Abstract
This study aimed to optimise ozone treatment and storage temperature conditions for preserving the quality of Kazakhstani Early-Maturing walnuts. The experiments examined ozone concentration (0.50 and 1.0 mg/m3), treatment duration (30 and 60 min), and storage temperature (+10 and +25 °C). Organoleptic, physicochemical, and microbiological characteristics were assessed using standard methods, with statistical analysis performed via regression and multifactorial approaches. Results showed that ozone treatment did not adversely affect sensory qualities but significantly reduced yeast microflora counts by 2–3 times (p < 0.05), improving microbiological stability. Oxidative degradation was inhibited, and rancidity was prevented. The optimal parameters were determined as an ozone concentration of 0.50 mg/m3, treatment duration of 30 min, and storage at +10 °C. Under these conditions, the acid value was 4.40 mg KOH/100 g, the peroxide value 21.0 mmol ½O/kg, and the moisture content 2.0%, all within acceptable limits. These findings confirm that ozone treatment is an effective and eco-friendly method for extending walnut shelf life and maintaining product quality during storage.
1. Introduction
Walnut (Juglans regia L.) is among the most valuable oil crops, with high nutritional, biological, and commercial significance. Due to its unique composition, including essential fatty acids (primarily linoleic and α-linolenic), complete proteins, vitamins (E, B, C), antioxidants (polyphenols, tocopherols), and minerals (magnesium, potassium, iron, zinc), walnut is widely used in the production of functional and dietary foods, as well as in the pharmaceutical and cosmetic industries [1,2]. However, high biological activity and instability of the lipid fraction, as well as susceptibility to microbiological spoilage, determine strict requirements for storage conditions. Nuts are prone to rapid oxidation of their contained fats, which leads to the formation of toxic peroxide decomposition products, changes in organoleptic characteristics, and loss of nutritional value. In addition, high humidity and temperature promote active growth of mould fungi and bacteria, including potentially pathogenic species, which also limits the shelf life and transportation of the product [3,4,5].
A promising method aimed at increasing the shelf life and preserving the quality of nut crops is ozone treatment, or ozonation, which has complex effects. Ozone is a strong oxidiser and disinfectant that can effectively inhibit the growth of a wide range of micro-organisms, including mould fungi, yeast, bacteria, and spores [6,7]. This is especially important when storing oil-containing products like walnuts, which are sensitive to microbiological spoilage and the development of mycotoxins, especially in conditions of high humidity and temperature. Recent studies have demonstrated the potential of ozone for controlling fungal contamination and improving the safety of nuts. For instance, Seyedabadi et al. [8] showed that ozone treatment effectively reduced pest infestation in walnuts without negatively affecting their organoleptic properties. Similarly, Ali and Abdallah [9] confirmed the antifungal and antiaflatoxigenic potential of ozone in nuts, while maintaining their nutritional quality. In addition, Ferreira et al. [10] reported that ozonation of Brazil nuts at different pH levels significantly inactivated Aspergillus flavus and influenced nut colour and lipid profile. Due to its properties, ozone not only provides a sterilisation effect but also helps remove surface moisture due to its weak drying effect, which further reduces the risk of mould formation and lipid oxidation. Unlike many chemical preservatives, ozone breaks down into oxygen, so it does not leave harmful residues in the product, making it environmentally friendly and acceptable for use in the food industry, including organic production [11,12].
A key consideration in the application of ozone technology is the correct selection of treatment parameters, primarily ozone concentration, exposure time, and frequency of treatment. An insufficient dose may not provide the desired antimicrobial effect, whereas excessive exposure can adversely affect the lipid fraction of nuts, accelerating rancidity, reducing nutritional value, and deteriorating sensory quality. Therefore, numerous studies have emphasised the importance of optimising ozone dosage and treatment regimens individually for each type of nut and storage condition. Establishing these parameters is essential to achieve an effective balance between microbial control and the preservation of physicochemical integrity.
In addition to the gaseous environment, the temperature regime is a decisive factor determining the stability of oil-rich products during storage. Lowering the storage temperature effectively slows down biochemical processes such as the autooxidation of unsaturated fatty acids, degradation of vitamins, and activity of lipolytic enzymes. Previous research has indicated that the optimal temperature range for the storage of nuts lies between 0 and 5 °C [13,14]. Maintaining these conditions ensures the preservation of nutritional and sensory quality while minimising oxidative deterioration.
The combination of ozone treatment with controlled storage temperatures can therefore provide a synergistic effect, maximising the nutritional, microbiological, and organoleptic stability of walnuts. This integrated approach offers a promising and environmentally friendly alternative to conventional preservation methods, ensuring both product safety and the retention of its functional properties [15,16].
However, maintaining refrigeration is not always feasible, particularly during long-distance transportation or in regions with warm climates. In such cases, additional stabilisation methods are required to extend shelf life and ensure product quality. Promising solutions include ozone treatment, modified atmosphere packaging, or the use of combined preservation technologies. Traditional methods—such as vacuum sealing, inert gas application (nitrogen, carbon dioxide), or drying to a residual moisture content below 5%—are widely employed to slow down degradation processes [17,18]. Nevertheless, these approaches may be insufficient for long-term storage and transport, and are often associated with higher operational costs and lower environmental sustainability. In this context, the use of ozone presents an attractive and effective complementary technology for improving the storage stability of walnuts and other nut products.
The aim of this study was to optimise the ozonation and storage temperature conditions of walnuts to preserve their nutritional and functional value, to improve their microbiological safety, and to increase their shelf life. The study assesses the main biochemical, microbiological, and organoleptic indicators of walnuts under various storage conditions and offers recommendations for the use of this technology in the food industry.
2. Materials and Methods
Walnuts of the Kazakhstani Early-Maturing variety were grown in the Almaty region and collected at the stage of full maturity. The nuts did not undergo any preliminary processing and met the regulatory requirements for moisture and quality indicators, allowing them to be used for storage and subsequent experimental treatment.
This variety is characterised by agronomically significant features such as early ripening, stable yield formation even under a shortened growing season, high frost resistance ensuring adaptability to continental climatic conditions, and medium-sized fruits with a thin, easily cracked shell, making it suitable for both industrial processing and direct consumption.
For the experiment, walnuts were selected from a single homogeneous batch and carefully sorted by size and maturity degree to ensure uniformity of the material. The selected nuts were placed in airtight food-grade plastic containers with a volume of 3 L, each containing a 2 kg batch of walnuts (Figure 1). Ozonation was carried out in a chamber sealed with a sealed lid using ozone from pure oxygen at a volumetric flow rate of 2 L/min. The selected ozone concentrations correspond to ozone flow rates of 1 × 10−6 and 2 × 10−6 L/min. The containers were not mechanically agitated during treatment; ozonation was performed as a static surface treatment, where the gas mixture circulated naturally throughout the chamber. The walnuts were arranged in a single layer at the bottom of the container to ensure uniform exposure of all samples to ozone. All samples originated from the same homogeneous batch that met regulatory requirements for food safety and microbiological quality at the start of the experiment. The control samples (without ozone treatment) were stored under the same temperature and humidity conditions as the experimental ones, which allowed us to assess the effect of ozone on the natural microflora development during storage.
