Development of Rock-Based Geopolymers for Oilwell Cementing Applications—Utilizing Brazilian Rock Precursor
Abstract
1. Introduction
2. Materials and Methods
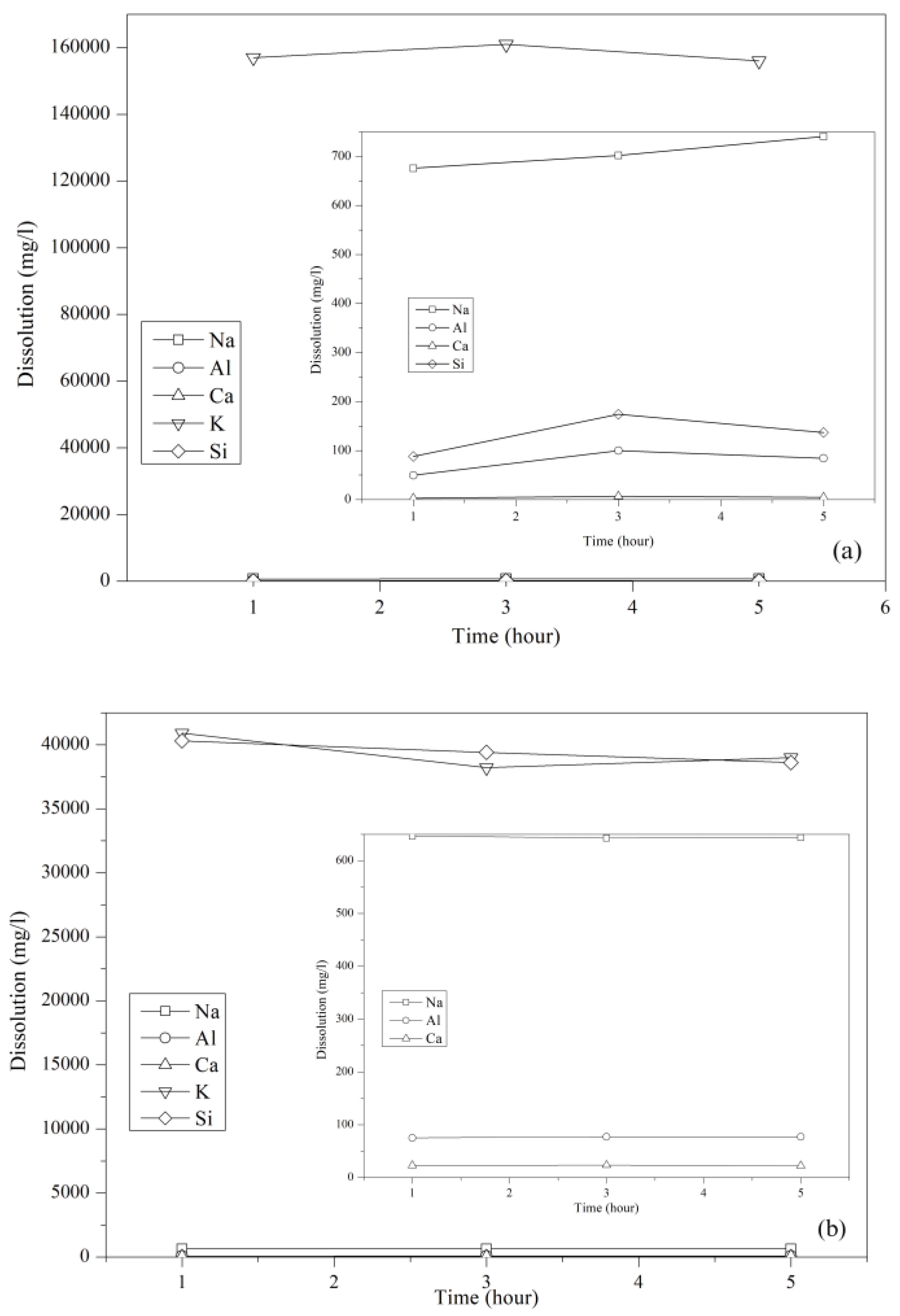
2.1. Dissolution Rate of Precursor
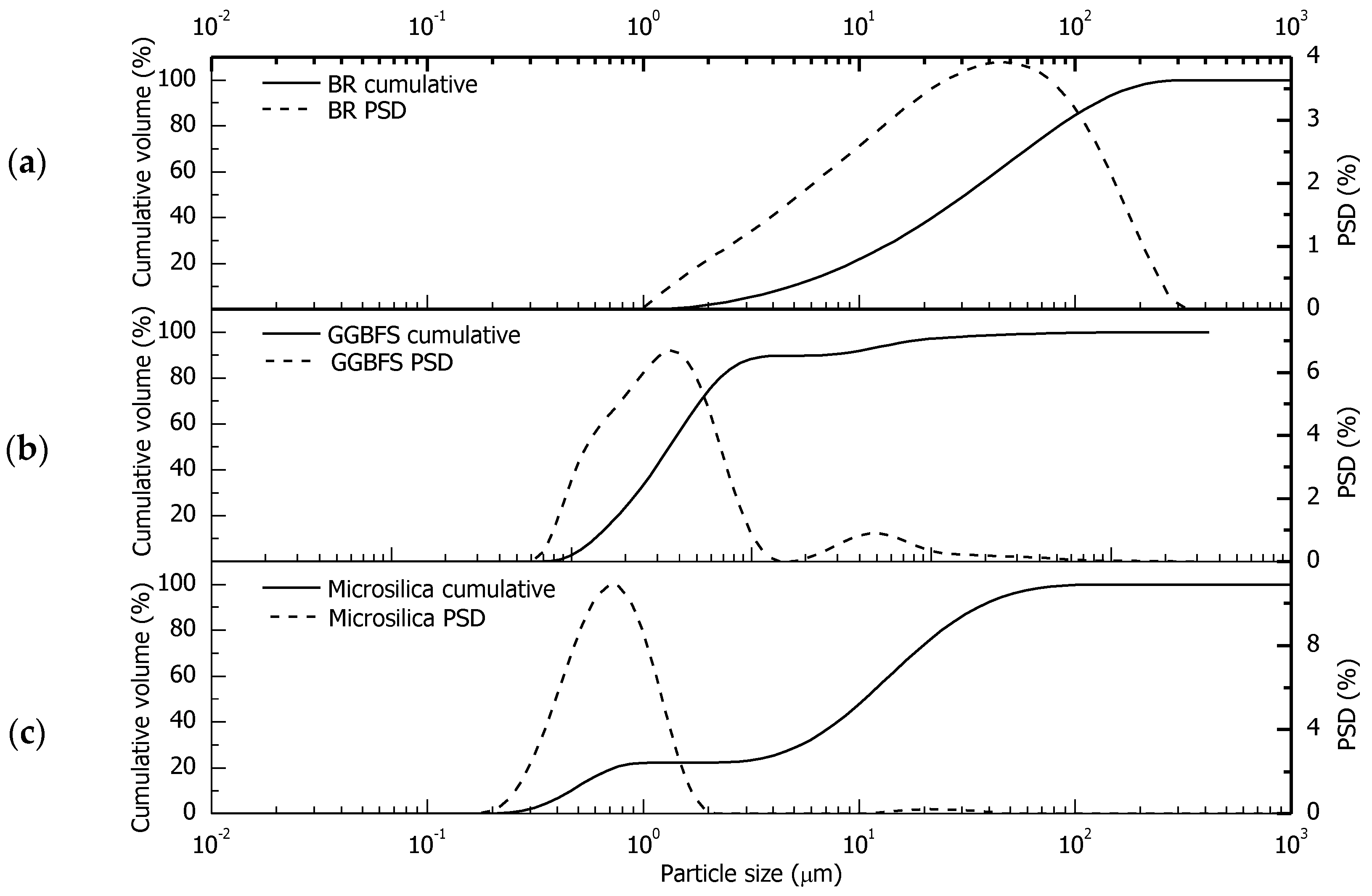
2.2. Slurries Preparation and Characterization
