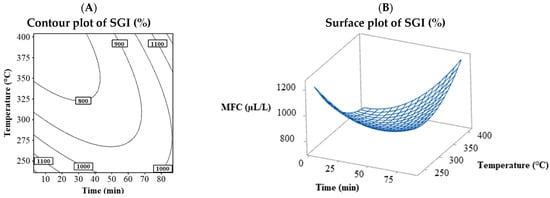
Figure 4.
Contour (A) and surface (B) plots for the MFC (µL/L) variable of G. persicaria combining the process variables (temperature and time).
3.2. Optimization
The optimal extraction process conditions considered all adjustable response variables (Table 2), including all the plant pathogens and inhibition types (% MGI, % SGI, and MFC). Despite variations in mycelial and germ tube inhibition, the optimal process conditions were effective for both fungal structures.
Table 3 reports the optimizer function results, aiming to maximize all % MGI and % SGI for all the plant pathogens, minimize the MFC for G. persicaria, and maximize yield. The optimal MOEO extraction process conditions were 46.27 min, 329.34 °C, and an 80.35 g/L solid/liquid ratio. This combination yielded multiple adjustments for response variables, each with respective SE and 95% confidence interval (CI), achieving a global desirability of 0.86.

Table 3.
Report of prediction conditions for process variables and estimation of response variables.
During the optimization of the extraction conditions that maximize the fungicidal and oomyceticidal effects, these conditions were largely concentrated in the central part of the experimental design (Figure 2 and Figure 3), indicating that increasing time, temperature, and solid/liquid ratio values reaches a maximum potential but further increases result in a decrease in fungicidal and oomyceticidal potential [9]. This occurs because the MOEO’s chemical composition varies with different extraction conditions [9,16]. This research showed that the concentration of major compounds in MOEO (Table 1) (thymol and carvacrol) is responsible for greater or lesser inhibition of these plant pathogens, both for mycelium and spore germination inhibition. Treatments with low compound content, like runs 4 and 6 with 47.60 and 46.33% thymol, respectively, exhibited the lowest inhibition in nearly all assays and plant pathogens.
