Electro-Oxidation of Biofloc Aquaculture Effluent Through a DoE-Driven Optimization in a Batch Reactor
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Equipment
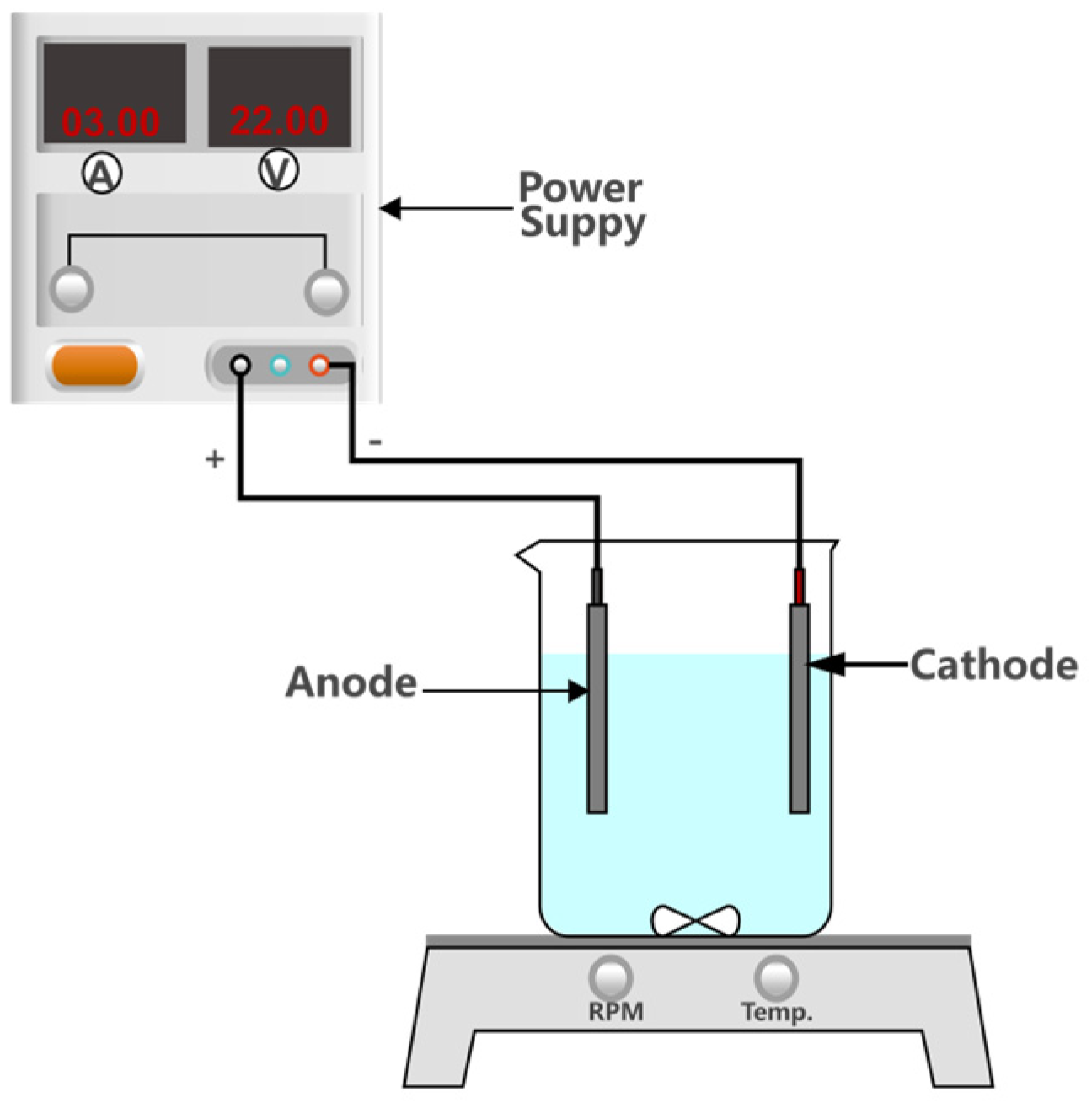
2.2. Experimental Set-Up
2.3. Analytical Methods
2.4. Taguchi Experimental Design
2.5. Operating Cost
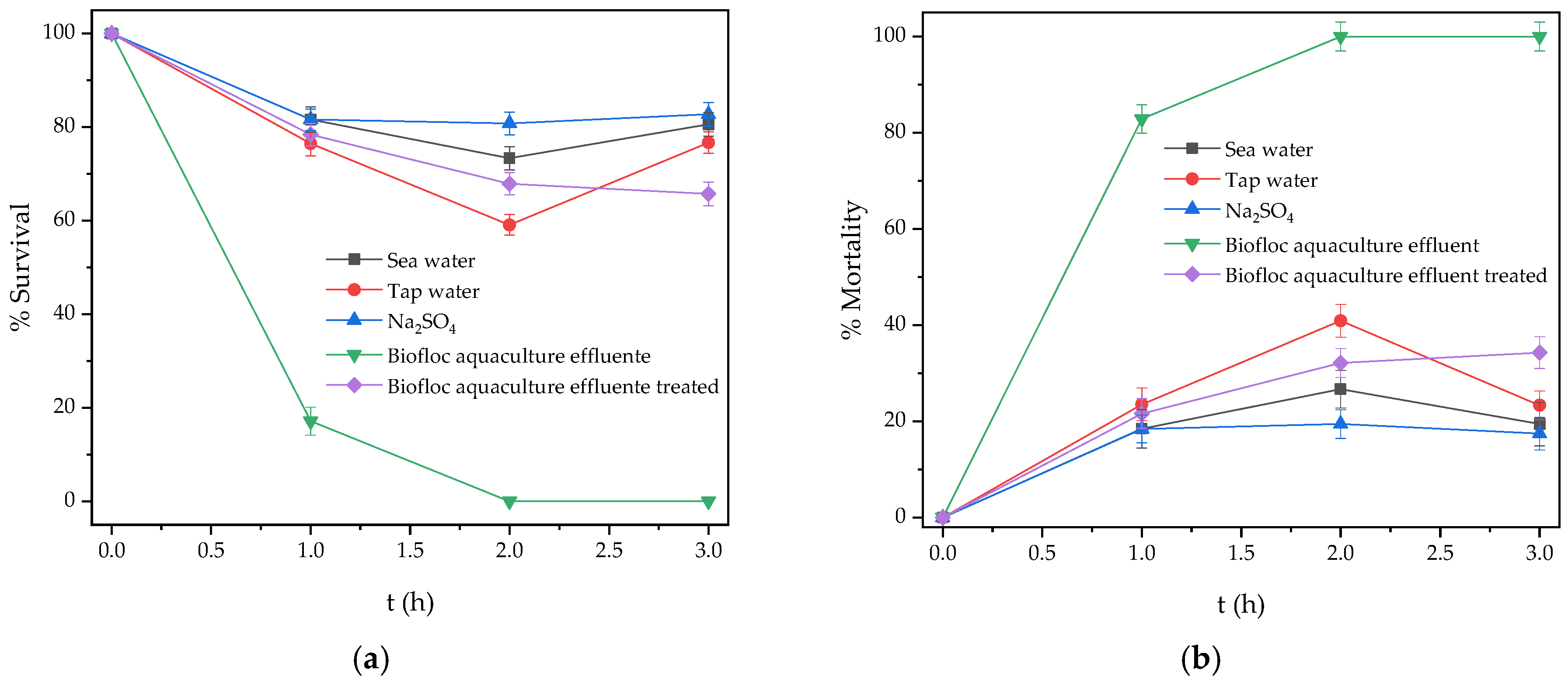
2.6. Phytotoxicity and Bioassays Test
2.6.1. Phytotoxicity
2.6.2. Bioassays with Artemia Salina
3. Results and Discussion
