Biofuels from Microalgae: A Review on Microalgae Cultivation, Biodiesel Production Techniques and Storage Stability
Abstract
1. Introduction
2. Biochemical Composition and Lipid Synthesis Pathway in Microalgae
| S. No. | Strain/Microalgae Biomass | Biochemical Composition | Elemental Composition | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Carbohydrate | Protein | Lipid | Carbon | Hydrogen g | Nitrogen | Sulfur | Oxygen | ||
| 1 | T. dimorphous | 26.15 | 36.63 | 28.33 | 49.94 | 7.58 | 6.60 | 0.52 | 35.35 |
| 2 | T. obliquus | 27.94 | 46.37 | 18.33 | 49.31 | 7.46 | 7.01 | 0.55 | 35.67 |
| 3 | Chlorella sp. | 34.61 | 31.13 | 21.66 | 53.91 | 7.71 | 6.01 | 0.43 | 31.95 |
| 4 | C. sorokiniana | 36.56 | 39.38 | 15.83 | 52.80 | 7.74 | 5.32 | 0.17 | 33.79 |
| 5 | P. cruentum | 13.89 | 42.90 | 14.67 | - | - | - | - | - |
| 6 | M. coccoides | 40.65 | 20.65 | 17 | 35.55 | 6.01 | 5.67 | - | - |
| 7 | Desmodesmus sp. | - | 18.6 | 38.8 | 25.8 | 5.4 | 6.2 | 0.6 | - |
| 8 | Nanofrustulum sp. 2 | - | 12..52 | 12.52 | 27.45 | 4.3 | 2.90 | 0.96 | - |
| 9 | Scenedesmus sp. | - | - | 50.6 | 43.8 | 5.7 | 8.1 | - | - |
| 10 | Chroococcidiopsis sp. | 45.40 | 36.72 | 5.6 | - | - | - | - | - |
3. Factors Responsible for Enhancing Lipid Production in Microalgae
3.1. Light
3.2. Temperature
3.3. Growth Nutrients
3.4. Phosphorous
3.5. Trace Metals
3.6. Cell Density and Mixing
3.7. Genetic and Metabolic Regulation
| Microalgae | Cultivation Mode | Cultivation Conditions | Bioreactor/Open Ponds | Biomass Productivity | Lipid Productivity | Biodiesel Yield | Ref. |
|---|---|---|---|---|---|---|---|
| Spirulina | Growth medium—ASN III Temp—25–27 °C, Time—14 days, Light intensity—94.5 μmol−2 s−1 | PBR | - | - | 99% | [79] | |
| Golenkinia sp. SDEC-16s algae | Autotrophic | Growth medium—seawater supplemented with monosodium glutamate wastewater Time—10 days | PBR | 0.26 g/L/d | 98.99 mg/L/d | 98% | [80] |
| Chlorella sp. | Heterotrophic | Growth medium—BBM Carbon source—Glycerol (4 g/L) | PBR | 446.50 mg/L/d | 165.15 mg/L/d | >20% | [81] |
| Chlorella vulgaris ESP | Autotrophic | Growth medium—Basal Carbon source −2% CO2, Artificial light | PBR | 250 mg/L/d | 56.2 mg/L/d | ||
| Scenedesmus obliquus | Heterotrophic | Growth medium—BG-11 Carbon source—acetate | EMF | 92.3 | 56 | 60.56% | [82,83] |
| Chlorella vulgaris | Autotrophic | Growth medium—Commercial fertilizer Outdoor conditions, pH = 7 | Open raceway ponds | 77 mg/L/d | 11.52 mg/L/d | 84.01% | [6] |
| Chlorella minutissima | Autotrophic | Growth medium—Commercial fertilizer Outdoor conditions, pH = 7 | Open raceway ponds | 162.0 mg/L/day | 31.43 mg/L/day | 90.21% | [5] |
| Ettlia sp. YC001 | Mixotrophic | Growth medium—BG-11 Carbon source—2% CO2–mixed air + Glucose; Artificial light | PBR | 410 mg/L/day | - | 15.5% | [84] |
| Chlorella protothecoides | Heterotrophic | Growth medium—BG-11 Carbon source—Glucose | EMF | 1656.25 mg/L/day | 687.5 mg/L/day | 73.2% | [85] |
| Chlorella pyrenoidosa | Heterotrophic | glucose and B.Peptone concentrations—~13–24 g/L and 1.3 ± 0.1 g/L | stir-tank bioreactor | - | - | 76% | [86] |
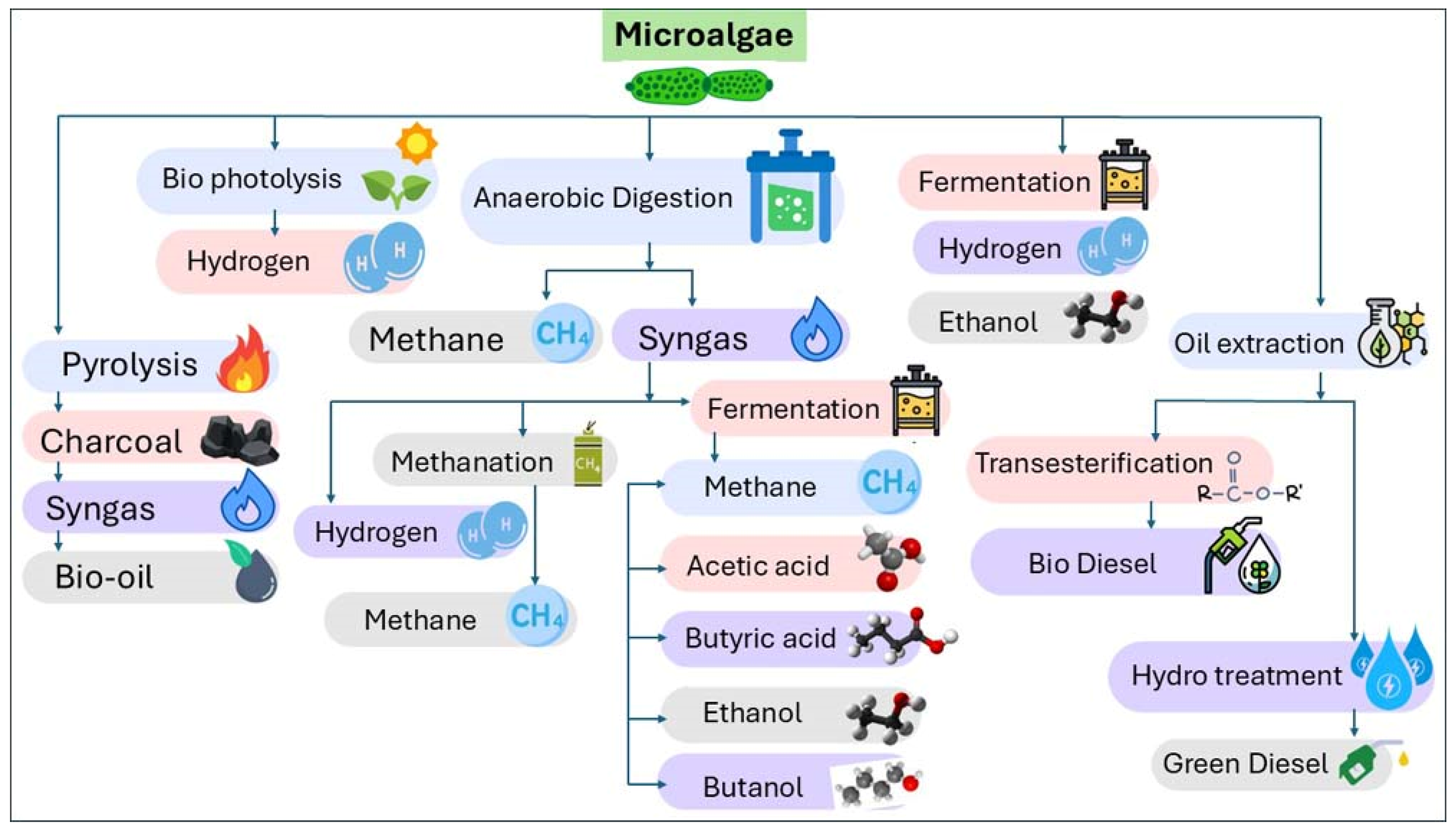
4. Upstream Process for Biodiesel Production
4.1. Growth Techniques
4.1.1. Open Ponds System
4.1.2. Closed System
4.2. Utilization of Wastewater for Microalgae Cultivation
4.3. Microalgae Harvesting
5. Downstream Processes
5.1. Conventional Processes of Microalgae Biomass
5.1.1. Lipid Extraction from Microalgae
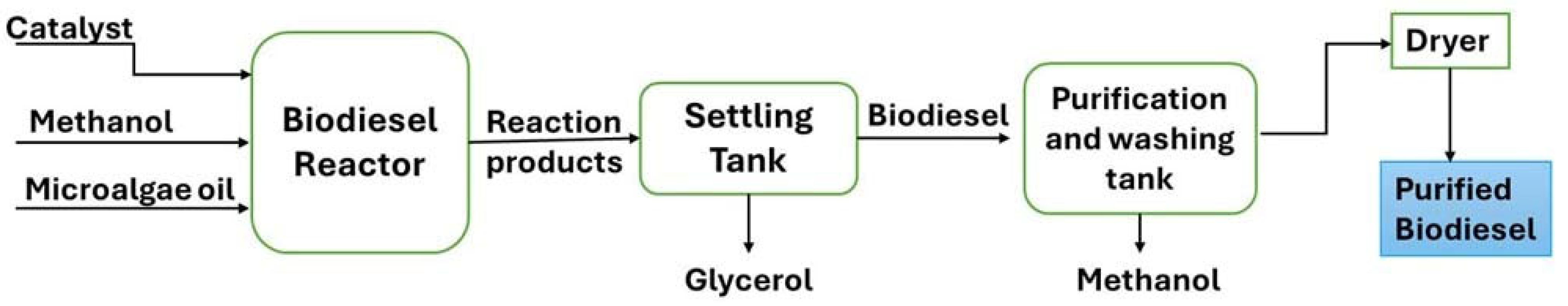
5.1.2. Transesterification of Extracted Lipids
5.2. In Situ Transesterification of Microalgae Biomass
5.3. Fuel Properties and Storage Stability of Microalgae
| S. No. | Parameters | Method | Microalgae Biodiesel Chlorella vulgaris | Chlorella minutissima | Spirulina platensis | Chlorella emersonii |
|---|---|---|---|---|---|---|
| 1. | Calorific value (MJ/kg) | D240 | 39.45 | 39.15 | 45.63 | 36.9 |
| 2. | Density at 15 °C (kg/m3) | D7777 | 889 | 0.877 | 0.8637 | nd |
| 3 | Viscosity at 40 °C (mm2/s) or cSt | D445 | 5.72 | 4.75 | 12.4 | 3.96 |
| Flash point (°C) | D93 | 155 | - | 189 | 212 | |
| 4 | Pour point (°C) | D97 | −12 | −3.76 | −9 | 7 |
| 5 | Copper strip corrosion | D130 | 1 | - | 1a | nd |
| 6 | Cetane number | 57.03 | - | 70 | 52.5 | |
| 7 | Acid number (mg KOH/g) | D664 | 0.49 | - | 0.75 | nd |
| 8 | Water and sediment (%) | D2709 | 0.03 | - | 3.9 | nd |
| 9 | Methyl linolenate (%) | EN 14103 | 7.54 | 7.45% | nd | nd |
| 10 | Unsaturated ester (>4 double bonds) % | Internal method—GC | 0 | nd | nd | nd |
| 11 | Oxidation stability (IP, at 140 °C, h) | ASTM-D7545 and prEN16091 | 3.08 | 2.80 (min) | nd | nd |
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sharma, A.K.; Sharma, P.K.; Chintala, V.; Khatri, N.; Patel, A. Environment-Friendly Biodiesel/Diesel Blends for Improving the Exhaust Emission and Engine Performance to Reduce the Pollutants Emitted from Transportation Fleets. Int. J. Environ. Res. Public Health 2020, 17, 3896. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Agrawal, S.; Nawaz, T.; Pan, S.; Selvaratnam, T. A Review of Algae-Based Produced Water Treatment for Biomass and Biofuel Production. Water 2020, 12, 2351. [Google Scholar] [CrossRef]
- Khoo, C.G.; Dasan, Y.K.; Lam, M.K.; Lee, K.T. Algae Biorefinery: Review on a Broad Spectrum of Downstream Processes and Products. Bioresour. Technol. 2019, 292, 121964. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wang, Y.; He, Y.; Liu, B.; Mou, H.; Chen, F.; Yang, S. Microalgae-Derived Pigments for the Food Industry. Mar. Drugs 2023, 21, 82. [Google Scholar] [CrossRef]
