Selective Recovery of Rare Earth Elements from Electric Motors in End-of-Life Vehicles via Copper Slag for Sustainability
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
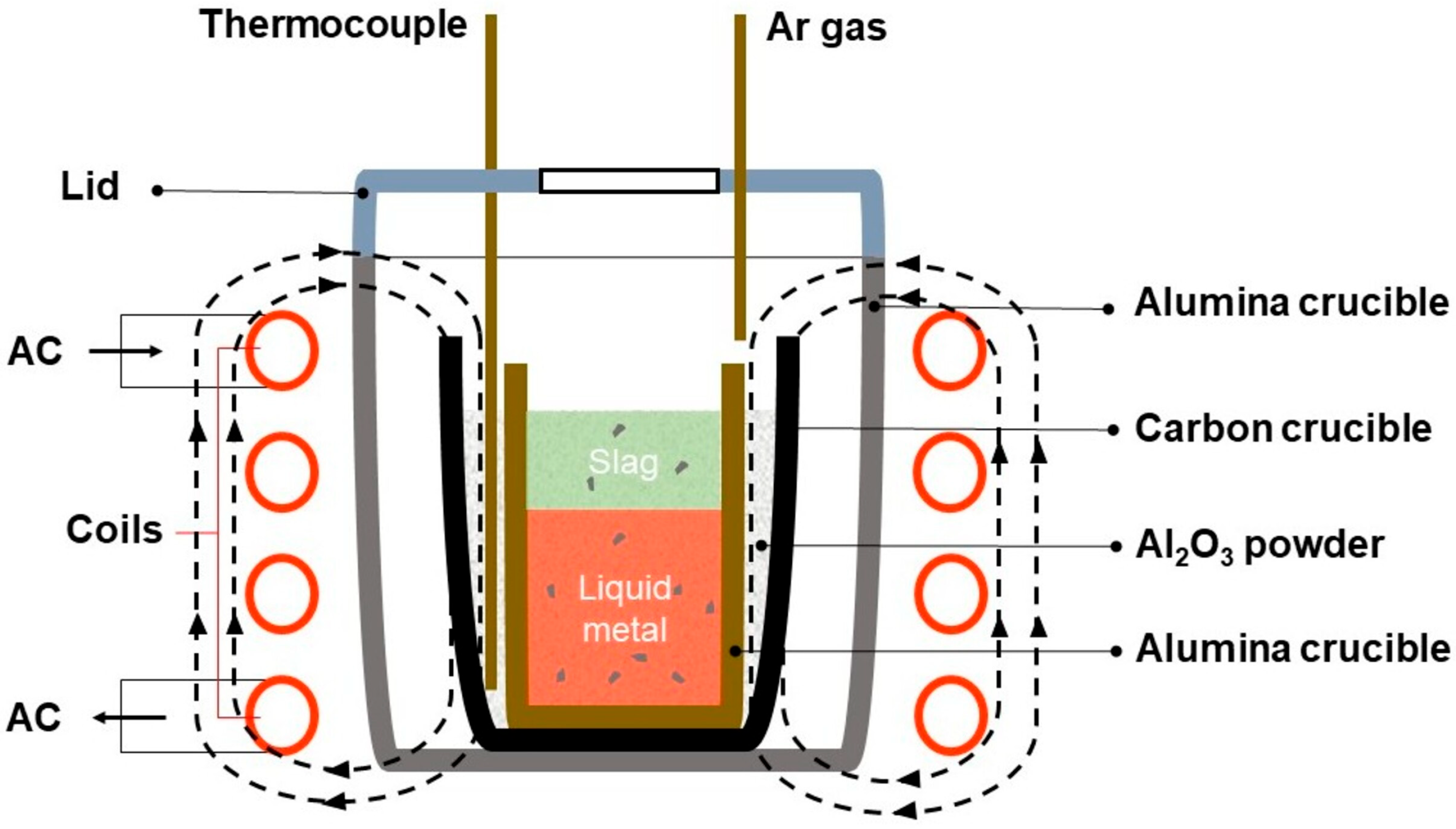
2.2. Experimental Apparatus and Procedure
2.3. Analytical Methods
2.3.1. Chemical and Morphological Analysis
2.3.2. Thermodynamic Simulation
2.3.3. Element Recovery Calculation
3. Results
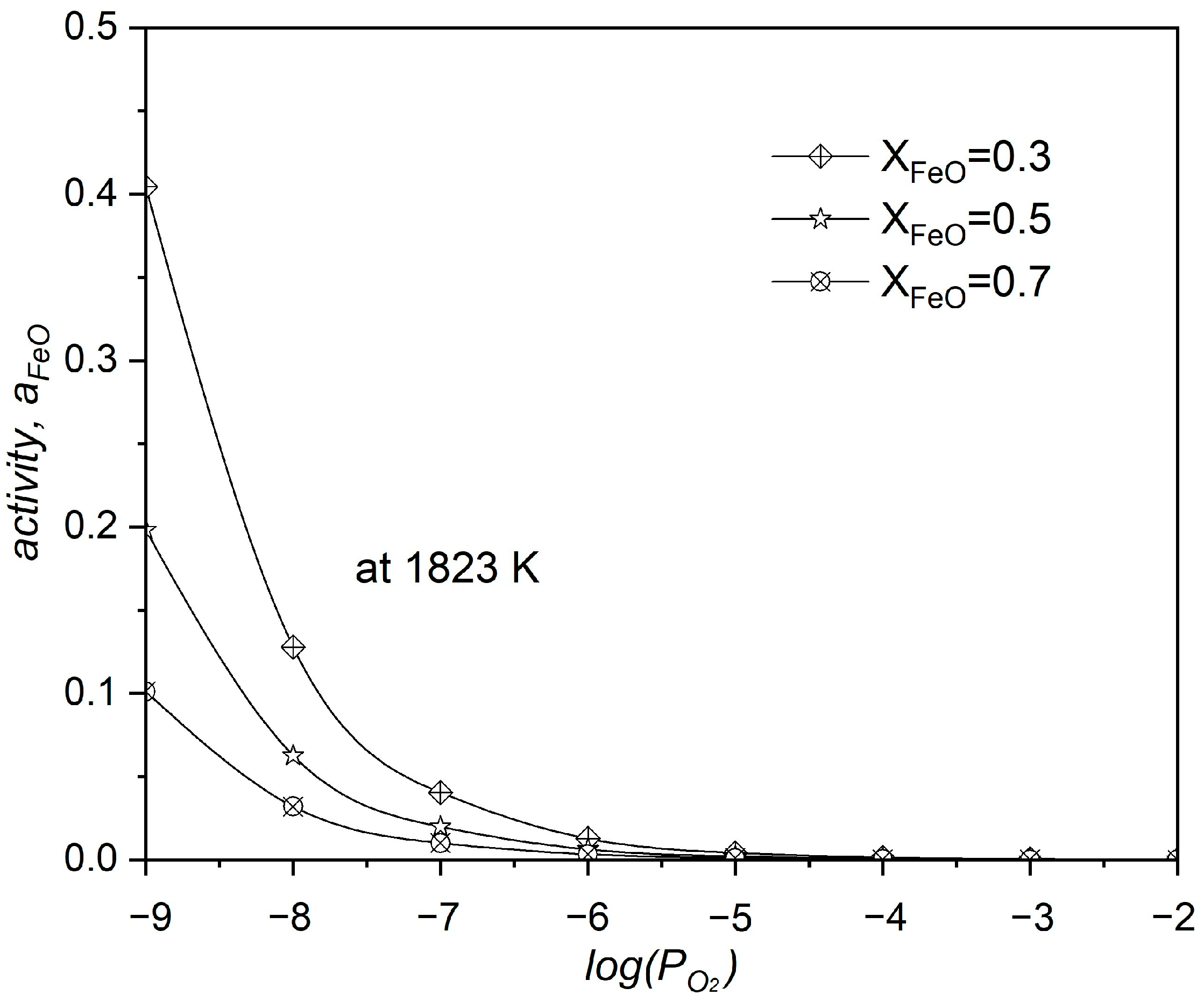
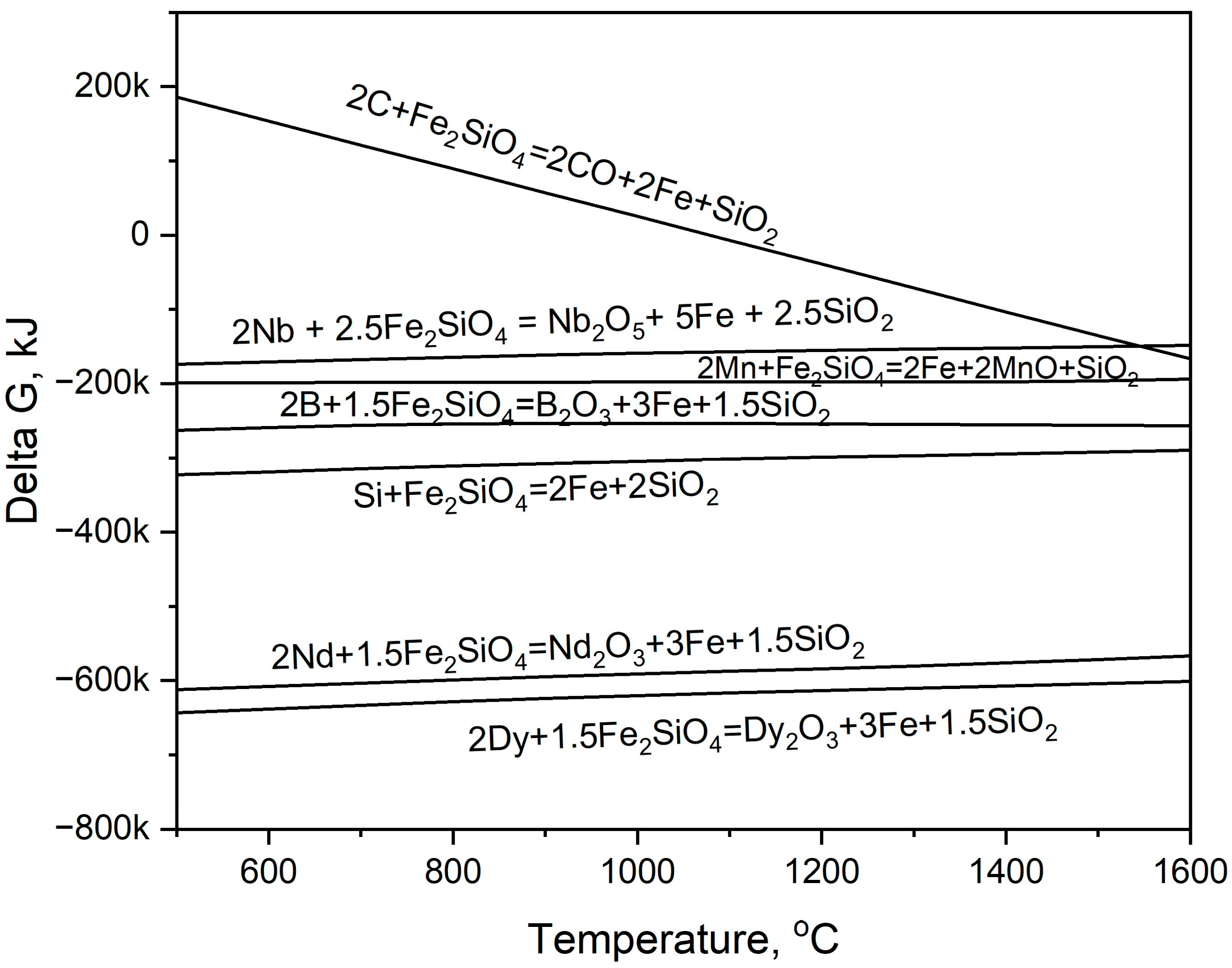
3.1. Calculation and Equilibrium Simulation of Steel and Magnet Smelting
3.2. Thermodynamic Modeling of Steel and Magnet Smelting with Copper Slag Flux
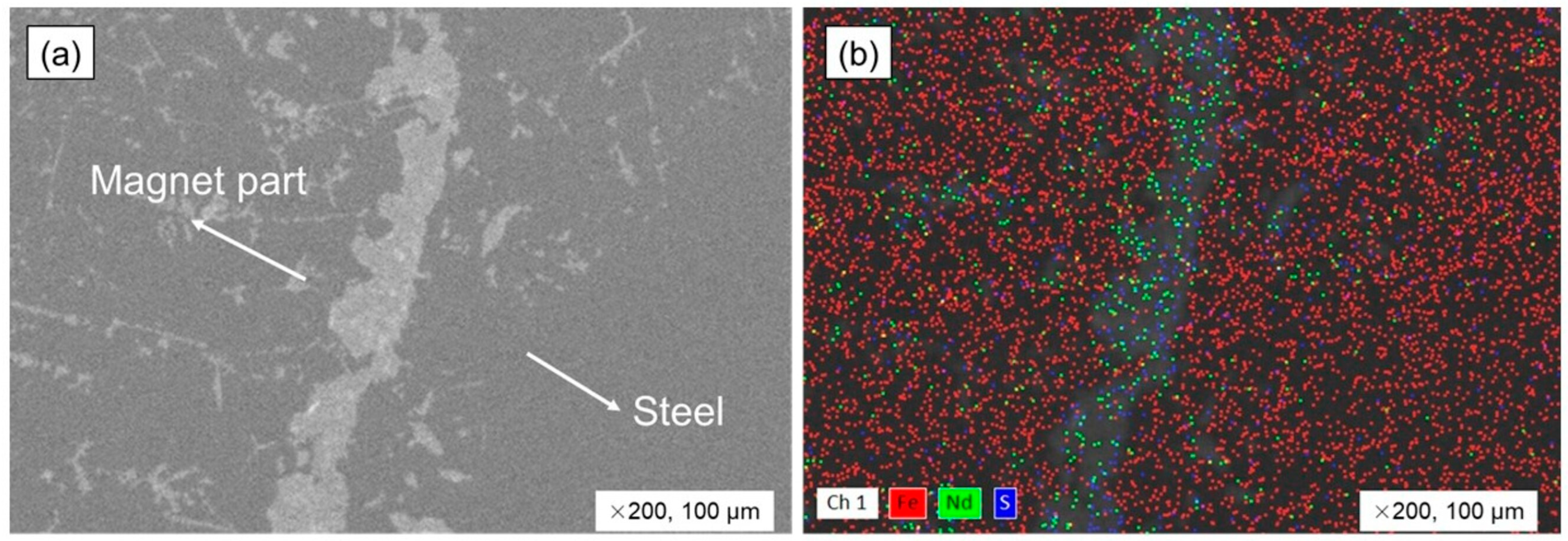
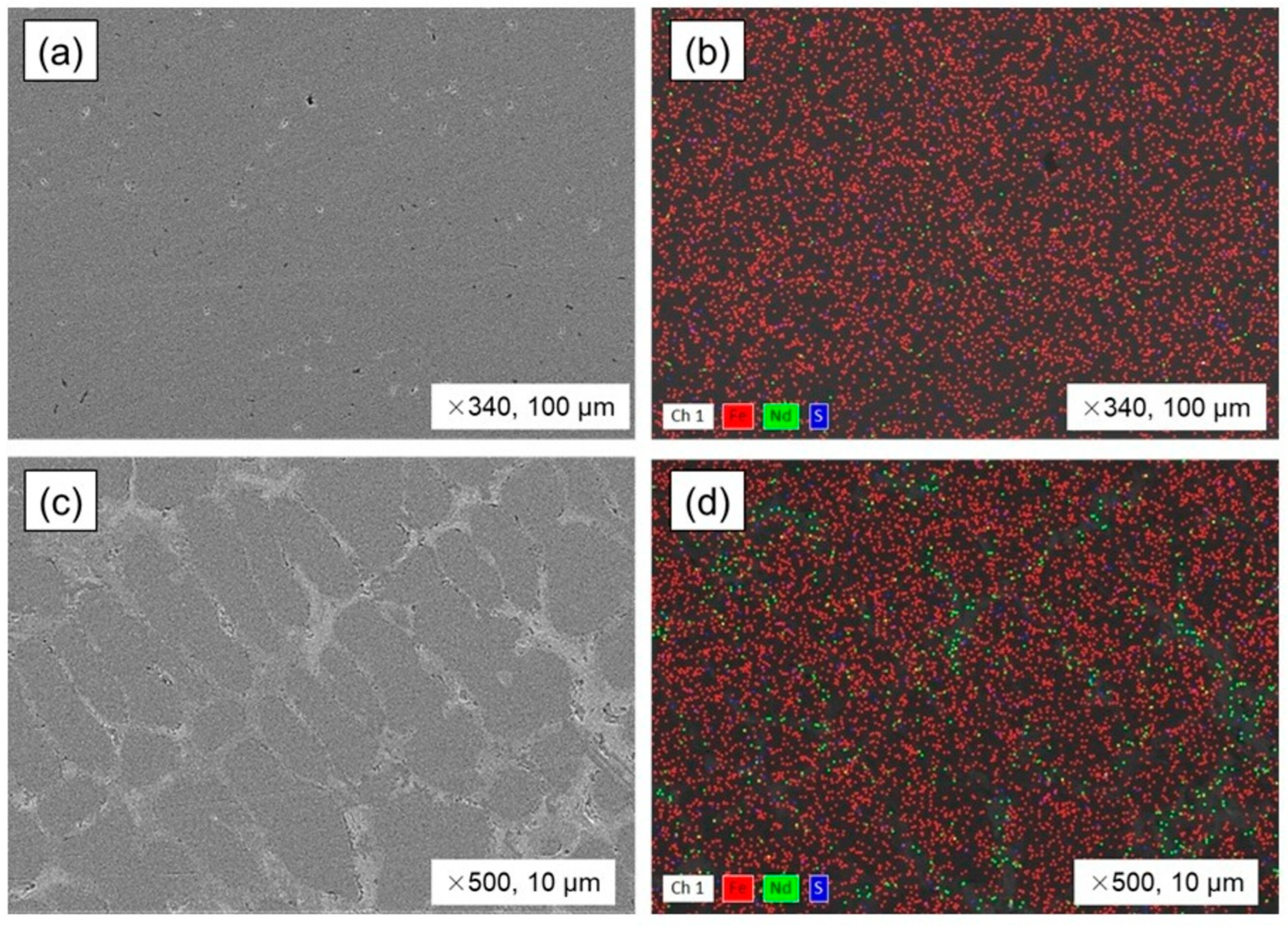
3.3. Steel and Magnet Smelting with Copper Slag Flux
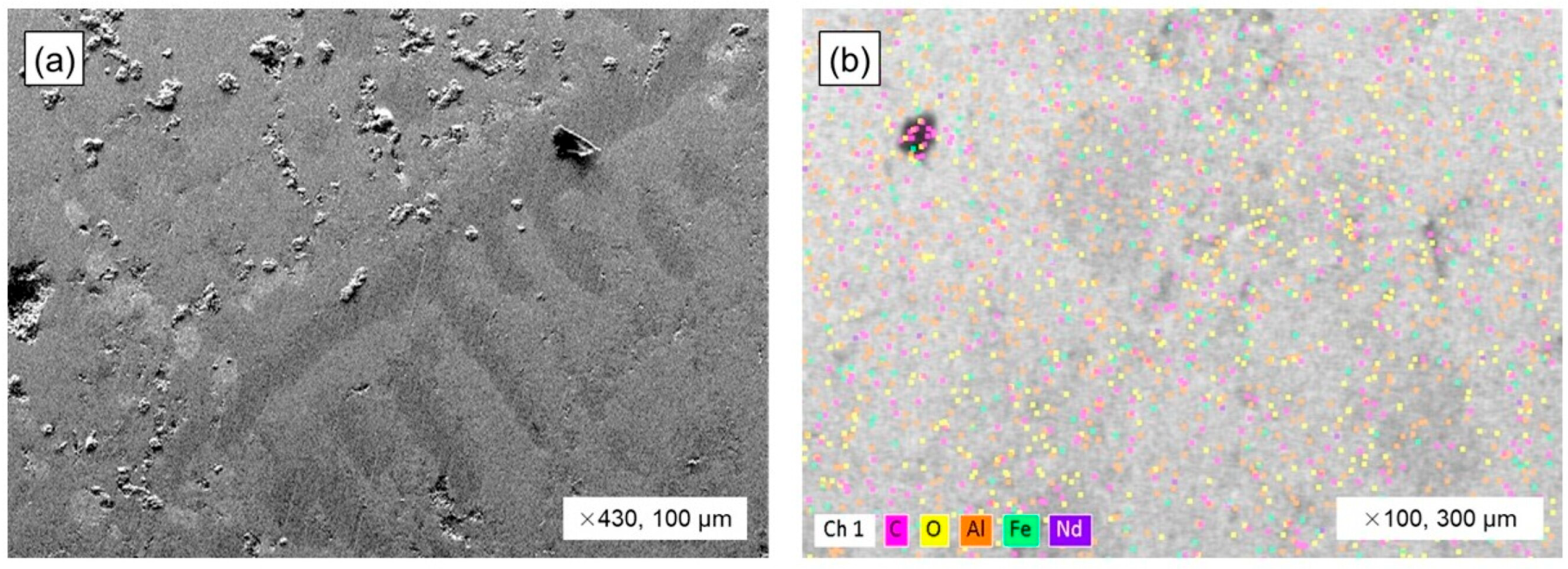
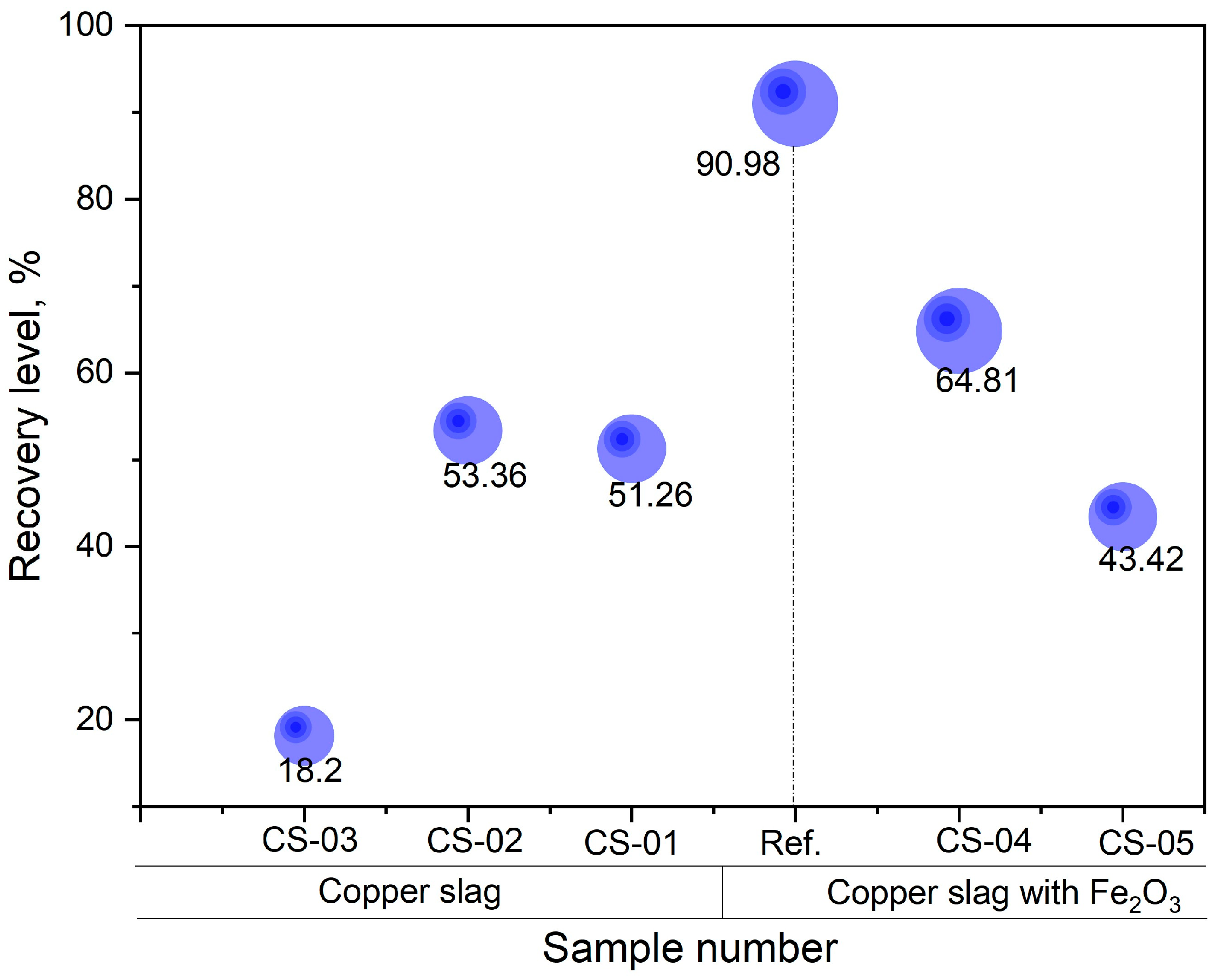
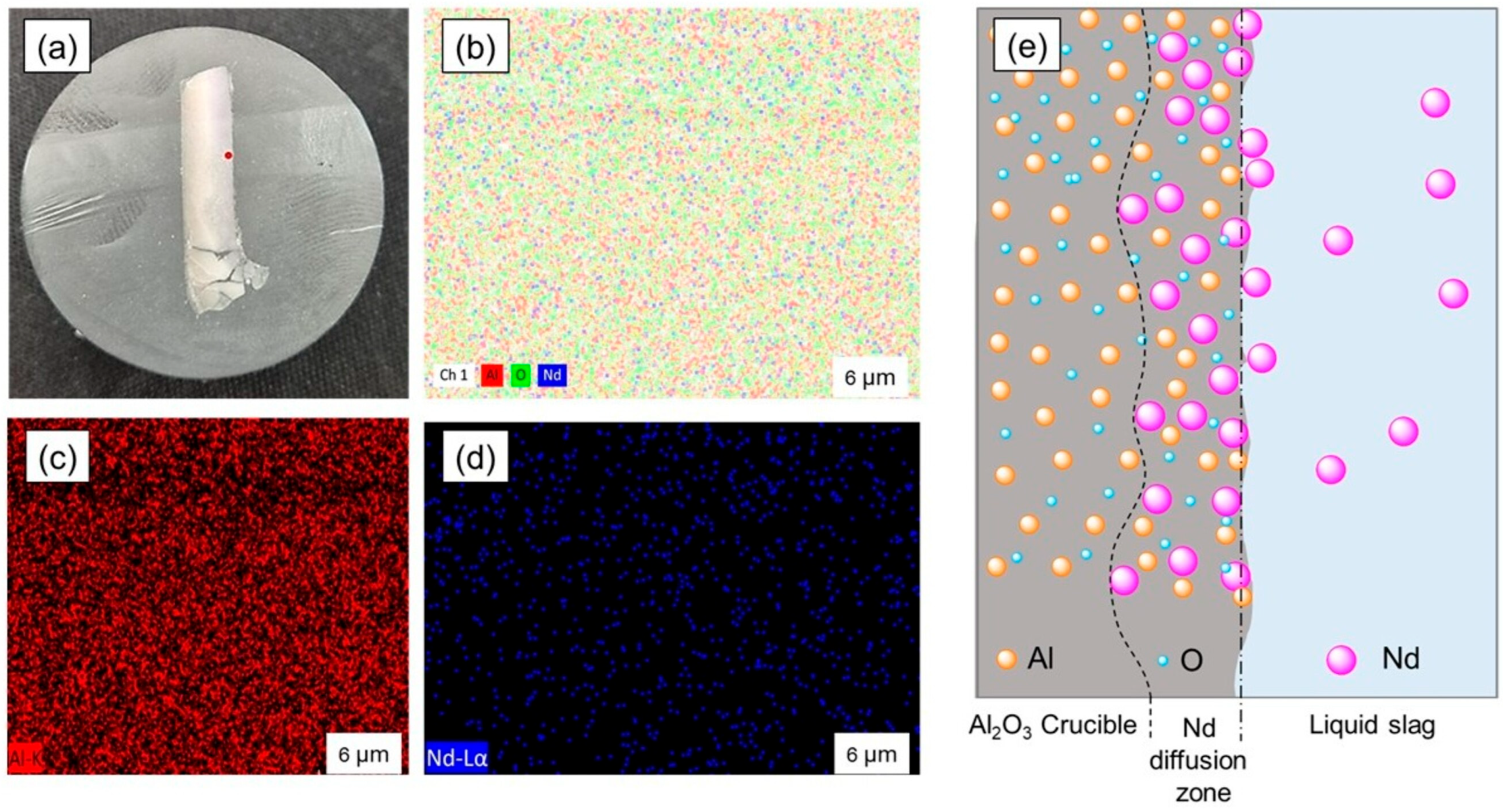
3.4. The Neodymium Recovery Within the Slag
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Emmanuel, O.O.; Ethan, S.; Amin, M. A comparative state-of-technology review and future directions for rare earth element separation. Renew. Sustain. Energy Rev. 2021, 143, 110917. [Google Scholar] [CrossRef]
