1. Introduction
Soil contamination with heavy metals such as lead (Pb), cadmium (Cd), mercury (Hg), arsenic (As), chromium (Cr), and nickel (Ni) represents a pervasive and critical environmental challenge worldwide, particularly in regions with extensive industrial and mining activities [
1]. These contaminants, introduced through anthropogenic activities including mining, industrial operations, and agricultural practices [
2], pose significant risks to ecosystem health, agricultural productivity, and human well-being due to their persistence, toxicity, and potential for bioaccumulation within the food chain [
3,
4]. The detrimental effects of metal pollution on plant growth, microbial activity, and overall ecosystem functionality necessitate effective, sustainable remediation strategies to rehabilitate contaminated lands and mitigate associated risks [
5].
Conventional remediation techniques, such as soil excavation, chemical treatment, and incineration, are often prohibitively expensive, environmentally disruptive, and energy-intensive, generating secondary pollution and driving interest in more eco-friendly alternatives [
6,
7]. In contrast, phytoremediation, the use of plants to extract, stabilize, degrade, or volatilize environmental contaminants, has emerged as a powerful green technology to address this challenge [
8]. This approach leverages natural plant processes, such as root uptake (phytoextraction), stabilization (phytostabilization), and microbial degradation in the rhizosphere to remediate polluted sites in situ. It offers a non-destructive, cost-effective strategy that avoids the secondary pollution (e.g., airborne dust from excavation, volatile organic compounds from incineration) associated with conventional methods, while concurrently promoting ecosystem restoration through root-mediated soil stabilization and enhanced biodiversity [
9,
10]. Furthermore, a successfully implemented phytoremediation system can restore ecosystem services by preventing erosion and enhancing biodiversity [
11].
The efficacy of phytoremediation hinges on identifying plant species with the inherent capacity to tolerate, accumulate, and translocate high concentrations of heavy metals without severe physiological stress—traits exemplified by hyperaccumulators [
12,
13]. An ideal phytoremediator should possess characteristics such as rapid growth, high biomass production, a deep and extensive root system, and an inherent capacity to accumulate contaminants in its harvestable tissues [
14,
15]. Hemp (
Cannabis sativa L.) has garnered significant attention as a promising candidate for phytoremediation, fulfilling these criteria [
16]. Notably, hemp demonstrates remarkable resilience to abiotic stress, a fast growth rate enabling multiple harvests, and a robust root system capable of accessing contaminants in deeper soil strata [
17]. Crucially, its non-selective nutrient uptake mechanism, potentially explained by the “inadvertent uptake hypothesis” [
18], allows it to absorb and hyperaccumulate various heavy metals into its aerial tissues without severe impacts on its development, making it an effective phytoextractor for the removal of contaminants from the site upon harvest [
19,
20].
While the general potential of hemp in phytoremediation is recognized, optimizing its efficacy requires a deeper understanding of the factors that influence metal uptake and translocation. These factors are critical because the success of any phytoremediation effort is fundamentally governed by soil properties and the bioavailability of contaminants, which directly determine the extent of metal absorption by plant roots. Factors such as soil pH, organic matter content, clay minerals, and the presence of competing ions critically influence the solubility and thus the plant-availability of metals [
21,
22]. Therefore, comprehensive soil analysis is an indispensable first step. Furthermore, key strategies to enhance bioavailability and subsequent accumulation include the application of soil amendments such as chelating agents to mobilize metals and nutrient supplementation and to support plant health and biomass under stress conditions [
23]. Comparative studies between short-term shock treatments and full-lifecycle growth are essential to evaluate the efficiency and practical applicability of these strategies for large-scale remediation projects, particularly in regions like South Africa, where mining activities have left a legacy of degraded and infertile lands [
24].
This study aims to comprehensively evaluate the phytoremediation potential of hemp in soils contaminated with a complex mixture of heavy metals, specifically from an evaporation dam associated with a steel production facility. This site was selected as representing a typical legacy contamination scenario, characterized by a complex, aged mixture of heavy metals and radionuclides, providing a rigorous test for any phytoremediation candidate. By integrating a detailed analysis of soil metal content and properties with an investigation into the plant’s metal uptake efficiency and translocation patterns, this research seeks to (a) characterize the native metal content of contaminated soils, (b) determine the impact of soil treatments (citric acid chelation and NPK nutrient supplementation) on hemp growth and health, and (c) elucidate the metal uptake patterns in plant tissues over both short-term (blasto-filtration) and full-lifecycle experiments. The findings will contribute to developing optimized, scalable, and sustainable hemp-based remediation protocols for the rehabilitation of polluted environments.
2. Materials and Methods
2.1. Soil Collection and Physicochemical Characterization
Surface soil samples (0–20 cm depth) were collected from a decommissioned evaporation dam at a historical steel production facility located in Vanderbijlpark, South Africa (26°37′51.2″ S 27°49′39.6″ E). The site was chosen due to its well-documented history of industrial use and its representative profile of multi-metal contamination, which includes elevated levels of both essential nutrients and phytotoxic elements, mirroring conditions found at many post-industrial and mining sites. To ensure representativeness, a composite sample was created from twenty (20) individual subsamples collected from random points across the site using a systematic grid-based approach. The composite soil was air-dried at ambient temperature, thoroughly homogenized using a mechanical soil grinder (RETSCH GmbH, Haan, Germany), and sieved through a 2 mm mesh to remove stones and large organic debris, obtaining the fine earth fraction for all experiments. The total elemental composition of the homogenized soil was determined in triplicate (n = 3) via ICP-MS following microwave-assisted acid digestion.
The total elemental composition of the soil was determined to assess the extent of metal contamination. For digestion, 0.5 g of soil was treated with a 3:1 (
v/
v) mixture of concentrated nitric acid (HNO
3, 69%, TraceMetal™ Grade, Fisher Scientific, Waltham, MA, USA) and hydrochloric acid (HCl, 37%, TraceMetal™ Grade, Fisher Scientific) using a microwave assisted digestion system (MARS 6, CEM Corporation, Matthews, NC, USA) following USEPA Method 3051A [
25]. The microwave digestion program for soil samples (USEPA Method 3051A) was as follows: the temperature was ramped to 175 °C over 5.5 min and held at 175 ± 5 °C for 4.5 min, with a maximum operating pressure of 350 psi. Concentrations of elements, including target heavy metals, were quantified using Inductively Coupled Plasma Mass Spectrometry (ICP-MS, Agilent 7900, Santa Clara, CA, USA) [
26]. Quality assurance and quality control (QA/QC) were maintained by including certified reference material (CRM) NIST 2711a (Montana II Soil, National Institute of Standards and Technology, Gaithersburg, MD, USA) and method blanks in each digestion batch. Analytical recovery for the CRMs ranged between 85 and 115% for all reported elements.
2.2. Experimental Design and Plant Cultivation
Seeds of industrial hemp (
Cannabis sativa L., variety ‘Felina 32’) were surface sterilized with 10% (
v/
v) H
2O
2 for 10 min and pre-germinated on moist filter paper in the dark at 25 °C for 48 h [
27,
28]. Two independent greenhouse experiments were conducted: a short-term blasto-filtration assay and a long-term full-cycle trial. A six-month duration was selected for the full-cycle trial to allow the plants to complete their primary vegetative growth phase, enabling a robust assessment of total biomass yield and cumulative metal uptake under sustained stress conditions.
2.2.1. Short-Term Blasto-Filtration Assay
A six-week pot experiment was designed to evaluate short-term metal phytoextraction efficiency. Pre-germinated seeds were transplanted into pots containing 2 kg of the homogenized contaminated soil. The experiment comprised three treatments, each with five replicates, arranged in a completely randomized design:
Control: Soil with no amendments.
Metal-spiked: Soil amended with a 50 mM aqueous solution of citric acid (C6H8O7, ≥99.5%, Sigma-Aldrich, St. Louis, MO, USA) as a chelating agent. The solution was applied to achieve and maintain the soil pH within the optimal range for hemp (5.8–6.7) throughout the trial.
NPK + spike: Soil amended with both the 50 mM citric acid solution and a balanced NPK fertilizer (20:10:10 N:P:K, applied at a rate of 200 kg·ha−1).