Additionally, inhibition behavior was consistent across pathogens (Figure 2 and Figure 3), as a treatment’s effectiveness depended on extraction conditions, varying inhibition percentage by pathogen due to structural differences among pathogen species, even within different species of the same genus [16].
Prediction Validation
The predicted process conditions (Table 3) were replicated five times to verify the model’s adequacy and reproducibility, followed by a Student’s t-test to check for significant differences. Table 4 confirms no significant differences (p > 0.05), indicating the prediction was adequate and reproducible. Once the optimal extraction conditions for the MOEO were obtained, the concentrations of thymol and carvacrol were determined, as well as the EC50 for both mycelium and spores, in addition to determining the MIC and MFC.

Table 4.
Student’s t-test of experimental values (n = 5) and fitted values of optimization response variables.
3.3. Chemical Profile of Optimized MOEO
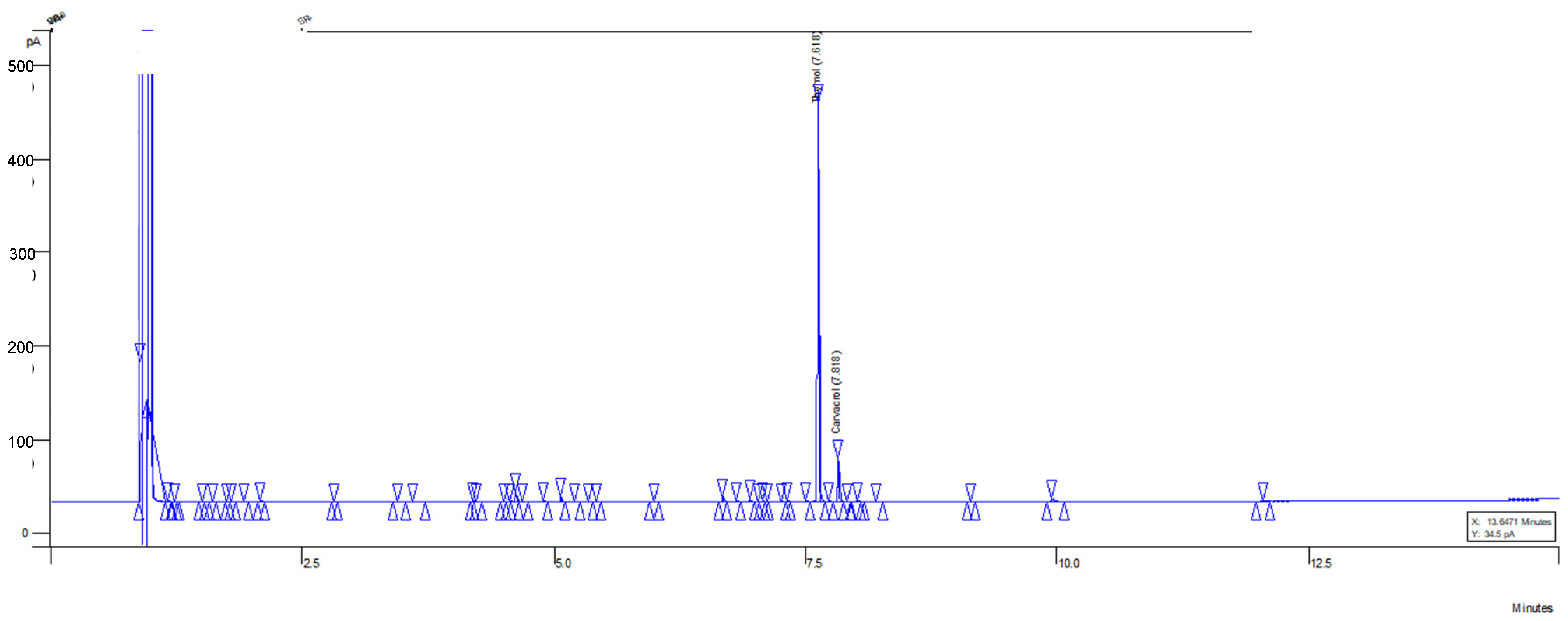
The optimized MOEO is notable for the presence of phenolic monoterpenes, such as thymol and carvacrol, where their combined concentration represents the majority of the chemical composition. The thymol and carvacrol concentrations were 70.4 ± 5.3% and 6.9 ± 0.5%, respectively. These compounds elute from the capillary column at 7.62 and 7.82 min, respectively (Figure 5), using helium as the carrier gas. To ensure the correct elution and quantification of thymol and carvacrol, a linear regression of the thymol and carvacrol standard was performed, where concentration (mg/mL) vs. peak size was plotted (Figures S7 and S8). Chromatograms of these points on the curve were obtained (Figures S1–S6), obtaining an R2 > 0.997, which is considered a good fit, ensuring the reliability of sample quantification (n = 3, Figure S9). This thymol and carvacrol content is higher than in multiple regions where the plant was also grown under greenhouse conditions, as in this research [17,18]. This variation may be due to various factors, such as temperature, humidity, altitude, radiation, irrigation frequency, nutrition, biotic stress, etc. [18,19]. In addition to differences in the cultivation methods of L. graveolens, it is noteworthy that the optimization process plays a significant role. In different runs of the optimization design, such as run 4 and 6, the thymol and carvacrol concentration is similar to that mentioned in other studies. Hence, it is important to carry out optimization processes that improve the extraction of these chemotypes with effects on phytopathogens.
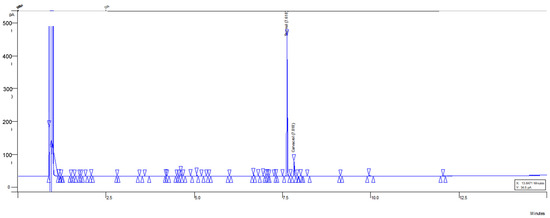
Figure 5.
Chromatogram of optimized MOEO sample (thymol and carvacrol). The blue peaks indicate the compounds identified and quantified with standards. The triangles indicate other signals of unquantified but minor compounds.
3.4. In Vitro Fungicidal and Oomyceticidal Activity Assays
As shown in Table 5, in vitro sensitivity assays for the four plant pathogens compared mean differences to identify the most sensitive pathogen depending on the structure, either mycelium or spores.

Table 5.
EC50, MIC, and MFC of the optimized MOEO in G. persicaria, A. rolfsii, P. capsica, and C. gloeosporioides.
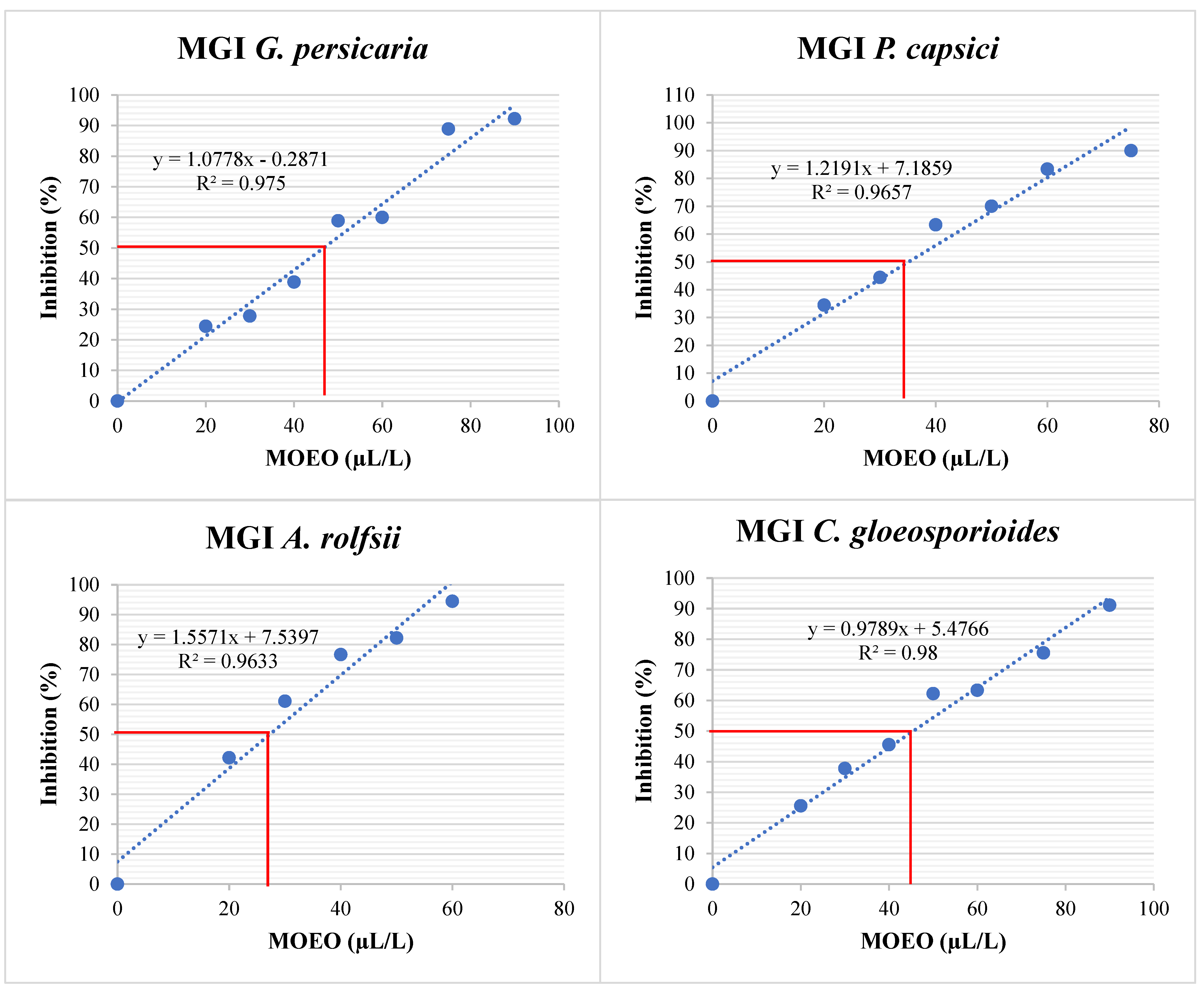
The different % MGI values were obtained at various MOEO concentrations (Figure 6), leading to a linear regression (Figure 7). This regression determined the EC50 for each plant pathogen (Table 5). The most sensitive species was A. rolfsii, followed by P. capsici, with similar effects on C. gloeosporioides and G. persicaria, with no significant differences (p = 0.957).
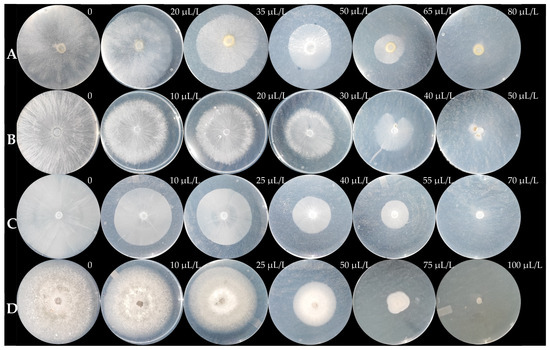
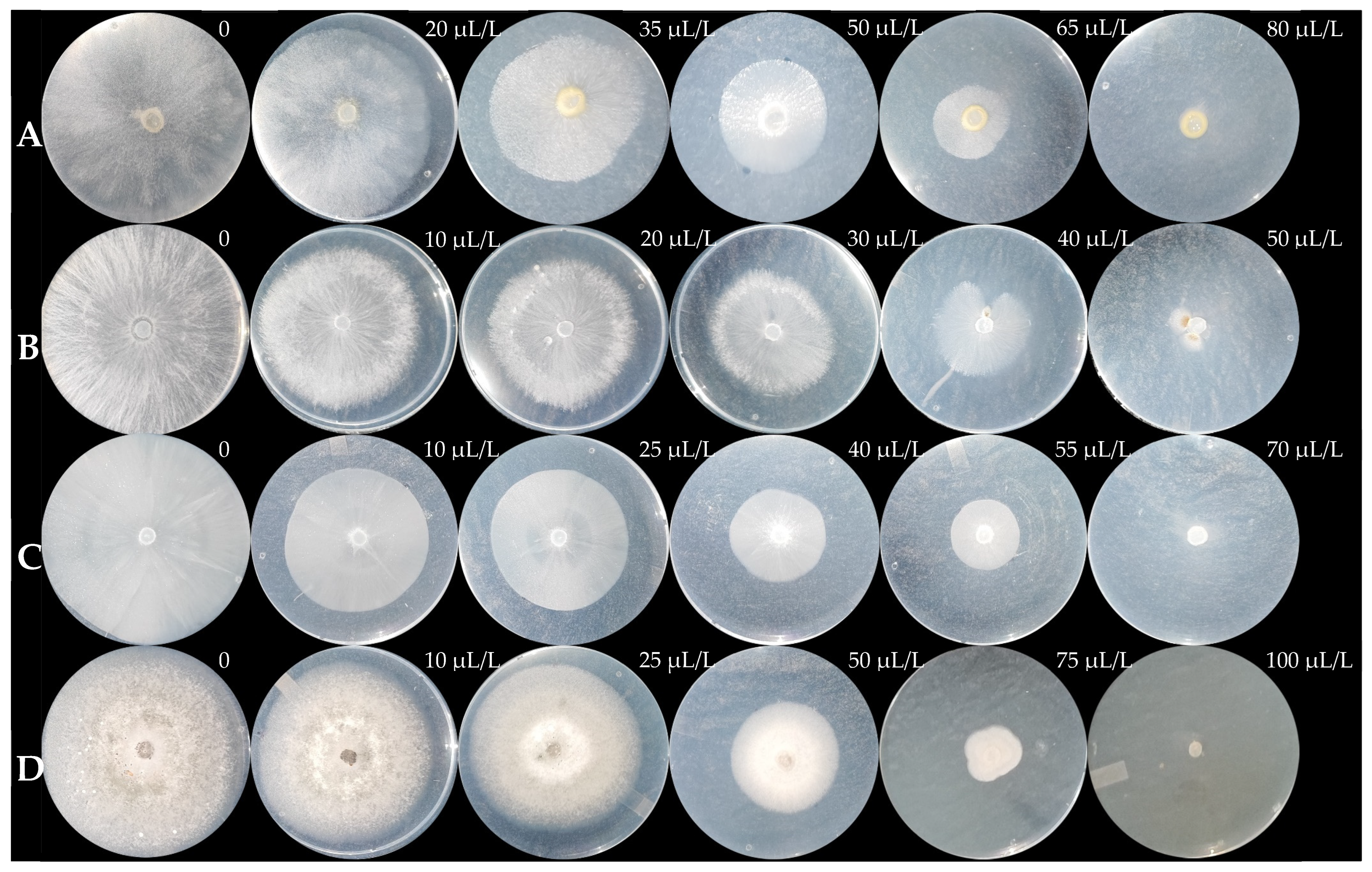
Figure 6.
