The Effect of Fermentation with Saccharomyces cerevisiae on the Release of Bound Phenolic Compounds from Wheat Bran and Its Effect on Antioxidant Capacity
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Raw Wheat Bran
2.2. Microbial Fermentation of Wheat Bran
2.3. Free Phenolic Compound Extraction
2.4. Bound Phenolic Compound Extraction
2.5. Determination of Total Phenolic Compounds
2.6. Trolox Equivalent Antioxidant Capacity (TEAC) Quantification of Phenolic Compounds
2.6.1. ABTS Decolorization Assay
2.6.2. DPPH Assay
2.6.3. FRAP Assay
2.7. Evaluation of the Release of Phenolic Compounds with an In Vitro Gastrointestinal System
2.8. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Free and Bound Phenolic Compounds Determination
3.2. ABTS Decolorization Assay
3.3. DPPH Assay
3.4. FRAP Assay
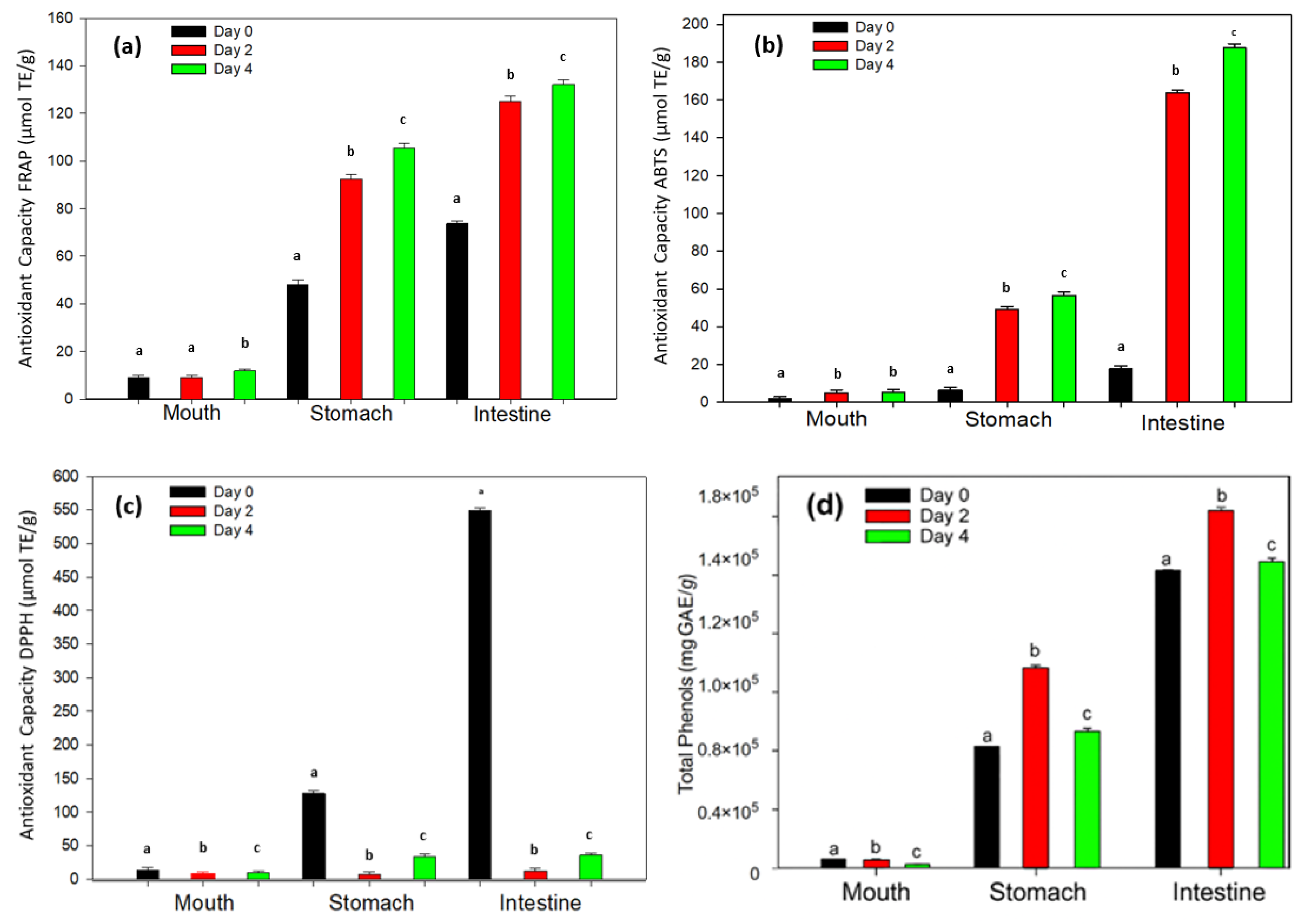
3.5. Evaluation of the Release of Phenolic Compounds with an In Vitro Gastrointestinal System
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| WB | wheat bran |
| AOX | antioxidant capacity |
| PCs | phenolic compounds |
| ABTS | 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) |
| TEAC | Trolox equivalent antioxidant capacity |
| FRAP | ferric-reducing ability of plasma |
| SD | standard deviation |
References
- Verma, R.; Chauhan, N.; Bhat, F.M.; Anand, A.; Dhaliwal, Y.S. Role of cereals in food security. In Cereal Grains; CRC Press: Boca Raton, FL, USA, 2023; pp. 15–24. [Google Scholar]
- Borneo, R.; León, A.E. Whole grain cereals: Functional components and health benefits. Food Funct. 2012, 3, 110–119. [Google Scholar] [CrossRef]
- Ricci, G.; Andreozzi, L.; Cipriani, F.; Giannetti, A.; Gallucci, M.; Caffarelli, C. Wheat allergy in children: A comprehensive update. Medicin 2019, 55, 400. [Google Scholar] [CrossRef] [PubMed]
- Spaggiari, M.; Calani, L.; Folloni, S.; Ranieri, R.; Dall’Asta, C.; Galaverna, G. The impact of processing on the phenolic acids, free betaine and choline in Triticum spp. L. whole grains and milling by-products. Food Chem. 2020, 311, 125940. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Suzauddula, M.; Bender, R.; Li, C.; Li, Y.; Sun, X.S.; Wang, W. Functional properties and potential applications of wheat bran extracts in food and cosmetics: A review of antioxidant, enzyme-inhibitory, and anti-aging benefits. Foods 2025, 14, 515. