Techno-Economic Assessment of Biogas-to-Methanol Processes Coupled with Low-Carbon H2 Production Technologies
Abstract
1. Introduction
Literature Review
2. Material and Methods
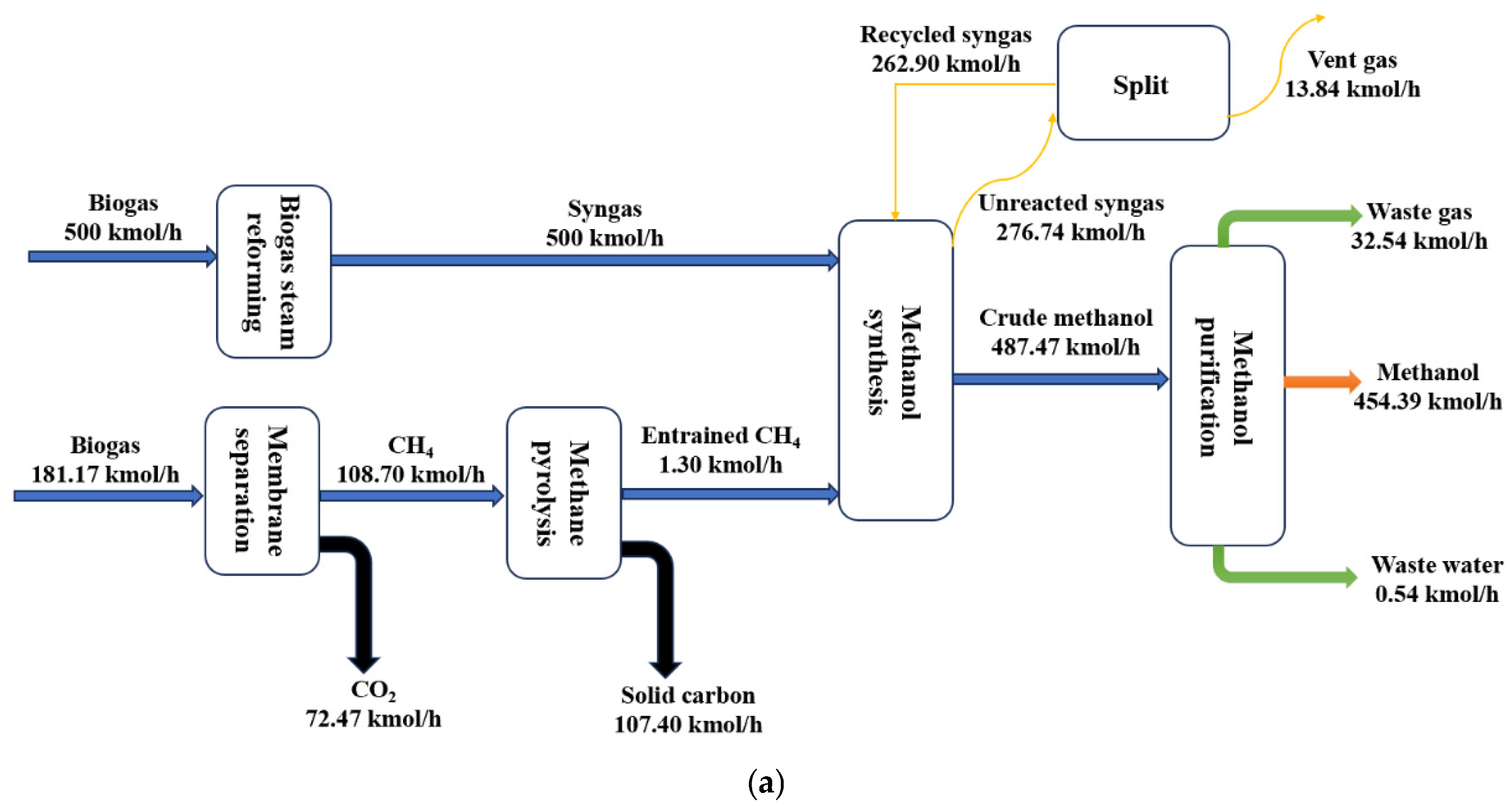
2.1. Process Description
- (1)
- The Peng–Robinson equation of state is chosen as the thermodynamic model owing to its better prediction ability for polar and nonpolar compounds [22].
- (2)
- The biogas is made up of CH4 (60 mol%) and CO2 (40 mol%). Its operating conditions are assumed to be 25 °C and 1 bar, respectively.
- (3)
- The isentropic efficiency of the compressor is set at 75%. The pressure drop of the reactor and heat exchangers is not considered. Additionally, the heat loss in the system is ignored.