- Sharma, A.K.; Sharma, A.; Singh, Y.; Chen, W.-H. Production of a Sustainable Fuel from Microalgae Chlorella minutissima Grown in a 1500 L Open Raceway Ponds. Biomass Bioenergy 2021, 149, 106073. [Google Scholar] [CrossRef]
- Sharma, A.K.; Sahoo, P.K.; Singhal, S.; Joshi, G. Exploration of Upstream and Downstream Process for Microwave Assisted Sustainable Biodiesel Production from Microalgae Chlorella vulgaris. Bioresour. Technol. 2016, 216, 793–800. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jung, J.-M.; Jung, S.; Park, Y.-K.; Tsang, Y.F.; Lin, K.-Y.A.; Choi, Y.-E.; Kwon, E.E. Biodiesel from Microalgae: Recent Progress and Key Challenges. Prog. Energy Combust. Sci. 2022, 93, 101020. [Google Scholar] [CrossRef]
- Shah, S.H.; Raja, I.A.; Rizwan, M.; Rashid, N.; Mahmood, Q.; Shah, F.A.; Pervez, A. Potential of Microalgal Biodiesel Production and Its Sustainability Perspectives in Pakistan. Renew. Sustain. Energy Rev. 2018, 81, 76–92. [Google Scholar] [CrossRef]
- Li, K.; Liu, Q.; Fang, F.; Luo, R.; Lu, Q.; Zhou, W.; Huo, S.; Cheng, P.; Liu, J.; Addy, M. Microalgae-Based Wastewater Treatment for Nutrients Recovery: A Review. Bioresour. Technol. 2019, 291, 121934. [Google Scholar] [CrossRef]
- Elrayies, G.M. Microalgae: Prospects for Greener Future Buildings. Renew. Sustain. Energy Rev. 2018, 81, 1175–1191. [Google Scholar] [CrossRef]
- Naik, B.; Mishra, R.; Kumar, V.; Mishra, S.; Gupta, U.; Rustagi, S.; Gupta, A.K.; Preet, M.S.; Bhatt, S.C.; Rizwanuddin, S. Micro-Algae: Revolutionizing Food Production for a Healthy and Sustainable Future. J. Agric. Food Res. 2024, 15, 100939. [Google Scholar] [CrossRef]
- Yang, C.; Li, R.; Zhang, B.; Qiu, Q.; Wang, B.; Yang, H.; Ding, Y.; Wang, C. Pyrolysis of Microalgae: A Critical Review. Fuel Process. Technol. 2019, 186, 53–72. [Google Scholar] [CrossRef]
- Jiménez-Llanos, J.; Ramírez-Carmona, M.; Rendón-Castrillón, L.; Ocampo-López, C. Sustainable Biohydrogen Production by Chlorella sp. Microalgae: A Review. Int. J. Hydrogen Energy 2020, 45, 8310–8328. [Google Scholar] [CrossRef]
- Katiyar, R.; Gurjar, B.R.; Biswas, S.; Pruthi, V.; Kumar, N.; Kumar, P. Microalgae: An Emerging Source of Energy Based Bio-Products and a Solution for Environmental Issues. Renew. Sustain. Energy Rev. 2017, 72, 1083–1093. [Google Scholar] [CrossRef]
- Patel, A.K.; Choi, Y.Y.; Sim, S.J. Emerging Prospects of Mixotrophic Microalgae: Way Forward to Sustainable Bioprocess for Environmental Remediation and Cost-Effective Biofuels. Bioresour. Technol. 2020, 300, 122741. [Google Scholar] [CrossRef]
- Sharma, A.K.; Kumar Ghodke, P.; Manna, S.; Chen, W.-H. Emerging Technologies for Sustainable Production of Biohydrogen Production from Microalgae: A State-of-the-Art Review of Upstream and Downstream Processes. Bioresour. Technol. 2021, 342, 126057. [Google Scholar] [CrossRef]
- Yin, Z.; Zhu, L.; Li, S.; Hu, T.; Chu, R.; Mo, F.; Hu, D.; Liu, C.; Li, B. A Comprehensive Review on Cultivation and Harvesting of Microalgae for Biodiesel Production: Environmental Pollution Control and Future Directions. Bioresour. Technol. 2020, 301, 122804. [Google Scholar] [CrossRef]
- Sharma, A.K.; Ghodke, P.; Sharma, P.K.; Manna, S.; Pugazhendhi, A.; Matsakas, L.; Patel, A. Holistic Utilization of Chlorella pyrenoidosa Microalgae for Extraction of Renewable Fuels and Value-Added Biochar through in situ Transesterification and Pyrolysis Reaction Process. Biomass Convers. Biorefin. 2022, 14, 5261–5274. [Google Scholar] [CrossRef]
- Moshood, T.D.; Nawanir, G.; Mahmud, F. Microalgae Biofuels Production: A Systematic Review on Socioeconomic Prospects of Microalgae Biofuels and Policy Implications. Environ. Chall. 2021, 5, 100207. [Google Scholar] [CrossRef]
- Deshmukh, S.; Kumar, R.; Bala, K. Microalgae Biodiesel: A Review on Oil Extraction, Fatty Acid Composition, Properties and Effect on Engine Performance and Emissions. Fuel Process. Technol. 2019, 191, 232–247. [Google Scholar] [CrossRef]
- Ananthi, V.; Raja, R.; Carvalho, I.S.; Brindhadevi, K.; Pugazhendhi, A.; Arun, A. A Realistic Scenario on Microalgae Based Biodiesel Production: Third Generation Biofuel. Fuel 2021, 284, 118965. [Google Scholar] [CrossRef]
- Goh, B.H.H.; Ong, H.C.; Cheah, M.Y.; Chen, W.H.; Yu, K.L.; Mahlia, T.M.I. Sustainability of Direct Biodiesel Synthesis from Microalgae Biomass: A Critical Review. Renew. Sustain. Energy Rev. 2019, 107, 59–74. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from Microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Dismukes, G.C.; Carrieri, D.; Bennette, N.; Ananyev, G.M.; Posewitz, M.C. Aquatic Phototrophs: Efficient Alternatives to Land-Based Crops for Biofuels. Curr. Opin. Biotechnol. 2008, 19, 235–240. [Google Scholar] [CrossRef]
- Javed, F.; Aslam, M.; Rashid, N.; Shamair, Z.; Khan, A.L.; Yasin, M.; Fazal, T.; Hafeez, A.; Rehman, F.; Rehman, M.S.U.; et al. Microalgae-Based Biofuels, Resource Recovery and Wastewater Treatment: A Pathway towards Sustainable Biorefinery. Fuel 2019, 255, 115826. [Google Scholar] [CrossRef]
- Neo, Y.T.; Chia, W.Y.; Lim, S.S.; Ngan, C.L.; Kurniawan, T.A.; Chew, K.W. Smart Systems in Producing Algae-Based Protein to Improve Functional Food Ingredients Industries. Food Res. Int. 2023, 165, 112480. [Google Scholar] [CrossRef]
- Tokuşoglu, O.; üUnal, M.K. Biomass Nutrient Profiles of Three Microalgae: Spirulina platensis, Chlorella vulgaris, and Isochrisis galbana. J. Food Sci. 2003, 68, 1144–1148. [Google Scholar] [CrossRef]
