1. Overview
1.1. Demand and Supply of High-Tech Metals
Metals are the anchor of modern civilization and are constituents from infrastructure to electronics. Among the metals, the classification ‘high-tech metals’ refers to those metals supporting the rapidly growing high-tech industries, fueled by overwhelming demand and an environmentally sustainable future.
One of the high-tech metal groups, the rare earth metal (REM) Nd, plays a vital role in the manufacturing of efficient magnets. The REMs have enhanced the performance and efficiency of devices significantly and hold an essential role to drive technological innovation and advancement [1]. With the EU’s commitment to reducing carbon emissions, the demand for high-tech metals is expected to rise, as they are crucial in energy-efficient devices like electric motors and generators [2,3].
Today, a reliable supply of the high-tech metals, including those used in small quantities for a variety of economically significant (e.g., smart phones, laptops, solar cells, electric vehicles, etc.), and national defense applications is at risk. To address this challenge of metals supply risk, the European Commission and the United States have compiled lists of critical raw materials (CRMs). China dominates the production of some of the critical metals globally but also faces its own supply vulnerabilities, which highlights the complexity of the global metals supply chain. In general, the CRMs lists, which are subject to review and update from time to time, reflect raw materials of high economic importance and of high supply risk [4,5,6].
1.2. Secondary Sources and Optimization of Existing Primary Production Processes
The important role of CRMs in the high-tech industry and the challenges of processing poor-grade CRM minerals due to depletion effects in the past decades combined with their scarcity within high-tech economies have pushed CRM recoveries from waste and industrial side streams. Several secondary resources, such as spent catalysts, Nd-Fe-B permanent magnets, spent batteries, super-alloy scrap, dust, sludge, etc., are rich in valuable metals, including REMs. However, until recently, most of the valuable metals in such waste have been lost in different unrecoverable fractions, and the recovery rate of the REMs is generally very low. Dust, sludge, and other waste generated in the metallurgical processes are good options as they contain a lot of valuable metals, based on the situation of the mining site and the primary ores composition [7]. Therefore, the efficient recovery of metals from e-waste and industrial side streams is a key enabler of the circular economy of valuable metals.
1.3. Environmental Aspect
Residues, dust, sludge, and other waste generated in the metal-making industries and e-waste pose a significant environmental threat if not properly disposed of and treated. The disposal and conventional treatment of these wastes is costly [7]. Thus, the comprehensive utilization of wastes generated in metallurgical processes and municipal waste streams has drawn worldwide attention from governments, industries, and academics. This can reduce the environmental challenges associated with metallurgical waste and hazardous municipal waste streams [5,8,9].
1.4. Low and Intermediate Temperature Extraction Processes
Pyrometallurgical, hydrometallurgical, or electrochemical processing routes can be applied to recover valuable metals from waste streams. Hydrometallurgical techniques are widely used for the recovery of valuable metals from secondary raw materials as well as low-grade ore minerals [10].
For the recovery of certain metals, such as the REEs, pyrometallurgical processes are difficult to realize in industrial applications. The typical hydrometallurgical processes that have been widely promoted include the hydrochloric acid total leaching, hydrochloric acid selective leaching, and sulfuric acid leaching methods [8].
Hydrometallurgy processes generally involve two major processing steps: leaching and precipitation [11]. In the leaching stage, an acid solution is used to dissolve components from secondary sources, where the leachate solution contains valuable metals together with other impurities. After the removal of residues from the aqueous solution by filtration, the valuable metal is separated from the bulk solution by precipitation with alkali sulfates, such as sodium sulfates [12].
Intermediate-temperature processes like roasting and pyrolysis are applied as pretreatments of complex raw materials for a subsequent hydrometallurgical recovery of metals [9]. For example, sulfation roasting has been applied in the metallurgical treatment of complex minerals. Sulfation roasting involves converting metal-oxides in complex minerals into metal-sulfate salts. These metallic sulfate salts can then be leached to produce the valuable metals [13]. While several processing routes have been explored for the extraction and recovery of critical materials, they face challenges related to feedstock compositional variability, low target material concentrations, toxic contaminant fractions, poor resource grades, low selectivity, limited recovery efficiencies, high processing costs, and complex separation techniques required for the recovery of high-purity metals [5].
2. Review of Contributions
This Special Issue on the “Extraction and Recovery of Valuable Metals from Waste and Mineral Materials” aimed to frame a comprehensive discussion and sharing of data on the progress of the sustainable metallurgical processing of primary and secondary resources. Submissions on scientific discoveries and emerging technologies that enable the sustainable extraction, processing, and separation of valuable metals from unconventional sources were invited. The contributions to this Special Issue came from 14 different prestigious institutions and companies located in Europe, Asia, and Africa, as illustrated in Figure 1.