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Mense, A.L.; Brewer, L.R.; Shi, Y.C. Wheat bran layers: Composition, structure, fractionation, and potential uses in foods. Crit. Rev. Food Sci. Nutr. 2023, 64, 6636–6659. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J.D. Insoluble-Bound Phenolics in Food. Molecules 2016, 21, 1216. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Mateo-Anson, N.; Hemery, Y.M.; Bast, A.; Haenen, G.R.M.M. Optimizing the bioactive potential of wheat bran by processing. Food Funct. 2012, 3, 362–375. [Google Scholar] [CrossRef]
- Urias-Orona, V.; Heredia, J.B.; Muy-Rangel, D.; Niño-Medina, G. Ácidos fenólicos con actividad antioxidante en salvado de maíz y salvado de trigo. Rev. De La Univ. Juárez Aut. De Tabasco 2016, 30, 43–50. [Google Scholar]
- Bourais, I.; Elmarrkechy, S.; Taha, D.; Mourabit, Y.; Bouyahya, A.; El Yadini, M.; Iba, N. A review on medicinal uses, nutritional value, and antimicrobial, antioxidant, anti-inflammatory, antidiabetic, and anticancer potential related to bioactive compounds of J. Regia. Food Rev. Int. 2023, 39, 6199–6249. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, P.; Cheng, G.; Zhang, Y. A brief review of phenolic compounds identified from plants: Their extraction, analysis, and biological activity. Nat. Prod. Commun. 2022, 17, 1934578X211069721. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. Therapeutic potential of phenolic compounds in medicinal plants—Natural health products for human health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef] [PubMed]
- Gimeno-Creus, E.G. Compuestos fenólicos. Offarm 2004, 23, 80–84. [Google Scholar]
- Gulcin, I. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Roche, L.; Martínez-Sánchez, G.; Díaz-Batista, A. Determinación de marcadores de estrés oxidativo en pacientes con enfermedades cardiovasculares. Acta Bioquím. Clín. Latinoam. 2009, 43, 307–313. [Google Scholar]
- Nascimento, A.P.S.; Barros, A.N. Sustainable Innovations in Food Microbiology: Fermentation, Biocontrol, and Functional Foods. Foods 2025, 14, 2320. [Google Scholar] [CrossRef]
- Katileviciute, A.; Plakys, G.; Budreviciute, A.; Onder, K.; Damiati, S.; Kodzius, R. A sight to wheat bran high value-added products. Biomolecules 2019, 9, 887. [Google Scholar] [CrossRef]
- Zhao, M.; Ma, J.; Zhang, L.; Qi, H. Engineering strategies for enhanced heterologous protein production by Saccharomyces cerevisiae. Microb. Cell Factories 2024, 23, 32. [Google Scholar] [CrossRef]
