Hydrothermal Synthesis of Zeolites from Volcanic Ash from Ubinas and Its Application in Catalytic Pyrolysis of Plastic Waste
Abstract
1. Introduction
2. Materials and Methods
2.1. Analysis of Ash and Zeolite Produced
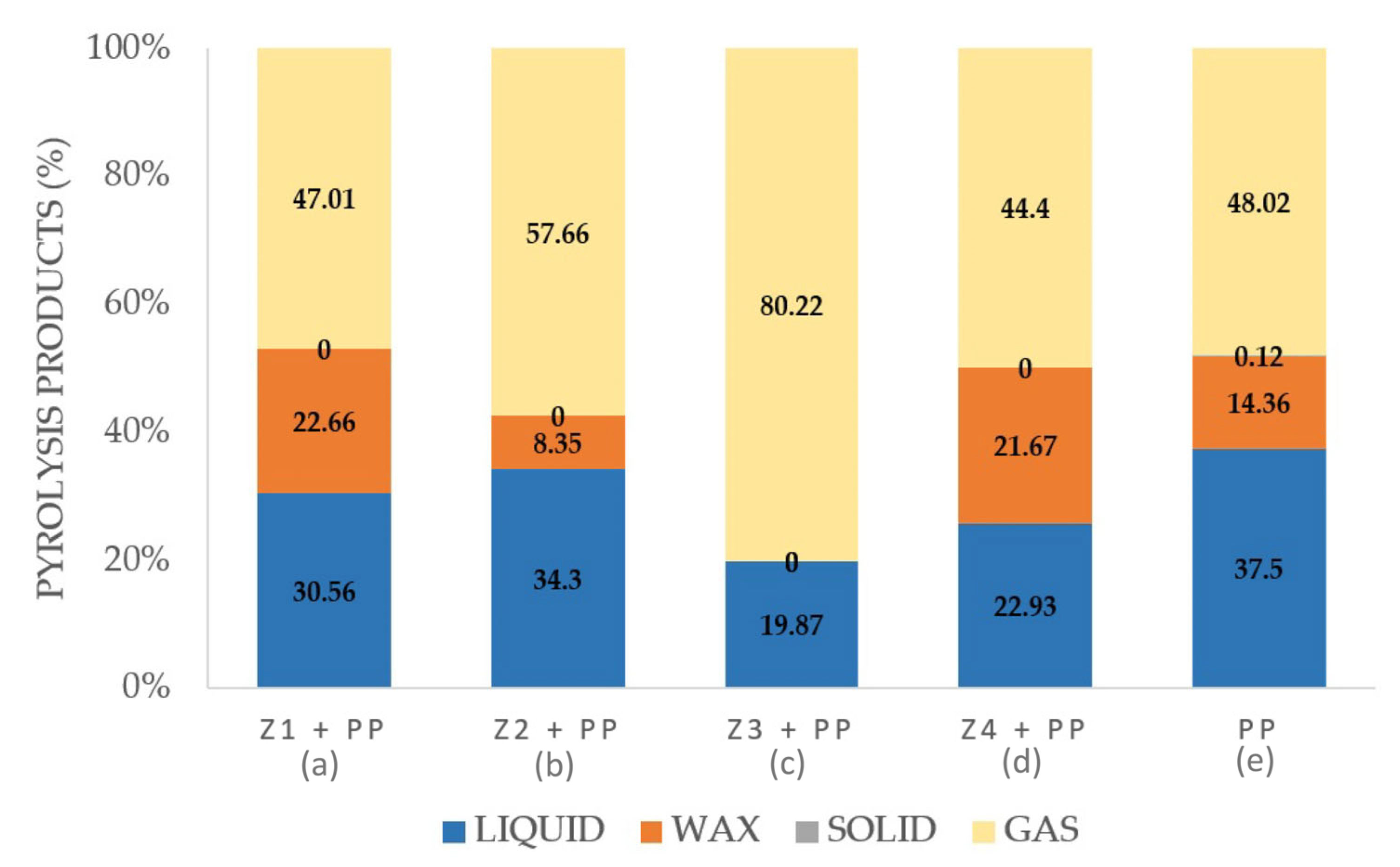
2.2. Catalytic Pyrolysis of Polypropylene Using Zeolites as Catalysts
3. Results and Discussion
3.1. Chemical Composition of Volcanic Ash
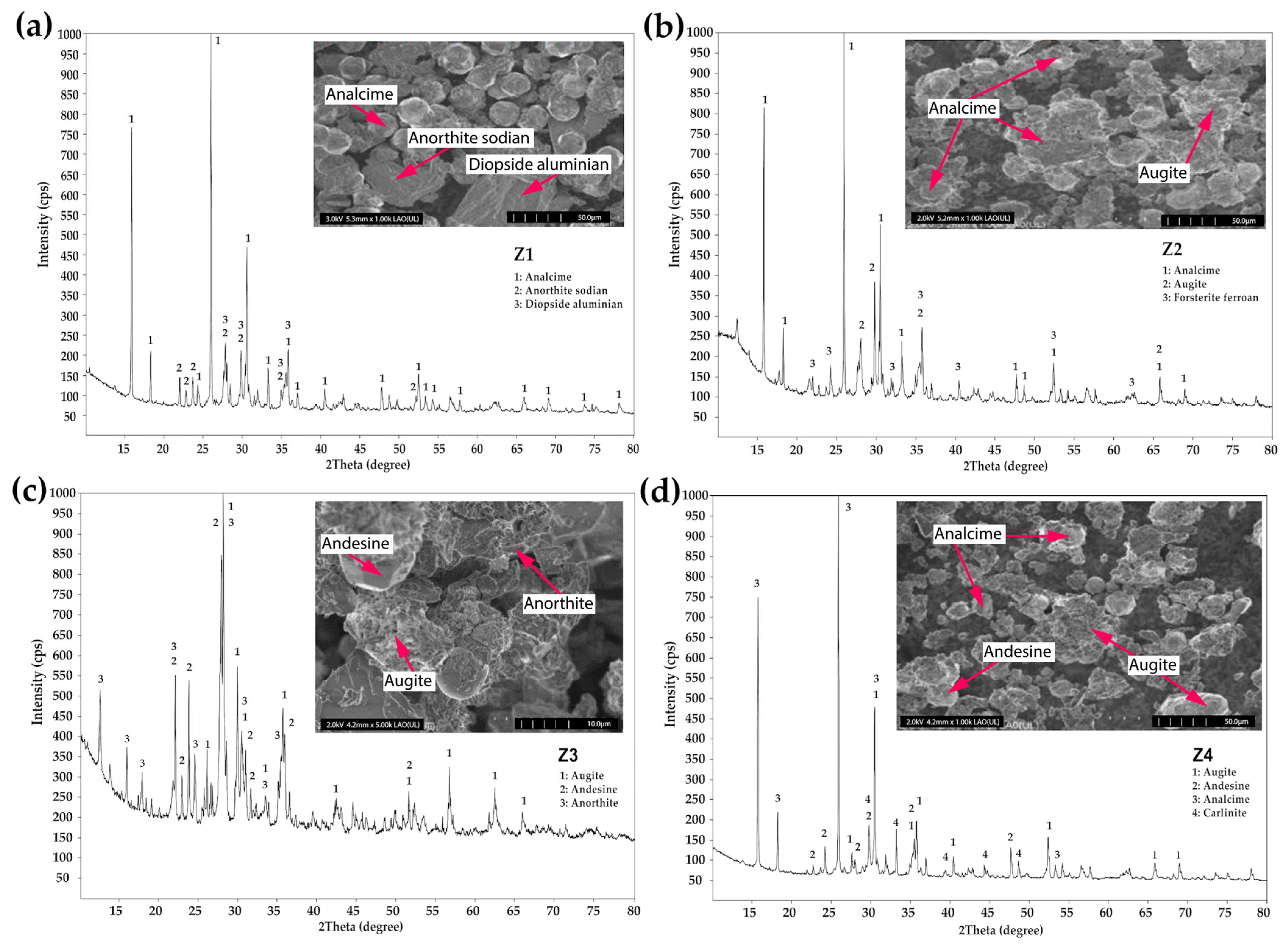
3.2. Morphological and Phase Analysis
3.3. Characterization of Zeolites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BET | Brunauer–Emmett–Teller |
| XRD | X-ray diffraction |
| FTIR | Fourier Transform Infrared Spectroscopy |
| SBET | Specific surface area |
| SEM | Scanning Electron Microscopy |
| TGA | Thermogravimetric Analysis |
| XRF | X-ray Fluorescence Spectrometry |
References
- Tupayachy-Quispe, D.; Apaza, F.; Churata, R.; Almirón, J.; Hermoza-Gutiérrez, M.; Velasco, F.; Torres-Carrasco, M. Chemical and microstructural characterization of ash from the Ubinas and Sabancaya volcanoes in Peru. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2021; Volume 1150, p. 12010. [Google Scholar] [CrossRef]