- Leal Filho, W.; Kotter, R.; Özuyar, P.G.; Abubakar, I.R.; Eustachio, J.H.P.P.; Matandirotya, N.R. Understanding Rare Earth Elements as Critical Raw Materials. Sustainability 2023, 15, 1919. [Google Scholar] [CrossRef]
- Wei, C.-W.; Deng, M.; Xu, C.; Chakhmouradian, A.R.; Smith, M.P.; Kynicky, J.; Song, W.-L.; Chen, W.; Fu, B. Mineralization of the Bayan Obo Rare Earth Element Deposit by Recrystallization and Decarbonation. Econ. Geol. 2022, 117, 1327–1338. [Google Scholar] [CrossRef]
- Michael, A.; Broom-Fendley, S.; Wei, C. Formation of Rare Earth Deposits in Carbonatites. Elements 2021, 17, 327–332. [Google Scholar] [CrossRef]
- Wei, C.-W.; Xu, C.; Deng, M.; Song, W.-L.; Shi, A.; Li, Z.; Fan, C.; Kuang, G. Origin of metasomatic fluids in the Bayan Obo rare-earth-element deposit. Ore Geo. Rev. 2022, 141, 104654. [Google Scholar] [CrossRef]
- Imarc. Rare Earth Elements Market Size, Share, Trends and Forecast by Application and Region, 2025–2033. Available online: https://www.imarcgroup.com/rare-earth-industry (accessed on 11 February 2025).
- Markets & Markets. Rare Earth Metals Market by Type (Lanthanum Oxide, Cerium Oxide, Neodymium Oxide, Europium Oxide, Terbium Oxide), Application (Permanent Magnets, Catalysts, Glass Polishing, Phosphors, Metal Alloys, Ceramics), and Region-Global Forecast