Citric acid was selected as the chelating agent due to its proven effectiveness in mobilizing a range of heavy metals and radionuclides, combined with its biodegradability and lower environmental persistence compared to synthetic alternatives like EDTA. This aligns with the goal of developing an environmentally sustainable remediation strategy.
2.2.2. Long-Term Greenhouse Full-Cycle Trial
A six-month experiment was conducted to assess long-term growth patterns, metal translocation, and the effect of combined fertilization and chelation. Plants were grown in the same soil under three treatments with six replicates each [
29]:
Control: Soil with no amendments.
NPK-amended: Soil amended with NPK fertilizer (200 kg·ha−1).
NPK + Spike: Soil amended with both NPK fertilizer (200 kg·ha−1) and the 50 mM citric acid solution (applied to maintain soil pH within 5.8–6.7).
All other growth conditions were identical to those described for the short-term assay (
Section 2.2.1).
2.3. Plant Harvest, Sample Preparation, and Analytical Procedures
Upon termination of the experiments, plant height was measured. Plants were carefully uprooted, and the root system was rinsed thoroughly with deionized water to remove adhering soil particles. For the full-cycle trial, plants were separated into roots, stems, and leaves. All plant material was oven-dried at 70 °C until a constant dry weight was achieved, and the biomass was recorded [
29].
Dried plant tissues were ground to a fine powder using a stainless-steel mill, RETSCH GmbH, Haan, Germany. For acid digestion, approximately 0.2–0.5 g of ground material was accurately weighed and placed into a digestion vessel. A two-step acid digestion procedure was employed [
29], adding 5–10 mL of concentrated HNO
3 to break down the organic matrix, followed by 1–2 mL of H
2O
2 as an oxidizing agent to complete the digestion of any remaining organic matter. Digestion was performed using a microwave digestion system (MARS 6, CEM Corporation, USA). The digestion program for plant tissues (adapted from USEPA Method 3052) was as follows: the temperature was ramped to 180 °C over 10 min and held at 180 ± 5 °C for 10 min, with a maximum operating pressure of 350 psi. The resulting digestates were cooled, filtered through Whatman filter paper (RETSCH GmbH, Haan, Germany), and diluted to 50 mL with deionized water [
30]. The resulting digestates were cooled, filtered through Whatman filter paper, and diluted to 50 mL with deionized water. Metal concentrations were determined by ICP-MS [
26]. The ICP-MS (Agilent 7900, USA) was calibrated using a series of multi-element calibration standards (Agilent Technologies) prepared in the same acid matrix as the samples. The calibration curve exhibited a linear response with a correlation coefficient (R
2) of >0.999 for all analyzed elements. Instrument tuning was performed daily with a tuning solution (Li, Y, Ce, Tl) to optimize sensitivity and minimize oxide and doubly charged ion formation. Internal standards (
45Sc,
89Y,
115In,
159Tb,
209Bi) were online added to all samples, blanks, and standards to correct for matrix effects and instrumental drift. The QA/QC protocol included the analysis of CRM NIST 1573a (Tomato Leaves) and procedural blanks. Recovery rates for the CRM were within acceptable limits (90–110%).
2.4. Calculation of Bioaccumulation and Translocation Factors
The efficiency of metal uptake and translocation was evaluated using standard indices:
The Bioaccumulation Factor (BAF) was calculated to quantify the plant’s ability to accumulate metals from the soil [
31]:
where C
plant is the metal concentration in the plant tissue (mg·kg
−1 dry weight) and C
soil is the total metal concentration in the soil (mg·kg
−1). A BAF > 1 indicates net accumulation.
The Translocation Factor (TF) was calculated to assess the efficiency of metal transfer from roots to shoots [
31]:
where C
shoot and C
root are the metal concentrations in the shoot and root tissues, respectively. A TF > 1 indicates preferential translocation to aerial parts.
2.5. Statistical Analysis
All data are presented as mean ± standard deviation (SD) of biological replicates (n = 5 for the 6-week trial, n = 6 for the 6-month full-cycle trial). Statistical analyses were performed using SPSS Statistics software (Version 26.0, IBM Corp., Armonk, NY, USA) [
32]. Data were tested for normality (Shapiro–Wilk test) and homogeneity of variances (Levene’s test) prior to analysis. Significant differences between treatment means were determined using a one-way analysis of variance (ANOVA) followed by Tukey’s Honestly Significant Difference (HSD) post hoc test [
33]. Differences were considered statistically significant at
p < 0.05.
2.6. Generative AI Statement
Generative artificial intelligence (GenAI, ChatGPT,
https://chatgpt.com/) was used during the preparation of this manuscript for superficial editing of grammar, spelling, and formatting. The authors reviewed and edited the content as needed, and they take full responsibility for the publication’s content.
3. Results and Discussion
3.1. Soil Metal Composition and Contamination Profile
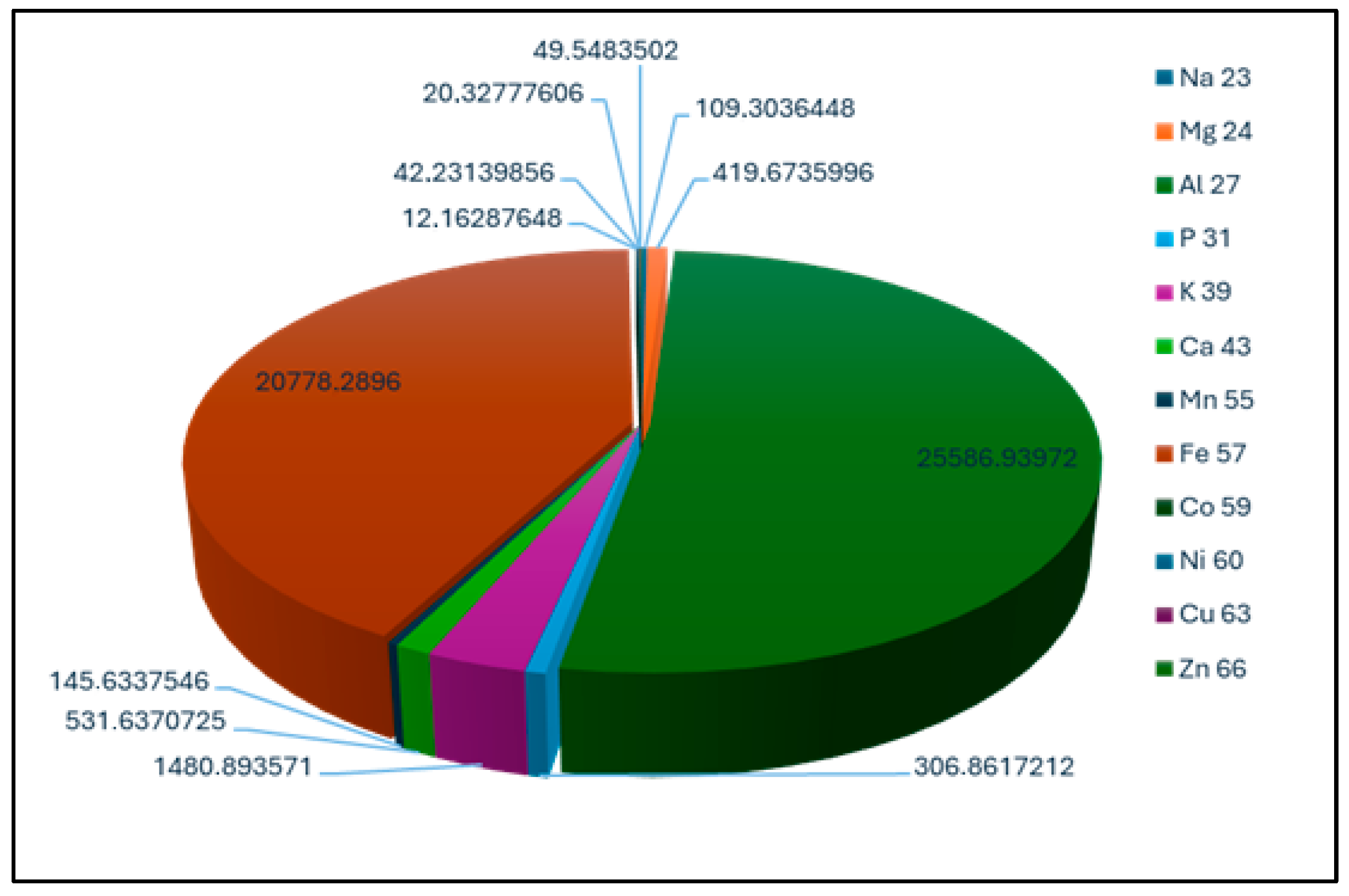
Inductively coupled plasma mass spectrometry (ICP-MS) analysis revealed a complex metal profile in the evaporation dam soil, characterized by both elevated essential nutrient concentrations and significant multi-metal contamination (
Figure 1). The soil showed exceptionally high concentrations of essential macronutrients, with iron (25,478 ± 1289 mg·kg
−1), magnesium (12,142 ± 876 mg·kg
−1), and potassium (8367 ± 542 mg·kg
−1) dominating the elemental composition [
34,
35,
36]. These elements provide crucial support for plant metabolic functions, including chlorophyll synthesis, enzyme activation, and osmoregulation.
