Abstract
Astringency in persimmon fruit is often eliminated by treatment with gaseous carbon dioxide, dry ice, or alcohol. However, these methods are time-consuming and labor-intensive, and astringency may recur after heat treatment. In this study, a method for easily reducing astringency was investigated by taking advantage of the benefits of combining proteins and polysaccharides. In the first experiment, the protein materials with strong astringency-reducing effects were screened from among 15 protein-rich foods using astringent persimmon juice (APJ), and collagen peptides were found to be highly effective. However, syneresis was observed when 1% collagen peptide powder was added to the astringent persimmon paste (AP). Therefore, in the second experiment, 0.5% collagen peptides (protein) were applied to reduce heating-induced astringency and reversion and 0.5% polysaccharides (guar, and xanthan gums) to maintain color and suppress syneresis. The results demonstrate that the combination of collagen peptide and polysaccharides is optimal for removing astringency in persimmon, inhibiting its recurrence by heating, and maintaining product quality. The results of this study may reduce the labor required for the astringency removal process, broaden the uses of AP, and facilitate the effective utilization of discarded astringent persimmons that do not meet the standards.
1. Introduction
Persimmons (Diospyros kaki Thunb.) are rich in tannins. Sweet varieties contain insoluble tannins and lack an astringent taste. In contrast, astringent varieties contain soluble tannins [1]. The astringency of persimmons can be eliminated by treating the fruits with reagents, such as carbon dioxide gas (dry ice) and alcohol [2,3,4,5,6,7], which can eliminate soluble tannins. In the carbon dioxide method, astringent persimmons are placed in a bag, filled with 1–2% dry ice by weight relative to the weight of the persimmons, which is sealed. In the alcohol method, persimmons are soaked in an ethanol solution of a 35% or higher concentration and placed in a bag, which is sealed. The carbon dioxide method takes ~3–5 days, and the alcohol method takes ~7 days, requiring labor and time. In Japan, persimmon fruit paste or puree is widely used to prepare jelly, pudding, cake, and cookies. To produce a non-astringent paste, the fruit should be ground after astringency removal. However, this method is time-consuming and labor-intensive, thereby limiting its application in the food industry, specifically considering the large quantities of astringent persimmons harvested in a short period.
Previous studies have explored the interactions between proteins and tannins [8,9,10]. The interaction of tannins with proline-associated proteins in saliva can be perceived as astringent [11,12]. Models describing protein–tannin (P–T) interaction have been proposed [11,13,14]. The protein-to-tannin ratio is a crucial factor in determining the particles in an interaction-produced precipitate [15]. The tannin-imparted astringent taste is ameliorated by using well-known protein materials. For example, the addition of soymilk and condensed milk can effectively eliminate astringency and prevent recurrence [16]. However, when AP with added protein was subjected to heat treatment or frozen storage, its physical quality was significantly reduced. Specifically, the paste turned brown, dull, and less smooth, with significant syneresis [16]. In contrast, adding a polysaccharide maintained the AP color and prevented syneresis, resulting in a smooth paste even after freezing, with guar and xanthan gums being particularly effective [17]. However, the ability of these gums to prevent astringency recurrence was lower than that of the protein materials [17]. In this study, it was hypothesized that mixing proteins and polysaccharides may address the limitations of both materials, while still exploiting their advantages. Interactions between tannins and polysaccharides have been reported, although not as extensively as those involving proteins [17]. Persimmon tannins and pectin form complexes [18,19] through hydrophobic and hydrogen bonds [18]. Haze formation resulting from P–T complex formation is challenging [20]. In the bovine serum albumin (BSA)/gelatin–epigallocatechin gallate (EGCG) system, polysaccharides (pectin, xanthan gum, and guar gum) compete with BSA/gelatin to bind to EGCG and protect against BSA/gelatin–EGCG haze formation [20]. Additionally, the effects of gum arabic, pectin, and polygalacturonic acid on salivary protein/grape seed procyanidins have been studied, with pectin reported to be the most efficient in inhibiting protein/tannin precipitation [21].
This study aimed to develop a method to produce high-quality pastes that are non-astringent, have good color and smoothness, and do not undergo syneresis or alterations after heating or freezing. To achieve this aim, polysaccharides and proteins were used. Ultimately, it is hoped that this will lead to effective use of discarded astringent persimmons.
2. Materials and Methods
2.1. Materials
2.1.1. Materials for Astringent Persimmon Juice (APJ) and Astringent Persimmon Paste (AP)
Astringent persimmons (variety Saijo) were obtained from the Shimane Agricultural Technology Center (Izumo City, Japan). The persimmon fruit used in this experiment was a fruit that did not meet the standards and would have been discarded.
2.1.2. Protein-Rich Foods
A total of 15 protein-rich foods were used to eliminate soluble tannins from APJ. Gelatin (New Silver) and two types of collagen peptides (CP3100: SCP-3100 and CP5100: SCP-5100) were provided by Nitta Gelatin Inc. (Osaka City, Japan). Collagen peptide (NP100: Nippi Collagen 100) was provided by NIPPI Inc. (Adachi-ku, Japan). Soybean dietary fiber, treated soymilk, and powdered milk were provided by Fuji Oil Co., Ltd. (Izumisano City, Japan). Silk powder (silk protein hydrolysate; Kaetsu So-go Shinkou Inc., Yosano Town, Japan), weak flour (Nissin Violet, Nissin Seifun Group Inc., Chiyoda-ku, Japan), medium-strength flour (Nissin Snow, Nissin Seifun Group Inc., Chiyoda-ku, Japan), extra-strength flour (Eagle, Nissin Seifun Group Inc., Chiyoda-ku, Japan), durum wheat flour (Semola Rimacinata di Grano Duro, Mininni Inc., Altamura, Italy), and whey protein powder (Sabbath whey protein 100; Meiji Holdings Co., Ltd., Chuo-ku, Japan) were purchased from their respective suppliers. Lecthin (from soybeans) and casein (from milk) powders were purchased from Nacalai Tesque Inc. (Nakagyo-ku, Japan), and albumin (from egg whites) was purchased from Tokyo Chemical Industry Co., Ltd. (Chuo-ku, Japan); these three materials were of reagent grade. All of these 15 protein-rich foods were in dried powder form.
2.1.3. Polysaccharide
Guar (GR-10), and xanthan gums (V-10) were provided by Ina Food Industry (Ina City, Japan).
2.1.4. Reagants for STC Assay
Folin–Ciocalteu reagent (2 N), methanol (>99.8%, guaranteed reagent), and sodium carbonate (guaranteed reagent) were obtained from Wako Pure Chemicals Industries Ltd. (Osaka City, Japan).
2.2. Astringent Persimmon Juice Liquid and Astringent Persimmon Paste Processing
The calyxes and seeds were removed, and the persimmon flesh was ground into a paste using a 1 L capacity osterizer 16-speed blender (Sunbeam Oster, Boca Raton, FL, USA) for 2 min, and AP was obtained. APJ was obtained by squeezing AP and filtering it through a filter cloth. The initial concentration of soluble tannins in APJ was 820 mg catechin (CTN) equivalent (eq)/100 g fresh weight (FW).
2.3. Complex Formation with Soluble Tannins in APJ
To investigate the protein material that most efficiently reduces the astringency of persimmons, 15 protein-rich foods (see Section 2.1.2) were added to APJ. All 15 protein-rich foods were in powder form and were added in an amount equivalent to 1% (w/v) to APJ. In other words, 0.2 g of protein-rich foods was added to 20 mL of APJ. To facilitate complex formation between the soluble tannins and protein-rich food items, the solutions were stirred for 2 min using a homogenizer (AHG-160D & HT1008, AS-ONE Co. Ltd., Nishi-ku, Japan).
2.4. Addition of Polysaccharide and Protein Combination and Heat/Freeze Treatment
As a result of the preliminary experiment, in this study, 0.5% polysaccharide (guar or xanthan gum) powder and 0.5% collagen peptide powder (CP3100, CP5100, or NP100) were added to the AP total weight. This means that 1 g of polysaccharide powder and 1 g of collagen peptide powder were added to 200 g of AP. Six types of mixed persimmon pastes were prepared using combinations of two types of polysaccharides (guar or xanthan gum) and three types of collagen peptides (CP3100, CP5100, or NP100). To facilitate complex formation between the soluble tannins, polysaccharide powder, and collagen peptide powder, these mixed samples were stirred for 2 min using a homogenizer. These mixed pastes were then subjected to heating and freezing treatments.
Heating conditions similar to those used for food processing were also considered. Astringent persimmon paste or mixed paste (50 g) was placed in a heat-resistant bag (B-1318; Meiwa Pax Co., Ltd., Kashiwara City, Japan) and heated to 100 °C using an induction heater (MR-B20; Toshiba Co., Minato-ku, Japan) for 40 min. Heat treatment is a common method for sterilizing paste foods under normal pressure. Astringent persimmon paste or mixed paste (50 g) was sealed in an aluminum pouch (AL14; Seisannipponsha Ltd., Chiyoda-ku, Japan) and heated to 121 °C in an autoclave (BS-245; TOMY SEIKO Co., Ltd., Nerima-ku, Japan) for 4 min. These represent the standard conditions for sterilizing food in retort pouches. Freezing treatment was assessed because fruit pastes are often distributed in the frozen form. Each sample was placed in a plastic bag (B-1318; Meiwa Pax Co., Ltd., Kashiwara City, Japan) and stored at −25 °C. Three replicates were performed per treatment.