- Anccasi Figueroa, R.M.; Taipe Maquerhua, E.L.; Mariño Salazar, J.; Apaza Choquehuayta, F.E.; Miranda Cruz, R.; Ramos Palomino, D.A.; Acosta Martinez, J. Caracterización del proceso eruptivo del volcán Ubinas 2013–2017. In Proceedings of the Libro de Resúmenes IX Foro Internacional de Peligros Volcánicos–IX FIPVO, Arequipa, Peru, 2–4 November 2022; Volume C90, pp. 25–29. Available online: https://repositorio.ingemmet.gob.pe/handle/20.500.12544/3780 (accessed on 1 August 2025).
- Lemougna, P.N.; Wang, K.T.; Tang, Q.; Nzeukou, A.N.; Billong, N.; Melo, U.C.; Cui, X.M. Review on the use of volcanic ashes for engineering applications. Resour. Conserv. Recycl. 2018, 137, 177–190. [Google Scholar] [CrossRef]
- Alraddadi, S.; Assaedi, H. Characterization and potential applications of different powder volcanic ash. J. King Saud Univ.-Sci. 2020, 32, 2969–2975. [Google Scholar] [CrossRef]
- Apaza Choquehuayta, F.E.; Churata Añasco, R.D.; Tupayachi, P.; Almiron Baca, J.J.; Pérez, L.; Hermosa, M.; Velazco, F. La Ceniza Volcánica en la Obtención de Geopolimeros Como Alternativa en la Industria de la Construcción. In Proceedings of the XIX Congreso Peruano de Geología, Geología: Ciencia para el Desarrollo Económico Sostenible, Lima, Peru, 23–26 September 2018; Available online: https://hdl.handle.net/20.500.12544/4226 (accessed on 1 August 2025).
