Towards Circularity and Sustainability: Phytoremediation Approaches, Legislative Challenges, and Bioenergy Potential in South African Mine Tailings Remediation
Abstract
1. Introduction
2. Methodology
3. Mine Tailings Management in South Africa
3.1. Evolution of the Legal Frameworks Governing Mine Tailings Management in South Africa
3.2. Re-Mining of Mine Tailings
3.3. Rehabilitation/Remediation/Revegetation of TSF
4. Solving the Conundrum: Achieving Sustainability Goals—Opportunities and Barriers
4.1. Inflexible Legal Framework
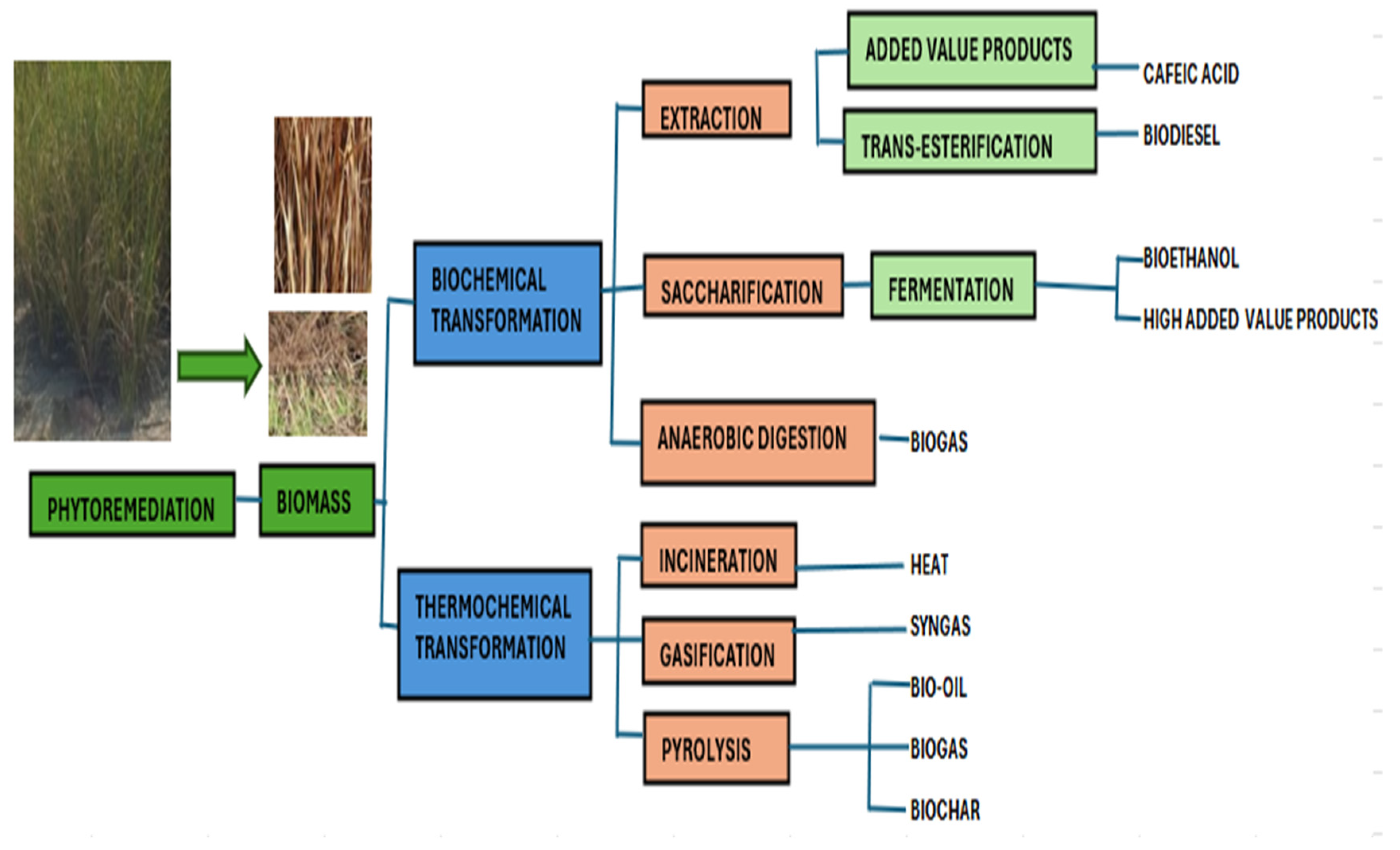
4.2. TSF as a Resource for Bioenergy Production
Vetiver Grass: A Multi-Faceted Solution
5. Theoretical Contributions, Practical Contributions and Limitations of the Study
6. Conclusions and Recommendations
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Festin, E.S.; Tigabu, M.; Chileshe, M.N.; Syampungani, S.; Odén, P.C. Progresses in restoration of post-mining landscape in Africa. J. For. Res. 2019, 30, 381–396. [Google Scholar] [CrossRef]
- Edraki, M.; Baumgartl, T.; Manlapig, E.; Bradshaw, D.; Franks, D.M.; Moran, C.J. Designing mine tailings for better environmental, social and economic outcomes: A review of alternative approaches. J. Clean. Prod. 2014, 84, 411–420. [Google Scholar] [CrossRef]
- Sonter, L.J.; Dade, M.C.; Watson, J.E.; Valenta, R.K. Renewable energy production will exacerbate mining threats to biodiversity. Nat. Commun. 2020, 11, 4174. [Google Scholar] [CrossRef] [PubMed]
- Gil-Loaiza, J.; White, S.A.; Root, R.A.; Solís-Dominguez, F.A.; Hammond, C.M.; Chorover, J.; Maier, R.M. Phytostabilization of mine tailings using compost-assisted direct planting: Translating greenhouse results to the field. Sci. Total Environ. 2016, 565, 451–461. [Google Scholar] [CrossRef] [PubMed]
- European Environmental Bureau (EEB). The Environmental Performance of the Mining Industry and the Action Necessary to Strengthen European Legislation in the Wake of the Tisza-Danube Pollution; EEB Document No 2000/016; European Environmental Bureau (EEB): Brussels, Belgium, 2000. [Google Scholar]
- Marin, O.A.; Kraslawski, A.; Cisternas, L.A. Estimating processing cost for the recovery of valuable elements from mine tailings using dimensional analysis. Miner. Eng. 2022, 184, 107629. [Google Scholar] [CrossRef]
- Adiansyah, J.S.; Rosano, M.; Vink, S.; Keir, G. A framework for a sustainable approach to mine tailings management: Disposal strategies. J. Clean. Prod. 2015, 108, 1050–1062. [Google Scholar] [CrossRef]
- Tang, L.; Liu, X.; Wang, X.; Liu, S.; Deng, H. Statistical analysis of tailings ponds in China. J. Geochem. Explor. 2020, 216, 106579. [Google Scholar] [CrossRef]
- Wang, L.; Ji, B.; Hu, Y.; Liu, R.; Sun, W. A review on in situ phytoremediation of mine tailings. Chemosphere 2017, 184, 594–600. [Google Scholar] [CrossRef]
- Werner, T.T.; Bach, P.M.; Yellishetty, M.; Amirpoorsaeed, F.; Walsh, S.; Miller, A.; Roach, M.; Schnapp, A.; Solly, P.; Tan, Y.; et al. A geospatial database for effective mine rehabilitation in Australia. Minerals 2020, 10, 745. [Google Scholar] [CrossRef]
- Lottermoser, B.G. Tailings. In Mine Wastes: Characterization, Treatment and Environmental Impacts; Springer: Berlin/Heidelberg, Germany, 2010; pp. 205–241. [Google Scholar]
- Sikaundi, G. The Copper Mining Industry in Zambia: Environmental Challenges. 2013. Available online: https://unstats.un.org/unsd/environment/envpdf/UNSD_UNEP_ECA%20Workshop/Session%2008-5%20Mining%20in%20Zambia%20(Zambia).pdf (accessed on 11 September 2025).
