Abstract
Blue hydrogen technology, generated from natural gas through carbon capture and storage (CCS) technology, is a promising solution to mitigate greenhouse gas emissions and meet the growing demand for clean energy. To improve the sustainability of blue hydrogen, it is crucial to explore alternative feedstocks, production methods, and improve the efficiency and economics of carbon capture, storage, and utilization strategies. Two established technologies for hydrogen synthesis are Steam Methane Reforming (SMR) and Autothermal Reforming (ATR). The choice between SMR and ATR depends on project specifics, including the infrastructure, energy availability, environmental goals, and economic considerations. ATR-based facilities typically generate hydrogen at a lower cost than SMR-based facilities, except in cases where electricity prices are elevated or the facility has reduced capacity. Both SMR and ATR are methods used for hydrogen production from methane, but ATR offers an advantage in minimizing CO2 emissions per unit of hydrogen generated due to its enhanced energy efficiency and unique process characteristics. ATR provides enhanced utility and flexibility regarding energy sources due to its autothermal characteristics, potentially facilitating integration with renewable energy sources. However, SMR is easier to run but may lack flexibility compared to ATR, necessitating meticulous management. Capital expenditures for SMR and ATR hydrogen reactors are similar at the lower end of the capacity spectrum, but when plant capacity exceeds this threshold, the capital costs of SMR-based hydrogen production surpass those of ATR-based facilities. The less profitably scaled-up SMR relative to the ATR reactor contributes to the cost disparity. Additionally, individual train capacity constraints for SMR, CO2 removal units, and PSA units increase the expenses of the SMR-based hydrogen facility significantly.
1. Introduction
In the next 30 years, energy transition is expected to emerge as a critical concern due to the imperative of reducing global greenhouse gas emissions. Operators are increasingly driven to seek energy-efficient plant design solutions to reduce energy consumption and environmental pollutants. The prevalent aspiration to mitigate global warming has prompted a focused endeavor to develop efficient techniques for CO2 sequestration and capture. In recent years, extensive research has been undertaken on carbon capture and storage technology. A variety of techniques, including gas-separation membranes, adsorption, absorption, and cryogenic distillation, can be employed to facilitate the capture process. Captured CO2 is typically compressed into a liquid or supercritical state for storage and transit. The deep ocean and geological formations that have contained fluids for millennia serve as prime examples of storage solutions. Biological approaches offer an alternative approach to extracting CO2 from the atmosphere, complementing point-source collection and storage. Biological carbon sequestration approaches encompass the production of biochar, the modification of land management practices to enhance subsurface biomass, and the augmentation of carbon sinks by reforestation [1].
A synthesis of technical and biological strategies may be essential to effectively reduce atmospheric carbon dioxide levels and the related effects of climate change [2,3].
The shift to a sustainable, low-carbon energy system has become a global necessity, and hydrogen has surfaced as a viable method to tackle this issue. Methods for hydrogen production, especially green and blue hydrogen, have attracted considerable interest due to their capacity to diminish greenhouse gas emissions and improve energy security [4,5]. Hydrogen has historically been generated by multiple methods, many of which are environmentally unsustainable. The scientific community is now investigating and developing novel technologies for the decarbonization of hydrogen generation.
The generation of low-carbon hydrogen can be accomplished in multiple ways, each possessing distinct advantages and constraints. Green hydrogen, generated via water electrolysis utilizing renewable energy, is acknowledged as the most environmentally friendly and sustainable option. This method presents considerable potential, as it can enhance energy security by diminishing reliance on fossil fuels and provides a feasible substitute for ecologically harmful hydrogen production techniques [6,7]. Nonetheless, the substantial expenses related to green hydrogen production and the restricted availability of the renewable energy producing capacity have presented considerable obstacles to its extensive adoption.
Conversely, blue hydrogen, generated from natural gas utilizing carbon capture and storage (CCS) technology, is regarded as a more economically feasible alternative. Although blue hydrogen signifies a notable advancement compared to conventional “gray” hydrogen derived from fossil fuels, apprehensions regarding its comprehensive environmental impact persist. The carbon capture and storage (CCS) technology essential for blue hydrogen production is not yet fully developed, and there are doubts regarding the long-term reliability and scalability of CCS systems.
Life cycle assessment studies indicate that the climate change impact of hydrogen production can be minimal when utilizing specific biogenic resources or water electrolysis powered by low-carbon electricity. However, the climate change impact of blue hydrogen remains less clear. Some studies suggest that even with carbon capture and storage, blue hydrogen may still generate considerable carbon dioxide emissions, highlighting the necessity for a shift towards genuinely “green” hydrogen production.
Ultimately, achieving a sustainable hydrogen economy necessitates a multifaceted strategy, involving the ongoing research and development of green hydrogen production technologies, alongside the establishment of suitable policies and financial incentives to address existing obstacles to broader adoption [4].
A significant technological achievement propelling the evolution of blue hydrogen production is pre-combustion technology. This procedure entails the gasification of natural gas, succeeded by the separation and capture of carbon dioxide prior to the combustion of hydrogen [6,7]. Pre-combustion techniques enable more efficient and cost-effective carbon capture by isolating carbon dioxide before combustion, in contrast to post-combustion technologies [7,8].
The extensive implementation of low-carbon hydrogen production encounters numerous obstacles. The production expenses of green and blue hydrogen can be considerably greater than those of hydrogen derived from fossil fuels lacking carbon capture and storage, posing a substantial obstacle to market entry. Moreover, the infrastructure necessary for the storage, transportation, and distribution of hydrogen remains nascent, with numerous projects now in the planning phase rather than the final investment decision stage [8,9,10].
Confronting these difficulties necessitates a multifaceted strategy, encompassing specific legislative measures, financial incentives, and ongoing research and development. Effective regulations, including carbon pricing and renewable energy standards, can provide equitable conditions and encourage the implementation of low-carbon hydrogen production techniques. Moreover, investment in hydrogen infrastructure and technological advancement can decrease production costs and improve the overall feasibility of the hydrogen economy [11,12,13].