2.3. Rheology Measurements
2.4. Consistency and Thickening Time
2.5. Mechanical Behavior of the Mix Designs
Curing Samples and Uniaxial Compressive Strength Test
3. Results
3.1. Dissolution Rate of Precursor
3.2. Characterization of Mix Designs
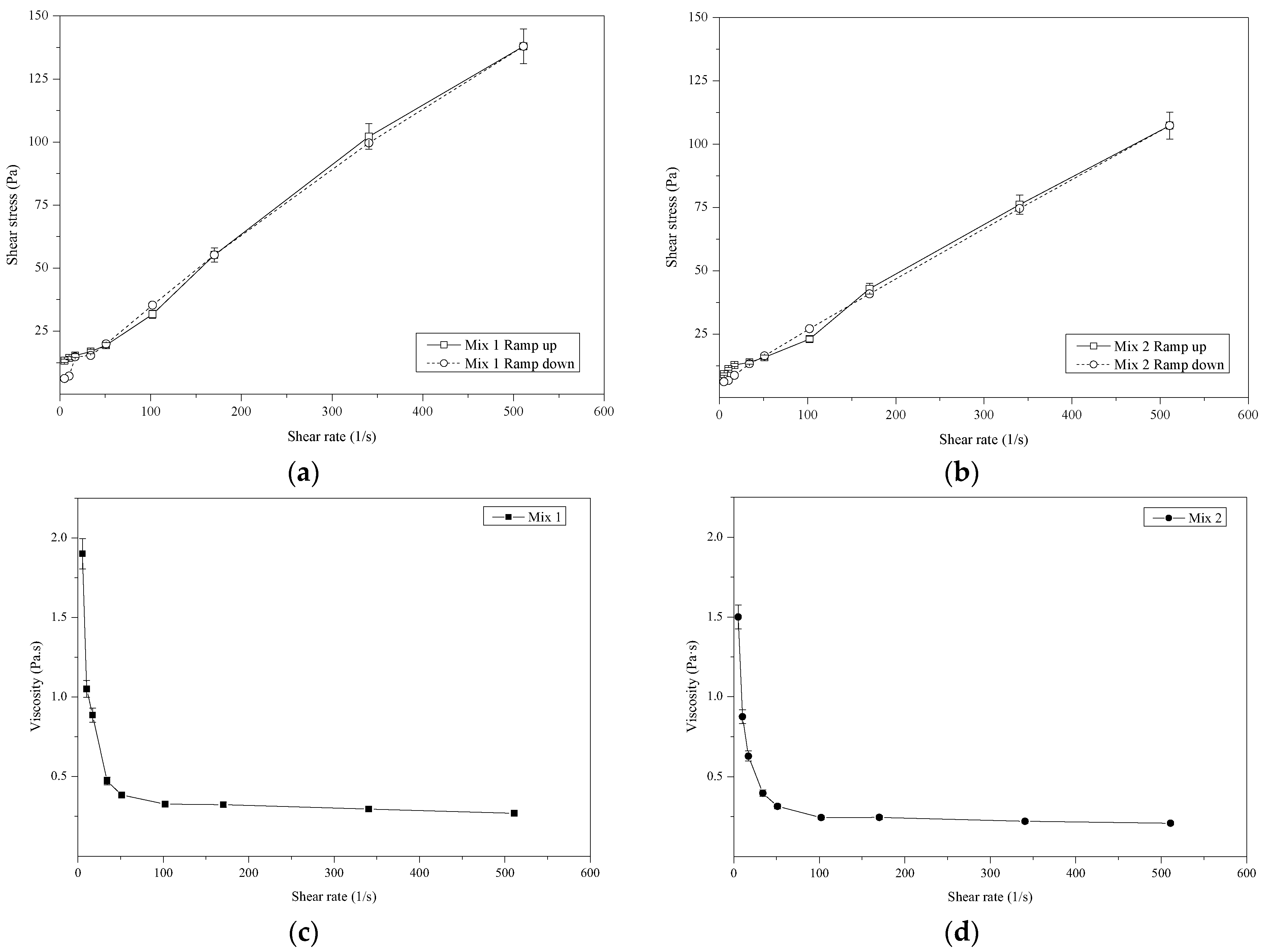
3.2.1. Rheological Behavior
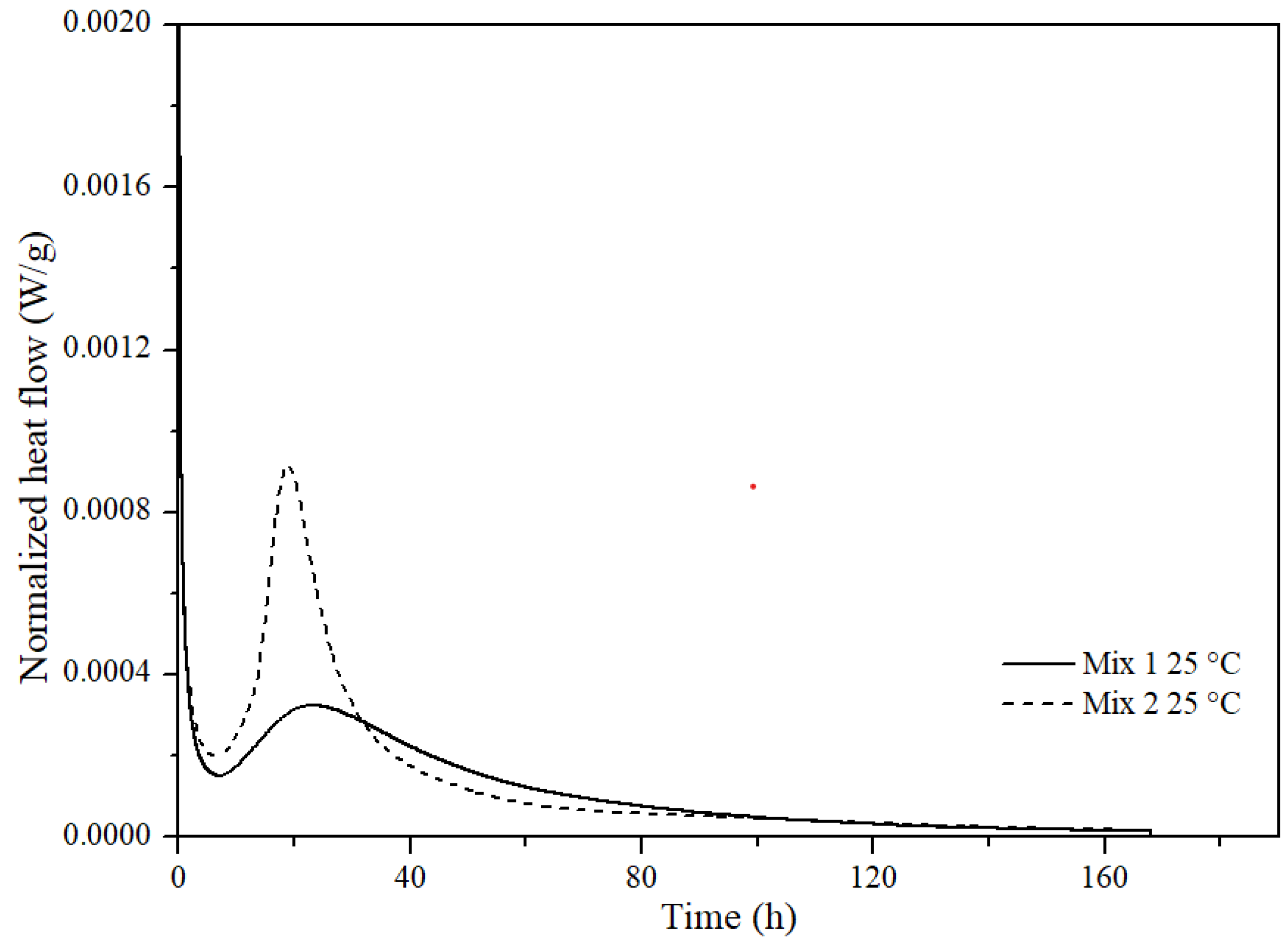