- Apaza Choquehuayta, F.E.; Churata Añasco, R.D.; Tupayachi, P.; Almiron Baca, J.J.; Velasco López, F.J. Estudio de la ceniza de volcanes peruanos como materia prima para la industria de la construcción. In Proceedings of the Foro Internacional: Los Volcanes y su Impacto, Arequipa, Peru, 26–27 April 2018. [Google Scholar]
- Gambo, S.; Sanda, U.M.; Ibrahim, A.G.; Usman, J.; Mohammad, U.H. Strength properties of ordinary Portland cement concrete containing high volume recycled coarse aggregate and volcanic ash. Mater. Today Proc. 2023, 86, 140–144. [Google Scholar] [CrossRef]
- Hamada, H.M.; Abed, F.; Beddu, S.; Humada, A.M.; Majdi, A. Effect of Volcanic Ash and Natural Pozzolana on mechanical properties of sustainable cement concrete: A comprehensive review. Case Stud. Constr. Mater. 2023, 19, e02425. [Google Scholar] [CrossRef]
- Vargas, M.; Hermoza-Gutierrez, M.; Tupayachy-Quispe, D.P.; Almirón, J.; Huanca, Z.; Paul, K.; Velasco-López, F.J. Manufacture of geopolymeric mortars from ash coming from the ubinas volcano, assessment of its mechanical, physical and microstructural properties. Rev. Boliv. Quím. 2020, 37, 148–175. [Google Scholar] [CrossRef]
- Gagliano, E.; Sgroi, M.; Falciglia, P.P.; Belviso, C.; Cavalcante, F.; Lettino, A.; Roccaro, P. Removal of ammonium from wastewater by zeolite synthetized from volcanic ash: Batch and column tests. J. Environ. Chem. Eng. 2022, 10, 107539. [Google Scholar] [CrossRef]
- Belviso, C.; Abdolrahimi, M.; Peddis, D.; Gagliano, E.; Sgroi, M.; Lettino, A.; Cavalcante, F. Synthesis of zeolite from volcanic ash: Characterization and application for cesium removal. Microporous Mesoporous Mater. 2021, 319, 111045. [Google Scholar] [CrossRef]
- Valdivia, M.R. Síntesis hidrotermal de zeolitas a partir de ceniza volcánica mediante tratamiento alcalino y su potencial aplicación en la remoción de NH4+, Pb2+, Zn2+ y Mn2+. Matéria 2022, 27, e13132. [Google Scholar] [CrossRef]
- El Bojaddayni, I.; Küçük, M.E.; El Ouardi, Y.; Jilal, I.; El Barkany, S.; Moradi, K.; Repo, E.; Laatikainen, K.; Ouammou, A. A review on synthesis of zeolites from natural clay resources and waste ash: Recent approaches and progress. Miner. Eng. 2023, 198, 108086. [Google Scholar] [CrossRef]
- Szerement, J.; Szatanik-Kloc, A.; Jarosz, R.; Bajda, T.; Mierzwa-Hersztek, M. Contemporary applications of natural and synthetic zeolites from fly ash in agriculture and environmental protection. J. Clean. Prod. 2021, 311, 127461. [Google Scholar] [CrossRef]
- Luévano-Hipólito, E.; Torres-Martínez, L.M.; Fernández-Trujillo, A. Ternary ZnO/CuO/Zeolite composite obtained from volcanic ash for photocatalytic CO2 reduction and H2O decomposition. J. Phys. Chem. Solids 2021, 151, 109917. [Google Scholar] [CrossRef]
- Salahudeen, N. A review on zeolite: Application, synthesis and effect of synthesis parameters on product properties. Chem. Afr. 2022, 5, 1889–1906. [Google Scholar] [CrossRef]
- Terzano, R.; D’Alessandro, C.; Spagnuolo, M.; Romagnoli, M.; Medici, L. Facile zeolite synthesis from municipal glass and aluminum solid wastes. CLEAN—Soil Air Water 2015, 43, 133–140. [Google Scholar] [CrossRef]