The shift to a low-carbon hydrogen future is a difficult undertaking, presenting both benefits and problems that require cautious navigation [7,9]. By harnessing the benefits of green and blue hydrogen and overcoming the technological, economic, and geopolitical challenges, policymakers and industry stakeholders may realize the revolutionary potential of hydrogen and develop a more sustainable and resilient energy system [8,14,15].
2. Research Objectives
The advancement of blue hydrogen technology has garnered considerable interest in recent years as a prospective remedy to alleviate the environmental effects of conventional energy sources. Blue hydrogen is a viable solution for diminishing greenhouse gas emissions while meeting the rising need for clean energy. To enhance the research and application of blue hydrogen, several critical areas require investigation.
A crucial aspect is the enhancement of the hydrogen manufacturing process using innovative technologies and methodologies. This encompasses enhancing the efficiency and cost-effectiveness of carbon capture, storage, and utilization techniques, alongside investigating alternate feedstocks and manufacturing methods to augment the overall sustainability of blue hydrogen.
The scalability and replicability of blue hydrogen technology in many geographies and settings should be a primary priority.
The study domains for blue hydrogen technologies encompass several technological, economic, environmental, utility-related, and operational flexibilities. By focusing on these essential aspects, the capacity of blue hydrogen to aid in the global energy transition can be more comprehensively actualized. In addition this can provide a guide for the selection between SMR and ATR technologies.
3. Blue Hydrogen, CCS Technologies, and Production Methods
The global energy landscape is seeing a significant transformation towards sustainability, with blue hydrogen and carbon capture and storage (CCS) technologies emerging as viable solutions to the issues of energy transition [5,8,16]. Blue hydrogen, generated from natural gas utilizing carbon capture and storage (CCS) technologies, provides an effective means to diminish the carbon footprint of hydrogen generation, an essential advancement in establishing a hydrogen economy [5,8]. On Earth, less than 1% of hydrogen occurs as a stable diatomic molecule (H2), which is the favored form of hydrogen for use as a fuel and energy carrier. The majority of hydrogen exists in a chemically bonded form within water and organic substances, such as hydrocarbons. Table 1 presents the primary physicochemical features of hydrogen fuel in comparison to other fuels. Due to its physical characteristics (i.e., diminutive molecule size, low viscosity, low molecular weight, and high diffusivity), H2 is particularly prone to leakage from transportation and storage systems, presenting an environmental and safety hazard [17].


Table 1.
Principal physicochemical properties of hydrogen fuel in comparison to alternative fuels [5].
Table 1.
Principal physicochemical properties of hydrogen fuel in comparison to alternative fuels [5].
| Property | Hydrogen | Gasoline | Methane |
|---|---|---|---|
| Chemical formula | H2 | C8H18 | CH4 |
| Flammability limits (Φ) | 0.1–7.1 | 0.7–4 | 0.4–1.6 |
| Minimum ignition energy (mJ) | 0.02 | 0.25 | 0.28 |
| Laminar flame speed at NTP (m/s) | 1.90 | 0.37–0.43 | 0.38 |
| Flame velocity (m/s) | 2.65–3.25 | 0.30–0.50 | 0.4–0.6 |
| Quench distance at NTP (mm) | |||
| Density at 1 atm and 300 K (kg/m3) | 29.53 | ||
| Stoichiometric composition in air (% by volume) | 29.53 | ||
| Stoichiometric fuel/air mass ratio | 29.53 | ||
| High heating value (MJ/kg) | 141.7 |
In the context of the energy transition, blue hydrogen has gained significant attention as a potential solution to decarbonize various sectors, such as transportation, industry, and power generation. The review paper by Manish et al. highlights the challenges and opportunities associated with green and blue hydrogen, emphasizing the need for a comprehensive approach to address the technical, economic, and geopolitical implications of a hydrogen-based economy [5]. One of the key advantages of blue hydrogen is its ability to leverage the existing natural gas infrastructure, which can facilitate a more cost-effective and efficient transition towards a low-carbon energy system as outlined in Table 2.
The role of CCS technologies in the production of blue hydrogen is crucial, as it enables the capture and storage of the carbon dioxide emitted during the production process. As outlined in the excerpt from the paper by Bellona, blue hydrogen can significantly reduce the carbon emissions compared to traditional “gray” hydrogen production methods, although it still results in some residual emissions [8,18].
To fully realize the potential of blue hydrogen, however, it is essential to ensure that the carbon capture and storage systems are highly efficient and effective. The review by Manish et al. highlights the need for appropriate policies and financial incentives to support the development and deployment of CCS technologies, as well as the importance of addressing the challenges related to hydrogen transport and storage.
Furthermore, the paper by Leeson et al. provides insights into the comparative environmental impacts of different hydrogen production methods, including gray, blue, and green hydrogen. The analysis suggests that blue hydrogen, while offering significant improvements over gray hydrogen, still has a carbon footprint that necessitates the long-term transition towards “green” hydrogen, which is produced using renewable energy sources and water electrolysis with minimal carbon emissions.
Momentum is gathering with a succession of commitments to hydrogen by various companies and governments. For example, in June 2020, Germany announced a EUR 9 billion hydrogen strategy [19], and the International Energy Agency says that “now is the time to scale up technologies and bring down costs to allow hydrogen to become widely used” [5,16].
Over the last three years, the number of companies in the international Hydrogen Council, which predicts a tenfold increase in hydrogen demand by 2050 [20], has jumped from 13 to 81 and includes oil and gas companies, automobile manufacturers, trading companies, and banks.
In 2018, global hydrogen production was 70 Mt/y [21]. Today’s demand is split between upgrading refined hydrocarbon products and being used as a feedstock in ammonia production for nitrogen fertilizers. Nearly all production comes from fossil fuels; it accounts for 6% of natural gas and 2% of coal consumption. If hydrogen is to contribute to carbon neutrality, it needs to be produced on a much larger scale and with far lower emission levels [22].
In the long term, the answer is likely to be “green” hydrogen, which is produced from the electrolysis of water powered by renewable energy. This supports the integration of renewable electricity generation by decoupling production from use. Hydrogen becomes a convertible currency enabling electrical energy to be stored and used as an emissions-free fuel and chemical feedstock [23]. This is currently expensive, and there is insufficient renewable energy available to support large-scale green hydrogen production. To put the scale of the task into perspective, meeting today’s hydrogen demand through electrolysis would require 3600 TWh of electricity, which exceeds the EU’s annual electricity use [24]. Moreover, using the current EU electricity mix would produce gray hydrogen from electrolysis with 2.2 times the greenhouse gas emissions of producing gray hydrogen from natural gas.