- Montero-Lobato, Z.; Fuentes, J.L.; Garbayo, I.; Ascaso, C.; Wierzchos, J.; Vega, J.M.; Vílchez, C. Identification, Biochemical Composition and Phycobiliproteins Production of Chroococcidiopsis sp. from Arid Environment. Process Biochem. 2020, 97, 112–120. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, J.; Pan, M.; Hao, Y.; Hu, R.; Xiao, W.; Li, G.; Lyu, T. Valorisation of Microalgae Residues after Lipid Extraction: Pyrolysis Characteristics for Biofuel Production. Biochem. Eng. J. 2022, 179, 108330. [Google Scholar] [CrossRef]
- Hait, S.; Gawali, S.; Awathare, P.; Nawaj Alam, S.; Singh, B.; Guldhe, A. Integrated Approach for Microalgal Biomass Generation Using Poultry Litter and Wastewater-Based Media and Its Application for Bio-Oil Production. Energy Convers. Manag. 2024, 313, 118610. [Google Scholar] [CrossRef]
- Ardiles, P.; Cerezal-Mezquita, P.; Salinas-Fuentes, F.; Órdenes, D.; Renato, G.; Ruiz-Domínguez, M.C. Biochemical Composition and Phycoerythrin Extraction from Red Microalgae: A Comparative Study Using Green Extraction Technologies. Processes 2020, 8, 1628. [Google Scholar] [CrossRef]
- Arif, M.; Li, Y.; El-Dalatony, M.M.; Zhang, C.; Li, X.; Salama, E.-S. A Complete Characterization of Microalgal Biomass through FTIR/TGA/CHNS Analysis: An Approach for Biofuel Generation and Nutrients Removal. Renew. Energy 2021, 163, 1973–1982. [Google Scholar] [CrossRef]
- Ampofo, J.; Abbey, L. Microalgae: Bioactive Composition, Health Benefits, Safety and Prospects as Potential High-Value Ingredients for the Functional Food Industry. Foods 2022, 11, 1744. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.J.L.B.; Laurens, L.M.L. Microalgae as Biodiesel & Biomass Feedstocks: Review & Analysis of the Biochemistry, Energetics & Economics. Energy Environ. Sci. 2010, 3, 554. [Google Scholar] [CrossRef]
- Liu, B.; Benning, C. Lipid Metabolism in Microalgae Distinguishes Itself. Curr. Opin. Biotechnol. 2013, 24, 300–309. [Google Scholar]
- Khoo, K.S.; Chew, K.W.; Yew, G.Y.; Leong, W.H.; Chai, Y.H.; Show, P.L.; Chen, W.-H. Recent Advances in Downstream Processing of Microalgae Lipid Recovery for Biofuel Production. Bioresour. Technol. 2020, 304, 122996. [Google Scholar] [CrossRef]
- Dong, M.; Zhang, Y.; Yu, Q.; Liu, Q.; Zhou, X.; Jin, C. Regulation of Light Quality on Lipid Production, Biodiesel Quality, and Nutritional Quality of Phaeodactylum tricornutum. Aquac. Int. 2023, 31, 1231–1251. [Google Scholar] [CrossRef]
- Mazzella, N.; Vrba, R.; Moreira, A.; Creusot, N.; Eon, M.; MillanNavarro, D.; Lavoie, I.; Morin, S. Mixed Light Photoperiod and Biocide Pollution Affect Lipid Profiles of Periphyton Communities in Freshwater Ecosystems. J. Hazard. Mater. Adv. 2023, 12, 100378. [Google Scholar] [CrossRef]
- Chin, G.J.W.L.; Andrew, A.R.; Abdul-Sani, E.R.; Yong, W.T.L.; Misson, M.; Anton, A. The Effects of Light Intensity and Nitrogen Concentration to Enhance Lipid Production in Four Tropical Microalgae. Biocatal. Agric. Biotechnol. 2023, 48, 102660. [Google Scholar] [CrossRef]
- Rosenberg, J.N.; Oyler, G.A.; Wilkinson, L.; Betenbaugh, M.J. A Green Light for Engineered Algae: Redirecting Metabolism to Fuel a Biotechnology Revolution. Curr. Opin. Biotechnol. 2008, 19, 430–436. [Google Scholar] [CrossRef]
- Hijazi, R.M.; Mounsef, J.R.; Kanaan, H.Y. Comparison of Light Intensity Effect on Microalgal Growth in Cactus-like and Cylindrical Photo Bioreactors. Processes 2024, 12, 1664. [Google Scholar] [CrossRef]
- Li, S.; Xing, D.; Sun, C.; Jin, C.; Zhao, Y.; Gao, M.; Guo, L. Effect of Light Intensity and Photoperiod on High-Value Production and Nutrient Removal Performance with Bacterial-Algal Coupling System. J. Environ. Manag. 2024, 356, 120595. [Google Scholar] [CrossRef] [PubMed]
- Esteves, A.F.; Salgado, E.M.; Vilar, V.J.P.; Gonçalves, A.L.; Pires, J.C.M. A Growth Phase Analysis on the Influence of Light Intensity on Microalgal Stress and Potential Biofuel Production. Energy Convers. Manag. 2024, 311, 118511. [Google Scholar] [CrossRef]
- Minhas, A.K.; Gaur, S.; Adholeya, A. Influence of Light Intensity and Photoperiod on the Pigment and, Lipid Production of Dunaliella tertiolecta and Nannochloropsis oculata under Three Different Culture Medium. Heliyon 2023, 9, e12801. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huff, J.; Crunkleton, D.W.; Johannes, T.W. Light Intensity and Spectral Quality Modulation for Improved Growth Kinetics and Biochemical Composition of Chlamydomonas reinhardtii. J. Biotechnol. 2023, 375, 28–39. [Google Scholar] [CrossRef]
- Liu, W.; Ji, Y.; Long, Y.; Huang, W.; Zhang, C.; Wang, H.; Xu, Y.; Lei, Z.; Huang, W.; Liu, D. The Role of Light Wavelengths in Regulating Algal-Bacterial Granules Formation, Protein and Lipid Accumulation, and Microbial Functions. J. Environ. Manag. 2023, 337, 117750. [Google Scholar] [CrossRef]
- Shang, X.; Yang, Y.; Zan, Y.; Sun, Z.; Lu, Z.; Sun, J. Effects of Temperature on the Growth, Total Lipid Content and Fatty Acid Composition of Skeletonema dohrnii. Front. Mar. Sci. 2024, 11, 1361157. [Google Scholar] [CrossRef]
- Wang, D.; Gao, X.; Wang, X.; Yuan, X.; Guo, X.; Zhang, Y.; Xu, K.; Li, Z. Diverse Thermal Responses of the Growth, Photosynthesis, Lipid and Fatty Acids in the Terrestrial Oil-Producing Microalga Vischeria Sp. WL1. J. Appl. Phycol. 2024, 36, 29–39. [Google Scholar] [CrossRef]
- Zheng, S.; Sun, S.; Zou, S.; Song, J.; Hua, L.; Chen, H.; Wang, Q. Effects of Culture Temperature and Light Regimes on Biomass and Lipid Accumulation of Chlamydomonas reinhardtii under Carbon-Rich and Nitrogen-Limited Conditions. Bioresour. Technol. 2024, 399, 130613. [Google Scholar] [CrossRef]