However, this natural fertility was compromised by substantial contamination with phytotoxic elements. Aluminum (5218 ± 321 mg·kg
−1) and chromium (2147 ± 198 mg·kg
−1) were present at concentrations known to inhibit plant growth through disruption of root development and nutrient uptake mechanisms [
37,
38]. Particularly concerning was the detection of radioactive elements, including thorium (312 ± 28 mg·kg
−1) and uranium (158 ± 14 mg·kg
−1), which indicate historical industrial contamination. Uranium poses significant environmental risks due to its mobility under acidic conditions and potential for bioaccumulation in food chains [
39,
40,
41,
42].
The simultaneous presence of essential nutrients and toxic metals creates a complex soil environment that demands careful management for successful phytoremediation. While the macronutrient content provides a foundation for plant establishment, the toxic metal load requires species with exceptional tolerance mechanisms. Industrial hemp (
Cannabis sativa L.) has demonstrated such capabilities in previous studies, making it an ideal candidate for evaluating phytoremediation potential in this challenging environment. While other plants may hyperaccumulate specific metals more effectively, hemp was chosen for its balanced and synergistic combination of robust growth, high total biomass, deep roots, broad-spectrum metal tolerance, and potential for valorization of the harvested biomass. This makes it a more comprehensive and scalable solution for the multi-metal contamination profile encountered in this research [
43].
This contamination profile establishes both the challenges and opportunities for remediation efforts. Natural fertility may support initial plant growth, while the diverse metal load provides a rigorous test of hemp’s phytoextraction capabilities under multi-metal stress conditions.
Despite this inherent fertility, the soils were characterized by elevated levels of phytotoxic metals. Aluminum (Al) and chromium (Cr) were present in significantly high concentrations. Aluminum toxicity, particularly in its soluble trivalent form (Al
3+), is a major constraint to plant growth in acidic soils, primarily through the inhibition of root elongation and subsequent disruption of water and nutrient uptake [
37]. Chromium, especially in its hexavalent state (Cr(VI)), is highly mobile and toxic, impairing plant development by disrupting nutrient absorption, causing oxidative stress, and reducing photosynthetic efficiency [
38]. The co-occurrence of these metals creates a compounded abiotic stress, often manifesting as stunted growth, chlorosis, and reduced biomass in non-tolerant species [
44].
Furthermore, the analysis detected the presence of radioactive elements, including uranium (U) and thorium (Th), as well as barium (Ba) (
Figure 2). The presence of U and Th is highly indicative of long-term industrial contamination and is uncommon in agricultural settings [
40]. Uranium is of particular concern due to its chemotoxic and radiotoxic properties and its potential mobility, especially under acidic or chelated conditions, which raises significant concerns regarding groundwater contamination and entry into the food chain [
39]. The soil also contained moderate levels of essential micronutrients such as zinc (Zn) and copper (Cu), which are crucial for numerous enzymatic functions and photosynthetic processes [
41,
42]. However, their bioavailability to plants was likely compromised by the antagonistic effects of the prevalent toxic metals and the overall complex soil chemistry, potentially leading to nutritional imbalances that impose additional physiological stress on plants [
44].
In conclusion, the soil composition presents a dual nature: it possesses a sufficient reservoir of macronutrients to sustain basic plant functions, yet it is simultaneously dominated by a complex cocktail of heavy metals and radionuclides. This juxtaposition underscores the necessity for a phytoremediation candidate that possesses not only high metal tolerance but also the ability to produce substantial biomass under stressful conditions. Industrial hemp (
Cannabis sativa L.), known for its fast growth, deep root system, and resilience to contaminants, meets these criteria and was therefore selected for this remediation study [
43].
3.2. Plant Growth Responses to Soil Amendments
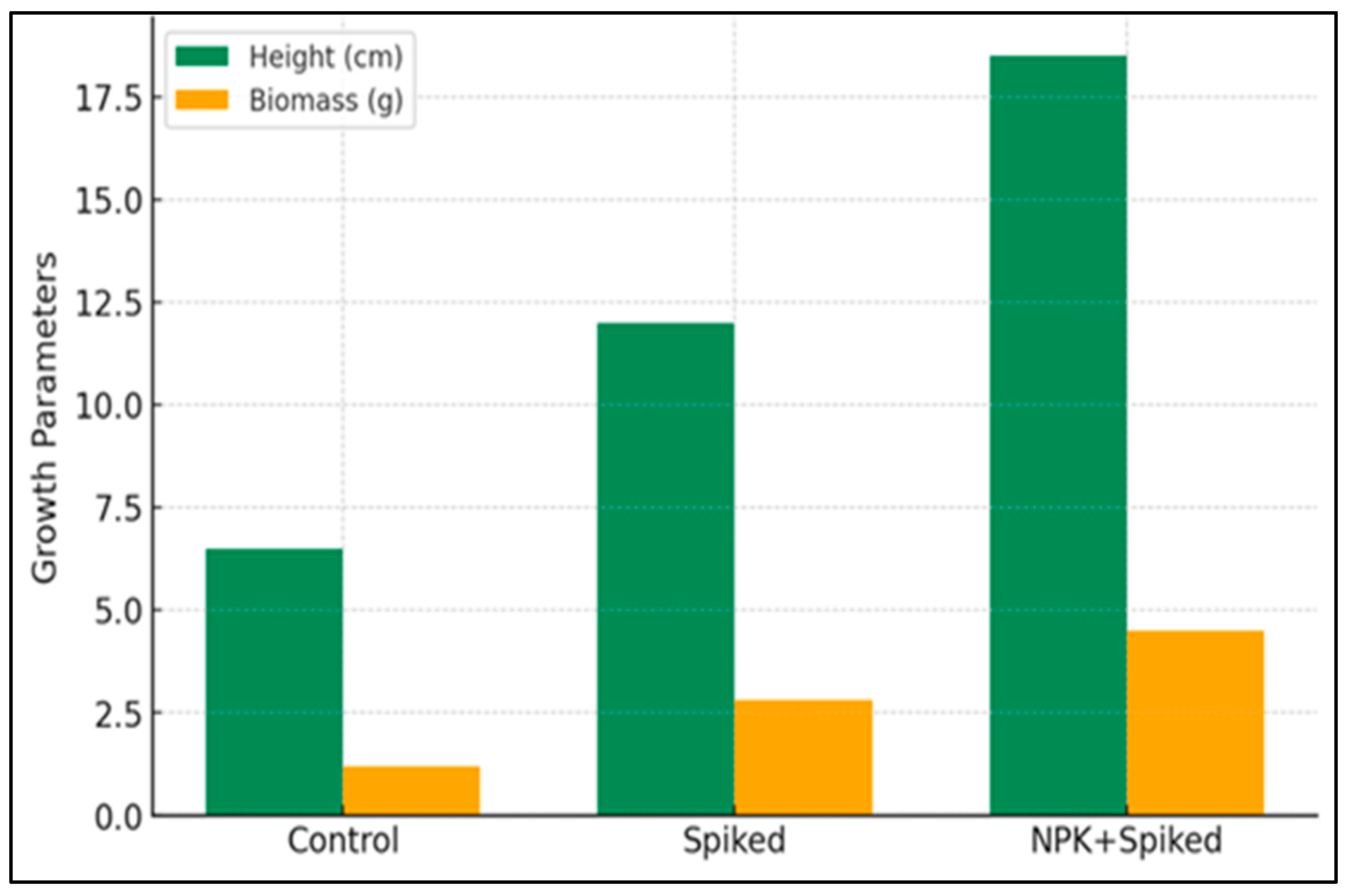
The growth of industrial hemp was significantly influenced by soil treatment, reflecting both the inherent toxicity of the uncontaminated soil and the capacity of amendments to mitigate metal-induced stress. In the control treatment (unamended soil), plants exhibited severely constrained growth, with average heights remaining below 7 cm and a mean dry biomass of approximately 1.2 g after six weeks (
Figure 3). This stunted morphology—characterized by reduced leaf expansion, chlorosis, and limited stem elongation—is a classic symptom of phytotoxicity in multi-metal contaminated environments [
44]. The combined presence of aluminum (Al) and chromium (Cr) likely played a key role in this response; Al disrupts root development and nutrient uptake [
37], while Cr(VI) induces oxidative stress and inhibits photosynthetic pigments [
38], collectively restricting carbon assimilation and biomass production.