An alternative is blue hydrogen produced from natural gas along with CCUS. Hydrogen production via electrolysis has a similar efficiency to blue hydrogen production, but the levelized cost of production is significantly higher for electrolysis at EUR 66/MWh compared with EUR 47/MWh for SMR–CCUS [24] In addition, it is widely acknowledged that scaling up blue hydrogen production will be easier than delivering green hydrogen. For example, the EU strategy [19] says that “other forms of low carbon hydrogen [i.e., blue] are needed, primarily to rapidly reduce emissions and support the parallel and future uptake of renewable [green] hydrogen”. The strategy goes on to say that neither green nor blue hydrogen production is cost-competitive against gray hydrogen production; the hydrogen costs estimated for the EU are EUR 1.5/kg for gray, EUR 2.0/kg for blue, and up to EUR 5.5/kg for green. With the cost of CO2 at USD 25–35/t, blue hydrogen becomes competitive against gray, albeit the higher capital costs, and green hydrogen may still be more than double the price of blue hydrogen by 2030 as shown in Figure 1 [20,24].
Blue H2 is defined as the type of H2 that is produced from fossil fuels (normally from natural gas) as outlined in Table 2. However, in this case, the resulting CO2 emissions are captured and stored in subsurface formations (e.g., natural gas reservoirs) using CCS techniques, aiming for the maximum elimination of greenhouse gas (GHG) emissions.


Table 2.
CO2 emissions for various types of hydrogen [20].
Table 2.
CO2 emissions for various types of hydrogen [20].
| Type | Primary Source/Process | CO2 Emissions |
|---|---|---|
| Brown H2 | Fossil fuels (coal) | CO2 is released into the environment |
| Gray H2 | Fossil fuels (mostly from natural gas) | CO2 is released into the environment |
| Blue H2 | Fossil fuels (mostly from natural gas) | CCS techniques are applied (minimal CO2 emissions) |
| Green H2 | Water electrolysis using electricity produced from renewable energy | Zero or minimal CO2 emissions |
| Turquoise H2 | Fossil fuels (mostly from natural gas) | Solid carbon production |
| Gold H2 | Biologically produced from residual hydrocarbons using microbes in the subsurface (i.e., depleted oil reservoirs) | Carbon neutral |
| Purple H2 | Water electrolysis based on nuclear energy | Zero or minimal CO2 emissions |
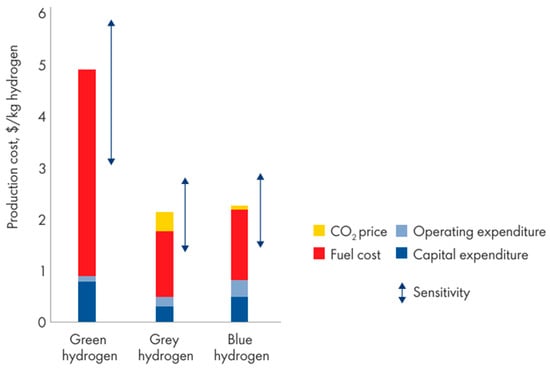
Figure 1.
Hydrogen production expenses in 2030 [20].
In summary, blue hydrogen, in conjunction with carbon capture and storage technologies, signifies a vital advancement in the pursuit of a sustainable energy future. Although it provides a more rapid remedy for decarbonizing several sectors, the ultimate objective should be to shift into green hydrogen, which can genuinely eradicate the environmental consequences of hydrogen generation.
The advancement and implementation of blue hydrogen and carbon capture and storage technologies, bolstered by suitable regulations and financial incentives, can facilitate a more robust and environmentally sustainable energy system.
Blue hydrogen denotes hydrogen generated from natural gas or other fossil fuels using steam methane reforming (SMR) or autothermal reforming (ATR). Blue hydrogen is characterized by the incorporation of carbon capture and storage (CCS) technology to reduce carbon dioxide (CO2) emissions generated during hydrogen production. This is an overview of blue hydrogen generation techniques and carbon capture and storage systems.
4. Methods for Producing Blue Hydrogen
4.1. Steam Methane Reforming (SMR)
SMR is the predominant technique for hydrogen production from natural gas.
The process involves the reaction of natural gas, mostly methane (CH4), with high-temperature steam (about 700–1100 °C) in the presence of a catalyst that typically consists of nickel, resulting in the production of hydrogen (H2) and carbon monoxide (CO) as shown in Figure 2.
CCS integration: Carbon dioxide is extracted from the reformate gas stream with a carbon capture device, typically an amine-based solvent or membrane system.
Steam methane reforming (SMR) is a process that involves heating methane from natural gas with steam, typically in the presence of a catalyst, to generate a mixture of carbon monoxide and hydrogen utilized in organic synthesis and as fuel.
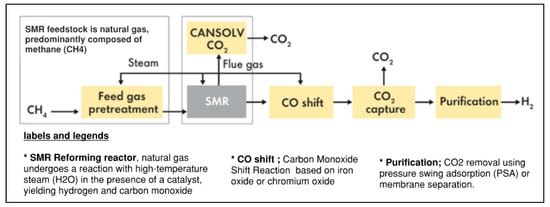
Figure 2.
Comprehensive flow diagram for SMR technology [20].
- Feedstock Preparation:
Natural gas: The principal feedstock for Steam Methane Reforming (SMR) is natural gas, which predominantly consists of methane (CH4). Natural gas is generally extracted from subterranean reserves and conveyed through pipes to steam methane reforming (SMR) plants.
- 2.
- Steam Reforming Reaction:
Reaction chemistry: In the reforming reactor, natural gas undergoes a reaction with high-temperature steam (H2O) in the presence of a catalyst, yielding hydrogen and carbon monoxide as per the following equations: CH4 + H2O CO + 3H2; and CO + H2O CO2 + H2.
- 3.