- Department of Environment and Tourism. South Africa Environment Outlook. A Report on the State of Environment; Department of Environmental affairs and Tourism: Pretoria, South Africa, 2008. [Google Scholar]
- Venkateswarlu, K.; Nirola, R.; Kuppusamy, S.; Thavamani, P.; Naidu, R.; Megharaj, M. Abandoned metalliferous mines: Ecological impacts and potential approaches for reclamation. Rev. Environ. Sci. Bio/Technol. 2016, 15, 327–354. [Google Scholar] [CrossRef]
- Pappu, A.; Saxena, M.; Asolekar, S.R. Solid wastes generation in India and their recycling potential in building materials. Build. Environ. 2007, 42, 2311–2320. [Google Scholar] [CrossRef]
- Pancaldi, F.; Trindade, L.M. Marginal lands to grow novel bio-based crops: A plant breeding perspective. Front. Plant Sci. 2020, 11, 227. [Google Scholar] [CrossRef]
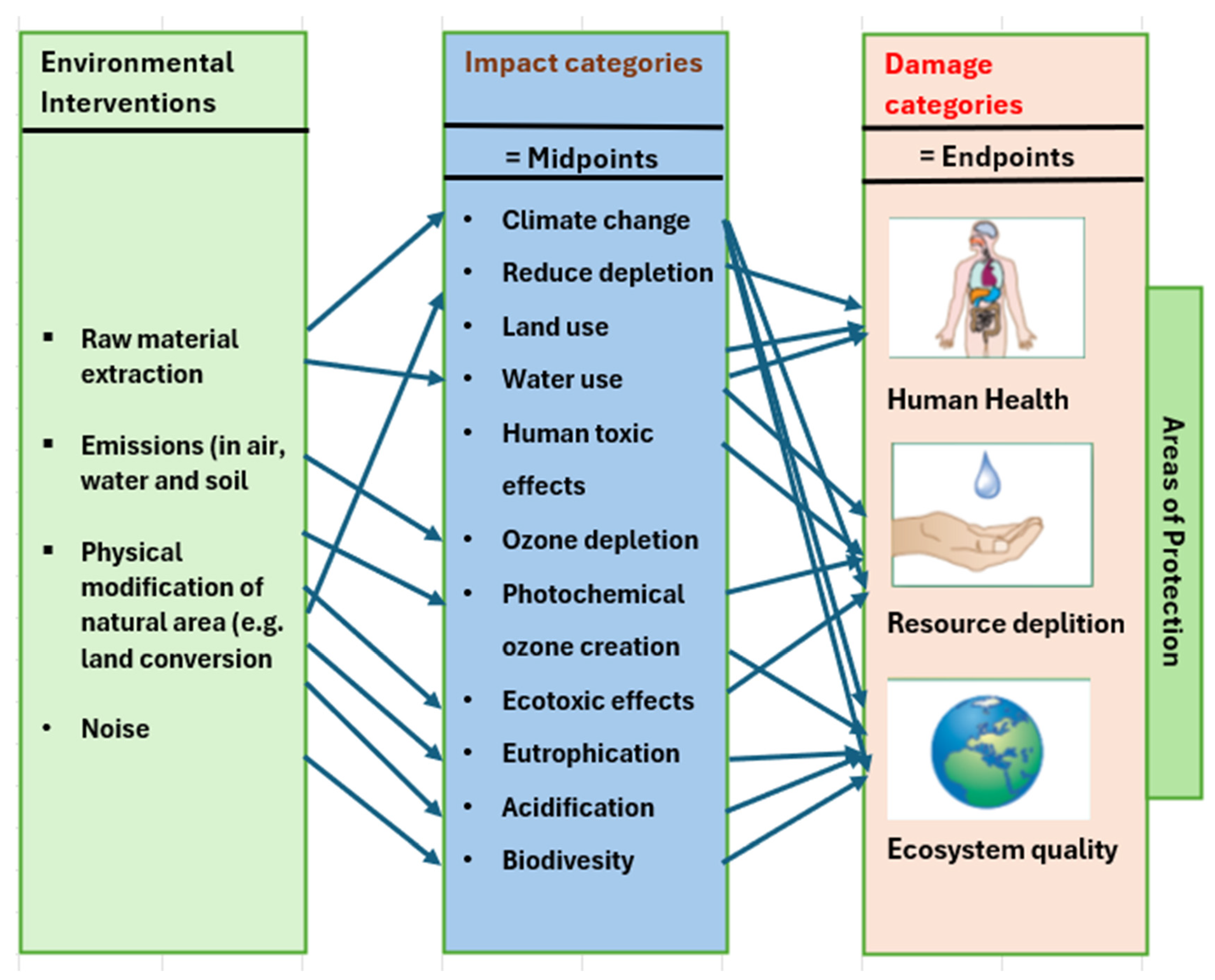
- Jolliet, O.; Brent, A.; Goedkoop, M.; Itsubo, N.; Mueller-Wenk, R.; Peña, C.; Schenk, R.; Stewart, M.; Weidema, B.; Bare, J.; et al. Final Report of the LCIA Definition Study, Life Cycle Impact Assessment Programme of the Life Cycle Initiative; HSG Publications: Hong Kong, China, 2003. [Google Scholar]
- Schandl, H.; Fischer-Kowalski, M.; West, J.; Giljum, S.; Dittrich, M.; Eisenmenger, N.; Geschke, A.; Lieber, M.; Wieland, H.; Schaffartzik, A.; et al. Global material flows and resource productivity: Forty years of evidence. J. Ind. Ecol. 2018, 22, 827–838. [Google Scholar] [CrossRef]
- Schaffartzik, A.; Mayer, A.; Gingrich, S.; Eisenmenger, N.; Loy, C.; Krausmann, F. The global metabolic transition: Regional patterns and trends of global material flows, 1950–2010. Glob. Environ. Change 2014, 26, 87–97. [Google Scholar] [CrossRef]
- de Araújo, S.N.; Ramos, S.J.; Martins, G.C.; Teixeira, R.A.; de Souza, E.S.; Sahoo, P.K.; Fernandes, A.R.; Gastauer, M.; Caldeira, C.F.; Souza-Filho, P.W.M.; et al. Copper mining in the eastern Amazon: An environmental perspective on potentially toxic elements. Environ. Geochem. Health 2022, 44, 1767–1781. [Google Scholar] [CrossRef]
- World Resources Institute. African Countries Launch AFR100 to Restore 100 Million Hectares of Land. 2016. Available online: http://www.wri.org/news/2015/12/release-african-countries-launch-afr100-restore-100-million-hectares-land (accessed on 16 September 2025).