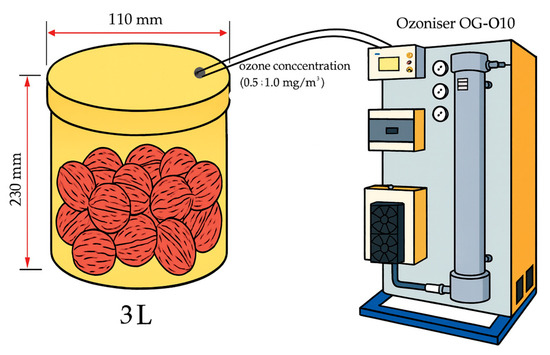
Figure 1.
Schematic of the experimental setup.
Ozonation was carried out using an industrial ozone generator OG-O10 (Figure 2), capable of producing ozone concentrations of up to 10 g/h.

Figure 2.
Ozoniser OG-O10.
Ozonation was carried out by varying the ozone concentration (0.5 and 1.0 mg/m3) and exposure time (30 and 60 min). The selected ozone levels were based on preliminary experiments and the published literature, indicating that such concentrations are effective for microbial reduction and oxidation control without negatively affecting the physicochemical quality of food products [19,20,21,22]. After treatment and sealing the containers, the samples were divided into groups and stored under different temperature conditions, either in a cool room at +10 °C or at room temperature of about +25 °C. The experimental plan is shown in Table 1.

Table 1.
Three-factor intervals and levels of variation in ozone treatment and storage temperature.
The experiment was designed as a three-factor, three-level system (coded levels −1, 0, +1), where each factor was varied within a defined interval. The coded values (0.25 and 0.75) presented in Table 1 represent the variation intervals and zero-level (central point) positions used for constructing the regression model. This design allows for the quantitative evaluation of the influence of ozone concentration (x1), processing time (x2), and storage temperature (x3) on walnut quality parameters.
Based on this factorial design, 8 treatment variants were generated and analysed to obtain statistically reliable regression equations and surface response models.
Based on the results of the conducted studies, regression equations were obtained that adequately (according to the Fisher criterion) describe the influence of the parameters of nut storage (Co, τ, t) on the listed indicators of nut quality after storage.
Data processing and all necessary calculations were carried out using the PLAN sequential regression analysis programme developed at the Odessa National Technological University (Odessa, Ukraine). To determine the variance of errors (reproducibility), 3 parallel experiments were carried out. Calculation of regression coefficients was carried out using matrices in natural dimensions and, accordingly, the equations themselves were also obtained in natural dimensions.
The general form of regression equations for 3 factors is given by Equation (1).
where yi are the i-th quality indicators, CO is the ozone concentration (Co·10−9 mg/m3), τ is the ozonation duration (min), and t is the storage temperature (°C).
yi = b0 + b1 Co + b2τ + b3t + b12 Coτ + b13 Cot + b23τt
At the end of the 45-day storage period, the quality of the walnuts was assessed through organoleptic evaluation, determination of physicochemical parameters, and microbiological analysis. This experimental design enabled a comprehensive evaluation of the effect of ozone concentration, exposure time, and temperature on the preservation of walnut quality during storage.
Standard and generally accepted analytical methods were used for all determinations. Organoleptic indicators (appearance, colour, smell, and taste) were assessed according to GOST 32874-2014 [23]. The physicochemical parameters included mass fraction of moisture (GOST 32874-2014) [24], fat content (GOST 29033-91) [25], ash content (GOST 25555.4-91) [26], peroxide value (GOST 26593-95) [27], acid value (GOST 31933-2012) [28], iodine value (GOST ISO 3961-2020) [29], and heavy metal content (Pb, Cd) (GOST 30178-96) [30].
The microbiological analysis included the determination of yeast and mould microflora according to GOST 10444.12-2013 [31], with additional methodological clarifications to ensure reproducibility. Each analysis was performed in triplicate using five representative walnut samples (n = 5) from each treatment group. From each sample, 10 g of kernel and shell fragments were aseptically transferred into sterile stomacher bags containing 90 mL of sterile 0.1% peptone water. The mixture was homogenised for 2 min to obtain a 10−1 dilution, followed by preparation of serial decimal dilutions.
Aliquots of 1.0 mL from appropriate dilutions were inoculated onto Dichloran Rose Bengal Chloramphenicol (DRBC) agar medium using the pour plate technique. The plates were incubated in an incubator at 25 ± 1 °C for 5–7 days. After incubation, colonies typical of yeasts and moulds were enumerated manually, and results were expressed as colony-forming units per gram of sample (CFU/g). The average counts from three replicates were used for statistical analysis.
This approach ensured reliable quantification of fungal contamination and allowed evaluation of the effectiveness of ozone treatment in reducing yeast and mould counts in stored walnuts.
Each analysis was conducted in triplicate using five representative walnut samples (n = 5) from each treatment group.
Statistical data processing was carried out using Microsoft Excel for Windows 2010 and the PLAN software (version 2.1) package developed by Stankevich et al. [32], applying standard methods of mathematical and regression analysis. The results were expressed as mean ± standard deviation (SD), and all measurements were performed in triplicate. The normality of data distribution was verified prior to analysis. The significance of differences between treatments was determined using analysis of variance (ANOVA) with a confidence level of 95% (p ≤ 0.05).
To evaluate the combined influence of the experimental factors—ozone concentration, exposure duration, temperature, and storage period—a multifactorial experimental design was applied. Regression equations were developed using the PLAN algorithm, allowing determination of regression coefficients, verification of their statistical significance, and assessment of the adequacy and predictive capability of the resulting mathematical models.
3. Results
The dependence of the walnut quality characteristics on the ozonation and storage parameters, including ozone concentration (Co, mg/m3), processing time (τ, min), and storage temperature (°C), was analysed and compared to control samples without ozonation.
The organoleptic property analysis showed that all organoleptic indicators remained unchanged, indicating the absence of a negative effect of ozonation on the appearance, taste, smell, or consistency of the product after storage. Five principle organoleptic parameter characteristics were assessed:
- Shell appearance. In all samples, the shell retained its integrity and natural colour, without mechanical damage, contamination, traces of mould, or pest damage.
- Kernel condition. The kernels had a uniform structure, were well formed, without signs of creases, spots, or surface defects. A slight natural shine indicated the high quality of the product.
- Smell and taste. The characteristic aroma of walnuts was preserved without foreign odours. The taste was rich and typical for a fresh ripe product. Signs of rancidity or mustiness were not detected.
- Fruit maturity. The kernels were easily separated from the shell, indicating physiological maturity. The internal partitions had a typical darkening characteristic of fully ripened fruits.
- Kernel consistency. The kernels were dense, but not excessively hard, without signs of excessive fragility or looseness. The internal structure remained uniform, confirming the suitability of the nuts for storage and processing.
Thus, the study showed that ozone treatment under the selected conditions does not deteriorate the organoleptic properties of walnuts, which is an important condition for their further industrial use and long-term storage.
Ten walnut quality indicators were also determined, including moisture content (y1, %), fat content (y2, %), ash content (y3, %), peroxide value (y4, mmol ½O/kg), acid value (y5, mg KOH/kg), iodine value (y6, g/100 g), lead content (y7, mg/kg), cadmium content (y8, mg/kg), yeast content (y9, CFU/g), and mould content (y10, CFU/g).
Factors:
Co—ozone concentration (mg/m3);
τ—exposure time (min);
t—storage temperature (°C).
To optimise the number of experiments and to obtain reliable experimental research results, multifactorial experiment planning methods were used. To reduce the influence of uncontrolled factors on the experimental results, the experiments were randomised using random number tables. The experimental conditions and the obtained quality indicator results for nuts stored for 45 days in an ozone environment are given in Table 2.