to 2030. Available online: https://www.marketsandmarkets.com/Market-Reports/rare-earth-metals-market-121495310.html?gad_source=1&gad_campaignid=365563516&gbraid=0AAAAADxY7SynAn4UULwIuMmlhJq11KBcF&gclid=Cj0KCQjwuKnGBhD5ARIsAD19RsZpwhpjdNDO7YwNJnw1nceIGmqr-QMRwtUdxWKszS6w8-pd9BOnzwwaAuzkEALw_wcB (accessed on 1 July 2025).
- Statista. Rare Earth Mining Global Distribution 2024, by Country. Available online: https://www.statista.com/statistics/270277/mining-of-rare-earths-by-country/ (accessed on 25 February 2025).
- Nasdag. Top 10 Countries by Rare Earth Metal Production. Available online: https://www.nasdaq.com/articles/top-10-countries-rare-earth-metal-production (accessed on 1 March 2025).
- Ilankoon, I.M.S.K.; Dushyantha, N.P.; Mancheri, N.; Edirisinghe, P.M.; Neethling, S.J.; Ratnayake, N.P.; Rohitha, L.P.S.; Dissanayake, D.M.D.O.K.; Premasiri, H.M.R.; Abeysinghe, A.M.K.B.; et al. Constraints to rare earth elements supply diversification: Evidence from an industry survey. J. Clean. Prod. 2022, 331, 129932. [Google Scholar] [CrossRef]
- Mpila, M.N.; Randy, L.V.W. Rare earth elements: Sector allocations and supply chain considerations. J. Rare Earths 2025, 43, 1–8. [Google Scholar] [CrossRef]