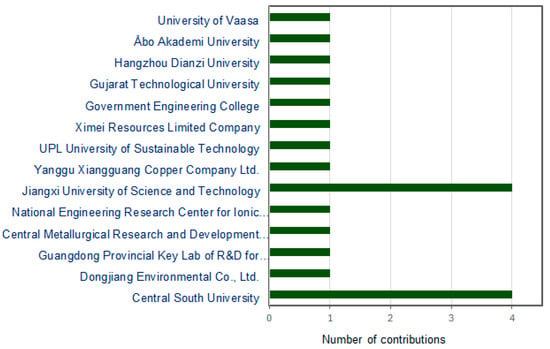
Figure 1.
Number of articles published in this Special Issue according to the affiliations of authors from different countries (China, India, Finland, and Egypt).
The peer-reviewed articles approved for publication under this special topic contribute significantly to the advancement of sustainable technologies in the extraction and recovery of metals, particularly those employing hydrometallurgical technologies. The contents of the articles should be of interest to a broad readership, like those considering a more sustainable production of valuable metals such as rare earth elements. The titles of the articles published under this topic are listed below and can be fully accessed via the journal’s page at: https://www.mdpi.com/journal/processes/special_issues/X011A6RNXP (accessed on 15 January 2025).
As the categorical data in Table 1 shows, most of the articles published are focused on developing hydrometallurgical techniques to extract/recover metals from the waste streams of the primary metals production routes.

Table 1.
Categorical data of the published contributions in this Special Issue.
The first article in the list, “Reaction Behavior of Kaolinite in Sulfur-Bearing Sodium Aluminate Solution under the Simulated Bayer Process” by Niu et al., proposed a novel idea of removing sulfur from high-sulfur bauxite, which poses hazards during the Bayer process. This process removes sulfur by having the silicon-containing minerals in bauxite react with sulfur species in a sodium aluminate solution to form sulfur-bearing desilication products (SDSP) for discharge with the red mud in the Bayer process. The authors experimentally investigated the reaction mechanism between kaolinite and different sulfur-containing ions under the conditions of the Bayer process, elucidating desulfurization rate variation and the formation mechanism of SDSPs. The authors also investigated the effect of increasing the temperature, amount, and duration of the reaction. In general, the work contributed to the efficient utilization of high-sulfur bauxite via the Bayer process.
The second article, entitled “Molecular Dynamics Calculation of the Coordination Behavior of Yb (III) in Sodium Carbonate Solution” by Lan et al., examined the coordination and hydration properties of the rare earth (REE) carbonates by using Yb (III) in high CO32− concentration solutions as a model for REEs. The authors also conducted dissolution experiments and molecular dynamics (MD) simulations of the actual solution, considering CO32− coordination and hydration actions simultaneously. According to their results, the main reason for the dissolution of Yb (III)’s carbonates is the coordination effect of CO32− on Yb (III). In general, the analysis method presented in the article provides guidance for understanding the coordination and hydration characteristics of oxyacid radicals to rare earth elements that help to improve extraction and recovery processes.
The article entitled “The Recovery of TiO2 from Ilmenite Ore by Ammonium Sulfate Roasting–Leaching Process” by Abdelgalil et al. proposes a processing route for TiO2 recovery from ilmenite ore via an ammonium sulfate roasting process. The authors experimentally studied the process for a low-temperature sulfation roasting application to chemically break down the crystal structure of ilmenite and generate metal-soluble sulfates simultaneously. Their roasted products were introduced to water leaching, and the residue was leached by diluted HCl acid, resulting in the enrichment of TiO2 in the leaching residue. Based on their experimental observations, authors suggest that the optimum roasting conditions are 500 °C, 210 min, an ilmenite-to-(NH4)2SO4 mass ratio of 1:7, and an ilmenite particle size of <43 µm. Under those conditions, optimized conditions, the TiO2 grade in the resulting synthetic rutile reached about 76 wt%.
The article entitled “Anodic Behavior of Hafnium in Anhydrous Electrodissolution-Coupled Hafnium Alkoxide Synthesis” by Li et al. investigated the anodic hafnium corrosion/dissolution electrochemical behavior of the Et4NCl or Et4NHSO4-based anhydrous system by employing green ethanol and Hf as feedstocks through electrochemical measurements combined with SEM observations. They reported that the Et4NCl-based anhydrous ethanol system exhibits an efficient passive film pitting corrosion breakdown and metal hafnium dissolution mechanisms, whereas the Et4NHSO4-based anhydrous ethanol system reflects a weaker corrosion mechanism of the anodic Hf under the passive film. According to the authors, the polarization resistance of the Et4NCl system was dramatically lower than that of the Et4NHSO4 system, indicating that the Et4NCl system has superior anodic Hf corrosion performance.