Amending the soil with citric acid (Spiked treatment) resulted in a moderate but significant improvement in plant performance. Average plant height increased to approximately 12 cm, and biomass nearly doubled to 2.8 g. As a low-molecular-weight organic acid, citric acid acts as a chelator, forming soluble complexes with metal ions and enhancing their mobility in the soil matrix [
45]. This chelation effect likely increased the bioavailability of both essential nutrients (e.g., Fe, Zn) and non-essential toxic metals. The net positive effect on growth suggests that the benefits of improved nutrient availability—particularly of micronutrients, otherwise bound in insoluble forms—outweighed the potential increase in toxicity risk under these experimental conditions. Nonetheless, the persistence of stress symptoms indicated that chelation alone was insufficient to fully counteract the soil’s high toxic load.
The most pronounced growth enhancement was observed in the NPK + Spike treatment, where plants achieved the greatest height (>18 cm) and highest biomass (≈4.5 g). This result highlights a clear synergistic effect between metal chelation and nutrient supplementation. The citric acid enhanced the solubility of soil-bound nutrients, while the application of NPK fertilizer directly addressed macronutrient deficiencies, supporting critical metabolic functions including photosynthesis, protein synthesis, and energy transfer [
46]. Nitrogen is integral to chlorophyll and amino acid production, phosphorus supports root development and energy metabolism (ATP) [
47,
48], and potassium regulates stomatal function and enzyme activation [
35]. The improved nutrient status likely enhanced plant tolerance to metal stress by promoting robust root systems and increasing the production of metal-chelating compounds (e.g., phytochelatins and organic acids) within plant tissues [
49].
The strong positive correlation between biomass production and metal extraction efficiency underscores the agronomic importance of balanced fertilization in phytoremediation strategies [
50]. By alleviating nutrient deficiencies and mitigating metal toxicity, the combined NPK and citric acid treatment maximized hemp’s potential for metal uptake and accumulation as a prerequisite for successful phytoextraction. The use of citric acid, a biodegradable chelator, was key to enhancing metal uptake while mitigating the long-term leaching risks associated with more persistent synthetic agents, thus upholding the principle of sustainable phytoremediation. The synergistic effect of NPK and citric acid on metal uptake can be explained by a division of labor: citric acid primarily enhanced metal mobility in the soil by forming soluble complexes, while NPK fertilization improved the plant’s physiological capacity for metal translocation. Adequate nutrition likely supported a stronger transpiration stream for xylem transport and the synthesis of internal chelators like phytochelatins, facilitating metal sequestration in aerial tissues and resulting in the higher observed translocation factors.
In conclusion, although hemp demonstrated a degree of innate tolerance to multi-metal contamination, its growth was markedly suppressed in unamended soil. Chelation with citric acid moderately improved growth by enhancing nutrient bioavailability, but the most effective strategy resulting in significantly greater biomass and plant vigor was the integration of chelation with NPK nutrition. These findings emphasize that effective phytoremediation of complexly contaminated sites requires integrated soil management strategies that address both toxicity and fertility constraints.
3.3. Metal Uptake and Bioaccumulation Patterns
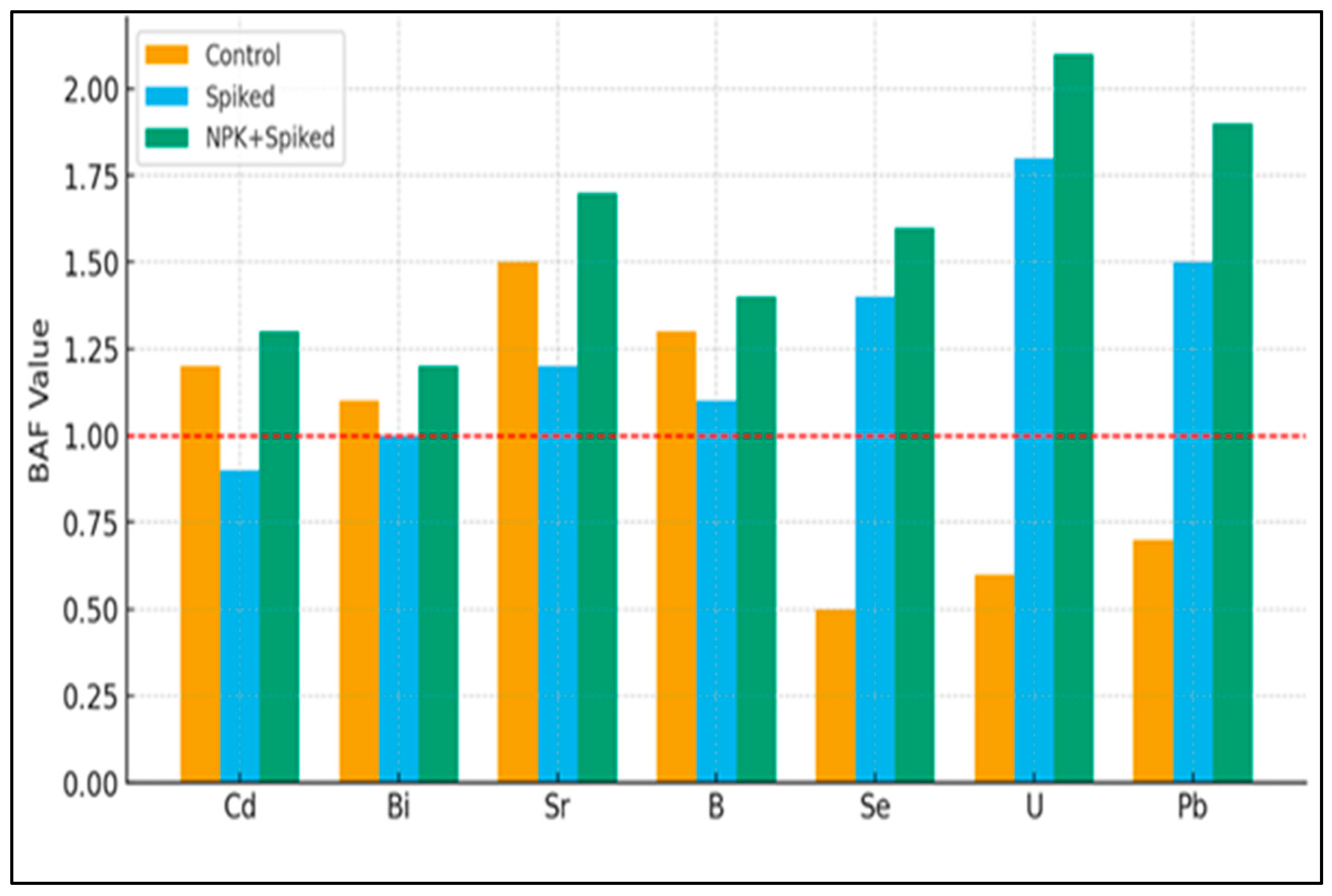
Analysis of bioaccumulation factors (BAF) revealed distinct metal uptake patterns across treatments, highlighting the interplay between soil chemistry, chelation, and nutrient availability. In control plants, BAF values exceeded unity (BAF > 1) for boron (B), strontium (Sr), cadmium (Cd), and bismuth (Bi) (
Figure 4). This pattern can be explained by the principle of ionic mimicry, where non-essential elements are absorbed by transporters for essential nutrients due to chemical similarity. Specifically, B is an essential micronutrient actively taken up by plants. Sr is a chemical analog of calcium (Ca) and is co-transported, Cd is mistaken for zinc (Zn) and iron (Fe) by divalent cation transporters, and Bi is likely absorbed via non-selective pathways. This inadvertent uptake, a consequence of hemp’s robust nutrient acquisition system, underpins its innate ability to accumulate these specific metals even in unamended soil. The accumulation of Sr likely occurs via calcium transport pathways due to their chemical similarity [
51,
52,
53,
54,
55], while Cd may be absorbed through divalent cation transporters, mistakenly mimicking essential nutrients like Zn or Fe. Despite these favorable BAF values, the severely restricted biomass of control plants limited the total amount of metals sequestered, underscoring that accumulation efficiency alone is insufficient for effective remediation without adequate plant growth [
56,
57,
58,
59].