- Visentin, T.G.; Guimarães, B.M.; Bastos, R.G. Effects of Temperature, PH, and C/N Ratio of Sugarcane Wastewater Processing (Vinasse) on Phormidium autumnale Heterotrophic Cultivation. Algal. Res. 2024, 77, 103349. [Google Scholar] [CrossRef]
- Ferrer-Ledo, N.; Stegemüller, L.; Janssen, M.; Wijffels, R.H.; Barbosa, M.J. Growth and Fatty Acid Distribution over Lipid Classes in Nannochloropsis oceanica Acclimated to Different Temperatures. Front. Plant Sci. 2023, 14, 1078998. [Google Scholar] [CrossRef] [PubMed]
- Daneshvar, E.; Wicker, R.J.; Show, P.-L.; Bhatnagar, A. Biologically-Mediated Carbon Capture and Utilization by Microalgae towards Sustainable CO2 Biofixation and Biomass Valorization–A Review. Chem. Eng. J. 2022, 427, 130884. [Google Scholar] [CrossRef]
- Yew, G.Y.; Lee, S.Y.; Show, P.L.; Tao, Y.; Law, C.L.; Nguyen, T.T.C.; Chang, J.S. Recent Advances in Algae Biodiesel Production: From Upstream Cultivation to Downstream Processing. Bioresour. Technol. Rep. 2019, 7, 100227. [Google Scholar] [CrossRef]
- Mohan, S.V.; Rohit, M.V.; Chiranjeevi, P.; Chandra, R.; Navaneeth, B. Heterotrophic Microalgae Cultivation to Synergize Biodiesel Production with Waste Remediation: Progress and Perspectives. Bioresour. Technol. 2015, 184, 169–178. [Google Scholar] [CrossRef]
- Wang, J.; Yang, H.; Wang, F. Mixotrophic Cultivation of Microalgae for Biodiesel Production: Status and Prospects. Appl. Biochem. Biotechnol. 2014, 172, 3307–3329. [Google Scholar] [CrossRef]
- Singh, S.K.; Sundaram, S.; Sinha, S.; Rahman, M.A.; Kapur, S. Recent Advances in CO2 Uptake and Fixation Mechanism of Cyanobacteria and Microalgae. Crit. Rev. Environ. Sci. Technol. 2016, 46, 1297–1323. [Google Scholar] [CrossRef]
- Pekkoh, J.; Ruangrit, K.; Aurepatipan, N.; Duangjana, K.; Sensupa, S.; Pumas, C.; Chaichana, C.; Pathom-aree, W.; Kato, Y.; Srinuanpan, S. CO2 to Green Fuel Converter: Photoautotrophic-Cultivation of Microalgae and Its Lipids Conversion to Biodiesel. Renew. Energy 2024, 222, 119919. [Google Scholar] [CrossRef]
- Wen, X.; Tao, H.; Peng, X.; Wang, Z.; Ding, Y.; Xu, Y.; Liang, L.; Du, K.; Zhang, A.; Liu, C.; et al. Sequential Phototrophic-Mixotrophic Cultivation of Oleaginous Microalga Graesiella Sp. WBG-1 in a 1000 m2 Open Raceway Pond. Biotechnol. Biofuels 2019, 12, 27. [Google Scholar] [CrossRef]
- Leong, W.H.; Saman, N.A.M.; Kiatkittipong, W.; Assabumrungrat, S.; Najdanovic-Visak, V.; Wang, J.; Khoo, K.S.; Lam, M.K.; Mohamad, M.; Lim, J.W. Photoperiod-Induced Mixotrophic Metabolism in Chlorella vulgaris for High Biomass and Lipid to Biodiesel Productions Using Municipal Wastewater Medium. Fuel 2022, 313, 123052. [Google Scholar] [CrossRef]
- Klamczynska, B.; Mooney, W.D. Heterotrophic Microalgae: A Scalable and Sustainable Protein Source. In Sustainable Protein Sources; Elsevier: Amsterdam, The Netherlands, 2017; pp. 327–339. [Google Scholar]
- Sharma, A.K.; Sahoo, P.K.; Singhal, S. Comparative Evolution of Biomass Production and Lipid Accumulation Potential of Chlorella Species Grown in a Bubble Column Photobioreactor. Biofuels 2016, 7, 1138040. [Google Scholar] [CrossRef]
- Sharma, A.K.; Sahoo, P.K.; Singal, S. Influence of Different Nitrogen and Organic Carbon Sources on Microalgae Growth and Lipid Production. IOSR J. Pharm. Biol. Sci. 2015, 10, 48–53. [Google Scholar]
- Sharma, A.K.; Sahoo, P.K.; Singhal, S.; Patel, A. Impact of Various Media and Organic Carbon Sources on Biofuel Production Potential from Chlorella spp. 3 Biotech 2016, 6, 116. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Liu, B.-F.; Kong, F.; Song, Q.; Ren, N.-Q.; Ren, H.-Y. Lipid Accumulation by a Novel Microalga Parachlorella kessleri R-3 with Wide PH Tolerance for Promising Biodiesel Production. Algal Res. 2023, 69, 102925. [Google Scholar] [CrossRef]
- Şirin, P.A.; Serdar, S. Effects of Nitrogen Starvation on Growth and Biochemical Composition of Some Microalgae Species. Folia Microbiol. 2024, 69, 889–902. [Google Scholar] [CrossRef]
- Nguyen, P.T.H.; Cao, P.; Vo, T. Effect of Phosphorus on the Growth, Pigmentation and Lipid Accumulation in Microalgae Picochlorum sp. Eur. J. Appl. Sci. Eng. Technol. 2024, 2, 151–159. [Google Scholar] [CrossRef]
- Li, D.-W.; Tan, J.-Z.; Li, Z.-F.; Ou, L.-J. Membrane Lipid Remodeling and Autophagy to Cope with Phosphorus Deficiency in the Dinoflagellate Prorocentrum shikokuense. Chemosphere 2024, 349, 140844. [Google Scholar] [CrossRef]
- Qian, W.; Yang, Y.; Chou, S.; Ge, S.; Li, P.; Wang, X.; Zhuang, L.-L.; Zhang, J. Effect of N/P Ratio on Attached Microalgae Growth and the Differentiated Metabolism along the Depth of Biofilm. Environ. Res. 2024, 240, 117428. [Google Scholar] [CrossRef]
- Manic, D.C.; Redil, R.D.; Rodriguez, I.B. Trace Metals in Phytoplankton: Requirements, Function, and Composition in Harmful Algal Blooms. Sustainability 2024, 16, 4876. [Google Scholar] [CrossRef]
- Tan, S.; Wen, F.; Liu, D.; Lu, H.; Li, L.; Zhu, L. Physiological Responses and Lipid Accumulation of Freshwater Microalgae Chlorella sorokiniana under Short-Term Zinc Stress in Water Solution. Algal Res. 2024, 80, 103528. [Google Scholar] [CrossRef]
- Liu, T.; Guo, H.; Yu, Q.; Wang, Y.; Liu, H.; Zeng, Y.; Wang, Y.; Liu, C.; Li, J. The Impact of Cr(III) and Cr(VI) on Lipid Accumulation in Chlorella pyrenoidosa. Processes 2024, 12, 905. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, J.; Zhang, Z.; Lan, C.Q. High Cell Density Culture of Microalgae in Horizontal Thin-Layer Algal Reactor: Modeling of Light Attenuation and Cell Growth Kinetics. Chem. Eng. J. 2024, 496, 154175. [Google Scholar] [CrossRef]