Mycelial growth of G. persicaria (A), A. rolfsii (B), P. capsici (C), and C. gloeosporioides (D) at different concentrations of optimized MOEO.
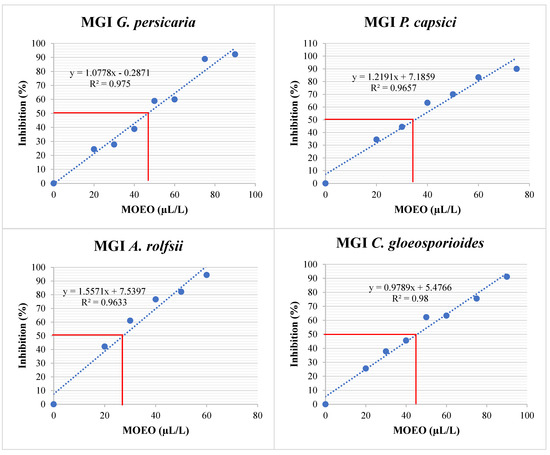
Figure 7.
MGI (%) of G. persicaria, A. rolfsii, P. capsici, and C. gloeosporioides at different concentrations of optimized MOEO. MGI: mycelial growth inhibition; MOEO: Mexican oregano essential oil. The red line indicates the intersection representing the concentration necessary to inhibit 50% of mycelial growth. The blue dashed line represents the linear regression fit. The blue dots in the figure indicate the treatments that were performed to obtain the linear regression fit.
3.4.1. MGI (%)
Regarding the MOEO, other essential oils show different effects against the same fungal pathogens evaluated in this research or compared to other fungi. For example, multiple essential oils effective against C. gloeosporioides were less effective than our optimal treatment, with Lippia alba, Aloysia citrodora, Cymbopogon winterianus, and Ocimum americanum showing an EC50 of 700, 410, 860, and 640 µL/L, respectively, compared to 43.67 µL/L in this study. This is due to the different chemical compositions of essential oils from various aromatic plants, with β-linalool, β-citral, cis-geraniol, and cineole being the major compounds, respectively [20]. Phenolic monoterpenes like carvacrol, thymol, and eugenol have shown superior fungicidal effects compared to most monoterpenes, explaining the significant difference in effects between the optimized MOEO and essential oils from multiple aromatic plants [21].
3.4.2. SGI (%)
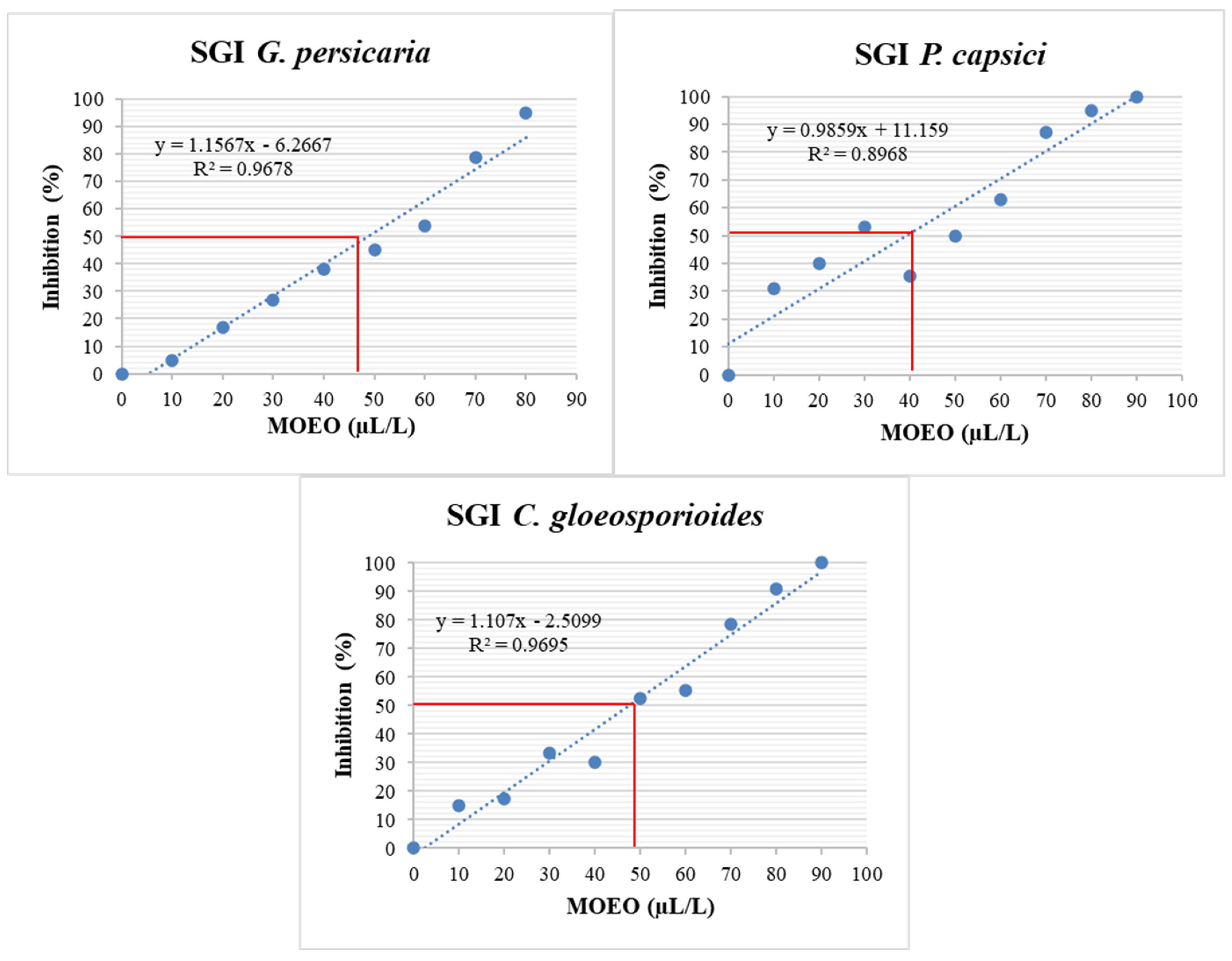
Similar to the MGI, a linear regression of the MOEO concentrations versus the % SGI was conducted (Figure 8). This data calculated the EC50 for different pathogens (Table 5), excluding A. rolfsii due to the absence of spore-producing structures. P. capsici was the most sensitive among the three remaining pathogen species for % SGI, followed by C. gloeosporioides and G. persicaria, with no significant differences (p = 0.642).
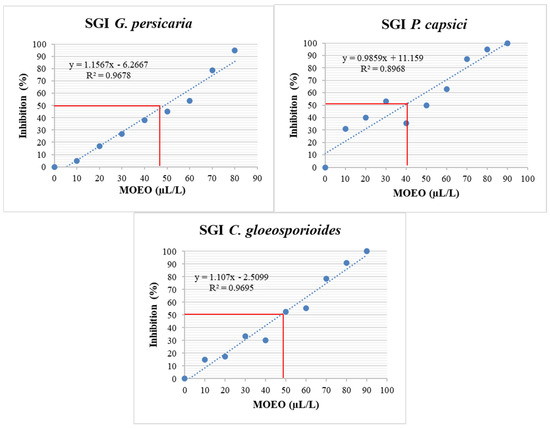
Figure 8.