- Melini, F.; Melini, V.; Luziatelli, F.; Ficca, A.G.; Ruzzi, M. Health-promoting components in fermented foods: An up-to-date systematic review. Nutrients 2019, 11, 1189. [Google Scholar] [CrossRef]
- Balli, D.; Bellumori, M.; Pucci, L.; Gabriele, M.; Longo, V.; Paoli, P.; Melani, F.; Mulinacci, N.; Innocenti, M. Does fermentation really increase the phenolic content in cereals? A study on millet. Foods 2020, 9, 303. [Google Scholar] [CrossRef]
- Tsafrakidou, P.; Michaelidou, A.-M.; Biliaderis, C.G. Fermented Cereal-based Products: Nutritional Aspects, Possible Impact on Gut Microbiota and Health Implications. Foods 2020, 9, 734. [Google Scholar] [CrossRef]
- Hur, S.J.; Lim, B.O.; Decker, E.A.; McClements, D.J. In vitro human digestion models for food applications. Food Chem. 2011, 125, 1–12. [Google Scholar] [CrossRef]
- Tomé-Sánchez, I.; Martín-Diana, A.B.; Peñas, E.; Frias, J.; Rico, D.; Jiménez-Pulido, I.; Martínez-Villaluenga, C. Bioprocessed wheat ingredients characterization, bioaccessibility of phenolic compounds, and bioactivity during in vitro digestion. Front. Plant Sci. 2021, 12, 790898. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.M.; Dias, T.; Hassimotto, N.M.A.; Naves, M.M.V. Ascorbic acid and phenolic contents, antioxidant capacity and flavonoids composition of Brazilian Savannah native fruits. Food Sci. Technol. 2017, 37, 564–569. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants: A comprehensive review. Arch. Toxicol. 2025, 99, 1–105. [Google Scholar] [CrossRef]
- Rico, D.; Cano, A.B.; Álvarez Álvarez, S.; Río Briones, G.; Martín Diana, A.B. Study of the total antioxidant capacity (TAC) in native cereal–pulse flours and the influence of the baking process on TAC using a combined Bayesian and support vector machine modeling approach. Foods 2023, 12, 3208. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Evaluation of the antioxidant capacity of food products: Methods, applications and limitations. Processes 2022, 10, 2031. [Google Scholar] [CrossRef]
- Reyes-Pérez, F.; Salazar-García, M.G.; Romero-Baranzini, A.L.; Islas-Rubio, A.R.; Ramírez-Wong, B. Estimated glycemic index and dietary fiber content of cookies elaborated with extruded wheat bran. Plant Foods Hum. Nutr. 2013, 68, 52–56. [Google Scholar] [CrossRef]
- Călinoiu, L.F.; Cătoi, A.F.; Vodnar, D.C. Solid-state yeast fermented wheat and oat bran as a route for delivery of antioxidants. Antioxidants 2019, 8, 372. [Google Scholar] [CrossRef]