Table 2.
Experimental conditions and quality indicators of walnuts treated with ozone.
Statistical analysis of the data presented in Table 2 was performed using one-way analysis of variance (ANOVA) to determine the significance of differences between the treatments. Results are expressed as mean ± standard deviation (SD), and different superscript letters within the same column indicate statistically significant differences at p ≤ 0.05. This analysis confirmed that the applied ozone concentrations and exposure durations had a significant influence on the main physicochemical parameters of walnuts, including moisture, peroxide, and acid values, compared to the untreated control samples.
Summary data on the obtained regression equations in natural variables are given in Table 3, which also shows the standard errors of the experiments (Se) and inadequacy (Sinad), as well as the calculated (Fc) and critical (Fcr) values of the Fisher criterion at a confidence level of p = 0.05. More detailed data on the statistical characteristics of the obtained regression equations are given in the listings of their calculations.

Table 3.
Regression equations in natural variables and statistical characteristics of the dependencies of the quality indicators of walnuts treated with ozone on the factors influencing them (Co, τ, t).
Analysis of the obtained regression equations shows that, of the 10 studied walnut quality indicators, the equations for the mass fraction of fat (y2) and for the cadmium content (y8) are inadequate for the experimental data. It is also evident that the indicators y1, y2, y3, y6, and y10 do not depend on the processing parameters. The indicator y5 does not depend on Co, and the rest depend on all 3 mode factors.
The compiled regression equations are mathematical models that allow the nut quality indicators to be predicted depending on the values of their processing and storage parameters (factors Co, τ, and t).
To optimise the ozone processing parameters, the acid number was chosen as the target function (Equation (2)):
y5 = 6.713333 − 0.052833τ − 0.152333t + 0.002633τt → max
Equation (2) shows that the acid number is affected by only two parameters (τ and t). At the same time, the factors τ and t have not only a linear effect, but also a joint mutual influence. Therefore, it is difficult to make an unambiguous conclusion about the individual influence of each of the considered factors on the acid number y5 using Equation (2). A more visual representation of this influence can be obtained from the response surface shown in Figure 3.
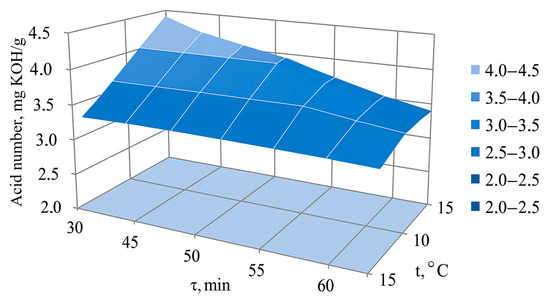
Figure 3.
Response surface of the combined influence of factors τ and t on the acid number y5 (measured at Co = 0.50 mg/m3).
It is evident from the figure that, due to the mutual influence of the factors Co and t, the acid number increases to 4.40 mg KOH/kg with a simultaneous decrease in τ and t.
Optimisation of the ozone processing parameters was carried out using the multivariate dichotomy method. The optimal ozonation conditions at Co 0.50 mg/m3 were 30 min duration (τ) with storage at 10 °C (t), under which the target objective function (acid number) was 4.40 mg KOH/kg. The values of the remaining quality indicators under the optimal processing conditions are given in Table 4.

Table 4.
Walnut quality indicator values under optimal ozone treatment and storage conditions.
Thus, ozone treatment under the defined optimal conditions ensured the maximum acid number value of 4.64 mg KOH/100 g and maintained the studied walnut quality indicators within acceptable limits.
Thus, under the optimal treatment conditions, all quality parameters of walnuts remained within the acceptable limits established by food safety and quality standards: moisture content ≤ 5.0% (GOST 32874-2014); fat content 50–70% (typical range, no strict limit); ash content ≤ 3.0% (GOST 32874-2014); peroxide value (PV) ≤ 25 mmol ½O/kg fat (GOST 26593-95); acid value (AV) ≤ 4.5 mg KOH/g fat (GOST 31933-2012; ISO 660:2020 [33]); iodine value–informational only (no limit) (GOST ISO 3961-2020); lead (Pb) ≤ 0.10 mg/kg (GOST 30178-96); cadmium (Cd) ≤ 0.05 mg/kg (GOST 30178-96); yeasts ≤ 10 CFU/g (GOST 10444.12-2013); and moulds ≤ 10 CFU/g (GOST 10444.12-2013).
4. Discussion
The results of the present study showed that ozone treatment at a concentration of 0.50 mg/m3 for 30 min, combined with storage at +10 °C, significantly inhibited lipid oxidation processes in walnuts. Under these conditions, the acid value reached 4.40 mg KOH/100 g, indicating limited accumulation of free fatty acids and effective preservation of the fat fraction. This effect is attributed to the oxidation–reduction potential of ozone, which disrupts microbial cell walls and inactivates enzymes responsible for lipid hydrolysis. Similar trends were observed by Seyedabadi et al. [8] and Ferreira et al. [10], who found that ozone concentrations between 0.5 and 2.0 mg/m3 with exposures of 30–60 min effectively controlled fungal growth and lipid oxidation in walnuts and Brazil nuts [34,35].
Control samples without ozone treatment exhibited a higher level of mould and yeast microflora, along with an increase in acid and peroxide values during storage. This confirms that ozone, due to its strong bactericidal and fungicidal effects, provides more stable storage conditions. Comparable results were obtained by Ali and Abdallah [9], who reported that ozone treatment at 1.0 mg/m3 reduced Aspergillus and Penicillium contamination in nuts while maintaining nutritional quality [36]. Pandiselvam et al. [37] also demonstrated that ozone treatment of oilseeds extended shelf life by decreasing microbial load and oxidative rancidity [38].
Even after increasing exposure time to 60 min, no deterioration was observed in organoleptic characteristics such as taste, smell, colour, or texture. This agrees with İbanoğlu [39] (2023) and Meneses-Espinosa et al. [40], who confirmed that ozone levels below 2 mg/m3 preserve the natural sensory attributes of nuts and seeds without causing oxidative off-flavours.
A comparison of storage conditions revealed that oxidation products accumulated more rapidly at +25 °C than at +10 °C, where the quality indicators remained stable throughout storage. Low-temperature storage combined with ozone exposure demonstrated a synergistic effect, consistent with results by Vijay Rakesh Reddy et al. [41] and Ferreira et al. [10], who found that refrigeration (8–12 °C) coupled with ozone treatment reduced mould counts and oxidative degradation during long-term storage of nuts [40,41].
Our results align with studies showing that moderate gaseous ozone suppresses fungal/yeast growth and improves storability of nuts without deteriorating sensory quality. For Brazil nuts, optimised ozonation suppressed Aspergillus flavus while maintaining quality when exposure was controlled; overly long exposures increased oxidative markers. Similar trends have been reported for pistachios, where extended gassing/stirring can accelerate oxidation of lipids if parameters are not optimised. Reviews on fruits/vegetables likewise report microbial reductions and shelf-life extension with minimal nutrient loss under appropriate conditions. Together with our data (yeast counts ↓2–3×; acceptable acid/peroxide values at 0.50 mg/m3, 30 min, +10 °C), these works support ozone’s efficacy when time–dose–temperature are balanced [42,43,44].