The addition of citric acid (Spiked treatment) significantly altered uptake dynamics. Chelation enhanced the accumulation of selenium (Se), uranium (U), and palladium (Pd), in addition to Cd. Citric acid functions as an effective chelator, forming soluble complexes with metal ions, thereby increasing their phytoavailability and mobility in the soil solution [
45,
55]. This mechanism was particularly consequential for U, a radionuclide typically characterized by low solubility and bioavailability in soil [
39]. The pronounced uptake of U highlights the potential of chelate-assisted phytoextraction for remediating radionuclide-contaminated sites. The use of citric acid, a biodegradable chelator, was a conscious choice to mitigate the long-term leaching risks associated with persistent synthetic agents like EDTA. Furthermore, the synergistic effect observed in the NPK + Spike treatment, where enhanced plant growth and transpiration are likely concurrent with metal uptake, presents a strategy to maximize phytoextraction while potentially minimizing the pool of mobile metals available for leaching. Nevertheless, field-scale validation, including leaching assessment, remains an essential next step. However, this approach necessitates careful management to mitigate the risk of metal leaching and subsequent groundwater contamination [
55].
The most effective strategy for enhancing both metal uptake and total extraction was the NPK + Spike treatment. This combination yielded the highest BAF values for several metals, including Cd (BAF = 1.3), U (BAF = 2.1), Pb (BAF = 1.9), and Sr (BAF = 1.7). The synergistic effect of chelation and nutrition is evident: citric acid mobilized soil-bound metals, while NPK fertilization alleviated nutrient deficiencies, promoting robust root development and overall plant vigor [
46]. Enhanced root biomass and vitality increase the soil volume explored, thereby improving access to bioavailable metal complexes [
25]. Furthermore, improved plant physiological status likely supported more efficient translocation, compartmentalization, and storage of metals in aerial tissues [
49], as evidenced by the higher BAF values. Consequently, this treatment achieved the highest total metal removal due to the combination of superior accumulation efficiency and significantly greater biomass production [
57].
In summary, hemp demonstrates a natural propensity to accumulate specific metals, a capacity that can be markedly enhanced through soil amendment. Chelation with citric acid effectively mobilizes poorly soluble elements like U, broadening the spectrum of metals available for uptake. However, the integration of NPK fertilization with chelation proves to be the most effective strategy, as it simultaneously optimizes plant growth and bioaccumulation efficiency. This combined approach maximizes the total phytoextraction capacity of hemp, reinforcing its suitability for the remediation of soils with complex multi-metal contamination.
3.4. Tissue-Specific Metal Accumulation and Translocation
The distribution of metals within hemp plants was highly tissue-specific, with leaves consistently exhibiting higher concentrations of most metals compared to stems, establishing them as the primary sink for metal sequestration (
Table 1,
Figure 5). Quantitative analysis revealed that concentrations of cadmium (Cd) and lead (Pb) in leaves were nearly double those in stems, yielding stem-to-leaf translocation factors (TF
leaf/stem) of 2.08 and 2.00, respectively (
Table 1). A similar pattern was observed for uranium (U, TF = 2.00) and selenium (Se, TF = 2.20), indicating efficient translocation from vascular tissues into leaf mesophyll. Statistical analysis confirmed that metal concentrations in leaves were significantly higher than in stems for all these elements (
p < 0.05).
This pronounced translocation to aerial tissues is driven by the transpiration stream, where metals absorbed by the roots are mobilized through the xylem and ultimately deposited in leaves [
51]. The high transpiration rates in leaves, essential for cooling and nutrient flow, result in the accumulation of metals that are not readily redistributed via the phloem [
51]. From a phytoremediation perspective, this is a highly desirable trait. The preferential accumulation of contaminants in harvestable above-ground biomass, rather than in roots, greatly enhances the feasibility and efficiency of phytoextraction strategies [
58].
The consistent TFleaf/stem values > 1 for Cd, Pb, U, and Se demonstrate that hemp possesses efficient physiological mechanisms for translocating a diverse range of contaminants, including both heavy metals and radionuclides. This broad-spectrum translocation capacity is a key attribute for remediating sites with complex multi-metal contamination.
A notable exception to this pattern was observed for silver (Ag), which was preferentially retained in stem tissues. This suggests a different detoxification strategy, whereby the plant sequesters particularly toxic or non-essential elements in less metabolically active tissues to protect sensitive photosynthetic machinery in the leaves [
59]. Such compartmentalization is a well-documented tolerance mechanism in metal hyperaccumulators and tolerant species, preventing cellular damage in critical organelles [
49].
In conclusion, the general tendency for metals to accumulate in leaves underscores hemp’s strong potential for phytoextraction, as the primary contaminated biomass is easily harvestable. The concurrent, metal-specific strategy of stem sequestration for elements like Ag also illustrates the plant’s sophisticated internal detoxification mechanisms, which contribute to its overall resilience in highly contaminated environments. These findings highlight the importance of considering tissue-specific metal distribution when designing harvesting protocols for phytoremediation campaigns.