- INN. Rare Earths Market Update: H1 2025 in Review. Available online: https://investingnews.com/rare-earths-forecast/ (accessed on 14 August 2025).
- Orlova, S.; Rassõlkin, A. Permanent Magnets in Sustainable Energy: Comparative Life Cycle Analysis. Energies 2024, 17, 6384. [Google Scholar] [CrossRef]
- Usman, A.; Saxena, A. Technical Roadmaps of Electric Motor Technology for Next Generation Electric Vehicles. Machines 2025, 13, 156. [Google Scholar] [CrossRef]
- Ranjan, P.; Kalla, U.K. Comprehensive Study on Various Types of Magnets Used in Permanent Magnet Electrical Motors. In Proceedings of the 2024 IEEE Region 10 Symposium (TENSYMP), New Delhi, India, 27–29 September 2024; pp. 1–7. [Google Scholar] [CrossRef]
- KtM. The Role of Sintered NdFeB Magnets in Electric Vehicles. Available online: https://www.ktmagnet.com/the-role-of-sintered-ndfeb-magnets-in-electric-vehicles.html (accessed on 31 January 2024).
- Heim, J.W.; Vander Wal, R.L. NdFeB Permanent Magnet Uses, Projected Growth Rates and Nd Plus Dy Demands across End-Use Sectors through 2050: A Review. Minerals 2023, 13, 1274. [Google Scholar] [CrossRef]
- Wang, H.; Lamichhane, T.N.; Paranthaman, M.P. Review of additive manufacturing of permanent magnets for electrical machines: A prospective on wind turbine. Mater. Today Phys. 2022, 24, 100675. [Google Scholar] [CrossRef]