- Maest, A.S. Remining for renewable energy metals: A review of characterization needs, resource estimates, and potential environmental effects. Minerals 2023, 13, 1454. [Google Scholar] [CrossRef]
- Navarro-Ramos, S.E.; Sparacino, J.; Rodríguez, J.M.; Filippini, E.; Marsal-Castillo, B.E.; García-Cannata, L.; Renison, D.; Torres, R.C. Active revegetation after mining: What is the contribution of peer-reviewed studies? Heliyon 2022, 8, e09179. [Google Scholar] [CrossRef]
- Sekhohola-Dlamini, L.M.; Keshinro, O.M.; Masudi, W.L.; Cowan, A.K. Elaboration of a phytoremediation strategy for successful and sustainable rehabilitation of disturbed and degraded land. Minerals 2022, 12, 111. [Google Scholar] [CrossRef]
- Haigh, M.J. Land rehabilitation. In Land Use, Land Cover and Soil Sciences; EOLSS-Encyclopedia of Life Support Systems Section 24, Act 108 of 1996; UNESCO: Paris, France, 2007. [Google Scholar]
- Munnik, V. The Social and Environmental Consequence of Coal Mining in South Africa: A Case Study; Environmental Monitoring Group: Cape Town, South Africa, 2010. [Google Scholar]
- Laker, M.C. Environmental impacts of gold mining—With special reference to South Africa. Mining 2023, 3, 205–220. [Google Scholar] [CrossRef]
- Swart, E. The South African legislative framework for mine closure. J. South. Afr. Inst. Min. Metall. 2003, 103, 489–492. [Google Scholar]
- Yıldız, T.D.; Güner, M.O.; Kural, O. The effects of the mineral waste regulation in Turkey on the mining sector. In Proceedings of the 25th International Mining Congress and Exhibition, Antalya, Turkey, 11–14 April 2017; pp. 457–472. [Google Scholar]
- Payne, E.G.I.; Hatt, B.E.; Deletic, A.; Dobbie, M.F.; McCarthy, D.T.; Chandrasena, G.I. Adoption Guidelines for Stormwater Biofiltration Systems—Summary Report; Cooperative Research Centre for Water Sensitive Cities: Melbourne, Australia, 2015. [Google Scholar]
- Schoeman, J.L.; van Deventer, P. Soils and the environment: The past 25 years. South Afr. J. Plant Soil 2004, 21, 369–387. [Google Scholar] [CrossRef]
- Hattingh, R.P.; Lake, J.; Boer, R.H.; Aucamp, P.; Viljoen, C. Rehabilitation of Contaminated Gold Tailings Dam Footprints; Report, (1001/1), 03; Water Research Commission: Pretoria, South Africa, 2003. [Google Scholar]
- Rösner, T. The Environmental Impact of Seepage from Gold Mine Tailings Dams Near Johannesburg; University of Pretoria: Pretoria, South Africa, 1999. [Google Scholar]
- Carbutt, C.; Kirkman, K. Ecological grassland restoration—A South African perspective. Land 2022, 11, 575. [Google Scholar] [CrossRef]
- Coaltech Guidelines for the Rehabilitation of Mined Lands. 2007. Available online: https://coaltech.co.za/wp-content/uploads/2019/10/Task-12.1-Guideline-for-the-Rehabilitation-of-Mined-Land-2007.pdf (accessed on 23 July 2025).