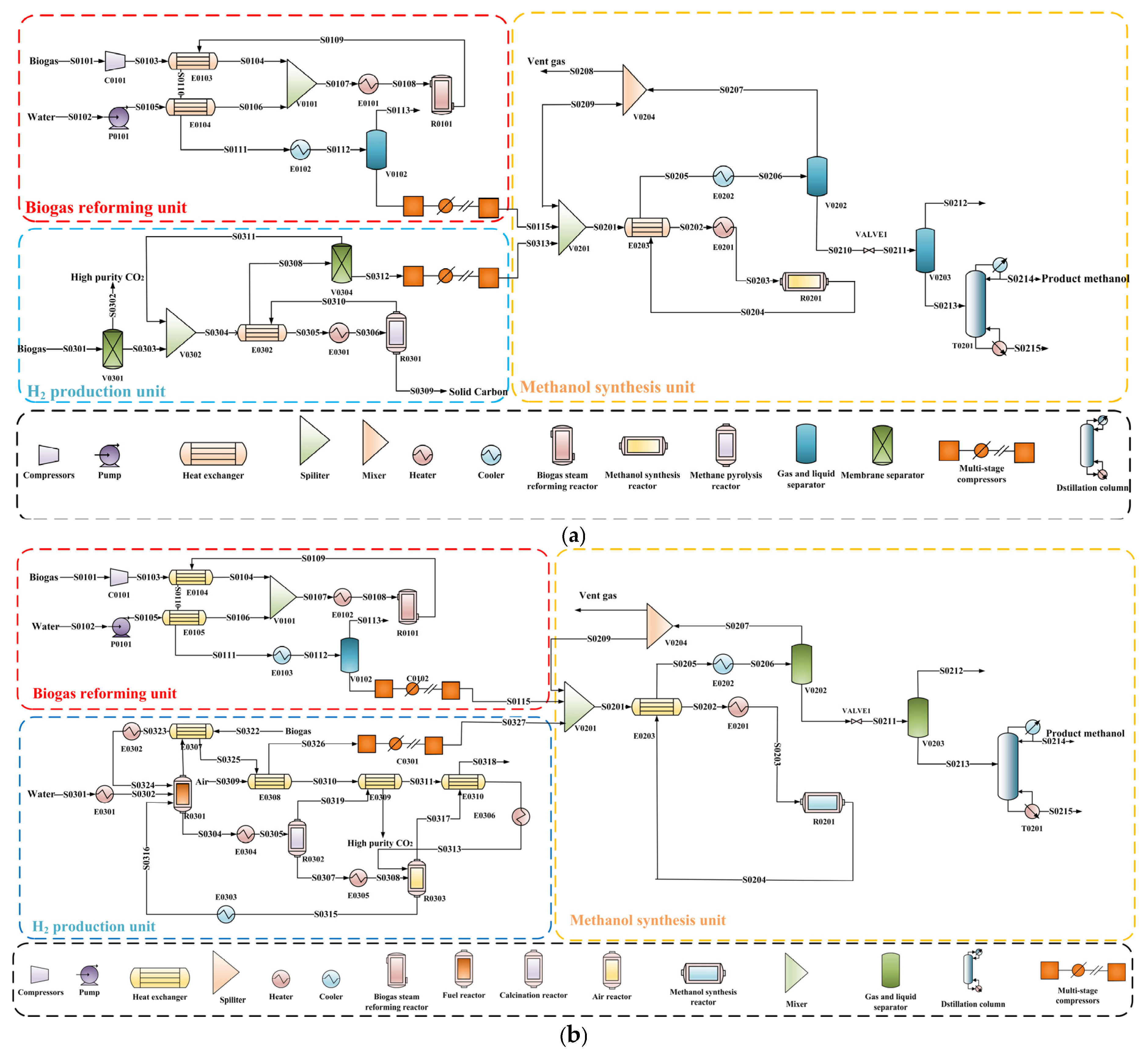
2.1.1. Biogas Reforming Unit
2.1.2. H2 Production Unit
- (1)
- Methane pyrolysis unit
- (2)
- Chemical looping reforming unit
2.1.3. Methanol Synthesis Unit
2.2. Technical Analysis Methods
2.2.1. Carbon Conversion Efficiency
2.2.2. Energy Efficiency
2.2.3. Unit Carbon Emissions
2.3. Economic Evaluation Methods
2.3.1. Annual Capital Investment
2.3.2. Total Operating Cost
3. Results and Discussion
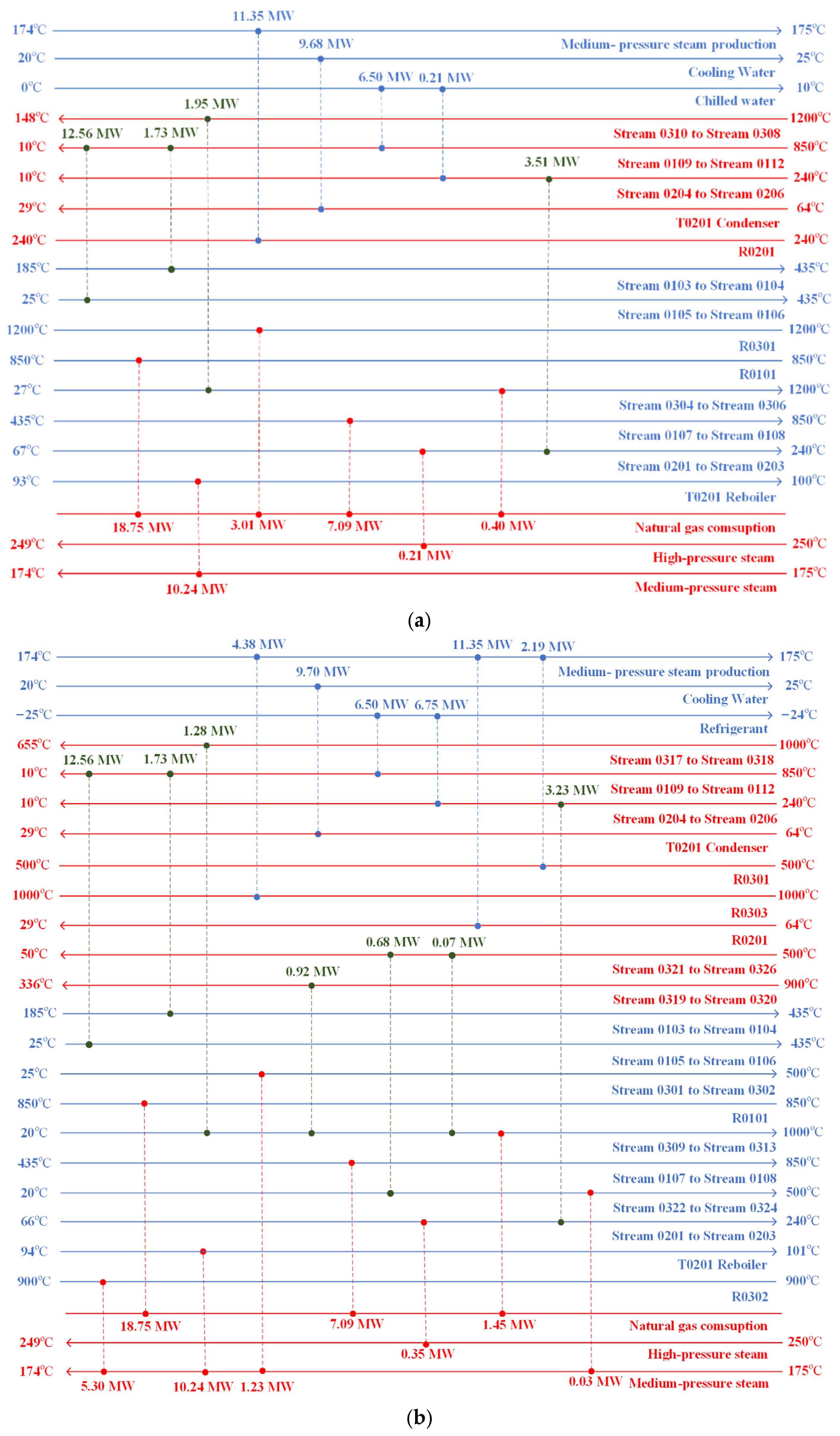
3.1. Technical Performance Analysis
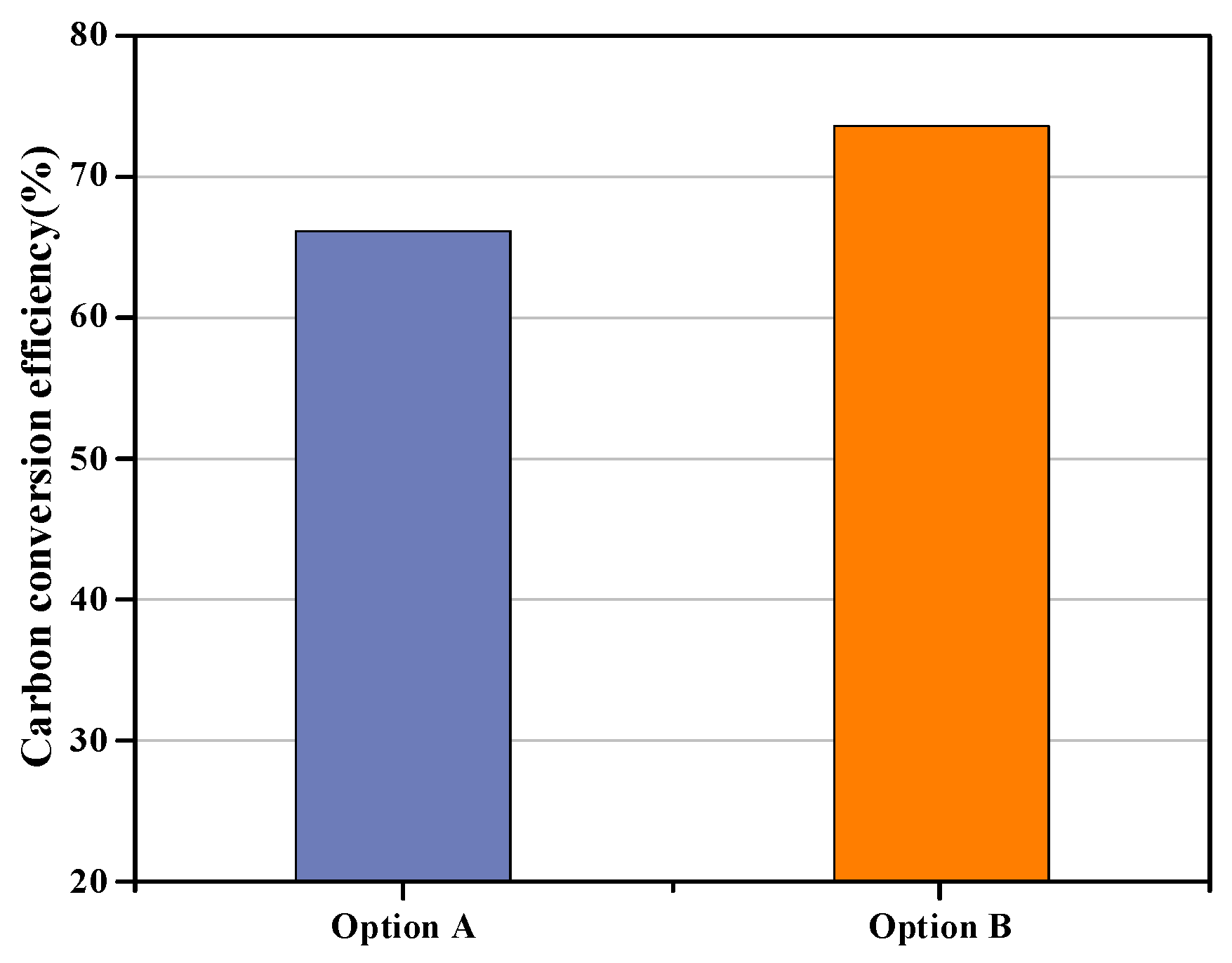
3.1.1. Carbon Conversion Efficiency
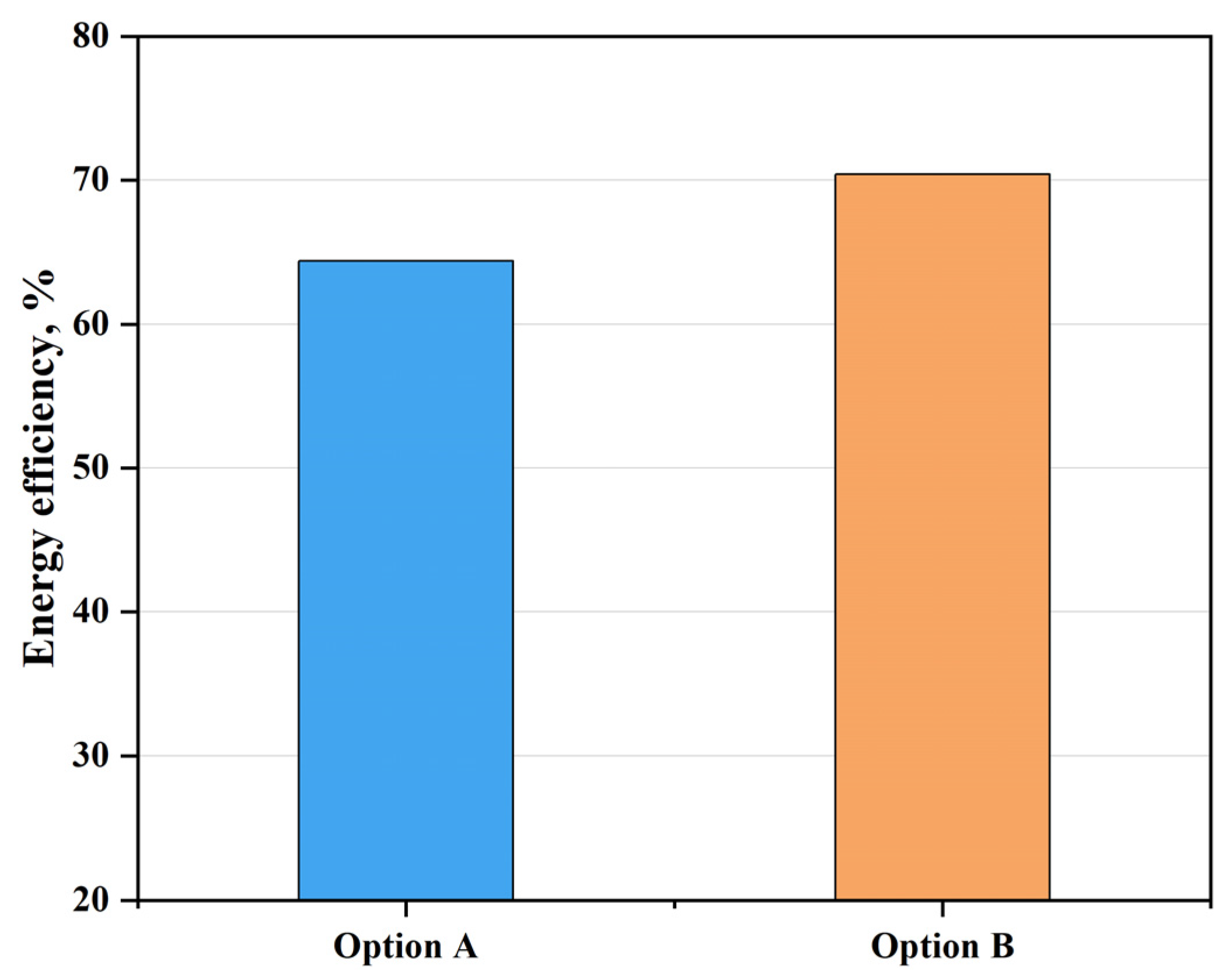
3.1.2. Energy Efficiency
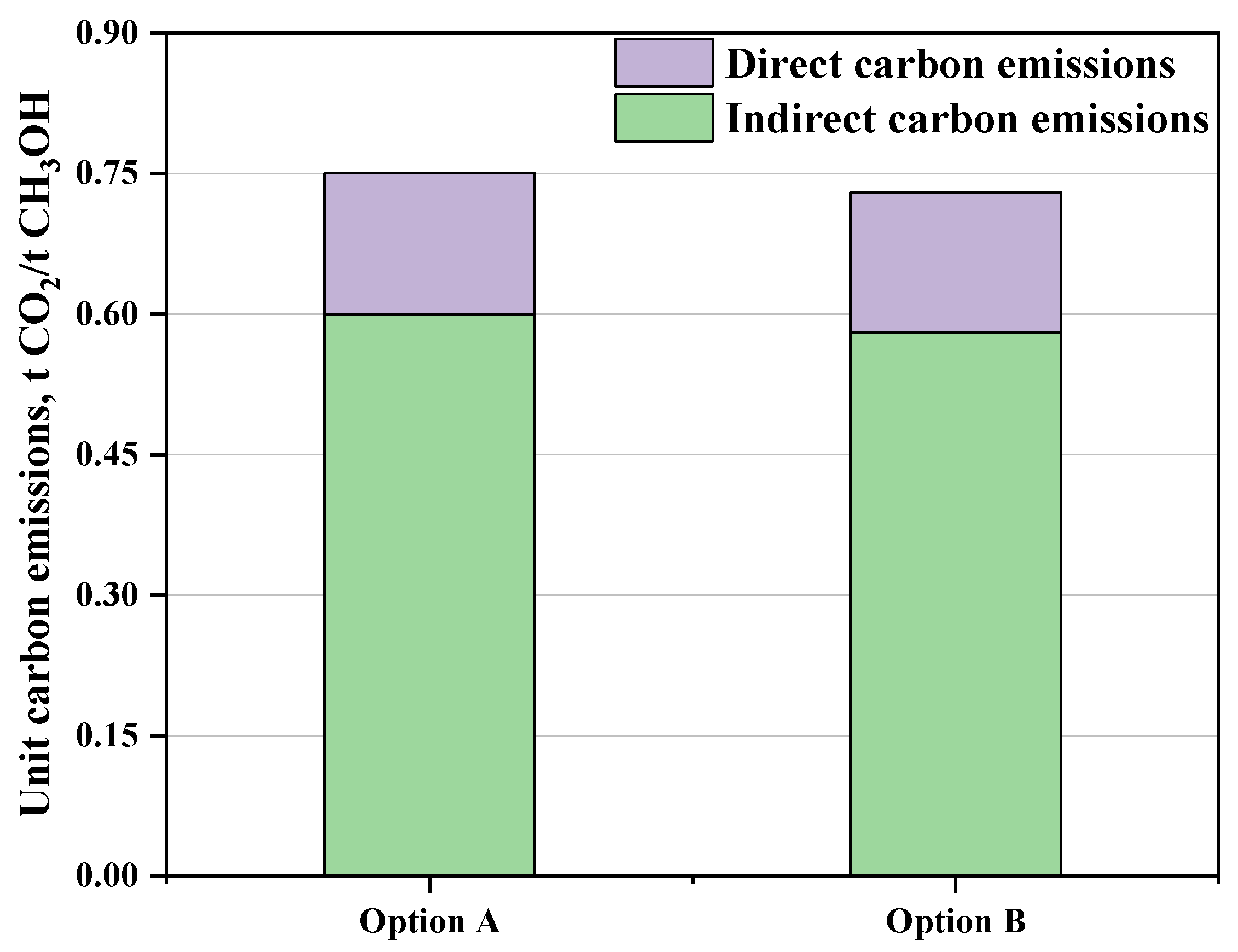
3.1.3. Unit Carbon Emissions
3.2. Economic Performance Analysis
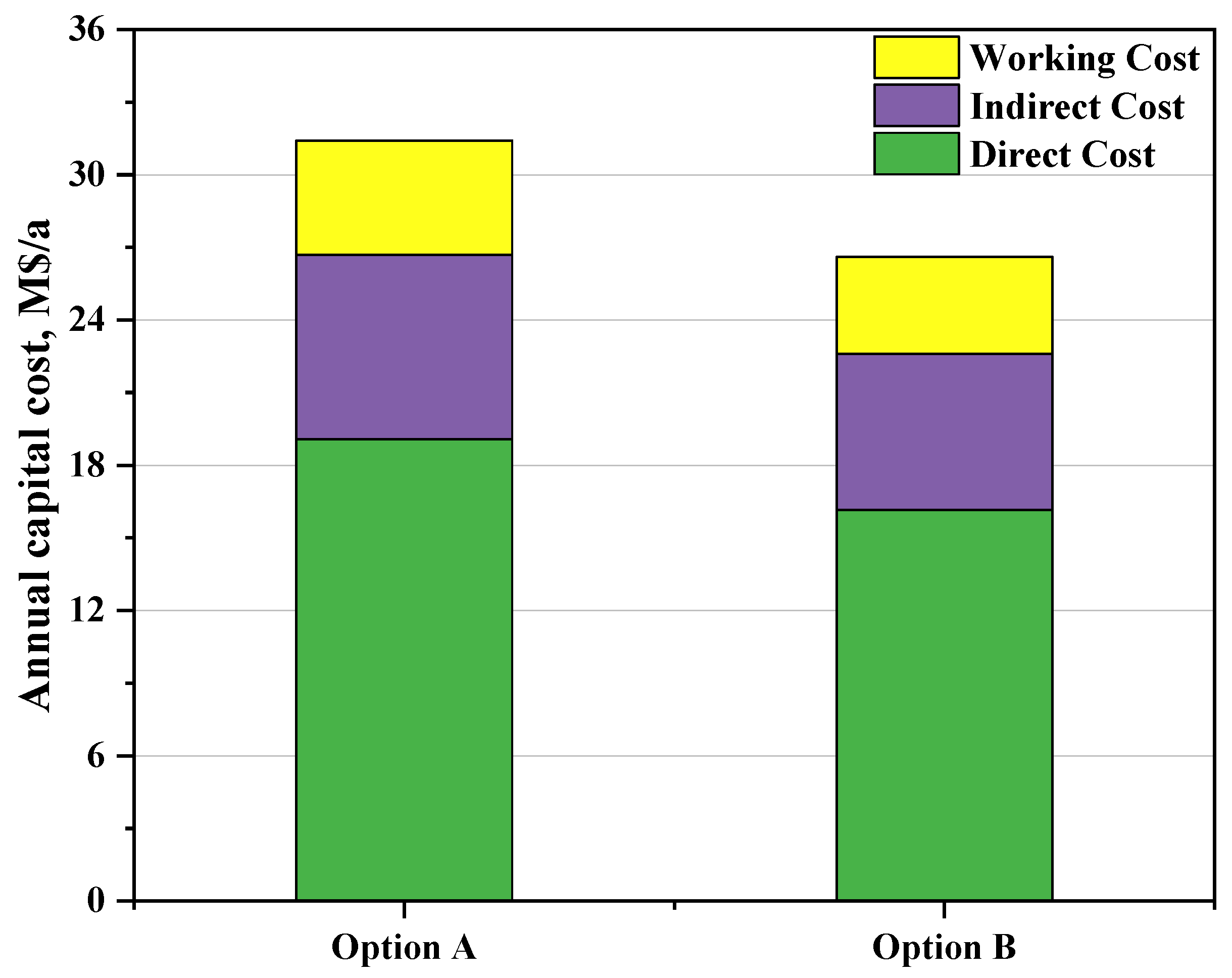
3.2.1. Annual Capital Investment Estimation
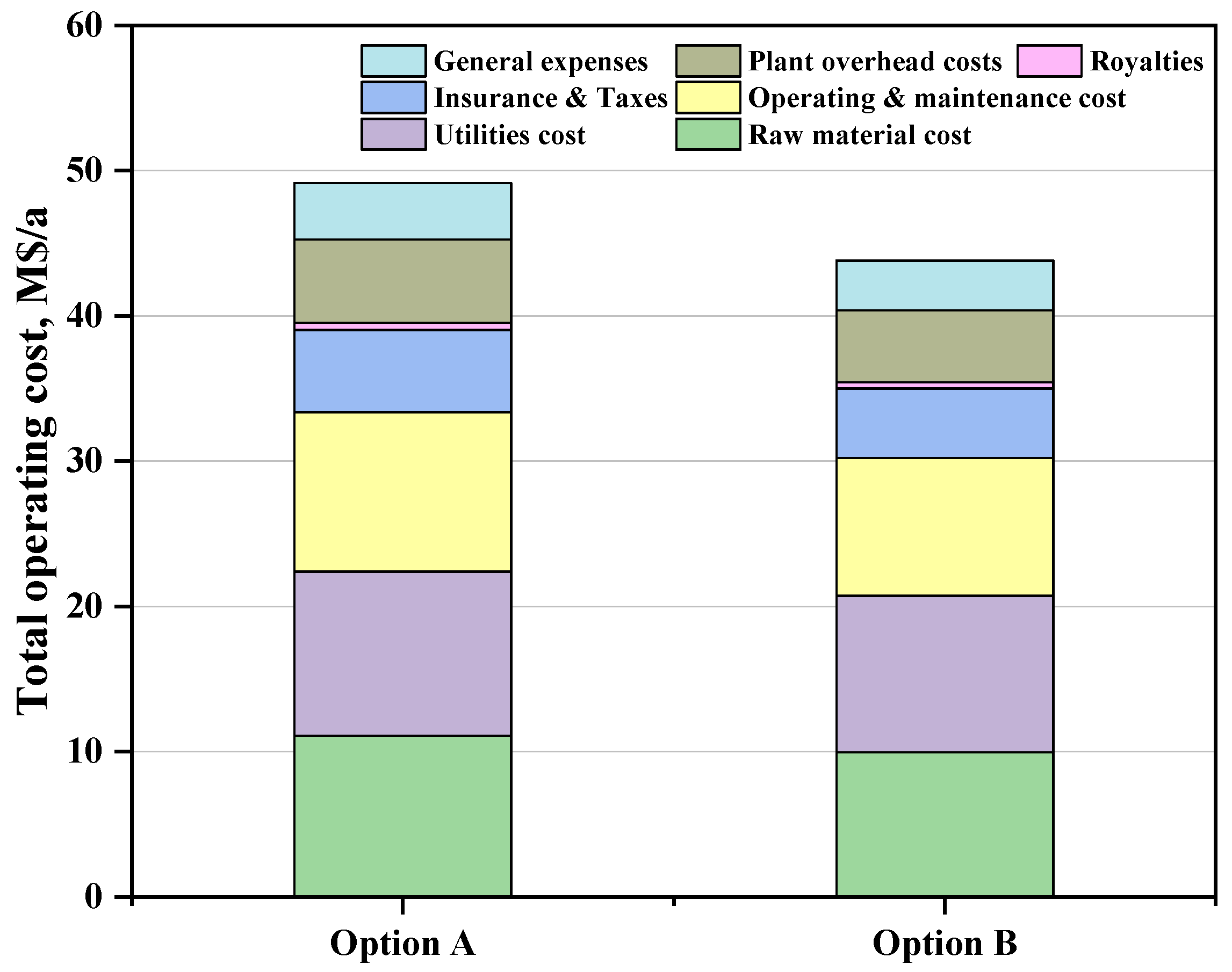
3.2.2. Total Operating Cost Estimation
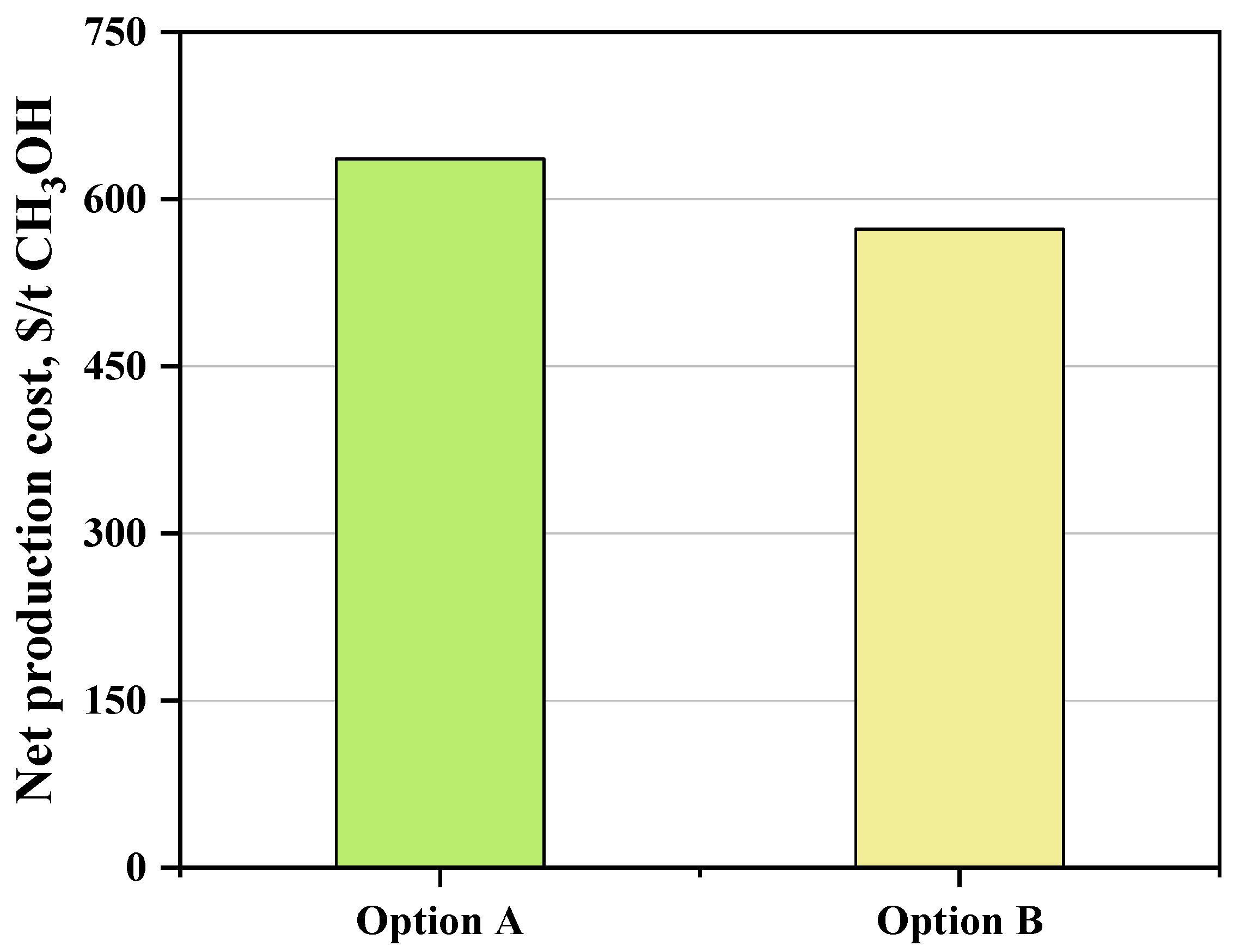
3.2.3. Net Production Cost Estimation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Işık, C.; Kuziboev, B.; Ongan, S.; Saidmamatov, O.; Mirkhoshimova, M.; Rajabov, A. The volatility of global energy uncertainty: Renewable alternatives. Energy 2024, 297, 131250. [Google Scholar] [CrossRef]
- Arutyunov, V.; Nikitin, A.; Strekova, L.; Savchenko, V.; Sedov, I. Utilization of renewable sources of biogas for small-scale production of liquid fuels. Catal. Today 2021, 379, 23–27. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Wahid, M.A. Development of biogas combustion in combined heat and power generation. Renew. Sustain. Energy Rev. 2014, 40, 868–875. [Google Scholar] [CrossRef]
- Chen, C.; Yang, A. Power-to-methanol: The role of process flexibility in the integration of variable renewable energy into chemical production. Energy Convers. Manag. 2021, 228, 113673. [Google Scholar] [CrossRef]