- Stanford Magnets. Application of Neodymium Magnets in Wind Turbine Generators. Available online: https://www.stanfordmagnets.com/application-of-neodymium-magnets-in-wind-turbine-generators.html#:~:text=The%20Curie%20temperature%20of%20the,dimensional%20tolerances%20and%20magnetic%20properties (accessed on 16 April 2024).
- Divyani, J.; Sunil, K.; Shally, V. Introduction to artificial intelligence-empowered electric vehicles in smart grids. In Artificial Intelligence-Empowered Modern Electric Vehicles in Smart Grid Systems; Aparna, K., Sudeep, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 3–31. [Google Scholar] [CrossRef]
- Arévalo, P.; Ochoa-Correa, D.; Villa-Ávila, E. A Systematic Review on the Integration of Artificial Intelligence into Energy Management Systems for Electric Vehicles: Recent Advances and Future Perspectives. World Electr. Veh. J. 2024, 15, 364. [Google Scholar] [CrossRef]
- Evs & Beyond. 2024 Breaks Global EV Sales Record with over 17 Million Units Sold. Available online: https://evsandbeyond.co.nz/2024-breaks-global-ev-sales-record-with-over-17-million-units-sold/#:~:text=Electric%20vehicle%20(EV)%20sales%20reached,1.3%20million%20(+27%25) (accessed on 16 January 2025).