3.1. Removal of COD, TAN, and Turbidity
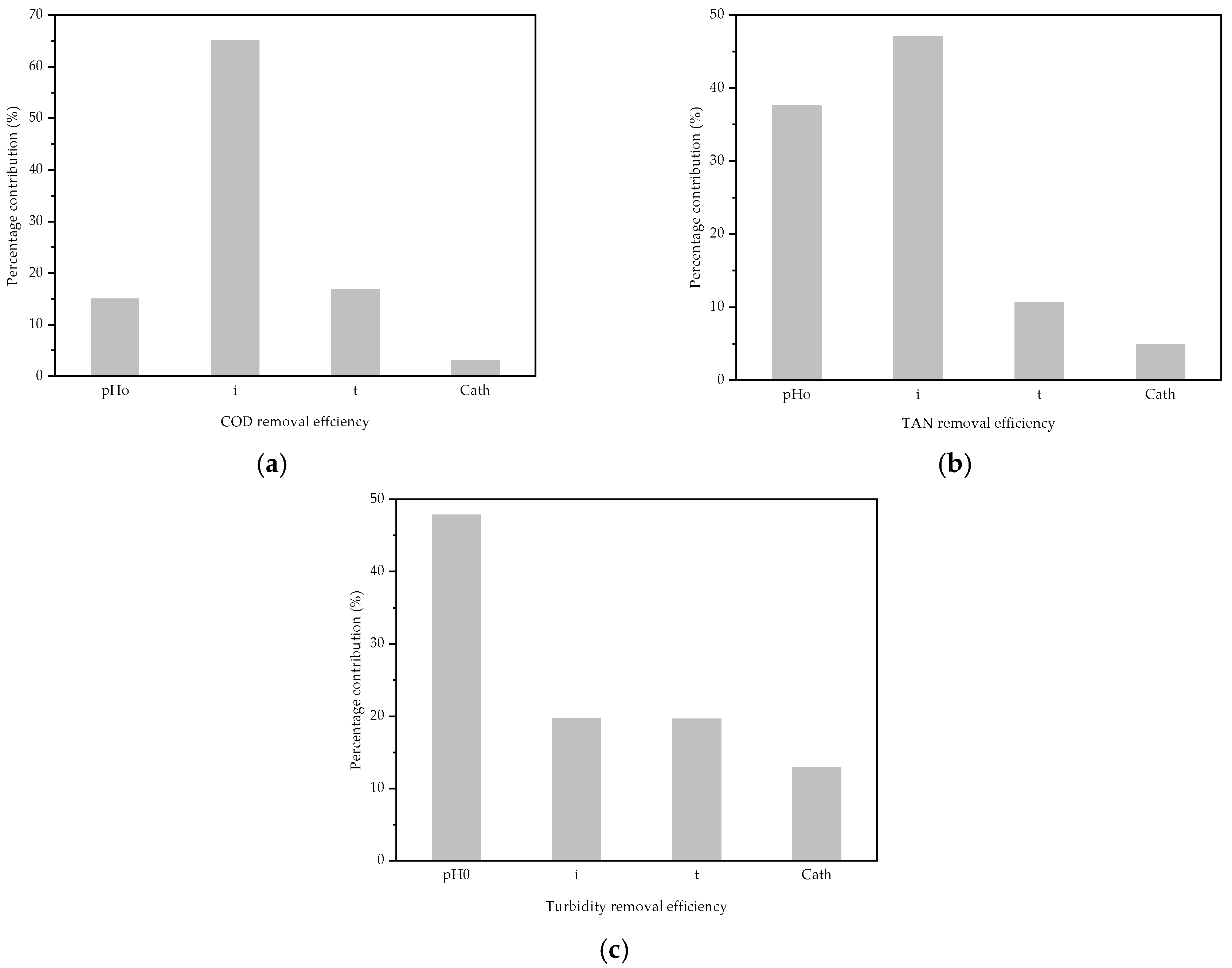
3.2. ANOVA Analysis
3.3. Optimization Process
3.4. Experimental Validation of Optimal Operating Conditions
3.5. Total Operating Cost
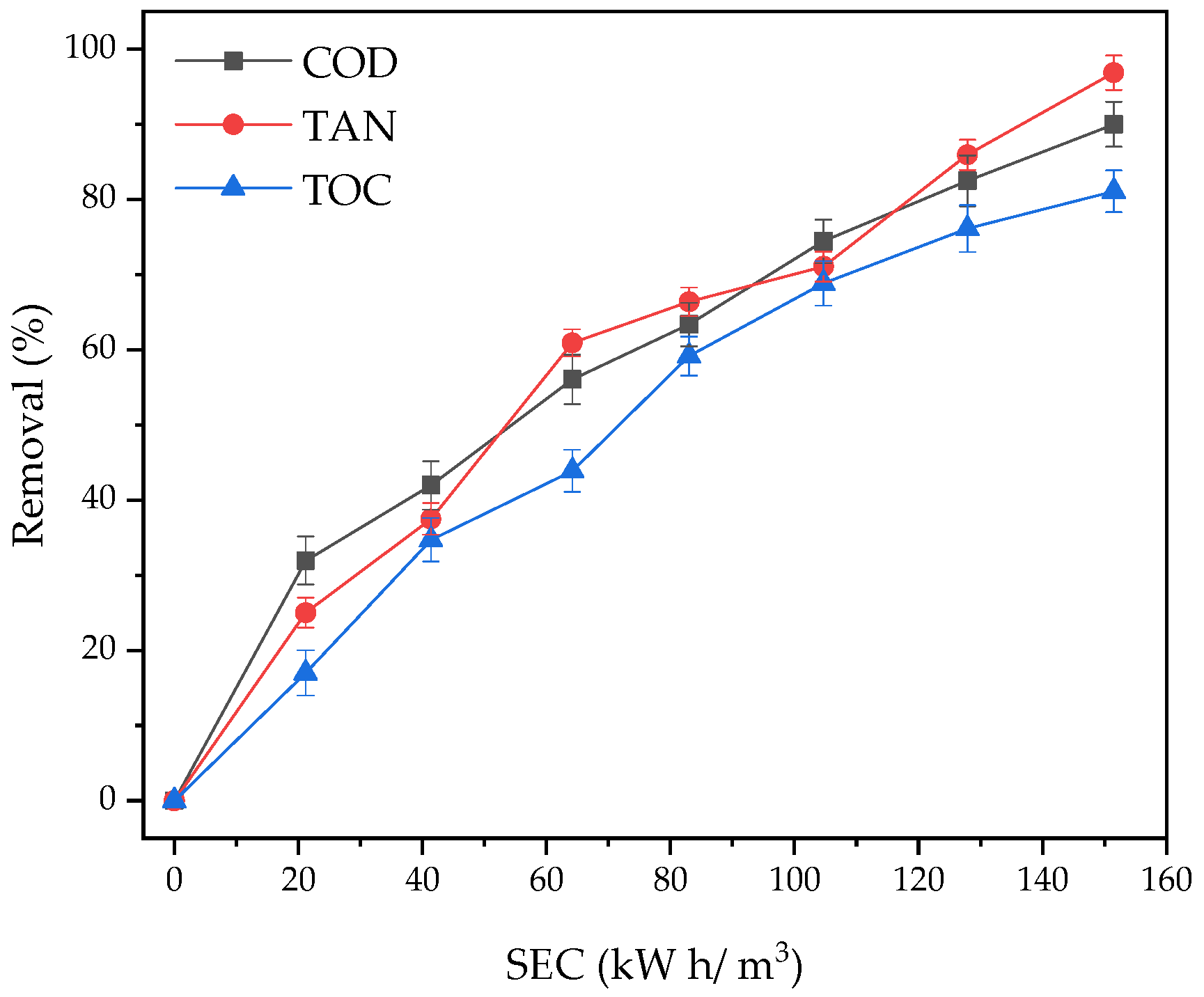
3.6. Removal Profiles
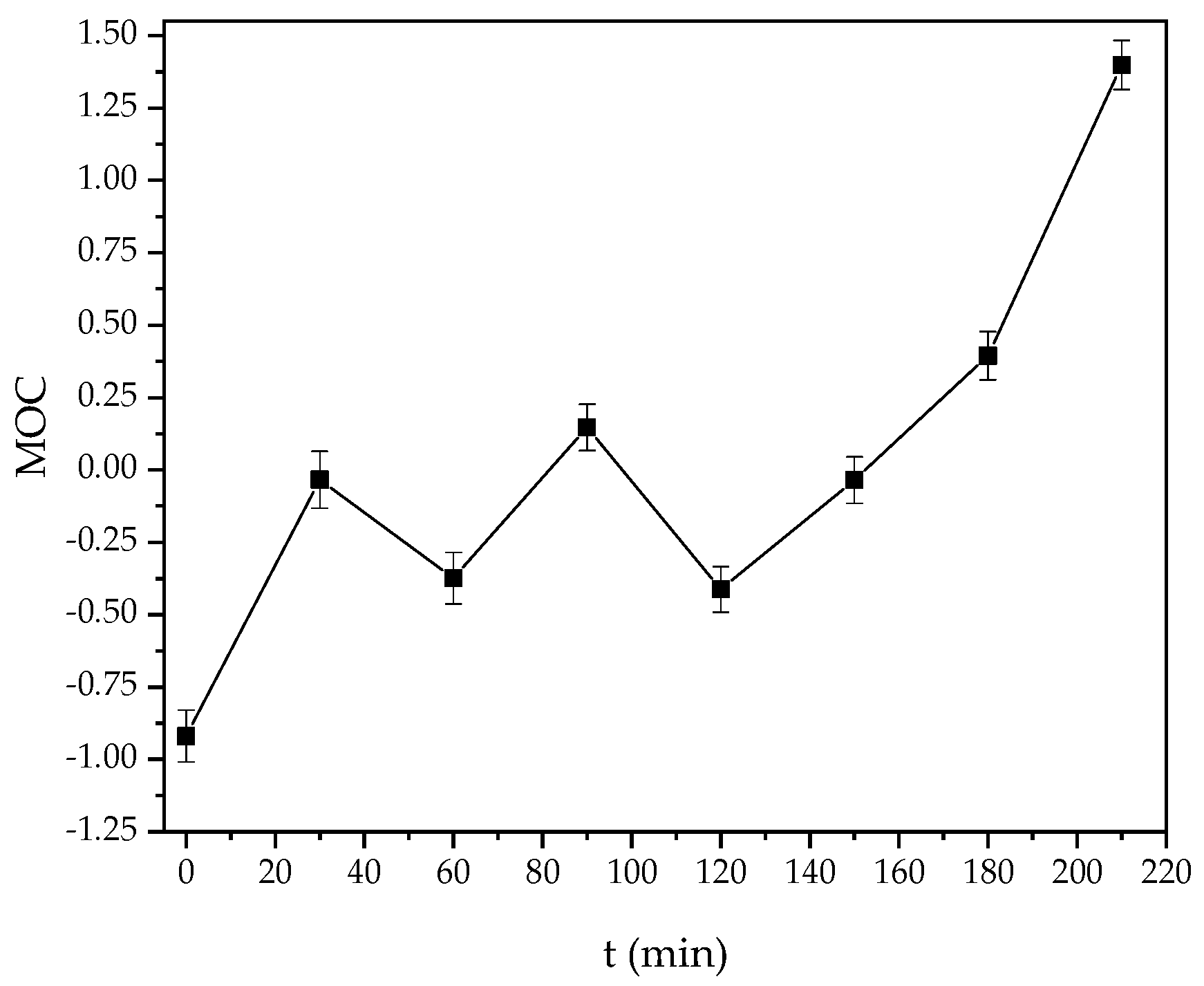
3.7. Mean Oxidation Number of Carbon (MOC)
3.8. Phytotoxicity Assays Test
3.9. Bioassay Test
3.10. Results Comparison with the Literature
4. Conclusions
- Electrochemical oxidation proved to be an efficient and effective alternative for the treatment of tilapia biofloc effluent in a batch reactor.
- The optimal operating conditions were identified as an initial pH of 11.5, a current intensity of 2 A, and a treatment time of 3.5 h using a titanium cathode. Under these parameters, removal efficiencies reached 96.57% for chemical oxygen demand (COD), 67.68% for turbidity, and 99.06% for total ammonia nitrogen (TAN), in compliance with Mexican discharge regulations (NOM-001-SEMARNAT-2021).
- Phytotoxicity assays using Vigna radiata and bioassays with Artemia salina confirmed significant detoxification of the treated effluent.
- The process demonstrated an energy consumption of 150 kWh/m3 and an estimated operating cost of USD 1 per liter, suggesting strong potential for scale-up due to expected cost reductions at the industrial level.