3.5. Temporal Dynamics of Metal Uptake: Short-Term vs. Long-Term Trials
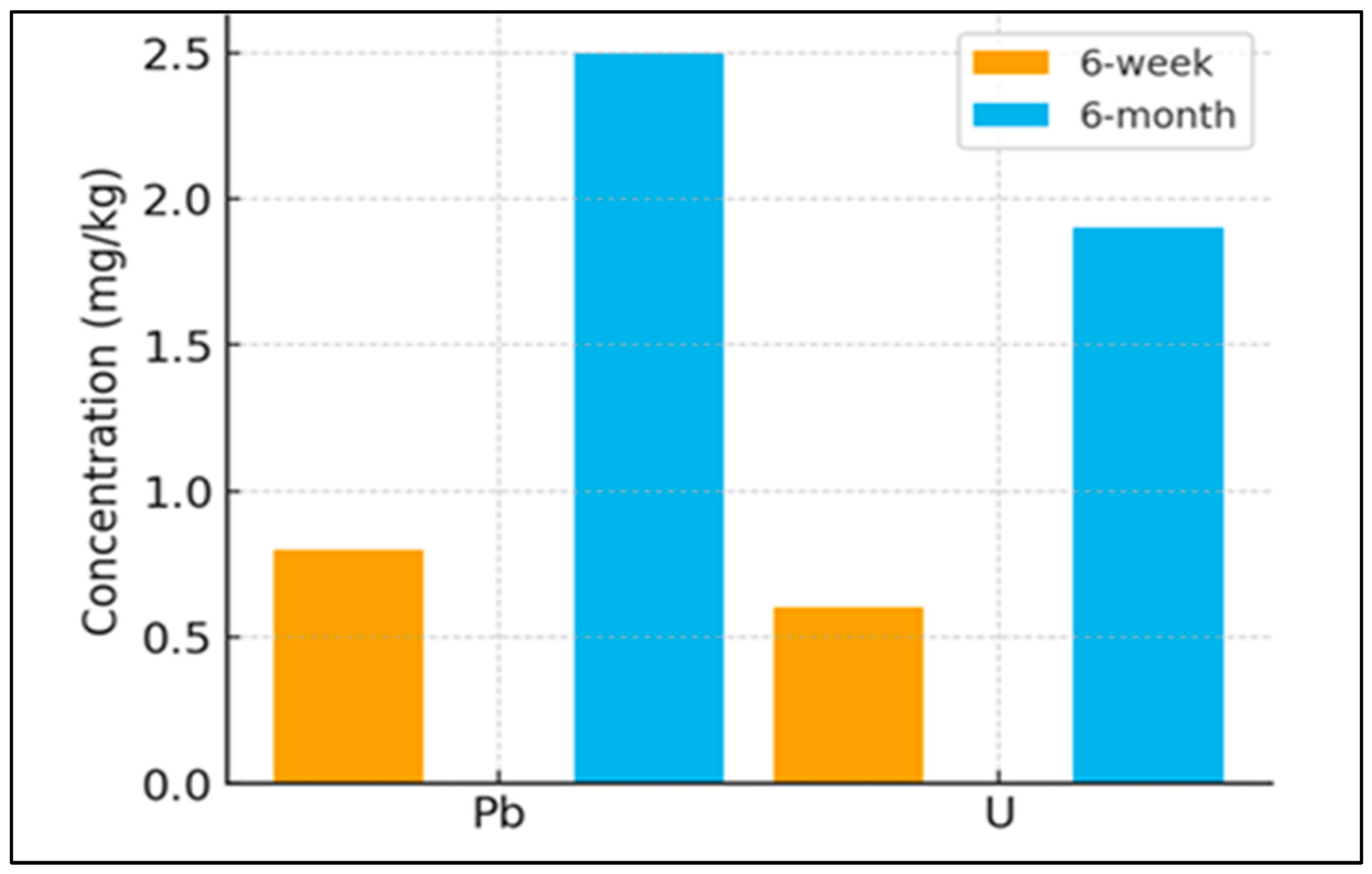
A comparative analysis of the six-week blasto-filtration trial and the six-month full-cycle greenhouse trial (
Figure 6) revealed significant temporal dynamics in metal uptake patterns and biomass development, underscoring the importance of exposure duration in phytoremediation efficacy.
In the six-week trial, hemp plants exhibited a broader spectrum of metal uptake, with low concentrations (generally <1.0 mg·kg
−1) of numerous trace elements detected. This pattern is characteristic of the initial phase of phytoextraction, where readily soluble and bioavailable metals in the soil solution are rapidly absorbed by the root system [
60]. The presence of a citric acid chelator in the “spiked” treatments further enhanced this early-stage mobility, allowing for the detection of elements like uranium and palladium. However, the limited duration of the trial restricted both plant growth and the translocation process, resulting in low absolute metal concentrations and modest total sequestration per plant, despite the diversity of elements accumulated.
In contrast, the six-month trial demonstrated a more focused and intense accumulation profile. The substantial increase in metal concentration and total extraction in the six-month trial underscores the necessity of full-lifecycle studies for accurately quantifying phytoremediation efficacy, as short-term assays primarily reflect initial bioavailability and underestimate total potential. While the number of metals detected was narrower, the concentrations of accumulated metals were substantially higher. For instance, lead (Pb) and uranium (U) reached concentrations of approximately 2.5 mg·kg
−1 and 1.9 mg·kg
−1, respectively—several times greater than those observed in the short-term experiment. This outcome can be attributed to two key factors: (1) the development of an extensive root system, which explored a larger soil volume and accessed less readily available metal pools over time [
61], and (2) a significant increase in biomass, which provided a greater sink for metal storage and dilution [
62]. The extended growth period also allowed for repeated cycles of transpiration, progressively enhancing the translocation and sequestration of metals in above-ground tissues, particularly leaves [
51].
These findings highlight a critical, time-dependent trade-off in phytoremediation: short-term trials are highly effective for screening the potential of plant species and amendments to uptake a wide range of metals quickly, providing valuable data on bioavailability and initial tolerance [
63]. However, they may underestimate the total extraction capacity. Long-term trials, while potentially showing uptake of fewer elements, provide a more accurate representation of the total metal removal achievable in a field setting, as they account for the effects of sustained growth, increased biomass, and progressive accumulation [
64].
In practical terms, these results suggest that an optimized phytoremediation strategy could employ a two-phase approach: an initial rapid screening of promising cultivars and soil amendments using short-term pot trials, followed by the implementation of long-term cultivation with the most effective candidates to maximize contaminant removal from the soil at scale.
3.6. Visual Growth Response to Soil Treatments
Qualitative morphological assessments provided a clear visual corroboration of the quantitative growth and biomass data, reinforcing the physiological impact of the different soil treatments. The observed phenotypes directly reflected the degree of metal stress and the efficacy of the applied amendments. The visual superiority of the plants in the NPK + Spiked treatment is a direct result of the synergistic effect of the combined amendments. This is clearly demonstrated in
Figure 7, which compares plant morphology and quantifies height across the three treatments. The figure shows that the combined amendment resulted in the tallest plants, with statistically significant differences in growth.
Control plants, grown in the unamended contaminated soil, exhibited severe stunting and chlorosis. Their leaves were small, pale green, and showed signs of interveinal yellowing, a classic symptom of nutrient deficiency and metal toxicity [
44]. Stem development was poor, resulting in spindly and weak architecture with limited branching. This phenotype is consistent with the known effects of aluminum (Al) and chromium (Cr) toxicity, which inhibit root development and disrupt the uptake and translocation of essential nutrients like magnesium and iron, leading to impaired chlorophyll synthesis [
37,
38]. The overall appearance confirmed the high level of abiotic stress imposed by the multi-metal contaminated soil in its untreated state.
Plants in the Spiked treatment (citric acid only) showed a moderate improvement in vigor. Foliage was a darker green compared to the control, and leaves were slightly larger, indicating a partial alleviation of nutrient deficiencies likely due to the chelation of micronutrients such as Fe and Zn [
52]. Stem elongation was also more pronounced. However, these plants still displayed symptoms of stress, including occasional necrotic spots on older leaves and somewhat uneven growth. This suggests that while chelation improved the availability of some essential nutrients, it concurrently increased the bioavailability of toxic metals like Cd and U, imposing a new physiological burden that manifested as residual toxicity symptoms [
45].
The most robust and healthy morphology was observed in the NPK + Spiked treatment. These plants were visibly taller, with strong, thick stems and a well-branched architecture. The leaves were broad, a deep green color, and showed no visible signs of chlorosis or necrosis. This vigorous canopy development is indicative of high photosynthetic capacity and efficient carbon assimilation [
61]. The visual superiority of these plants is a direct result of the synergistic effect of the combined amendments: the citric acid enhanced the solubility of both nutrients and metals, while the NPK fertilization provided a readily available source of macronutrients that supported fundamental metabolic processes, including photosynthesis, protein synthesis, and cell expansion [
46]. This balanced nutritional support allowed the plants to better tolerate the uptake and sequestration of toxic metals.
These qualitative observations provide a compelling visual narrative that aligns perfectly with the quantitative data on biomass and height. They underscore a critical principle for phytoremediation: successful plant establishment and growth on contaminated sites requires more than just metal tolerance. An integrated soil management strategy that addresses both the mobilization of desired elements (via chelation) and the supplementation of limiting nutrients is essential to unlock the full phytoremediation potential of a species like hemp, enabling it to thrive under conditions of multi-metal stress.