- RHO Motion. Over 17 Million EVs Sold in 2024—Record Year. Available online: https://rhomotion.com/news/over-17-million-evs-sold-in-2024-record-year/#:~:text=Over%2017%20million%20EVs%20sold%20in%202024,*%20Rest%20of%20World:%201.3%20million%2C%20+27% (accessed on 14 January 2025).
- Mohamed, K.; Nassar, Y.; El-Khozondar, H.J.; Monaem, E.R.Z.; Yaghoubi, E.; Yaghoubi, E. Electric Vehicles in China, Europe, and the United States: Current Trend and Market Comparison. Int. J. Electr. Eng. Sustain. 2024, 2, 1–20. Available online: https://ijees.org/index.php/ijees/article/view/70/36. (accessed on 9 January 2024).
- IEA.org. The Global Electric Vehicle Fleet Is Set to Grow Twelve-Fold by 2035 Under Stated Policies. Available online: https://www.iea.org/reports/global-ev-outlook-2024/outlook-for-electric-mobility (accessed on 23 April 2024).
- Danouche, M.; Bounaga, A.; Oulkhir, A.; Boulif, R.; Zeroual, Y.; Benhida, R.; Lyamlouli, K. Advances in bio/chemical approaches for sustainable recycling and recovery of rare earth elements from secondary resources. Sci. Total Environ. 2024, 912, 168811. [Google Scholar] [CrossRef]
- Cherkezova-Zheleva, Z.; Burada, M.; Sobetkii, A.E.; Paneva, D.; Fironda, S.A.; Piticescu, R.R. Green and Sustainable Rare Earth Element Recycling and Reuse from End-of-Life Permanent Magnets. Metals 2024, 14, 658. [Google Scholar] [CrossRef]
- Urtnasan, E.; Park, J.-H.; Chung, Y.-J.; Wang, J.-P. Pyrometallurgical Recycling of Electric Motors for Sustainability in End-of-Life Vehicle Metal Separation Planning. Processes 2025, 13, 1729. [Google Scholar] [CrossRef]
- Li, Z.; Hamidi, A.S.; Yan, Z.; Sattar, A.; Hazra, S.; Soulard, J.; Guest, C.; Ahmed, S.H.; Tailor, F. A circular economy approach for recycling Electric Motors in the end-of-life Vehicles: A literature review. Resour. Conserv. Recycl. 2024, 205, 107582. [Google Scholar] [CrossRef]
- Tiwari, D.; Miscandlon, J.; Tiwari, A.; Jewell, G.W. A Review of Circular Economy Research for Electric Motors and the Role of Industry 4.0 Technologies. Sustainability 2021, 13, 9668. [Google Scholar] [CrossRef]
- Chang, M.M.L.; Ong, S.K.; Nee, A.Y.C. Approaches and Challenges in Product Disassembly Planning for Sustainability. Procedia Cirp 2017, 60, 506–511. [Google Scholar] [CrossRef]
- Mitrouchev, P.; Wang, C.G.; Lu, L.X.; Li, G.O. Selective disassembly sequence generation based on lowest level disassembly graph method. Int. J. Adv. Manuf. Technol. 2015, 80, 141–159. [Google Scholar] [CrossRef]
- Katsunori, Y. Rare Earth Element Recovery Technology for Motor Magnets for Electric Vehicles That Aims to Reduce Costs in a Short Time. Science Technology Innovation Japan. Available online: https://sj.jst.go.jp/stories/2022/s0301-01a.html (accessed on 1 March 2022).
- Saito, T.; Sato, H.; Ozawa, S.; Yu, J.; Motegi, T. The extraction of Nd from waste Nd–Fe–B alloys by the glass slag method. J. Alloys Compd. 2003, 353, 189–193. [Google Scholar] [CrossRef]
- Yang, Y.; Abrahami, S.T.; Xiao, Y. Recovery of rare earth elements from EOL permanent magnets with molten slag extraction. In The 3rd International Slag Valorisation Symposium; ACCO: Leuven, Belgium, 2013; pp. 249–252. [Google Scholar]
- Kim, K.O.; Jung, Y.H.; Park, J.C.; Lim, M.S. Comparative Study of Mechanical and Electrical Characteristics of High-Strength and Conventional Electrical Steel for EV Traction High-Speed Multilayer IPMSM Using Rare-Earth Free PM. IEEE Trans. Magn. 2023, 59, 8102205. [Google Scholar] [CrossRef]
- Lobo, J.A.; Geiger, G.H. Thermodynamics and solubility of carbon in ferrite and ferritic Fe-Mo alloys. Metall. Trans. A 1976, 7, 1347–1357. [Google Scholar] [CrossRef]
- Liu, B.; Zhu, N.; Li, Y.; Wu, P.; Dang, Z.; Ke, Y. Efficient recovery of rare earth elements from discarded NdFeB magnets. Process Saf. Environ. Prot. 2019, 124, 317–325. [Google Scholar] [CrossRef]
- Aarti, K.; Raman, R.; Randhawa, N.S.; Sushanta, K.S. Energy efficient process for recovery of rare earths from spent NdFeB magnet by chlorination roasting and water leaching. Hydrometallurgy 2021, 201, 105581. [Google Scholar] [CrossRef]
- Masahiro, I.; Koji, M.; Ken-ichi, M. Novel rare earth recovery process on NdFeB magnet scrap by selective chlorination using NH4Cl. J. Alloys Compd. 2009, 477, 484–487. [Google Scholar] [CrossRef]
- Yuuki, M.; Naoto, T.; Katsuyasu, S. Selective Recovery of Rare Earth Elements from Dy containing NdFeB Magnets by Chlorination. ACS Sustain. Chem. Eng. 2013, 1, 655–662. [Google Scholar] [CrossRef]
- Shirayama, S.; Okabe, T.H. Selective Extraction and Recovery of Nd and Dy from Nd-Fe-B Magnet Scrap by Utilizing Molten MgCl2. Met. Mater. Trans. B 2018, 49, 1067–1077. [Google Scholar] [CrossRef]
- Tomohiko, A.; Yu, M.; Tomonori, S.; Masahide, O.; Toru, H.O. Optimum conditions for extracting rare earth metals from waste magnets by using molten magnesium. J. Alloys Compd. 2017, 703, 337–343. [Google Scholar] [CrossRef]