2.5. STC Assay Method
STC was used as an index of astringency because of its high correlation with organoleptic (sensory) astringency in humans [3]. Since the polyphenols in persimmon are mainly tannins, with few low-molecular-weight polyphenols, the values obtained by the Folin–Ciocalteu method were used as STC, as in previous studies [3]. To analyze the STC, 80% methanol was added to 5 g of the paste and mixed [3]. Each sample solution was appropriately diluted, and the STC was measured using the Folin–Ciocalteu method [22] and analyzed by partially modifying the method proposed by Chung et al. [23].
More specifically, the extract (90 µL), 1 N Folin–Ciocalteu reagent (90 µL), and 10% sodium carbonate (90 µL) were mixed in a 96-well microplate. After incubation for 60 min at room temperature, the reaction color was measured using a microplate reader (SH-9000Lab, CORONA ELECTRIC Co., Ltd., Hitachinaka City, Japan) at 690 nm. The STC was expressed as mg catechin (CTN) eq/100 g FW.
2.6. External Appearance and Syneresis Rate
To assess the effects of polysaccharide or protein addition and heat treatment or freezing on AP appearance, each sample was placed in a stainless-steel dish (diameter of 40 mm, height of 15 mm) and photographed using a digital camera (WG-40W; Ricoh Co., Ltd., Minato-ku, Japan). To assess syneresis (liquid loss from the gel matrix), 15 g of each paste sample (W0) was placed in a 50 mL tube, centrifuged (50B-7; Sakuma Manufacturing Co., Ltd., Ota-ku, Japan) at 3000 rpm for 5 min, and photographed using a digital camera (WG-40W; Ricoh Co., Ltd., Minato-ku, Japan). The supernatant was carefully collected using a Pasteur pipette (Fisher Scientific, Pittsburgh, PA, USA), and its weight (W1) was measured to calculate syneresis as W1/W0 × 100 [24].
2.7. Statistical Analysis
Statistical analysis was performed using SPSS version 28.0 (IBM Inc, Chicago, IL, USA). One-way analysis of variance (ANOVA) was used to determine differences among groups, followed by Tukey’s Honestly Significant Difference (HSD) test to assess significance at the 5% level.
3. Results and Discussion
3.1. Exploring Protein Materials That Bind to Tannins
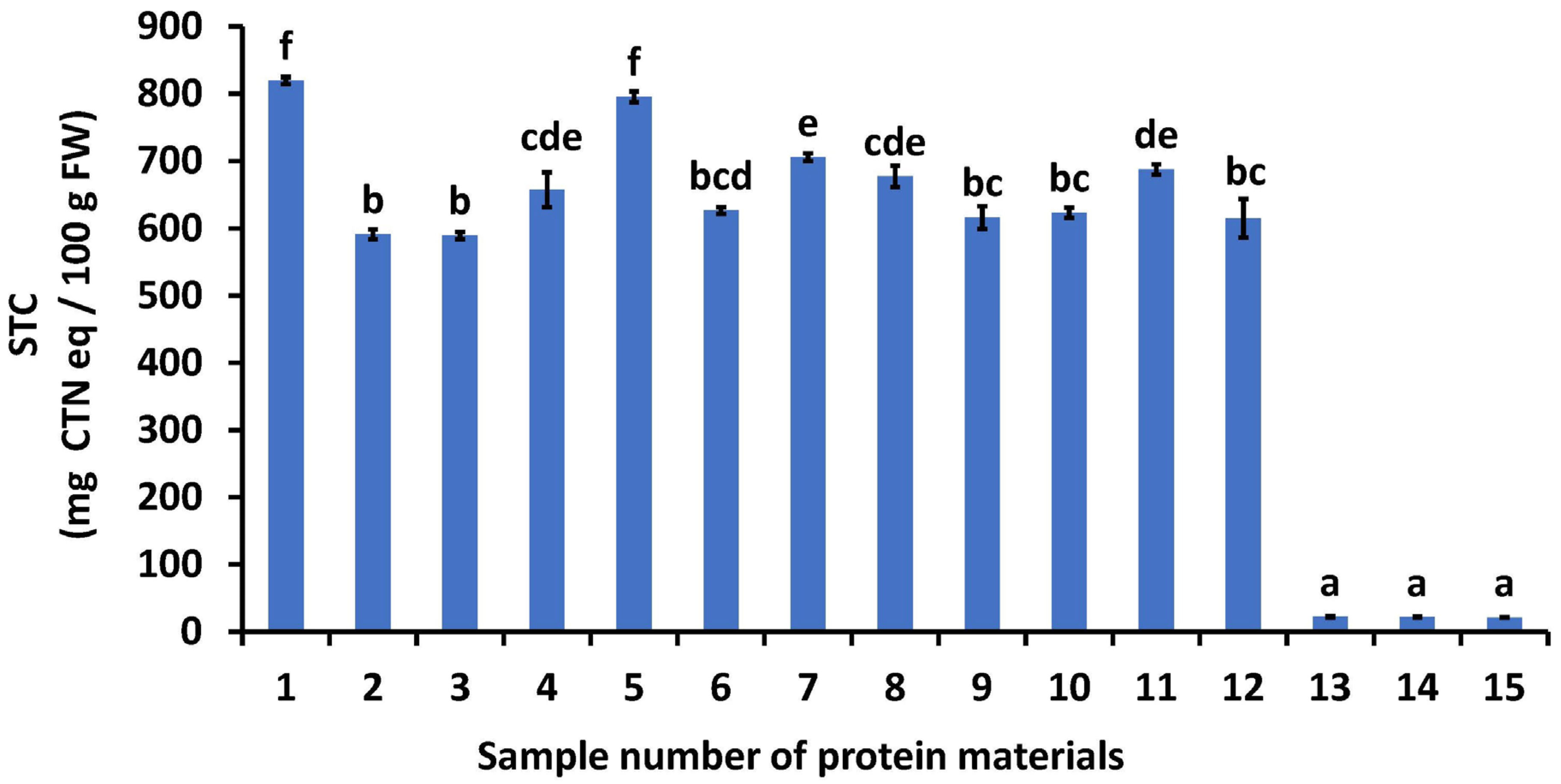
Screening was carried out based on Section 2.3 for protein materials that bound most strongly with tannin and reduced STC. Figure 1 illustrates the effect of various protein-rich foods on STC. STC is highly correlated with human sensory astringency, and it is said that astringency is not observed when there is less than 100 mg CTN eq/100 g FW [3]. For this reason, STC was used as an indicator of astringency in this study.
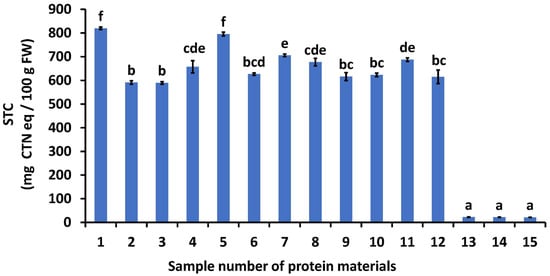
Figure 1.
Effects of various protein materials on soluble tannin content. 1: astringent persimmon juice, 2: weak flour, 3: medium-strength flour, 4: extra-strength flour, 5: casein powder, 6: whey protein powder, 7: soybean dietary fiber, 8: lecithin powder, 9: albumin, 10: treated soymilk and powdered milk, 11: silk powder, 12: gelatin, 13: collagen peptide (SCP-3100), 14: collagen peptide (SCP-5100), and 15: collagen peptide (Nippi Collagen 100). STC: soluble tannin content, FW: fresh weight, CTN: catechin, AP: astringent persimmon paste. Mean values are presented as vertical bars indicating standard error (n = 6). The results were obtained using Tukey’s test for multiple comparisons. Different letters indicate significant differences at p < 0.05.
The P–T complex formation resulted in the elimination of the astringent taste, whereas the taste remained in the absence of the P–T complex. Therefore, identifying proteins that bind strongly to tannins is crucial to eliminate astringency. The STC of the control (no added protein) was 820 mg CTN eq/100 g FW, and it decreased when protein-rich foods were added. The STC values of the treated samples ranged from 21 to 795 mg CTN eq/100 g FW. Among the added food items, CP3100, CP5100, and NP100 exhibited the lowest STC values, indicating that these collagen peptides strongly bind persimmon soluble tannins and are effective in reducing astringency in persimmons.