- Verhelst, S.; Turner, J.W.G.; Sileghem, L.; Vancoillie, J. Methanol as a fuel for internal combustion engines. Prog. Energy Combust. Sci. 2019, 70, 43–88. [Google Scholar] [CrossRef]
- Santos, R.O.d.; Santos, L.d.S.; Prata, D.M. Simulation and optimization of a methanol synthesis process from different biogas sources. J. Clean. Prod. 2018, 186, 821–830. [Google Scholar] [CrossRef]
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An overview of hydrogen production technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Hassan, N.S.; Jalil, A.A.; Rajendran, S.; Khusnun, N.F.; Bahari, M.B.; Johari, A.; Kamaruddin, M.J.; Ismail, M. Recent review and evaluation of green hydrogen production via water electrolysis for a sustainable and clean energy society. Int. J. Hydrogen Energy 2024, 52, 420–441. [Google Scholar] [CrossRef]
- Riley, J.; Atallah, C.; Siriwardane, R.; Stevens, R. Technoeconomic analysis for hydrogen and carbon Co-Production via catalytic pyrolysis of methane. Int. J. Hydrogen Energy 2021, 46, 20338–20358. [Google Scholar] [CrossRef]
- Murugan, A.; Thursfield, A.; Metcalfe, I.S. A chemical looping process for hydrogen production using iron-containing perovskites. Energy Environ. Sci. 2011, 4, 4639–4649. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, R.; Tang, Z.; Zhang, C.; Jun, K.-W.; Ki Kim, S.; Zhao, T.; Wan, H.; Guan, G. Boosting carbon utilization efficiency for sustainable methanol production from biomass: Techno-economic and environmental analysis. Energy Convers. Manag. 2024, 311, 118504. [Google Scholar] [CrossRef]
- Brouwer, A.S.; van den Broek, M.; Seebregts, A.; Faaij, A. Impacts of large-scale Intermittent Renewable Energy Sources on electricity systems, and how these can be modeled. Renew. Sustain. Energy Rev. 2014, 33, 443–466. [Google Scholar] [CrossRef]