SGI (%) of G. persicaria, P. capsici, and C. gloeosporioides at different concentrations of optimized MOEO. SGI: spore germination inhibition; MOEO: Mexican oregano essential oil. The red line indicates the intersection representing the concentration necessary to inhibit 50% of spore germination. The blue dashed line represents the linear regression fit. The blue dots in the figure indicate the treatments that were performed to obtain the linear regression fit.
3.4.3. MIC
The MIC results (Table 5) differed from the MGI and SGI sequences. The MIC values were similar for G. persicaria, A. rolfsii, and P. capsici, except for C. gloeosporioides, which was the least sensitive.
Regarding Colletotrichum sp., essential oils from L. sidoides and L. gracilis caused 100% inhibition at 300 µL/L, which is also less effective than our optimal treatment [22]. Oliveira et al. [23] also observed this behavior, where L. gracilis oil inhibited 100% of C. gloeosporioides at 310 µL/L.
These inferior effects could be attributed to the major components reported in L. gracilis: carvacrol at 48.57% and thymol at 7.78%, with differing composition and lower combined amounts than the optimized MOEO treatment, with 70.41 ± 5.31% thymol and 6.89 ± 0.52% carvacrol, favoring thymol as the major component, proven to have a greater effect than carvacrol.
Our optimal treatment was superior in inhibiting C. gloeosporioides compared to Lippia scaberrima essential oil, which achieved 100% inhibition at 1600–2400 µL/L versus 128.33 µL/L of the optimized MOEO. This significant difference is due to different Lippia species compositions, with the optimized MOEO rich in thymol and L. scaberrima abundant in carvone, limonene, and 1,8-cineole [24]. On the other hand, Antonia et al. [25] evaluated L. sidoides oil against C. gloeosporioides, finding an EC50 of 46.83 µL/L, comparable to the optimized MOEO treatment at 43.67 µL/L (p = 0.118), and an MIC of 125 µL/L for L. sidoides and 128.33 µL/L for the optimal treatment, with no significant difference (p = 0.635), both having thymol as the major component, explaining the equal inhibition of C. gloeosporioides.