Collagen peptides were the most effective protein-rich foods for reducing astringency owing to various factors. First is the three-dimensional structure of collagen. Collagen—a fibrous protein—has a long, fiber-like shape and triple-helix structure (one of the two basic supercoiled protein motifs) [25]. The fibrous protein may have an exposed functional group on its surface that can bind to tannins. Second, collagen is rich in basic amino acids, and tannins are rich in hydroxyl groups. Matsuo and Ito [26] reported that soluble tannins in persimmons consist of oligomers of catechin, catechin-3-gallate, gallocatechin, gallocatechin-3-gallate, and unknown terminal residues. These tannins are B-type proanthocyanidins possessing a carbon–carbon inter-flavan linkage between C-7 of one unit and C-6 or C-8 of another. Additionally, they contain numerous hydroxyl groups. Goto et al. [27] reported that the amino groups of collagens bind easily to the hydroxyl groups of soluble tannins in astringent persimmons, indicating that the binding was because of electrostatic interactions. Basic proteins containing more amino groups are preferable for astringency removal. Third is the amount of proline in the collagen. Tannins have been reported to bind easily to proline [11]. Butler et al. [28] reported that sorghum tannins can bind strongly to dietary proteins, with the highest affinity for such binding observed in large, proline-rich proteins with an open structure. Collagen and collagen peptides are rich in proline residues. The combination of these three factors likely resulted in the addition of 1% (w/v) collagen peptides to the APJ, thereby reducing the STC to a level where astringency was not noticeable.
Based on the above results, high-quality persimmon paste was manufactured by combining the protein ingredients in Figure 1, Sample No. 13–15 (SCP3100, SCP5100, or NP100), with polysaccharides (guar gum or xanthan gum) [17] that have been reported to reduce astringency.
3.2. Appearance of the Paste
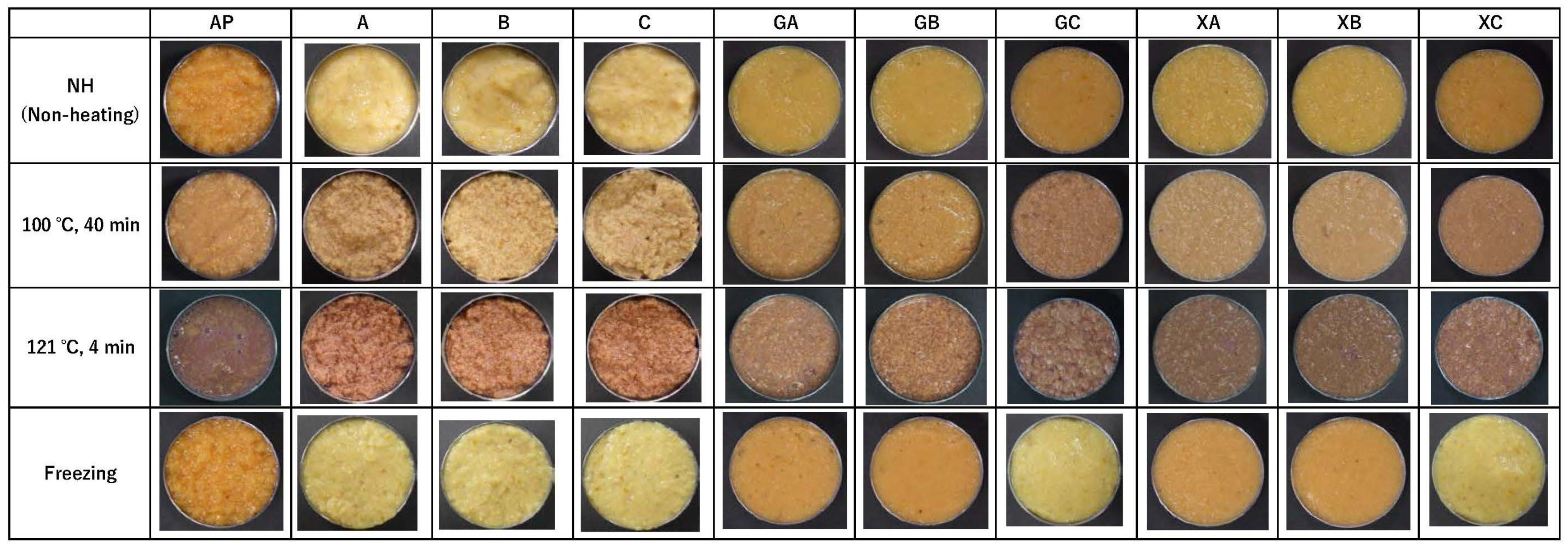
From Section 3.1, it was clear that collagen peptides were the most effective at lowering the STC, so the next step was to investigate the combined effects of collagen peptides and polysaccharides on appearance. Figure 2 illustrates the appearance of the paste after the treatments presented in Section 2.4. In the NH and frozen samples, AP was dull and uneven. In images A–C, for which the paste only contained collagen peptides, the paste changed to a yellow color, rather than the original orange color of persimmons, although it was smooth. However, pastes treated with a combination of polysaccharides and proteins were smooth, had a good appearance, exhibited less browning and dullness, and appeared bright yellow or orange. Treatments with guar gum + CP3100 (GA), guar gum + CP5100 (GB), xanthan gum + CP3100 (XA), and xanthan gum + CP5100 (XB) resulted in a bright, strong yellow paste. In contrast, the paste treated with guar gum + NP100 (GC) and xanthan gum + NP100 (XC) had a strong orange color similar to that of the untreated AP. Following heat treatment at 100 °C for 40 min, the GC pastes were slightly dark, whereas the GA, GB, XA, XB, and XC pastes were bright. Heat treatment at 121 °C for 4 min resulted in browning, agglutination, and non-uniform paste formation in all samples.

Figure 2.
Effect of polysaccharide and collagen peptide addition and heat/freeze treatment on the appearance of astringent persimmon paste. AP: astringent persimmon paste, A: SCP-3100 (1.0%), B: SCP-5100 (1.0%), C: Nippi Collagen 100 (1.0%), GA: guar gum (0.5%) + SCP-3100 (0.5%), GB: guar gum (0.5%) + SCP-5100 (0.5%), GC: guar gum (0.5%) + Nippi Collagen 100 (0.5%), XA: xanthan gum (0.5%) + SCP-3100 (0.5%), XB: xanthan gum (0.5%) + SCP-5100 (0.5%), and XC: xanthan gum (0.5%) + Nippi Collagen 100 (0.5%).
From the above, it was found that the guar gum + NP100 (GC) and xanthan gum + NP100 (XC) treatments could improve the appearance of AP in non-heated and frozen treatments. In addition, GA, GB, XA, XB, and XC were more effective than GC at improving the appearance of the AP when heated at 100 °C for 40 min. However, when treated at 121 °C for 4 min, the appearance of AP could not be improved even when treated with a combination of collagen peptides and polysaccharides.