Ozone (a powerful oxidant) inactivates microorganisms via multi-target oxidative damage: lipid peroxidation of membranes, oxidation of cell-wall components, and damage to intracellular enzymes and nucleic acids. In clean systems, relatively low concentrations and short contact times are sufficient; efficacy is reduced in matrices with high ozone demand (rich in oxidizable substances). These mechanisms explain the strong antifungal/anti-yeast effect we observed on walnuts’ textured surfaces [45,46].
In the U.S., ozone is recognised by FDA as GRAS for food treatment/storage/processing (final rule, 26 June 2001) and is permitted by USDA/FSIS, provided good manufacturing practices and off-gas destruction are used. Occupational exposure must respect OSHA limits (e.g., 0.1 ppm 8 h TWA; 0.3 ppm 15 min ceiling). Our setup used sealed containers with controlled dosing and post-treatment aeration, minimising residual ozone [47,48].
If overdosed (high concentration and/or long exposure), ozone can:
- -
- accelerate lipid oxidation (↑ peroxide/acid values, off-flavours);
- -
- alter colour/texture and some phytochemicals;
- -
- cause excessive mass loss or quality defects.
The literature on nuts shows that very long exposures can reduce lipid and protein fractions and drive rancidity; reviews on produce highlight sensory/nutrient losses when parameters are not tailored to the commodity. Our optimisation (0.50 mg/m3, 30 min, +10 °C) was chosen specifically to avoid these effects while achieving microbial control [49].
Because ozone efficacy depends on concentration × time, gas distribution, product load, relative humidity, and temperature, scale-up requires validated monitoring (in-chamber ozone sensors), uniform airflow, and verified off-gas destruction. Process windows should be defined to keep oxidative indices (acid/peroxide values) within specification while meeting microbial targets [50]. This is consistent with current technical guidance on ozone handling in food systems.
The key scientific novelty of this work lies in its integrated approach to the study of the parameters of ozone treatment in combination with storage temperature conditions, as applied to the Kazakhstani Early-Maturing walnut variety. Such studies have been scarce in Central Asia. The results obtained expand our understanding of the potential of using ozone both as an antiseptic and as a stabilising factor of the lipid complex to extend shelf life. From a practical point of view, this technology allows the use of chemical preservatives to be replaced, meeting modern requirements for environmentally friendly and safe food products. In addition, the use of ozone on an industrial scale is justified due to its availability, low cost, and high efficiency with minimal impact on the organoleptic characteristics of the product. In global practice, ozone is used widely to disinfect fruits, vegetables, and nuts, but data on its use in walnuts remain limited. Our results are both consistent with earlier studies [39,40,41], which showed the positive effect of ozone on the preservation of hazelnuts and almonds, and complement them by assessing varieties grown in the specific climatic conditions of Kazakhstan.
The present results provide a scientific basis for the practical use of ozone treatment in the storage of walnuts under controlled temperature conditions. However, further research is needed to ensure the transition of this technology from the laboratory to industrial applications. Future studies should focus on scaling up ozone treatment systems for continuous or semi-continuous processing of nuts, determining the uniformity of ozone distribution in large storage chambers, and assessing long-term storage stability over different harvest seasons. In addition, more comprehensive analyses of ozone interaction with lipids and phenolic compounds are required to confirm the absence of sublethal oxidative effects during extended storage.
At the commercial scale, the next step involves developing pilot systems for controlled ozonation integrated with existing nut storage and packaging lines. The economic efficiency of ozone treatment should be evaluated in comparison with conventional preservation methods such as modified atmosphere packaging or nitrogen flushing. Moreover, future work should explore the applicability of this approach to other oil-containing crops (e.g., almonds, hazelnuts, and pistachios) grown in different climatic regions. Successful industrial adaptation of controlled ozone–temperature treatment could provide a sustainable, residue-free, and energy-efficient technology for ensuring the safety and extended shelf life of nut products.
5. Conclusions
Controlled ozonation combined with temperature regulation effectively preserves the sensory and physicochemical quality of Kazakhstani Early-Maturing walnuts. The treatment maintained normal taste, aroma, and texture, while reducing yeast counts by 2–3 times and preventing oxidative spoilage. The optimal conditions were established as 0.50 mg/m3 ozone concentration, 30 min exposure, and +10 °C storage temperature. Under these parameters, key quality indicators such as acid and peroxide values remained within permissible ranges. Ozone treatment can therefore be recommended as a sustainable alternative to chemical preservatives, offering enhanced storage safety and longer shelf life. Future studies should focus on scaling up the process and assessing its applicability to other nut and oilseed varieties.
Author Contributions
Conceptualisation, data curation and formal analysis, A.I. and G.U.; funding acquisition, investigation and methodology P.M. and F.D.; resources, software and writing—review and editing, M.Y. and A.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan, grant number AP19174427, “Development of technology for safe long-term storage of walnuts.” The authors express their gratitude to the management of Almaty Technological University for their support in conducting the research.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author. Additional files are provided in Appendix A.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A. Calculation of Regression Coefficients for Describing the Quality Indicators of Walnuts Treated with Ozone (Almaty Region)