- Lim, D.; Lee, B.; Lee, H.; Byun, M.; Lim, H. Projected cost analysis of hybrid methanol production from tri-reforming of methane integrated with various water electrolysis systems: Technical and economic assessment. Renew. Sustain. Energy Rev. 2022, 155, 111876. [Google Scholar] [CrossRef]
- Moioli, E.; Schildhauer, T. Eco-Techno-Economic Analysis of Methanol Production from Biogas and Power-to-X. Ind. Eng. Chem. Res. 2022, 61, 7335–7348. [Google Scholar] [CrossRef]
- Prabowo, J.; Lai, L.; Chivers, B.; Burke, D.; Dinh, A.H.; Ye, L.; Wang, Y.; Wang, Y.; Wei, L.; Chen, Y. Solid carbon co-products from hydrogen production by methane pyrolysis: Current understandings and recent progress. Carbon 2024, 216, 118507. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, S.; Aziz, M. Highly integrated system for ammonia and electricity production from biomass employing direct chemical looping: Exergy and exergoeconomic analyses. Energy Convers. Manag. 2022, 251, 115013. [Google Scholar] [CrossRef]
- Chisalita, D.-A.; Cormos, C.-C. Techno-economic assessment of hydrogen production processes based on various natural gas chemical looping systems with carbon capture. Energy 2019, 181, 331–344. [Google Scholar] [CrossRef]
- Ostadi, M.; Bromberg, L.; Cohn, D.R.; Gençer, E. Flexible methanol production process using biomass/municipal solid waste and hydrogen produced by electrolysis and natural gas pyrolysis. Fuel 2023, 334, 126697. [Google Scholar] [CrossRef]
- Okeke, I.; Mani, S. Techno-economic assessment of biogas to liquid fuels conversion technology via Fischer-Tropsch synthesis: Cost of producing liquid biofuels from biogas. Biofuels Bioprod. Biorefining 2017, 11, 472–487. [Google Scholar] [CrossRef]
- Xiang, D.; Li, P.; Yuan, X.; Cui, P.; Huang, W. Highly efficient carbon utilization of coal-to-methanol process integrated with chemical looping hydrogen and air separation technology: Process modeling and parameter optimization. J. Clean. Prod. 2020, 258, 120910. [Google Scholar] [CrossRef]