3.4.4. MFC
The MFC differences among plant pathogens (Table 5) did not follow the same pattern as the MGI, SGI, and MIC. While P. capsici and A. rolfsii were the most sensitive, with no significant differences (p = 0.501), significant differences (p < 0.0001) were observed between C. gloeosporioides and G. persicaria, disrupting the observed SGI pattern. This significant difference separated G. persicaria from the other plant pathogens, breaking the consistency observed in other assays.
Once the optimized extraction conditions were demonstrated, the optimal treatment showed fungicidal and oomyceticidal effects on various plant pathogens (Table 5). Generally, the effect pattern was similar across species, with A. rolfsii (Basidiomycota), P. capsici (Oomycota), G. persicaria (Mucoromycota), and C. gloeosporioides (Ascomycota) being, respectively, more sensitive.
This variation is mainly due to the cell structure of each plant pathogenic species, differing among fungal phyla, most notably between Basidiomycota and Ascomycota, with the main difference being the chitin/glucan ratio, with Basidiomycota members containing more glucans [26,27,28,29,30].
However, the largest difference in cell structural composition occurs between fungi and oomycetes, as fungal cell walls are composed of chitin, glucans, and cell membranes primarily containing ergosterol. In contrast, oomycetes have cell walls composed of cellulose, glucans, and cell membranes primarily containing phytosterols different from ergosterol [30,31,32]. This could explain why fungi, such as C. gloeosporioides, are less sensitive compared to the oomycete P. capsici. However, in some cases, there was no significant difference between P. capsici and A. rolfsii.
Therefore, considering the difference between the Basidiomycota and Ascomycota phyla and the occasional similarity between the Oomycota and Basidiomycota phyla, it can be highlighted that chitin content is essential in making the organism less susceptible to the MOEO, as there was a significant difference in all trials between the Ascomycota and Oomycota phyla.
The obtained results met the objective of this research, which was to determine the fungicidal and oomyceticidal effects of MOEO on different plant pathogens. We considered applying this extract to various cultivated plants to minimize pathogen damage once the extraction process was optimized, considering the parameters of the MGI, SGI, MIC, and MFC.
4. Conclusions
The optimal extraction conditions (time, temperature, and solid/liquid ratio) for MOEO were found to maximize yield and fungicidal and oomyceticidal effects against G. persicaria, P. capsici, A. rolfsii, and C. gloeosporioides. The variation in extraction conditions influenced all four plant pathogens; however, inhibition values depended on each plant pathogenic species due to their morphological differences. These conditions were validated, resulting in an adequate and reproducible model. Generally, the most sensitive plant pathogen to the optimized MOEO effect was A. rolfsii, followed by P. capsici, G. persicaria, and C. gloeosporioides.
These results provide important findings, optimizing the extraction process of MOEO and maximizing its fungicidal/oomicidal effects against various phytopathogens. However, they are limited to in vitro studies, which must be considered as a preliminary stage in determining the true potential for using MOEO to eradicate diseases with organic treatments.
To address these limitations, it is suggested to use MOEO in in vivo conditions against various phytopathogens, as well as to scale up the industrial extraction process of MOEO. The aim is to apply this treatment against diseases that devastate agricultural crops and to be able to create an optimal formulation for its use as a botanical product.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr13020490/s1. Figure S1. Chromatogram of level 1 of the thymol and carvacrol standard curve; Figure S2. Chromatogram of level 2 of the thymol and carvacrol standard curve; Figure S3. Chromatogram of level 3 of the thymol and carvacrol standard curve; Figure S4. Chromatogram of level 4 of the thymol and carvacrol standard curve; Figure S5. Chromatogram of level 5 of the thymol and carvacrol standard curve; Figure S6. Chromatogram of thymol and carvacrol standard mix levels; Figure S7. Linear regression of the carvacrol standard curve; Figure S8. Linear regression of the thymol standard curve; Figure S9. Chromatogram of optimized MOEO (n = 3) of thymol and carvacrol; Table S1. Analysis of Variance, R2 and regression equation of the Yield variable; Table S2. Analysis of Variance of the Carvacrol (%) variable; Table S3. Analysis of Variance of the Thymol (%) variable; Table S4. Analysis of Variance of the Thymol + Carvacrol (%) variable; Table S5. Analysis of Variance, R2 and regression equation of the % MGI of G. persicaria variable; Table S6. Analysis of Variance, R2 and regression equation of the % MGI of A. rolfsii variable; Table S7. Analysis of Variance, R2 and regression equation of the % MGI of P. capsici variable; Table S8. Analysis of Variance, R2 and regression equation of the % MGI of C. gloeosporioides variable; Table S9. Analysis of Variance, R2 and regression equation of the % SGI of G. persicaria variable; Table S10. Analysis of Variance, R2 and regression equation of the % SGI of C. gloeosporioides variable; Table S11. Analysis of Variance of the MIC of G. persicaria variable; Table S12. Analysis of Variance of the MIC of C. gloeosporioides variable; Table S13. Analysis of Variance, R2 and regression equation of the MFC of G. persicaria variable; Table S14. Analysis of Variance of the MFC of C. gloeosporioides variable; Figure S10. Contour (A) and surface (B) plots for the % MGI of G. persicaria combining process variables missing elements in the main text; Figure S11. Contour (A) and surface (B) plots for the % MGI of A. rolfsii combining process variables missing elements in the main text; Figure S12. Contour (A) and surface (B) plots for the % MGI of P. capsici combining process variables missing elements in the main text; Figure S13. Contour (A) and surface (B) plots for the % MGI of C. gloeosporioides combining process variables missing elements in the main text; Figure S14. Contour (A) and surface (B) plots for the % SGI of G. persicaria combining process variables missing elements in the main text; Figure S15. Contour (A) and surface (B) plots for the % SGI of C. gloeosporioides combining process variables missing elements in the main text; Figure S16. Contour (A) and surface (B) plots for the MFC of G. persicaria combining process variables missing elements in the main text.