- Espejel-Ayala, F.; Ramírez, Z.R.M. Production process of zeolite X using alum sludge issued from drinking water treatment plants. MRS Online Proc. Libr. 2011, 1380, 1–6. [Google Scholar] [CrossRef]
- Espejel-Ayala, F.; Schouwenaars, R.; Durán-Moreno, A.; Ramírez-Zamora, R.M. Use of drinking water sludge in the production process of zeolites. Res. Chem. Intermed. 2014, 40, 2919–2928. [Google Scholar] [CrossRef]
- Behin, J.; Bukhari, S.S.; Dehnavi, V.; Kazemian, H.; Rohani, S. Using coal fly ash and wastewater for microwave synthesis of LTA zeolite. Chem. Eng. Technol. 2014, 37, 1532–1540. [Google Scholar] [CrossRef]
- Kokloku, B.K.; Awudza, J.A.; Mensah, M.B.; Rockson, M.A.; Kemausuor, F. Characterization of Ghanaian Clays as Raw Materials for the Synthesis of ZSM-5 Zeolite. Sci. Afr. 2025, 27, e02570. [Google Scholar] [CrossRef]
- Martínez-del-Pozo, I.; Esbrí, J.M.; García-Lorenzo, L.; López-Andrés, S. Synthesis of zeolites from volcanic ash (Tajogaite, Spain) for the remediation of waters contaminated by fluoride. Environ. Sci. Pollut. Res. 2024, 31, 7058–7072. [Google Scholar] [CrossRef] [PubMed]
- Collins, F.; Rozhkovskaya, A.; Outram, J.G.; Millar, G.J. A critical review of waste resources, synthesis, and applications for Zeolite LTA. Microporous Mesoporous Mater. 2020, 291, 109667. [Google Scholar] [CrossRef]
- Zhou, Q.; Jiang, X.; Qiu, Q.; Zhao, Y.; Long, L. Synthesis of High-Quality NaP1 Zeolite from Municipal Solid Waste Incineration Fly Ash by Microwave-Assisted Hydrothermal Method and Its Adsorption Capacity. Sci. Total Environ. 2023, 855, 158741. [Google Scholar] [CrossRef]
- Galindo Valbuena, H.M.; Medina, A.F.; Vargas, J.C.; Hernández Fandiño, O. Synthesis of Zeolites Na-A, Na-X, and Analcime from Crushed Stone Waste and Their Applications in Heavy Metal Removal in Aqueous Media. Chem. Eng. Res. Des. 2023, 197, 159–172. [Google Scholar] [CrossRef]
- Serna-Galvis, E.A.; Martínez-Mena, Y.L.; Arboleda-Echavarría, J.; Hoyos-Ayala, D.A.; Echavarría-Isaza, A.; Torres-Palma, R.A. Zeolite 4A Activates Peroxymonosulfate toward the Production of Singlet Oxygen for the Selective Degradation of Organic Pollutants. Chem. Eng. Res. Des. 2023, 193, 121–131. [Google Scholar] [CrossRef]
- Boer, D.G.; Langerak, J.; Pescarmona, P.P. Zeolites as Selective Adsorbents for CO2 Separation. ACS Appl. Energy. Mater. 2023, 6, 2634–2656. [Google Scholar] [CrossRef]
- Yuan, H.; Li, C.; Shan, R.; Zhang, J.; Wu, Y.; Chen, Y. Recent developments on the zeolites catalyzed polyolefin plastics pyrolysis. Fuel Process. Technol. 2022, 238, 107531. [Google Scholar] [CrossRef]
- Yang, G.; Peng, P.; Guo, H.; Song, H.; Li, Z. The catalytic pyrolysis of waste polyolefins by zeolite-based catalysts: A critical review on the structure-acidity synergies of catalysts. Polym. Degrad. Stab. 2024, 222, 110712. [Google Scholar] [CrossRef]
- Valencia-Huaman, A.G.; Fuentes-Mamani, S.H.; Mamani-De La Cruz, L.F.; Velasco, F.; Churata, R.; Silva-Vela, A.; Mamani-Quispe, J.; Almirón, J. Obtaining Zeolites from Natural Materials of Volcanic Origin for Application in Catalytic Pyrolysis for the Sustainable Chemical Recycling of Polymers. Sustainability 2024, 16, 5910. [Google Scholar] [CrossRef]