3.2.2. Consistency Profile
3.3. Mechanical Behavior
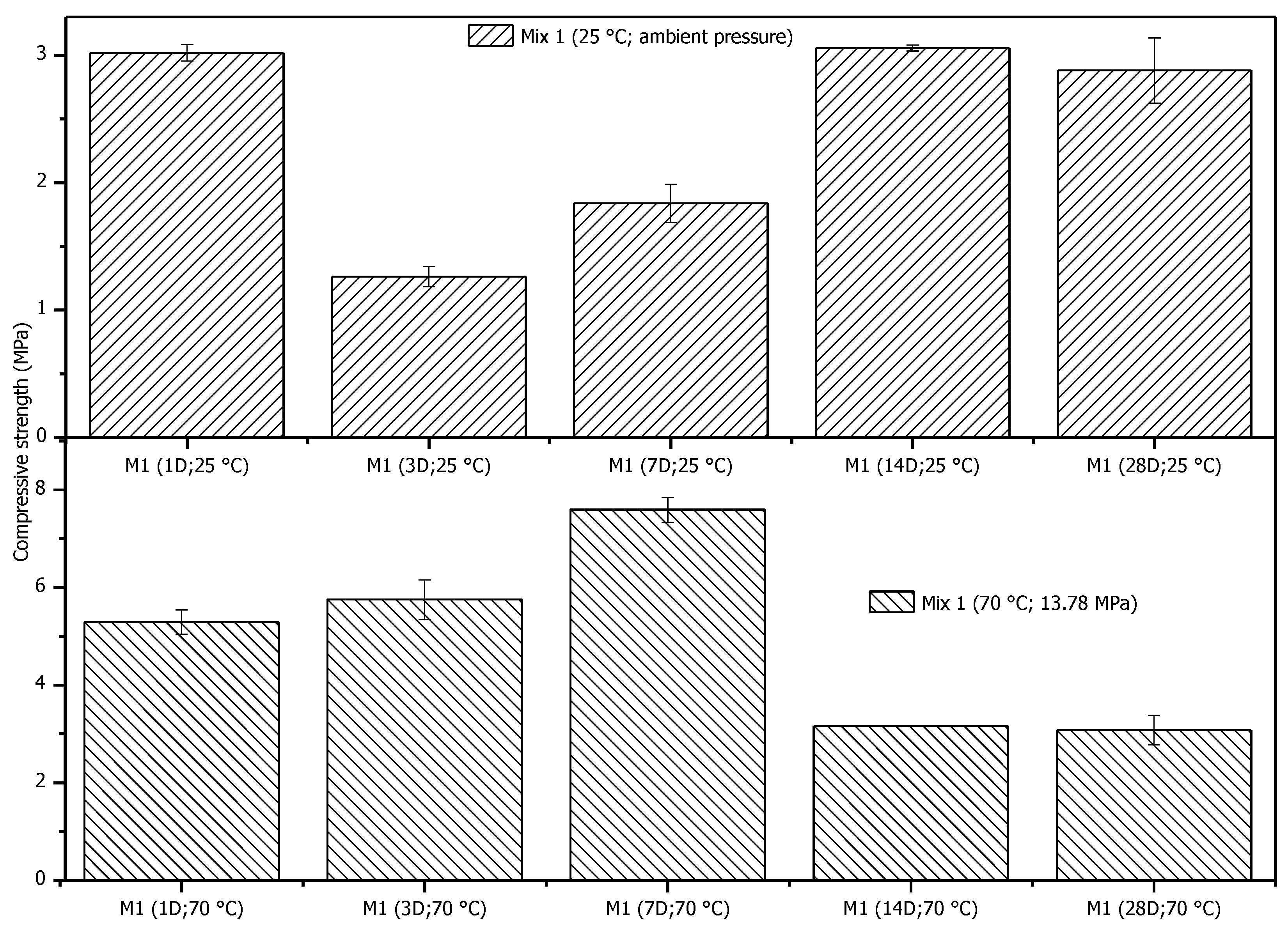
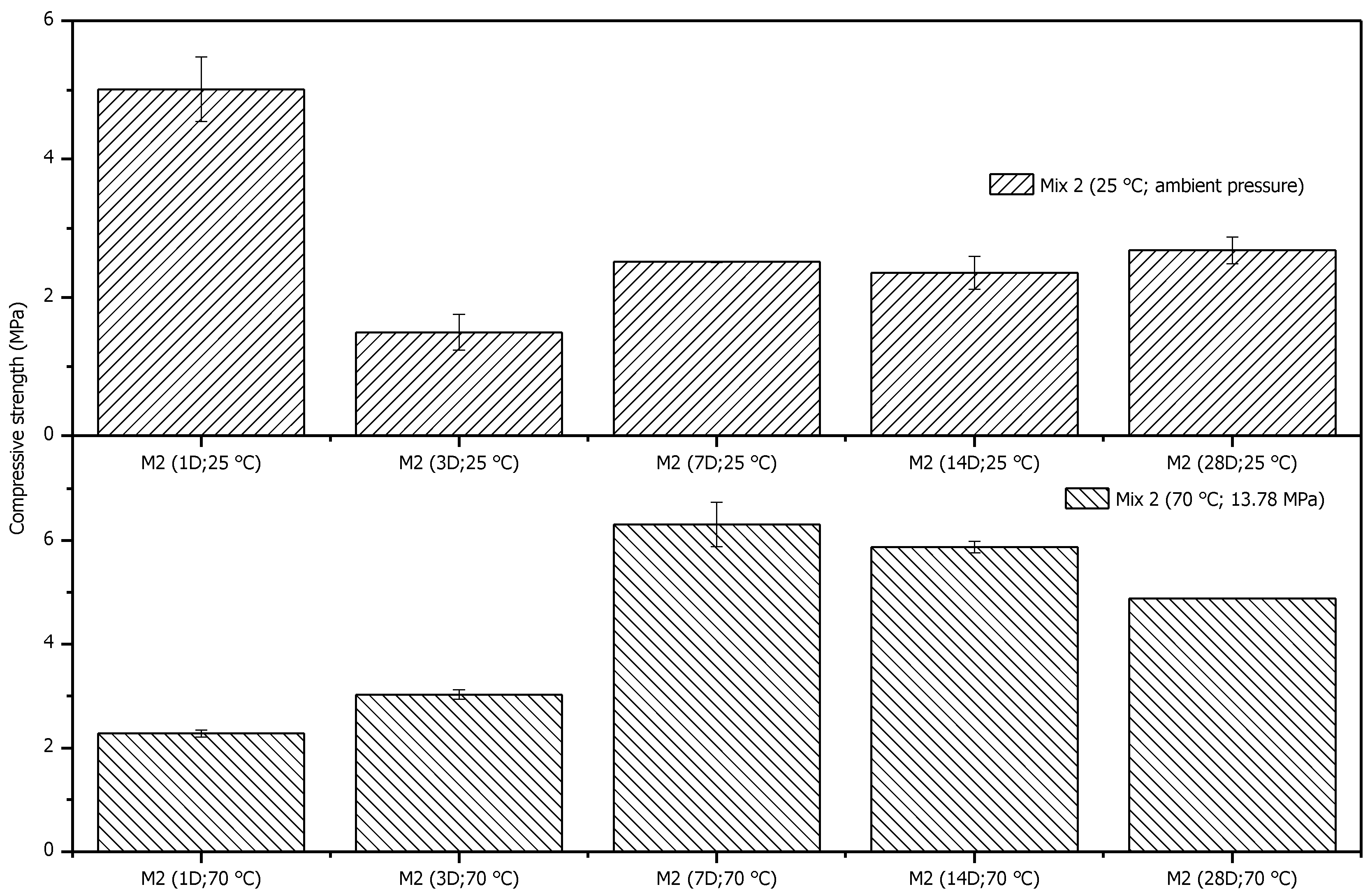
3.3.1. Uniaxial Compressive Strength
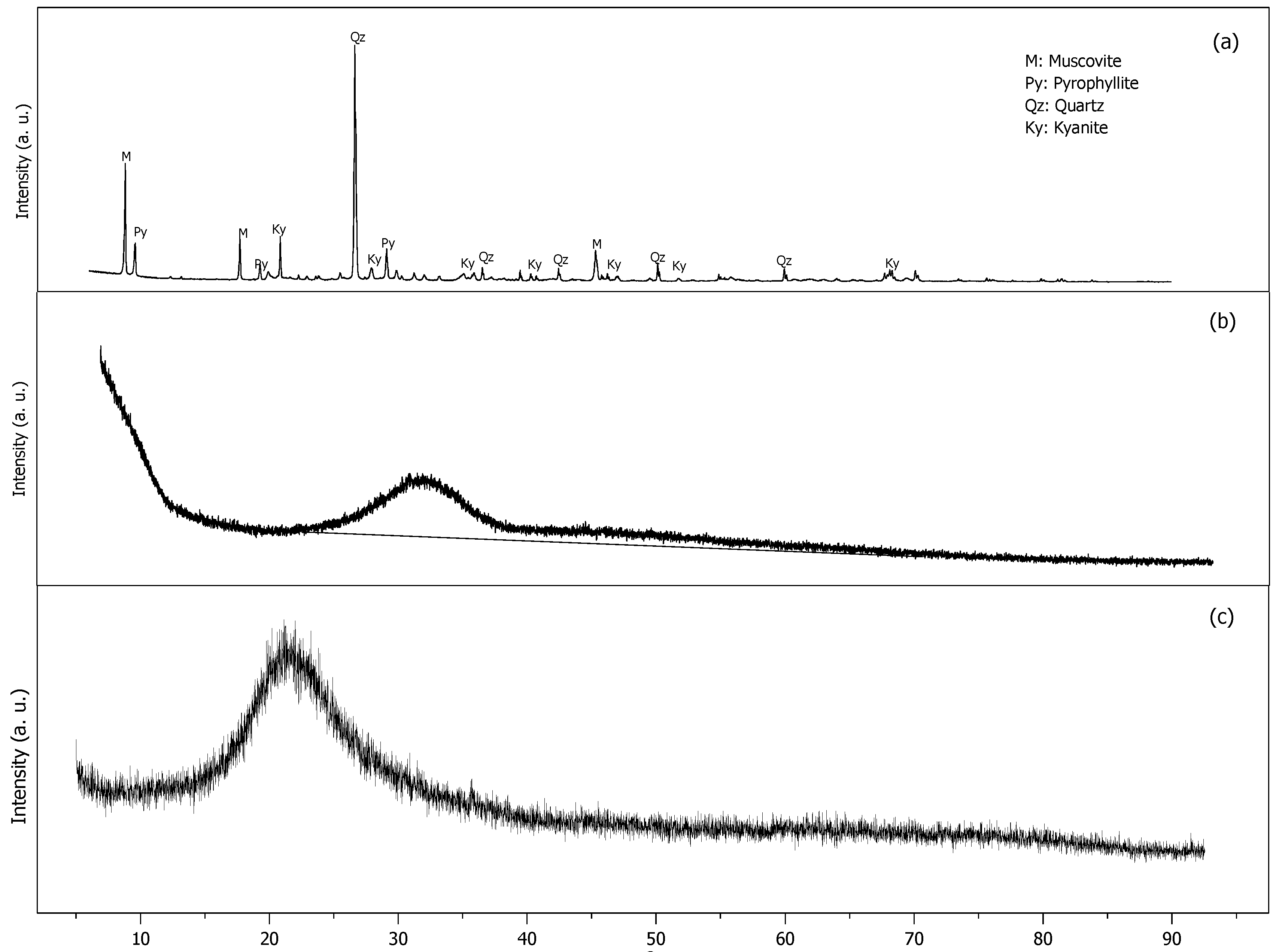
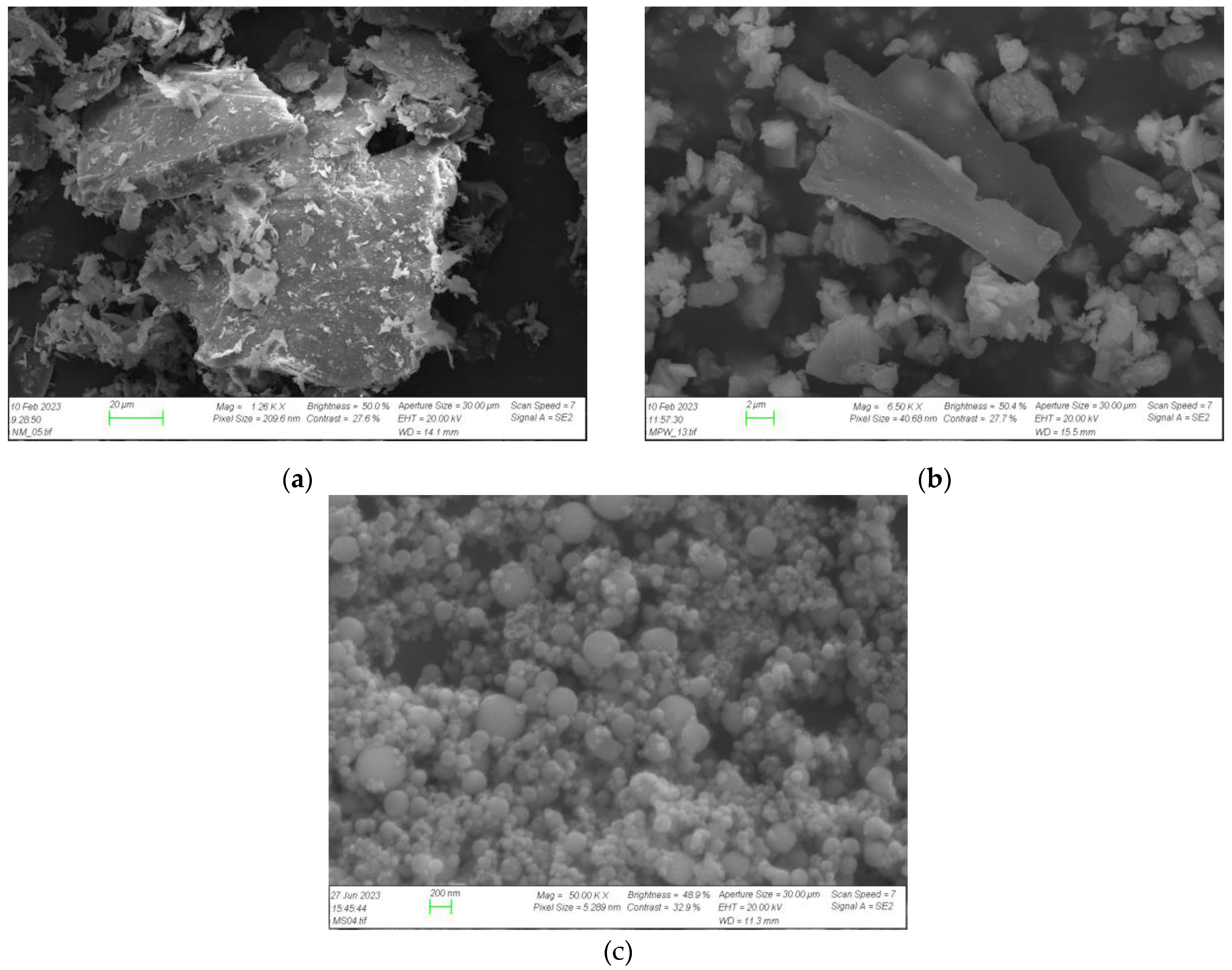
3.3.2. Microstructural Characterization
4. Conclusions
- The geopolymers exhibited Herschel-Bulkley rheological behavior, and the addition of microsilica resulted in a reduction in viscosity.
- Temperature and pressure significantly influence the pumpability of the slurries. The geopolymer’s pumping time is strongly influenced by pressure; increasing it to 13.8 MPa reduces the time by 50%.