- Zhang, D.; Duan, R.; Li, H.; Yang, Q.; Zhou, H. Optimal design, thermodynamic, cost and CO2 emission analyses of coal-to-methanol process integrated with chemical looping air separation and hydrogen technology. Energy 2020, 203, 117876. [Google Scholar] [CrossRef]
- Vita, A.; Italiano, C.; Previtali, D.; Fabiano, C.; Palella, A.; Freni, F.; Bozzano, G.; Pino, L.; Manenti, F. Methanol synthesis from biogas: A thermodynamic analysis. Renew. Energy 2018, 118, 673–684. [Google Scholar] [CrossRef]
- Gao, R.; Zhang, C.; Lee, Y.-J.; Kwak, G.; Jun, K.-W.; Kim, S.K.; Park, H.-G.; Guan, G. Sustainable production of methanol using landfill gas via carbon dioxide reforming and hydrogenation: Process development and techno-economic analysis. J. Clean. Prod. 2020, 272, 122552. [Google Scholar] [CrossRef]
- Keipi, T.; Li, T.; Løvås, T.; Tolvanen, H.; Konttinen, J. Methane thermal decomposition in regenerative heat exchanger reactor: Experimental and modeling study. Energy 2017, 135, 823–832. [Google Scholar] [CrossRef]
- Meng, W.X.; Banerjee, S.; Zhang, X.; Agarwal, R.K. Process simulation of multi-stage chemical-looping combustion using Aspen Plus. Energy 2015, 90, 1869–1877. [Google Scholar] [CrossRef]
- Kasemanand, S.; Im-orb, K.; Tippawan, P.; Wiyaratn, W.; Arpornwichanop, A. Exergy analysis of the biogas sorption-enhanced chemical looping reforming process integrated with a high-temperature proton exchange membrane fuel cell. Energy Convers. Manag. 2017, 149, 485–494. [Google Scholar] [CrossRef]
- Bisotti, F.; Fedeli, M.; Prifti, K.; Galeazzi, A.; Dell’Angelo, A.; Manenti, F. Impact of Kinetic Models on Methanol Synthesis Reactor Predictions: In Silico Assessment and Comparison with Industrial Data. Ind. Eng. Chem. Res. 2022, 61, 2206–2226. [Google Scholar] [CrossRef]