- Ghosh, G.; Atta, A.; Chakraborty, S. Multiscale Effects of Radial Mixing on Mixotrophic Microalgal Growth and Macromolecular Synthesis in Tubular Bubble-Column Photobioreactors. Algal Res. 2024, 80, 103518. [Google Scholar] [CrossRef]
- Muñoz, C.F.; Südfeld, C.; Naduthodi, M.I.S.; Weusthuis, R.A.; Barbosa, M.J.; Wijffels, R.H.; D’Adamo, S. Genetic Engineering of Microalgae for Enhanced Lipid Production. Biotechnol. Adv. 2021, 52, 107836. [Google Scholar] [CrossRef]
- Khoo, K.S.; Ahmad, I.; Chew, K.W.; Iwamoto, K.; Bhatnagar, A.; Show, P.L. Enhanced Microalgal Lipid Production for Biofuel Using Different Strategies Including Genetic Modification of Microalgae: A Review. Prog. Energy Combust. Sci. 2023, 96, 101071. [Google Scholar] [CrossRef]
- Takahashi, K.; Ide, Y.; Hayakawa, J.; Yoshimitsu, Y.; Fukuhara, I.; Abe, J.; Kasai, Y.; Harayama, S. Lipid Productivity in TALEN-Induced Starchless Mutants of the Unicellular Green Alga Coccomyxa sp. Strain Obi. Algal Res 2018, 32, 300–307. [Google Scholar] [CrossRef]
- Chang, K.S.; Kim, J.; Park, H.; Hong, S.-J.; Lee, C.-G.; Jin, E. Enhanced Lipid Productivity in AGP Knockout Marine Microalga Tetraselmis sp. Using a DNA-Free CRISPR-Cas9 RNP Method. Bioresour. Technol. 2020, 303, 122932. [Google Scholar] [CrossRef]
- Jeon, S.; Lim, J.-M.; Lee, H.-G.; Shin, S.-E.; Kang, N.K.; Park, Y.-I.; Oh, H.-M.; Jeong, W.-J.; Jeong, B.; Chang, Y.K. Current Status and Perspectives of Genome Editing Technology for Microalgae. Biotechnol. Biofuels 2017, 10, 267. [Google Scholar] [CrossRef]
- Mittal, V.; Ghosh, U.K. Optimization of Biodiesel Production from Spirulina Microalgae via Nanocatalytic Transesterification Process. Bioresour. Technol. Rep. 2023, 23, 101504. [Google Scholar] [CrossRef]
- Yu, Z.; Hou, Q.; Liu, M.; Xie, Z.; Ma, M.; Chen, H.; Pei, H. From Lab to Application: Cultivating Limnetic Microalgae in Seawater Coupled with Wastewater for Biodiesel Production on a Pilot Scale. Water Res. 2023, 229, 119471. [Google Scholar] [CrossRef]
- Katiyar, R.; Gurjar, B.R.; Bharti, R.K.; Kumar, A.; Biswas, S.; Pruthi, V. Heterotrophic Cultivation of Microalgae in Photobioreactor Using Low-Cost Crude Glycerol for Enhanced Biodiesel Production. Renew. Energy 2017, 113, 1359–1365. [Google Scholar] [CrossRef]
- Shen, X.-F.; Liu, J.-J.; Chu, F.-F.; Lam, P.K.S.; Zeng, R.J. Enhancement of FAME Productivity of Scenedesmus obliquus by Combining Nitrogen Deficiency with Sufficient Phosphorus Supply in Heterotrophic Cultivation. Appl. Energy 2015, 158, 348–354. [Google Scholar] [CrossRef]
- Shen, X.-F.; Hu, H.; Ma, L.-L.; Lam, P.K.S.; Yan, S.-K.; Zhou, S.-B.; Zeng, R.J. FAMEs Production from Scenedesmus Obliquus in Autotrophic, Heterotrophic and Mixotrophic Cultures under Different Nitrogen Conditions. Environ. Sci. 2018, 4, 461–468. [Google Scholar] [CrossRef]
- Rashid, N.; Ryu, A.J.; Jeong, K.J.; Lee, B.; Chang, Y.-K. Co-Cultivation of Two Freshwater Microalgae Species to Improve Biomass Productivity and Biodiesel Production. Energy Convers. Manag. 2019, 196, 640–648. [Google Scholar] [CrossRef]
- Wang, T.; Ge, H.; Liu, T.; Tian, X.; Wang, Z.; Guo, M.; Chu, J.; Zhuang, Y. Salt Stress Induced Lipid Accumulation in Heterotrophic Culture Cells of Chlorella Protothecoides: Mechanisms Based on the Multi-Level Analysis of Oxidative Response, Key Enzyme Activity and Biochemical Alteration. J. Biotechnol. 2016, 228, 18–27. [Google Scholar] [CrossRef]
- Jacob, A.; Ashok, B.; Usman, K.M. Production of Chlorella pyrenoidosa Biodiesel by Heterotrophic Pathway to Improve CI Engine Output Characteristics Using Statistical Approaches. Process. Saf. Environ. Prot. 2022, 160, 478–490. [Google Scholar] [CrossRef]
- Simonazzi, M.; Pezzolesi, L.; Guerrini, F.; Vanucci, S.; Samorì, C. Bioresource Technology Use of Waste Carbon Dioxide and Pre-Treated Liquid Digestate from Biogas Process for Phaeodactylum tricornutum Cultivation in Photobioreactors and Open Ponds. Bioresour. Technol. 2019, 292, 121921. [Google Scholar] [CrossRef]
- Magalhães, I.B.; de Paula Pereira, A.S.A.; Silva, T.A.; Ferreira, J.; Braga, M.Q.; Couto, E.A.; Assemany, P.P.; Calijuri, M.L. Advancements in High-Rate Algal Pond Technology for Enhanced Wastewater Treatment and Biomass Production: A Review. J. Water Process Eng. 2024, 66, 105929. [Google Scholar] [CrossRef]
- Benemann, J. Microalgae for Biofuels and Animal Feeds. Energies 2013, 6, 5869–5886. [Google Scholar] [CrossRef]
- Alami, A.H.; Alasad, S.; Ali, M.; Alshamsi, M. Investigating Algae for CO2 Capture and Accumulation and Simultaneous Production of Biomass for Biodiesel Production. Sci. Total Environ. 2021, 759, 143529. [Google Scholar] [CrossRef]
- Acién, F.G.; Molina, E.; Reis, A.; Torzillo, G.; Zittelli, G.C.; Sepúlveda, C.; Masojídek, J. Photobioreactors for the Production of Microalgae. In Microalgae-Based Biofuels and Bioproducts; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–44. [Google Scholar]
- Sharma, A.K.; Sahoo, P.K.; Singhal, S. Feasibility of Biodiesel Production from Chlorella vulgaris grown in Flat Plate Photobioreactor under Outdoor Conditions. Int. J. Chemtech. Res. 2015, 8, 671–678. [Google Scholar]
- Rahman, M.R.; Hellgardt, K. Flat-Plate Photobioreactors for Renewable Resources Production. In Algal Bioreactors; Elsevier: Amsterdam, The Netherlands, 2025; pp. 423–447. [Google Scholar]