- Rethman, N. Approaches to biodiversity on rehabilitated minelands in South Africa. Trop. Grassl. 2000, 34, 251–253. [Google Scholar]
- Thatcher, F.M. A Study of the Vegetation Established on the Slimes Dams of the Witwatersrand. Ph.D. Thesis, University of the Witwatersrand, Johannesburg, South Africa, 1979; pp. 89–169, unpublished. [Google Scholar]
- Weiersbye, I.M.; Witkowski, E.T.F.; Reichardt, M. Floristic composition of gold and uranium tailings dams, and adjacent polluted areas, on South Africa’s deep-level mines. Bothalia 2006, 36, 101–127. [Google Scholar] [CrossRef]
- Mendez, M.O.; Maier, R.M. Phytostabilization of mine tailings in arid and semiarid environments—An emerging remediation technology. Environ. Health Perspect. 2008, 116, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Akinpelu, E.A.; Nchu, F. Advancements in Phytoremediation Research in South Africa (1997–2022). Appl. Sci. 2024, 14, 7660. [Google Scholar] [CrossRef]
- Schachtschneider, K.; Chamier, J.; Somerset, V. Phytostabilization of metals by indigenous riparian vegetation. Water SA 2017, 43, 177–185. [Google Scholar] [CrossRef]
- El-Mahrouk, E.S.M.; Eisa, E.A.H.; Hegazi, M.A.; Abdel-Gayed, M.E.S.; Dewir, Y.H.; El-Mahrouk, M.E.; Naidoo, Y. Phytoremediation of cadmium-, copper-, and lead-contaminated soil by Salix mucronata (Synonym Salix safsaf). HortScience 2019, 54, 1249–1257. [Google Scholar] [CrossRef]
- Banda, M.F.; Mokgalaka, N.S.; Combrinck, S.; Regnier, T. Five-weeks pot trial evaluation of phytoremediation potential of Helichrysum splendidum Less. for copper-and lead-contaminated soils. Int. J. Environ. Sci. Technol. 2022, 19, 1837–1848. [Google Scholar] [CrossRef]
- Liphadzi, M.S.; Kirkham, M.B.; Paulsen, G.M. Auxin-enhanced root growth for phytoremediation of sewage-sludge amended soil. Environ. Technol. 2006, 27, 695–704. [Google Scholar] [CrossRef]
- Liphadzi, M.S.; Kirkham, M.B. Availability and plant uptake of heavy metals in EDTA-assisted phytoremediation of soil and composted biosolids. South Afr. J. Bot. 2006, 72, 391–397. [Google Scholar] [CrossRef]
- Badejo, A.A.; Sridhar, M.K.; Coker, A.O.; Ndambuki, J.M.; Kupolati, W.K. Phytoremediation of water using Phragmites karka and Veteveria nigritana in constructed wetland. Int. J. Phytoremediat. 2015, 17, 847–852. [Google Scholar] [CrossRef]
- Masinire, F.; Adenuga, D.O.; Tichapondwa, S.M.; Chirwa, E.M. Phytoremediation of Cr (VI) in wastewater using the vetiver grass (Chrysopogon zizanioides). Miner. Eng. 2021, 172, 107141. [Google Scholar] [CrossRef]
- Robinson, B.H.; Brooks, R.R.; Howes, A.W.; Kirkman, J.H.; Gregg, P.E.H. The potential of the high-biomass nickel hyperaccumulator Berkheya coddii for phytoremediation and phytomining. J. Geochem. Explor. 1997, 60, 115–126. [Google Scholar] [CrossRef]
- Abed, S.N.; Almuktar, S.A.; Scholz, M. Phytoremediation performance of floating treatment wetlands with pelletized mine water sludge for synthetic greywater treatment. J. Environ. Health Sci. Eng. 2019, 17, 581–608. [Google Scholar] [CrossRef]
- Akinbile, B.J.; Matsinha, L.C.; Ambushe, A.A.; Makhubela, B.C. Catalytic conversion of CO2 to formate promoted by a biochar-supported nickel catalyst sourced from nickel phytoextraction using cyanogen-rich cassava. ACS Earth Space Chem. 2021, 5, 2846–2854. [Google Scholar] [CrossRef]
- Ndlovu, S.; Pullabhotla, R.V.; Ntuli, N.R. Agro-morphological changes caused by the accumulation of lead in Corchorus olitorius, a leafy vegetable with phytoremediation properties. J. Appl. Bot. Food Qual. 2019, 92, 371–377. [Google Scholar]
- Okem, A.; Kulkarni, M.G.; Van Staden, J. Enhancing phytoremediation potential of Pennisetum clandestinum Hochst in cadmium-contaminated soil using smoke-water and smoke-isolated karrikinolide. Int. J. Phytoremediat. 2015, 17, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Atagana, H.I. Bioremediation of co-contamination of crude oil and heavy metals in soil by phytoremediation using Chromolaena odorata (L) King & HE Robinson. Water Air Soil Pollut. 2011, 215, 261–271. [Google Scholar]