3.3. Syneresis Rate of AP
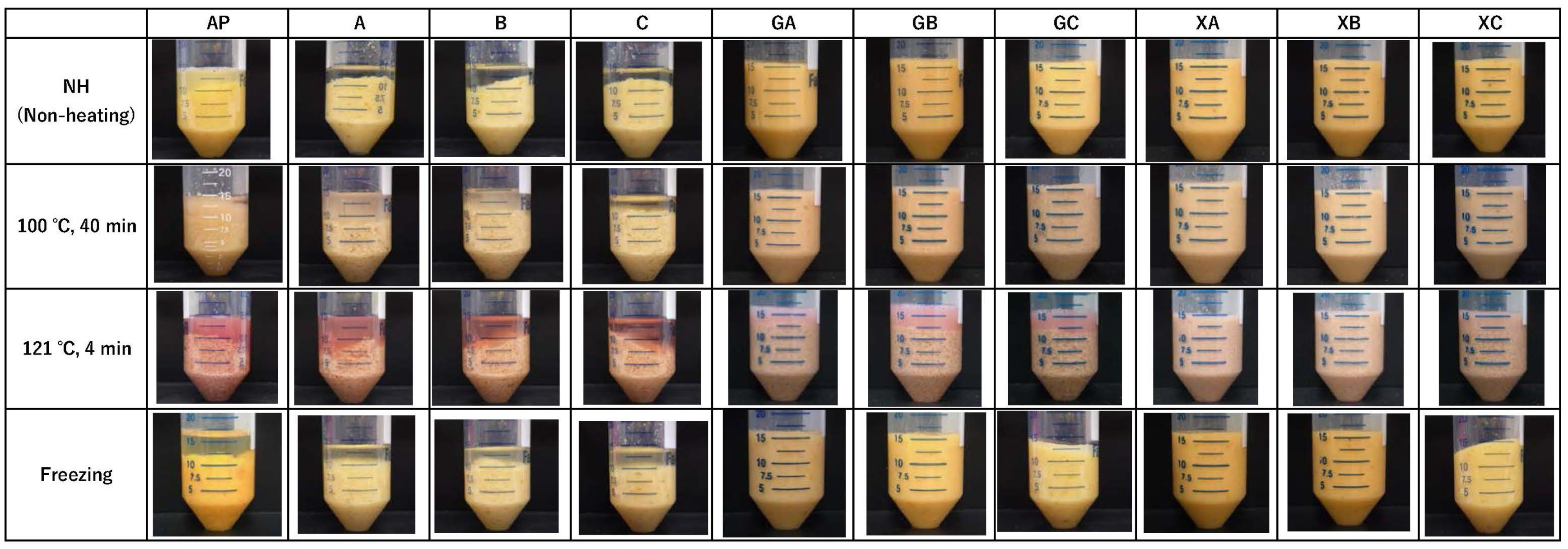
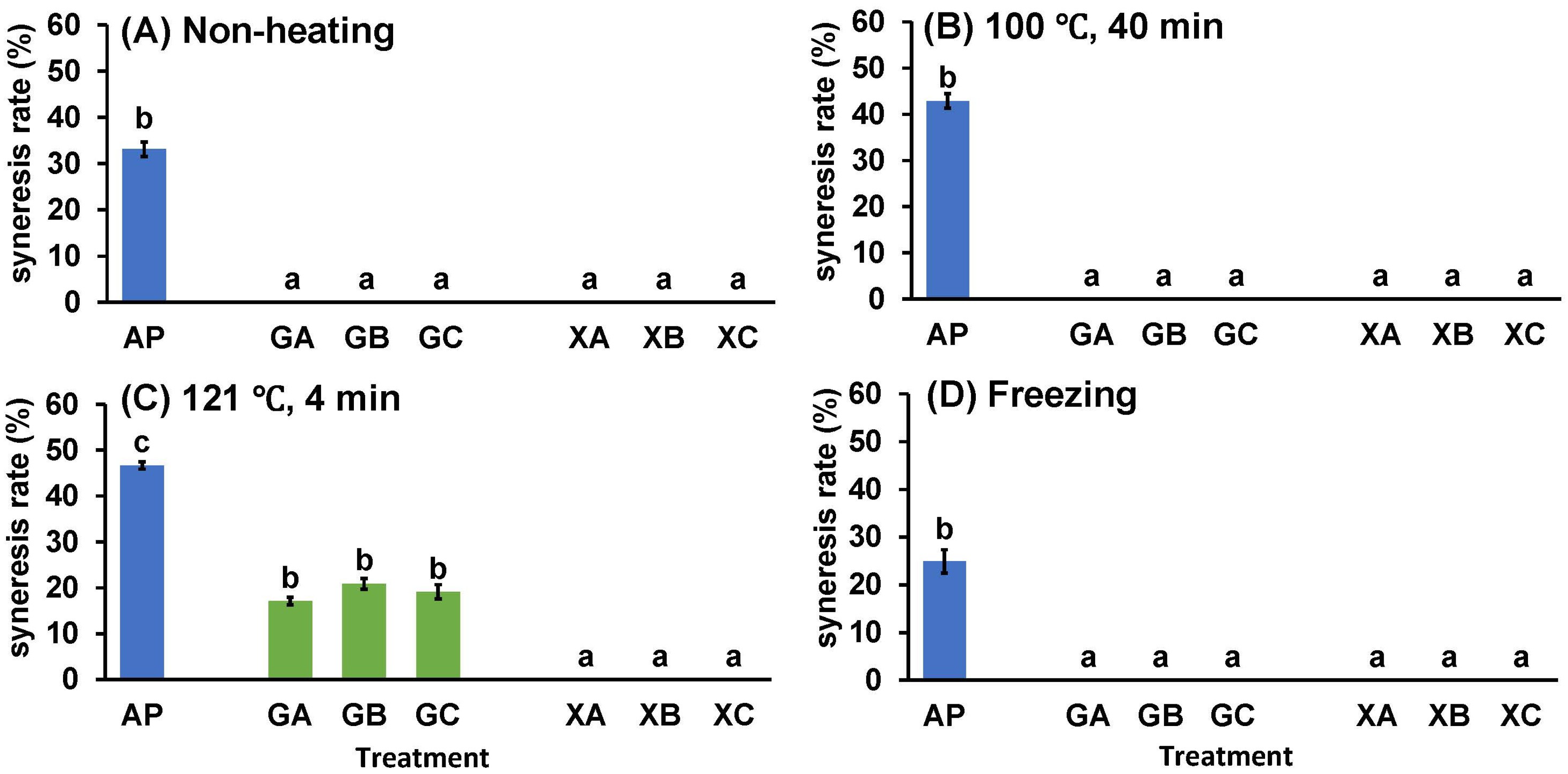
The effect of combining collagen peptides and polysaccharides on the syneresis of AP was investigated. Figure 3 presents images of the paste after different processing methods and centrifugation, whereas Figure 4 illustrates the syneresis rate. The images indicate remarkable syneresis in all cases involving non-heating (NH), heating, and freezing treatments for AP, CP3100 (A), CP5100 (B), and NP100 (C) (Figure 3). In contrast, syneresis was suppressed by combined treatment with polysaccharides and proteins (GA, GB, GC, XA, XB, and XC). However, certain heating treatment groups in the combined treatment (GA, GB, GC, XA, XB, and XC) exhibited water separation. Therefore, the syneresis rate was measured for the combined treatment of AP, proteins, and polysaccharides to accurately evaluate how effectively syneresis can be suppressed by combining polysaccharides and proteins. AP exhibited remarkable syneresis rates of 33.1% ± 1.6% at NH, 42.9% ± 1.6% at 100 °C for 40 min, 46.7% ± 0.8% at 121 °C for 40 min, and 24.9% ± 2.5% under freezing conditions. Compared with AP, the syneresis rate was significantly suppressed in all treatment groups (GA, GB, GC, XA, XB, and XC). During the high-temperature treatment (121 °C for 4 min), guar gum treatments (GA, GB, and GC) exhibited water separation rates of 17.1% ± 0.8%, 20.9% ± 1.2%, and 19.1% ± 1.6%, respectively. However, syneresis was completely suppressed by NH treatment, heating treatment at 100 °C for 40 min, and freezing. In contrast, xanthan gum completely suppressed syneresis in all treatment groups (XA, XB, and XC): NH, 100 °C for 40 min, 121 °C for 4 min, and freezing. This combined processing of proteins and polysaccharides demonstrated that xanthan gum is more effective than guar gum in preventing syneresis, and it can suppress syneresis caused by high-temperature processing. When considering the sterilization of fruit paste by retorting for long-term distribution, combined collagen peptide and xanthan gum treatment, which suppresses syneresis at 121 °C for 4 min, is highly effective.
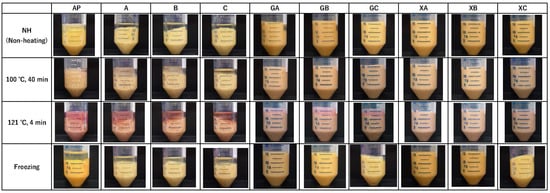
Figure 3.
Images of the paste after different processing methods and centrifugation. AP: astringent persimmon paste, A: SCP-3100 (1.0%), B: SCP-5100 (1.0%), C: Nippi Collagen 100 (1.0%), GA: guar gum (0.5%) + SCP-3100 (0.5%), GB: guar gum (0.5%) + SCP-5100 (0.5%), GC: guar gum (0.5%) + Nippi Collagen 100 (0.5%), XA: xanthan gum (0.5%) + SCP-3100 (0.5%), XB: xanthan gum (0.5%) + SCP-5100 (0.5%), and XC: xanthan gum (0.5%) + Nippi Collagen 100 (0.5%).
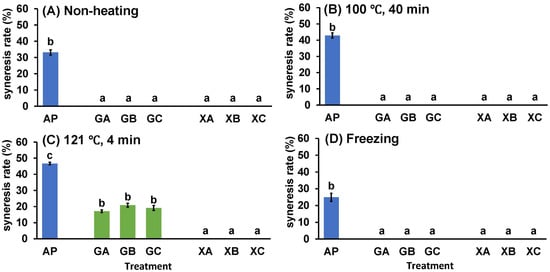
Figure 4.
Effect of polysaccharide and collagen peptide addition to (A) non-heating, heating treatment at (B) 100 °C or (C) 121 °C, or (D) freeze treatment on syneresis of astringent persimmon paste. Mean values are presented as vertical bars indicating standard error (n = 6). The results were obtained using Tukey’s test for multiple comparisons. Letters indicate significant differences (p < 0.05). AP: astringent persimmon paste, GA: guar gum (0.5%) + SCP-3100 (0.5%), GB: guar gum (0.5%) + SCP-5100 (0.5%), GC: guar gum (0.5%) + Nippi Collagen 100 (0.5%), XA: xanthan gum (0.5%) + SCP-3100 (0.5%), XB: xanthan gum (0.5%) + SCP-5100 (0.5%), and XC: xanthan gum (0.5%) + Nippi Collagen 100 (0.5%).