- Reaction Parameters:
The reforming processes generally transpire at temperatures between 700°C and 1100 °C (1292 °F to 2012 °F), contingent upon the particular catalyst and process configuration.
High pressure, typically ranging from 20 to 40 bar, is sustained within the reforming reactor to promote the equilibrium favoring hydrogen production. The steam-to-carbon ratio (S/C ratio) is meticulously regulated to enhance hydrogen production and reduce the generation of by-products such as carbon dioxide (CO2) and methane.
- ○
- Catalyst: Nickel-based catalysts are commonly used in the reforming reactor to facilitate these reactions. The catalyst enhances the rate of reaction and allows for operation at lower temperatures compared to non-catalytic methods.
- 4.
- Reaction Conditions:
- ○
- Temperature: The reforming reactions typically occur at temperatures ranging from 700 °C to 1100 °C (1292 °F to 2012 °F), depending on the specific catalyst and process design.
- ○
- Pressure: High pressure (usually between 20 and 40 bar) is maintained inside the reforming reactor to favor the equilibrium towards hydrogen production.
- ○
- Steam-to-carbon ratio: The steam-to-carbon ratio (often denoted as S/C ratio) is carefully controlled to optimize hydrogen yield and minimize the formation of by-products like carbon dioxide (CO2) and methane.
- 5.
- Product Gas Composition:
- ○
- Syngas: The primary product of SMR is a mixture of hydrogen (H2) and carbon monoxide (CO) known as syngas. The composition of syngas can vary depending on the operating conditions, but it typically consists of approximately 75–85% hydrogen and 10–15% carbon monoxide.
- 6.
- Carbon Monoxide Shift Reaction:
- ○
- Shift conversion: To further increase the hydrogen content and reduce carbon monoxide levels, the syngas undergoes a water–gas shift (WGS) reaction as follows: CO + H2O→CO2 + H2CO + H_2O\CO_2 + H_2CO + H2O→CO2 + H2
- ○
- Catalyst: A shift catalyst, often based on iron oxide or chromium oxide, facilitates this reaction at lower temperatures (200 °C to 400 °C) compared to the reforming reactor.
- 7.
- Gas Purification:
- ○
- CO2 removal: Any remaining carbon dioxide (CO2) is removed from the shifted gas using various methods such as pressure swing adsorption (PSA) or membrane separation.
- ○
- Final purification: The purified hydrogen gas is then compressed and dried to meet product specifications for purity (typically 99.9% or higher).
- 8.
- Advantages of SMR
- Efficiency: SMR is a mature and efficient technology capable of producing large volumes of hydrogen.
- Economic viability: It benefits from the existing infrastructure and economies of scale, making it cost-effective compared to newer technologies.
- Flexibility: SMR can adjust production rates quickly to meet the fluctuating demand for hydrogen.
- 9.
- Challenges
- Carbon emissions: SMR without carbon capture and storage (CCS) releases CO2, contributing to greenhouse gas emissions.
- Energy intensive: High-temperature operations require significant energy input, impacting overall efficiency.
- Environmental impact: Emissions of pollutants and the energy intensity of the process are significant environmental considerations.
In summary, steam methane reforming (SMR) is a well-established and widely used method for industrial hydrogen production from natural gas. It plays a crucial role in current hydrogen supply chains but faces challenges related to carbon emissions and energy efficiency as the demand for low-carbon hydrogen intensifies.
4.2. Autothermal Reforming (ATR)
ATR integrates partial oxidation and steam reforming within a singular reactor to generate hydrogen.
The process involves the partial combustion of natural gas and oxygen (or air) to generate heat and produce syngas, a mixture of hydrogen, carbon monoxide, and carbon dioxide. Steam is subsequently introduced to the syngas to transform CO into CO2 and produce further hydrogen as shown in Figure 3.
CCS integration: Like SMR, ATR necessitates a CCS unit for the capture of CO2 emissions.
Autothermal reforming (ATR) is a significant technique for generating hydrogen (H2) from natural gas or alternative hydrocarbon feedstocks. In contrast to steam methane reforming (SMR), which depends exclusively on steam for the reforming process, autothermal reforming (ATR) integrates partial oxidation with steam reforming within a single reactor. This combination facilitates efficient heat generation and hydrogen production. Below is a comprehensive overview of the autothermal reforming (ATR) process.
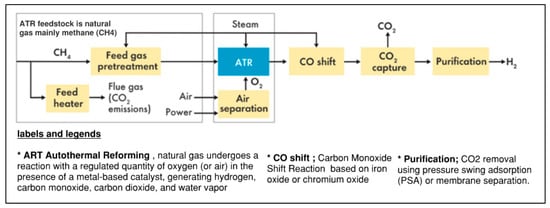
Figure 3.
Comprehensive flow diagram for ATR technology [20].
- Feedstock Preparation:
Natural gas: Like SMR, the principal feedstock for ATR is natural gas, predominantly consisting of methane (CH4). Natural gas is generally pre-heated before entering the reformer to enhance reaction conditions.
- 2.
- Partial Oxidation Reaction:
Reaction chemistry: In the initial phase of autothermal reforming (ATR), natural gas is partially combusted with a regulated quantity of oxygen (or air) in the presence of a catalyst, resulting in the generation of heat and the formation of a gas mixture comprising hydrogen, carbon monoxide, carbon dioxide, and water vapor
- ○
- Catalyst: A transition metal-based catalyst (e.g., nickel or platinum) is often used to promote the partial oxidation reactions at temperatures typically ranging from 800 °C to 1100 °C.
- 3.
- Steam Reforming Reaction:
- ○
- Integration: The heat generated from the partial oxidation reaction provides the energy needed for the subsequent steam reforming reaction.
- ○
- Temperature and pressure: The reforming reactions occur under high temperature (typically 800 °C to 1100 °C) and moderate pressure (usually around 20 to 40 bar) to favor hydrogen production.
- 4.
- Autothermal Operation:
- ○
- Synergistic effects: ATR operates in a self-sustaining manner, where the exothermic partial oxidation reactions provide the necessary heat for the endothermic steam reforming reactions.
- ○
- Efficiency: This integration of partial oxidation and steam reforming results in improved overall process efficiency compared to separate SMR and partial oxidation processes.