The fifth article, entitled “Co-Pyrolysis Behavior, Kinetic and Mechanism of Waste-Printed Circuit Board with Biomass” by Prajapati et al. demonstrated experimentally pyrolysis as a thermal degradation process for the treatment of waste-printed circuit boards (WPCBs) with biomass. They utilized a cotton stalk (CS) as an additive for the pyrolysis experiments on different samples; WPCBs, CSs, and their blends. They employed the thermogravimetric analyzer (TGA) technique at three different heating rates. They also applied three model-free methods: Kissinger–Akahira–Sunose (KAS), Flynn–Wall–Ozawa (FWO), and Starink, to calculate the apparent activation energy (Eα) and pre-exponential factor (A). The authors also calculated Eα values, and the possible decomposition mechanism was probed using the Criado method. The resulting pyrolysis oils from the experiments were also analyzed using Fourier-transform infrared spectroscopy analysis. The pyrolysis kinetics of the main thermal decomposition processes observed in this study promote the prediction and understanding of the pyrolysis behavior of a WPCB and help to design a suitable reactor and perform their mathematical modeling for process optimization.
The article entitled “Leaching Behavior of the Main Metals from Copper Anode Slime during the Pretreatment Stage of the Kaldor Furnace Smelting Process” by Zeng et al. reported an experimental observation that promotes the pretreatment process of the copper anode slime in the removal of impurities before Kaldor furnace reduction smelting. They conducted an experimental study to determine the leaching behavior of each metal in copper anode slime. They quantitatively determined the phase composition of Cu, Te, Pb, Bi, As, Sb, Se, Ag, and Au. They introduced hydrogen peroxide to enhance the leaching of impurities. Their results demonstrate that the Cu and Te in the copper anode slime mainly exist in the form of CuO/CuSO4 and Te/AuTe2, respectively. According to their experimental data, >99% Cu and 97% Te were leached out using 250 g/L H2SO4 and 28.8 g/L H2O2 with a leaching pressure of 0.8 MPa at 150 °C for 2 h, while the leaching of Au and Ag was <0.03%. This showed that the removal of Cu and Te and the enrichment of precious metals were achieved. The results reported in the article promote the optimization of the Kaldor furnace process.
The seventh article, entitled “Comparison of the Preparation Process of Rare Earth Oxides from the Water Leaching Solution of Waste Nd-Fe-B Magnets’ Sulfate Roasting Products”, by Chen et al. presented experimental investigation results that attempted to address the economic and efficient preparation of rare earth oxides (RExOy) with higher purity from water leaching solutions. Their work optimized the sodium carbonate precipitation process and compared it with the oxalic acid precipitation and double sulfate precipitation processes. The recovery efficiency of the REEs and the purity of the RExOy obtained from the sodium carbonate precipitation–calcination method were 99.12% and 98.33%, respectively. In general, the research results reported promote more economical, environmentally friendly, and efficient REE recovery and separation processes.
Data Availability Statement
Not applicable.
Acknowledgments
This work was partly supported by the K.H. Renlund Foundation in Finland under the project “Innovative e-waste recycling processes for greener and more efficient recoveries of critical metals and energy” at Åbo Akademi University.
Conflicts of Interest
The authors declare no conflicts of interest.
List of Contributions
- Niu, F.; Liu, G.; Zhu, J.; Pan, J.; Qi, T.; Wang, S.; Li, X.; Wang, S.; Yang, Y. Reaction Behavior of Kaolinite in Sulfur-Bearing Sodium Aluminate Solution under the Simulated Bayer Process. Processes 2023, 11, 2630. https://doi.org/10.3390/pr11092630
- Lan, Q.; Yang, Y.; Xie, Z.; Guo, H.; Liu, D.; Zhang, X. Molecular Dynamics Calculation of the Coordination Behavior of Yb (III) in Sodium Carbonate Solution. Processes 2023, 11, 2624. https://doi.org/10.3390/pr11092624
- Abdelgalil, M.S.; El-Barawy, K.; Ge, Y.; Xia, L. The Recovery of TiO2 from Ilmenite Ore by Ammonium Sulfate Roasting–Leaching Process. Processes 2023, 11, 2570. https://doi.org/10.3390/pr11092570
- Li, S.; Yang, S.; Zhao, P.; Chen, Y.; Tang, C.; Lai, Y.; Deng, C.; Wang, C. Anodic Behavior of Hafnium in Anhydrous Electrodissolution-Coupled Hafnium Alkoxide Synthesis. Processes 2023, 11, 564. https://doi.org/10.3390/pr11020564
- Prajapati, S.B.; Gautam, A.; Gautam, S.; Yao, Z.; Tesfaye, F.; Lü, X. Co-Pyrolysis Behavior, Kinetic and Mechanism of Waste-Printed Circuit Board with Biomass. Processes 2023, 11, 229. https://doi.org/10.3390/pr11010229
- Zeng, H.; Liu, F.; Zhou, S.; Liao, C.; Chen, F.; Zeng, Y. Leaching Behavior of the Main Metals from Copper Anode Slime during the Pretreatment Stage of the Kaldor Furnace Smelting Process. Processes 2022, 10, 2510. https://doi.org/10.3390/pr10122510
- Chen, F.; Liu, F.; Wang, L.; Wang, J. Comparison of the Preparation Process of Rare Earth Oxides from the Water Leaching Solution of Waste Nd-Fe-B Magnets’ Sulfate Roasting Products. Processes 2022, 10, 2310. https://doi.org/10.3390/pr10112310
References
- Orlova, S.; Rassõlkin, A. Permanent Magnets in Sustainable Energy: Comparative Life Cycle Analysis. Energies 2024, 17, 6384. [Google Scholar] [CrossRef]
- European Commission. A European Green Deal. Available online: https://commission.europa.eu/strategy-and-policy/priorities-2019-2024/european-green-deal_en (accessed on 15 December 2024).