- Silambarasan, S.; Logeswari, P.; Valentine, A.; Cornejo, P.; Kannan, V.R. Pseudomonas citronellolis strain SLP6 enhances the phytoremediation efficiency of Helianthus annuus in copper contaminated soils under salinity stress. Plant Soil 2020, 457, 241–253. [Google Scholar] [CrossRef]
- Cooke, J.A.; Johnson, M.S. Ecological restoration of land with particular reference to the mining of metals and industrial minerals: A review of theory and practice. Environ. Rev. 2002, 10, 41–71. [Google Scholar] [CrossRef]
- Koch, J. The Alteration of Mine Tailings through Chemical, Physical and Biological Amelioration Aimed at Improving Soil Aggregation. Ph.D. Thesis, North-West University, Potchefstroom, South Africa, 2022; 379p. [Google Scholar]
- Washaya, S.; Mupangwa, J.; Muchenje, V. Effects of supplementing a basal diet of Chloris gayana hay with protein-rich forage legume hays on chevon quality of Xhosa goats. Anim. Feed Sci. Technol. 2025, 321, 116255. [Google Scholar] [CrossRef]
- Negawo, A.T.; Muktar, M.S.; Assefa, Y.; Hanson, J.; Sartie, A.M.; Habte, E.; Jones, C.S. Genetic diversity and population structure of a Rhodes grass (Chloris gayana) collection. Genes 2021, 12, 1233. [Google Scholar] [CrossRef]
- van Coller, C.; do Amaral Filho, J.R.; Smart, M.; Harrison, S.T. Bioaugmentated Technosols as a Nature-Based Strategy for Mine-Site Rehabilitation. In Conference of Metallurgists; Springer Nature: Cham, Switzerland, 2024; pp. 1165–1170. [Google Scholar] [CrossRef]
- Marshall, V.M.; Lewis, M.M.; Ostendorf, B. Buffel grass (Cenchrus ciliaris) as an invader and threat to biodiversity in arid environments: A review. J. Arid Environ. 2012, 78, 1–12. [Google Scholar] [CrossRef]
- Mohammed, R.; Al-Gburi, H.F.; Alotaibi, M.F.; Almuqati, N.S.; Alsufyani, S.J.; Almoiqli, M.S.; Albarq, M.M.; Alharbi, K.N. Bioaccumulation and translocation of radionuclides heavy metals in Cynodon dactylon: A phytoremediation approach in Al-Dora refinery. J. Radiat. Res. Appl. Sci. 2024, 17, 100953. [Google Scholar] [CrossRef]
- Lion, G.N.; Olowoyo, J.O.; Modise, T.A. Trace metals bioaccumulation potentials of three indigenous grasses grown on polluted soils collected around mining areas in Pretoria, South Africa. West Afr. J. Appl. Ecol. 2016, 24, 43–51. [Google Scholar]
- Okereafor, G.U.; Makhatha, M.E.; Mekuto, L.; Mavumengwana, V. Assessing the effectiveness of Hyparrhenia hirta in the rehabilitation of the ecosystem of a gold mine dump. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2020; Volume 158, p. 04004. [Google Scholar] [CrossRef]
- Vurayai, R.; Nkoane, B.; Moseki, B.; Chaturvedi, P. Phytoremediation potential of Jatropha curcas and Pennisetum clandestinum grown in polluted soil with and without coal fly ash: Selibe-Phikwe, Botswana. Botswana. J. Biodiv. Environ. Sci 2017, 10, 193–206. [Google Scholar]
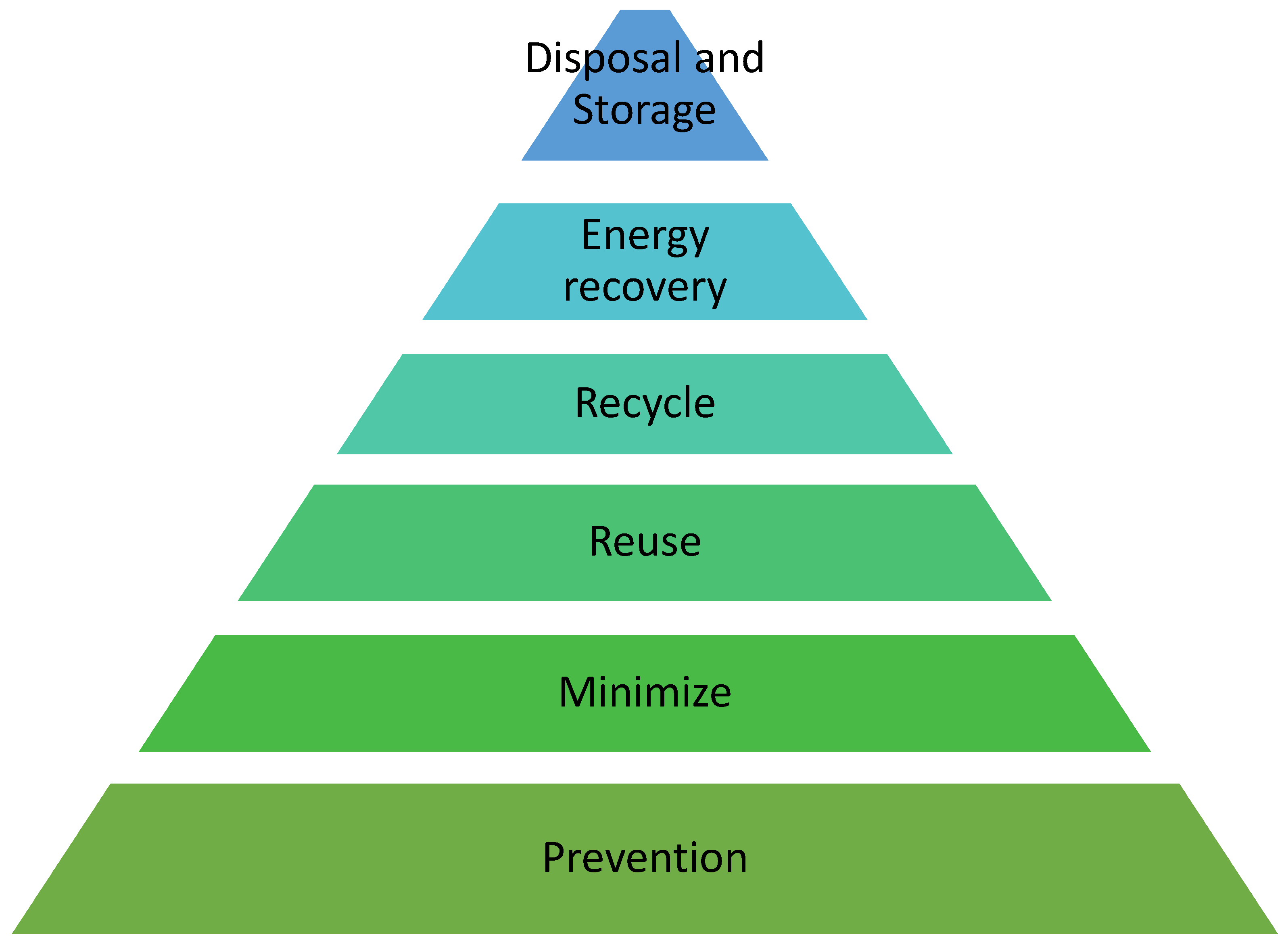
- Yıldırım, S.; Kantarcı, T. A review on sustainability policies of businesses: Recycling and waste reduction. J. Recycl. Econ. Sustain. Policy 2022, 1, 1–9. [Google Scholar]
- Van Ewijk, S.; Stegemann, J.A. Limitations of the waste hierarchy for achieving absolute reductions in material throughput. J. Clean. Prod. 2016, 132, 122–128. [Google Scholar] [CrossRef]
- Kinnunen, P.H.M.; Kaksonen, A.H. Towards circular economy in mining: Opportunities and bottlenecks for tailings valorization. J. Clean. Prod. 2019, 228, 153–160. [Google Scholar] [CrossRef]
- International Council on Mining and Metals (ICMM). Planning for Integrated Mine Closure: Toolkit. 2008. Available online: https://resourcegovernance.org/sites/default/files/Planning-for-Integrated-Closure-Toolkit---Final.pdf (accessed on 16 March 2025).
- International Council on Mining and Metals (ICMM). Mining and Metals and the Circular Economy. 2016. Available online: https://www.icmm.com/website/publications/pdfs/responsible-sourcing/icmm-circular-economy-1-.pdf (accessed on 16 March 2025).