- Overall, the results confirm that electrochemical oxidation is a viable technology for improving aquaculture effluent quality and advancing sustainable water management in intensive farming systems.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| An | Anode |
| BDD | Boron doped diamond |
| Cat | Cathode |
| CE | Energy consumption, kWh |
| OC | Operating cost, USD/L |
| COD | Chemical Oxygen Demand, mg/L |
| Cu | Copper |
| EO | Electrooxidation |
| i | Current intensity, A |
| pH | Potential of hydrogen |
| SEC | Specific energy consumption kWh/g COD |
| t | Time, h |
| TAN | Total Ammoniacal Nitrogen, mg/L |
| Ti | Titanium |
| TOC | Total Organic Carbon, mg/L |
References
- Bhatt, P.; Huang, J.-Y.; Brown, P.; Shivaram, K.B.; Yakamercan, E.; Simsek, H. Electrochemical Treatment of Aquaculture Wastewater Effluent and Optimization of the Parameters Using Response Surface Methodology. Environ. Pollut. 2023, 331, 121864. [Google Scholar] [CrossRef]
- Kashem, A.H.M.; Das, P.; Hawari, A.H.; Mehariya, S.; Thaher, M.I.; Khan, S.; Abduquadir, M.; Al-Jabri, H. Aquaculture from Inland Fish Cultivation to Wastewater Treatment: A Review. Rev. Environ. Sci. Biotechnol. 2023, 22, 969–1008. [Google Scholar] [CrossRef]
- Díaz, V.; Ibáñez, R.; Gómez, P.; Urtiaga, A.M.; Ortiz, I. Kinetics of Electro-Oxidation of Ammonia-N, Nitrites and COD from a Recirculating Aquaculture Saline Water System Using BDD Anodes. Water Res. 2011, 45, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Wijesekara, R.G.S.; Nomura, N.; Matsumura, M. Electrochemical Removal of Ammonia, Chemical Oxygen Demand and Energy Consumption from Aquaculture Waters Containing Different Marine Algal Species. J. Chem. Technol. Biotechnol. 2005, 80, 1408–1415. [Google Scholar] [CrossRef]
- Mook, W.T.; Chakrabarti, M.H.; Aroua, M.K.; Khan, G.M.A.; Ali, B.S.; Islam, M.S.; Abu Hassan, M.A. Removal of Total Ammonia Nitrogen (TAN), Nitrate and Total Organic Carbon (TOC) from Aquaculture Wastewater Using Electrochemical Technology: A Review. Desalination 2012, 285, 1–13. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Advanced Oxidation Process-Mediated Removal of Pharmaceuticals from Water: A Review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef]
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical Advanced Oxidation Processes: A Review on Their Application to Synthetic and Real Wastewaters. Appl. Catal. B 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Candia-Onfray, C.; Espinoza, N.; Sabino da Silva, E.B.; Toledo-Neira, C.; Espinoza, L.C.; Santander, R.; García, V.; Salazar, R. Treatment of Winery Wastewater by Anodic Oxidation Using BDD Electrode. Chemosphere 2018, 206, 709–717. [Google Scholar] [CrossRef]
- Souli, I.; Fernandes, A.; Lopes, A.; Gomes, I.; Afonso, A.; Labiadh, L.; Ammar, S. Treatment of Cheese Whey Wastewater by Electrochemical Oxidation Using BDD, Ti/RuO2-TiO2, and Ti/RuO2-IrO2-Pt Anodes: Ecotoxicological and Energetic Evaluation. Environ. Sci. Pollut. Res. 2025, 32, 7058–7069. [Google Scholar] [CrossRef]
- Quan, F.; Zhan, G.; Zhou, B.; Ling, C.; Wang, X.; Shen, W.; Li, J.; Jia, F.; Zhang, L. Electrochemical Removal of Ammonium Nitrogen in High Efficiency and N2 Selectivity Using Non-Noble Single-Atomic Iron Catalyst. J. Environ. Sci. 2023, 125, 544–552. [Google Scholar] [CrossRef]
- Qing, G.; Anari, Z.; Abolhassani, M.; Foster, S.L.; Matlock, M.; Thoma, G.; Greenlee, L.F. Electrochemical Ammonia Removal and Disinfection of Aquaculture Wastewater Using Batch and Flow Reactors Incorporating PtRu/Graphite Anode and Graphite Cathode. Aquac. Eng. 2021, 93, 102155. [Google Scholar] [CrossRef]
- Romano, A.; Ortiz, I.; Urtiaga, A.M. Comprehensive Kinetics of Electrochemically Assisted Ammonia Removal in Marine Aquaculture Recirculating Systems. J. Electroanal. Chem. 2021, 897, 115619. [Google Scholar] [CrossRef]
- Ruan, Y.; Lu, C.; Guo, X.; Deng, Y.; Zhu, S. Electrochemical Treatment of Recirculating Aquaculture Wastewater Using a Ti/RuO2-IrO2 Anode for Synergetic Total Ammonia Nitrogen and Nitrite Removal and Disinfection. Trans. ASABE 2016, 59, 1831–1840. [Google Scholar] [CrossRef]
- Roy, R.K. A Primer on the Taguchi Method, 2nd ed.; Society of Manufacturing Engineers: Southfield, MI, USA, 2010; ISBN 9780872638648. [Google Scholar]
- NORMA Oficial Mexicana NOM-001-SEMARNAT-2021, Que Establece Los Límites Permisibles de Contaminantes En Las Descargas de Aguas Residuales En Cuerpos Receptores Propiedad de La Nación. 2021. Available online: http://legismex.mty.itesm.mx/normas/ecol/semarnat001-2022_03.pdf (accessed on 13 May 2015).