- 5.
- Gas Composition:
- ○
- Syngas formation: The primary product of ATR is a syngas mixture consisting of hydrogen (H2), carbon monoxide (CO), carbon dioxide (CO2), and water vapor (H2O). The composition of the syngas depends on the feedstock composition and process conditions.
- 6.
- Gas Cleanup and Purification:
- ○
- CO shift reaction: Similarly to SMR, the syngas undergoes a water–gas shift (WGS) reaction to increase the hydrogen content and reduce carbon monoxide levels.
- ○
- Gas purification: CO2 and other impurities are typically removed using pressure swing adsorption (PSA) or other purification techniques to obtain high-purity hydrogen gas.
- 7.
- Advantages of ATR
- Efficiency: ATR combines partial oxidation and steam reforming in a single reactor, leading to improved overall efficiency and reduced energy consumption compared to separate processes.
- Flexibility: ATR can be adjusted to varying feedstock compositions and operating conditions, making it adaptable for different hydrogen production scenarios.
- Heat Integration: The integration of exothermic and endothermic reactions allows for better heat management and process control.
- 8.
- Challenges Are the Following
- Complexity: ATR processes are more complex to design and operate compared to conventional SMR due to the integration of multiple reactions and the need for precise control of temperature, pressure, and catalyst activity.
- Carbon emissions: Without carbon capture and storage (CCS), ATR still produces CO2 emissions, contributing to greenhouse gas emissions
- Cost: ATR systems may have higher initial capital costs and operational expenses compared to SMR, particularly when considering the integration of partial oxidation and steam reforming.
In summary, autothermal reforming (ATR) is a sophisticated hydrogen production process that integrates partial oxidation and steam reforming to effectively produce hydrogen from natural gas or alternative hydrocarbon feedstocks. The integration of reactions provides benefits in efficiency and flexibility; however, it necessitates meticulous attention.
5. Simulation of Both SMR and ATR
5.1. Methodology
The pre-reforming reactions were considered in light go the following general equation [21].
Cn Hm + nH2O ⇌ nCO + (m2 + n) H2
Additionally, the SR, DSR, and WGSR reactions were considered within the SR [21].
CH4 + H2O ⇌ CO + 3H2
CH4 + 2H2O ⇌ CO2 + 4H2
CO + H2O ⇌ CO2 + H2
The sole reaction addressed in the shift reactors is the water–gas-shift reaction, as illustrated in the above equation. Table 3 below presents the complete list of equations, together with their designated type and reaction set within HYSYS as per simulation flow sheets in Figure 4 and Figure 5.

Table 3.
Simulation basis and assumptions.
- Assumptions Made
- Steady state and isothermal process.
- Ideal gas behavior since the impact will be almost the same for both SMR and ART.
- No heat losses were considered since the ambient conditions will be same for both SMR and ART in the target comparison, leading in minimal impact on the analysis of this study.
- Reverse reactions were not considered, except for WGSR (equilibrium-based).
- Pressure drops were only considered within heaters, coolers, and shift reactors (200 kPa for all).
- Outlet pressure of mixing streams set to be equal to the lowest of the inlet streams.
- Feed stream considered to be completely de-sulfurized.
- All streams with names “0”, ”00”, and ”000” have no molar flow.
- Streams “H2O-1” and “H2O-2” are pure steams, with “H2O-3” being pure liquid water.
- A total pf 5% of hydrogen product becomes adsorbed within PSA (95% H2 recovery).
- PSA can achieve a purity of 99.9+%
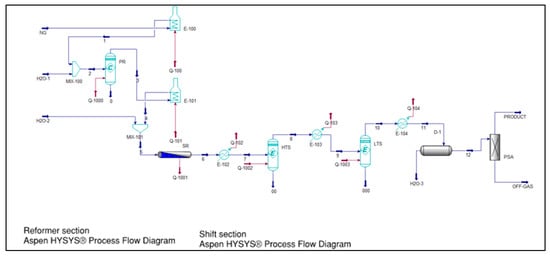
Figure 4.
SMR simulation flow sheet [25].
Natural gas compositions vary widely; therefore, the feed composition must be clearly defined. The feed NG composition was chosen to be representative of raw natural gas from Saudi Arabia. The composition of the feed stream is listed in Table 4.

Table 4.
Composition of natural gas feeds.
The inlet conditions for the natural gas and steam streams are presented in Table 5.

Table 5.
Natural gas feed process conditions.
5.2. Simulation Method
The entire hydrogen production process is designed and simulated using the commercial Aspen Plus software (v 7.2) as specified in Figure 4 and Figure 5, which includes existing features and built-in modules, serving as a valuable tool for chemical engineering process design. The property approach employed by Aspen Plus is PR-BM (Peng-Robinson with Boston-Mathias), which is suitable for methane reforming and yields precise findings for hydrogen production. The solar thermal reactor is conceptualized as a black model for idealization purposes.
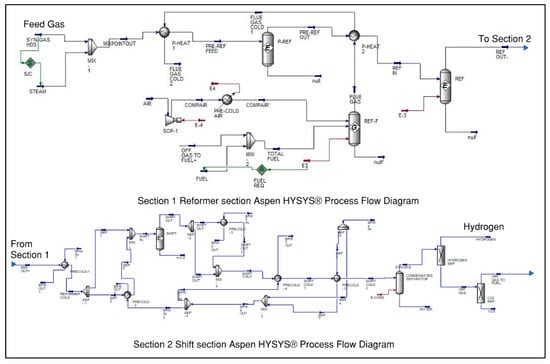

Figure 5.
ATR simulation flow sheet [25].
6. Results and Analysis
The capital expenditures and hydrogen production expenses for a steam methane reforming (SMR) and autothermal reforming (ATR) facility, equipped with carbon capture and storage (CCS), at various hydrogen plant capacities, can be determined using the previously mentioned maximum individual train capabilities. The capital expenditures encompass carbon capture and compression, excluding pipeline and sequestration expenses. Figure 6 and Figure 7 encapsulate this analysis.
