Green Valorization of Pitaya (Hylocereus polyrhizus) Peels by Ultrasound-Assisted Extraction and Encapsulation of Bioactive Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pitaya Peel Extracts
2.3. Freeze-Dried Particles Produced from the Optimized Pitaya Peel Extract
2.4. Determination of Betalain Content
2.5. Determination of Total Phenolic Content (TPC)
2.6. Antioxidant Activity (AA)
2.7. Physicochemical Characterization of Freeze-Dried Pitaya Particles
2.8. Microstructure of Freeze-Dried Pitaya Particles
2.9. In Vitro Simulated Digestion
2.10. Statistical Analysis
3. Results and Discussion
3.1. RCCD Model
3.2. TPC and Betalains Content of Optimized Pitaya Peel Extract (PAE) and Freeze-Dried Pitaya Particles
3.3. Characterization of Freeze-Dried Pitaya Particles
3.4. Morphology
3.5. Multivariate Analysis
3.6. Simulated In Vitro Digestion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Antioxidant Activity |
| DW | Dry weight |
| FD-PAE | Freeze-dried pitaya aqueous extract |
| FRAP | Ferric reducing antioxidant power |
| G100 | Powder formulated with 100% GA |
| GA | Gum arabic |
| GAE | Gallic acid equivalent |
| M100 | Powder formulated with 100% MD |
| M25G | Powder formulated with 25% MD and 75% GA |
| M50G | Powder formulated with 50% MD and 50% GA |
| M75G | Powder formulated with 75% MD and 25% GA |
| MD | Maltodextrin |
| PAE | Pitaya aqueous extract |
| RCCD | Rotatable Central Composite Design |
| SGF | Simulated gastric fluid |
| SIF | Simulated intestinal fluid |
| SSF | Simulated salivary fluid |
| TPC | Total phenolic content |
| UAE | Ultrasound-assisted extraction |
References
- Shah, K.; Chen, J.; Chen, J.; Qin, Y. Pitaya Nutrition, Biology, and Biotechnology: A Review. Int. J. Mol. Sci. 2023, 24, 13986. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, W.; Li, X.; Shu, C.; Jiang, W.; Cao, J. Nutrition, Phytochemical Profile, Bioactivities and Applications in Food Industry of Pitaya (Hylocereus Spp.) Peels: A Comprehensive Review. Trends Food Sci. Technol. 2021, 116, 199–217. [Google Scholar] [CrossRef]