- Rinaldi, R.; Lombardelli, G.; Gatti, M.; Visconti, C.G.; Romano, M.C. Techno-economic analysis of a biogas-to-methanol process: Study of different process configurations and conditions. J. Clean. Prod. 2023, 393, 136259. [Google Scholar] [CrossRef]
- Baena-Moreno, F.M.; Pastor-Pérez, L.; Wang, Q.; Reina, T.R. Bio-methane and bio-methanol co-production from biogas: A profitability analysis to explore new sustainable chemical processes. J. Clean. Prod. 2020, 265, 121909. [Google Scholar] [CrossRef]
- Peters, J.I. Review of: “ Plant Design and Economics for Chemical Engineers ” Max S. Peters: McGraw-Hill Book Company, New York, 1958. 511 pp. $11.00. Eng. Econ. 2007, 5, 27–30. [Google Scholar] [CrossRef]
- Gao, R.; Zhang, L.; Wang, L.; Zhang, C.; Jun, K.-W.; Kim, S.K.; Park, H.-G.; Zhao, T.; Wan, H.; Guan, G. Efficient utilization of CO2 in power-to-liquids/power-to-gas hybrid processes: An economic-environmental assessment. J. CO2 Util. 2023, 68, 102376. [Google Scholar] [CrossRef]
- Gao, R.; Zhang, L.; Wang, L.; Zhang, X.; Zhang, C.; Jun, K.-W.; Ki Kim, S.; Park, H.-G.; Gao, Y.; Zhu, Y.; et al. A comparative study on hybrid power-to-liquids/power-to-gas processes coupled with different water electrolysis technologies. Energy Convers. Manag. 2022, 263, 115671. [Google Scholar] [CrossRef]
- Tang, Z.; Zhang, L.; Wang, L.; Gao, R.; Jun, K.-W.; Kim, S.K.; Zhang, C.; Yang, Y.; Wan, H.; Guan, G. Carbon negative methanol production for CO2 utilization: Process design and 4E analysis. Energy 2024, 313, 134064. [Google Scholar] [CrossRef]

| Classification | RF |
|---|---|
| (1) Delivered purchased equipment | 1 |
| (2) Equipment and installation cost | 1.47 |
| (3) Auxiliary system | 1.15 |
| (4) Building and land cost | 0.98 |
| (5) Project construction cost | 0.63 |
| (6) Supervision and contingency cost | 0.81 |
| (7) Working capital cost | 0.89 |
| (8) Fixed capital cost | (2) + (3) + (4) + (5) + (6) + (7) |