- The geopolymer samples demonstrated initial development in compressive strength. However, after seven days of curing, specific reactions occurred that decreased compressive strength. Further investigation is required to better understand how aging affects the compressive strength of settled materials.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1
Appendix A.2

References
- Chukwuemeka, A.O.; Oluyemi, G.; Mohammed, A.I.; Njuguna, J. Plug and abandonment of oil and gas wells—A comprehensive review of regulations, practices, and related impact of materials selection. Geoenergy Sci. Eng. 2023, 226, 211718. [Google Scholar] [CrossRef]
- Nelson, E.B.; Guillot, D. Well Cementing; Schlumberger: Houston, TX, USA, 2006. [Google Scholar]
- Johnson, C.R.; Shindgikar, N.D.; Beurel, M.M. Overcoming Environmental and Technical Challenges for Well Cementing: A Global Perspective. SPE Prod. Oper. 2017, 32, 12–27. [Google Scholar] [CrossRef]
- Lécolier, E.; Rivereau, A.; Le Saoût, G.; Audibert-Hayet, A. Durability of Hardened Portland Cement Paste used for Oilwell Cementing. Oil Gas Sci. Technol.-Rev. IFP 2007, 62, 335–345. [Google Scholar] [CrossRef]
- Haustveit, K.; Haffener, J.; Young, S.; Dwyer, J.; Glaze, G.; Green, B.; Ketter, C.; Williams, T.; Brinkley, K.; Elliott, B. Cementing: The Good, the Bad, and the Isolated—Techniques to Measure Cement Quality and its Impact on Well Performance. In Proceedings of the SPE Hydraulic Fracturing Technology Conference and Exhibition, The Woodlands, TX, USA, 6–8 February 2024. [Google Scholar] [CrossRef]
- Joseph, S.; Cizer, Ö. Hydration of Hybrid Cements at Low Temperatures: A Study on Portland Cement-Blast Furnace Slag—Na2SO4. Materials 2022, 15, 1914. [Google Scholar] [CrossRef]
- Wolterbeek, T.; Hangx, S. The thermal properties of set Portland cements—A literature review in the context of CO2 injection well integrity. Int. J. Greenh. Gas Control 2023, 126, 103909. [Google Scholar] [CrossRef]
- Korczak, K.; Kochański, M.; Skoczkowski, T. Mitigation options for decarbonization of the non-metallic minerals industry and their impacts on costs, energy consumption and GHG emissions in the EU-Systematic literature review. J. Clean. Prod. 2022, 358, 132006. [Google Scholar] [CrossRef]
- Cheng, D.; Reiner, D.M.; Yang, F.; Cui, C.; Meng, J.; Shan, Y.; Liu, Y.; Tao, S.; Guan, D. Projecting future carbon emissions from cement production in developing countries. Nat. Commun. 2023, 14, 8213. [Google Scholar] [CrossRef]
- Khaiyum, M.Z.; Sarker, S.; Kabir, G. Evaluation of Carbon Emission Factors in the Cement Industry: An Emerging Economy Context. Sustainability 2023, 15, 15407. [Google Scholar] [CrossRef]
- Miller, S.A.; Habert, G.; Myers, R.J.; Harvey, J.T. Achieving net zero greenhouse gas emissions in the cement industry via value chain mitigation strategies. One Earth 2021, 4, 1398–1411. [Google Scholar] [CrossRef]
- Chaudhury, R.; Sharma, U.; Thapliyal, P.; Singh, L. Low-CO2 emission strategies to achieve net zero target in cement sector. J. Clean. Prod. 2023, 417, 137466. [Google Scholar] [CrossRef]
- Imtiaz, L.; Rehman, S.K.; Ali Memon, S.; Khizar Khan, M.; Faisal Javed, M. A Review of Recent Developments and Advances in Eco-Friendly Geopolymer Concrete. Appl. Sci. 2020, 10, 7838. [Google Scholar] [CrossRef]
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Jwaida, Z.; Dulaimi, A.; Mashaan, N.; Othuman Mydin, M.A. Geopolymers: The Green Alternative to Traditional Materials for Engineering Applications. Infrastructures 2023, 8, 98. [Google Scholar] [CrossRef]
- Salehi, S.; Khattak, M.J.; Ali, N.; Ezeakacha, C.; Saleh, F.K. Study and Use of Geopolymer Mixtures for Oil and Gas Well Cementing Applications. ASME J. Energy Resour. Technol. 2017, 140, 012908. [Google Scholar] [CrossRef]
- Ji, Z.; Zhang, G.; Liu, R.; Qu, J.; Liu, H. Potential applications of solid waste-based geopolymer materials: In wastewater treatment and greenhouse gas emission reduction. J. Clean. Prod. 2024, 443, 141144. [Google Scholar] [CrossRef]
- Mishra, J.; Nanda, B.; Patro, S.K.; Krishna, R.S. A comprehensive review on compressive strength and microstructure properties of GGBS-based geopolymer binder systems. Constr. Build. Mater. 2024, 417, 135242. [Google Scholar] [CrossRef]
- Xu, H.; Van Deventer, J.S.J. The geopolymerisation of alumino-silicate minerals. Int. J. Miner. Process 2000, 59, 247–266. [Google Scholar] [CrossRef]
- Panagiotopoulou, C.; Kontori, E.; Perraki, T.; Kakali, G. Dissolution of aluminosilicate minerals and by-products in alkaline media. J. Mater. Sci. 2007, 42, 2967–2973. [Google Scholar] [CrossRef]
- Zakira, U.; Zheng, K.; Xie, N.; Birgisson, B. Development of high-strength geopolymers from red mud and blast furnace slag. J. Clean. Prod. 2023, 383, 135439. [Google Scholar] [CrossRef]
- Wang, Y.S.; Peng, K.D.; Alrefaei, Y.; Dai, J.G. The bond between geopolymer repair mortars and OPC concrete substrate: Strength and microscopic interactions. Cem. Concr. Compos. 2021, 119, 103991. [Google Scholar] [CrossRef]
- Abdellatief, M.; Alanazi, H.; Radwan, M.K.; Tahwia, A.M. Multiscale Characterization at Early Ages of Ultra-High Performance Geopolymer Concrete. Polymers 2022, 14, 5504. [Google Scholar] [CrossRef]
- Arunachelam, N.; Maheswaran, J.; Chellapandian, M.; Murali, G.; Vatin, N.I. Development of High-Strength Geopolymer Concrete Incorporating High-Volume Copper Slag and Micro Silica. Sustainability 2022, 14, 7601. [Google Scholar] [CrossRef]
- Oliveira, L.B.; Azevedo, A.R.G.; Marvila, M.T.; Pereira, E.C.; Fediuk, R.; Vieira, C.M.F. Durability of geopolymers with industrial waste. Case Stud. Constr. Mater. 2022, 16, e00839. [Google Scholar] [CrossRef]
- Long, Q.; Zhao, Y.; Zhang, B.; Yang, H.; Luo, Z.; Li, Z.; Zhang, G.; Liu, K. Interfacial Behavior of Slag, Fly Ash, and Red Mud-Based Geopolymer Mortar with Concrete Substrate: Mechanical Properties and Microstructure. Buildings 2024, 14, 652. [Google Scholar] [CrossRef]
- Mohsen, K.; Yousif, I.A.; Sadek, E.F.; Morsy, K.M. Experimental study of interface Behaviour of geopolymer concrete. Sci. Rep. 2025, 15, 32696. [Google Scholar] [CrossRef]