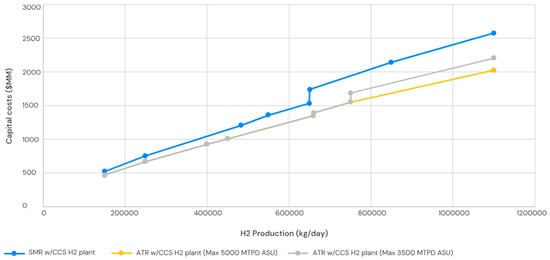
Figure 6.
Capital costs for the H2 plant.
The CAPEX of SMR is highlighted in Figure 6, and the CAPEX is calculated for the main five units as follows:
- Reactor and catalyst system (35% of the total CAPEX).
- Heat recovery system (25% of the total CAPEX).
- Water–gas shift reactor (10% of the total CAPEX).
- CO2 capture unit (if included) (20% of the total CAPEX).
- Pressure swing adsorption (PSA) (10% of the total CAPEX).
The CAPEX of ATR is highlighted in Figure 6, and the CAPEX is calculated for the main five units as follows:
- Reactor and catalyst system (40% of the total CAPEX).
- Oxygen supply system (20% of the total CAPEX).
- Water–gas shift reactor (10% of the total CAPEX).
- CO2 capture unit (if included) (20% of the total CAPEX).
- Pressure swing adsorption (PSA) (10% of the total CAPEX).
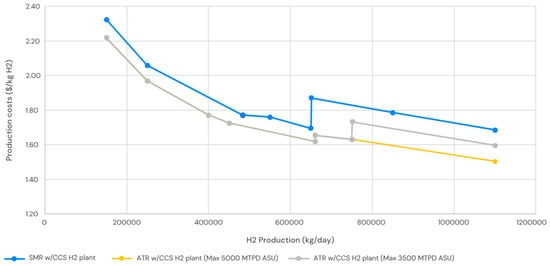
Figure 7.
Operating costs for the H2 plant.
The OPEX of SMR are highlighted in Figure 7; the OPEX are calculated for the main five units as follows:
- Feedstock (70% of the total OPEX).
- Energy system (15% of the total OPEX).
- Catalyst replacement (10% of the total OPEX).
- Maintenance (5% of the total OPEX).
The OPEX of ATR are highlighted in Figure 7; the OPEX are calculated for the main five units as follows:
- Feedstock (65% of the total OPEX).
- Energy system (15% of the total OPEX).
- Catalyst replacement (10% of the total OPEX).
- Maintenance (10% of the total OPEX).
The capital expenditures for SMR and ATR hydrogen reactors are similar at the lower end of the capacity spectrum considered in this study (150,000 kg/day or 62.5 MMSCFD H2). However, when the plant capacity exceeds this basic threshold, the capital costs of SMR-based hydrogen production surpass those of ATR-based facilities. The less profitably scaled-up SMR relative to the ATR reactor contributes to the cost disparity. Furthermore, as the individual train capacity constraints for the SMR, CO2 removal units—including the expensive flue gas CO2 capture unit—and the PSA units are simultaneously attained, the expenses of the SMR-based hydrogen facility escalate significantly at 650,000 kg/d hydrogen (270 MMSCFD). If the ASU’s maximum capacity is restricted to 145,833.3 kg/h O2 (3500 MTPD O2), a supplementary ASU train would be required, resulting in an increase in the ATR-based hydrogen plant’s costs to over 750,000 kg/d H2 (312 MMSCFD H2).
The elevated power costs are outweighed by the reduced capital expenditure of the ATR-based facility and its superior thermal efficiency, leading to a modestly decreased hydrogen production cost for the ATR-based plant at this diminished capacity. Nonetheless, the SMR and ATR hydrogen reactors exhibit comparable production costs at the lower spectrum of the capacity range under consideration (150,000 kg/day or 62.5 MMSCFD H2). The projected energy price of USD 60/MWh, employed in this study, significantly influences the production cost disparity and will fluctuate according to the facility’s location.
Our analysis indicates that this very insignificant cost disparity is mostly sustained until the threshold capacity of 650,000 kg/d H2 (270 MSCFD H2) is reached. At this juncture, the production expenses of the ATR-based hydrogen facility begin to diminish significantly. This is mostly due to the previously noted increase in capital costs for the SMR-based H2 plant at this capacity barrier.
When the maximum capacity of the ASU is restricted to 145,833.3 kg/h O2 (3500 MTPD O2), the production cost of the ATR-based hydrogen facility escalates considerably to around 750,000 kg/d H2 (312 MMSCFD H2). A second ASU train would be required, incurring supplementary capital expenses. Nonetheless, despite the constraint of a reduced ASU maximum individual train capacity, the ATR-based hydrogen plant continues to produce hydrogen at a lower cost than the SMR-based plant at and above this capacity.
Our research indicates that ATR-based hydrogen production facilities typically produce hydrogen at a lower cost than SMR-based facilities, particularly in cases of smaller capacity facilities and/or places with higher energy costs, and Table 6 below summarizes the results of the analysis.

Table 6.
SMR and ATR summary for CAPRX and OPEX.
6.1. Environment
The production of hydrogen has emerged as a vital component of the energy sector, with numerous reforming methods competing for significance. Steam methane reforming and autothermal reforming are two significant techniques, each with distinct benefits and limitations.
Steam methane reformation is a recognized technique that produces synthesis gas with elevated hydrogen concentrations and reduced carbon oxide levels. This method is extremely energy-intensive, necessitating the combustion of natural gas to provide the required energy for the reforming reaction. This supplementary energy usage results in heightened carbon dioxide emissions, which constitute a major environmental issue.
Conversely, autothermal reforming is a contemporary technology designed to mitigate the energy efficiency and emission limitations of steam methane reforming. This process employs the heat produced by the exothermic partial oxidation reaction to facilitate the endothermic steam reforming reaction, hence diminishing the requirement for external energy and perhaps decreasing overall carbon dioxide emissions.
Thermodynamic research indicates that dry reforming, a form of autothermal reforming, is especially advantageous for the use of biogas, which generally comprises elevated levels of carbon dioxide. By integrating this greenhouse gas into the reforming process, dry reforming may diminish net carbon dioxide emissions relative to steam methane reforming.