| Calculation of regression coefficients by the least squares method according to the linear plan taking into account interfactorial interactions Number of experiments N = 8, coefficients KK = 7, factors KF = 3 X1-Co, mg/m3; x2-tau, min; x3-t, °C y1 Mass fraction of moisture, % Number of replicates of experiments m = 1 m0 = 0 Regression coefficients (b) and their trust errors (e): b0 = 2.103333 b1 = −0.046667 b2 = −0.001556 b3 = 0.000667 b12 = 0.001333 b13 = −0.001333 b23 =−0.000044 e0 = 1.432355 e1 = 1.578606 e2 = 0.026310 e3 = 0.058364 e12 = 0.026779 e13 = 0.053558 e23 = 0.000893 Significant regression coefficients: b0 = 2.002500 e0 = 0.100422 ------------------------------------------------------ N X1 X2 X3 Yav Yc styp ------------------------------------------------------ 1 1.00 60.00 25.00 1.97 2.00 1.65 2 0.50 60.00 25.00 1.95 2.00 2.69 3 1.00 30.00 25.00 1.99 2.00 0.63 4 0.50 30.00 25.00 2.03 2.00 1.35 5 1.00 60.00 10.00 2.00 2.00 0.13 6 0.50 60.00 10.00 2.01 2.00 0.37 7 1.00 30.00 10.00 2.04 2.00 1.84 8 0.50 30.00 10.00 2.03 2.00 1.35 ------------------------------------------------------ min 0.500 30.000 10.000 2.00 max 0.500 30.000 10.000 2.00 Statistical indicators: Student’s criterion tcr = 4.304 variance of error of experience and inadequacy s2y = 0.0044 s2ag = 0.0010 standard deviation sy = 0.0660 sag = 0.0315 number of degrees of freedom Ns2y = 2 Ns2ag = 7 Fisher criterion Fc = 4.39 Fcr = 4.74 Calculation of regression coefficients by the least squares method according to the linear plan taking into account interfactorial interactions Number of experiments N = 8, coefficients KK = 7, factors KF = 3 X1-Co, mg/m3; x2-tau, min; x3-t, °C Y2 Mass fraction of fat, % Number of replicates of experiments m = 1 m0= 0 Regression coefficients (b) and their trust errors (e): b0 = 58.037917 b1 = −0.295000 b2 = −0.003139 b3 = −0.004167 b12 = 0.003667 b13 = 0.006000 b23 = −0.000011 e0 = 31.468400 e1 = 34.681501 e2 = 0.578025 e3 = 1.282234 e12 = 0.588329 e13 = 1.176659 e23 = 0.019611 Significant regression coefficients: b0 = 57.796250 e0 = 2.206235 ------------------------------------------------------ N X1 X2 X3 Yav Yc styp ------------------------------------------------------ 1 1.00 60.00 25.00 57.81 57.80 0.02 2 0.50 60.00 25.00 57.76 57.80 0.06 3 1.00 30.00 25.00 57.79 57.80 0.01 4 0.50 30.00 25.00 57.82 57.80 0.04 5 1.00 60.00 10.00 57.78 57.80 0.03 6 0.50 60.00 10.00 57.80 57.80 0.01 7 1.00 30.00 10.00 57.78 57.80 0.03 8 0.50 30.00 10.00 57.83 57.80 0.06 ------------------------------------------------------ min 0.500 30.000 10.000 57.80 max 0.500 30.000 10.000 57.80 Statistical indicators: Student’s criterion tcr = 4.304 variance of error of experience and inadequacy s2y= 2.1025 s2ag = 0.0005 standard deviation sy = 1.4500 sag = 0.0233 number of degrees of freedom Ns2y = 2 Ns2ag = 7 Fisher criterion Fc = 3885.81 Fcr = 4.74 Calculation of regression coefficients by the least squares method according to the linear plan taking into account interfactorial interactions Number of experiments N = 8, coefficients KK = 7, factors KF = 3 X1-Co, mg/m3; x2-tau, min; x3-t, °C Y3 Ash content, % Number of replicates of experiments m = 1 m0= 0 Regression coefficients (b) and their trust errors (e): b0 = 1.855000 b1 = 0.033333 b2 = 0.001111 b3 = 0.000000 b12 = −0.001333 b13 = 0.002667 b23 = −0.000044 e0 = 0.737880 e1 = 0.813221 e2 = 0.013554 e3 = 0.030066 e12 = 0.013795 e13 = 0.027591 e23 = 0.000460 Significant regression coefficients: b0 = 1.885000 e0 = 0.051732 ------------------------------------------------------ N X1 X2 X3 Yav Yc styp ------------------------------------------------------ 1 1.00 60.00 25.00 1.88 1.89 0.27 2 0.50 60.00 25.00 1.86 1.89 1.34 3 1.00 30.00 25.00 1.91 1.89 1.31 4 0.50 30.00 25.00 1.89 1.89 0.26 5 1.00 60.00 10.00 1.87 1.89 0.80 6 0.50 60.00 10.00 1.89 1.89 0.26 7 1.00 30.00 10.00 1.90 1.89 0.79 8 0.50 30.00 10.00 1.88 1.89 0.27 ------------------------------------------------------ min 0.500 30.000 10.000 1.89 max 0.500 30.000 10.000 1.89 Statistical indicators: Student’s criterion tcr = 4.304 variance of error of experience and inadequacy s2y = 0.0012 s2ag = 0.0003 standard deviation sy = 0.0340 sag = 0.0160 number of degrees of freedom Ns2y = 2 Ns2ag = 7 Fisher criterion Fc = 4.50 Fcr = 4.74 Calculation of regression coefficients by the least squares method according to the linear plan taking into account interfactorial interactions Number of experiments N = 8, coefficients KK = 7, factors KF = 3 X1-Co, mg/m3; x2-tau, min; x3-t, °C Y4 Peroxide value, mmol ½O/kg Number of replicates of experiments m = 1 m0 = 0 Regression coefficients (b) and their trust errors (e): b0 = 22.948750 b1 = 4.895000 b2 = 0.150806 b3 = −0.454500 b12 = −0.259000 b13 = 0.398000 b23 = 0.000411 e0 = 10.417126 e1 = 11.480773 e2 = 0.191346 e3 = 0.424464 e12 = 0.194757 e13 = 0.389515 e23 = 0.006492 Significant regression coefficients: b0 = 26.296250 b2 = 0.110459 b3 = −0.509953 b12 = −0.195612 b13 = 0.496604 e0 = 2.870193 e2 = 0.106176 e3 = 0.254442 e12 = 0.125806 e13 = 0.313428 ------------------------------------------------------ N X1 X2 X3 Yav Yc styp ------------------------------------------------------ 1 1.00 60.00 25.00 20.51 20.85 1.67 2 0.50 60.00 25.00 20.95 20.51 2.08 3 1.00 30.00 25.00 23.54 23.41 0.56 4 0.50 30.00 25.00 19.91 20.13 1.13 5 1.00 60.00 10.00 21.08 21.05 0.13 6 0.50 60.00 10.00 24.32 24.44 0.49 7 1.00 30.00 10.00 24.11 23.61 2.08 8 0.50 30.00 10.00 23.65 24.06 1.73 ------------------------------------------------------ min 0.500 30.000 25.000 20.13 max 0.500 60.000 10.000 24.44 Statistical indicators: Student’s criterion tcr = 4.304 variance of error of experience and inadequacy s2y= 0.2304 s2ag = 0.2700 standard deviation sy = 0.4800 sag = 0.5196 number of degrees of freedom Ns2y = 2 Ns2ag = 3 Fisher criterion Fc = 1.17 Fcr = 19.16 Calculation of regression coefficients by the least squares method according to the linear plan taking into account interfactorial interactions Number of experiments N = 8, coefficients KK = 7, factors KF = 3 X1-Co, mg/m3; x2-tau, min; x3-t, °C Y5 Acid number, mg KOH/kg Number of replicates of experiments m = 1 m0= 0 Regression coefficients (b) and their trust errors (e): b0 = 7.702083 b1 = −1.318333 b2 = −0.073083 b3 = −0.115833 b12 = 0.027000 b13 = −0.048667 b23 = 0.002633 e0 = 3.906422 e1 = 4.305290 e2 = 0.071755 e3 = 0.159174 e12 = 0.073034 e13 = 0.146068 e23 = 0.002434 Significant regression coefficients: b0 = 6.713333 