- Chen, C.; Yeh, K.; Aisyah, R.; Lee, D.; Chang, J. Bioresource Technology Cultivation, Photobioreactor Design and Harvesting of Microalgae for Biodiesel Production: A Critical Review. Bioresour. Technol. 2010, 102, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Vajdi, S.; Aslani, A. Design and Techno-Economic Analysis of Direct CO2 Capturing with Integrated Photobioreactors as a Building Façade. Sustain. Energy Technol. Assessments 2023, 56, 103068. [Google Scholar] [CrossRef]
- Silva, T.A.; de Paula Pereira, A.S.A.; Ferreira, J.; Lorentz, J.F.; de Assis, M.L.; Assemany, P.P.; dos Reis, A.J.D.; Calijuri, M.L. Enhancing Microalgae Biomass Production: Exploring Improved Scraping Frequency in a Hybrid Cultivation System. J. Environ. Manag. 2024, 355, 120505. [Google Scholar] [CrossRef] [PubMed]
- Rörig, L.R.; Gressler, P.D.; Tramontin, D.P.; de Souza Schneider, R.D.C.; Derner, R.B.; de Oliveira Bastos, E.; de Souza, M.P.; Oliveira, C.Y.B. Biomass Productivity and Characterization of Tetradesmus obliquus Grown in a Hybrid Photobioreactor. Bioprocess Biosyst. Eng. 2024, 47, 367–380. [Google Scholar] [CrossRef]
- Nishshanka, G.K.S.H.; Liyanaarachchi, V.C.; Premaratne, M.; Nimarshana, P.H.V.; Ariyadasa, T.U.; Kornaros, M. Wastewater-Based Microalgal Biorefineries for the Production of Astaxanthin and Co-Products: Current Status, Challenges and Future Perspectives. Bioresour. Technol. 2021, 342, 126018. [Google Scholar] [CrossRef]
- Daneshvar, E.; Zarrinmehr, M.J.; Koutra, E.; Kornaros, M.; Farhadian, O.; Bhatnagar, A. Sequential Cultivation of Microalgae in Raw and Recycled Dairy Wastewater: Microalgal Growth, Wastewater Treatment and Biochemical Composition. Bioresour. Technol. 2019, 273, 556–564. [Google Scholar] [CrossRef]
- Bora, A.; Gurusamy, S.; Veleeswaran, A.; Thondi Rajan, A.S.; Rathinam, Y.; Ramalingam, K.R.; Alagarsamy, A. Simultaneous Biodiesel and Bioelectricity Generation Utilizing Dairy and Rice Mill Wastewater by Freshwater Microalgal Isolate: An Integrated Energy-Efficient Approach. Process Saf. Environ. Prot. 2024, 190, 149–161. [Google Scholar] [CrossRef]
- Vellaiyan, S. An Integrated Approach for Wastewater Treatment and Algae Cultivation: Nutrient Removal Analysis, Biodiesel Extraction, and Energy and Environmental Metrics Enhancement. J. Environ. Manag. 2024, 354, 120410. [Google Scholar] [CrossRef]
- Bagchi, S.K.; Patnaik, R.; Rawat, I.; Prasad, R.; Bux, F. Beneficiation of Paper-Pulp Industrial Wastewater for Improved Outdoor Biomass Cultivation and Biodiesel Production Using Tetradesmus obliquus (Turpin) Kützing. Renew. Energy 2024, 222, 119848. [Google Scholar] [CrossRef]
- Nagarajan, D.; Lee, D.J.; Chen, C.Y.; Chang, J.S. Resource Recovery from Wastewaters Using Microalgae-Based Approaches: A Circular Bioeconomy Perspective. Bioresour. Technol. 2020, 302, 122817. [Google Scholar] [CrossRef]
- Weschler, M.K.; Barr, W.J.; Harper, W.F.; Landis, A.E. Bioresource Technology Process Energy Comparison for the Production and Harvesting of Algal Biomass as a Biofuel Feedstock. Bioresour. Technol. 2014, 153, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.O.; Serrano, J.G.; Montero, R.D.; Sampaio, I.C.F.; de Morais, E.G. Harvesting Microalgae: Overcoming the Bottlenecks in Microalgae-Based Wastewater Treatment Through Industrial-Scale Gravity Sedimentation and Thickening. In Microalgal Bioengineering; Springer: Cham, Switzerland, 2024; pp. 83–102. [Google Scholar]
- Najjar, Y.S.H.; Abu-Shamleh, A. Harvesting of Microalgae by Centrifugation for Biodiesel Production: A Review. Algal Res. 2020, 51, 102046. [Google Scholar] [CrossRef]
- Tan, J.S.; Lee, S.Y.; Chew, K.W.; Lam, M.K.; Lim, J.W.; Ho, S.H.; Show, P.L. A Review on Microalgae Cultivation and Harvesting, and Their Biomass Extraction Processing Using Ionic Liquids. Bioengineered 2020, 11, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Peng, C.; Zhang, S.; Zhang, M.; Li, D.; Wang, X.; Mao, B. Comprehensive Analysis of the Combined Flocculation and Filtration Process for Microalgae Harvesting at Various Operating Parameters. Sci. Total Environ. 2023, 857, 159658. [Google Scholar] [CrossRef] [PubMed]
- Mirzayanti, Y.W.; Marlinda, L.; Irawan, H.; Al Muttaqii, M.; Ma’sum, Z.; Asri, N.P.; Chern, J.-M. Performance of In-Situ Stirring Batch Reactor Transesterification of Nannochloropsis Sp Microalgae into Biodiesel. Int. J. Technol. 2024, 15, 859–869. [Google Scholar] [CrossRef]
- Mittal, V.; Ghosh, U.K. Potential of Microalgae for Phytoremediation of Various Wastewaters for Nutrient Removal and Biodiesel Production through Nanocatalytic Transesterification. Asia-Pacific J. Chem. Eng. 2023, 18, e2847. [Google Scholar] [CrossRef]
- Wang, X.; Jin, X.; Wang, H.; Wang, Y.; Zuo, L.; Shen, B.; Yang, J. Catalytic Pyrolysis of Microalgal Lipids to Liquid Biofuels: Metal Oxide Doped Catalysts with Hierarchically Porous Structure and Their Performance. Renew. Energy 2023, 212, 887–896. [Google Scholar] [CrossRef]
- Rawat, J.; Jaiswal, K.K.; Das, N.; Kumar, S.; Gururani, P.; Bisht, B.; Vlaskin, M.S.; Nayak, M.; Kumar, V. Hydrothermal Liquefaction of Freshwater Microalgae Biomass Using Fe3O4 Nanoparticle as a Catalyst. Energy Sources Part A Recover. Util. Environ. Effects 2023, 45, 12988–13000. [Google Scholar]
- Ummalyma, S.B.; Chiang, A.; Herojit, N.; Arumugam, M. Sustainable Microalgal Cultivation in Poultry Slaughterhouse Wastewater for Biorefinery Products and Pollutant Removal. Bioresour. Technol. 2023, 374, 128790. [Google Scholar] [CrossRef]