Although steam methane reforming and autothermal reforming possess distinct advantages, a comparative comparison of their CO2 emissions underscores the potential benefits of autothermal reforming, especially regarding sustainable hydrogen production.
- SMR: Generally releases approximately 9000–10,000 kg of CO2 for each 1000 kg of hydrogen generated, accounting for both direct and indirect emissions from the procedure.
- ATR: Typically produces lower CO2 emissions per unit of hydrogen than SMR, owing to superior energy efficiency and the possibility of combination with CO2 capture technologies. The precise decrease in CO2 emissions may fluctuate but is frequently substantial in comparison to SMR.
- Table 7 below shows that carbon capture can add significant costs to both SMR and ATR.
 Table 7. SMR and ATR summary for CCUS impact on cost.
Table 7. SMR and ATR summary for CCUS impact on cost.
The reduction in CO2 emissions in ATR is influenced by the following factors:
- Energy efficiency: The autothermal characteristics of ATR enhance the energy efficiency of methane, resulting in reduced CO2 emissions per hydrogen unit produced.
- Process integration: ATR may combine CO2 capture and utilization technologies more efficiently than SMR because of its syngas composition and process attributes.
In conclusion, whereas both SMR and ATR are techniques used for generating hydrogen from methane, ATR generally provides benefits in terms of reduced CO2 emissions per hydrogen unit generated, owing to its superior energy efficiency and process attributes. The incorporation of CO2 collection technologies significantly amplifies ATR’s capability to diminish greenhouse gas emissions relative to conventional SMR methods.
6.2. Table 7
- SMR: SMR plants often necessitate external energy sources, such as natural gas or electricity, to supply the heat required for the reforming reaction. This may render SMR less adaptable regarding energy sources and perhaps lead to increased operational expenses based on energy prices.
- ATR: ATR is autothermal, signifying that it produces its own heat via the partial oxidation process. This diminishes dependence on external energy sources and augments the process’s utility and adaptability. ATR can potentially facilitate integration with renewable energy sources or waste heat recovery systems, thereby enhancing its applicability across diverse energy contexts.
6.3. Operational Flexibility
- SMR: SMR units are well-established and provide relatively uncomplicated operation and control. Nonetheless, they may exhibit constraints in their ability to adjust to fluctuating feedstocks or altering operational conditions without compromising performance or catalyst longevity.
- ATR: ATR is more complex to operate than SMR because of the dual nature of the process, which involves both partial oxidation and steam reforming. The effective management of oxygen supply, temperature regulation, and syngas composition is essential for optimal operation. Nonetheless, ATR’s capacity for self-generated heat and its potential for enhanced energy efficiency can offer operating flexibility and adaptability to fluctuating feedstock conditions.
7. Conclusions
The selection between SMR and ATR is contingent upon the specific project specifications, encompassing economic factors, environmental objectives (such as the mitigation of CO2 emissions), and the accessibility of energy resources and infrastructure.
Our research indicates that ATR-based hydrogen production facilities typically produce hydrogen at a lower cost than SMR-based facilities, particularly in cases of smaller capacity facilities and/or places with higher energy costs.
Both SMR and ATR are technologies used for hydrogen production from methane; however, ATR generally provides benefits in terms of reduced CO2 emissions per hydrogen unit produced, attributable to its superior energy efficiency and process attributes. The incorporation of CO2 collection technologies significantly amplifies ATR’s capability to diminish greenhouse gas emissions relative to conventional SMR methods.
Evaluating steam methane reforming (SMR) and autothermal reforming (ATR) in terms of cost, CO2 emissions, utility, and operational flexibility, the following conclusions can be drawn:
- Cost
SMR generally incurs reduced initial capital expenditures, although ATR may provide diminished operational expenses in the long term, owing to enhanced energy efficiency.
- 2.
- CO2 Emissions
ATR typically generates lower CO2 emissions per unit of hydrogen produced in comparison to SMR.
- 3.
- Utility
ATR provides enhanced utility and flexibility regarding energy sources due to its autothermal characteristics, potentially facilitating integration with renewable energy sources.
- 4.
- Operational Flexibility
SMR is easier to run but may lack flexibility compared to ATR, which necessitates meticulous management yet has advantages in self-sufficiency and the possibility for enhanced efficiency.
The choice between SMR and ATR hydrogen reactors depends on project specifics, infrastructure, energy availability, environmental goals, and economic considerations. ATR-based facilities typically generate hydrogen at a lower cost, but SMR incurs reduced initial capital expenditures. ATR offers lower CO2 emissions per unit of hydrogen and may provide diminished operational expenses in the long term due to enhanced energy efficiency. More studies for integrated technology FA between SMR and ATR are warranted in the future.