3.4. Effect of Protein and Polysaccharide Addition on the STC of AP
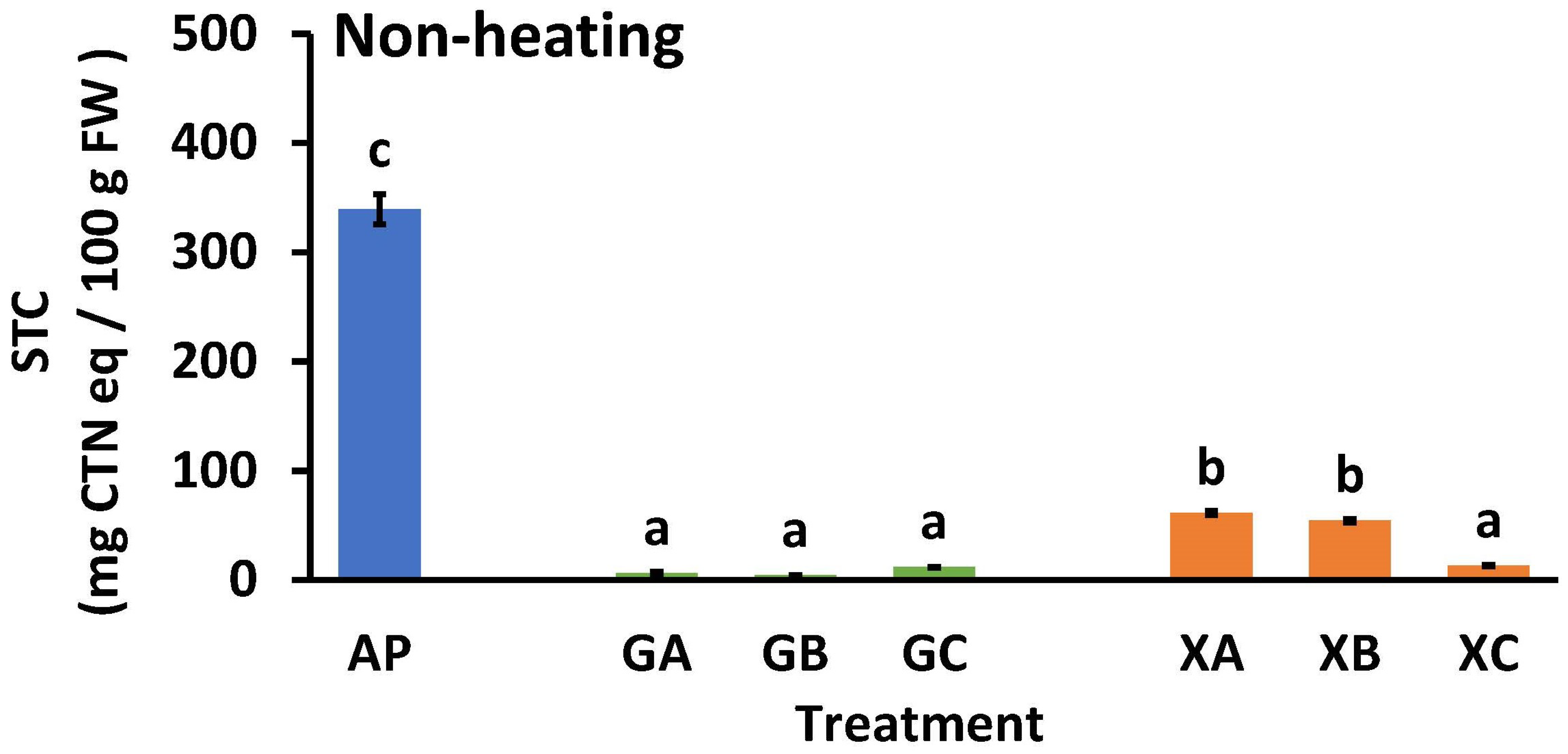
Figure 5 illustrates the effects of adding collagen peptides and polysaccharides to the STC of AP. The STC of non-heat-treated AP was significantly high (339.5 ± 13.8 mg CTN eq/100 g FW). In contrast, the STC values for GA, GB, and GC were significantly low at 6.5 ± 0.8, 4.3 ± 0.3, and 11.9 ± 0.1 mg CTN eq/100 g FW, respectively. The STC values of XA, XB, and XC were 61 ± 1.9, 54 ± 1.4, and 13.3 ± 0.6 mg CTN eq/100 g FW, respectively, which were significantly lower than that of AP. The STC levels of the guar gum samples (GA and GB) were lower than those of the xanthan gum samples (XA and XB) when CP3100 (A) and CP5100 (B) were added. Although not illustrated in Figure 5, the STC values for NH samples with only guar gum (1%) or xanthan gum (1%) were 52.7 ± 4.4 and 84.8 ± 1.3 mg CTN equivalents/100 g FW, respectively.
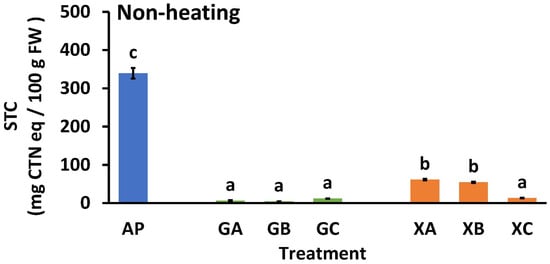
Figure 5.
Effect of polysaccharide and collagen peptide addition on the soluble tannin content of astringent persimmon paste. Mean values are presented as vertical bars indicating standard error (n = 6). The results were obtained using Tukey’s test for multiple comparisons. Letters indicate significant differences (p < 0.05). STC: soluble tannin content, FW: fresh weight, CTN: catechin, AP: astringent persimmon paste, GA: guar gum (0.5%) + SCP-3100 (0.5%), GB: guar gum (0.5%) + SCP-5100 (0.5%), GC: guar gum (0.5%) + Nippi Collagen 100 (0.5%), XA: xanthan gum (0.5%) + SCP-3100 (0.5%), XB: xanthan gum (0.5%) + SCP-5100 (0.5%), and XC: xanthan gum (0.5%) + Nippi Collagen 100 (0.5%).
The difference in astringency elimination effects between the guar and xanthan gums may explain the variation in the STC levels of the samples. The STC value of GC was similar to that of GA and GB, but the value of XC was significantly lower than that of XA and XB. This indicated that even in small amounts, NP100 (C) was the most effective in eliminating astringency in persimmons. Typically, at an STC value of 100 mg CTN eq/100 g FW [3,29,30,31,32,33], the paste does not have an astringent taste. This indicates that astringency was eliminated in all treatments except for AP.
3.5. Effect of Heat Treatment on the STC of AP
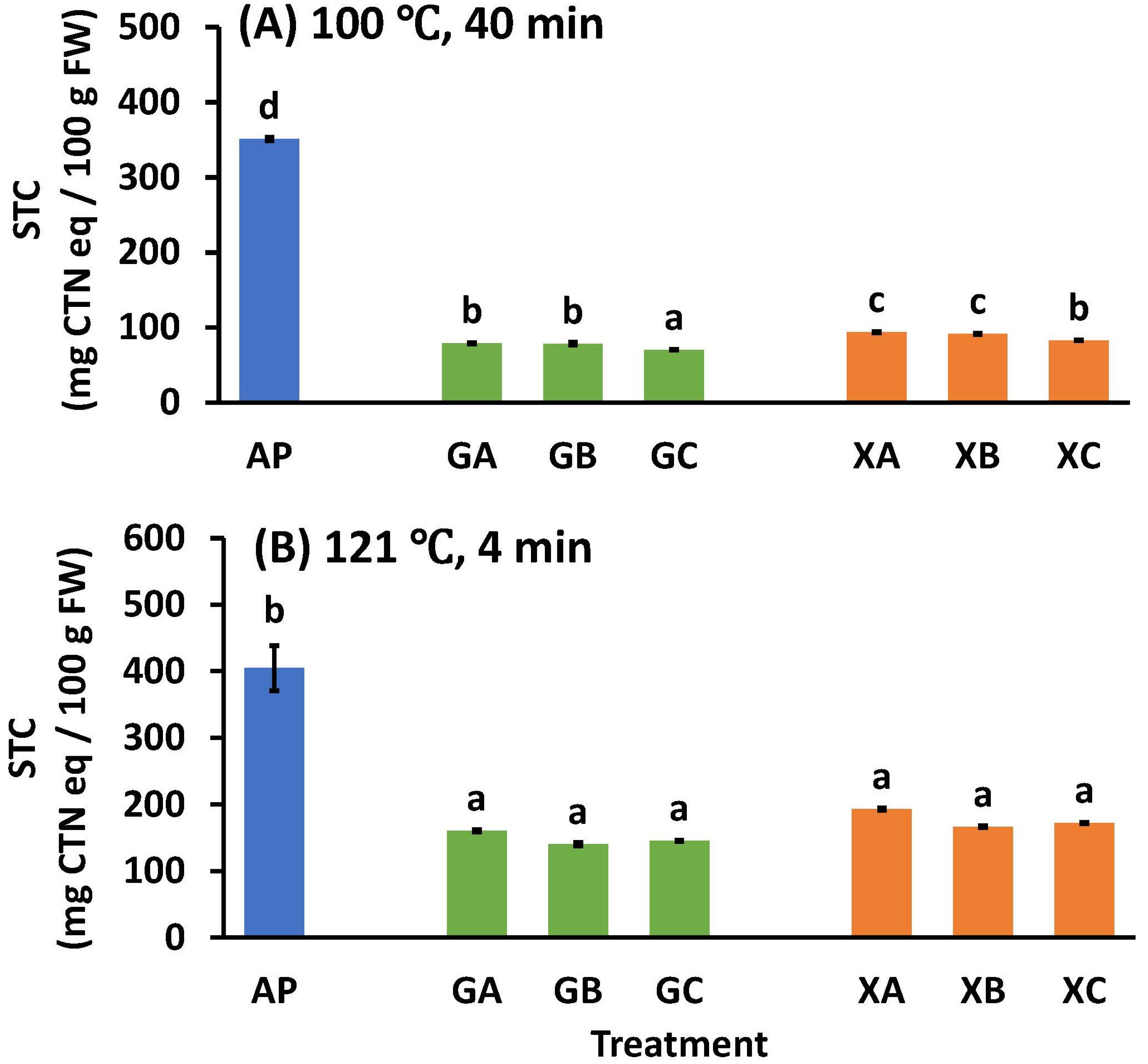
Even if the astringency is removed by carbon dioxide or ethanol, the astringency recurs when heated, which is a problem for the manufacture of persimmon processed products. Therefore, the effect of using collagen peptides and polysaccharides on the stringency recurrence during heating was investigated. The STC levels after heat treatment at 100 °C for 40 min and 121 °C for 4 min are shown in Figure 6A and Figure 6B, respectively. When comparing GA with XA, GB with XB, and GC with XC, the STC values of the guar gum samples were significantly lower than those of the xanthan gum samples. Additionally, in the guar and xanthan gum samples, the levels were GA = GB > GC, XA = XB > XC, respectively. In summary, guar gum was more effective than xanthan gum for astringency elimination, and NP100 was more effective than CP3100 and CP5100 (Figure 6A).