- Joshi, M.; Prabhakar, B. Phytoconstituents and Pharmaco-therapeutic Benefits of Pitaya: A Wonder Fruit. J. Food Biochem. 2020, 44, e13260. [Google Scholar] [CrossRef]
- Song, H.; Chu, Q.; Xu, D.; Xu, Y.; Zheng, X. Purified Betacyanins from Hylocereus undatus Peel Ameliorate Obesity and Insulin Resistance in High-Fat-Diet-Fed Mice. J. Agric. Food Chem. 2016, 64, 236–244. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Xu, C.-Y.; Mazhar, M.S.; Naiker, M. Nutritional Value and Therapeutic Benefits of Dragon Fruit: A Comprehensive Review with Implications for Establishing Australian Industry Standards. Molecules 2024, 29, 5676. [Google Scholar] [CrossRef]
- Barroso-Torres, N.; Lobo, M.G.; Dorta, E. Bibliometric Analysis of Papaya and Dragon Fruit By-Products. Foods 2025, 14, 2275. [Google Scholar] [CrossRef]
- Hay, T.O.; Nastasi, J.R.; Prakash, S.; Fitzgerald, M.A. Comparison of Gidyea Gum, Gum Arabic, and Maltodextrin in the Microencapsulation and Colour Stabilisation of Anthocyanin-Rich Powders Using Freeze-Drying and Spray-Drying Techniques. Food Hydrocoll. 2025, 163, 111023. [Google Scholar] [CrossRef]
- Pham, V.T.; Vu, N.D.; Nguyen, T.N.P.; Minh Truong, N.; Bui, Q.M.; Bui, T.T.T.; Phan, N.Q.T. Study of Using Ultrasonic Waves in the Producing Dried Dragon Fruit Peel Processes. Int. J. Food Sci. 2024, 2024, 8619783. [Google Scholar] [CrossRef]
- Martins, I.R.; Martins, L.H.D.S.; Chisté, R.C.; Picone, C.S.F.; Joele, M.R.S.P. Betalains from Vegetable Peels: Extraction Methods, Stability, and Applications as Natural Food Colorants. Food Res. Int. 2024, 195, 114956. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; He, X.; Tang, Y.; Li, Z.; Li, C.; Zeng, Y.; Tang, J.; Sun, J. Betacyanins and Anthocyanins in Pulp and Peel of Red Pitaya (Hylocereus polyrhizus Cv. Jindu), Inhibition of Oxidative Stress, Lipid Reducing, and Cytotoxic Effects. Front. Nutr. 2022, 9, 894438. [Google Scholar] [CrossRef]