- Hua, Z.; Wang, J.; Wang, L.; Zhao, Z.; Li, X.; Xiao, Y.; Yang, Y. Selective Extraction of Rare Earth Elements from NdFeB Scrap by Molten Chlorides. ACS Sustain. Chem. Eng. 2014, 2, 2536–2543. [Google Scholar] [CrossRef]
- Abbasalizadeh, A.; Malfliet, A.; Seetharaman, S.; Sietsma, J.; Yang, Y.X. Electrochemical Recovery of Rare Earth Elements from Magnets: Conversion of Rare Earth Based Metals into Rare Earth Fluorides in Molten Salts. Mater. Trans. 2017, 58, 400–405. [Google Scholar] [CrossRef]
- Sun, M.; Hu, X.; Peng, L.; Fu, P.; Ding, W.; Peng, Y. On the production of Mg-Nd master alloy from NdFeB magnet scraps. J. Mater. Process. Technol. 2015, 218, 57–61. [Google Scholar] [CrossRef]
- Takeda, O.; Okabe, T.H.; Umetsu, Y. Phase equilibrium of the system Ag–Fe–Nd, and Nd extraction from magnet scraps using molten silver. J. Alloys Compd. 2004, 379, 305–313. [Google Scholar] [CrossRef]
- Martina, M.; Annett, G.; Mihai, S.; Margitta, U.; Wolfgang, L. A route for recycling Nd from Nd-Fe-B magnets using Cu melts. J. Alloys Compd. 2015, 647, 997–1006. [Google Scholar] [CrossRef]
- Ching-Chien, H.; Chih-Chieh, M. Vacuum processing temperature effect on highly coercive recycled NdFeB permanent magnets with Pr-based alloy addition. Mater. Chem. Phys. 2024, 325, 129648. [Google Scholar] [CrossRef]
- Bian, Y.; Guo, S.; Jiang, L. Extraction of Rare Earth Elements from Permanent Magnet Scraps by FeO–B2O3 Flux Treatment. J. Sustain. Metall. 2015, 1, 151–160. [Google Scholar] [CrossRef]
- Chung, H.; Stopic, S.; Emil-Kaya, E.; Gürmen, S.; Friedrich, B. Recovery of Rare Earth Elements from Spent NdFeB-Magnets: Separation of Iron through Reductive Smelting of the Oxidized Material (Second Part). Metals 2022, 12, 1615. [Google Scholar] [CrossRef]
- Blenau, L.W.; Vogt, D.; Lonski, O.; Abrar, A.; Fabrichnaya, O.; Charitos, A. Development of a Process to Recycle NdFeB Permanent Magnets Based on the CaO-Al2O3-Nd2O3 Slag System. Processes 2023, 11, 1783. [Google Scholar] [CrossRef]






| Element, wt.% | C | Si | Mn | P | S | Cr | Mo | Ni | Cu | Al | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rotor steel | 0.05 | 3.0 | 0.45 | 0.03 | 0.03 | - | - | - | - | 0.05 | Bal. |
| Cast iron flakes * | 3.48 | 2.61 | 0.58 | 0.34 | 0.13 | 0.51 | 0.014 | 0.027 | 0.03 | <0.001 | Bal. |
| Oxid | Fe2O3 | SiO2 | Al2O3 | MgO | CaO | CuO | ZnO | K2O | SO3 | TiO2 | MoO3 | PbO | Cr2O3 | NiO | MnO | P2O5 | Nd * |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wt.% | 54.44 | 30.61 | 4.81 | 1.93 | 1.85 | 1.41 | 1.16 | 1.05 | 0.83 | 0.49 | 0.48 | 0.42 | 0.12 | 0.11 | 0.10 | 0.10 | ND |
| Element, wt.% | C | Si | Mn | P | S | Fe | Weight, kg | |
| Cast Iron, | Total | |||||||
| Motor steel | 0.05 | 3.0 | 0.45 | 0.03 | 0.03 | Bal. | 3.0 | 37.8 |
| Cast iron addition, wt.% | ||||||||
| 10 wt.% | 0.60 | 2.93 | 0.47 | 0.08 | 0.05 | Bal. | 6.78 | 41.58 |
| 20 wt.% | 0.85 | 2.91 | 0.48 | 0.10 | 0.05 | Bal. | 10.56 | 45.36 |
| 30 wt.% | 1.05 | 2.89 | 0.49 | 0.12 | 0.06 | Bal. | 14.34 | 49.14 |
| Materials | Weight, kg | wt.% | |
|---|---|---|---|
| Motor materials | Steel | 34.8 | 67.92 |
| Nd magnets | 2.1 | 4.10 | |
| Cast iron | 3.0 | 5.85 | |
| Cast iron addition with 30 wt.% | 11.34 | 22.13 | |
| Total | 51.24 | 100.0 | |
| Element | C | O | Nd | Fe | Al | Sum. |
|---|---|---|---|---|---|---|
| Wt.% | 9.25 | 35.47 | 28.11 | 26.59 | 0.58 | 100.0 |
| Element | Fe | C | Si | Al | Nd | Mg | S | Ca | O | Sum. |
|---|---|---|---|---|---|---|---|---|---|---|
| Metal bulk | 88.3 | 4.86 | 6.65 | 0.19 | - | - | - | - | - | 100.0 |
| Inclusions | 15.2 | 3.45 | 2.52 | 3.45 | 35.2 | 1.2 | 0.8 | 0.68 | 37.5 | 100.0 |
| Element, wt.% | C | Si | Mn | P | S | Al | B | Nd | Nb | Dy | Fe | Weight of Products, g | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Liq_ Steel | Nd Solid | Slag_liq | Re2O3_liq | ||||||||||||
| without flux | 1.01 | 2.78 | 0.33 | 0.11 | 4.3 | 0.18 | 0.04 | 0.97 | 0.04 | 0.04 | 94.46 | 99.68 | 0.31 | - | - |
| with 10 wt.% flux | 0.99 | 1.67 | 0.23 | 0.11 | 0.05 | 6 × 10−5 | 0.01 | 6 × 10−8 | 0.04 | 7 × 10−7 | 96.65 | 101.53 | 1.80 | 6.54 | 0.109 |
| with 20 wt.% flux | 0.97 | 0.31 | 0.08 | 0.11 | 0.06 | 1 × 10−5 | 2 × 10−3 | 8 × 10−9 | 0.04 | 1 × 10−8 | 98.01 | 104.04 | 1.81 | 14.03 | 0.102 |
| with 30 wt.% flux | 0.97 | 6 × 10−3 | 4 × 10−3 | 0.11 | 0.06 | 6 × 10−7 | 9 × 10−5 | 6 × 10−10 | 0.03 | 6 × 10−10 | 98.22 | 103.47 | 1.57 | 24.81 | 0.13 |
| Element, wt.% | SiO2 | FeO | Fe2O3 | Al2O3 | MgO | MnO | B2O3 | KAlO2 | ZnO | CaO | K2O |
|---|---|---|---|---|---|---|---|---|---|---|---|
| with 10 wt.% slag flux | 82.12 | 0.54 | 2 × 10−5 | 5.16 | 3.57 | 2.06 | 1.51 | 3.03 | 5 × 10−4 | 1.91 | 0.05 |
| with 20 wt.% slag flux | 81.60 | 1.71 | 1 × 10−4 | 5.29 | 3.18 | 2.48 | 0.97 | 2.94 | 3 × 10−3 | 1.7 | 0.03 |
| with 30 wt.% slag flux | 74.06 | 13.96 | 0.02 | 3.29 | 1.37 | 0.97 | 0.38 | 4.8 | 0.53 | 0.72 | 0.07 |
| Element | Fe | Nd | S | Sum. |
|---|---|---|---|---|
| Wt.% | 86.25 | 11.32 | 4.62 | 100.0 |