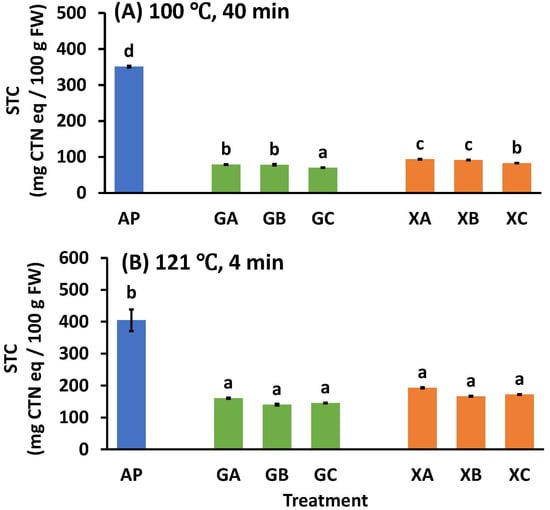
Figure 6.
Effect of heating treatment at (A) 100 °C or (B) 121 °C on the soluble tannin content of astringent persimmon paste. Mean values are presented as vertical bars indicating standard error (n = 6). The results were obtained using Tukey’s test for multiple comparisons. Letters indicate significant differences (p < 0.05). STC: soluble tannin content, FW: fresh weight, CTN: catechin, AP: astringent persimmon paste, GA: guar gum (0.5%) + SCP-3100 (0.5%), GB: guar gum (0.5%) + SCP-5100 (0.5%), GC: guar gum (0.5%) + Nippi Collagen 100 (0.5%), XA: xanthan gum (0.5%) + SCP-3100 (0.5%), XB: xanthan gum (0.5%) + SCP-5100 (0.5%), and XC: xanthan gum (0.5%) + Nippi Collagen 100 (0.5%).
After heat treatment of the pastes at 121 °C for 4 min, there was no significant difference between the treatments using polysaccharides or any collagen peptides. The STC values of GA, GB, GC, XA, XB, and XC were 160.5 ± 2.2, 140.3 ± 2.7, 145.1 ± 1.1, 193.2 ± 2.2, 166.7 ± 1.6, and 172.3 ± 1.3 mg CTN eq/100 g FW, respectively (Figure 6B). The effect of polysaccharides alone was determined in a previous study, with STC values of 252.1 ± 7.3 and 293.7 ± 39.1 mg CTN eq/100 g FW for guar and xanthan gum 1% (w/w) treatments at 121 °C for 4 min, respectively [17]. The combination treatment of 0.5% (w/w) collagen peptide and 0.5% (w/w) polysaccharide was more effective than that of polysaccharides alone.
As discussed (see Section 3.4), when the STC value is <100 mg CTN eq/100 g FW [3] [29,30,31,32,33], the paste has no astringent taste. Under the heating conditions of 100 °C for 40 min, the GA, GB, GC, XA, XB, and XC treatment groups all achieved STC values of <100 mg CTN eq/100 g FW, and the astringent taste of the astringent persimmons was suppressed to the extent that it could not be perceived. However, in the case of the high-temperature heating treatment at 121 °C for 4 min, the STC values for GA, GB, GC, XA, XB, and XC were all >100 mg CTN eq/100 g FW, and the amount of soluble polyphenols increased to the extent that astringency was perceived. In conclusion, there are limits to the effectiveness of heating in suppressing astringency when using a combination of protein and polysaccharide processing, and while it is possible to use it for heat sterilization at a normal pressure (100 °C, 40 min), there are limits to its applicability for pressurized sterilization (121 °C, 4 min), as the astringency returns to a level that can be perceived by humans.
3.6. Effect of Freeze Treatment on the STC of AP
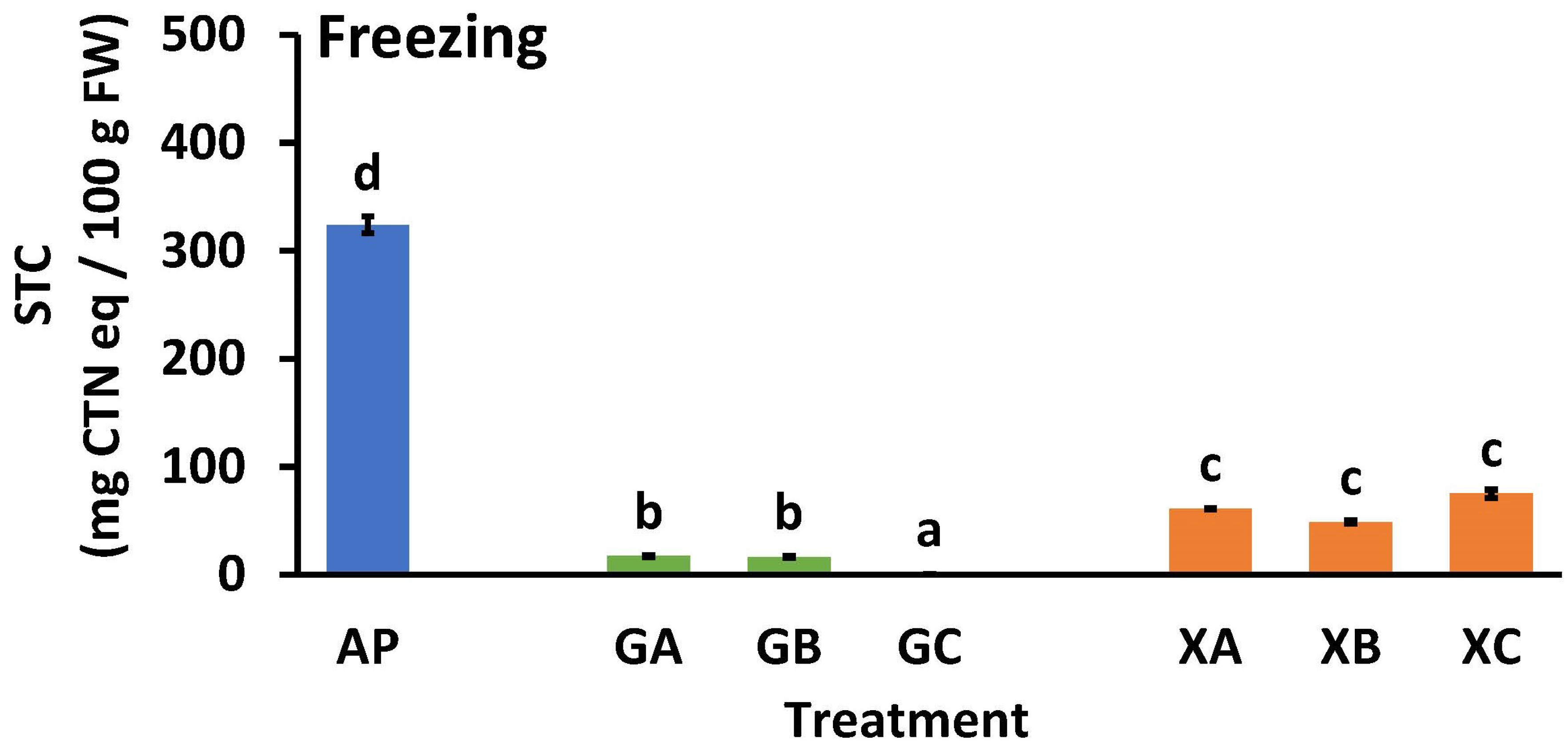
In the long-term distribution of AP, freezing is necessary, so the effect of the combined use of collagen peptides and polysaccharides on the STC of AP during freezing was investigated. Figure 7 illustrates the effect of freezing on the STC of AP. The STC values of untreated AP before and after freezing were 339.5 ± 13.8 (Figure 5) and 324.0 ± 7.9 (Figure 7), respectively. Soluble tannins become insoluble during freezing [34]; in this study, soluble tannins became partially insoluble during freezing. However, the STC level of AP after freezing did not decrease to a level that was not noticeable to humans (<100 mg CTN eq/100 g FW). A comparison of STC values for guar gum (GA, GB, and GC) and xanthan gum (XA, XB, and XC) samples with same the collagen peptide revealed that the STC values for guar gum were significantly lower than those of xanthan gum (GA < XA, GB < XB, and GC < XC). In the guar gum samples, the values were as follows: GA = GB > GC. In the xanthan gum samples, the type of collagen peptide added did not significantly affect the STC values.