- Javed, F.; Zimmerman, W.B.; Fazal, T.; Hafeez, A.; Mustafa, M.; Rashid, N.; Rehman, F. Green Synthesis of Biodiesel from Microalgae Cultivated in Industrial Wastewater via Microbubble Induced Esterification Using Bio-MOF-Based Heterogeneous Catalyst. Chem. Eng. Res. Design 2023, 189, 707–720. [Google Scholar] [CrossRef]
- Lu, T.; Sun, Y.; Shi, M.; Ding, D.; Ma, Z.; Pan, Y.; Yuan, Y.; Liao, W.; Sun, Y. Ni Dopped MgAl Hydrotalcite Catalyzed Hydrothermal Liquefaction of Microalgae for Low N, O Bio-Oil Production. Fuel 2023, 333, 126437. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, M.; Saraiva, J.A.; Martins, A.P.; Pinto, C.A.; Prieto, M.A.; Simal-Gandara, J.; Cao, H.; Xiao, J.; Barba, F.J. Extraction of Lipids from Microalgae Using Classical and Innovative Approaches. Food Chem. 2022, 384, 132236. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Prasad, P.; Shang, X.; Keum, Y.-S. Advances in Lipid Extraction Methods—A Review. Int. J. Mol. Sci. 2021, 22, 13643. [Google Scholar] [CrossRef] [PubMed]
- Sati, H.; Mitra, M.; Mishra, S.; Baredar, P. Microalgal Lipid Extraction Strategies for Biodiesel Production: A Review. Algal Res. 2019, 38, 101413. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Rashd, J.A.; Lalung, J.; Kassim, M.A.; Wijaya, D.; Allzrag, A.M.M.; Shaah, M.A. Kinetics and Thermodynamic Studies on Biodiesel Synthesis via Soxhlet Extraction of Scenedesmus parvus Algae Oil. Energy Convers. Manag. X 2024, 23, 100633. [Google Scholar] [CrossRef]
- Aravind, S.; Barik, D. Taguchi Optimization on the Biooil Extraction from Fresh Water Algae (Spirogyra) Using Soxhlet Apparatus. Int. J. Energy Res. 2023, 2023, 6213851. [Google Scholar] [CrossRef]
- Lee, S.Y.; Cho, J.M.; Chang, Y.K.; Oh, Y.-K. Cell Disruption and Lipid Extraction for Microalgal Biorefineries: A Review. Bioresour. Technol. 2017, 244, 1317–1328. [Google Scholar] [CrossRef]
- Shrivastav, G.; Prava Jyoti, T.; Chandel, S.; Singh, R. Eco-Friendly Extraction: Innovations, Principles, and Comparison with Traditional Methods. Sep. Purif. Rev. 2024, 1–17. [Google Scholar] [CrossRef]
- Shankar, M.; Chhotaray, P.K.; Gardas, R.L.; Tamilarasan, K.; Rajesh, M. Application of Carboxylate Protic Ionic Liquids in Simultaneous Microalgal Pretreatment and Lipid Recovery from Marine Nannochloropsis sp. and Chlorella sp. Biomass Bioenergy 2019, 123, 14–24. [Google Scholar] [CrossRef]
- He, Y.; Zhang, B.; Guo, S.; Guo, Z.; Chen, B.; Wang, M. Sustainable Biodiesel Production from the Green Microalgae Nannochloropsis: Novel Integrated Processes from Cultivation to Enzyme-Assisted Extraction and Ethanolysis of Lipids. Energy Convers. Manag. 2020, 209, 112618. [Google Scholar] [CrossRef]
- Jacob, A.; Ashok, B.; Alagumalai, A.; Chyuan, O.H.; Le, P.T.K. Critical Review on Third Generation Micro Algae Biodiesel Production and Its Feasibility as Future Bioenergy for IC Engine Applications. Energy Convers. Manag. 2021, 228, 113655. [Google Scholar] [CrossRef]
- Bošnjaković, M.; Sinaga, N. The Perspective of Large-Scale Production of Algae Biodiesel. Appl. Sci. 2020, 10, 8181. [Google Scholar] [CrossRef]
- Sharma, A.K.; Sahoo, P.K.; Singhal, S. Screening and Optimization of Culture Media for Chlorella sp. as a Raw Material for Biodiesel Production. Int. J. Pharma Bio. Sci. 2015, 6, B251-62. [Google Scholar]
- Singh, R.; Kumar, A.; Chandra Sharma, Y. Biodiesel Production from Microalgal Oil Using Barium–Calcium–Zinc Mixed Oxide Base Catalyst: Optimization and Kinetic Studies. Energy Fuels 2019, 33, 1175–1184. [Google Scholar] [CrossRef]
- Ruhul, A.M.; Kalam, M.A.; Masjuki, H.H.; Fattah, I.M.R.; Reham, S.S.; Rashed, M.M. State of the Art of Biodiesel Production Processes: A Review of the Heterogeneous Catalyst. RSC Adv. 2015, 5, 101023–101044. [Google Scholar] [CrossRef]
- Liu, H.; Liu, T.-J.; Guo, H.-W.; Wang, Y.-J.; Ji, R.; Kang, L.-L.; Wang, Y.-T.; Guo, X.; Li, J.-G.; Jiang, L.-Q.; et al. A Review of the Strategy to Promote Microalgae Value in CO2 Conversion-Lipid Enrichment-Biodiesel Production. J. Clean. Prod. 2024, 436, 140538. [Google Scholar] [CrossRef]
- Makareviciene, V.; Gumbyte, M.; Skorupskaite, V.; Sendzikiene, E. Biodiesel Fuel Production by Enzymatic Microalgae Oil Transesterification with Ethanol. J. Renew. Sustain. Energy 2017, 9, 23101. [Google Scholar] [CrossRef]
- Go, A.W.; Sutanto, S.; Ong, L.K.; Tran-Nguyen, P.L.; Ismadji, S.; Ju, Y.H. Developments in In-Situ (Trans) Esterification for Biodiesel Production: A Critical Review. Renew. Sustain. Energy Rev. 2016, 60, 284–305. [Google Scholar] [CrossRef]
- Qu, S.; Chen, C.; Guo, M.; Jiang, W.; Lu, J.; Yi, W.; Ding, J. Microwave-Assisted in-Situ Transesterification of Spirulina platensis to Biodiesel Using PEG/MgO/ZSM-5 Magnetic Catalyst. J. Clean. Prod. 2021, 311, 127490. [Google Scholar] [CrossRef]
- Oliva, G.; Buonerba, A.; Grassi, A.; Hasan, S.W.; Korshin, G.V.; Zorpas, A.A.; Belgiorno, V.; Naddeo, V.; Zarra, T. Microalgae to Biodiesel: A Novel Green Conversion Method for High-Quality Lipids Recovery and in-situ Transesterification to Fatty Acid Methyl Esters. J. Environ. Manag. 2024, 357, 120830. [Google Scholar] [CrossRef] [PubMed]
- Mathimani, T.; Le, T.; Salmen, S.H.; Ali Alharbi, S.; Jhanani, G.K. Process Optimization of One-Step Direct Transesterification and Dual-Step Extraction-Transesterification of the Chlorococcum-Nannochloropsis Consortium for Biodiesel Production. Environ. Res. 2024, 240, 117580. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.astm.org/d6751-20a.html (accessed on 30 September 2024).