- Kasprzhitskii, A.S.; Kruglikov, A.A. Molecular Insights into Adhesion at Interface of Geopolymer Binder and Cement Mortar. Int. J. Mol. Sci. 2024, 25, 8374. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Aughenbaugh, K.; Lee, H.; Nair, S.; van Oort, E. Geopolymer-Synthetic Based Mud Hybrid Cements for Primary Cementing and Lost Circulation Control. In Proceedings of the SPE International Conference on Oilfield Chemistry, Montgomery, TX, USA, 3–5 April 2017. [Google Scholar] [CrossRef]
- Kalyoncu Erguler, G.; Dahi Taleghani, A. Geopolymers for integrity of geothermal and CO2 sequestration wells: A state-of-the-art review. Geoenergy Sci. Eng. 2025, 255, 214081. [Google Scholar] [CrossRef]
- Luo, Y.; Klima, K.; Melzer, S.; Brouwers, H.; Yu, Q. Uncover the thermal behavior of geopolymer: Insights from in-situ high temperature exposure. Cem. Concr. Compos. 2025, 164, 106282. [Google Scholar] [CrossRef]
- Aslani, F.; Zhang, Y.; Manning, D.; Valdez, L.C.; Manning, N. Additive and alternative materials to cement for well plugging and abandonment: A state-of-the-art review. J. Pet. Sci. Eng. 2022, 215, 110728. [Google Scholar] [CrossRef]
- Patel, Y.J.; Shah, N. Enhancement of the properties of Ground Granulated Blast Furnace Slag based Self Compacting Geopolymer Concrete by incorporating Rice Husk Ash. Constr. Build. Mater. 2018, 171, 654–662. [Google Scholar] [CrossRef]
- Ahdaya, M.; Imqam, A. Investigating geopolymer cement performance in presence of water based drilling fluid. J. Pet. Sci. Eng. 2019, 176, 934–942. [Google Scholar] [CrossRef]
- Mohd Basri, M.S.; Mustapha, F.; Mazlan, N.; Ishak, M.R. Rice Husk Ash-Based Geopolymer Binder: Compressive Strength, Optimize Composition, FTIR Spectroscopy, Microstructural, and Potential as Fire-Retardant Material. Polymers 2021, 13, 4373. [Google Scholar] [CrossRef]
- Yousefi Oderji, S.; Chen, B.; Ahmad, M.R.; Shah, S.F.A. Fresh and hardened properties of one-part fly ash-based geopolymer binders cured at room temperature: Effect of slag and alkali activators. J. Clean. Prod. 2019, 225, 1–10. [Google Scholar] [CrossRef]
- Wan-En, O.; Yun-Ming, L.; Cheng-Yong, H.; Abdullah, M.M.A.B.; Li, L.Y.; Ho, L.N.; Loong, F.K.; Shee-Ween, O.; Hui-Teng, N.; Yong-Sing, N.; et al. Comparative mechanical and microstructural properties of high calcium fly ash one-part geopolymers activated with Na2SiO3-anhydrous and NaAlO2. J. Mater. Res. Technol. 2021, 15, 3850–3866. [Google Scholar] [CrossRef]
- Xiong, G.; Guo, X. Effects and mechanism of superplasticizers and precursor proportions on the fresh properties of fly ash–slag powder based geopolymers. Constr. Build. Mater. 2022, 350, 128734. [Google Scholar] [CrossRef]
- Zarębska, K.; Szczurowski, J.; Muszyńska, J.; Baran, P. Geopolymer Materials from Fly Ash—A Sustainable Approach to Hazardous Waste Management. Materials 2024, 17, 3515. [Google Scholar] [CrossRef]
- Yazid, M.H.; Faris, M.A.; Abdullah, M.M.; Ibrahim, M.S.; Razak, R.A.; Burduhos Nergis, D.D.; Burduhos Nergis, D.P.; Benjeddou, O.; Nguyen, K. Mechanical Properties of Fly Ash-Based Geopolymer Concrete Incorporation Nylon66 Fiber. Materials 2022, 15, 9050. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Castel, A. Developing Geopolymer Concrete by Using Ferronickel Slag and Ground-Granulated Blast-Furnace Slag. Ceramics 2023, 6, 1861–1878. [Google Scholar] [CrossRef]
- Zhang, Z.; Su, T.; Zhang, L.; Zheng, R.; Ma, K.; Zhang, L.; Amaechi, C.V.; Wang, C. The Influence of Fly Ash and Slag on the Mechanical Properties of Geopolymer Concrete. Buildings 2024, 14, 2720. [Google Scholar] [CrossRef]
- Arunachelam, N.; Maheswaran, J.; Chellapandian, M.; Ozbakkaloglu, T. Effective Utilization of Copper Slag for the Production of Geopolymer Concrete with Different NaOH Molarity under Ambient Curing Conditions. Sustainability 2022, 14, 16300. [Google Scholar] [CrossRef]
- Maniarasan, S.K.; Chandrasekaran, P.; Jayaprakash, S.; Ravindran, G. Influence of Slag-Based Geopolymer Concrete on the Seismic Behavior of Exterior Beam Column Joints. Sustainability 2023, 15, 2327. [Google Scholar] [CrossRef]
- Azimi, Z.; Toufigh, V. Influence of Blast Furnace Slag on Pore Structure and Transport Characteristics in Low-Calcium Fly-Ash-Based Geopolymer Concrete. Sustainability 2023, 15, 13348. [Google Scholar] [CrossRef]
- Yang, K.H.; Song, J.K.; Lee, J.S. Properties of alkali-activated mortar and concrete using lightweight aggregates. Mater. Struct./Mater. Constr. 2010, 43, 403–416. [Google Scholar] [CrossRef]
- Kim, M.S.; Jun, Y.; Lee, C.; Oh, J.E. Use of CaO as an activator for producing a price-competitive non-cement structural binder using ground granulated blast furnace slag. Cem. Concr. Res. 2013, 54, 208–214. [Google Scholar] [CrossRef]
- Abdel-Gawwad, H.A.; Abo-El-Enein, S.A. A novel method to produce dry geopolymer cement powder. HBRC J. 2016, 12, 13–24. [Google Scholar] [CrossRef]
- Adeleke, B.O.; Kinuthia, J.M.; Oti, J.; Ebailila, M. Physico-Mechanical Evaluation of Geopolymer Concrete Activated by Sodium Hydroxide and Silica Fume-Synthesised Sodium Silicate Solution. Materials 2023, 16, 2400. [Google Scholar] [CrossRef]
- Cao, B.; Li, Y.; Li, P. Synergistic Effect of Blended Precursors and Silica Fume on Strength and High Temperature Resistance of Geopolymer. Materials 2024, 17, 2975. [Google Scholar] [CrossRef]
- Baltazar, L.G. Performance of Silica Fume-Based Geopolymer Grouts for Heritage Masonry Consolidation. Crystals 2022, 12, 288. [Google Scholar] [CrossRef]
- Caldas, P.H.C.H.; De Azevedo, A.R.G.; Marvila, M.T. Silica fume activated by NaOH and KOH in cement mortars: Rheological and mechanical study. Constr. Build. Mater. 2023, 400, 132623. [Google Scholar] [CrossRef]
- Sothornchaiwit, K.; Dokduea, W.; Tangchirapat, W.; Keawsawasvong, S.; Thongchom, C.; Jaturapitakkul, C. Influences of Silica Fume on Compressive Strength and Chemical Resistances of High Calcium Fly Ash-Based Alkali-Activated Mortar. Sustainability 2022, 14, 2652. [Google Scholar] [CrossRef]