- Nitisuk, P.; Wanyo, P.; Chamsai, T.; Charoenjit, K. Sustainable Valorization of Tropical Fruit Peels for Sustainable Production of Natural Antioxidants and Functional Food Ingredients. Sustain. Food Technol. 2025, 3, 1189–1202. [Google Scholar] [CrossRef]
- Taharuddin, N.H.; Jumaidin, R.; Mansor, M.R.; Hazrati, K.Z.; Tarique, J.; Asyraf, M.R.M.; Razman, M.R. Unlocking the Potential of Lignocellulosic Biomass Dragon Fruit (Hylocereus polyrhizus) in Bioplastics, Biocomposites and Various Commercial Applications. Polymers 2023, 15, 2654. [Google Scholar] [CrossRef]
- Haider, M.W.; Abbas, S.M.; Saeed, M.A.; Farooq, U.; Waseem, M.; Adil, M.; Javed, M.R.; Haq, I.U.; Osei Tutu, C. Environmental and Nutritional Value of Fruit and Vegetable Peels as Animal Feed: A Comprehensive Review. Anim. Res. One Health 2025, 3, 149–164. [Google Scholar] [CrossRef]
- Pal, P.; Singh, A.K.; Srivastava, R.K.; Rathore, S.S.; Sahoo, U.K.; Subudhi, S.; Sarangi, P.K.; Prus, P. Circular Bioeconomy in Action: Transforming Food Wastes into Renewable Food Resources. Foods 2024, 13, 3007. [Google Scholar] [CrossRef]
- Nurhadi, B.; Qonit, M.A.H.; Mubarok, S.; Saputra, R.A. Enhancing Betacyanin Stability: Comparison of Dragon Fruit (Hylocereus Polyrhizus) Pulp and Peel Powders through Encapsulation Technology during Storage. Food Sci. Nutr. 2024, 12, 3251–3264. [Google Scholar] [CrossRef]
- Pudžiuvelytė, L.; Petrauskaitė, E.; Stabrauskienė, J.; Bernatonienė, J. Spray-Drying Microencapsulation of Natural Bioactives: Advances in Sustainable Wall Materials. Pharmaceuticals 2025, 18, 963. [Google Scholar] [CrossRef]
- Baltrusch, K.L.; Torres, M.D.; Domínguez, H. Optimizing Ultrasound-Assisted Extraction with Custom Design and Response Surface Methodology: A Case Study Using Ulva Spp. Ultrason. Sonochem. 2025, 120, 107443. [Google Scholar] [CrossRef]
- Loan, L.T.K.; Thao, L.T.N.; Vinh, B.T.; Mansamut, C.; Tai, N.V. Enhancing Antioxidant Extraction Efficiency from Red Dragon Fruit Peel by Green Approach Using Novel Optimization Technique. Curr. Res. Green Sustain. Chem. 2025, 11, 100474. [Google Scholar] [CrossRef]