- Belrhazi, I.; Sair, S.; Ousaleh, H.A.; Abdellaoui, Y.; Zahouily, M. Catalytic transformation of plastic waste: Harnessing zeolite for enhanced energy product yield in pyrolysis. Energy Convers. Manag. 2024, 318, 118897. [Google Scholar] [CrossRef]
- Zeleke, M.A.; Belay, N.C.; Štangar, U.L.; Kwapinski, W.; Mequanint, K. Pyrolysis Liquid Fuel Production from Waste Plastics using Spherical-shape Zeolite Catalyst. J. Environ. Chem. Eng. 2025, 13, 115543. [Google Scholar] [CrossRef]
- Dong, Z.; Chen, W.; Xu, K.; Liu, Y.; Wu, J.; Zhang, F. Understanding the structure–activity relationships in catalytic conversion of polyolefin plastics by zeolite-based catalysts: A critical review. ACS Catal. 2022, 12, 14882–14901. [Google Scholar] [CrossRef]
- Han, S.; Yan, X.; Han, H.; Lin, W.; He, H.; Xie, J.; Mondal, A.K.; Zou, Q.; Huang, F. Study the implication of different SiO2/Al2O3 ratios on the pore size and acidity of Beta zeolite and its catalytic pyrolysis mechanism of Kraft lignin. J. Anal. Appl. Pyrolysis 2024, 183, 106730. [Google Scholar] [CrossRef]
- Otieno, S.O.; Kengara, F.O.; Kowenje, C.O.; Mokaya, R. Hydrothermal synthesis of zeolites using silica extracted from tropical volcanic ash. Mater. Adv. 2023, 4, 2292–2300. [Google Scholar] [CrossRef]
- Almirón, J.; Vargas, M.; Tupayachy-Quispe, D.; Duquesne, S.; Roudet, F.; Silva-Vela, A. Influence of the process of synthesis of zeolites from volcanic ash in its synergistic action as a flame-retardant for polypropylene composites. Buildings 2021, 12, 24. [Google Scholar] [CrossRef]
- Choi, J.; Han, Y.; Kim, D.; Park, S.; Park, J.; Park, J.; Kim, H. Synthesis and characterization of mesoporous silica from anorthite-clay mineral: Role of mechanical activation. Mater. Trans. 2014, 55, 1895–1899. [Google Scholar] [CrossRef]
- Gajek, M.; Rapacz-Kmita, A.; Stodolak-Zych, E.; Zarzecka-Napierała, M.; Wilk, M.; Magdziarz, A.; Dudek, M. Microstructure and mechanical properties of diopside and anorthite glazes with high abrasion resistance. Ceram. Int. 2022, 48, 6792–6798. [Google Scholar] [CrossRef]
- Byrappa, K.; Kumar, B.S. Characterization of zeolites by infrared spectroscopy. Asian J. Chem. 2007, 19, 4933. [Google Scholar]
- Ma, Y.K.; Rigolet, S.; Michelin, L.; Paillaud, J.L.; Mintova, S.; Khoerunnisa, F.; Daou, T.J.; Ng, E.P. Facile and fast determination of Si/Al ratio of zeolites using FTIR spectroscopy technique. Microporous Mesoporous Mater. 2021, 311, 110683. [Google Scholar] [CrossRef]
- Sandoval-Díaz, L.E.; González-Amaya, J.A.; Trujillo, C.A. General aspects of zeolite acidity characterization. Microporous Mesoporous Mater. 2015, 215, 229–243. [Google Scholar] [CrossRef]
- Iqbal, A.; Sattar, H.; Haider, R.; Munir, S. Synthesis and characterization of pure phase zeolite 4A from coal fly ash. J. Clean. Prod. 2019, 219, 258–267. [Google Scholar] [CrossRef]
- Breck, D.W. Zeolite Molecular Sieves: Structure, Chemistry, and Use; John Wiley and Sons: Hoboken, NJ, USA, 1974. [Google Scholar]