- Chiremba, C.; Taylor, J.R.N.; Rooney, L.W.; Beta, T. Phenolic acid content of sorghum and maize cultivars varying in hardness. Food Chem. 2012, 134, 81–88. [Google Scholar] [CrossRef]
- Guo, W.; Beta, T. Phenolic acid composition and antioxidant potential of insoluble and soluble dietary fibre extracts derived from select whole-grain cereals. Food Res. Int. 2013, 51, 518–525. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A., Jr. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Zugazua-Ganado, M.; Bordagaray, A.; Ezenarro, J.; Garcia-Arrona, R.; Ostra, M.; Vidal, M. Adaptation of the Folin–Ciocalteu and Fast Blue BB spectrophotometric methods to digital image analysis for the determination of total phenolic content: Reduction of reaction time, interferences and sample analysis. LWT 2024, 193, 115756. [Google Scholar] [CrossRef]
- Frangu, A.; Ashrafi, A.M.; Sýs, M.; Arbneshi, T.; Metelka, R.; Adam, V.; Vlček, M.; Richtera, L. Determination of Trolox equivalent antioxidant capacity in berries using amperometric tyrosinase biosensor based on multi-walled carbon nanotubes. Appl. Sci. 2020, 10, 2497. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Rubio, C.P.; Hernández-Ruiz, J.; Martinez-Subiela, S.; Tvarijonaviciute, A.; Ceron, J.J. Spectrophotometric assays for total antioxidant capacity (TAC) in dog serum: An update. BMC Vet. Res. 2016, 12, 166. [Google Scholar] [CrossRef]
- Rioja-Antezana, A.P.; Vizaluque, B.E.; Aliaga-Rossel, E.; Tejeda, L.; Book, O.; Mollinedo, P.; Pearrieta, J.M. Determinación de la capacidad antioxidante total, fenoles totales y la actividad enzimática en una bebida no láctea en base a granos de Chenopodium quinoa. Rev. Boliv. Quim. 2018, 35, 168–176. [Google Scholar]
- Pérez-Pérez, L.M.; Huerta-Ocampo, J.A.; Ruiz-Cruz, S.; Cinco-Moroyoqui, F.J.; Wong-Corral, F.J.; Rascón-Valenzuela, L.A.; Robles-Garcia, M.A.; Gonzalez-Vega, R.I.; Rosas-Burgos, E.C.; Corella-Madueño, M.A.G.; et al. Evaluation of quality, antioxidant capacity, and digestibility of chickpea (Cicer arietinum L. cv Blanoro) stored under N2 and CO2 atmospheres. Molecules 2021, 26, 2773. [Google Scholar] [CrossRef]
- Finnie, S.; Atwell, W.A. Wheat Flour; Handbook Series; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–16. ISBN 978-1-891127-90-8. [Google Scholar]