- Lim, S.D.; Yusof, Y.A.; Chin, N.L.; Talib, R.A. Effect of Extraction Parameters on the Yield of Betacyanins from Pitaya Fruit (Hylocereus polyrhyzus) Pulps. J. Food Agric. Environ. 2011, 9, 158–162. [Google Scholar]
- Obanda, M.; Owuor, P.O.; Taylor, S.J. Flavanol Composition and Caffeine Content of Green Leaf as Quality Potential Indicators of Kenyan Black Teas. J. Sci. Food Agric. 1997, 74, 209–215. [Google Scholar] [CrossRef]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant Activity of Dietary Polyphenols As Determined by a Modified Ferric Reducing/Antioxidant Power Assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef]
- Cai, Y.Z.; Corke, H. Production and Properties of Spray-dried Amaranthus Betacyanin Pigments. J. Food Sci. 2000, 65, 1248–1252. [Google Scholar] [CrossRef]
- Cano-Chauca, M.; Stringheta, P.C.; Ramos, A.M.; Cal-Vidal, J. Effect of the Carriers on the Microstructure of Mango Powder Obtained by Spray Drying and Its Functional Characterization. Innov. Food Sci. Emerg. Technol. 2005, 6, 420–428. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues Vieira, T.R.; Lima, A.B.; Ribeiro, C.M.C.M.; De Medeiros, P.V.Q.; Converti, A.; Dos Santos Lima, M.; Maciel, M.I.S. Red Pitaya (Hylocereus polyrhizus) as a Source of Betalains and Phenolic Compounds: Ultrasound Extraction, Microencapsulation, and Evaluation of Stability. LWT 2024, 196, 115755. [Google Scholar] [CrossRef]
- Gali, L.; Bedjou, F.; Ferrari, G.; Donsì, F. Formulation and Characterization of Zein/Gum Arabic Nanoparticles for the Encapsulation of a Rutin-Rich Extract from Ruta chalepensis L. Food Chem. 2022, 367, 129982. [Google Scholar] [CrossRef]
- Šturm, L.; Osojnik Črnivec, I.G.; Istenič, K.; Ota, A.; Megušar, P.; Slukan, A.; Humar, M.; Levic, S.; Nedović, V.; Kopinč, R.; et al. Encapsulation of Non-Dewaxed Propolis by Freeze-Drying and Spray-Drying Using Gum Arabic, Maltodextrin and Inulin as Coating Materials. Food Bioprod. Process. 2019, 116, 196–211. [Google Scholar] [CrossRef]