4. Study Limitations
This study provides valuable insights into the synergistic effects of chelation and fertilization on hemp’s phytoremediation potential; however, several limitations should be considered. The experiments were conducted under controlled greenhouse conditions, which do not fully replicate field variability such as fluctuating climate, soil heterogeneity, microbial activity, and plant competition. Consequently, the proposed NPK + chelation strategy requires validation under field-scale conditions to confirm its real-world applicability.
The potential for metal leaching presents another limitation. Although citric acid is a biodegradable chelating agent, it may enhance metal mobility and increase the risk of leaching, particularly in high-rainfall environments or in soils with low vegetative cover. Since leachate monitoring was not included in the experimental design, this risk remains unquantified and warrants further investigation prior to large-scale application.
Additionally, the absence of an “NPK-only” treatment limited the ability to isolate the individual effects of fertilization and chelation on plant growth and metal uptake. Future studies should adopt a full factorial design to better quantify these independent and interactive effects.
Physiological responses were primarily evaluated through growth and visual assessments. Including biochemical and physiological indicators, such as photosynthetic performance, oxidative stress markers, and antioxidant enzyme activity, would strengthen the mechanistic understanding of metal stress alleviation.
Finally, the fate of the harvested, metal-enriched biomass was not examined. Assessing its safe disposal, treatment, or valorization (e.g., for bioenergy or metal recovery) is essential for evaluating the environmental and economic feasibility of the remediation cycle.
Overall, while the present study advances understanding of hemp-based phytoremediation, future research should address these limitations to ensure sustainable, scalable applications in contaminated soils.
5. Conclusions
This study comprehensively assessed the phytoremediation potential of industrial hemp (Cannabis sativa L.) for multi-metal contaminated soil collected from a historical evaporation dam. The contaminated substrate displayed a dual nature of high inherent fertility—owing to elevated macronutrients such as Fe, K, and Mg—and severe pollution from toxic metals (Al, Cr) and radionuclides (U, Th). The results demonstrated that while hemp exhibits intrinsic tolerance and metal uptake capacity, its growth in untreated soil was severely inhibited by phytotoxic metal concentrations. The pronounced stunting and chlorosis observed in the control treatment confirmed the toxicity of the contaminated matrix. In contrast, treatments incorporating nutrient (NPK) and chelation (citric acid) amendments markedly improved plant vigor and metal uptake, with the combined NPK + citric acid treatment yielding the greatest biomass and bioaccumulation of key metals including Cd, Pb, and U.
The observed synergy between NPK fertilization and citric acid chelation underscores the importance of integrated amendment strategies. Citric acid alone enhanced nutrient and metal mobility but risked increased metal stress, while NPK alone did not fully overcome limited metal bioavailability. Their combined application, however, simultaneously alleviated nutrient deficiencies and reduced metal-induced stress, thereby enabling robust growth and efficient metal extraction. This integrative approach effectively transformed hemp from a stress-tolerant species into a high-performing phytoextractor.
Nevertheless, several limitations temper the interpretation of these results. The controlled greenhouse environment cannot fully replicate field conditions, including climatic fluctuations, soil heterogeneity, and microbial dynamics. The absence of an NPK-only treatment in some trials constrained the ability to isolate treatment effects. Furthermore, the potential for metal leaching under chelation-enhanced conditions and the fate of harvested, metal-rich biomass remain critical research gaps.
In light of these constraints, future field-scale studies should validate the efficacy and safety of the NPK + chelation protocol under natural conditions, incorporating leachate monitoring and long-term soil assessments. Moreover, the sustainable management or valorization of contaminated biomass—through bioenergy conversion or metal recovery—should be prioritized to ensure environmental and economic viability. Overall, this study demonstrates that coupling nutrient enrichment with mild chelation can unlock hemp’s full potential as a sustainable, scalable tool for the remediation of multi-metal contaminated soils.
Author Contributions
Conceptualization, M.J.K.; methodology, C.J.R. and M.J.K.; software, M.J.K.; validation, M.J.K.; formal analysis, C.J.R. and M.J.K.; investigation, C.J.R. and M.J.K.; resources, M.J.K.; data curation, M.J.K.; writing—original draft preparation, C.J.R. and M.J.K.; writing—review and editing, M.J.K.; visualization, M.J.K.; supervision, M.J.K.; project administration, M.J.K.; funding acquisition, M.J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Sciences Department, Faculty of Applied and Computer Science, Vaal University of Technology South Africa.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol. 2011, 2011, 402647. [Google Scholar] [CrossRef]
- Alloway, B.J. Sources of heavy metals and metalloids in soils. In Heavy Metals in Soils, 2nd ed.; Alloway, B.J., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 11–50. [Google Scholar]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. In Molecular, Clinical and Environmental Toxicology; Luch, A., Ed.; Springer: Basel, Switzerland, 2012; pp. 133–164. [Google Scholar]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef]
- Mahar, A.; Wang, P.; Ali, A.; Awasthi, M.K.; Lahori, A.H.; Wang, Q.; Li, R.; Zhang, Z. Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: A review. Ecotoxicol. Environ. Saf. 2016, 126, 111–121. [Google Scholar] [CrossRef]
- Cunningham, S.D.; Ow, D.W. Promises and prospects of phytoremediation. Plant Physiol. 1996, 110, 715–719. [Google Scholar] [CrossRef]
- Salt, D.E.; Smith, R.D.; Raskin, I. Phytoremediation. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 643–668. [Google Scholar] [CrossRef]
- Pilon-Smits, E. Phytoremediation. Annu. Rev. Plant Biol. 2005, 56, 15–39. [Google Scholar] [CrossRef]
- Garbisu, C.; Alkorta, I. Phytoextraction: A cost-effective plant-based technology for the removal of metals from the environment. Bioresour. Technol. 2001, 77, 229–236. [Google Scholar] [CrossRef]
- Rascio, N.; Navari-Izzo, F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci. 2011, 180, 169–181. [Google Scholar] [CrossRef]
- van der Ent, A.; Baker, A.J.M.; Reeves, R.D.; Pollard, A.J.; Schat, H. Hyperaccumulators of metal and metalloid trace elements: Facts and fiction. Plant Soil 2013, 362, 319–334. [Google Scholar] [CrossRef]
- McGrath, S.P.; Zhao, F.J. Phytoextraction of metals and metalloids from contaminated soils. Curr. Opin. Biotechnol. 2003, 14, 277–282. [Google Scholar] [CrossRef]
- Linger, P.; Müssig, J.; Fischer, H.; Kobert, J. Industrial hemp (Cannabis sativa L.) growing on heavy metal contaminated soil: Fibre quality and phytoremediation potential. Ind. Crops Prod. 2002, 16, 33–42. [Google Scholar] [CrossRef]
- Ahmad, R.; Tehsin, Z.; Malik, S.T.; Asad, S.A.; Shahzad, M.; Bilal, M.; Shah, M.M.; Khan, S.A. Phytoremediation potential of hemp (Cannabis sativa L.): Identification and characterization of heavy metals responsive genes. Clean Soil Air Water 2016, 44, 195–201. [Google Scholar] [CrossRef]
- Zia ur Rehman, M.; Rizwan, M.; Ali, S.; Ok, Y.S.; Ishaque, W.; Saifullah; Nawaz, M.F.; Akmal, F.; Waqar, M. Remediation of Heavy Metal Contaminated Soils by Using Solanum nigrum: A Review. Ecotoxicol. Environ. Saf. 2017, 143, 236–248. [Google Scholar] [CrossRef]
- Citterio, S.; Santagostino, A.; Fumagalli, P.; Prato, N.; Ranalli, P.; Sgorbati, S. Heavy metal tolerance and accumulation of Cd, Cr and Ni by Cannabis sativa L. Plant Soil 2003, 256, 243–252. [Google Scholar] [CrossRef]
- Shi, G.; Cai, Q. Cadmium tolerance and accumulation in eight potential energy crops. Biotechnol. Adv. 2009, 27, 555–561. [Google Scholar] [CrossRef]
- Appel, C.; Ma, L. Concentration, pH, and surface charge effects on cadmium and lead sorption in three tropical soils. J. Environ. Qual. 2002, 31, 581–589. [Google Scholar] [CrossRef]
- Sauvé, S.; Martinez, C.E.; McBride, M.; Hendershot, W. Adsorption of free lead (Pb2+) by pedogenic oxides, ferrihydrite, and leaf compost. Soil Sci. Soc. Am. J. 2000, 64, 595–599. [Google Scholar] [CrossRef]
- Evangelou, M.W.H.; Ebel, M.; Schaeffer, A. Chelate assisted phytoextraction of heavy metals from soil: Effect, mechanism, toxicity, and fate of chelating agents. Chemosphere 2007, 68, 989–1003. [Google Scholar] [CrossRef] [PubMed]
- Meadows, M.E.; Hoffman, T.M. The nature, extent and causes of land degradation in South Africa: Legacy of the past, lessons for the future? Area 2002, 34, 428–437. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (USEPA). Method 3051A: Microwave Assisted Acid Digestion of Sediments, Sludges, Soils, and Oils; USEPA: Washington, DC, USA, 2007.