Author Contributions
Conceptualization, O.V.-B. and R.S.G.-E.; methodology, O.V.-B., J.P.M.-Q., J.M.T.-P., P.d.J.B.-B., I.C.-L. and J.B.H.; formal analysis, O.V.-B. and R.S.G.-E.; investigation, O.V.-B.; writing—original draft preparation, O.V.-B.; writing—review and editing, O.V.-B., R.S.G.-E., J.M.T.-P., P.d.J.B.-B., I.C.-L. and J.B.H.; visualization, O.V.-B. and R.S.G.-E.; supervision, R.S.G.-E. and J.M.T.-P.; project administration, R.S.G.-E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Acknowledgments
O.V.-B. thanks CONAHCYT for the scholarship granted for its graduate studies.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Agrios, G.N. Plant Pathology; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Carmona, M.; Sautua, F. La problemática de la resistencia de hongos a fungicidas. Causas y efectos en cultivos extensivos. Agron. Ambiente 2017, 37, 1. [Google Scholar]
- Ortiz, G.; Pacheco-Moises, F.; Macías-Islas, M.; Jiménez-Gil, F.; Miranda, A.; Flores-Alvarado, L.; Cruz-Ramos, J.; Morales-Sánchez, E.; Ramírez-Ramírez, V.; Alatorre-Jiménez, M.; et al. Toxicidad de plaguicidas y su asociación con la enfermedad de Parkinson. Arch. Neurocienc. 2011, 16, 33. [Google Scholar]
- Belhi, Z.; Boulenouar, N.; Abdelkrim, C. The use of natural products against Fusarium oxysporum: A review. Nat. Prod. J. 2022, 12, 27–37. [Google Scholar] [CrossRef]
- Cespedes, C.L.; Alarcon, J.; Aqueveque, P.M.; Lobo, T.; Becerra, J.; Balbontin, C.; Avila, J.G.; Kubo, I.; Seigler, D.S. New environmentally-friendly antimicrobials and biocides from Andean and Mexican biodiversity. Environ. Res. 2015, 142, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Chemat, S.; Cherfouh, R.; Meklati, B.Y.; Belanteur, K. Composition and microbial activity of thyme (Thymus algeriensis genuinus) essential oil. J. Essent. Oil Res. 2012, 24, 5–11. [Google Scholar] [CrossRef]
- Do, T.C.V.; Vu, T.C. Optimization of essential oil production from Cymbopogon citratus in Vietnam by hydro-distillation. Indones. J. Chem. 2024, 24, 459–469. [Google Scholar] [CrossRef]
- Garzoli, S.; Pirolli, A.; Vavala, E.; Di Sotto, A.; Sartorelli, G.; Božović, M.; Angiolella, L.; Mazzanti, G.; Pepi, F.; Rngno, R. Multidisciplinary approach to determine the optimal time and period for extracting the essential oil from Mentha suaveolens Ehrh. Molecules 2015, 20, 9640–9655. [Google Scholar] [CrossRef]
- Stankovic, M.; Cakic, M.; Nikolic, N.; Nikolić, G. The effect of the hydrodistillation technique on the yield and composition of the essential oil from the leaves of Mentha verticillata L. Hem. Ind. 2001, 55, 389–393. [Google Scholar]
- Sharma, N.; Tripathi, A. Fungitoxicity of the essential oil of Citrus sinensis on post-harvest pathogens. World J. Microb. Biot. 2006, 22, 587–593. [Google Scholar] [CrossRef]
- Stupar, M.; Grbić, M.L.; Džamić, A.; Unković, N.; Ristić, M.; Jelikić, A.; Vukojević, J. Antifungal activity of selected essential oils and biocide benzalkonium chloride against the fungi isolated from cultural heritage objects. S. Afr. J. Bot. 2014, 93, 118–124. [Google Scholar] [CrossRef]
- Hänel, H.; Raether, W. A more sophisticated method of determining the fungicidal effect of water-insoluble preparations with a cell harvester, using miconazole as an example./eine verbesserte methode zur bestimmung der fungizidie von wasserunlöslichen präparaten mit hilfe eines zellerntegerätes am beispiel von miconazol. Mycoses 1988, 31, 148–154. [Google Scholar] [PubMed]
- Wei, C.; Wan, C.; Huang, F.; Guo, T. Extraction of Cinnamomum longepaniculatum deciduous leaves essential oil using solvent-free microwave extraction: Process optimization and quality evaluation. Oil Crop Sci. 2023, 8, 7–15. [Google Scholar] [CrossRef]
- Zhao, T.; Gao, F.; Zhou, L.; Song, T. Essential oil from Inula britannica extraction with SF-CO2 and its antifungal activity. J. Integr. Agric. 2013, 12, 1791–1798. [Google Scholar] [CrossRef]
- Chen, C.; Wan, C.; Peng, X.; Chen, Y.; Chen, M.; Chen, J. Optimization of antifungal extracts from Ficus hirta fruits using response surface methodology and antifungal activity tests. Molecules 2015, 20, 19647–19659. [Google Scholar] [CrossRef]