- Sztupecki, W.; Rhazi, L.; Depeint, F.; Aussenac, T. Functional and nutritional characteristics of natural or modified wheat bran non-starch polysaccharides: A literature review. Foods 2023, 12, 2693. [Google Scholar] [CrossRef]
- Merali, Z.; Collins, S.R.; Elliston, A.; Wilson, D.R.; Käsper, A.; Waldron, K.W. Characterization of cell wall components of wheat bran following hydrothermal pretreatment and fractionation. Biotechnol. Biofuels 2015, 8, 23. [Google Scholar] [CrossRef]
- Li, C.; Wang, L.; Chen, Z.; Li, Y.; Li, J. Facile and green preparation of diverse arabinoxylan hydrogels from wheat bran by combining subcritical water and enzymatic crosslinking. Carbohydr. Polym. 2020, 241, 116317. [Google Scholar] [CrossRef]
- Hassan, M.A.; Abdelshafy, A.M.; Hussien, S.M.; Sorour, M.A.; Mahmoud, E.A. Improving wheat bran properties using potential bioprocesses for application in functional bread production. Food Saf. Health 2025, 3, 482–491. [Google Scholar] [CrossRef]
- Abdel-Aty, A.M.; Barakat, A.Z.; Bassuiny, R.I.; Mohamed, S.A. Improved production of antioxidant-phenolic compounds and certain fungal phenolic-associated enzymes under solid-state fermentation of chia seeds with Trichoderma reesei: Response surface methodology-based optimization. Food Meas. 2022, 16, 3488–3500. [Google Scholar] [CrossRef]
- Saroj, R.; Malik, M.A.; Kaur, D. Effect of solid-state yeast fermentation on the physicochemical properties, antioxidant and anti-nutritional activity of wheat bran. Cereal Res. Commun. 2025, 53, 1659–1675. [Google Scholar] [CrossRef]
- Chakraborty, M.; Bhowal, J. Prospects of Health Beneficial Functional Xylooligosaccharides Produced by Enzymatic Hydrolysis of Xylan and Application in Food Industry: A Comprehensive Review. Food Rev. Int. 2025, 41, 643–670. [Google Scholar] [CrossRef]
- Islam, S.; Miah, M.A.S.; Islam, M.F.; Tisa, K.J.; Bhuiyan, M.H.R.; Bhuiyan, M.N.I.; Hossain, M.H. Fermentation with lactic acid bacteria enhances the bioavailability of bioactive compounds of whole wheat flour. Appl. Food Res. 2024, 4, 100610. [Google Scholar] [CrossRef]
- Ramos-Enríquez, J.R.; Ramírez-Wong, B.; Robles-Sánchez, R.M.; Robles-Zepeda, R.E.; González-Aguilar, G.A.; Gutiérrez-Dorado, R. Effect of extrusion conditions and the optimization of phenolic compound content and antioxidant activity of wheat bran using response surface methodology. Plant Foods Hum. Nutr. 2018, 73, 228–234. [Google Scholar] [CrossRef]