- Thomas, R. Practical Guide to ICP-MS: A Tutorial for Beginners, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Jones, J.B., Jr.; Case, V.W. Sampling, handling, and analyzing plant tissue samples. In Soil Testing and Plant Analysis, 3rd ed.; Westerman, R.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1990; pp. 389–427. [Google Scholar]
- United States Environmental Protection Agency (USEPA). Method 3052: Microwave Assisted Acid Digestion of Siliceous and Organically Based Matrices; USEPA: Washington, DC, USA, 1996.
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows, version 26.0; IBM Corp.: Armonk, NY, USA, 2019. [Google Scholar]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Statistics in Biological Research, 4th ed.; W.H. Freeman and Company: New York, NY, USA, 2012. [Google Scholar]
- Briat, J.F.; Curie, C.; Gaymard, F. Iron utilization and metabolism in plants. Curr. Opin. Plant Biol. 2007, 10, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Amtmann, A.; Armengaud, S. Effects of N, P, K and S on metabolism: New knowledge gained from multi-level analysis. Curr. Opin. Plant Biol. 2009, 12, 275–283. [Google Scholar] [CrossRef]
- Chandrasekaran, U.S.; Sangweme, R.S. Magnesium and plant growth and development. In Magnesium Nutrient Management in Plants; Springer: Berlin, Germany, 2020; pp. 15–28. [Google Scholar]
- Kochian, L.V.; Piñeros, M.A.; Liu, J. Plant adaptation to acid soils: The molecular basis for crop aluminum resistance. Annu. Rev. Plant Biol. 2015, 66, 571–598. [Google Scholar] [CrossRef]
- Shanker, A.K.; Cervantes, C.; Loza-Tavera, H.; Avudainayagam, S. Chromium toxicity in plants. Environ. Int. 2005, 31, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Gray, C.W. Uranium in the Environment: Behavior and Toxicity; Springer: Berlin, Germany, 2016. [Google Scholar]
- Sheppard, C.J. Uranium and Thorium: Mineralogy, Geochemistry, and the Environment; De Gruyter: Berlin, Germany, 2019. [Google Scholar]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in plants. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets–iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Liu, C. Hemp: A more sustainable annual energy crop for climate and energy policy. Energy Policy 2022, 163, 112828. [Google Scholar]
- Sharma, S.S.; Dietz, K.J.; Mimura, T. Vacuolar compartmentalization as indispensable component of heavy metal detoxification in plants. Plant Cell Environ. 2016, 39, 1112–1126. [Google Scholar] [CrossRef]
- Saleem, H.; Rehman, K.; Arslan, M.; Afzal, M. Enhanced degradation of phenol in floating treatment wetlands by plant-bacterial synergism. Int J. Phytoremediation 2018, 20, 692–698. [Google Scholar] [CrossRef]
- White, P.J.; Brown, P.H. Plant nutrition for sustainable development and global health. Ann. Bot. 2010, 105, 1073–1080. [Google Scholar] [CrossRef]
- MacDonald, G.K.; Bennett, E.M.; Potter, P.A. Agronomic phosphorus imbalances across the world’s croplands. Proc. Natl. Acad. Sci. USA 2011, 108, 3086–3091. [Google Scholar] [CrossRef]
- Hawkesford, M.J. Reducing the reliance on nitrogen fertilizer for wheat production. J. Cereal Sci. 2014, 59, 276–283. [Google Scholar] [CrossRef]
- Clemens, S. Molecular mechanisms of plant metal tolerance and homeostasis. Planta 2001, 212, 475–486. [Google Scholar] [CrossRef] [PubMed]
- van der Ent, A.; Baker, A.J.M.; Reeves, R.D.; Chaney, R.L.; Anderson, C.W.N. Agromining: Farming for metals in the future? Environ. Sci. Technol. 2015, 49, 4773–4780. [Google Scholar] [CrossRef] [PubMed]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Clemens, S. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 2006, 88, 1707–1719. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Zhang, S.; Zhou, W.; Liu, Y.; Liu, Y.; Wu, Y. The effects of exogenous malic acid in relieving aluminum toxicity in Pinus massoniana. Int. J. Phytoremediation 2020, 22, 669–678. [Google Scholar] [CrossRef]
- Robinson, B.H.; Fernández, J.E.; Madejón, P.; Marañón, T.; Murillo, J.M.; Green, S.R.; Clothier, B.E. Phytoextraction: Where’s the action? J. Geochem. Explor. 2015, 151, 34–40. [Google Scholar] [CrossRef]
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Rehim, A.; Hussain, S. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef]
- Greger, M.; Landberg, T. Role of rhizosphere mechanisms in metal uptake by plants. In Phytoremediation of Metal-Contaminated Soils; Springer: Berlin, Germany, 2008; pp. 97–132. [Google Scholar]
- Chaney, R.L.; Malik, M.; Li, Y.M.; Brown, S.L.; Brewer, E.P.; Angle, J.S.; Baker, A.J.M. Phytoremediation of soil metals. Curr. Opin. Biotechnol. 1997, 8, 279–284. [Google Scholar] [CrossRef]
- Baker, A.J.M. Accumulators and excluders-strategies in the response of plants to heavy metals. J. Plant Nutr. 1981, 3, 643–654. [Google Scholar] [CrossRef]
- Morel, J.L.; Echevarria, G.; Goncharova, N. Phytoextraction of heavy metals: Principles and applications. In Phytoremediation of Metal-Contaminated Soils; NATO Science Series; Springer: Berlin, Germany, 2006; pp. 1–25. [Google Scholar]
- Mench, M.; Vangronsveld, J.; Didier, V.; Clijsters, H. Progress in remediation and revegetation of the barren Jales gold mine spoil after in situ treatments. Plant Soil 2003, 249, 187–202. [Google Scholar] [CrossRef]
- Baker, A.J.M.; Brooks, R.R. Terrestrial higher plants which hyperaccumulate metallic elements. A review of their distribution, ecology and phytochemistry. Biorecovery 1989, 1, 81–126. [Google Scholar]
- McGrath, S.P.; Chaudri, A.M.; Giller, K.E. Long-term changes in the extractability and bioavailability of zinc and cadmium after sludge application. J. Environ. Qual. 2000, 29, 875–883. [Google Scholar] [CrossRef]
- Reeves, R.D.; Baker, A.J.M. Metal-accumulating plants. In Phytoremediation of Toxic Metals: Using Plants to Clean Up the Environment; Raskin, I., Ensley, B.D., Eds.; Wiley: New York, NY, USA, 2000; pp. 193–229. [Google Scholar]
- Marschner, H. Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK, 2011. [Google Scholar]
- Brown, T.; Taylor, G. Atomic Spectroscopy Techniques: A Guide to AAS and AES; Cambridge University Press: Cambridge, UK, 2018. [Google Scholar]
- Smith, J. Principles of Instrumental Analysis, 7th ed.; HarperCollins: New York, NY, USA, 2020. [Google Scholar]
- Keller, C.; Marchetti, M.; Rossi, L.; Lugon-Moulin, N. Reduction of Cadmium Availability to Tobacco (Nicotiana tabacum) Plants using Soil Amendments in Low Cadmium Contaminated Agricultural Soils: A Pot Experiment. Plant Soil 2005, 276, 69–84. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).