- Soto-Armenta, L.C.; Sacramento-Rivero, J.C.; Acereto-Escoffié, P.O.; Peraza-González, E.E.; Reyes-Sosa, C.F.; Rocha-Uribe, J.A. Extraction yield of essential oil from Lippia graveolens leaves by steam distillation at laboratory and pilot scales. J. Essent. Oil Bear. Plants 2017, 20, 610–621. [Google Scholar] [CrossRef]
- Bueno-Durán, A.Y.; Cervantes-Martínez, J.; Obledo-Vázquez, E.N. Composition of essential oil from Lippia graveolens. Relationship between spectral light quality and thymol and carvacrol content. J. Essent. Oil Res. 2014, 26, 153–160. [Google Scholar] [CrossRef]
- Souza, L.M.; da Fonseca, F.S.A.; Silva, J.C.R.L.; Martins, E.R. Seasonal variation of essential oil of germplasm of Lippia origanoides Kunth (Verbenaceae). Boletín Latinoam. Y Del Caribe De Plantas Med. Y Aromáticas 2022, 21, 716–724. [Google Scholar] [CrossRef]
- Araújo, E.R.; Costa-Carvalho, R.R.; Fontes, M.G.; Laranjeira, D.; Blank, A.F.; Alves, P.B. Antifungal activity of essential oils of Lippia species of Colletotrichum sp. in vitro. Acta Hortic. 2012, 1198, 9–15. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, Z.; Wei, M.; Xie, Y.; He, S.; Shi, H.; Lin, Z. Evaluation of the antifungal activity of individual and combined monoterpenes against Rhizopus stolonifer and Absidia coerulea. Environ. Sci. Pollut. Res. 2019, 26, 7804–7809. [Google Scholar] [CrossRef] [PubMed]
- Leal, L.E.; Alarcón, A.A.; Ortega-Baes, P.; Cayo, F.; Alarcón, R. Effects of essential oils from two Lippia species on growth of phytopathogenic fungi. Bol. latinoam. Caribe Plantas Med. Aromát. 2018, 17, 30–35. [Google Scholar]
- Oliveira, T.N.S.; Silva, C.M.S.; Malveira, E.A.; Aguiar, T.K.B.; Santos, H.S.; Albuquerque, C.C.; Morais, M.B.; Teixeira, E.H.; Vasconcelos, M.A. Antifungal and antibiofilm activities of the essential oil of leaves from Lippia gracilis Schauer against phytopathogenic fungi. J. Appl. Microbiol. 2021, 130, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Regnier, T.; Combrinck, S.; Du, W. Improvement of postharvest quality of subtropical fruits using Lippia Scaberrima essential oil. Acta Hortic. 2009, 877, 1567–1573. [Google Scholar] [CrossRef]
- Antonia, B.D.; Oliveira, J.; da Silva, P.P.M.; Biazotto, A.D.; de Toledo, N.M.V.; da Glória, E.M.; Spoto, M.H.F. Positive effect of Lippia sidoides essential oil associated with carboxymethylcellulose in the control of anthracnose in avocado. Food Prod. Process. Nutr. 2024, 6, 39. [Google Scholar] [CrossRef]
- Gow Neil, A.R.; Latge, J.P.; Munro Carol, A. The fungal cell wall: Structure, biosynthesis, and function. Microbiol. Spectr. 2017, 5, 10–1128. [Google Scholar]
- Hernández, T.; Canales, M.; García, A.M.; Duran, A.; Meráz, S.; Dávila, P.; Ávila, J.G. Antifungal activity of the essential oils of two Verbenaceae: Lantana achyranthifolia and Lippia graveolens of Zapotitlan de las Salinas, Puebla (Mexico). Bol. Latinoam. Caribe Plantas Med. Aromát. 2008, 7, 203–207. [Google Scholar]
- Linde, J.H.; Combrinck, S.; Regnier, T.J.C.; Virijevic, S. Chemical composition and antifungal activity of the essential oils of Lippia rehmannii from South Africa. S. Afr. J. Bot. 2010, 76, 37–42. [Google Scholar] [CrossRef]
- Roberson, R.W. Subcellular structure and behaviour in fungal hyphae. J. Microsc. 2020, 280, 75–85. [Google Scholar] [CrossRef]
- Feofilova, E.P. The kingdom fungi: Heterogeneity of physiological and biochemical properties and relationships with plants, animals, and prokaryotes (Review). Appl. Biochem. Microbiol. 2001, 37, 124–137. [Google Scholar] [CrossRef]
- Hurdeal, V.G.; Gentekaki, E.; Hyde, K.D.; Jeewon, R. Where are the basal fungi? Current status on diversity, ecology, evolution, and taxonomy. Biologia 2021, 76, 421–440. [Google Scholar] [CrossRef]
- Ruiz-Herrera, J.; Ortiz-Castellanos, L. Analysis of the phylogenetic relationships and evolution of the cell walls from yeasts and fungi. FEMS Yeast Res. 2010, 10, 225–243. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).