- European Commission. Fit for 55: Delivering the EU’s 2030 Climate Target on the Way to Climate Neutrality. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52021DC0550 (accessed on 18 December 2024).
- European Commission—Press Release. Closing the Loop: Commission Adopts Ambitious New Circular Economy Package to Boost Competitiveness, Create Jobs and Generate Sustainable Growth. Available online: http://europa.eu/rapid/press-release_IP-15-6203_en.htm (accessed on 2 January 2025).
- Iloeje, C.O.; Tesfaye, F.; Anderson, A.E.; Hamuyuni, J. Recovery of Rare Earth and Critical Metals from Unconventional Sources. JOM 2022, 74, 990–992. [Google Scholar] [CrossRef]
- Van Gosen, B.S.; Verplanck, P.L.; Seal, R.R., II; Long, K.R.; Gambogi, J. Rare-earth elements. In Critical Mineral Resources of the United States—Economic and Environmental Geology and Prospects for Future Supply, 1st ed.; Schulz, K.J., DeYoung, J.H., Jr., Seal, R.R., II, Bradley, D.C., Eds.; Professional Paper 1802; USGS: Reston, VA, USA, 2017; pp. O1–O31. [Google Scholar]
- Sethurajan, M.; Lens, P.N.L.; Horn, H.A.; Figueiredo, L.H.A.; van Hullebusch, E.D. Leaching and Recovery of Metals. In Sustainable Heavy Metal Remediation, 1st ed.; Rene, E., Sahinkaya, E., Lewis, A., Lens, P., Eds.; Springer: Cham, Switzerland, 2017; Volume 2, pp. XII, 278. [Google Scholar]
- Chen, F.; Liu, F.; Wang, L.; Wang, J. Comparison of the Preparation Process of Rare Earth Oxides from the Water Leaching Solution of Waste Nd-Fe-B Magnets’ Sulfate Roasting Products. Processes 2022, 10, 2310. [Google Scholar] [CrossRef]
- Prajapati, S.B.; Gautam, A.; Gautam, S.; Yao, Z.; Tesfaye, F.; Lü, X. Co-Pyrolysis Behavior, Kinetic and Mechanism of Waste-Printed Circuit Board with Biomass. Processes 2023, 11, 229. [Google Scholar] [CrossRef]
- Maroufi, S.; Nekouei, R.K.; Hossain, R.; Assefi, M.; Sahajwalla, V. Recovery of Rare Earth (i.e., La, Ce, Nd, and Pr) Oxides from End-of-Life Ni-MH Battery via Thermal Isolation. ACS Sustain. Chem. Eng. 2018, 6, 11811. [Google Scholar] [CrossRef]
- Tunsu, C.; Petranikova, M.; Gergoric, M.; Ekberg, C.; Retegan, T. Reclaiming rare earth elements from end-of-life products: A review of the perspectives for urban mining using hydrometallurgical unit operations. Hydrometallurgy 2015, 156, 239. [Google Scholar] [CrossRef]
- Wickleder, M.S. Inorganic Lanthanide Compounds with Complex Anions. Chem. Rev. 2002, 102, 2011–2087. [Google Scholar] [CrossRef]
- Abdelgalil, M.S.; El-Barawy, K.; Ge, Y.; Xia, L. The Recovery of TiO2 from Ilmenite Ore by Ammonium Sulfate Roasting–Leaching Process. Processes 2023, 11, 2570. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).