- Rezende, Y.R.R.S.; Nogueira, J.P.; Narain, N. Microencapsulation of Extracts of Bioactive Compounds Obtained from Acerola (Malpighia emarginata DC) Pulp and Residue by Spray and Freeze Drying: Chemical, Morphological and Chemometric Characterization. Food Chem. 2018, 254, 281–291. [Google Scholar] [CrossRef]
- Yamashita, C.; Chung, M.M.S.; Dos Santos, C.; Mayer, C.R.M.; Moraes, I.C.F.; Branco, I.G. Microencapsulation of an Anthocyanin-Rich Blackberry (Rubus Spp.) by-Product Extract by Freeze-Drying. LWT 2017, 84, 256–262. [Google Scholar] [CrossRef]
- Cortés-Rojas, D.F.; Souza, C.R.F.; Oliveira, W.P. Optimization of Spray Drying Conditions for Production of Bidens pilosa L. Dried Extract. Chem. Eng. Res. Des. 2015, 93, 366–376. [Google Scholar] [CrossRef]
- Syamaladevi, R.M.; Insan, S.K.; Dhawan, S.; Andrews, P.; Sablani, S.S. Physicochemical Properties of Encapsulated Red Raspberry (Rubus idaeus) Powder: Influence of High-Pressure Homogenization. Dry. Technol. 2012, 30, 484–493. [Google Scholar] [CrossRef]
- Alam, M.; Sid, S.; Giri, S.; Das, R.; Kishore, A.; Kumar, N. Encapsulated Kinnow Peel Powder Using Freeze Drying: Effect of Maltodextrin and Gum Arabic Concentrations on Physiochemical, Functional and Thermal Properties. Food Humanity 2025, 4, 100546. [Google Scholar] [CrossRef]
- Saikia, S.; Mahnot, N.K.; Mahanta, C.L. Optimisation of Phenolic Extraction from Averrhoa Carambola Pomace by Response Surface Methodology and Its Microencapsulation by Spray and Freeze Drying. Food Chem. 2015, 171, 144–152. [Google Scholar] [CrossRef]
- Che Man, Y.; Irwandi, J.; Abdullah, W. Effect of Different Types of Maltodextrin and Drying Methods on Physico-Chemical and Sensory Properties of Encapsulated Durian Flavour. J. Sci. Food Agric. 1999, 79, 1075–1080. [Google Scholar] [CrossRef]
- Tonon, R.V.; Brabet, C.; Pallet, D.; Brat, P.; Hubinger, M.D. Physicochemical and Morphological Characterisation of Açai (Euterpe oleraceae Mart.) Powder Produced with Different Carrier Agents. Int. J. Food Sci. Technol. 2009, 44, 1950–1958. [Google Scholar] [CrossRef]