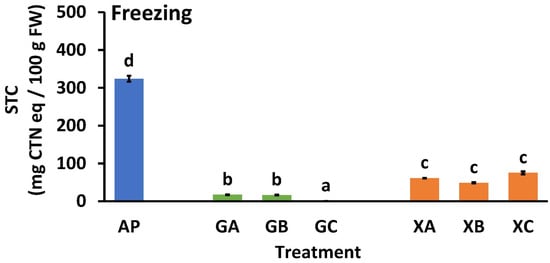
Figure 7.
Effect of freezing treatment on the soluble tannin content of astringent persimmon paste. Mean values are presented as vertical bars indicating standard error (n = 6). The results were obtained using Tukey’s test for multiple comparisons. Letters indicate significant differences (p < 0.05). STC: soluble tannin content, FW: fresh weight, CTN: catechin, AP: astringent persimmon paste, GA: guar gum (0.5%) + SCP-3100 (0.5%), GB: guar gum (0.5%) + SCP-5100 (0.5%), GC: guar gum (0.5%) + Nippi Collagen 100 (0.5%), XA: xanthan gum (0.5%) + SCP-3100 (0.5%), XB: xanthan gum (0.5%) + SCP-5100 (0.5%), and XC: xanthan gum (0.5%) + Nippi Collagen 100 (0.5%).
The combined use of protein and polysaccharide showed that the astringency removal effect of AP (Figure 5) and re-astringency preventive effect of heating (Figure 6) were maintained even when freezing (Figure 7). The polysaccharide used, guar gum, had a higher de-astringent effect than xanthan gum (Figure 7), which is consistent with the results shown in Figure 5. In addition, when collagen peptides were studied as a protein material without freezing (Figure 5), the STC values were GA = GB = GC, XA = XB > XC; after freezing, the values were GA = GB > GC, XA = XB = XC, and although only slightly, the effect of NP100 was higher than that of CP3100 and CP5100. In this experiment, guar gum was more effective as a polysaccharide, and NP100 was more effective as a collagen peptide. However, the reasons for this remain unclear and require further study.
The astringency of persimmons can be removed by treating the fruit with reagents capable of removing soluble tannins, such as carbon dioxide (dry ice) or alcohol [2,3,4,5,6,7]. However, it takes ~3–5 days for the astringency to disappear using the carbon dioxide method, and ~7 days for the alcohol method. Research has also been conducted into freezing persimmon fruit to remove their bitterness, and it has been found that storing the fruit at −20 °C or −80 °C for 60 days is an effective way to remove the astringency from persimmons [34]. Air drying of ‘Rojo Brillante’ persimmons for 20 days has also been reported to reduce the soluble tannin content [35]. As shown above, there are several methods for removing the astringency of persimmons, but all of them require time and effort. In comparison, the method used in this study is an excellent method that requires little time, as it only requires mixing AP, collagen peptides, and polysaccharide. It is also known that heating leads the taste of astringent persimmons to recur, which prevents their use in processed persimmon products [36]. One paper reports that adding 1% polysaccharide to persimmon paste removes the astringent taste [37]; however, it was also reported that the effect of heating in removing the astringent taste was lower than when 1% gelatin was added [17]. In this study, it was found that the combination of collagen peptides and polysaccharides prevented the recurrence of astringency even after heating at 100 °C for 40 min, and it was shown that this is a useful method for removing astringency while maintaining the quality of PA and suppressing the recurrence of astringency due to heating.
4. Conclusions
In this study, a novel and straightforward method for eliminating astringent components from AP by adding collagen peptides and polysaccharides was developed. Additionally, the color retention and syneresis of the mixed pastes (AP, collagen peptides, and polysaccharides) after heating or freezing were assessed. Specifically, 0.5% collagen peptide and 0.5% polysaccharide materials were added to the paste, with proteins used to effectively reduce astringency and reversion caused by heating, and polysaccharides used to maintain color and suppress syneresis. The STC was reduced to the extent that no astringency was observed with non-heating, heating at 100 °C for 40 min, and freezing. Additionally, the addition of 0.5% collagen peptide and 0.5% polysaccharide was effective in retaining the color and preventing syneresis. Combinations of three types of collagen peptides (CP3100, CP5100, and NP100) and two types of polysaccharides (guar and xanthan gums) demonstrated similar levels of STC reduction, color retention, and syneresis inhibition.
This study is anticipated to increase the use of persimmon paste because it can be produced using a straightforward method with good quality. Approximately 30% of astringent persimmons are discarded as substandard fruit. Producing a high-quality, astringency-free persimmon paste that is resistant to heat freezing would significantly reduce waste. The results of this study will make a significant contribution to the food processing industry because the findings have implications for reducing food waste, enhancing the utility of substandard astringent persimmons, and advancing sustainable food-processing techniques. As such, the findings have the potential to impact food waste management and agricultural sustainability.
However, only one ratio of collagen peptides to polysaccharides was assessed, highlighting the need for further experiments using different ratios. In addition, the color of the paste was only evaluated by visual observation using photographs, with an evaluation by panelists or instrumental analysis necessary in future studies.
Funding
This study was funded by the KAKENHI Grant-in-Aid for Scientific Research (B) (21H00808), Ministry of Education, Science, and Culture of Japan.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
I would like to thank Misaki Onda, Sae Kumagai, and Michiko Hirose for providing technical assistance.