b2 = −0.052833 b3 = −0.152333 b23 = 0.002633 e0 = 2.198614 e2 = 0.046351 e3 = 0.115477 e23 = 0.002434 ------------------------------------------------------ N X1 X2 X3 Yav Yc styp ------------------------------------------------------ 1 1.00 60.00 25.00 3.49 3.69 5.59 2 0.50 60.00 25.00 3.88 3.69 5.03 3 1.00 30.00 25.00 2.83 3.30 16.43 4 0.50 30.00 25.00 3.76 3.30 12.37 5 1.00 60.00 10.00 3.52 3.60 2.27 6 0.50 60.00 10.00 3.68 3.60 2.17 7 1.00 30.00 10.00 4.18 4.40 5.14 8 0.50 30.00 10.00 4.61 4.40 4.66 ------------------------------------------------------ min 0.500 30.000 25.000 3.30 max 0.500 30.000 10.000 4.40 Statistical indicators: Student’s criterion tcr = 4.304 variance of error of experience and inadequacy s2y = 0.0324 s2ag = 0.1534 standard deviation sy = 0.1800 sag = 0.3917 number of degrees of freedom Ns2y = 2 Ns2ag = 4 Fisher criterion Fc = 4.74 Fcr = 19.25 Calculation of regression coefficients by the least squares method according to the linear plan taking into account interfactorial interactions Number of experiments N = 8, coefficients KK = 7, factors KF= 3 X1-Co, mg/m3; x2-tau, min; x3-t, °C Y6 iodine value, g/100 g Number of replicates of experiments m = 1 m0= 0 Regression coefficients (b) and their trust errors (e): b0 = 121.000000 b1 = −5.333333 b2 = −0.466667 b3 = 1.400000 b12 = 0.733333 b13 = −1.066667 b23 = −0.006667 e0 = 93.320083 e1 = 102.848588 e2 = 1.714143 e3 = 3.802487 e12 = 1.744701 e13 = 3.489401 e23 = 0.058157 Significant regression coefficients: b0 = 126.000000 e0 = 6.542628 ------------------------------------------------------ N X1 X2 X3 Yav Yc styp ------------------------------------------------------ 1 1.00 60.00 25.00 130.00 126.00 3.08 2 0.50 60.00 25.00 124.00 126.00 1.61 3 1.00 30.00 25.00 127.00 126.00 0.79 4 0.50 30.00 25.00 132.00 126.00 4.55 5 1.00 60.00 10.00 131.00 126.00 3.82 6 0.50 60.00 10.00 117.00 126.00 7.69 7 1.00 30.00 10.00 125.00 126.00 0.80 8 0.50 30.00 10.00 122.00 126.00 3.28 ------------------------------------------------------ min 0.500 30.000 10.000 126.00 max 0.500 30.000 10.000 126.00 Statistical indicators: Student’s criterion tcr = 4.304 variance of error of experience and inadequacy s2y= 18.4900 s2ag = 25.7143 standard deviation sy = 4.3000 sag = 5.0709 number of degrees of freedom Ns2y = 2 Ns2ag = 7 Fisher criterion Fc = 1.39 Fcr = 19.35 Calculation of regression coefficients by the least squares method according to the linear plan taking into account interfactorial interactions Number of experiments N = 8, coefficients KK = 7, factors KF = 3 X1-Co, mg/m3; x2-tau, min; x3-t, °C Y7 lead, mg/kg Number of replicates of experiments m = 1 m0 = 0 Regression coefficients (b) and their trust errors (e): b0 = 0.016458 b1 = −0.003500 b2 = −0.000114 b3 = −0.000083 b12 = 0.000033 b13 = −0.000200 b23 = 0.000006 e0 = 0.006728 e1 = 0.007415 e2 = 0.000124 e3 = 0.000274 e12 = 0.000126 e13 = 0.000252 e23 = 0.000004 Significant regression coefficients: b0 = 0.013666 b2 = −0.000086 b13 = −0.000298 b23 = 0.000005 e0 = 0.001926 e2 = 0.000046 e13 = 0.000093 e23 = 0.000002 ----------------------------------------------------- N X1 X2 X3 Yav Yc styp ------------------------------------------------------ 1 1.00 60.00 25.00 0.009 0.009 1.34 2 0.50 60.00 25.00 0.013 0.013 1.15 3 1.00 30.00 25.00 0.008 0.008 4.20 4 0.50 30.00 25.00 0.011 0.011 3.58 5 1.00 60.00 10.00 0.009 0.009 2.57 6 0.50 60.00 10.00 0.010 0.010 2.60 7 1.00 30.00 10.00 0.009 0.010 8.06 8 0.50 30.00 10.00 0.012 0.011 6.52 ------------------------------------------------------ min 1.000 30.000 25.000 0.008 max 0.500 60.000 25.000 0.013 Statistical indicators: Student’s criterion tcr = 4.304 variance of error of experience and inadequacy s2y = 0.0000 s2ag = 0.0000 standard deviation sy = 0.0003 sag = 0.0006 number of degrees of freedom Ns2y = 2 Ns2ag = 4 Fisher criterion Fc = 4.07 Fcr = 19.25 Calculation of regression coefficients by the least squares method according to the linear plan taking into account interfactorial interactions Number of experiments N = 8, coefficients KK = 7, factors KF = 3 X1-Co, mg/m3; x2-tau, min; x3-t, °C Y8 cadmium, mg/kg Number of replicates of experiments m = 1 m0 = 0 Regression coefficients (b) and their trust errors (e): b0 = −0.004583 b1 = 0.008333 b2 = 0.000061 b3 = 0.000033 b12 = −0.000133 b13 = −0.000133 b23 = 0.000002 e0 = 0.000456 e1 = 0.000502 e2 = 0.000008 e3 = 0.000019 e12 = 0.000009 e13 = 0.000017 e23 = 0.000000 ------------------------------------------------------ N X1 X2 X3 Yav Yc styp ------------------------------------------------------ 1 1.00 60.00 25.00 0.000 0.000 2 0.50 60.00 25.00 0.002 0.002 12.50 3 1.00 30.00 25.00 0.001 0.001 25.00 4 0.50 30.00 25.00 0.000 0.000 5 1.00 60.00 10.00 0.000 −0.000 6 0.50 60.00 10.00 0.000 0.000 7 1.00 30.00 10.00 0.001 0.001 25.00 8 0.50 30.00 10.00 0.000 −0.000 ------------------------------------------------------ min 1.000 60.000 10.000 −0.000 max 0.500 60.000 25.000 0.002 Statistical indicators: Student’s criterion tcr = 4.304 variance of error of experience and inadequacy s2y = 0.0000 s2ag = 0.0000 standard deviation sy = 0.0000 sag = 0.0007 number of degrees of freedom Ns2y = 2 Ns2ag = 1 Fisher criterion Fc = 1133.79 Fcr = 18.51 Calculation of regression coefficients by the least squares method according to the linear plan taking into account interfactorial interactions Number of experiments N = 8, coefficients KK = 7, factors KF = 3 X1-Co, mg/m3; x2-tau, min; x3-t, °C Y9 yeast, CFU/g Number of replicates of experiments m = 1 m0 = 0 Regression coefficients (b) and their trust errors (e): b0 = 4.333333 b1 = −0.000000 b2 = −0.055556 b3 = −0.133333 b12 = 0.000000 b13 = 0.000000 b23 = 0.002222 e0 = 1.128522 e1 = 1.243750 e2 = 0.020729 e3 = 0.045984 e12 = 0.021099 e13 = 0.042197 e23 = 0.000703 Significant regression coefficients: b0 = 4.333333 b2 = −0.055556 b3 = −0.133333 b23 = 0.002222 e0 = 0.635155 e2 = 0.013390 e3 = 0.033360 e23 = 0.000703 ------------------------------------------------------ N X1 X2 X3 Yav Yc styp ------------------------------------------------------ 1 1.00 60.00 25.00 1.000 1.000 0.00 2 0.50 60.00 25.00 1.000 1.000 0.00 3 1.00 30.00 25.00 1.000 1.000 0.00 4 0.50 30.00 25.00 1.000 1.000 0.00 5 1.00 60.00 10.00 1.000 1.000 0.00 6 0.50 60.00 10.00 1.000 1.000 0.00 7 1.00 30.00 10.00 2.000 2.000 0.00 8 0.50 30.00 10.00 2.000 2.000 0.00 ------------------------------------------------------ min 0.500 30.029 25.000 1.000 max 0.500 30.000 10.000 2.000 Statistical indicators: Student’s criterion tcr = 4.304 variance of error of experience and inadequacy s2y = 0.0027 s2ag = 0.0000 standard deviation sy = 0.0520 sag = 0.0000 number of degrees of freedom Ns2y = 2 Ns2ag = 4 Fisher criterion Fc = 8221.86 Fcr = 0 |