- Adebo, O.A.; Medina-Meza, I.G. Impact of fermentation on the phenolic compounds and antioxidant activity of whole cereal grains: A mini review. Molecules 2020, 25, 927. [Google Scholar] [CrossRef]
- Sandhu, K.S.; Punia, S.; Kaur, M. Effect of duration of solid-state fermentation by Aspergillus awamorinakazawa on antioxidant properties of wheat cultivars. LWT-Food Sci. Technol. 2016, 71, 323–328. [Google Scholar] [CrossRef]
- Bayat, E.; Moosavi-Nasab, M.; Fazaeli, M.; Majdinasab, M.; Mirzapour-Kouhdasht, A.; Garcia-Vaquero, M. Wheat germ fermentation with Saccharomyces cerevisiae and Lactobacillus plantarum: Process optimization for enhanced composition and antioxidant properties in vitro. Foods 2022, 11, 1125. [Google Scholar] [CrossRef]
- Zhou, J.; Du, G.; Chen, J. Novel fermentation processes for manufacturing plant natural products. Curr. Opin. Biotechnol. 2014, 25, 17–23. [Google Scholar] [CrossRef]
- Đorđević, T.M.; Šiler-Marinković, S.S.; Dimitrijević-Branković, S.I. Effect of fermentation on antioxidant properties of some cereals and pseudo cereals. Food Chem. 2010, 119, 957–963. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Antioxidant property of coffee components: Assessment of methods that define mechanisms of action. Molecules 2014, 19, 19180–19208. [Google Scholar] [CrossRef]
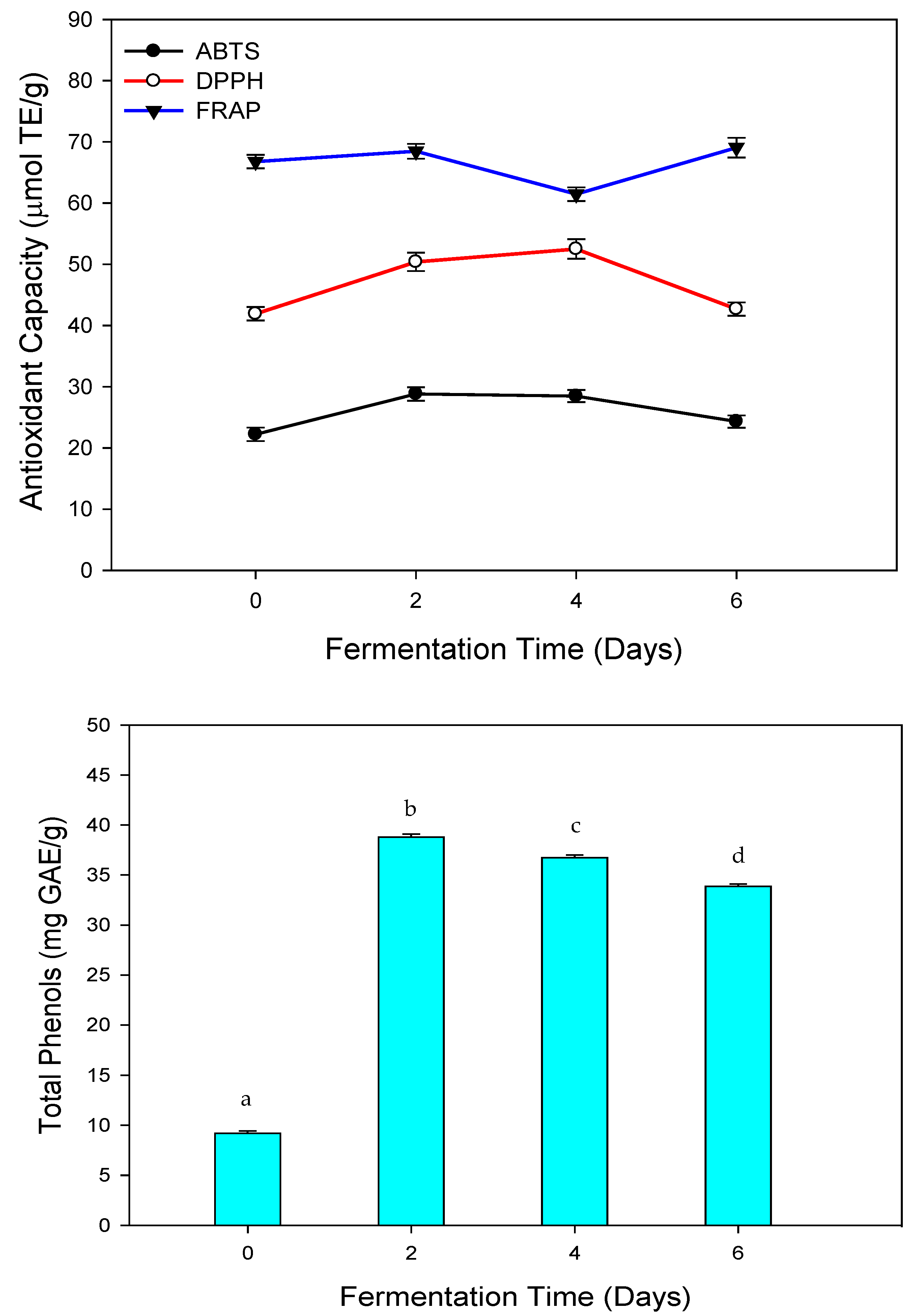
| Fermentation Time (Days) | Free Phenolic Compounds (mgGAE/g) | Bound Phenolic Compounds (mgGAE/g) | Total Phenolic Compounds (mgGAE/g) |
|---|---|---|---|
| 0 (Control) | 1.40 ± 0.04 a | 7.79 ± 0.2 b | 9.19 ± 0.13 c |
| 2 | 8.56 ± 0.05 d | 30.24 ± 0.06 e | 38.81 ± 0.06 f |
| 4 | 9.55 ± 0.01 g | 27.18 ± 0.40 h | 36.73 ± 0.21 i |
| 6 | 5.46 ± 0.00 j | 28.41 ± 0.40 k | 33.87 ± 0.20 l |
| Fermentation Time (Days) | Free Phenolic Compounds (μmolTE/g) | Bound Phenolic Compounds (μmolTE/g) | Total Phenolic Compounds (μmolTE/g) |
|---|---|---|---|
| 0 (Control) | 2.08 ± 0.00 a | 20.14 ± 0.22 b | 22.22 ± 0.11 c |
| 2 | 2.61 ± 0.00 d | 26.20 ± 0.43 e | 28.81 ± 0.22 f |
| 4 | 2.54 ± 0.03 g | 25.93 ± 0.96 h | 28.47 ± 0.50 i |
| 6 | 2.56 ± 0.04 j | 21.75 ± 0.46 k | 24.31 ± 0.25 l |
| Fermentation Time (Days) | Free Phenolic Compounds (μmolTE/g) | Bound Phenolic Compounds (μmolTE/g) | Total Phenolic Compounds (μmolTE/g) |
|---|---|---|---|
| 0 (Control) | 6.36 ± 0.18 a | 35.54 ± 0.11 b | 41.91 ± 0.15 c |
| 2 | 4.66 ± 0.05 d | 45.72 ± 0.02 e | 50.38 ± 0.03 f |
| 4 | 5.12 ± 0.06 g | 47.38 ± 0.07 h | 52.50 ± 0.06 i |