- Musyoka, N.M.; Petrik, L.F.; Hums, E.; Kuhnt, A.; Schwieger, W. Thermal stability studies of zeolites A and X synthesized from South African coal fly ash. Res. Chem. Intermed. 2015, 41, 575–582. [Google Scholar] [CrossRef]
- Colella, C. Use of thermal analysis in zeolite research and application. In Characterization Techniques of Glasses and Ceramics; Springer: Berlin/Heidelberg, Germany, 1999; pp. 112–137. [Google Scholar] [CrossRef]
- Ríos, C.A.; Williams, C.D.; Roberts, C.L. A comparative study of the synthesis of zeolites from volcanic ash and kaolinite. Appl. Clay Sci. 2012, 52, 488–495. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Poulopoulos, S.G. Adsorption, Ion Exchange and Catalysis: Design of Operations and Environmental Applications. J. Hazard. Mater. 2006, B135, 201–209. [Google Scholar] [CrossRef]
- Musyoka, N.M.; Petrik, L.F.; Gitari, W.M.; Hums, E. Hydrothermal conversion of fly ash into zeolites: A review. Microporous Mesoporous Mater. 2015, 214, 1–26. [Google Scholar] [CrossRef]
- Guerra-González, Y.; Almenares-Reyes, R.S.; Tito-Robles, W.; Aldino-Utria, A.; Danguillecourt-Álvarez, E. Influencia de la temperatura en la descomposición térmica de las tobas zeolitizadas del yacimiento caimanes. Rev. Cuba. Quím. 2021, 33, 117–137. [Google Scholar]
- Pál-Borbély, G. Thermal analysis of zeolites. In Molecular Sieves: Characterization II; Springer: Berlin/Heidelberg, Germany, 2005; pp. 67–101. [Google Scholar] [CrossRef]
- Bastardo-González, E.; Bellorín, M.C. Síntesis y caracterización de zeolitas ZSM-5 preparadas a partir de una arcilla caolinita activada con ácido. Av. Quím. 2018, 13, 15–20. [Google Scholar]
- Franus, W.; Wdowin, M.; Franus, M. Synthesis and characterization of zeolites prepared from industrial fly ash. Environ. Monit. Assess. 2014, 186, 5721–5729. [Google Scholar] [CrossRef]
- Rimoli, M.G.; Rabaioli, M.R.; Melisi, D.; Curcio, A.; Mondello, S.; Mirabelli, R.; Abignente, E. Synthetic zeolites as a new tool for drug delivery. J. Biomed. Mater. Res. A 2008, 87, 156–164. [Google Scholar] [CrossRef]
- Khaleque, A.; Alam, M.M.; Hoque, M.; Mondal, S.; Haider, J.B.; Xu, B.; Johir, M.; Karmakar, A.K.; Zhou, J.; Ahmed, M.B.; et al. Zeolite synthesis from low-cost materials and environmental applications: A review. Environ. Adv. 2020, 2, 100019. [Google Scholar] [CrossRef]
- Lovera, R. Caracterización textural de adsorbentes. Rev. Chil. Ing Concep. 2003, 6, 24–28. [Google Scholar]
- García Franco, R.; Hernández, M.Á.; Portillo Reyes, R.; Petranovskii, V.; Rubio, E.; Quiroz Estrada, K.F. Adsorción de CO2, H2 y CH4 en zeolitas naturales de poro angosto. Rev. Int. Contam. Ambient. 2018, 34, 685–696. [Google Scholar] [CrossRef]
- Curi, A.; Granda, W.J.; Lima, H.M.; Sousa, W.T. Las zeolitas y su aplicación en la descontaminación de efluentes mineros. Inf. Tecnol. 2006, 17, 111–118. [Google Scholar] [CrossRef]
- Novembre, D.; Gimeno, D. Synthesis and characterization of analcime (ANA) zeolite using a kaolinitic rock. Sci. Rep. 2021, 11, 13373. [Google Scholar] [CrossRef]
- Elias, S.; Alderton, D. Encyclopedia of Geology; Academic Press: Cambridge, MS, USA, 2020. [Google Scholar]
- Tsuchiyama, A.; Takahashi, E. Melting kinetics of a plagioclase feldspar. Contrib. Mineral. Petrol. 1983, 84, 345–354. [Google Scholar] [CrossRef]