References
- Raja, G.; Shaker, I.A.; Inampudi, M. Nutritional analysis of nuts extract of Juglans regia. Int. J. Bioassays 2012, 1, 68. [Google Scholar] [CrossRef][Green Version]
- Das, N.; Tandukar, P.; Niraula, M.; Gautam, D.R.; Pathak, I. Phytochemical Analysis and Biological Activities of Different Extracts of Walnut (Juglans regia Linn.) Kernels. J. Nepal Chem. Soc. 2024, 44, 78–89. [Google Scholar] [CrossRef]
- Bechelova, A. Macro and Micronutrients Contents in Walnut Leaves (Juglans regia L.) (Southern Kyrgyzstan). Bull. Sci. Pract. 2023, 1, 81–88. [Google Scholar] [CrossRef]
- Sharma, M.; Sharma, M.; Sharma, M. A comprehensive review on ethnobotanical, medicinal and nutritional potential of walnut (Juglans regia L.). Proc. Indian Natl. Sci. Acad. 2022, 88, 601–616. [Google Scholar] [CrossRef]
- Elouafy, Y.; El Yadini, A.; El Moudden, H.; Harhar, H.; Alshahrani, M.M.; Awadh, A.A.A.; Goh, K.W.; Ming, L.C.; Bouyahya, A.; Tabyaoui, M. Influence of the Extraction Method on the Quality and Chemical Composition of Walnut (Juglans regia L.) Oil. Molecules 2022, 27, 7681. [Google Scholar] [CrossRef] [PubMed]
- Antos, P.; Piechowiak, T.; Balawejder, M.; Kowalczyk, K.; Tereszkiewicz, K. Impact of gaseous ozone treatment of fish carcasses (Gadus morhua) on the microbiological load and their quality. PLoS ONE 2025, 20, e0327866. [Google Scholar] [CrossRef]
- Leontieff, D.A.; Ikehata, K.; Inanaga, Y.; Furukawa, S. Ozone for Industrial Wastewater Treatment: Recent Advances and Sector Applications. Processes 2025, 13, 2331. [Google Scholar] [CrossRef]
- Seyedabadi, E.; Aran, M.; Moghaddam, R.M. Application of ozone against the larvae of Plodia interpunctella (Hübner) and its impacts on the organoleptic properties of walnuts. J. Food Prot. 2021, 84, 147–151. [Google Scholar] [CrossRef]
- Ali, E.M.; Abdallah, B.M. The potential use of ozone as an antifungal and antiaflatoxigenic agent in nuts and its effect on nutritional quality. Braz. J. Biol. 2022, 84, e263814. [Google Scholar] [CrossRef]
- Ferreira, W.F.D.S.; de Alencar, E.R.; Blum, L.E.B.; Ferreira, M.D.A.; Mendonca, M.A.; Racanicci, A.M.C.; Urruchi, W.M.I. Ozonation of Brazil nuts in aqueous media at different pH levels: Ozone decomposition, Aspergillus flavus inactivation, and effects on nut color and crude oil lipid profile. Ozone Sci. Eng. 2021, 43, 351–362. [Google Scholar] [CrossRef]
- Leeuwen, J.; van Pandiselvam, R.; Jeevarathinam, G. Cost Estimation for the Preservation of Selected Food/Crop Products with Ozone. J. Food Process Eng. 2024, 47, e14772. [Google Scholar] [CrossRef]
- Dhami, J.S.; Jyakhwo, S.; Thapa, B.B.; Tyata, R.B. Ozone Generation Via DBD Plasma for Water and Curd Treatment. J. Sci. Eng. 2025, 12, 57–61. [Google Scholar] [CrossRef]
- Alkhair, A.; Tveritnikova, I.S.; Kirsh, I.A.; Filinskaya, Y.A.; Melesse, I.; Bannikova, O.A. Optimizing pistachio packaging to extend shelf life and ensure quality. Food Process. Ind. 2024, 2, 44–48. [Google Scholar] [CrossRef]
- Ndlovu, N. Nuts and diabetes management: Nutritional and metabolic benefits. Bioact. Compd. Health Dis. 2025, 8, 318–333. [Google Scholar] [CrossRef]
- Lv, Z.; Wang, W.; Yang, D.; He, X.; Qu, Z.; Jing, W.; Mei, Y.; Huang, H.; Su, B.; Zhuang, Y. Optimal Design and Tests of a Pulsating Roll-Cleaning Device for Tiger Nuts. Agriculture 2024, 14, 1673. [Google Scholar] [CrossRef]
- Brusa, V.; Restovich, V.; Cap, M.; Chiapparoli, V.; Grigioni, G.; Giannuzzi, L.; Vaudagna, S.; Leotta, G. Effect of ozone application on bovine carcasses in abattoir cold chambers. PLoS ONE 2025, 20, e0321146. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, J.; Lv, H.; Zeng, X.; Ling, Z.; Ding, L.; Jin, C. Advances in Ozone Technology for Environmental, Energy, Food and Medical Applications. Processes 2025, 13, 1126. [Google Scholar] [CrossRef]
- Paravathi, C.; Lisha, S.; Harshitha, S.; Archana, S. Sustainable Food Preservation Using Integrated Methods. Int. J. Sci. Res. Eng. Manag. 2025, 9, 1–7. [Google Scholar] [CrossRef]
- Mohammad, A.A.; Salem, S.H.; Amer, H.M.; Hussein, M.S. Ozone as a postharvest treatment to maintain the quality characteristics of fresh-cut plants. J. Food Meas. Charact. 2025, 19, 4325–4336. [Google Scholar] [CrossRef]
- Anchang, M.M.; Imamou Hassani, M.; Okoyeuzu, C.F.; Karimidastjerd, A.; Bono, G.; Okpala, C.O.R. Ozone treatment and quality control of shrimp. In Postharvest Technologies and Quality Control of Shrimp; Elsevier: Amsterdam, The Netherlands, 2025; pp. 251–275. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Rathnakumar, K.; Nickhil, C.; Charles, A.P.R.; Falsafi, S.R.; Rostamabadi, H.; Sofia, A.; Aydar, A.Y.; Priya, V.; Malik, S.; et al. Ozone-Based Oxidation Treatment to Enhance Food Drying Rate and Quality: Mechanisms, Current Knowledge, and Future Outlook. Food Bioprocess Technol. 2025, 18, 5038–5057. [Google Scholar] [CrossRef]
- Sverhun, Z.G.; Horiuk, Y.V. Assessment of bactericidal action of aqueos ozone to disinfected the surface of eggs. Sci. Messenger LNU Vet. Med. Biotechnol. 2025, 27, 89–95. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Q.; Han, Y.; Zong, W.; Ge, Z.; Wei, X. Alternaria mycotoxin degradation and quality evaluation of orange juice by ozone treatment. J. Food Process Eng. 2024, 47, e14703. [Google Scholar] [CrossRef]
- GOST 32874-2014; Walnuts. Specifications. (UNECE Standard DDP-01:2013, MOD). Standartinform: Moscow, Russia, 2015; pp. 1–15. Available online: https://internet-law.ru/gosts/gost/58311/ (accessed on 15 March 2025).
- GOST 29033-91; Grain and Its Processed Products. Method for Determination of Fat. IPC, Publishing House of standards: Moscow, Russia, 2004; pp. 1–6. Available online: https://files.stroyinf.ru/Data2/1/4294825/4294825650.pdf (accessed on 21 March 2025).
- GOST 25555.4-91; Processed Products of Fruits and Vegetables. Methods for Determination of Ash and Alkalinity of Total and Water-Soluble Ash. Standartinform: Moscow, Russia, 2011; pp. 1–6. Available online: https://internet-law.ru/gosts/gost/10506/ (accessed on 3 April 2025).