| 6 | 4.57 ± 0.00 j | 38.11 ± 0.01 k | 42.69 ± 0.01 l |
| Fermentation Time (Days) | Free Phenolic Compounds (μmolTE/g) | Bound Phenolic Compounds (μmolTE/g) | Total Phenolic Compounds (μmolTE/g) |
|---|---|---|---|
| 0 (Control) | 10.56 ± 1.65 ai | 56.22 ± 0.13 b | 66.78 ± 0.89 c |
| 2 | 11.87 ± 0.03 a | 56.59 ± 0.07 d | 68.46 ± 0.05 e |
| 4 | 13.02 ± 0.04 f | 48.43 ± 0.07 g | 61.45 ± 0.06 h |
| 6 | 11.69 ± 0.12 i | 57.38 ± 0.10 j | 69.06 ± 0.11 k |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ocaño-Higuera, V.M.; López-Avilés, G.; Almendariz-Tapia, F.J.; Del-Toro-Sánchez, C.L.; Tapia-Hernández, J.A.; Garzón-García, A.M.; Dublán-García, O.; Vergel-Alfonso, A.A.; González-Aguilar, G.A.; Valdez-Hurtado, S.; et al. The Effect of Fermentation with Saccharomyces cerevisiae on the Release of Bound Phenolic Compounds from Wheat Bran and Its Effect on Antioxidant Capacity. Processes 2025, 13, 3506. https://doi.org/10.3390/pr13113506
Ocaño-Higuera VM, López-Avilés G, Almendariz-Tapia FJ, Del-Toro-Sánchez CL, Tapia-Hernández JA, Garzón-García AM, Dublán-García O, Vergel-Alfonso AA, González-Aguilar GA, Valdez-Hurtado S, et al. The Effect of Fermentation with Saccharomyces cerevisiae on the Release of Bound Phenolic Compounds from Wheat Bran and Its Effect on Antioxidant Capacity. Processes. 2025; 13(11):3506. https://doi.org/10.3390/pr13113506
Chicago/Turabian StyleOcaño-Higuera, Víctor Manuel, Guadalupe López-Avilés, Francisco Javier Almendariz-Tapia, Carmen Lizette Del-Toro-Sánchez, José Agustín Tapia-Hernández, Alba Mery Garzón-García, Octavio Dublán-García, Ariel Alain Vergel-Alfonso, Gustavo Adolfo González-Aguilar, Santiago Valdez-Hurtado, and et al. 2025. "The Effect of Fermentation with Saccharomyces cerevisiae on the Release of Bound Phenolic Compounds from Wheat Bran and Its Effect on Antioxidant Capacity" Processes 13, no. 11: 3506. https://doi.org/10.3390/pr13113506
APA StyleOcaño-Higuera, V. M., López-Avilés, G., Almendariz-Tapia, F. J., Del-Toro-Sánchez, C. L., Tapia-Hernández, J. A., Garzón-García, A. M., Dublán-García, O., Vergel-Alfonso, A. A., González-Aguilar, G. A., Valdez-Hurtado, S., Barrales-Cureño, H. J., Ramos-Enríquez, J. R., & Canizales-Rodríguez, D. F. (2025). The Effect of Fermentation with Saccharomyces cerevisiae on the Release of Bound Phenolic Compounds from Wheat Bran and Its Effect on Antioxidant Capacity. Processes, 13(11), 3506. https://doi.org/10.3390/pr13113506