| Item | |
|---|---|
| (1) Raw materials | Biogas 4 USD/GJ [19] |
| Feed water 0.53 USD/tonne [31] | |
| (2) Utilities | Nonrenewable electricity 0.063 USD/kWh [32] |
| Natural gas 8.80 USD/MMBtu [33] | |
| Cooling water 0.033 USD/m3 [31] | |
| (3) Operating and maintenance | |
| (3.1) Operating labor | 5 labors/shift, 3 shift/day, 60,000 USD/labor/a |
| (3.2) Operating supervision and laboratory charges | 35% of operating labor |
| (3.3) Maintenance and repairs | 3% of fixed capital investment |
| (3.4) Operating supplies | 15% of maintenance and repairs |
| (4) Other costs | |
| (4.1) Insurance and taxes | 2% of fixed capital investment |
| (4.2) Royalties | 1% of operating cost |
| (4.3) Plant overhead costs | 60% of labor, supervision and maintenance |
| (4.4) General expenses | |
| (4.4.1) Administration | 20% of labor, supervision and maintenance |
| (4.4.2) Distribution and selling | 2% of operating cost |
| (4.4.3) Research and development | 2% of operating cost |
| Total operating cost | (1) + (2) + (3) + (4) |
| Item | Option A | Option B | Unit | |
|---|---|---|---|---|
| Direct carbon emissions | Waste gas | 1.78 | 1.86 | tonne/h |
| Tail gas | 0.35 | 0.35 | tonne/h | |
| Indirect carbon emissions | Electricity consumption | 3.35 | 3.26 | tonne/h |
| Heat consumption | 5.31 | 5.02 | tonne/h | |
| Total carbon emissions | 10.79 | 10.50 | tonne/h | |
| Unit carbon emissions | 0.75 | 0.73 | t CO2/t CH3OH |
| No. | Equipment | Option A | Option B | Unit |
|---|---|---|---|---|
| 1 | Heat exchangers | 6.58 | 7.56 | M$ |
| 2 | Compressors and pumps | 6.95 | 6.79 | |
| 3 | Biogas reforming reactor | 19.10 | 19.10 | |
| 4 | Methanol synthesis reactor | 6.27 | 6.09 | |
| 5 | Membrane separator | 2.98 | / | |
| 6 | Methanol separation column | 0.41 | 0.41 | |
| 7 | Flash tanks | 0.49 | 0.48 | |
| 8 | Methane pyrolysis reactor | 8.23 | / | |