- GOST 26593-95; Vegetable Oils. Method for Measuring Peroxide Value. Standartinform: Moscow, Russia, 1999; pp. 1–5. Available online: https://files.stroyinf.ru/Data2/1/4294827/4294827842.pdf (accessed on 2 April 2025).
- GOST 31933-2012; Vegetable Oils. Methods for Determining Acid Value. Standartinform: Moscow, Russia, 2019; pp. 1–14. Available online: https://files.stroyinf.ru/Data2/1/4293781/4293781264.pdf (accessed on 2 April 2025).
- GOST ISO 3961-2020; Animal and Vegetable Fats and Oils. Determination of Iodine Value. Standartinform: Moscow, Russia, 2020; pp. 1–18. Available online: https://internet-law.ru/gosts/gost/73886/ (accessed on 1 April 2025).
- GOST 30178-96; Raw Materials and Food Products. Atomic Absorption Method for Determining Toxic Elements. Standartinform: Moscow, Russia, 2010; pp. 1–10. Available online: https://internet-law.ru/gosts/gost/9123 (accessed on 22 March 2025).
- GOST 10444.12-2013; Microbiology of Food Products and Animal Feed. Methods for Detecting and Counting the Amount of Yeast and Mold Fungi. Standartinform: Moscow, Russia, 2014; pp. 1–12. Available online: https://internet-law.ru/gosts/gost/55923/ (accessed on 1 April 2025).
- Ostapchuk, N.V.; Kaminsky, V.D.; Stankevich, G.N.; Chuchuy, V.P. Mathematical modeling of food production processes. In Collection of Problems: Textbook; Vishcha Shkola: Kyiv, Ukraine, 1992; p. 175. [Google Scholar]
- ISO 660:2020; Animal and Vegetable Fats and Oils—Determination of Acid Value and Acidity. International Standard: Geneva, Switzerland, 2020; pp. 1–9. Available online: https://cdn.standards.iteh.ai/samples/75594/90e8488f0492461cb04d4ac36a77297e/ISO-660-2020.pdf (accessed on 6 September 2025).
- Çelebi, Y.; Koç, G.Ç.; Süfer, Ö.; Tekgül Barut, Y.; Şahin Ercan, S.; Yüksel, A.N.; Sezer, S.; Ramniwas, S.; Rastogi, S.; Chandra Khanashyam, A.; et al. Impact of Ozone Treatment on Lipid Oxidation in Foods: A Critical Review. Ozone Sci. Eng. 2024, 46, 430–454. [Google Scholar] [CrossRef]
- Anjali, K.U.; Reshma, C.; Sruthi, N.U.; Pandiselvam, R.; Kothakota, A.; Kumar, M.; Siliveru, K.; Marszałek, K.; Mousavi Khaneghah, A. Influence of ozone treatment on functional and rheological characteristics of food products: An updated review. Crit. Rev. Food Sci. Nutr. 2022, 64, 3687–3701. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, Z.; Barbara, R. Inactivation of microbes by ozone in the food industry: A review. Afr. J. Food Sci. 2021, 15, 113–120. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Subhashini, S.; Banuu Priya, E.P.; Kothakota, A.; Ramesh, S.V.; Shahir, S. Ozone based food preservation: A promising green technology for enhanced food safety. Ozone Sci. Eng. 2018, 41, 17–34. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Singh, A.; Agriopoulou, S.; Sachadyn-Król, M.; Aslam, R.; Gonçalves Lima, C.M.; Khanashyam, A.C.; Kothakota, A.; Atakan, O.; Kumar, M.; et al. A comprehensive review of impacts of ozone treatment on textural properties in different food products. Trends Food Sci. Technol. 2022, 127, 74–86. [Google Scholar] [CrossRef]
- İbanoğlu, Ş. Applications of ozonation in the food industry. In Non-Thermal Food Processing Operations; Elsevier: Amsterdam, The Netherlands, 2023; pp. 55–91. [Google Scholar] [CrossRef]
- Meneses-Espinosa, E.; Gálvez-López, D.; Rosas-Quijano, R.; Adriano-Anaya, L.; Vázquez-Ovando, A. Advantages and Disadvantages of Using Emerging Technologies to Increase Postharvest Life of Fruits and Vegetables. Food Rev. Int. 2023, 40, 1348–1373. [Google Scholar] [CrossRef]
- Vijay Rakesh Reddy, S.; Sudhakar Rao, D.V.; Sharma, R.R.; Preethi, P.; Pandiselvam, R. Role of Ozone in Post-Harvest Disinfection and Processing of Horticultural Crops: A Review. Ozone Sci. Eng. 2021, 44, 127–146. [Google Scholar] [CrossRef]
- De Oliveira, J.M.; de Alencar, E.R.; Blum, L.E.B.; de Souza Ferreira, W.F.; Botelho, S.d.C.C.; Racanicci, A.M.C.; dos Santos Leandro, E.; Mendonça, M.A.; Moscon, E.S.; dos Santos Bizerra, L.V.A.; et al. Ozonation of Brazil nuts: Decomposition kinetics, control of Aspergillus flavus and the effect on color and on raw oil quality. LWT 2020, 123, 109106. [Google Scholar] [CrossRef]
- Baia, G.M.; Freitas-Silva, O.; Junior, M.F. Understanding the Role of Chlorine and Ozone to Control Postharvest Diseases in Fruit and Vegetables: A Review. Curr. Nutr. Food Sci. 2020, 16, 455–461. [Google Scholar] [CrossRef]
- Botondi, R.; Barone, M.; Grasso, C. A Review into the Effectiveness of Ozone Technology for Improving the Safety and Preserving the Quality of Fresh-Cut Fruits and Vegetables. Foods 2021, 10, 748. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-G.; Yousef, A.E.; Dave, S. Application of Ozone for Enhancing the Microbiological Safety and Quality of Foods: A Review. J. Food Prot. 1999, 62, 1071–1087. [Google Scholar] [CrossRef] [PubMed]
- Epelle, E.I.; Macfarlane, A.; Cusack, M.; Burns, A.; Okolie, J.A.; Mackay, W.; Rateb, M.; Yaseen, M. Ozone application in different industries: A review of recent developments. Chem. Eng. J. 2023, 454, 140188. [Google Scholar] [CrossRef] [PubMed]
- Epelle, E.I.; Emmerson, A.; Nekrasova, M.; Macfarlane, A.; Cusack, M.; Burns, A.; Mackay, W.; Yaseen, M. Microbial Inactivation: Gaseous or Aqueous Ozonation? Ind. Eng. Chem. Res. 2022, 61, 9600–9610. [Google Scholar] [CrossRef]
- Xue, W.; Macleod, J.; Blaxland, J. The Use of Ozone Technology to Control Microorganism Growth, Enhance Food Safety and Extend Shelf Life: A Promising Food Decontamination Technology. Foods 2023, 12, 814. [Google Scholar] [CrossRef]
- Atakan, O.; Caner, C. Evaluation of different ozonation on aflatoxin degradation and physicochemical characteristics of hazelnuts. J. Food Process. Preserv. 2021, 45, e15276. [Google Scholar] [CrossRef]
- Brodowska, A.J.; Nowak, A.; Śmigielski, K. Ozone in the food industry: Principles of ozone treatment, mechanisms of action, and applications: An overview. Crit. Rev. Food Sci. Nutr. 2017, 58, 2176–2201. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).