Author Contributions
Conceptualization: W.E. and F.K.G. identified the research goals and targets considering the hydrogen market challenges. Methodology: W.E., F.K.G. and M.S. broke the plan down into steps and identified the methodology of the research. Software: W.E. conducted a simulation for all cases based on the different technologies used for hydrogen production; M.A.N. reviewed the simulation models. Validation: W.E. and F.K.G. identified and validated the simulation and the calculation related to hydrogen technologies. Formal analysis: W.E., F.K.G. and M.S. conducted a thorough review for the entire analysis; M.A.N. reviewed the analysis. Investigation: W.E. thoroughly benchmarked the simulation with real market conditions. Resources: Computer resources were provided by all authors. Visualization: W.E. prepared all photos and conducted the graphic analysis. Writing—original draft: W.E. prepared the original draft and shared it with F.K.G. for review and comments. Writing—review and editing: F.K.G. and M.S. reviewed and commented on the manuscript. Draft write-up for the paper: W.E. prepared the final version of the paper, accounting for all the comments. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
Author Waleed Elhefnawy was employed by the company Process Department Manger, KBR, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Klai, N.; John, R.P.; Song, Y.; Tyagi, R.D.; Surampalli, R.Y.; Zhang, T.C. Carbon Capture and Sequestration: Biological Technologies. In Carbon Capture and Storage: Physical, Chemical, and Biological Methods; American Society of Civil Engineers: Reston, VA, USA, 2015; pp. 65–111. [Google Scholar] [CrossRef]
- Benson, S.M.; Surles, T. Carbon Dioxide Capture and Storage: An Overview with Emphasis on Capture and Storage in Deep Geological Formations. Proc. IEEE 2006, 94, 1795–1805. [Google Scholar] [CrossRef]
- Pires, J.C.; Alvim-Ferraz, M.; Martins, F.; Simões, M. Carbon dioxide capture from flue gases using microalgae: Engineering aspects and biorefinery concept. Renew. Sustain. Energy Rev. 2012, 16, 3043–3053. [Google Scholar] [CrossRef]
- Maganza, A.; Gabetti, A.; Pastorino, P.; Zanoli, A.; Sicuro, B.; Barceló; Cesarani, A.; Dondo, A.; Prearo, M.; Esposito, G. Toward Sustainability: An Overview of the Use of Green Hydrogen in the Agriculture and Livestock Sector. Animals 2023, 13, 2561. [Google Scholar] [CrossRef] [PubMed]
- Noussan, M.; Raimondi, P.P.; Scita, R.; Häfner, M. The Role of Green and Blue Hydrogen in the Energy Transition—A Technological and Geopolitical Perspective. Sustainability 2020, 13, 298. [Google Scholar] [CrossRef]
- Vidas, L.; Castro, R. Recent Developments on Hydrogen Production Technologies: State-of-the-Art Review with a Focus on Green-Electrolysis. Appl. Sci. 2021, 11, 11363. [Google Scholar] [CrossRef]
- Bauer, C.; Treyer, K.; Antonini, C.; Bergerson, J.; Gazzani, M.; Gençer, E.; Gibbins, J.; Mazzotti, M.; McCoy, S.; McKenna, R.; et al. On the climate impacts of blue hydrogen production. Sustain. Energy Fuels 2022, 6, 66–75. [Google Scholar] [CrossRef]
- Ingersoll, J.G. The Renewable Hydrogen–Methane (RHYME) Transportation Fuel: A Practical First Step in the Realization of the Hydrogen Economy. Hydrogen 2022, 3, 84–111. [Google Scholar] [CrossRef]
- Dash, S.K.; Chakraborty, S.; Elangovan, D. A Brief Review of Hydrogen Production Methods and Their Challenges. Energies 2023, 16, 1141. [Google Scholar] [CrossRef]
- OECD. Green Hydrogen Opportunities for Emerging and Developing Economies; OECD: Paris, France, 2022. [Google Scholar] [CrossRef]
- Jovana, D.; Svetlana, S. Review on Compressed Hydrogen as Contemporary Renewable Energy Resource. Univers. J. Manag. 2017, 5, 313–319. [Google Scholar] [CrossRef]
- Martino, M.; Ruocco, C.; Meloni, E.; Pullumbi, P.; Palma, V. Main Hydrogen Production Processes: An Overview. Catalysts 2021, 11, 547. [Google Scholar] [CrossRef]
- Yadav, V.; Vinoth, R.; Yadav, D. Bio-hydrogen production from waste materials: A review. MATEC Web Conf. 2018, 192, 02020. [Google Scholar] [CrossRef]
- Diewvilai, R.; Audomvongseree, K. Possible Pathways toward Carbon Neutrality in Thailand’s Electricity Sector by 2050 through the Introduction of H2 Blending in Natural Gas and Solar PV with BESS. Energies 2022, 15, 3979. [Google Scholar] [CrossRef]
- Dissanayake, P.D.; Sonne, C.; Cho, W.; Kim, C.H.; Ok, Y.S. South Korea’s big move to hydrogen society. Cogent Environ. Sci. 2020, 6, 1856459. [Google Scholar] [CrossRef]
- Lee, W.S.; Kim, Y.; Shinn, Y.J.; Wang, J.; Moon, B.S.; Park, H.; Chang, S.; Kwon, O. Role of Blue Hydrogen for Developing National Hydrogen Supply Infrastructure. J. Korean Soc. Miner. Energy Resour. Eng. 2021, 58, 503–520. [Google Scholar] [CrossRef]
- A Hydrogen Strategy for a Climate-Neutral Europe, European Commission. 2020. Available online: www.ec.europa.eu/energy/sites/ener/files/hydrogen_strategy.pdf (accessed on 20 April 2025).
- Lipman, T.; Shah, N. Ammonia as an Alternative Energy Storage Medium for Hydrogen Fuel Cells: Scientific and Technical Review for Near-Term Stationary Power Demonstration Projects, Final Report. Federal Reserve Bank of St. Louis. 2007. Available online: https://tsrc.berkeley.edu/publications/ammonia-alternative-energy-storage-medium-hydrogen-fuel-cells-scientific-and-technical (accessed on 20 April 2025).
- Ger. Gover. 2020. Available online: https://www.bundesregierung.de/bregen/service/archive/wasserstoffstrategie-kabinett-1758982 (accessed on 20 April 2025).
- Hydrogen Scaling Up. A Sustainable Pathway for the Global Energy Transition, Hydrogen Council. 2017. Available online: https://www.france-hydrogene.org/publication/hydrogen-scaling-up-a-sustainable-pathway-for-the-global-energy-transition/ (accessed on 20 April 2025).
- Available online: https://www.iea.org/energy-system/low-emission-fuels/hydrogen (accessed on 20 April 2025).
- Sadeghi, S.; Ghandehariun, S.; Rosen, M.A. Comparative economic and life cycle assessment of solar-based hydrogen production for oil and gas industries. Energy 2020, 208, 118347. [Google Scholar] [CrossRef]
- Saqlain, M. Sustainable Hydrogen Production: A Decision-Making Approach Using VIKOR and Intuitionistic Hypersoft Sets. J. Intell. Manag. Decis. 2023, 2, 130–138. [Google Scholar] [CrossRef]
- The Future of Hydrogen, International Energy Agency. 2019. Available online: https://www.iea.org/reports/the-future-of-hydrogen (accessed on 20 April 2025).
- Hydrogen from Natural Gas—The Key to Deep Decarbonisation, Pöyry Management Consulting. 2020. Available online: www.poyry.com/sites/default/files/zukunft_erdgas_key_to_deep_decarbonisation_0.pdf (accessed on 20 April 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).