Conflicts of Interest
The author declares no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AP | Astringent persimmon paste |
| P–T | Protein–tannin |
| BSA | Bovine serum albumin |
| EGCG | Epigallocatechin gallate |
| APJ | Astringent persimmon juice |
| CTN | Catechin |
| eq | Equivalent |
| FW | Fresh weight |
| STC | Soluble tannin content |
| CP3100 | SCP-3100 |
| CP5100 | SCP-5100 |
| NP100 | Nippi Collagen 100 |
| GA | Guar gum + CP3100 |
| GB | Guar gum + CP5100 |
| GC | Guar gum + NP100 |
| XA | Xanthan gum + CP3100 |
| XB | Xanthan gum + CP5100 |
| XC | Xanthan gum + NP100 |
| NH | Non-heating |
References
- Giordani, E.; Doumett, S.; Nin, S.; Del Bubba, M. Selected primary and secondary metabolites in fresh persimmon (Diospyros kaki Thunb.): A review of analytical methods and current knowledge of fruit composition and health benefits. Food Res. Int. 2011, 44, 1752–1767. [Google Scholar] [CrossRef]
- Cortes, V.; Rodriguez, A.; Blasco, J.; Rey, B.; Besada, C.; Cubero, S.; Salvador, A.; Talens, P.; Aleixos, N. Prediction of the level of astringency in persimmon using visible and near-infrared spectroscopy. J. Food Eng. 2017, 204, 27–37. [Google Scholar] [CrossRef]
- Yamada, M.; Taira, S.; Ohtsuki, M.; Sato, A.; Iwanami, H.; Yakushiji, H.; Wang, R.Z.; Yang, Y.; Li, G.C. Varietal differences in the ease of astringency removal by carbon dioxide gas and ethanol vapor treatments among Oriental astringent persimmons of Japanese and Chinese origin. Sci. Hortic. 2002, 94, 63–72. [Google Scholar] [CrossRef]
- Salvador, A.; Arnal, L.; Besada, C.; Larrea, V.; Hernando, I.; Perez-Munuera, I. Reduced effectiveness of the treatment for removing astringency in persimmon fruit when stored at 15 degrees C: Physiological and micro structural study. Postharvest Biol. Technol. 2008, 49, 340–347. [Google Scholar] [CrossRef]
- Monteiro, M.F.; AGUILA, J.S.D.; Pessoa, C.D.O.; Kluge, R.A. Vacuum packaging is efficient to remove astringency and to maintain the firmness of ‘Giombo’ persimmon. Rev. Bras. De Frutic. 2017, 39, e-129. [Google Scholar] [CrossRef]
- Zhu, Q.G.; Wang, M.M.; Gong, Z.Y.; Fang, F.; Sun, N.J.; Li, X.; Grierson, D.; Yin, X.R.; Chen, K.S. Involvement of DkTGA1 Transcription Factor in Anaerobic Response Leading to Persimmon Fruit Postharvest De-Astringency. PLoS ONE 2016, 11, 13. [Google Scholar] [CrossRef]
- Edagi, F.K.; Chiou, D.G.; Terra, F.d.A.M.; Sestari, I.; Kluge, R.A. Astringency removal of ‘Giombo’ persimmon with ethanol sub-doses. Ciênc. Rural. 2009, 39, 2022–2028. [Google Scholar] [CrossRef]
- Lu, Y.; Bennick, A. Interaction of tannin with human salivary proline-rich proteins. Arch. Oral Biol. 1998, 43, 717–728. [Google Scholar] [CrossRef]
- Soares, S.; García-Estévez, I.; Ferrer-Galego, R.; Brás, N.F.; Brandão, E.; Silva, M.; Teixeira, N.; Fonseca, F.; Sousa, S.F.; Ferreira-da-Silva, F. Study of human salivary proline-rich proteins interaction with food tannins. Food Chem. 2018, 243, 175–185. [Google Scholar] [CrossRef]
- Soares, S.; Brandão, E.; Guerreiro, C.; Soares, S.; Mateus, N.; de Freitas, V. Tannins in Food: Insights into the Molecular Perception of Astringency and Bitter Taste. Molecules 2020, 25, 2590. [Google Scholar] [CrossRef]
- Poncet-Legrand, C.; Edelmann, A.; Putaux, J.-L.; Cartalade, D.; Sarni-Manchado, P.; Vernhet, A. Poly (L-proline) interactions with flavan-3-ols units: Influence of the molecular structure and the polyphenol/protein ratio. Food Hydrocoll. 2006, 20, 687–697. [Google Scholar] [CrossRef]
- Edelmann, A.; Lendl, B. Toward the optical tongue: Flow-through sensing of tannin−protein interactions based on FTIR spectroscopy. J. Am. Chem. Soc. 2002, 124, 14741–14747. [Google Scholar] [CrossRef] [PubMed]
- Jöbstl, E.; O’Connell, J.; Fairclough, J.P.A.; Williamson, M.P. Molecular model for astringency produced by polyphenol/protein interactions. Biomacromolecules 2004, 5, 942–949. [Google Scholar] [CrossRef]
- Mercurio, M.D.; Smith, P.A. Tannin quantification in red grapes and wine: Comparison of polysaccharide-and protein-based tannin precipitation techniques and their ability to model wine astringency. J. Agric. Food Chem. 2008, 56, 5528–5537. [Google Scholar] [CrossRef]
- Siebert, K.J. Haze formation in beverages. LWT Food Sci. Technol. 2006, 39, 987–994. [Google Scholar] [CrossRef]
- Tsurunaga, Y.; Onda, M. Effects of soy milk and condensed milk on astringency removal, astringency recurrence, appearance, and syneresis in persimmon paste. Acta Hortic. 2022, 1338, 365–374. [Google Scholar] [CrossRef]
- Tsurunaga, Y.; Takahashi, T.; Kanou, M.; Onda, M.; Ishigaki, M. Removal of astringency from persimmon paste via polysaccharide treatment. Heliyon 2022, 8, e10716. [Google Scholar] [CrossRef]
- Mamet, T.; Ge, Z.-Z.; Zhang, Y.; Li, C.-M. Interactions between highly galloylated persimmon tannins and pectins. Int. J. Biol. Macromol. 2018, 106, 410–417. [Google Scholar] [CrossRef]
- Mamet, T.; Yao, F.; Li, K.-K.; Li, C.-M. Persimmon tannins enhance the gel properties of high and low methoxyl pectin. LWT 2017, 86, 594–602. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, S.; Liao, T.; Shu, X.; Guo, D.; Huang, Y.; Yang, X.; Wang, Q.; Chen, X. Polysaccharide selection and mechanism for prevention of protein–polyphenol haze formation in beverages. J. Food Sci. 2020, 85, 3776–3785. [Google Scholar] [CrossRef]
- Soares, S.; Mateus, N.; de Freitas, V. Carbohydrates Inhibit Salivary Proteins Precipitation by Condensed Tannins. J. Agric. Food Chem. 2012, 60, 3966–3972. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.L.; Swain, T. The inhibition of enzymes by tannins. Phytochemistry 1965, 4, 185–192. [Google Scholar] [CrossRef]
- Chung, H.S.; Kim, H.S.; Lee, Y.G.; Seong, J.H. Effect of deastringency treatment of intact persimmon fruits on the quality of fresh-cut persimmons. Food Chem. 2015, 166, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, T.; Dufour, D.; Moreno, I.X.; Ceballos, H. Comparison of Pasting and Gel Stabilities of Waxy and Normal Starches from Potato, Maize, and Rice with Those of a Novel Waxy Cassava Starch under Thermal, Chemical, and Mechanical Stress. J. Agric. Food Chem. 2010, 58, 5093–5099. [Google Scholar] [CrossRef] [PubMed]
- Beck, K.; Brodsky, B. Supercoiled protein motifs: The collagen triple-helix and the α-helical coiled coil. J. Struct. Biol. 1998, 122, 17–29. [Google Scholar] [CrossRef]
- Matsuo, T.; Ito, S. The chemical structure of kaki-tannin from immature fruit of the persimmon (Diospyros kaki L.). Agric. Biol. Chem. 1978, 42, 1637–1643. [Google Scholar]
- Goto, Y.; Watanabe, O. Development of inhibitory technique for astringency recurrence in astringent persimmon fruit. Nippon. Shokuhin Kagaku Kogaku Kaishi = J. Jpn. Soc. Food Sci. Technol. 2010, 57, 220–223. [Google Scholar] [CrossRef]
- Butler, L.G.; Riedl, D.J.; Lebryk, D.; Blytt, H. Interaction of proteins with sorghum tannin: Mechanism, specificity and significance. J. Am. Oil Chem. Soc. 1984, 61, 916–920. [Google Scholar] [CrossRef]
- Amorim, C.; Alves, E.G.; Rodrigues, T.H.S.; Bender, R.J.; Canuto, K.M.; Garruti, D.S.; Antoniolli, L.R. Volatile compounds associated to the loss of astringency in ‘Rama Forte’ persimmon fruit. Food Res. Int. 2020, 136, 109570. [Google Scholar] [CrossRef]
- Del Bubba, M.; Giordani, E.; Pippucci, L.; Cincinelli, A.; Checchini, L.; Galvan, P. Changes in tannins, ascorbic acid and sugar content in astringent persimmons during on-tree growth and ripening and in response to different postharvest treatments. J. Food Compos. Anal. 2009, 22, 668–677. [Google Scholar] [CrossRef]
- Inari, T.; Tomoyeda, M. Consideration on the Removal of Astringency in the Persimmons (Part 2) Insolubility of Tannins and Changes of the Related Enzyme Activities. J. Home Econ. Jpn. 1992, 43, 271–276. [Google Scholar]
- Noypitak, S.; Terdwongworakul, A.; Krisanapook, K.; Kasemsumran, S. Evaluation of astringency and tannin content in ‘Xichu’ persimmons using near infrared spectroscopy. Int. J. Food Prop. 2015, 18, 1014–1028. [Google Scholar] [CrossRef]
- Taira, S. Astringency in persimmon. In Fruit Analysis; Springer: Berlin/Heidelberg, Germany, 1996; pp. 97–110. [Google Scholar]
- Das, P.R.; Eun, J.-B. Removal of astringency in persimmon fruits (Diospyros kaki) subjected to different freezing temperature treatments. J. Food Sci. Technol. 2021, 58, 3154–3163. [Google Scholar] [CrossRef] [PubMed]
- González, C.M.; Gil, R.; Moraga, G.; Salvador, A. Natural drying of astringent and non-astringent persimmon “Rojo Brillante”. Drying kinetics and physico-chemical properties. Foods 2021, 10, 647. [Google Scholar] [CrossRef] [PubMed]
- Tsurunaga, Y.; Onda, M.; Takahashi, T. Effect of heating methods on astringency recurrence, syneresis, and physical properties of persimmon paste. J. Food Sci. Technol. 2021, 58, 4616–4625. [Google Scholar] [CrossRef]
- Taboada, D.; García-Hernández, J.; Ortolá, M.D.; Castelló, M.L. Astringent and non-astringent persimmon cremogenates made with different thickeners. Int. J. Food Sci. Technol. 2024, 59, 1051–1062. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).