- Sharma, S.; Simsek, H. Treatment of Canola-Oil Refinery Effluent Using Electrochemical Methods: A Comparison between Combined Electrocoagulation + Electrooxidation and Electrochemical Peroxidation Methods. Chemosphere 2019, 221, 630–639. [Google Scholar] [CrossRef]
- Abbas, R.N.; Abbas, A.S. The Taguchi Approach in Studying and Optimizing the Electro-Fenton Oxidation to Reduce Organic Contaminants in Refinery Wastewater Using Novel Electrodes. Eng. Technol. Appl. Sci. Res. 2022, 12, 8928–8935. [Google Scholar] [CrossRef]
- Luong, X.T.H.; Liang, C. Evaluation of Atrazine Degradation by Iron Persulfate Activation Process in Aqueous Phase Using Taguchi Approach. Environ. Eng. Res. 2023, 29, 230137. [Google Scholar] [CrossRef]
- Asath Murphy, M.S.; Jane, D.J.; Leenus, S.S.; Riju, S.R.; Jegathambal, P.; Kalivel, P. Electrochemical Treatment of Textile Wastewater Using Copper Electrodes. J. Environ. Sci. Health Part A 2023, 58, 971–980. [Google Scholar] [CrossRef]
- Selvinsimpson, S.; Eva Gnana Dhana Rani, S.; Ganesh Kumar, A.; Rajaram, R.; Sharmila Lydia, I.; Chen, Y. Photocatalytic Activity of SnO2/Fe3O4 Nanocomposites and the Toxicity Assessment of Vigna Radiata, Artemia Salina and Danio Rerio in the Photodegraded Solution. Environ. Res. 2021, 195, 110787. [Google Scholar] [CrossRef] [PubMed]
- Baía, A.; Lopes, A.; Nunes, M.J.; Ciríaco, L.; Pacheco, M.J.; Fernandes, A. Removal of Recalcitrant Compounds from Winery Wastewater by Electrochemical Oxidation. Water 2022, 14, 750. [Google Scholar] [CrossRef]
- Flox, C.; Cabot, P.-L.; Centellas, F.; Garrido, J.A.; Rodríguez, R.M.; Arias, C.; Brillas, E. Solar Photoelectro-Fenton Degradation of Cresols Using a Flow Reactor with a Boron-Doped Diamond Anode. Appl. Catal. B 2007, 75, 17–28. [Google Scholar] [CrossRef]
- Donoso, G.; Dominguez, J.R.; González, T.; Correia, S.; Cuerda-Correa, E.M. Electrochemical and Sonochemical Advanced Oxidation Processes Applied to Tartrazine Removal. Influence of Operational Conditions and Aqueous Matrix. Environ. Res. 2021, 202, 111517. [Google Scholar] [CrossRef]
- Panizza, M.; Michaud, P.A.; Cerisola, G.; Comninellis, C. Anodic Oxidation of 2-Naphthol at Boron-Doped Diamond Electrodes. J. Electroanal. Chem. 2001, 507, 206–214. [Google Scholar] [CrossRef]
- Cabeza, A.; Urtiaga, A.M.; Ortiz, I. Electrochemical Treatment of Landfill Leachates Using a Boron-Doped Diamond Anode. Ind. Eng. Chem. Res. 2007, 46, 1439–1446. [Google Scholar] [CrossRef]