| 9 | Chemical looping reforming reactor | / | 2.76 | |
| Total equipment cost | 51.00 | 43.20 |
| Category | Option A | Option B | Unit |
|---|---|---|---|
| (1) Raw materials | 11.11 | 9.96 | M$/a |
| (1.1) Biogas | 11.05 | 9.90 | |
| (1.2) Process water | 0.06 | 0.07 | |
| (2) Utilities | 11.29 | 10.77 | |
| (2.1) Nonrenewable electricity | 3.55 | 3.46 | |
| (2.2) Natural gas | 6.63 | 6.27 | |
| (2.3) Cooling water | 1.11 | 1.03 | |
| (3) Operating and maintenance | 10.97 | 9.48 | |
| (4) Other cost | 15.76 | 13.59 | |
| (4.1) Insurance and taxes | 5.66 | 4.79 | |
| (4.2) Royalties | 0.49 | 0.44 | |
| (4.3) Plant overhead costs | 5.74 | 4.96 | |
| (4.4) General expenses | 3.88 | 3.41 | |
| Operating cost | 49.13 | 43.80 |
| Item | Option A | Option B | Unit |
|---|---|---|---|
| Solid carbon | 20.61 | / | USD/t CH3OH |
| Highly purified CO2 | 5.88 | 10.23 | |
| Total production revenue | 26.49 | 10.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Fan, J.; Zhang, L.; Gao, R.; Zhang, C. Techno-Economic Assessment of Biogas-to-Methanol Processes Coupled with Low-Carbon H2 Production Technologies. Processes 2025, 13, 313. https://doi.org/10.3390/pr13020313
Yang Y, Fan J, Zhang L, Gao R, Zhang C. Techno-Economic Assessment of Biogas-to-Methanol Processes Coupled with Low-Carbon H2 Production Technologies. Processes. 2025; 13(2):313. https://doi.org/10.3390/pr13020313
Chicago/Turabian StyleYang, Yang, Jiayu Fan, Leiyu Zhang, Ruxing Gao, and Chundong Zhang. 2025. "Techno-Economic Assessment of Biogas-to-Methanol Processes Coupled with Low-Carbon H2 Production Technologies" Processes 13, no. 2: 313. https://doi.org/10.3390/pr13020313
APA StyleYang, Y., Fan, J., Zhang, L., Gao, R., & Zhang, C. (2025). Techno-Economic Assessment of Biogas-to-Methanol Processes Coupled with Low-Carbon H2 Production Technologies. Processes, 13(2), 313. https://doi.org/10.3390/pr13020313