| Element, wt.% | Fe | Nd | S | Sum. |
|---|---|---|---|---|
| Steel bulk | 98.54 | 1.35 | 0.11 | 100.0 |
| Magnet part | 89.84 | 9.95 | 0.21 | 100.0 |
| Sample | Holding Time, min | Element of Metal, wt.% | Nd * | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | Si | Mn | P | S | Cu | Ni | Mo | Etc. | Fe | ||||
| Ref. [29] | With fayalite for 30 min | 1.08 | 0.18 | 0.05 | 0.05 | 0.015 | 0.1 | – | – | 0.25 | 98.27 | 0.008 | |
| with 30 wt.% copper slag flux | |||||||||||||
| CS-01 | 30 | 0.88 | 1.69 | 0.11 | 0.15 | 0.26 | 0.3 | 0.03 | 0.08 | 0.57 | 95.93 | 0.081 | |
| CS-02 | 20 | 0.91 | 1.6 | 0.07 | 0.14 | 0.14 | 0.31 | 0.03 | 0.10 | 0.50 | 96.21 | 0.019 | |
| CS-03 | 10 | 0.89 | 0.97 | 0.07 | 0.11 | 0.09 | 0.3 | 0.03 | 0.09 | 0.45 | 97.0 | 0.002 | |
| with 2 wt.% of Fe2O3 and 30 wt.% of copper slag flux | |||||||||||||
| CS-04 | 30 | 0.87 | 0.7 | 0.07 | 0.12 | 0.17 | 0.43 | 0.03 | 0.08 | 0.48 | 97.05 | 0.005 | |
| CS-05 | 20 | 0.91 | 0.79 | 0.09 | 0.12 | 0.18 | 0.44 | 0.02 | 0.07 | 0.51 | 96.87 | 0.003 | |
| Sample | SiO2 | Fe2O3 | Al2O3 | MnO | MgO | CaO | K2O | TiO2 | Na2O | Cr2O3 | SO3 | ZnO | SrO | Nd * |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ref. [29] | 52.6 | 24.49 | 13.6 | 2.2 | - | 0.12 | - | 0.2 | - | 0.25 | - | - | - | 4.0 |
| with 30 wt.% copper slag flux | ||||||||||||||
| CS-01 | 42.62 | 26.42 | 22.6 | 1.48 | 1.65 | 2.08 | 1.2 | 0.75 | 0.51 | 0.36 | 0.17 | 0.02 | 0.01 | 2.45 |
| CS-02 | 42.49 | 24.42 | 24.89 | 1.41 | 1.52 | 2.12 | 1.22 | 0.78 | 0.48 | 0.37 | 0.18 | 0.02 | 0.04 | 2.53 |
| CS-03 | 48.22 | 15.63 | 27.79 | 1.55 | 1.52 | 2.12 | 1.25 | 0.77 | 0.56 | 0.42 | 0.07 | 0.02 | 0.01 | 0.96 |
| with 2 wt.% Fe2O3 and 30 wt.% copper slag flux | ||||||||||||||
| CS-04 | 45.37 | 23.0 | 21.84 | 1.79 | 1.78 | 2.29 | 1.35 | 0.75 | 0.55 | 0.46 | 0.08 | 0.1 | 0.03 | 2.97 |
| CS-05 | 47.96 | 16.73 | 26.4 | 1.87 | 1.66 | 2.13 | 1.33 | 0.74 | 0.5 | 0.41 | 0.07 | 0.04 | 0.03 | 2.1 |
| Holding Time, min | Nd in Magnet, g | Slag Weight, g | Nd in Slag, wt.% | Nd in Slag, g |
|---|---|---|---|---|
| with 30 wt.% of copper slag flux | ||||
| 30 | 4.08 | 85.03 | 2.46 | 2.09 |
| 20 | 3.89 | 82.05 | 2.53 | 2.08 |
| 10 | 3.93 | 74.53 | 0.96 | 0.72 |
| with 2 wt.% of Fe2O3 and 30 wt.% of copper slag flux | ||||
| 30 | 3.95 | 86.25 | 2.97 | 2.56 |
| 20 | 4.17 | 86.22 | 2.10 | 1.81 |
| Technique | Raw Material | Chemical | Temperature, °C | Ref. |
|---|---|---|---|---|
| Carbonization/hydrogenation | NdFeB magnet scrap |
| 1400 | Bowen et al. [39] |
| Chlorination | NdFeB magnet scrap |
| 950 300 1000 | Aarti et al. [40] Masahiro et al. [41] Yuuki et al. [42] |
| Molten salts | NdFeB magnet scrap |
| 1000 1200 950 | Shirayama et al. [43], Tomohiko et al. [44] Zhongsheng et al. [45] Abbasalizadeh et al. [46] |
| Liquid metal extraction | NdFeB magnet scrap |
| 830 1090 Arc melting 1085 | Ming et al. [47] Takeda et al. [48]. Martina et al. [49] Huang et al. [50] |
| Glass slag | NdFeB magnet scrap |
| 1300 1500 | Bain et al. [51] Saito et al. [35] |
| Smelting with slag |
|
| 1550 1400 1700 1500 | Erdenebold et al. [29] Katsunori et al. [34] Blenau et al. [53] Chung et al. [52] |
| Input | Output | |||
|---|---|---|---|---|
| Material | Weight Per Motor, kg | Product | Weight Per Motor, kg | Recovery Rate, % |
| Steel | 34.8 | Copper | 8.33 | 98 |
| Cast iron | 3.0 | Aluminum | 13.40 | 95 |
| Copper | 8.5 | REEs | 0.57 | 91 |
| Aluminum | 14.1 | Slag | 11.56 | 78 |
| NdFeB magnet | 2.1 | Fe-C alloy from | 55.77 | – |
| Cast iron addition | 12.0 | – steel and cast iron | 49.8 | 100 |
| Copper slag flux | 14.94 | – Fe from magnet | 1.26 | 60 |
| Fe2O3 oxidzer | 0.80 | – Fe from Fe2O3 – Fe from copper slag | 0.56 3.99 | 69 70 |
| Energy consumption | kWh/motor | – Cu from copper slag | 0.16 | 100 |
| for copper melting | 4.93 | |||
| for aluminum melting | 5.64 | |||
| for steel and magnet smelting Total | 33.46 44.03 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urtnasan, E.; Kim, C.-J.; Chung, Y.-J.; Wang, J.-P. Selective Recovery of Rare Earth Elements from Electric Motors in End-of-Life Vehicles via Copper Slag for Sustainability. Processes 2025, 13, 3502. https://doi.org/10.3390/pr13113502
Urtnasan E, Kim C-J, Chung Y-J, Wang J-P. Selective Recovery of Rare Earth Elements from Electric Motors in End-of-Life Vehicles via Copper Slag for Sustainability. Processes. 2025; 13(11):3502. https://doi.org/10.3390/pr13113502
Chicago/Turabian StyleUrtnasan, Erdenebold, Chang-Jeong Kim, Yeon-Jun Chung, and Jei-Pil Wang. 2025. "Selective Recovery of Rare Earth Elements from Electric Motors in End-of-Life Vehicles via Copper Slag for Sustainability" Processes 13, no. 11: 3502. https://doi.org/10.3390/pr13113502
APA StyleUrtnasan, E., Kim, C.-J., Chung, Y.-J., & Wang, J.-P. (2025). Selective Recovery of Rare Earth Elements from Electric Motors in End-of-Life Vehicles via Copper Slag for Sustainability. Processes, 13(11), 3502. https://doi.org/10.3390/pr13113502