- Vogel, F.; Harf, J.; Hug, A.; von Rohr, P.R. The Mean Oxidation Number of Carbon (MOC)—A Useful Concept for Describing Oxidation Processes. Water Res. 2000, 34, 2689–2702. [Google Scholar] [CrossRef]


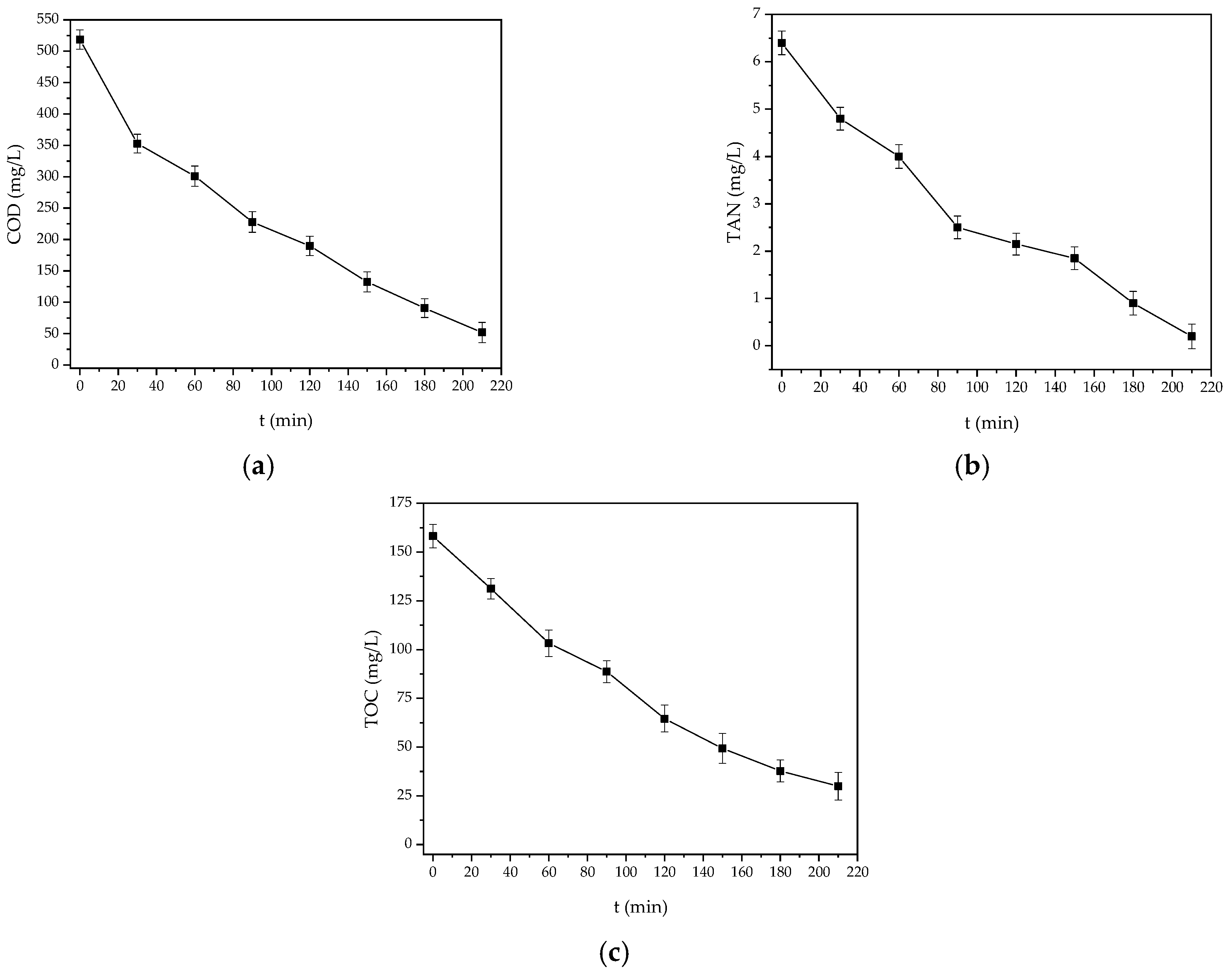
| Parameters | Mean Values |
|---|---|
| Conductivity (mS/cm) | 4.93 |
| TDS (mg/L) | 2416 |
| Turbidity (NTU) | 7.29 |
| COD (mg/L) | 518.52 |
| TAN (mg/L) | 6.4 |
| TOC (mg/L) | 158.10 |
| Factor | Levels (−1, 0, +1) |
|---|---|
| A: pH0 | 5.5, 8.5, 11.5 |
| B: i (A) | 1.0, 1.5, 2 |
| C: t (h) | 2.5, 3.0, 3.5 |
| D: Cathode | BDD, Cu, Ti |
| Factors | Responses | ||||||
|---|---|---|---|---|---|---|---|
| Run | A | B | C | D | Removal (%) | ||
| COD | Turbidity | TAN | |||||
| 1 | 8.5 | 1.5 | 3.5 | BDD | 82.09 | 60.78 | 98.17 |
| 2 | 5.5 | 2.0 | 3.5 | Cu | 82.84 | 53.26 | 95.83 |
| 3 | 8.5 | 2.0 | 2.5 | Ti | 79.74 | 67.12 | 98.04 |
| 4 | 11.5 | 1.0 | 3.5 | Ti | 77.04 | 73.28 | 94.149 |
| 5 | 11.5 | 2.0 | 3.0 | BDD | 90.68 | 67.61 | 99.01 |
| 6 | 5.5 | 1.5 | 3.0 | Ti | 78.36 | 61.89 | 91.08 |
| 7 | 11.5 | 1.5 | 2.5 | Cu | 76.84 | 60.15 | 92.88 |
| 8 | 8.5 | 1.0 | 3.0 | Cu | 62.43 | 75.39 | 94.00 |
| 9 | 5.5 | 1.0 | 2.5 | BDD | 54.91 | 56.11 | 90.01 |
| Source | Sum of Squares | Degree of Freedom | Mean Square |
|---|---|---|---|
| COD Removal | |||
| A-pH0 | 143.10 | 2 | 71.55 |
| B-i | 618.13 | 2 | 309.06 |
| C-t | 159.83 | 2 | 79.92 |
| D-Cath | 28.50 | 2 | 14.25 |
| Core total | 949.56 | 8 | |
| TAN Removal | |||
| A-pH0 | 30.85 | 2 | 15.42 |
| B-i | 38.71 | 2 | 19.36 |
| C-t | 8.75 | 2 | 4.38 |
| D-Cath | 3.95 | 2 | 1.98 |
| Core total | 82.27 | 8 | |
| Turbidity Removal | |||
| A-pH0 | 213.09 | 2 | 106.55 |
| B-i | 87.87 | 2 | 43.94 |
| C-t | 87.43 | 2 | 43.72 |
| D-Cath | 57.44 | 2 | 28.72 |
| Core total | 445.84 | 8 | |
| Response | Objective | Limits | Importance | |
|---|---|---|---|---|
| Low | High | |||
| pH0 | In range | 5.50 | 11.5 | +++ |
| i | In range | 1.00 | 2.00 | +++ |
| t | In range | 2.50 | 3.50 | +++ |
| Cath | In range | BDD | Cu | +++ |
| COD | Maximizer | 54.91 | 90.68 | +++ |
| TAN | Maximizer | 90.00 | 99.01 | +++ |
| Turbidity | Maximizer | 53.26 | 75.39 | +++ |
| Removal Kinetic | Orden | Kapp | R2 |
|---|---|---|---|
| COD | Pseudo-first | 0.0101 L/min | 0.9726 |
| TAN | Pseudo-zero | 0.0276 mg/L min | 0.9586 |
| TOC | Pseudo-first | 0.0081 L/min | 0.9944 |
| Variable | Control | Biofloc Aquaculture Wastewater | |||
|---|---|---|---|---|---|
| Tap Water | Distilled Water | Untreated Effluent Without Na2SO4 | Untreated Effluent with Na2SO4 | Treated Effluent with Na2SO4 | |