- Prasad, M.N.V. Holistic approach to bioremediation-derived biomass and biorefineries for accelerating bioeconomy, circular bioeconomy, and carbon neutrality. In Bioremediation and Bioeconomy; Elsevier: Amsterdam, The Netherlands, 2024; pp. 33–58. [Google Scholar]
- Çelik, Ö.; Elbeyli, I.Y.; Piskin, S. Utilization of gold tailings as an additive in Portland cement. Waste Manag. Res. 2006, 24, 215–224. [Google Scholar] [CrossRef]
- Rashad, A.M.; Essa, G.M.; Mokhtar, M.M.; Mohamed, R.A.E. Valorization of calcined Egyptian marble waste as a reactive CaO additive for fortifying alkali-activated slag cement. J. Eng. Appl. Sci. 2025, 72, 167. [Google Scholar] [CrossRef]
- Ahmari, S.; Zhang, L. Durability and leaching behavior of mine tailings-based geopolymer bricks. Constr. Build. Mater. 2013, 44, 743–750. [Google Scholar] [CrossRef]
- Ahmari, S.; Zhang, L. Utilization of cement kiln dust (CKD) to enhance mine tailings-based geopolymer bricks. Constr. Build. Mater. 2013, 40, 1002–1011. [Google Scholar] [CrossRef]
- Haywood, L.K.; De Wet, B.; de Lange, W.; Oelofse, S. Legislative challenges hindering mine waste being reused and repurposed in South Africa. Extr. Ind. Soc. 2019, 6, 1079–1085. [Google Scholar] [CrossRef]
- Godfrey, L.K.; Oelofse, S.H.; Phiri, A.; Nahman, A.; Hall, J. Mineral Waste: The Required Governance Environment to Enable Re-Use. 2007. Available online: http://hdl.handle.net/10204/3541 (accessed on 11 April 2025).
- Harrison, S.; Rumjeet, S.; Mabasa, X.; Verster, B. Towards Resilient Futures: Can Fibre-Rich Plants Serve the Joint Role of Remediation of Degraded Mine Land and Fuelling of a Multi-Product Value Chain? IDEAS: London, UK,, 2019. [Google Scholar]
- Mlalazi, N.; Chimuka, L.; Simatele, M.D. Synergistic effect of compost and moringa leaf extract biostimulants on the remediation of gold mine tailings using chrysopogon zizanioides. Sci. Afr. 2024, 26, e02358. [Google Scholar] [CrossRef]
- Mlalazi, N.; Chimuka, L.; Simatele, M.D. The effect of compost and moringa leaf extract biostimulant on the phytoremediation of gold mine tailing in South Africa using Chrysopogon Zizanioides (l.) roberty. Nat. Based Solut. 2025, 8, 100266. [Google Scholar] [CrossRef]
- Hauptvogl, M.; Kotrla, M.; Prčík, M.; Pauková, Ž.; Kováčik, M.; Lošák, T. Phytoremediation potential of fast-growing energy plants: Challenges and perspectives–a review. Pol. J. Environ. Stud. 2019, 29, 505–516. [Google Scholar] [CrossRef]
- Acharya, R.N.; Perez-Pena, R. Role of comparative advantage in biofuel policy adoption in Latin America. Sustainability 2020, 12, 1411. [Google Scholar] [CrossRef]
- Singh, S.; Jaiswal, D.K.; Krishna, R.; Mukherjee, A.; Verma, J.P. Restoration of degraded lands through bioenergy plantations. Restor. Ecol. 2020, 28, 263–266. [Google Scholar] [CrossRef]
- Pulighe, G.; Bonati, G.; Colangeli, M.; Morese, M.M.; Traverso, L.; Lupia, F.; Khawaja, C.; Janssen, R.; Fava, F. Ongoing and emerging issues for sustainable bioenergy production on marginal lands in the Mediterranean regions. Renew. Sustain. Energy Rev. 2019, 103, 58–70. [Google Scholar] [CrossRef]
- Bauddh, K.; Singh, B.; Korstad, J. (Eds.) Phytoremediation Potential of Bioenergy Plants; Springer: Singapore, 2017. [Google Scholar]
- Witters, N.; Mendelsohn, R.; Van Slycken, S.; Weyens, N.; Schreurs, E.; Meers, E.; Tack, F.; Carleer, R.; Vangronsveld, J. Phytoremediation, a sustainable remediation technology? Conclusions from a case study. I: Energy production and carbon dioxide abatement. Biomass Bioenergy 2012, 39, 454–469. [Google Scholar] [CrossRef]
- Brandão, P.C.; de Souza, A.L.; Rousset, P.; Simas, F.N.B.; de Mendonça, B.A.F. Forest biomass as a viable pathway for sustainable energy supply in isolated villages of Amazonia. Environ. Dev. 2021, 37, 100609. [Google Scholar] [CrossRef]
- Shukla, N.; Sahoo, D.; Remya, N. Biochar from microwave pyrolysis of rice husk for tertiary wastewater treatment and soil nourishment. J. Clean. Prod. 2019, 235, 1073–1079. [Google Scholar] [CrossRef]
- Kalt, G.; Mayer, A.; Theurl, M.C.; Lauk, C.; Erb, K.H.; Haberl, H. Natural climate solutions versus bioenergy: Can carbon benefits of natural succession compete with bioenergy from short rotation coppice? Gcb Bioenergy 2019, 11, 1283–1297. [Google Scholar] [CrossRef]
- Leirpoll, M.E.; Næss, J.S.; Cavalett, O.; Dorber, M.; Hu, X.; Cherubini, F. Optimal combination of bioenergy and solar photovoltaic for renewable energy production on abandoned cropland. Renew. Energy 2021, 168, 45–56. [Google Scholar] [CrossRef]
- Strassburg, B.B.N.; Iribarrem, A.; Beyer, H.L.; Cordeiro, C.L.; Crouzeilles, R.; Jakovac, C.C.; Junqueira, A.B.; Lacerda, E.; Latawiec, A.E.; Balmford, A.; et al. Global priority areas for ecosystem restoration. Nature 2020, 586, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Næss, J.S.; Cavalett, O.; Cherubini, F. The land–energy–water nexus of global bioenergy potentials from abandoned cropland. Nat. Sustain. 2021, 4, 525–536. [Google Scholar] [CrossRef]
- Ionata, E.; Caputo, E.; Mandrich, L.; Marcolongo, L. Moving towards biofuels and high-value products through phytoremediation and biocatalytic processes. Catalysts 2024, 14, 118. [Google Scholar] [CrossRef]
- Danelli, T.; Sepulcri, A.; Masetti, G.; Colombo, F.; Sangiorgio, S.; Cassani, E.; Anelli, S.; Adani, F.; Pilu, R. Arundo donax L. biomass production in a polluted area: Effects of two harvest timings on heavy metals uptake. Appl. Sci. 2021, 11, 1147. [Google Scholar] [CrossRef]