- Lorenzoni Nunes, G.; Marques Da Silva, T.; Tasch Holkem, A.; Da Cunha Schley, V.; Ragagnin De Menezes, C. Microencapsulação de culturas probióticas: Princípios do método de spray drying. Ciênc. E Nat. 2015, 37, 132. [Google Scholar] [CrossRef]
- De Carvalho Alves, J.N.; Oliveira, N.L.; De Oliveira Meira, A.C.F.; Pio, L.A.S.; De Resende, J.V. Valorization of the Peel of Pitaya’s Fruit (Hylocereus polyrhizus) Producing Betalain-Rich Freeze-Dried Microparticles. Waste Biomass Valorization 2024, 15, 1097–1111. [Google Scholar] [CrossRef]
- Widianto, R. Encapsulation of Betacyanin Extract from Red Dragon Fruit Peel with Maltodextrin and Inulin: Storage Stability and Simulated Gastrointestinal Digestion. Food Biosci. 2024, 61, 104566. [Google Scholar] [CrossRef]
- Peanparkdee, M.; Janchai, P.; Aksornsri, T.; Vaithanomsat, P. Design and Evaluation of Bromelain-Encapsulated Alginate Beads Reinforced with Gum Arabic: Formulation, Characterization, and Stability in Simulated Gastrointestinal Conditions. J. Agric. Food Res. 2025, 19, 101698. [Google Scholar] [CrossRef]
- De Oliveira, G.; De Lima Costa, I.H.; Dos Santos Lima, M.; Macedo Dantas, A.; Guerra Dias, A.R.; Da Silva Campelo Borges, G. Pitaya (Hylocereus polyrhizus) Peel Powder: A Source of Pigments, Phenolic and Antioxidants Activity for Use in Food Hydrocolloids. Food Biosci. 2025, 68, 106512. [Google Scholar] [CrossRef]
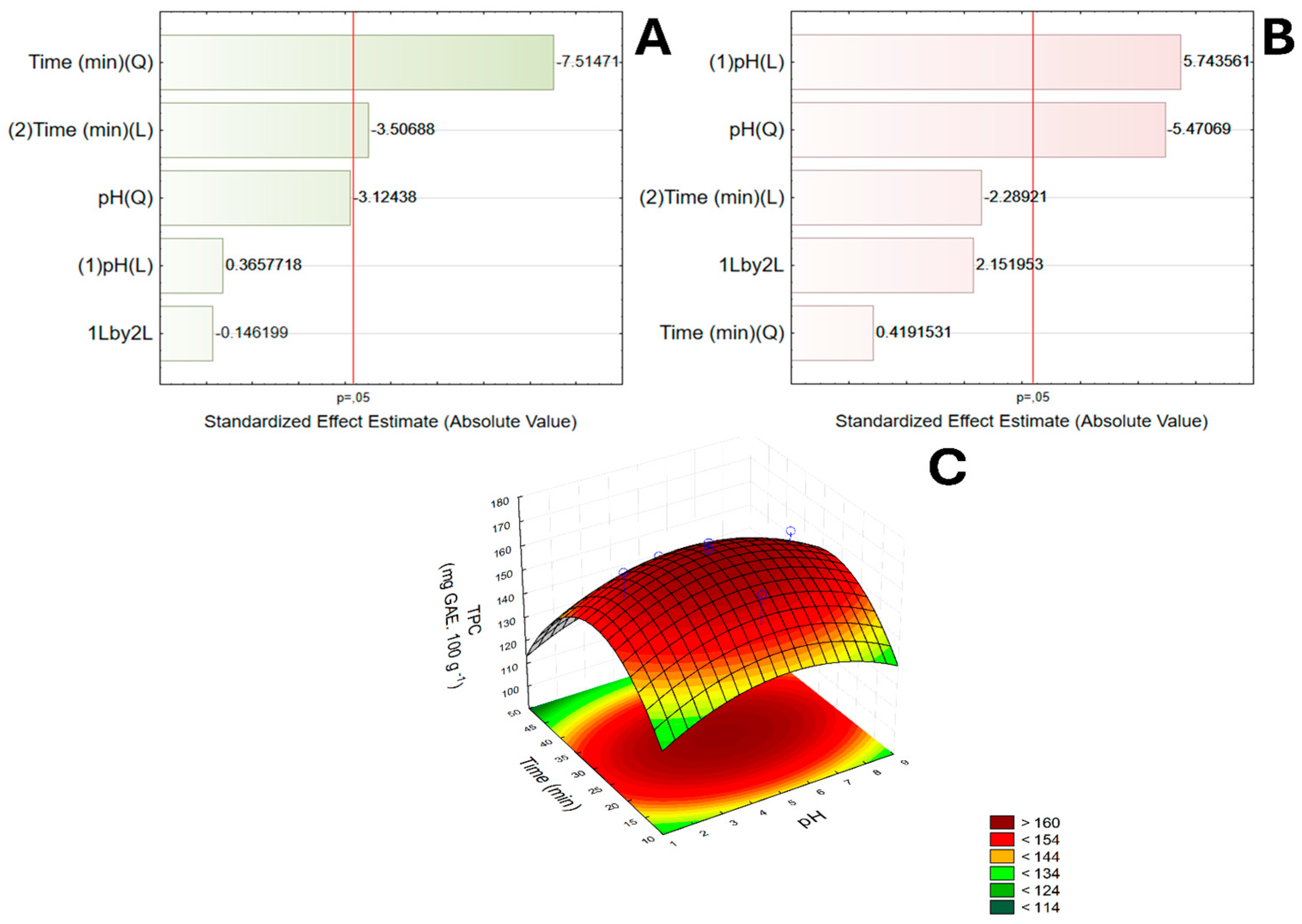


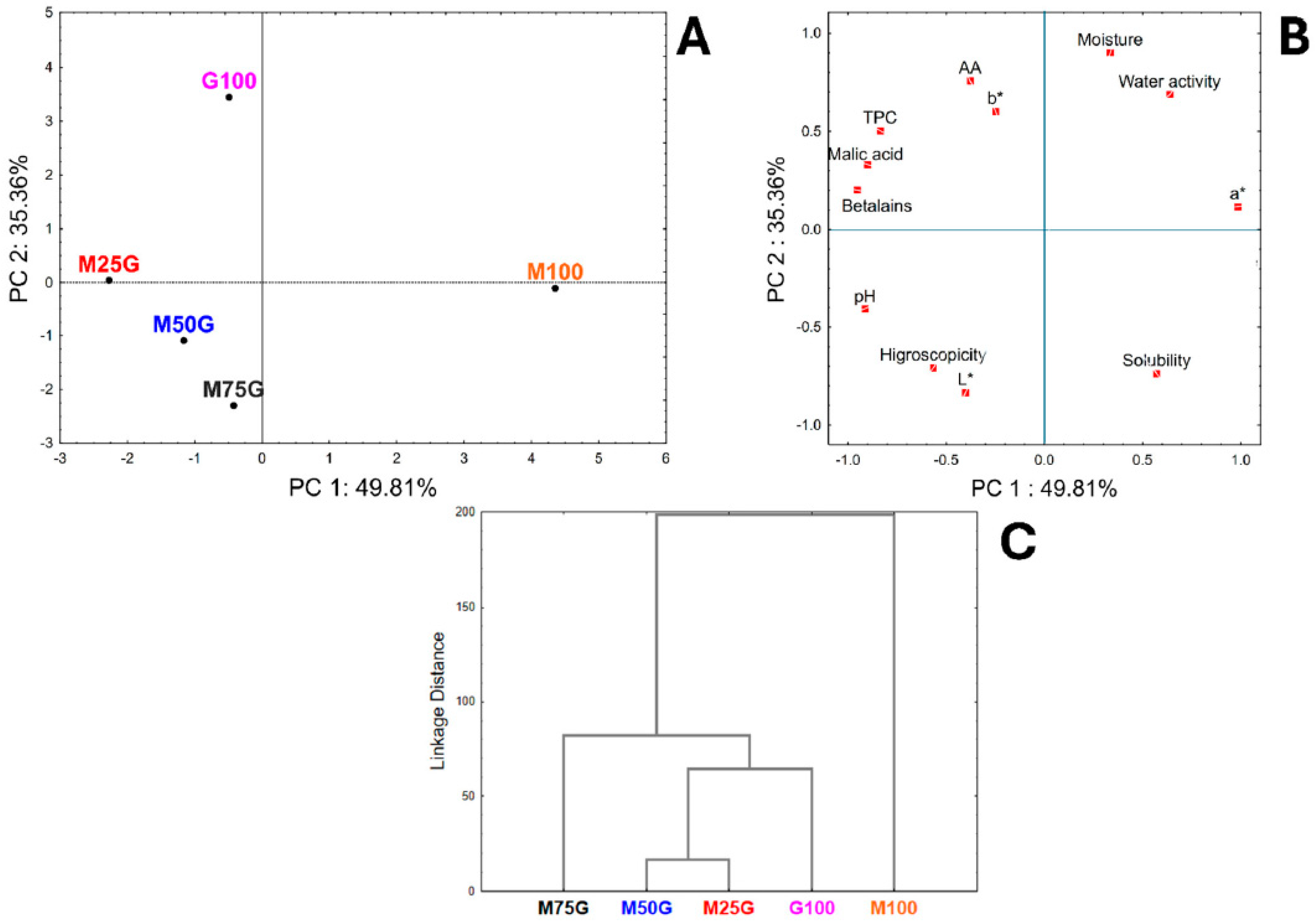
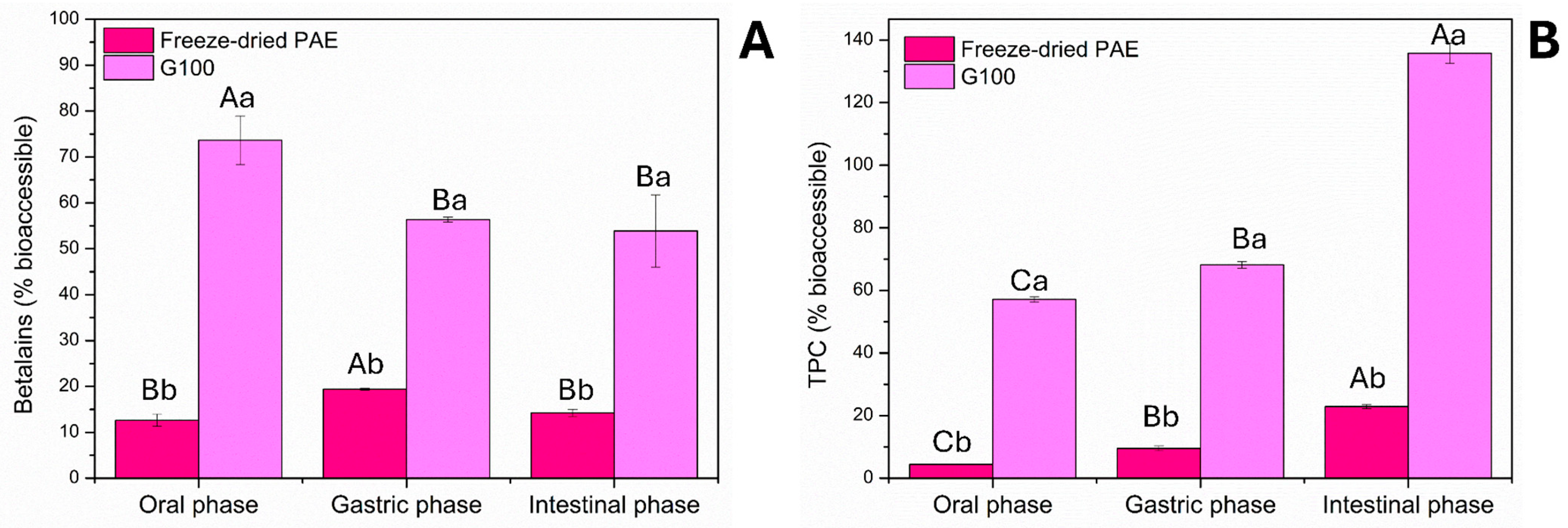
| Tests | Levels of Coded Variables | Levels of Real Variables (%) | ||
|---|---|---|---|---|
| X1 | X2 | pH | Time (min) | |
| 1 | (−1) | (−1) | 3.00 | 20.00 |
| 2 | (−1) | (+1) | 3.00 | 40.00 |
| 3 | (+1) | (−1) | 7.00 | 20.00 |
| 4 | (+1) | (+1) | 7.00 | 40.00 |
| 5 | (−1.41) | (0) | 2.17 | 30.00 |
| 6 | (+1.41) | (0) | 7.83 | 30.00 |
| 7 | (0) | (−1.41) | 5.00 | 15.86 |
| 8 | (0) | (+1.41) | 5.00 | 44.14 |
| 9 © | (0) | (0) | 5.00 | 30.00 |