- Zhou, C.; Alshameri, A.; Yan, C.; Qiu, X.; Wang, H.; Ma, Y. Characteristics and evaluation of synthetic 13X zeolite from Yunnan’s natural halloysite. J. Porous Mater. 2013, 20, 587–594. [Google Scholar] [CrossRef]
- Otieno, S.; Kowenje, C.; Kengara, F.; Mokaya, R. Effect of kaolin pre-treatment method and NaOH levels on the structure and properties of kaolin-derived faujasite zeolites. Mater. Adv. 2021, 2, 5997–6010. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, T.; Lv, Y.; Jing, T.; Gao, X.; Gu, Z.; Li, S.; Ao, H.; Fang, D. Recent progress on the synthesis and applications of zeolites from industrial solid wastes. Catalysts 2024, 14, 734. [Google Scholar] [CrossRef]
- Liu, X.D.; Wang, Y.P.; Cui, X.M.; He, Y.; Mao, J. Influence of synthesis parameters on NaA zeolite crystals. Powder Technol. 2013, 243, 184–193. [Google Scholar] [CrossRef]
- Jung, S.H.; Cho, M.H.; Kang, B.S.; Kim, J.S. Pyrolysis of a fraction of waste polypropylene and polyethylene for the recovery of BTX aromatics using a fluidized bed reactor. Fuel Process. Technol. 2010, 91, 277–284. [Google Scholar] [CrossRef]
- Demirbas, A. Pyrolysis of municipal plastic wastes for recovery of gasoline-range hydrocarbons. J. Anal. Appl. Pyrolysis 2004, 72, 97–102. [Google Scholar] [CrossRef]
- Lopez-Urionabarrenechea, A.; De Marco, I.; Caballero, B.M.; Laresgoiti, M.F.; Adrados, A. Catalytic stepwise pyrolysis of packaging plastic waste. J. Anal. Appl. Pyrolysis 2012, 96, 54–62. [Google Scholar] [CrossRef]
- Syamsiro, M.; Cheng, S.; Hu, W.; Saptoadi, H.; Pratama, N.N.; Trisunaryanti, W.; Yoshikawa, K. Liquid and Gaseous Fuels from Waste Plastics by Sequential Pyrolysis and Catalytic Reforming Processes over Indonesian Natural Zeolite Catalysts. Waste Technol. 2014, 2, 44–51. [Google Scholar] [CrossRef]
- Abbas-Abadi, M.S.; Haghighi, M.N.; Yeganeh, H.; McDonald, A.G. Evaluation of pyrolysis process parameters on polypropylene degradation products. J. Anal. Appl. Pyrolysis 2014, 109, 272–277. [Google Scholar] [CrossRef]
- Aisien, E.T.; Otuya, I.C.; Aisien, F.A. Thermal and catalytic pyrolysis of waste polypropylene plastic using spent FCC catalyst. Environ. Technol. Innov. 2021, 22, 101455. [Google Scholar] [CrossRef]
- Inayat, A.; Inayat, A.; Schwieger, W.; Sokolova, B.; Lestinsky, P. Enhancing aromatics and olefins yields in thermo-catalytic pyrolysis of LDPE over zeolites: Role of staged catalysis and acid site density of HZSM-5. Fuel 2022, 314, 123071. [Google Scholar] [CrossRef]
- Kamble, V.M.; Samarth, N.B.; Mahanwar, P.A. Thermo-catalytic pyrolysis of waste high-density polyethylene: Effect of γ-ray irradiation on degradation. Energy Sources Part A Recovery Util. Environ. Eff. 2017, 39, 1102–1108. [Google Scholar] [CrossRef]
- Cheng, Y.T.; Wang, Z.; Gilbert, C.J.; Fan, W.; Huber, G.W. Production of p--xylene from biomass by catalytic fast pyrolysis using ZSM--5 catalysts with reduced pore openings. Angew. Chem.-Int. Ed. 2012, 51, 11097. [Google Scholar] [CrossRef]