- Hunce, S.Y.; Clemente, R.; Bernal, M.P. Energy production potential of phytoremediation plant biomass: Helianthus annuus and Silybum marianum. Ind. Crops Prod. 2019, 135, 206–216. [Google Scholar] [CrossRef]
- González-Chávez, M.D.C.A.; Carrillo-González, R.; Hernández Godínez, M.I.; Evangelista Lozano, S. Jatropha curcas and assisted phytoremediation of a mine tailing with biochar and a mycorrhizal fungus. Int. J. Phytoremediat. 2017, 19, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Mleczek, M.; Rutkowski, P.; Rissmann, I.; Kaczmarek, Z.; Golinski, P.; Szentner, K.; Strażyńska, K.; Stachowiak, A. Biomass productivity and phytoremediation potential of Salix alba and Salix viminalis. Biomass Bioenergy 2010, 34, 1410–1418. [Google Scholar] [CrossRef]
- Osman, H.E.; Quronfulah, A.S.; El-Morsy, M.H.; Alamoudi, W.M.; El-Hamid, H.T.A. Bioenergy crop rotation for phytoremediation of heavy metal contaminated soils at Mahd AD’Dahab mine, Kingdom of Saudi Arabia. J. Taibah Univ. Sci. 2024, 18, 2357257. [Google Scholar] [CrossRef]
- Zhuang, P.; Yang, Q.W.; Wang, H.B.; Shu, W.S. Phytoextraction of heavy metals by eight plant species in the field. Water, Air, and Soil Pollution 2007, 184, 235–242. [Google Scholar] [CrossRef]
- Jha, A.B.; Misra, A.N.; Sharma, P. Phytoremediation of heavy metal-contaminated soil using bioenergy crops. In Phytoremediation Potential Bioenergy Plants; Springer: Singapore, 2017; pp. 63–96. [Google Scholar] [CrossRef]
- Paniego, N.; Heinz, R.; Fernandez, P.; Talia, P.; Nishinakamasu, V.; Esteban Hopp, H. Sunflower. In Genome Mapping and Molecular Breeding in Plants; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 2. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, F.; Liu, S.; Wang, L.; Qiu, L.; Alexandrov, G.; Jothiprakash, V. Bioenergy production and environmental impacts. Geosci. Lett. 2018, 5, 14. [Google Scholar] [CrossRef]
- Ge, X.; Xu, F.; Vasco-Correa, J.; Li, Y. Giant reed: A competitive energy crop in comparison with miscanthus. Renew. Sustain. Energy Rev. 2016, 54, 350–362. [Google Scholar] [CrossRef]
- Baioni e Silva, G.; Manicardi, T.; Longati, A.A.; Lora, E.E.; Milessi, T.S. Parametric comparison of biodiesel transesterification processes using non-edible feedstocks: Castor bean and jatropha oils. Biofuels Bioprod. Biorefining 2023, 17, 297–311. [Google Scholar] [CrossRef]
- Cheban, I.; Dibrova, A. Development of liquid biofuel market: Impact assessment of the new support system in Ukraine. J. Int. Stud. 2020, 13, 262–278. [Google Scholar] [CrossRef]
- Naik, S.N.; Saxena, D.K.; Dole, B.R.; Khare, S.K. Potential and perspective of castor biorefinery. In Waste Biorefinery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 623–656. [Google Scholar]
- Alherbawi, M.; McKay, G.; Al-Ansari, T. Development of a hybrid biorefinery for jet biofuel production. Energy Convers. Manag. 2023, 276, 116569. [Google Scholar] [CrossRef]
- Kaur, R.; Gera, P.; Jha, M.K.; Bhaskar, T. Hydrothermal treatment of pretreated castor residue for the production of bio-oil. BioEnergy Res. 2023, 16, 517–527. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Z.; Shi, L.; Dai, L.; Liu, R.; Zhang, L.; Lyu, B.; Zhao, S.; Thakur, V.K. From Castor Oil-Based Multifunctional Polyols to Waterborne Polyurethanes: Synthesis and Properties. Macromol. Mater. Eng. 2023, 308, 2200662. [Google Scholar] [CrossRef]
- Rheay, H.T.; Omondi, E.C.; Brewer, C.E. Potential of hemp (Cannabis sativa L.) for paired phytoremediation and bioenergy production. GCB Bioenergy 2021, 13, 525–536. [Google Scholar] [CrossRef]
- Witzel, C.P.; Finger, R. Economic evaluation of Miscanthus production–A review. Renew. Sustain. Energy Rev. 2016, 53, 681–696. [Google Scholar] [CrossRef]
- Pidlisnyuk, V.; Stefanovska, T.; Lewis, E.E.; Erickson, L.E.; Davis, L.C. Miscanthus as a productive biofuel crop for phytoremediation. Crit. Rev. Plant Sci. 2014, 33, 1–19. [Google Scholar] [CrossRef]
- van Wilgen, B.W.; Richardson, D.M.; Le Maitre, D.C.; Marais, C.; Magadlela, D. The economic consequences of alien plant invasions: Examples of impacts and approaches to sustainable management in South Africa. Environ. Dev. Sustain. 2001, 3, 145–168. [Google Scholar] [CrossRef]
- Montes, C.; Rendon-Martos, M.; Varela, L.; Cappa, M. Mediterranean Wetland Restoration Manual; Ministry of Environment: Seville, Spain, 2007. [Google Scholar]
- Rodríguez-Echeverría, S. The legume-rhizobia symbiosis in invasion ecology: Facilitation of the invasion and disruption of native mutualisms? Asp. Appl. Biol. 2009, 98, 113–115. [Google Scholar]
- Leguizamo, M.A.O.; Gómez, W.D.F.; Sarmiento, M.C.G. Native herbaceous plant species with potential use in phytoremediation of heavy metals, spotlight on wetlands—A review. Chemosphere 2017, 168, 1230–1247. [Google Scholar] [CrossRef]
- Kiamarsi, Z.; Kafi, M.; Soleimani, M.; Nezami, A.; Lutts, S. Evaluating the bio-removal of crude oil by vetiver grass (Vetiveria zizanioides L.) in interaction with bacterial consortium exposed to contaminated artificial soils. Int. J. Phytoremediat. 2022, 24, 483–492. [Google Scholar] [CrossRef]
- Dhawan, S.S.; Gupta, P.; Lal, R.K. Cultivation and Breeding of Commercial Perfumery Grass Vetiver. In Medicinal Plants: Domestication, Biotechnology and Regional Importance; Springer: Cham, Switzerland, 2021; pp. 415–433. [Google Scholar] [CrossRef]
- Grimshaw, D. A Visit to Southern Africa. Available online: https://www.vetiver.org/SAVN_visit.htm#:~:text=There%20are%20two%20species%20of,Natal%20as%20early%20as%201860 (accessed on 21 June 2025).