| 10 © | (0) | (0) | 5.00 | 30.00 |
| 11 © | (0) | (0) | 5.00 | 30.00 |
| 12 © | (0) | (0) | 5.00 | 30.00 |
| Samples | MD (w/w %) | GA (w/w %) |
|---|---|---|
| M100 | 100 | 0 |
| G100 | 0 | 100 |
| M25G | 25 | 75 |
| M50G | 50 | 50 |
| M75G | 75 | 25 |
| Runs | TPC (mg GAE · 100 g−1) | Betalains (mg · g−1) |
|---|---|---|
| 1 | 146.64 ± 4.57 | 42.10 ± 1.15 |
| 2 | 145.32 ± 2.30 | 30.20 ± 1.89 |
| 3 | 150.67 ± 7.25 | 43.30 ± 2.45 |
| 4 | 148.54 ± 1.38 | 38.30 ± 3.86 |
| 5 | 168.17 ± 3.14 | 36.30 ± 2.10 |
| 6 | 165.07 ± 2.75 | 48.14 ± 1.47 |
| 7 | 165.50 ± 4.78 | 47.38 ± 5.15 |
| 8 | 148.51 ± 2.89 | 51.99 ± 6.20 |
| 9 © | 170.00 ± 7.56 | 47.36 ± 1.20 |
| 10 © | 167.31 ± 3.14 | 46.10 ± 1.45 |
| 11 © | 164.25 ± 5.77 | 44.10 ± 2.37 |
| 12 © | 164.25 ± 4.52 | 44.10 ± 2.54 |
| ANOVA | ||
| R2 | 0.57 | 0.50 |
| p-value | 0.004 | 0.703 |
| Calculated F value | 4.67 | 3.21 |
| Critical F value (5%) | 4.26 | 4.26 |
| Lack of fit | 0.017 | 0.013 |
| Samples | TPC (mg GAE · 100 g−1 DW) | Betalains Content (mg · 100 g−1 DW) | AA (µmol Fe2+ eq·g−1 DW) |
|---|---|---|---|
| PAE | 736.00 ± 25.40 a | 42.61 ± 2.36 a | ND |
| M100 | 155.52 ± 6.90 d | 5.57 ± 0.09 d | 6.63 ± 0.12 b |
| G100 | 316.33 ± 6.60 b | 17.58 ± 2.24 b | 17.43 ± 4.90 a |
| M25G | 298.03 ± 7.49 b | 21.25 ± 0.89 b | 13.74 ± 6.13 ab |
| M50G | 285.18 ± 33.13 b | 18.94 ± 0.44 b | 4.61 ± 0.22 b |
| M75G | 210.60 ± 4.27 c | 12.87 ± 1.15 c | 9.12 ± 1.46 ab |
| M100 | G100 | M25G | M50G | M75G | |
|---|---|---|---|---|---|
| Moisture (%) | 5.24 ± 0.35 b | 6.85 ± 0.11 a | 3.43 ± 0.52 c | 4.11 ± 0.39 c | 2.41 ± 0.27 d |
| Solubility (%) | 80.03 ± 0.60 a | 75.62 ± 3.68 a | 78.11 ± 0.67 a | 77.57 ± 1.07 a | 79.16 ± 0.59 a |
| Hygroscopicity (%) | 0.35 ± 0.12 c | 0.29 ± 0.08 c | 1.34 ± 0.27 a | 0.83 ± 0.16 b | 1.56 ± 0.18 a |
| Water activity | 0.16 ± 0.00 a | 0.18 ± 0.00 a | 0.04 ± 0.00 c | 0.08 ± 0.00 b | 0.07 ± 0.00 b |
| Color parameters | |||||
| L* | 64.46 ± 1.18 a | 64.09 ± 1.13 a | 65.17 ± 1.16 a | 66.75 ± 1.19 a | 66.65 ± 1.02 a |