- Available online: https://standards.iteh.ai/catalog/standards/cen/d3475989-a401-45ea-a3b3-27c9b71ab152/en-14214-2008?srsltid=AfmBOopd3m9PzoGGYTexJq7mhfDSZWyE1fvBMnW5x-gJOHdRdzmnJ_fq (accessed on 30 September 2024).
- Available online: https://www.services.bis.gov.in/php/BIS_2.0/bisconnect/standard_review/Standard_review/Isdetails?ID=MjczMDQ%3D (accessed on 30 September 2024).
- Masudi, A.; Muraza, O.; Jusoh, N.W.C.; Ubaidillah, U. Improvements in the Stability of Biodiesel Fuels: Recent Progress and Challenges. Environ. Sci. Pollut. Res. 2022, 30, 14104–14125. [Google Scholar] [CrossRef] [PubMed]
- Jemima Romola, C.V.; Meganaharshini, M.; Rigby, S.P.; Ganesh Moorthy, I.; Shyam Kumar, R.; Karthikumar, S. A Comprehensive Review of the Selection of Natural and Synthetic Antioxidants to Enhance the Oxidative Stability of Biodiesel. Renew. Sustain. Energy Rev. 2021, 145, 111109. [Google Scholar] [CrossRef]
- Devi, A.; Das, V.K.; Deka, D. A Green Approach for Enhancing Oxidation Stability Including Long Storage Periods of Biodiesel via Thuja oreantalis L. as an Antioxidant Additive. Fuel 2019, 253, 1264–1273. [Google Scholar] [CrossRef]
- Zhou, J.; Xiong, Y.; Xu, S. Evaluation of the Oxidation Stability of Biodiesel Stabilized with Antioxidants Using the PetroOXY Method. Fuel 2016, 184, 808–814. [Google Scholar] [CrossRef]
- Rajpoot, A.S.; Choudhary, T.; Chelladurai, H.; Shukla, A.K.; Sinha, A.A. Comparative Analysis of Energy, Exergy, Emission, and Sustainability Aspects of Third Generation Microalgae Biodiesels in a Diesel Engine. Process Saf. Environ. Prot. 2024, 188, 1026–1036. [Google Scholar] [CrossRef]
- Mostafa, S.S.M.; El-Gendy, N.S. Evaluation of Fuel Properties for Microalgae Spirulina platensis Bio-Diesel and Its Blends with Egyptian Petro-Diesel. Arab. J. Chem. 2017, 10, S2040–S2050. [Google Scholar] [CrossRef]
- Available online: https://www.astm.org/d7545-14r19e01.html (accessed on 30 September 2024).


| Microalgae | Cultivation Conditions | Reaction Conditions | Yield (%) | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| Catalyst Type | Catalyst | Alcohol: Oil (mol/mol) | Temperature (°C) | Time (h) | ||||
| Nannochloropsis | Artificial growth medium - | Hydrotalcite catalyst | CaO | 1:1 Hexane/ Methanol | 60 °C | 4 h | Olefine (61.49%) Gasoline (38.51%) | [109] |
| Tetraselmis indica | Wastewater growth medium 16:8 light/dark period beneath the light intensity of 94.5 μmol m−2 s−1 for 14 days | Nanocatalyst | Lithium-impregnated calcium oxide | - | - | - | 87.25% | [110] |
| Spirulina sp. | Artificial Temperature: (25–27 °C) Time: 14 days Light intensity: 94.5 μ/mol/s 16:08 dark and light cycle | Nanocatalyst | Ca(OCH)2 | 30:1 M/O | 80 °C | 3 h | 99% | [79] |
| Nannochloropsis sp. | - | Composite catalysts | 2:1 Dichloromethane and methanol | 80 °C | 4 h | ~80.0% | [111] | |
| Chlorella sorokiniana | Artificial growth medium 25 ± = 2 °C (300 µmol m−2 s−1) | Magnetic nanoparticles | (Fe3O4) | - | 350.0 °C | 45 min | 28.0% | [112] |
| Chlorella pyrenoidosa | Artificial, fertilizer nutrient media Outdoor conditions (20–252 °C) | Homogenous | KOH | 4:1 | 65.0 °C | 7 min | 15.5% of dry wt. | [18] |
| Chlorella minutissima | commercial fertilizer Outdoor conditions (20–252 °C) | Homogenous | KOH | 10.5:1 | 63.9 °C | 6 min | 90.2% | [5] |
| Neochloris sp. SK57 and Chlorella sp. SL7A | Slaughterhouse wastewater as growth media 25 ± 2 °C and a day-night cycle of 11:13 | Homogenous | 2% H2SO4 | - | 100.0 °C | 6 h | - | [113] |
| C.vulgaris | Textile industry wastewater as growth media 4600 lux, 30 ± 5 °C | Heterogenous | [HMIM][HSO4]/ Bio-MOF | 15:1 | 70.0 °C | 30 min | 92.0% | [114] |
| Scenedesmus sp. | - | Ni dopped MgAl layered double hydroxides | NixMg2Al-LDH | - | 300.0 °C | 1 h | 31.4% | [115] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, A.K.; Jaryal, S.; Sharma, S.; Dhyani, A.; Tewari, B.S.; Mahato, N. Biofuels from Microalgae: A Review on Microalgae Cultivation, Biodiesel Production Techniques and Storage Stability. Processes 2025, 13, 488. https://doi.org/10.3390/pr13020488
Sharma AK, Jaryal S, Sharma S, Dhyani A, Tewari BS, Mahato N. Biofuels from Microalgae: A Review on Microalgae Cultivation, Biodiesel Production Techniques and Storage Stability. Processes. 2025; 13(2):488. https://doi.org/10.3390/pr13020488
Chicago/Turabian StyleSharma, Amit Kumar, Shivangi Jaryal, Shubham Sharma, Archana Dhyani, Bhagya Sindhu Tewari, and Neelima Mahato. 2025. "Biofuels from Microalgae: A Review on Microalgae Cultivation, Biodiesel Production Techniques and Storage Stability" Processes 13, no. 2: 488. https://doi.org/10.3390/pr13020488
APA StyleSharma, A. K., Jaryal, S., Sharma, S., Dhyani, A., Tewari, B. S., & Mahato, N. (2025). Biofuels from Microalgae: A Review on Microalgae Cultivation, Biodiesel Production Techniques and Storage Stability. Processes, 13(2), 488. https://doi.org/10.3390/pr13020488