- Santella, C.; Cafiero, L.; De Angelis, D.; La Marca, F.; Tuffi, R.; Ciprioti, S.V. Thermal and catalytic pyrolysis of a mixture of plastics from small waste electrical and electronic equipment (WEEE). Waste Manag. 2016, 54, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Miandad, R.; Barakat, M.A.; Aburiazaiza, A.S.; Rehan, M.; Nizami, A.S. Catalytic pyrolysis of plastic waste: A review. Process Saf. Environ. Prot. 2016, 102, 822–838. [Google Scholar] [CrossRef]
- Smit, B.; Maesen, T.L. Towards a molecular understanding of shape selectivity. Nature 2008, 451, 671–678. [Google Scholar] [CrossRef]
- Fermanelli, C.S.; Pierella, L.B.; Saux, C. Comparative study of zeolites matrices in bio-wastes pyrolytic valorization. Process Saf. Environ. Prot. 2021, 147, 808–817. [Google Scholar] [CrossRef]
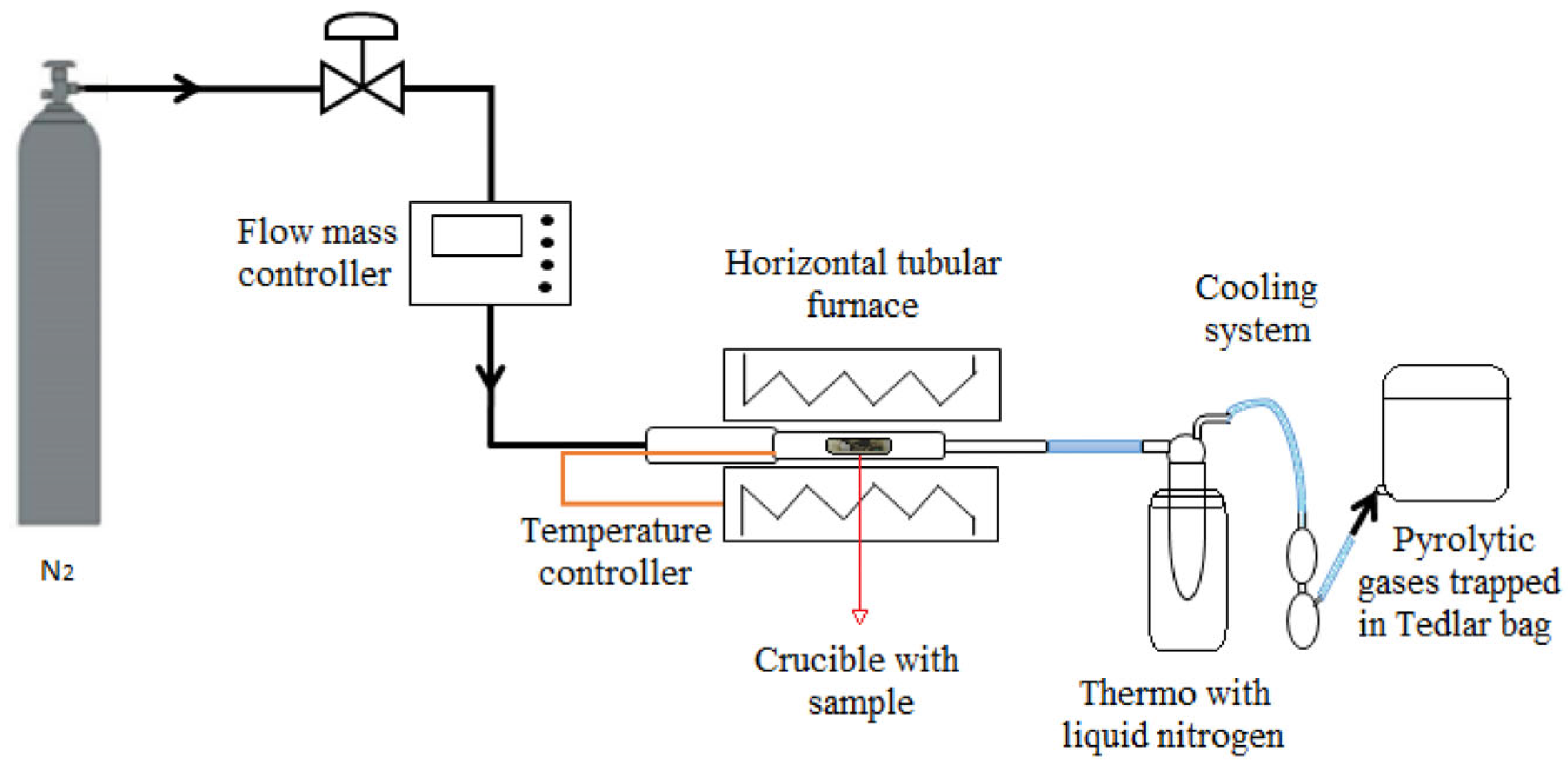
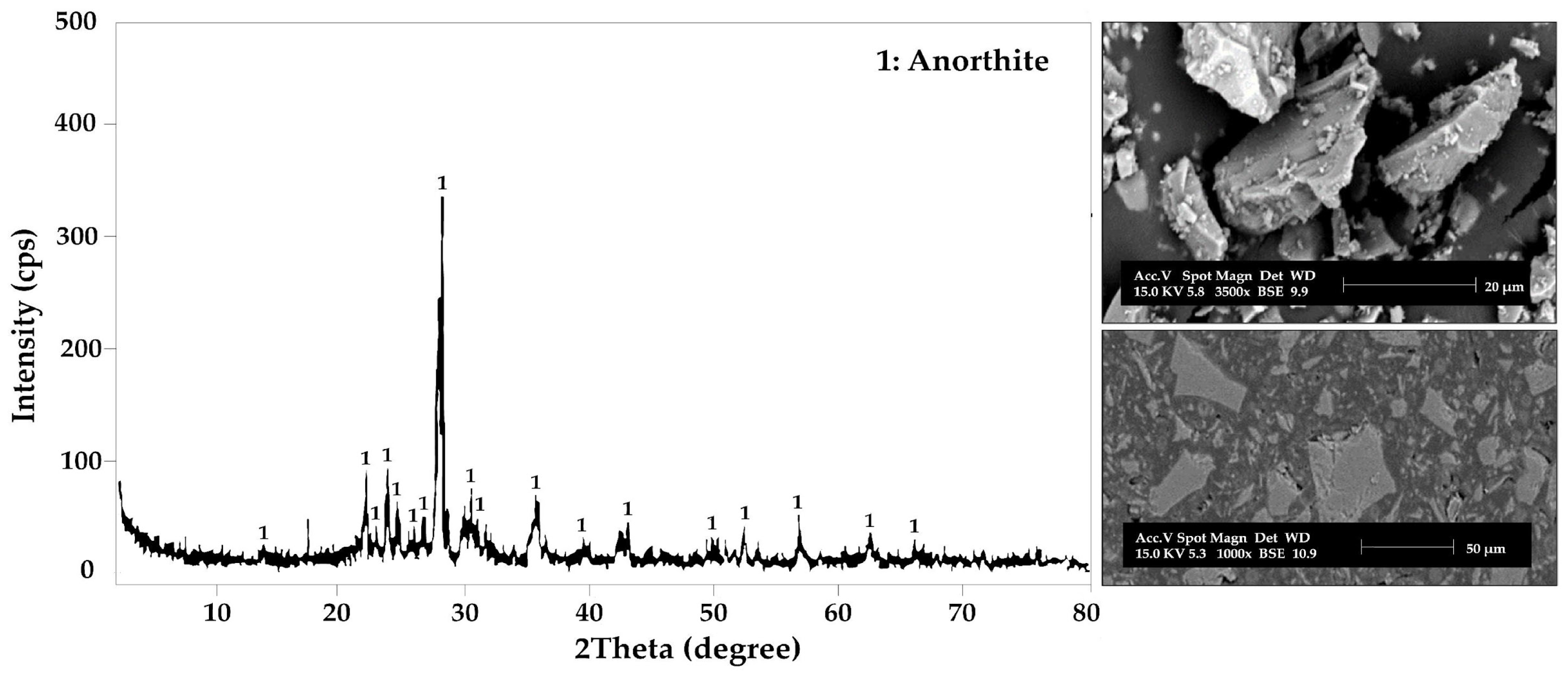

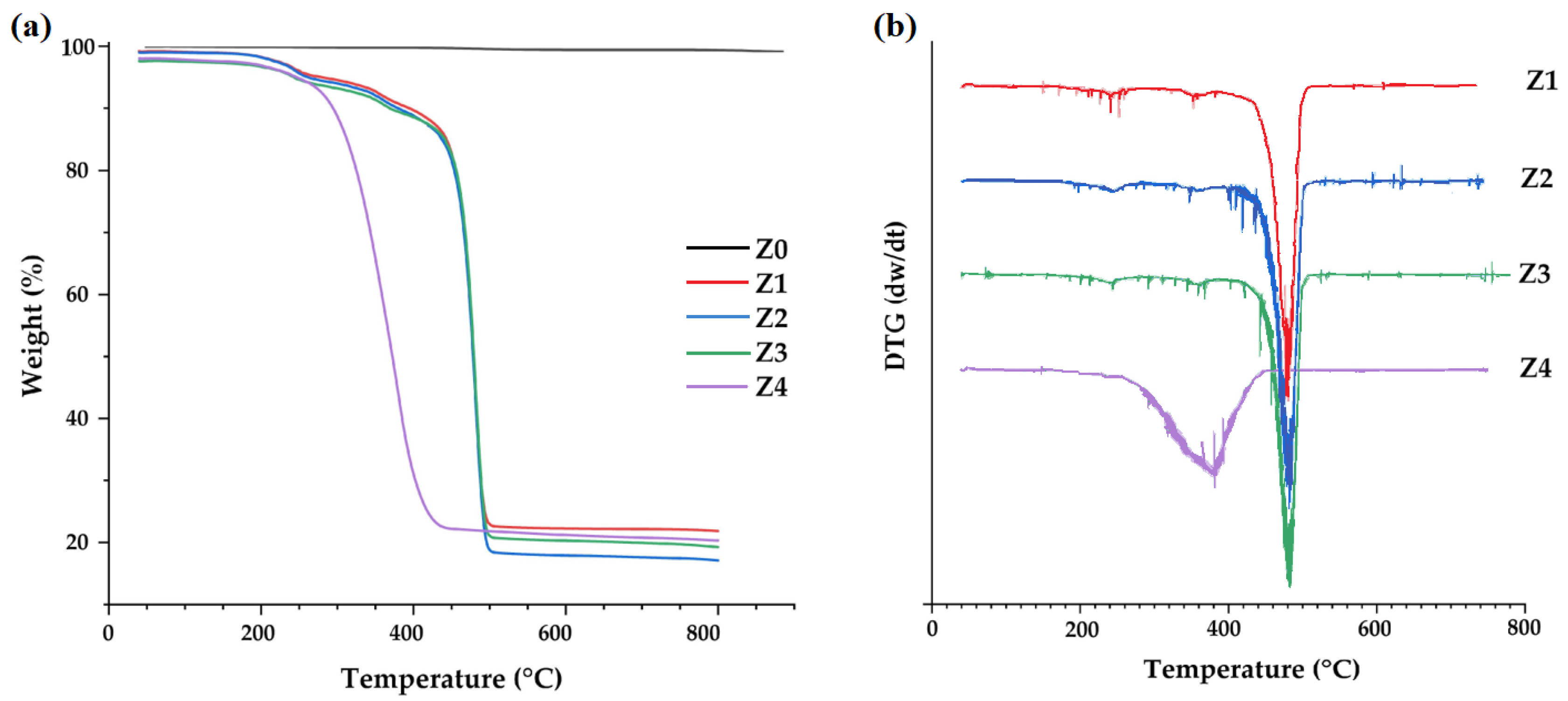
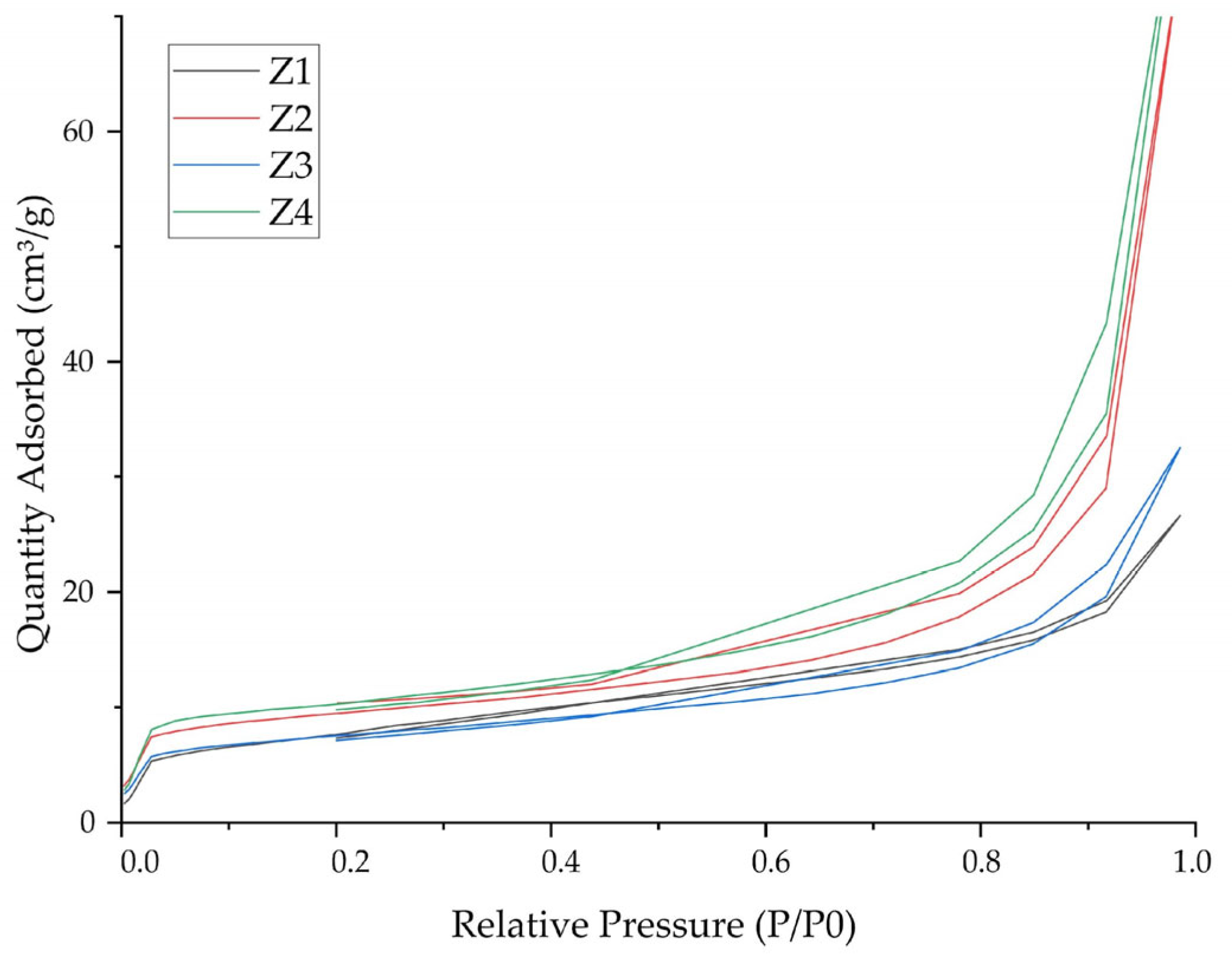
| N° | Code | T (°C) | [NaOH] (M) | t (h) |
|---|---|---|---|---|
| 0 | Z0 (volcanic ash) | - | - | - |
| 1 | Z1 | 120 | 1.5 | 12 |
| 2 | Z2 | 120 | 3 | 12 |
| 3 | Z3 | 150 | 1.5 | 12 |
| 4 | Z4 | 150 | 3 | 12 |
| Oxides | Al2O3 | CaO | Fe2O3 | K2O | MgO | MnO | Na2O | P2O5 | SiO2 | TiO2 | LOI* |
|---|---|---|---|---|---|---|---|---|---|---|---|
| % | 14.58 | 5.17 | 6.23 | 2.15 | 3.15 | 0.08 | 2.94 | 0.30 | 61.69 | 1.11 | 2.34 |
| N° | Code | SBET (m2/g) | Pore Volume (cm3/g) |
|---|---|---|---|
| 0 | Z0 | 2.06 | 0.008 |
| 1 | Z1 | 27.85 | 0.04 |
| 2 | Z2 | 32.44 | 0.12 |
| 3 | Z3 | 25.91 | 0.05 |
| 4 | Z4 | 35.60 | 0.13 |
| Type of Zeolite | Percentage in Weight (wt%) | ||||||
|---|---|---|---|---|---|---|---|
| Analcime | Anorthite | Diopside | Augite | Forsterite | Andesine | Carlinite | |
| Z1 | 67 | 17 | 16 | - | - | - | - |
| Z2 | 82 | - | - | 14 | 4 | - | - |
| Z3 | - | 38 | - | 23 | - | 39 | - |
| Z4 | 66 | - | - | 15 | - | 13 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almirón, J.; Churata, R.; Vargas, M.; Roudet, F.; Valverde-Ponce, K.; Gordillo-Andia, C.; Tupayachy-Quispe, D. Hydrothermal Synthesis of Zeolites from Volcanic Ash from Ubinas and Its Application in Catalytic Pyrolysis of Plastic Waste. Processes 2025, 13, 3376. https://doi.org/10.3390/pr13113376
Almirón J, Churata R, Vargas M, Roudet F, Valverde-Ponce K, Gordillo-Andia C, Tupayachy-Quispe D. Hydrothermal Synthesis of Zeolites from Volcanic Ash from Ubinas and Its Application in Catalytic Pyrolysis of Plastic Waste. Processes. 2025; 13(11):3376. https://doi.org/10.3390/pr13113376
Chicago/Turabian StyleAlmirón, Jonathan, Rossibel Churata, María Vargas, Francine Roudet, Katia Valverde-Ponce, Carlos Gordillo-Andia, and Danny Tupayachy-Quispe. 2025. "Hydrothermal Synthesis of Zeolites from Volcanic Ash from Ubinas and Its Application in Catalytic Pyrolysis of Plastic Waste" Processes 13, no. 11: 3376. https://doi.org/10.3390/pr13113376
APA StyleAlmirón, J., Churata, R., Vargas, M., Roudet, F., Valverde-Ponce, K., Gordillo-Andia, C., & Tupayachy-Quispe, D. (2025). Hydrothermal Synthesis of Zeolites from Volcanic Ash from Ubinas and Its Application in Catalytic Pyrolysis of Plastic Waste. Processes, 13(11), 3376. https://doi.org/10.3390/pr13113376