| Germination (%) | 93.33 | 93.33 | 90.00 | 76.67 | 86.67 |
| Root size (cm) | 7.93 | 8.51 | 7.88 | 5.22 | 4.38 |
| Stem size (cm) | 23.46 | 23.91 | 20.61 | 1.48 | 1.10 |
| Wastewater Source | Operating Reaction Conditions | Removal Efficiency (%) | SEC (kw h /m3) | OC (USD/L) | Ref. |
|---|---|---|---|---|---|
| Tilapia biofloc aquaculture effluent | Optimized [COD]0 = 518 mg/L [TAN]0 = 6.4 mg/L [TOC]0 = 158.10 mg/L An: 1-Cath: 2 j = 0.125 A/cm2 A = 16 cm2 Average U = 10.74 V t = 3.5 h Vs = 500 mL | COD: 96.57 TAN: 99.06 Turbidity: 67.68 TOC: 81.09 | 150 | 1.0 | This work |
| Synthetic aquaculture wastewater | Non optimized = 20 mg/L An: 3-Cath: 4 j = 7.5 × 10−4 A/cm2 A = 4 cm2 U = 1.89 V t = 2 h Vs = 30 mL | : 99.3 | --- | --- | [10] |
| Aquaculture wastewater | Non optimized = 15 mg/L An: 3-Cath: 4 j = 7.5 × 10−4 A/cm2 A = 4 cm2 U = 1.89 V t = 2 h Vs = 30 mL | : 96.7 | |||
| Synthetic aquaculture wastewater | Non optimized [NH3-N]0 = 50 mg/L An: 5-Cath: 4 j = 0.025 A/cm2 A = 8.06 cm2 U = n.a. t = 2 h Vs = 400 mL | NH3-N: 100.0 | --- | --- | [11] |
| Aquaculture wastewater | Non optimized [NH3-N]0 = 7 mg/L An: 5-Cath: 4 j = 0.025 A/cm2 A = 8.06 cm2 U = n.a. t = 2 h Vs = 400 mL | NH3-N: 100.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peralta-Reyes, E.; Gómez-Gómez, G.; Gallardo-Collí, A.; Meraz, J.F.; Pérez-Rostro, C.I.; Espinoza-Montero, P.J.; Regalado-Méndez, A. Electro-Oxidation of Biofloc Aquaculture Effluent Through a DoE-Driven Optimization in a Batch Reactor. Processes 2025, 13, 3377. https://doi.org/10.3390/pr13113377
Peralta-Reyes E, Gómez-Gómez G, Gallardo-Collí A, Meraz JF, Pérez-Rostro CI, Espinoza-Montero PJ, Regalado-Méndez A. Electro-Oxidation of Biofloc Aquaculture Effluent Through a DoE-Driven Optimization in a Batch Reactor. Processes. 2025; 13(11):3377. https://doi.org/10.3390/pr13113377
Chicago/Turabian StylePeralta-Reyes, Ever, Gina Gómez-Gómez, Alfredo Gallardo-Collí, Juan F. Meraz, Carlos Iván Pérez-Rostro, Patricio J. Espinoza-Montero, and Alejandro Regalado-Méndez. 2025. "Electro-Oxidation of Biofloc Aquaculture Effluent Through a DoE-Driven Optimization in a Batch Reactor" Processes 13, no. 11: 3377. https://doi.org/10.3390/pr13113377
APA StylePeralta-Reyes, E., Gómez-Gómez, G., Gallardo-Collí, A., Meraz, J. F., Pérez-Rostro, C. I., Espinoza-Montero, P. J., & Regalado-Méndez, A. (2025). Electro-Oxidation of Biofloc Aquaculture Effluent Through a DoE-Driven Optimization in a Batch Reactor. Processes, 13(11), 3377. https://doi.org/10.3390/pr13113377