| a* | 20.19 ± 0.86 a | 17.41 ± 0.66 b | 16.48 ± 0.38 b | 16.59 ± 0.48 b | 16.99 ± 0.81 b |
| b* | 8.44 ± 0.41 a | 8.59 ± 0.30 a | 8.71 ± 0.32 a | 8.27 ± 0.27 a | 8.35 ± 0.38 a |
| C* | 21.88 ± 0.95 a | 19.41 ± 0.72 b | 18.64 ± 0.49 b | 18.53 ± 0.54 b | 18.93 ± 0.87 b |
| H | 26.68 ± 0.14 b | 26.26 ± 0.11 c | 27.86 ± 0.34 a | 26.50 ± 0.31 c | 26.19 ± 0.71 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sampaio, L.M.F.; Rebouças, C.R.d.S.; Soares, L.M.; Lima, A.C.d.S.; Lima, A.R.N.; Ferreira, N.L.d.S.; Carvalho, J.D.G.; Pedrini, M.R.d.S.; Hoskin, R.T.; Oliveira, L.d.S. Green Valorization of Pitaya (Hylocereus polyrhizus) Peels by Ultrasound-Assisted Extraction and Encapsulation of Bioactive Compounds. Processes 2025, 13, 3628. https://doi.org/10.3390/pr13113628
Sampaio LMF, Rebouças CRdS, Soares LM, Lima ACdS, Lima ARN, Ferreira NLdS, Carvalho JDG, Pedrini MRdS, Hoskin RT, Oliveira LdS. Green Valorization of Pitaya (Hylocereus polyrhizus) Peels by Ultrasound-Assisted Extraction and Encapsulation of Bioactive Compounds. Processes. 2025; 13(11):3628. https://doi.org/10.3390/pr13113628
Chicago/Turabian StyleSampaio, Lorena Maria Freire, Cinthia Regina da Silva Rebouças, Lara Mota Soares, Antonia Carlota de Souza Lima, Amélia Ruth Nascimento Lima, Nayanne Lima dos Santos Ferreira, Juliane Doering Gasparin Carvalho, Márcia Regina da Silva Pedrini, Roberta Targino Hoskin, and Luciana de Siqueira Oliveira. 2025. "Green Valorization of Pitaya (Hylocereus polyrhizus) Peels by Ultrasound-Assisted Extraction and Encapsulation of Bioactive Compounds" Processes 13, no. 11: 3628. https://doi.org/10.3390/pr13113628
APA StyleSampaio, L. M. F., Rebouças, C. R. d. S., Soares, L. M., Lima, A. C. d. S., Lima, A. R. N., Ferreira, N. L. d. S., Carvalho, J. D. G., Pedrini, M. R. d. S., Hoskin, R. T., & Oliveira, L. d. S. (2025). Green Valorization of Pitaya (Hylocereus polyrhizus) Peels by Ultrasound-Assisted Extraction and Encapsulation of Bioactive Compounds. Processes, 13(11), 3628. https://doi.org/10.3390/pr13113628