- Ilcan, H.; Sahin, O.; Unsal, Z.; Ozcelikci, E.; Kul, A.; Cağatay Demiral, N.; Ozkan Ekinci, M.; Sahmaran, M. Effect of industrial waste-based precursors on the fresh, hardened and environmental performance of construction and demolition wastes-based geopolymers. Constr. Build. Mater. 2023, 394, 132265. [Google Scholar] [CrossRef]
- Widnyana, I.N.S.; Salain, I.M.A.K.; Sutarja, I.N.; Widiarsa, I.B.R. Setting time of coconut fiber ash-based geopolymer binder. IOP Conf. Ser. Earth Environ. Sci. 2022, 1117, 012001. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Lee, B.J.; Saraswathy, V.; Kwon, S.J. Strength and durability performance of alkali-activated rice husk ash geopolymer mortar. Sci. World J. 2014, 2014, 209584. [Google Scholar] [CrossRef]
- Sturm, P.; Gluth, G.J.G.; Brouwers, H.J.H.; Kühne, H.C. Synthesizing one-part geopolymers from rice husk ash. Constr. Build. Mater. 2016, 124, 961–966. [Google Scholar] [CrossRef]
- Handayani, L.; Aprilia, S.; Rahmawati, C.; Aulia, T.B.; Ludvig, P.; Ahmad, J. Sodium Silicate from Rice Husk Ash and Their Effects as Geopolymer Cement. Polymers 2022, 14, 2920. [Google Scholar] [CrossRef] [PubMed]
- Panitsa, O.A.; Kioupis, D.; Kakali, G. Advancing the Sustainability of Geopolymer Technology through the Development of Rice Husk Ash Based Solid Activators. Sustainability 2024, 16, 7243. [Google Scholar] [CrossRef]
- Basri, M.S.; Mustapha, F.; Mazlan, N.; Ishak, M.R. Optimization of Rice Husk Ash-Based Geopolymers Coating Composite for Enhancement in Flexural Properties and Microstructure Using Response Surface Methodology. Coatings 2020, 10, 165. [Google Scholar] [CrossRef]
- Hossain, S.S.; Roy, P.; Bae, C. Utilization of waste rice husk ash for sustainable geopolymer: A review. Constr. Build. Mater. 2021, 310, 125218. [Google Scholar] [CrossRef]
- Van Jaarsveld, J.G.S.; Van Deventer, J.S.J.; Lukey, G.C. The effect of composition and temperature on the properties of fly ash-and kaolinite-based geopolymers. Chem. Eng. J. 2002, 89, 63–73. [Google Scholar] [CrossRef]
- Li, T.; Feng, J.; He, R.; Chen, X.; Xu, C.; Liao, J.; Jiang, Q. Optimization of compressive strength and exploration of enhancement mechanism of coal gangue-based geopolymers using the Taguchi method. Mod. Phys. Lett. B 2025, 39, 2550216. [Google Scholar] [CrossRef]
- Kurt, Z.; Ustabaş, İ.; Cakmak, T. Novel binder material in geopolymer mortar production: Obsidian stone powder. Struct. Concr. 2023, 24, 5600–5613. [Google Scholar] [CrossRef]
- Al-dujaili, M.A.A.; Al-hydary, I.A.D.; Hassan, Z.Z. Physical Characteristics and Compressive Strength of Na-Geopolymer Paste Designed by a Taguchi Method. IOP Conf. Ser. Earth Environ. Sci. 2021, 877, 012036. [Google Scholar] [CrossRef]
- Hu, Y.; Yao, Y.; Zhang, L.; Hu, X.; Yang, X. Green Low-Temperature Activation and Curing for High-Toughness Geopolymer Binders from Diabase Tailings. Materials 2025, 18, 3815. [Google Scholar] [CrossRef] [PubMed]
- Pitaloka, D.A.A.; Salain, I.M.A.K.; Widiarsa, I.B.R. The Effect of Precursor-Activator Ratio and Activator Type on the Bulk Density, Compressive Strength, and Microstructure of Abuan Pumice-Based Geopolymer Binder. J. Ilm. Tek. Sipil 2024, 28, 211–221. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Lin, C. Compressive and Flexural Properties of Ultra-Fine Coal Gangue-Based Geopolymer Gels and Microscopic Mechanism Analysis. Gels 2022, 8, 145. [Google Scholar] [CrossRef]
- Tsaousi, G.M.; Douni, I.; Panias, D. Characterization of the properties of perlite geopolymer pastes. Mater. Constr. 2016, 66, e102. [Google Scholar] [CrossRef]
- Owsiak, Z.; Szczykutowicz, K. Physical and mechanical properties of meta-halloysite-based geopolymer mortars. Cem.-Wapno-Beton = Cem. Lime Concr. 2023, 28, 351–361. [Google Scholar] [CrossRef]
- Hájková, P. Kaolinite Claystone-Based Geopolymer Materials: Effect of Chemical Composition and Curing Conditions. Minerals 2018, 8, 444. [Google Scholar] [CrossRef]
- Adjei, S.; Elkatatny, S.; Aggrey, W.N.; Abdelraouf, Y. Geopolymer as the future oilwell cement: A review. J. Pet. Sci. Eng. 2022, 208, 109485. [Google Scholar] [CrossRef]
- Hajiabadi, S.H.; Khalifeh, M.; van Noort, R.; Silva Santos Moreira, P.H. Review on Geopolymers as Wellbore Sealants: State of the Art Optimization for CO2 Exposure and Perspectives. ACS Omega 2023, 8, 23320–23345. [Google Scholar] [CrossRef]
- Rahmatullah, I.K.; Ahmed, A.; Elkatatny, S.; El Fattah, A.M.A. Utilizing Saudi volcanic scoria in lightweight geopolymer for enhanced wellbore cementing. Sci. Rep. 2025, 15, 20965. [Google Scholar] [CrossRef]
- Nana, A.; Tomé, S.; Anensong, S.C.D.; Venyite, P.; Djobo, J.N.Y.; Ngouné, J.; Kamseu, E.; Bignozzi, M.C.; Leonelli, C. Mechanical Performance, Phase Evolution and Microstructure of Natural Feldspathic Solid Solutions Consolidated via Alkali Activation: Effect of NaOH Concentration. Silicon 2022, 14, 4107–4120. [Google Scholar] [CrossRef]
- Khalifeh, M. Materials for Optimized P&A Performance Potential Utilization of Geopolymers. Ph.D. Thesis, University of Stavanger, Stavanger, Norway, 2016. [Google Scholar]
- da Silva, R.R.; de Oliveira Freitas, J.C.; Muniz Moreira, R.P.; Braga, R.M.; Khalifeh, M. Development of a Rock-Based Geopolymer for Well Abandonment Applications—Utilizing Brazilian Rock Precursors. In Proceedings of the Offshore Technology Conference Brasil, OTCB 2023, Rio de Janeiro, Brazil, 24–26 October 2023. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; van Deventer, J.S.J. Dissolution Behaviour of source materials for synthesis of geopolymer binders: A kinetic approach. Int. J. Miner. Process. 2016, 153, 80–86. [Google Scholar] [CrossRef]
- Kuenzel, C.; Ranjbar, N. Dissolution mechanism of fly ash to quantify the reactive aluminosilicates in geopolymerisation. Resour. Conserv. Recycl. 2019, 150, 104421. [Google Scholar] [CrossRef]
- Küçük, M.E.; Kinnarinen, T.; Häkkinen, A. Dissolution kinetics of aluminosilicates from biomass ashes in alkaline solutions. Ceram. Int. 2021, 47, 11714–11726. [Google Scholar] [CrossRef]