- Leknoi, U.; Yiengthaisong, A.; Likitlersuang, S. Promoting use of vetiver grass for landslide protection: A pathway to achieve Sustainable Development Goals in Thailand. Environ. Dev. 2025, 54, 101155. [Google Scholar] [CrossRef]
- Danh, L.T.; Truong, P.; Mammucari, R.; Tran, T.; Foster, N. Vetiver grass, Vetiveria zizanioides: A choice plant for phytoremediation of heavy metals and organic wastes. Int. J. Phytoremediat. 2009, 11, 664–691. [Google Scholar] [CrossRef]
- Andra, S.S.; Datta, R.; Sarkar, D.; Saminathan, S.K.; Mullens, C.P.; Bach, S.B. Analysis of phytochelatin complexes in the lead tolerant vetiver grass [Vetiveria zizanioides (L.)] using liquid chromatography and mass spectrometry. Environ. Pollut. 2009, 157, 2173–2183. [Google Scholar] [CrossRef]
- Truong, T.T.V.; Pinners, E.; Truong, P. Vetiver System Applications Technical Reference Manual; The Vetiver Network International: San Antonio, TX, USA, 2016. [Google Scholar]
- Melato, F.A.; Mokgalaka, N.S.; McCrindle, R.I. Adaptation and detoxification mechanisms of Vetiver grass (Chrysopogon zizanioides) growing on gold mine tailings. Int. J. Phytoremediat. 2016, 18, 509–520. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, B.; Xia, H. Effect of cadmium on growth, photosynthesis, mineral nutrition, and metal accumulation of bana grass and vetiver grass. Ecotoxicol. Environ. Saf. 2014, 106, 102–108. [Google Scholar] [CrossRef]
- Banerjee, R.; Goswami, P.; Pathak, K.; Mukherjee, A. Vetiver grass: An environment clean-up tool for heavy metal contaminated iron ore mine-soil. Ecol. Eng. 2016, 90, 25–34. [Google Scholar] [CrossRef]
- Banerjee, R.; Goswami, P.; Lavania, S.; Mukherjee, A.; Lavania, U.C. Vetiver grass is a potential candidate for phytoremediation of iron ore mine spoil dumps. Ecol. Eng. 2019, 132, 120–136. [Google Scholar] [CrossRef]
- Truong, P.N. Vetiver grass technology for mine tailings rehabilitation. In Proceedings of the First Asia Pacific Conference on Ground and Water Bio-Engineering for Erosion Control and Slope Stabilization, Manila, Philippines, April 1999. [Google Scholar]
- Datta, R.; Quispe, M.A.; Sarkar, D. Greenhouse study on the phytoremediation potential of vetiver grass, Chrysopogon zizanioides L., in arsenic-contaminated soils. Bull. Environ. Contam. Toxicol. 2011, 86, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Lal, R.K.; Gupta, P.; Gupta, V.; Sarkar, S.; Singh, S. Genetic variability and character associations in vetiver (Vetiveria zizanioides L. Nash). Ind. Crops Prod. 2013, 49, 273–277. [Google Scholar] [CrossRef]

| Phytoremediation Technology and Pollutants Targeted | Species | References |
|---|---|---|
| Phytostabilization and Phytoextraction of Al, Fe, and Mn | Cyperus haspan, Schoenoplectus corymbosus, Typha capensis, Phragmites australis, Cynodon dactylon, Cyperus marginatus, and Juncus effusus | [41] |
| Phytostabilization and Phytoextraction of Cd, Cu, and Pb | Salix mucronata | [42] |
| Phytostabilization and Phytoextraction of Pb and Cu | Helichrysum splendidum | [43] |
| Phytoextraction of Cd, Cu, Fe, Mn, Ni, Pb, and Zn | Helianthus annuus | [44] |
| [45] | ||
| Phytoextraction of Fe, Mn, Pb, Mg, and Cr | Phragmites karka and Veteveria nigritana | [46] |
| Phytoextraction of Cr | Chrysopogon zizanioides | [47] |
| Phytoextraction of Ni | Berkhya coddii | [48] |
| Phytoextraction of B, Cd, Cr, Cu, Mg, Ni, and Zn | Phragmites australis | [49] |
| Phytoextraction of Ni | Manihot esculenta | [50] |
| Phytoextraction of Pb | Corchorus olitorius | [51] |
| Phytoextraction of Cd | Pennisetum clandestinum | [52] |
| Phytoextraction of Crude oil, Cd, Ni, and Zn | Chromolaena odorata | [53] |
| Phytostabilisation of Cu | Helianthus annuus | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mlalazi, N.; Mbohwa, C.; Ramuhaheli, S.; Chimwani, N. Towards Circularity and Sustainability: Phytoremediation Approaches, Legislative Challenges, and Bioenergy Potential in South African Mine Tailings Remediation. Processes 2025, 13, 3400. https://doi.org/10.3390/pr13113400
Mlalazi N, Mbohwa C, Ramuhaheli S, Chimwani N. Towards Circularity and Sustainability: Phytoremediation Approaches, Legislative Challenges, and Bioenergy Potential in South African Mine Tailings Remediation. Processes. 2025; 13(11):3400. https://doi.org/10.3390/pr13113400
Chicago/Turabian StyleMlalazi, Nkanyiso, Charles Mbohwa, Shumani Ramuhaheli, and Ngonidzashe Chimwani. 2025. "Towards Circularity and Sustainability: Phytoremediation Approaches, Legislative Challenges, and Bioenergy Potential in South African Mine Tailings Remediation" Processes 13, no. 11: 3400. https://doi.org/10.3390/pr13113400
APA StyleMlalazi, N., Mbohwa, C., Ramuhaheli, S., & Chimwani, N. (2025). Towards Circularity and Sustainability: Phytoremediation Approaches, Legislative Challenges, and Bioenergy Potential in South African Mine Tailings Remediation. Processes, 13(11), 3400. https://doi.org/10.3390/pr13113400