- Provis, J.L.; Van Deventer, J.S.J. Geopolymers: Structure, Processing, Properties and Industrial Applications; Woodhead Publishing: Sawston, UK, 2009. [Google Scholar]
- Myers, R.J.; Bernal, S.A.; San Nicolas, R.; Provis, J.L. Generalized structural description of calcium–aluminosilicate hydrate gels: The cross-linked substituted tobermorite model. Langmuir 2015, 31, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Rees, C.A.; Provis, J.L.; Lukey, G.C.; Van Deventer, J.S.J. The mechanism of geopolymer gel formation investigated through the development of a charge-balanced model. Colloids Surf. A 2008, 318, 97–105. [Google Scholar] [CrossRef]
- Power, D.; Zamora, M. Drilling Fluid Yield Stress: Measurement Techniques for Improved Understanding of Critical Drilling Fluid Parameters. 2003. Available online: https://www.aade.org/application/files/7515/7304/4604/AADE-03-NTCE-35-Power.pdf (accessed on 28 August 2025).
- Szcześniak, A.; Siwiński, J.; Stolarski, A.; Piekarczuk, A.; Nasiłowska, B. The Influence of the Addition of Microsilica and Fly Ash on the Properties of Ultra-High-Performance Concretes. Materials 2025, 18, 28. [Google Scholar] [CrossRef] [PubMed]
- Kamali, M.; Khalifeh, M.; Eid, E.; Saasen, A. Experimental Study of Hydraulic Sealability and Shear Bond Strength of Cementitious Barrier Materials. ASME J. Energy Resour. Technol. 2022, 144, 023007. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; Van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Rovnaník, P. Effect of curing temperature on the development of hard structure of metakaolin-based geopolymer. Constr. Build. Mater. 2010, 24, 1176–1183. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Geopolymer foam concrete: An emerging material for sustainable construction. Constr. Build. Mater. 2012, 56, 113–127. [Google Scholar] [CrossRef]
- Castillo, H.; Collado, H.; Droguett, T.; Sánchez, S.; Vesely, M.; Garrido, P.; Palma, S. Factors affecting the compressive strength of geopolymers: A review. Minerals 2021, 11, 1317. [Google Scholar] [CrossRef]
- Wang, L.; Geddes, D.A.; Walkley, B.; Provis, J.L.; Mechtcherine, V.; Tsang, D.C.W. The role of zinc in metakaolin-based geopolymers. Cem. Concr. Res. 2020, 136, 106194. [Google Scholar] [CrossRef]
- Smoleń, W.; Marczyk, J.; Łach, M.; Nguyen, T.; Korniejenko, K. Effect of microsilica addition on properties of geopolymer composites. J. Achiev. Mater. Manuf. Eng. 2023, 121, 46–59. [Google Scholar] [CrossRef]
- Khater, H.M. Effect of silica fume on the characterization of the geopolymer materials. Int. J. Adv. Struct. Eng. 2013, 5, 12. [Google Scholar] [CrossRef]




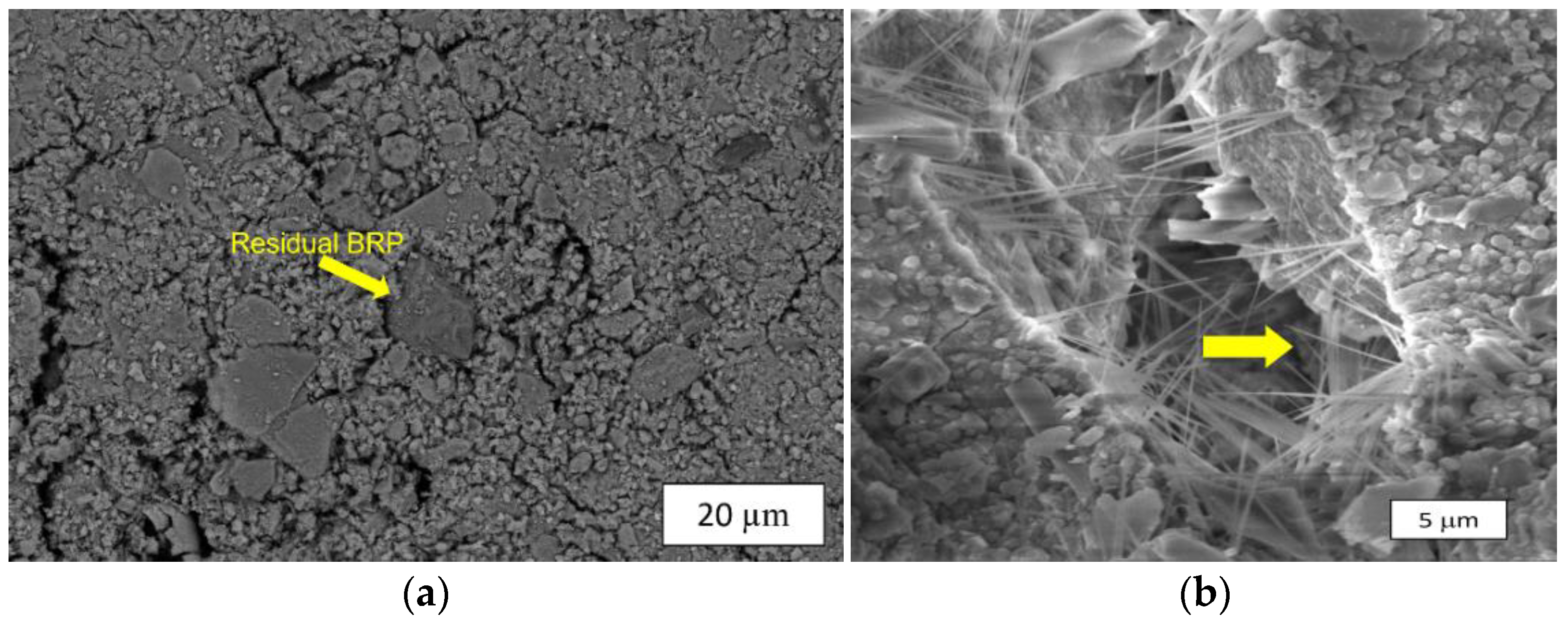
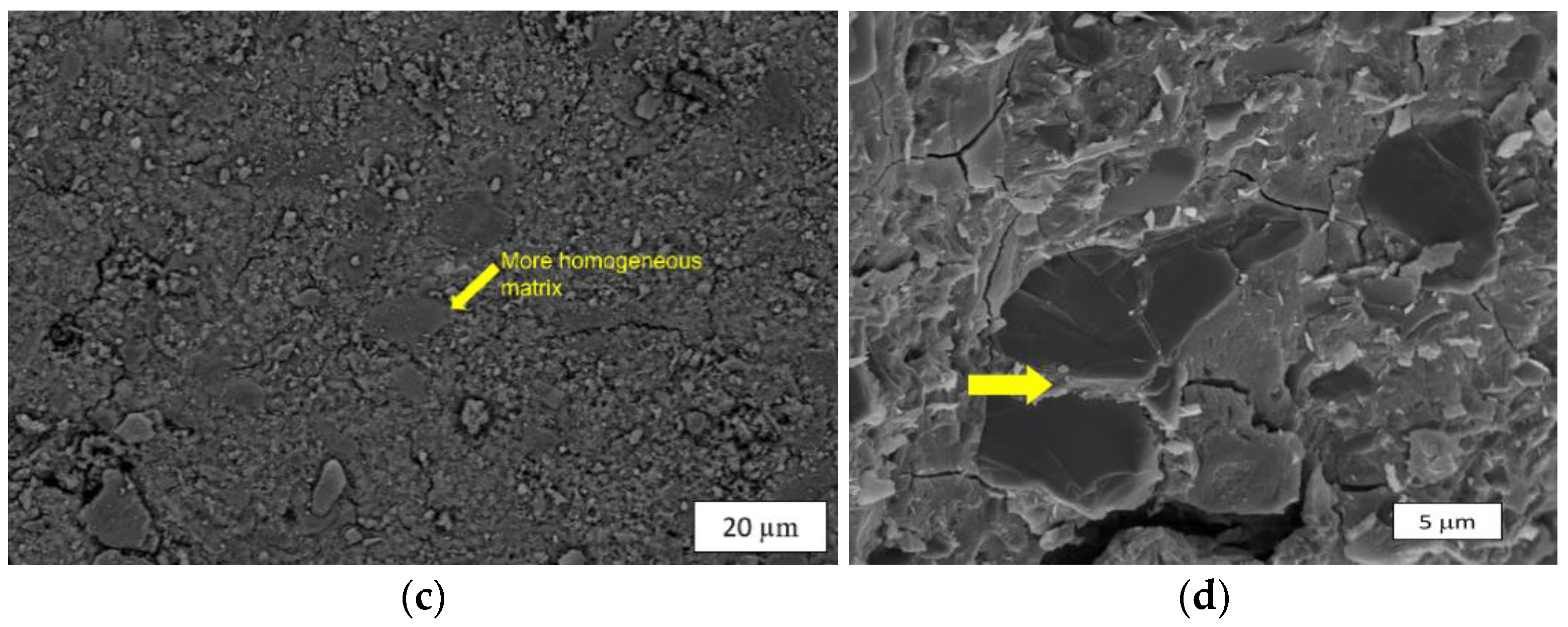
| Reference | Rock-Based Precursor | Activator System | UCS Reported | Curing Temperature (BHST) |
|---|---|---|---|---|
| [74] | Saudi volcanic scoria | NaOH | 12.4 MPa | Not reported |
| [64] | Volcanic glass mixes (obsidian) | 12 M NaOH solution | 93.7 MPa | 90 °C |
| [75] | Trachyte, Pegmatite, and Granite | Sodium hydroxide and Sodium silicate | 108 Mpa after 28 days | NR |
| [66] | Diabase tailings (crystalline igneous) | Na-based activator | 42.3 Mpa after 7 days | 20 °C |
| [69] | Perlite (glassy volcanic) | NaOH 2–5 M | Strength optimized by low NaOH | ~90 °C |
| Chemical (wt.%) | SiO2 | Al2O3 | CaO | K2O | Na2O | MgO | TiO2 | Fe2O3 | Cl | SO3 | ZrO2 | Mn2O3 | SrO | L.O.I. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BR | 66.68 | 26.46 | - | 2.40 | 0.77 | 1.60 | 0.35 | 0.18 | 0.16 | 0.07 | 0.03 | 1.00 | - | 0.4 |
| GGBFS | 29.36 | - | 59.08 | 5.49 | - | - | 1.23 | 2.2 | - | 0.23 | - | 2.09 | 0.16 | - |
| Microsilica | 95.1 | 0.58 | 0.4 | 1.0 | 0.4 | 0.5 | - | 0.3 | - | - | - | - | - | 1.8 |
| Components | Mix | |
|---|---|---|
| 1 | 2 | |
| Brazilian rock | 65.52 | 61.99 |
| GGBFS | 16.38 | 16.38 |
| Microsilica | - | 3.52 |
| Solid activator | 17.00 | 17.00 |
| Superplasticizer | 1.11 | 1.11 |
| Activator to solid ratio | 17.00 | 17.00 |
| Sample | (lbf/100 ft2) | R2 | ||
|---|---|---|---|---|
| Mix 1 | 18.14 | 0.045 | 0.58 | 0.97 |
| Mix 2 | 13.34 | 0.055 | 0.59 | 0.97 |
| Mix 1 | Mix 2 | |
|---|---|---|
| Plastic Viscosity (Pa.s) | 0.26 | 0.19 |
| 10 sec gel (Pa) | 7.6 | 7.6 |
| 10 min gel (Pa) | 19.6 | 32.7 |
| Mineral Phase | Mineral Group | Mix 1_1D | Mix 2_1D | Mix 1_28 D | Mix 2_28 D |
|---|---|---|---|---|---|
| Biotite | Mica | 2.57% | 3.574 | 2.65 | 3.287 |
| Microcline intermediate | K-Feldspar | 10.09% | 9.728 | 9.92 | 9.95 |
| Microcline maximum | K-Feldspar | 13.27% | 13.843 | 10.73 | 9.96 |
| Quartz, SiO2 | Quartz | 14.10% | 12.01 | 70.63 | 9.968 |
| Chlorite | Chlorite | 14.29% | 12.15 | 13.88 | 13.05 |
| Muscovite | Mica | 8.69% | 10.3 | 10.87 | 10.19 |
| Clinochlore | Chlorite | 28.91% | 30.96 | 31.42 | 34.02 |
| Pyrophyllite | Pyrophyllite | 8.09% | 7.43 | 9.9 | 9.87 |
| GOF * | 1.63% | 1.86 | 1.73 | 1.69 | |
| Rwp | 7.36% | 8.15 | 7.67 | 7.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro da Silva, R.; de Oliveira Freitas, J.C.; Khalifeh, M.; Martins Braga, R. Development of Rock-Based Geopolymers for Oilwell Cementing Applications—Utilizing Brazilian Rock Precursor. Processes 2025, 13, 3624. https://doi.org/10.3390/pr13113624
Ribeiro da Silva R, de Oliveira Freitas JC, Khalifeh M, Martins Braga R. Development of Rock-Based Geopolymers for Oilwell Cementing Applications—Utilizing Brazilian Rock Precursor. Processes. 2025; 13(11):3624. https://doi.org/10.3390/pr13113624
Chicago/Turabian StyleRibeiro da Silva, Raphael, Julio Cezar de Oliveira Freitas, Mahmoud Khalifeh, and Renata Martins Braga. 2025. "Development of Rock-Based Geopolymers for Oilwell Cementing Applications—Utilizing Brazilian Rock Precursor" Processes 13, no. 11: 3624. https://doi.org/10.3390/pr13113624
APA StyleRibeiro da Silva, R., de Oliveira Freitas, J. C., Khalifeh, M., & Martins Braga, R. (2025). Development of Rock-Based Geopolymers for Oilwell Cementing Applications—Utilizing Brazilian Rock Precursor. Processes, 13(11), 3624. https://doi.org/10.3390/pr13113624






